- 1Department of Biochemistry, Center of Excellence in Molecular Biology and Regenerative Medicine (CEMR), JSS Academy of Higher Education & Research (JSS AHER), Mysore, India
- 2AU College of Pharmaceutical Sciences, Andhra University, Visakhapatnam, India
- 3Special Interest Group in Cancer Biology and Cancer Stem Cells (SIG-CBCSC), JSS Medical College, JSS Academy of Higher Education & Research (JSS AHER), Mysore, India
- 4I. M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University), Moscow, Russia
- 5Department of Normal and Topographic Anatomy, M.V. Lomonosov Moscow State University, Moscow, Russia
- 6Research Institute of Human Morphology, Moscow, Russia
- 7Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
- 8Institute of Physiologically Active Compounds, Russian Academy of Sciences, Moscow, Russia
- 9GALLY International Research Institute, San Antonio, TX, United States
Severe Acute Respiratory Syndrome-Corona Virus-2 (SARS-CoV-2) induced Coronavirus Disease - 19 (COVID-19) cases have been increasing at an alarming rate (7.4 million positive cases as on June 11 2020), causing high mortality (4,17,956 deaths as on June 11 2020) and economic loss (a 3.2% shrink in global economy in 2020) across 212 countries globally. The clinical manifestations of this disease are pneumonia, lung injury, inflammation, and severe acute respiratory syndrome (SARS). Currently, there is no vaccine or effective pharmacological agents available for the prevention/treatment of SARS-CoV2 infections. Moreover, development of a suitable vaccine is a challenging task due to antibody-dependent enhancement (ADE) and Th-2 immunopathology, which aggravates infection with SARS-CoV-2. Furthermore, the emerging SARS-CoV-2 strain exhibits several distinct genomic and structural patterns compared to other coronavirus strains, making the development of a suitable vaccine even more difficult. Therefore, the identification of novel small molecule inhibitors (NSMIs) that can interfere with viral entry or viral propagation is of special interest and is vital in managing already infected cases. SARS-CoV-2 infection is mediated by the binding of viral Spike proteins (S-protein) to human cells through a 2-step process, which involves Angiotensin Converting Enzyme-2 (ACE2) and Transmembrane Serine Protease (TMPRSS)-2. Therefore, the development of novel inhibitors of ACE2/TMPRSS2 is likely to be beneficial in combating SARS-CoV-2 infections. However, the usage of ACE-2 inhibitors to block the SARS-CoV-2 viral entry requires additional studies as there are conflicting findings and severe health complications reported for these inhibitors in patients. Hence, the current interest is shifted toward the development of NSMIs, which includes natural antiviral phytochemicals and Nrf-2 activators to manage a SARS-CoV-2 infection. It is imperative to investigate the efficacy of existing antiviral phytochemicals and Nrf-2 activators to mitigate the SARS-CoV-2-mediated oxidative stress. Therefore, in this review, we have reviewed structural features of SARS-CoV-2 with special emphasis on key molecular targets and their known modulators that can be considered for the development of NSMIs.
Introduction
Global Burden of COVID-19
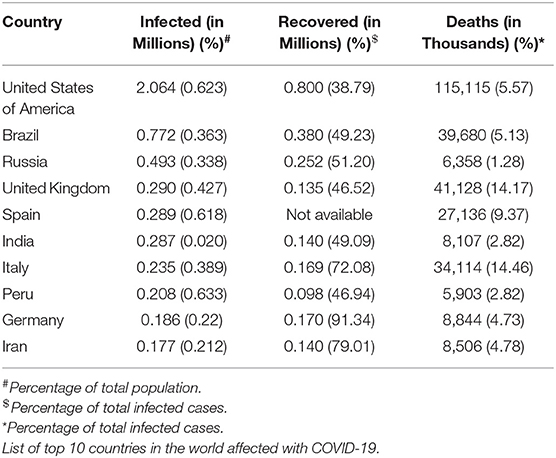
COVID-19 is a devastating disease caused by a coronavirus related to the one that caused outbreaks of Severe Acute Respiratory Syndrome (SARS) in the year 2002 (1, 2). Middle East Respiratory Syndrome (MERS)-related coronavirus is an infamous member of this cohort. COVID-19, which is caused by the SARS-CoV-2 infection, was detected in Wuhan, China in December 2019. The World Health Organization (WHO) declared this infection a pandemic on March 11 2020 due to its severity and rapid spread across the globe. As of June 11 2020, SARS-CoV-2 had infected 7.4 million individuals, and caused 4,17,956 deaths across 212 countries worldwide (Table 1).
Structural Features of SARS-CoV-2
Coronaviruses (CoV) belongs to a family of single-stranded RNA viruses (+RNA) that can infect a variety of mammals such as bats and humans (3). SARS-CoV-2 contains RNA of 29,891-nucleotide length, which codes for 9,860 amino acids (4). The RNA has a 5' cap and 3' poly-A tail and produces a poly-protein 1a/1ab (pp1a/pp1ab) in the host (4). SARS-CoV-2 belongs to beta CoV category and appears in a crown shape with a size of ~60–140 nm (Figure 1).
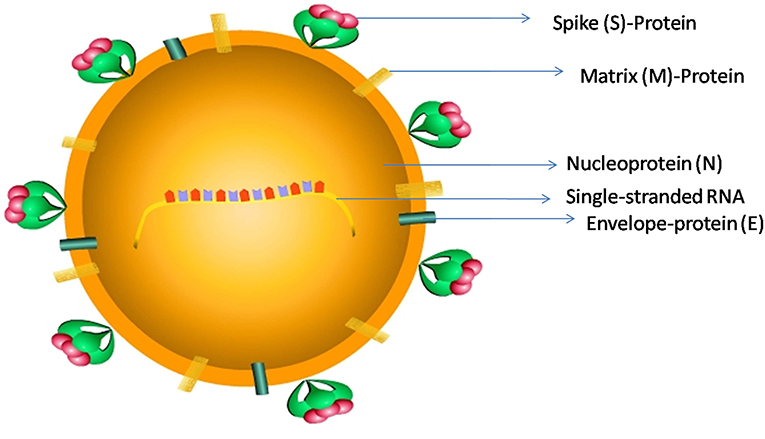
Figure 1. The schematic representation of SARS-CoV-2 structure: SARS-CoV-2 has a size ranging from 60 to 140 nm, and is a spherical to elliptical shaped virus with a crown-like appearance; it consists of a single-stranded RNA genome, a Spike protein (S), a Matrix protein (M), a nucleoprotein (N), and an Envelope-protein (E).
Gene sequencing data revealed that SARS-CoV-2 has 89 and 82% sequence similarity with bat SARS-like-CoV-ZXC21 and human SARS-CoV, respectively (4, 5). The spike (S) protein-coding gene mutation in the nsp2 and nsp3 regions results in the replacement of glycine (G) with serine (S) at 723 position (G723S), and an isoleucine (I) replaced with proline (P) at 1010 amino acid position (I1010P). Due to these mutations, the invading potential of SARS-CoV-2 has increased significantly toward host tissues. This virus can also be transmitted through the respiratory droplets from coughs and sneezes of infected individuals (4). This mode of aerosol transmission is possible, especially, when protracted exposure occurs in closed areas (4). The incubation time of the virus varies significantly from individual to individual. In general it takes about 6 days from the day of infection to the first appearance of symptoms. However, in a few cases the symptoms may appear only after 2 weeks (6).
SARS-CoV2 Infection and Pulmonary Pathogenesis
Members of Coronaviridae are known to induce respiratory complications in humans (7, 8). At first, SARS-CoV, MERS-CoV, and SARS-CoV-2 varieties were transmitted from animals to humans which triggered severe respiratory diseases (9–11). However, subsequent transmission occurred among humans primarily due to physical contact. Hence, conventional preventive measures such as physical isolation were implemented to avoid propagation of early infection across the human population (1, 12). Similar to the SARS-CoV, the pathological manifestations of SARS-CoV-2 could induce lung malfunction in humans as indicated by the severe acute respiratory syndrome and pneumonia (12). Recent studies reported that SARS-CoV-2 infection can induce mild, moderate, and severe illness in infected patients (4). Clinical manifestations of this infection include chronic pneumonia, sepsis, septic shock, fever, and dry cough (4). A progressive respiratory failure during this infection may lead to sudden death (4). Mild illness resulting from a SARS-CoV-2 infection is characterized by the presence of malaise, headache, low fever and dyspnea. In the case of moderate illness from SARS-CoV-2, the complication is manifested by the presence of cough and mild pneumonia. Severe illness from SARS-CoV-2 is associated with chronic pneumonia, cough, SARS, hypoxia, and tachypnea (in children) followed by respiratory, and cardiovascular system failure (4). The autopsy and biopsy reports of SARS-CoV-2 patients revealed severe edema with pulmonary tissue exudates, focal reactive hyperplasia, damage to pneumocytes as well as alveolar macrophages, and patchy cellular infiltration (13).
Coronavirus-induced lung damage has been demonstrated experimentally by several investigators in animal models (14). For instance, the Sialodacryoadenitis virus and Parker's RCoV were shown to induce damage to alveolar type-I cells through the expression of pro-inflammatory cytokines, and chemokines such as CINC-2, CINC-3, LIX, MIP-3α, and fractalkines (15–21). For example, fractalkine promotes the infiltration of cytotoxic lymphocytes in the alveolar epithelium thereby inducing a severe inflammatory response (15, 22). Similarly, MIP-3α confers the chemotaxis of immune cells via IL-1β and TNF-α inflammatory mediators (17, 22–25). Therefore, these animal models could be used to develop effective pharmacological agents against SARS-CoV-2 infections.
Molecular Mechanisms of SARS-CoV-2 Infection
Studies from several laboratories have demonstrated that the entry of SARS-CoV-2 into human cells is facilitated by ACE-2 (26). ACE-2 is a member of the Renin-angiotensin system (RAS), which plays a vital role in cardiovascular and renal homeostasis. ACE-2 and TMPRSS2 facilitates the entry of the virus into host cells during SARS-CoV-2 infection (7). In addition, there are other proteases such as aminopeptidase N (APN) which plays a prominent role for the entry of HCoV-NL63 and HCoV-229E into host cells (27–30). APN is a membrane-bound glycoprotein that mediates the zinc-dependent protease activity during the entry and or replication of coronavirus strains into host cells (29, 31, 32). Hence, the ACE-2 receptor's down-modulation may prevent SARS-CoV-2 viral entry/replication (33). The S-protein of SARS-CoV and other coronavirus strains are different in their structural and functional domains (3). S-protein can bind to the N-terminus of ACE-2 receptors on the outer surface of host cells including respiratory epithelium of the lungs (34–36). Identifying the key amino acid residues in S-protein of the SARS-CoV-2 strain may benefit virologists and medical scientists to develop better therapeutic agents. However, to date these details are not known, hence, there is an immediate requirement to identify the amino acids involved in binding S-proteins to ACE-2 receptors on host cell surfaces. Furthermore, investigations should also focus on establishing the structural similarities of S-protein motifs that are interacting with the ACE-2 receptors of other coronavirus strains (37–41). These investigations might help in deciphering molecular strategies to target receptor binding sites of ACE-2 proteins with SARS-CoV-2 using novel therapeutics and vaccines to avoid membrane fusion process and viral entry (7).
The TMPRSS2 protease can foster the entry of the SARS-CoV-2 virus by activating the S-protein for virus-host cell membrane fusion, consequently enhancing viral replication in the host cells (7, 42–46). TMPRSS2 plays a vital role in generating inflammatory cytokines and chemokines in lung epithelial cells by cleaving S-protein during coronavirus infections including SARS-CoV-2. Hence, TMPRSS2 is another potential therapeutic target to consider for the novel drug development against SARS-CoV-2 (46–48).
Novel Small Molecule Inhibitors (NSMIs) in the Prevention and Treatment of SARS-CoV-2 Infections
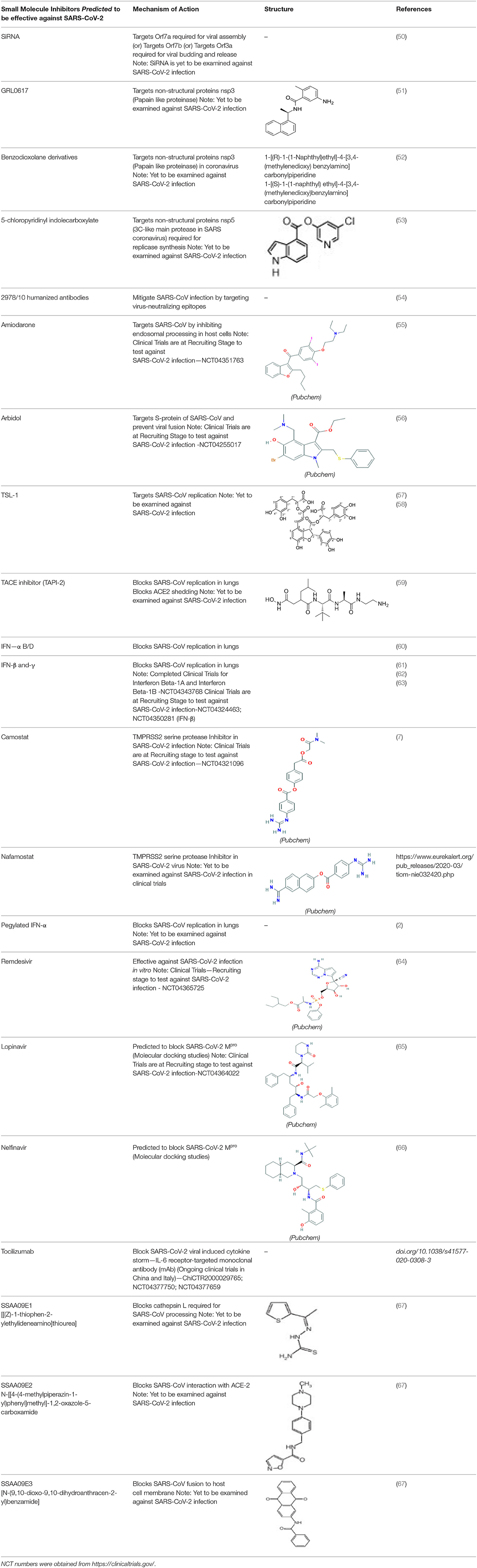
Prevention and treatment of SARS-CoV-2 infections are achieved at different levels (49). The primary approach involves physical isolation to prevent the spread of virus from individual to individual; the second approach involves inhibiting the entry of virus into human cells and the third method includes treating the infected individuals to minimize inflammatory reactions and pulmonary damage. Although physical isolation is the ideal way of limiting the spread, in reality this approach is difficult to execute, hence, many pharmacological companies are actively involved in developing small molecule inhibitors to prevent the entry of the virus into human hosts (7, 49). In this regard several NSMIs have been investigated to treat SARS-CoV; but, significant breakthroughs are yet to come for treating SARS-CoV-2 (48) (Table 2).
Viral Entry Inhibitors vs. SARS-CoV-2
Adedeji et al. (49) reported the discovery and characterization of novel inhibitors to block SARS-CoV replication via different mechanisms. One mechanism uses screening of small molecule inhibitors using “HIV-1 pseudotyped with SARS-CoV surface glycoprotein S (SARS-S)” (49, 68). “SSAA09E2” is a novel small molecule inhibitor, which blocks the interaction of CoV SARS-S with ACE-2 receptors, thus blocking the viral entry (49). Another NSMI is “SSAA09E1” reported to be involved in blocking the cathepsin L, which is required for CoV-SARS-S processing to mediate viral entry into the host cell (49). SSAA09E3 is another NSMI, which can block the fusion of viral membranes with host cell surfaces (49) (Figure 2). Since the pathological aspects and genomic similarity of SARS-CoV-2 virus with SARS-CoV, the above strategies of inhibition may be considered for developing potent pharmacological agents to prevent SARS-CoV-2 infections (49). However, the prospective research should address the efficacy of these inhibitors against SARS-CoV-2 infections.

Figure 2. Molecular pathogenesis of SARS-CoV-2 in human lung cells. Binding of S-protein of SARS-CoV-2 to the ACE-2 receptors triggers the processing of ACE-2 through ADAM-17/TNF-α-converting enzyme and induces the “ACE-2 shedding” into the extracellular space and facilitates uptake of SARS-CoV-2 followed by the development of SARS. Alternatively, the entry of SARS-CoV-2 by membrane TMPRSS2 serine protease'/HAT (Human Airway Trypsin-like protease)-mediated cleavage of ACE2 can facilitate SARS-CoV S-glycoprotein-mediated virus entry. Even though, several NSMIs targeting these processes were described and their mode of action against coronavirus were delineated, their efficacy against SARS-CoV-2 is yet to be tested.
Kinase Inhibitors vs. SARS-CoV-2
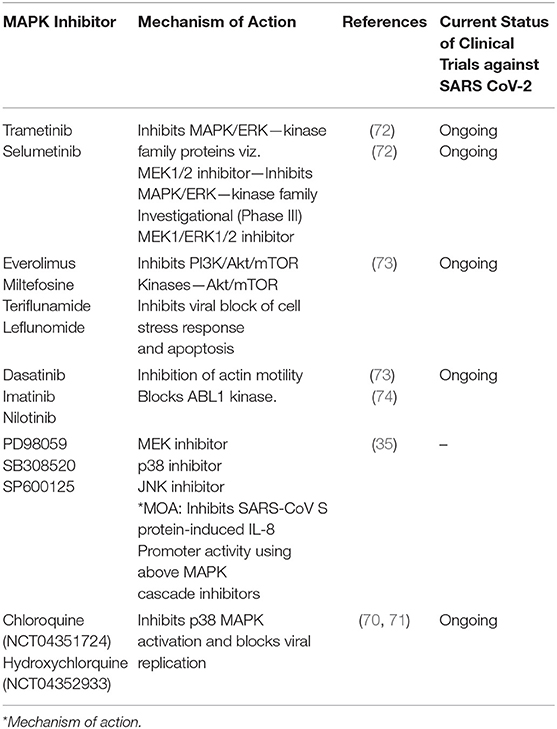
Cytokine storm was predominantly reported during SARS-CoV-2 infection. Targeting cytokine-mediated inflammatory responses induced by SARS-CoV-2 is another viable approach for mitigating the complications of viral infection. In this regard, Chang et al. (35), documented the inflammatory cascades mediated through intracellular signaling pathways conferred by the SARS-CoV in both lung epithelial cells and fibroblasts. Authors of this study have reported that S-protein of SARS-CoV efficiently mediate the IL-8 release in the infected lung cells by activating MAPKinases, and activator protein-1 (AP-1) without intervention of NF-kB cascade (35). This study suggested a promising lead for novel rational drug design through the identification of a “specific sequence motif of S-protein functional domain,” which is responsible for inducing IL-8-mediated inflammatory response in lungs (35). Baricitinib is a pharmacological agent, which was reported to block the SARS-CoV-2 viral entry and inflammation through the inhibition of AP2-associated protein kinase 1 (AAK1), cyclin g-associated kinase, and janus kinase-1 and 2 (69). Chloroquine (CQ) and hydroxychloroquine (HCQ) were reported to be effective in mitigating the coronaviral load (70, 71). CQ and HCQ not only inhibit the entry of SARS-CoV-2 but also change the pH of acidic intracellular organelles such as endosomes and lysosomes thereby preventing membrane fusion reactions. However, many contradictions and queries prevail pertaining to the use of HCQ for the treatment of COVID-19. At the time of the submission of this review, results of many clinical trials are yet to be announced, hence, the efficacy of HCQ for inhibiting SARS-CoV-2 infection is still a possibility.
Prospective studies should focus on testing the FDA approved inhibitors of “ABL-1 kinases,” “PI3K/Akt/mTOR” signaling, and “MAPKinase” pathways against SARS CoV-2. Since these pathways are involved in cell survival, inflammatory cytokines production, and proliferation of cells, targeted downregulation of these pathways is likely to mitigate the exacerbations induced by coronavirus. In this direction, many of these inhibitors are currently being tested against SARS-CoV-2 (Table 3) (72–74). For instance, sorafenib, which inhibits RAF, is being experimented in preclinical models and early clinical trials (72). Likewise, the efficacy of IL-1 receptor antagonists and TNF-α receptor antagonists for blocking the rat coronavirus-mediated chemokine production was already proven effective in animal models (15, 75, 76). Further studies testing the safety and efficacy are warranted before considering these inhibitory agents for treating individuals infected with SARS-CoV-2 (76).
However, the concept of “One Drug to Treat All” should be followed to combat several devastating viral infections (77, 78). For instance, the Ebola, Marburg, and SARS-CoV-2 are undoubtedly devastating viral pathogens, which can induce high mortality as they transmit rapidly via air and body fluids (78). Outbreaks of these viruses occur sporadically and currently there are no clinically approved NSMIs available to combat these viruses. A recent report by Taylor et al. (79) demonstrated the efficacy of a synthetic adenosine analog, BCX4430 in blocking a broad spectrum of viral species viz., “coronaviruses, paramyxoviruses, and bunyaviruses” as these viruses could induce SARS, measles, and mumps. BCX4430 could efficiently block both Ebola and Marburg viral titers in non-human primate models by targeting viral RNA polymerase (78, 79). Hence, this molecule should be tested for further studies against SARS-CoV2 infections in humans.
Membrane Protease Inhibitors vs. SARS-CoV-2
Targeting the membrane protease involved in viral S-protein processing and the viral entry into host cells is another approach in mitigating SARS-CoV-2. The host cellular proteases viz., “trypsin,”, “miniplasmin,” “human airway trypsin like protease,” “tryptase Clara,” and “TMPRSS2” could cleave the HA glycoprotein located in influenza A virus and thereby promote viral entry into lung cells (80). The usage of serine protease inhibitors such as Camostat and Aprotinin significantly blocked the replication of influenza virus in epithelial cells of lungs and bronchioles (81). In addition, these NSMIs could block the release of inflammatory mediators such as cytokines, IL-6 and TNF-α, during this infection (81).
TMPRSS2 is a key protein involved in the pathogenesis of several seasonal viral infections including influenza, H1N1, H3N2, and H7N9 (82–85). TMPRSS2 cleaves the S-protein of coronavirus to produce unlocked, fusion-catalyzing viral forms and binds to the host cell surface thereby enhancing rapid viral entry (43, 44, 86–90). Both SARS-CoV and MERS-CoV could rapidly enter into the host cells as TMPRSS2 can facilitate viral binding to the cell surface (42, 43, 45, 87, 91, 92). TMPRSS2 also plays a vital role in the immuno-pathology of coronavirus infections including SARS-CoV-2 across lungs by inducing lung fibrosis (46). Hence, the emerging research should promote the development of NSMIs to target these proteases thereby hindering the entry of SARS-CoV-2 into host cells.
A proof-of-concept study by Iwata-Yoshikawa et al. (46) reported that SARS-CoV failed to replicate in the bronchioles and lungs of TMPRSS2 knockout mice. Authors of this study reported elevated expression of TLR3-mRNA expression in the lungs of “SARS-CoV-inoculated TMPRSS2-deficient mice” and showed enhanced TLR-3 mediated localization of dsRNA into endosomes (46). In this study, TMPRSS2 knockout has resulted in downregulation of inflammatory cytokines and chemokine expression, which are involved in the bronchiolitis obliterans organizing pneumonia (BOOP), SARS, and pulmonary fibrosis in SARS-CoV infection (46, 93, 94).
Interferon Therapy vs. SARS-CoV-2
The intricate SARS-CoV-2 pathogenesis is similar to that of SARS-CoV. Studies have reported the efficacy of IFNs to block SARS-CoV in cell line models but not against SARS-CoV-2. Among IFN- α/ -β/ and -γ, the IFN-β was reported to be the most potent blocker of SARS-CoV growth (3, 95–98). Furthermore, IFN-β and-γ have a synergistic effect in blocking SARS-CoV viral replication (62, 63). However, the effect of this combination against SARS-CoV-2 is not yet reported. Therefore, future studies should focus on determining the efficacy of IFN- α/ -β/ and –γ against SARS-CoV-2 infections.
SiRNAs vs. SARS-CoV-2
Unlike small molecule inhibitors, siRNAs are specific and can be designed to mitigate SARS-CoV associated structural proteins by targeting ORF4 (99, 100), ORF5 (101, 102), ORF9a (50, 103, 104), and ORF7a (50, 105–107). For example, siRNAs siSC2 and siSC5 have shown success in cultured cells as well as in preclinical mouse models in inhibiting the SARS infection without causing toxicity (108). Several other reports have also recently demonstrated the efficacy of SiRNAs to inhibit the expression of SARS-CoV genes coding for 3CL protease in cell line models (108–114). The activity of SARS-CoV 3CL protease is essential for viral replication as this protein is involved in the processing of viral proteins (114). Selective optimization and screening of hexa-chlorophene analogs can be “active 3CL protease inhibitors” during a SARS-CoV infection (114). Hence, the pharmacological agents/SiRNAs targeting these pathways may likely produce effective clinical outcomes in SARS-CoV-2 infections. However, clinical studies should test the utility of these agents/siRNA in reducing the burden of infections caused by SARS-CoV-2 (111).
Monoclonal Antibodies (MABs) and Other NSMIs vs. SARS-CoV-2
The genome of coronaviruses is reported to be significantly involved in coding both structural proteins, and non-structural proteins (nsp's) for the effective viral replication (115). The nsp's (nsp8C and nsp7) are required for novice CoV viral particle formation through viral ORF 1ab polyprotein processing (115). Several NSMIs were reported to target these non-structural proteins in coronavirus infections to treat SARS (115). For instance, GRL0617, a bendioxolane derivative, could target papain-like proteinases like nsp3 (51, 52, 116, 117), whereas 5-choloropyridinyl indolecarboxylate targets nsp5 (53, 118–120) and a “combination of zinc derivatives with pyrithione” targets nsp12 (121, 122); ranitidine bismuth citrate targets nsp13 (123–127). Monoclonal antibodies; CR3014 (128), mAb-201 (129), mDEF-201 (130), ampligen (131), polyICLC (61, 132), stinging nettle lectin (131), and TAPI-2 (a TACE-inhibitor) (59) are anti-coronaviral agents tested in vivo models of SARS. For instance, a study showed that Amiodarone (a known anti-arrhythmic agent) effectively targets coronaviral spreading in in vitro models (55). Working in a similar fashion, 2878/10 humanized antibodies can neutralize coronaviruses thereby reduce the complications caused by viral infections (54). However, the above NSMIs should be tested against SARS-CoV-2 viral associated proteins and against the activity of nsp's to derive an effective therapeutic intervention. Prospective research must focus on the development of novel “helicase inhibitors, viral attachment inhibitors, and activity of Rhesus θ-defensin” that block SARS-CoV-2 infection using in vitro, in vivo, and clinical studies (115). Hence, the development of NSMIs to target the synthesis of nsp's in SARS-CoV-2 may deliver cellular antiviral responses by blocking their replication in host cells (115, 133, 134).
Drug Repurposing Strategies (DRS) vs. SARS-CoV-2
Repurposing existing drugs is another strategy widely under consideration to target key proteins involved in the SARS-CoV-2 infection. In this regard, the existing NSMIs viz., antivirals (umefenovir, remdesivir, Nitazoxanide, favipiravir, ritonavir, lopinavir, IFNs), anticytokines, antimalaria drugs (chloroquine, hydroxychloroquine), and passive antibody therapies are currently being evaluated to improve clinical outcomes in SARS-CoV-2 infected patients (3, 47, 64, 135, 136). However, these agents require additional experimental and clinical validations before being tested in SARS-CoV-2 infections. For example, hydroxychloroquine (anti-malarial drug) and the tocilizumab (immunosuppressive drug) are preferred currently to mitigate viral entry and cytokine production in the SARS-CoV-2 infection. These drugs are being tested in ongoing trails in China and Italy (135, 137).
Priming the Spike (S)-protein of coronavirus by host cells using membrane proteases is a necessary process for viral entry and replication, which further determines zoonotic potential of coronaviruses (138). A recent report by Markus Hoffmann et al. (7) investigated the protease dependence of SARS-CoV-2 for its entry into cells. For example, SARS-CoV-2 uses the TMPRSS2 protease for its priming (7). Inhibition of TMPRSS2 using Camostat mesylate retarded the viral entry into Caco-2 cells (7). Camostat mesylate could be recommended as an NSMI for human clinical trials to combat the SARS-CoV-2 virus (7). This report delineated the ability of neutralizing antibody responses against S-protein to block the SARS-CoV-2 entry into host cells (139). The serum antibody responses raised to combat the “SARS-S protein/ACE-2 interface” during the SARS-CoV-2 infection indicates that the vaccination strategy may be an effective therapeutic modality against the COVID-19 infection (7).
Conflicting Reports About the ACE-2 Inhibitors Usage for Treating SARS-CoV-2 Infections
ACE-2 catalytic efficacy is significantly higher than ACE for Angiotensin-II (140). Several compounds, such as MLN-4760, were screened according to structure-based/substrate-based studies through virtual screening for inhibiting ACE-2 activity (140–143). ACE-2 is predominantly expressed in lungs, brain, heart, blood vessels, and renal organs (144, 145). ACE-2 is essential for cardiovascular homeostasis, and CNS homeostasis as ACE-2 confer redox homeostasis by mitigating Ang-II-induced oxidative stress (146). However, in COVID-19, ACE-2 acts as receptor on human respiratory epithelial cells for SARS-CoV-2 binding (7). A recent report by Markus Hoffmann et al. (7) provided evidence that the SARS-CoV-2 strain use its spike (S)-protein to bind to ACE-2. Authors of this paper have also demonstrated the efficiency of TMPRSS2 in SARS-CoV-2 viral strain priming in host cells (7). Therefore, targeting ACE-2 could be a viable strategy to prevent the entry of SARS-CoV-2 into the human system. However, a recent report by Guan et al. (147) cautioned that the administration of ACE inhibitors significantly induced adverse clinical outcomes in COVID-19 patients due to severe hypertension, coronary artery disease, and chronic renal failure; hence, further use of ACE inhibitors to treat COVID-19 infections was halted (147–149). In another report Diaz (149) hypothesized that COVID-19 patients receiving I.V. infusions of ACEIs and ARBs (AT1- Receptor Blockers) are at a higher risk of attaining severe disease pathogenesis. Hence, they supported the development of NSMIs such as “TMPRSS2 inhibitors to treat SARS-CoV-2 infections (7)”.
Reasons for the Failure of Current Therapeutic Modalities Against SARS-CoV-2
The failure of disease management and lack of selective therapies could be due to the intricate COVID-19 pathogenesis induced by the SARS-CoV-2 infection. Hence, the early recognition of disease is essential for effective management of COVID-19 (48).
Although, several reports delineated the efficacy of certain NSMIs viz., ribavirin, promazine, and IMP dehydrogenase inhibitors to inhibit in vivo models of SARS- CoV replication, later, they were proven ineffective (60, 150–152). A report by Reghunathan et al. (153) showed that the immune response produced against SARS-CoV may be different from other viral infections as indicated by the lack of upregulation in MHC-I genes, cytokines, and IFNs or complement-mediated cytolysis in peripheral blood mononuclear cells (PBMCs). The failure in the development of a vaccine is due to antibody-dependent enhancement and Th-2 immunopathology (154–156).
Pegylated IFN-α inhibits viral replication of SARS-CoV and offers protection against type I pneumocytes in lungs (2). A significant reason for the failure or lack of selective therapies against SARS-CoV-2-induced SARS is the intricate immune system mediated pathophysiology (4). Other reports by Law et al., also detailed similar mechanisms (157, 158). SARS-CoV can evade host IFN-mediated viral growth inhibition by activating IFN-regulatory factor 3 (157). Furthermore, SARS-CoV could induce apoptosis in lymphocytes in vitro using “ORF 7a, ORF 3a, and ORF 3b, E protein, and N protein” (159–162). For instance, the SARS-CoV can evade immunity as indicated by the decline in CD4 and CD8 T cells (163). Therefore, it is necessary to uncover the complement-based cytolysis in human patients in response to the SARS-CoV-2 strain as this virus executes unusual mechanisms to evade the human immune system consequently inducing pathogenesis and mortality. The prospective research should focus on this viral-mediated immune signaling with respect to SARS-CoV for developing effective NSMIs.
Intravenous (IV) hyperimmune globulin therapy is one of the immunotherapies known to downmodulate pro-inflammatory cytokines and mitigate the severity of infection in COVID-19 patients. IV infusion of immunoglobulins composed of a high dose of antibodies, which can bind to a number of inhibitory receptors viz., Fc gamma receptor IIB (FcγRIIB) (164, 165) and FcγRIIC (166) and confer anti-inflammatory responses against SARS-CoV-2 (Completed Clinical Trials: hyperimmune plasma NCT04321421).
Nrf-2 Modulators vs. SARS-CoV-2 Induced Oxidative Stress
Oxidative stress is significantly induced by several viral infections inside the lungs through the downregulation of redox regulator nuclear factor-erythroid 2 related factor 2 (Nrf-2) (167). Nrf-2 is a leucine-zipper transcription factor (167) expressed predominantly in nasal epithelium, epithelial cells of lungs, and alveolar macrophages (168, 169). Disruption of Nrf-2 and Keap1 interaction triggers the activation of the anti-oxidant defense mechanism (169). For instance, Nrf-2 activation offers protection against inflammation and lung injury induced by influenza viral infections and respiratory syncytial virus (RSV) through the anti-oxidant defense pathway (170). Several viral proteins in the host cells can foster optimum levels of ROS-mediated oxidative stress to facilitate viral metabolism and the viral replication cycle without killing host cells (168, 171–174). Recent seminal studies described the active role of viruses in inhibiting the Nrf-2 pathway (175–177). For instance, the positive regulation of Nrf-2 in modulating the thiol redox system and oxidative stress for the survival of infected astrocytes was observed in Moloney murine leukemia virus and HIV virus (178). The HCV virus could induce the downregulation of Nrf-2 dependent NQO1, GCLC, and GPx and modulate oxidative stress (179, 180). An RSV infection mediates proteasomal degradation, deacetylation, and SUMOylation of Nrf-2 consequently causing the downregulation of NQO1, CAT, and SOD1 gene expression (181). Hence, Nrf-2 activators are potential anti-viral agents, which can be tested against the SARS-CoV-2 infection (167). Future research is highly imperative in unraveling the underlying activity of Nrf-2 for emerging SARS-CoV-2 survival by analyzing Nrf2 target genes NQO1, GCLC, and GPx. In addition, the SARS-CoV-2 mediated expression of serine and cysteine proteases in different cell lines should be investigated in relation to Nrf-2 activation, which is a beneficial strategy to combating SARS-CoV-2 pathogenesis. However, in the case of certain viral infections, it is imperative to develop Nrf-2 inhibitors to protect the host cells (182). For instance, the Marburg virus (a causative agent for lethal hemorrhagic fever) can modulate oxidative stress by activating Nrf-2 dependent signaling through the blockade of “VP-24 viral protein” binding to KEAP1 (183). Therefore, VP-24 dependent Nrf2 activation can mediate the upregulation of genes HO (heme oxygenase)-1, NQO1, and GCLM (183). In the case of Dengue virus, the viral particles could induce ER stress and activate Nrf-2 signaling, which then lead to TNF-α secretion (184). In this scenario, it is crucial to uncover any underlying mechanisms of emerging SARS-CoV-2 survival through the modulation of oxidative stress via Nrf-2 signaling in different cells of different organs including lungs (183). The prospective research studies should focus on the development of Nrf-2 modulators against SARS-CoV-2.
Natural Nrf-2 Modulators vs. Viral Infections
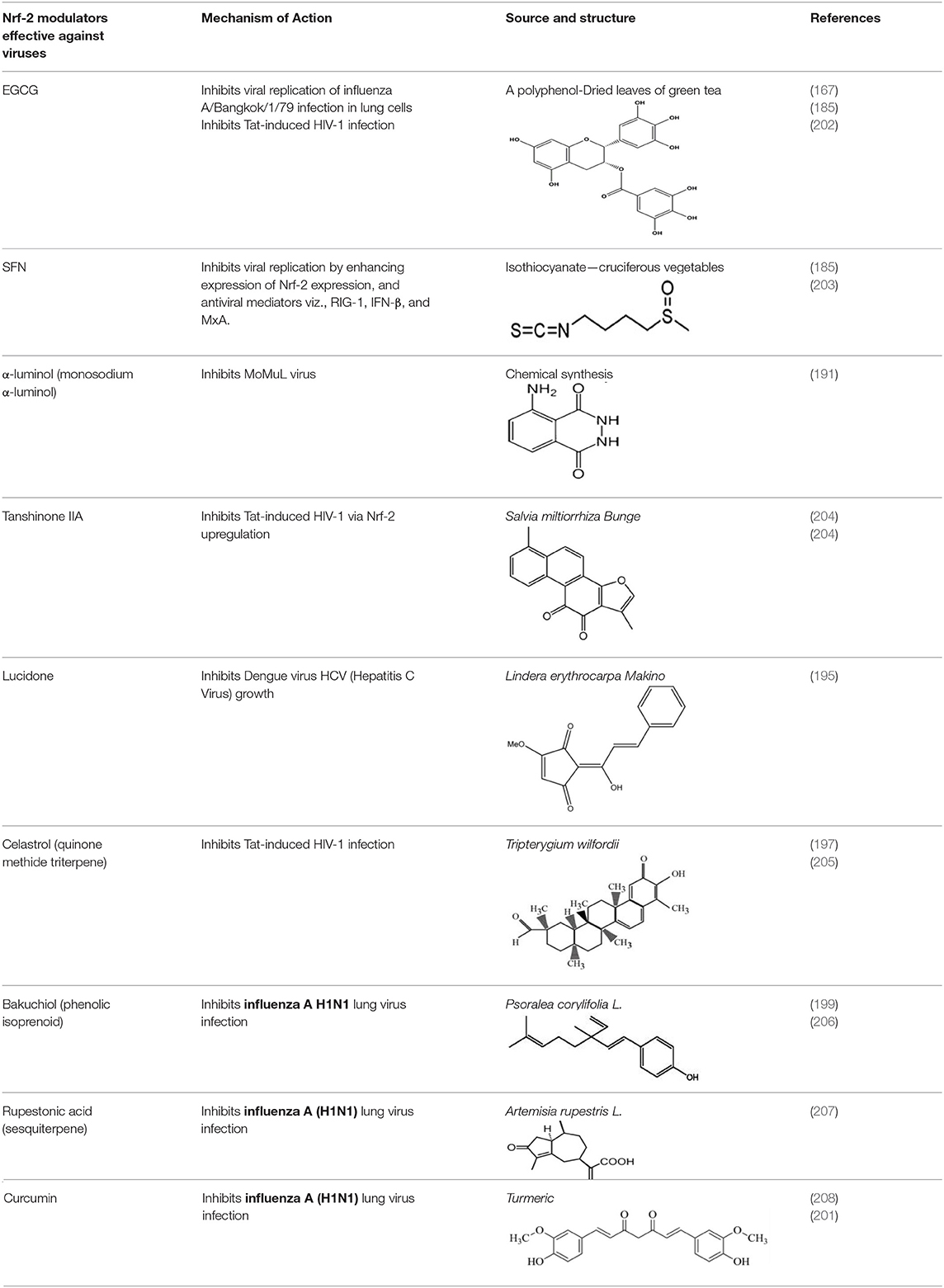
Natural products were proven to offer protection against virus-induced oxidative stress by modulating anti-oxidant defense pathways (185, 186). For instance, the administration of EGCG has mitigated viral replication of “influenza A/Bangkok/1/79 infection” by activating Nrf-2 to attenuate virus-induced oxidative stress, inflammation, and apoptosis in lung cells (186). Similarly, the cytoprotective and antioxidant efficacy of Nrf-2 was reported against PR8 influenza-A viral infection in AT-I and AT-II cells (186). Prospective research should focus on testing the efficacy of several natural products to block SARS-CoV-2 viral replication by ascertaining Nrf-2 mediated antioxidant responses. Studies have also shown the activation of host cellular transmembrane proteases (for example, serine proteases, cysteine proteases), which can further foster a prompt viral entry and viral replication in host cells by reducing Nrf-2 expression (4). Decline in proteolysis of the above proteases can actuate the propagation of several human viruses viz., Influenza, HIV, NIpah, Ebola, and Coronaviruses (SARS-CoV, MERS-CoV, SARS-CoV-2) (42, 90, 187–189). In this scenario, similar to influenza-A virus (190), it is highly important to unravel the influence of Nrf-2 expression on TMPRSS2, and human airway trypsin-like protease during SARS-CoV-2-mediated inflammatory conditions and oxidative stress in lungs. The downregulation of the Nrf-2 gene is correlated to serine protease activity and consequent influenza viral entry (185). Recent studies have demonstrated the efficacy of natural Nrf-2 activators viz., EGCG and sulforaphane (SFN) for blocking viral entry/viral replication as well as promoting antiviral mediators RIG-I, IFN-β, and MxA (185). In this context, it is essential to demonstrate the effects of nutritional interventions like SFN and EGCG against SARS-CoV-2 induced oxidative stress by modulating Nrf-2 signaling.
Evidence has demonstrated the use of naturally occurring Nrf2 activators for mitigating viral infections/post-viral infection induced complications. For example, α-luminol is a natural Nrf-2 activator, which confers the protection of astrocytes against the MoMuL virus (191). EGCG enhances nuclear Nrf-2 levels during Tat-induced HIV-1 infection and offers protection against virus induced oxidative stress (192). Tanshinone II A can induce upregulation of Nrf-2 expression and mitigates ROS production during Tat-induced HIV-1 infection via modulating AMPK/Nampt/SIRT1 signaling in host cells (193). SFN enhances the phagocytic function of “HIV-infected alveolar macrophages in lungs” by activating Nrf-2 signaling, which further induces downstream antioxidant cascades (194). Lucidone is effective for the Nrf-2 mediated blockade of Dengue virus by inducing heme oxygenase-1 (195); rographolide could induce Nrf-2 induced antioxidant defenses against influenza A in lung cells (196); celastrol can mediate Nrf-2 induced antioxidant defenses against HIV-1 Tat-induced inflammation (197). Broccoli sprouts containing SFN acts as a Nrf-2 activator to reduce influenza-induced infection in lung cells (198). Bakuchiol and Rupestonic acid are phytoconstituents that confer Nrf-2 activation thereby promoting NQO1 gene expression and HO-1-mediated interferon activity to enhance antioxidant response against influenza virus in lung cells (199, 200). Curcumin is another significant compound that can modulate Nrf-2 signaling and enhance the generation of IFN-β to offer protection against the influenza virus (201). Curcumin can mitigate this viral infection by modulating TLR2/4, p38/JNK MAPK, and NF-κB pathways (201). However, studies are required to decipher the activity of these phytochemicals against SARS-CoV-2 (Table 4). A recent report by Drăgoi (209) hypothesized that the potent natural Nrf-2 activators viz., resveratrol, SFN, curcumin, and Asea redox should be evaluated in different combinations with conventional drugs against SARS-CoV-2 infection in both in vitro and in vivo models and to further deduce a correlation between Nrf-2 activity and SARS-CoV-2 viral entry/replication.
Naturally Occurring Small Molecule Inhibitors vs. SARS-CoV-2
SARS-CoV-2 is progressively inducing a high mortality rate across the globe due to the lack of selective therapeutic interventions or vaccination. Recent reports by Lu et al. (210) and Xu et al. (148) delineated that the S-protein of SARS-CoV-2 and SARS Co-V exhibit similar 3-D pharmacophore in the receptor binding domain (RBD) of ACE-2 of human cells. COVID-19 patients are characterized by the severe viral pathogenesis due to extensive cytokine storm viz., TNF-α, IL-1β, IL-10, IFNγ, and MCP-1 in infected lung tissues (48). A report by Chen and Du (211) hypothesized that the phyto-constituents such as “baicalin, scutellarin, hesperetin, nicotianamine, and glycyrrhizin” may deliver anti-SARS-CoV-2 effects. Hesperetin glycoside abundant in citrus fruits, which can inhibit the SARS-CoV 3CLpro (212). The activity of this molecule must be examined against serine/cysteine proteases, which support SARS-CoV-2 viral entry/replication. Traditional citrus flavonoids were reported to have a potential to act against SARS-CoV-2 as studied by molecular docking studies. Molecular docking simulations, LC-MS studies described the efficacy of citrus flavonoids (ex. naringenin) in binding to ACE-2, and mitigating inflammation-induced lung injury by the SARS-CoV-2 virus (213). Further studies should evaluate these compounds in preclinical models to determine the safety and efficacy against the SARS-CoV-2 infection.
Natural products such as di/tri-terpenoids, lignoids were proven to inhibit the viral replication of coronaviruses in vitro (214); griffithsin could block coronaviral entry by binding to the SARS-CoV spike glycoprotein (215). TSL-1 can block coronaviral entry/replication; Leaf extracts of Toona sinesis Roem effectively blocked SARS-CoV replication (57). Betulinic acid, savinin can act as competitive inhibitors against SARS-CoV 3CL protease to block viral entry (214). The research gap must be filled to develop nutritional therapeutic interventions by investigating the efficacy of these phytochemicals against SARS-CoV-2 viral entry. Seeds of Psorelia corylifolia exhibit inhibitory effects against the SARS-CoV papain-like protease required for coronavirus entry/replication. The efficacy of these molecules should be examined against SARS-CoV-2 (216) (Table 5). The active site pockets of main proteases such as 6LU7 and 2 GTB in SARS-CoV-2 are reported to be involved in conferring viral entry/fusion; hence, these sites should be considered as the potential drug targets against SARS-CoV-2 (66). A molecular docking study by Khaerunnisa et al. (65) reported the predicted efficacy of bioactive compounds against above SARS-CoV-2 main protease (Mpro) sites viz., “nelfinavir, lopinavir, kaempferol, quercetin, luteolin-7-glucoside, demethoxycurcumin, naringenin, apigenin-7-glucoside, oleuropein, curcumin, catechin, epicatechin-gallate, zingerol, gingerol, and allicin” (Table 5).
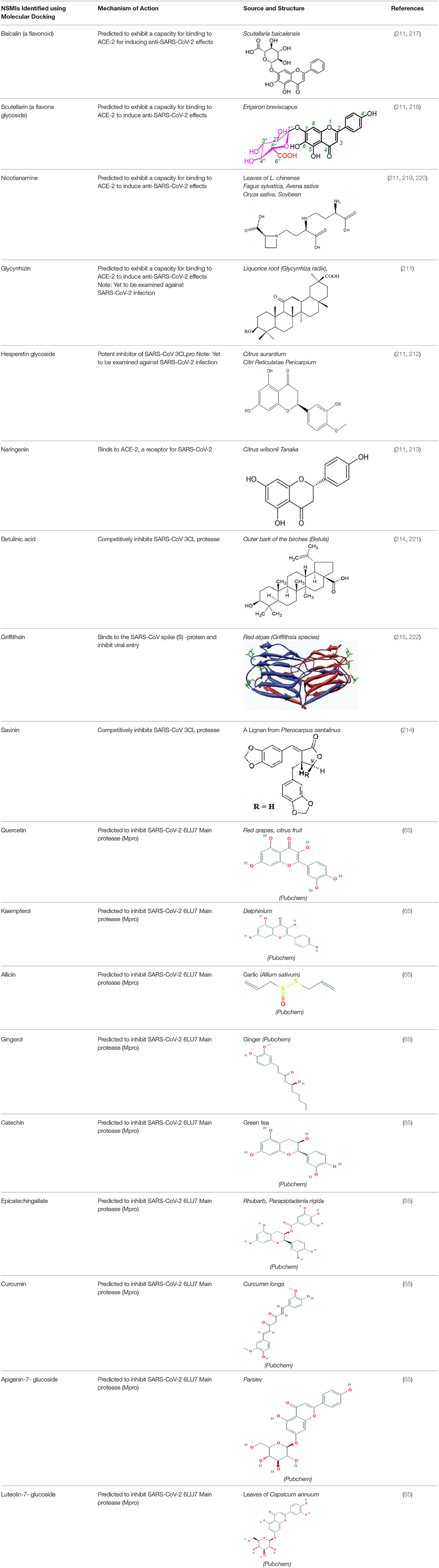
Table 5. Structure and mechanism of action of NSMIs identified against SARS-CoV2 using molecular docking studies.
Conclusions
The life-threatening consequences of the COVID-19 pandemic remain high due to lack of selective targeted therapies and vaccination strategies. This is primarily due to extreme genomic variability of RNA viruses as well as variations in the host-cell invading mechanisms. Hence, this review benefits virologists, medical scientists, and cell biologists to ascertain and develop NSMIs, Nrf-2 modulators, and clinically viable vaccines to combat this devastating SARS-CoV-2 strain. However, many more preclinical and clinical studies are required to uncover the therapeutic efficacy of potential phytochemicals, natural Nrf2 modulators, and several NSMIs against the SARS-CoV-2 infection. Furthermore, studies are also warranted to overcome ADE responses, and Th-2 immunopathology for the development of safe and efficacious vaccines against SARS-CoV-2. In summary, this review provides an overview on the existing knowledge and shows directions to various areas that require immediate attention.
Author Contributions
NB, SS, and SM: idea development, data collection, manuscript preparation, writing, and proof reading. AS, VN, and LM: cross referencing, data collection, and proof reading. SM, GA, and RR: editing, literature search, and proof reading. SS: execution of the literature search. All authors contributed to the article and approved the submitted version.
Conflict of Interest
GA is employed by GALLY International Biomedical Research & Consulting LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
RTIs, Respiratory tract infections; ORF, Open reading frame; TMPRSS2, Transmembrane serine protease; ADAM17, Disintegrin and metallopeptinase-containing domain 17; SARS-CoV, Severe respiratory syndrome-coronavirus; SARS-CoV-2, Severe respiratory syndrome-Coronavirus-2 (COVID-19); GM-CSF: Granulocyte–macrophage colony-stimulating factor.
References
1. Guan Y, Zheng B, He Y, Liu X, Zhuang Z, Cheung C, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. (2003) 302:276–8. doi: 10.1126/science.1087139
2. Haagmans BL, Kuiken T, Martina BE, Fouchier RA, Rimmelzwaan GF, Van Amerongen G, et al. Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. (2004) 10:290–3. doi: 10.1038/nm1001
3. Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. (2005) 69:635–64. doi: 10.1128/MMBR.69.4.635-664.2005
4. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19). StatPearls Publishing (2020).
5. Chan JF-W, Kok K-H, Zhu Z, Chu H, To K-W, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. (2020) 9:221–36. doi: 10.1080/22221751.2020.1719902
6. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. Ne Engl J Med. (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
7. Hoffmann M, Kleine-Weber H, Krüger N, Mueller MA, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. (2020). doi: 10.1101/2020.01.31.929042
8. Corman V, Lienau J, Witzenrath M. Coronaviruses as the cause of respiratory infections. Der Internist. (2019) 60:1136–45. doi: 10.1007/s00108-019-00671-5
9. Fehr AR, Channappanavar R, Perlman S. Middle East respiratory syndrome: emergence of a pathogenic human coronavirus. Ann Rev Med. (2017) 68:387–99. doi: 10.1146/annurev-med-051215-031152
10. Organization WH. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance. World Health Organization (2020).
11. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. (2016) 14:523. doi: 10.1038/nrmicro.2016.81
12. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. (2020) 395:470–3. doi: 10.1016/S0140-6736(20)30185-9
13. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao S-Y. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. (2020). doi: 10.20944/preprints202002.0220.v2
14. Menachery VD, Yount BL, Josset L, Gralinski LE, Scobey T, Agnihothram S, et al. Attenuation and restoration of severe acute respiratory syndrome coronavirus mutant lacking 2′-O-methyltransferase activity. J Virol. (2014) 88:4251–64. doi: 10.1128/JVI.03571-13
15. Miura TA, Wang J, Holmes KV, Mason RJ. Rat coronaviruses infect rat alveolar type I epithelial cells and induce expression of CXC chemokines. Virol. (2007) 369:288–98. doi: 10.1016/j.virol.2007.07.030
16. Abu-Harb M, Bell F, Finn A, Rao W, Nixon L, Shale D, et al. IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur Respir J. (1999) 14:139–43. doi: 10.1034/j.1399-3003.1999.14a23.x
17. Tumpey TM, García-Sastre A, Taubenberger JK, Palese, Swayne DE, Pantin-Jackwood MJ, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. (2005) 79:14933–44. doi: 10.1128/JVI.79.23.14933-14944.2005
18. Turner RB. The role of neutrophils in the pathogenesis of rhinovirus infections. Pediatr Infect Dis J. (1990) 9:832–5.
19. Wang S, Xu H, Wraith A, Bowden J, Alpers J, Forsyth K. Neutrophils induce damage to respiratory epithelial cells infected with respiratory syncytial virus. Eur Respir J. (1998) 12:612–8. doi: 10.1183/09031936.98.12030612
20. Zhang Y, Luxon BA, Casola A, Garofalo RP, Jamaluddin M, Brasier AR. Expression of respiratory syncytial virus-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J Virol. (2001) 75:9044–58. doi: 10.1128/JVI.75.19.9044-9058.2001
21. Zhu Z, Tang W, Gwaltney JM Jr., Wu Y, Elias JA. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-κB. Am J Physiol Lung Cell Mol Physiol. (1997) 273:L814–24. doi: 10.1152/ajplung.1997.273.4.L814
22. Stievano L, Piovan E, Amadori A. C and CX 3 C chemokines: cell sources and physiopathological implications. Crit Rev Immunol. (2004) 24:205–28. doi: 10.1615/CritRevImmunol.v24.i3.40
23. Vanderbilt JN, Mager EM, Allen L, Sawa T, Wiener-Kronish J, Gonzalez R, et al. CXC chemokines and their receptors are expressed in type II cells and upregulated following lung injury. Am J Respir Cell Mol Biol. (2003) 29:661–8. doi: 10.1165/rcmb.2002-0227OC
24. Londhe VA, Belperio JA, Keane MP, Burdick MD, Xue YY, Strieter RM. CXCR2 is critical for dsRNA-induced lung injury: relevance to viral lung infection. J Inflamm. (2005) 2:4. doi: 10.1186/1476-9255-2-4
25. Yen Y-T, Liao F, Hsiao C-H, Kao C-L, Chen Y-C, Wu-Hsieh BA. Modeling the early events of severe acute respiratory syndrome coronavirus infection in vitro. J Virol. (2006) 80:2684–93. doi: 10.1128/JVI.80.6.2684-2693.2006
26. South AM, Diz D, Chappell MC. COVID-19, ACE2 and the Cardiovascular Consequences. Rockville, MD: American Physiological Society (2020).
27. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. (2003) 426:450–4. doi: 10.1038/nature02145
28. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. (2005) 11:875–9. doi: 10.1038/nm1267
29. Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pöhlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci USA. (2005) 102:7988–93. doi: 10.1073/pnas.0409465102
30. Yeager CL, Ashmun RA, Williams RK, Cardellichio CB, Shapiro LH, Look AT, et al. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. (1992) 357:420–2. doi: 10.1038/357420a0
31. van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, et al. Identification of a new human coronavirus. Nat Med. (2004) 10:368–73. doi: 10.1038/nm1024
32. Kolb AF, Hegyi A, Siddell SG. Identification of residues critical for the human coronavirus 229E receptor function of human aminopeptidase N. J Gen Virol. (1997) 78:2795–802. doi: 10.1099/0022-1317-78-11-2795
33. Michel N, Allespach I, Venzke S, Fackler OT, Keppler OT. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell-surface CCR5 and CD4. Curr Biol. (2005) 15:714–23. doi: 10.1016/j.cub.2005.02.058
34. Wevers BA, van der Hoek L. Renin–angiotensin system in human coronavirus pathogenesis. Fut Virol. (2010) 5:145–161. doi: 10.2217/fvl.10.4
35. Chang Y-J, Liu CY-Y, Chiang B-L, Chao Y-C, Chen C-C. Induction of IL-8 release in lung cells via activator protein-1 by recombinant baculovirus displaying severe acute respiratory syndrome-coronavirus spike proteins: identification of two functional regions. J Immunol. (2004) 173:7602–14. doi: 10.4049/jimmunol.173.12.7602
36. Tripet B, Howard MW, Jobling M, Holmes RK, Holmes KV, Hodges RS. Structural characterization of the SARS-coronavirus spike S fusion protein core. J Biol Chem. (2004) 279:20836–49. doi: 10.1074/jbc.M400759200
37. Pyrc K, Dijkman R, Deng L, Jebbink MF, Ross HA, Berkhout B, et al. Mosaic structure of human coronavirus NL63, one thousand years of evolution. J Mol Biol. (2006) 364:964–73. doi: 10.1016/j.jmb.2006.09.074
38. Li W, Sui J, Huang I-C, Kuhn JH, Radoshitzky SR, Marasco WA, et al. The S proteins of human coronavirus NL63 and severe acute respiratory syndrome coronavirus bind overlapping regions of ACE2. Virology. (2007) 367:367–74. doi: 10.1016/j.virol.2007.04.035
39. Wu K, Li W, Peng G, Li F. Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor. Proc Natl Acad Sci USA. (2009) 106:19970–74. doi: 10.1073/pnas.0908837106
40. Haga S, Yamamoto N, Nakai-Murakami C, Osawa Y, Tokunaga K, Sata T, et al. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc Natl Acad Sci USA. (2008) 105:7809–14. doi: 10.1073/pnas.0711241105
41. Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. (2010) 84:1198–205. doi: 10.1128/JVI.01248-09
42. Matsuyama S, Nagata N, Shirato K, Kawase M, Takeda M, Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol. (2010) 84:12658–64. doi: 10.1128/JVI.01542-10
43. Shirato K, Kawase M, Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol. (2013) 87:12552–61. doi: 10.1128/JVI.01890-13
44. Bertram S, Dijkman R, Habjan M, Heurich A, Gierer S, Glowacka I, et al. TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium. J Virol. (2013) 87:6150–60. doi: 10.1128/JVI.03372-12
45. Gierer S, Bertram S, Kaup F, Wrensch F, Heurich A, Krämer-Kühl A, et al. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. J Virol. (2013) 87:5502–11. doi: 10.1128/JVI.00128-13
46. Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. (2019) 93:e01815–18. doi: 10.1128/JVI.01815-18
47. Ko W-C, Rolain J-M, Lee N-Y, Chen P-L, Huang C-T, Lee P-I, et al. Arguments in favor of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. (2020) 55:105933. doi: 10.1016/j.ijantimicag.2020.105933
48. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
49. Adedeji AO, Severson W, Jonsson C, Singh K, Weiss SR, Sarafianos SG. Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms. J Virol. (2013) 87:8017–28. doi: 10.1128/JVI.00998-13
50. Åkerström S, Mirazimi A, Tan Y-J. Inhibition of SARS-CoV replication cycle by small interference RNAs silencing specific SARS proteins, 7a/7b, 3a/3b and S. Antiviral Res. (2007) 73:219–27. doi: 10.1016/j.antiviral.2006.10.008
51. Ratia K, Pegan S, Takayama J, Sleeman K, Coughlin M, Baliji S, et al. A noncovalent class of papain-like protease/deubiquitinase inhibitors blocks SARS virus replication. Proc Natl Acad Sci USA. (2008) 105:16119–24. doi: 10.1073/pnas.0805240105
52. Ghosh AK, Takayama J, Rao KV, Ratia K, Chaudhuri R, Mulhearn DC, et al. Severe acute respiratory syndrome coronavirus papain-like novel protease inhibitors: design, synthesis, protein– ligand X-ray structure and biological evaluation. J Med Chem. (2010) 53:4968–79. doi: 10.1021/jm1004489
53. Ghosh AK, Gong G, Grum-Tokars V, Mulhearn DC, Baker SC, Coughlin M, et al. Design, synthesis and antiviral efficacy of a series of potent chloropyridyl ester-derived SARS-CoV 3CLpro inhibitors. Bioorg Med Chem Lett. (2008) 18:5684–8. doi: 10.1016/j.bmcl.2008.08.082
54. Rogers J, Schoepp R, Schröder O, Clements T, Holland T, Li J, et al. Rapid discovery and optimization of therapeutic antibodies against emerging infectious diseases. Protein Eng Design Select. (2008) 21:495–505. doi: 10.1093/protein/gzn027
55. Stadler K, Ha HR, Ciminale V, Spirli C, Saletti G, Schiavon M, et al. Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level. Am J Respir Cell Mol Biol. (2008) 39:142–9. doi: 10.1165/rcmb.2007-0217OC
56. Khamitov R, Loginova S, Shchukina V, Borisevich S, Maksimov V, Shuster A. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Voprosy Virusol. (2008) 53:9–13.
57. Chen C-J, Michaelis M, Hsu H-K, Tsai C-C, Yang KD, Wu Y-C, et al. Toona sinensis Roem tender leaf extract inhibits SARS coronavirus replication. J Ethnopharmacol. (2008) 120:108–11. doi: 10.1016/j.jep.2008.07.048
58. Gong J, Ju A, Zhou D, Li D, Zhou W, Geng W, et al. Salvianolic acid Y: a new protector of PC12 cells against hydrogen peroxide-induced injury from Salvia officinalis. Molecules. (2015) 20:683–92. doi: 10.3390/molecules20010683
59. Haga S, Nagata N, Okamura T, Yamamoto N, Sata T, Yamamoto N, et al. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antiviral Res. (2010) 85:551–5. doi: 10.1016/j.antiviral.2009.12.001
60. Barnard DL, Day CW, Bailey K, Heiner M, Montgomery R, Lauridsen L, et al. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antiviral Chem Chemother. (2006) 17:275–84. doi: 10.1177/095632020601700505
61. Kumaki Y, Day CW, Bailey KW, Wandersee MK, Wong M-H, Madsen JR, et al. Induction of interferon-γ-inducible protein 10 by SARS-CoV infection, interferon alfacon 1 and interferon inducer in human bronchial epithelial Calu-3 cells and BALB/c mice. Antiviral Chem Chemother. (2010) 20:169–77. doi: 10.3851/IMP1477
62. Scagnolari C, Vicenzi E, Bellomi F, Stillitano MG, Pinna D, Poli G, et al. Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferons. Antivir Ther. (2004) 9:1003–11.
63. Sainz B Jr., Mossel EC, Peters C, Garry RF. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV). Virology. (2004) 329:11–7. doi: 10.1016/j.virol.2004.08.011
64. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. (2020) 30:269–71. doi: 10.1038/s41422-020-0282-0
65. Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto S. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Europe PMC. (2020) 1:1–4. doi: 10.20944/preprints202003.0226.v1
66. Xu Z, Peng C, Shi Y, Zhu Z, Mu K, Wang X, et al. Nelfinavir was predicted to be a potential inhibitor of 2019-nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation. BioRxiv. (2020). doi: 10.1101/2020.01.27.921627. Available online at: https://europepmc.org/article/ppr/ppr110446
67. Adedeji AO, Sarafianos SG. Antiviral drugs specific for coronaviruses in preclinical development. Curr Opin Virol. (2014) 8:45–53. doi: 10.1016/j.coviro.2014.06.002
68. Wong SK, Li W, Moore MJ, Choe H, Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. (2004) 279:3197–201. doi: 10.1074/jbc.C300520200
69. Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. (2020) 395:e30–31. doi: 10.1016/S0140-6736(20)30304-4
70. Kono M, Tatsumi K, Imai AM, Saito K, Kuriyama T, Shirasawa H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antiviral Res. (2008) 77:150–2. doi: 10.1016/j.antiviral.2007.10.011
71. Colson P, Rolain J-M, Lagier J-C, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. (2020) 55:105932. doi: 10.1016/j.ijantimicag.2020.105932
72. Kindrachuk J, Ork B, Hart BJ, Mazur S, Holbrook MR, Frieman MB, et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrobial Agents Chemother. (2015) 59:1088–99. doi: 10.1128/AAC.03659-14
73. Schor S, Einav S. Repurposing of kinase inhibitors as broad-spectrum antiviral drugs. DNA Cell Biol. (2018) 37:63–9. doi: 10.1089/dna.2017.4033
74. Dyall J, Coleman CM, Hart BJ, Venkataraman T, Holbrook MR, Kindrachuk J, et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. (2014) 58:4885–93. doi: 10.1128/AAC.03036-14
75. Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. (1996) 87:2095–147. doi: 10.1182/blood.V87.6.2095.bloodjournal8762095
76. Dinarello CA. Differences between anti-tumor necrosis factor-alpha monoclonal antibodies and soluble TNF receptors in host defense impairment. J Rheumatol Suppl. (2005) 74:40–47.
77. Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Van Tongeren SA, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. (2014) 508:402–5. doi: 10.1038/nature13027
78. Hiv LLSF. Designing defenses against deadly viruses. Cell. (2014) 157:279–81. doi: 10.1016/j.cell.2014.03.033
79. Taylor R, Kotian P, Warren T, Panchal R, Bavari S, Julander J, et al. BCX4430–a broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J Infect Public Health. (2016) 9:220–6. doi: 10.1016/j.jiph.2016.04.002
80. Kido H, Okumura Y, Takahashi E, Pan H-Y, Wang S, Chida J, et al. Host envelope glycoprotein processing proteases are indispensable for entry into human cells by seasonal and highly pathogenic avian influenza viruses. J Mol Genet Med. (2009) 3:167. doi: 10.4172/1747-0862.1000029
81. Yamaya M, Shimotai Y, Hatachi Y, Kalonji NL, Tando Y, Kitajima Y, et al. The serine protease inhibitor camostat inhibits influenza virus replication and cytokine production in primary cultures of human tracheal epithelial cells. Pulmon Pharmacol Ther. (2015) 33:66–74. doi: 10.1016/j.pupt.2015.07.001
82. Hatesuer B, Bertram S, Mehnert N, Bahgat MM, Nelson PS, Pöhlman S, et al. Tmprss2 is essential for influenza H1N1 virus pathogenesis in mice. PLoS Pathog. (2013) 9:e1003774. doi: 10.1371/journal.ppat.1003774
83. Tarnow C, Engels G, Arendt A, Schwalm F, Sediri H, Preuss A, et al. TMPRSS2 is a host factor that is essential for pneumotropism and pathogenicity of H7N9 influenza A virus in mice. J Virol. (2014) 88:4744–51. doi: 10.1128/JVI.03799-13
84. Sakai K, Ami Y, Tahara M, Kubota T, Anraku M, Abe M, et al. The host protease TMPRSS2 plays a major role in in vivo replication of emerging H7N9 and seasonal influenza viruses. J Virol. (2014) 88:5608–16. doi: 10.1128/JVI.03677-13
85. Sakai K, Sekizuka T, Ami Y, Nakajima N, Kitazawa M, Sato Y, et al. A mutant H3N2 influenza virus uses an alternative activation mechanism in TMPRSS2 knockout mice by loss of an oligosaccharide in the hemagglutinin stalk region. J Virol. (2015) 89:5154–8. doi: 10.1128/JVI.00124-15
86. Bertram S, Heurich A, Lavender H, Gierer S, Danisch S, Perin P, et al. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS ONE. (2012) 7:e35876. doi: 10.1371/journal.pone.0035876
87. Glowacka I, Bertram S, Müller MA, Allen P, Soilleux E, Pfefferle S, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. (2011) 85:4122–34. doi: 10.1128/JVI.02232-10
88. Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. (2014) 88:1293–307. doi: 10.1128/JVI.02202-13
89. Shirato K, Kawase M, Matsuyama S. Wild-type human coronaviruses prefer cell-surface TMPRSS2 to endosomal cathepsins for cell entry. Virology. (2018) 517:9–15. doi: 10.1016/j.virol.2017.11.012
90. Shulla A, Heald-Sargent T, Subramanya G, Zhao J, Perlman S, Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. (2011) 85:873–82. doi: 10.1128/JVI.02062-10
91. Bertram S, Glowacka I, Müller MA, Lavender H, Gnirss K, Nehlmeier I, et al. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J Virol. (2011) 85:13363–72. doi: 10.1128/JVI.05300-11
92. Simmons G, Zmora P, Gierer S, Heurich A, Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antiviral Res. (2013) 100:605–14. doi: 10.1016/j.antiviral.2013.09.028
93. Lappi-Blanco E, Soini Y, Kinnula V, Pääkkö P. VEGF and bFGF are highly expressed in intraluminal fibromyxoid lesions in bronchiolitis obliterans organizing pneumonia. J Pathol. (2002) 196:220–7. doi: 10.1002/path.1038
94. van den Brand JM, Smits SL, Haagmans BL. Pathogenesis of Middle East respiratory syndrome coronavirus. J Pathol. (2015) 235:175–84. doi: 10.1002/path.4458
95. Chen F, Chan K, Jiang Y, Kao R, Lu H, Fan K, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. (2004) 31:69–75. doi: 10.1016/j.jcv.2004.03.003
96. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr H. Treatment of SARS with human interferons. Lancet. (2003) 362:293–4. doi: 10.1016/S0140-6736(03)13973-6
97. Ströher U, DiCaro A, Li Y, Strong JE, Aoki F, Plummer F, et al. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-α. J Infect Dis. (2004) 189:1164–7. doi: 10.1086/382597
98. Zheng B, He M-L, Wong K-L, Lum CT, Poon LL, Peng Y, et al. Potent inhibition of SARS-associated coronavirus (SCOV) infection and replication by type I interferons (IFN-α/β) but not by type II interferon (IFN-γ). J Interferon Cytok Res. (2004) 24:388–90. doi: 10.1089/1079990041535610
99. Satija N, Lal SK. The molecular biology of SARS coronavirus. Ann N Y Acad Sci. (2007) 1102:26–38. doi: 10.1196/annals.1408.002
100. Chen S, Luo H, Chen L, Chen J, Shen J, Zhu W, et al. An overall picture of SARS coronavirus (SARS-CoV) genome-encoded major proteins: structures, functions and drug development. Curr Pharma Design. (2006) 12:4539–53. doi: 10.2174/138161206779010459
101. Zhao G, Shi S-Q, Yang Y, Peng J-P. M and N proteins of SARS coronavirus induce apoptosis in HPF cells. Cell Biol Toxicol. (2006) 22:313–22. doi: 10.1007/s10565-006-0077-1
102. Chan C-M, Ma C-W, Chan W-Y, Chan HYE. The SARS-Coronavirus Membrane protein induces apoptosis through modulating the Akt survival pathway. Arch Biochem Biophys. (2007) 459:197–207. doi: 10.1016/j.abb.2007.01.012
103. Saikatendu KS, Joseph JS, Subramanian V, Neuman BW, Buchmeier MJ, Stevens RC, et al. Ribonucleocapsid formation of severe acute respiratory syndrome coronavirus through molecular action of the N-terminal domain of N protein. J Virol. (2007) 81:3913–21. doi: 10.1128/JVI.02236-06
104. Chen C-Y, Chang C-k, Chang Y-W, Sue S-C, Bai H-I, Riang L, et al. Structure of the SARS coronavirus nucleocapsid protein RNA-binding dimerization domain suggests a mechanism for helical packaging of viral RNA. J Mol Biol. (2007) 368:1075–86. doi: 10.1016/j.jmb.2007.02.069
105. Huang C, Ito N, C.-Tseng TK, Makino S. Severe acute respiratory syndrome coronavirus 7a accessory protein is a viral structural protein. J Virol. (2006) 80:7287–94. doi: 10.1128/JVI.00414-06
106. Yuan X, Wu J, Shan Y, Yao Z, Dong B, Chen B, et al. SARS coronavirus 7a protein blocks cell cycle progression at G0/G1 phase via the cyclin D3/pRb pathway. Virology. (2006) 346:74–85. doi: 10.1016/j.virol.2005.10.015
107. Vasilenko N, Moshynskyy I, Zakhartchouk A. SARS coronavirus protein 7a interacts with human Ap 4 A-hydrolase. Virol J. (2010) 7:31. doi: 10.1186/1743-422X-7-31
108. Li B-j, Tang Q, Cheng D, Qin C, Xie FY, Wei Q, et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat Med. (2005) 11:944–51. doi: 10.1038/nm1280
109. Li T, Zhang Y, Fu L, Yu C, Li X, Li Y, et al. siRNA targeting the leader sequence of SARS-CoV inhibits virus replication. Gene Ther. (2005) 12:751–61. doi: 10.1038/sj.gt.3302479
110. Yi S, De Hua Y, Xiong J, Jie J, Huang B, Jin YX. Inhibition of genes expression of SARS coronavirus by synthetic small interfering RNAs. Cell Res. (2005) 15:193–200. doi: 10.1038/sj.cr.7290286
111. Wang Z, Ren L, Zhao X, Hung T, Meng A, Wang J, et al. Inhibition of severe acute respiratory syndrome virus replication by small interfering RNAs in mammalian cells. J Virol. (2004) 78:7523–7. doi: 10.1128/JVI.78.14.7523-7527.2004
112. Wu C-J, Huang H-W, Liu C-Y, Hong C-F, Chan Y-L. Inhibition of SARS-CoV replication by siRNA. Antiviral Res. (2005) 65:45–8. doi: 10.1016/j.antiviral.2004.09.005
113. Zheng B-j, Guan Y, Tang Q, Du C, Xie FY, He M-L, et al. Prophylactic and therapeutic effects of small interfering RNA targeting SARS coronavirus. Antiviral Ther. (2004) 9:365–74.
114. Liu Y-C, Huang V, Chao T-C, Hsiao C-D, Lin A, Chang M-F, et al. Screening of drugs by FRET analysis identifies inhibitors of SARS-CoV 3CL protease. Biochem Biophys Res Commun. (2005) 333:194–9. doi: 10.1016/j.bbrc.2005.05.095
115. Barnard DL, Kumaki Y. Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy. Fut Virol. (2011) 6:615–31. doi: 10.2217/fvl.11.33
116. Barretto N, Jukneliene D, Ratia K, Chen Z, Mesecar AD, Baker SC. Deubiquitinating activity of the SARS-CoV papain-like protease. Nidoviruses. (2006) 581:37–41. doi: 10.1007/978-0-387-33012-9_5
117. Lindner HA, Lytvyn V, Qi H, Lachance P, Ziomek E, Ménard R. Selectivity in ISG15 and ubiquitin recognition by the SARS coronavirus papain-like protease. Arch Biochem Biophys. (2007) 466:8–14. doi: 10.1016/j.abb.2007.07.006
118. Yang H, Bartlam M, Rao Z. Drug design targeting the main protease, the Achilles' heel of coronaviruses. Curr Pharma Design. (2006) 12:4573–90. doi: 10.2174/138161206779010369
119. Liang P-H. Characterization and inhibition of SARS-coronavirus main protease. Curr Top Med Chem. (2006) 6:361–76. doi: 10.2174/156802606776287090
120. Graziano V, McGrath WJ, DeGruccio AM, Dunn JJ, Mangel WF. Enzymatic activity of the SARS coronavirus main proteinase dimer. FEBS Lett. (2006) 580:2577–83. doi: 10.1016/j.febslet.2006.04.004
121. Imbert I, Guillemot JC, Bourhis JM, Bussetta C, Coutard B, Egloff MP, et al. A second, non-canonical RNA-dependent RNA polymerase in SARS Coronavirus. Embo J. (2006) 25:4933–42. doi: 10.1038/sj.emboj.7601368
122. Te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. (2010) 6:e1001176. doi: 10.1371/journal.ppat.1001176
123. Prentice E, McAuliffe J, Lu X, Subbarao K, Denison MR. Identification and characterization of severe acute respiratory syndrome coronavirus replicase proteins. J Virol. (2004) 78:9977–86. doi: 10.1128/JVI.78.18.9977-9986.2004
124. Fan Z, Peng K, Tan X, Yin B, Dong X, Qiu F, et al. Molecular cloning, expression, and purification of SARS-CoV nsp13. Prot Exp Purif. (2005) 41:235–40. doi: 10.1016/j.pep.2004.08.003
125. Ivanov KA, Thiel V, Dobbe JC, van der Meer Y, Snijder EJ, Ziebuhr J. Multiple enzymatic activities associated with severe acute respiratory syndrome coronavirus helicase. J Virol. (2004) 78:5619–32. doi: 10.1128/JVI.78.11.5619-5632.2004
126. Yang N, Tanner JA, Wang Z, Huang J-D, Zheng B-J, Zhu N, et al. Inhibition of SARS coronavirus helicase by bismuth complexes. Chem Commun. (2007) 4413–15. doi: 10.1039/b709515e
127. Yang N, Tanner JA, Zheng BJ, Watt RM, He ML, Lu LY, et al. Bismuth complexes inhibit the SARS coronavirus. Angew Chem Int Ed. (2007) 46:6464–8. doi: 10.1002/anie.200701021
128. ter Meulen J, Bakker AB, van den Brink EN, Weverling GJ, Martina BE, Haagmans BL, et al. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. (2004) 363:2139–41. doi: 10.1016/S0140-6736(04)16506-9
129. Kumaki Y, Ennis J, Rahbar R, Turner JD, Wandersee MK, Smith AJ, et al. Single-dose intranasal administration with mDEF201 (adenovirus vectored mouse interferon-alpha) confers protection from mortality in a lethal SARS-CoV BALB/c mouse model. Antiviral Res. (2011) 89:75–82. doi: 10.1016/j.antiviral.2010.11.007
130. Kumaki Y, Day CW, Wandersee MK, Schow BP, Madsen JS, Grant D, et al. Interferon alfacon 1 inhibits SARS-CoV infection in human bronchial epithelial Calu-3 cells. Biochem Biophys Res Commun. (2008) 371:110–3. doi: 10.1016/j.bbrc.2008.04.006
131. Day CW, Baric R, Cai SX, Frieman M, Kumaki Y, Morrey JD, et al. A new mouse-adapted strain of SARS-CoV as a lethal model for evaluating antiviral agents in vitro and in vivo. Virology. (2009) 395:210–22. doi: 10.1016/j.virol.2009.09.023
132. Zhu X, Nishimura F, Sasaki K, Fujita M, Dusak JE, Eguchi J, et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. J Transl Med. (2007) 5:10. doi: 10.1186/1479-5876-5-10
133. Xiao Y, Ma Q, Restle T, Shang W, Svergun DI, Ponnusamy R, et al. Nonstructural proteins 7 and 8 of feline coronavirus form a 2: 1 heterotrimer that exhibits primer-independent RNA polymerase activity. J Virol. (2012) 86:4444–54. doi: 10.1128/JVI.06635-11
134. Li S, Zhao Q, Zhang Y, Zhang Y, Bartlam M, Li X, et al. New nsp8 isoform suggests mechanism for tuning viral RNA synthesis. Protein Cell. (2010) 1:198–204. doi: 10.1007/s13238-010-0028-8
135. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. (2020) 14:72–3. doi: 10.5582/bst.2020.01047
136. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. (2020) 382:1787–99. doi: 10.1056/NEJMoa2001282
137. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. (2020) 26:450–2. doi: 10.1038/s41591-020-0820-9
138. Menachery VD, Dinnon KH, Yount BL, McAnarney ET, Gralinski LE, Hale A, et al. Trypsin treatment unlocks barrier for zoonotic bat coronavirus infection. J Virol. (2020) 94:e01774-19. doi: 10.1128/JVI.01774-19
139. Liu W, Fontanet A, Zhang P-H, Zhan L, Xin Z-T, Baril L, et al. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis. (2006) 193:792–5. doi: 10.1086/500469
140. Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. (2002) 277:14838–43. doi: 10.1074/jbc.M200581200
141. Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. (2004) 383:45–51. doi: 10.1042/BJ20040634
142. Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. (2004) 25:291–4. doi: 10.1016/j.tips.2004.04.001
143. Rella M, Rushworth CA, Guy JL, Turner AJ, Langer T, Jackson RM. Structure-based pharmacophore design and virtual screening for novel angiotensin converting enzyme 2 inhibitors. J Chem Inform Model. (2006) 46:708–16. doi: 10.1021/ci0503614
144. Patel VB, Zhong J-C, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 axis of the renin–angiotensin system in heart failure. Circ Res. (2016) 118:1313–26. doi: 10.1161/CIRCRESAHA.116.307708
145. Warner FJ, Lew RA, Smith AI, Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem. (2005) 280:39353–62. doi: 10.1074/jbc.M508914200
146. Xia H, Suda S, Bindom S, Feng Y, Gurley S. ACE2-mediated reduction of oxidative stress in the central nervous system is associated with improvement of autonomic function. PLoS ONE. (2011) 6:e22682. doi: 10.1371/journal.pone.0022682
147. Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 18:1708–20. doi: 10.1101/2020.02.06.20020974
148. Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. (2020) 63:457–60. doi: 10.1007/s11427-020-1637-5
149. Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med. (2020) 27. doi: 10.1093/jtm/taaa041
150. Barnard D, Day C, Wandersee M, Kumaki Y, Salazar A. Evaluation of interferon inducers, ribavirin and mouse hyperimmune serum in a pathogenesis/lethal mouse model using a mouse-adapted SARS-CoV. Antiviral Res. (2008) 2:A20. doi: 10.1016/j.antiviral.2008.01.025
151. Barnard DL, Day CW, Bailey K, Heiner M, Montgomery R, Lauridsen L, et al. Enhancement of the infectivity of SARS-CoV in BALB/c mice by IMP dehydrogenase inhibitors, including ribavirin. Antiviral Res. (2006) 71:53–63. doi: 10.1016/j.antiviral.2006.03.001
152. Barnard DL, Day CW, Bailey K, Heiner M, Montgomery R, Lauridsen L, et al. Is the anti-psychotic, 10-(3-(dimethylamino) propyl) phenothiazine (promazine), a potential drug with which to treat SARS infections?: Lack of efficacy of promazine on SARS-CoV replication in a mouse model. Antiviral Res. (2008) 79:105–13. doi: 10.1016/j.antiviral.2007.12.005
153. Reghunathan R, Jayapal M, Hsu L-Y, Chng H-H, Tai D, Leung BP, et al. Expression profile of immune response genes in patients with severe acute respiratory syndrome. BMC Immunol. (2005) 6:2. doi: 10.1186/1471-2172-6-2
154. Eroshenko N, Gill T, Keaveney MK, Church GM, Trevejo JM, Rajaniemi H. Implications of antibody-dependent enhancement of infection for SARS-CoV-2 countermeasures. Nat Biotechnol. (2020) 38:789–91. doi: 10.1038/s41587-020-0577-1
155. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. (2017) 39:529–39. doi: 10.1007/s00281-017-0629-x
156. Tseng C-T, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE. (2012) 7:e35421. doi: 10.1371/journal.pone.0035421
157. Spiegel M, Pichlmair A, Martínez-Sobrido L, Cros J, García-Sastre A, Haller O, et al. Inhibition of beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J Virol. (2005) 79:2079–86. doi: 10.1128/JVI.79.4.2079-2086.2005
158. Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, et al. Chemokine up-regulation in sars-coronavirus–infected, monocyte-derived human dendritic cells. Blood. (2005) 106:2366–74. doi: 10.1182/blood-2004-10-4166
159. Surjit M, Liu B, Jameel S, Chow VT, Lal SK. The SARS coronavirus nucleocapsid protein induces actin reorganization and apoptosis in COS-1 cells in the absence of growth factors. Biochem J. (2004) 383:13–8. doi: 10.1042/BJ20040984
160. Law PT, Wong C-H, Au TC, Chuck C-P, Kong S-K, Chan PK, et al. The 3a protein of severe acute respiratory syndrome-associated coronavirus induces apoptosis in Vero E6 cells. J General Virol. (2005) 86:1921–30. doi: 10.1099/vir.0.80813-0
161. Tan Y-J, Fielding BC, Goh P-Y, Shen S, Tan TH, Lim SG, et al. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J Virol. (2004) 78:14043–7. doi: 10.1128/JVI.78.24.14043-14047.2004
162. Yuan X, Shan Y, Zhao Z, Chen J, Cong Y. G0/G1 arrest and apoptosis induced by SARS-CoV 3b protein in transfected cells. Virol J. (2005) 2:66. doi: 10.1186/1743-422X-2-66
163. Li T, Qiu Z, Zhang L, Han Y, He W, Liu Z, et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis. (2004) 189:648–51. doi: 10.1086/381535
164. Dhodapkar KM, Banerjee D, Connolly J, Kukreja A, Matayeva E, Veri MC, et al. Selective blockade of the inhibitory Fcγ receptor (FcγRIIB) in human dendritic cells and monocytes induces a type I interferon response program. J Exp Med. (2007) 204:1359–69. doi: 10.1084/jem.20062545
165. Chan KR, Zhang SL-X, Tan HC, Chan YK, Chow A, Lim APC, et al. Ligation of Fc gamma receptor IIB inhibits antibody-dependent enhancement of dengue virus infection. Proc Natl Acad Sci USA. (2011) 108:12479–84. doi: 10.1073/pnas.1106568108
166. Nagelkerke SQ, Kuijpers TW. Immunomodulation by IVIg and the role of Fc-gamma receptors: classic mechanisms of action after all? Front Immunol. (2015) 5:674. doi: 10.3389/fimmu.2014.00674
167. Lee C. Therapeutic modulation of virus-induced oxidative stress via the Nrf2-dependent antioxidative pathway. Oxid Med Cell Longev. (2018) 2018:6208067. doi: 10.1155/2018/6208067
168. Isaguliants M, Smirnova O, Ivanov AV, Kilpelainen A, Kuzmenko Y, Petkov S, et al. Oxidative stress induced by HIV-1 reverse transcriptase modulates the enzyme's performance in gene immunization. Hum Vaccines Immunother. (2013) 9:2111–9. doi: 10.4161/hv.25813
169. Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. (2008) 45:549–61. doi: 10.1016/j.freeradbiomed.2008.05.004
170. Cho H-Y, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, et al. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am J Respir Crit Care Med. (2009) 179:138–50. doi: 10.1164/rccm.200804-535OC
171. Lee Y-H, Lai C-L, Hsieh S-H, Shieh C-C, Huang L-M, Wu-Hsieh BA. Influenza A virus induction of oxidative stress and MMP-9 is associated with severe lung pathology in a mouse model. Virus Res. (2013) 178:411–22. doi: 10.1016/j.virusres.2013.09.011
172. Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. (2002) 122:366–75. doi: 10.1053/gast.2002.30983
173. Olagnier D, Peri S, Steel C, van Montfoort N, Chiang C, Beljanski V, et al. Cellular oxidative stress response controls the antiviral and apoptotic programs in dengue virus-infected dendritic cells. PLoS Pathog. (2014) 10:e1004566. doi: 10.1371/journal.ppat.1004566
174. Fukuyama S, Kawaoka Y. The pathogenesis of influenza virus infections: the contributions of virus and host factors. Curr Opin Immunol. (2011) 23:481–6. doi: 10.1016/j.coi.2011.07.016
175. Hosakote YM, Jantzi PD, Esham DL, Spratt H, Kurosky A, Casola A, et al. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. (2011) 183:1550–60. doi: 10.1164/rccm.201010-1755OC
176. Komaravelli N, Ansar M, Garofalo RP, Casola A. Respiratory syncytial virus induces NRF2 degradation through a promyelocytic leukemia protein-ring finger protein 4 dependent pathway. Free Radic Biol Med. (2017) 113:494–504. doi: 10.1016/j.freeradbiomed.2017.10.380
177. Staitieh BS, Ding L, Neveu WA, Spearman P, Guidot DM, Fan X. HIV-1 decreases Nrf2/ARE activity and phagocytic function in alveolar macrophages. J Leukoc Biol. (2017) 102:517–25. doi: 10.1189/jlb.4A0616-282RR
178. Qiang W, Kuang X, Liu J, Liu N, Scofield VL, Reid AJ, et al. Astrocytes survive chronic infection and cytopathic effects of the ts1 mutant of the retrovirus Moloney murine leukemia virus by upregulation of antioxidant defenses. J Virol. (2006) 80:3273–84. doi: 10.1128/JVI.80.7.3273-3284.2006
179. Carvajal-Yepes M, Himmelsbach K, Schaedler S, Ploen D, Krause J, Ludwig L, et al. Hepatitis C virus impairs the induction of cytoprotective Nrf2 target genes by delocalization of small Maf proteins. J Biol Chem. (2011) 286:8941–51. doi: 10.1074/jbc.M110.186684
180. Machida K, Cheng KT-H, Lai C-K, Jeng K-S, Sung VM-H, Lai MM. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. J Virol. (2006) 80:7199–207. doi: 10.1128/JVI.00321-06
181. Komaravelli N, Tian B, Ivanciuc T, Mautemps N, Brasier AR, Garofalo RP, et al. Respiratory syncytial virus infection down-regulates antioxidant enzyme expression by triggering deacetylation-proteasomal degradation of Nrf2. Free Radic Biol Med. (2015) 88:391–403. doi: 10.1016/j.freeradbiomed.2015.05.043
182. Johnson B, Li J, Adhikari J, Edwards MR, Zhang H, Schwarz T, et al. Dimerization controls Marburg virus VP24-dependent modulation of host antioxidative stress responses. J Mol Biol. (2016) 428:3483–94. doi: 10.1016/j.jmb.2016.07.020
183. Page A, Volchkova VA, Reid SP, Mateo M, Bagnaud-Baule A, Nemirov K, et al. Marburgvirus hijacks nrf2-dependent pathway by targeting nrf2-negative regulator keap1. Cell Rep. (2014) 6:1026–36. doi: 10.1016/j.celrep.2014.02.027
184. Cheng Y-L, Lin Y-S, Chen C-L, Tsai T-T, Tsai C-C, Wu Y-W, et al. Activation of Nrf2 by the dengue virus causes an increase in CLEC5A, which enhances TNF-α production by mononuclear phagocytes. Sci Rep. (2016) 6:32000. doi: 10.1038/srep32000
185. Kesic MJ, Simmons SO, Bauer R, Jaspers I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic Biol Med. (2011) 51:444–453. doi: 10.1016/j.freeradbiomed.2011.04.027
186. Kosmider B, Messier EM, Janssen WJ, Nahreini P, Wang J, Hartshorn KL, et al. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir Res. (2012) 13:43. doi: 10.1186/1465-9921-13-43
187. Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. (2005) 308:1643–5. doi: 10.1126/science.1110656
188. Matsuyama S, Taguchi F. Two-step conformational changes in a coronavirus envelope glycoprotein mediated by receptor binding and proteolysis. J Virol. (2009) 83:11133–41. doi: 10.1128/JVI.00959-09
189. Simmons G, Reeves JD, Rennekamp AJ, Amberg SM, Piefer AJ, Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc Natl Acad Sci USA. (2004) 101:4240–5. doi: 10.1073/pnas.0306446101
190. Böttcher E, Matrosovich T, Beyerle M, Klenk H-D, Garten W, Matrosovich M. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J Virol. (2006) 80:9896–8. doi: 10.1128/JVI.01118-06
191. Jiang Y, Scofield VL, Yan M, Qiang W, Liu N, Reid AJ, et al. Retrovirus-induced oxidative stress with neuroimmunodegeneration is suppressed by antioxidant treatment with a refined monosodium α-luminol (Galavit). J Virol. (2006) 80:4557–69. doi: 10.1128/JVI.80.9.4557-4569.2006
192. Zhang H-S, Wu T-C, Sang W-W, Ruan Z. EGCG inhibits Tat-induced LTR transactivation: role of Nrf2, AKT, AMPK signaling pathway. Life Sci. (2012) 90:747–54. doi: 10.1016/j.lfs.2012.03.013
193. Zhang HS, Chen XY, Wu TC, Zhang FJ. Tanshinone II A inhibits tat-induced HIV-1 transactivation through redox-regulated AMPK/Nampt pathway. J Cell Physiol. (2014) 229:1193–201. doi: 10.1002/jcp.24552
194. Fan X, Staitieh BS, Jensen JS, Mould KJ, Greenberg JA, Joshi PC, et al. Activating the Nrf2-mediated antioxidant response element restores barrier function in the alveolar epithelium of HIV-1 transgenic rats. Am J Physiol Lung Cell Mol Physiol. (2013) 305:L267–77. doi: 10.1152/ajplung.00288.2012
195. Chen W-C, Tseng C-K, Lin C-K, Wang S-N, Wang W-H, Hsu S-H, et al. Lucidone suppresses dengue viral replication through the induction of heme oxygenase-1. Virulence. (2018) 9:588–603. doi: 10.1080/21505594.2017.1421893
196. Chen J-X, Xue H-J, Ye W-C, Fang B-H, Liu Y-H, Yuan S-H, et al. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol Pharma Bull. (2009) 32:1385–91. doi: 10.1248/bpb.32.1385
197. Youn GS, Kwon D-J, Ju SM, Rhim H, Bae YS, Choi SY, et al. Celastrol ameliorates HIV-1 Tat-induced inflammatory responses via NF-kappaB and AP-1 inhibition and heme oxygenase-1 induction in astrocytes. Toxicol Appl Pharmacol. (2014) 280:42–52. doi: 10.1016/j.taap.2014.07.010
198. Noah TL, Zhang H, Zhou H, Glista-Baker E, Müller L, Bauer RN, et al. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: a randomized, double-blind study. PLoS ONE. (2014) 9:e98671. doi: 10.1371/journal.pone.0098671
199. Shoji M, Arakaki Y, Esumi T, Kohnomi S, Yamamoto C, Suzuki Y, et al. Bakuchiol is a phenolic isoprenoid with novel enantiomer-selective anti-influenza A virus activity involving Nrf2 activation. J Biol Chem. (2015) 290:28001–17. doi: 10.1074/jbc.M115.669465
200. Yin J, Ma L, Wang H, Yan H, Hu J, Jiang W, et al. Chinese herbal medicine compound Yi-Zhi-Hao pellet inhibits replication of influenza virus infection through activation of heme oxygenase-1. Acta Pharma Sin B. (2017) 7:630–7. doi: 10.1016/j.apsb.2017.05.006
201. Dai J, Gu L, Su Y, Wang Q, Zhao Y, Chen X, et al. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int Immunopharmacol. (2018) 54:177–87. doi: 10.1016/j.intimp.2017.11.009
202. Snitsarev V, Young MN, Miller RM, Rotella DP. The spectral properties of (-)-epigallocatechin 3-O-gallate (EGCG) fluorescence in different solvents: Dependence on solvent polarity. PLoS ONE. (2013) 8:e79834. doi: 10.1371/journal.pone.0079834
203. Ahn Y-H, Hwang Y, Liu H, Wang XJ, Zhang Y, Stephenson KK, et al. Electrophilic tuning of the chemoprotective natural product sulforaphane. Proc Natl Acad Sci USA. (2010) 107:9590–5. doi: 10.1073/pnas.1004104107
204. Wang Y, Yan J, Li S, Cai X, Wang W, Luo K, et al. Pharmacokinetics and tissue distribution study of tanshinone IIA after oral administration of Bushen Huoxue Qubi granules to rats with blood stasis syndrome. Pharmacogn Mag. (2014) 10:285. doi: 10.4103/0973-1296.137369
205. Cascão R, Fonseca JE, Moita LF. Celastrol: a spectrum of treatment opportunities in chronic diseases. Front Med. (2017) 4:69. doi: 10.3389/fmed.2017.00069
206. Alalaiwe A, Hung C-F, Leu Y-L, Tahara K, Chen H-H, Hu K-Y, et al. The active compounds derived from Psoralea corylifolia for photochemotherapy against psoriasis-like lesions: the relationship between structure and percutaneous absorption. Eur J Pharma Sci. (2018) 124:114–26. doi: 10.1016/j.ejps.2018.08.031
207. He Y-W, Dong C-Z, Zhao J-Y, Ma L-L, Li Y-H, Aisa HA. 1, 2, 3-Triazole-containing derivatives of rupestonic acid: click-chemical synthesis and antiviral activities against influenza viruses. Eur J Med Chem. (2014) 76:245–55. doi: 10.1016/j.ejmech.2014.02.029
208. Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives–a review. J Tradit Complement Med. (2017) 7:205–33. doi: 10.1016/j.jtcme.2016.05.005
209. Drăgoi A-L. Potent NRF2-activating dietary supplements (like resveratrol, curcumin, sulforaphane, “Asea redox supplement” [ARS]) should be clinically tested as adjuvants in all types of medium and severe cases of aggressive respiratory viral infections (including Influenza A/B/C, SARS, MERS, COVID-19, measles, avian influenza etc.) based on their extrapolated cytoprotective antioxidant effects (especially on vital organs), including the cytoprotection offered by ARS on the cardiac muscle of DMD patients which can be extrapolated to the lungs - A very short medical communication. (2019). doi: 10.13140/RG.2.2.33764.12163G.2.2.33764.12163
210. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
211. Chen H, Du Q. Potential natural compounds for preventing 2019-nCoV infection. Europe PMC [preprint]. (2020). doi: 10.20944/preprints202001.0358.v3
212. Lin C-W, Tsai F-J, Tsai C-H, Lai C-C, Wan L, Ho T-Y, et al.-Chao DL. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. (2005) 68:36–42. doi: 10.1016/j.antiviral.2005.07.002
213. Cheng LP, Zheng WK, Li M, Huang J, Bao SZ, Xu Q, et al. Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2. Preprints. (2020) 2020:1–13.
214. Wen C-C, Kuo Y-H, Jan J-T, Liang P-H, Wang S-Y, Liu H-G, et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. (2007) 50:4087–95. doi: 10.1021/jm070295s
215. O'Keefe BR, Giomarelli B, Barnard DL, Shenoy SR, Chan PK, McMahon JB, et al. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J Virol. (2010) 84:2511–21. doi: 10.1128/JVI.02322-09
216. Kim DW, Seo KH, Curtis-Long MJ, Oh KY, Oh J-W, Cho JK, et al. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J Enzyme Inhibit Med Chem. (2014) 29:59–63. doi: 10.3109/14756366.2012.753591
217. Moghaddam E, Teoh B-T, Sam S-S, Lani R, Hassandarvish P, Chik Z, et al. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci Rep. (2014) 4:5452. doi: 10.1038/srep05452
218. Liu Q, Li X, Ouyang X, Chen D. Dual effect of glucuronidation of a Pyrogallol-type phytophenol antioxidant: a comparison between scutellarein and scutellarin. Molecules. (2018) 23:3225. doi: 10.3390/molecules23123225
219. Fushiya S, Takahashi K, Nakatsuyama S, Sato Y, Nozoe S, Takagi S-I. Co-occurrence of nicotianamine and avenic acids in Avena sativa and Oryza sativa. Phytochemistry. (1982) 21:1907–8. doi: 10.1016/0031-9422(82)83012-4
220. Kristensen I, Larsen PO. Azetidine-2-carboxylic acid derivatives from seeds of Fagus silvatica L. and a revised structure for nicotianamine. Phytochemistry. (1974) 13:2791–8. doi: 10.1016/0031-9422(74)80243-8
221. Hordyjewska A, Ostapiuk A, Horecka A, Kurzepa J. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem Rev. (2019) 18:929–51. doi: 10.1007/s11101-019-09623-1
222. Xue J, Hoorelbeke B, Kagiampakis I, Demeler B, Balzarini J, LiWang PJ. The griffithsin dimer is required for high-potency inhibition of HIV-1: evidence for manipulation of the structure of gp120 as part of the griffithsin dimer mechanism. Antimicrob Agents Chemother. (2013) 57:3976–89. doi: 10.1128/AAC.00332-13
Keywords: SARS-CoV, SARS-CoV-2, COVID-19, natural Nrf-2 modulators, NSMIs
Citation: Beeraka NM, Sadhu SP, Madhunapantula SV, Rao Pragada R, Svistunov AA, Nikolenko VN, Mikhaleva LM and Aliev G (2020) Strategies for Targeting SARS CoV-2: Small Molecule Inhibitors—The Current Status. Front. Immunol. 11:552925. doi: 10.3389/fimmu.2020.552925
Received: 17 April 2020; Accepted: 18 August 2020;
Published: 18 September 2020.
Edited by:
Denise L. Doolan, James Cook University, AustraliaReviewed by:
Rong Hai, University of California, Riverside, United StatesKatie Louise Flanagan, RMIT University, Australia
Copyright © 2020 Beeraka, Sadhu, Madhunapantula, Rao Pragada, Svistunov, Nikolenko, Mikhaleva and Aliev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: SubbaRao V. Madhunapantula, bXZzc3RzdWJiYXJhbyYjeDAwMDQwO2pzc3VuaS5lZHUuaW4=; Gjumrakch Aliev, YWxpZXYwMyYjeDAwMDQwO2dtYWlsLmNvbQ==; Y29iYWx0NTUmI3gwMDA0MDtnYWxseWludGVybmF0aW9uYWwuY29t
†These authors have contributed equally to this work
 Narasimha M. Beeraka
Narasimha M. Beeraka Surya P. Sadhu2†
Surya P. Sadhu2† Gjumrakch Aliev
Gjumrakch Aliev