- 1The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
- 2Union Hospital Affiliated with Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China
- 3Department of Neurology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, China
- 4School of Public Health, Bengbu Medical College, Bengbu, China
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is spreading worldwide. Measuring the prevention and control of the disease has become a matter requiring urgent focus.
Objective: Based on coronavirus disease 2019 (COVID-19) clinical data from Wuhan, we conducted an in-depth analysis to clarify some of the pathological mechanisms of the disease and identify simple measures to predict its severity early on.
Methods: A total of 230 patients with non-mild COVID-19 were recruited, and information on their clinical characteristics, inflammatory cytokines, and T lymphocyte subsets was collected. Risk factors for severity were analyzed by binary logistic regression, and the associations of neutrophil-to-lymphocyte ratios (N/LRs) with illness severity, disease course, CT grading, inflammatory cytokines, and T lymphocyte subsets were evaluated.
Results: Our results showed that the N/LRs were closely related to interleukin (IL)-6 and IL-10 (P < 0.001, P = 0.024) and to CD3+ and CD8+ T lymphocytes (P < 0.001, P = 0.046). In particular, the N/LRs were positively correlated with the severity and course of the disease (P = 0.021, P < 0.001). Compared to the values at the first test after admission, IL-6 and IL-10 were significantly decreased and increased, respectively, as of the last test before discharge (P = 0.006, P < 0.001). More importantly, through binary logistic regression, we found that male sex, underlying diseases (such as cardiovascular disease), pulse, and N/LRs were all closely related to the severity of the disease (P = 0.004, P = 0.012, P = 0.013, P = 0.028).
Conclusions: As a quick and convenient marker of inflammation, N/LRs may predict the disease course and severity level of non-mild COVID-19; male sex, cardiovascular disease, and pulse are also risk factors for the severity of non-mild COVID-19.
Introduction
At present, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (1) is largely under control in China, but it is still spreading rapidly worldwide, seriously threatening and damaging global health (2). The prevention and control of coronavirus disease 2019 (COVID-19) is currently the most urgent issue facing the globe.
Many studies have confirmed that inflammatory storms are the key pathological basis determining the clinical symptoms, course, and prognosis of COVID-19 (3). According to the diagnosis and treatment protocol for COVID-19 (seventh edition) (4), the pathologic anatomy of patients with COVID-19 revealed that the lungs, spleen, cardiovascular system, liver, kidney, and other organs were markedly infiltrated by a large number of inflammatory cells, which resulted in functional damage and functional failure. Many studies have confirmed that the impairment of immune function directly affects the course and prognosis of COVID-19 (5). By autopsy, a decrease was observed in the numbers of CD4+ and CD8+ T cells in the spleen and lymph nodes, and denaturation and necrosis of the spleen and lymph nodes were also found, in addition to the proliferation of spleen macrophages. It has been speculated that T lymphocytes and macrophages may also be important targets for COVID-19 (6).
Severe pneumonia and acute respiratory distress syndrome are the worst outcomes in patients with COVID-19, resulting in cytokine release syndrome and multiorgan failure; the role of IL-6 in the pathological development of patients with COVID-19 has been a focus of research (7, 8), but the function of IL-6 in the course of the disease is still controversial (9–12). Lymphocytopenia is a common feature of COVID-19 and may be a key factor associated with disease severity and mortality (13). It is critically important to identify at the early stage of the infection those patients who would be prone to developing the most adverse effects. Based on clinical data from Wuhan (which had a severe outbreak of COVID-19 early in the pandemic), we conducted an in-depth analysis to clarify some of the pathological mechanisms of the disease and identify simple measures to predict its severity early on.
Materials and Methods
Ethical Approval and Consent to Participate
This study was approved by the Research Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China (USTC) and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants gave their informed consent prior to their inclusion in this study. Consent was obtained directly from each patient or from a family member or other legal guardian. If a patient was considered incapable of giving informed consent, a family member or other legal guardian was contacted to give informed consent on behalf of the patient. All patients or their close relations were informed of the purpose of the study and signed informed consent. The consent procedures were approved by the ethics committee.
Subjects
We collected the clinical data of all inpatients with COVID-19 in the cancer center of Wuhan Union Hospital from February 2, 2020 to March 17, 2020.
Inclusion and Exclusion Criteria
The diagnostic criteria for suspected cases of COVID-19 pneumonia included the following (4): (1) Epidemiological history included: (i) pre-onset history of travel or residence in Wuhan or other areas where local cases continue to spread, (ii) fever or respiratory symptoms in patients who were from Wuhan City or other areas with continued local spread and were exposed 14 days before onset, and (iii) a cluster or epidemiological association with COVID-19 infection; (2) the following clinical features were present: (i) fever, (ii) imaging features indicative of pneumonia, and (iii) a normal or decreased total number of white blood cells in the early stage of the disease or a reduced lymphocyte count; or (3) if there was any epidemiological history, a suspected case could be diagnosed in the presence of any two of the listed clinical manifestations.
The inclusion criteria were as follows: (1) The case met two criteria: (i) suspected COVID-19 and (ii) detection of COVID-19 nucleic acids by real-time fluorescent RT-PCR using sputum, pharyngeal swabs, or lower respiratory tract exudate; (2) non-mild severity.
The exclusion criteria were as follows: mild cases were defined according to the National Protocol: “the clinical symptoms were mild, and there was no sign of pneumonia on imaging” (4). Given the state of emergency at the time, only patients with moderate or severe cases were admitted to the cancer center of Wuhan Union Hospital; patients with mild symptoms were admitted to the mobile cabin hospital (14).
Clinical data on 230 patients with COVID-19 pneumonia in the isolation ward of the cancer center of Wuhan Union Hospital who met the above criteria were collected from February 2 to March 17, 2020.
Clinical Classification of Disease Severity
All confirmed patients were clinically classified according to the “Novel Coronavirus Pneumonia Treatment Scheme of the National Health Commission of the People’s Republic of China (version 7)” at the time of admission (4), as follows: (1) general type: symptoms such as fever and respiratory tract complaints were present, and manifestations of pneumonia could be seen on imaging; (2) serious type: any of the following criteria were met: (1) respiratory distress, respiratory rate (RR) ≥ 30 times/min; (2) resting oxygen saturation ≤ 93%; or (3) arterial partial oxygen pressure (PaO2)/oxygen absorption concentration (FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa); (3) critical type: any of the following criteria were met: (1) respiratory failure and a need for mechanical ventilation, (2) shock, or (3) a combination of factors with other organ failure requiring ICU care.
Course of Disease and CT Grading
The course of disease was defined as the time (days) between the onset of clinical symptoms of COVID-19 and the time of discharge from the hospital (symptoms recovery). All images were independently read by three senior radiological specialists. The location, shape, number, and size of the abnormalities on chest CTs were carefully observed and recorded. In case of discordant reading, consensus was reached during another reading session. CT grading was defined according to the “Novel Coronavirus Pneumonia Treatment Scheme of the National Health Commission of the People’s Republic of China (version 7)”and Chinese expert consensus (4, 15).
Testing Methods
Real-Time Reverse Transcription Polymerase Chain Reaction Tests
The confirmation of COVID-19 was achieved by real-time reverse transcription polymerase chain reaction (RT-PCR) testing of throat sputum, pharyngeal swabs, and lower respiratory tract exudates of suspected patients. Following the recommendation of the China National Centre for Disease Control, two target genes were targeted as described previously (16), namely, open reading frame 1ab (ORF1ab) and nucleocapsid protein (N), and these genes were simultaneously amplified and tested during the real-time RT-PCR assay. The primers for target 1 (ORF1ab) were forward primer CCCTGTGGGTTTTACACTTAA; reverse primer ACGATTGTGCATCAGCTGA; and probe 5′-FAM-CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1-3′. The primers for target 2 (N) were forward primer GGGGAACT TCTCCTGCTAGAAT; reverse primer CAGACATTTTGCT CTCAAGCTG; and probe 5′-FAMTTGCTGCTGCTTGACAG ATT-TAMRA-3′. A cycle threshold value (Ct value) less than 37 was defined as a positive result, and a Ct value exceeding 40 was defined as a negative test.
Inflammatory Cytokines and General Laboratory Tests
Venous blood was taken in the emergency department at admission and sent to the laboratory for examination of routine blood parameters and inflammatory cytokines. However, T lymphocyte subsets and some biochemical indicators were obtained by testing fasting blood the morning after admission. Serum inflammatory cytokines were detected by enzyme-linked immunosorbent assays for IL-2, IL-4, IL-6, IL-6, IL-10, and tumor necrosis factor-α (TNF-α). The tests were carried out strictly according to the instructions, and the test kits were purchased from eBioscience Company (EPX650-16500-901).
Flow Cytometry Assay
In order to quantify T lymphocyte subsets, 100 μl of whole blood was incubated in 900 μl of Tris-NH4Cl buffer (Thermo Fisher Scientific) at room temperature for 5 min to lyse erythrocytes. After two washes with phosphate-buffered saline (PBS), cells were incubated with CD3-APC, CD4-PerCP, and CD8-FITC antibodies (5 μg/ml each, all from BD Biosciences) for 15 min on ice. After another two washes with PBS, cells were resuspended in 500 μl of PBS. Samples were analyzed on a BD FACS Canto Plus flow cytometer. Among all collected events, single events were gated between forward scatter (FSC)-A and FSC-H. Cell debris was excluded, and intact cells were then gated from single events based on FSC-A and side scatter (SSC). Each cell population was then detected based on antibody staining.
Statistical Analysis
All statistical analyses were conducted with Statistical Package for the Social Sciences version 16.0 (SPSS, Company, Chicago, IL, United States). All continuous data were tested for normal distributions using the Kolmogorov–Smirnov test. Normally distributed variables were described as the mean ± standard deviation (M ± SD), and non-normally distributed variables were presented as the median and the interquartile range [median (IQR)]. Categorical variables were expressed as constituent ratios and percentages in each category. One-way ANOVA or Kruskal–Wallis analysis was applied to continuous variables, and the chi-square test or Fisher’s exact test was applied to categorical variables. Differences in cytokine and T lymphocyte subsets tested twice for each patient were assessed by paired t-test or Wilcoxon signed rank test. Correlation analysis was used to determine the relationship between N/LRs and inflammatory cytokines, T lymphocyte subsets, severity of illness, course of disease, or CT grading. N/LRs in different patient condition groups were tested by Kruskal–Wallis analysis and the Jonckheere–Terpstra trend test. Binary logistic regression analysis was used to test the risk factors for the severity level of patients with COVID-19. A P value < 0.05 was considered statistically significant.
Results
(1) Clinical characteristics of patients with COVID-19.
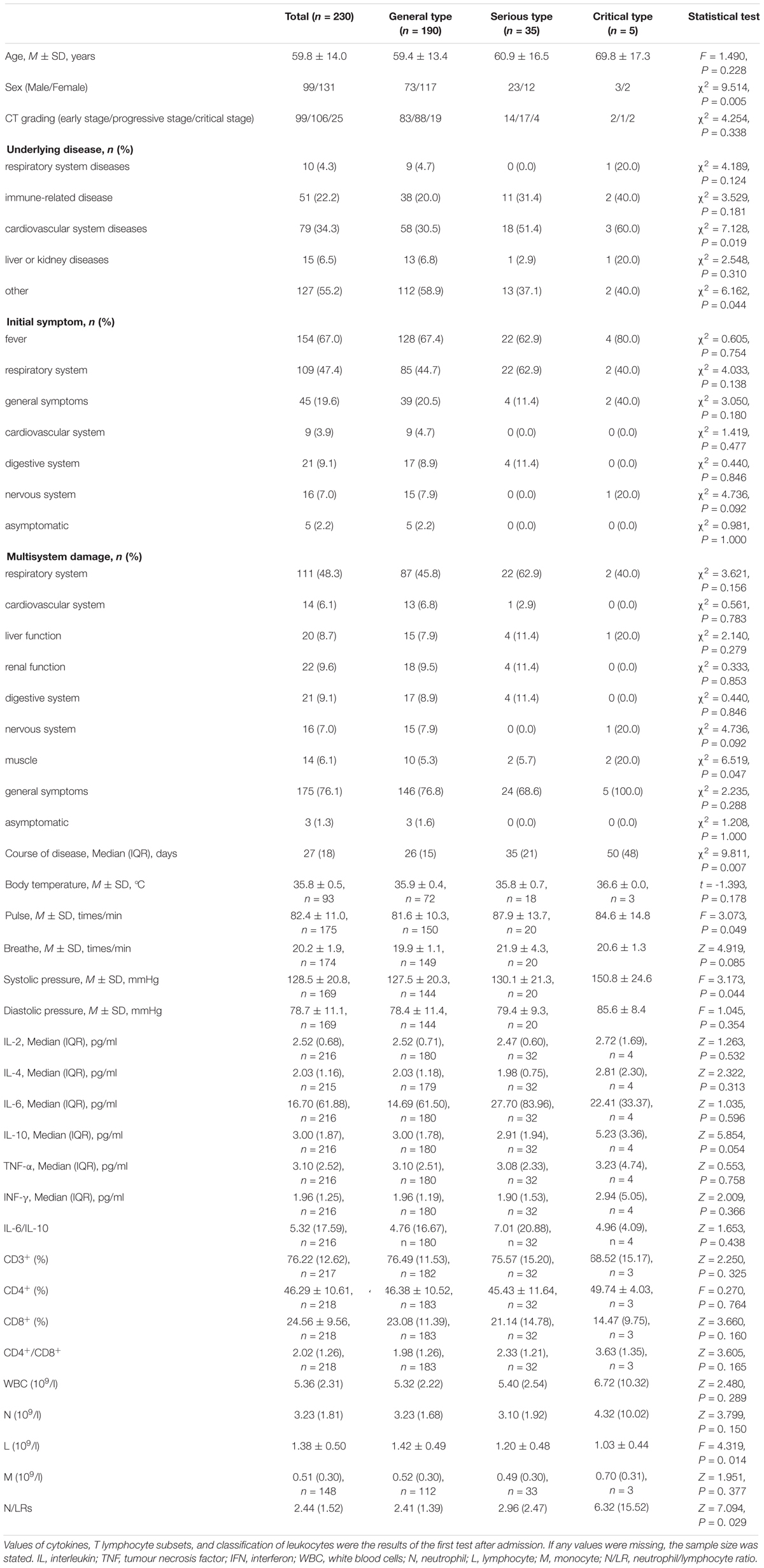
We collected 230 patients with COVID-19; 99 (43%) patients were male, the average age was 62 (16) years, and the total course of disease was 27 (18) days. The distribution of severity categories was as follows: 190 (82.6%) patients had the general type, 35 (15.2%) patients had the serious type, and five (2.2%) patients had the critical type. According to CT grading, 99 (43.0%) patients were in the early stage, nearly half (106 patients, 46.1%) were in the progressive stage, and 25 (10.9%) were in the critical stage. The underlying (comorbid) diseases of the patients were as follows: 10 (4.3%) patients had respiratory system diseases, 51 (22.2%) patients had immune-related diseases, 79 (34.3%) patients had cardiovascular system diseases, 15 (6.5%) patients had liver or kidney diseases, and 127 (55.2%) patients had other comorbidities. The patients’ initial symptoms were as follows: 154 (67.0%) patients had fever, 109 (47.4%) patients had respiratory system symptoms, 45 (19.6%) patients had general symptoms, nine (3.9%) patients had cardiovascular system symptoms, 21 (9.1%) patients had digestive system symptoms, 16 (7.0%) patients had nervous system symptoms, and five (2.2%) patients were asymptomatic. The damage to physiological systems was as follows (Multiple system damage were directly caused by COVID-19): 111 (48.3%) patients had respiratory system damage, 14 (6.1%) patients had cardiovascular system damage, 20 (8.7%) patients had liver function damage, 22 (9.6%) patients had renal function damage, 21 (9.1%) patients had digestive system damage, 16 (7.0%) patients had nervous system damage, and 14 (6.1%) patients had muscle damage. A total of 175 (76.1%) patients had general symptoms. Of the patients, three (1.3%) were asymptomatic. The variables with statistical significance were identified by univariate analysis. These variables were sex (male) (P = 0.005), underlying disease (cardiovascular system diseases) (P = 0.019), multisystem damage (muscle) (P = 0.047), course of disease (P = 0.007), pulse (P = 0.049), systolic pressure (P = 0.044), L (P = 0. 014), and N/LRs (P = 0. 029) (see Table 1).
(2) There were significant differences between the first test and last test regarding the cytokines and classification of leukocytes.
We collected the data regarding the cytokines and T lymphocyte subsets and classification of leukocytes at the time of admission (first test) and the time of discharge (last test), and there were statistically significant differences in cytokine levels between admission and discharge. For example, IL-6 and IL-10 were significantly decreased and increased, respectively (P = 0.006, P < 0.001). The IL-6/IL-10 ratio was also significantly different (P < 0.001). Additionally, there were statistically significant differences in lymphocytes and N/LRs between admission and discharge (P = 0.002, P < 0.001). However, there were no statistically significant differences in T lymphocyte subsets between admission and discharge (Table 2).
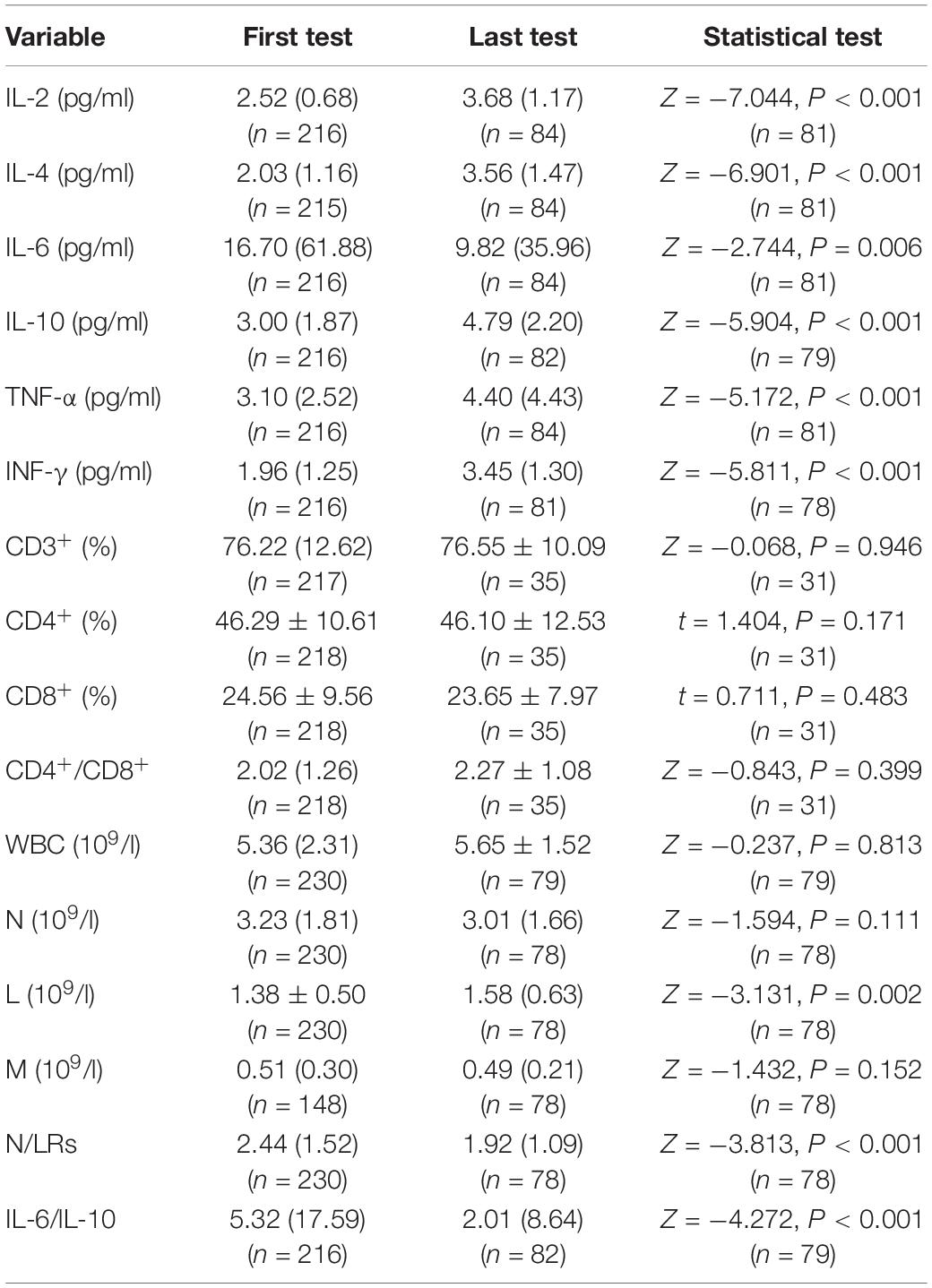
Table 2. Cytokines, T lymphocyte subsets, and classification of leukocytes in patients with COVID-19 (N = 230).
(3) Risk factors for the severity of COVID-19.
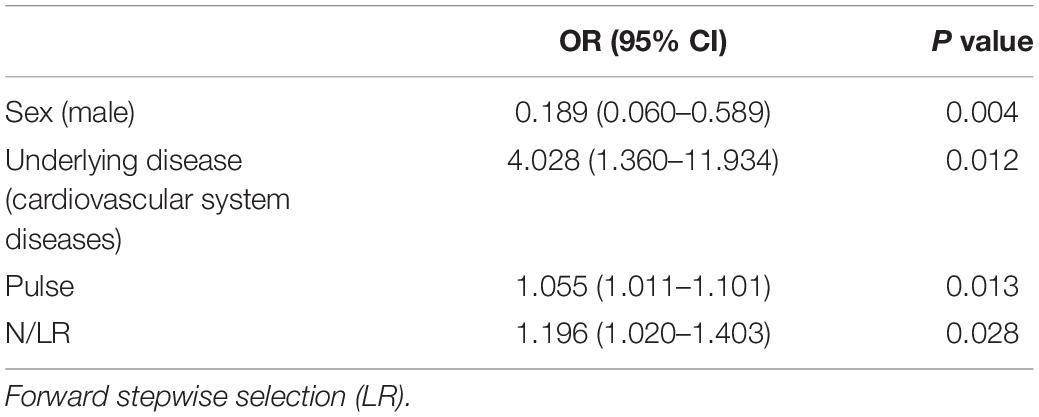
The risk factors for the severity of COVID-19 were analyzed by binary logistic regression. Due to the small number of critical-type samples, the critical type and the serious type were grouped together. Variables with statistically significant differences among different groups of diseases by univariate analysis were substituted into the regression equation, and finally, four variables with statistical significance were selected: male sex, underlying disease (cardiovascular disease), pulse, and N/LRs were all closely related to the severity of the disease (P = 0.004, P = 0.012, P = 0.013, P = 0.028) (Table 3).
(4) N/LRs are closely related to severity level and disease course.
We found that N/LRs were closely related to the severity level and disease course of COVID-19 according to the Kruskal–Wallis test, P = 0.021 and P < 0.001, respectively (Table 4).

Table 4. The relationship between N/LRs and the severity of illness, course of disease, and CT grading (N = 230).
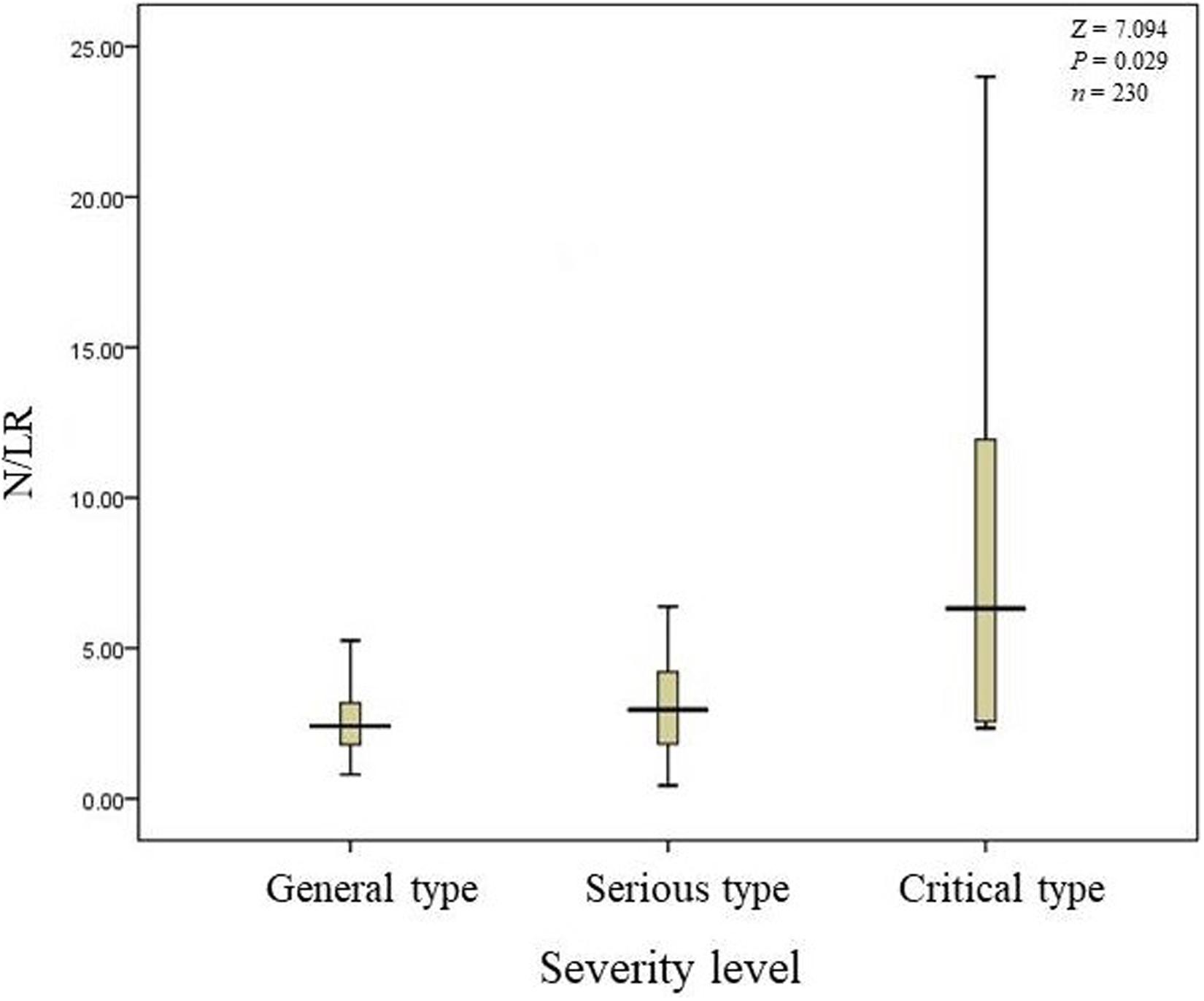
(5) Patients with higher N/LRs had higher severity and a longer disease course.
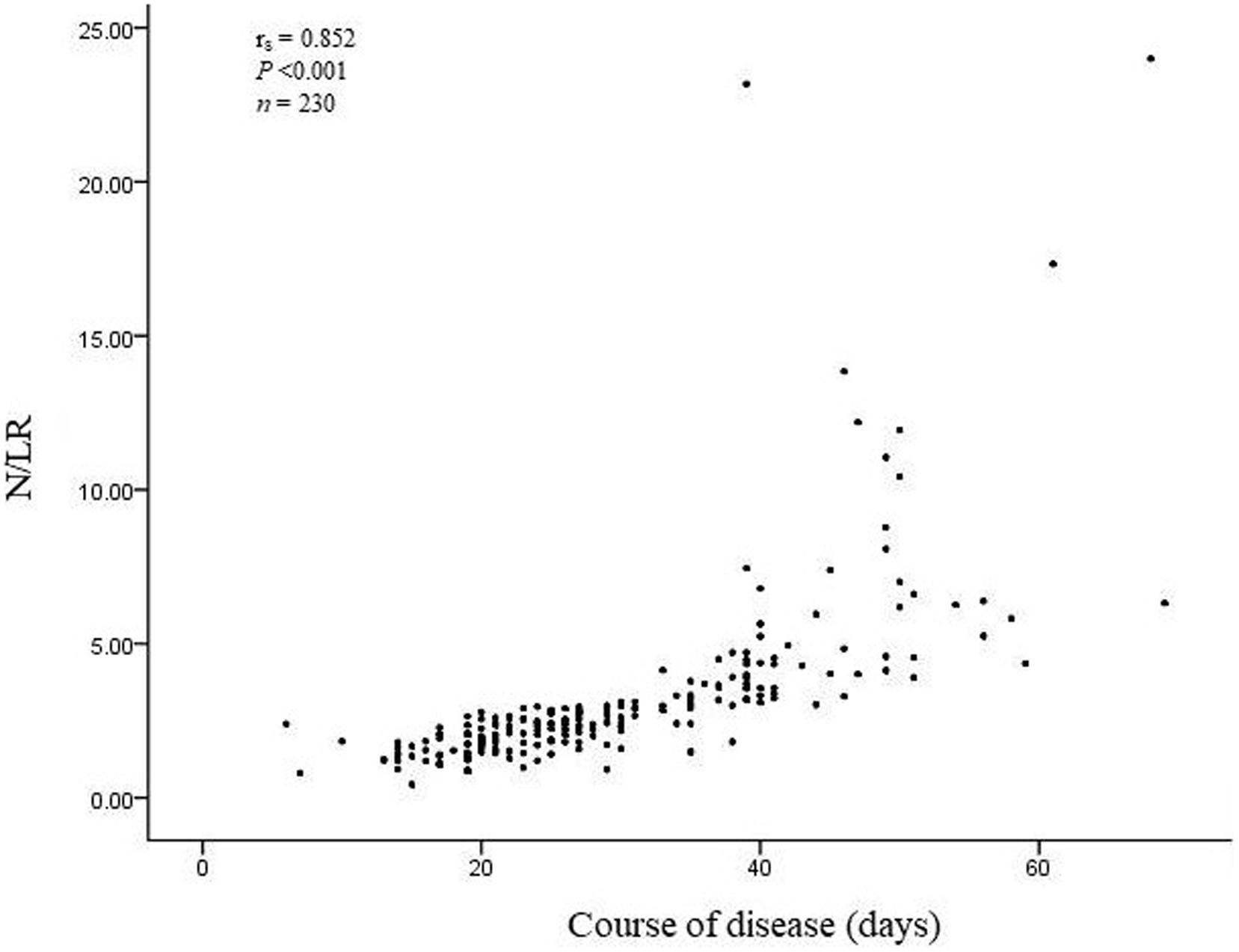
The total number of COVID-19 patients was 230. 190 patients’ N/LRs were 2.41 (1.39), with general symptoms; 35 patients’ N/LRs were 2.96 (2.47), with serious symptoms; and five patients’ N/LRs were 6.32 (15.52), with critical symptoms, according to the Kruskal-Wallis test (Z = 7.094, P = 0.029) (Table 1). The difference between each pair of groups was statistically significant. Patients with higher N/LRs had more severe disease. A Jonckheere-Terpstra trend test was performed and showed a statistically significant difference between each group; the more severe the disease, the higher the N/LR (Figure 1). Patients with higher N/LRs had longer disease courses (Figure 2) (P < 0.001).
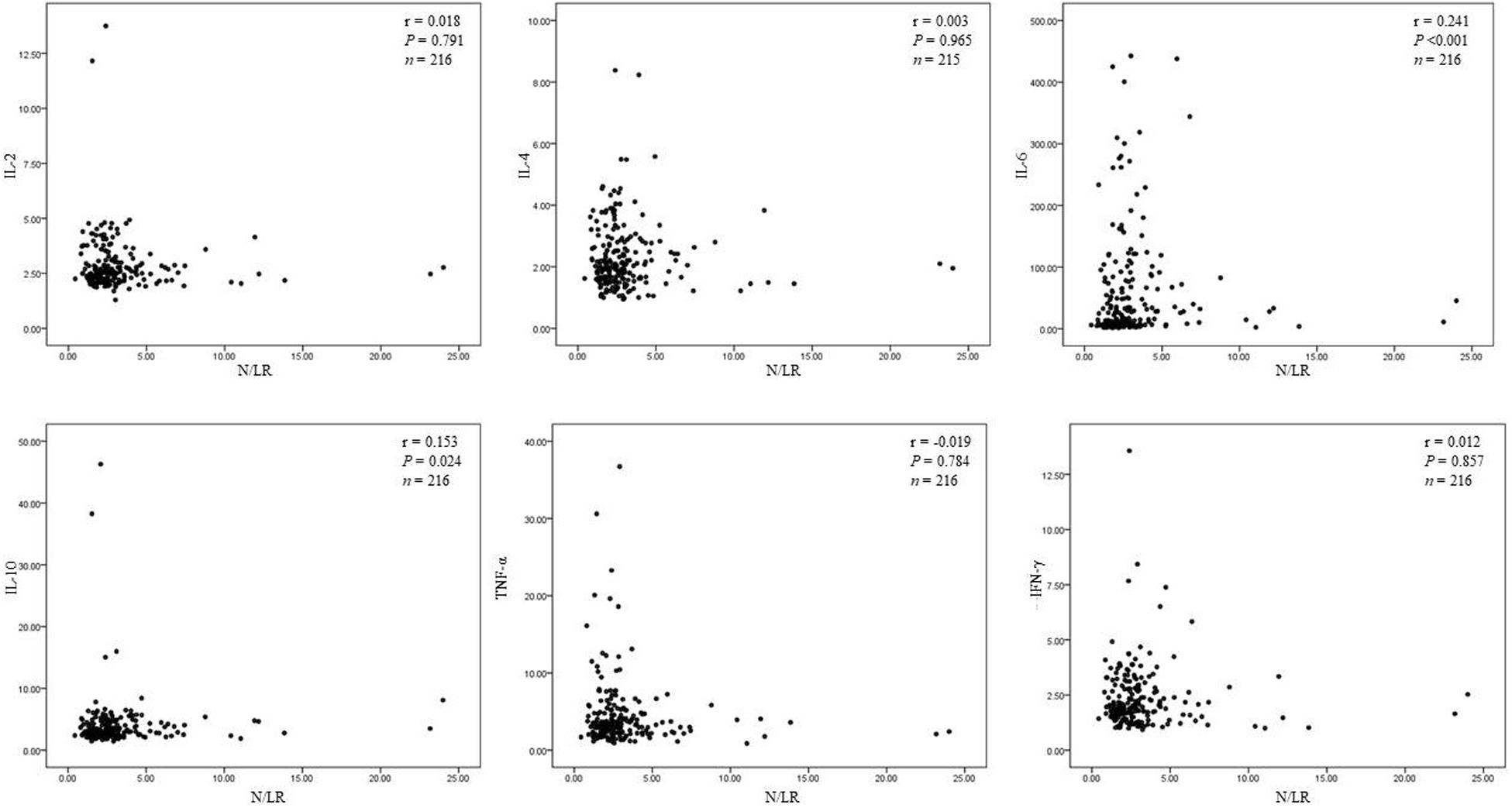
(6) N/LRs were closely related to IL-6 and IL-10.
We found that N/LRs were positively related to IL-6 and IL-10, P < 0.001 and P = 0.024, respectively. However, there was no significant correlation with other cytokines (Figure 3). The trend of the relationship between N/LRs and different inflammatory cytokines is shown in Figure 3.
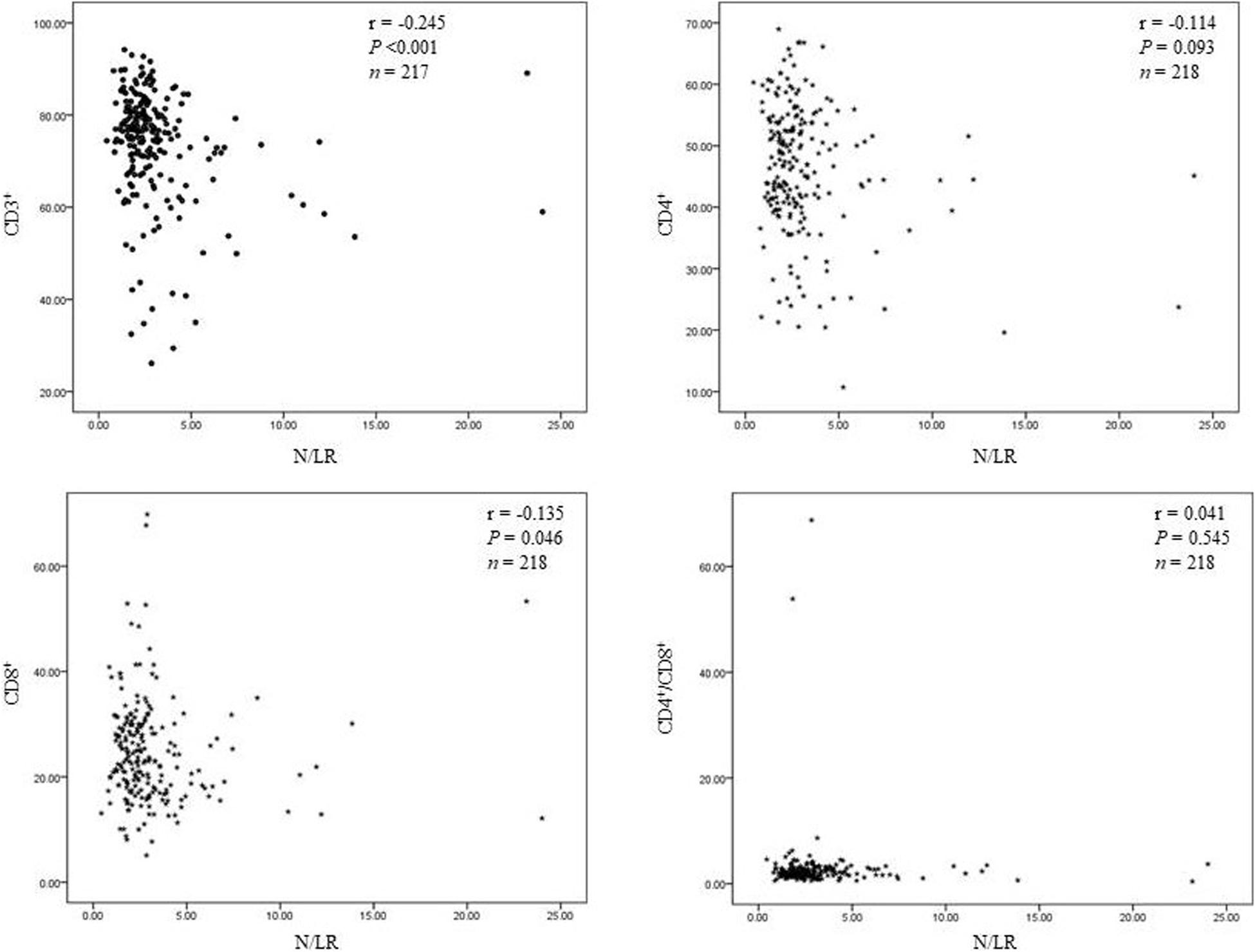
(7) N/LRs are closely related to CD3+ and CD8+ cells (T lymphocyte subsets).
We found that N/LRs were negatively related to CD3 + and CD8 + T lymphocytes (P < 0.001 and P = 0.046, respectively; Figure 4). The trend of the relationship between N/LRs and different T lymphocyte subsets is shown in Figure 4.
Discussion
In this study, we analyzed the clinical data of 230 patients diagnosed with non-mild COVID-19 in the cancer center of Wuhan Union Hospital and summarized the clinical characteristics of this disease. We found the following: (1) N/LRs, male sex, underlying disease (cardiovascular disease), and pulse were all closely related to the severity of the disease; (2) N/LRs were positively correlated with the levels of cytokines (IL-6, IL-10); (3) N/LRs were negatively correlated with the proportion of CD3+ and CD8+ T lymphocyte subsets; (4) N/LRs were positively correlated with the severity of the disease, as the higher N/LRs were, the more serious the disease; (5) N/LRs were related to the course of disease, as the higher N/LRs were, the longer the course of disease; and (6) most patients had multisystem symptoms and injury and had underlying multisystem comorbidities.
As the COVID-19 epidemic continues to spread across the globe, effective treatment is urgent, and it is particularly crucial to explore the pathogenesis of the disease. COVID-19 infection activates innate and adaptive immune responses. However, uncontrolled damage from the natural inflammatory response and adaptive immune response may lead to local and systemic tissue damage. Lymphocytopenia is a common feature in patients with severe COVID-19, with a dramatic decrease in the number of CD4+ and CD8+ T cells, B cells, and natural killer (NK) cells (5, 17–19) and lower proportions of monocytes, eosinophils, and basophils among leukocytes (18, 20); in particular, patients with higher N/LRs have poorer outcomes (20). Yang et al. found that NLRs can be considered independent biomarkers for indicating poor clinical outcomes (21). Consistent with the above data, our research found that the NLRs were closely related to the severity and course of patients with non-mild COVID-19 and that higher N/LRs were related to longer courses and more severe non-mild COVID-19.
Currently, N/LRs are a well-known marker of systemic inflammation and infection, and they have been studied as predictors of bacterial infection, showing superior predictive value over conventional inflammatory markers (22–24). In addition, the N/LRs have displayed good predictive power for pneumonia, as well as dose-response information relating to the burden of community-acquired pneumonia, such as pneumonia severity or mortality (25, 26). Neutrophilia and lymphocytopenia are physiological responses of the innate immune system to systemic inflammation. Lymphocytopenia consists of accelerated apoptosis and margination of lymphocytes within the reticuloendothelial system, liver, and splanchnic lymphatic system and of the redistribution of lymphocytes within the lymphatic system (27–29). As immune changes and inflammation are the core pathological basis of COVID-19, compared with imaging and biochemical examination, N/LRs may be a simpler and more specific way to determine the prognosis of COVID-19.
Most patients with severe COVID-19 show significantly elevated serum levels of inflammatory cytokines, such as IL-6 and IL-1, as well as IL-2, IL-17, G-GSF, GM-GSF, IP-10, MCP1, MIP-1a (also known as CCL3), and TNF, known as a cytokine storm (5, 17–19). In our research, we found that cytokines, such as serum IL-2, IL-4, IL-6, IL-10, TNF-α, and INF-γ, were significantly elevated at admission, and in some patients, these inflammatory cytokines were reviewed before discharge. There were statistically significant differences in cytokine levels at admission and discharge. Interestingly, we found that in the process of treatment, the levels of some inflammatory cytokines were decreased, while others were increased. This phenomenon may be because some inflammatory cytokines are pro-inflammatory and others are anti-inflammatory; these changes may reflect the dynamic transformation process of inflammation from damage to repair during homeostasis and inflammation (30). More importantly, correlation analysis indicated that N/LRs were closely related to IL-6 and IL-10. A recent study showed that M1 macrophages produce pro-inflammatory cytokines, such as IL-6, and that M2 macrophages produce anti-inflammatory cytokines, such as IL-10 (30). Our research found that, compared to those at the first test, IL-10 significantly increased and IL-6 significantly decreased at the last test (P < 0.001), which described the patient’s transition from the acute phase to the convalescent phase, and these phenomena may indicate that N/LRs were closely related to injury in the acute stage and repair in the recovery stage in patients with COVID-19. The source, function, and interaction of various inflammatory cytokines are complex. Severe acute respiratory syndrome coronavirus 2-infected macrophages demonstrate upregulation of IL-6 production and low expression of interferons (31, 32). Th1 cell-derived IFN-γ is essential for an effective antiviral immune response. However, IL-6 may inhibit Th1 polarization by stimulating CD4 + cells to differentiate into Th2 cells or by suppressing IFN-γ expression (33, 34). However, IL-6 can cooperate with transforming growth factor (TGF)-β to induce IL-10 production in Th17 cells (35); IL-10 is also produced by regulatory T cells (Tregs), and TGF-β is critical to enable human Tregs to express IL-10 (36). IL-10 can stimulate lymphocytes, leading to a decrease in the secretion of IL-2 and IFN-γ by T cells (37). Studying the complex inflammatory cytokine network after COVID-19 infection is of great significance for the treatment and prediction of the disease.
Most patients with COVID-19 exhibit mild to moderate symptoms, but approximately 15% progress to severe pneumonia, and approximately 5% eventually develop acute respiratory distress syndrome (ARDS), septic shock, and/or multiple organ failure (5, 17). Wei’s group study showed that after COVID-19 infection, CD4+ T lymphocytes are rapidly activated to become pathogenic T helper (Th)1 cells and generate GM-CSF. The cytokine environment induces inflammatory CD14+CD16+ monocytes with high expression of IL-6 and accelerates inflammation (3). These aberrant pathogenic Th1 cells and inflammatory monocytes may enter the pulmonary circulation in large numbers and play an immune damaging role to cause lung functional disability and quick mortality (5, 38). Their team launched a clinical trial using tocilizumab to block inflammatory storms (IL-6 receptor inhibitor) in severely ill patients and achieved some results (3). Partly corresponding to Wei’s team (3), our clinical study found that the serum level of IL-6 decreased significantly before discharge, which may reflect the pro-inflammatory role of IL-6 in the course of COVID-19 from another perspective. However, our team did not find that inflammatory cytokines (such as IL-6) were significantly different among different severity level groups. Part of the explanation is that there are no mild cases in this study (see section “Materials and Methods”). More importantly, although the role of IL-6 in the pathological development of COVID-19 has attracted much attention (3), its specific role remains controversial (9–12), and several experimental models of viral lung infections suggest that IL-6 demonstrates either pathogenic (39)or protective (40) effects in vivo; the consequences of IL-6 induction in COVID-19 may vary depending on the stage of infection and the immune status of the host. The role of these cytokines in SARS-CoV-2 infection should be carefully evaluated.
Severe acute respiratory syndrome coronavirus 2 might act mainly on lymphocytes, especially T lymphocytes (18), and decreases in the levels of CD3+ and CD4+ T lymphocytes are associated with immunosuppression (41). Our research found that N/LRs were negatively related to CD3+ and CD8+ T lymphocyte levels, suggesting that the elevated N/LRs reflect the degree of lymphatic impairment, which may support the hypothesis that N/LRs are a sensitive and simple biomarker of immune function in patients with COVID-19.
Additionally, our results suggest that male sex, underlying disease (cardiovascular disease), and pulse are risk factors for COVID-19. The risk associated with male sex raises a topic of great interest: the effects of estrogens on IL-6 and on COVID-19 progression. Estrogens can suppress lipopolysaccharide (LPS)-mediated IL-6 expression in mouse macrophages by both blocking nuclear factor kappa B (NF-kB) activation (42, 43) and inhibiting p38 mitogen-activated protein kinase (MAPK) phosphorylation (43) by acting on estrogen receptors to decrease the production of pro-inflammatory cytokines (42). However, the immunomodulatory effects of estrogens in COVID-19 require further study. Cardiovascular disease and pulse are risk factors for COVID-19, which may suggest that cardiovascular disease has a critical impact on COVID-19 patient mortality (44) and requires more attention as a comorbidity. We found that there were statistically significant differences in cytokines and N/LRs at admission and discharge, while there were no differences in T lymphocyte subsets (CD3+/CD4+/CD8+) in the research. We wanted to know whether the indicators of lymphocyte subset recovery lagged relatively over time and what their significance was. Further work is needed to understand the specific characteristics of the various inflammatory cytokines and T cell subsets in COVID-19, the specific relationship between N/LRs and different inflammatory cytokines and T cell subsets in COVID-19, and the prognostic value for the disease, especially during immunotherapy.
Conclusion
Post-COVID-19 inflammation is a very complex network system that has a decisive influence on the prognosis of patients. Some inflammatory cytokines (such as IL-6), whose mechanisms and effects are still controversial, need further study. As a quick and convenient marker of inflammation, N/LRs may predict the disease course and severity level of non-mild COVID-19; male sex, cardiovascular disease, and pulse are also risk factors for the severity of non-mild COVID-19.
Limitations
First, the sample size of critical type patients was relatively small, which may have influenced the results. Second, due to the emergency situation of the epidemic outbreak, there is a certain lack of clinical data, including inflammatory cytokines. We are also collating clinical data from other centers and investigating the mechanisms of inflammatory factors in animal models.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
SQ was involved in the study design, data interpretation, and manuscript writing and was a recipient of the obtained funding. JZ participated in the analysis, interpretation, and collection of the data. FH participated in the study design and the statistical analysis. ZL, JW, JC, HG, CZ, YM, YZ, DX, YiW, HH, YoW, MF, YY, MZ, and YX were involved in the data collection. YuW and JC participated in the data analysis and data collection. XH participated in the data analysis. WG participated in the data analysis and manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Key Research and Development Project of Anhui Province (No. 202004j07020014) and the Hefei Science and Technology Bureau “Borrow, Transfer, and Supplement” Project (No. J2019Y01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
ZL, JW, JC, HG, CZ, YM, YZ, DX, YiW, HH, YoW, MF, YY, MZ, YX, and JZ are all members of the Chinese Anhui Medical Team who aid to fight the novel coronavirus pneumonia in Wuhan, and all the information was collected by them.
References
1. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet (Lond Engl). (2020) 395:470–3. doi: 10.1016/S0140-6736(20)30185-9
2. Arshad Ali S, Baloch M, Ahmed N, Arshad Ali A, Iqbal A. The outbreak of coronavirus disease 2019 (COVID-19)-an emerging global health threat. J Infect Public Health. (2020) 13:644–6. doi: 10.1016/j.jiph.2020.02.033
3. Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. (2020) 13:nwaa041. doi: 10.1093/nsr/nwaa041
4. National Health Commission. National Health Commission of the People’s Republic of China. The Guideline on Diagnosis and Treatment of the Novel Coronavirus Pneumonia (NCP): Revised Version of the 7th Edition. (2020). Available online at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml (accessed March 3, 2020).
5. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet. Respirator Med. (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X
6. Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, et al. [A pathological report of three COVID-19 cases by minimally invasive autopsies]. Zhongh Bing Xue Zhi Chinese J Pathol. (2020) 49:E009.
7. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. (2020) 55:105954. doi: 10.1016/j.ijantimicag.2020.105954
8. Soy M, Keser G. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. (2020) 39:2085–94. doi: 10.1007/s10067-020-05190-5
9. Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nature reviews. Rheumatology. (2017) 13:399–409. doi: 10.1038/nrrheum.2017.83
10. Böttcher JP, Schanz O, Garbers C, Zaremba A, Hegenbarth S, Kurts C, et al. IL-6 trans-signaling-dependent rapid development of cytotoxic CD8+ T cell function. Cell Rep. (2014) 8:1318–27. doi: 10.1016/j.celrep.2014.07.008
11. Eskilsson A, Mirrasekhian E, Dufour S, Schwaninger M. Immune-induced fever is mediated by IL-6 receptors on brain endothelial cells coupled to STAT3-dependent induction of brain endothelial prostaglandin synthesis. J Neurosci. (2014) 34:15957–61. doi: 10.1523/JNEUROSCI.3520-14.2014
12. Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nature reviews. Immunology. (2015) 15:335–49. doi: 10.1038/nri3843
13. Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet (Lond Engl). (2020) 395:514–23. doi: 10.1016/S0140-6736(20)30154-9
14. Ye L, Zhou J, Feng A. Practice and Reflection on the management of mobile cabin hospital during the COVID-19 epidemic. Chinese General Practice Nursing. General Care. (2020) 18:1077–9.
15. Chinese Science Press. CT Early Signs and Differential Diagnosis of COVID-19. Beijing: Chinese Science Press (2020).
16. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients With 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
17. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (Lond Engl). (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
18. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. (2020) 112:ciaa248.
19. Shi Y, Tan M, Chen X, Liu Y, Huang J, Ou J, et al. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. medRxiv. (2020). [Preprint]. 2020.03.12.20034736. doi: 10.1101/2020.03.12.20034736
20. Zhang B, Zhou X, Zhu C, Feng F, Qiu Y, Feng J, et al. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. medRxiv. (2020). [Preprint]. 2020.03.12.20035048. doi: 10.1101/2020.03.12.20035048
21. Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. (2020) 84:106504. doi: 10.1016/j.intimp.2020.106504
22. Curbelo J, Luquero Bueno S, Galván-Román JM, Ortega-Gómez M, Rajas O, Fernández-Jiménez G, et al. Inflammation biomarkers in blood as mortality predictors in community-acquired pneumonia admitted patients: Importance of comparison with neutrophil count percentage or neutrophil-lymphocyte ratio. PLoS One. (2017) 12:e0173947. doi: 10.1371/journal.pone.0173947
23. de Jager CPC, Wever PC, Gemen EFA, Kusters R, van Gageldonk-Lafeber AB, van der Poll T, et al. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One. (2012) 7:e46561. doi: 10.1371/journal.pone.0046561
24. Sari R, Karakurt Z. Neutrophil to lymphocyte ratio as a predictor of treatment response and mortality in septic shock patients in the intensive care unit. Turk J Med Sci. (2019) 49:1336–49. doi: 10.3906/sag-1901-105
25. Cataudella E, Giraffa CM, Di Marca S, Pulvirenti A, Alaimo S, Pisano M, et al. Neutrophil-to-lymphocyte ratio: an emerging marker predicting prognosis in elderly adults with community-acquired pneumonia. J Am Geriatr Soc. (2017) 65:1796–801. doi: 10.1111/jgs.14894
26. Lee J-H, Song S, Yoon S-Y, Lim CS, Song J-W, Kim H-S. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as diagnostic markers for pneumonia severity. Br J Biomed Sci. (2016) 73:140–2. doi: 10.1080/09674845.2016.1209898
27. Ayala A, Herdon CD, Lehman DL, Ayala CA, Chaudry IH. Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency, and the nature of the mediators. Blood. (1996) 87:4261–75. doi: 10.1182/blood.V87.10.4261.bloodjournal87104261
28. Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Critical Care Med. (1999) 27:1230–51. doi: 10.1097/00003246-199907000-00002
29. Unsinger J, Kazama H, McDonough JS, Hotchkiss RS, Ferguson TA. Differential lymphopenia-induced homeostatic proliferation for CD4+ and CD8+ T cells following septic injury. J Leukocyte Biol. (2009) 85:382–90. doi: 10.1189/jlb.0808491
30. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. (2018) 33:6425–40. doi: 10.1002/jcp.26429
31. Chen Y, Feng Z, Diao B, Wang R, Wang G, Wang C, et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv. (2020). [Preprint]. 2020.03.27.20045427. doi: 10.1101/2020.03.27.20045427
32. Tseng CT, Perrone LA, Zhu H, Makino S, Peters CJ. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J Immunol. (2005) 174:7977–85. doi: 10.4049/jimmunol.174.12.7977
33. Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. The role of interleukin 6 during viral infections. Front Microbiol. (2019) 10:1057. doi: 10.3389/fmicb.2019.01057
34. Wu W, Dietze KK, Gibbert K, Lang KS, Trilling M, Yan H, et al. TLR ligand induced IL-6 counter-regulates the anti-viral CD8(+) T cell response during an acute retrovirus infection. Sci Rep. (2015) 5:10501. doi: 10.1038/srep10501
35. Jin JO, Han X, Yu Q. Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation. J Autoimmun. (2013) 40:28–44. doi: 10.1016/j.jaut.2012.07.009
36. Bonacci B, Edwards B, Jia S, Williams CB, Hessner MJ, Gauld SB, et al. Requirements for growth and IL-10 expression of highly purified human T regulatory cells. J Clin Immunol. (2012) 32:1118–28. doi: 10.1007/s10875-012-9701-4
37. Poujol F, Monneret G, Gallet-Gorius E, Pachot A, Textoris J, Venet F. Ex vivo stimulation of lymphocytes with IL-10 mimics sepsis-induced intrinsic T-cell alterations. Immunol Invest. (2018) 47:154–68. doi: 10.1080/08820139.2017.1407786
38. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. (2020) 15:700–4. doi: 10.1016/j.jtho.2020.02.010
39. Percopo CM, Ma M, Brenner TA, Krumholz JO, Break TJ, Laky K, et al. Critical adverse impact of IL-6 in acute pneumovirus infection. J Immunol. (2019) 202:871–82. doi: 10.4049/jimmunol.1800927
40. Yang ML, Wang CT, Yang SJ, Leu CH, Chen SH, Wu CL, et al. IL-6 ameliorates acute lung injury in influenza virus infection. Sci Rep. (2017) 7:43829. doi: 10.1038/srep43829
41. Feng HX, Cheng Y, Zhu W, Jiang LL, Dong XF, Gui Q, et al. T-lymphocyte subsets as a predictive biomarker for stroke-associated pneumonia. Am J Transl Res. (2018) 10:4367–75.
42. Biswas DK, Singh S, Shi Q, Pardee AB, Iglehart JD. Crossroads of estrogen receptor and NF-kappaB signaling. Sciences Stke. (2005) 2005:e27. doi: 10.1126/stke.2882005pe27
43. Liu L, Zhao Y, Xie K, Sun X, Jiang L, Gao Y, et al. Estrogen inhibits LPS-induced IL-6 production in macrophages partially via the nongenomic pathway. Immunol Investigat. (2014) 43:693–704. doi: 10.3109/08820139.2014.917095
Keywords: Neutrophil-to-lymphocyte ratios, inflammation, cytokines, immune damage, severity and course of non-mild COVID-19
Citation: Qun S, Wang Y, Chen J, Huang X, Guo H, Lu Z, Wang J, Zheng C, Ma Y, Zhu Y, Xia D, Wang Y, He H, Wang Y, Fei M, Yin Y, Zheng M, Xu Y, Ge W, Hu F and Zhou J (2020) Neutrophil-to-Lymphocyte Ratios Are Closely Associated With the Severity and Course of Non-mild COVID-19. Front. Immunol. 11:2160. doi: 10.3389/fimmu.2020.02160
Received: 20 May 2020; Accepted: 07 August 2020;
Published: 02 September 2020.
Edited by:
Delmiro Fernandez-Reyes, University College London, United KingdomReviewed by:
Yasser Mohamed El-Sherbiny, Nottingham Trent University, United KingdomAna Maria Teixeira, University of Coimbra, Portugal
Copyright © 2020 Qun, Wang, Chen, Huang, Guo, Lu, Wang, Zheng, Ma, Zhu, Xia, Wang, He, Wang, Fei, Yin, Zheng, Xu, Ge, Hu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Ge, Z3cxMDAzQDE2My5jb20=; Fuyong Hu, NTcyODg5MjhAcXEuY29t; Jian Zhou, emhqaWFuMDI3QDEyNi5jb20=
†These authors share first authorship
 Sen Qun
Sen Qun Yulan Wang1†
Yulan Wang1† Changcheng Zheng
Changcheng Zheng