
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 04 August 2020
Sec. Alloimmunity and Transplantation
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.01584
This article is part of the Research Topic Transplantation of Marginal Organs: Immunological Aspects and Therapeutic Perspectives View all 17 articles
 Judith Kahn1,2
Judith Kahn1,2 Gudrun Pregartner3
Gudrun Pregartner3 Alexander Avian3
Alexander Avian3 Daniela Kniepeiss1,2
Daniela Kniepeiss1,2 Helmut Müller1,2
Helmut Müller1,2 Peter Schemmer1,2*
Peter Schemmer1,2*Background: Transplant centers are forced to use livers of extended criteria donors for transplantation due to a dramatic organ shortage. The outcome effect of extended donor criteria (EDCs) remains unclear. Thus, this study was designed to assess the impact of EDCs on outcome including immunological aspects after liver transplantation (LT).
Patients and Methods: Between November 2016 and March 2018, 49 patients (85.7% male) with a mean age of 57 ± 11 years underwent LT. The impact of EDCs on outcome after LT was assessed retrospectively using both MedOcs and ENIS (Eurotransplant Network Information System).
Results: About 80% of grafts derived from extended criteria donors. Alanine aminotransferase/aspartate aminotransferase (AST/ALT) levels elevated more than three times above normal values in organ donors was the only significant risk factor for primary dysfunction (PDF) and primary non-function (PNF)/Re-LT and early non-anastomotic biliary strictures (NAS). Balance of risk (BAR) score did not differ between EDC and non-EDC recipients. PDF (14.3% of all patients) and PNF (6.1% of all patients) occurred in 23.1% of EDC-graft recipients and in 10.0% of non-EDC-graft recipients (RR 2.31, p = 0.663). The 90-day mortality was 3.6%. There was no difference of early non-anastomotic biliary tract complications and biopsy proven rejections (BPR). There was no correlation of PDF/PNF with BPR and NAS, respectively; however, 66.7% of the patients with BPR also developed early NAS (p < 0.001).
Conclusion: With the Graz liver allocation strategy, excellent survival can be achieved selecting livers with no more than 2 not outcome-relevant EDCs for patients with MELD >20. Further, BPR is associated with biliary complications.
Organ shortage has driven transplant centers to extend their criteria for organ acceptance. Donors have become increasingly older and multi-morbid. Allocation strategies for liver transplantation (LT) as well as the acceptance criteria for donor organs in order to expand the entire pool of available organs (1, 2) are continuously being adapted. Various extended donor criteria (EDC) for each organ have been defined; however, the impact of these criteria on outcome after LT is still under debate. Apart from that, there is no definite answer to the question of how to measure the advantages and disadvantages of LT with EDC-grafts. Is it more adequate to judge the waitlist mortality, or the cumulative patient and graft survival, when assessing a LT program? What is the primary aim of LT? To make a long answer short: it is the utility, the generation of a maximum of life years through an optimized allocation of this scarce resource.
To better predict the mortality risk of patients on the waiting list, the model of end-stage liver disease (MELD) system, which is based on three laboratory values including serum creatinine, bilirubin, and international normalized ratio (INR) (laboratory (lab)MELD score), was introduced and adopted by many LT programs worldwide in order to prioritize patients for transplantation by urgency. This “sickest first” allocation policy shows conflicting results, and it has induced medical, ethical, and socio-economic debates. Several risk scores combining donor and/or recipient risk factors predicting outcome after LT have been developed, like the donor risk index (DRI, Eurotransplant [ET]-DRI), and balance of risk (BAR) score including 6 variables (donor age, recipient age, recipient MELD score, re-transplantation, pretransplant life support, and cold ischemic time), University of California, Los Angeles (UCLA), acute-on-chronic liver failure (ACLF), survival outcome following LT score (SOFT) using 18 risk factors, Pedi-SOFT and D-MELD (donor age × recipient MELD) scores, which are models for matching EDC grafts with low-risk recipients and vice versa in order to find a balance between urgency and utility and benefit. Allocation of an EDC graft to a high-risk recipient with a high MELD score should be avoided because of the risk of short-term mortality. Those patients were shown to benefit from high-quality grafts (3). Comorbidities that are not categorically evaluated in the above mentioned scores should also be exceptionally considered to accurately predict post-LT outcome, as a combination of comorbidities like age and aggravation of comorbidities like cardiovascular disease, and frailty can potentially lead to deleterious outcomes after LT.
Apart from that, a score can never replace subjective surgical experience when inspecting a graft during organ retrieval and directly prior to transplantation after having reviewed a particular recipient's condition at the time of transplant.
EDC-grafts have been widely used in the Eurotransplant (ET) region. Good results can be achieved using such liver grafts. An increased risk for biliary tract complications, primarily non-anastomotic biliary strictures (NAS), as well as vascular complications associated with the various types of EDC, as well as an potential increase of early malfunction, i.e., primary dysfunction (PPF) and primary non-function (PNF), have been reported after LT using EDC-grafts (4). Implications on acute and chronic graft rejection have been proposed (5), representing a link between the degree of ischemia reperfusion injury (IRI) and activation of innate immunity (6). EDC in LT is a hot topic. Various EDC have a different impact on outcome after LT.
Here the impact of the Graz allocation strategy (no acceptance of potentially outcome-relevant EDCs in >20 MELD recipients; i.e., >3-fold elevation of normal ranges of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) or cold ischemic time (CIT > 10.5 h) on outcome after LT has been assessed considering immunological aspects in a low volume transplant center (≤40 LT/year) in a non-MELD based allocation system.
All clinical, demographic, surgical, and post-surgical follow-up data were analyzed from all consecutive primary LT performed between November 2016 and March 2018 in a single transplant center. Based on the definition of EDC by the executive committee of the German Federal Medical Society and the ET definition the following donor criteria were assessed as EDC: donor age >65 years, ventilation >7 days, >3-fold elevation of normal ranges AST or ALT, bilirubin >3 mg/dl, peak serum sodium >165 mmol/l, biopsy-proven macrovesicular steatosis >40%, prolonged hypotensive episodes in the donor (>1 h, <60 mmHg, inotropic drug use, e.g., dopamine >14 μg/kg/min) or donor cardiac arrest, body mass index (BMI) >30, CIT >14 h, history of extrahepatic malignancy, previous drug abuse, positive hepatitis B serology (anti-hepatitis B core [HBc] antibody and/or hepatitis B surface [HBs] antigen positive) and donation after cardiac death (DCD) grafts. The concept of EDC LT was explained to the patients on the wait list for LT, and IC was obtained in all patients prior to LT except in high urgent recipients. The presence of any EDC was assessed as well as the number of EDC, if present. The following recipient criteria were assessed: demographics, indication for LT, labMELD score, post-LT laboratory parameters for liver function and liver injury (AST, ALT, alkaline phosphatase [AP], bilirubin, γ-glutamyl transferase [GGT]), PDF, PNF, ICU stay, re-LT, biliary complications within the first 3 post-operative months, vascular complications including hepatic artery thrombosis (HAT), portal vein or hepatic vein thrombosis, bleeding requiring further surgical interventions, and rejection episodes. Primary dysfunction (PDF) and primary non-function (PNF) were defined as AST and ALT >1,500 U/l and AST >2,500 U/l, respectively, during the first 72 h after LT or re-LT/graft failure (7).
The surgical technique of LT included cavo-caval end-to-side (Piggyback technique) or side-to-side anastomosis (Belghiti modified Piggyback technique). The immunosuppressive regimen was tacrolimus based together with mycophenolic acid and a cortison taper for 3 months. Induction therapy was administered in patients <40 years of age, patients suffering from autoimmune hepatitis (AIH), patients with renal insufficiency with a glomerular filtration rate of <60 ml/min, grafts from donors after cardiac death (DCD).
This retrospective analysis was based on both MedOcs and ENIS (ET Network Information System) electronic data. The study protocol has been approved by the local ethics committee, Medical University of Graz, Austria (Ethic Committee number 30-426 ex 17/18).
Continuous data are presented as mean ± standard deviation or median and range, as appropriate. Categorical data are presented as absolute and relative frequencies. For continuous data differences between groups were analyzed using t-test, Mann Whitney U-test. Differences in the distribution of categorical data were analyzed using χ2 -test or Fisher's exact test. For risk factor analysis relative risks and corresponding 95% confidence intervals (95%CI) were calculated. R version 3.4.4 and SPSS 26.0.0.0 (IBM Corp., Armonk, NY, USA) were used for these analyses.
Forty-nine patients (85.7% male) with a mean age of 57 ± 11 years underwent LT for alcoholic liver cirrhosis (45%), hepatocellular carcinoma (HCC) (41%), primary sclerosing cholangitis (PSC) or AIH (10%), HBV-associated liver cirrhosis (2%), and acute liver failure (ALF) (2%) (Table 1). The median labMELD score of the patients was 15 (range 7–32), and 24.5% of patients presented with a labMELD score of <10, 61.2% of cases have shown a labMELD score of 10–20, 10.2% were identified with a labMELD score of 21–30, and 4.1% of patients were documented with a labMELD score of >30 (labMELD categories 1–4, Figure 1). Three patients underwent re-LT, one of which for PNF and the other 2 cases for HAT. Re-LT were excluded from further analysis, and 79.6% of grafts have shown up to three EDCs (Figure 2); the categories of EDCs are shown in Table 2. Of those, 51.3% had 1 EDC, 38.5% had 2 EDCs, and 10.2% had 3 or 4 EDCs (Table 3). No EDC existed for 20.4% of grafts. Patients were classified in EDC and non-EDC recipients. These 2 groups were comparable based on demographics, indication for LT, and labMELD score. In labMELD category 1 and 2, 16.7% of the patients (MELD score ≤ 20) received a non-EDC organ, 83.3% received an EDC organ; 57.1% of the patients in labMELD category 3 and 4 (labMELD score >20) received a non-EDC organ, 42.9% received an EDC organ with no more than 2 EDC categories.
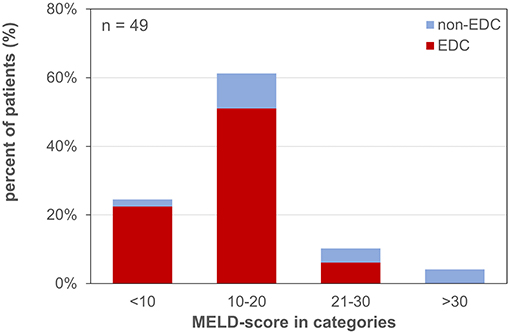
Figure 1. MELD-score categories of patients waiting for liver transplantation. MELD-score was categorized into 4 groups: 1: MELD <10, 2: MELD 10–20, 3: MELD 21–30, 4: MELD >30. EDC (extended donor criteria) vs. non-EDC (non-extended donor criteria) graft recipients.
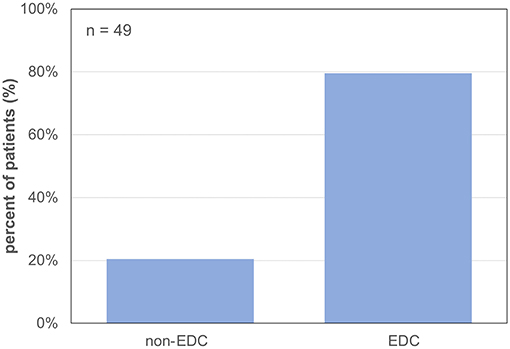
Figure 2. Percentage of grafts with extended donor criteria and non-extended donor criteria. 79.6% EDC (extended donor criteria) grafts and 20.4% non-EDC (non-extended donor criteria) grafts.
BAR-score was 6.1 (±2.4) in EDC and 7.6 (±3.2) in non-EDC recipients (n.s.) (Table 4).
Median follow-up time of the patients was 22 months [range 13–31 months]. One-year patient survival was 96.4% with a 90-day mortality of 3.6%. While one patient died after acute pulmonary embolism on post-operative day (POD) 7 the other cause of death was due to septic multi-organ failure. Both deaths occurred after EDC LT.
Laboratory findings reflecting both graft injury and graft function were comparable between groups. All early (first post-operative 3 months) but one (HAT after non-EDC LT), and all late surgical re-interventions due to bleeding, vascular complication, and incisional hernia (IH) repair were performed in EDC-graft recipients. Of all EDC-graft recipients, 20.5% had 1 re-intervention, 5.1% had 2 re-interventions, and 2.6% had 3 re-interventions.
EDC LT had no significant impact on both the ICU stay and ventilation time. The median ICU stay was 2 days in both groups; however, the range of ventilation time with 1–60 days was higher in EDC-graft recipients as compared to 1–9 days after non-EDC LT; 25.6% of EDC-graft recipients requiring more than 4 days of ICU in contrast to 10.0% after non-EDC LT (RR 1.19; p = 0.419).
While the median ventilation time after LT was comparable after both EDC and non-EDC LT with 11 and 15 h, respectively; the range was higher after EDC LT with 5–179 h as compared to 8–65 h after non-EDC LT. Only 7.9% of EDC-graft recipients required a post-operative ventilation of more than 24 h. This is in contrast to 22.2% after non-EDC LT (RR 0.72; p = 0.240).
Temporary post-operative hemodialysis was necessary in 12.2% of all patients with no difference between groups (EDC-graft recipients: 12.8%, non-EDC-graft recipients: 10.0%; RR of 1.28; p = 1).
PDF (14.3% of all patients), PNF and the necessity for re-LT (PNF/Re-LT; 6.1% of all patients) occurred in 23.1% of cases after EDC LT and in 10.0% after non-EDC LT (RR 2.31; p = 0.663; Figure 3).
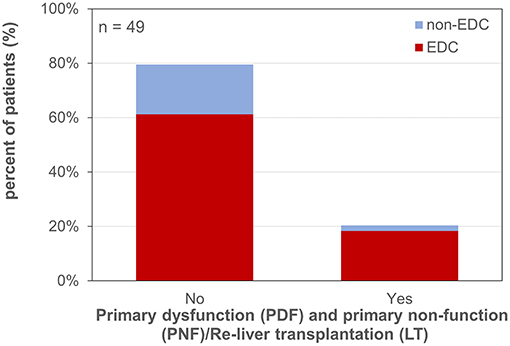
Figure 3. Primary dysfunction and primary non-function/re-liver transplantation. EDC (extended donor criteria) vs. non-EDC (non-extended donor criteria) graft recipients.
Early NAS (12.2% of all patients during the first 3 months) occurred in 12.8% of EDC-graft recipients and in 10.0% after non-EDC LT (RR of 1.28; p = 1; Figure 4).
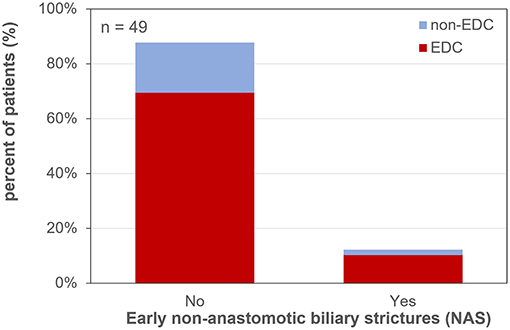
Figure 4. Early non-anastomotic biliary strictures after liver transplantation. EDC (extended donor criteria) vs. non-EDC (non-extended donor criteria) graft recipients.
Six patients developed BPR after a median follow up of 106.5 days (6–177 days) post-LT. BPR occurred in 12.8% in EDC recipients (grafts with 1 EDC: 3 patients; grafts with 3 or 4 EDCs: 2 patients, respectively) and in 10.0% in non-EDC recipients (RR of 1.28; p = 1; Figure 5). There was a coincidence of BPR with PDF/PNF in 33.3% of cases (p = 0.588), and 66.7% of the patients with BPR also developed early NAS (p < 0.001).
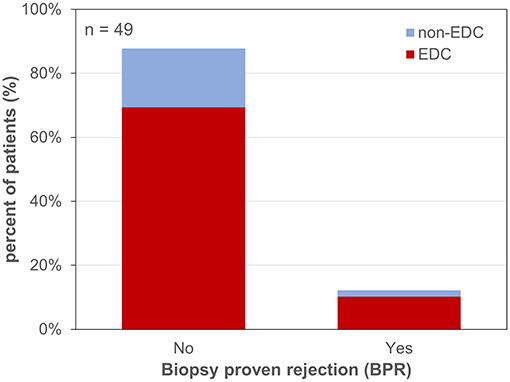
Figure 5. Biopsy proven rejection after liver transplantation. EDC (extended donor criteria) vs. non-EDC (non-extended donor criteria) graft recipients.
Intra-patient tacrolimus trough level variability within the first post-LT year did not differ between EDC- and non-EDC-graft recipients (42.5 ± 1.9% vs. 49.9 ± 10.8%, respectively; p = 1).
AST/ALT serum levels in organ donors more than three times increased above normal limits was a significant risk factor for PDF and PNF/Re-LT (RR 4.15, 95%CI 1.39–12.41; p = 0.024) as well as for early NAS (RR 5.54, 95%CI 1.62–18.99; p = 0.003). A CIT of > 10.5 h was the second strongest risk factor for PDF and PNF/Re-LT (RR 3.33, 95%CI 0.95–11.71) and for early NAS (RR 2.08, 95%CI 0.64–6.77).
All patients with a labMELD score >20 received either non-EDC grafts or EDC grafts with no more than 2 EDCs which did not include increased AST/ALT levels or prolonged CIT (Figures 6, 7).
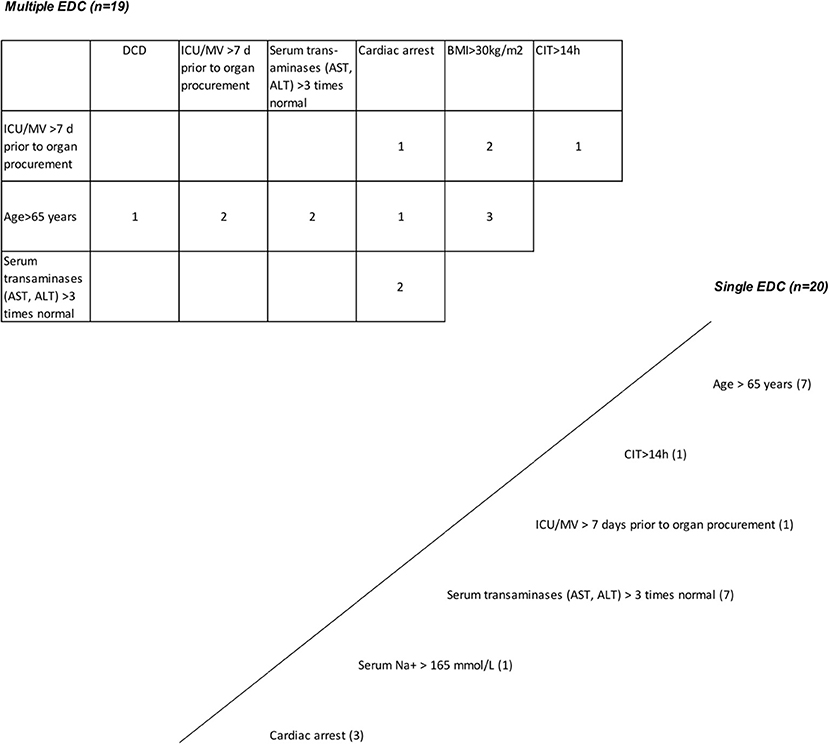
Figure 6. Grafts with extended donor criteria (EDCs). Single EDC—multiple EDC. A total of 49 primary cadaveric LTs were analyzed. Up to four EDCs were present in 79.6% of all grafts. Grafts with single EDC are shown below and right to the oblique line. Nineteen grafts had more than one extended criterion (table above and left to the oblique line; multiple EDCs [n = 19]). The number within the boxes represents how many grafts had the corresponding combined EDC. Prolonged cold ischemia time >14 h and ICU stay with ventilation >7 days, and cardiac arrest and alanine aminotransferase/aspartate aminotransferase (AST/ALT) levels >3 times normal were present in 2 grafts from donation after cardiac death (DCD) donors >65 years; AST/ALT levels >3 times normal in combination with cardiac arrest and positive hepatitis B virus (HBV) serology*, and in combination with hypernatremia >165 mmol/l and intensive care unit (ICU) stay with ventilation >7 days were present in 2 grafts; a history of extrahepatic malignancy* in combination with donor age >65 years, cardiac arrest and hypernatremia >165 mmol/l was present in 1 graft, in combination with ICU stay with ventilation >7 days and a prolonged cold ischemic time (CIT) in another (4 grafts with 3 or more functionally relevant EDCs). *Not relevant for post-transplant graft function.
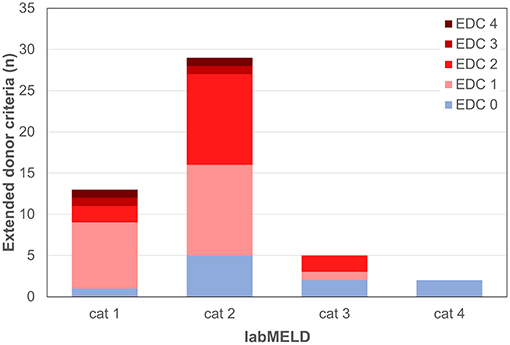
Figure 7. Extended donor criteria (EDCs)/laboratory model of end stage liver disease (labMELD) categories (cat 1-4). Distribution of grafts with no EDCs/EDCs (n=0-4) among recipients at different risk/labMELD category 1 (labMELD <10), category 2 (labMELD 10–20), category 3 (labMELD 21–30), and category 4 (labMELD >30). In category 3 and 4 grafts with >3-fold increased AST/ALT levels and CIT > 10.5 h were not used.
Due to the increasing lack of organs, the criteria that define donor organs suitable for LT are constantly being expanded. While older donor age or resuscitation of the donor, for example, were absolute contraindications for LT 30 years ago, today this is at most a relative contraindication. Nevertheless, survival after LT has steadily increased over the years. One-year survival is currently more than 90%, 5-year survival 80%, and 10-year survival more than 70% according to the European Liver Transplant Registry (ELTR) (8), EASL Clinical Practice Guidelines (CPG) LT (9). What are the current challenges? These are primarily early functional disorders of the graft such as PDF and PNF in 2–15% of cases (10, 11), as well as the long-term consequences of the immunosuppression (IS). The primary aim of LT is the generation of a maximum of life years through an optimized allocation of this scarce resource (12).
There is no unique definition for EDC. But, there are two categories: (i) factors directly influencing post-transplant-function and (ii) factors not influencing post-transplant-function. There was a consensus conference in 2007 on extended donor criteria, and those were defined as donor age, macrosteatosis, elevated liver enzymes, hemodynamic instability of the donor, hypernatremia, CIT, DCD, split LT, transmission of malignancy, and infections (13). Other attempts to sum up the main EDC criteria are the ET score, the German Medical Association (BÄK) score, and the UNOS definition score (14–16).
Concerning donor factors potentially influencing post-transplant graft function, one of the most important challenges is the fact that the age of donors is constantly increasing. Potential risks of LT using aged grafts are higher rates of transplant failure PNF and PDF with potentially increased mortality, and a higher degree of ischemia reperfusion injury (IRI) due to less potential to regenerate. The risk in hepatitis C positive aged grafts is even higher, as well as the damage due to longer CIT in combination with aged grafts. Some studies confirmed the negative consequences of such grafts (17–19), especially in the context of hepatitis C positivity, but many studies to date have confirmed no disadvantages for patients receiving aged liver grafts in large cohorts (up to 23,763 patients) (20, 21). Macrosteatosis of >60% of the donor liver is an unacceptable risk for graft failure, while 30–60% macrosteatosis can achieve acceptable outcomes in select donor-recipient combinations (9). The balance of risk score (BAR) is one attempt to combine the strongest donor and recipient risk predictors to generate a risk score predicting less survival for a BAR score of >18 (22). Elevated liver enzymes AST and ALT of donor livers were shown to achieve good results after LT (23), whereas elevated GGT and INR were shown to be associated with inferior results (13). Hypotensive episodes in organ donors as well as donor resuscitation were associated with non-inferior LT results (24). The need of catecholamines (norepinephrine, dopamine >10 mcg/kg/min) was shown to be a risk factor for graft failure (25). Lower patient and graft survival with donor hypernatremia >155 mEq/l was reported in several studies (26, 27), whereas most recent studies on donor hypernatremia showed no influence on patient and graft survival (28, 29). CIT of more than 8 h leads to impaired 5-year-survival after LT (7), and with each additional hour of CIT the risk for PNF increases by 1% (30).
LT after DCD has steadily increased over the years, with a DCD rate of >20% in the UK and around 6% in the United States (31). Associated risks with DCD LT include biliary tract complications (i.e., ischemic type biliary lesions [ITBL]), vascular complications like HAT, as well as PDF and PNF potentially necessitating re-LT. An increased rate of biliary tract complications of more than 30% was reported by various groups (32, 33), whereas similar 1-, 3-, and 5-year survival was reported by Kollmann et al. (34), and similar 1- and 10-year survival, but inferior 5-year-survival by Blok et al. (35) analyzing ET data. One recent study even showed better results in DCD LT with donor age <50 years and CIT <6 h than in DBD LT using grafts from donors >60 years (36).
The other donor criteria which were defined as “extended” like split LT, transmission of infections, and malignancy do not directly have a potential impact on graft function, PDF, and PNF.
The experienced transplant surgeon is responsible for accepting the best possible match. General rules include that EDC organs shall not be used for the sickest patients, since these patients do not have any reserves to survive primary dysfunction or primary non-function. Further, according to the literature, the combination of 3 or more than 3 EDC factors decreases outcome quality after transplantation (37). The number of EDCs was higher in patients with lower labMELD scores, which is based on the opinion that a recipient in a good clinical condition can better tolerate an EDC graft than a patient with a higher labMELD score. This is in line with other publications (4, 38, 39). Hence, according to our data and other reports in the literature, patient and graft outcomes were not different (1, 2, 4, 12). The BAR-score, which is available before decision making of accepting or not an organ for a specific recipient, was reported to have the potential to detect unfavorable combinations of donor and recipient factors (22). It was also applied in this patient cohort. In this small volume center within a non-MELD-based allocation system, the MELD scores were generally low among patients on the waiting list for LT with only 14.3% of the patients with a labMELD score of >20, as were the BAR scores (6.1 [±2.4] in EDC and 7.6 [±3.2] in non-EDC recipients). According to findings in the literature (2, 4, 13, 27, 40, 41) the Graz allocation system was established avoiding outcome-relevant EDCs for high risk patients; patients with labMELD scores >20 received grafts with no more than 2 EDCs excluding >3-fold increased AST/ALT levels or prolonged CIT > 10.5 h which were most relevant for outcome after LT. Risk factor analysis revealed that AST/ALT levels elevated more than three times above normal values in organ donors was the only significant risk factor for primary dysfunction (PDF) and primary non-function (PNF)/Re-LT and early non-anastomotic biliary strictures (NAS).
In EDC-kidney transplantation (KT), Aubert et al. found an EDC-graft survival comparable to that of patients receiving a SDC transplant in KT recipients, whereby patients receiving EDC transplants who presented with circulating donor specific antibodies (DSA) at the time of transplantation had significantly worse allograft survival after 7 years than patients receiving EDC kidneys without circulating DSA at transplantation (44 vs. 85%). Recipients of EDC kidneys with circulating DSA showed a 5.6-fold increased risk of graft loss compared with all other transplant therapies [p < 0.001; (42)]. According to this large KT analysis including 6,891 patients allocation policies to avoid DSA and CIT could promote wider implementation of EDC transplantation in the context of organ shortage and improve its prognosis. No comparable results are available from LT cohorts, whereas allocation policies for EDC liver grafts have been modified accordingly. The so-called rescue allocation (RA) is one strategy for LT that has been implemented within the ET area mainly for this reason. Liver grafts are considered for RA when the regular organ allocation is declined by at least 3 centers or is averted because of donor instability or other unfavorable logistical reasons. Thus, such a donor enters a competitive or a single-recipient rescue organ offer procedure, respectively. The accepting center has the advantage to select a recipient from its own waiting list for these RA grafts (1), which is not common practice in all countries within ET.
According to the Collaborative Transplant Study (CTS) positive lymphocytotoxic T-cell crossmatches have been shown to be associated with significantly decreased graft survival in first kidney transplants performed from 1990 to 1999, but not from 2000 to 2007, in kidney re-transplants regardless of transplant period and in heart and liver transplants. Positive B-cell crossmatches were associated with significantly decreased kidney and heart, but not liver transplant survival (43). According to consensus guidelines on the testing and clinical management associated with HLA and non-HLA antibodies, a KT can be performed in the absence of a prospective crossmatch if single-antigen bead screening for antibodies to all class I and II HLA loci is negative. The presence of DSA HLA antibodies should be avoided in heart and lung transplantation and considered a risk factor for liver, intestinal, and islet cell transplantation (44).
Biliary complications after LT have a constant incidence of 10–15%. Anastomotic biliary strictures (AS) are more related to technical aspects as bile leaks, or HAT, whereas NAS are related to risk factors including immunologic, IRI, or consequences of infectious complications (45). ITBL (46) is one of the major post-operative complications accounting for up to 38% of morbidity and mortality rates of all biliary complications.
In the longer term, NAS potentially result from the use of grafts with various EDC and can be a consequence of profound IRI, as well as an increased incidence of acute and chronic rejection (4–6, 47). EDC-liver grafts are more susceptible to cold and warm IRI and develop more easily ITBL than normal livers (48), as ischemic cholangiopathy is more common with the use of DCD grafts and prolonged warm ischemic time (WIT) (49). Several studies link ITBL to various immunologically mediated processes such as AB0-incompatible liver transplants, PSC, PBC, and AIH (50).
Immunological risk factors like PBC, crossmatch positivity, and acute and chronic rejection were found to be important variables associated with the development of biliary strictures after LT in a retrospective analysis of 273 DBD LT (45), independent from IRI. An immunological component causing ITBL could be confirmed by the detection of DSA HLA antibodies in LT recipients (51).
Organ age has been linked to higher acute rejection rates (52). Experimental data show that age-associated epigenetic changes that result in hypermethylation of the CpG regions or hypomethylation of the non-CpG regions (53) may increase the immunogenicity of the DNA; hypomethylation of aged DNA has been reported to induce a stronger activation of dendritic cells (DCs) compared to DNA from young donors (54). Old DCs have also been shown to secrete more inflammatory cytokines upon stimulation, possibly via decreased activation of PI3K-signaling pathways and reduced suppression of p38-MAPK activation (55). Although immunosenescence leads to an overall decline of immune function, enhanced antigen-presenting capacities have been reported (56). Older endothelial cells express higher levels of VCAM-1 and MCP-1, facilitating leukocyte adhesion and infiltration and thereby contribute to enhanced immunogenicity (57).
The compromised repairing capacity of aged organs may also play an important role for an aggravated immune response. Cell death via apoptosis is a physiological part of the aging process and older grafts contain more apoptotic cells representing a significant source of local inflammation (54, 55). As a consequence of impaired repairing capacity, old parenchymal cells express more MHC molecules (58).
Non-specific injuries like IRI, and a mechanical trauma during explantation, induce a proinflammatory milieu which can activate the innate immune response and initiate the adaptive immune response. This can be aggravated by longer CIT, also potentially leading to an increased rate of acute rejection (59, 60).
Activation and recruitment of recipient's dendritic cells (DCs) into the graft activating recipient's T cells via the indirect pathway, together with increased apoptosis and antigen presentation augmenting the immune response, represents an important link between injury and immune response (56). It has been shown that IRI enhances the immunogenicity of allograft-derived DCs via toll-like receptor 4 and nuclear factor-kappa B activation (59).
In steatotic livers, the increased volume of the hepatocytes leads to microcirculatory impairment and thereby to an increased susceptibility to IRI with an immunological impact as mentioned above.
In conclusion, the immune response against steatotic grafts and older grafts can be enhanced relative to younger grafts with cryptic self-antigens exposed during necrotic cell death involved (56).
Results of our data are in accordance with previous findings, that excellent survival can be achieved with careful selection of EDC-liver grafts and appropriate recipient matching (EDC grafts for low-risk recipients and vice versa). However, there is an increased risk for biliary complications associated with the various types of EDC, and there is an indication that there may be implications in rejection, but without increased mortality risk. We also found no significant difference with respect to biliary complications, PDF/PNF, and rejection between EDC- and non-EDC-graft recipients. Altogether, the Graz allocation strategy has been proven to be safe and effective within a non-MELD based allocation system.
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed to anVkaXRoLmthaG5AbWVkdW5pZ3Jhei5hdA==.
The studies involving human participants were reviewed and approved by ethics commission of the Medical University of Graz. The patients/participants provided their written informed consent to participate in this study.
JK performed the study and wrote the manuscript. GP and AA analyzed the data. PS designed and performed the study, and wrote the manuscript. DK and HM edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Preliminary results were presented at the Austrotransplant congress 2018 with an abstract published in Transplant International (Proceedings) (61).
EDC, extended donor criteria; LT, liver transplantation; AST, alanine aminotransferase; ALT, aspartate aminotransferase; HBV, hepatitis B virus anti-HBc antibody, anti-hepatitis B core antibody HBs antigen, hepatitis B surface antigen; PDF, primary dysfunction; PNF, primary non-function; BPR, biopsy proven rejections; NAS, non-anastomotic biliary strictures; UCLA, University of California, Los Angeles; INR, international normalized ratio; MELD, model of end stage liver disease; labMELD, laboratory model of end stage liver disease; DRI, donor risk index; ET-DRI, Eurotransplant donor risk index; BAR score, balance of risk score; ACLF, acute-on-chronic liver failure; SOFT, survival outcome following liver transplantation score; Pedi-SOFT, pediatric survival outcome following liver transplantation score; BMI, body mass index; CIT, cold ischemic time; AP, alkaline phosphatase; GGT, γ-glutamyl transferase; HAT, hepatic artery thrombosis; IC, informed consent; DCD, donation after cardiac death; AIH, autoimmune hepatitis; ALF, acute liver failure; ELTR, European Liver Transplant Registry; EASL CPG, European association of the study of the liver Clinical Practice Guidelines; IS, immunosuppression; ITBL, ischemic type biliary lesions; ICU, intensive care unit; IRI, ischemia reperfusion injury; BÄK, German Medical Association; RA, rescue allocation; CTS, Collaborative Transplant Study; KT, kidney transplantation; DSA, donor specific antibodies; DBD, donation after brain death; WIT, warm ischemic time; DC, dendritic cells.
1. Schemmer P, Nickkholgh A, Gerling T, Weitz J, Buchler MW, Schmidt J. Rescue allocation for liver transplantation within Eurotransplant: the Heidelberg experience. Clin Transplant. (2009) 23(Suppl. 21):42–8. doi: 10.1111/j.1399-0012.2009.01109.x
2. Schemmer P, Nickkholgh A, Hinz U, Gerling T, Mehrabi A, Sauer P, et al. Extended donor criteria have no negative impact on early outcome after liver transplantation: a single-center multivariate analysis. Transplant Proc. (2007) 39:529–34. doi: 10.1016/j.transproceed.2006.12.002
3. Petrowsky H, Rana A, Kaldas FM, Sharma A, Hong JC, Agopian VG, et al. Liver transplantation in highest acuity recipients: identifying factors to avoid futility. Ann Surg. (2014) 259:1186–94. doi: 10.1097/SLA.0000000000000265
4. Lozanovski VJ, Khajeh E, Fonouni H, Pfeiffenberger J, von Haken R, Brenner T, et al. The impact of major extended donor criteria on graft failure and patient mortality after liver transplantation. Transpl Int. (2018) 403:719–31. doi: 10.1007/s00423-018-1704-z
5. Nunez K, Thevenot P, Afadhli A, Cohen A. Complement activation in liver transplantation: role of donor macrosteatosis and implications in delayed graft function. Int J Mol Sci. (2018) 19:1750. doi: 10.3390/ijms19061750
6. Uehara M, Bahmani B, Jiang L, Jung S, Banouni N, Kasinath V, et al. Nanodelivery of mycophenolate mofetil to the organ improves transplant vasculopathy. ACS Nano. (2019) 13:12393–407. doi: 10.1021/acsnano.9b05115
7. Kniepeiss D, Iberer F, Grasser B, Schaffellner S, Stadlbauer V, Tscheliessnigg KH. A single-center experience with retrograde reperfusion in liver transplantation. Transpl Int. (2003) 16:730–5. doi: 10.1111/j.1432-2277.2003.tb00232.x
8. Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. (2012) 57:675–88. doi: 10.1016/j.jhep.2012.04.015
9. European Association for the Study of the Liver. EASL clinical practice guidelines: liver transplantation. J Hepatol. (2016) 64:433–85. doi: 10.1016/j.jhep.2015.10.006
10. Uemura T, Randall HB, Sanchez EQ, Ikegami T, Narasimhan G, McKenna GJ, et al. Liver retransplantation for primary nonfunction: analysis of a 20-year single-center experience. Liver Transpl. (2007) 13:227–33. doi: 10.1002/lt.20992
11. Das S, Swain SK, Addala PK, Balasubramaniam R, Gopakumar CV, Zirpe D, et al. Initial poor function and primary nonfunction in deceased-donor orthotopic liver transplantation maintaining short cold ischemic time. Prog Transpl. (2016) 26:340–7. doi: 10.1177/1526924816663516
12. Linecker M, Krones T, Berg T, Niemann CU, Steadman RH, Dutkowski P, et al. Potentially inappropriate liver transplantation in the era of the “sickest first” policy - a search for the upper limits. J Hepatol. (2018) 68:798–813. doi: 10.1016/j.jhep.2017.11.008
13. Durand F, Renz JF, Alkofer B, Burra P, Clavien PA, Porte RJ, et al. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl. (2008) 14:1694–707. doi: 10.1002/lt.21668
14. Bundesärztekammer D. Richtlinien zur Organtransplantation gemäß § 16 TPG. Bekanntmachungen Bundesärztekammer. (2004) 3:1.
15. Briceno J, Solorzano G, Pera C. A proposal for scoring marginal liver grafts. Transpl Int. (2000) 13(Suppl.):S249–52. doi: 10.1007/s001470050334
16. Busuttil RW, Koichi T. The utility of marginal donors in liver transplantation. Liver Transpl. (2003) 9:651–63. doi: 10.1053/jlts.2003.50105
17. Uemura T, Nikkel LE, Hollenbeak CS, Ramprasad V, Schaefer E, Kadry Z. How can we utilize livers from advanced aged donors for liver transplantation for hepatitis C? Transpl Int. (2012) 25:671–9. doi: 10.1111/j.1432-2277.2012.01474.x
18. Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, et al. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg. (2005) 241:905–16; discussion 16–8. doi: 10.1097/01.sla.0000164077.77912.98
19. Frühauf NR, Fischer-Fröhlich C-L, Kutschmann M, Schmidtmann I, Kirste G. Joint impact of donor and recipient parameters on the outcome of liver transplantation in Germany. Transplantation. (2011) 92:1378–84. doi: 10.1097/TP.0b013e318236cd2f
20. Segev DL, Maley WR, Simpkins CE, Locke JE, Nguyen GC, Montgomery RA, et al. Minimizing risk associated with elderly liver donors by matching to preferred recipients. Hepatology. (2007) 46:1907–18. doi: 10.1002/hep.21888
21. Gao Q, Mulvihill MS, Scheuermann U, Davis RP, Yerxa J, Yerokun BA, et al. Improvement in liver transplant outcomes from older donors: a US National analysis. Ann Surg. (2019) 270:333–9. doi: 10.1097/SLA.0000000000002876
22. Dutkowski P, Oberkofler CE, Slankamenac K, Puhan MA, Schadde E, Mullhaupt B, et al. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. (2011) 254:745–53; discussion: 53. doi: 10.1097/SLA.0b013e3182365081
23. Radunz S, Paul A, Nowak K, Treckmann JW, Saner FH, Mathe Z. Liver transplantation using donor organs with markedly elevated liver enzymes: how far can we go? Liver Int. (2011) 31:1021–7. doi: 10.1111/j.1478-3231.2011.02525.x
24. Hoyer DP, Paul A, Saner F, Gallinat A, Mathe Z, Treckmann JW, et al. Safely expanding the donor pool: brain dead donors with history of temporary cardiac arrest. Liver Int. (2015) 35:1756–63. doi: 10.1111/liv.12766
25. Saidi RF, Kenari SKH. Challenges of organ shortage for transplantation: solutions and opportunities. Int J Org Transplant Med. (2014) 5:87–96.
26. Figueras J, Busquets J, Grande L. The deleterious effect of donor high plasma sodium and extended preservation in liver transplantation: a multivariate analysis. Transplantation. (1996) 61:410–3. doi: 10.1097/00007890-199602150-00016
27. Silberhumer GR, Rahmel A, Karam V, Gonen M, Gyoeri G, Kern B, et al. The difficulty in defining extended donor criteria for liver grafts: the Eurotransplant experience. Transpl Int. (2013) 26:990–8. doi: 10.1111/tri.12156
28. Tector AJ, Magnus R, Chestovich P, Vianna R, Fridell J, Milgrom ML, et al. Use of extended criteria livers decreases wait time for liver transplantation without adversely impacting posttransplant survival. Ann Surg. (2006) 244:439–50. doi: 10.1097/01.sla.0000234896.18207.fa
29. Goldaracena N, Quinonez E, Mendez P, Anders M, Orozco Ganem F, Mastai R, et al. Extremely marginal liver grafts from deceased donors have outcome similar to ideal grafts. Transplant Proc. (2012) 44:2219–22. doi: 10.1016/j.transproceed.2012.07.113
30. Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. (2006) 6:783–90. doi: 10.1111/j.1600-6143.2006.01242.x
31. Broomhead RH, Patel S, Fernando B, O'Beirne J, Mallett S. Resource implications of expanding the use of donation after circulatory determination of death in liver transplantation. Liver Transpl. (2012) 18:771–8. doi: 10.1002/lt.23406
32. Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, et al. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg. (2011) 253:259–64. doi: 10.1097/SLA.0b013e318204e658
33. Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. (2011) 253:817–25. doi: 10.1097/SLA.0b013e3182104784
34. Kollmann D, Sapisochin G, Goldaracena N, Hansen BE, Rajakumar R, Selzner N, et al. Expanding the donor pool: donation after circulatory death and living liver donation do not compromise the results of liver transplantation. Liver Transpl. (2018) 24:779–89. doi: 10.1002/lt.25068
35. Blok JJ, Detry O, Putter H, Rogiers X, Porte RJ, van Hoek B, et al. Longterm results of liver transplantation from donation after circulatory death. Liver Transpl. (2016) 22:1107–14. doi: 10.1002/lt.24449
36. Scalea JR, Redfield RR, Foley DP. Liver transplant outcomes using ideal donation after circulatory death livers are superior to using older donation after brain death donor livers. Liver Transpl. (2016) 22:1197–204. doi: 10.1002/lt.24494
37. Nickkholgh A, Weitz J, Encke J, Sauer P, Mehrabi A, Büchler MW, et al. Utilization of extended donor criteria in liver transplantation: a comprehensive review of the literature. Nephrol Dial Transplant. (2007) 22(Suppl. 8):viii29–36. doi: 10.1093/ndt/gfm654
38. Schrem H, Reichert B, Frühauf N, Kleine M, Zachau L, Becker T, et al. Extended donor criteria defined by the German medical association: study on their usefulness as prognostic model for early outcome after liver transplantation. Chirurg. (2012) 83:980–8. doi: 10.1097/00007890-201211271-00742
39. Schrem H, Platsakis AL, Kaltenborn A, Koch A, Metz C, Barthold M, et al. Value and limitations of the BAR-score for donor allocation in liver transplantation. Langenbecks Arch Surg. (2014) 399:1011–9. doi: 10.1007/s00423-014-1247-x
40. Nemes B, Gámán G, Polak WG, Gelley F, Hara T, Ono S, et al. Extended criteria donors in liver transplantation Part I: reviewing the impact of determining factors. Expert Rev Gastroenterol Hepatol. (2016) 10:827–39. doi: 10.1586/17474124.2016.1149061
41. Nemes B, Gámán G, Polak WG, Gelley F, Hara T, Ono S, et al. Extended-criteria donors in liver transplantation Part II: reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Expert Rev Gastroenterol Hepatol. (2016) 10:841–59. doi: 10.1586/17474124.2016.1149062
42. Aubert O, Kamar N, Vernerey D, Viglietti D, Martinez F, Duong-Van-Huyen JP, et al. Long term outcomes of transplantation using kidneys from expanded criteria donors: prospective, population based cohort study. BMJ. (2015) 351:h3557. doi: 10.1136/bmj.h3557
43. Opelz G, Döhler B, Süsal C. Analysis of positive kidney, heart, and liver transplant crossmatches reported to the collaborative transplant study. Hum Immunol. (2009) 70:627–30. doi: 10.1016/j.humimm.2009.04.009
44. Tait BD, Süsal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. (2013) 95:19–47. doi: 10.1097/TP.0b013e31827a19cc
45. Mocchegiani F, Vincenzi P, Lanari J, Montalti R, Nicolini D, Baroni GS, et al. Immunological risk factors in biliary strictures after liver transplantation. Ann Transplant. (2015) 20:218–24. doi: 10.12659/AOT.892393
46. Pascher A, Gerlach U, Neuhaus P. Bile duct strictures after liver transplantation. Curr Opin Gastroenterol. (2014) 30:320–5. doi: 10.1097/MOG.0000000000000061
47. Attia M, Silva MA, Mirza DF. The marginal liver donor - an update. Transpl Int. (2008) 21:713–24. doi: 10.1111/j.1432-2277.2008.00696.x
48. Cursio R, Guggenheim J. Ischemia-reperfusion injury and ischemic-type biliary lesions following liver transplantation. J Transplant. (2012) 2012:164329. doi: 10.1155/2012/164329
49. Mourad MM, Algarni A, Liossis C, Bramhall SR. Aetiology and risk factors of ischaemic cholangiopathy after liver transplantation. World J Gastroenterol. (2014) 20:6159–69. doi: 10.3748/wjg.v20.i20.6159
50. Guichelaar MM. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. (2003) 3:885–90. doi: 10.1034/j.1600-6143.2003.00165.x
51. Schulte K, Puhl G, Gül S, Schönemann C, Lachmann N, Pratschke J. Donor specific HLA antibodies as a trigger for ischemic type biliary lesions (ITBL) after orthotopic liver transplantation-a case controlled study. J Liver Disease Transplant. (2017) 6, 1–5. doi: 10.4172/2325-9612.1000147
52. Tullius SG, Milford E. Kidney allocation and the aging immune response. N Engl J Med. (2011) 364:1369–70. doi: 10.1056/NEJMc1103007
53. Christensen BC, Houseman EA, Marsit CJ, Zheng S, Wrensch MR, Wiemels JL, et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG Island Context. PLoS Genet. (2009) 5:e1000602. doi: 10.1371/journal.pgen.1000602
54. Agrawal A, Tay J, Yang EE, Agrawal S, Gupta S. Age-associated epigeneic modifications in human DNA increase its immunogenicity. Aging. (2010) 2:93–100. doi: 10.18632/aging.100121
55. Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. (2007) 178:6912–22. doi: 10.4049/jimmunol.178.11.6912
56. Slegtenhorst BR, Dor FJ, Elkhal A, Rodriguez H, Yang X, Edtinger K, et al. Mechanisms and consequences of injury and repair in older organ transplants. Transplantation. (2014) 97:1091–9. doi: 10.1097/TP.0000000000000072
57. Rippe C, Blimline M, Magerko KA, Lawson BR, LaRocca TJ, Donato AJ, et al. MicroRNA changes in human arterial endothelial cells with senescence: relation to apoptosis, eNOS and inflammation. Exp Gerontol. (2012) 47:45–51. doi: 10.1016/j.exger.2011.10.004
58. Oberhuber R, Ge X, Tullius SG. Donor age-specific injury and immune responses. Am J Transplant. (2012) 12:38–42. doi: 10.1111/j.1600-6143.2011.03798.x
59. Jurewicz M, Ueno T, Azzi J, Tanaka K, Murayama T, Yang S, et al. Donor antioxidant strategy prolongs cardiac allograft survival by attenuating tissue dendritic cell immunogenicity(dagger). Am J Transplant. (2011) 11:348–55. doi: 10.1111/j.1600-6143.2010.03360.x
60. Goes N, Urmson J, Ramassar V, Halloran PF. Ischemic acute tubular necrosis induces an extensive local cytokine response: evidence for induction of interferon-gamma, transforming growth factor-beta 1, granulocyte-macrophage colony stimulating factor, interleukin-2 and interleukin-10. Transplantation. (1995) 59:565–72.
Keywords: extended donor criteria, immunological aspects, liver transplantation, liver allocation, outcome
Citation: Kahn J, Pregartner G, Avian A, Kniepeiss D, Müller H and Schemmer P (2020) The Graz Liver Allocation Strategy—Impact of Extended Criteria Grafts on Outcome Considering Immunological Aspects. Front. Immunol. 11:1584. doi: 10.3389/fimmu.2020.01584
Received: 20 September 2019; Accepted: 15 June 2020;
Published: 04 August 2020.
Edited by:
Eric Spierings, Utrecht University, NetherlandsReviewed by:
Mahzad Akbarpour, Northwestern University, United StatesCopyright © 2020 Kahn, Pregartner, Avian, Kniepeiss, Müller and Schemmer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Schemmer, cGV0ZXIuc2NoZW1tZXJAbWVkdW5pZ3Jhei5hdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.