
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 24 June 2020
Sec. Inflammation
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.01289
This article is part of the Research Topic Long-term Consequences of Sepsis and Severe Trauma on Innate and Adaptive Immunity View all 16 articles
 Dijoia B. Darden1
Dijoia B. Darden1 Julie A. Stortz1
Julie A. Stortz1 McKenzie K. Hollen1
McKenzie K. Hollen1 Michael C. Cox1
Michael C. Cox1 Camille G. Apple1
Camille G. Apple1 Russell B. Hawkins1
Russell B. Hawkins1 Jaimar C. Rincon1
Jaimar C. Rincon1 Maria-Cecilia Lopez2
Maria-Cecilia Lopez2 Zhongkai Wang3
Zhongkai Wang3 Eduardo Navarro1
Eduardo Navarro1 Jennifer E. Hagen4
Jennifer E. Hagen4 Hari K. Parvataneni4
Hari K. Parvataneni4 Maigan A. Brusko5
Maigan A. Brusko5 Michael Kladde6
Michael Kladde6 Rhonda Bacher3
Rhonda Bacher3 Babette A. Brumback3
Babette A. Brumback3 Scott C. Brakenridge1
Scott C. Brakenridge1 Henry V. Baker2
Henry V. Baker2 Christopher R. Cogle7
Christopher R. Cogle7 Alicia M. Mohr1
Alicia M. Mohr1 Philip A. Efron1*
Philip A. Efron1*Older adults have significantly worse morbidity and mortality after severe trauma than younger cohorts. The competency of the innate immune response decreases with advancing age, especially after an inflammatory insult. Subsequent poor outcomes after trauma are caused in part by dysfunctional leukocytes derived from the host's hematopoietic stem and progenitor cells (HSPCs). Our objective was to analyze the bone marrow (BM) HSPC transcriptomic [mRNA and microRNA (miR)] responses to trauma in older and younger adults. BM was collected intraoperatively <9 days after initial injury from trauma patients with non-mild injury [ISS ≥ 9] or with shock (lactate ≥ 2, base deficit ≥ 5, MAP ≤ 65) who underwent operative fixation of a pelvic or long bone fracture. Samples were also analyzed based on age (<55 years and ≥55 years), ISS score and transfusion in the first 24 h, and compared to age/sex-matched controls from non-cancer elective hip replacement or purchased healthy younger adult human BM aspirates. mRNA and miR expression patterns were calculated from lineage-negative enriched HSPCs. 924 genes were differentially expressed in older trauma subjects vs. age/sex-matched controls, while 654 genes were differentially expressed in younger subjects vs. age/sex-matched control. Only 68 transcriptomic changes were shared between the two groups. Subsequent analysis revealed upregulation of transcriptomic pathways related to quantity, function, differentiation, and proliferation of HSPCs in only the younger cohort. miR expression differences were also identified, many of which were associated with cell cycle regulation. In summary, differences in the BM HSPC mRNA and miR expression were identified between older and younger adult trauma subjects. These differences in gene and miR expression were related to pathways involved in HSPC production and differentiation. These differences could potentially explain why older adult patients have a suboptimal hematopoietic response to trauma. Although immunomodulation of HSPCs may be a necessary consideration to promote host protective immunity after host injury, the age related differences further highlight that patients may require an age-defined medical approach with interventions that are specific to their transcriptomic and biologic response. Also, targeting the older adult miRs may be possible for interventions in this patient population.
Traumatic injury remains one of the leading causes of morbidity and mortality in the United States and the world, despite advances in the management of these patients (1, 2). The majority of trauma patients are known to recover rapidly; however, 3-year mortality remains ~16%, and about 20% of trauma patients develop chronic critical illness (CCI) (3–6), defined as > 14 days in ICU with continued organ dysfunction (7–9). Importantly, studies have revealed that risk factors for poor post-traumatic outcomes include age and injury severity score (ISS) at admission as well as total blood transfusion in the first 12 h after injury (3, 10–13).
A dysfunctional host immune system is responsible in part for post-traumatic morbidity and mortality (8). This includes innate immune cells originating from the host's hematopoietic stem and progenitor cells (HSPC) in the setting of unresolving organ failure—leukocytes derived from these HSPCs are not able to adequately resolve secondary, post-traumatic infectious insults (8, 13–15).
Importantly, advanced age is associated with an attenuated acute peripheral leukocyte response and age is known to be one of the strongest risk factors for poor outcome after severe trauma with hemorrhagic shock (6, 11, 16). Older adults have a baseline dysfunction in their immune system (immunosenescence) and low grade systemic inflammation (inflammaging) that in part can contribute to their suboptimal immune response to inflammation, increasing poor outcomes after severe injury (17). Our laboratory, as well as others, have demonstrated that elderly mice after severe injury are unable to mount an effective immune response as compared to juvenile mice. This is secondary, in part, to a failure of bone marrow progenitors to effectively respond to trauma (18). In a murine trauma model, this resulted in increased mortality with delivery of post-trauma Pseudomonas pneumonia (18–21).
Numerous attempts at pharmacological and therapeutic interventions to prevent or attenuate post-traumatic insults in this high risk group of older adults have not sufficiently taken into account their unique response to severe injury (22–25). This includes the HSPC response to trauma, as well as the epigenome that in part controls the transcription of these host cells. Specifically, microRNAs (miRs), a class small, non-coding RNAs that regulate gene expression, are important to cellular transcriptional/epigenetic modification of multiple processes such as cell development and differentiation (26). miRs can functions in several ways, including RNA silencing and post-transcriptional regulation of gene expression, and are known to modulate immune responses (27). Modulating miRs has emerged as powerful therapeutic target for cell specific therapy in personalized medicine (28).
We hypothesized that the bone marrow (BM) HSPC transcriptomic and miR response to trauma would be dependent upon the age of the subject and could in part explain the post-trauma dysfunctional myelopoiesis in these individuals, and reveal potential therapeutic targets.
Approval was obtained from the University of Florida (UF) Institutional Review Board and was performed from 2014 to 2019 at UF Health Shands Hospital, a 996-bed academic quaternary-care referral center. The study was registered with clinicaltrials.gov (NCT02577731) and conducted by the Sepsis and Critical Illness Research Center at UF. In every case, signed, informed consent was obtained from the individual patient or their designated legal representative. If informed consent was obtained from the legal representative, the patient was re-consented after they had achieved a clinical state where they could provide informed consent. If written informed consent could not be obtained from the patient or their legal representative within 96 h of study enrollment, the patient was removed from the study and all collected biologic samples and clinical data were destroyed.
We enrolled adult trauma patients (age ≥18) that were admitted to UF Health Shands Hospital according to the UF Institutional Review Board protocol #201601386. Patients with blunt and/or penetrating trauma resulting in long bone or pelvic fractures requiring open reduction and internal fixation or closed reduction, percutaneous pinning) were selected if they demonstrated an injury severity score (ISS) ≥ 9 or hemorrhagic shock (HS; defined by systolic blood pressure ≤ 90 mmHg or mean arterial pressure ≤ 65 mmHg or base deficit (BD) ≥ 5 meq or lactate ≥ 2) within the first 24 h of admission. Patients were excluded if pregnant, prisoners, expected survival was <48 h, receiving chronic corticosteroids or immunosuppression therapies, previous bone marrow transplantation, previous diagnosis of End Stage Renal Disease, or any pre-existing hematological disease.
Age/sex-matched controls were either purchased whole human bone marrow (Lonza, Biosciences, Berkshire, U.K.) or bone marrow collected from non-cancer, non-infectious elective hip repair patients. Control patients were enrolled according to the same IRB protocol above and were excluded if pregnant, prisoners, receiving chronic corticosteroids or immunosuppression therapies, previous chemotherapy or radiation therapy, previous bone marrow transplantation, previous diagnosis of end stage renal disease, or any pre-existing hematological disease, pathological fractures, cancer, HIV or connective tissue disease.
A 10 ml aspirate sample of whole bone marrow was collected intraoperatively. Of note, this aspirate contained tissue and not just cellular material. This tissue was considered a waste sample as it would have to be removed to make room for the hardware being placed by the orthopedic surgeons. Bone marrow was collected into a heparinized tube, placed on ice and transferred to the laboratory and processed <12 h after collection (29).
Bone marrow collection and bone marrow cell isolation were conducted as previously published by our laboratory (29). HSPCs from either trauma, control or purchased whole human bone marrow were negatively isolated from bone marrow via magnetic separation using a lineage-positive, cell depletion kit according to protocol (Miltenyi Biotec). Total RNA was isolated using QIAGEN RNeasy™ Mini Kit (Qiagen) and labeled and hybridized onto GeneChip® Human Transcriptome Array 2.0 (Affymetrix, Santa Clara, CA) and processed following manufacturer's instructions. BRBArray Tools® was used to identify significant microarray gene expression differences. Fold expression changes of the significant genes were calculated vs. age/sex-matched controls. Three separate analyses were performed based on: (1) Patient age: younger <55 years or old ≥ 55 years; (2) ISS score; and (3) blood transfusion.
The significant, differentially expressed genes were further analyzed with Ingenuity Pathway Analysis (IPA) software™ and Gene Ontology™ (GO) enrichment analysis. IPA software was employed to make downstream functional predictions from these groups of genes with a Z-score greater than two indicating significance. GO enrichment analysis identifies significant representative pathways/biogroups that are over-represented, indicating that their expression is influenced by the intervention. We selected pathways with p ≤ 0.005, determined by the LS/KS permutation test and Efron-Tibshirani's GSA maxmean test utilized in GO software to find significant gene sets.
miRNA (miR) profiling was performed using GeneChip™ miRNA 4.0 Array (ThermoFisher Scientific), covering 2,578 human microRNAs annotated in miRBase V2.0. miR expression patterns were calculated with a log2-transformed expression matrix with significant expression differences (fold expression changes over age/sex-matched control) identified using BRBArrayTools® (p < 0.05). Predicted target genes of the differentially expressed miR were identified with TargetScan Human 7.2, which predicts biological targets of miRs, by searching for conserved 8-mer, 7-mer and 6-mer sites matching the seed region of each miR (30).
Results for continuous variables are reported as mean ± SD for normally distributed variables or median ± interquartile range for non-normally distributed variables. Normality was checked via the Shapiro-Wilk test. Student's t-test or nonparametric Mann-Whitney test was used to compare normal or non-normal variables respectively between different groups or time points. Tukey's multiple comparison procedure was used to adjust p-values for multiple comparisons. Data were analyzed using Prism 7 (GraphPad Software, CA) and SAS 9.4 (SAS Institute Inc., Cary NC).
The overall and age-defined characteristics of the younger and older adult trauma patient cohorts of this study are displayed in Table 1. Previous studies have demonstrated that age ≥55 years is associated with worse outcomes after severe trauma (11, 31, 32). Younger and older adult groups were relatively similar with the exception of a higher percentage of trauma-related falls in the older group, as opposed to zero in the younger adult group. ISS, lactate, blood transfusion, and Apache II score from the first 24 h from admission did not significantly differ between the two groups (p = 0.48, p = 0.64, p = 0.85, and p = 0.58, respectively) (33–36).
Trauma bone marrow samples were obtained during long bone or pelvic fracture repair <9 days following blunt trauma (3.5 ± 2.4 days), and control samples (mean age 60.0 ± 9.1 years) were obtained at the time of elective surgery or from a commercial vendor (mean age 28.3 ± 4.4 years).
Total RNA was isolated from negatively-isolated bone marrow HSPCs for transcriptomic analysis. In an analysis of all trauma patients (n = 33) vs. healthy controls (n = 16), 1,845 genes were differentially expressed by enriched HSPC populations (p < 0.001). Sub-group analysis revealed that the severity of injury (ISS categories) did not significantly influence HSPC genome-wide expression (p = 0.083) (Figure 1). Transcriptomic differences were also not detectable between patients with or without blood transfusion (n = 7 and 26, respectively) within 24 h after injury. However, HSPC genome-wide expression did vary after trauma in older vs. younger adult patients. Older adult trauma patients (vs. age-matched controls) had a total of 924 probe sets representing 749 unique genes that were differentially expressed (p < 0.005; Figures 2A,B). Analysis of HSPCs in younger trauma vs. age-matched controls, however, revealed differential expression of 709 probe sets representing 654 unique genes (p < 0.0005; Figures 2C,D).
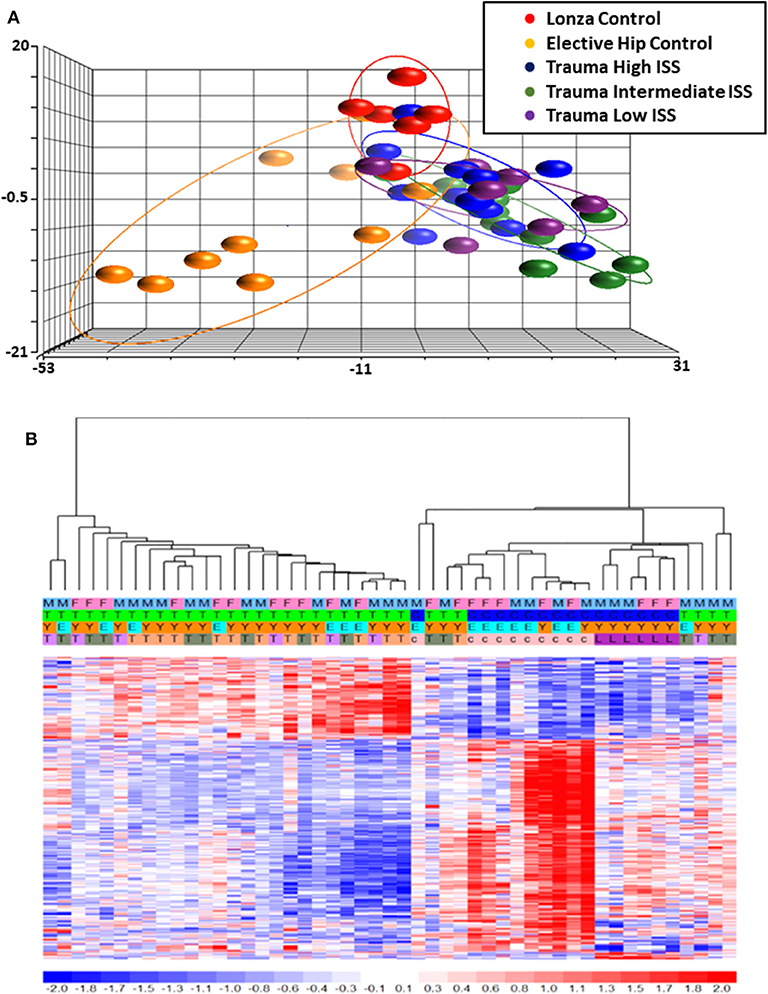
Figure 1. Microarray Transcriptomic Analysis of Leukocytes from Trauma Patients with Low, Intermediate and High ISS and Healthy Control Subjects. The genomic response of isolated leukocyte RNA in healthy controls and trauma patients and healthy controls. (A) Conditional principal component analysis of ISS and healthy control leukocyte gene expression patterns. (B) Heat map (log2) of the hierarchical clustering of leukocyte gene expression patterns and variation between trauma patients with differing ISS healthy control subjects. M, Male; F, Female; Y, Younger group; E, Older group; T, Trauma subject (three colors on row four represent three different ISS groups); c, Elective hip control subject; L, Lonza control subject.
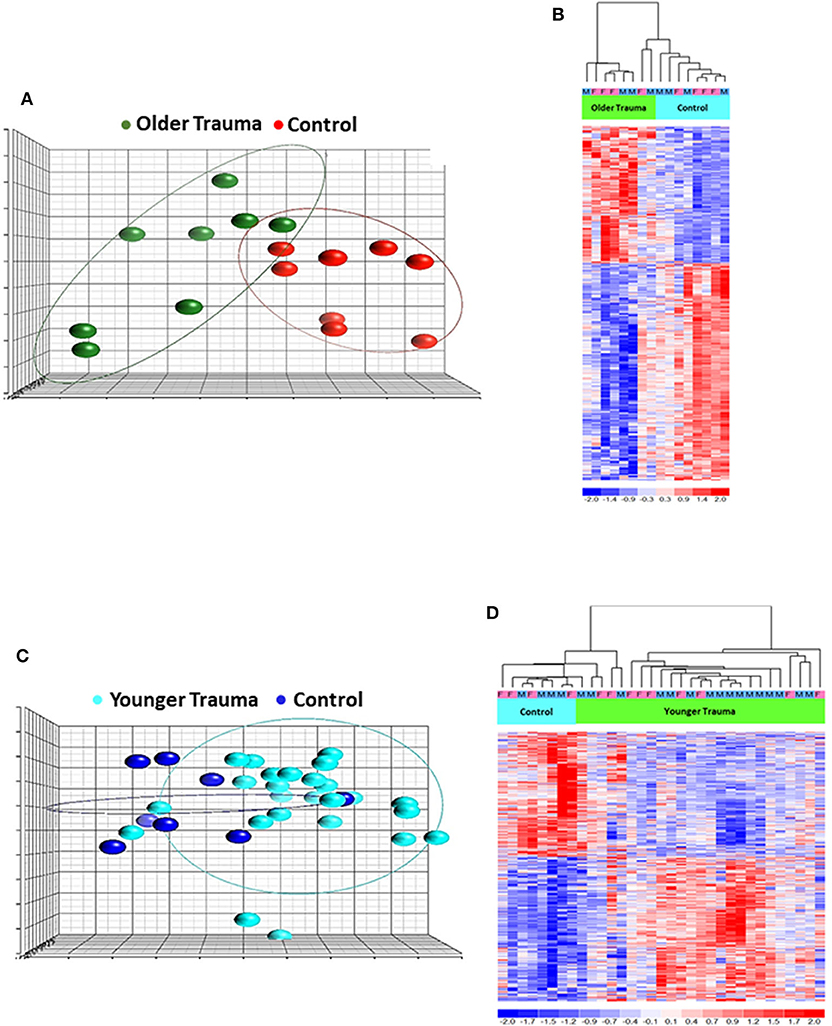
Figure 2. Microarray Transcriptomic Analysis of Leukocytes from Older Trauma Patients and Younger Trauma Patients vs. Healthy Control Subjects. The genomic response of isolated leukocyte RNA in healthy controls and trauma patients and healthy controls. (A) Conditional principal component analysis of older adult trauma patients and healthy control leukocyte gene expression patterns. (B) Heat map (log2) of the hierarchical clustering of leukocyte gene expression patterns and variation between older adult trauma patients and healthy control subjects. (C) Conditional principal component analysis of younger adult trauma patients and healthy control leukocyte gene expression patterns. (D) Heat map (log2) of the hierarchical clustering of leukocyte gene expression patterns and variation between younger adult trauma patients and healthy control subjects. M, Male; F, Female.
Interestingly, we determined that the majority of genes with significant up or down regulation after trauma (vs. control) were dissimilar between the older and younger adult trauma patients (Table 2). Of the 749 and 654 genes identified after trauma in older and younger adult trauma patients, respectively, only 68 genes were observed to be in common (~10%). In this set of common genes between older and younger adult trauma, the directionality of the change was identical, implying their importance to the common mammalian response to severe injury. Fifty-six genes were found to be downregulated, while the remaining 12 genes were upregulated (Table 3). Many of these genes are known to be important in inflammation and innate immunity, such as MYCT1, NRIP1, FLT3, TNFAIP2, and BCL2 (37–40).
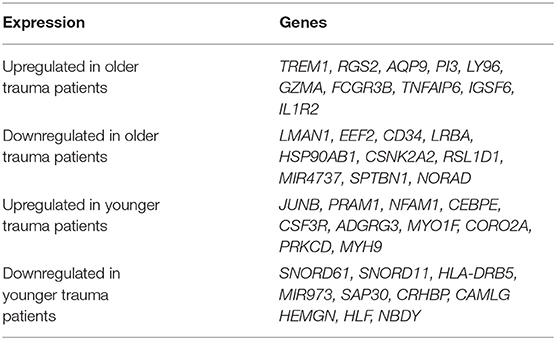
Table 2. Top 10 genes with the greatest significant expression changes in only older or younger adult trauma patients (relative to/vs. age-matched controls) in bone marrow HSPCs.
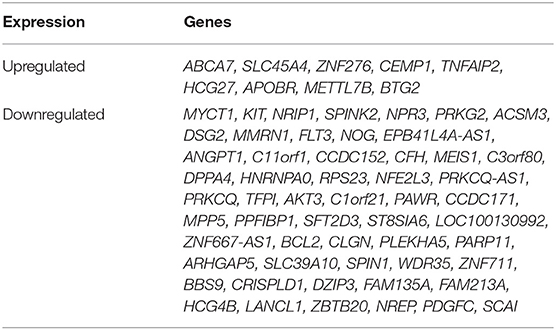
Table 3. Genes with common transcriptomic up or down regulation in HSPCs from older trauma and younger trauma patients (as compared to their age/sex matched healthy controls).
The older adult trauma cohort was noted to have significant downregulation in the expression of genes involved in hematopoiesis, not seen in younger trauma patients, when compared to age/sex-matched controls (p < 0.001). This included, but was not limited to, CD34, CASP2, CDK6, CXCL12, SMARCA2, and SATB1 (Table 4) (37, 41–44). Analysis of genes that were only significant in younger trauma HSPCs (vs. age/sex-matched controls) revealed significant upregulation of genes for receptors important to HSPC proliferation, migration and differentiation, e.g., IL-8Rα, GM-CSFRα, and G-CSFR.
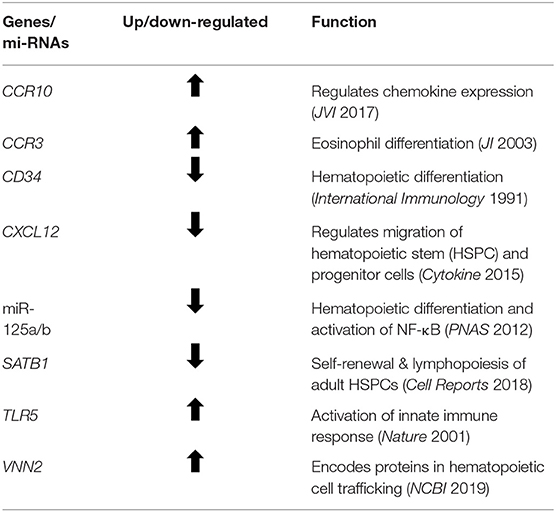
Table 4. Prominent genes and miRs found to be significantly altered in old, but not young, bone marrow HSPCs following severe trauma vs. age/sex-matched healthy controls.
We utilized IPA and GO for further overall and pathway analysis of our genomic data. The use of these software allows greater biological insight into the functional processes activated or inhibited in each trauma group. IPA functional pathways revealed that bone marrow HSPCs from older adult trauma patients had an attenuated transcriptomic/epigenetic response to severe trauma, as displayed in Figure 3. In addition, only HSPCs from younger trauma patients demonstrated significant (z-score > |2|) upregulation of hematopoiesis pathways of function, quantity, and differentiation (Figure 4; Supplemental Tables 1, 2; Supplemental Figure 1).
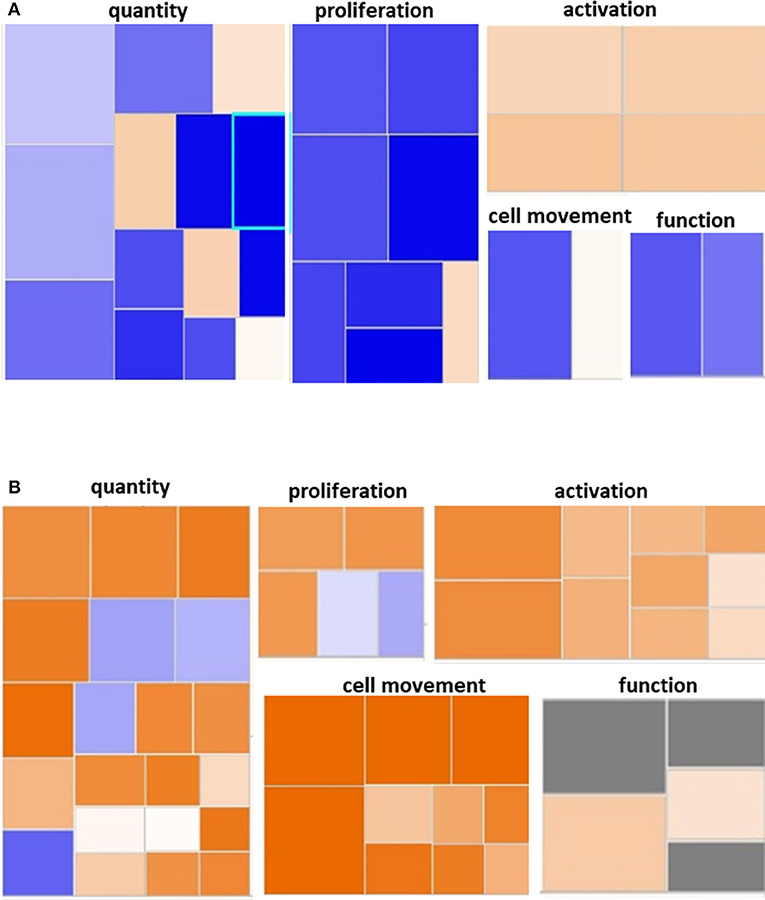
Figure 3. Hematological System Development and Function Pathway from Younger and Older Trauma Patients vs. Age-Matched Controls. Ingenuity Pathway Analysis engendered figure illustrating significant (A) down regulation of many genes in the hematological system development and function pathways in older trauma patients as opposed to (B) upregulation in younger trauma patients. Orange to red = upregulation, green to blue = downregulation.
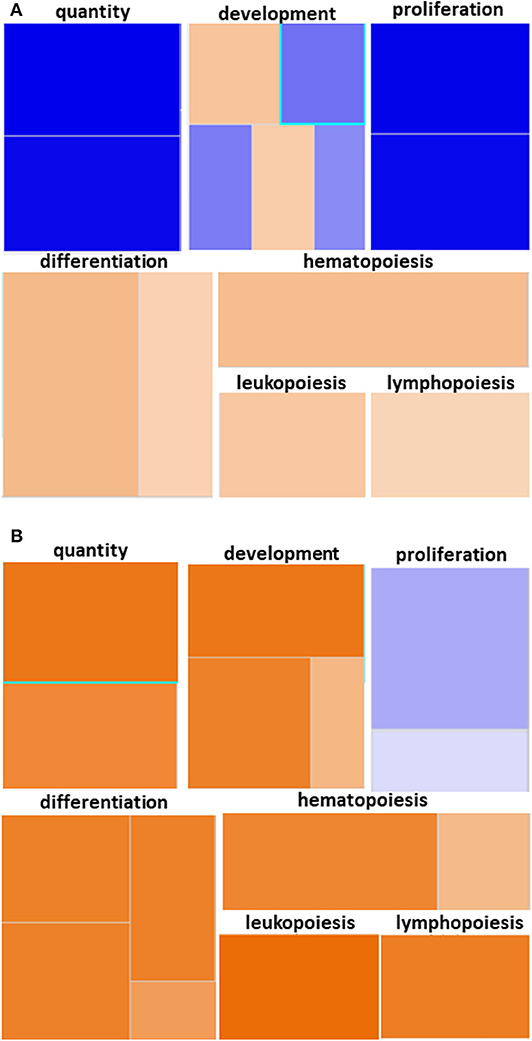
Figure 4. Hematopoiesis Diseases and Functions Pathway from Younger and Older Trauma Patients vs. Age-Matched Controls. Ingenuity Pathway Analysis engendered figure illustrating significant (A) down regulation of genes in the hematopoiesis diseases and function pathways in old trauma patients as opposed to (B) upregulation in younger trauma patients. Orange to red = upregulation, green to blue = downregulation.
GO analysis of the differentially expressed genes illustrated involvement of the older adult HSPC transcriptome mainly in biological processes related to energy and protein metabolism (Supplementary Table 3). Regulation of IL-10 production was only statistically represented in older adult trauma patients, supporting the concept that the inflammatory response of each age group is dissimilar and potentially dysregulated in older vs. younger adults (Figure 5A). The younger trauma patients were predicted to have over-representation of several biological process categories important for immune response, such as myeloid and neutrophil mediated immunity (Figures 5B,C), which was not seen in the older patients (Supplementary Table 4). This post-analysis provided further insights into the important pathways potentially involved in younger and older trauma HSPCs.
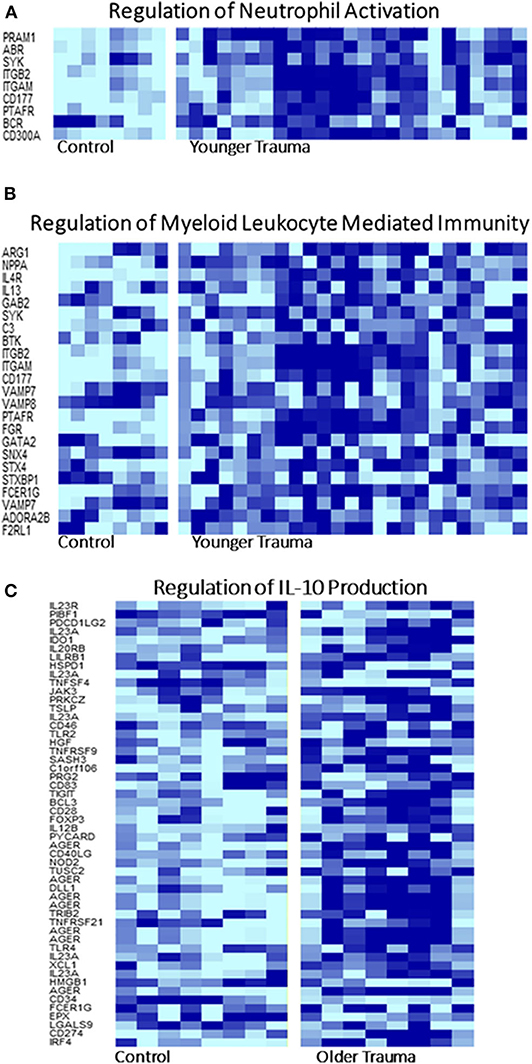
Figure 5. Selected Gene Ontology Pathway Heat Maps in Younger and Older Trauma Patients. Gene ontology pathway analysis demonstrated that several pathways involved in innate and adaptive immunity, (A) neutrophil activation and (B) myeloid leukocyte mediated immunity, only in the younger trauma patients were significantly different from controls. (C) Only older trauma patients had over-representation of the regulation of IL-10 production. Dark blue = upregulation, light blue = down regulation.
A comparison of bone marrow HSPC miR expression from all trauma (n = 27) and control (n = 16) subjects revealed 60 miRs that were differentially expressed (p < 0.005). The expression of 27 miRs were significantly downregulated and 33 were significantly upregulated. Fifteen of these miRs from trauma patients demonstrated at least a 2-fold positive or negative change in expression when compared with control subjects (Table 5). Among these were miRs 125a/b and 146a of which are known to influence HSPC function persistence (45, 46).
Interestingly, miR-125a/b and −146a were amongst the 8 miRs with the highest fold-change difference between expressions of bone marrow HSPC miR from older trauma vs. younger trauma patients. The elderly trauma patients had markedly greater down-regulation of these miRs. Additionally among the miR with the highest fold-change differences, miR-7515 and miR-3128 (47, 48), both implicated in tumor suppression, were upregulated in older trauma patients and down-regulated in younger trauma patients (Table 6).
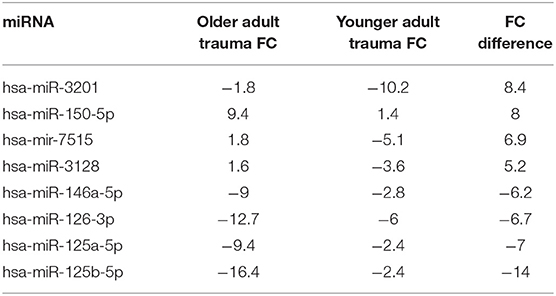
Table 6. miRNA in Human HSPCs from older vs. younger adult trauma patients with largest absolute fold-change (FC) difference.
Analysis using TargetScan revealed that the predicted gene targets of the expressed miR with the largest fold changes from HSPCs isolated from older trauma patients overlap with the genes found significantly expressed in the same subjects (Table 7). The predicted gene targets for the up-regulated miRs had a higher overlap with the genes down-regulated in HSPCs of older trauma patients. These included genes associated with HSPC proliferation and function like AREG, CXCR1, KIT, and MGAM.
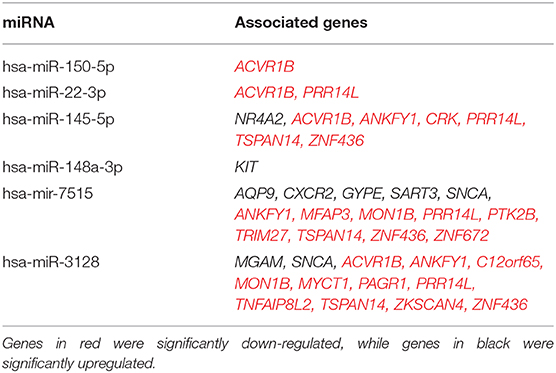
Table 7. List of select significant upregulated HSPC miRNAs from old trauma patients and their predicted targets of significant genes of the same cells as analyzed by Target Scan software.
Advanced age is a known risk factor for poor outcomes in trauma patients (11). Increased mortality in the elderly is largely attributed to their diminished physiologic and immunologic reserves, resulting in higher rates of nosocomial infections and sepsis post-trauma (11, 12). Previous murine studies by our laboratory demonstrate that the increased incidence of infection in the elderly is also related to HSPC failure in the aged host after severe injury (18). Therefore, we sought to determine if similar hematopoietic defects are present in humans. While there was no difference in genomic expression patterns based on the magnitude of the traumatic injury (ISS) nor based on blood transfusion in the first 24 h, age was associated with significantly different genomic expression patterns when compared to younger trauma patients of equivalent severity.
Transcriptomic evaluation of bone marrow HSPC from older adult trauma patients illustrates a diminished functional status as well as a blunted capacity for the terminal differentiation of myeloid cells (49). Many genes important in HSPC proliferation, differentiation and mobilization, such as CD34, CXCL12, miR-125a/b, SATB1, APEX1, and PRDX1, were all found to be downregulated and only differentially expressed in older trauma HSPCs (50, 51). Interestingly, the genes found differentially expressed only in younger trauma patients were predicted by IPA to have increased activation of hematopoietic function pathways not seen in older trauma patients (Supplemental Tables 1, 2). Pathway analysis also revealed significantly down regulated IL-4 and IL-8 signaling in HSPCs from older trauma patients compared to the younger patients (Supplemental Figure 2).
The older trauma patients show a more blunted and down regulation of many genes in the CXCR4 pathway leading presumably to decreased capacity for cell migration in comparison to the younger trauma patient with significant upregulation in this signaling pathway (Supplemental Figure 3). A few studies have demonstrated that CXCL12 expressed by surrounding cells in the bone marrow niche promotes HSPC quiescence and retention in the bone marrow via the CXCR4 signaling pathway (52–54). Although, our data demonstrates a downregulation of CXCL12 in HSPCs of trauma patients in this study, Ding et al. showed that deletion of CXCL12 in HSPCs had no effect on HSPC mobilization (55). Downregulation of CXCR4 has been noted in the literature to increase mobilization of HSPCs (56–58). However, we did not find a significant change in CXCR4 gene expression in our study. Additionally, many studies have noted modulation of CXCL12 expression via epigenetics (59–62). Specifically, miR-23a, noted to be upregulated in old trauma patient HSPCs in our study, down-regulates CXCL12 mRNA and protein expression (59). The role of endogenous CXCL12 is not completely understood, but these points highlight the complexity of the bone marrow niche in the regulation of the HSPC response to certain stressors. More research to study the effect of trauma on specific pathways in the bone marrow niche, its crosstalk with HSPCs, and HSPC migration from the bone marrow is warranted.
Our laboratory previously determined that elderly patients with complicated outcomes have significantly decreased plasma cytokine and chemokine concentrations 0–4 days after severe injury and hemorrhage (11). Interestingly, HSPC genomic analysis in this study revealed many pro-inflammatory related genes differentially expressed and upregulated only in older trauma patients such as TLR5, TLR8, TNFAIP6, TGFA, IL1R2, IL23A, IL7R, CCR3, and IL10RA. However, GO analysis of the differentially expressed genes in the older trauma patients revealed over-representation of biological process categories related to only one inflammatory molecule—IL-10. Additionally, GO revealed over-representation of many processes involved in immune activation in the younger trauma HPSC mRNAs not seen in the older adult patients (Supplementary Tables 3, 4). Taken together, this data suggests that older patients fail to activate the appropriate immune response early after severe injury and show a dysregulated inflammatory response.
miR analysis also revealed differential HSPC regulation after trauma dependent upon age, with significant differences in expression patterns of miRs known to be important for HSPC function. This included increased expression of miR-143/145 in the young, as well as decreased expression of miR-125a/b in the old, all of which are vital to HSPC function (45, 46). Under-expression of miR-143 and−145 have been noted in many malignancies, and overexpression in these malignant cell lines impair cell growth, differentiation, and cause apoptosis of CD34+ HSPCs (63, 64). Expression of miR-125a has been noted to increase the number of HSPCs (65), while overexpression of miR-125b in hematopoietic stem cells has been noted to promote self-renewal, differentiation and expansion (66). Additionally, expression of miR-125a/b inhibits TNFAIP3, leading to activation of the NF-κB pathway (45). Evidence indicates that NF-κB signaling leads to a loss of HSPC quiescence and increased differentiation (67). The markedly lowered expression of miR-125a/b seen in HSPCs from the older trauma patients may translate to a markedly decreased NF-κB activity, which in turn contributes to the continued quiescence of HSPCs after traumatic injury.
Our study was limited in several ways. First, HSPCs were negatively isolated by depletion of lineage-committed cells. This is due to the limited amount of bone marrow that can be safely be obtained from these patients, and with hematopoietic stem cells being an extremely rare population. Therefore, the transcriptomics in this study represent all lineage-negative cells including hematopoietic stem and early progenitor cells. HSPCs are known to expand after trauma and also with advanced age, so the expression patterns likely reflect this phenomena as well (18, 49, 68). However, multi-potent progenitor cells (MPPs) and some other progenitor cells, like HSCs, do represent early cell populations present that are host-capable of engendering differentiated hematopoietic cells (69). However, the number of lineage negative cells that we could isolate was limited due to being a rare population, constricting our ability to perform detailed fluorescence-activated cell sorting/flow cytometry. Future studies using novel technology such as single cell RNAseq, Cellular Indexing of Transcriptomes and Epitopes by Sequencing (CITE-Seq), and Assay for Transposase Accessible Chromatin using sequencing (ATAC-Seq) are currently underway in our laboratory and will be required to better comprehend how each cell type may contribute to the suboptimal aged response to injury (70). In addition, sample size and the number of lineage negative cells that we could isolate limited our ability to perform several sub-analyses such as methylcellulose colony assays and functional assays to evaluate mobilization of HSPCs into peripheral blood. Additionally, age-specific data analysis of miR arrays was unable to be performed due to the small sample size of the older adult patient cohort. This may have led to a bias toward the younger adult arrays in the analysis of miR in all trauma patients.
However, trends can still be seen that suggest age-specific differences in epigenetic expression in response to trauma. In addition, we were unable to perform multi-variant logistic regression analysis on all risk factors between older and younger adults (e.g., co-morbidities on arrival) so that age could be determined to be independently associated with the HSPC differences. However, there are limitations to how many bone marrow samples can be obtained in these patients. In addition, in a somewhat practical analysis of older subjects, this is the HSPC response present after trauma for patients ≥55 years old, regardless of other factors (11).
Bone marrow HSPCs from older human trauma patients have a unique, and in some ways, subdued mRNA/miR response to trauma compared to younger patients. Independent of injury severity and blood transfusion requirement, advanced age may be the key driver of post-traumatic bone marrow HSPC transcriptomic and some epigenetic changes. The regulation of vital miRs and genes involved in HSPC production and differentiation may suggest why older patients have a blunted hematopoietic response to trauma, contributing to their subsequent immune dyscrasia. Although immunomodulation of HSPCs is possible, elderly patients may not respond well to standard cytokines or growth factors. Precision medicine may require epigenetic manipulation to modify HSPC protective immunity to improve long-term outcomes in the elderly.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
The studies involving human participants were reviewed and approved by University of Florida Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Project conception and design: DD, JS, MH, MC, CA, RH, JR, JH, MB, MK, RB, BB, SB, HB, CC, AM, and PE. Data collection: DD, JS, MH, MC, CA, EN, JH, and HP. Data analysis and interpretation: DD, JS, MH, M-CL, ZW, BB, and HB. Original manuscript drafting: DD, MH, and PE. Manuscript review and editing: DD, JS, MH, MC, CA, RH, JR, M-CL, MB, MK, RB, SB, HB, CC, AM, and PE. Manuscript final approval: DD, SB, HB, CC, AM, and PE. All authors contributed to the article and approved the submitted version.
This work was supported in part by the following National Institutes of Health grants: NIGMS: R01 GM-040586, R01 GM-104481, R01 GM-113945, P50 GM-111152, T32 GM-008721, and NIA: 1P30AG028740.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to acknowledge Ricardo Ungaro, Marvin L. Dirain, and Dina C. Nacionales for their assistance in technical support. Thank you to Drs. Frederick A. Moore, Lyle L. Moldawer, Azra Bihorac, and Todd Brusko for advice with the project concept and design. We would also like to thank Drs. Frederick A. Moore and Lyle L. Moldawer for assistance in editing the manuscript in its final form.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.01289/full#supplementary-material
1. DiMaggio C, Ayoung-Chee P, Shinseki M, Wilson C, Marshall G, Lee DC, et al. Traumatic injury in the United States: in-patient epidemiology 2000-2011. Injury. (2016) 47:1393–403. doi: 10.1016/j.injury.2016.04.002
2. Murphy SL, Xu J, Kochanek KD, Arias E. Mortality in the United States, 2017. NCHS Data Brief . (2018) 1–8.
3. Gardner AK, Ghita GL, Wang Z, Ozrazgat-Baslanti T, Raymond SL, Mankowski RT, et al. The development of chronic critical illness determines physical function, quality of life, and long-term survival among early survivors of sepsis in surgical ICUs. Crit Care Med. (2019) 47:566–73. doi: 10.1097/CCM.0000000000003655
4. Brakenridge SC, Efron PA, Stortz JA, Ozrazgat-Baslanti T, Ghita G, Wang Z, et al. The impact of age on the innate immune response and outcomes after severe sepsis/septic shock in trauma and surgical intensive care unit patients. J Trauma Acute Care Surg. (2018) 85:247–55. doi: 10.1097/TA.0000000000001921
5. Efron PA, Mohr AM, Bihorac A, Horiguchi H, Hollen MK, Segal MS, et al. Persistent inflammation, immunosuppression, and catabolism and the development of chronic critical illness after surgery. Surgery. (2018) 164:178–84. doi: 10.1016/j.surg.2018.04.011
6. Mira JC, Cuschieri J, Ozrazgat-Baslanti T, Wang Z, Ghita GL, Loftus TJ, et al. The epidemiology of chronic critical illness after severe traumatic injury at two level-one trauma centers. Crit Care Med. (2017) 45:1989–96. doi: 10.1097/CCM.0000000000002697
7. Kahn JM, Le T, Angus DC, Cox CE, Hough CL, White DB, et al. The epidemiology of chronic critical illness in the United States. Crit Care Med. (2015) 43:282–7. doi: 10.1097/CCM.0000000000000710
8. Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, Ungaro R, Davis R, Cuenca AG, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. (2014) 76:21–30. doi: 10.1097/TA.0b013e3182ab1ab5
9. Davidson GH, Hamlat CA, Rivara FP, Koepsell TD, Jurkovich GJ, Arbabi S. Long-term survival of adult trauma patients. JAMA. (2011) 305:1001–7. doi: 10.1001/jama.2011.259
10. Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. (1996) 40:501–10. doi: 10.1097/00005373-199604000-00001
11. Vanzant EL, Hilton RE, Lopez CM, Zhang J, Ungaro RF, Gentile LF, et al. Host response to injury, advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care. (2015) 19:77. doi: 10.1186/s13054-015-0788-x
12. Taylor MD, Tracy JK, Meyer W, Pasquale M, Napolitano LM. Trauma in the elderly: intensive care unit resource use and outcome. J Trauma. (2002) 53:407–14. doi: 10.1097/00005373-200209000-00001
13. Rosenthal MD, Kamel AY, Rosenthal CM, Brakenridge S, Croft CA, Moore FA. Chronic critical illness: application of what we know. Nutr Clin Pract. (2018) 33:39–45. doi: 10.1002/ncp.10024
14. Warren HS, Elson CM, Hayden DL, Schoenfeld DA, Cobb JP, Maier RV, et al. Inflammation, and P. Host response to injury large scale collaborative research, a genomic score prognostic of outcome in trauma patients. Mol Med. (2009) 15:220–7. doi: 10.2119/molmed.2009.00027
15. de Kruijf EFM, Fibbe WE, van Pel M. Cytokine-induced hematopoietic stem and progenitor cell mobilization: unraveling interactions between stem cells and their niche. Ann N Y Acad Sci. (2019) 1466:24–38. doi: 10.1111/nyas.14059
16. Hashmi A, Ibrahim-Zada I, Rhee P, Aziz H, Fain MJ, Friese RS, et al. Predictors of mortality in geriatric trauma patients: a systematic review and meta-analysis. J Trauma Acute Care Surg. (2014) 76:894–901. doi: 10.1097/TA.0b013e3182ab0763
17. Horiguchi H, Loftus TJ, Hawkins RB, Raymond SL, Stortz JA, Hollen MK, et al. Critical illness research center, innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front Immunol. (2018) 9:595. doi: 10.3389/fimmu.2018.00595
18. Nacionales DC, Szpila B, Ungaro R, Lopez MC, Zhang J, Gentile LF, et al. A detailed characterization of the dysfunctional immunity and abnormal myelopoiesis induced by severe shock and trauma in the aged. J Immunol. (2015) 195:2396–407. doi: 10.4049/jimmunol.1500984
19. Chen MM, Palmer JL, Plackett TP, Deburghgraeve CR, Kovacs EJ. Age-related differences in the neutrophil response to pulmonary pseudomonas infection. Exp Gerontol. (2014) 54:42–6. doi: 10.1016/j.exger.2013.12.010
20. Nacionales DC, Gentile LF, Vanzant E, Lopez MC, Cuenca A, Cuenca AG, et al. Aged mice are unable to mount an effective myeloid response to sepsis. J Immunol. (2014) 192:612–22. doi: 10.4049/jimmunol.1302109
21. Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med. (2011) 208:2691–703. doi: 10.1084/jem.20111490
22. Flierl MA, Perl M, Rittirsch D, Bartl C, Schreiber H, Fleig V, et al. The role of C5a in the innate immune response after experimental blunt chest trauma. Shock. (2008) 29:25–31. doi: 10.1097/shk.0b013e3180556a0b
23. Mock CN, Dries DJ, Jurkovich GJ, Maier RV. Assessment of two clinical trials: interferon-gamma therapy in severe injury. Shock. (1996) 5:235–40. doi: 10.1097/00024382-199604000-00001
24. Rosenthal MD, Patel J, Staton K, Martindale RG, Moore FA, Upchurch GR Jr. Can specialized pro-resolving mediators deliver benefit originally expected from fish oil? Curr Gastroenterol Rep. (2018) 20:40. doi: 10.1007/s11894-018-0647-4
25. Huber-Lang M, Gebhard F, Schmidt CQ, Palmer A, Denk S, Wiegner R. Complement therapeutic strategies in trauma, hemorrhagic shock and systemic inflammation - closing Pandora's box? Semin Immunol. (2016) 28:278–84. doi: 10.1016/j.smim.2016.04.005
26. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. (2017) 16:203–22. doi: 10.1038/nrd.2016.246
27. Fleshner M, Crane CR. Exosomes, DAMPs and miRNA: features of stress physiology and immune homeostasis. Trends Immunol. (2017) 38:768–76. doi: 10.1016/j.it.2017.08.002
28. Baue AE. Sepsis, systemic inflammatory response syndrome, multiple organ dysfunction syndrome, and multiple organ failure: are trauma surgeons lumpers or splitters? J Trauma. (2003) 55:997–8. doi: 10.1097/01.TA.0000094631.54198.07
29. Loftus TJ, Mira JC, Miller ES, Kannan KB, Plazas JM, Delitto D, et al. The postinjury inflammatory state and the bone marrow response to anemia. Am J Respir Crit Care Med. (2018) 198:629–38. doi: 10.1164/rccm.201712-2536OC
30. Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. (2015) 4:e05005. doi: 10.7554/eLife.05005
31. Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA, Lezotte DC. Early predictors of postinjury multiple organ failure. Arch Surg. (1994) 129:39–45. doi: 10.1001/archsurg.1994.01420250051006
32. Demetriades D, Chan LS, Velmahos G, Berne TV, Cornwell EE 3rd, Belzberg H, et al. TRISS methodology in trauma: the need for alternatives. Br J Surg. (1998) 85:379–84. doi: 10.1046/j.1365-2168.1998.00610.x
33. Gale SC, Kocik JF, Creath R, Crystal JS, Dombrovskiy VY. A comparison of initial lactate and initial base deficit as predictors of mortality after severe blunt trauma. J Surg Res. (2016) 205:446–55. doi: 10.1016/j.jss.2016.06.103
34. Odom SR, Howell MD, Silva GS, Nielsen VM, Gupta A, Shapiro NI, et al. Lactate clearance as a predictor of mortality in trauma patients. J Trauma Acute Care Surg. (2013) 74:999–1004. doi: 10.1097/TA.0b013e3182858a3e
35. Callaway DW, Shapiro NI, Donnino MW, Baker C, Rosen CL. Serum lactate and base deficit as predictors of mortality in normotensive elderly blunt trauma patients. J Trauma. (2009) 66:1040–4. doi: 10.1097/TA.0b013e3181895e9e
36. Vandromme MJ, Griffin RL, Weinberg JA, Rue LW 3rd, Kerby JD. Lactate is a better predictor than systolic blood pressure for determining blood requirement and mortality: could prehospital measures improve trauma triage? J Am Coll Surg. (2010) 210:861–7:867–9. doi: 10.1016/j.jamcollsurg.2010.01.012
37. Holmfeldt P, Ganuza M, Marathe H, He B, Hall T, Kang G, et al. Functional screen identifies regulators of murine hematopoietic stem cell repopulation. J Exp Med. (2016) 213:433–49. doi: 10.1084/jem.20150806
38. Jia L, Shi Y, Wen Y, Li W, Feng J, Chen C. The roles of TNFAIP2 in cancers and infectious diseases. J Cell Mol Med. (2018) 22:5188–95. doi: 10.1111/jcmm.13822
39. Chung HT. RIP140, a Janus metabolic switch involved in defense functions. Cell Mol Immunol. (2013) 10:7–9. doi: 10.1038/cmi.2012.53
40. Parigi SM, Czarnewski P, Das S, Steeg C, Brockmann L, Fernandez-Gaitero S, et al. Flt3 ligand expands bona fide innate lymphoid cell precursors in vivo. Sci Rep. (2018) 8:154. doi: 10.1038/s41598-017-18283-0
41. Brown J, Greaves MF, Molgaard HV. The gene encoding the stem cell antigen, CD34, is conserved in mouse and expressed in haemopoietic progenitor cell lines, brain, and embryonic fibroblasts. Int Immunol. (1991) 3:175–84. doi: 10.1093/intimm/3.2.175
42. Mendt M, Cardier JE. Role of SDF-1 (CXCL12) in regulating hematopoietic stem and progenitor cells traffic into the liver during extramedullary hematopoiesis induced by G-CSF, AMD3100 and PHZ. Cytokine. (2015) 76:214–21. doi: 10.1016/j.cyto.2015.05.004
43. Doi Y, Yokota T, Satoh Y, Okuzaki D, Tokunaga M, Ishibashi T, et al. Variable SATB1 levels regulate hematopoietic stem cell heterogeneity with distinct lineage fate. Cell Rep. (2018) 23:3223–35. doi: 10.1016/j.celrep.2018.05.042
44. Laurenti E, Frelin C, Xie S, Ferrari R, Dunant CF, Zandi S, et al. CDK6 levels regulate quiescence exit in human hematopoietic stem cells. Cell Stem Cell. (2015) 16:302–13. doi: 10.1016/j.stem.2015.01.017
45. Kim SW, Ramasamy K, Bouamar H, Lin AP, Jiang D, Aguiar RC. MicroRNAs miR-125a and miR-125b constitutively activate the NF-kappaB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20). Proc Natl Acad Sci USA. (2012) 109:7865–70. doi: 10.1073/pnas.1200081109
46. Zhao JL, Starczynowski DT. Role of microRNA-146a in normal and malignant hematopoietic stem cell function. Front Genet. (2014) 5:219. doi: 10.3389/fgene.2014.00219
47. Lee JM, Yoo JK, Yoo HY, Jung H, Lee DR, Jeong HC, et al. The novel miR-7515 decreases the proliferation and migration of human lung cancer cells by targeting c-Met. Mol Cancer Res. (2013) 11:43–53. doi: 10.1158/1541-7786.MCR-12-0355
48. Luo J, Guo Y, Liu X, Yang X, Xiao F, Zhou M. Long non-coding RNA LINC01410 promotes colon cancer cell proliferation and invasion by inhibiting miR-3128. Exp Ther Med. (2018) 16:4824–30. doi: 10.3892/etm.2018.6806
49. Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. (2011) 108:20012–7. doi: 10.1073/pnas.1116110108
50. Xie JY, Li MX, Xiang DB, Mou JH, Qing Y, Zeng LL, et al. Elevated expression of APE1/Ref-1 and its regulation on IL-6 and IL-8 in bone marrow stromal cells of multiple myeloma. Clin Lymphoma Myeloma Leuk. (2010) 10:385–93. doi: 10.3816/CLML.2010.n.072
51. Venezia TA, Merchant AA, Ramos CA, Whitehouse NL, Young AS, Shaw CA, et al. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. (2004) 2:e301. doi: 10.1371/journal.pbio.0020301
52. Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. (2002) 195:1145–54. doi: 10.1084/jem.20011284
53. Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. (2006) 25:977–88. doi: 10.1016/j.immuni.2006.10.016
54. Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. (2008) 205:777–83. doi: 10.1084/jem.20072513
55. Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. (2013) 495:231–5. doi: 10.1038/nature11885
56. Ratajczak MZ, Adamiak M, Plonka M, Abdel-Latif A, Ratajczak J. Mobilization of hematopoietic stem cells as a result of innate immunity-mediated sterile inflammation in the bone marrow microenvironment-the involvement of extracellular nucleotides and purinergic signaling. Leukemia. (2018) 32:1116–23. doi: 10.1038/s41375-018-0087-z
57. Adamiak M, Ratajczak MZ. Innate immunity and mobilization of hematopoietic stem cells. Curr Stem Cell Rep. (2017) 3:172–80. doi: 10.1007/s40778-017-0087-3
59. Arabanian LS, Fierro FA, Stolzel F, Heder C, Poitz DM, Strasser RH, et al. MicroRNA-23a mediates post-transcriptional regulation of CXCL12 in bone marrow stromal cells. Haematologica. (2014) 99:997–1005. doi: 10.3324/haematol.2013.097675
60. Qu R, Sun Y, Li Y, Hu C, Shi G, Tang Y, et al. MicroRNA-130a-3p suppresses cell viability, proliferation and invasion in nasopharyngeal carcinoma by inhibiting CXCL12. Am J Transl Res. (2017) 9:3586–98.
61. Cioffi M, Trabulo SM, Vallespinos M, Raj D, Kheir TB, Lin ML, et al. The miR-25-93-106b cluster regulates tumor metastasis and immune evasion via modulation of CXCL12 and PD-L1. Oncotarget. (2017) 8:21609–25. doi: 10.18632/oncotarget.15450
62. Yu PF, Huang Y, Xu CL, Lin LY, Han YY, Sun WH, et al. Downregulation of CXCL12 in mesenchymal stromal cells by TGFbeta promotes breast cancer metastasis. Oncogene. (2017) 36:840–9. doi: 10.1038/onc.2016.252
63. Iio A, Nakagawa Y, Hirata I, Naoe T, Akao Y. Identification of non-coding RNAs embracing microRNA-143/145 cluster. Mol Cancer. (2010) 9:136. doi: 10.1186/1476-4598-9-136
64. Hartmann JU, Brauer-Hartmann D, Kardosova M, Wurm AA, Wilke F, Schodel C, et al. MicroRNA-143 targets ERK5 in granulopoiesis and predicts outcome of patients with acute myeloid leukemia. Cell Death Dis. (2018) 9:814. doi: 10.1038/s41419-018-0837-x
65. Guo S, Lu J, Schlanger R, Zhang H, Wang JY, Fox MC, et al. MicroRNA miR-125a controls hematopoietic stem cell number. Proc Natl Acad Sci USA. (2010) 107:14229–34. doi: 10.1073/pnas.0913574107
66. Shaham L, Binder V, Gefen N, Borkhardt A, Izraeli S. MiR-125 in normal and malignant hematopoiesis. Leukemia. (2012) 26:2011–8. doi: 10.1038/leu.2012.90
67. Nakagawa MM, Rathinam CV. Constitutive activation of the canonical NF-kappaB pathway leads to bone marrow failure and induction of erythroid signature in hematopoietic stem cells. Cell Rep. (2018) 25:2094–109.e4. doi: 10.1016/j.celrep.2018.10.071
68. Beerman I, Maloney WJ, Weissmann IL, Rossi DJ. Stem cells and the aging hematopoietic system. Curr Opin Immunol. (2010) 22:500–6. doi: 10.1016/j.coi.2010.06.007
69. Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. (2010) 2:640–53. doi: 10.1002/wsbm.86
Keywords: hematopoietic stem and progenitor cell, bone marrow, trauma, age, transcriptome, RNA, microRNA
Citation: Darden DB, Stortz JA, Hollen MK, Cox MC, Apple CG, Hawkins RB, Rincon JC, Lopez M-C, Wang Z, Navarro E, Hagen JE, Parvataneni HK, Brusko MA, Kladde M, Bacher R, Brumback BA, Brakenridge SC, Baker HV, Cogle CR, Mohr AM and Efron PA (2020) Identification of Unique mRNA and miRNA Expression Patterns in Bone Marrow Hematopoietic Stem and Progenitor Cells After Trauma in Older Adults. Front. Immunol. 11:1289. doi: 10.3389/fimmu.2020.01289
Received: 15 February 2020; Accepted: 21 May 2020;
Published: 24 June 2020.
Edited by:
Thomas Griffith, University of Minnesota Twin Cities, United StatesReviewed by:
Jason M. Butler, Hackensack Meridian Health, United StatesCopyright © 2020 Darden, Stortz, Hollen, Cox, Apple, Hawkins, Rincon, Lopez, Wang, Navarro, Hagen, Parvataneni, Brusko, Kladde, Bacher, Brumback, Brakenridge, Baker, Cogle, Mohr and Efron. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philip A. Efron, cGhpbGlwLmVmcm9uQHN1cmdlcnkudWZsLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.