
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Immunol. , 10 June 2020
Sec. Cytokines and Soluble Mediators in Immunity
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.01179
This article is part of the Research Topic Purinergic Signaling and Inflammation View all 10 articles
Unique structural features characterize the P2X7 receptor with respect to other P2X family members. Dual gating by eATP and regulated expression of P2X7 can imprint distinct outcomes to the T cell depending on the metabolic fitness and/or developmental stage. In the thymus, signaling by P2X7 contributes to γδ T cell lineage choice. In secondary lymphoid organs, P2X7 stimulation promotes Th1/Th17 polarization of CD4+ naïve cells, Tregs conversion to Th17 cells and cell death of Tfh cells that are not stimulated by cognate antigen. Moreover, P2X7 stimulation in eATP rich microenvironments, such as damaged and/or inflamed tissues as well as tumors, induces cell death of various T cell effector subsets.
Signaling by adenosine triphosphate (ATP) emerged very early in evolution and is involved in the regulation of highly diverse biologic functions. Trimeric ATP-gated ionotropic P2X receptors are amongst the most ancient signaling channels, having been present in single-cell protozoa and algae (1). The first evidence of T cell responsiveness to extracellular ATP (eATP) dates back to 1989, when Di Virgilio et al. showed that eATP induced plasma membrane depolarization and permeability to low MW dyes, possibly leading to cell death (2). It was then hypothesized that endogenously generated eATP promoted the effector function of cytotoxic T cells via purinergic receptors (3). Subsequent experiments indicated that activation of P2X receptors in T cells could contribute to the outcome of TCR stimulation both in murine and human cells (4, 5). As in other cells of the immune system, the P2X7 receptor subtype stands out among P2X family members as the most important regulator of T cell function. It is a non-selective cationic channel characterized by dual gating: receptor exposure to low concentrations of ATP (e.g., micromolar range) results in small-amplitude currents, whereas stimulation with ATP in the hundreds micromolar range leads to opening of a cytolytic pore and cell death (6). Cryoelectron microscopy of the rat receptor in apo (closed pore) and ATP-bound (open pore) states has unraveled structural insights into P2X7 architecture, which confer the functional peculiarities that distinguish it from the other P2X family members, namely low affinity for ATP, lack of desensitization and cell death initiation (7). In particular, P2X7 combines a P2X domain with a unique “C-cysteine anchor” intra-cytoplasmic motif and a C-terminal cytoplasmic ballast domain (which contains a Zn coordinating cysteine motif and a GDP-binding region), both of which are not present in other P2X receptors. The C-terminal region of P2X7 has been recently hypothesized to originate from the capture of a ballast domain by a P2X gene in ancestral jawed vertebrates (8).
The human and mouse genes encoding for P2X7 are located in syntenic regions of chromosome 12 and 5, respectively, in close proximity with the gene encoding the P2X4 receptor. Numerous splice variants have been identified for the P2X7 receptor in different species, however, the functional characterization of the various protein isoforms is largely incomplete [reviewed in (9)]. The P2RX7 gene is highly polymorphic and single nucleotide polymorphisms (SNPs) can significantly influence the functional properties of the receptor (10). Genetic association studies support non-synonymous SNPs (NS-SNPs) in the P2RX7 gene as an important genetic factor that alters the susceptibility of individuals to various pathological conditions. The predominant expression of P2X7 in cells of the immune system correlates with detection of NS-SNPs in diseases, in which immune system cells play a pivotal role in the pathogenesis [reviewed in (11)].
In addition to eATP, non-nucleotide agonists, including cathelicidins, amyloidogenic peptide β, and serum amyloid, have been suggested to activate P2X7 or act as positive allosteric effectors (10). Moreover, the murine P2X7 receptor can be ADP-ribosylated by the ADP-ribosyltransferase 2.2 (ART2.2) that catalyzes the transfer of ribose from nicotinamide adenine dinucleotide (NAD+) to R125 in the ectodomain of the P2X7 receptor, resulting in its activation (12). In T cells, P2X7 activation by ADP-ribosylation causes calcium flux, phosphatidylserine exposure, shedding of L-selectin (CD62L), cell shrinkage, pore formation and propidium iodide uptake (13). This alternate mechanism of P2X7 activation is not observed in humans, which lack ART2.1 and ART2.2 (14), and is particularly relevant in murine T cells compared to other cells because of the specific expression of a P2X7 splice variant, that is sensitive to activation by ADP-ribosylation (15–17). The high sensitivity of immunosuppressive T regulatory cells (Tregs) to depletion by NAD+ released during cell damage or inflammation led to hypothesize a function for the ART2-P2X7 pathway in murine Tregs homeostasis (18). An important consequence of P2X7 gating by ADP-ribosylation is the “spontaneous” P2X7 activation of T cells (19) and reduced vitality of Tregs, tissue-resident memory (Trm) (20) and natural killer T cells (21) that co-express high levels of ART2.2 and P2X7, during the isolation procedure from mice. This phenomenon has been successfully counteracted by the injection of ART2.2-blocking nanobodies prior to organ harvesting (20, 22). The shedding of CD62L mentioned above as well as of CD27 and IL-6 receptor (IL-6R) by P2X7 stimulation, are due to P2X7-mediated activation of metalloproteases, such as ADAM10 and ADAM17 (23–25). Since CD62L promotes T cell homing to secondary lymphoid organs (SLOs), P2X7 activation in naïve T cells stimulated by cognate antigen might promote their egress from SLOs. Interestingly, Tregs expressing the ATP-degrading enzyme ectonucleoside triphosphate diphosphohydrolase-1 (CD39) ameliorated contact hypersensitivity reactions by suppressing ATP-induced CD62L shedding and promoting CD8+ cells retention in skin-draining lymph nodes (LNs) (26). Another possible important target of P2X7 induced metalloprotease activation in T cells is CD27, a member of the tumor necrosis factor receptor family, which supports antigen-specific expansion and T cell memory generation (27, 28). Since CD27 activation by interaction with its ligand CD70 is crucial for the outcome of T cell response (29), P2X7-mediated shedding of CD27 might contribute to the regulation of adaptive immunity and/or immunopathology. Along another line, the induction of IL-6R shedding by P2X7 could condition T cell polarization toward pro-inflammatory vs. immunosuppressive programs. These observations indicate the pleiotropic role this P2X7 feature might have in conditioning T cell function.
αβ and γδ T cell development in the thymus is characterized by transition of thymocytes through multiple checkpoints, most of which are regulated by the rearrangement status and specificity of the clonotypic TCR. Whereas, γδ cells develop from CD4−8− double negative (DN) thymocytes, αβ cells progress from DN to mature MHCI and MHCII restricted CD8+ and CD4+ T cells, respectively, through an intermediate CD4+8+ double positive (DP) stage, in which TCR specificity dictates either positive or negative selection of cells (30). The analysis of the dynamics of changes in cytosolic Ca2+ elicited by eATP in thymocytes via P2X7 receptor showed significant variations between individual cells that were dependent on the developmental stage. It was hypothesized that eATP could promote differentiation of most immature DN cells in the outer cortex; conversely, progression to the DP stage in the inner cortex would correspond to loss of responsiveness to eATP via P2X7, thus protecting positively selected cells from eATP released during massive apoptosis of neglected or negatively selected DP cells (31). More recently, this phenomenon was explained by the demonstration of the direct binding of histone deacetylase (HDAC) 3 to the P2rx7 enhancer and repression of P2X7 signaling in DP cells (32). Nevertheless, protection of DP cells from death by pharmacological P2X antagonism could suggest some function of P2X7 in the elimination of neglected DP cells [(33); Figure 1A].
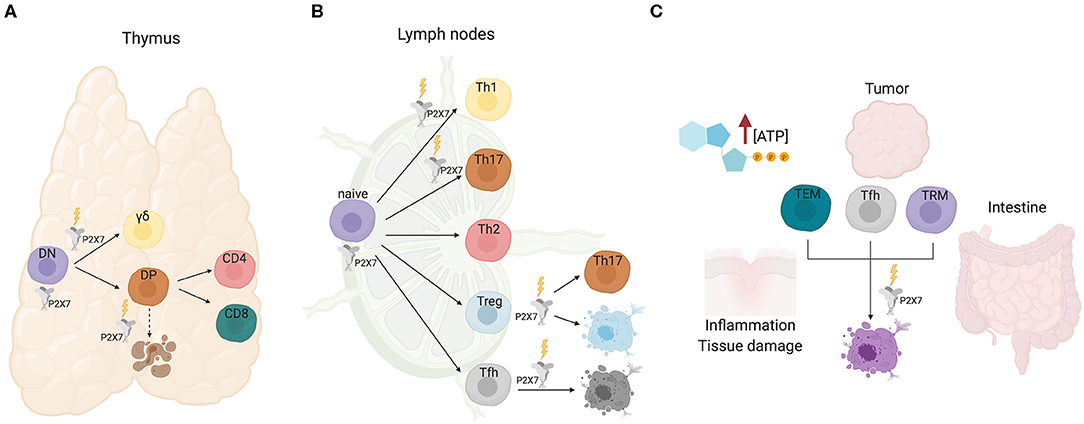
Figure 1. P2X7 activity in T cell development and function. (A) In the thymus, P2X7 activity could promote differentiation of immature DN cells. Signaling by P2X7 contributes to γδ lineage choice by promoting ERK phosphorylation and induction of early growth response (Egr) transcripts. Conversely, progression to the DP and single positive CD4 or CD8 stages is not influenced by P2X7, albeit P2X7 might function in cell death induction of neglected DP cells. (B) In secondary lymphoid organs, P2X7 stimulation promotes Th1/Th17 polarization of CD4+ naïve cells, Tregs conversion to Th17 cells and cell death of Tfh cells that are not stimulated by cognate antigen. (C) Sensitivity of TEM, Tfh, and tissue resident memory T cells to P2X7 mediated cell death in eATP rich microenvironments.
TCR signal strength is a crucial determinant in T cell fate. Increased signal strength of γδTCR with respect to pre-TCR results in induction of the γδ differentiation program. P2X7 signaling contributes to γδ lineage choice by promoting ERK phosphorylation and induction of early growth response (Egr) transcripts. Moreover, the impairment of the ERK-Egr-inhibitor of differentiation 3 (Id3) signaling pathway in γδ cells from P2rx7−/− mice resulted in diversion of γδ T cells to “innate-like” NK1.1-expressing cells with limited TCR diversity (34). These experiments suggest a function of P2X7 in shaping the γδ T cells repertoire, whereas lineage choice and differentiation to mature CD4+ or CD8+ αβ thymocytes do not seem to be affected by P2X7 expression (Figure 1A). Whether and how P2X7 activity might influence cell metabolism in conditioning γδ thymocytes differentiation has not been addressed so far.
In T cells, the increase in the concentration of cytosolic Ca2+ that follows TCR stimulation by peptide/MHC complex is accompanied by mitochondrial uptake of Ca2+. This phenomenon avoids cellular Ca2+ overload, and contribute to a rapid clearing of Ca2+ in spatially restricted areas, such as near Ca2+ channels in the plasma membrane or the ER (35). Moreover, mitochondrial uptake of Ca2+ stimulates the aerobic synthesis of ATP (36, 37). TCR triggering of naïve T cells results in ATP release via pannexin-1 hemichannels and autocrine stimulation of P2X receptors in the plasma membrane. Murine naïve CD4 T cells express P2rx1, P2rx4 transcripts, and higher levels of P2rx7. The ATP released upon naïve T cell activation functions as an autocrine stimulus and sustains MAPK signaling and induction of pro-inflammatory programs via P2X receptors stimulation (Figure 1B). Accordingly, pharmacological antagonism of P2X activity promoted T cell anergy and showed beneficial effects in autoimmune conditions (38). These effects were also favored by the conversion of naïve CD4 T cells into immunosuppressive T regulatory cells (Tregs) (39). Autocrine signaling by eATP via P2X7 receptor was shown to contribute to TCR-mediated Ca2+ influx, NFAT activation and IL-2 production in human CD4 T cells; blocking of P2X7 signaling inhibited T cell activation, suggesting P2X7 receptor is required for effective T cell activation (40). Importantly, expression of CD39 and CD73, the ecto-5′-nucleotidase that degrades extracellular AMP into adenosine, by other immune and tissue resident cells can dramatically condition the outcome of T cell responses (41–43). The P2xr7 gene is robustly upregulated in T effector/memory (TEM) cells. P2X7 activity seems to play different functions in regulating the proliferative response of naïve vs. TEM cells upon TCR stimulation. Murine P2rx7−/− CD4 naïve cells did not show any difference in cell proliferation as compared to WT cells upon TCR stimulation, suggesting that P2X1 and/or P2X4 could compensate for the lack of P2X7 activity, an observation made also in human T cells (44). In contrast, stimulation of P2rx7−/− TEM cells revealed a peculiar enhancement of cell cycling activity with respect to the WT counterpart (our unpublished observations). This phenomenon could be due to the sustained generation of mitochondrial reactive oxygen species (ROS) that was associated to P2X7 activity in T cells (45), and induction of premature cellular senescence.
Extracellular ATP is virtually absent in the interstitium of tissues in physiological conditions with the notable exception of the intestine, where eATP generated by the microbiota can permeate enterocytes (46). In contrast, damaged and/or inflamed tissues as well as tumors' microenvironment (TME) are characterized by eATP concentrations that can reach the millimolar range (47–49). Therefore, P2X7 expression can crucially impact the outcome of local immune system response. In this respect, we have shown that P2X7 stimulation in immunosuppressive T regulatory cells (Tregs) can result in conversion into pro-inflammatory IL-17 secreting cells, thereby possibly worsening the inflammatory tissue damage in pathological conditions [(39); Figure 1B]. Analogously, P2X7 receptor inhibition promoted long-term cardiac transplant survival in murine recipients of fully mismatched allograft by reducing T cell activation and Th1/Th17 differentiation (50).
In T follicular helper (Tfh) cells, conversely, P2X7 stimulation restricts the expansion of aberrant cells and the generation of self-reactive antibodies in experimental murine lupus, but its activity is dispensable for regulation of antigen-specific Tfh cells during parenteral vaccination. P2X7 stimulation likely controls the development of pathogenic ICOS+ IFN-γ-secreting Tfh cells, which characterize systemic lupus erythematosus (SLE), by inducing pyroptosis via caspase-mediated activation of gasdermin D (Figure 1B). Notably, SLE patients are characterized by reduced P2X7 activity in circulating Tfh cells (51). Acute TCR stimulation of Tfh cells robustly downregulates P2rx7 expression, thus protecting antigen responding T cell from cell death (52). Similar results have been obtained in tissue resident memory T cells, suggesting that selective downregulation of P2rx7 in T cells that productively respond to cognate antigen would ensure the amplification of pathogen-destructing cells during infections (53). In contrast, P2X7 activity is required for the establishment and maintenance of long-lived central and tissue-resident memory CD8 T cells in mice, probably reflecting the function of P2X7 as ion channel in promoting mitochondrial function and metabolic fitness (54).
The intestinal microbiota influences host physiology, metabolism, and immune system homeostasis. The interaction between microbes and mammalian immune system results in the selection and “tolerance” of beneficial species. Within this inter-kingdom relationship, eATP plays an important role as a released bacterial metabolite capable of modulating immune system function. The first evidence that commensal bacteria-derived ATP could condition host immune system was provided by Atarashi et al. by showing that a CD70highCD11clow subset of lamina propria cells could be activated by intestinal ATP and induce the differentiation of pro-inflammatory Th17 cells (55). Extracellular ATP was shown to activate dendritic cells (DCs) via P2X7, thereby polarizing the T cell response in a number of physiological and pathophysiological conditions (48, 49, 56–60). However, whether P2X7 stimulation in DCs was responsible for the induction of Th17 cells by intestinal microbiota-derived eATP was not established. Signaling by P2X7 is responsible for cell death of Tfh cells in the Peyer's patches of the small intestine by bacteria-derived ATP, a mechanism important in ensuring controlled generation of T cell dependent secretory IgA (52) and a beneficial shaping of gut microbiota composition (61). The intestinal microenvironment profoundly influences the sensitivity of intraepithelial CD8 cells, both the CD8αβ and CD8αα expressing subset, to P2X7 mediated cell death. In fact, retinoic acid causes up-regulation of P2X7 on purified CD8 T cells and induces responsiveness to extracellular nucleotides. Accordingly, lack of P2X7 led to enhanced CD8+ T cell responses in the intestinal mucosa, thus defining P2X7 as a regulatory element in the control of CD8+ T cells in the intestinal mucosa (62). The induction of P2X7 upregulation by retinoic acid was observed also in CD4+ effector T cells. Hashimoto-Hill et al. showed retinoic acid receptor α binding to an intragenic enhancer region of the P2rx7 gene (63). Probably, this transcriptional control is responsible for the robust expression of P2X7 on most intestinal αβ and γδ T cells, including T-helper type 1 (Th1) and Th17 cells as well as invariant NKT cells (64). Intestinal effector T cells are effectively deleted by P2X7 mediated cell death and P2X7 activation suppressed T-cell-induced colitis in lymphopenic mice (Figure 1C). Results obtained with vitamin A-deficient and P2rx7−/− mice indicate that the retinoic acid-P2X7 pathway is important in preventing expansion of aberrantly activated T cells, as observed with “P2X7-hypoactive” Tfh cells in SLE (51). Therefore, it appears that retinoic acid controls intestinal effector T-cell populations by inducing P2X7 expression. This pathway is likely responsible also for P2X7 mediated control of Tfh cells response to oral vaccination, thereby limiting the generation of high-affinity secretory IgA (46).
Dual gating and regulated expression of P2X7 can imprint distinct outcomes to the T cell depending on the metabolic fitness and/or developmental stage via autocrine signaling or microenvironment's clues, like eATP or other factors (e.g., NAD+ in mice) conditioning P2X7 activity. The peculiarity of P2X7 function as cationic channel and cytolytic pore could be responsible for some apparently contradictory findings on P2X7 dependent responses in particular T cell subsets in different experimental settings. It would be important to define molecular mechanisms that could affect P2X7 activity in T cells (e.g., gene polymorphism, RNA splicing, microRNAs, long non-coding RNAs) in different physiological and pathophysiological contexts.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
The author confirms being the sole contributor of this work and has approved it for publication.
This research was funded by Swiss National Fund, Grant No. 310030_192531.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Verkhratsky A, Burnstock G. Biology of purinergic signalling: its ancient evolutionary roots, its omnipresence and its multiple functional significance. Bioessays. (2014) 36:697–705. doi: 10.1002/bies.201400024
2. Di Virgilio F, Bronte V, Collavo D, Zanovello P. Responses of mouse lymphocytes to extracellular adenosine 5′-triphosphate (ATP). Lymphocytes with cytotoxic activity are resistant to the permeabilizing effects of ATP. J Immunol. (1989) 143:1955–60.
3. Filippini A, Taffs RE, Sitkovsky MV. Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc Natl Acad Sci USA. (1990) 87:8267–71.
4. Baricordi OR, Ferrari D, Melchiorri L, Chiozzi P, Hanau S, Chiari E, et al. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood. (1996) 87:682–90.
5. Loomis WH, Namiki S, Ostrom RS, Insel PA, Junger WG. Hypertonic stress increases T cell interleukin-2 expression through a mechanism that involves ATP release, P2 receptor, and p38 MAPK activation. J Biol Chem. (2003) 278:4590–6. doi: 10.1074/jbc.M207868200
6. Khadra A, Tomic M, Yan Z, Zemkova H, Sherman A, Stojilkovic SS. Dual gating mechanism and function of P2X7 receptor channels. Biophys J. (2013) 104:2612–21. doi: 10.1016/j.bpj.2013.05.006
7. McCarthy AE, Yoshioka C, Mansoor SE. Full-Length P2X7 structures reveal how palmitoylation prevents channel desensitization. Cell. (2019) 179:659–70. doi: 10.1016/j.cell.2019.09.017
8. Rump A, Smolander OP, Ruutel Boudinot S, Kanellopoulos JM, Boudinot P. Evolutionary origin of the P2X7 c-ter region: capture of an ancient ballast domain by a P2X4-like gene in ancient jawed vertebrates. Front Immunol. (2020) 11:113. doi: 10.3389/fimmu.2020.00113
10. Di Virgilio F, Giuliani AL, Vultaggio-Poma V, Falzoni S, Sarti AC. Non-nucleotide agonists triggering P2X7 receptor activation and pore formation. Front Pharmacol. (2018) 9:39. doi: 10.3389/fphar.2018.00039
11. Caseley EA, Muench SP, Roger S, Mao HJ, Baldwin SA, Jiang LH. Non-synonymous single nucleotide polymorphisms in the P2X receptor genes: association with diseases, impact on receptor functions and potential use as diagnosis biomarkers. Int J Mol Sci. (2014) 15:13344–71. doi: 10.3390/ijms150813344
12. Adriouch S, Bannas P, Schwarz N, Fliegert R, Guse AH, Seman M, et al. ADP-ribosylation at R125 gates the P2X7 ion channel by presenting a covalent ligand to its nucleotide binding site. FASEB J. (2008) 22:861–9. doi: 10.1096/fj.07-9294com
13. Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, et al. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity. (2003) 19:571–82. doi: 10.1016/s1074-7613(03)00266-8
14. Rissiek B, Haag F, Boyer O, Koch-Nolte F, Adriouch S. P2X7 on mouse t cells: one channel, many functions. Front Immunol. (2015) 6:204. doi: 10.3389/fimmu.2015.00204
15. Hong S, Schwarz N, Brass A, Seman M, Haag F, Koch-Nolte F, et al. Differential regulation of P2X7 receptor activation by extracellular nicotinamide adenine dinucleotide and ecto-ADP-ribosyltransferases in murine macrophages and T cells. J Immunol. (2009) 183:578–92. doi: 10.4049/jimmunol.0900120
16. Schwarz N, Drouot L, Nicke A, Fliegert R, Boyer O, Guse AH, et al. Alternative splicing of the N-terminal cytosolic and transmembrane domains of P2X7 controls gating of the ion channel by ADP-ribosylation. PLoS ONE. (2012) 7:e41269. doi: 10.1371/journal.pone.0041269
17. Xu XJ, Boumechache M, Robinson LE, Marschall V, Gorecki DC, Masin M, et al. Splice variants of the P2X7 receptor reveal differential agonist dependence and functional coupling with pannexin-1. J Cell Sci. (2012) 125:3776–89. doi: 10.1242/jcs.099374
18. Hubert S, Rissiek B, Klages K, Huehn J, Sparwasser T, Haag F, et al. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J Exp Med. (2010) 207:2561–8. doi: 10.1084/jem.20091154
19. Scheuplein F, Schwarz N, Adriouch S, Krebs C, Bannas P, Rissiek B, et al. NAD+ and ATP released from injured cells induce P2X7-dependent shedding of CD62L and externalization of phosphatidylserine by murine T cells. J Immunol. (2009) 182:2898–908. doi: 10.4049/jimmunol.0801711
20. Rissiek B, Danquah W, Haag F, Koch-Nolte F. Technical advance: a new cell preparation strategy that greatly improves the yield of vital and functional tregs and NKT cells. J Leukoc Biol. (2014) 95:543–9. doi: 10.1189/jlb.0713407
21. Borges da Silva H, Wang H, Qian LJ, Hogquist KA, Jameson SC. ARTC2.2/P2RX7 signaling during cell isolation distorts function and quantification of tissue-resident CD8+ T cell and invariant NKT subsets. J Immunol. (2019) 202:2153–63. doi: 10.4049/jimmunol.1801613
22. Rissiek B, Lukowiak M, Raczkowski F, Magnus T, Mittrucker HW, Koch-Nolte F. In vivo blockade of murine ARTC2.2 during cell preparation preserves the vitality and function of liver tissue-resident memory T cells. Front Immunol. (2018) 9:1580. doi: 10.3389/fimmu.2018.01580
23. Garbers C, Janner N, Chalaris A, Moss ML, Floss DM, Meyer D, et al. Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J Biol Chem. (2011) 286:14804–11. doi: 10.1074/jbc.M111.229393
24. Gu B, Bendall LJ, Wiley JS. Adenosine triphosphate-induced shedding of CD23 and L-selectin (CD62L) from lymphocytes is mediated by the same receptor but different metalloproteases. Blood. (1998) 92:946–51.
25. Jamieson GP, Snook MB, Thurlow PJ, Wiley JS. Extracellular ATP causes of loss of L-selectin from human lymphocytes via occupancy of P2Z purinocepters. J Cell Physiol. (1996) 166:637–42. doi: 10.1002/SICI1097-4652199603166:3637::AID-JCP193.0.CO;2-3
26. Mahnke K, Useliene J, Ring S, Kage P, Jendrossek V, Robson SC, et al. Down-Regulation of CD62L shedding in T cells by CD39(+) regulatory T cells leads to defective sensitization in contact hypersensitivity reactions. J Invest Dermatol. (2017) 137:106–14. doi: 10.1016/j.jid.2016.08.023
27. Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. (2000) 1:433–40. doi: 10.1038/80877
28. Moon H, Na HY, Chong KH, Kim TJ. P2X7 receptor-dependent ATP-induced shedding of CD27 in mouse lymphocytes. Immunol Lett. (2006) 102:98–105. doi: 10.1016/j.imlet.2005.08.004
29. Nolte MA, van Olffen RW, van Gisbergen KP, van Lier AR. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev. (2009) 229:216–31. doi: 10.1111/j.1600-065X.2009.00774.x
30. von Boehmer H. Selection of the T-cell repertoire: receptor-controlled checkpoints in T-cell development. Adv Immunol. (2004) 84:201–38. doi: 10.1016/S0065-2776(04)84006-9
31. Ross PE, Ehring GR, Cahalan MD. Dynamics of ATP-induced calcium signaling in single mouse thymocytes. J Cell Biol. (1997) 138:987–98. doi: 10.1083/jcb.138.5.987
32. Philips RL, McCue SA, Rajcula MJ, Shapiro VS. Cutting edge: HDAC3 protects double-positive thymocytes from P2X7 receptor-induced cell death. J Immunol. (2019) 202:1033–38. doi: 10.4049/jimmunol.1801438
33. Freedman BD, Liu QH, Gaulton G, Kotlikoff MI, Hescheler J, Fleischmann BK. ATP-evoked Ca2+ transients and currents in murine thymocytes: possible role for P2X receptors in death by neglect. Eur J Immunol. (1999) 29:1635–46. doi: 10.1002/SICI1521-414119990529:051635::AID-IMMU16353.0.CO;2-B
34. Frascoli M, Marcandalli J, Schenk U, Grassi F. Purinergic P2X7 receptor drives T cell lineage choice and shapes peripheral gammadelta cells. J Immunol. (2012) 189:174–80. doi: 10.4049/jimmunol.1101582
35. Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci STKE. (2004) 2004: re1. doi: 10.1126/stke.2152004re1
36. Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. (1995) 82:415–24.
37. Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. (1999) 96:13807–12.
38. Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. (2008) 1: ra6. doi: 10.1126/scisignal.1160583
39. Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. (2011) 4: ra12. doi: 10.1126/scisignal.2001270
40. Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. Faseb J. (2009) 23:1685–93. doi: 10.1096/fj.08-126458
41. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. (2007) 204:1257–65. doi: 10.1084/jem.20062512
42. Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. (2007) 110:1225–32. doi: 10.1182/blood-2006-12-064527
43. Takenaka MC, Robson S, Quintana FJ. Regulation of the T cell response by CD39. Trends Immunol. (2016) 37:427–39. doi: 10.1016/j.it.2016.04.009
44. Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, et al. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. (2010) 116:3475–84. doi: 10.1182/blood-2010-04-277707
45. Foster JG, Carter E, Kilty I, MacKenzie AB, Ward SG. Mitochondrial superoxide generation enhances P2X7R-mediated loss of cell surface CD62L on naive human CD4+ T lymphocytes. J Immunol. (2013) 190:1551–9. doi: 10.4049/jimmunol.1201510
46. Proietti M, Perruzza L, Scribano D, Pellegrini G, D'Antuono R, Strati F, et al. ATP released by intestinal bacteria limits the generation of protective IgA against enteropathogens. Nat Commun. (2019) 10:250. doi: 10.1038/s41467-018-08156-z
47. Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS ONE. (2008) 3:e2599 doi: 10.1371/journal.pone.0002599
48. Weber F, Esser C, Muller PR, Ganesan T, Pellegatti J, Simon P, et al. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J Exp Med. (2010) 207:2609–19. doi: 10.1084/jem.20092489
49. Wilhelm K, Ganesan J, Muller T, Durr C, Grimm M, Beilhack A, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. (2010) 16:1434–8. doi: 10.1038/nm.2242
50. Vergani A, Tezza S, D'Addio F, Fotino C, Liu K, Niewczas M, et al. Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation. (2013) 127:463–75. doi: 10.1161/CIRCULATIONAHA.112.123653
51. Faliti CE, Gualtierotti R, Rottoli E, Gerosa M, Perruzza L, Romagnani A, et al. P2X7 receptor restrains pathogenic Tfh cell generation in systemic lupus erythematosus. J Exp Med. (2019) 216:317–36. doi: 10.1084/jem.20171976
52. Proietti M, Cornacchione V, Rezzonico Jost T, Romagnani A, Faliti CE, Perruzza L, et al. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer's patches to promote host-microbiota mutualism. Immunity. (2014) 41:789–801. doi: 10.1016/j.immuni.2014.10.010
53. Stark R, Wesselink TH, Behr FM, Kragten NAM, Arens R, Koch-Nolte F, et al. T RM maintenance is regulated by tissue damage via P2RX7. Sci Immunol. (2018) 3:eaau1022. doi: 10.1126/sciimmunol.aau1022
54. Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, et al. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8(+) T cells. Nature. (2018) 559:264–8. doi: 10.1038/s41586-018-0282-0
55. Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. (2008) 455:808–12. doi: 10.1038/nature07240
56. Mutini C, Falzoni S, Ferrari D, Chiozzi P, Morelli A, Baricordi OR, et al. Mouse dendritic cells express the P2X7 purinergic receptor: characterization and possible participation in antigen presentation. J Immunol. (1999) 163:1958–65.
57. Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G, et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. (2010) 70:855–8. doi: 10.1158/0008-5472.CAN-09-3566
58. Killeen ME, Ferris L, Kupetsky EA, Falo L, Mathers AR. Signaling through purinergic receptors for ATP induces human cutaneous innate and adaptive Th17 responses: implications in the pathogenesis of psoriasis. J Immunol. (2013) 190:4324–36. doi: 10.4049/jimmunol.1202045
59. Sáez PJ, Vargas P, Shoji KF, Harcha PA, Lennon-Duménil AM, Sáez JC. ATP promotes the fast migration of dendritic cells through the activity of pannexin 1 channels and P2. Sci Signal. (2017) 10:eaah7107. doi: 10.1126/scisignal.aah7107
60. Fan ZD, Zhang YY, Guo YH, Huang N, Ma HH, Huang H, et al. Involvement of P2X7 receptor signaling on regulating the differentiation of Th17 cells and type II collagen-induced arthritis in mice. Sci Rep. (2016) 6:35804. doi: 10.1038/srep35804
61. Perruzza L, Gargari G, Proietti M, Fosso B, D'Erchia AM, Faliti CE, et al. T follicular helper cells promote a beneficial gut ecosystem for host metabolic homeostasis by sensing microbiota-derived extracellular AT. Cell Rep. (2017) 18:2566–75. doi: 10.1016/j.celrep.2017.02.061
62. Heiss K, Janner N, Mahnss B, Schumacher V, Koch-Nolte F, Haag F, et al. High sensitivity of intestinal CD8+ T cells to nucleotides indicates P2X7 as a regulator for intestinal T cell responses. J Immunol. (2008) 181:3861–9. doi: 10.4049/jimmunol.181.6.3861
63. Hashimoto-Hill S, Friesen L, Kim M, Kim CH. Contraction of intestinal effector T cells by retinoic acid-induced purinergic receptor P2X7. Mucosal Immunol. (2017) 10:912–23. doi: 10.1038/mi.2016.109
Keywords: P2X7, T cell, extracellular ATP (eATP), T cell effector function, mucosal immunology, T cell development
Citation: Grassi F (2020) The P2X7 Receptor as Regulator of T Cell Development and Function. Front. Immunol. 11:1179. doi: 10.3389/fimmu.2020.01179
Received: 31 March 2020; Accepted: 13 May 2020;
Published: 10 June 2020.
Edited by:
Marco Idzko, Medical University of Vienna, AustriaReviewed by:
Silvia Piconese, Sapienza University of Rome, ItalyCopyright © 2020 Grassi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabio Grassi, ZmFiaW8uZ3Jhc3NpQGlyYi51c2kuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.