- Department of Rheumatology, University College London Hospital (UCLH), London, United Kingdom
Tumor necrosis factor-α inhibitors (TNFis) have revolutionized the management of rheumatoid arthritis (RA), however despite considerable progress, only a small proportion of patients maintain long-term clinical response. Selection of, and switching between, biologics is mainly empirical, experiential, and not evidence-based. Most biopharmaceutical proteins (BP) can induce an immune response against the foreign protein component. Immunogenicity and the development of anti-drug antibodies (ADAs) is considered one of the main reasons for loss of therapeutic efficacy (secondary failure). ADAs may neutralize and/or promote clearance of circulating BP with resultant low serum drug levels, loss of clinical response, poor drug survival and adverse events, such as infusion reactions. ADA identification is technically difficult and not standardized, making interpretation of immunogenicity data from published clinical studies challenging. Trough TNFi drug levels correlate with clinical outcomes, exhibiting a “concentration-response” relationship. Measurement of ADA and drug levels may improve patient care and improve cost-effectiveness of BP use. However, in the absence of clinically-validated, reliable assays and consensus guidelines, therapeutic drug monitoring (TDM) and immunogenicity testing have not been widely adopted in routine clinical practice in Rheumatology. Here we discuss the utility and relevance of TDM and immunogenicity testing of TNFis in RA (focusing on the most widely used TNFis globally, with the most available data, i.e., infliximab, adalimumab, and etanercept), the limitations of currently available assays and potential future immunopharmacological strategies to personalize disease management.
Introduction
Biologic agents, such as TNF-α inhibitors (TNFis), have revolutionized the treatment of rheumatoid arthritis (RA), but despite this advance, not all patients respond favorably. Up to 40% of RA patients do not respond to the first biologic (primary failure) or lose response over time (secondary failure). Drug survival (the time to discontinuation of a drug) is influenced by many factors including lack or loss of efficacy, adverse events (AEs), and poor adherence. Immunogenicity is defined as the ability of biopharmaceutical products (BPs) to induce an immune response, resulting in the generation of anti-drug antibodies (ADAs). ADAs are considered an important (albeit not the only) mechanism of secondary treatment failure and limited drug survival, due to effects on pharmacokinetics and bioavailability. ADAs are also implicated in treatment-related AEs, such as infusion and injection-site reactions (1). Immunogenicity testing is a mandatory, regulatory requirement for BP drug licensing, as part of the safety profile package required by both the US Food and Drug Administration (2) (FDA) and the European Medicines Agency (3) (EMA).
Therapeutic drug monitoring (TDM) and immunogenicity testing, using trough drug levels and ADAs, have the potential to improve clinical decision-making, by influencing drug selection, dose, and frequency of administration. This may allow clinicians to reduce under- and over- treatment for patients in clinical relapse or remission. There are currently no consensus guidelines recommending the use of BP drug levels and immunogenicity testing in RA, and as such, their use in clinical practice is widely variable.
TNFis (in combination with methotrexate and as monotherapy) are often selected as first-line biologic therapy in patients with RA who are refractory to non-biologic disease-modifying antirheumatic drugs (DMARDs), due to the availability of long-term data from clinical trials and extensive real world experience. Moreover, costs have recently lowered due to the advent of biosimilar TNFis. Infliximab, adalimumab, and etanercept (in bio-original and biosimilar forms) are the most frequently used TNFis, with the most available data. Here, we discuss the utility and clinical relevance of TDM and immunogenicity testing of TNFis in patients with RA, and potential future immunopharmacological strategies to personalize disease management.
Immunogenicity of TNFis in RA
Consequences of Immunogenicity
Immunogenicity can impact both the efficacy and safety of BPs. ADAs may reduce the clinical efficacy of TNFis by competing with the cytokine binding site (neutralizing antibodies) or by accelerating drug clearance leading to subtherapeutic drug levels (non-neutralizing/binding antibodies; with formation of immune complexes), hence both neutralizing and non-neutralizing ADAs may be clinically relevant. Trough TNFi drug levels exhibit a “concentration-response” relationship (4) (an inverse correlation with clinical outcomes), which forms the basis for the rationale for TDM in RA. This has been observed in studies of the key TNFis used in clinical practice—including infliximab (5–10), adalimumab (11), etanercept (12, 13), golimumab (14), and certolizumab (15, 16).
ADAs are associated with low trough drug levels and loss of drug efficacy, although the association appears to be stronger for infliximab, adalimumab, and golimumab, than for etanercept and certolizumab (4). ADAs in isolation do not always correlate with poor clinical outcomes, as the antibody titer may be insufficient to reduce the active drug level below the therapeutic threshold. Furthermore, the risk of immunogenicity is not sufficient to predict loss of drug efficacy e.g., although adalimumab is more immunogenic than etanercept, some studies report only a small difference in drug survival (17, 18).
ADAs have been linked to several AEs including infusion/injection site hypersensitivity reactions, serum sickness, and arthus reactions (1, 19). The pathogenic mechanisms are yet to be fully elucidated and may involve complement-mediated events, cytokine release, formation of immune complexes, and production of IgE antibodies. Reassuringly, switching from bio-original to biosimilar BP, has not been associated with greater AEs or immunogenicity concerns thus far (20).
Factors Influencing Immunogenicity
Historically, the foreign (murine) components of the drug were thought to be mainly culpable for the development of ADAs, which led to a drive to minimize non-human elements to reduce immunogenicity. It soon became apparent that even fully human BPs could provoke an immune response, due to TNF-binding idiotypes that are not part of the normal human antibody repertoire, and multiple factors influencing immunogenicity are now emerging. TNFis may be chimeric (e.g., infliximab), humanized (e.g., certolizumab), fully human (e.g., adalimumab and golimumab), or fusion proteins containing antibody fragments (e.g., etanercept). Infliximab is considered the most immunogenic TNFi, particularly when it is used without concomitant methotrexate (21, 22). ADAs have been reported in up to 53% of patients treated with infliximab within the first 6 months of treatment (5, 8, 23–25). By contrast, in the same timeframe, up to 19% of patients receiving adalimumab develop ADAs (8, 24, 26). Etanercept, a receptor construct, does not express idiotypes and thus is the least immunogenic out of the three; ADAs to etanercept are minimal, usually transient and non-neutralizing with a reported incidence of 0–7% (21, 27, 28).
Effective detection of ADAs is dependent upon several factors—the type of the assay used, the timing of the blood sample in relation to drug dosing (usually trough levels, taken before a scheduled dose) and the duration of treatment. In addition, assay results are affected by the relative amount of drug and antibody: excess serum drug levels can prevent the detection of free ADAs; equal drug and antibody levels can prevent measurement of both; and excess ADA usually permits only the detection of free antibodies (29).
Mechanisms leading to immunogenicity are complex and multifactorial; related to the drug (e.g., purity and aggregations) and its production process (e.g., contaminants), the patient and treatment (1, 30). Patient-related factors include genetic predisposition (31), disease activity (32), obesity (32), smoking (32), and indication (33) for biologic treatment. It is tempting to speculate that ADAs are more likely to be evoked in classical autoimmune diseases, where B-lymphocytes are implicated in disease pathogenesis, e.g., a trend toward higher frequency of ADAs is found in patients with RA compared with psoriasis, when treated with the same biologic (4). However, ADAs are clinically relevant in non-antibody-mediated rheumatic conditions e.g., axial spondyloarthritis (34) and are extensively described in inflammatory bowel disease (IBD). This concept was exemplified in a study of patients with spondyloarthritis (n = 294) and rheumatoid arthritis (n = 276) with secondary TNFi failure, where significantly more patients with spondyloarthritis (31.3%) had anti-infliximab antibodies, compared with those that had RA (21.1%; p = 0.014) (33). Treatment-related factors include the dose, frequency, route, and continuity of administration, prior drug exposures as well as concomitant immunomodulators (35). In general higher doses of the BP or a loading regimen (36) followed by continuous rather than episodic dosing (37), the intravenous (compared with subcutaneous) (38, 39) route of administration and concomitant immunosuppression (28, 40) are associated with a lower frequency of ADAs. However, there are some caveats—subcutaneous delivery (relatively more immunogenic and usually the preferred route of administration for most BPs) of tocilizumab (an anti-interleukin (IL)-6 receptor monoclonal antibody) is not more immunogenic than its intravenous administration (41) and whilst concomitant immunosuppressants reduce immunogenicity in RA and Crohns disease (28, 40), evidence for this strategy is not valid across all indications e.g., methotrexate co-prescription does not significantly influence drug survival of TNFis in psoriatic arthritis populations (42).
Limitations of Immunogenicity Testing
The clinical application and interpretation of immunogenicity data is challenging as studies of TNFis show wide variation in the prevalence of ADAs, as well as their impact on serum drug concentrations and clinical outcomes. These observations may be due to heterogeneous patient populations and differences in study design, duration of follow-up, drug dosage, use of concurrent DMARDs and timing of blood sampling. Comparisons between publications are difficult due to inter-laboratory variability and inconsistent (and occasionally absent) reporting of assay methods and characteristics. Furthermore, it is very difficult to make comparisons between different assays for different BPs, due to the reliance of each method on the specific positive control used (43).
Even if detection methods are reliable, most available assays do not evaluate the in vivo functionality of drug and ADAs, i.e., the amount of active circulating drug or the neutralizing capability of the ADA, which could limit the clinical application of the results.
ADA detection involves either a bridging ELISA (most commonly), or a radioimmunoassay (RIA). Available RAIs include the antigen binding test (radiolabelled therapeutic TNFi antibodies bind to free ADAs in serum samples) or pulldown assays (ADAs are coupled to a high-capacity solid substrate). Both ELISAs and RIAs are only able to detect free ADAs; therefore, high drug levels, with formation of ADA-drug complexes, can lead to false negative results. This is known as “drug interference/tolerance,” where ADAs are only detected if their amount exceeds the level of the circulating drug. ELISAs can further underestimate the presence of ADAs, as they do not identify IgG4 ADAs [which are more likely to be neutralizing (44)] and are less drug-tolerant than RIAs. RIAs are more specific than bridging ELISA, are less prone to interference by drug and rheumatoid factor and can capture clinically relevant IgG1 and IgG4 ADA. RIAs are more sensitive than ELISAs when using random blood samples [with better concordance between the assays when ADA titres are high (45)], which would be more convenient for patients, however their widespread use is limited by the cost and complexity associated with radioisotopes.
From a practical perspective, TDM and immunogenicity testing can be difficult. Ease of access to tests is variable, and it may be difficult to obtain accurately timed blood samples for trough drug levels. Newer drug-tolerant assays that measure both free and complexed ADAs, including the pH-shift anti-idiotype binding tests (PIA), may be more suited to random blood sampling, but these tests are expensive, may only be available in specialized centers and have as yet, undetermined clinical utility (46).
Current Clinical Practice
Current options for managing TNFi failures in RA include cycling within class, i.e., to an alternative TNFi, or switching between class i.e., to a drug with a different mechanism of action. Published recommendations provide little guidance to determine the best strategy (47, 48). Both options are supported by data from randomized controlled trials and the real world, therefore the decision is generally empirical and based on physician discretion. This dilemma was summarized in a recent review (29). In the open-label, 52 weeks randomized Rotation or Change (ROC) trial, the treating physician selected between a second TNFi and a non-TNFi in patients with primary TNFi failure (49). The ROC trial results concluded that the reasons for improved drug survival when switching to a second TNFi was better efficacy, and with switching to a non-TNFi was reduced AEs. Further evidence from a prospective study, suggests better outcomes can be achieved using an algorithm based on trough drug levels and ADAs, compared with “empirical switching” (50).
Current treatment recommendations for RA endorse combination therapy with a biologic and DMARD (47, 48), which is consistently more effective than biologic monotherapy, possibly due to effects on immunogenicity. Methotrexate significantly increases adalimumab trough concentrations (51, 52), and in a dose- dependent manner, reduces immunogenicity (51), and improves clinical outcomes in early disease (53).
Given the limitations regarding assay diversity and data interpretation, and the lack of conclusive support for cost- effectiveness, routine use of TDM and ADA testing has not been widely adopted in British Rheumatology practice (54). There are exceptions, with local management algorithms for RA incorporating these tests (55, 56), but overall the use and interpretation of TDM and ADAs is inconsistent. By contrast, The British Society for Gastroenterology guidelines for the management of IBD includes clear, algorithmic recommendations for measurement of drug levels (±ADA) (57). In IBD, clinical decision making using drug levels and ADAs in secondary non-responders is more cost-effective when compared to empirical drug escalation (58, 59). The recent prospective, observational personalized anti-TNF therapy in Crohn's disease study (PANTS), demonstrated that low concentrations of adalimumab and infliximab at week 14 were associated with primary non-response, non-remission at week 54 and the development of ADAs (32). ADAs predicted subsequent low drug levels and concomitant immunomodulators (thiopurine or methotrexate) mitigated the risk of developing ADAs (32).
Potential Immunopharmacological Algorithm
In time, readily available, accurate assays to measure drug levels and ADA titer, will hopefully arm clinicians with powerful tools to optimize the management of RA, especially in patients with secondary loss of response. A potential algorithm that could be used in future management strategies is shown in Figure 1.
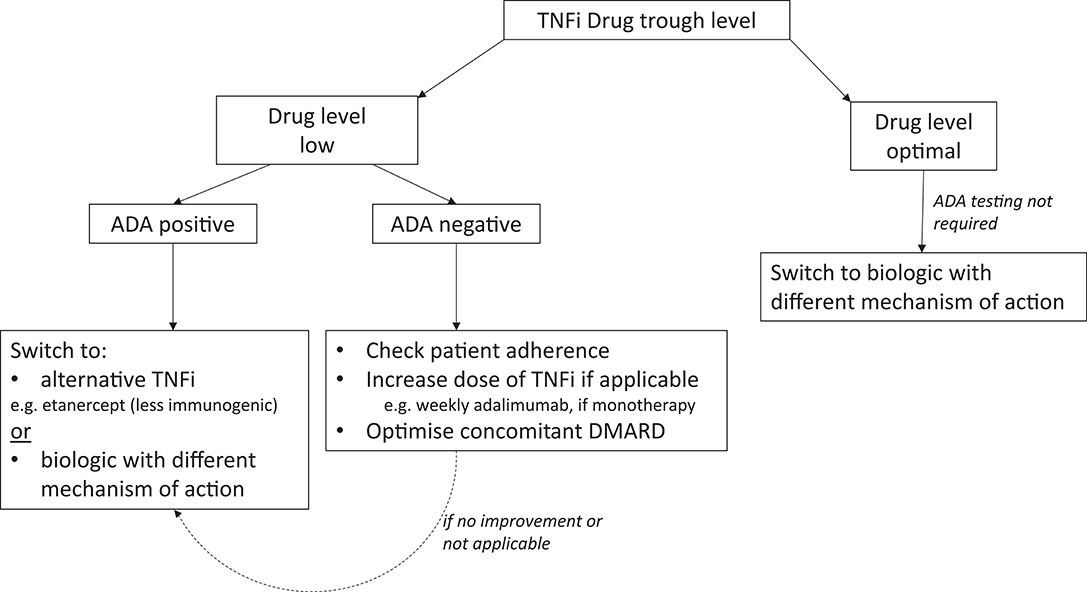
Figure 1. Potential algorithm for RA patients with secondary failure to TNFis. ADA, Anti-drug antibody; BP, Biopharmaceutical product; TNFi, Tumor Necrosis Factor inhibitor.
Measurement of trough drug levels is the most valuable test in the first instance to identify patients with low or optimal (therapeutic) circulating drug. Using ADAs for the first branching in the algorithm is probably inappropriate, as ADAs are not always clinically relevant (especially if present at low-titer) if there is sufficient circulating drug. In cases of treatment failure, supplementary knowledge of ADAs (and perhaps the titer) may be helpful in determining the etiology of suboptimal drug levels. Low drug levels without ADAs may be due to factors such poor adherence to therapy (as most biologics are self-administered injections), a higher BMI and/or faster drug metabolism, which would require different strategies compared with those for patients with detectable ADAs. To overcome this problem, optimizing the dose of biologic by reducing the interval of administration, e.g., changing adalimumab monotherapy from fortnightly to weekly [as permitted by the National Institute of Health and Care Excellence (NICE) in the U.K. (60)], or optimizing dose of concomitant immunosuppressants may recapture a response (61, 62). Emerging evidence suggests that efficacy can be re-established in ADA positive patients with secondary failure, by addition of methotrexate to infliximab treatment in IBD (63), although there is limited support for this approach in the RA literature. If these strategies are unsuccessful or not applicable, switching BP should be considered.
If ADAs are detected in the context of a low drug level, switching to a less immunogenic drug within the same class (e.g., etanercept) could be beneficial, especially if the patient has previously responded to a TNFi. Switching to a second TNFi may be successful due to differences in drug molecular structure, immunological action, immunogenicity, and pharmacokinetics, as well as different underlying disease pathogenesis (24). There is an argument however, to switch to a biologic with a different mechanism of action, as although ADAs are not cross-reactive, patients with ADAs to the first failed TNFi are more likely to seroconvert and produce ADAs with subsequent TNFis (64–67) and are thus less likely to respond to a second TNFi, especially if this is a monoclonal antibody (64, 66). Of note, ADAs to bio-originals are reactive to the corresponding biosimilars, and therefore after detection of ADA, switching a bio-original to its biosimilar version would not be recommended (68). It is plausible to suggest that a patient with ADAs, refractory to multiple biologics, may benefit from a treatment with a less or minimally immunogenic drug, e.g., a receptor fusion protein e.g., abatacept (69) or a small molecule [JAK inhibitor (70)]. In the case of non-responders with optimal drug levels, the presence/absence of ADAs is unlikely to influence subsequent management. These patients have a lower probability of response to an agent within the same class and therefore we would postulate that they are most likely to benefit from switching to a drug with a different mechanism of action (64).
Given the high cost and potential AEs associated with biologic therapies, strategies have been proposed to taper biologics (by reducing drug doses or increasing dosing intervals) in patients with sustained clinical remission, thereby reducing risks and costs overtreatment. In some studies, correlation between DAS28 (disease activity score; a composite measure of disease activity in RA) improvement and serum drug trough levels has been verified up to a threshold of drug level, above which no significant DAS28 changes occur (71). A recent study using certolizumab found that a drug level above a defined threshold was not associated with any additional clinical benefit, and therefore it may be possible in the future to use TDM to titrate treatment (15). Withdrawal of treatment in disease quiescence is an area of active research and currently there is insufficient evidence to draw meaningful conclusions about the role of TDM and immunogenicity testing. Data from ongoing, randomized controlled trials (72) using TDM or ADA to guide withdrawal strategies may inform future practice. It is reasonable to hypothesize that drug withdrawal may be possible in patients with inactive disease and undetectable drug levels or high ADA titres, as remission is probably not being maintained by treatment with the BP.
Future Directions and Unanswered Questions
The increasing and earlier use of BPs in RA is likely to lead to a greater proportion of patients receiving these therapies. Efforts are expanding to predict, reduce and reverse BP immunogenicity to mitigate the impact on drug development, which was summarized in a recent review (73). Strategies to reduce the immunogenic potential of BPs include “de-immunizing” approaches through protein engineering e.g., rational amino acid substitutions and/or addition of epitope-masking moieties, as well as induction of peripheral tolerance (73). There are emerging concerns that immunogenicity may limit the development of newer investigational medicinal products such as the bispecific antibodies.
Despite long-standing interest and accrual of data, we are still unable to predict responses to TNFi. Prospective, longitudinal studies of BP-naïve patients may provide mechanistic information and address a critical unanswered question—why BPs are immunogenic in some patients, but tolerogenic in others. Prediction of immunogenicity may allow mitigation and management strategies to be implemented to prevent or minimize the generation of ADAs (73). Other strategies to personalize biologic selection, include pharmacogenetic testing to identify genetic factors that may predict lack of response to, or toxicities from, TNFi (74).
Further research is needed to develop standardized, clinically-validated assays for both drug and ADA testing. These tests could then be incorporated into evidence-based guidelines to optimize treatment decisions along the patient pathway: for patients with active disease about to start treatment, not responding to treatment (primary or secondary failure) or for those in remission, to permit drug tapering strategies. Taken together this may help to improve the long-term efficacy, safety profile and cost-effectiveness of BPs.
Author Contributions
PM and JM co-wrote this manuscript and both authors approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Jani M, Dixon WG, Chinoy H. Drug safety and immunogenicity of tumour necrosis factor inhibitors: the story so far. Rheumatology. (2018) 57:1896–907. doi: 10.1093/rheumatology/kex434
2. Food and Drug Administration (FDA). Immunogenicity Testing of Therapeutic Protein Products – Developing and Validating Assays for Anti-Drug Antibody Detection. Guidance for Industry. Rockville, MD: Food and Drug Administration (FDA) (2019).
3. Agency EM. Guideline on Immunogenicity Assessment of Biotechnology-Derived Therapeutic Proteins. (2017). Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_~guideline/2017/06/WC500228861.pdf
4. Strand V, Balsa A, Al-Saleh J, Barile-Fabris L, Horiuchi T, Takeuchi T, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs. (2017) 31:299–316. doi: 10.1007/s40259-017-0231-8
5. Bendtzen K, Geborek P, Svenson M, Larsson L, Kapetanovic MC, Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum. (2006) 54:3782–9. doi: 10.1002/art.22214
6. Mulleman D, Chu Miow Lin D, Ducourau E, Emond P, Ternant D, Magdelaine-Beuzelin C, et al. Trough infliximab concentrations predict efficacy and sustained control of disease activity in rheumatoid arthritis. Ther Drug Monit. (2010) 32:232–6. doi: 10.1097/FTD.0b013e3181cc6fef
7. Pascual-Salcedo D, Plasencia C, Ramiro S, Nuno L, Bonilla G, Nagore D, et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology. (2011) 50:1445–52. doi: 10.1093/rheumatology/ker124
8. Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, van Riel PL, et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis. (2009) 68:1739–45. doi: 10.1136/ard.2008.092833
9. St Clair EW, Wagner CL, Fasanmade AA, Wang B, Schaible T, Kavanaugh A, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. (2002) 46:1451–9. doi: 10.1002/art.10302
10. Wolbink GJ, Voskuyl AE, Lems WF, de Groot E, Nurmohamed MT, Tak PP, et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. (2005) 64:704–7. doi: 10.1136/ard.2004.030452
11. Pouw MF, Krieckaert CL, Nurmohamed MT, van der Kleij D, Aarden L, Rispens T, et al. Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis. (2015) 74:513–8. doi: 10.1136/annrheumdis-2013-204172
12. Jamnitski A, Krieckaert CL, Nurmohamed MT, Hart MH, Dijkmans BA, Aarden L, et al. Patients non-responding to etanercept obtain lower etanercept concentrations compared with responding patients. Ann Rheum Dis. (2012) 71:88–91. doi: 10.1136/annrheumdis-2011-200184
13. Jani M, Chinoy H, Warren RB, Griffiths CE, Plant D, Fu B, et al. Clinical utility of random anti-tumor necrosis factor drug-level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis. Arthritis Rheumatol. (2015) 67:2011–9. doi: 10.1002/art.39169
14. Kneepkens EL, Plasencia C, Krieckaert CL, Pascual-Salcedo D, van der Kleij D, Nurmohamed MT, et al. Golimumab trough levels, antidrug antibodies and clinical response in patients with rheumatoid arthritis treated in daily clinical practice. Ann Rheum Dis. (2014) 73:2217–9. doi: 10.1136/annrheumdis-2014-205983
15. Gehin JE, Goll GL, Warren DJ, Syversen SW, Sexton J, Strand EK, et al. Associations between certolizumab pegol serum levels, anti-drug antibodies and treatment response in patients with inflammatory joint diseases: data from the NOR-DMARD study. Arthritis Res Ther. (2019) 21:256. doi: 10.1186/s13075-019-2009-5
16. Jani M, Isaacs JD, Morgan AW, Wilson AG, Plant D, Hyrich KL, et al. High frequency of antidrug antibodies and association of random drug levels with efficacy in certolizumab pegol-treated patients with rheumatoid arthritis: results from the BRAGGSS cohort. Ann Rheum Dis. (2017) 76:208–13. doi: 10.1136/annrheumdis-2015-208849
17. Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. (2010) 62:22–32. doi: 10.1002/art.27227
18. Krieckaert CL, Jamnitski A, Nurmohamed MT, Kostense PJ, Boers M, Wolbink G. Comparison of long-term clinical outcome with etanercept treatment and adalimumab treatment of rheumatoid arthritis with respect to immunogenicity. Arthritis Rheum. (2012) 64:3850–5. doi: 10.1002/art.34680
19. Murdaca G, Spano F, Contatore M, Guastalla A, Penza E, Magnani O, et al. Immunogenicity of infliximab and adalimumab: what is its role in hypersensitivity and modulation of therapeutic efficacy and safety? Expert Opin Drug Saf. (2016) 15:43–52. doi: 10.1517/14740338.2016.1112375
20. Jorgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. (2017) 389:2304–16. doi: 10.1016/s0140-6736(17)30068-5
21. Anderson PJ. Tumor necrosis factor inhibitors: clinical implications of their different immunogenicity profiles. Semin Arthritis Rheum. (2005) 34(5 Suppl. 1):19–22. doi: 10.1016/j.semarthrit.2005.01.005
22. Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. (2008) 117:244–79. doi: 10.1016/j.pharmthera.2007.10.001
23. Finckh A, Dudler J, Wermelinger F, Ciurea A, Kyburz D, Gabay C, et al. Influence of anti-infliximab antibodies and residual infliximab concentrations on the occurrence of acquired drug resistance to infliximab in rheumatoid arthritis patients. Joint Bone Spine. (2010) 77:313–8. doi: 10.1016/j.jbspin.2010.02.021
24. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. (2017) 13:707–18. doi: 10.1038/nrrheum.2017.187
25. Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. (1998) 41:1552–63. doi: 10.1002/1529-0131(199809)41:9<1552::Aid-art5>3.0.Co;2-w
26. Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. (2011) 305:1460–8. doi: 10.1001/jama.2011.406
27. Dore RK, Mathews S, Schechtman J, Surbeck W, Mandel D, Patel A, et al. The immunogenicity, safety, and efficacy of etanercept liquid administered once weekly in patients with rheumatoid arthritis. Clin Exp Rheumatol. (2007) 25:40–6.
28. Garces S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis. (2013) 72:1947–55. doi: 10.1136/annrheumdis-2012-202220
29. Rubbert-Roth A, Szabo MZ, Kedves M, Nagy G, Atzeni F, Sarzi-Puttini P. Failure of anti-TNF treatment in patients with rheumatoid arthritis: the pros and cons of the early use of alternative biological agents. Autoimmun Rev. (2019) 18:102398. doi: 10.1016/j.autrev.2019.102398
30. van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol. (2013) 9:164–72. doi: 10.1038/nrrheum.2013.4
31. Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, Reppell M, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn's disease. Gastroenterology. (2020) 158:189–99. doi: 10.1053/j.gastro.2019.09.041
32. Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. (2019) 4:341–53. doi: 10.1016/s2468-1253(19)30012-3
33. Balsa A, Sanmarti R, Rosas J, Martin V, Cabez A, Gomez S, et al. Drug immunogenicity in patients with inflammatory arthritis and secondary failure to tumour necrosis factor inhibitor therapies: the REASON study. Rheumatology. (2018) 57:688–93. doi: 10.1093/rheumatology/kex474
34. Senabre Gallego JM, Rosas J, Marco-Mingot M, Garcia-Gomez JA, Santos-Soler G, Salas-Heredia E, et al. Clinical relevance of monitoring serum adalimumab levels in axial spondyloarthritis. Rheumatol Int. (2019) 39:841–9. doi: 10.1007/s00296-019-04288-7
35. Murdaca G, Negrini S, Greco M, Schiavi C, Giusti F, Borro M, et al. Immunogenicity of infliximab and adalimumab. Expert Opin Drug Saf. (2019) 18:343–5. doi: 10.1080/14740338.2019.1602117
36. Rutgeerts P, D'Haens G, Targan S, Vasiliauskas E, Hanauer SB, Present DH, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn's disease. Gastroenterology. (1999) 117:761–9. doi: 10.1016/s0016-5085(99)70332-x
37. Rutgeerts P, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. (2004) 126:402–13. doi: 10.1053/j.gastro.2003.11.014
38. Porter S. Human immune response to recombinant human proteins. J Pharm Sci. (2001) 90:1–11. doi: 10.1002/1520-6017(200101)90:1<1::aid-jps1>3.0.co;2-k
39. Ross C, Clemmesen KM, Svenson M, Sorensen PS, Koch-Henriksen N, Skovgaard GL, et al. Immunogenicity of interferon-beta in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Danish Multiple Sclerosis Study Group Ann Neurol. (2000) 48:706–12. doi: 10.1002/1531-8249(200011)
40. Jani M, Barton A, Warren RB, Griffiths CE, Chinoy H. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology. (2014) 53:213–22. doi: 10.1093/rheumatology/ket260
41. Burmester GR, Rubbert-Roth A, Cantagrel A, Hall S, Leszczynski P, Feldman D, et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). A2nn Rheum Dis. (2014) 73:69–74. doi: 10.1136/annrheumdis-2013-203523
42. Mease PJ, Collier DH, Saunders KC, Li G, Kremer JM, Greenberg JD. Comparative effectiveness of biologic monotherapy versus combination therapy for patients with psoriatic arthritis: results from the Corrona registry. RMD Open. (2015) 1:e000181. doi: 10.1136/rmdopen-2015-000181
43. Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco-Kent D, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. (2008) 48:1267–81. doi: 10.1016/j.jpba.2008.09.020
44. Sethu S, Govindappa K, Alhaidari M, Pirmohamed M, Park K, Sathish J. Immunogenicity to biologics: mechanisms, prediction and reduction. Arch Immunol Ther Exp. (2012) 60:331–44. doi: 10.1007/s00005-012-0189-7
45. Jani M, Isaacs JD, Morgan AW, Wilson AG, Plant D, Hyrich KL, et al. Detection of anti-drug antibodies using a bridging ELISA compared with radioimmunoassay in adalimumab-treated rheumatoid arthritis patients with random drug levels. Rheumatology. (2016) 55:2050–5. doi: 10.1093/rheumatology/kew299
46. Bloem K, van Leeuwen A, Verbeek G, Nurmohamed MT, Wolbink GJ, van der Kleij D, et al. Systematic comparison of drug-tolerant assays for anti-drug antibodies in a cohort of adalimumab-treated rheumatoid arthritis patients. J Immunol Methods. (2015) 418:29–38. doi: 10.1016/j.jim.2015.01.007
47. Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. (2016) 68:1–26. doi: 10.1002/art.39480
48. Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. (2017) 76:960–77. doi: 10.1136/annrheumdis-2016-210715
49. Gottenberg JE, Brocq O, Perdriger A, Lassoued S, Berthelot JM, Wendling D, et al. Non-TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: a randomized clinical trial. JAMA. (2016) 316:1172–80. doi: 10.1001/jama.2016.13512
50. Garces S, Antunes M, Benito-Garcia E, da Silva JC, Aarden L, Demengeot J. A preliminary algorithm introducing immunogenicity assessment in the management of patients with RA receiving tumour necrosis factor inhibitor therapies. Ann Rheum Dis. (2014) 73:1138–43. doi: 10.1136/annrheumdis-2013-203296
51. Krieckaert CL, Nurmohamed MT, Wolbink GJ. Methotrexate reduces immunogenicity in adalimumab treated rheumatoid arthritis patients in a dose dependent manner. Ann Rheum Dis. (2012) 71:1914–5. doi: 10.1136/annrheumdis-2012-201544
52. Vogelzang EH, Pouw MF, Nurmohamed M, Kneepkens EL, Rispens T, Wolbink GJ, et al. Adalimumab trough concentrations in patients with rheumatoid arthritis and psoriatic arthritis treated with concomitant disease-modifying antirheumatic drugs. Ann Rheum Dis. (2015) 74:474–5. doi: 10.1136/annrheumdis-2014-206588
53. Burmester GR, Kivitz AJ, Kupper H, Arulmani U, Florentinus S, Goss SL, et al. Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis. (2015) 74:1037–44. doi: 10.1136/annrheumdis-2013-204769
54. Excellence NIHC. Diagnostics Assessment Programme: Therapeutic Monitoring of TNF-alpha Inhibitors in Rheumatoid Arthritis. (2019). Available online at: https://www.nice.org.uk/guidance/dg36/documents/overview
55. Clyde NGG. Rheumatology Biologic Drug Monitoring Recommendations. (2017). Available online at: https://www.nhsggc.org.uk/media/246324/rheumatology-biologic-drug-monitoring-recommendations-september-2017.pdf
56. Group GMMM. High Cost Drugs Pathway For Rheumatoid Arthritis. (2017). Available online at: http://gmmmg.nhs.uk/docs/guidance/GMMMG-RA-pathway-FINAL-v4-1.pdf
57. Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. (2019) 68:s1–106. doi: 10.1136/gutjnl-2019-318484
58. Steenholdt C, Brynskov J, Thomsen OO, Munck LK, Fallingborg J, Christensen LA, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn's disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. (2014) 63:919–27. doi: 10.1136/gutjnl-2013-305279
59. Velayos FS, Kahn JG, Sandborn WJ, Feagan BG. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn's disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol. (2013) 11:654–66. doi: 10.1016/j.cgh.2012.12.035
60. Excellence NIC. (2016). Adalimumab, Etanercept, Infliximab, Certolizumab Pegol, Golimumab, Tocilizumab and Abatacept for Rheumatoid Arthritis Not Previously Treated With DMARDs or After Conventional DMARDs Only Have Failed. Available online at: https://www.nice.org.uk/guidance/ta375
61. Sidiropoulos P, Bertsias G, Kritikos HD, Kouroumali H, Voudouris K, Boumpas DT. Infliximab treatment for rheumatoid arthritis, with dose titration based on the Disease Activity Score: dose adjustments are common but not always sufficient to assure sustained benefit. Ann Rheum Dis. (2004) 63:144–8. doi: 10.1136/ard.2003.015933
62. Stern R, Wolfe F. Infliximab dose and clinical status: results of 2 studies in 1642 patients with rheumatoid arthritis. J Rheumatol. (2004) 31:1538–45. Available online at: http://www.jrheum.org/content/31/8/1538.long
63. Ben-Horin S, Waterman M, Kopylov U, Yavzori M, Picard O, Fudim E, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. (2013) 11:444–7. doi: 10.1016/j.cgh.2012.10.020
64. Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: a cohort study. Ann Rheum Dis. (2010) 69:817–21. doi: 10.1136/ard.2009.112847
65. Frederiksen MT, Ainsworth MA, Brynskov J, Thomsen OO, Bendtzen K, Steenholdt C. Antibodies against infliximab are associated with de novo development of antibodies to adalimumab and therapeutic failure in infliximab-to-adalimumab switchers with IBD. Inflamm Bowel Dis. (2014) 20:1714–21. doi: 10.1097/mib.0000000000000138
66. Jamnitski A, Bartelds GM, Nurmohamed MT, van Schouwenburg PA, van Schaardenburg D, Stapel SO, et al. The presence or absence of antibodies to infliximab or adalimumab determines the outcome of switching to etanercept. Ann Rheum Dis. (2011) 70:284–8. doi: 10.1136/ard.2010.135111
67. van der Bijl AE, Breedveld FC, Antoni CE, Kalden JR, Kary S, Burmester GR, et al. An open-label pilot study of the effectiveness of adalimumab in patients with rheumatoid arthritis and previous infliximab treatment: relationship to reasons for failure and anti-infliximab antibody status. Clin Rheumatol. (2008) 27:1021–8. doi: 10.1007/s10067-008-0866-4
68. Ruiz-Arguello MB, Maguregui A, Ruiz Del Agua A, Pascual-Salcedo D, Martinez-Feito A, Jurado T, et al. Antibodies to infliximab in Remicade-treated rheumatic patients show identical reactivity towards biosimilars. Ann Rheum Dis. (2016) 75:1693–6. doi: 10.1136/annrheumdis-2015-208684
69. Alten R, Kaine J, Keystone E, Nash P, Delaet I, Genovese MC. Long-term safety of subcutaneous abatacept in rheumatoid arthritis: integrated analysis of clinical trial data representing more than four years of treatment. Arthritis Rheumatol. (2014) 66:1987–97. doi: 10.1002/art.38687
70. Taylor PC. Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology. (2019) 58:i17–26. doi: 10.1093/rheumatology/key225
71. Krieckaert CL, Nair SC, Nurmohamed MT, van Dongen CJ, Lems WF, Lafeber FP, et al. Personalised treatment using serum drug levels of adalimumab in patients with rheumatoid arthritis: an evaluation of costs and effects. Ann Rheum Dis. (2015) 74:361–8. doi: 10.1136/annrheumdis-2013-204101
72. Emery P, Burmester GR, Naredo E, Zhou Y, Hojnik M, Conaghan PG. Design of a phase IV randomised, double-blind, placebo-controlled trial assessing the impact of residual inflammation detected via imaging techniques, drug levels and patient characteristics on the outcome of dose tapering of adalimumab in clinical remission rheumatoid arthritis (RA) patients (PREDICTRA). BMJ Open. (2018) 8:e019007. doi: 10.1136/bmjopen-2017-019007
73. Pratt KP. Anti-drug antibodies: emerging approaches to predict, reduce or reverse biotherapeutic immunogenicity. Antibodies.(2018) 7:19. doi: 10.3390/antib7020019
Keywords: immunogenicity, anti-drug antibodies, biopharmaceutical products, TNF-inhibitors, rheumatoid arthritis
Citation: Mehta P and Manson JJ (2020) What Is the Clinical Relevance of TNF Inhibitor Immunogenicity in the Management of Patients With Rheumatoid Arthritis? Front. Immunol. 11:589. doi: 10.3389/fimmu.2020.00589
Received: 28 January 2020; Accepted: 13 March 2020;
Published: 07 April 2020.
Edited by:
Zuben E. Sauna, United States Food and Drug Administration, United StatesReviewed by:
Francesco Puppo, University of Genoa, ItalyGiuseppe Murdaca, University of Genoa, Italy
Copyright © 2020 Mehta and Manson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica J. Manson, amVzc2ljYS5tYW5zb24mI3gwMDA0MDtuaHMubmV0
 Puja Mehta
Puja Mehta Jessica J. Manson
Jessica J. Manson