
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 07 May 2020
Sec. Immunological Tolerance and Regulation
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.00563
This article is part of the Research Topic Tolerating Factor VIII: Novel Strategies to Prevent and Reverse Anti-FVIII Inhibitors View all 20 articles
 A. Abdi1
A. Abdi1 M. R. Bordbar2
M. R. Bordbar2 S. Hassan3
S. Hassan3 F. R. Rosendaal3
F. R. Rosendaal3 J. G. van der Bom3,4
J. G. van der Bom3,4 J. Voorberg5,6
J. Voorberg5,6 K. Fijnvandraat1,5
K. Fijnvandraat1,5 S. C. Gouw1,3*
S. C. Gouw1,3*Objectives: In hemophilia A the presence of non-neutralizing antibodies (NNAs) against Factor VIII (FVIII) may predict the development of neutralizing antibodies (inhibitors) and accelerate the clearance of administrated FVIII concentrates. This systematic review aimed to assess: (1) the prevalence and incidence of NNAs in patients with congenital hemophilia without inhibitors and (2) the association between NNAs and patient and treatment characteristics.
Methods: We conducted a search in MEDLINE, Embase, Web of Science and the Cochrane database. We included cross-sectional and longitudinal studies reporting on NNAs in patients with hemophilia A and B, who were inhibitor-negative at the start of the observation period. Data were extracted on: hemophilia type and severity, patient and treatment characteristics, NNA prevalence and incidence, NNA assays and inhibitor development. Two independent reviewers performed study selection, data extraction and risk of bias assessment, using adapted criteria of the Joanna Briggs Institute. Studies were classified as high-quality when ≥5/9 criteria were met. NNA assays were classified as high-quality when both quality criteria were met: (1) use of positive controls and (2) competition with FVIII to establish FVIII-specificity. We reported NNA prevalence and incidence for each study. The pooled NNA prevalence was assessed for well-designed studies in previously treated patients, employing high-quality NNA assays.
Results: We included data from 2,723 inhibitor-negative patients with hemophilia A, derived from 28 studies. Most studies were cross-sectional (19/28) and none reported on NNAs in hemophilia B. Study design was of high quality in 16/28 studies and the NNA assay quality was high in 9/28 studies. Various NNA assays were used, predominantly ELISA (18/28) with different cut-off values. We found a large variety in NNA prevalence (Range, 0–100%). The pooled NNA prevalence in high-quality studies was 25% (95% CI, 16–38%). The incidence of new NNA development was reported in one study (0.01 NNA per person-exposure day).
Conclusion: This systematic review identified studies that were heterogeneous in study design, patient population and NNA assay type, with NNA prevalence ranging from 0 to 100% in inhibitor-negative patients with hemophilia A. The pooled NNA prevalence was 25% in high-quality studies including only previously treated patients and performing high-quality NNA assays.
The development of neutralizing antibodies (inhibitors) against Factor VIII (FVIII) or Factor IX (FIX) is a major complication of the treatment of hemophilia patients with clotting factor concentrates. Inhibitors impair the pro-coagulant effect of FVIII or FIX concentrates, rendering replacement therapy ineffective and increasing the susceptibility to major bleeding episodes (1). It is estimated that about 30% of patients with severe and 13% of patients with non-severe hemophilia A develop an inhibitor during the treatment course (2–4). Inhibitor prevalence in hemophilia B has been reported to be 1.5–3% overall and 9–23% in severe patients (5, 6). Therefore, inhibitor development is associated with considerable morbidity and mortality (2, 7, 8).
Previous studies report that non-neutralizing antibodies (NNAs) against FVIII may also be detected in a considerable number of patients with hemophilia A, as well as in healthy individuals (9–14). NNAs are usually of the immunoglobulin G (IgG) isotype, frequently directed toward the heavy-chain and especially the B-domain of FVIII (9, 10, 15). NNAs of the IgM and IgA isotype have also been reported in recent studies (9, 10, 16).
The significance of NNAs is not well-understood. It has been suggested that these antibodies are a predictor for future inhibitor development (17, 18). Furthermore, NNAs may also increase the clearance of administrated FVIII concentrate from the circulation, thereby reducing the plasma concentration of FVIII and limiting effective hemostasis to control bleeding (15, 19). In a study among 42 patients with severe and moderate hemophilia A, the presence of high-titer FVIII-specific NNAs was associated with reduced FVIII half-life in comparison to patients without NNAs (median 7.8 h, IQR 6.6–9.2 vs. 10.4 h, IQR 8.9–13.8) (20).
Whereas, the prevalence of inhibitors is well-known, this is less precisely defined for NNAs. In contrast with inhibitors that are measured by standardized assays (Bethesda or Nijmegen-modified Bethesda assay), there is no standardized assay to detect NNAs (21, 22). Consequently, a variety of laboratory methods are used (10, 13, 23). In addition to other differences in study design and patient populations, this contributes to the widely varying reports of NNA prevalence.
In this systematic review we aimed: (1) to obtain more precise estimates of the prevalence and incidence of NNAs in patients with congenital hemophilia without inhibitors and (2) to assess the association between the presence of NNAs and patient and treatment characteristics.
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (www.prisma-statement.org) (24). The inclusion criteria and the methodological quality criteria were specified and documented in a protocol in advance.
Cross-sectional or longitudinal studies reporting the prevalence or incidence of NNAs in congenital hemophilia, published as an article or letter in a peer-reviewed journal, were eligible for inclusion, without restriction on publication date or language. Studies not clearly reporting the method employed to measure NNAs and studies including fewer than 10 patients, were excluded.
Eligible for inclusion were patients with congenital hemophilia A or B who were inhibitor-negative at the start of the study observation period, regardless of previous clotting factor treatment. Patients that received previous treatment with clotting factor concentrate, were defined as previously treated patients, regardless of the cumulative number of exposure days. Patients that had not yet received any previous treatment with clotting factor concentrate at study entry, were defined as previously untreated patients. Absence of an inhibitor needed to be confirmed with a Bethesda assay, according to the cut-off value used by the investigators of the original studies.
The primary endpoints were the prevalence and incidence of NNAs. The secondary endpoints were the prevalence and incidence of NNAs, stratified by immunoglobulin (Ig) isotype and IgG subclass. The presence of NNAs was defined as having a positive antibody titer according to the NNA assay (Anti-Drug Antibody assay) and the cut-off value used by the original publication, in patients who were inhibitor-negative based on a Bethesda assay (25).
Studies were identified by searching the following electronic databases: MEDLINE, Embase, Web of Science and the Cochrane database. The reference lists of the retrieved publications were searched to identify additional relevant publications. We used the following search terms to search all databases: hemophilia A, factor VIII, factor 8, hemophilia B, factor 9, factor IX, non-neutralizing, antibodies, neutralizing. The full search is listed in Supplementary Data 1. The search was designed and supervised by an experienced librarian. The first search was conducted on July 12, 2018. An update of the search in MEDLINE was run on September 11, 2019.
Two of the authors (AA and MB) screened the titles and abstracts independently to select relevant articles. The full-text of selected articles were reviewed to assess their eligibility for inclusion. In case of any doubt for eligibility or disagreement between the reviewers, this was discussed with a methodological expert (SG).
We excluded duplicate studies by checking the authors' names, authors' affiliations and catchment areas. When studies included overlapping patient cohorts, assessed during the same time period, we included the study containing the highest number of patients. Studies that included 2 or more cohorts were included, when data extraction was possible for each cohort.
The following data were extracted from each included study: study characteristics (i.e., year of publication, study period, study design), population characteristics (i.e., number of inhibitor-negative patients, hemophilia type, hemophilia severity), patient characteristics (i.e., treatment history, inhibitor development), laboratory characteristics (type of NNA and inhibitor assay and cut-off values for positivity) and the prevalence and incidence of NNAs (overall and for each Ig class and IgG subclass).
Critical appraisal of studies was assessed by two reviewers independently (AA and MB). The Joanna Briggs Institute (JBI) checklist for prevalence studies was adapted and used to assess the methodological quality of each included study (Supplementary Data 2) (26). Using the formula provided by the JBI guideline, a sample size of ≥139 was considered adequate. Studies were classified as high-quality when at least 5 of the 9 criteria of the adapted JBI checklist were met.
In compliance with the most recent regulatory guideline, we defined two criteria to assess the quality of the various laboratory methods used to detect NNAs: (1) the use of positive controls as an internal standard and (2) the measurement of FVIII-specificity by means of a competition assay (27). NNA assays were classified as high-quality, when they met both of the quality criteria. The quality assessment of NNA assays, was included into the JBI checklist (Supplementary Data 2, question 6).
The patient and treatment characteristics were described using median and interquartile range (IQR) or range (R) for continues variables and count and percentage for categorical variables. Exact 95% Confidence Intervals (95% CI) of the reported prevalence and incidence rates were calculated by means of the Wilson method, using an online tool for the analysis of epidemiologic data (http://epitools.ausvet.com.au).
For cross-sectional studies, in inhibitor-negative patients, the prevalence of NNAs was determined by calculating the proportion of the number of NNA-positive patients of the total number of patients. For longitudinal studies, the prevalence was calculated using the patient numbers at the end of follow-up.
Depending on the way it was reported in the original study, we reported the incidence of NNAs as the cumulative incidence (the proportion of cases in a given time-period) or as the incidence rate (the rate of new cases per person-exposure day). The association between NNA status and subsequent inhibitor development was assessed by calculating the incidence rate ratio of inhibitor formation in NNA-positive patients, compared to NNA-negative patients for each study.
We pooled the prevalence of NNAs in the studies including only previously treated patients and employing high-quality NNA assays. In advance, we hypothesized that NNA incidence and prevalence differs between previously treated patients and previously untreated patients. Therefore, in order to provide a meaningful estimate of NNA prevalence, we pooled the data of studies including only previously treated patients.
Because conventional methods for meta-analysis can be biased when the outcome NNA prevalence is rare and when continuity corrections are used, we applied the Binomial-Normal model for the meta-analysis of NNA prevalence (28, 29). We explored heterogeneity by estimating the between-study variance (τ2) and by visually assessing the extent to which the 95% CIs of the individual studies overlapped. The meta-analysis was performed in R (version 3.6.1), using the metafor package (28, 30).
In these same studies, we also investigated whether NNA prevalence differed according to severity of disease and inhibitor history. When appropriate, meta-regression analysis was performed.
To evaluate whether small study data trends were present, all studies were sorted in a forest plot, according to sample size and asymmetry of the forest plots was visually assessed (31).
The flow chart of the study selection process is presented in Figure 1. Using the above search strategy, we identified a total of 2,047 unique articles. After title and abstract screening, 73 articles were identified as being potentially relevant. After full text reading and application of the inclusion criteria, 28 studies were eligible for inclusion. The reasons for exclusion after full-text screening were: small sample size (n = 4), duplicate publication of results (n = 2), unclear methods or insufficient data (n = 7), or not meeting the inclusion criteria (n = 32). Supplementary Table 1 summarizes the studies that appeared to meet eligibility criteria but on further inspection did not.
The study and patient characteristics are summarized in Table 1. Studies were all published in English, between 1994 and 2019. Seventeen studies were (partly) conducted in Europe and the majority had a cross-sectional design (19/28). The studies included a total of 3,208 patients with congenital hemophilia A, including 2,723 inhibitor-negative patients. In 14 studies, data on inhibitor history were available, involving 1,583 inhibitor-negative patients, of whom 118 had had an inhibitor in the past. The majority of patients were adult previously treated patients, with severe hemophilia A. In eight of the 11 studies that included information on FVIII product-type, recombinant FVIII (rFVIII) was the most used product. There were no studies with information on NNA prevalence or incidence in patients with hemophilia B. Nor did the cohorts of excluded articles provide information on patients with hemophilia B.
The characteristics of the NNA and inhibitor assays are provided in Table 2, including the results of the quality assessment of the NNA assays. An ELISA was used in 18 of 28 studies. Other studies employed fluorescence based assay (FLI, n = 4), multiplexed assay (X-MAP, n = 2), immunoprecipitation (IP, n = 2), and flow cytometry (FC, n = 1). In one study, the NNA assay was not reported (14). Finally, in one study FC and ELISA were compared. As the focus of this study was on the FC NNA detection method, the ELISA assay was not further described (47). A wide range of cut-off values for NNA-positivity was used, generally (12/28 studies) based on healthy controls (+2SD, +3SD). Four studies quantified the FVIII-binding affinity of detected NNAs, measured by ELISA (n = 3) or IP (n = 1) (17, 20, 46).
In nine studies both quality criteria for the NNA assay were met, including ELISA (n = 6), IP (n = 2), and FC (n = 1) assays (9, 10, 17, 20, 23, 33, 34, 46, 47). In the other studies, one (n = 10) or both (n = 9) quality criteria were not met. In most of these studies, FVIII-specificity had not been evaluated.
The methodological quality assessment is summarized in Table 3. The methodological quality was high in 16/28 studies, as these studies met at least five quality criteria of the adapted JBI check list. None of the 28 included studies met all the quality criteria. Most frequently, this was because the mode of sampling was not described (n = 16) or the sample size was smaller than 139 (n = 21). Furthermore, in 27 studies, the sample coverage and response rate were unclear.
Overall, the prevalence of NNAs in inhibitor-negative patients ranged from 0 to 100%, with a straight unweighted average prevalence of 25% (95% CI, 4–46) (Table 4). In the nine studies with a high-quality NNA assay, the NNA prevalence ranged from 7.8 to 40% (Figure 2). Two of these studies involved previously untreated patients and NNAs were measured with ELISA and IP. Six studies were performed in previously treated patients and NNAs were detected with ELISA (n = 4), IP (n = 1), or FC (n = 1). One study included both previously treated and previously untreated patients and used ELISA to detect NNAs.
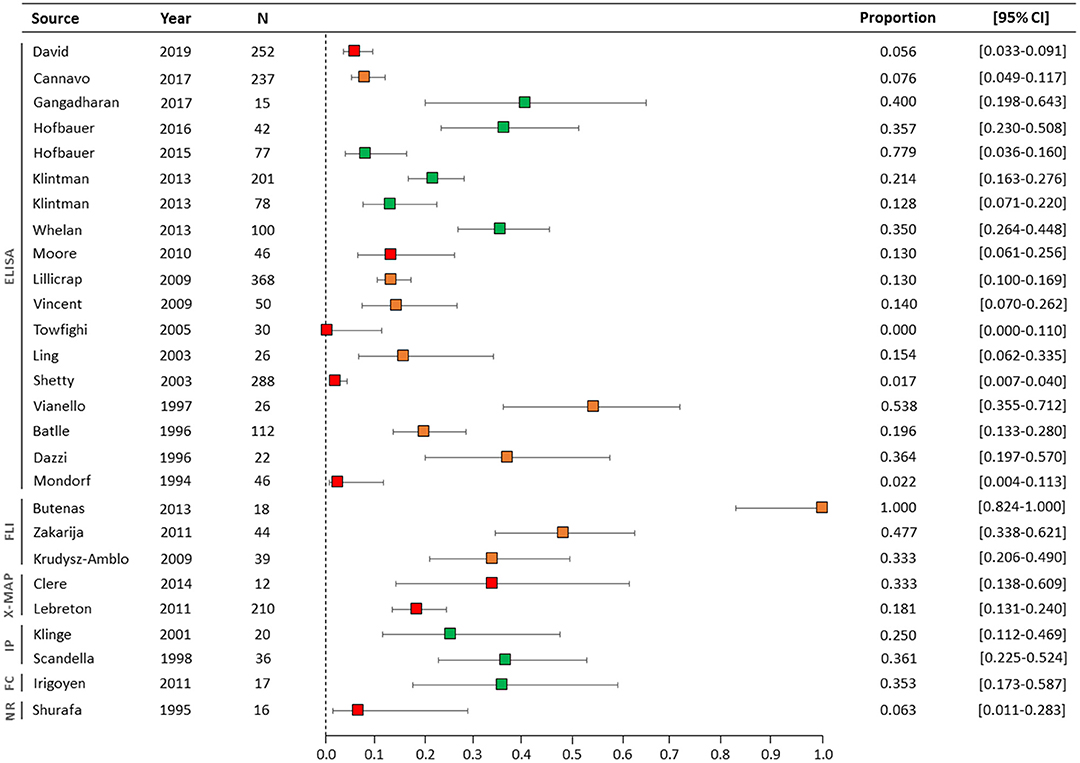
Figure 2. Forest plot of NNA prevalence in all studies. The NNA assay types are illustrated on the left side of the figure. The colors of the boxes represent the quality of the NNA assays: green (high-quality), orange (intermediate-quality), and red (low-quality). N, number of inhibitor-negative patients; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; FLI, Fluorescence based assay; IP, immunoprecipitation; X-MAP, multiplexed assay; FC, Flow cytometry; NR, name of assay not reported.
Table 5 summarizes the results of studies in which prevalence of FVIII-specific IgG subclasses or of FVIII-specific IgA or IgM isotypes were reported. In the six studies with IgG subclasses, IgG1 was the most prevalent with the prevalence ranging up to 40% (95% CI, 19.8–64.3%). NNAs of the IgG4 subclass were the least prevalent (range: 0–6.2%).
Four high-quality studies that only included previously treated patients, were included in the meta-analysis of NNA prevalence (Figure 3) (9, 23, 34, 47). The NNA prevalence in these four studies ranged from 13 to 35%. The pooled NNA prevalence was 25% (95% CI 16–38%). The high-quality studies of Hofbauer et al. were not included in the meta-analysis, due to probable overlap in patient cohorts with the study of Whelan et al. (9, 10, 20). The latter study was included, as it included the largest number of patients.
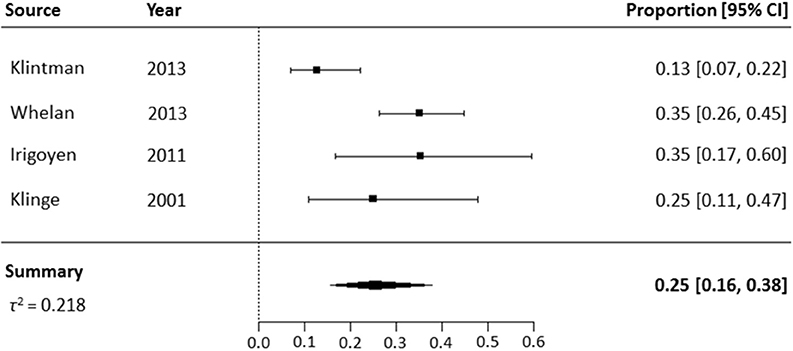
Figure 3. Meta-analysis of NNA prevalence in high-quality studies including previously treated patients.
In the four high-quality studies, the majority of patients (199/215) had severe hemophilia A. In two studies reporting on inhibitor history, 27 of 178 patients had had an inhibitor in the past (9, 34). NNA prevalence was higher i.e., 24% (95% CI, 18–31%) in patients with a negative inhibitor history vs. 33% (95% CI, 19–52%) in patients with a positive inhibitor history, who had all been successfully treated with ITI.
Only one study reported on the incidence of NNAs (17). In this study, 15 previously untreated patients were followed during the first 50 exposure days to treatment with rFVIII. Six of the 15 patients developed NNAs, all of IgG1 subclass with low apparent affinity, detected on at least 2 time points (NNA incidence rate: 0.01 per person-exposure day). In one of the six patients, the low-affinity IgG1 NNA was later accompanied by non-neutralizing high-affinity IgG1 NNA. The other 5 patients did not develop high-affinity NNAs and switching to other IgG subclasses was not observed.
One study evaluated the incidence of inhibitor development in patients who were NNA-positive and NNA-negative at baseline before any FVIII treatment (18). In this study, 237 previously untreated patients were followed for 50 exposure days to FVIII or 3 years, whichever came first. Patients with NNAs at baseline had an 83% higher risk of inhibitor development than patients without NNAs (hazard ratio, 1.83; 95% CI 0.84–3.99). The cumulative incidence of inhibitor development was 45.4% (95% CI, 19.5–71.3%) in NNA-positive patients and 34.0% (95% CI, 27.1–40.9%) in NNA-negative patients.
To explore the potential presence of small study data trends, the forest plot was arranged by study sample size. Asymmetry in the forest plot could be identified, due to relatively high NNA prevalences in studies with small sample sizes (Supplementary Figure 1).
In this systematic review, we summarized the data of 2,723 inhibitor-negative patients with hemophilia A from 28 studies to estimate the prevalence and incidence of NNAs. We found a large variety in reported NNA prevalences, ranging from 0 to 100%. In the subset of high-quality studies that included previously treated patients, the pooled NNA prevalence was 25% (95% CI, 16–38%). IgG1 was the most prevalent NNA isotype. The incidence of NNAs in inhibitor-negative patients was only given in one paper.
This study is, to our knowledge, the first comprehensive systematic overview of NNA prevalence and incidence available to date. The strengths of our study were the systematic search of the literature and the extensive quality assessment of included studies, appraising the quality of both the study methodology and the NNA assay. Studies that used high-quality NNA assays and involved only previously treated patients were subsequently included in a meta-analysis, in order to provide a more reliable estimate of NNA prevalence in this subset of patients.
However, our study had several limitations. A limited number of studies reporting on the NNA prevalence was identified, including a significant number with methodological weaknesses. NNA measurement has not yet been frequently included in clinical and translational studies, because knowledge on the clinical significance of NNAs is still limited. Another limitation was the significant study heterogeneity regarding study and patient characteristics and type and quality of NNA assays. Consequently, we could only include four high-quality studies on previously treated patients in the meta-analysis, limiting the precision of the pooled estimate. Furthermore, various studies used different methods to determine cut-off values of NNA positivity. Depending on the cut-off definition, this may have led to misclassification of NNA status and over- or underestimation of the NNA prevalence. Also, the majority of studies were conducted in patients with severe hemophilia A, which limits the generalizability of the results to patients with moderate or mild hemophilia. Therefore, further research among patients with non-severe hemophilia is needed.
Our systematic review yielded only limited insight on the NNA incidence, as only one study reported on this. Furthermore, no studies on NNA occurrence in hemophilia B were identified.
When evaluating only studies that used a high-quality NNA assay, there was more consistency in NNA prevalence. In studies that reported more extreme NNA prevalences, the quality assessment of the NNA assay was intermediate or low. The prevalence of 0% (95% IC, 0–11%) reported by one study was probably caused by the fact that this study used different cut-off values for each Ig isotype, as NNAs of IgG and IgM isotype were indeed detected in 2 and 3 patients, respectively (16). The very high prevalence of NNAs (100%, 95% CI 82.4–100%) reported by another study may have resulted from lack of evaluating FVIII-specificity, since competition with FVIII was not performed as part of the assay (43).
Use of the validated ELISA-based assay may be considered in clinical practice, because this assay meets all quality criteria and also because costs and processing time are acceptable (9).
Several patient- and treatment related determinants for anti-FVIII inhibitor development have been described in the literature, including hemophilia severity, mutation type, and FVIII treatment (product type and intensity) (2–4, 48, 58, 59). Based on recent reports, we hypothesize that the FVIII immune response is a continuum between non-neutralizing antibodies and neutralizing antibodies and therefore the determinants of both may be similar (10, 18).
We were not able to analyze the association between hemophilia severity and the presence of NNAs due to the low number of moderate and mild patients included in the four high-quality studies. A recent study in 210 patients did not demonstrate an association between disease severity and the presence of NNAs (15).
In patients with a negative inhibitor history NNA prevalence was 24 vs. 33% in patients with a positive inhibitor history successfully treated with ITI. As there were only 2 studies that reported on inhibitor history, including a relatively low number of patients, many other study or patient characteristics might explain this observed difference in NNA prevalence (9, 33). Therefore, meta-regression analysis was not performed (60).
It is not known whether the preexisting NNAs persist after inhibitor eradication, or whether ITI itself induces new NNA formation. In one study, it has been suggested that ITI changes the subclass distribution of NNAs. In high-titer inhibitor patients undergoing ITI, a rise in the contribution of anti-FVIII IgG4 was demonstrated, independent of changes in inhibitor titer (61). Further study is needed to evaluate the association between NNA characteristics and ITI outcome and to determine if NNA presence after ITI is associated with inhibitor recurrence.
In this systematic review, 9 studies also reported on NNA prevalence in healthy subjects (n = 2,010, NNA prevalence IQR 1.14–17%). Data are summarized in Supplementary Table 2.
The clinical significance of low-affinity NNAs in healthy individuals is incompletely understood. Previous reports indicate that low-affinity self-reactive antibodies may have a role in regulating the immune hemostasis (62, 63). In line with this, FVIII-specific NNAs in healthy individuals are hypothesized to be involved in the maintenance of peripheral immune tolerance toward FVIII (9, 10).
Many questions remain regarding the epitope specificity, FVIII binding affinity and clinical significance of NNAs. Previous studies in patients with hemophilia as well as healthy subjects have found NNAs mostly directed against epitopes on A1, A3, and B domains of the FVIII molecule (11, 64, 65). Furthermore, Lebreton et al. demonstrated a clear immune-dominance of the complete heavy chain (A1, A2, and B-domains) in the epitope profile of NNAs, independent of hemophilia severity (15). The exact NNA epitopes remain, however, elusive and need to be characterized in future studies.
The possible effect of infused FVIII on pharmacokinetic parameters remains to be fully elucidated. Dazzi et al. demonstrated an increase in clearance rates of infused FVIII concentrate in three of 22 NNA-positive patients with negative Bethesda assays (12). This finding was supported by Hofbauer et al. who reported that high-titer NNAs modulate FVIII half-life, independent of VWF antigen level and age (20). The NNA presence was not associated with a reduced FVIII in vivo recovery in these inhibitor-negative patients, which is in line with two previous reports (20, 66, 67). If further studies confirm the effect of NNAs on FVIII half-life, the screening for NNAs may be considered to guide pharmacokinetic measurements.
It has been hypothesized that NNAs could serve as biomarkers for future inhibitor development. The presence of NNAs at baseline was recently demonstrated to confer an increased risk of inhibitor development (hazard ratio, 1.83; 95% CI 0.84–3.99) (18). This observation is supported by the presence of high-affinity IgG1 and IgG4 NNAs, that could be detected in an inhibitor-positive patient, in samples taken 1.5 years before the inhibitor appeared (10). It has been postulated that the affinity of NNAs could provide information on the underlying regulatory pathways involved in their generation. Hence, high-affinity NNAs of the IgG or IgA isotype are thought to be produced by long-lived plasma cells, originating from follicular differentiation pathways in germinal centers (68, 69). In line with this, Hofbauer and colleagues have suggested that NNA affinity is of more importance than NNA titers when considering the risk for inhibitor development, because even low titers of high-affinity IgG4 might indicate an evolving inhibitor (10). Adequately powered clinical studies and strict NNA monitoring are required to investigate whether high-affinity NNAs might provide an opportunity to predict and eventually prevent inhibitor development.
We found a wide range of NNA prevalences in patients with hemophilia A, which resulted from considerable heterogeneity in study design with regard to disease-specific patient characteristics and type of assays used to detect NNAs. The pooled NNA prevalence was 25% in high-quality studies that included only previously treated patients and performed high-quality NNA assays. As NNA incidence was only reported in one study, more longitudinally designed studies are needed to better assess the incidence of NNAs and to further elucidate the clinical significance of these antibodies.
The datasets analyzed for this study are available from the corresponding author (S. C. Gouw).
AA, MB, SH, and SG: design of study. AA, MB, and SH: collection of data. AA, SH, and SG: statistical analysis. AA and JV: quality assessment NNA assays. AA, SG, and KF: redaction of manuscript. AA, MB, SH, FR, JB, JV, KF, and SG: critical review of manuscript.
JB is consultant for Bayer. JV is an inventor on FVIII-related patients and has received research funding from Novo Nordisk and has acted as an advisor for Biotest. The institution of KF has received unrestricted research grants from CSL Behring and Novo Nordisk and consultancy fees from Grifols, Takeda, Novo Nordisk and Roche.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank P. Batty for providing additional information and N. van der Werf for expert support in designing the literature search.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.00563/full#supplementary-material
1. Ananyeva NM, Lacroix-Desmazes S, Hauser CA, Shima M, Ovanesov MV, Khrenov AV, et al. Inhibitors in hemophilia A: mechanisms of inhibition, management and perspectives. Blood Coagul Fibrinolysis. (2004) 15:109–24. doi: 10.1097/00001721-200403000-00001
2. Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. (2003) 9:418–35. doi: 10.1046/j.1365-2516.2003.00780.x
3. Gouw SC, Van Den Berg HM, Fischer K, Auerswald G, Carcao M, Chalmers E, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: The RODIN study. Blood. (2013) 121:4046–55. doi: 10.1182/blood-2012-09-457036
4. Eckhardt CL, Van Velzen AS, Peters M, Astermark J, Brons PP, Castaman G, et al. Factor VIII gene (F8) mutation and risk of inhibitor development in nonsevere hemophilia A. Blood. (2013) 122:1954–62. doi: 10.1182/blood-2013-02-483263
5. DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol. (2007) 138:305–15. doi: 10.1111/j.1365-2141.2007.06657.x
6. Santoro C, Quintavalle G, Castaman G, Baldacci E, Ferretti A, Riccardi F, et al. Inhibitors in Hemophilia B. Semin Thromb Hemost. (2018) 44:578–89. doi: 10.1055/s-0038-1660817
7. Eckhardt CL, Loomans JI, van Velzen AS, Peters M, Mauser-Bunschoten EP, Schwaab R, et al. Inhibitor development and mortality in non-severe hemophilia A. J Thromb Haemost. (2015) 13:1217–25. doi: 10.1111/jth.12990
8. Leissinger C, Cooper DL, Solem CT. Assessing the impact of age, race, ethnicity and inhibitor status on functional limitations of patients with severe and moderately severe haemophilia A. Haemophilia. (2011) 17:884–9. doi: 10.1111/j.1365-2516.2011.02509.x
9. Whelan SFJ, Hofbauer CJ, Horling FM, Allacher P, Wolfsegger MJ, Oldenburg J, et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. (2013) 121:1039–48. doi: 10.1182/blood-2012-07-444877
10. Hofbauer CJ, Whelan SFJ, Hirschler M, Allacher P, Horling FM, Lawo J, et al. Affinity of FVIII-specific antibodies reveals major differences between neutralizing and nonneutralizing antibodies in humans. Blood. (2015) 125:1180–9. doi: 10.1182/blood-2014-09-598268
11. Batlle J, Gómez E, Rendal E, Torea J, Lourés E, Couselo M, et al. Antibodies to factor VIII in plasma of patients with hemophilia A and normal subjects. Ann Hematol. (1996) 72:321–6. doi: 10.1007/s002770050179
12. Dazzi F, Tison T, Vianello F, Radossi P, Zerbinati P, Carraro P, et al. High incidence of anti-FVIII antibodies against non-coagulant epitopes in haemophilia A patients: a possible role for the half-life of transfused FVIII. Br J Haematol. (1996) 93:688–93. doi: 10.1046/j.1365-2141.1996.d01-1705.x
13. Krudysz-Amblo J, Parhami-Seren B, Butenas S, Brummel-Ziedins KE, Gomperts ED, Rivard GE, et al. Quantitation of anti-factor VIII antibodies in human plasma. Blood. (2009) 113:2587–94. doi: 10.1182/blood-2008-08-174987
14. Shurafa M, Kithier K. IgG antibodies against factor VIII in normal individuals. J Thromb Thrombolysis. (1995) 2:113–5. doi: 10.1007/BF01064378
15. Lebreton A, Lapalud P, Chambost H, Biron-Andreani C, Morange PE, Combescure C, et al. Prevalence and epitope specificity of non-neutralising antibodies in a large cohort of haemophilia A patients without inhibitors. Thromb Haemost. (2011) 105:954–61. doi: 10.1160/TH10-10-0668
16. Towfighi F, Gharagozlou S, Sharifian RA, Kazemnejad A, Esmailzadeh K, Managhchi MR, et al. Comparative measurement of anti-factor VIII antibody by Bethesda assay and ELISA reveals restricted isotype profile and epitope specificity. Acta Haematol. (2005) 114:84–90. doi: 10.1159/000086580
17. Gangadharan B, Reipert B, Berg V, Scheiflinger F, Blatný J, Fijnvandraat K, et al. Data coming out of the human inhibitor PUP study (HIPS) reveal 4 subgroups of patients with distinct antibody signatures. Blood. (2018) 132:3774. doi: 10.1182/blood-2018-99-115979
18. Cannavò A, Valsecchi C, Garagiola I, Palla R, Mannucci PM, Rosendaal FR, et al. Nonneutralizing antibodies against factor VIII and risk of inhibitor development in severe hemophilia A. Blood. (2017) 129:1245–50. doi: 10.1182/blood-2016-06-720086
19. Montalvão SAL, Tucunduva AC, Siqueira LH, Sambo ALA, Medina SS, Ozelo MC. A longitudinal evaluation of anti-FVIII antibodies demonstrated IgG4 subclass is mainly correlated with high-titre inhibitor in haemophilia A patients. Haemophilia. (2015) 21:686–92. doi: 10.1111/hae.12646
20. Hofbauer CJ, Kepa S, Schemper M, Quehenberger P, Reitter-Pfoertner S, Mannhalter C, et al. FVIII-binding IgG modulates FVIII half-life in patients with severe and moderate hemophilia A without inhibitors. Blood. (2016) 128:293–6. doi: 10.1182/blood-2015-10-675512
21. Verbruggen B, Novakova I, Wessels H, Boezeman J, Van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb Haemost. (1995) 73:247–51. doi: 10.1055/s-0038-1653759
22. Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. (2014) 12:1935–9. doi: 10.1111/jth.12672
23. Klinge J, Auerswald G, Budde U, Klose H, Kreuz W, Lenk H, et al. Detection of all anti-factor VIII antibodies in haemophilia A patients by the Bethesda assay and a more sensitive immunoprecipitation assay. Haemophilia. (2001) 7:26–32. doi: 10.1046/j.1365-2516.2001.00456.x
24. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
25. Rup B, Pallardy M, Sikkema D, Albert T, Allez M, Broet P, et al. Standardizing terms, definitions and concepts for describing and interpreting unwanted immunogenicity of biopharmaceuticals: recommendations of the innovative medicines initiative ABIRISK consortium. Clin Exp Immunol. (2015) 181:385–400. doi: 10.1111/cei.12652
26. Munn Z, Moola S, Lisy K, Rittano D. The systematic review of prevalence and incidence data. In: Joanna Briggs Institute Reviewers' Manual: 2014 Edition/Supplement. Adelaide: Joanna Briggs Institute (2014). p. 32–5.
27. FDA (2019). Immunogenicity Testing of Therapeutic Protein Products - Developing and Validating Assays for Anti-Drug Antibody Detection. Available online at: https://www.fda.gov/media/119788/download
28. Stijnen T, Hamza TH, Özdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. (2010) 29:3046–67. doi: 10.1002/sim.4040
29. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. (2004) 23:1351–75. doi: 10.1002/sim.1761
30. The metafor Package: A Meta-analysis Package for R. Binomial-Normal Model for the Meta-Analysis of Proportions. Available online at: http://www.metafor-project.org/doku.php/analyses:stijnen2010 (updated 2018 December 08; cited 2020 Febraruy 14).
31. Weckmann G, Chenot J-F, Reber KC. A practical approach to reading and interpreting meta-analyses | Metaanalysen lesen und interpretieren: Eine praktische Anleitung. Z Allgemeinmed. (2015) 91:469–73. doi: 10.3238/zfa.2015.0469–0473
32. David S, Mathews NS, Singh GS, Korula A, Aboobacker FN, Abraham A, et al. Evaluation of nonneutralizing antibodies against factor VIII in severe haemophilia A patients from India. Blood Coagul Fibrinolysis. (2019) 30:337–40. doi: 10.1097/MBC.0000000000000843
33. Klintman J, Hillarp A, Donfield S, Berntorp E, Astermark J. Antibody formation and specificity in Bethesda-negative brother pairs with haemophilia A. Haemophilia. (2013) 19:106–12. doi: 10.1111/j.1365-2516.2012.02903.x
34. Klintman J, Hillarp A, Berntorp E, Astermark J. Long-term anti-FVIII antibody response in Bethesda-negative haemophilia A patients receiving continuous replacement therapy. Br J Haematol. (2013) 163:385–92. doi: 10.1111/bjh.12540
35. Moore GW, Maloney JC, Christie A, Rangarajan S, Bevan DH. Prevalence of non-inhibitory FVIII antibodies. Haemophilia. (2010) 16:408–12. doi: 10.1111/j.1365-2516.2010.02202.x
36. Lillicrap D, Tuttle A, Crawford E, Vincent A-M, Rivard GE. The prevalence of non-neutralizing anti-FVIII antibodies in the canadian hemophilia population. Blood. (2009) 114:1291. doi: 10.1182/blood.V114.22.1291.1291
37. Vincent AM, Lillicrap D, Boulanger A, Meilleur C, Amesse C, St-louis J, et al. Non-neutralizing anti-FVIII antibodies: different binding specificity to different recombinant FVIII concentrates. Haemophilia. (2009) 15:374–6. doi: 10.1111/j.1365-2516.2008.01909.x
38. Ling M, Duncan EM, Rodgers SE, Street AM, Lloyd JV. Low detection rate of antibodies to non-functional epitopes on factor VIII in patients with hemophilia A and negative for inhibitors by bethesda assay. J Thromb Haemost. (2003) 1:2548–53. doi: 10.1111/j.1538-7836.2003.tb04204.x
39. Shetty S, Ghosh K, Mohanty D. An ELISA assay for the detection of factor VIII antibodies - Comparison with the conventional Bethesda assay in a large cohort of haemophilia samples. Acta Haematol. (2003) 109:18–22. doi: 10.1159/000067272
40. Vianello F, Radossi P, Tison T, Dazzi F, Tagariello G, Davoli PG, et al. Prevalence of anti-FVIII antibodies in severe haemophilia A patients with inversion of intron 22. Br J Haematol. (1997) 97:807–9. doi: 10.1046/j.1365-2141.1997.1082922.x
41. Mondorf W, Ehrenforth S, Vigh Z, Last J, Tippmann G, Kreuz W, et al. Screening of F.VIII:C antibodies by an enzyme-linked immunosorbent assay. Vox Sang. (1994) 66:8–13. doi: 10.1159/000462463
42. Boylan B, Rice AS, Miller CH, Dunn AL, Abshire TC, Kempton CL, et al. Characterization of the anti-factor VIII immunoglobulin profile in patients with hemophilia A by use of a fluorescence-based immunoassay. J Thromb Haemost. (2015) 13:47–53. doi: 10.1111/jth.12768
43. Butenas S, Krudysz-Amblo J, Rivard GE, Mann GK. Product-dependent anti-factor VIII antibodies. Haemophilia. (2013) 19:619–25. doi: 10.1111/hae.12127
44. Zakarija A, Harris S, Rademaker AW, Brewer J, Krudysz-Amblo J, Butenas S, et al. Alloantibodies to factor VIII in haemophilia. Haemophilia. (2011) 17:636–40. doi: 10.1111/j.1365-2516.2010.02468.x
45. Clere AS, Diaz I, Lebreton A, Lavigne-Lissalde G, Schved JF, Biron-Andréani C. Are low-density lipoprotein receptor-related protein 1 or non-neutralizing antibodies predictors of FVIII in vivo recovery in haemophilia A patients? Haemophilia. (2014) 20:e406–8. doi: 10.1111/hae.12508
46. Scandella D, Mondorf W, Klinge J. The natural history of the immune response to exogenous factor VIII in severe haemophilia A. Haemophilia. (1998) 4:546–51. doi: 10.1046/j.1365-2516.1998.440546.x
47. Irigoyen MB, Primiani L, Felippo M, Candela M, Bianco RP, De Bracco MMDE, et al. A flow cytometry evaluation of anti-FVIII antibodies: correlation with ELISA and Bethesda assay. Haemophilia. (2011) 17:267–74. doi: 10.1111/j.1365-2516.2010.02406.x
48. Peyvandi F, Mannucci PM, Garagiola I, El-Beshlawy A, Elalfy M, Ramanan V, et al. A randomized trial of factor VIII and neutralizing antibodies in Hemophilia A. N Engl J Med. (2016) 374:2054–64. doi: 10.1056/NEJMoa1516437
49. Astermark J, Berntorp E, White GC, Kroner BL. The Malmo International Brother Study (MIBS): further support for genetic predisposition to inhibitor development in hemophilia patients. Haemophilia. (2001) 7:267–72. doi: 10.1046/j.1365-2516.2001.00510.x
50. Berntorp E, Astermark J, Donfield SM, Nelson GW, Oldenburg J, Shapiro AD, et al. Haemophilia Inhibitor Genetics Study - evaluation of a model for studies of complex diseases using linkage and association methods. Haemophilia. (2005) 11:427–9. doi: 10.1111/j.1365-2516.2005.01119.x
51. Bray GL, Gomperts ED, Courter S, Gruppo R, Gordon EM, Manco-Johnson M, et al. A multicenter study of recombinant factor VIII (recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. Blood. (1994) 83:2428–35.
52. Kreuz W, Escuriola Ettingshausen C, Zyschka A, Oldenburg J, Martinez Saguer I, Ehrenforth S, et al. Inhibitor development in previously untreated patients with hemophilia A: a prospective long-term follow-up comparing plasma-derived and recombinant products. Semin Thromb Hemost. (2002) 28:285–90. doi: 10.1055/s-2002-32664
53. Cannavò A, Valsecchi C, Garagiola I, Palla, R, Mannucci PM, Rosendaal FR, et al. Nonneutralizing antibodies against factor VIII and risk of inhibitor development in severe hemophilia A. Blood. (2017) 129:1245–50. doi: 10.1182/blood-2016-06-720086
54. Shurafa M, Kithier K. A new approach to immunologic identification of factor VIII antibodies. Haemophilia. (1995) 1:175–7. doi: 10.1111/j.1365-2516.1995.tb00063.x
55. Jaki T, Lawo JP, Wolfsegger MJ, Singer J, Allacher P, Horling F. A formal comparison of different methods for establishing cut points to distinguish positive and negative samples in immunoassays. J Pharm Biomed Anal. (2011) 55:1148–56. doi: 10.1016/j.jpba.2011.04.006
56. Lavigne-Lissalde G, Tarrade C, Lapalud P, Chtourou S, Schved JF, Granier C, et al. Simultaneous detection and epitope mapping of anti-factor VIII antibodies. Thromb Haemost. (2008) 99:1090–6. doi: 10.1160/TH07-08-0497
57. Thompson AR, Murphy MEP, Liu M, Saenko EL, Healey JF, Lollar P, et al. Loss of tolerance to exogenous and endogenous factor VIII in a mild hemophilia A patient with an Arg593 to cys mutation. Blood. (1997) 90:1902–10. doi: 10.1182/blood.V90.5.1902
58. Gouw SC, Van Den Berg HM, Oldenburg J, Astermark J, De Groot PG, Margaglione M, et al. F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood. (2012) 119:2922–34. doi: 10.1182/blood-2011-09-379453
59. van Velzen AS, Eckhardt CL, Peters M, Leebeek FWG, Escuriola-Ettingshausen C, Hermans C, et al. Intensity of factor VIII treatment and the development of inhibitors in nonsevere hemophilia A patients: results of the INSIGHT case-control study. J Thromb Haemost. (2017) 15:1422–9. doi: 10.1111/jth.13711
60. Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med. (2002) 21:1559–73. doi: 10.1002/sim.1187
61. Van Helden PMW, Van Den Berg HM, Gouw SC, Kaijen PHP, Zuurveld MG, Mauser-Bunschoten EP, et al. IgG subclasses of anti-FVIII antibodies during immune tolerance induction in patients with hemophilia A. Br J Haematol. (2008) 142:644–52. doi: 10.1111/j.1365-2141.2008.07232.x
62. Cohen IR. Biomarkers, self-antigens and the immunological homunculus. J Autoimmunity. (2007) 29:246–9. doi: 10.1016/j.jaut.2007.07.016
63. Kaveri SV. Intravenous immunoglobulin: exploiting the potential of natural antibodies. Autoimmunity Rev. (2012) 11:792–4. doi: 10.1016/j.autrev.2012.02.006
64. Di Giambattista M, Branckaert T, Laub R. Mapping of natural anti-factor VIII antibodies in plasma pools from healthy donors: use of rationally designed synthetic peptides. Biologicals. (2001) 29:229–32. doi: 10.1006/biol.2001.0295
65. Hay CRM, Ludlam CA, Colvin BT, Hill FGH, Preston FE, Wasseem N, et al. Factor VIII inhibitors in mild and moderate-severity haemophilia A. Thromb Haemost. (1998) 79:762–6. doi: 10.1055/s-0037-1615061
66. Kempton CL, Meeks SL, Donald Harvey R, Abshire TC. Evaluation of factor VIII pharmacokinetics and anti-factor VIII antibodies in four boys with haemophilia A and a poor clinical response to factor VIII. Haemophilia. (2011) 17:155–6. doi: 10.1111/j.1365-2516.2010.02345.x
67. Mondorf W, Klinge J, Luban NLC, Bray G, Saenko E, Scandella D. Low factor VIII recovery in haemophilia A patients without inhibitor titre is not due to the presence of anti-factor VIII antibodies undetectable by the Bethesda assay. Haemophilia. (2001) 7:13–9. doi: 10.1046/j.1365-2516.2001.00463.x
68. Oracki SA, Walker JA, Hibbs ML, Corcoran LM, Tarlinton DM. Plasma cell development and survival. Immunol Rev. (2010) 237:140–59. doi: 10.1111/j.1600-065X.2010.00940.x
Keywords: hemophilia, FVIII, FIX, non-neutralizing antibodies, anti-drug antibodies, ADA assay, inhibitors
Citation: Abdi A, Bordbar MR, Hassan S, Rosendaal FR, van der Bom JG, Voorberg J, Fijnvandraat K and Gouw SC (2020) Prevalence and Incidence of Non-neutralizing Antibodies in Congenital Hemophilia A— A Systematic Review and Meta-Analysis. Front. Immunol. 11:563. doi: 10.3389/fimmu.2020.00563
Received: 17 December 2019; Accepted: 12 March 2020;
Published: 07 May 2020.
Edited by:
Sébastien Lacroix-Desmazes, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Rolf Ljung, Lund University, SwedenCopyright © 2020 Abdi, Bordbar, Hassan, Rosendaal, van der Bom, Voorberg, Fijnvandraat and Gouw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. C. Gouw, cy5jLmdvdXdAbHVtYy5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.