
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 21 February 2020
Sec. Inflammation
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.00249
This article is part of the Research Topic Immunomodulatory Roles of Tryptophan Metabolites in Inflammation and Cancer View all 18 articles
 Lukas Lanser1
Lukas Lanser1 Patricia Kink1
Patricia Kink1 Eva Maria Egger1
Eva Maria Egger1 Wolfgang Willenbacher2,3
Wolfgang Willenbacher2,3 Dietmar Fuchs4
Dietmar Fuchs4 Guenter Weiss1
Guenter Weiss1 Katharina Kurz1*
Katharina Kurz1*Many patients with cancer suffer from anemia, depression, and an impaired quality of life (QoL). These patients often also show decreased plasma tryptophan levels and increased kynurenine concentrations in parallel with elevated concentrations of Th1 type immune activation marker neopterin. In the course of anti-tumor immune response, the pro-inflammatory cytokine interferon gamma (IFN-γ) induces both, the enzyme indoleamine 2,3-dioxygenase (IDO) to degrade tryptophan and the enzyme GTP-cyclohydrolase I to form neopterin. High neopterin concentrations as well as an increased kynurenine to tryptophan ratio (Kyn/Trp) in the blood of cancer patients are predictive for a worse outcome. Inflammation-mediated tryptophan catabolism along the kynurenine pathway is related to fatigue and anemia as well as to depression and a decreased QoL in patients with solid tumors. In fact, enhanced tryptophan breakdown might greatly contribute to the development of anemia, fatigue, and depression in cancer patients. IDO activation and stimulation of the kynurenine pathway exert immune regulatory mechanisms, which may impair anti-tumor immune responses. In addition, tumor cells can degrade tryptophan to weaken immune responses directed against them. High IDO expression in the tumor tissue is associated with a poor prognosis of patients. The efficiency of IDO-inhibitors to inhibit cancer progression is currently tested in combination with established chemotherapies and with immune checkpoint inhibitors. Inflammation-mediated tryptophan catabolism and its possible influence on the development and persistence of anemia, fatigue, and depression in cancer patients are discussed.
Cancer is a leading cause of death and disability worldwide with an increasing prevalence. Patients with malignant diseases often have sustained systemic immune activation, which is linked to tumor progression and a poor clinical outcome (1, 2). Initially, immune activation is an important mechanism to prevent carcinogenesis. However, this mechanism does not seem to work properly in patients with advanced cancer. Tumor cells are able to escape immune-mediated elimination by leukocytes due to loss of antigenicity and/or immunogenicity but also by creating an immunosuppressive microenvironment and by blocking anti-tumor immune response (3). Tryptophan (Trp) metabolism appears to play an important role within the tumor microenvironment (4).
In fact, enhanced Trp breakdown, reflected by decreased Trp and elevated kynurenine (Kyn) concentrations in the peripheral blood, is often observed in cancer patients and related to tumor progression, poor clinical outcome (Table 1) and an impaired quality of life (QoL) (58, 85). Trp breakdown in patients with malignancies is primarily mediated by increased tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenase 1 (IDO1) activities (86). The latter is primarily activated by pro-inflammatory cytokines of the T helper 1 (Th1) type immune response, particularly interferon gamma (IFN-γ) (87). IFN-γ also stimulates the formation of reactive oxygen species (ROS) as well as the expression of GTP-cyclohydrolase I (GCH-1) in target cells. In human monocytes/macrophages, this enzyme subsequently degrades GTP to form the pteridine neopterin, which has been established as a clinically useful marker for Th1 driven immune activation (88).
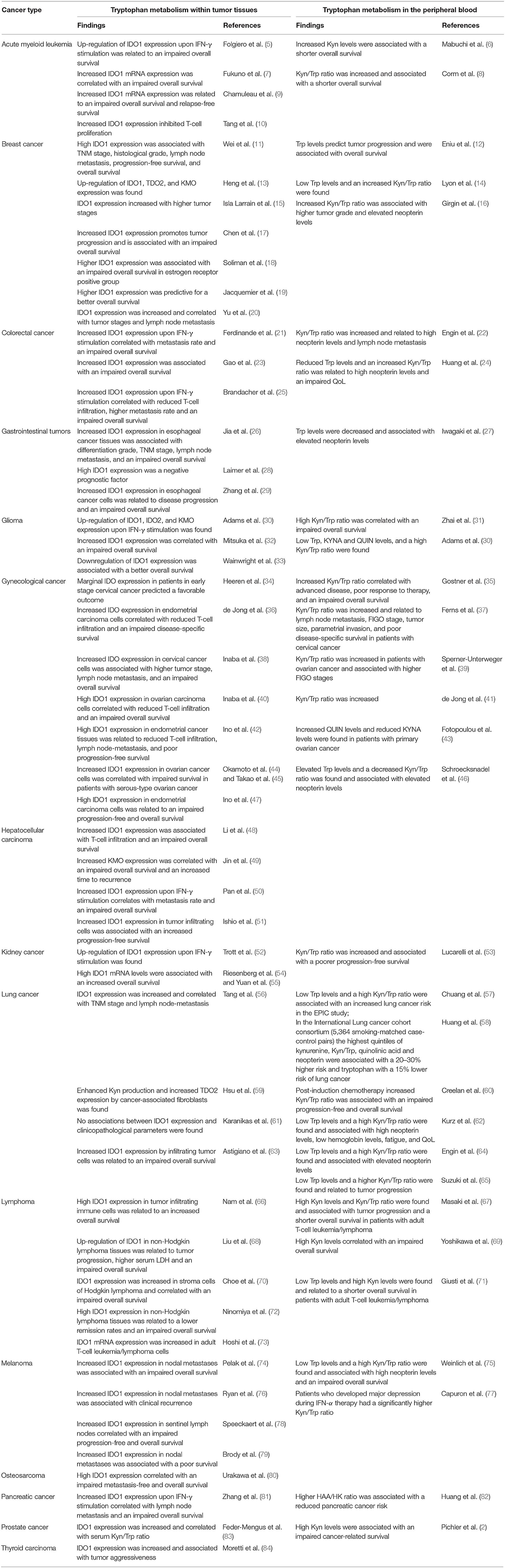
Table 1. Altered tryptophan metabolism in different cancer types and its relations to disease severity, progression, and survival.
Higher neopterin concentrations mostly coincide with increased IDO-activation as reflected by a higher Kyn/Trp ratio (24, 46, 89, 90) and are related to tumor progression and an increased mortality rate (1, 91) in patients with malignant diseases.
Trp is essential for the growth and proliferation of all kinds of cells; therefore, local inflammation-induced Trp depletion is initially a defense mechanism of the immune system to limit growth of microbes but also of proliferating malignant cells (92). However, tumor cells seem to develop countermeasures via degradation of Trp, allowing them to escape this defense mechanism. Moreover, stimulation of IDO1 and Trp breakdown also impacts on Trp availability for immune cells over time and leads to the accumulation of Trp metabolites such as the kynurenines, which can directly modulate anti-tumor immune responses (93).
Apart from an activated immune system and enhanced Trp breakdown, patients with malignancies frequently suffer from anemia (94). Anemia is a main contributor to sustained fatigue (95), which is the most frequently reported symptom in cancer patients (96), affecting up to 78% (97). Actually, activities of daily living are mostly affected by cancer related fatigue (CRF) (98). Another common comorbidity is depression, affecting ~20% of cancer patients (99–101). All these comorbidities have been related to immune activation and the associated Trp breakdown.
This review discusses the current knowledge on and consequences of immune activation and Trp breakdown for the development and persistence of anemia, fatigue, and depression in cancer patients. Moreover, it gives an overview of possible therapeutic options for the treatment of comorbidities. At the beginning, a brief depiction of Trp metabolism and its relations to immune activation will be given.
Trp is an essential amino acid that is required for protein biosynthesis. Therefore, it is essential for the growth and proliferation of cells. Trp must be supplied by diet or obtained from protein degradation, since it cannot be synthesized by human cells. The required daily amount for adults lies between 175 and 250 mg. Yet, the average daily intake for many individuals lies between 900 and 1,000 mg (102, 103). Thus, decreased Trp concentrations are suggested to be primarily caused by enhanced Trp breakdown.
Trp is also an important precursor for several bioactive metabolites including tryptamine, serotonin, melatonin, kynurenine (Kyn) and quinolinic acid (QUIN) and kynurenic acid (KYNA) as well as for the coenzyme NAD+. These metabolites are mainly generated by two different biochemical pathways.
First, Trp can be catabolized by the enzyme tryptophan 5-hydroxylase (TPH) to 5-hydroxytryptophan (5-HTP) (Figure 1). 5-HTP is converted into 5-methoxytryptophan (5-MTP) by the hydroxyindole-O-methyltransferase (HIOMT) (104) and subsequently decarboxylated to 5-hydroxytryptamine (5-HT) by the vitamin B6 dependent aromatic-L-amino-acid decarboxylase (AADC) (105). 5-HT, better known as serotonin, is an important neurotransmitter that modulates numerous neuropsychological processes including mood, anxiety, anger, reward, and cognition (106). It is also involved in important processes outside the central nervous system (CNS), including regulatory functions in the gastrointestinal (GI) tract as well as cardiovascular and pulmonary system. Actually, over 90% of the total body serotonin is synthesized in the GI tract (107).
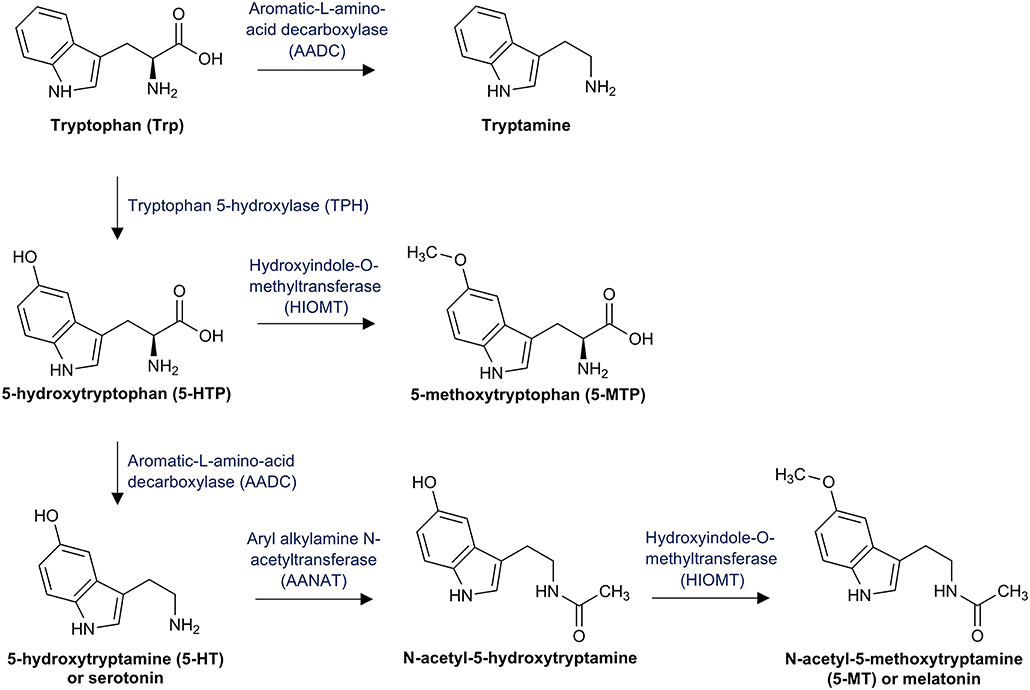
Figure 1. Tryptophan pathway to serotonin and melatonin: This figure illustrates tryptophan breakdown to serotonin via the intermediate product 5-hydroxytryptophan (5-HTP) and the further conversion to melatonin via the intermediate product 5-acetyl-5-hydroxytryptamine.
Although only 1% of the available Trp is converted by the Trp/5-HT pathway in healthy individuals, decreased Trp availability is associated with decreased serotonin concentrations and consequently with neuropsychologic disorders (105). In the pineal gland, aryl alkylamine N-acetyltransferase (AANAT) converts 5-HT into N-acetyl-5-hydroxytryptamine, which is further catabolyzed by the HIOMT to N-acetyl-5-methoxytryptamine (5-MT), better known as melatonin (108). Melatonin is primarily secreted at night and regulates the circadian rhythm under normal light/dark conditions (109). Finally, Trp can be directly decarboxylated by the AADC to tryptamine, which is an important neuromodulator of serotonin (110).
The second and quantitatively most important pathway is the decay to Kyn (Figure 2). Approximately 90% of the available Trp is oxidized to N-formylkynurenine by either tryptophan 2,3-dioxygenase (TDO; EC 1.13.11.11), indoleamine 2,3-dioxygenase 1 (IDO1; EC 1.13.11.52), or indoleamine 2,3-dioxygenase 2 (IDO2; 1.13.11.-). N-formylkynurenine is then subsequently hydrolyzed to Kyn by kynurenine formamidase. Kyn is further catalyzed by one of the four kynurenine aminotransferases (KATs) to KYNA. It can also be hydroxylated to 3-hydroxykynurenine (3-HK) by kynurenine 3-monooxygenase (KMO) and then converted to 3-hydroxyanthralinic acid (3-HAA) by the kynureninase (KYNU). Another important enzyme of the Kyn pathway, namely 3-hydroxyanthranilic acid dioxygenase (HAD), converts 3-HAA into 2-amino-3-carboxymuconate semialdehyde, which decays non-enzymatically into QUIN. Finally, phosphoribosyl transferase (QPRT) converts QUIN into nicotinamide, which is an important component of NAD+ and NADP+ being necessary for energy production (111).
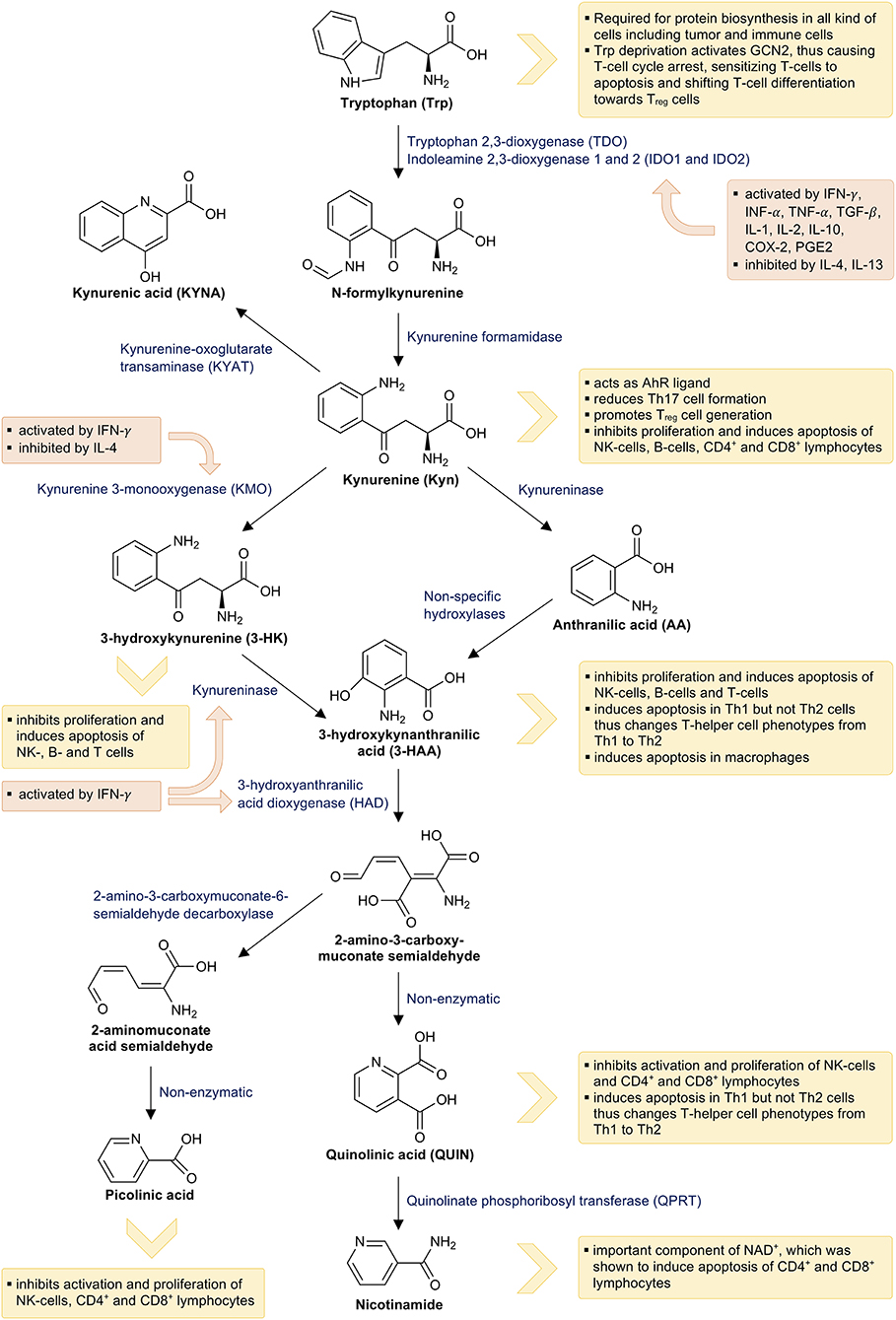
Figure 2. Tryptophan breakdown via the kynurenine pathway and its interactions with the immune system: This figure illustrates tryptophan breakdown via the kynurenine pathway. The orange boxes indicate the effects of immune mediators on the kynurenine pathway and the yellow boxes indicate the effects of tryptophan metabolites on the immune system.
TDO, IDO1, and IDO2 are heme-containing enzymes and catalyze the first and rate-limiting step in Trp breakdown. TDO is mainly expressed in the liver and oxidizes excess Trp, thereby generating ATP and especially NAD+. In mammals, NAD+ is synthesized from Trp via the Preiss-Handler pathway in liver and kidney (112). Actually, the Trp concentration in the diet has been shown to influence the liver NAD+ levels (113). TDO expression is stimulated by its substrate Trp (114) as well as by heme (115) and corticosteroids (116). NAD+ inhibits TDO expression, thus forming a negative feedback loop (117). IDO1 can be expressed by many different cells, including antigen-presenting cells (APCs) like monocyte-derived macrophages, dendritic cells (DCs) and fibroblasts. Its expression is mainly induced by inflammatory stimuli such as IFN-γ, tumor necrosis factor alpha (TNF-α), IL-1, and IL-2 secreted by Th1 type cells, inflammatory cytokines of innate immune cells as well as TGF-β, IL-10, and adenosine secreted by regulatory T cells (Treg) (118). IDO1 expression is further stimulated by its own product Kyn via the aryl hydrocarbon receptor (AhR) (119–121) as well as by the cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) (122). Contrary to this, IDO1 expression is inhibited by the anti-inflammatory cytokines IL-4 and IL-13 (123, 124). Little is known about the physiological functions of the recently detected IDO2. It is primarily expressed in the liver, kidney, brain, placenta, and APCs including DCs and B cells; yet, IDO2 is significantly less active when compared to IDO1 (125). Similar to IDO1, IDO2 expression is stimulated by AhR activation (120). Interestingly, IDO2 negatively regulates IDO1 activity by competing for heme-binding (126). IFN-γ also stimulates KMO, KYNU and HAD activity (127).
An immunologicaly privileged milieu with a decreased reactivity to allogeneic (non-self) antigens is found in certain parts of the human body (e.g., brain, eye, testis, placenta). This immune tolerance prevents fetal rejection and immune responses against immunogenic sperms. An enhanced expression of TDO, IDO1, and IDO2, with a subsequent accumulation of Trp metabolites, is found in several parts of the human body including the placenta (128, 129), maternal and embryonic tissues in early conceptions (130, 131) as well as in the epididymis (132–134). Therefore, these enzymes are suggested to play an important role in immune tolerance. Immune tolerizing effects are also observed in the local tumor microenvironment. An enhanced Trp catabolism via Kyn pathway seems to be involved in immune paralysis against tumor cells. This may be primarily mediated by increased IDO1 expression and subsequent accumulation of Trp metabolites, since IDO1 is either expressed by many tumor cells themselves (see Table 1) or by tumor associated cells such as DCs or endothelial cells (ECs) (118).
Nearly all metabolites of the Kyn pathway affect immune activity via several mechanisms (Figure 2). Trp depletion slows down protein biosynthesis in immune cells and induces cell cycle arrest of T cells via eIF-2-alpha kinase GCN2, thus making them highly susceptible to Fas-ligand-mediated apoptosis (135, 136). Activation of GCN2 further promotes the generation of regulatory phenotypes (Treg) in naive CD4+ T cells (137). Activation of AhR by its endogenous ligand Kyn results in reduced T helper 17 (Th17) cell differentiation, while promoting the generation of Treg cells (138, 139). Treg cells, in turn, induce IDO1 expression in DCs, thus expanding their own population and forming a positive regulatory feedback loop (137). Th17 cells upregulate KMO expression, which reduces the availability of Kyn for AhR activation and consequently results in a reduced Th17 formation in the sense of a negative regulatory feedback loop (140). Finally, several metabolites of Trp breakdown such as Kyn, 3-HK, 3-HAA, QUIN, and picolinic acid were demonstrated to suppress the proliferation of CD4+ lymphocytes, CD8+ lymphocytes, and natural killer (NK) cells. Furthermore, they induce apoptosis of these cells probably mediated by oxygen free radicals (141–144), while 3-HAA induces apoptosis of monocytes/macrophages (145). However, apoptosis primarily occurs in Th1 cells and not in Th2 cells, thereby forming a negative feedback loop and preventing an excessive Th1 activation (141). In addition, the final product of the Kyn pathway NAD+ also induces apoptosis in CD4+ and CD8+ lymphocytes (146).
Apart from immune modulating properties, Kyn metabolites may also help tumors to “optimize their microenvironment”: Formation of QUIN by glioma cells was described to promote resistance to oxidative stress (147). Additionally, tumor cells might enhance their own IDO activity via an autocrine AhR-IL-6-STAT3 signaling loop (148), thereby suppressing T-cell proliferation. Upregulation of the tryptophanyl-tRNA synthetase WARS may protect Trp-degrading cancer cells from excessive intracellular Trp depletion via IFNγ and/or GCN2- signaling (149).
On the other hand, 5-MTP, which is produced by mesenchymal cells such as fibroblasts via 5-HTP, inhibits migration of cancer cells, tumor growth and cancer metastasis. This effect is probably mediated by 5-MTP derived inhibition of COX-2, which is constitutively overexpressed in cancer cells and promotes carcinogenesis (150). Therefore, reduced 5-MTP formation due to decreased Trp availability can contribute to tumor growth and cancer metastasis.
IDO1 expression is a counter-regulatory mechanism to slow down potentially harmful over-activated immune responses. However, when the immune system attempts to fight a tumor, this counter-regulation is highly undesirable (151). In the majority of studies, an upregulation of IDO1 expression was associated with a poor clinical outcome (Table 1). Only in a small number of tumor entities, increased IDO1 activity was associated with a favorable prognosis (19, 54). The apparent inflammation-induced IDO1 expression in these patients probably indicates a stronger innate anti-tumor immune response.
It is suggested that IDO1 takes different positions in the three phases of cancer immunoediting: elimination, equilibrium, and escape (118). In the first phase (elimination), most tumor cells are recognized, and destroyed by the immune system. Low-level IDO1 production in the tumor microenvironment contributes to this tumor defense by inhibiting tumor proliferation (152). During the second phase (equilibrium), heterogeneity, and genetic instability progress in tumor cells that survived the elimination phase, thus enabling tumor cells to resist the immune response (153). In the last phase (escape), the tumor cells themselves as well as the tolerogenic immune cells produce large quantities of IDO1 (154), which results in immune tolerance described above (155, 156).
Due to these findings, inhibition of IDO1 as a therapeutic approach in cancer treatment has gained increasing attention in immuno-oncology. A recent study found that limitation of programmed cell death protein 1 (PD-1) inhibition might be due to an immunosuppressive tumor microenvironment based on IDO1 activation within macrophages (157). This suggests that IDO inhibition can be a potential therapeutic target in cancer patients, specifically in those who do not respond to immune checkpoint inhibitors. By now, clinical trials testing IDO1 inhibitors in combination with other chemotherapeutic or immunotherapeutic agents seem more promising than administration of IDO1 inhibitors alone. So far, five IDO1 inhibitors were studied as potential therapeutic options in cancer patients: indoximod [IDO pathway modulator; 1-methyl-D-tryptophan (1-MT)], epacadostat (selective IDO1 inhibitor; INCB024360), navoximod (GDC-0919), BMS-986205, and IDO1-targeting vaccines. All these IDO1 inhibitors were shown to be safe and well-tolerated (158–161). Epacadostat is the clinically most advanced IDO1 inhibitor and has been shown to inhibit tumor growth in mice models (162).
In human patients, epacadostat monotherapy was not effective (163, 164), while combined administration with PD-1 or cytotoxin T-lymphocyte-associated protein 4 (CTLA-4) inhibitors showed promising clinical activity in phase I/II studies (165–168). Unfortunately, a recent trial with combined administration of epacadostat with pembrolizumab found no superiority over pembrolizumab alone (169). Despite this setback, several ongoing trials investigate the effect of other (also structurally new) IDO1 inhibitors in combination with different immunotherapies (162).
Until now, IDO2 has been investigated far less than IDO1. Although IDO2 is expressed by cancer cells, it does not contribute to the accumulation of Trp metabolites to the same extent as IDO1 (170, 171). However, it was recently implicated that IDO2 affects B cell-mediated autoimmunity (172), and also contributes to carcinogenesis in models of pancreatic cancers (173). Interestingly, IDO2-deficiency was predictive for disease-free survival in patients receiving adjuvant radiotherapy (173).
Recent studies revealed that TDO may also be involved in tumor immune-escape. It was demonstrated that TDO is expressed in various tumors including glial tumors (174), breast cancers (175), lung cancers (59), colorectal carcinomas (176), melanomas, bladder carcinomas, and hepatocellular carcinomas (177). In glial tumors, TDO activity suppressed the anti-tumor immune responses via increased Kyn production (174). TDO was shown to be a promising therapeutic target to improve immune response to cancer cells (178). A recent study by Schramme et al. demonstrated that TDO inhibition increases the antitumor efficacy of immune checkpoint inhibitors (179).
Also, KMO activity may be involved in tumor immune tolerance. Recent studies have shown that its overexpression is related to rapid cancer progression and a poor prognosis (49, 180). Similar to inflammatory-induced IDO1 expression, KMO expression is induced by inflammatory stimuli (181, 182). Interestingly enough, the non-steroidal anti-inflammatory drug diclofenac is capable of binding human KMO, thereby inhibiting its activity (183). Since there is evidence that diclofenac also exerts anti-cancer effects (184), a possible explanation might be its interaction with Trp metabolism. Diclofenac inhibits COX-2 related IDO1 expression and KMO expression, thus reducing the accumulation of Trp catabolites.
Cancer related fatigue (CRF) is a complex multi-dimensional phenomenon that affects physical, cognitive and emotional activity, and behavior (185). It is associated with the cancer and its comorbidities themselves and often deteriorates during treatment (186). Actually, persisting fatigue limits the adherence of patients to cancer therapy (187). Chronic inflammation is proposed to be a leading cause of CRF. Higher inflammatory markers including IL-6, TNF-α, CRP, and neopterin were shown to correlate with fatigue in cancer patients prior to treatment, during treatment and also after treatment (62, 188–190).
Patients with lung cancer and moderate or severe fatigue are presented with lower Trp and hemoglobin concentrations, but with higher inflammatory markers (62). They furthermore assessed their QoL worse, and decreased QoL was associated with higher inflammatory markers and lower Trp concentrations. These results in 50 patients with lung cancer are well in line with earlier data showing significant correlations between fatigue/decreased QoL and immune-mediated Trp degradation in patients with different malignant diseases (85) as well as in patients with HIV-infection (191). Interestingly, correlations between inflammatory markers and decreased QoL were only seen in patients without antidepressant therapy in both HIV-infected and lung cancer patients. Also, in patients with colorectal cancer increased neopterin and decreased Trp levels correlated significantly with a decreased survival; QoL was worse in patients with low Trp (192).
A recent study in patients with solid tumors excluded patients with known depression or antidepressant treatment or established infection (90). Again, an association between immune activation and the QoL of patients as well as their depression susceptibility became evident. Fatigue was present in a high percentage of patients and was significantly associated with a decreased QoL, with decreased Trp and hemoglobin values (90). As low Trp or increased Kyn/Trp concentrations were associated with fatigue and decreased QoL, respectively, in several studies, this data indicates that immune activation and immune-mediated Trp degradation might contribute to the development of fatigue. Also, Kim and co-workers suggested a key role of inflammation-induced IDO-activation in CRF (193).
It is of importance that treatment with corticosteroids or anti-inflammatory drugs like celecoxib reduces fatigue in patients with advanced cancer (194, 195), suggesting that anti-inflammatory therapy improves fatigue by interfering with immune activation. A causal relationship between fatigue and immune activation has also been proposed in patients with other autoimmune diseases and infection (196) and treatment with TNF-α antagonists significantly reduces fatigue in patients with rheumatoid arthritis and psoriasis (197, 198).
Fatigue is one of the main symptoms of depression, which is another common comorbidity in subjects suffering from malignancies, affecting ~20% of the patients (99–101). Depression is probably not only due to emotional distress but also due to immunological mechanisms, which might negatively affect the QoL and increase all-cause mortality (199–201). Enhanced Trp breakdown as a consequence of immune activation has been proposed to play a crucial role in the development of depression in cancer patients (202–204).
Recently, correlations between inflammation markers (neopterin and CRP) and depression scores in a population of patients with solid tumors were reported, and particularly in male patients, lower Trp levels were associated with higher depression scores and stronger fatigue (90).
This clinical data fit well with results from animal experiments: Depressive-like behavior related to immune activation was demonstrated to be associated with an upregulation of IDO1 (205–207) as well as KMO (208–210). Peripheral administration of lipopolysaccharide activated IDO, resulting in a distinct depressive-like behavioral syndrome (205). Interestingly, IDO inhibition prevented the development of depressive-like behavior (211), while Kyn administration dose dependently induced depressive-like behavior. Also the anti-inflammatory cytokine IL-10 was able to normalize IDO1 expression, thus relieving depressive-like behavior in mice (212).
Depression is also related to enhanced Trp breakdown and immune activation in patients with HIV-infection (191, 213), as well as in patients receiving immunotherapy [e.g., IL-2 or INF-α; (77, 214)].
Immune activation probably affects the development of CRF and depression also by other mechanisms: Pro-inflammatory cytokines, for one thing, directly affect basal ganglia and dopamine function and, for another, activate sensory nerves. This results in production of pro-inflammatory cytokines and prostaglandins by microglia in the CNS, which then affect the functionality of neurons, thereby contributing to fatigue (215). Immune activation furthermore influences the biosynthesis of the catecholamines dopamine, epinephrine and norepinephrine and the neurotransmitter serotonin (216).
There are several pathophysiological mechanisms, which might explain how Trp metabolites cause CRF or neurobehavioral symptoms related to CRF such as depression.
Trp is a crucial amino acid in brain homeostasis and a precursor for serotonin and melatonin synthesis. It can cross the blood-brain barrier; therefore, reduced Trp availability may contribute to serotonin dysregulation and neurobehavioral manifestations (217, 218). However, also the accumulation of downstream metabolites of the Kyn pathway is suggested to trigger neurobehavioral symptoms (204, 205).
QUIN, which is primarily produced by monocytes/macrophages and microglia, generates free radicals, causes structural changes, and is a selective agonist at the glutamate receptor sensitive to N-methyl-D-aspartate (NMDA receptor) (219). Its accumulation results in excitotoxicity, neuronal cell death and disturbs glutamatergic transmission (220). QUIN cannot cross the blood brain barrier, which is why only QUIN synthesized by microglia or monocytes/macrophages migrated to the CNS influences neuroimmunology (221). On the contrary, KYNA is considered as a neuroprotective Trp metabolite, because it acts as antagonist at the NMDA and other glutamate receptors (222). Previous studies have demonstrated that KYNA can protect against QUIN related neuronal damage (223). This balance between neurotoxic and neuroprotective effects is expressed by the QUIN/KYNA ratio and related to the grade of pathway activity, but also immune activation (224). It was shown that depressed patients have a higher QUIN/KYNA ratio compared to healthy controls, thus moving the balance toward the neurodegenerative effects (225). The imbalance of neurotoxic and neuroprotective Trp metabolites is suggested to play a major role in the development of neuropsychiatric symptoms including CRF and depression (226). 3-HK also exerts neurotoxic effects by causing lipid peroxidation (227).
Although immune system activation frequently coincides with fatigue or depression in cancer patients, it has to be kept in mind, that fatigue or depression also can develop isolatedly in patients with other predisposing conditions (like anxiety or little social support). Probably the development of neuropsychiatric disturbances and depression is alleviated in the presence of an activated immune system and accelerated Trp breakdown, but it must not necessarily lead to depressed mood. Maybe the handling of bad news is impaired if Trp and thus serotonin availability is low.
Additionally, also other factors, like psychosocial aspects including demographical factors (age, gender, culture/ethnicity and social support), behavior/well-being (composed of stress/distress—including spiritual, anxiety, sleep disturbance, coping style, and pain) but also functional status (performance status, physical activity level, physical functioning, and productivity/work) contribute to the development, severity, and duration of CRF and depression. Moreover, an imbalance in the autonomic nervous system, disturbances in the hypothalamic-pituitary-adrenal axis and circadian rhythm as well as hypoxia or anemia are key players in the pathophysiology of CRF and depression (228, 229). These factors might in fact enforce vicious circles, such as e.g., psychosocial stress triggers oxidative stress and inflammation, and thus tumor progression (201).
Experiments in mice demonstrated that the IDO pathway modulator indoximod inhibits depressive-like behavior (consecutive to bacterial infection) without altering the infectious immune response (211, 230). Moreover, the specific IDO1 inhibitor epacadostat was shown to reverse chronic social defeat in mice (231). Another interesting compound, which might target IDO, is the antibiotic minocycline, which was demonstrated to reduce IDO activation and thus prevent depressive-like behavior in animal studies (232–234). Minocycline was also able to decrease IDO expression and the formation of pro-inflammatory cytokines in LPS-treated monocytic human microglial cells (235–237), suggesting that IDO inhibition might be responsible for the anti-depressive effects of minocycline. Also, in humans a large and statistically significant antidepressant effect of minocycline has been observed when comparing to placebo [see review and meta-analysis by Rosenblat and McIntyre (238)]. Due to the good tolerability, future larger RCTs investigating the potential of minocycline (238), but also of other anti-inflammatory treatments (239) are considered. Contrary to these findings, a recent study with mice showed no improvement of cancer-related behavioral symptoms when inhibiting IDO1 (either by an unspecific or a specific IDO inhibitor). Mice treated with 1-MT even had slightly more treatment-associated burrowing deficits. Genetic deletion of IDO on the other hand had no effect on the behavior of mice, but was associated with a worse tumor outcome (240). In consideration of these conflicting data, more studies investigating effects of IDO inhibition in cancer are needed. Clinical trials targeting TDO revealed antidepressant effects as well as amelioration of neurodegeneration following TDO inhibition, and seem to be a promising therapeutic target in cancer patients, especially with neurobehavioral symptoms (241, 242).
Inhibition of KMO also seems to be a possible therapeutic approach in the treatment of fatigue and depression by shifting Kyn metabolism toward the enhanced production of neuroprotective KYNA while decreasing production of neurotoxic QUIN. A recent mice trial revealed that KMO gene deletion substantially reduces 3-HK and QUIN concentrations while elevating KYNA concentrations (243). It was further shown to ameliorate neurodegeneration in patients with Alzheimer's and Parkinson's diseases (242). Therefore, KMO inhibition may be a promising therapeutic target in inflammation-related fatigue or depression by reducing generation of the neurotoxic Trp metabolites 3-HK and QUIN.
Another recent study showed decreased IDO1 and KMO expression in the murine brain as well as decreased IDO1 and IDO2 expression in human peripheral blood mononuclear cells as a consequence of antidepressant treatment (244, 245). This, in turn, demonstrates that reduction of psychosocial stress can also reduce inflammation-related factors.
Monoaminergic antidepressants and also omega-3 fatty acids were demonstrated to reduce neurotoxic effects related to Trp breakdown (246). Omega-3 fatty acids contribute to the beneficial effects of the Mediterranean diet, which is regarded as anti-inflammatory diet (247). High adherence to this diet is linked to a lower risk of developing cancer and to a reduced cancer mortality in observational studies (248). A “Western” diet rich in refined sugars and long chain fatty acids and with low fiber content on the other hand enforces a type 1 pro-inflammatory state (249). Mouse experiments furthermore showed that Western diet exposure exacerbated hippocampal and hypothalamic proinflammatory cytokine expression and brain IDO activation after immune stimulation with LPS (250). Inflammation-induced Trp degradation in humans might then further intensify subdued psychosocial factors such as mood, negative thoughts and lack of energy or simply make patients more susceptible to them.
In fact, diet and the gut microbiome may influence inflammation and Trp metabolism by several ways (251): Microbiota metabolize phytochemicals (e.g., in vegetables) to indoles, which activate AhR as ligands, while other microbial-derived metabolites such as the short chain fatty acids butyrate, propionate, and acetate importantly mediate the crosstalk between host-microbiota and thereby have immune modulating effects (251). Actually Trp metabolic pathways are regarded as key biochemical pathways influencing the microbiota-neural-immune axis by translating information on the nutritional, inflammatory, microbial, and emotional state of the organism to the immune system (252–254) and by modulating intestinal immune response (251).
A recent review by Weber et al. proposed that preclinical and several clinical studies argued for the use of a ketogenic diet (KD) in combination with standard therapies in patients with cancer (255): KD had the potential to enhance the antitumor effects of classic chemo- and radiotherapy and to increase the QoL of patients (255). However, the heterogeneity between studies investigating these effects and low adherence to diet limit the current evidence (256). Interestingly, KD was shown to positively influence the Kyn pathway in rats (257). Increased β-hydroxybutyrate concentrations and an increased production of the neuroprotective KYNA were found in rat brain structures as a consequence of KD (258, 259). Also, a recent study in children revealed that Kyn levels significantly decreased and KYNA levels significantly increased 3 months after starting a KD (260).
Significant differences regarding Trp metabolism were reported between a low-glycemic load dietary pattern (characterized by whole grains, legumes, fruits, and vegetables) and a diet high in refined grains and added sugars on inflammation and energy metabolism pathways (261). In line with results of this study, a Mediterranean diet and other plant-based diets have been proposed to reduce fatigue in cancer survivors (262).
As cancer cells are very vulnerable to nutrient deprivation (especially glucose), fasting or fasting-mimicking diets (FMDs) might be another effective strategy to generate environments that can reduce the capability of cancer cells to adapt and survive and thus improve the effects of cancer therapies (263). Further studies investigating the effects of FMDs on Trp catabolism in the tumor microenvironment might therefore provide interesting new insights for future treatment approaches.
Besides, treatment with probiotics might be beneficial for cancer patients: In colorectal cancer survivors, probiotics (Lactobacillus acidophilus and rhamnosus) improved CRF, irritable bowel syndromes and QoL significantly in a double-blind placebo-controlled study (264); furthermore, probiotics and also melatonin supplementation appear to alleviate side effects of radiation therapy (265). Probiotic supplementation with Lactobacillus plantarum in combination with SSRI treatment improved cognitive performance and decreased Kyn concentrations in patients with major depression [compared to SSRI treatment alone, (266)]. Supplementation with a multispecies probiotic had a beneficial effect on Trp metabolism in trained athletes (267) and influenced Trp degradation and gut bacteria composition in patients with Alzheimer's disease (268). Additionally, highly adaptive lactobacilli where shown to produce the AhR ligand indole-3-aldehyde, which enabled IL-22 transcription for the fine tuning of host mucosal reactivity (269). Conclusively, these studies indicate that beneficial effects of probiotics on fatigue or depression might be due to alterations of Trp metabolism or anti-inflammatory effects [see review by (270)]. However, evidence is limited due to the heterogeneity of clinical trials. Therefore, further well-designed longitudinal placebo-controlled studies are desperately needed (271, 272).
Also, a recent review of clinical trials that assessed nutritional interventions for preventing and treating CRF suggests that supplementation with probiotics but also ginseng, or ginger may improve cancer survivors' energy levels and that nutritional interventions, alone or in combination with other interventions should be considered as therapy for fatigue in cancer survivors. Nevertheless, there is lacking evidence to determine the optimal diet to improve CRF in cancer patients (262, 273). Furthermore, also physical activity, psychosocial, mind-body, and pharmacological treatments have been proven to be effective (187).
Physical exercise also affects Trp metabolism and thereby might improve fatigue and depression. As this subject has been discussed elsewhere recently (274, 275), it will be discussed only briefly hereafter. Physical activity increases Trp availability in the brain, which results in an increased 5-HT synthesis and anti-depressant effects (276). Increased muscle use of branched-chain amino acids (BCAAs) favors the passing of Trp through the blood-brain barrier (277). In addition, endurance exercise increases concentrations of circulating free fatty acids, which displaces Trp from albumin, thus increasing free Trp concentrations (278). Additionally, physical activity increases the expression of kynurenine aminotransferases, which enhance the conversion of Kyn into KYNA (unable to cross the blood-brain barrier), thus protecting the brain from stress-induced changes (279). Interestingly, intense physical exercise induces the formation of several pro-inflammatory cytokines (280), which in turn activate IDO1 and Trp breakdown.
Another common comorbidity in cancer causing fatigue is anemia (95, 281). Anemic cancer patients have a worse QoL, an adverse outcome as well as a reduced rate of local tumor control compared to non-anemic cancer patients (282, 283).
Anemia is the most common “hematological complication,” found in ~40–64% of patients with malignant diseases (94) and is mostly due to anemia of chronic disease (ACD) (284). ACD is caused by enhanced formation of pro-inflammatory cytokines, which can on the one hand directly inhibit erythropoiesis and on the other hand restrict the availability of iron for erythropoiesis. The latter is caused by an increased uptake and retention of iron within the cells of the reticuloendothelial system together with a suppression of iron absorption in the duodenum. The master regulator of iron homeostasis, hepcidin, has a decisive role in these processes. Similarly to Trp breakdown, this is initially a protective mechanism of the immune system to restrict available iron from microbes or tumor cells (285, 286).
IFN-γ, one of the main cytokines of Th1 type immune response, activates IDO and neopterin formation in hematopoietic stem cells and also exerts an influence on the proliferation of various stem cell populations (287). The intravenous injection of neopterin into mice resulted in a prolonged decrease in the number of erythroid progenitor cells and increased the number of myeloid progenitor cells (CFU-GMs) by activating stromal cells (288).
Trp metabolites like Kyn, on the other hand, increase hepcidin expression and inhibit erythropoietin (EPO) production by activating AhR (289). AhR competes with hypoxia-inducible factor 2α (HIF-2α), the key regulator of EPO production, for binding with HIF-1β (289, 290). Well in line with this finding, Kyn/Trp and neopterin were shown earlier to be associated inversely with hemoglobin concentrations and positively with hepcidin concentrations in patients with HIV-infection before antiretroviral therapy (287). Antiretroviral treatment slowed down immune-mediated Trp catabolism and improved iron metabolism and anemia (287).
Interestingly, in patients with different malignant diseases, increased Kyn/Trp and neopterin concentrations also coincided with lower hemoglobin values (85). Also, recent data confirms that anemic cancer patients present with higher inflammatory markers and a higher Kyn/Trp than non-anemic individuals (90). The same is also true for patients with anemia due to inflammation (291) and for HIV-infected patients (191).
Also, QUIN was shown to inhibit EPO production (292) by stimulating the production of nitric oxide (NO) (293) and inducing HIF-1α degradation (294).
In patients with myelodysplastic syndromes, a fundamental role for Trp metabolized along the serotonin pathway in normal erythropoiesis and in the physiopathology of MDS-related anemia was demonstrated recently: Decreased blood serotonin levels were related with impaired erythroid proliferating capacities, and treatment with fluoxetine, a common antidepressant, was effective in increasing serotonin levels and the number of erythroid progenitors (295).
Low serotonin concentrations are also associated with the development of depression. Vulser et al. actually showed a considerable association between anemia and depression in otherwise healthy adults (296). Increased Trp degradation might therefore be a connection between anemia and depression.
These findings show that impaired Trp availability but also accumulation of Trp metabolites, may affect erythropoiesis. In cancer patients, tumor cells produce TDO and IDO1, and both are equally capable of producing Kyn (174). However, they may only contribute to local Trp degradation and do not influence systemic Trp breakdown. On the other hand, IDO1 activity is also stimulated by the activated immune system and thereby contributes to systemic Trp catabolism. Therefore, inflammation-induced IDO1 activation and consecutive Trp breakdown might influence erythropoiesis. The most common symptom of anemia is fatigue, which is why both ACD and inflammation-induced Trp breakdown may be major contributors to overall-fatigue in patients with malignant diseases.
Inflammation-induced Trp breakdown in cancer patients is considered to play a key role in the pathophysiology of tumor immune tolerance. Accumulation of Trp metabolites as well as impaired Trp availability suppress the tumor immune response and may also greatly contribute to the development of comorbidities such as fatigue, depression, or anemia, which are all common in patients with malignancies. Although anemia is primarily caused by the enhanced immune response itself, inflammatory-induced Trp degradation may also be involved strongly. Studies have shown that inhibition of Trp breakdown might be a promising therapeutic option in cancer patients to counteract the immunosuppressive tumor microenvironment. Especially cancer patients with no response to immune checkpoint inhibitors might benefit from an additional IDO1 inhibition. Moreover, there is evidence that inhibition of IDO1, TDO, and KMO or other interventions targeting Trp metabolism (like diet or probiotics) may further improve neurobehavioral manifestations including CRF or depression. Further studies investigating the effects of IDO1, TDO, or KMO inhibition on tumor immune response should also take the impact on neurobehavioral manifestations into consideration.
LL and KK wrote the manuscript. PK, EE, WW, DF, and GW critically read and revised the paper. All authors listed approved the submitted version for publication.
This study received funding from Medizinische Universität Innsbruck.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We hereby thank M.Sc. Simon Geisler and PD Johanna Gostner for technical support and interesting discussions.
1. Rieder J, Lirk P, Hoffmann G. Neopterin as a potential modulator of tumor cell growth and proliferation. Med Hypotheses. (2003) 60:531–4. doi: 10.1016/S0306-9877(03)00002-1
2. Pichler R, Fritz J, Heidegger I, Steiner E, Culig Z, Klocker H, et al. Predictive and prognostic role of serum neopterin and tryptophan breakdown in prostate cancer. Cancer Sci. (2017) 108:663–70. doi: 10.1111/cas.13171
3. Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. (2015) 21:687–92. doi: 10.1158/1078-0432.CCR-14-1860
4. Opitz CA, Somarribas Patterson LF, Mohapatra SR, Dewi DL, Sadik A, Platten M, et al. The therapeutic potential of targeting tryptophan catabolism in cancer. Br J Cancer. (2020) 122:30–44. doi: 10.1038/s41416-019-0664-6
5. Folgiero V, Goffredo BM, Filippini P, Masetti R, Bonanno G, Caruso R, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity in leukemia blasts correlates with poor outcome in childhood acute myeloid leukemia. Oncotarget. (2014) 5:2052–64. doi: 10.18632/oncotarget.1504
6. Mabuchi R, Hara T, Matsumoto T, Shibata Y, Nakamura N, Nakamura H, et al. High serum concentration of L-kynurenine predicts unfavorable outcomes in patients with acute myeloid leukemia. Leuk Lymphoma. (2016) 57:92–8. doi: 10.3109/10428194.2015.1041388
7. Fukuno K, Hara T, Tsurumi H, Shibata Y, Mabuchi R, Nakamura N, et al. Expression of indoleamine 2,3-dioxygenase in leukemic cells indicates an unfavorable prognosis in acute myeloid leukemia patients with intermediate-risk cytogenetics. Leuk Lymphoma. (2015) 56:1398–405. doi: 10.3109/10428194.2014.953150
8. Corm S, Berthon C, Imbenotte M, Biggio V, Lhermitte M, Dupont C, et al. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients' sera by HPLC and is inducible by IFN-gamma. Leuk Res. (2009) 33:490–4. doi: 10.1016/j.leukres.2008.06.014
9. Chamuleau ME, van de Loosdrecht AA, Hess CJ, Janssen JJ, Zevenbergen A, Delwel R, et al. High INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica. (2008) 93:1894–8. doi: 10.3324/haematol.13112
10. Tang XQ, Zhao ZG, Wang HX, Li QB, Lu J, Zou P. [Indoleamine 2, 3-dioxygenase activity in acute myeloid leukemia cells contributing to tumor immune escape]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2006) 14:539–42.
11. Wei L, Zhu S, Li M, Li F, Wei F, Liu J, et al. High indoleamine 2,3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer. Front Immunol. (2018) 9:724. doi: 10.3389/fimmu.2018.00724
12. Eniu DT, Romanciuc F, Moraru C, Goidescu I, Eniu D, Staicu A, et al. The decrease of some serum free amino acids can predict breast cancer diagnosis and progression. Scandinav J Clin Lab Investig. (2019) 79:17–24. doi: 10.1080/00365513.2018.1542541
13. Heng B, Lim CK, Lovejoy DB, Bessede A, Gluch L, Guillemin GJ. Understanding the role of the kynurenine pathway in human breast cancer immunobiology. Oncotarget. (2016) 7:6506–20. doi: 10.18632/oncotarget.6467
14. Lyon DE, Walter JM, Starkweather AR, Schubert CM, McCain NL. Tryptophan degradation in women with breast cancer: a pilot study. BMC Research Notes. (2011) 4:156. doi: 10.1186/1756-0500-4-156
15. Isla Larrain MT, Rabassa ME, Lacunza E, Barbera A, Cretón A, Segal-Eiras A, et al. IDO is highly expressed in breast cancer and breast cancer-derived circulating microvesicles and associated to aggressive types of tumors by in silico analysis. Tumor Biol. (2014) 35:6511–9. doi: 10.1007/s13277-014-1859-3
16. Girgin G, Sahin TT, Fuchs D, Kasuya H, Yuksel O, Tekin E, et al. Immune system modulation in patients with malignant and benign breast disorders: tryptophan degradation and serum neopterin. Int J Biol Markers. (2009) 24:265–71. doi: 10.1177/172460080902400408
17. Chen JY, Li CF, Kuo CC, Tsai KK, Hou MF, Hung WC. Cancer/stroma interplay via cyclooxygenase-2 and indoleamine 2,3-dioxygenase promotes breast cancer progression. Breast Cancer Res. (2014) 16:410. doi: 10.1186/s13058-014-0410-1
18. Soliman H, Rawal B, Fulp J, Lee JH, Lopez A, Bui MM, et al. Analysis of indoleamine 2-3 dioxygenase (IDO1) expression in breast cancer tissue by immunohistochemistry. Cancer Immunol Immunother. (2013) 62:829–37. doi: 10.1007/s00262-013-1393-y
19. Jacquemier J, Bertucci F, Finetti P, Esterni B, Charafe-Jauffret E, Thibult ML, et al. High expression of indoleamine 2,3-dioxygenase in the tumour is associated with medullary features and favourable outcome in basal-like breast carcinoma. Int J Cancer. (2012) 130:96–104. doi: 10.1002/ijc.25979
20. Yu J, Sun J, Wang SE, Li H, Cao S, Cong Y, et al. Upregulated expression of indoleamine 2, 3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Clin Dev Immunol. (2011) 2011:469135. doi: 10.1155/2011/469135
21. Ferdinande L, Decaestecker C, Verset L, Mathieu A, Moles Lopez X, Negulescu AM, et al. Clinicopathological significance of indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. Br J Cancer. (2012) 106:141–7. doi: 10.1038/bjc.2011.513
22. Engin A, Gonul II, Engin AB, Karamercan A, Sepici Dincel A, Dursun A. Relationship between indoleamine 2,3-dioxygenase activity and lymphatic invasion propensity of colorectal carcinoma. World J Gastroenterol. (2016) 22:3592–601. doi: 10.3748/wjg.v22.i13.3592
23. Gao YF, Peng RQ, Li J, Ding Y, Zhang X, Wu XJ, et al. The paradoxical patterns of expression of indoleamine 2,3-dioxygenase in colon cancer. J Transl Med. (2009) 7:71. doi: 10.1186/1479-5876-7-71
24. Huang A, Fuchs D, Widner B, Glover C, Henderson DC, Allen-Mersh TG. Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. Br J Cancer. (2002) 86:1691–6. doi: 10.1038/sj.bjc.6600336
25. Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. (2006) 12:1144–51. doi: 10.1158/1078-0432.CCR-05-1966
26. Jia Y, Wang H, Wang Y, Wang T, Wang M, Ma M, et al. Low expression of Bin1, along with high expression of IDO in tumor tissue and draining lymph nodes, are predictors of poor prognosis for esophageal squamous cell cancer patients. Int J Cancer. (2015) 137:1095–106. doi: 10.1002/ijc.29481
27. Iwagaki H, Hizuta A, Tanaka N, Orita K. Decreased serum tryptophan in patients with cancer cachexia correlates with increased serum neopterin. Immunol Invest. (1995) 24:467–78. doi: 10.3109/08820139509066843
28. Laimer K, Troester B, Kloss F, Schafer G, Obrist P, Perathoner A, et al. Expression and prognostic impact of indoleamine 2,3-dioxygenase in oral squamous cell carcinomas. Oral Oncol. (2011) 47:352–7. doi: 10.1016/j.oraloncology.2011.03.007
29. Zhang G, Liu WL, Zhang L, Wang JY, Kuang MH, Liu P, et al. Involvement of indoleamine 2,3-dioxygenase in impairing tumor-infiltrating CD8 T-cell functions in esophageal squamous cell carcinoma. Clin Dev Immunol. (2011) 2011:384726. doi: 10.1155/2011/384726
30. Adams S, Teo C, McDonald KL, Zinger A, Bustamante S, Lim CK, et al. Involvement of the kynurenine pathway in human glioma pathophysiology. PLoS ONE. (2014) 9:e112945. doi: 10.1371/journal.pone.0112945
31. Zhai L, Dey M, Lauing KL, Gritsina G, Kaur R, Lukas RV, et al. The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy. J Clin Neurosci. (2015) 22:1964–8. doi: 10.1016/j.jocn.2015.06.018
32. Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery. (2013) 72:1031–8; discussion: 1038–9. doi: 10.1227/NEU.0b013e31828cf945
33. Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. (2012) 18:6110–21. doi: 10.1158/1078-0432.CCR-12-2130
34. Heeren AM, van Dijk I, Berry D, Khelil M, Ferns D, Kole J, et al. Indoleamine 2,3-dioxygenase expression pattern in the tumor microenvironment predicts clinical outcome in early stage cervical cancer. Front Immunol. (2018) 9:1598. doi: 10.3389/fimmu.2018.01598
35. Gostner JM, Obermayr E, Braicu IE, Concin N, Mahner S, Vanderstichele A, et al. Immunobiochemical pathways of neopterin formation and tryptophan breakdown via indoleamine 2,3-dioxygenase correlate with circulating tumor cells in ovarian cancer patients- A study of the OVCAD consortium. Gynecol Oncol. (2018) 149:371–80. doi: 10.1016/j.ygyno.2018.02.020
36. de Jong RA, Kema IP, Boerma A, Boezen HM, van der Want JJ, Gooden MJ, et al. Prognostic role of indoleamine 2,3-dioxygenase in endometrial carcinoma. Gynecol Oncol. (2012) 126:474–80. doi: 10.1016/j.ygyno.2012.05.034
37. Ferns DM, Kema IP, Buist MR, Nijman HW, Kenter GG, Jordanova ES. Indoleamine-2,3-dioxygenase (IDO) metabolic activity is detrimental for cervical cancer patient survival. Oncoimmunology. (2015) 4:e981457. doi: 10.4161/2162402X.2014.981457
38. Inaba T, Ino K, Kajiyama H, Shibata K, Yamamoto E, Kondo S, et al. Indoleamine 2,3-dioxygenase expression predicts impaired survival of invasive cervical cancer patients treated with radical hysterectomy. Gynecol Oncol. (2010) 117:423–8. doi: 10.1016/j.ygyno.2010.02.028
39. Sperner-Unterweger B, Neurauter G, Klieber M, Kurz K, Meraner V, Zeimet A, et al. Enhanced tryptophan degradation in patients with ovarian carcinoma correlates with several serum soluble immune activation markers. Immunobiology. (2011) 216:296–301. doi: 10.1016/j.imbio.2010.07.010
40. Inaba T, Ino K, Kajiyama H, Yamamoto E, Shibata K, Nawa A, et al. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol Oncol. (2009) 115:185–92. doi: 10.1016/j.ygyno.2009.07.015
41. de Jong RA, Nijman HW, Boezen HM, Volmer M, Ten Hoor KA, Krijnen J, et al. Serum tryptophan and kynurenine concentrations as parameters for indoleamine 2,3-dioxygenase activity in patients with endometrial, ovarian, and vulvar cancer. Int J Gynecol Cancer. (2011) 21:1320–7. doi: 10.1097/IGC.0b013e31822017fb
42. Ino K, Yamamoto E, Shibata K, Kajiyama H, Yoshida N, Terauchi M, et al. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin Cancer Res. (2008) 14:2310–7. doi: 10.1158/1078-0432.CCR-07-4144
43. Fotopoulou C, Sehouli J, Pschowski R, VON Haehling S, Domanska G, Braicu EI, et al. Systemic changes of tryptophan catabolites via the indoleamine-2,3-dioxygenase pathway in primary cervical cancer. Anticancer Res. (2011) 31:2629–35.
44. Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. (2005) 11:6030–9. doi: 10.1158/1078-0432.CCR-04-2671
45. Takao M, Okamoto A, Nikaido T, Urashima M, Takakura S, Saito M, et al. Increased synthesis of indoleamine-2,3-dioxygenase protein is positively associated with impaired survival in patients with serous-type, but not with other types of, ovarian cancer. Oncol Rep. (2007) 17:1333–9. doi: 10.3892/or.17.6.1333
46. Schroecksnadel K, Winkler C, Fuith LC, Fuchs D. Tryptophan degradation in patients with gynecological cancer correlates with immune activation. Cancer Lett. (2005) 223:323–9. doi: 10.1016/j.canlet.2004.10.033
47. Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K, et al. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer. (2006) 95:1555–61. doi: 10.1038/sj.bjc.6603477
48. Li S, Han X, Lyu N, Xie Q, Deng H, Mu L, et al. Mechanism and prognostic value of indoleamine 2,3-dioxygenase 1 expressed in hepatocellular carcinoma. Cancer Sci. (2018) 109:3726–36. doi: 10.1111/cas.13811
49. Jin H, Zhang Y, You H, Tao X, Wang C, Jin G, et al. Prognostic significance of kynurenine 3-monooxygenase and effects on proliferation, migration, and invasion of human hepatocellular carcinoma. Sci Rep. (2015) 5:10466. doi: 10.1038/srep10466
50. Pan K, Wang H, Chen MS, Zhang HK, Weng DS, Zhou J, et al. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol. (2008) 134:1247–53. doi: 10.1007/s00432-008-0395-1
51. Ishio T, Goto S, Tahara K, Tone S, Kawano K, Kitano S. Immunoactivative role of indoleamine 2,3-dioxygenase in human hepatocellular carcinoma. J Gastroenterol Hepatol. (2004) 19:319–26. doi: 10.1111/j.1440-1746.2003.03259.x
52. Trott JF, Kim J, Abu Aboud O, Wettersten H, Stewart B, Berryhill G, et al. Inhibiting tryptophan metabolism enhances interferon therapy in kidney cancer. Oncotarget. (2016) 7:66540–57. doi: 10.18632/oncotarget.11658
53. Lucarelli G, Rutigliano M, Ferro M, Giglio A, Intini A, Triggiano F, et al. Activation of the kynurenine pathway predicts poor outcome in patients with clear cell renal cell carcinoma. Urol Oncol. (2017) 35:461.e15–461.e27. doi: 10.1016/j.urolonc.2017.02.011
54. Riesenberg R, Weiler C, Spring O, Eder M, Buchner A, Popp T, et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin Cancer Res. (2007) 13:6993–7002. doi: 10.1158/1078-0432.CCR-07-0942
55. Yuan F, Liu Y, Fu X, Chen J. Indoleamine-pyrrole 2,3-dioxygenase might be a prognostic biomarker for patients with renal cell carcinoma. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2012) 37:649–55. doi: 10.3969/j.issn.1672-7347.2012.07.001
56. Tang D, Yue L, Yao R, Zhou L, Yang Y, Lu L, et al. P53 prevent tumor invasion and metastasis by down-regulating IDO in lung cancer. Oncotarget. (2017) 8:54548–57. doi: 10.18632/oncotarget.17408
57. Chuang SC, Fanidi A, Ueland PM, Relton C, Midttun O, Vollset SE, et al. Circulating biomarkers of tryptophan and the kynurenine pathway and lung cancer risk. Cancer Epidemiol Biomarkers Prev. (2014) 23:461–8. doi: 10.1158/1055-9965.EPI-13-0770
58. Huang JY, Larose TL, Luu HN, Wang R, Fanidi A, Alcala K, et al. Circulating markers of cellular immune activation in prediagnostic blood sample and lung cancer risk in the Lung Cancer Cohort Consortium (LC3). Int J Cancer. (2019). doi: 10.1002/ijc.32555. [Epub ahead of print].
59. Hsu YL, Hung JY, Chiang SY, Jian SF, Wu CY, Lin YS, et al. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. (2016) 7:27584–98. doi: 10.18632/oncotarget.8488
60. Creelan BC, Antonia S, Bepler G, Garrett TJ, Simon GR, Soliman HH. Indoleamine 2,3-dioxygenase activity and clinical outcome following induction chemotherapy and concurrent chemoradiation in Stage III non-small cell lung cancer. Oncoimmunology. (2013) 2:e23428. doi: 10.4161/onci.23428
61. Karanikas V, Zamanakou M, Kerenidi T, Dahabreh J, Hevas A, Nakou M, et al. Indoleamine 2,3-dioxygenase (IDO) expression in lung cancer. Cancer Biol Ther. (2007) 6:1258–62. doi: 10.4161/cbt.6.8.4446
62. Kurz K, Fiegl M, Holzner B, Giesinger J, Pircher M, Weiss G, et al. Fatigue in patients with lung cancer is related with accelerated tryptophan breakdown. PLoS ONE. (2012) 7:e36956. doi: 10.1371/journal.pone.0036956
63. Astigiano S, Morandi B, Costa R, Mastracci L, D'Agostino A, Ratto GB, et al. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia. (2005) 7:390–6. doi: 10.1593/neo.04658
64. Engin AB, Ozkan Y, Fuchs D, Yardim-Akaydin S. Increased tryptophan degradation in patients with bronchus carcinoma. Eur J Cancer Care. (2010) 19:803–8. doi: 10.1111/j.1365-2354.2009.01122.x
65. Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, et al. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer. (2010) 67:361–5. doi: 10.1016/j.lungcan.2009.05.001
66. Nam SJ, Kim S, Paik JH, Kim TM, Heo DS, Kim CW, et al. An increase in indoleamine 2,3-dioxygenase-positive cells in the tumor microenvironment predicts favorable prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone. Leuk Lymphoma. (2016) 57:1956–60. doi: 10.3109/10428194.2015.1117610
67. Masaki A, Ishida T, Maeda Y, Suzuki S, Ito A, Takino H, et al. Prognostic significance of tryptophan catabolism in adult T-cell leukemia/lymphoma. Clin Cancer Res. (2015) 21:2830–9. doi: 10.1158/1078-0432.CCR-14-2275
68. Liu XQ, Lu K, Feng LL, Ding M, Gao JM, Ge XL, et al. Up-regulated expression of indoleamine 2,3-dioxygenase 1 in non-Hodgkin lymphoma correlates with increased regulatory T-cell infiltration. Leuk Lymphoma. (2014) 55:405–14. doi: 10.3109/10428194.2013.804917
69. Yoshikawa T, Hara T, Tsurumi H, Goto N, Hoshi M, Kitagawa J, et al. Serum concentration of L-kynurenine predicts the clinical outcome of patients with diffuse large B-cell lymphoma treated with R-CHOP. Eur J Haematol. (2010) 84:304–9. doi: 10.1111/j.1600-0609.2009.01393.x
70. Choe JY, Yun JY, Jeon YK, Kim SH, Park G, Huh JR, et al. Indoleamine 2,3-dioxygenase (IDO) is frequently expressed in stromal cells of Hodgkin lymphoma and is associated with adverse clinical features: a retrospective cohort study. BMC Cancer. (2014) 14:335. doi: 10.1186/1471-2407-14-335
71. Giusti RM, Maloney EM, Hanchard B, Morgan OS, Steinberg SM, Wachter H, et al. Differential patterns of serum biomarkers of immune activation in human T-cell lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis, and adult T-cell leukemia/lymphoma. Cancer Epidemiol Biomarkers Prev. (1996) 5:699–704.
72. Ninomiya S, Hara T, Tsurumi H, Hoshi M, Kanemura N, Goto N, et al. Indoleamine 2,3-dioxygenase in tumor tissue indicates prognosis in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol. (2011) 90:409–16. doi: 10.1007/s00277-010-1093-z
73. Hoshi M, Ito H, Fujigaki H, Takemura M, Takahashi T, Tomita E, et al. Indoleamine 2,3-dioxygenase is highly expressed in human adult T-cell leukemia/lymphoma and chemotherapy changes tryptophan catabolism in serum and reduced activity. Leuk Res. (2009) 33:39–45. doi: 10.1016/j.leukres.2008.05.023
74. Pelak MJ, Snietura M, Lange D, Nikiel B, Pecka KM. The prognostic significance of indoleamine-2,3-dioxygenase and the receptors for transforming growth factor beta and interferon gamma in metastatic lymph nodes in malignant melanoma. Pol J Pathol. (2015) 66:376–82. doi: 10.5114/pjp.2015.57249
75. Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology. (2007) 214:8–14. doi: 10.1159/000096906
76. Ryan M, Crow J, Kahmke R, Fisher SR, Su Z, Lee WT. FoxP3 and indoleamine 2,3-dioxygenase immunoreactivity in sentinel nodes from melanoma patients. Am J Otolaryngol. (2014) 35:689–94. doi: 10.1016/j.amjoto.2014.08.009
77. Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, et al. Interferon-alpha-induced changes in tryptophan metabolism: relationship to depression and paroxetine treatment. Biol Psychiatry. (2003) 54:906–14. doi: 10.1016/S0006-3223(03)00173-2
78. Speeckaert R, Vermaelen K, van Geel N, Autier P, Lambert J, Haspeslagh M, et al. Indoleamine 2,3-dioxygenase, a new prognostic marker in sentinel lymph nodes of melanoma patients. Eur J Cancer. (2012) 48:2004–11. doi: 10.1016/j.ejca.2011.09.007
79. Brody JR, Costantino CL, Berger AC, Sato T, Lisanti MP, Yeo CJ, et al. Expression of indoleamine 2,3-dioxygenase in metastatic malignant melanoma recruits regulatory T cells to avoid immune detection and affects survival. Cell Cycle. (2009) 8:1930–4. doi: 10.4161/cc.8.12.8745
80. Urakawa H, Nishida Y, Nakashima H, Shimoyama Y, Nakamura S, Ishiguro N. Prognostic value of indoleamine 2,3-dioxygenase expression in high grade osteosarcoma. Clin Exp Metast. (2009) 26:1005–12. doi: 10.1007/s10585-009-9290-7
81. Zhang T, Tan XL, Xu Y, Wang ZZ, Xiao CH, Liu R. Expression and prognostic value of indoleamine 2,3-dioxygenase in pancreatic cancer. Chin Med J. (2017) 130:710–6. doi: 10.4103/0366-6999.201613
82. Huang JY, Butler LM, Midttun O, Ulvik A, Wang R, Jin A, et al. A prospective evaluation of serum kynurenine metabolites and risk of pancreatic cancer. PLoS ONE. (2018) 13:e0196465. doi: 10.1371/journal.pone.0196465
83. Feder-Mengus C, Wyler S, Hudolin T, Ruszat R, Bubendorf L, Chiarugi A, et al. High expression of indoleamine 2,3-dioxygenase gene in prostate cancer. Eur J Cancer. (2008) 44:2266–75. doi: 10.1016/j.ejca.2008.05.023
84. Moretti S, Menicali E, Voce P, Morelli S, Cantarelli S, Sponziello M, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) is up-regulated in thyroid carcinoma and drives the development of an immunosuppressant tumor microenvironment. J Clin Endocrinol Metab. (2014) 99:E832–840. doi: 10.1210/jc.2013-3351
85. Schroecksnadel K, Fiegl M, Prassl K, Winkler C, Denz HA, Fuchs D. Diminished quality of life in patients with cancer correlates with tryptophan degradation. J Cancer Res Clin Oncol. (2007) 133:477–85. doi: 10.1007/s00432-007-0191-3
86. Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. (2019) 18:379–401. doi: 10.1038/s41573-019-0016-5
87. Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. (2005) 54:721–8. doi: 10.1007/s00262-004-0653-2
88. Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. (2002) 3:175–87. doi: 10.2174/1389200024605082
89. Denz H, Orth B, Weiss G, Herrmann R, Huber P, Wachter H, et al. Weight loss in patients with hematological neoplasias is associated with immune system stimulation. Clin Investig. (1993) 71:37–41. doi: 10.1007/BF00210961
90. Kink P, Egger E, Klaunzner M, Weiss G, Willenbacher W, Kasseroler M, et al. Tryptophan and phenylalanine metabolism and depression in patients with solid tumors. In: Pteridines, 38th International Winter-Workshop Clinical, Chemical and Biochemical Aspects of Pteridines and Related Topics Innsbruck, February 26th–March 1st, 2019. Innsbruck (2019).
91. Melichar B, Spisarova M, Bartouskova M, Krcmova LK, Javorska L, Studentova H. Neopterin as a biomarker of immune response in cancer patients. Ann Transl Med. (2017) 5:280. doi: 10.21037/atm.2017.06.29
92. Carlin JM, Ozaki Y, Byrne GI, Brown RR, Borden EC. Interferons and indoleamine 2,3-dioxygenase: role in antimicrobial and antitumor effects. Experientia. (1989) 45:535–41. doi: 10.1007/BF01990503
93. Timosenko E, Hadjinicolaou AV, Cerundolo V. Modulation of cancer-specific immune responses by amino acid degrading enzymes. Immunotherapy. (2017) 9:83–97. doi: 10.2217/imt-2016-0118
94. Gaspar BL, Sharma P, Das R. Anemia in malignancies: pathogenetic and diagnostic considerations. Hematology. (2015) 20:18–25. doi: 10.1179/1607845414Y.0000000161
95. Sobrero A, Puglisi F, Guglielmi A, Belvedere O, Aprile G, Ramello M, et al. Fatigue: a main component of anemia symptomatology. Semin Oncol. (2001) 28(2 Suppl. 8):15–8. doi: 10.1016/S0093-7754(01)90207-6
96. Stone P, Richards M, Hardy J. Fatigue in patients with cancer. Eur J Cancer. (1998) 34:1670–76. doi: 10.1016/S0959-8049(98)00167-1
97. Narayanan V, Koshy C. Fatigue in cancer: a review of literature. Indian J Palliat Care. (2009) 15:19–25. doi: 10.4103/0973-1075.53507
98. LaVoy EC, Fagundes CP, Dantzer R. Exercise, inflammation, and fatigue in cancer survivors. Exerc Immunol Rev. (2016) 22:82–93.
99. Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. (2011) 12:160–74. doi: 10.1016/S1470-2045(11)70002-X
100. Ng CG, Boks MP, Zainal NZ, de Wit NJ. The prevalence and pharmacotherapy of depression in cancer patients. J Affect Disord. (2011) 131:1–7. doi: 10.1016/j.jad.2010.07.034
101. Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. (2012) 141:343–51. doi: 10.1016/j.jad.2012.03.025
102. Peters JC. Tryptophan nutrition and metabolism: an overview. Adv Exp Med Biol. (1991) 294:345–58. doi: 10.1007/978-1-4684-5952-4_32
103. Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-Tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res. (2009) 2:45–60. doi: 10.4137/IJTR.S2129
104. Cheng HH, Kuo CC, Yan JL, Chen HL, Lin WC, Wang KH, et al. Control of cyclooxygenase-2 expression and tumorigenesis by endogenous 5-methoxytryptophan. Proc Natl Acad Sci USA. (2012) 109:13231–6. doi: 10.1073/pnas.1209919109
105. Jacobsen JP, Medvedev IO, Caron MG. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. (2012) 367:2444–59. doi: 10.1098/rstb.2012.0109
106. Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. (2007) 10:1103–9. doi: 10.1038/nn1964
107. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. (2009) 60:355–66. doi: 10.1146/annurev.med.60.042307.110802
108. Slominski A, Baker J, Rosano TG, Guisti LW, Ermak G, Grande M, et al. Metabolism of serotonin to N-acetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. J Biol Chem. (1996) 271:12281–6. doi: 10.1074/jbc.271.21.12281
109. Claustrat B, Leston J. Melatonin: physiological effects in humans. Neurochirurgie. (2015) 61:77–84. doi: 10.1016/j.neuchi.2015.03.002
110. Jones RS. Tryptamine: a neuromodulator or neurotransmitter in mammalian brain? Prog Neurobiol. (1982) 19:117–39. doi: 10.1016/0301-0082(82)90023-5
111. Nishizuka Y, Hayaishi O. Enzymic synthesis of niacin nucleotides from 3-hydroxyanthranilic acid in mammalian liver. J Biol Chem. (1963) 238:483–5.
112. Liu L, Su X, Quinn WJ III, Hui S, Krukenberg K, Frederick DW, et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell metabolism. (2018) 27:1067–80.e5. doi: 10.1016/j.cmet.2018.03.018
113. Powanda MC, Wannemacher RW Jr. Evidence for a linear correlation between the level of dietary tryptophan and hepatic NAD concentration and for a systematic variation in tissue NAD concentration in the mouse and the rat. J Nutr. (1970) 100:1471–8. doi: 10.1093/jn/100.12.1471
114. Mehler AH, Knox WE. The conversion of tryptophan to kynurenine in liver. II. The enzymatic hydrolysis of formylkynurenine. J Biol Chem. (1950) 187:431–8.
115. Ren S, Correia MA. Heme: a regulator of rat hepatic tryptophan 2,3-dioxygenase? Arch Biochem Biophys. (2000) 377:195–203. doi: 10.1006/abbi.2000.1755
116. Danesch U, Gloss B, Schmid W, Schutz G, Schule R, Renkawitz R. Glucocorticoid induction of the rat tryptophan oxygenase gene is mediated by two widely separated glucocorticoid-responsive elements. EMBO J. (1987) 6:625–30. doi: 10.1002/j.1460-2075.1987.tb04800.x
117. Badawy AAB. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. (2017) 10:1178646917691938. doi: 10.1177/1178646917691938
118. Hornyak L, Dobos N, Koncz G, Karanyi Z, Pall D, Szabo Z, et al. The role of indoleamine-2,3-dioxygenase in cancer development, diagnostics, and therapy. Front Immunol. (2018) 9:151. doi: 10.3389/fimmu.2018.00151
119. Pallotta MT, Fallarino F, Matino D, Macchiarulo A, Orabona C. AhR-mediated, non-genomic modulation of IDO1 function. Front Immunol. (2014) 5:497. doi: 10.3389/fimmu.2014.00497
120. Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. (2008) 375:331–5. doi: 10.1016/j.bbrc.2008.07.156
121. Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA. (2010) 107:19961–6. doi: 10.1073/pnas.1014465107
122. Hennequart M, Pilotte L, Cane S, Hoffmann D, Stroobant V, Plaen E, et al. Constitutive IDO1 expression in human tumors is driven by cyclooxygenase-2 and mediates intrinsic immune resistance. Cancer Immunol Res. (2017) 5:695–709. doi: 10.1158/2326-6066.CIR-16-0400
123. Musso T, Gusella GL, Brooks A, Longo DL, Varesio L. Interleukin-4 inhibits indoleamine 2,3-dioxygenase expression in human monocytes. Blood. (1994) 83:1408–11. doi: 10.1182/blood.V83.5.1408.1408
124. Chaves AC, Ceravolo IP, Gomes JA, Zani CL, Romanha AJ, Gazzinelli RT. IL-4 and IL-13 regulate the induction of indoleamine 2,3-dioxygenase activity and the control of Toxoplasma gondii replication in human fibroblasts activated with IFN-gamma. Eur J Immunol. (2001) 31:333–44. doi: 10.1002/1521-4141(200102)31:2<333::aid-immu333>3.0.co;2-x
125. Prendergast GC, Malachowski WJ, Mondal A, Scherle P, Muller AJ. Indoleamine 2,3-dioxygenase and its therapeutic inhibition in cancer. Int Rev Cell Mol Biol. (2018) 336:175–203. doi: 10.1016/bs.ircmb.2017.07.004
126. Lee YK, Lee HB, Shin DM, Kang MJ, Yi EC, Noh S, et al. Heme-binding-mediated negative regulation of the tryptophan metabolic enzyme indoleamine 2,3-dioxygenase 1 (IDO1) by IDO2. Exp Mol Med. (2014) 46:e121. doi: 10.1038/emm.2014.69
127. Mandi Y, Vecsei L. The kynurenine system and immunoregulation. J Neural Transm. (2012) 119:197–209. doi: 10.1007/s00702-011-0681-y
128. Minatogawa Y, Suzuki S, Ando Y, Tone S, Takikawa O. Tryptophan pyrrole ring cleavage enzymes in placenta. Adv Exp Med Biol. (2003) 527:425–34. doi: 10.1007/978-1-4615-0135-0_50
129. Kudo Y, Boyd CA, Spyropoulou I, Redman CW, Takikawa O, Katsuki T, et al. Indoleamine 2,3-dioxygenase: distribution and function in the developing human placenta. J Reprod Immunol. (2004) 61:87–98. doi: 10.1016/j.jri.2003.11.004
130. Suzuki S, Tone S, Takikawa O, Kubo T, Kohno I, Minatogawa Y. Expression of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in early concepti. Biochem J. (2001) 355(Pt 2), 425–9. doi: 10.1042/bj3550425
131. Badawy AA. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep. (2015) 35:e00261. doi: 10.1042/BSR20150197
132. Britan A, Maffre V, Tone S, Drevet JR. Quantitative and spatial differences in the expression of tryptophan-metabolizing enzymes in mouse epididymis. Cell Tissue Res. (2006) 324:301–10. doi: 10.1007/s00441-005-0151-7
133. Fukunaga M, Yamamoto Y, Kawasoe M, Arioka Y, Murakami Y, Hoshi M, et al. Studies on tissue and cellular distribution of indoleamine 2,3-dioxygenase 2: the absence of IDO1 upregulates IDO2 expression in the epididymis. J Histochem Cytochem. (2012) 60:854–60. doi: 10.1369/0022155412458926
134. Jrad-Lamine A, Henry-Berger J, Damon-Soubeyrand C, Saez F, Kocer A, Janny L, et al. Indoleamine 2,3-dioxygenase 1 (ido1) is involved in the control of mouse caput epididymis immune environment. PLoS ONE. (2013) 8:e66494. doi: 10.1371/journal.pone.0066494
135. Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. (2002) 107:452–60. doi: 10.1046/j.1365-2567.2002.01526.x
136. Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. (2005) 22:633–42. doi: 10.1016/j.immuni.2005.03.013
137. Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, et al. Tryptophan catabolism generates autoimmune-preventive regulatory T cells. Transpl Immunol. (2006) 17:58–60. doi: 10.1016/j.trim.2006.09.017
138. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. (2010) 185:3190–8. doi: 10.4049/jimmunol.0903670
139. de Araujo EF, Feriotti C, Galdino NAL, Preite NW, Calich VLG, Loures FV. The IDO-AhR axis controls Th17/Treg immunity in a pulmonary model of fungal infection. Front Immunol. (2017) 8:880. doi: 10.3389/fimmu.2017.00880
140. Stephens GL, Wang Q, Swerdlow B, Bhat G, Kolbeck R, Fung M. Kynurenine 3-monooxygenase mediates inhibition of Th17 differentiation via catabolism of endogenous aryl hydrocarbon receptor ligands. Eur J Immunol. (2013) 43:1727–34. doi: 10.1002/eji.201242779
141. Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. (2002) 9:1069–77. doi: 10.1038/sj.cdd.4401073
142. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. (2002) 196:459–68. doi: 10.1084/jem.20020121
143. Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. (2002) 196:447–57. doi: 10.1084/jem.20020052
144. Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L, et al. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci USA. (2007) 104:18619–24. doi: 10.1073/pnas.0709261104
145. Morita T, Saito K, Takemura M, Maekawa N, Fujigaki S, Fujii H, et al. L-tryptophan-kynurenine pathway metabolite 3-hydroxyanthranilic acid induces apoptosis in macrophage-derived cells under pathophysiological conditions. Adv Exp Med Biol. (1999) 467:559–63. doi: 10.1007/978-1-4615-4709-9_69
146. Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, et al. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity. (2003) 19:571–82. doi: 10.1016/S1074-7613(03)00266-8
147. Sahm F, Oezen I, Opitz CA, Radlwimmer B, von Deimling A, Ahrendt T, et al. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res. (2013) 73:3225–34. doi: 10.1158/0008-5472.CAN-12-3831
148. Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M, et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. (2014) 5:1038–51. doi: 10.18632/oncotarget.1637
149. Adam I, Dewi DL, Mooiweer J, Sadik A, Mohapatra SR, Berdel B, et al. Upregulation of tryptophanyl-tRNA synthethase adapts human cancer cells to nutritional stress caused by tryptophan degradation. Oncoimmunology. (2018) 7:e1486353. doi: 10.1080/2162402X.2018.1486353
150. Wu KK, Cheng HH, Chang TC. 5-methoxyindole metabolites of L-tryptophan: control of COX-2 expression, inflammation and tumorigenesis. J Biomed Sci. (2014) 21:17. doi: 10.1186/1423-0127-21-17
151. Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. (2016) 37:193–207. doi: 10.1016/j.it.2016.01.002
152. Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. (2008) 222:206–21. doi: 10.1111/j.1600-065X.2008.00610.x
153. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. (2004) 22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803
154. Shou D, Wen L, Song Z, Yin J, Sun Q, Gong W. Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies. Oncotarget. (2016) 7:64505–11. doi: 10.18632/oncotarget.11352
155. Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. (2002) 168:3771–6. doi: 10.4049/jimmunol.168.8.3771
156. Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. (2011) 17:6985–91. doi: 10.1158/1078-0432.CCR-11-1331
157. Toulmonde M, Penel N, Adam J, Chevreau C, Blay JY, Le Cesne A, et al. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: a phase 2 clinical trial. JAMA Oncol. (2018) 4:93–7. doi: 10.1001/jamaoncol.2017.1617
158. Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res. (2014) 20:221–32. doi: 10.1158/1078-0432.CCR-13-1560
159. Soliman HH, Jackson E, Neuger T, Dees EC, Harvey RD, Han H, et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget. (2014) 5:8136–46. doi: 10.18632/oncotarget.2357
160. Perez RP, Riese MJ, Lewis KD, Saleh MN, Daud A, Berlin J, et al. Epacadostat plus nivolumab in patients with advanced solid tumors: preliminary phase I/II results of ECHO-204. J Clin Oncol. (2017) 35(Suppl. 15):3003. doi: 10.1200/JCO.2017.35.15_suppl.3003
161. Luke JJ, Tabernero J, Joshua A, Desai J, Varga AI, Moreno V, et al. BMS-986205, an indoleamine 2, 3-dioxygenase 1 inhibitor (IDO1i), in combination with nivolumab (nivo): Updated safety across all tumor cohorts and efficacy in advanced bladder cancer (advBC). J Clin Oncol. (2019) 37(Suppl. 7):358. doi: 10.1200/JCO.2019.37.7_suppl.358
162. Chen S, Song Z, Zhang A. Small-molecule immuno-oncology therapy: advances, challenges and new directions. Curr Top Med Chem. (2019) 19:180–5. doi: 10.2174/1568026619666190308131805
163. Michelagnoli M, Whelan J, Forsyth S, Otis Trial Management Group SI. A phase II study to determine the efficacy and safety of oral treosulfan in patients with advanced pre-treated Ewing sarcoma ISRCTN11631773. Pediatr Blood Cancer. (2015) 62:158–9. doi: 10.1002/pbc.25156
164. Kristeleit R, Davidenko I, Shirinkin V, El-Khouly F, Bondarenko I, Goodheart MJ, et al. A randomised, open-label, phase 2 study of the IDO1 inhibitor epacadostat (INCB024360) versus tamoxifen as therapy for biochemically recurrent (CA-125 relapse)-only epithelial ovarian cancer, primary peritoneal carcinoma, or fallopian tube cancer. Gynecol Oncol. (2017) 146:484–90. doi: 10.1016/j.ygyno.2017.07.005
165. Spira AI, Hamid O, Bauer TM, Borges VF, Wasser JS, Smith DC, et al. Efficacy/safety of epacadostat plus pembrolizumab in triple-negative breast cancer and ovarian cancer: Phase I/II ECHO-202 study. J Clin Oncol. (2017) 35:1103. doi: 10.1200/JCO.2017.35.15_suppl.1103
166. Mitchell TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Olszanski AJ, et al. Epacadostat plus pembrolizumab in patients with advanced solid tumors: phase i results from a multicenter, open-label phase I/II Trial (ECHO-202/KEYNOTE-037). J Clin Oncol. (2018) 36:3223–30. doi: 10.1200/JCO.2018.78.9602
167. Gibney GT, Hamid O, Lutzky J, Olszanski AJ, Mitchell TC, Gajewski TF, et al. Phase 1/2 study of epacadostat in combination with ipilimumab in patients with unresectable or metastatic melanoma. J Immunother Cancer. (2019) 7:80. doi: 10.1186/s40425-019-0562-8
168. Jung KH, LoRusso P, Burris H, Gordon M, Bang YJ, Hellmann MD, et al. Phase I study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) administered with PD-L1 inhibitor (atezolizumab) in advanced solid tumors. Clin Cancer Res. (2019) 25:3220–8. doi: 10.1158/1078-0432.CCR-18-2740
169. Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. (2019) 20:1083–97. doi: 10.1016/S1470-2045(19)30274-8
170. Lob S, Konigsrainer A, Zieker D, Brucher BL, Rammensee HG, Opelz G, et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. (2009) 58:153–7. doi: 10.1007/s00262-008-0513-6
171. Hascitha J, Priya R, Jayavelu S, Dhandapani H, Selvaluxmy G, Sunder Singh S, et al. Analysis of Kynurenine/Tryptophan ratio and expression of IDO1 and 2 mRNA in tumour tissue of cervical cancer patients. Clin Biochem. (2016) 49:919–24. doi: 10.1016/j.clinbiochem.2016.04.008
172. Merlo LMF, Pigott E, DuHadaway JB, Grabler S, Metz R, Prendergast GC, et al. IDO2 is a critical mediator of autoantibody production and inflammatory pathogenesis in a mouse model of autoimmune arthritis. J Immunol. (2014) 192:2082–90. doi: 10.4049/jimmunol.1303012
173. Nevler A, Muller AJ, Sutanto-Ward E, DuHadaway JB, Nagatomo K, Londin E, et al. Host IDO2 gene status influences tumor progression and radiotherapy response in KRAS-driven sporadic pancreatic cancers. Clin Cancer Res. (2019) 25:724–34. doi: 10.1158/1078-0432.CCR-18-0814
174. Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. (2011) 478:197–203. doi: 10.1038/nature10491
175. D'Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, et al. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. (2015) 75:4651–64. doi: 10.1158/0008-5472.CAN-15-2011
176. Chen IC, Lee KH, Hsu YH, Wang WR, Chen CM, Cheng YW. Expression pattern and clinicopathological relevance of the indoleamine 2,3-dioxygenase 1/tryptophan 2,3-dioxygenase protein in colorectal cancer. Dis Markers. (2016) 2016:8169724. doi: 10.1155/2016/8169724
177. Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frederick R, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA. (2012) 109:2497–502. doi: 10.1073/pnas.1113873109
178. Hua S, Chen F, Wang X, Wang Y, Gou S. Pt(IV) hybrids containing a TDO inhibitor serve as potential anticancer immunomodulators. J Inorg Biochem. (2019) 195:130–40. doi: 10.1016/j.jinorgbio.2019.02.004
179. Schramme F, Crosignani S, Frederix K, Hoffmann D, Pilotte L, Stroobant V, et al. Inhibition of tryptophan-dioxygenase activity increases the antitumor efficacy of immune checkpoint inhibitors. Cancer Immunol Res. (2020) 8:32–45. doi: 10.1158/2326-6066.CIR-19-0041
180. Chiu YH, Lei HJ, Huang KC, Chiang YL, Lin CS. Overexpression of kynurenine 3-monooxygenase correlates with cancer malignancy and predicts poor prognosis in canine mammary gland tumors. J Oncol. (2019) 2019:6201764. doi: 10.1155/2019/6201764
181. Connor TJ, Starr N, O'Sullivan JB, Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-gamma? Neurosci Lett. (2008) 441:29–34. doi: 10.1016/j.neulet.2008.06.007
182. Jones SP, Franco NF, Varney B, Sundaram G, Brown DA, de Bie J, et al. Expression of the kynurenine pathway in human peripheral blood mononuclear cells: implications for inflammatory and neurodegenerative disease. PLoS ONE. (2015) 10:e0131389. doi: 10.1371/journal.pone.0131389
183. Shave S, McGuire K, Pham NT, Mole DJ, Webster SP, Auer M. Diclofenac identified as a kynurenine 3-monooxygenase binder and inhibitor by molecular similarity techniques. ACS Omega. (2018) 3:2564–8. doi: 10.1021/acsomega.7b02091
184. Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing Drugs in Oncology (ReDO)-diclofenac as an anti-cancer agent. Ecancermedicalscience. (2016) 10:610. doi: 10.3332/ecancer.2016.610
185. Radbruch L, Strasser F, Elsner F, Goncalves JF, Loge J, Kaasa S, et al. Fatigue in palliative care patients – an EAPC approach. Palliat Med. (2008) 22:13–32. doi: 10.1177/0269216307085183
186. Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. (2015) 13:1012–39. doi: 10.6004/jnccn.2015.0122
187. Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. (2014) 11:597–609. doi: 10.1038/nrclinonc.2014.127
188. Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. (2002) 64:604–11. doi: 10.1097/00006842-200207000-00010
189. Saligan LN, Kim HS. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain Behav Immun. (2012) 26:830–48. doi: 10.1016/j.bbi.2012.05.004
190. Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. (2013) 30(Suppl.): S48–57. doi: 10.1016/j.bbi.2012.06.011
191. Schroecksnadel K, Sarcletti M, Winkler C, Mumelter B, Weiss G, Fuchs D, et al. Quality of life and immune activation in patients with HIV-infection. Brain Behav Immun. (2008) 22:881–9. doi: 10.1016/j.bbi.2007.12.011
192. Huang A, Fuchst D, Widnert B, Glover C, Henderson DC, Allen-Mersh TG. Tryptophan and quality of life in colorectal cancer. In: Allegri G, Costa CVL, Ragazzi E, Steinhart H, Varesio L, editors. Developments in Tryptophan and Serotonin Metabolism. Boston, MA: Springer (2003). p. 353–8. doi: 10.1007/978-1-4615-0135-0_39
193. Kim S, Miller BJ, Stefanek ME, Miller AH. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: relevance to cancer-related fatigue. Cancer. (2015) 121:2129–36. doi: 10.1002/cncr.29302
194. Yennurajalingam S, Frisbee-Hume S, Palmer JL, Delgado-Guay MO, Bull J, Phan AT, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. (2013) 31:3076–82. doi: 10.1200/JCO.2012.44.4661
195. Guo Q, Li Q, Wang J, Liu M, Wang Y, Chen Z, et al. A comprehensive evaluation of clinical efficacy and safety of celecoxib in combination with chemotherapy in metastatic or postoperative recurrent gastric cancer patients: a preliminary, three-center, clinical trial study. Medicine. (2019) 98:e16234. doi: 10.1097/MD.0000000000016234
196. Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends Neurosci. (2014) 37:39–46. doi: 10.1016/j.tins.2013.10.003
197. Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. (2006) 367:29–35. doi: 10.1016/S0140-6736(05)67763-X
198. Yount S, Sorensen MV, Cella D, Sengupta N, Grober J, Chartash EK. Adalimumab plus methotrexate or standard therapy is more effective than methotrexate or standard therapies alone in the treatment of fatigue in patients with active, inadequately treated rheumatoid arthritis. Clin Exp Rheumatol. (2007) 25:838–46.
199. Mystakidou K, Tsilika E, Parpa E, Katsouda E, Galanos A, Vlahos L. Assessment of anxiety and depression in advanced cancer patients and their relationship with quality of life. Qual Life Res. (2005) 14:1825–33. doi: 10.1007/s11136-005-4324-3
200. Mols F, Husson O, Roukema JA, van de Poll-Franse LV. Depressive symptoms are a risk factor for all-cause mortality: results from a prospective population-based study among 3,080 cancer survivors from the PROFILES registry. J Cancer Surviv. (2013) 7:484–92. doi: 10.1007/s11764-013-0286-6
201. Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G, et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat Rev. (2017) 52:58–70. doi: 10.1016/j.ctrv.2016.11.004
202. Kurz K, Schroecksnadel S, Weiss G, Fuchs D. Association between increased tryptophan degradation and depression in cancer patients. Curr Opin Clin Nutr Metab Care. (2011) 14:49–56. doi: 10.1097/MCO.0b013e328340d849
203. Barreto FS, Chaves Filho AJ, de Araujo M, de Moraes MO, de Moraes ME, Maes M, et al. Tryptophan catabolites along the indoleamine 2,3-dioxygenase pathway as a biological link between depression and cancer. Behav Pharmacol. (2018) 29:165–80. doi: 10.1097/FBP.0000000000000384
204. Sforzini L, Nettis MA, Mondelli V, Pariante CM. Inflammation in cancer and depression: a starring role for the kynurenine pathway. Psychopharmacology. (2019) 236:2997–3011. doi: 10.1007/s00213-019-05200-8
205. O'Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. (2009) 14:511–22. doi: 10.1038/sj.mp.4002148
206. Norden DM, Devine R, Bicer S, Jing R, Reiser PJ, Wold LE, et al. Fluoxetine prevents the development of depressive-like behavior in a mouse model of cancer related fatigue. Physiol Behav. (2015) 140:230–5. doi: 10.1016/j.physbeh.2014.12.045
207. Doolin K, Allers KA, Pleiner S, Liesener A, Farrell C, Tozzi L, et al. Altered tryptophan catabolite concentrations in major depressive disorder and associated changes in hippocampal subfield volumes. Psychoneuroendocrinology. (2018) 95:8–17. doi: 10.1016/j.psyneuen.2018.05.019
208. Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PS, Victor TA, et al. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav Immun. (2015) 46:55–9. doi: 10.1016/j.bbi.2015.02.007
209. Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, et al. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun. (2016) 53:39–48. doi: 10.1016/j.bbi.2015.11.003
210. Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O'Connor JC. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry. (2016) 6:e918. doi: 10.1038/tp.2016.200
211. O'Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. (2009) 182:3202–12. doi: 10.4049/jimmunol.0802722
212. Laumet G, Edralin JD, Chiang AC, Dantzer R, Heijnen CJ, Kavelaars A. Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology. (2018) 43:2597–605. doi: 10.1038/s41386-018-0154-1
213. Martinez P, Tsai AC, Muzoora C, Kembabazi A, Weiser SD, Huang Y, et al. Reversal of the Kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J Acquir Immune Defic Syndr. (2014) 65:456–62. doi: 10.1097/QAI.0000000000000062
214. Maes M, Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, et al. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol Psychiatry. (2001) 6:475–80. doi: 10.1038/sj.mp.4000872
215. Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. (2012) 33:315–27. doi: 10.1016/j.yfrne.2012.09.003
216. Neurauter G, Schrocksnadel K, Scholl-Burgi S, Sperner-Unterweger B, Schubert C, Ledochowski M, et al. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab. (2008) 9:622–7. doi: 10.2174/138920008785821738
217. Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science. (1971) 173:149–52. doi: 10.1126/science.173.3992.149
218. Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. (2005) 10:538–44. doi: 10.1038/sj.mp.4001600
219. Lugo-Huitron R, Ugalde Muniz P, Pineda B, Pedraza-Chaverri J, Rios C, Perez-de la Cruz V. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid Med Cell Longev. (2013) 2013:104024. doi: 10.1155/2013/104024
220. Bender DA, McCreanor GM. Kynurenine hydroxylase: a potential rate-limiting enzyme in tryptophan metabolism. Biochem Soc Trans. (1985) 13:441–3. doi: 10.1042/bst0130441
221. Foster AC, Miller LP, Oldendorf WH, Schwarcz R. Studies on the disposition of quinolinic acid after intracerebral or systemic administration in the rat. Exp Neurol. (1984) 84:428–40. doi: 10.1016/0014-4886(84)90239-5
222. Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. (1982) 247:184–7. doi: 10.1016/0006-8993(82)91048-4
223. Foster AC, Vezzani A, French ED, Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett. (1984) 48:273–8. doi: 10.1016/0304-3940(84)90050-8
224. Braidy N, Grant R. Kynurenine pathway metabolism and neuroinflammatory disease. Neural Regen Res. (2017) 12:39–42. doi: 10.4103/1673-5374.198971
225. Myint AM. Kynurenines: from the perspective of major psychiatric disorders. FEBS J. (2012) 279:1375–85. doi: 10.1111/j.1742-4658.2012.08551.x
226. Myint AM, Schwarz MJ, Steinbusch HW, Leonard BE. Neuropsychiatric disorders related to interferon and interleukins treatment. Metab Brain Dis. (2009) 24:55–68. doi: 10.1007/s11011-008-9114-5
227. Crozier-Reabe KR, Phillips RS, Moran GR. Kynurenine 3-monooxygenase from Pseudomonas fluorescens: substrate-like inhibitors both stimulate flavin reduction and stabilize the flavin-peroxo intermediate yet result in the production of hydrogen peroxide. Biochemistry. (2008) 47:12420–33. doi: 10.1021/bi8010434
228. Barsevick AM, Irwin MR, Hinds P, Miller A, Berger A, Jacobsen P, et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. (2013) 105:1432–40. doi: 10.1093/jnci/djt242
230. Souza LC, Jesse CR, de Gomes MG, Del Fabbro L, Goes ATR, Donato F, et al. Activation of brain indoleamine-2,3-dioxygenase contributes to depressive-like behavior induced by an intracerebroventricular injection of streptozotocin in mice. Neurochem Res. (2017) 42:2982–95. doi: 10.1007/s11064-017-2329-2
231. Fuertig R, Azzinnari D, Bergamini G, Cathomas F, Sigrist H, Seifritz E, et al. Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: Both effects are reversed by inhibition of indoleamine 2,3-dioxygenase. Brain Behav Immun. (2016) 54:59–72. doi: 10.1016/j.bbi.2015.12.020
232. Xie W, Cai L, Yu Y, Gao L, Xiao L, He Q, et al. Activation of brain indoleamine 2,3-dioxygenase contributes to epilepsy-associated depressive-like behavior in rats with chronic temporal lobe epilepsy. J Neuroinflamm. (2014) 11:41. doi: 10.1186/1742-2094-11-41
233. Liu Y-N, Peng Y-L, Liu L, Wu T-Y, Zhang Y, Lian Y-J, et al. TNFα mediates stress-induced depression by upregulating indoleamine 2,3-dioxygenase in a mouse model of unpredictable chronic mild stress. Eur Cytok Netw. (2015) 26:15–25. doi: 10.1684/ecn.2015.0362
234. Reis DJ, Casteen EJ, Ilardi SS. The antidepressant impact of minocycline in rodents: a systematic review and meta-analysis. Sci Rep. (2019) 9:261–261. doi: 10.1038/s41598-018-36507-9
235. Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflamm. (2008) 5:15. doi: 10.1186/1742-2094-5-15
236. Bahrami Z, Firouzi M, Hashemi-Monfared A, Zahednasab H, Harirchian MH. The effect of minocycline on indolamine 2, 3 dioxygenase expression and the levels of kynurenic acid and quinolinic acid in LPS-activated primary rat microglia. Cytokine. (2018) 107:125–9. doi: 10.1016/j.cyto.2017.12.013
237. Clemens V, Regen F, Le Bret N, Heuser I, Hellmann-Regen J. Anti-inflammatory effects of minocycline are mediated by retinoid signaling. BMC Neurosci. (2018) 19:58. doi: 10.1186/s12868-018-0460-x
238. Rosenblat JD, McIntyre RS. Efficacy and tolerability of minocycline for depression: a systematic review and meta-analysis of clinical trials. J Affect Disord. (2018) 227:219–25. doi: 10.1016/j.jad.2017.10.042
239. Kohler O, Krogh J, Mors O, Benros ME. Inflammation in depression and the potential for anti-inflammatory treatment. Curr Neuropharmacol. (2016) 14:732–42. doi: 10.2174/1570159X14666151208113700
240. Vichaya EG, Vermeer DW, Budac D, Lee A, Grossberg A, Vermeer PD, et al. Inhibition of indoleamine 2,3 dioxygenase does not improve cancer-related symptoms in a murine model of human papilloma virus-related head and neck cancer. Int J Tryptophan Res. 12:1178646919872508. doi: 10.1177/1178646919872508
241. Salter M, Hazelwood R, Pogson CI, Iyer R, Madge DJ. The effects of a novel and selective inhibitor of tryptophan 2,3-dioxygenase on tryptophan and serotonin metabolism in the rat. Biochem Pharmacol. (1995) 49:1435–42. doi: 10.1016/0006-2952(95)00006-L
242. Breda C, Sathyasaikumar KV, Sograte Idrissi S, Notarangelo FM, Estranero JG, Moore GG, et al. Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc Natl Acad Sci USA. (2016) 113:5435–40. doi: 10.1073/pnas.1604453113
243. Giorgini F, Huang SY, Sathyasaikumar KV, Notarangelo FM, Thomas MA, Tararina M, et al. Targeted deletion of kynurenine 3-monooxygenase in mice: a new tool for studying kynurenine pathway metabolism in periphery and brain. J Biol Chem. (2013) 288:36554–66. doi: 10.1074/jbc.M113.503813
244. Brooks AK, Janda TM, Lawson MA, Rytych JL, Smith RA, Ocampo-Solis C, et al. Desipramine decreases expression of human and murine indoleamine-2,3-dioxygenases. Brain Behav Immun. (2017) 62:219–29. doi: 10.1016/j.bbi.2017.02.010
245. Duda W, Curzytek K, Kubera M, Connor TJ, Fagan EM, Basta-Kaim A, et al. Interaction of the immune-inflammatory and the kynurenine pathways in rats resistant to antidepressant treatment in model of depression. Int Immunopharmacol. (2019) 73:527–38. doi: 10.1016/j.intimp.2019.05.039
246. Borsini A, Alboni S, Horowitz MA, Tojo LM, Cannazza G, Su KP, et al. Rescue of IL-1beta-induced reduction of human neurogenesis by omega-3 fatty acids and antidepressants. Brain Behav Immun. (2017) 65:230–8. doi: 10.1016/j.bbi.2017.05.006
247. Román GC, Jackson RE, Gadhia R, Román AN, Reis J. Mediterranean diet: the role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev Neurol. (2019) 175:724–41. doi: 10.1016/j.neurol.2019.08.005
248. Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. Adherence to mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. (2017) 9:E1063. doi: 10.3390/nu9101063
249. Myles IA. Fast food fever: reviewing the impacts of the Western diet on immunity. Nutr J. (2014) 13:61. doi: 10.1186/1475-2891-13-61
250. André C, Dinel AL, Ferreira G, Layé S, Castanon N. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun. (2014) 41:10–21. doi: 10.1016/j.bbi.2014.03.012
251. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. (2018) 23:716–24. doi: 10.1016/j.chom.2018.05.003
252. Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. (2018) 6:133–48. doi: 10.1016/j.jcmgh.2018.04.003
253. Rankin LC, Artis D. Beyond host defense: emerging functions of the immune system in regulating complex tissue physiology. Cell. (2018) 173:554–67. doi: 10.1016/j.cell.2018.03.013
254. Wang G, Huang S, Wang Y, Cai S, Yu H, Liu H, et al. Bridging intestinal immunity and gut microbiota by metabolites. Cell Mol Life Sci. (2019) 76:3917–37. doi: 10.1007/s00018-019-03190-6
255. Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer - where do we stand? Mol Metab. (2019). doi: 10.1016/j.molmet.2019.06.026. [Epub ahead of print].
256. Sremanakova J, Sowerbutts AM, Burden S. A systematic review of the use of ketogenic diets in adult patients with cancer. J Hum Nutr Diet. (2018) 31:793–802. doi: 10.1111/jhn.12587
257. Heischmann S, Gano LB, Quinn K, Liang LP, Klepacki J, Christians U, et al. Regulation of kynurenine metabolism by a ketogenic diet. J Lipid Res. (2018) 59:958–66. doi: 10.1194/jlr.M079251
258. Zarnowski T, Choragiewicz T, Tulidowicz-Bielak M, Thaler S, Rejdak R, Zarnowski I, et al. Ketogenic diet increases concentrations of kynurenic acid in discrete brain structures of young and adult rats. J Neural Transm. (2012) 119:679–84. doi: 10.1007/s00702-011-0750-2
259. Zarnowski T, Tulidowicz-Bielak M, Zarnowska I, Mitosek-Szewczyk K, Wnorowski A, Jozwiak K, et al. Kynurenic acid and neuroprotective activity of the ketogenic diet in the eye. Curr Med Chem. (2017) 24:3547–58. doi: 10.2174/0929867324666170509120257
260. Zarnowska I, Wróbel-Dudzinska D, Tulidowicz-Bielak M, Kocki T, Mitosek-Szewczyk K, Gasior M, et al. Changes in tryptophan and kynurenine pathway metabolites in the blood of children treated with ketogenic diet for refractory epilepsy. Seizure. (2019) 69:265–72. doi: 10.1016/j.seizure.2019.05.006
261. Navarro SL, Tarkhan A, Shojaie A, Randolph TW, Gu H, Djukovic D, et al. Plasma metabolomics profiles suggest beneficial effects of a low-glycemic load dietary pattern on inflammation and energy metabolism. Am J Clin Nutr. (2019) 110:984–92. doi: 10.1093/ajcn/nqz169
262. Inglis JE, Lin PJ, Kerns SL, Kleckner IR, Kleckner AS, Castillo DA, et al. Nutritional interventions for treating cancer-related fatigue: a qualitative review. Nutr Cancer. (2019) 71:21–40. doi: 10.1080/01635581.2018.1513046
263. Nencioni A, Caffa I, Cortellino S, Longo VD. Fasting and cancer: molecular mechanisms and clinical application. Nat Rev Cancer. (2018) 18:707–19. doi: 10.1038/s41568-018-0061-0
264. Lee JY, Chu SH, Jeon JY, Lee MK, Park JH, Lee DC, et al. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Dig Liver Dis. (2014) 46:1126–32. doi: 10.1016/j.dld.2014.09.004
265. Stubbe CE, Valero M. Complementary strategies for the management of radiation therapy side effects. J Adv Pract Oncol. (2013) 4:219–31. doi: 10.6004/jadpro.2013.4.4.3
266. Rudzki L, Ostrowska L, Pawlak D, Malus A, Pawlak K, Waszkiewicz N, et al. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. (2019) 100:213–22. doi: 10.1016/j.psyneuen.2018.10.010
267. Strasser B, Geiger D, Schauer M, Gostner JM, Gatterer H, Burtscher M, et al. Probiotic supplements beneficially affect tryptophan-kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: a randomized, double-blinded, placebo-controlled trial. Nutrients. (2016) 8:E752. doi: 10.3390/nu8110752
268. Leblhuber F, Steiner K, Schuetz B, Fuchs D, Gostner JM. Probiotic supplementation in patients with Alzheimer's dementia - an explorative intervention study. Curr Alzheimer Res. (2018) 15:1106–13. doi: 10.2174/1389200219666180813144834
269. Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. (2013) 39:372–85. doi: 10.1016/j.immuni.2013.08.003
270. Park C, Brietzke E, Rosenblat JD, Musial N, Zuckerman H, Ragguett RM, et al. Probiotics for the treatment of depressive symptoms: an anti-inflammatory mechanism? Brain Behav Immun. (2018) 73:115–24. doi: 10.1016/j.bbi.2018.07.006
271. Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry. (2017) 16:14. doi: 10.1186/s12991-017-0138-2
272. Nikolova V, Zaidi SY, Young AH, Cleare AJ, Stone JM. Gut feeling: randomized controlled trials of probiotics for the treatment of clinical depression: systematic review and meta-analysis. Ther Adv Psychopharmacol. (2019) 9:2045125319859963. doi: 10.1177/2045125319859963
273. Baguley BJ, Skinner TL, Wright ORL. Nutrition therapy for the management of cancer-related fatigue and quality of life: a systematic review and meta-analysis. Br J Nutr. (2019) 122:527–41. doi: 10.1017/S000711451800363X
274. Metcalfe AJ, Koliamitra C, Javelle F, Bloch W, Zimmer P. Acute and chronic effects of exercise on the kynurenine pathway in humans - a brief review and future perspectives. Physiol Behav. (2018) 194:583–7. doi: 10.1016/j.physbeh.2018.07.015
275. Gostner JM, Geisler S, Stonig M, Mair L, Sperner-Unterweger B, Fuchs D. Tryptophan metabolism and related pathways in psychoneuroimmunology: the impact of nutrition and lifestyle. Neuropsychobiology. 79:89–99. doi: 10.1159/000496293
276. Wegner M, Helmich I, Machado S, Nardi AE, Arias-Carrion O, Budde H. Effects of exercise on anxiety and depression disorders: review of meta- analyses and neurobiological mechanisms. CNS Neurol Dis Drug Targets. (2014) 13:1002–14. doi: 10.2174/1871527313666140612102841
277. Meeusen R. Exercise, nutrition and the brain. Sports Med. (2014) 44(Suppl. 1):S47–56. doi: 10.1007/s40279-014-0150-5
278. Valente-Silva P, Ruas JL. Tryptophan-kynurenine metabolites in exercise and mental health. In: Spiegelman B, editor. Hormones, Metabolism and the Benefits of Exercise. Cham: Springer (2017). p. 83–91. doi: 10.1007/978-3-319-72790-5_7
279. Agudelo LZ, Femenía T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. (2014) 159:33–45. doi: 10.1016/j.cell.2014.07.051
280. Sprenger H, Jacobs C, Nain M, Gressner AM, Prinz H, Wesemann W, et al. Enhanced release of cytokines, interleukin-2 receptors, and neopterin after long-distance running. Clin Immunol Immunopathol. (1992) 63:188–95. doi: 10.1016/0090-1229(92)90012-D
281. Kallich JD, Tchekmedyian NS, Damiano AM, Shi J, Black JT, Erder MH. Psychological outcomes associated with anemia-related fatigue in cancer patients. Oncology. (2002) 16:117–24.
282. Sabbatini P. The relationship between anemia and quality of life in cancer patients. Oncologist. (2000) 5(Suppl. 2):19–23. doi: 10.1634/theoncologist.5-suppl_2-19
283. Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. (2004) 116(Suppl. 7A):11–26S. doi: 10.1016/j.amjmed.2003.12.008
284. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. (2005) 352:1011–23. doi: 10.1056/NEJMra041809
286. Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. (2019) 133:40–50. doi: 10.1182/blood-2018-06-856500
287. Kurz K, Schroecksnadel S, Seifert M, Sarcletti M, Fuchs D, Weiss G, et al. Iron metabolic changes and immune activation in patients with HIV-1 infection before and after ART. Pteridines. (2010) 21:47. doi: 10.1515/pteridines.2010.21.1.29
288. Tsuboi I, Harada T, Hirabayashi Y, Kanno J, Inoue T, Aizawa S. Inflammatory biomarker, neopterin, predominantly enhances myelopoiesis, which suppresses erythropoiesis via activated stromal cells. Immunobiology. (2010) 215:348–55. doi: 10.1016/j.imbio.2009.05.004
289. Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, Stefanidis I. Kynurenine, by activating aryl hydrocarbon receptor, decreases erythropoietin and increases hepcidin production in HepG2 cells: a new mechanism for anemia of inflammation. Exp Hematol. (2016) 44:60–7.e1. doi: 10.1016/j.exphem.2015.08.010
290. Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. (2012) 72:5435–40. doi: 10.1158/0008-5472.CAN-12-0569
291. Weiss G, Schroecksnadel K, Mattle V, Winkler C, Konwalinka G, Fuchs D. Possible role of cytokine-induced tryptophan degradation in anaemia of inflammation. Eur J Haematol. (2004) 72:130–4. doi: 10.1046/j.0902-4441.2003.00197.x
292. Pawlak D, Koda M, Pawlak S, Wolczynski S, Buczko W. Contribution of quinolinic acid in the development of anemia in renal insufficiency. Am J Physiol Renal Physiol. (2003) 284:F693–700. doi: 10.1152/ajprenal.00327.2002
293. Braidy N, Grant R, Adams S, Brew BJ, Guillemin GJ. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox Res. (2009) 16:77–86. doi: 10.1007/s12640-009-9051-z
294. Huang LE, Willmore WG, Gu J, Goldberg MA, Bunn HF. Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide and nitric oxide. Implications for oxygen sensing and signaling. J Biol Chem. (1999) 274:9038–44. doi: 10.1074/jbc.274.13.9038
295. Sibon D, Coman T, Rossignol J, Lamarque M, Kosmider O, Bayard E, et al. Enhanced renewal of erythroid progenitors in myelodysplastic anemia by peripheral serotonin. Cell Rep. (2019) 26:3246–56.e4. doi: 10.1016/j.celrep.2019.02.071
Keywords: inflammation, tryptophan, kynurenine, cancer, anemia, fatigue, depression
Citation: Lanser L, Kink P, Egger EM, Willenbacher W, Fuchs D, Weiss G and Kurz K (2020) Inflammation-Induced Tryptophan Breakdown is Related With Anemia, Fatigue, and Depression in Cancer. Front. Immunol. 11:249. doi: 10.3389/fimmu.2020.00249
Received: 02 October 2019; Accepted: 30 January 2020;
Published: 21 February 2020.
Edited by:
Heiko Mühl, Goethe University Frankfurt, GermanyReviewed by:
Leah M. Pyter, The Ohio State University, United StatesCopyright © 2020 Lanser, Kink, Egger, Willenbacher, Fuchs, Weiss and Kurz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katharina Kurz, a2F0aGFyaW5hLmt1cnpAaS1tZWQuYWMuYXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.