
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 07 February 2020
Sec. Immunological Tolerance and Regulation
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.00150
This article is part of the Research Topic Tolerating Factor VIII: Novel Strategies to Prevent and Reverse Anti-FVIII Inhibitors View all 20 articles
Anti-drug antibodies to coagulation factor VIII (fVIII), often termed inhibitors, present the greatest economical and treatment related obstacle in the management of hemophilia A. Although several genetic and environmental risk factors associated with inhibitor development have been identified, the precise mechanisms responsible for the immune response to exogenous fVIII therapies remain undefined. Clinical trials suggest there is an increased immunogenic potential of recombinant fVIII compared to plasma-derived products. Additional biochemical and immunological studies have demonstrated that changes in recombinant fVIII production and formulation can alter fVIII structure and immunogenicity. Recently, one study demonstrated increased immunogenicity of the recombinant fVIII product Helixate in hemophilia A mice following oxidation with hypochlorite (ClO−). It is widely reported that protein aggregates within drug products can induce adverse immune reactions in patients. Several studies have therefore investigated the prevalence of molecular aggregates in commercial recombinant products with and without use-relevant stress and agitation. To investigate the potential link between oxidation-induced immunogenicity and molecular aggregation, we analyzed the recombinant fVIII product, Helixate, via sedimentation velocity analytical ultracentrifugation following oxidation with ClO−. At 80 μM ClO−, a concentration that reduced the specific-activity by 67%, no detectable increase in large molecular aggregates (s > 12 S) was observed when compared to non-oxidized fVIII. This lack of aggregates was demonstrated both in commercial excipient as well as a HEPES buffered saline formulation. These data suggest that oxidation induced immunogenicity is independent of aggregate-mediated immune response. Therefore, our data support multiple, independent mechanisms underlying fVIII immunogenicity.
The standard of care for patients with hemophilia A is prophylactic treatment with concentrated exogenous factor VIII (fVIII) products to prevent and control bleeding and mitigate joint damage. Commercial fVIII products, whether plasma-derived or recombinant, result in a significant incidence of anti-drug antibodies (ADAs), termed inhibitors, which prevent fVIII activation (1), interfere with co-factor function to factor IX (1), abrogate phospholipid and/or platelet binding (2), or enhance drug clearance (3). These inhibitors can occur in upwards of 40% of previously untreated patients (4–6) and recent clinical studies have suggested that recombinant products may be more immunogenetic (7–9). Current theories for immune complex formation within recombinant products include (i) the absence of the immuno-protective effect of von Willebrand factor, (ii) altered glycosylation patterns and structures due to various heterologous expression systems, and (iii) increased molecular aggregation of recombinant products prior to infusion. While the precise pathogenic mechanisms responsible for fVIII inhibitor development remain unclear, a multifactorial combination of genetic and non-genetic risk factors are suggested to influence the anti-fVIII immune response (10–13).
Non-genetic factors, such as inflammation, hemarthrosis, reactive oxygen species (ROS), and redox states are postulated to stimulate the anti-fVIII immune response. Hemarthrosis prior to and during factor infusion was associated with an increased inhibitor response in rats with severe hemophilia A (14), suggesting that on-demand treatment may correlate with increased risk. Additionally, sites of endothelial damage during hemarthrosis precede the release of ROS species (15) which influence the local redox milieu. A recent investigation of the effect of oxidation demonstrated an increased immune response in hemophilia A mice when fVIII was oxidized with ClO− prior to administration (16). ClO− oxidation of fVIII compromised its procoagulant activity but did not abrogate VWF binding, demonstrating that oxidation alters the fVIII structure outside of the VWF binding sites and that VWF did not protect from an immune response to the oxidized fVIII species. The concentration of coagulation factors at sites of injury and inflammation may therefore result in undesirable structural changes which alter the immunogenicity of the protein.
Oxidation is widely reported to increase the immunogenicity of proteins including interferon alpha2b (17), collagen type II (18), and ovalbumin (19). A link between oxidative induced aggregate formation and subsequent immunogenicity has been demonstrated with human interferon-β (20). Protein aggregates in commercial products, as a result of altered structure and assemblies, also increase the risk of ADAs in patients (21, 22). These altered assemblies can result from chemical reactions as previously described, or result from accumulated misfolded protein. Recombinant fVIII is produced in heterologous expressions systems and it has been reported that 2nd generation products Helixate and Kogenate are more immunogenic than the 3rd generation product Advate (23, 24). It has also been demonstrated that these products contain significantly higher levels of aggregates before and after use-related stress as measured by size exclusion high-performance liquid chromatography, dynamic light scattering, and sedimentation velocity analytical ultracentrifugation (SV-AUC) (25–27). In the present study, we use SV-AUC to investigate the effect of oxidation of recombinant fVIII product Helixate on the formation of molecular aggregates.
Sodium hypochlorite (NaOCl) was purchased from Sigma-Aldrich (St. Louis, MO). NaOCl concentration was determined using an extinction coefficient of 350 M−1 cm−1 at 292 nm in water. Zeba Spin desalting columns and Amicon Ultra centrifugal filters were purchased from Thermo Fisher Scientific (Waltham, MA) and Millipore Sigma (Burlington, MA), respectively. Helixate FS (CSL Behring, Kankakee, IL, USA; Lots 270PP4J and 27N1VK1) was purchased from the manufacturer and reconstituted using sterile water in accordance with the kits and instructions provided.
For some experiments, Helixate was exchanged into 0.15 M NaCl, 0.02 M HEPES, 5 mM CaCl2, 0.01% polysorbate 80 (w/w), pH 7.4 (HBS/Ca/PS-80) with a Zeba Spin desalting column or by repeated filtration using Amicon Ultra-15 Ultracel-30K centrifugal filter, which was passivated with (HBS/Ca/PS-80) prior to buffer exchange. Helixate was oxidized by addition of sodium hypochlorite for ~10 min followed by buffer exchange.
Specific-activity of Helixate was determined by one-stage coagulation assay using a Diagnostica Stago Start (Parsippany, NJ) viscosity-based hemostasis analyzer and referenced to pooled citrated normal plasma (FACT). Activity was normalized to A280 mass determination following extinction coefficient corrections using an extinction coefficient of 1.2 (mg/mL)−1 cm−1 based on tyrosine, tryptophan and cysteine composition and molar mass. Specific-activities were measured immediately following addition of ClO− and remained stable over 24 h. fVIII deficient plasma and FACT reference were purchased from George King Biomedical (Overland Park, KS). Automated APTT reagent was purchased from Trinity Biotech (Wicklow, Ireland).
SV experiments were performed at 105,000 g (38,000 rpm) at 20°C in a Beckman Coulter ProteomeLab XLI analytical ultracentrifuge. Scanning was done at 280 nm in an An-60 rotor equipped with 12 mm pathlength double sector cells and sapphire windows. Sample and reference buffer volumes were 0.40 mL each. Scans were initiated in continuous mode at ~4 min intervals using a radial spacing of 0.003 cm after reaching the target rotor speed and were acquired at ~3 min intervals.
Data were analyzed using the continuous c(s) distribution model in SEDFIT, version 16.1c (28, 29), or the hybrid local continuous/global discrete species model in SEDPHAT, version 15.2b (http://analyticalultracentrifugation.com). These models produce a least squares fit of the absorbance signal as a function of radial position and time to a set of Lamm equations corresponding to a user-defined range and increments of sedimentation coefficients, yielding a sedimentation coefficient distribution, c(s), and a signal-average frictional ratios, f/fo. The simplex algorithm and Marquardt-Levenberg algorithms were used for both programs. The meniscus position, baseline, and time-invariant noise also were fitted. Continuous c(s) distribution fitting was done using maximum entropy regularization with a confidence interval of 0.68. Hybrid local continuous/global discrete species modeling was done using Tikhonov regularization with a confidence interval of 0.68.
The molar mass, M, of the dominant species in formulated Helixate was estimated using
where η is the solvent viscosity, ρ is the solvent density, is the partial specific volume of fVIII, and Na is Avogadro's number. A value of 0.719 mL/g was used for the partial specific volume of fVIII (25).
The sedimentation coefficients were converted to the standard condition of water as reference solvent at 20°C ((sw)20,w) as described by Svedberg and Pedersen (30). The viscosity and density of the formulation buffer and HBS/Ca/Tw were measured in a Lovis 2000M viscometer and an Anton Paar DMA4500 densitometer. SV graphs were plotted using GUSSI version 1.4.2 (31).
Peyron et al. recently demonstrated that oxidation of Helixate reduced procoagulant function and increased immunogenicity without affecting binding to VWF (16). While the mechanism of the observed immunity is not yet understood, oxidation is suggested to alter the structure and/or assembly of the protein. To analyze the formation of protein aggregates in the fVIII product, Helixate, by SV AUC, dose-finding studies were conducted to determine the concentration of ClO− required to abrogate procoagulant function and therefore alter the physical structure of fVIII. To ensure that active and/or inactive fVIII mass was not removed following buffer exchange, A280 measurements were taken before and after oxidation and specific-activity (IU/mg) is reported. Exposure of fVIII to concentrations of 50, 60, and 80 μM ClO− in 2x formulation buffer for 10 min prior to buffer exchange to remove ClO− resulted in 47, 58, and 67% reduction in specific-activity (Figure 1). These concentrations represent a 55–88-fold molar excess of ClO− over fVIII. The concentrations required to reduce Helixate specific activity in 2x formulation buffer were roughly double those required in HBS/Ca/PS-80 (data not shown), likely due to the presence of the anti-oxidant histidine (Table 1). As a control against subsequent structural changes and loss of coagulant activity because of the 8 h SV AUC process, specific-activity of samples were tested immediately following centrifugation. Samples demonstrated no change in specific activity 8 h after initial testing (Figure 1).
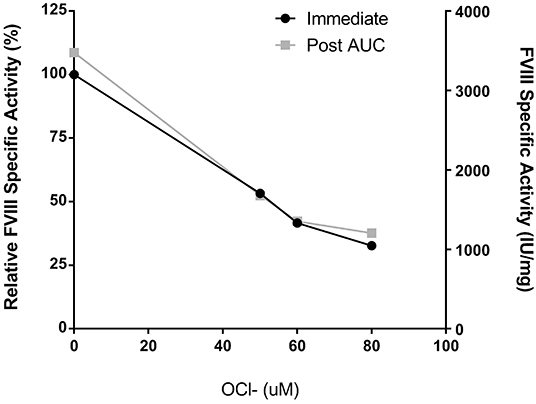
Figure 1. Helixate specific-activity reduction following oxidation. Helixate was reconstituted in ½ volume of Sterile Water for Injection resulting in 2x formulation buffer. Following oxidation with molar excess ClO−, Helixate was exchanged into HBS/Ca/PS-80 and specific-activity was determined (black circles). FVIII specific-activity was measured by one-stage coagulation activity and plotted as specific activity relative to the absence of ClO- as well as absolute specific activity. Specific-activity of oxidized Helixate was also measured following completion of AUC (gray squares) to confirm no loss of activity during AUC.
To assess the formation of aggregates following oxidation, Helixate was desalted into HBS/Ca/PS-80, and exposed to 80 μM ClO− or control buffer for 10 min. Helixate then underwent buffer exchange into HBS/Ca/PS-80 to remove ClO− and was subjected to SV AUC. Figure 2A shows an overlay of the 80 μM ClO− and control Helixate sedimentation coefficient distributions. Diffuse aggregates are evident in the region between 12 and 100 S in the control sample as observed previously for Helixate (25). The extent of aggregation was similar in the oxidized sample. Aggregates in the 12–100 S represented 13.4 and 13.3% of the signal relative to the region between 5 and 100 S for the control and 80 μM samples, respectively.
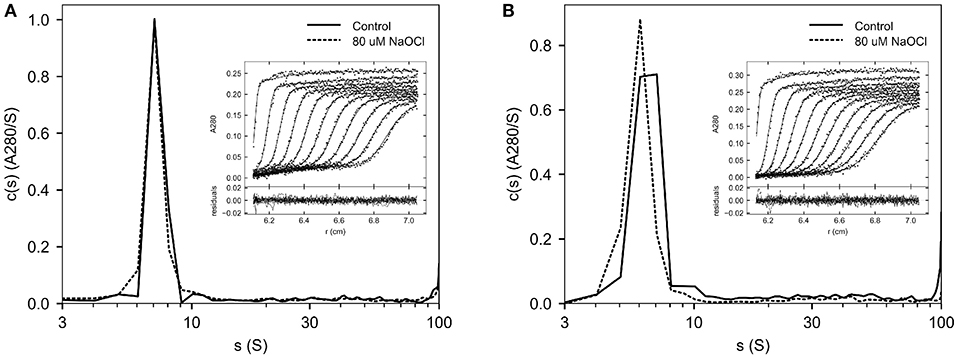
Figure 2. SV AUC of oxidized Helixate. Helixate in HBS/Ca/PS-80 (A) or 2x formulation buffer (B) was treated with either 80 μM ClO− (dashed curve) or control buffer (solid curve). Following buffer exchange using a desalting column, samples were subjected to SV AUC at 105,000 g at 20°C. A280 scans were fitted to a continuous c(s) distribution from 0 to 100 S at 1 S increments. The insets show the fitted data for control samples. Only every fourth scan and every other data point are shown for clarity. The lower panels in the insets show the residuals of the fitted data.
For therapeutic use, Helixate is reconstituted with sterile water for injection, which produces a solution with excipient and stabilizer concentrations listed in Table 1. To evaluate the oxidation of Helixate in the excipient/stabilizer buffer system, and to increase the 280 nm absorbance signal for SV AUC for increased potential of detecting a differential increase in aggregates due to oxidation, Helixate was reconstituted at twice the concentration of the therapeutic formulation and exposed to control buffer or 80 μM ClO−. Figure 2B shows that aggregation was similar between oxidized and control fVIII. Aggregates in the 12–100 S region were 14.5 and 9.2% relative to the region between 5 and 100 S for the control and 80 μM samples, respectively.
As an additional control, we determined whether aggregates were removed from Helixate during buffer exchange procedure. This potentially would produce a false negative result in which oxidized fVIII aggregates were selectively removed by a solid phase matrix. Figure 3 shows the c(s) distributions of Helixate in formulation buffer and following desalting into HBS/Ca/PS-80, revealing a major peak with an (Sw)20, w-value of 7.6 S. Aggregate levels were 18.9% and 15.9% in formulation buffer and HBS/Ca/PS-80, respectively, indicating that aggregates do not become trapped on the solid phase matrix. To further confirm this, SV AUC was performed on oxidized Helixate without removal of HOCl, and no increase in diffuse aggregates was observed (data not shown).

Figure 3. SV AUC of Helixate in formulation buffer and HBS/Ca/PS-80. Helixate was reconstituted in Sterile Water for Injection to produce its therapeutic formulation (solid curve) or exchanged into HBS/Ca/PS 80 (dashed curve) and immediately subjected to SV AUC at 105,000 g at 20°C. A280 scans were fitted to a continuous c(s) distribution from 0 to 200 S.
The integration range for Helixate in formulation buffer was 15–100 S instead of 12–100 S because dominant species in Helixate sediments faster in formulation buffer than in HBS/Ca/PS-80 (Figure 3). This is surprising because the nominal excipients and stabilizers in Helixate produce a ~0.3 M glycine/0.03 M sucrose solution. Therefore, the predicted density and viscosity of formulation buffer would be greater than HBS/Ca/PS-80 and thus slow the sedimentation of fVIII. To investigate this phenomenon, the density and viscosity of Helixate in formulation buffer was measured and SV AUC was performed on the same sample. The density and viscosity of the formulation buffer were 1.0147 g/mL and 0.01095 Poise, respectively, compared to 1.0062 g/mL and 0.01028 Poise of HBS/Ca/PS-80.
Figure 4 shows the fitted sedimentation profiles and c(s) distribution of Helixate in formulation buffer. The shaded region in Figure 4B was integrated, producing a signal-average sedimentation coefficient, sw, of 9.14 S. This corresponds to a value at 20°C in water, (sw)20,w, of 10.43 S. In contrast, the (sw)20,w of the fVIII heterodimer in Helixate is 7.6 S in HBS/Ca/PS-80 (Figure 3). Continuous c(s) distribution analysis, which produces an estimate of the signal-average frictional ratio, f/fo, of the entire sedimenting population (29), yielded a value of 1.29. To exclude the possibility that aggregates were significantly contributing to the frictional ratio, the hybrid local continuous/global discrete species model in SEDPHAT, which provides an estimate of the frictional ratio in user-defined regions within the sedimentation coefficient distribution range of interest (32), was implemented and produced an estimated frictional ratio of 1.28. In contrast, the frictional ratio of the fVIII heterodimer in HBS/Ca/PS-80 is 1.91. The frictional ratio is a measure of the departure of the sedimenting particle from spherical symmetry and is inversely related to the sedimentation coefficient. The sedimentation coefficient and frictional ratio, combined with the solvent density and viscosity and partial specific volume of the protein, produced an estimated signal-average molar mass 213,000 g/mol for molecule(s) corresponding to the shaded region in Figure 4B as described in Methods. This is close to the mass estimated for the fVIII heterodimer in HBS/Ca/PS-80 (25). These results are consistent with the dominant species in formulated Helixate being the fVIII heterodimer that sediments faster in formulation buffer due to a dramatic decrease in the frictional ratio. The frictional ratio of fVIII in formulation buffer is typical of a globular protein whereas the value in HBS/Ca/PS-80 of 1.9 is due to a significant departure from spherical symmetry. Scanning transmission electron microscopy studies indicate that the B domain projects from the body of porcine fVIII as a long stalk (33), which could explain the relatively large frictional ratio of the fVIII heterodimer. Conceivably, the B domain packs next to the body of fVIII in HBS/Ca/PS-80 buffer, reducing its frictional ratio.
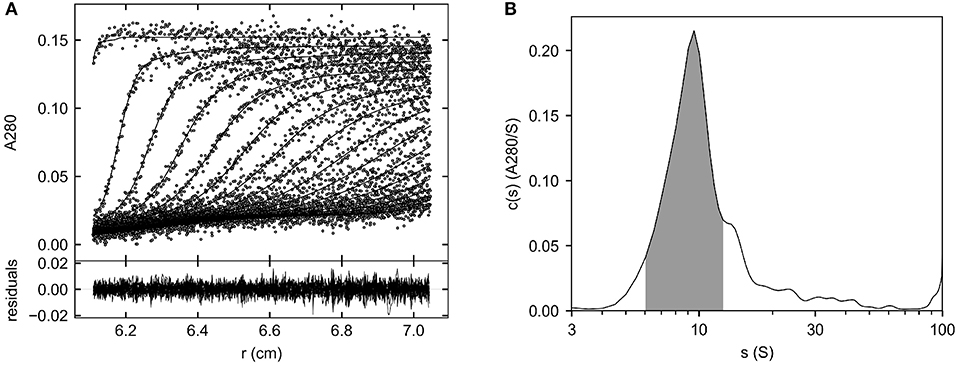
Figure 4. Major species of Helixate in formulation buffer determined by SV AUC. Helixate was reconstituted in Sterile Water for Injection to produce its therapeutic formulation. The density and viscosity of the formulated solution were measured as described in Methods and the sample was subjected to SV AUC at 105,000 g at 20°C. (A) Fitted absorbance scans. Only every fourth scan and every other data point are shown for clarity. Lower panel, residuals of the fitted data. (B) Continuous c(s) distribution from 0 to 100 S. The shaded area corresponds to the region used to determine the signal average sedimentation coefficient of the dominant species.
This study demonstrates that oxidation of Helixate, although damaging to procoagulant function, does not result in increased large molecular weight aggregates. SV AUC analysis following oxidation in a HEPES buffer or manufacturer's formulation buffer revealed an equivalent proportion of aggregates compared to non-oxidized Helixate, which was similar to previous reports (25). Buffer exchange using a desalting column did not remove aggregates from Helixate and the total A280 was unchanged before and after oxidation. The immunogenicity of fVIII is independent of its procoagulant function (34, 35) but is, in part, regulated by association with von Willebrand factor (VWF) (34, 36). Oxidation of Helixate with ClO− was previously shown not to affect VWF binding (16). Therefore, the mechanism by which oxidized fVIII induces an increased immune response remains unanswered, however, it is not protected by VWF binding. Aggregates within protein biologics, including fVIII (37), are known to increase the immunogenic potential of the protein drug. It is conceivable that the structural and chemical changes caused by oxidation of Helixate result in increased or novel epitope exposure or altered antigen recognition following administration. Modifications to the protein which are not detected as changes in sedimentation rate, size, or frictional ratio must be occurring due to the loss of procoagulant activity, and these deformations must be further investigate for a better understanding of fVIII immunogenicity. Taken together, this study supports multiple independent mechanisms of immunogenicity that contribute to the complexity of ADA formation.
Herein we highlight the importance of formulation composition for biologic drugs with heterogeneous populations, such as fVIII. Within marketed recombinant fVIII products, buffers, and stabilizers have changed significantly across product generations without sufficient analysis into their propensity to aggregate, until recently (26). In this study, we demonstrate a significant difference in fVIII conformation in HBS/Ca/PS80 buffer compared to 1x or 2x formulation buffer, measured by frictional ratio (Figure 3). This resulted in the dominant species of fVIII in formulation buffer to distribute over a larger c(s) range (Figure 4). In light of recent studies suggesting increased immunogenicity in recombinant products compared to plasma derived (38, 39), the stability and uniformity of recombinant fVIII products prior to administration is of great importance. One such complication is the inconsistent concentration of excipient stabilizers across lots of a single recombinant product, normalized to fVIII IU/ml (25). Excess or deficient solute within the formulation buffer alters the density, viscosity, and frictional ratio which therefore alters the homogeneity of the fVIII product. Furthermore, absence of sufficient redox protectants can increase the propensity to form ADA following administration. To this effect, we also measured fVIII activity in NaHPO4 buffer following OCl-oxidation (data not shown). In this buffer devoid of alternative oxidation targets such as HEPES, PS-80, histidine, glycine, etc., the IC50 was determined at only 5-fold molar excess compared to 55-fold in 2x formulation buffer. Therefore, while the mechanism of oxidation-induced immunity is not driven by aggregate formation and remains unexplained, the inclusion of improved buffers, stabilizers, and antioxidants may contribute to a reduced incidence of anti-fVIII inhibitors.
All datasets for this study are included in the article/supplementary material.
PZ, IS, JH, and PL planned and performed experiments, interpreted data, and participated in writing the paper. DL interpreted data and participated in writing the paper.
This work was supported by the National Institutes of Health/National Heart, Lung and Blood Institute (award number U54HL141981 to PL) and the Canadian Institute of Health Research (award number FDN 154285 to DL).
PL is inventor on patents owned by Emory University claiming compositions of matter that include modified FVIII proteins with reduced reactivity with anti-FVIII antibodies. DL receives grant support from Bayer, Bioverativ, CSL, and Octapharma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Markovitz RC, Healey JF, Parker ET, Meeks SL, Lollar P. The diversity of the immune response to the A2 domain of human factor VIII. Blood. (2013) 121:2785–95. doi: 10.1182/blood-2012-09-456582
2. Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Antihuman factor VIII C2 domain antibodies in hemophilia A mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation. Blood. (2007) 110:4234–42. doi: 10.1182/blood-2007-06-096842
3. Batsuli G, Ito J, Mercer R, Baldwin WH, Cox C, Parker ET, et al. Anti-C1 domain antibodies that accelerate factor VIII clearance contribute to antibody pathogenicity in a murine hemophilia A model. J Thromb Haemost. (2018) 16:1779–88. doi: 10.1111/jth.14233
4. Gouw SC, van den Berg HM, Fischer K, Auerswald G, Carcao M, Chalmers E, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood. (2013) 121:4046–55. doi: 10.1182/blood-2012-09-457036
5. Gouw SC, van der Bom JG, Ljung R, Escuriola C, Cid AR, Claeyssens-Donadel S, et al. Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med. (2013) 368:231–9. doi: 10.1056/NEJMoa1208024
6. Lusher JM, Arkin S, Abildgaard CF, Schwartz RS. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and development of inhibitors. Kogenate Previously Untreated Patient Study Group. N Engl J Med. (1993) 328:453–9. doi: 10.1056/NEJM199302183280701
7. Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. (2003) 9:418–35. doi: 10.1046/j.1365-2516.2003.00780.x
8. Goudemand J, Rothschild C, Demiguel V, Vinciguerrat C, Lambert T, Chambost H, et al. Influence of the type of factor VIII concentrate on the incidence of factor VIII inhibitors in previously untreated patients with severe hemophilia A. Blood. (2006) 107:46–51. doi: 10.1182/blood-2005-04-1371
9. Calvez T, Chambost H, d'Oiron R, Dalibard V, Demiguel V, Doncarli A, et al. Analyses of the FranceCoag cohort support differences in immunogenicity among one plasma-derived and two recombinant factor VIII brands in boys with severe hemophilia A. Haematologica. (2018) 103:179–89. doi: 10.3324/haematol.2017.174706
10. Gouw SC, van den Berg HM, Oldenburg J, Astermark J, de Groot PG, Margaglione M, et al. F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood. (2012) 119:2922–34. doi: 10.1182/blood-2011-09-379453
11. Astermark J, Altisent C, Batorova A, Diniz MJ, Gringeri A, Holme PA, et al. Non-genetic risk factors and the development of inhibitors in haemophilia: a comprehensive review and consensus report. Haemophilia. (2010) 16:747–66. doi: 10.1111/j.1365-2516.2010.02231.x
12. Bardi E, Astermark J. Genetic risk factors for inhibitors in haemophilia A. Eur J Haematol. (2015) 94(Suppl 77):7–10. doi: 10.1111/ejh.12495
13. Álvarez T, Soto I, Astermark J. Non-genetic risk factors and their influence on the management of patients in the clinic. Eur J Haematol. (2015) 94(Suppl 77):2–6. doi: 10.1111/ejh.12494
14. Lövgren KM, Søndergaard H, Skov S, Wiinberg B. Joint bleeds increase the inhibitor response to human factor VIII in a rat model of severe haemophilia A. Haemophilia. (2016) 22:772–9. doi: 10.1111/hae.13014
15. Alom-Ruiz SP, Anilkumar N, Shah AM. Reactive oxygen species and endothelial activation. Antioxid Redox Signal. (2008) 10:1089–100. doi: 10.1089/ars.2007.2007
16. Peyron I, Dimitrov JD, Delignat S, Gangadharan B, Srivastava A, Kaveri SV, et al. Oxidation of factor VIII increases its immunogenicity in mice with severe hemophilia A. Cell Immunol. (2018) 325:64–8. doi: 10.1016/j.cellimm.2018.01.008
17. Hermeling S, Schellekens H, Maas C, Gebbink MF, Crommelin DJ, Jiskoot W. Antibody response to aggregated human interferon alpha2b in wild-type and transgenic immune tolerant mice depends on type and level of aggregation. J Pharm Sci. (2006) 95:1084–96. doi: 10.1002/jps.20599
18. Shahab U, Ahmad S, Moinuddin, Dixit K, Habib S, Alam K, et al. Hydroxyl radical modification of collagen type II increases its arthritogenicity and immunogenicity. PLoS ONE. (2012) 7:e31199. doi: 10.1371/journal.pone.0031199
19. Prokopowicz ZM, Arce F, Biedron R, Chiang CL, Ciszek M, Katz DR, et al. Hypochlorous acid: a natural adjuvant that facilitates antigen processing, cross-priming, and the induction of adaptive immunity. J Immunol. (2010) 184:824–35. doi: 10.4049/jimmunol.0902606
20. van Beers MM, Sauerborn M, Gilli F, Brinks V, Schellekens H, Jiskoot W. Oxidized and aggregated recombinant human interferon beta is immunogenic in human interferon beta transgenic mice. Pharm Res. (2011) 28:2393–402. doi: 10.1007/s11095-011-0451-4
21. Roberts CJ. Therapeutic protein aggregation: mechanisms, design, and control. Trends Biotechnol. (2014) 32:372–80. doi: 10.1016/j.tibtech.2014.05.005
22. Moussa EM, Panchal JP, Moorthy BS, Blum JS, Joubert MK, Narhi LO, et al. Immunogenicity of therapeutic protein aggregates. J Pharm Sci. (2016) 105:417–30. doi: 10.1016/j.xphs.2015.11.002
23. Collins PW, Palmer BP, Chalmers EA, Hart DP, Liesner R, Rangarajan S, et al. Factor VIII brand and the incidence of factor VIII inhibitors in previously untreated UK children with severe hemophilia A, 2000-2011. Blood. (2014) 124:3389–97. doi: 10.1182/blood-2014-07-580498
24. Calvez T, Chambost H, Claeyssens-Donadel S, d'Oiron R, Goulet V, Guillet B, et al. Recombinant factor VIII products and inhibitor development in previously untreated boys with severe hemophilia A. Blood. (2014) 124:3398–408. doi: 10.1182/blood-2014-07-586347
25. Healey JF, Parker ET, Lollar P. Identification of aggregates in therapeutic formulations of recombinant full-length factor VIII products by sedimentation velocity analytical ultracentrifugation. J Thromb Haemost. (2018) 16:303–15. doi: 10.1111/jth.13917
26. Anzengruber J, Lubich C, Prenninger T, Gringeri A, Scheiflinger F, Reipert BM, et al. Comparative analysis of marketed factor VIII products: recombinant products are not alike vis-a-vis soluble protein aggregates and subvisible particles. J Thromb Haemost. (2018) 16:1176–81. doi: 10.1111/jth.14125
27. De Cristofaro R, Sacco M, Lancellotti S, Berruti F, Garagiola I, Valsecchi C, et al. Molecular aggregation of marketed recombinant FVIII products: biochemical evidence and functional effects. TH Open. (2019) 3:e123–31. doi: 10.1055/s-0039-1688413
28. Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys J. (2000) 78:1606–19. doi: 10.1016/S0006-3495(00)76713-0
29. Dam J, Schuck P. Calculating sedimentation coefficient distributions by direct modeling of sedimentation velocity concentration profiles. Methods Enzymol. (2004) 384:185–212. doi: 10.1016/S0076-6879(04)84012-6
30. Svedberg T, Pedersen KO. The Ultracentrifuge. New York, NY: Oxford at the Clarendon Press (1940).
31. Brautigam CA. Calculations and publication-quality illustrations for analytical ultracentrifugation data. Methods Enzymol. (2015) 562:109–33. doi: 10.1016/bs.mie.2015.05.001
32. Zhao H, Brautigam CA, Ghirlando R, Schuck P. Overview of current methods in sedimentation velocity and sedimentation equilibrium analytical ultracentrifugation. Curr Protoc Protein Sci. (2013) Chapter 20:Unit20.12. doi: 10.1002/0471140864.ps2012s71
33. Mosesson MW, Fass DN, Lollar P, DiOrio JP, Parker CG, Knutson GJ, et al. Structural model of porcine factor VIII and factor VIIIa molecules based on scanning transmission electron microscope (STEM) images and stem mass analysis. J Clin Invest. (1990) 145:1310–1. doi: 10.1172/JCI114662
34. Meeks SL, Cox CL, Healey JF, Parker ET, Doshi BS, Gangadharan B, et al. A major determinant of the immunogenicity of factor VIII in a murine model is independent of its procoagulant function. Blood. (2012) 120:2512–20. doi: 10.1182/blood-2012-02-412361
35. Gangadharan B, Delignat S, Ollivier V, Gupta N, Mackman N, Kaveri SV, et al. Role of coagulation-associated processes on factor VIII immunogenicity in a mouse model of severe hemophilia A. J Thromb Haemost. (2014) 12:2065–9. doi: 10.1111/jth.12740
36. Pipe SW, Montgomery RR, Pratt KP, Lenting PJ, Lillicrap D. Life in the shadow of a dominant partner: the FVIII-VWF association and its clinical implications for hemophilia A. Blood. (2016) 128:2007–16. doi: 10.1182/blood-2016-04-713289
37. Pisal DS, Kosloski MP, Middaugh CR, Bankert RB, Balu-Iyer SV. Native-like aggregates of factor VIII are immunogenic in von Willebrand factor deficient and hemophilia a mice. J Pharm Sci. (2012) 101:2055–65. doi: 10.1002/jps.23091
38. Canis K, Anzengruber J, Garenaux E, Feichtinger M, Benamara K, Scheiflinger F, et al. In-depth comparison of N-glycosylation of human plasma-derived factor VIII and different recombinant products: from structure to clinical implications. J Thromb Haemost. (2018) 16:1592–603. doi: 10.1111/jth.14204
39. Peyvandi F, Mannucci PM, Garagiola I, Elalfy M, El-Beshlawy A, Ramanan MV, et al. Source of factor VIII replacement (PLASMATIC OR RECOMBINANT) and incidence of inhibitory alloantibodies in previously untreated patients with severe hemophilia a: the multicenter randomized sippet study. Blood. (2015) 126:5. doi: 10.1182/blood.V126.23.5.5
Keywords: hemophilia A, factor VIII, oxidation, immunogenicity, analytical ultracentrifugation
Citation: Zakas PM, Healey JF, Smith IW, Lillicrap D and Lollar P (2020) Sedimentation Velocity Analytical Ultracentrifugation of Oxidized Recombinant Full-Length Factor VIII. Front. Immunol. 11:150. doi: 10.3389/fimmu.2020.00150
Received: 24 November 2019; Accepted: 20 January 2020;
Published: 07 February 2020.
Edited by:
Sébastien Lacroix-Desmazes, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Sathy Balu-Iyer, University at Buffalo, United StatesCopyright © 2020 Zakas, Healey, Smith, Lillicrap and Lollar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pete Lollar, amxvbGxhckBlbW9yeS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.