- 1Guangdong Provincial Key Laboratory of Medical Molecular Diagnostics, The Scientific Research Center of Dongguan, College of Pharmacy, Institute of Clinical Laboratory Medicine, Guangdong Medical University, Dongguan, China
- 2Marine Medical Research Institute of Guangdong Zhanjiang, Zhanjiang, China
- 3Biosafety Level-3 Laboratory, Guangdong Provincial Key Laboratory of Tropical Disease Research, School of Public Health, Southern Medical University, Guangzhou, China
- 4Key Laboratory for Tropical Diseases Control of the Ministry of Education, Department of Microbiology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China
- 5School of Biomedical and Pharmaceutical Science, Guangdong University of Technology, Guangzhou, China
- 6Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
CD47 is an immunoglobulin that is overexpressed on the surface of many types of cancer cells. CD47 forms a signaling complex with signal-regulatory protein α (SIRPα), enabling the escape of these cancer cells from macrophage-mediated phagocytosis. In recent years, CD47 has been shown to be highly expressed by various types of solid tumors and to be associated with poor patient prognosis in various types of cancer. A growing number of studies have since demonstrated that inhibiting the CD47-SIRPα signaling pathway promotes the adaptive immune response and enhances the phagocytosis of tumor cells by macrophages. Improved understanding in this field of research could lead to the development of novel and effective anti-tumor treatments that act through the inhibition of CD47 signaling in cancer cells. In this review, we describe the structure and function of CD47, provide an overview of studies that have aimed to inhibit CD47-dependent avoidance of macrophage-mediated phagocytosis by tumor cells, and assess the potential and challenges for targeting the CD47-SIRPα signaling pathway in anti-cancer therapy.
Introduction
Despite rapid improvements in anti-cancer therapies, the incidence of cancer and the rates of morbidity and mortality in cancer patients remain unacceptably high. In 1957, Burnet and Thomas proposed the “immuno-monitoring” hypothesis, suggesting that the immune system can monitor and eliminate “exotic” entities to maintain a homeostatic environment, including malignant cells that express various tumor-specific as well as non-tumor-specific antigens (1). Tumor immunosurveillance is an important process of limiting tumor growth and macrophages play a major role in the recognition and removal of tumor cells from the body (2). T-cells and natural killer (NK) cells are other important effector cells of the immune system, which also play important roles in anti-tumor immunity (2). The evasive behavior of tumor cells from immune recognition and clearance depends on a multitude of processes and includes induction of an immunosuppressive tumor microenvironment and reductions in tumor cell immunogenicity (3, 4). One key mechanism of tumor cell immune escape is through overexpression of the immunosuppressive signaling molecule, CD47 (5–7). Widely regarded as a “don't eat me” signal, CD47 helps maintain immunotolerance by non-malignant cells under physiological conditions (7), but this same molecule can aid in the survival of cancer cells in various cancer types (5, 6). In many cancer types, CD47 binding to signal-regulatory protein α (SIRPα) initiates an inhibitory signaling pathway that leads to the evasion of malignant cells from phagocytosis by macrophages (8).
Immunotherapy aims to reduce tumor propagation through activation or modulation of the innate or adaptive immune systems (9). To improve understanding of the mechanisms used by immune cells to detect and eliminate cancer cells, several molecules and signaling pathways have been investigated (10). Given that the binding of CD47 with SIRPα in tumor cells limits the anti-cancer immune response, it is possible that therapies that inhibit CD47 signaling in cancer cells would promote the phagocytosis of tumor cells by macrophages and thereby limit tumor growth (11). For example, immune cells or antibodies that inhibit tumor cell-expressed CD47 could be used to reduce the growth and spread of tumors with high expression of CD47, providing a feasible immunological target for anti-tumor therapies (12). However, the CD47-mediated regulation of phagocytotic removal of different types of cancer cells remains incompletely understood. The aims of this review are to: (i) describe the structure and function of CD47; (ii) provide an overview of studies that attempt to promote macrophage-mediated tumor cell phagocytosis through antagonism of CD47 signaling; and (iii) discuss the potential and challenges for targeting CD47-SIRPα signaling in anti-cancer therapies.
Characterization of CD47 Structure and Ligands
CD47 is a transmembrane protein that is glycosylated on the surface of a variety of different cell types (13, 14). Lindberg et al. isolated and purified CD47, which is also known as integrin-associated protein (IAP) (13). CD47 belongs to the immunoglobulin superfamily and is a supramolecular complex composed of integrins, G protein, and cholesterol (15). The structure of CD47 includes an amino terminal extracellular variable region, a transmembrane region formed of highly hydrophobic transmembrane segment, and a hydrophilic carboxy-terminal cytoplasmic tail that interacts with the corresponding ligands (15) to mediate a series of processes such as cell proliferation, migration, phagocytosis, and apoptosis, as well as immune homeostasis and inhibition of NO signaling (16, 17).
The ligands of CD47 include SIRPα, thrombospondin-1 (TSP-1), and integrins including αvβ3 and α2β1 (15). SIRPα, also known as SHPS-1, is a transmembrane protein with an extracellular region containing three immunoglobulin superfamily-like regions: one NH2-terminal V-like structure domain and two C1-like IgSF domains, with the NH2 terminal domain able to bind to CD47 (Figure 1A). Examination of the CD47/SIRPα complex revealed that the anti-human SIRPα antibody, KWAR23, binds SIRPα at an epitope overlapping with the CD47/SIRPα interface, indicating a basis for competitive antagonism of the CD47/SIRPα interaction (Figure 1B). SIRPα is highly expressed on the membrane of myeloid cells, such as macrophages, granulocytes, monocytes, and myeloid dendritic cells (20). It regulates cell migration and phagocytic activity as well as immune homeostasis and neural network formation (16, 21). TSP-1 is a homotrimeric multi-domain extracellular matrix glycoprotein belonging to a family of extracellular secreted proteins that consist of a variety of domains known to bind extracellular matrix components and cell surface receptors (12). TSP-1 is secreted by platelets, monocytes, macrophages, and a variety of other non-hematopoietic cell types (22). TSP-1 binding to CD47 leads to changes in the concentration of intracellular calcium ions and cyclic adenosine/cyclophosphinoside that regulate cell survival and migration, and TSP-1 also induces cellular responses to tissue damage (12, 17).
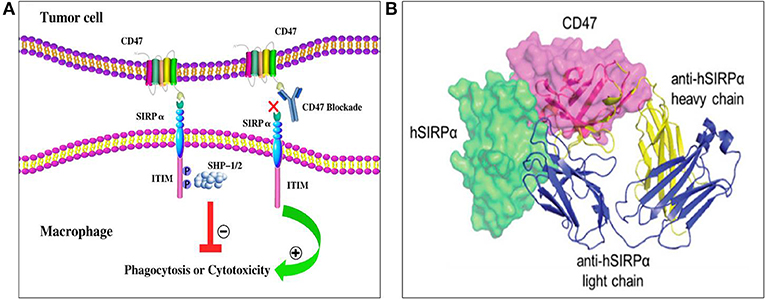
Figure 1. Structure and interaction of CD47 and SIRPα. (A) CD47 contains one N-terminal extracellular IgV-like domain and five transmembrane spanning segments. SIRPα contains three extracellular IgSF domains, one transmembrane spanning region, and an intracellular domain with ITIM motifs. After the binding of CD47 to SIRPα, two ITIMs in the cytoplasmic tail of SIRPα become phosphorylated then recruit and activate phosphatases including SHP-1 and SHP-2. Ultimately, CD47-SIRPα binding inhibits the host cell from being targeted for phagocytosis, while anti-CD47 antibodies can block the suppression signal and promote phagocytosis (18). (B) The anti-SIRPα antibody, KWAR23 (depicted as ribbons), binds SIRPα at an epitope overlapping with the CD47/SIRPα interface (19). Abbreviations: CD47, cluster of differentiation 47; ITIM, immunoreceptor tyrosine-based inhibitory motif; SHPS-1/2, protein tyrosine phosphatase substrate-1/2; SIRPα, signal-regulatory protein α.
Pathophysiological Function of CD47
The expression of CD47 is used by macrophages to distinguish between “self” or “non-self” (7). CD47 is expressed on the surface of non-malignant cells as well as multiple types of cancer cells and can bind to the SIRPα transmembrane protein on myeloid cells (especially macrophages) to form the CD47-SIRPα signaling complex (23). The extracellular IgV domain of SIRPα binds to CD47, leading to tyrosine phosphorylation on the intracellular ITIM motif; SIRPα also binds to the SH2 domain-containing tyrosine phosphatases, both of which inhibit the accumulation of myosin IIA in phagocytic synapses and facilitate the release of “don't eat me” signals that inhibit macrophage-mediated phagocytosis and protect normal cells from being damaged by the immune system (8, 23). Conversely, when the surface expression of CD47 is reduced, for example in aged cells, the CD47-SIRPα signaling pathway is weakened (24, 25), and macrophages can move toward and phagocytose these cells (26). CD47 on normal erythrocytes binds to SIRPα on the surface of macrophages, producing an inhibitory signal that prevents phagocytosis (27), but when erythrocytes undergo senescence, the expression level of CD47 is decreased, and CD47-deficient senescent erythrocytes are regarded as foreign and rapidly cleared by macrophages in the spleen (27). Ishikawa-Sekigami et al. transferred normal erythrocytes into mice lacking intracellular ITIM motifs and found that these erythrocytes were rapidly phagocytosed, confirming that the CD47-SIRPα signaling pathway plays a major role in the phagocytosis of erythrocytes (28). CD47 and its ligands not only regulate the immune response, but also mediate various pathophysiological processes such as neutrophil chemotaxis and nervous system development, as well as playing a regulatory role in immune tolerance and T-cell activation (11).
CD47 Expression in Cancer Cells and Tumors
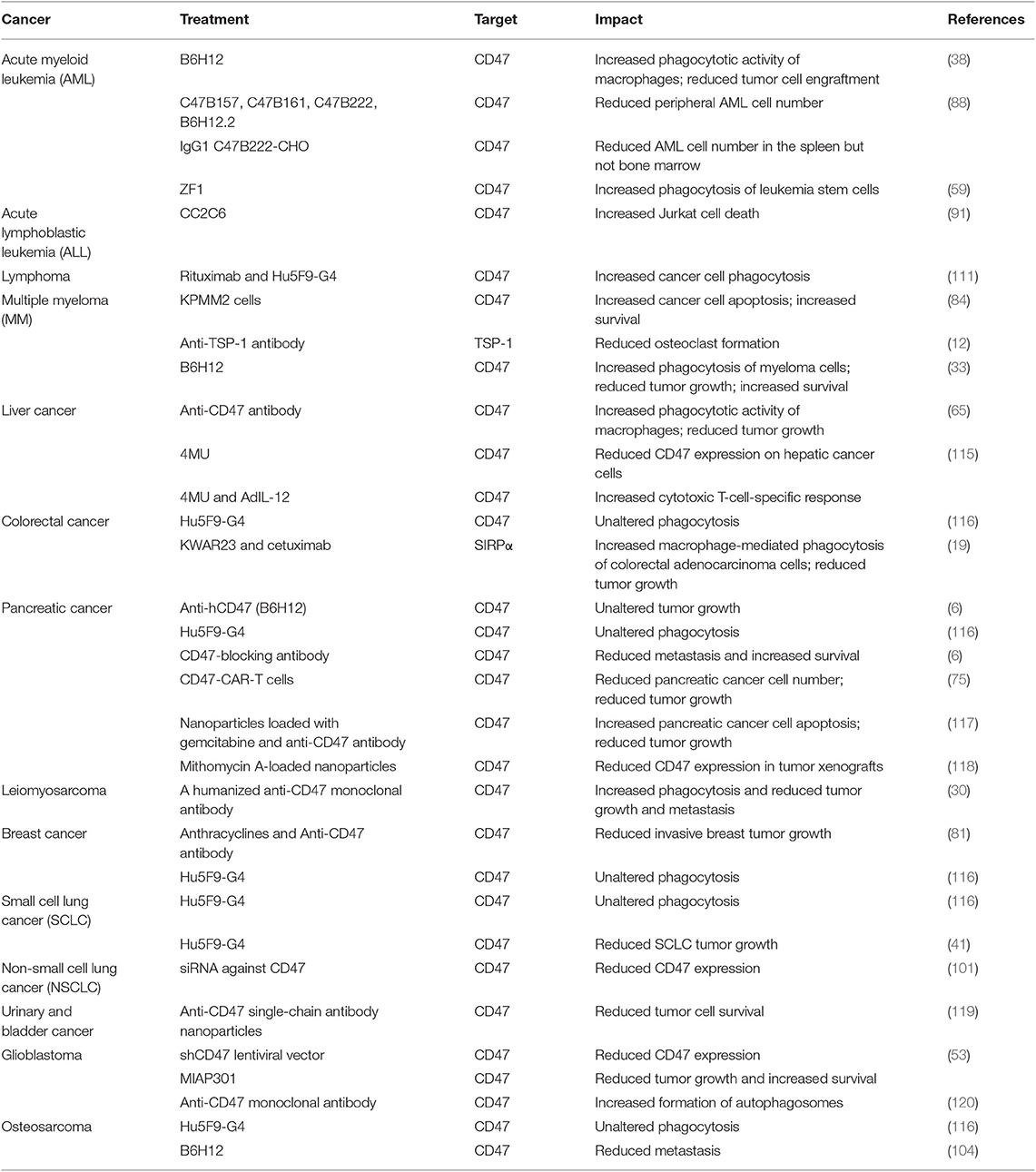
Cancer cells exploit the “don't eat me” function of CD47 by expressing higher levels of CD47 on their surface compared with non-malignant cells; numerous studies have shown that CD47 is overexpressed in different types of tumors (16), including in myeloma (29), leiomyosarcoma (30), acute lymphocytic leukemia (31), non-Hodgkin's lymphoma (32), breast cancer (33), osteosarcoma (34), and head and neck squamous cell carcinoma (23). For example, studies by Russ et al. demonstrated that CD47 is overexpressed in acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) cells when compared with normal myeloid cells from healthy mice or humans; these authors also showed that higher CD47 expression levels were associated with worsened therapeutic response and prognosis (35). Under inflammatory conditions and during cytokine mobilization, the expression of CD47 in normal hematopoietic stem cells is upregulated (36), and leukemia progenitor cells use this mechanism to evade macrophage-mediated phagocytosis (37). Majeti et al. (38) and Yang et al. (39) demonstrated that CD47 mRNA and protein levels in leukemia stem cells from AML patients were higher than those in normal healthy stem cells and that these increases were associated with poor prognosis. Galli et al. (40) found that CD47 is highly expressed in 25% of primary AML samples and that the expression of CD47 is inversely related to treatment response and favorable prognosis. Mohanty et al. found that the expression of CD47 in human osteosarcoma samples was higher than that of normal bone samples (34). Weiskopf et al. found that small cell lung cancer (SCLC) cell lines (NCI-H1688, NCI-H128, NCI-H524, NCI-H82, NCI-H69, and NCI-H196) expressed high levels of CD47 mRNA and protein (41). Compared to both normal peripheral blood and germinal center B cells, CD47 is more highly expressed on a large subset of primary patient samples from multiple B cell non-Hodgkin lymphoma (NHL) subtypes, including diffuse large B cell lymphoma (DLBCL), B cell chronic lymphocytic leukemia (B-CLL), mantle cell lymphoma (MCL), follicular lymphoma (FL), marginal zone lymphoma (MZL), and pre-B acute lymphoblastic leukemia (pre-B ALL), while high expression of CD47 is predictive of poor prognosis in NHL patients (42). CD47 is also overexpressed on myeloma cells, and its expression increases with the progression of multiple melanoma (MM) (43). Rendtlew Danielsen et al. (44) analyzed the expression of CD47 and TSP-1 and TSP-2 in plasmocytes from MM patients using fluorescence-activated cell sorting and found that CD47 and TSP-1 and TSP-2 ligands are highly up-regulated in the MM interstitial environment. Furthermore, CD47 is overexpressed on the surface of hepatocellular carcinoma (HCC) cells, and high expression of CD47 is associated with enhanced metastasis of liver cancer cells in transplanted mice (45). In a study of CD47 expression in four cholangiocarcinoma (CCA) cell lines (KKU-100, KKU-055, KKU-213, and HuCCT1) and 54 CCA tissues samples, high expression of CD47 was found in 3 out of 4 of the CCAs cell lines (KKU-055, KKU-213, and HuCCT1) and in 50 out of 54 of the CCA tissue samples (46). Wang et al. (47) used immunohistochemistry to detect CD47 in ovarian clear cell carcinoma tissues and showed that the survival rate of patients with low CD47 is higher than that of high-level patients, while CD47 level is predictive of the patient's disease stage, chemotherapy resistance, and prognosis. Li et al. (48) demonstrated that ovarian cancer patients with low expression of CD47 in their tumors have a better therapeutic response to standard treatment and overall survival compared with the patients with higher CD47 expression. Brightwell et al. (49) used the cancer genome atlas to test the association between CD47 expression and clinical outcome in epithelial ovarian cancer patients; immunohistochemical analysis of 265 patient specimens showed that CD47 expression was found in 210 of 265 (79%) patients, and that of these patients, high levels of CD47 protein expression was found in 129 of 265 (49%) patients. Nagahara et al. (50) evaluated CD47 and SIRPα mRNA levels in the bone marrow and peripheral blood of 738 breast cancer patients; these authors showed the survival rate of patients with high CD47 expression in their tumors was significantly lower than those with low CD47 expression levels. CD47 protein and mRNA levels are also overexpressed on the surfaces of lung cancer cells, and by SCLC tumors and non-small cell lung cancer (NSCLC) tumors (51). It has also been reported that CD47 is expressed on more than 80% of the surface of bladder cancer cells, and that the expression of CD47 in muscular invasive bladder cancer (MIBC) and non-muscle invasive bladder cancer (NMIBC) is significantly higher than that in normal urothelial cells (52). Li et al. (53) found that CD47 is highly expressed on glioma cells and glioma stem cells, while the levels of CD47 in 5 distinct patient-derived pancreatic ductal adenocarcinoma (PDAC) cell lines (T366, T395, T449, T608, and T738) are variable (54). In studies of Epstein-Barr virus-associated gastric carcinoma (EBVaGC), CD47 expression was increased in EBVaGC tissue samples compared to EBV-negative gastric cancer tissue samples (55). High expression of CD47 is also associated with poor prognosis in EBVaGC (55). Yoshida et al. (56) showed that gastric cancer cells with high CD47 expression show higher proliferation and spheroid colony formation than those expressing low levels of CD47. Conversely, another study indicated that there is no difference in the expression of CD47 mRNA between primary gastric cancer and normal gastric tissue (57). Furthermore, increased expression of CD47 mRNA is not an unfavorable prognostic factor in primary gastric tumors, suggesting that there may be post-transcriptional differences leading to increased expression of CD47 protein and that the reliance of disease progression upon CD47 expression could vary between different cancer types and patient populations. Future studies should continue to attempt to elucidate the respective role(s) of CD47 in different cancer types including pancreatic and gastric cancer (57).
Mechanisms of Action and Impact of Targeting CD47-SIRPα Signaling in Anti-Tumor Therapy
Overexpression of CD47 on the tumor cell surface can help these cells escape monitoring and clearance by immune cells, making CD47 a plausible target in the development of novel anti-tumor drugs (18). For example, anti-CD47 treatments have been used to block CD47-SIRPα inhibitory signaling and promote the phagocytosis of tumor cells by macrophages, but anti-CD47 treatments can exert their anti-tumor impact through a number of different mechanisms (Figure 2) (58).
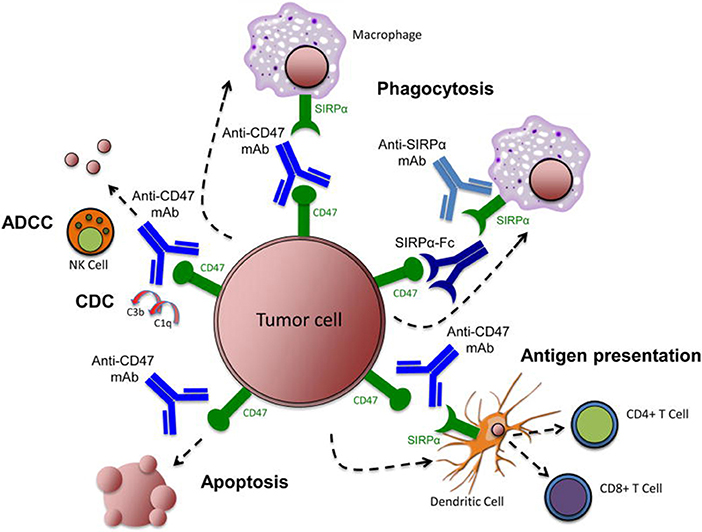
Figure 2. Targeting the CD47-SIRPα pathway in cancer. Therapeutic targeting of the CD47-SIRPα pathway can cause elimination of cancer cells through multiple mechanisms. First, inhibition of the CD47-SIRPα interaction using an anti-CD47 antibody, an anti-SIRPα antibody, or a recombinant SIRPα protein, leads to phagocytic uptake of tumor cells by macrophages. Second, anti-CD47 antibodies enable phagocytic uptake of tumor cells by dendritic cells and subsequent antigen presentation to CD4+ and CD8+ T-cells, thereby stimulating an anti-tumor adaptive immune response. Third, anti-CD47 antibodies eliminate tumor cells through natural killer cell-mediated antibody-dependent cytotoxicity and complement dependent cytotoxicity. Fourth, anti-CD47 antibodies stimulate apoptosis of tumor cells through a caspase-independent mechanism. Image reproduced with permission from Chao et al. (58). ADCC, antibody-dependent cell-mediated cytotoxicity; CDC, complement dependent cytotoxicity; mAb, monoclonal antibody; NK, natural killer; SIRPα, signal-regulatory protein α.
Impact of CD47/SIRPα Targeting on Macrophage-Mediated Phagocytosis
CD47 inhibition leads to stimulation of phagocytosis of cancer cells by macrophages (38, 41). Anti-CD47 antibodies enhance the phagocytosis of tumor cells by macrophages by directly blocking the binding of tumor cell-expressing CD47 to macrophage-expressing SIRPα (18). Treatment of mice with a macrophage-depleting chemical (clodronate) leads to abrogation of the anti-tumorigenic effects of CD47 blockade (38), supporting the role of macrophages in anti-CD47-mediated anti-tumor response. Zeng et al. (59) showed that the humanized anti-CD47 antibody, ZF1, blocks the interaction between CD47 and SIRPα, and enhances the phagocytosis of leukemia stem cells by macrophages. Through direct competition with SIRPα on the surface of macrophages, E. coli-constructed recombinant hSIRPext binds to CD47 on the surface of leukemia stem cells, which blocks CD47-SIRPα signaling (60). In their study, Lin et al. (60) combined the plasmid vector, pET32a, with the soluble extracellular domain of SIRPα, and imported the resulting construct into the E. coli strain, BL21, to obtain a CD47 fusion protein. Alternatively, they obtained another variant of the CD47 fusion protein by splicing the extracellular domain of human CD47 into a pET32a plasmid vector and importing this into the E. coli strain, BL21. Lin et al. (60) then co-incubated the 2 CD47 fusion proteins (Trx-hCD47ext and Trx-CD47ext) with Jurkat cells and showed that both the proteins enhance the phagocytosis of leukemia cells by macrophages in vitro. Other studies have shown that anti-CD47 antibodies promote macrophage phagocytic activity in human NHL cell-engrafted mice (61). Xu et al. (61) treated NHL mice with an anti-CD47 antibody and blinatumomab (which targets CD19 and CD3); this combination of therapies led to persistent control of lymphoma progression by inducing cancer cell phagocytosis and T-cell cytotoxicity. Hu5F9-G4 is a humanized IgG4 isotype monoclonal antibody against CD47 and a macrophage immunological checkpoint inhibitor that blocks CD47 to induce phagocytosis; previous studies have found that Hu5F9-G4 may act synergistically with rituximab to eliminate B-cell NHL cells by enhancing macrophage-mediated antibody-dependent phagocytosis (62). Weiskopf et al. (63) demonstrated that the anti-tumor synergistic impact of Hu5F9-G4 and rituximab treatment depends largely on macrophage-mediated tumor killing in a canine lymphoma xenograft model. Hu5F9-G4 also blocked the binding of SIRPα on the surface of lymphoma cells to CD47 on the surface of macrophages, thus attenuating the “don't eat me” signals within this cancer cell type. Compared to treatment with IgG control antibody, a monoclonal antibody against CD47, B6H12, enhanced the in vitro phagocytotic activity of human macrophages against cancer cells and prolonged the survival of mice with intraperitoneal metastatic cancer (56). Macrophage-mediated phagocytosis of liver cancer cells can be enhanced by treatment with an anti-CD47 antibody, a SIRPα blocking antibody, or by blocking the CD47-TSP-1 interaction (64, 65). Attenuation of CD47-SIRPα signaling in cholangiocarcinoma promotes the phagocytotic potential of a variety of macrophage subpopulations and inhibits cholangiocarcinoma growth and intrahepatic metastasis (66). Anti-SIRPα antibody treatment leads to enhanced macrophage phagocytic activity (67) and reduced tumor progression in a mouse model of colon cancer (67) and CD47-SIRPα signaling promotes the expansion and metastasis of colon cancer cells in tumor microenvironments that are rich in tumor-associated macrophages (68). Two xenograft models of leiomyosarcoma in mice (via LMS04 and LMS05 tumor cell transplant) have also been treated with a humanized anti-CD47 monoclonal antibody, which increases the levels of macrophage-mediated phagocytosis of leiomyosarcoma tumor cells and inhibits the growth of primary tumors and the formation of lung metastases after primary tumor graft resection (30). Ring et al. (19) incubated different colorectal adenocarcinoma cell lines with human macrophages after treatment with an anti-SIRPα antibody (KWAR23) in combination with cetuximab or panitumumab (two types of treatments targeting epidermal growth factor receptor); these authors found that KWAR23 alone enhances macrophage-mediated phagocytosis of DLD-1 colorectal adenocarcinoma cells, and that the combination of KWAR23 and cetuximab increases the macrophage-mediated phagocytosis of DLD-1, LS, 174T, HT-29, and HCT 116 colon adenocarcinoma cells. Notably, the effectiveness of KWAR23 in inducing macrophage-mediated tumor cell phagocytosis was dependent upon the concentration of the antibody used, suggesting that the dose of CD47-SIRPα-targeting antibodies should be carefully optimized during the development of novel treatments that aim to inhibit CD47-SIRPα signaling (19). In this regard, future studies should aim to generate sufficient yields of CD47 inhibitors with a view to clinical use. It should also be noted that phagocytosis is regulated by the balance of pro-phagocytotic and anti-phagocytic signals, so the net effect of pro-phagocytotic signaling and phagocytosis antagonism will impact upon macrophage phagocytosis (69).
Impact of CD47/SIRPα Targeting on Macrophage Recruitment and Polarization
As well as increasing the level of phagocytosis, it is possible that blocking CD47 increases macrophage recruitment to tumors. For example, phagocytosis following anti-CD47 treatment can cause the secretion of chemokines and cytokines that recruit additional immune cells to tumors; these factors secreted in response to CD47-blocking therapies include monocyte chemotactic protein 3 (41). The CD47-blocking antibody, Hu5F9-G4, inhibits the growth of SCLC tumors in vivo and stimulates the release of chemokines that promote macrophage recruitment and activation, thus contributing to the efficacy of CD47-blocking therapy (41). Macrophage polarization state may also be altered by anti-CD47 therapy and one study of glioblastoma found that CD47 blockade converts tumor-associated macrophages into an anti-tumor state and increases macrophage recruitment into the tumor (70).
Impact of CD47/SIRPα Targeting on the Adaptive Immune Response
CD47 blockade can promote the adaptive immune response, e.g., when treatment with an anti-CD47 antibody induced antigen-specific CD8+ T-cell proliferation and macrophage phagocytosis but reduced regulatory T-cell number in a colon cancer model, suggesting that anti-CD47 treatments can facilitate adaptive T-cell immune response (71). Similarly, a study by Liu et al. found that anti-CD47 antibody treatment inhibits tumor progression by enhancing the antigen-specific CD8+ T-cell response through dendritic cell-mediated presentation of tumor antigens to T-cells (72). In their study, Liu et al. also found using immunocompetent mouse models of lymphoma and lung cancer, that the anti-tumor responses to anti-CD47 treatment were partially dependent on an intact immune system (72). Furthermore, a separate study confirmed that anti-CD47 antibodies exert tumor-killing effects through the activation of CD8+ T-cells and dendritic cells (73), which phagocytose tumor cells and process specific antigens that lead to presentation of tumor cells to CD8+ T-cells, thereby activating tumor cell-specific adaptive immunity (73). Soto-Pantoja et al. have also shown that CD47 blockade induces a cytotoxic T-cell-dependent anti-tumor immune response in fibrosarcoma and that CD47 deletion in CD8+ T cells increases their anti-tumor activity, while raised CD47 expression was found to be associated with reduced infiltration of CD8+ T-cells in melanoma (74). In a study by Golubovskaya et al. (75), CD47-CAR-T cells that bind to CD47 were effective in killing pancreatic cancer cells. In addition, injection of CD47-CAR-T cells into immunodeficient mice blocked the growth of BxPC3 pancreatic xenograft tumors (75). Therefore, anti-tumorigenic immune cells directed to target CD47 could be used to inhibit tumorigenesis (76, 77). In another study, Zhou et al. (78) constructed tumor-activatable oxaliplatin prodrug vesicles, MPV-HOAD, and found that MPV-HOAD induced immunogenic cell death when combined with anti-CD47 antibody treatment and enhanced tumor immunogenicity; MPV-HOAD also induced the maturation of antigen presenting cells, thereby activating an anti-tumor immune response and effectively inhibiting the growth of both primary and abscopal tumors. In addition, after treatment with MPV-HOAD and CD47 blockade, CT26 colorectal tumor-bearing mice exhibited reduced tumor recurrence.
Impact of CD47/SIRPα Targeting on Cell-Mediated Cytotoxicity
Anti-CD47 treatments can activate the antibody-dependent cellular cytotoxicity-mediated innate immune response. For instance, a study of head-and-neck squamous cell carcinoma demonstrated that raised CD47 expression is associated with decreased natural NK cell-mediated cytotoxicity, while anti-CD47 antibody treatment enhanced NK cell-mediated cytotoxicity (79). In particular, anti-CD47 antibodies can cause NK cell-mediated antibody-dependent cellular cytotoxicity in an Fc receptor-dependent manner (42), while anti-CD47 antibodies or fusion proteins can also function through the Fc active domain leading to antibody-dependent cell-mediated cytotoxicity (80). Feliz-Mosquea et al. (81) performed a quantitative high-throughput screening of anti-cancer drugs and administered an anti-CD47 antibody to a mouse 4T1 breast cancer model and concluded that blocking CD47: (i) enhances anthracycline-mediated tumor cytotoxicity; (ii) enhances macrophage-mediated cytotoxicity of breast cancer cells; and (iii) prevents anthracycline-mediated cardiomyocyte and tissue toxicity. A separate study showed that CD47/SIRPα blockade augments neutrophil-mediated antibody-dependent cytotoxicity (82). Other studies have identified direct cytotoxicity of CD47-targeting therapies on CD47+ cancer cells (83–85), but therapies targeting the SIRPα binding epitope do not cause direct cytotoxicity on tumor cells (41, 63).
Impact of CD47/SIRPα Targeting on Tumor Cell Apoptosis
CD47 inhibition can also induce apoptosis of tumor cells; for instance, anti-CD47 antibodies increase tumor cell clearance by inducing apoptosis through a caspase-independent mechanism (71). Researchers have treated mice with KPMM2 cells, which are human myeloma cells transplanted with a mouse monoclonal single-chain antibody fragment targeting CD47 (84). Treatment with the KPMM2 cells induced cancer cell apoptosis and can be combined with other chemotherapies (83), thus providing a putative treatment for MM that may be used in combination with conventional chemotherapy (86). CD47 blockade using an antibody that is specific for an extracellular domain of CD47 augmented apoptosis of leukemia and myeloma cells (83, 84). Song et al. (87) transfected a CD47-siRNA lentiviral vector into the KG1a AML cell line to inhibit the expression of CD47 and found that this treatment reduced the expression of anti-apoptotic genes including Bcl-2, Bcl-xl and MCL-1. Pietsch et al. (88) assessed the anti-leukemia activity of several anti-CD47 antibodies in mice and cynomolgus monkeys; after transplantation of HL60, MV4-11, and Kasumi-3 AML cells, animals were treated with the Fc region of various anti-CD47 monoclonal antibodies (including C47B157, C47B161, C47B222, and B6H12.2) that led to reductions in the levels of peripheral AML cells. Interestingly, treatment of human or murine acute lymphoblastic leukemia (ALL) T-cells with a soluble CD47 agonist peptide induces changes in reactive oxygen species (ROS) production (89) and caspase-independent and calcium-dependent leukemia cell death; this study also showed that PKHB1-treated cells could be used as a prophylactic to prevent tumorigenesis (90). Leclair et al. (91) compared the Jurkat T-cell line (cells that expresses high levels of CD47) with the JC47-2-4 cell line (cells that expresses low levels of CD47). It was found that the anti-CD47 antibody, CC2C6, induces Jurkat cell death in a CD47-dependent manner. In addition to directly inducing cell death via blockade of CD47-SIRPα signaling, this study showed that CC2C6 synergizes with low-dose chemotherapeutic agents that induce classical apoptosis, resulting in an effective combination of treatments without the need for high-dose chemotherapy, which is often associated with long-term side-effects in pediatric ALL patients (91). A recent study has also shown that treatment of transgenic mice with the hyaluronan synthesis inhibitor, 4-methylumbelliferone (4MU), induces apoptosis and reduces inflammation, steatosis, and the expression of cancer stem cells markers (92). In the presence of cancer stem cells, 4MU downregulates the expression of CD47 on HCC cells and promotes phagocytosis of antigen presenting cells; in combination with adenovirus encoding interleukin-12 genes (AdIL-12), 4MU elicits a potent cytotoxic T-cell-specific response and prolongs survival in a hepatocellular carcinoma model established in fibrotic liver (93).
Impact of CD47/SIRPα Targeting on Tumor Cell Proliferation and Migration
Anti-CD47 therapies may inhibit tumor cell proliferation, given that CD47 promotes tumor cell proliferation and metastasis (94). In a mouse model of lymphoma, anti-CD47 antibodies prevent lymphoma cell proliferation and prolong survival (95). Treatment with a CD47-blocking antibody also leads to delayed progression of metastasis and prolonged survival in mice with pancreatic tumors (6, 96). CD47 binding with TSP-1 inhibits nitric oxide (NO) signaling and limits NO production, which accelerates osteoclast formation and activation, and in turn promotes tumor cell metastasis to bone (97). Meanwhile, blocking the interaction between CD47 and TSP-1 with anti-TSP-1 antibodies disrupts tumor-induced osteoclast formation (98), but deficiency of CD47 alone does not fully recapitulate the effects of antibody-mediated blockade of TSP-1 in either murine or human osteoclasts cells (12). Kim et al. (33) constructed a mouse model of myeloma cell transplantation and treated these mouse with B6H12, showing that B6H12 inhibits myeloma cell growth in the bone marrow microenvironment. Li et al. (53) showed that when a shCD47 lentiviral vector was used to reduce the expression of CD47 on glioma stem cells, the growth potential and differentiation of these cells were inhibited. Li et al. (53) also showed in immunocompetent mouse glioma models that blocking CD47 inhibits tumor growth and prolongs survival. Furthermore, synergistic activities of anti-CD47 therapies and chemotherapeutic drugs can enhance the efficacy of chemotherapy in patient-derived HCC xenograft mouse models and inhibit the growth and metastasis of liver cancer cells in vivo (99, 100). Using an antibody-free approach, the NSCLC cell lines, A549 and NCI-H520, have been transfected with siRNA against CD47 to reduce the endogenous expression of CD47 (101). Reduction of CD47 expression by siRNA treatment inhibited the migration and invasion of A549 and NCI-H520 cells on a microfluidic chip (102). Furthermore, CD47 overexpression in A549 and NCI-H520 cells by transfection with pcDNA3.1-3xFlag-CD47 conversely enhanced the migration and invasion potentials of A549 and NCI-H520 cells (5). These in vitro findings were confirmed in vivo using a mouse xenograft model of NSCLC (103). Given that NSCLC cell viability did not decrease following siRNA transfection to reduce CD47 expression, it appears that the treatment-dependent reductions in cell migration and invasion can occur without cytotoxicity. Xu et al. (104) treated a mouse osteosarcoma cell line (LM8) and a human osteosarcoma cell line (KRIB) with an anti-CD47 antibody (B6H12) or control IgG antibody; their study showed that in a mouse model of metastatic KRIB osteosarcoma, there is a positive correlation between CD47 expression in the KRIB cells and the extent of metastasis, and following treatment with the anti-CD47 antibody, osteosarcoma cells were less invasive than IgG-treated cells. By treating these mice with an anti-CD47 antibody (B6H12), spontaneous metastasis of xenograft osteosarcoma cancer cells to the lung was inhibited, and phagocytosis of the osteosarcoma cells by macrophages enhanced (104). Cell division control protein 42 (Cdc42), a member of the Rho family of small GTPases, has been identified as a regulator of metastasis and is activated downstream of CD47 to promote cell formation (105, 106). Cdc42 expression level is positively correlated with CD47 expression level in A549 and NCI-H520 lung cancer cell lines (107). In A549 and NCI-H520 lung cancer cells that ectopically express CD47, decreasing Cdc42 levels reduced cancer cell migration and invasion (108). Conversely, when the expression of Cdc42 is increased, the migration and invasion ability of lung tumor cells is enhanced (109). In 80 patients with advanced NSCLC, the expression levels of CD47 and Cdc42 were shown by immunohistochemistry to be positively correlated (5). If CD47 controls Cdc42 expression in NSCLC cells, and Cdc42 in turn mediates the invasive phenotype of these cancer cells, then blocking CD47 or inhibiting Cdc42 expression may become a new method of inhibiting the metastasis of NSCLC cells.
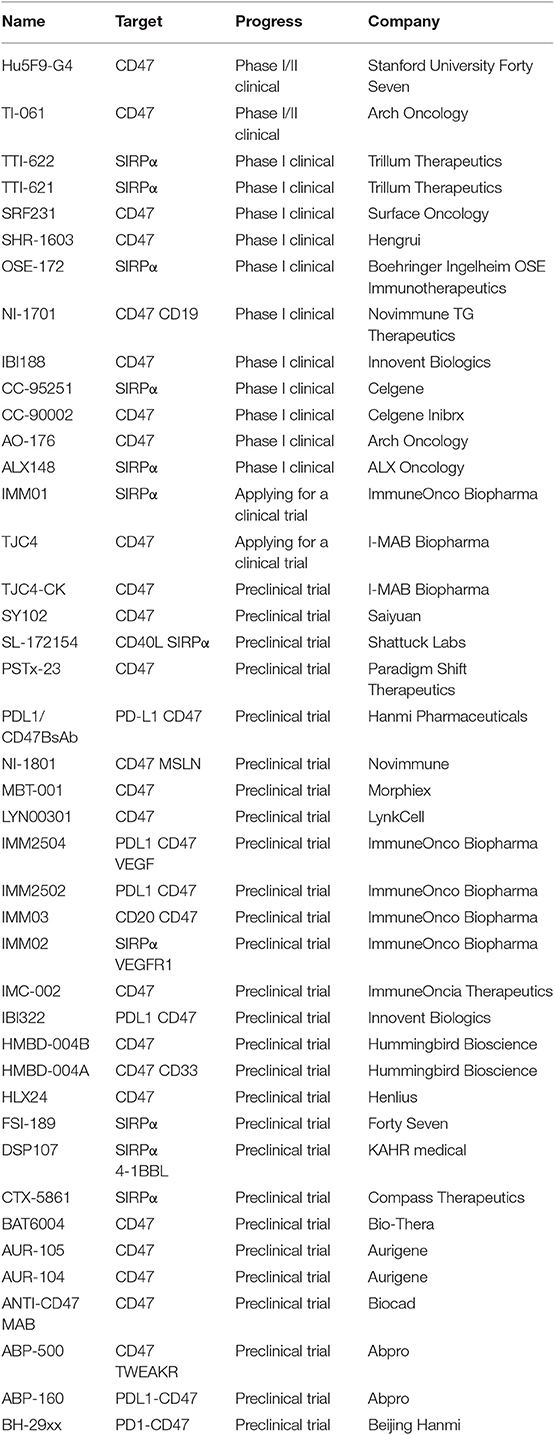
Anti-CD47 Therapies in Clinical Development and Their Tumor Specificity
While the CD47 signaling cascade remains incompletely understood, recent studies have improved understanding of CD47-dependent signaling and given rise to the development of CD47-targeting anti-cancer therapies that inhibit primary tumor growth and reduce metastasis in various types of cancer. Studies using an anti-CD47 antibody (IgG1 C47B222-CHO) in primary AML patients, however, showed that this treatment only affected the number of AML cells in the spleen without affecting the overall burden of myeloid leukemia (88). Nevertheless, several CD47-targeting antibodies or drugs have reached clinical trials (Table 1), including Hu5F9-G4 (Forty-Seven), CC-90002 (Celgene), TTI-621 (Trillium), ALX148 (Alexo Therapeutics), SRF231 (Surface Oncology), SHR-1603 (Hengrui), and IBI188 (Innovent Biologics). Among these, Hu5F9-G4, CC-90002, and IBI188 are anti-CD47 antibodies, while TTI-621 and ALX148 are SIRPα-Fc fusion proteins (18, 110). Advani et al. (111) recruited 22 patients with lymphoma (15 with DLBCL, 7 with follicular lymphoma, and 95% of whom had rituximab refractory disease) to carry out a phase 1b clinical trial; in patients with invasive and indolent lymphoma, Hu5F9-G4-associated phagocytosis was enhanced by the addition of rituximab; furthermore, adverse reactions experienced by the treated patients (e.g., anemia, nausea, diarrhea, and infusion-related reactions) occurred only in the first few weeks and were not followed by a significant increase in clinically safety events in the later stages of the trial. Recent studies have also proposed another method for reducing CD47 expression and decreasing tumor cell growth by using miRNA to inhibit the expression of CD47 (112, 113). Given that there is a negative correlation between miR-708 and CD47 in T-ALL patients (114), the recovery of miR-708 expression could regulate the expression of CD47. Furthermore, a combination of miR-708 and anti-CD47 antibody treatment increases the phagocytic activity of macrophages against leukemic CEM cells compared with either agent alone, and could therefore be used as a combinatorial anti-leukemia therapy in future studies (114).
Despite encouraging findings from studies of the impact of anti-CD47 therapy on cancer progression, the effectiveness of anti-CD47 treatments can vary between cancer types, different studies, and even different cancer types (Table 2); in other words, the therapeutic efficacy of anti-CD47 treatments may be tumor-specific. The different responses between tumor types could be a result of differences in: (i) drug delivery method (route, vehicle, frequency, efficiency); (ii) drug compartmentalization in the tumor cell; (iii) stage of cancer progression; (iv) capability of the immune system; and (v) acquired drug resistance. Potential factors contributing to drug resistance include (but are not limited to) tumor heterogeneity, changes in the tumor microenvironment, drug inactivation, decreased drug absorption or increased drug release from the tumor cells, activation of tumor cell survival pathways, and epigenetic changes (121, 122). In preclinical studies, the variation in response to anti-CD47 treatment between cancer type and individual studies could also be a result of differences in the type of tumor model(s) used to test treatment efficacy. For example, many of the studies that have been published to date make use of xenograft tumor models, while fewer studies have instead employed syngeneic tumor models; future studies should aim to develop and utilize tumor models that are as consistent as possible with the pathology of human tumors. The strengths and weaknesses of preclinical cancer models have been thoroughly reviewed elsewhere (123). While no single tumor model can fully recapitulate the pathogenesis and progression of malignancy in humans, consistent findings from a wide variety of different types of mouse models could ultimately lead to the development of novel CD47-targeting treatments for cancer patients.
Biosafety Problems and Future Perspectives
Preclinical studies using anti-CD47 antibodies in mice and macaques suggested that these therapeutics are well-tolerated (33, 62, 124). In 2017, however, Arch Oncology discontinued a phase I/II clinical trial of an anti-CD47 monoclonal antibody, Ti-061, while in 2018, Celgene discontinued a clinical trial of the anti-CD47 monoclonal antibody, CC-90002 for the treatment of AML. Given that CD47 is ubiquitously expressed, potential problems with using anti-CD47 antibodies as anti-cancer treatments include possible off-target effects such as anemia (73). For instance, CD47 is expressed by non-malignant cells of the hematopoietic system (28), including normal red blood cells, senescent red blood cells, and platelets (125, 126). Buatois et al. (127) showed that Hu47F9-G4 alone or in combination with other antibodies may cause accidental killing of normal red blood cells, potentially resulting in anemia. To alleviate this side-effect, one study (111) proposed to give short-term low-dose Hu5F9-G4 in combination with rituximab, which causes predictable and transient mild anemia due to selective elimination of aged red blood cells, followed by treatment with compensatory reticulocytes (127). The toxicity of anti-CD47 antibodies, however, appears to be Fc-dependent, given that anti-CD47 antibodies and SIRPα-Fc fusion proteins give rise to this toxicity, while high-affinity SIRPα monomers do not (33, 62, 124). These findings suggest that future studies should aim to optimize the structure of the anti-CD47 therapeutic when attempting to design novel drugs without unwanted side-effects. For example, next-generation SIRPα-Fc fusion protein variants have been generated to enhance their binding to CD47; these higher-affinity SIRPα variants bind to CD47 with greater potency compared to wild-type SIRPα (124), and were effective against hematologic and solid tumors in preclinical studies, but have not yet progressed to clinical trials (124, 128). In another recent study, Sim et al. describe their discovery of high affinity, pan-allelic, and pan-mammalian antibodies against SIRPα (129). Prior to this, Ho et al. described the production of high affinity CD47 ectodomain as an antagonist of SIRPα to increase antibody-dependent phagocytosis (130). As an aside to this, patient age should be considered in future studies of CD47/SIRPα-targeted therapies, given that older erythrocytes may be more susceptible to phagocytosis (131, 132). How to reduce or avoid damage to normal cells while exerting anti-tumor effects is one of the problems that needs to be considered when designing anti-CD47 therapies (Figure 3) (81). TTI-621 (Trilium) is an example of an antibody fusion protein consisting of the N-terminal V domain of human SIRPα fused to the human IgG1 Fc region that was developed to avoid damage to normal cells while still being able to increase the removal of tumor cells; although TTI-621 resulted in anemia and cytopenia in primates, healthy human erythrocytes showed minimal binding activity to TTI-621, enabling the therapy to be administered at low therapeutic doses whilst still maintaining sufficient receptor binding (133). The molecular structures and treatment regimens of ALX148 (134) and Hu5F9-G4 have also been designed with a view to reducing cytotoxicity.
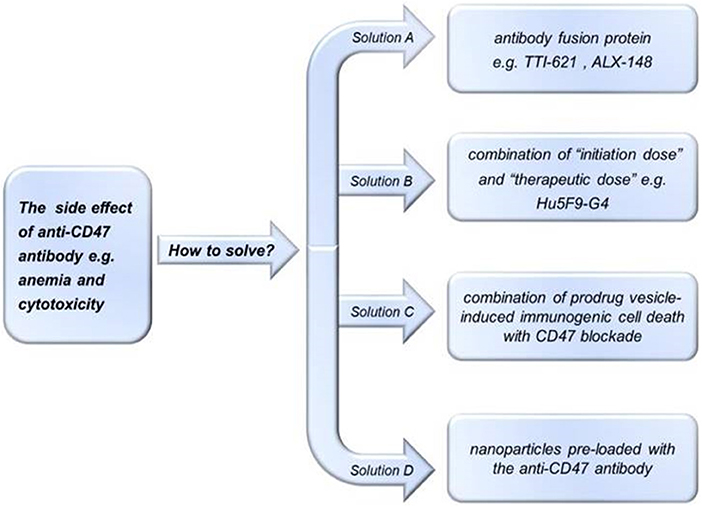
Figure 3. Potential methods of avoiding the side effects of anti-CD47 treatments. To reduce side effects of treatment with anti-CD47 antibodies, investigators could use: (A) antibody fusion proteins e.g., TTI-621 (133) or ALX-148 (134); (B) “initiation doses” followed by “therapeutic doses” e.g., Hu5F9-G4 (111); (C) pro-drug vesicle-induced immunogenic cell death combined with CD47 blockade (78); or (D) tumor-targeting nanoparticles loaded with anti-CD47 antibody (135).
The so-called “antigen sink” effect could also pose a problem in the development of anti-CD47 treatments. The ubiquitous expression of CD47 means that large initiation doses and/or frequent administrations may be required for a drug to achieve an effective therapeutic blockade of CD47. In a phase I trial of Hu5F9-G4, for example, an “initiation dose” was followed by a “therapeutic dose” (111). Alternatively, SIRPα has a more restricted histological distribution vs. CD47, which could lead to less toxicity and greater blockade when therapeutically targeted (128). SIRPα is highly expressed, however, on myeloid cells and central and peripheral nervous system cells (136), so the potential for neurological side-effects should be considered when using therapeutics that target SIRPα. Furthermore, cross-reactivity may occur between other SIRP family members (SIRPβ and SIRPγ) as a result of their sequence similarity, while that at least 10 polymorphisms of human SIRPα have been identified (137). The consequences of targeting these different isoforms of receptors is not yet clear (20, 137). In future studies, methods of targeting CD47 and its ligands specifically on tumor cells should be investigated; these could include novel drug delivery vehicles such as modified biomimetic nanoparticles (135) or quorum-sensing bacteria (138). For example, a recent study showed that a bio-responsive fibrin gel solution containing anti-CD47-conjugated nanoparticles modulate the immune response and induce the phagocytosis of tumor cells by blocking CD47; furthermore, this treatment resulted in an augmented T cell-mediated immune response and activation of tumor-associated macrophages (135). In another recent study by Chowdhury and colleagues, quorum-sensing bacteria were used to deliver a single-chain antibody fragment that targets CD47 and showed that this strategy leads to systemic anti-tumor immunity and reduced tumor progression (138). Multi-functional iron oxide magnetic nanoparticles have been developed as vehicles for selective treatment of pancreatic cancer, including for the simultaneous delivery of gemcitabine and anti-CD47 antibodies (117). The therapies delivered via nanoparticles did not have additional cytotoxicity and the nanoparticle-mediated delivery of the anti-CD47 antibody to tumor cells caused greater PDX models of PDAC apoptosis compared with free antibody in the treatment of pancreatic cancer (117). Mithomycin A-loaded nanoparticles can also be used to down-regulate the expression of CD47 and to increase therapeutic efficacy in BxPC-3 tumor xenografts in athymic mice (118). Furthermore, Davis et al. (139) combined anti-CD47 antibodies with SERS nanoparticles and showed by applied Raman spectroscopy that the antibody-loaded nanoparticles targeted ovarian cancer cells. Nanoparticle delivery methods could be further optimized in future studies, for example to prevent the circulating nanoparticles from being cleared by the reticuloendothelial system. If nanomedical technologies continue to advance at the current rate, it is possible that drug-loaded nanoparticles targeting CD47 will ultimately be developed for the treatment of CD47-overexpressing tumors. As well as new delivery methods, it is also possible that intra-tumoral drug administrations may lead to greater drug delivery and efficacy and reduced toxicity compared with intra-peritoneal or sub-cutaneous delivery routes.
As mentioned above, non-optimal tumor responses following anti-CD47 treatment have led to studies investigating the use of combination therapies including tumor cell-specific opsonizing antibodies and T-cell checkpoint inhibitors. In a mouse model of non-Hodgkin lymphoma, for example, an anti-CD47 antibody (BRIC126 or B6H12) combined with a clinically-approved anti-CD20 antibody (rituximab) resulted in human lymphoma cell ablation in xenograft models of tumor progression (42, 140). Such approaches can be used in attempts to achieve increased tumor specificity and decreased on-target toxicity to CD47-expressing non-malignant cells. In a similar approach, another bispecific antibody that targets CD47 and CD19 (NI-1701) was designed for B-cell lymphoma and refractory leukemia (127), while a fusion protein targeting CD47 and PD-L1 has also been shown to have anti-tumor efficacy by activating the adaptive immune response (141, 142). The phagocytotic and anti-tumor effects of high-affinity SIRPα proteins that block CD47 are also enhanced when combined with tumor-opsonizing antibodies including rituximab, trastuzumab, or cetuximab (124), or the anti-CD56 antibody, lorvotuzumab (41). Anti-SIRPα antagonists have also been combined with tumor-opsonizing antibodies such as rituximab to show anti-tumor efficacy in vitro (130) and in xenograft lymphoma and syngeneic colon cancer models (143), while bispecific agents have been produced whereby the binding domain of SIRPα is fused to a tumor-targeting antibody such as anti-CD33 (144). While clinical trials have begun to assess CD47-blocking agents in combination with rituximab, cetuximab, and trastuzumab (128), it is also possible that future improvements in cancer screening and precision medicine will enable the identification and stratification of specific tumor types and/or stages of cancer that would be most amenable to treatment with a certain type or types of anti-CD47 treatment.
Conclusions
Given that CD47-SIRPα signaling enables malignant cells to avoid macrophage-mediated phagocytosis, the inhibition of the CD47-SIRPα signaling axis represents a promising therapeutic strategy for the treatment of cancer, and multiple CD47 targeted drugs have entered the clinical trials. However, there are a series of biosafety problems with such treatments including anemia, and preclinical studies have demonstrated different effects of CD47-targeted drugs on different tumor types. In addition, the signaling mechanisms that are upstream and downstream of the CD47-SIRPα complex are incompletely understood. A better understanding of the mechanisms that allow tumor cells to avoid immune clearance along with improvements in the delivery of anti-CD47 agents could lead to the development of novel and effective anti-cancer treatments that enhance the phagocytosis of malignant cells.
Author Contributions
WZ, QH, and WX did the drafting. YZ, JP, HX, HZ, and JX were responsible for looking up and collecting information. CE and HJ were responsible for revising the manuscript.
Funding
This work was supported by the Science and Technology Project of Guangdong Province (No. 2017A010103019), National Natural Science Foundation of China (Grants 81572184), National Natural Science Foundation of China for Young Scientists (No. 81801649), China Postdoctoral Science Foundation (No. 2018M631026), and Special Funds for the Cultivation of Guangdong College Students' Scientific and Technological Innovation (Climbing Program Special Funds, No. pdjh2019b0224), Ph.D early development program of Guangdong Medical University (B2019012) and Group-type Special Supporting Project for Educational Talents in Universities (4SG19057G). CE received an American Heart Association Career Development Award (19CDA34500000).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. (2011) 331:1565–70. doi: 10.1126/science.1203486
2. Marcus A, Gowen BG, Thompson TW, Iannello A, Ardolino M, Deng W. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol. (2014) 122:91–128. doi: 10.1016/B978-0-12-800267-4.00003-1
3. Sick E, Jeanne A, Schneider C, Dedieu S, Takeda K, Martiny L. Cd47 update: a multifaceted actor in the tumour microenvironment of potential therapeutic interest. Br J Pharmacol. (2012) 167:1415–30. doi: 10.1111/j.1476-5381.2012.02099.x
4. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. (2015) 348:56–61. doi: 10.1126/science.aaa8172
5. Zhao H, Wang J, Kong X, Li E, Liu Y, Du X, et al. Cd47 promotes tumor invasion and metastasis in non-small cell lung cancer. Sci Rep. (2016) 6:29719. doi: 10.1038/srep29719
6. Cioffi M, Trabulo S, Hidalgo M, Costello E, Greenhalf W, Erkan M, et al. Inhibition of cd47 effectively targets pancreatic cancer stem cells via dual mechanisms. Clin Cancer Res. (2015) 21:2325–37. doi: 10.1158/1078-0432.CCR-14-1399
7. Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the cd47-sirpalpha signalling pathway. Trends Cell Biol. (2009) 19:72–80. doi: 10.1016/j.tcb.2008.12.001
8. McCracken MN, Cha AC, Weissman IL. Molecular pathways: activating t cells after cancer cell phagocytosis from blockade of cd47 “don't eat me” signals. Clin Cancer Res. (2015) 21:3597–601. doi: 10.1158/1078-0432.CCR-14-2520
9. Locy H, de Mey S, de Mey W, De Ridder M, Thielemans K, Maenhout SK. Immunomodulation of the tumor microenvironment: Turn foe into friend. Front Immunol. (2018) 9:2909. doi: 10.3389/fimmu.2018.02909
10. McGreal EP, Martinez-Pomares L, Gordon S. Divergent roles for c-type lectins expressed by cells of the innate immune system. Mol Immunol. (2004) 41:1109–21. doi: 10.1016/j.molimm.2004.06.013
11. Klimp AH, de Vries EG, Scherphof GL, Daemen T. A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol/Hematol. (2002) 44:143–61. doi: 10.1016/S1040-8428(01)00203-7
12. Koduru SV, Sun BH, Walker JM, Zhu M, Simpson C, Dhodapkar M, et al. The contribution of cross-talk between the cell-surface proteins cd36 and cd47-tsp-1 in osteoclast formation and function. J Biol Chem. (2018) 293:15055–69. doi: 10.1074/jbc.RA117.000633
13. Lindberg FP, Lublin DM, Telen MJ, Veile RA, Miller YE, Donis-Keller H, et al. Rh-related antigen cd47 is the signal-transducer integrin-associated protein. J Biol Chem. (1994) 269:1567–70.
14. Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ. In vivo expression of alternatively spliced forms of integrin-associated protein (cd47). J Cell Sci. (1995) 108(Pt 11):3419–25.
15. Brown EJ, Frazier WA. Integrin-associated protein (cd47) and its ligands. Trends Cell Biol. (2001) 11:130–5. doi: 10.1016/S0962-8924(00)01906-1
16. Ratnikova NM, Lezhnin YN, Frolova EI, Kravchenko JE, Chumakov SP. [cd47 receptor as a primary target for cancer therapy]. Mol Biol. (2017) 51:251–61. doi: 10.7868/S0026898417010153
17. Gao W, Xie XS. [the role of tsp-1-cd47 in ros-mediated pulmonary fibrosis induced by paraquat]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. (2018) 36:653–61. doi: 10.3760/cma.j.issn.1001-9391.2018.09.003
18. Matlung HL, Szilagyi K, Barclay NA, van den Berg Timo K. The cd47-sirpα signaling axis as an innate immune checkpoint in cancer. Immunol Rev. (2017) 276:145–64. doi: 10.1111/imr.12527
19. Ring NG, Herndler-Brandstetter D, Weiskopf K, Shan L, Volkmer JP, George BM, et al. Anti-sirpα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci USA. (2017) 114:E10578–85. doi: 10.1073/pnas.1710877114
20. Barclay Neil A, Brown Marion H. The sirp family of receptors and immune regulation. Nat Rev Immunol. (2006) 6:457–64. doi: 10.1038/nri1859
21. Ishikawa-Sekigami T, Kaneko Y, Okazawa H, Tomizawa T, Okajo J, Saito Y, et al. Shps-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood. (2006) 107:341–8. doi: 10.1182/blood-2005-05-1896
22. Jaffe EA, Ruggiero JT, Falcone DJ. Monocytes and macrophages synthesize and secrete thrombospondin. Blood. (1985) 65:79–84. doi: 10.1182/blood.V65.1.79.bloodjournal65179
23. Wu L, Yu GT, Deng WW, Mao L, Yang LL, Ma SR, et al. Anti-cd47 treatment enhances anti-tumor t-cell immunity and improves immunosuppressive environment in head and neck squamous cell carcinoma. Oncoimmunology. (2018) 7:e1397248. doi: 10.1080/2162402X.2017.1397248
24. Per-Arne O. Role of cd47 and signal regulatory protein alpha (sirpα) in regulating the clearance of viable or aged blood cells. Transfus Med Hemother. (2012) 39:315–20. doi: 10.1159/000342537
25. Pan Y, Wang F, Liu Y, Jiang J, Yang YG, Wang H. Studying the mechanism of cd47-sirpα interactions on red blood cells by single molecule force spectroscopy. Nanoscale. (2014) 6:9951–4. doi: 10.1039/C4NR02889A
26. Doyen V, Rubio M, Braun D, Nakajima T, Abe J, Saito H, et al. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J Exp Med. (2003) 198:1277–83. doi: 10.1084/jem.20030705
27. Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R. Cd47 functions as a molecular switch for erythrocyte phagocytosis. Blood. (2012) 119:5512–21. doi: 10.1182/blood-2011-10-386805
28. Ishikawa-Sekigami T, Kaneko Y, Saito Y, Murata Y, Okazawa H, Ohnishi H, et al. Enhanced phagocytosis of cd47-deficient red blood cells by splenic macrophages requires shps-1. Biochem Biophys Res Commun. (2006) 343:1197–200. doi: 10.1016/j.bbrc.2006.03.094
29. Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-cd47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. (2012) 26:2538–45. doi: 10.1038/leu.2012.141
30. Edris B, Weiskopf K, Volkmer AK, Volkmer JP, Willingham SB, Contreras-Trujillo H, et al. Antibody therapy targeting the cd47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci USA. (2012) 109:6656–61. doi: 10.1073/pnas.1121629109
31. Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, et al. Therapeutic antibody targeting of cd47 eliminates human acute lymphoblastic leukemia. Cancer Res. (2011) 71:1374–84. doi: 10.1158/0008-5472.CAN-10-2238
32. Chao MP, Tang C, Pachynski RK, Chin R, Majeti R, Weissman IL. Extranodal dissemination of non-hodgkin lymphoma requires cd47 and is inhibited by anti-cd47 antibody therapy. Blood. (2011) 118:4890–901. doi: 10.1182/blood-2011-02-338020
33. Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The cd47-signal regulatory protein alpha (sirpa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. (2012) 109:6662–7. doi: 10.1073/pnas.1121623109
34. Mohanty S, Yerneni K, Theruvath JL, Graef CM, Nejadnik H, Lenkov O, et al. Nanoparticle enhanced mri can monitor macrophage response to cd47 mab immunotherapy in osteosarcoma. Cell Death Dis. (2019) 10:36. doi: 10.1038/s41419-018-1285-3
35. Russ A, Hua AB, Montfort WR, Rahman B, Riaz IB, Khalid MU, et al. Blocking “don't eat me” signal of cd47-sirpα in hematological malignancies, an in-depth review. Blood Rev. (2018) 32:480–9. doi: 10.1016/j.blre.2018.04.005
36. Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. (2010) 31:212–9. doi: 10.1016/j.it.2010.04.001
37. Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. Cd47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. (2009) 138:271–85. doi: 10.1016/j.cell.2009.05.046
38. Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, et al. Cd47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. (2009) 138:286–99. doi: 10.1016/j.cell.2009.05.045
39. Yang K, Xu J, Liu Q, Li J, Xi Y. Expression and significance of cd47, pd1 and pdl1 in t-cell acute lymphoblastic lymphoma/leukemia. Pathol Res Pract. (2019) 215:265–71. doi: 10.1016/j.prp.2018.10.021
40. Galli S, Zlobec I, Schürch C, Perren A, Ochsenbein AF, Banz Y. Cd47 protein expression in acute myeloid leukemia: a tissue microarray-based analysis. Leuk Res. (2015) 39:749–56. doi: 10.1016/j.leukres.2015.04.007
41. Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, et al. Cd47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Investig. (2016) 126:2610–20. doi: 10.1172/JCI81603
42. Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-cd47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-hodgkin lymphoma. Cell. (2010) 142:699–713. doi: 10.1016/j.cell.2010.07.044
43. Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. (2014) 28:1122–8. doi: 10.1038/leu.2013.313
44. Rendtlew Danielsen JM, Knudsen LM, Dahl IM, Lodahl M, Rasmussen T. Dysregulation of cd47 and the ligands thrombospondin 1 and 2 in multiple myeloma. Br J Haematol. (2007) 138:756–60. doi: 10.1111/j.1365-2141.2007.06729.x
45. Rivera A, Fu X, Tao L, Zhang X. Expression of mouse cd47 on human cancer cells profoundly increases tumor metastasis in murine models. BMC Cancer. (2015) 15:964. doi: 10.1186/s12885-015-1980-8
46. Vaeteewoottacharn K, Kariya R, Pothipan P, Fujikawa S, Pairojkul C, Waraasawapati S, et al. Attenuation of cd47-sirpα signal in cholangiocarcinoma potentiates tumor-associated macrophage-mediated phagocytosis and suppresses intrahepatic metastasis. Transl Oncol. (2019) 12:217–25. doi: 10.1016/j.tranon.2018.10.007
47. Wang H, Tan M, Zhang S, Li X, Gao J, Zhang D, et al. Expression and significance of cd44, cd47 and c-met in ovarian clear cell carcinoma. Int J Mol Sci. (2015) 16:3391–404. doi: 10.3390/ijms16023391
48. Li Y, Lu S, Xu Y, Qiu C, Jin C, Wang Y, et al. Overexpression of cd47 predicts poor prognosis and promotes cancer cell invasion in high-grade serous ovarian carcinoma. Am J Transl Res. (2017) 9:2901–10.
49. Brightwell RM, Grzankowski KS, Lele S, Eng K, Arshad M, Chen H, et al. The cd47 “don't eat me signal” is highly expressed in human ovarian cancer. Gynecol Oncol. (2016) 143:393–7. doi: 10.1016/j.ygyno.2016.08.325
50. Nagahara M, Mimori K, Kataoka A, Ishii H, Tanaka F, Nakagawa T, et al. Correlated expression of cd47 and sirpa in bone marrow and in peripheral blood predicts recurrence in breast cancer patients. Clin Cancer Res. (2010) 16:4625–35. doi: 10.1158/1078-0432.CCR-10-0349
51. Liu L, Zhang L, Yang L, Li H, Li R, Yu J, et al. Anti-cd47 antibody as a targeted therapeutic agent for human lung cancer and cancer stem cells. Front Immunol. (2017) 8:404. doi: 10.3389/fimmu.2017.00404
52. Stone L. Bladder cancer: on the dot–targeted molecular imaging. Nat Rev Urol. (2015) 12:6. doi: 10.1038/nrurol.2014.318
53. Li F, Lv B, Liu Y, Hua T, Han J, Sun C, et al. Blocking the cd47-sirpα axis by delivery of anti-cd47 antibody induces antitumor effects in glioma and glioma stem cells. Oncoimmunology. (2018) 7:e1391973. doi: 10.1080/2162402X.2017.1391973
54. Michaels AD, Newhook TE, Adair SJ, Morioka S, Goudreau BJ, Nagdas S, et al. Cd47 blockade as an adjuvant immunotherapy for resectable pancreatic cancer. Clin Cancer Res. (2018) 24:1415–25. doi: 10.1158/1078-0432.CCR-17-2283
55. Abe H, Saito R, Ichimura T, Iwasaki A, Yamazawa S, Shinozaki-Ushiku A, et al. Cd47 expression in epstein-barr virus-associated gastric carcinoma: coexistence with tumor immunity lowering the ratio of cd8/foxp3 t cells. Virchows Archiv. (2018) 472:643–51. doi: 10.1007/s00428-018-2332-2
56. Yoshida K, Tsujimoto H, Matsumura K, Kinoshita M, Takahata R, Matsumoto Y, et al. CD47 is an adverse prognostic factor and a therapeutic target in gastric cancer. Cancer Med. (2015) 4, 1322–1333. doi: 10.1002/cam4.478
57. Sudo T, Takahashi Y, Sawada G, Uchi R, Mimori K, Akagi Y. Significance of cd47 expression in gastric cancer. Oncol Lett. (2017) 14:801–9. doi: 10.3892/ol.2017.6257
58. Chao MP, Weissman IL, Majeti R. The cd47-sirpα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. (2012) 24:225–32. doi: 10.1016/j.coi.2012.01.010
59. Zeng D, Sun Q, Chen A, Fan J, Yang X, Xu L, et al. A fully human anti-cd47 blocking antibody with therapeutic potential for cancer. Oncotarget. (2016) 7:83040–50. doi: 10.18632/oncotarget.13349
60. Lin Y, Yan XQ, Yang F, Yang XW, Jiang X, Zhao XC, et al. Soluble extracellular domains of human sirpα and cd47 expressed in escherichia coli enhances the phagocytosis of leukemia cells by macrophages in vitro. Protein Expr Purif . (2012) 85:109–16. doi: 10.1016/j.pep.2012.07.002
61. Xu L, Wang S, Li J, Li B. Cd47/sirpα blocking enhances cd19/cd3-bispecific t cell engager antibody-mediated lysis of b cell malignancies. Biochem Biophys Res Commun. (2019) 509:739–45. doi: 10.1016/j.bbrc.2018.12.175
62. Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, et al. Pre-clinical development of a humanized anti-cd47 antibody with anti-cancer therapeutic potential. PLoS ONE. (2015) 10:e0137345. doi: 10.1371/journal.pone.0137345
63. Weiskopf K, Anderson KL, Ito D, Schnorr PJ, Tomiyasu H, Ring AM, et al. Eradication of canine diffuse large b-cell lymphoma in a murine xenograft model with cd47 blockade and anti-cd20. Cancer Immunol Res. (2016) 4:1072–87. doi: 10.1158/2326-6066.CIR-16-0105
64. Roberts DD, Kaur S, Soto-Pantoja DR. Therapeutic targeting of the thrombospondin-1 receptor cd47 to treat liver cancer. J Cell Commun Signal. (2015) 9:101–2. doi: 10.1007/s12079-015-0283-9
65. Xiao Z, Chung H, Banan B, Manning PT, Ott KC, Lin S, et al. Antibody mediated therapy targeting cd47 inhibits tumor progression of hepatocellular carcinoma. Cancer Lett. (2015) 360:302–9. doi: 10.1016/j.canlet.2015.02.036
66. Ma YH, Dou WT, Pan YF, Dong LW, Tan YX, He XP, et al. Fluorogenic 2d peptidosheet unravels cd47 as a potential biomarker for profiling hepatocellular carcinoma and cholangiocarcinoma tissues. Adv Mater. (2017) 29:1604253. doi: 10.1002/adma.201604253
67. Abe T, Tanaka Y, Piao J, Tanimine N, Oue N, Hinoi T, et al. Signal regulatory protein alpha blockade potentiates tumoricidal effects of macrophages on gastroenterological neoplastic cells in syngeneic immunocompetent mice. Ann Gastroenterol Surg. (2018) 2:451–62. doi: 10.1002/ags3.12205
68. Zhang Y, Sime W, Juhas M, Sjölander A. Crosstalk between colon cancer cells and macrophages via inflammatory mediators and cd47 promotes tumour cell migration. Eur J Cancer. (2013) 49:3320–34. doi: 10.1016/j.ejca.2013.06.005
69. Hutter G, Theruvath J, Graef CM, Zhang M, Schoen MK, Manz EM, et al. Microglia are effector cells of cd47-sirpα antiphagocytic axis disruption against glioblastoma. Proc Natl Acad Sci USA. (2019) 116:997–1006. doi: 10.1073/pnas.1721434116
70. Zhang M, Hutter G, Kahn SA, Azad TD, Gholamin S, Xu CY, Liu J, et al. Anti-cd47 treatment stimulates phagocytosis of glioblastoma by m1 and m2 polarized macrophages and promotes m1 polarized macrophages in vivo. PLoS ONE. (2016) 11:e0153550. doi: 10.1371/journal.pone.0153550
71. Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, et al. Anti-cd47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor t-cell response. Proc Natl Acad Sci USA. (2013) 110:11103–8. doi: 10.1073/pnas.1305569110
72. Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, et al. Cd47 blockade triggers t cell-mediated destruction of immunogenic tumors. Nat Med. (2015) 21:1209–15. doi: 10.1038/nm.3931
73. Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by cd47. Sci Transl Med. (2010) 2:63ra94. doi: 10.1126/scitranslmed.3001375
74. Soto-Pantoja DR, Terabe M, Ghosh A, Ridnour LA, DeGraff WG, Wink DA, et al. Cd47 in the tumor microenvironment limits cooperation between antitumor t-cell immunity and radiotherapy. Cancer Res. (2014) 74:6771–83. doi: 10.1158/0008-5472.CAN-14-0037-T
75. Golubovskaya V, Berahovich R, Zhou H, Xu S, Harto H, Li L, et al. Cd47-car-t cells effectively kill target cancer cells and block pancreatic tumor growth. Cancers. (2017) 9:139. doi: 10.3390/cancers9100139
76. Ali AI, Oliver AJ, Samiei T, Chan JD, Kershaw MH, Slaney CY. Genetic redirection of t cells for the treatment of pancreatic cancer. Front Oncol. (2019) 9:56. doi: 10.3389/fonc.2019.00056
77. Jindal V, Arora E, Masab M, Gupta S. Chimeric antigen receptor t cell therapy in pancreatic cancer: from research to practice. Med Oncol. (2018) 35:84. doi: 10.1007/s12032-018-1145-0
78. Zhou F, Feng B, Yu H, Wang D, Wang T, Ma Y, et al. Tumor microenvironment-activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and cd47 blockade. Adv Mater. (2019) 31:e1805888. doi: 10.1002/adma.201805888
79. Kim MJ, Lee JC, Lee JJ, Kim S, Lee SG, Park SW, et al. Association of cd47 with natural killer cell-mediated cytotoxicity of head-and-neck squamous cell carcinoma lines. Tumour Biol. (2008) 29:28–34. doi: 10.1159/000132568
80. Saumet A, Slimane MB, Lanotte M, Lawler J, Dubernard V. Type 3 repeat/c-terminal domain of thrombospondin-1 triggers caspase-independent cell death through cd47/alphavbeta3 in promyelocytic leukemia nb4 cells. Blood. (2005) 106:658–67. doi: 10.1182/blood-2004-09-3585
81. Feliz-Mosquea YR, Christensen AA, Wilson AS, Westwood B, Varagic J, Meléndez GC, et al. Combination of anthracyclines and anti-cd47 therapy inhibit invasive breast cancer growth while preventing cardiac toxicity by regulation of autophagy. Breast Cancer Res Treat. (2018) 172:69–82. doi: 10.1007/s10549-018-4884-x
82. Zhao XW, van Beek EM, Schornagel K, Van der Maaden H, Van Houdt M, Otten MA. Cd47-signal regulatory protein-α (sirpα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci USA. (2011) 108:18342–7. doi: 10.1073/pnas.1106550108
83. Kikuchi Y, Uno S, Yoshimura Y, Otabe K, Iida S, Oheda M, et al. A bivalent single-chain fv fragment against cd47 induces apoptosis for leukemic cells. Biochem Biophys Res Commun. (2004) 315:912–8. doi: 10.1016/j.bbrc.2004.01.128
84. Kikuchi Y, Uno S, Kinoshita Y, Yoshimura Y, Iida S, Wakahara Y, et al. Apoptosis inducing bivalent single-chain antibody fragments against cd47 showed antitumor potency for multiple myeloma. Leuk Res. (2005) 29:445–50. doi: 10.1016/j.leukres.2004.09.005
85. Manna PP, Frazier WA. Cd47 mediates killing of breast tumor cells via gi-dependent inhibition of protein kinase a. Cancer Res. (2004) 64:1026–36. doi: 10.1158/0008-5472.CAN-03-1708
86. De Beck L, Melhaoui S, De Veirman K, Menu E, De Bruyne E, Vanderkerken K, et al. Epigenetic treatment of multiple myeloma mediates tumor intrinsic and extrinsic immunomodulatory effects. Oncoimmunology. (2018) 7:e1484981. doi: 10.1080/2162402X.2018.1484981
87. Song G, Yang L. Inhibited cd47 gene affects the clearance of acute myelogenous leukemia stem cells. J Cell Biochem. (2018) 120:10303–9. doi: 10.1002/jcb.28314
88. Pietsch EC, Dong J, Cardoso R, Zhang X, Chin D, Hawkins R, et al. Anti-leukemic activity and tolerability of anti-human cd47 monoclonal antibodies. Blood Cancer J. (2017) 7:e536. doi: 10.1038/bcj.2017.7
89. Martinez-Torres AC, Quiney C, Attout T, Boullet H, Herbi L, Vela L, et al. Cd47 agonist peptides induce programmed cell death in refractory chronic lymphocytic leukemia b cells via plcγ1 activation: Evidence from mice and humans. PLoS Med. (2015) 12:e1001796. doi: 10.1371/journal.pmed.1001796
90. Uscanga-Palomeque AC, Calvillo-Rodríguez KM, Gómez-Morales L, Lardé E, Denèfle T, Caballero-Hernández D, et al. Cd47 agonist peptide pkhb1 induces immunogenic cell death in t-cell acute lymphoblastic leukemia cells. Cancer Sci. (2019) 110:256–68. doi: 10.1111/cas.13885
91. Leclair P, Liu CC, Monajemi M, Reid GS, Sly LM, Lim CJ. Cd47-ligation induced cell death in t-acute lymphoblastic leukemia. Cell Death Dis. (2018) 9:544. doi: 10.1038/s41419-018-0601-2
92. Sukowati CHC, Anfuso B, Fiore E, Ie SI, Raseni A, Vascotto F, et al. Hyaluronic acid inhibition by 4-methylumbelliferone reduces the expression of cancer stem cells markers during hepatocarcinogenesis. Sci Rep. (2019) 9:4026. doi: 10.1038/s41598-019-40436-6
93. Rodríguez MM, Fiore E, Bayo J, Atorrasagasti C, García M, Onorato A, et al. 4mu decreases cd47 expression on hepatic cancer stem cells and primes a potent antitumor t cell response induced by interleukin-12. Mol Ther. (2018) 26:2738–50. doi: 10.1016/j.ymthe.2018.09.012
94. Boukhari A, Alhosin M, Bronner C, Sagini K, Truchot C, Sick E, et al. Cd47 activation-induced uhrf1 over-expression is associated with silencing of tumor suppressor gene p16ink4a in glioblastoma cells. Anticancer Res. (2015) 35:149–57.
95. Piccione EC, Juarez S, Tseng S, Liu J, Stafford M, Narayanan C, et al. Sirpα-antibody fusion proteins selectively bind and eliminate dual antigen-expressing tumor cells. Clin Cancer Res. (2016) 22:5109–19. doi: 10.1158/1078-0432.CCR-15-2503
96. Zhang JF, Hua R, Liu DJ, Liu W, Huo YM, Sun YW. Effect of cd74 on the prognosis of patients with resectable pancreatic cancer. Hepatobiliary Pancreat Dis Int. (2014) 13:81–6. doi: 10.1016/S1499-3872(14)60011-4
97. Uluçkan O, Becker SN, Deng H, Zou W, Prior JL, Piwnica-Worms D, et al. Cd47 regulates bone mass and tumor metastasis to bone. Cancer Res. (2009) 69:3196–204. doi: 10.1158/0008-5472.CAN-08-3358
98. Kukreja A, Radfar S, Sun BH, Insogna K, Dhodapkar MV. Dominant role of cd47-thrombospondin-1 interactions in myeloma-induced fusion of human dendritic cells: Implications for bone disease. Blood. (2009) 114:3413–21. doi: 10.1182/blood-2009-03-211920
99. Lo J, Lau EY, So FT, Lu P, Chan VS, Cheung VC, et al. Anti-cd47 antibody suppresses tumour growth and augments the effect of chemotherapy treatment in hepatocellular carcinoma. Liver Int. (2016) 36:737–45. doi: 10.1111/liv.12963
100. Takahashi S. Molecular functions of sirpα and its role in cancer. Biomed Rep. (2018) 9:3–7. doi: 10.3892/br.2018.1102
101. Wu J, Li Z, Yang Z, Guo L, Zhang Y, Deng H, et al. A glutamine-rich carrier efficiently delivers anti-cd47 sirna driven by a “glutamine trap” to inhibit lung cancer cell growth. Mol Pharm. (2018) 15:3032–45. doi: 10.1021/acs.molpharmaceut.8b00076
102. Cabrales P. Rrx-001 acts as a dual small molecule checkpoint inhibitor by downregulating cd47 on cancer cells and sirp-α on monocytes/macrophages. Transl Oncol. (2019) 12:626–32. doi: 10.1016/j.tranon.2018.12.001
103. Barrera L, Montes-Servín E, Hernandez-Martinez JM, García-Vicente MLÁ, Montes-Servín E, Herrera-Martínez M, et al. Cd47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer. (2017) 117:385–97. doi: 10.1038/bjc.2017.173
104. Xu JF, Pan XH, Zhang SJ, Zhao C, Qiu BS, Gu HF, et al. Cd47 blockade inhibits tumor progression human osteosarcoma in xenograft models. Oncotarget. (2015) 6:23662–70. doi: 10.18632/oncotarget.4282
105. Stengel K, Zheng Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signal. (2011) 23:1415–23. doi: 10.1016/j.cellsig.2011.04.001
106. Miyashita M, Ohnishi H, Okazawa H, Tomonaga H, Hayashi A, Fujimoto TT, et al. Promotion of neurite and filopodium formation by cd47: roles of integrins, rac, and cdc42. Mol Biol Cell. (2004) 15:3950–63. doi: 10.1091/mbc.e04-01-0019
107. Yoshida H, Tomiyama Y, Ishikawa J, Oritani K, Matsumura I, Shiraga M, et al. Integrin-associated protein/cd47 regulates motile activity in human b-cell lines through cdc42. Blood. (2000) 96:234–41. doi: 10.1182/blood.V96.1.234.013k06_234_241
108. Li Y, Zhang H, Gong H, Yuan Y, Li Y, Wang C, et al. Mir-182 suppresses invadopodia formation and metastasis in non-small cell lung cancer by targeting cortactin gene. J Exp Clin Cancer Res. (2018) 37:141. doi: 10.1186/s13046-018-0824-1
109. Lv J, Zeng J, Guo F, Li Y, Xu M, Cheng Y, et al. Endothelial cdc42 deficiency impairs endothelial regeneration and vascular repair after inflammatory vascular injury. Respir Res. (2018) 19:27. doi: 10.1186/s12931-018-0729-8
110. Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. (2018) 29:84–91. doi: 10.1093/annonc/mdx755
111. Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. Cd47 blockade by hu5f9-g4 and rituximab in non-hodgkin's lymphoma. N Engl J Med. (2018) 379:1711–21. doi: 10.1056/NEJMoa1807315
112. Zhang L, Huang H. Targeting the cancer biomarker cd47: a review on the diverse mechanisms of the cd47 pathway in cancer treatment. Anti-cancer Agents Med Chem. (2016) 16:658–67. doi: 10.2174/1871520615666151008123223
113. Yang SY, Choi SA, Lee JY, Park AK, Wang KC, Phi JH, et al. Mir-192 suppresses leptomeningeal dissemination of medulloblastoma by modulating cell proliferation and anchoring through the regulation of dhfr, integrins, and cd47. Oncotarget. (2015) 6:43712–30. doi: 10.18632/oncotarget.6227
114. Huang W, Wang WT, Fang K, Chen ZH, Sun YM, Han C, et al. Mir-708 promotes phagocytosis to eradicate t-all cells by targeting cd47. Mol Cancer. (2018) 17:12. doi: 10.1186/s12943-018-0768-2
115. Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med. (2005) 201:291–301. doi: 10.1084/jem.20041509
116. Barkal Amira A, Weiskopf K, Kao Kevin S, Gordon Sydney R, Rosental B, Yiu Ying Y, et al. Engagement of mhc class I by the inhibitory receptor lilrb1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. (2018) 19:76–84. doi: 10.1038/s41590-017-0004-z
117. Trabulo S, Aires A, Aicher A, Heeschen C, Cortajarena AL. Multifunctionalized iron oxide nanoparticles for selective targeting of pancreatic cancer cells. Biochim Biophys Acta. (2017) 1861:1597–605. doi: 10.1016/j.bbagen.2017.01.035
118. Liu XJ, Li L, Liu XJ, Li Y, Zhao CY, Wang RQ, et al. Mithramycin-loaded mpeg-plga nanoparticles exert potent antitumor efficacy against pancreatic carcinoma. Int J Nanomed. (2017) 12:5255–69. doi: 10.2147/IJN.S139507
119. Rezaei G, Habibi-Anbouhi M, Mahmoudi M, Azadmanesh K, Moradi-Kalbolandi S, Behdani M, et al. Development of anti-cd47 single-chain variable fragment targeted magnetic nanoparticles for treatment of human bladder cancer. Nanomedicine. (2017) 12:597–613. doi: 10.2217/nnm-2016-0302
120. Zhang X, Chen W, Fan J, Wang S, Xian Z, Luan J, et al. Disrupting cd47-sirpα axis alone or combined with autophagy depletion for the therapy of glioblastoma. Carcinogenesis. (2018) 39:689–99. doi: 10.1093/carcin/bgy041
121. Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: A brief review. Adv Pharm Bull. (2017) 7:339–48. doi: 10.15171/apb.2017.041
122. Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, et al. Drug resistance in cancer: an overview. Cancers. (2014) 6:1769–92. doi: 10.3390/cancers6031769
123. Day CP, Merlino G, Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell. (2015) 163:39–53. doi: 10.1016/j.cell.2015.08.068
124. Weiskopf K, Ring AM, Ho Chia Chi M, Volkmer J-P, Levin AM, Volkmer AK, et al. Engineered sirpα variants as immunotherapeutic adjuvants to anticancer antibodies. Science. (2013) 341:88–91. doi: 10.1126/science.1238856
125. Catani L, Sollazzo D, Ricci F, Polverelli N, Palandri F, Baccarani M, et al. The cd47 pathway is deregulated in human immune thrombocytopenia. Exp Hematol. (2011) 39:486–94. doi: 10.1016/j.exphem.2010.12.011
126. Oldenborg PA. Role of cd47 in erythroid cells and in autoimmunity. Leuk Lymphoma. (2004) 45:1319–27. doi: 10.1080/1042819042000201989
127. Buatois V, Johnson Z, Salgado-Pires S, Papaioannou A, Hatterer E, Chauchet X, et al. Preclinical development of a bispecific antibody that safely and effectively targets cd19 and cd47 for the treatment of b-cell lymphoma and leukemia. Mol Cancer Ther. (2018) 17:1739–51. doi: 10.1158/1535-7163.MCT-17-1095
128. Weiskopf K. Cancer immunotherapy targeting the cd47/sirpα axis. Euro J Cancer. (2017) 76:100–9. doi: 10.1016/j.ejca.2017.02.013
129. Sim J, Sockolosky JT, Sangalang E, Izquierdo S, Pedersen D, Harriman W, et al. Discovery of high affinity, pan-allelic, and pan-mammalian reactive antibodies against the myeloid checkpoint receptor sirpα. MAbs. (2019) 11:1036–52. doi: 10.1080/19420862.2019.1624123
130. Ho Chia Chi M, Guo N, Sockolosky JT, Ring Aaron M, Weiskopf K, Özkan E, et al. “Velcro” engineering of high affinity cd47 ectodomain as signal regulatory protein α (sirpα) antagonists that enhance antibody-dependent cellular phagocytosis. J Biol Chem. (2015) 290:12650–63. doi: 10.1074/jbc.M115.648220
131. Khandelwal S, van Rooijen N, Saxena RK. Reduced expression of cd47 during murine red blood cell (rbc) senescence and its role in rbc clearance from the circulation. Transfusion. (2007) 47:1725–32. doi: 10.1111/j.1537-2995.2007.01348.x
132. Anniss Angela M, Sparrow Rosemary L. Expression of cd47 (integrin-associated protein) decreases on red blood cells during storage. Transfus Apher Sci. (2002) 27:233–8. doi: 10.1016/S1473-0502(02)00070-8
133. Petrova PS, Viller NN, Wong M, Pang X, Lin GH, Dodge K, et al. Tti-621 (sirpαfc): a cd47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res. (2017) 23:1068–79. doi: 10.1158/1078-0432.CCR-16-1700
134. Kauder SE, Kuo TC, Harrabi O, Chen A, Sangalang E, Doyle L, et al. Alx148 blocks cd47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS ONE. (2018) 13:e0201832. doi: 10.1371/journal.pone.0201832
135. Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol. (2019) 14:89–97. doi: 10.1038/s41565-018-0319-4
136. Adams S, van der Laan LJ, Vernon-Wilson E, Renardel de Lavalette C, Döpp EA, Dijkstra CD, et al. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol. (1998) 161:1853–9.
137. Takenaka K, Prasolava Tatiana K, Wang Jean CY, Mortin-Toth Steven M, Khalouei S, Gan Olga I, et al. Polymorphism in sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. (2007) 8:1313–23. doi: 10.1038/ni1527
138. Chowdhury S, Castro S, Coker C, Hinchliffe Taylor E, Arpaia N, Danino T. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med. (2019) 25:1057–63. doi: 10.1038/s41591-019-0498-z
139. Davis RM, Campbell JL, Burkitt S, Qiu Z, Kang S, Mehraein M, et al. A raman imaging approach using cd47 antibody-labeled sers nanoparticles for identifying breast cancer and its potential to guide surgical resection. Nanomaterials. (2018) 8:953. doi: 10.3390/nano8110953
140. Piccione Emily C, Juarez S, Liu J, Tseng S, Ryan Christine E, Narayanan C, et al. A bispecific antibody targeting cd47 and cd20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs. (2015) 7:946–56. doi: 10.1080/19420862.2015.1062192
141. Liu B, Guo H, Xu J, Qin T, Guo Q, Gu N, et al. Elimination of tumor by cd47/pd-l1 dual-targeting fusion protein that engages innate and adaptive immune responses. MAbs. (2018) 10:315–24. doi: 10.1080/19420862.2017.1409319
142. Sockolosky Jonathan T, Dougan M, Ingram Jessica R, Ho Chia Chi M, Kauke Monique J, Almo Steven C, et al. Durable antitumor responses to cd47 blockade require adaptive immune stimulation. Proc Natl Acad Sci USA. (2016) 113:E2646–54. doi: 10.1073/pnas.1604268113
143. Yanagita T, Murata Y, Tanaka D, Motegi SI, Arai E, Daniwijaya EW, et al. Anti-sirpα antibodies as a potential new tool for cancer immunotherapy. JCI Insight. (2017) 2:e89140. doi: 10.1172/jci.insight.89140
Keywords: CD47, CD47/SIRPa axis, immunotherapy, phagocytosis, signal-regulatory protein α (SIRPα)
Citation: Zhang W, Huang Q, Xiao W, Zhao Y, Pi J, Xu H, Zhao H, Xu J, Evans CE and Jin H (2020) Advances in Anti-Tumor Treatments Targeting the CD47/SIRPα Axis. Front. Immunol. 11:18. doi: 10.3389/fimmu.2020.00018
Received: 03 October 2019; Accepted: 07 January 2020;
Published: 28 January 2020.
Edited by:
Nurit Hollander, Tel Aviv University, IsraelReviewed by:
Sharareh Gholamin, Stanford University, United StatesMichael Dougan, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2020 Zhang, Huang, Xiao, Zhao, Pi, Xu, Zhao, Xu, Evans and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Colin E. Evans, Y29saW5ldmFuc0Bub3J0aHdlc3Rlcm4uZWR1; Hua Jin, amluaHVhMDQxM0BnZG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Wenting Zhang1,2†
Wenting Zhang1,2† Colin E. Evans
Colin E. Evans Hua Jin
Hua Jin