
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Immunol. , 29 November 2019
Sec. Primary Immunodeficiencies
Volume 10 - 2019 | https://doi.org/10.3389/fimmu.2019.02735
This article is part of the Research Topic Application of Cytometry in Primary Immunodeficiencies View all 21 articles
 Enrico Attardi1,2†
Enrico Attardi1,2† Silvia Di Cesare1,3*†
Silvia Di Cesare1,3*† Donato Amodio1,3
Donato Amodio1,3 Carmela Giancotta1
Carmela Giancotta1 Nicola Cotugno3,4
Nicola Cotugno3,4 Cristina Cifaldi1
Cristina Cifaldi1 Maria Chiriaco1
Maria Chiriaco1 Paolo Palma4
Paolo Palma4 Andrea Finocchi1,3
Andrea Finocchi1,3 Gigliola Di Matteo1,3
Gigliola Di Matteo1,3 Paolo Rossi1,3
Paolo Rossi1,3 Caterina Cancrini1,3*
Caterina Cancrini1,3*Multiparametric flow cytometry (MFC) represents a rapid, highly reproducible, and sensitive diagnostic technology for primary immunodeficiencies (PIDs), which are characterized by a wide range of T cell perturbations and a broad clinical and genetic heterogeneity. MFC data from CD4+ and CD8+ T cell subsets were examined in 100 patients referred for Primary Immunodeficiencies to our center. Naïve, central memory, effector memory, and terminal effector memory cell differentiation stages were defined by the combined expression CD45RA/CD27 for CD4 and CD45RA/CCR7 for CD8. Principal component analysis (PCA), a non-hypothesis driven statistical analysis, was applied to analyze MFC data in order to distinguish the diverse PIDs. Among severe lymphopenic patients, those affected by severe combined and combined immunodeficiency (SCID and CID) segregated in a specific area, reflecting a homogenous, and a more severe T cell impairment, compared to other lymphopenic PID, such as thymectomized and partial DiGeorge syndrome patients. PID patients with predominantly antibody defects were distributed in a heterogeneous pattern, but unexpectedly PCA was able to cluster some patients' resembling CID, hence warning for additional and more extensive diagnostic tests and a diverse clinical management. In conclusion, PCA applied to T cell MFC data might help the physician to estimate the severity of specific PID and to diversify the clinical and diagnostic approach of the patients.
Primary Immunodeficiencies Disorders (PIDs) are a heterogeneous group of congenital disorders, caused by defects in development and/or function of the immune system, associated with an increased susceptibility to infections, immune-dysregulation, and a higher risk of malignancy (1, 2). Currently, about 340 genetic disorders responsible for defects in the immune system have been identified (3). The T cell compartment plays a key role in coordinating innate and adaptive immune responses upon antigen stimulation. Its impairment leads to a broad spectrum of immune diseases, which require rapid and defined diagnosis in order to adopt the targeted therapeutic management. Severe combined immunodeficiency (SCID) are caused by a severe defect in T cells differentiation, variably associated with B cell, natural killer (NK) cell, and/or myeloid lineage impairment, with the first symptoms usually manifesting within the first year of life and the only curative therapy is represented by haematopoietic stem cell transplantation and/or gene therapy for defined diseases (4, 5). Conversely, CID manifest later with a more heterogeneous clinical picture, often associated with immune-dysregulation manifestations (6, 7). Although few observational studies on CID are in progress, a commonly accepted clinical and diagnostic management for these patients has not been defined yet, and it often relies on the local center expertise, rather than on evidence based systematic experiences (8, 9). Moreover, humoral defects, classified as a predominantly antibody defects and common variable immunodeficiency (CVID) are characterized by recurrent infections, hypogammaglobulinemia, poor response to vaccines, and can be associated to diverse T cell abnormalities (10). Currently, the most accepted T cell maturation model suggests a progressive differentiation from naïve T cells to the memory phenotype, ending with the generation of T effector cells (11, 12). Multiparametric flow cytometry (MFC) allows an extensive and detailed characterization of lymphocytes subsets (13, 14). In this study, we analyzed by MFC the T cell immunophenotypes in a large group of PID patients, clinically classified according to ESID (European Society for Immunodeficiencies) criteria (15). T cell subsets frequencies were then investigated by principal component analysis (PCA) in order to test if this analysis could estimate the relative disease severity and could possibly support the clinical and diagnostic approach (16, 17).
Study cohort is composed of 100 patients affected by PID and 30 healthy donors followed at Bambino Gesù Childrens' Hospital between 2013 and 2017 and diagnosed for PID by ESID criteria (15). Patients' data were collected retrospectively and the study groups are described in Table 1 while their clinical and molecular characteristics are reported in Supplementary Tables 1a–c.
Moreover, the study cohort includes 10 patients affected, respectively, by: selective IgM deficiency, NEMO (NF-kappa-B essential modulator) deficiency, not determined agammaglobulinemia, undefined T-defect, DOCK8-deficiency (dedicator of cytokinesis 8 gene), XIAP deficiency (X-linked inhibitor of apoptosis), XL-HIGM1 (X-linked Hyper IgM type 1), STING (STimulator of INterferon Genes) associated vasculopathy with onset in infancy (SAVI). Patients did not receive any corticosteroid treatment or immunosuppressive therapy at enrollment. Patients' median age was 10 years (range 3,6 months−36 years) while healthy donors' median age was 6,1 years (range 5 months−30 years). Healthy donors were immunocompetent individuals. The work was conducted in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent, approved by the Ethical Committee of the Children's Hospital Bambino Gesù and Policlinico Tor Vergata, was obtained from either patients or their parents/legal guardians, if minors.
T cell development can be phenotypically assessed by the combined cell surface expression of CD45RA, CD31, CCR7, and CD27 molecules. CD4 subsets are identified as naïve (CD4+ TN: CD45RA+CD27+), central memory (CD4+ TCM: CD45RA-CD27+), effector memory (CD4+ TEM: CD45RA-CD27–) and terminally differentiated (CD4+ TEMRA: CD45RA+CD27-). Moreover, CD4+ naïve T cells coexpressing CD31+ are highly enriched in recent thymic emigrants (RTE), a naïve T CD4+ cell subpopulation that have just egressed the thymus and characterized by higher signal joint T-cell receptor excision circle (sjTREC) content (18, 19). Similarly CD8 subsets are defined by the expression of lymph node homing receptor CCR7 in naïve (CD8+ TN: CD45RA+CCR7+), central memory (CD8+ TCM: CD45RA-CCR7+), effector memory (CD8+ TEM: CD45RA-CCR7-), and terminally differentiated (CD8+ TEMRA: CD45RA+CCR7-). All flow cytometric analysis were performed on ethylenediamine tetraacetic acid (EDTA) blood samples within 24 h of venipuncture. After red blood cells lysis with ammonium chloride the lymphocytes were stained with the following previously titrated monoclonal Abs: CD3 PerCP (clone BW264/56, Miltenyi Biotec), CD45RA APC-H7 (clone T6D11, Miltenyi Biotec), CCR7 PE (clone 3D12, Ebioscience), CD4 APC (clone OKT4, Becton Dickinson), CD8 PE-Cy7 (clone RPA-T8, Becton Dickinson), CD19 PE-CY7 (clone SJ25C1, Becton Dickinson), CD16 PE (clone 3G8), CD56 (clone NCAM16.2) PE, CD27 FITC (clone M-T271, Becton Dickinson), TCR alpha-beta APC (clone T10B9, Becton Dickinson), TCR gamma-delta FITC (11F3, Miltenyi Biotec). Cells were incubated with the appropriate antibody cocktail for 30 min at 4°C, washed with PBS and suspended in PBS. At least 50,000 events in the lymphocyte live gate were acquired for each sample. Samples were acquired on FACSCANTO II (BD Biosciences, San Diego, CA, USA) and analyzed with FlowJo software (Tree Star Inc, version 8.8.6, Ashland, Ore). Some patients of our cohort presented with severe lymphopenia and to avoid this bias, we have considered the cell subsets frequencies, instead of absolute counts. Details of the gating strategy are shown in Supplementary Figure 1.
Unpaired t-test was used to compare the patients and controls for variables with normal distribution. For non-parametric variables, the unpaired two-tailed non-parametric Mann-Whitney test was used. All graphical representations and statistical analyses were performed using Prism 6.0 (GraphPad). The relative T subsets frequencies were subjected to PCA analysis, using PAST (PAleontological STatistics, version 3.22, University of Oslo) to visualize and to estimate the correlation among variables.
T subsets frequencies were diversely perturbed in most PIDs in univariate analysis, while those of STAT3, XLA, and SIgAD patients were all comparable to healthy donors. Moreover, no significant differences were evident between all lymphopenic groups (CID, DGS, TE) (Figure 1). T cell subsets frequencies were then interrogated by PCA: STAT3, XLA, and SIgAD groups did not show any evident alteration (Supplementary Figures S4A,B), with the exception of one SIgAD patient with severe autoimmune cytopenia clustering far from the SIgAD group (A13) and two XLA patients (X2 and X4) with TEM/EMRA CD8+ cell expansion (Supplementary Figure 4B).
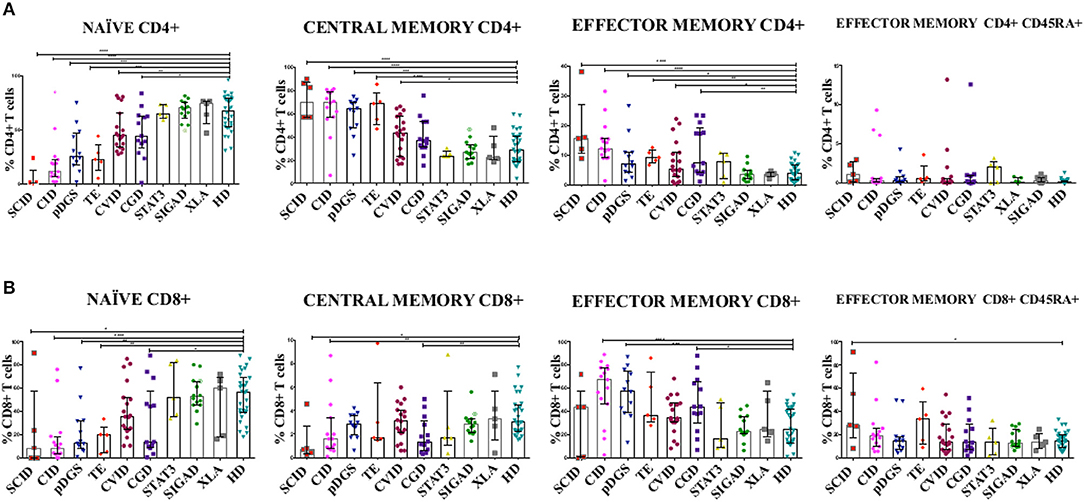
Figure 1. (A) CD4+ and (B) CD8+ T cell subsets in patients with primary immunodeficiency diseases. Unpaired two-tailed non-parametric Mann-Whitney test was used to compare the patients and controls. The columns and error bars indicate median and interquantile ranges (25°–75°). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. SCID and CID patients showed a severe reduction in frequency of TN CD4+ and CD8+ cells and a corresponding increase in TCM and TEM CD4+ and CD8+, while in CVID patients the same reduction is reported only in CD4+ subsets. DGS and Thymic-excision patients present the same distributions pattern, except for TCM and TEM CD8+. In CGD group there is a reduction in the frequency of TN CD4+ cells and an increase TEM CD4+ cells, while TN and TCM CD8+ T cell compartment are significantly reduced accompanied by TEM CD8+ expansion. Symbol with a cross in the SIgAD groups represents the SIgAD patient with the severe clinical presentation. SCID, Severe Combined Immunodeficiency; CID, Combined Immunodeficiency; TE, Thymic Excision; DGS, DiGeorge Syndrome; STAT3, Loss Of Function STAT3 (Autosomal Dominat Hyper-IgE Syndrome); CVID, Common Variable Immunodeficiency; CGD, Chronic Granulomatous Disease; XLA, X-linked Agammaglobulinemia; SIgAD, Selective IgA deficiency; HD, Healthy Donors.
Most of the CID patients clearly segregated far from healthy donors, similarly to SCID (Figures 2A,B and Supplementary Figures 2, 5A) and the main discriminating variables were the TCM CD4+ and TEM CD8+ and to a lesser extent TEMRA CD8+ cell subsets, as clearly evident in the CID patients (C9, C13, C14, C15) diagnosed as APDS (activated PI3K delta syndrome) (Figure 2B). While 13 patients out of 15 segregated uniformly, two patients (C3 and C7) classified as CID, with recurrent respiratory infections but in absence of immune dysregulation phenomena, segregated differently: C7 lied inside the HD area and C3 was skewed toward naive cells and low memory subsets in PCA (Figure 2B). Indeed, patient C3 had normal TREC levels, despite a reduction in CD4+CD31+CD45RA+ T cells and an increase in the CD4+CD31-CD27+CD45RA+ (>20%), suggesting a defective T cell maturation. Some other patients were clearly identifiable, like the DOCK8 (AR-HIES) deficiency patient (K1) showing a trend vs. TEMRA CD8+ expansion and the patient (R1) with an undefined T cell defect clustering toward TEM CD4+ differentiation. Conversely, the immunophenotype of the two XL-HIGM1 patients (L1, L2) and XIAP deficiency patient (P1) segregated more closely to HD, although their clinical picture mimicked a CID (Figure 2B). In patient S1, admitted at 14 months of age for a severe dermatitis, chronic diarrhea, and anemia associated with a profound alteration in T cell distribution, PCA showed clearly a peculiar localization near HD area and far from CID group, excluding a combined immunodeficiency. Later targeted next generation sequencing (NGS) analysis revealed a STING (STimulator of INterferon Genes) mutation, justifying her severe course due to a deficiency in the interferon pathway (20).
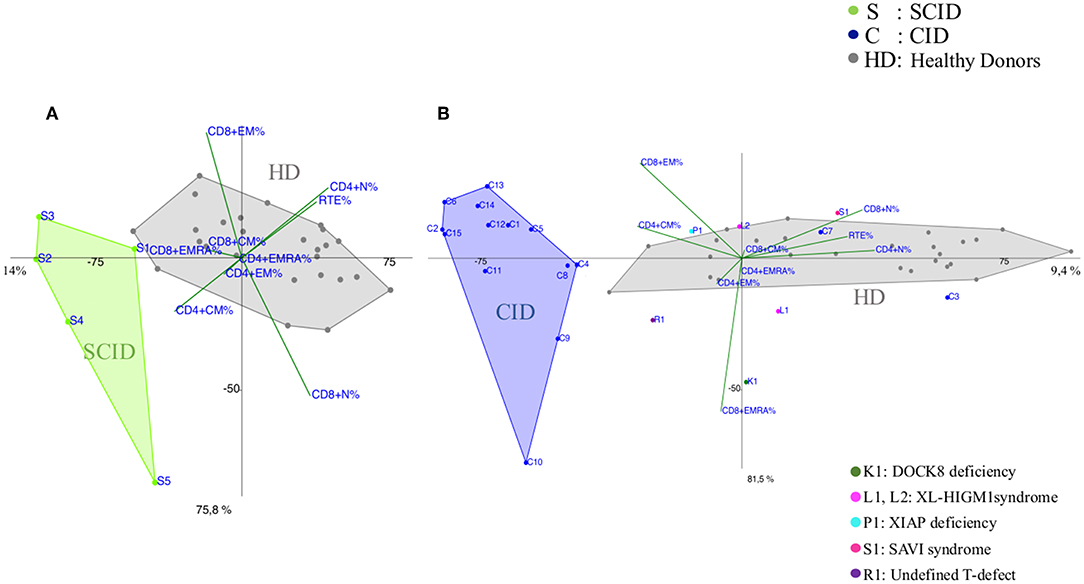
Figure 2. PCA Scatter plots of T cell subpopulations frequencies of (A) Severe Combined Immunodeficiency (SCID), (B) Combined Immunodeficiency (CID) patients compared to healthy donors (HD). Each sample (subject) is represented by the combination of variables (T subsets) and allocated in a Cartesian space. Samples are plotted, and similarities and differences can be visualized. The overlay of the 2D (2 Dimensional) plot of the scores (subjects) with the 2D plot of the loadings (combination of T subsets) allows the identification of the variables that most contribute to the characterization of a specific subject, since they lie in the same area of the graphs. PCA identifies directions, called principal components, along which the variation in the data is maximal, reported as percentages near the axis. SCID patients are indicated by green dots and the letter S, CID patients by blue dots and the letter C and healthy donors (HD) are represented by gray dots. Individual patients are represented as: C3 and C7 (blue dots) diagnosed as Combined Immunodeficiency patients (CID), K1 (dark green dot) is a DOCK8 Deficiency patient (AR-HIES), L1 and L2 (fuchsia dots) are X-Linked HyperIgM type1 patients (XL-HIGM1), P1 (light blue) is a patient with X-Linked Inhibitor of Apoptosis Protein deficiency (XIAP), S1 (magenta dot) is a SAVI patient (STING-Associated Vasculopathy with onset in Infancy: STING), R1 (purple dot) is a patient with a not determined T cell defect.
CVID immunophenotypes did not segregate uniformly (Figure 3), but when analyzed by age groups and compared to CID (Figures 4A,B and Supplementary Figure 3), PCA showed one 4-year-old patient (V1) with a CID-like clinical phenotype, characterized by recurrent infections, bronchiectasis, and facial dysmorfism that clustered far from HD near the CID age matched patients' area (Supplementary Figure 3). Furthermore, two 6–16-year-old CVID patients (V4 and V8, Figure 4A) presented a severe clinical course and could be distinguished by TEMRA CD8+ expansion in V4, probably related to persistent viral infections, and TCM CD4+ and TEM CD8+ in V8; patient V8 developed overtime a MAS (Macrophage Activated Syndrome), leading eventually to death. On the other hand, in the same 6–16 year age range three CVID patients (V2, V5, V10) with increased TN CD4+ clustered nearby but showed diverse molecular diagnosis: in V2 was reported a dominant heterozygous mutation c.2557CNT (p.Arg853*) in the NFKB2 gene, in V5 was detected a mutation in Transmembrane Activator and Calcium-Modulator and Cyclophilin-Ligand Interactor (TACI), while V10 is still without a definite diagnosis (Figure 4A).
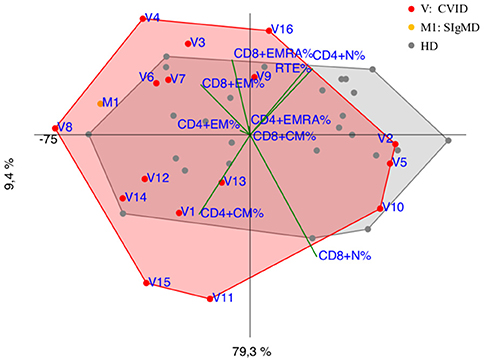
Figure 3. PCA Scatter plot of T cell subpopulations frequencies of CVID patients (shown as red dots and the letter V) and SIgMD patient (orange dot) compared to healthy donors (HD gray dots) show overlapping areas except for V3,V4, V8, V11, V15, V16 and M1.
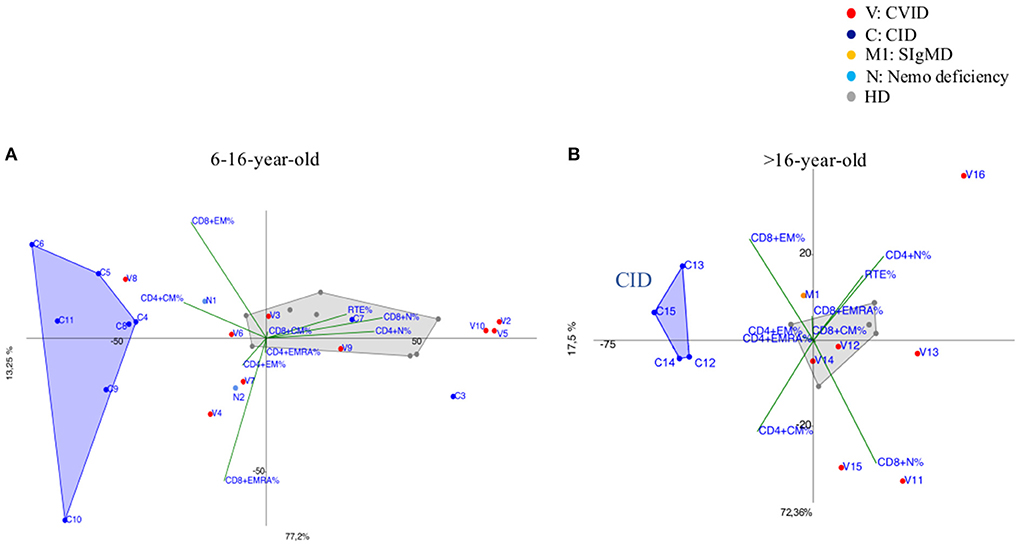
Figure 4. (A) 6–16-year-old CVID patients (shown as red dots and with the letter V) compared to 6–16 year old CID patients (distinguished by blue dots and the letter C) and age matched HDs (shown as gray dots). CVID patients (V2, V5, V10) showed immunoprofile with increased TN CD4+ although they do not have the same diagnosis. N1 and N2 (light blue dots) patients bearing a splice site mutation NEMO transcript are differently identified: N1 more vs. TCM CD4+ while N2 showed a trend vs. TEM/EMRA CD8+. (B) more than 16-year-old CVID patients (shown as red dots and the letter V) compared to more than 16-year CID patients (drawn as blue dots and the letter C) and age matched HD (shown as gray dots). CID patients (C13, C14, C15) diagnosed as APDS (activated PI3K delta syndrome) and C12 cluster together due to the main discriminating variables such as TCM CD4+ and TEM CD8+ and to TEMRA CD8+ cell subsets. M1 (orange dot) is a selective IgM deficiency (SIgMD) patient with an immunoprofile showing a trend vs. late memory differentiation in both CD4+ and CD8+ subsets.
The immunoprofile of two NEMO deficiency relatives patients in age range 6–16 year (N1 and N2) (Figure 4A) and bearing a splice site mutation in the 59 UTR of the NEMO transcript, was skewed differently: N1 more vs. TCM CD4+ while N2 showed a trend vs. TEM/EMRA CD8+.
The older than 16 year CVID patient (V16), mother of patient V2 and bearing the same NFKB2 mutation of her child, outlied far in the upper right quadrant, influenced by the highly increased TN CD4+ frequencies (Figure 4B). In the same age range two CVID patients (V11, V15), segregated according to their relative TCM CD4+ expansion, maintaining normal TN CD8+ frequencies: V15 carried a Cytotoxic T-Lymphocyte-Associated protein 4 deficiency (CTLA4) while patient V11 is still under investigation (Figure 4B). The single selective IgM deficiency patient (M1) was not characterized by a distinctive differentiation pattern, although segregating outside HD area (Figure 4B).
DGS and Thymic excision patients were distributed in a broad area between controls and CID group (Figure 5 and Supplementary Figure 5B) mirroring the reported variable T cell defect severity and highlighting those with a CID-like phenotype (D4 and D12). Consistently, patient D1, the only one affected by complete DGS, resembling a SCID phenotype, clustered close to SCID area.
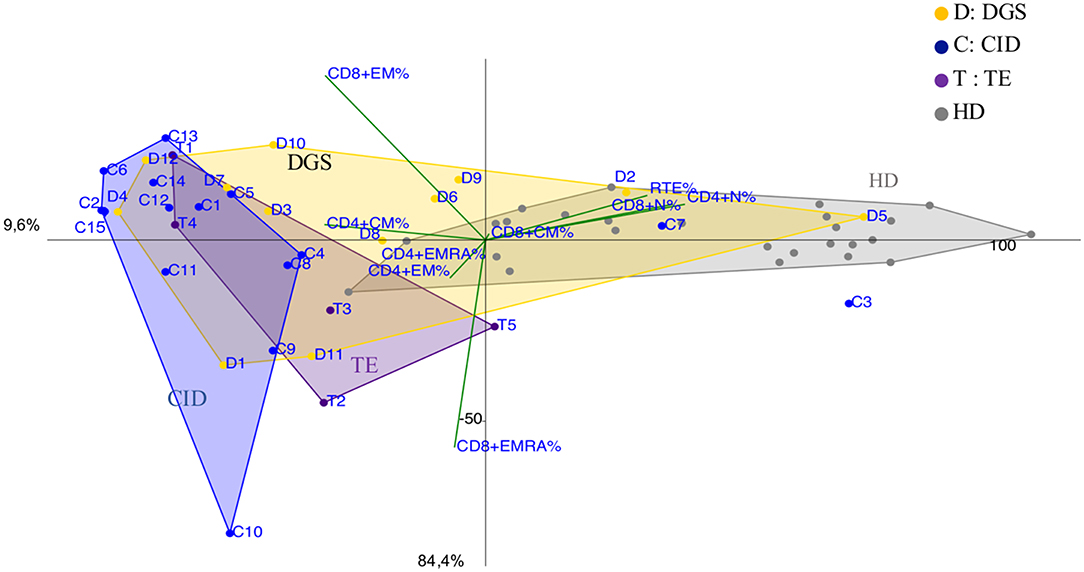
Figure 5. PCA Scatter plots of T cell subpopulations frequencies of DiGeorge Syndrome (DGS, yellow dots and letter D) patients, Thymic Excision patients (TE, distinguished by purple dots and letter T), Combined Immunodeficiency (CID, represented by blue dots and the letter C) patients and healthy donors are shown as gray dots. D1 is the complete DGS patient, D4 and D12 are partial DGS patients with a CID-like clinical presentation.
Notably, CGD patients showed a trend vs. effector memory subsets, more evident in older patients and in the younger ones (G5 and G6) with a more severe clinical presentation (Figure 6).
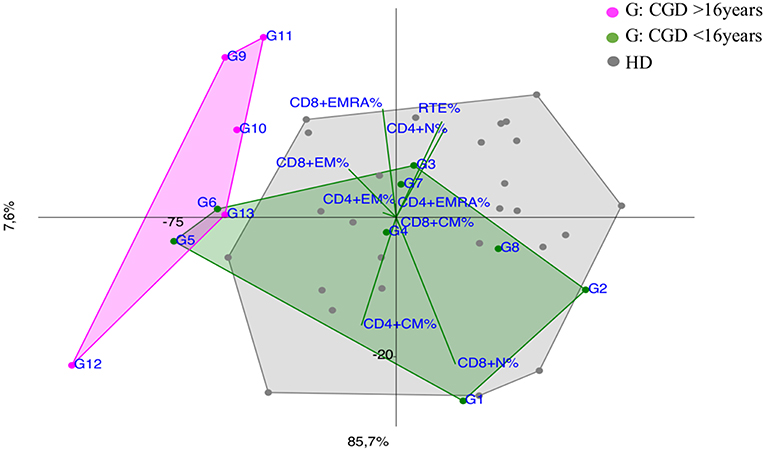
Figure 6. PCA Scatter plots of T cell subpopulations frequencies of Chronic Granulomatous Disease (CGD) patients divided by age: younger CGD patients (G1, G2, G3, G4, G5, G6, G7, G8 represented by green dots) are <16 year old; they cluster inside HD area with the exception of G5 and G6, that are two twin brothers with a severe clinical presentation. The older than 16 year CGD patients (G9, G10, G11, G12, G13 drawn as fuchsia dots), show a CID-like immunoprofile.
Several attempts have been used to categorize PIDs based on clinical manifestations, humoral immune defects and T cell phenotypes. Standardized disease definitions are still lacking in the current classification for PIDs, especially in pediatric age (15, 21). Unsupervised clustering methods applied to immunophenotype data might provide additional information regarding the diagnostic and clinical criteria of PIDs, which do not fulfill any classification. We particularly focused our analysis on specific categories of patients, as well as CID and CVID given their high clinical heterogeneity, which increases the complexity of the diagnostic approach and the clinical management. Indeed, according to PCA analysis, most of CID patients clearly segregated from healthy donors and the principal discriminating variables resulted the TCM CD4+ and TEM CD8+ and to a lesser extent TEMRA CD8+ cell subsets, suggesting an imbalance between CD4+ helper and CD8+ cytotoxic function in peripheral sites (22, 23). Their expansion in the majority of CID patients is partially explained by a lymphopenia-induced proliferation process (24, 25), but also revealed a trend to an accelerated T cell exhaustion leading to an inefficient immune response and the risk to develop immunedysregulation phenomena (6). This is particularly evident in APDS patients (C9, C13, C14, C15) (Figure 2B), in which their T cell senescence leads to a higher risk of chronic infection, such as EBV replication, and therefore to lymphoproliferative disease/malignancy susceptibility (26). At the same time PCA clearly identified two CID patients (C3, C7) with milder clinical course segregating far from CID area and close to HD (Figure 4A), distinguishing them from those at higher risk to develop severe complications.
A highly heterogeneous pattern of T cell abnormalities has been observed in CVID group (Figures 3, 4A,B and Supplementary Figure 3) and PCA clustered some CVID immunophenotypes in proximity to CID area, providing a clue for a deeper monitoring in these patients. Recent ESID Registry-Working Definitions for Clinical Diagnosis shows that some patients, previously diagnosed as CVID, were reclassified as CID and unclassified antibody deficiency (15, 27). Indeed, V8 patient, initially identified as CVID, showed a marked TCM CD4+ and TEM CD8+ expansion (Figure 4A), suggesting that a more aggressive treatment should have been considered, before developing fatal complications. In patient V15 segregating far from HD due to TCM CD4+ increase (Figure 4B) a CTLA4 haploinsufficiency responsible for a perturbed T CD4+ cell homeostasis was eventually confirmed by NGS (28).
Interestingly V2 (Figure 4A) and V16 patients (Figure 4B) currently diagnosed as CVID clustered far from either HD and CVID group reflecting their own peculiar NFKB2 driven differentiation defect (TN cell expansion) (29, 30). NEMO deficiency patients (N1, N2) (Figure 4A) showed a different trend vs. TCM CD4+ and TEM/EMRA CD8+ respectively, confirming the high variability in the clinical and immunological disease expression (31). IgM deficiency patient (M1) (Figure 4B) showed a trend vs. late memory differentiation in both CD4+ and CD8+ subsets that could influence B cell subset, as reported in a larger cohort (32).
As largely described (33), immunoprofiles of patients with partial DGS and Thymic excisions resulted extremely heterogeneuos in PCA (Figure 5), as well as in their clinical course. Patients who underwent to total thymic excision during cardiac surgery in neonatal age show a clinical improvement with age in terms of frequency/severity of infections, suggesting a peripheral recovery of the T-cell compartment (34). Interestingly two patients partial DGS (D4 and D12) with a more severe clinical phenotype (refractory autoimmune cytopenia and recurrent bacterial infections) clustered in CID area suggesting the need of a stricter follow up.
Although CGD is primarily a phagocytes disorder, recent evidence showed a defect in adaptive immunity in both T and B cell compartment (35–37), as shown in PCA by an early T cell senescence evident in older CGD patients (Figure 6), probably related to chronic inflammation. PCA could be useful to consider and to monitor an immunomodulating treatment whenever necessary in CGD patients to reduce the T cell exhaustion (38).
No evident alterations and specific segregation were detected in XL-HIGM1, XIAP, LOF STAT3 (AD-HIES), and SIgAD patients (Supplementary Figures 4A,B), except for one SIgAD patient (A13) with a severe autoimmune cytopenia which segregated outside the HD area (39–42). Although reduction in CD4+ memory T cell subsets was previously reported in XLA patients (43), this data was not confirmed in our limited cohort and a longer follow up is necessary. Only two XLA patients (X2 and X4) segregated accordingly to their TEM/EMRA CD8+ cell expansion, likely associated to recent infections (Supplementary Figure 4B).
In order to test the PCA potentiality, we applied it in three patients (K1, R1, and S1) with a severe clinical presentation not easily classifiable (Figure 2B and Table 1). Patient K1, with a history of endocarditis, vasculitis and sepsis, apparently normal T cell count, showed a high and evident terminal effector CD8+ T cells expansion and later DOCK8 deletion was detected using multiple genetic approaches (44, 45). Patient R1, with a history of interstitial pneumonia and lymphocytes lung infiltration, showed TEM CD4+ expansion, alerting us for a severe but still undefined T cell defect. Finally, child S1 with a picture mimicking a CID, in PCA segregated close to healthy donors' area, ruling out a severe immunodeficiency. Mutation in STING gene was detected by NGS targeted panel for autoinflammatory inborn error allowing the start of a specific treatment (46).
The multivariate data processing techniques could be used as a diagnostic and prognostic tool to identify peculiar immune profiles, to screen atypical PID with higher risk for severe disease progression and monitor the response to personalized therapeutic approaches.
The datasets generated for this study are available on request to the corresponding author.
SD performed the experiments, analyzed, and interpreted the results and wrote the manuscript. EA analyzed and interpreted the results and wrote the manuscript. GD and CCi performed the molecular analysis. EA, NC, DA, CG, CCi, PP, and AF provided or referred clinical samples and patient's clinical data. EA, SD, and CCa designed the work, participated to the study design and data interpretation. NC, DA, MC, GD, CCi, and PR revised critically the manuscript.
The study was supported by grants of the Italian Ministero della Salute (NET-2011-02350069) to CCa and Ricerca Corrente from Childrens' Hospital Bambino Gesù, Rome, Italy (201702P003966) to CCa. We are grateful to Yu Nee Lee for providing advice regarding the multivariate analysis and Prof. Notarangelo Luigi for helpful suggestions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.02735/full#supplementary-material
Supplementary Figure 1. Naiv¨e and memory T-cell subsets gating strategy. PBMC from lysed whole blood were gated on live lymphocytes and identified as CD45+. Then after sequential gating on CD3+CD4+ subset, the naiv¨e (TN) cells were distinguished as CD45RA+CD27+, central memory cells (TCM) as CD45RA-CD27+, effector memory cells (TEM) as CD45RA-CD27-, and terminally differentiated cells (TEMRA) as CD45RA+CD27-. Among CD3+CD4+ T cells, recent thymic emigrants (RTE) were identified as CD31++CD45RA+. Similarly after gating on CD3+CD8+ subset, the naiv¨e (TN) cells were gated as CD45RA+CCR7+, central memory cells (TCM) as CD45RA-CCR7+, effector memory cells (TEM) as CD45RA-CCR7-, and terminally differentiated cells (TEMRA) as CD45RA+CCR7-.
Supplementary Figure 2. PCA Scatter plot of T cell subpopulations frequencies in SCID patients and HD <6-year-old. Healthy donors <6-year-old are represented by gray dots and SCID patients by green dots and the letter S. PCA identifies directions along which the variation in the data is maximal, in this analysis represented by TCM CD4+ cells inversely correlated to TN CD4+ as variables that most contribute to the characterization of different subjects.
Supplementary Figure 3. Less than 6-year-old Common Variable Immunodeficiency (CVID) aged patient (V1) indicated by red dot and the letter V compared to aged matched Combined Immunodeficiency (CID) patients, represented by blue dots and the letter C and age matched healthy donors (HD) shown as gray dots.
Supplementary Figure 4. PCA Scatter plot of T cell subpopulations frequencies in (A) Loss of Function STAT3 (LOF STAT3) (Autosomal Dominant Hyper-IgE Syndrome) (AD-HIES) patients represented by green dots and the letter E and healthy donors by gray dots, showing the complete overlapping of the areas. (B) X-linked Agammaglobulinemia (XLA) patients by light blue dots and the letter X, Selective IgA deficiency (SIgAD) patients by yellow dots and the letter A, one not defined Agammaglobulinemia patient (n.d) by brown dot and letter Y, healthy donors are represented by gray dots. The X2 and X4 patients' immunoprofiles segregated outside HD area, accordingly to their TEM/EMRA CD8+ cell expansion. Not defined Agammaglobulinemia (Y1) patient clustered in the HD area, not revealing any peculiar pattern.
Supplementary Figure 5. PCA Scatter plots of T cell subpopulations frequencies of (A) Severe Combined Immunodeficiency (SCID) compared to Combined Immunodeficiency (CID) patients. SCID patients are indicated by green dots and the letter S and CID patients by blue dots and the letter C. (B) DiGeorge Syndrome (DGS) patients (represented by yellow dots and letter D), Thymic excision patients (TE) (by purple dots and letter T) compared to CID patients (by blue dots and the letter C).
Supplementary Tables 1a–c. Clinical and molecular diagnosis of the patients.
SCID, severe combined immunodeficiency; CID, combined immunodeficiency; TE, thymic excision; DGS, DiGeorge syndrome; LOF STAT3 (AD-HIES), loss of function STAT3 (autosomal dominant hyper-IgE syndrome); CVID, common variable immunodeficiency; CGD, chronic granulomatous disease; XLA, X-linked agammaglobulinemia; SIgAD, selective IgA deficiency; NEMO deficiency, nuclear factor-kappa B essential modulator deficiency; DOCK8 (AR-HIES) deficiency, dedicator Of cytokinesis 8 (autosomal recessive hyper-IgE syndrome) deficiency; XL-HIGM1, X-linked HyperIgM type1; SAVI, STING-associated vasculopathy with onset in infancy; XIAP, X-linked inhibitor of apoptosis protein; TACI, transmembrane activator and calcium-modulator and cyclophilin-ligand lnteractor, CTLA4, cytotoxic T-lymphocyte-associated protein 4.
1. Notarangelo LD. Primary immunodeficiencies. J Allergy Clin Immunol. (2010) 125:S182–94. doi: 10.1016/j.jaci.2009.07.053
2. Lehman HK. Autoimmunity and immune dysregulation in primary immune deficiency disorders. Curr Allergy Asthma Rep. (2015) 15:53. doi: 10.1007/s11882-015-0553-x
3. Bousfiha A, Jeddane L, Picard C, Ailal F, Bobby Gaspar H, Al-Herz W, et al. The 2017 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. (2018) 38:129–43. doi: 10.1007/s10875-017-0465-8
4. Fischer A. Severe Combined Immunodeficiencies (SCID). Clin Exp Immunol. (2000) 122:143–9. doi: 10.1046/j.1365-2249.2000.01359.x
5. Cifaldi C, Alessandro F, Caterina A, Email C. Hematopoietic Stem Cell Gene Therapy for the Cure of Blood Diseases: Primary Immunodeficiencies. Rendiconti Lincei. Scienze Fisiche e Naturali. Available online at: doi: 10.1007/s12210-018-0742-3 (accessed October 30, 2018).
6. Delmonte OM, Castagnoli R, Calzoni E, Notarangelo LD. Inborn errors of immunity with immune dysregulation: from bench to bedside. Front Pediatr. (2019) 7:353. doi: 10.3389/fped.2019.00353
7. Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency 2015. J Clin Immunol. (2015) 35:696–726. doi: 10.3389/fimmu.2014.00162
8. Speckmann C, Doerken S, Aiuti A, Albert MH, Al-Herz W, Allende LM, et al. A prospective study on the natural history of patients with profound combined immunodeficiency: an interim analysis. J Allergy Clin Immunol. (2017) 139:1302–10.e4. doi: 10.1016/j.jaci.2016.07.040
9. pCID study (DRKS00000497). Available online at: https://www.uniklinik-freiburg.de/cci/studien/p-cid.htm
10. Bateman EA, Ayers L, Sadler R, Lucas M, Roberts C, Woods A, et al. T cell phenotypes in patients with common variable immunodeficiency disorders: associations with clinical phenotypes in comparison with other groups with recurrent infections. Clin Exp Immunol. (2012) 170:202–11. doi: 10.1111/j.1365-2249.2012.04643.x
11. Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol. (2005) 175:6489–97. doi: 10.4049/jimmunol.175.10.6489
12. Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the human immunology project. Nat Rev Immunol. (2012) 12:191–200. doi: 10.1038/nri3229
13. Garcia-Prat M, Álvarez-Sierra D, Aguiló-Cucurull A, Salgado-Perandrés S, Briongos-Sebastian S, Franco-Jarava C, et al. Extended immunophenotyping reference values in a healthy pediatric population. Cytometry B Clin Cytom. (2019) 96:223–33. doi: 10.1002/cyto.b.21728
14. van der Burg M, Kalina T, Perez-Andres M, Vlkova M, Lopez-Granados E, Blanco E, et al. The EuroFlow PID orientation tube for flow cytometric diagnostic screening of primary immunodeficiencies of the lymphoid system. Front Immunol. (2019) 10:246. doi: 10.3389/fimmu.2019.00246
15. Seidel MG, Kindle G, Gathmann B, Quinti I, Buckland M, van Montfrans J, et al. The European Society for Immunodeficiencies (ESID) registry working definitions for the clinical diagnosis of inborn errors of immunity. J Allergy Clin Immunol Pract. (2019) 7:1763–70. doi: 10.1016/j.jaip.2019.02.004
16. Genser B, Cooper PJ, Yazdanbakhsh M, Barreto ML, Rodrigues LC. A guide to modern statistical analysis of immunological data. BMC Immunol. (2007) 8:27. doi: 10.1186/1471-2172-8-27
17. Lugli E, Roederer M, Cossarizza A. Data analysis in flow cytometry: the future just started. Cytometry A. (2010) 77:705–13. doi: 10.1002/cyto.a.20901
18. Hazenberg MD, Verschuren MC, Hamann D, Miedema F, van Dongen JJ. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J Mol Med. (2001) 79:631–40. doi: 10.1007/s001090100271
19. Junge S, Kloeckener-Gruissem B, Zufferey R, Keisker A, Salgo B, Fauchere JC, et al. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol. (2007) 37:3270–80. doi: 10.1002/eji.200636976
20. Crow YJ, Casanova JL. STING-associated vasculopathy with onset in infancy–a new interferonopathy. N Engl J Med. (2014) 371:568–71. doi: 10.1056/NEJMe1407246
21. Farmer JR, Ong MS, Barmettler S, Yonker LM, Fuleihan R, Sullivan KE, et al. Common variable immunodeficiency non-infectious disease endotypes redefined using unbiased network clustering in large electronic datasets. Front Immunol. (2018) 8:1740. doi: 10.3389/fimmu.2017.01740
22. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. (2004) 22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702
23. Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. (2005) 17:326–32. doi: 10.1016/j.coi.2005.04.010
24. Delphine S, Larsen M, Fastenackels S, Roux A, Gorochov G, Katlama C, et al. Lymphopenia-driven homeostatic regulation of naive T cells in elderly and thymectomized young adults. J Immunol. (2012) 189:5541–8. doi: 10.4049/jimmunol.1201235
25. Saidakova EV, Shmagel KV, Korolevskaya LB, Shmage NG, Chereshnev VA. Lymphopenia-induced proliferation of CD4 T-cells is associated with CD4 T-lymphocyte exhaustion in treated HIV-infected patients. Indian J Med Res. (2018) 147:376–83. doi: 10.4103/ijmr.IJMR_1801_15
26. Wentink MWJ, Mueller YM, Dalm VASH, Driessen GJ, van Hagen PM, van Montfrans JM, et al. Exhaustion of the CD8+ T cell compartment in patients with mutations in phosphoinositide 3-kinase delta. Front Immunol. (2018) 9:446. doi: 10.3389/fimmu.2018.00446
27. De Valles-Ibáñez G, Esteve-Solé A, Piquer M, González-Navarro EA, Hernandez-Rodriguez J, Laayouni H, et al. Evaluating the genetics of common variable immunodeficiency: monogenetic model and beyond. Front Immunol. (2018) 9:636. doi: 10.3389/fimmu.2018.00636
28. Schwab C, Gabrysch A, Olbrich P, Patiño V, Warnatz K, Wolff D, et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4-insufficient subjects. J Allergy Clin Immunol. (2018) 142:1932–46. doi: 10.1016/j.jaci.2018.02.055
29. Maccari E, Scarselli A, Di Cesare S, Floris M, Deodati A, et al. Severe Toxoplasma gondii infection in a member of a NFKB2-deficient family with T and B cell dysfunction. Clin Immunol. (2017) 183:273–7. doi: 10.1016/j.clim.2017.09.011
30. Klemann C, Camacho-Ordonez N, Yang L, Eskandarian Z, Rojas-Restrepo JL, Frede N, et al. Clinical and immunological phenotype of patients with primary immunodeficiency due to damaging mutations in NFKB2. Front Immunol. (2019) 10:297. doi: 10.3389/fimmu.2019.00297
31. Fusco F, Pescatore A, Conte MI, Mirabelli P, Paciolla M, Esposito E, et al. EDA-ID and IP, two faces of the same coin: how the same IKBKG/NEMO mutation affecting the NF-κB pathway can cause immunodeficiency and/or inflammation. Int Rev Immunol. (2015) 34:445–59. doi: 10.3109/08830185.2015.1055331
32. Louis AG, Agrawal S, Gupta S. Analysis of subsets of B cells, Breg, CD4Treg and CD8Treg cells in adult patients with primary selective IgM deficiency. Am J Clin Exp Immunol. (2016) 5:21–32.
33. Javier C, Rosenblatt HM, O'Brian Smith E, Shearer WT, Noroski LM. Long-term assessment of T-cell populations in DiGeorge syndrome. J Allergy Clin Immunol. (2003) 111:573–9. doi: 10.1067/mai.2003.165
34. van Gent R, Schadenberg AWL, Otto SA, Nievelstein RAJ, Sieswerda GT, Haas F, et al. Long-term restoration of the human T-cell compartment after thymectomy during infancy: a role for thymic regeneration? Blood. (2011) 118:627–34. doi: 10.1182/blood-2011-03-341396
35. Chiriaco M, Casciano F, Di Matteo G, Gentner B, Claps A, Di Cesare S, et al. Impaired X-CGD T cell compartment is gp91phox-NADPH oxidase independent. Clin Immunol. (2018) 193:52–9. doi: 10.1016/j.clim.2018.01.010
36. Albuquerque AS, Fernandes SM, Tendeiro R, Cheynier R, Lucas M, Silva SL, et al. Major CD4 T-Cell Depletion and Immune Senescence in a Patient with Chronic Granulomatous Disease. Front Immunol. (2017) 8:543. doi: 10.3389/fimmu.2017.00543
37. Cotugno N, Finocchi A, Cagigi A, Di Matteo G, Chiriaco M, Di Cesare S, et al. Defective B-cell proliferation and maintenance of long-term memory in patients with chronic granulomatous disease. J Allergy Clin Immunol. (2015) 135:753–61.e2. doi: 10.1016/j.jaci.2014.07.012
38. Weisser M, Demel UM, Stein S, Chen-Wichmann L, Touzot F, Santilli G, et al. Hyperinflammation in patients with chronic granulomatous disease leads to impairment of hematopoietic stem cell functions. J Allergy Clin Immunol. (2016) 138:219–28.e9. doi: 10.1016/j.jaci.2015.11.028
39. Egwuagu CE. STAT3 in CD4+ T helper cell differentiation and inflammatory diseases. Cytokine. (2009) 47:149–56. doi: 10.1016/j.cyto.2009.07.003
40. Ma CS, Chew GYJ, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. (2008) 205:1551–5. doi: 10.1084/jem.20080218
41. Lougaris V, Lanzi G, Baronio M, Gazzurelli L, Vairo D, Lorenzini T, et al. Progressive severe B cell and NK cell deficiency with T cell senescence in adult CD40L deficiency. Clin Immunol. (2018) 190:11–14. doi: 10.1016/j.clim.2018.02.008
42. Rebecca A. Marsh, MD, Bleesing JJ, Filipovich AH. Using flow cytometry to screen patients for X-linked lymphoproliferative disease due to SAP deficiency and XIAP deficiency. J Immunol Methods. (2010) 362:1–9. doi: 10.1016/j.jim.2010.08.010
43. Martini H, Enright V, Perro M, Workman S, Birmelin J, Giorda E, et al. Importance of B cell co-stimulation in CD4(+) T cell differentiation: X-linked agammaglobulinaemia, a human model. Clin Exp Immunol. (2011) 164:381–7. doi: 10.1111/j.1365-2249.2011.04377.x
44. Randall KL, Chan SSY, Ma CS, Fung I, Mei Y, Yabas M, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. (2011) 208:2305–20. doi: 10.1084/jem.20110345
45. Janssen E, Tsitsikov E, Al-Herz W, Lefranc G, Megarbane A, Dasouki M, et al. Flow cytometry biomarkers distinguish DOCK8 deficiency from severe atopic dermatitis. Clin Immunol. (2013) 150:220–4. doi: 10.1016/j.clim.2013.12.006
Keywords: flow cytometric immunophenotyping, T cell subsets, primary immunodeficiencies, multivariate data analysis, diagnostic markers
Citation: Attardi E, Di Cesare S, Amodio D, Giancotta C, Cotugno N, Cifaldi C, Chiriaco M, Palma P, Finocchi A, Di Matteo G, Rossi P and Cancrini C (2019) Phenotypical T Cell Differentiation Analysis: A Diagnostic and Predictive Tool in the Study of Primary Immunodeficiencies. Front. Immunol. 10:2735. doi: 10.3389/fimmu.2019.02735
Received: 17 June 2019; Accepted: 07 November 2019;
Published: 29 November 2019.
Edited by:
Tomas Kalina, Charles University, CzechiaReviewed by:
Eduardo Lopez-Granados, University Hospital La Paz, SpainCopyright © 2019 Attardi, Di Cesare, Amodio, Giancotta, Cotugno, Cifaldi, Chiriaco, Palma, Finocchi, Di Matteo, Rossi and Cancrini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Di Cesare, ZGkuY2VzYXJlQG1lZC51bmlyb21hMi5pdA==; Caterina Cancrini, Y2FuY3JpbmlAbWVkLnVuaXJvbWEyLml0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.