- 1Division of Nephrology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 2Unità Operativa Complessa di Nefrologia, Azienda Ospedaliera-Universitaria Parma, Dipartimento di Medicina e Chirurgia, Università di Parma, Parma, Italy
- 3Department of Experimental Diagnostic and Specialty Medicine (DIMES), Nephrology, Dialysis and Renal Transplant Unit, St. Orsola Hospital, University of Bologna, Bologna, Italy
- 4Department of Pathology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 5Renal Unit, Department of Medicine, University-Hospital of Verona, Verona, Italy
- 6Department of Surgery, Maimonides Medical Center, New York, NY, United States
- 7Division of Rheumatology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Current guidelines encourage administering pneumococcal vaccine Prevnar-13 to patients with lupus, but whether such vaccinations affect disease severity is unclear. To address this issue, we treated 3-month-old MRL-lpr mice, that spontaneously develop a lupus-like syndrome, with Prevnar-13 or vehicle control. After 3 months, we quantified circulating anti-Pneumococcal polysaccharide capsule (PPS) antibodies and signs of disease severity, including albuminuria, renal histology and skin severity score. We also compared immunophenotypes and function of T and B cells from treated and untreated animals. Prevnar-13 elicited the formation of anti-pneumococcal IgM and IgG. Prevnar-13 treated animals showed reduced albuminuria, renal histological lesions, and milder dermatitis compared to vehicle-treated controls. Mitigated disease severity was associated with reduced and increased T follicular helper cells (TFH) and T follicular regulatory cells (TFR), respectively, in Prevnar-treated animals. T cells from Prevnar-13 vaccinated mice showed differential cytokine production after aCD3/aCD28 stimulation, with significantly decreased IL-17 and IL-4, and increased IL-10 production compared to non-vaccinated mice. In conclusion, pneumococcal vaccination elicits anti-pneumococcal antibody response and ameliorates disease severity in MRL-lpr mice, which associates with fewer TFH and increased TFR. Together, the data support use of Prevnar vaccination in individuals with SLE.
Introduction
Systemic lupus erythematosus (SLE) is the prototypical systemic autoimmune disease with complex genetic and immune system pathogenic mechanisms operative throughout the disease course (1, 2). While several therapeutic targets have been identified, treatment of the most severe manifestations (e.g., lupus nephritis) consists of glucocorticoids and immunosuppressive/cytotoxic agents that have significant side effects and risks (3–5). Long-term use of immunosuppressive medications is a major risk factor for infection-related morbidity and mortality in SLE patients (6, 7). Bacteria are the most common pathogens seen, with Streptococcus pneumoniae being the most frequent microorganism involved in respiratory tract infections (8–11). Accordingly, international guidelines recommend pneumococcal vaccine administration to the majority of patients with SLE (12).
Despite recommendations, vaccination rates in patients with SLE are only 50–60% (13–15). Initial concerns centered on questions of vaccine efficacy, but numerous studies over the past 30 years confirm that vaccinations against Influenza and Pneumococcus are equally efficacious in patients with SLE (16). Pneumococcal conjugate vaccine (PCV; Prevnar-13) contains polysaccharides from the 13 most common pathological pneumococcal serotypes and is recommended for immunosuppressed individuals, including patients with SLE. Additional concerns that anti-pneumococcal vaccination might worsen disease severity have proven obstructive to enacting guideline recommendations. Although a study of 24 SLE patients showed no significant change in autoantibody titers or C3/C4 serum levels 2 months after anti-pneumococcal vaccination (17), onset of SLE has been described after immunization against tetanus (18, 19), hepatitis B (18, 20), and other vaccines (21), providing cause for concern.
Several mechanisms have been proposed to account for induction of post-vaccination autoantibody-mediated disease. Molecular mimicry of host structures by pathogens is a well-known theory offering an explanation for initial activation of autoreactive B cells that does not require autoantigen priming. Furthermore, it was demonstrated that normal post-vaccination anti-pneumococcal antibody responses in healthy individuals can express lupus-associated anti-DNA idiotypes (22). Similarly, a high percentage of anti-bacterial antibodies against Streptococcus pneumoniae produced in patients with lupus are capable of binding double-stranded DNA (23). These findings are cause for concern despite official recommendations.
Conversely, anti-pneumococcal vaccine may also exert anti-inflammatory effect. While polysaccharides in Prevnar vaccine elicit T cell independent B cell responses, the vaccine is conjugated to Diphtheria CRM197 protein, producing T cell dependent responses, as well (24, 25). In a murine model of allergic rhinitis and allergic airway disease it was shown that conjugated pneumococcal vaccine regulated allergen-specific TH2 responses and induced regulatory T cells (TREG) (26). Similarly capsular polysaccharide A (PSA) from Bacteroides fragilis was shown to play an important role in regulating demyelination in experimental allergic encephalomyelitis, an animal model of multiple sclerosis (27).
Despite justified concerns preventing guideline enaction, mechanistic studies investigating the effect of Prevnar-13 on immune function in SLE are lacking. To address this issue, herein we report effects of Prevnar-13 on immunological and clinical parameters of the lupus-like disease occurring in MRL-lpr mice.
Materials and Methods
Mice
MRL-Faslpr (MRL-lpr) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were housed at Icahn School of Medicine at Mount Sinai (New York, NY). Study protocols were approved by the Institutional Animal Care and Use Committee at Icahn School of Medicine at Mount Sinai.
Procedures
Twelve-week-old MRL-lpr male mice were injected with vehicle (PBS) or Prevnar-13 (pneumococcal 13-valent conjugate vaccine injection, suspension, Wyeth LLC, Pfizer Inc, NY) in a single dose of 50 μl/per mouse, i.p., and sacrificed after 12 weeks. In selected experiment, mice were also given mycophenolate mofetil (MMF; 0.563%) added to standard chow (28) for the entire duration of the experiment (months of age 3–6).
Pneumococcal Specific Indirect ELISA
Peripheral blood was collected through retro-orbital blood draw before vaccination with Prevnar, and after sacrifice by cardiac puncture. Serum was then isolated from the peripheral blood and analyzed for anti-PPS IgM and IgG antibodies. Serum was diluted to 1:100 in PBS and transferred to 96 well ELISA plates coated with 10 μg/mL per well of polysaccharides. ELISA was then carried out according to the procedure described in the WHO guidelines for PPS ELISA (https://www.vaccine.uab.edu/uploads/mdocs/ELISAProtocol(007sp).pdf) with some modifications. The goat anti-murine IgM antibody used to detect PPS IgM by custom indirect ELISA was purchased from Jackson ImmunoResearch (115–006-020; West Grove, PA). The anti-murine IgG1 kappa antibody used to detect PPS IgG by custom indirect ELISA was purchased from Sigma-Aldrich (M7894; St. Louis, MO).
Assessment of Dermatitis
MRL-lpr mice develop inflammatory skin lesions on the forehead, ears and dorsum of the neck, which were scored on a scale of 0–3, where 0 = no visible skin changes, 1 = minimal hair loss with redness and a few scattered lesions, 2 = redness, scabbing, and hair loss with a small area of involvement, and 3 = ulcerations with an extensive area of involvement as described previously (29).
Renal Histology
After confirmation of stage 3 anesthesia, mice were transcardially perfused with periodate-lysin-paraformaldehyde fixate at 4% in phosphate buffered saline (PBS), and the kidneys were dissected out. Kidney samples were either frozen in Optimal Cutting Temperature compound (Tissue Tek O.C.T., Sakura, CA) or fixed in 10% formalin and then embedded in paraffin.
Light Microscopy
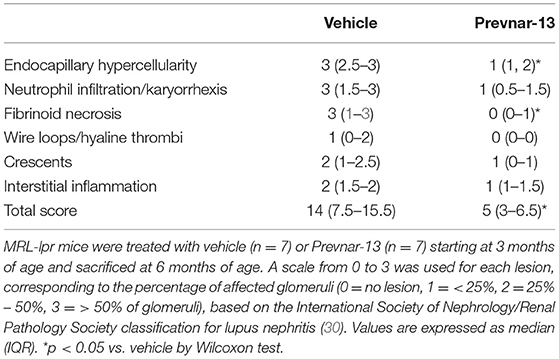
Mice kidneys were cut and stained with Periodic acid-Schiff (PAS) staining and subsequently were evaluated by a renal pathologist. Lesions were scored according to the National Institutes of Health (NIH) activity and chronicity index adapted by the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis (30). Scoring system is based on glomerular histopathological changes. The scoring criteria include these findings: endocapillary hypercellularity, neutrophils/karyorrhexis, fibrinoid necrosis, wire loop lesions and/or hyaline thrombi, cellular/fibrocellular crescents, and interstitial inflammation. A scale from 0 to 3 is used corresponding to how many glomeruli are affected, in which no lesion = 0, < 25% of glomeruli = 1, 25% – 50% of glomeruli = 2, or > 50% of glomeruli = 3.
Immunofluorescence Analysis in Renal Tissue
Samples were frozen in O.C.T. compound. For staining, 5 μm thick cryosections were incubated with PBS for 5 min then with blocking solution containing PBS, 2% bovine serum albumin, 2% fetal bovine serum, and 0.2% fish gelatin for 30 min at room temperature (RT). For renal tissue markers, the following antibodies were used: rat anti-mouse C3b (1:50; Hycult Biotech, Wayne, PA) incubation at 4°C overnight followed by indirect staining with secondary antibody (goat anti-rat Alexa fluor - 488) 1 h at RT; or rat anti-mouse IgG-FITC antibody (1:50; eBioscience, San Diego, CA) incubation at 4°C overnight. At least 65 glomeruli/section for each animal were randomly acquired using fluorescence non-confocal laser scanning microscopy (Zeiss AxioImager Z2M with ApoTome.2). Surface antibody expression was estimated by constructing a contour mask on the merged image. Software ImageJ was used to quantify C3b and IgG staining intensity.
Urine Albumin/Creatinine
Urine creatinine was quantified using commercial kits from Cayman Chemical (Ann Arbor, MI). Urine albumin was determined using a commercial assay from Bethyl Laboratory Inc (Houston, TX). Urine albumin excretion was expressed as the ratio of urine albumin to creatinine (A/C).
Splenic T and B Lymphocyte Cytokines
Single cell suspensions of splenic lymphocytes were separated using EasySep negative selection kits from Stem Cell Technologies (Vancouver, BC): T cell kits enriched CD3+ cells and B cell kits enriched CD19+ cells. CD3+ T cells were stimulated with Dynabeads Mouse T-activator CD3/CD28 (Invitrogen) in a 1:1 bead-to-cell ratio, and isolated CD19+ B cells were stimulated with 1 μg/mL of TLR7/8 agonist R848 (InvivoGen) or 20 ng/mL of LPS-EB (InvivoGen, San Diego, CA). Supernatants were collected after 1 day for T cells and after 5 days for B cells. Cytokine quantification of IL-4 (R&D Systems, MN), IFN-γ (BD Biosciences, CA), IL-17A (eBioscience), IL-10 (BD Biosciences), TNF-α (BD Biosciences), and IL-6 (R&D Systems) were carried out by sandwich ELISA per manufacturer's instructions.
Flow Cytometry
We used standard approaches for surface and intracellular staining as published (31). For surface staining, we used PE-Cy7 and BV510-anti-CD4 (clone RM4–5 and GK1.5 respectively), PE-Cy7-anti-CD8 (clone RPA-T8); FITC-anti-CD25 (clone 15F9); biotinylated anti-CXCR5 followed by Pacific Blue streptavidin, PerCP-Cy5.5-anti-TCRb (clone H57–597), PE-anti-ICOS (clone 15F9), PE-Cy7-anti-PD-1 (clone RMP1–30), PerCP-Cy 5.5-anti-B220 (clone RA 3–6 B2), APC-Cy7-anti-IgD (clone 11–26c), PE-Cy7-anti-IgM (clone eB121–15F9), PE-anti-FAS (clone Jo2), FITC-anti-GL7 (clone Ly-77). For intracellular staining, we used APC and FITC-anti-FoxP3 (clones MF23 and FJK-16S). All these antibodies were obtained from BD Pharmingen.
Data were acquired (10,000 to 100,000 events) on a three-laser Canto II flow cytometer (BD Biosciences) and analyzed using FlowJo (https://www.flowjo.com) software.
Statistical Analyses
Comparisons of continuous variables between two groups were analyzed by t-tests. For histological score comparisons, we used Wilcoxon test. Two-way repeated measures ANOVA test was used for multiple comparisons among treatment groups. P-values < 0.05 were considered significant. All statistical analyses were performed using GraphPad Prism (version 8 for Windows, GraphPad Software, Inc.).
Results
Prevnar-13 Vaccination Promotes an Anti-Pneumococcal IgG Response in MRL-lpr Mice
SLE, and medications used to treat it, may prevent effective vaccination responses. To test this, we injected 12-week-old MRL-lpr mice with Prevnar-13 or PBS as control. We quantified anti-PPS specific IgM and IgG antibodies 12 weeks later (Figure 1A). Anti-PPS IgM and IgG antibody responses were detected in all vaccinated mice (Figures 1B,C) while none were seen in control mice. To determine how immunosuppression affects antibody responses in SLE, we vaccinated MRL-lpr mice receiving mycophenolate mofetil (MMF), a commonly used treatment in human SLE. PPS-specific antibody levels in MMF treated mice were similar to those in mice receiving vaccine alone and significantly higher than in unvaccinated controls (Figures 1B,C). Together, these data show that Prevnar-13 vaccination elicits an anti-pneumococcal IgM and IgG response in MRL-lpr mice even under MMF immunosuppressive therapy.
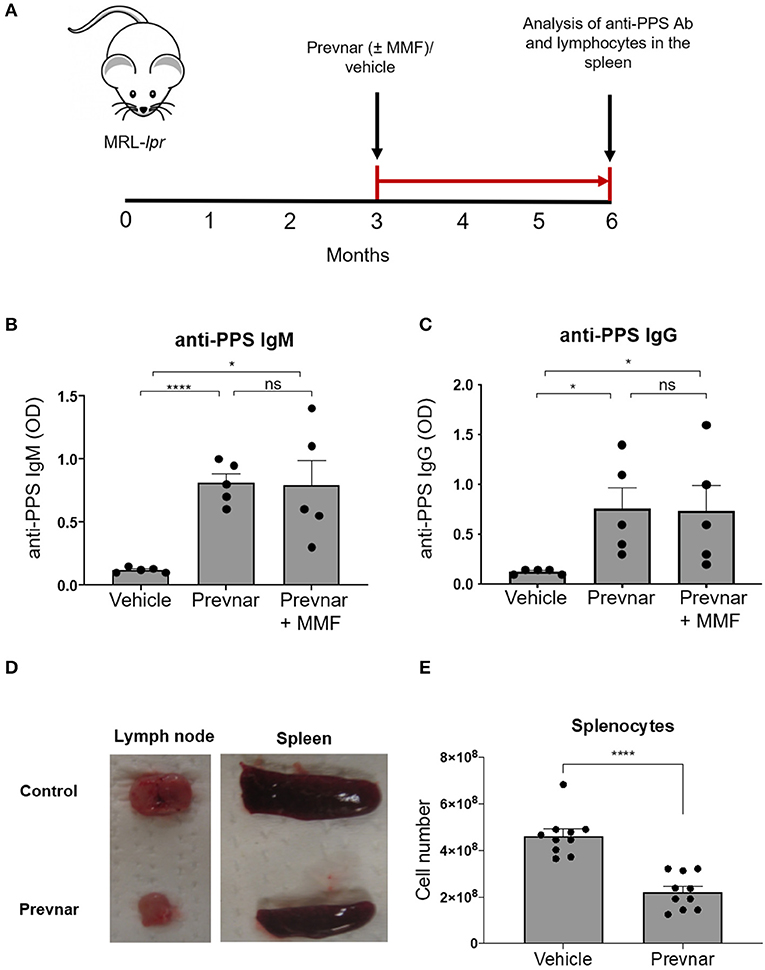
Figure 1. Prevnar-13 vaccination promotes an anti-pneumococcal IgG response and reduces lymphocyte numbers in MRL-lpr mice. Twelve-week-old MRL-lpr mice were given intraperitoneal injection with Prevnar-13 or vehicle control (A) with or without mycophenolate mofetil (MMF). Twelve weeks later, we measured serum anti-pneumococcal polysaccharide (anti-PPS) IgM (B) and IgG (C) levels. In a separate set of experiments, we treated animals with Prevnar or vehicle (no MMF) and we sacrificed them for quantification of disease severity and immunological studies. Representative pictures of lymph nodes and spleens (D) and splenocyte number (E) in Prevnar-13 or vehicle-treated MRL-lpr mice. Data are presented as mean ± SEM. They represent a total of 5 mice per group (B,C) or 10 mice per group (D,E) obtained over two or three independent experiment, respectively; *p < 0.05, ****p < 0.0001, ns, not significant by unpaired t-test.
Prevnar-13 Vaccination Reduces Lymphadenopathy in MRL-lpr Mice
We next determined whether Prevnar-13 vaccination affects autoimmune disease in lymph nodes, spleen and skin of MRL-lpr mice. Similar to many SLE patients with active disease, MRL-lpr mice spontaneously develop splenomegaly and lymphadenopathy. The incidence and severity of lymphadenopathy and splenomegaly and total number of splenocytes were reduced in vaccinated MRL-lpr mice compared to control mice (Figures 1D,E).
Prevnar-13 Vaccination Ameliorates Lupus Dermatitis and Nephritis
Worsening disease severity, including cutaneous and renal disease, is the most serious concern preventing widespread vaccination of SLE patients. Cutaneous lupus manifestations in the MRL-lpr strain include facial rash, lesions of the back and neck, ulceration and necrosis of the ears. We quantified development of skin lesions in MRL-lpr mice vaccinated with Prevnar-13 and in vehicle-treated ones. The incidence and severity of skin lesions were significantly reduced in Prevnar-13 vaccinated mice compared with the control (Figures 2A,B). MRL-lpr mice also develop kidney disease characterized by marked glomerular and interstitial inflammation. To evaluate the effect of Prevnar-13 vaccination on kidney disease, we quantified albuminuria and kidney pathology. Similar to its ameliorative effect on skin disease, Prevnar-13 vaccination decreased severity of kidney disease. Vaccinated mice had significantly less inflammatory infiltrates and proliferative glomerular lesions compared to controls (Figures 2C,D and Table 1). Consistently, Prevnar treatment was associated with significantly lower IgG (Figures 2E,F) and C3b (Figures 2G,H) deposition in the glomeruli and reduced albuminuria than in control mice (Figure 2I).
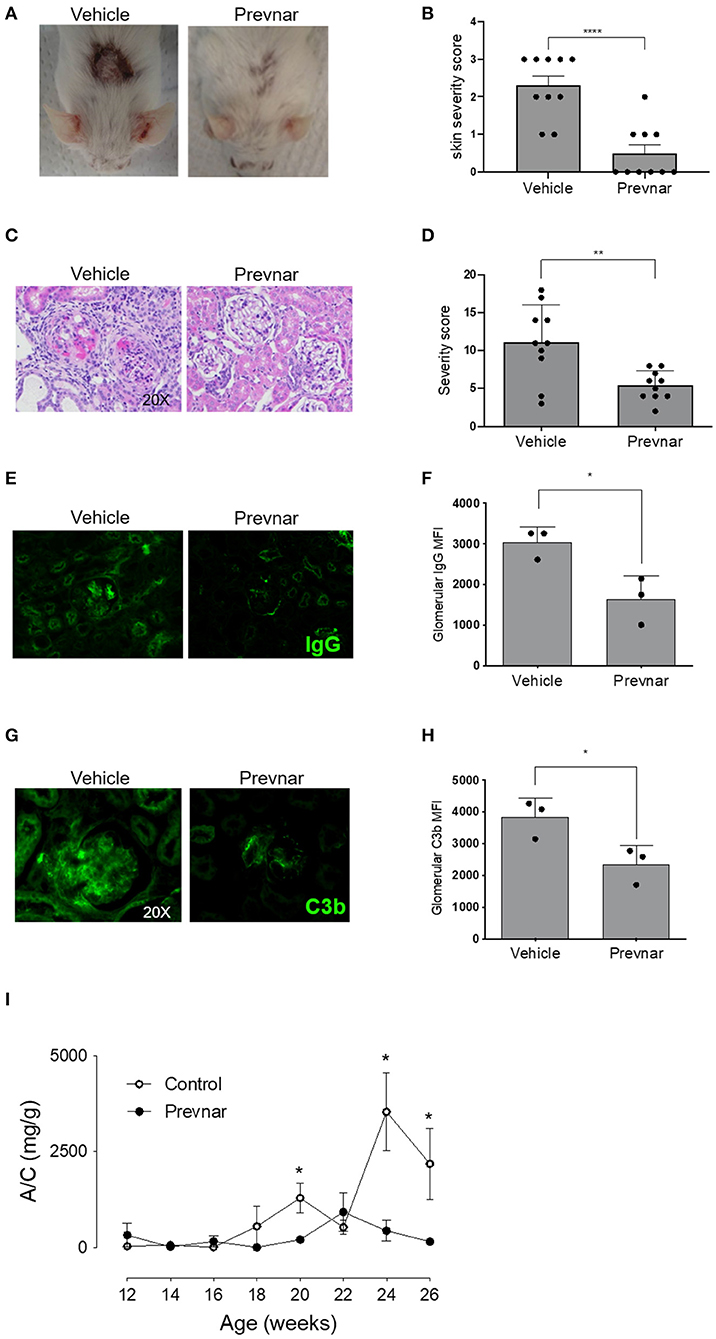
Figure 2. Prevnar-13 vaccination ameliorates lupus dermatitis and nephritis. Representative pictures (A) and score (B) of skin lesions in Prevnar-13 or vehicle treated MRL-lpr mice depicted in Figures 1D,E. Representative glomerular lesions (C) and histology severity score (D) of nephritis. Representative immunofluorescence staining for IgG (E) and C3b (G) and data quantification (F and H, respectively) in the glomeruli of 3 control and 3 Prevnar-13 vaccinated MRL-lpr mice (each dot represents the average of glomerular MFI for each mouse). Weekly changes in urinary albumin/creatinine ratio (A/C) (I). Data are presented as mean ± SEM and they are the total of three independent experiments including 3–4 mice per group each. Two mice per group died before 6 months of age; *p < 0.05, **p < 0.001, ****p < 0.0001 by Wilcoxon test (B,D), unpaired t-test (F,H), or two-way repeated measures ANOVA test (I; comparisons between the two groups at the same time-points).
Prevnar-13 Vaccination Differentially Regulates T and B Lymphocyte Cytokine Production in MRL-lpr Mice
Adaptive immune responses coordinated between T and B cells are essential to SLE pathology. We isolated CD3+ T cells and CD19+ B cells from spleens of control and Prevnar-13 vaccinated MRL-lpr mice to compare their function. Production of IL-17 and IL-4 by anti-CD3/anti-CD28 antibody-stimulated T cell was completely blocked in Prevnar-13 vaccinated mice (Figures 3A,B), while IL-10 production was significantly increased (Figure 3C). IFN-γ and TNF-α production was not affected (Figures 3D,E). We stimulated B cells with two toll like receptor ligands, namely R848 and LPS, that activate TLR7/8 and TLR4, respectively. Similar to T cells, stimulated B cells produced less cytokines (IL-6 and TNF-α) in Prevnar-13 vaccinated mice (Figures 3F–H).
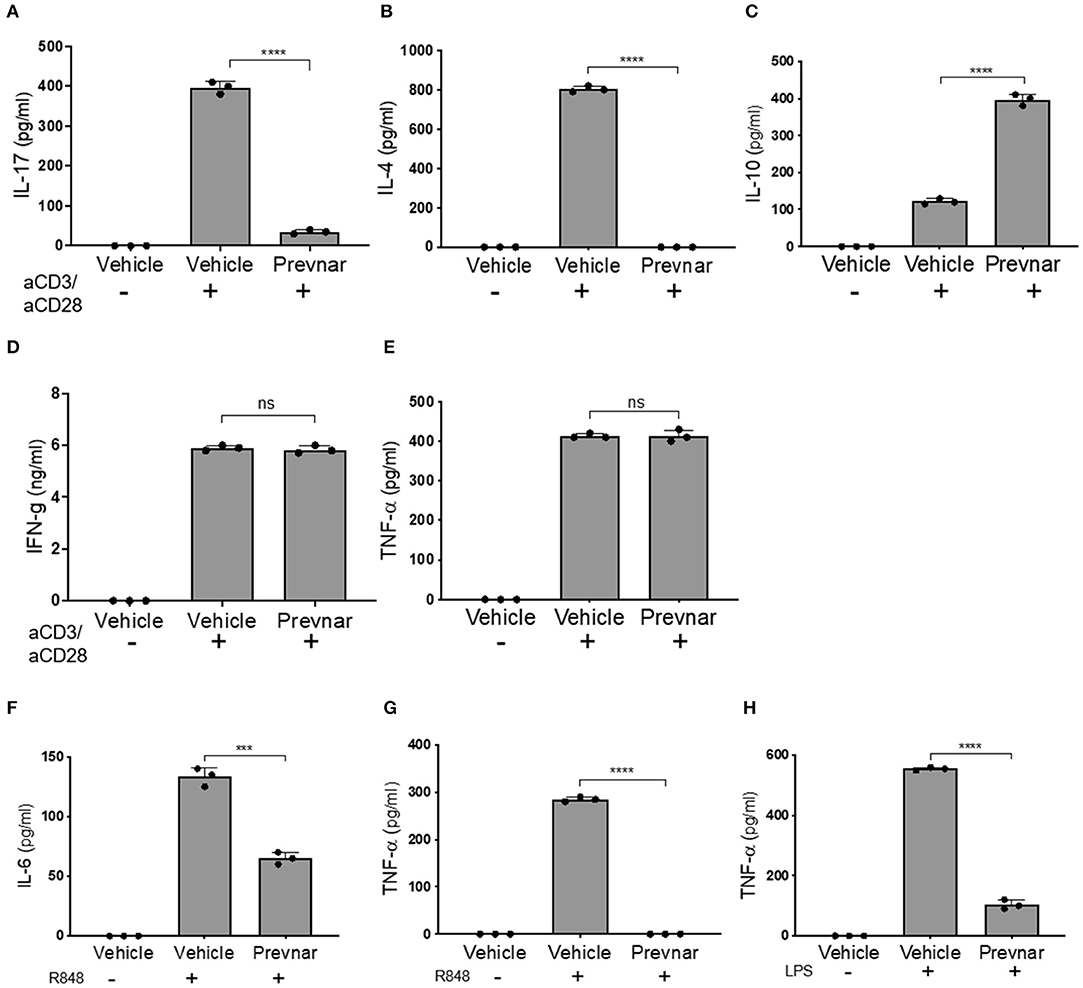
Figure 3. Prevnar-13 vaccination differentially regulates T and B lymphocyte cytokine production in MRL-lpr mice. Enriched CD3+CD4+ T cells isolated from the spleens of control or Prevnar-13 vaccinated MRL-lpr mice were stimulated with anti-CD3 and anti-CD28 antibodies for 4 h. Supernatants were harvested to measure cytokine production: IL-17 (A), IL-4 (B), IL-10 (C), and IFN-γ (D), TNF-α (E) (ELISA). Splenic B220+ B cells were enriched from the same mice and stimulated with the TLR7/8 ligand R848 (F,G) or TLR4 ligand LPS (H). At 1 day after T cell stimulation and 5 days after B cell stimulation, we measured IL-6 (F) and TNF-α (G,H) production in the supernatants (ELISA). Data are presented as mean ± SD and each dot represents the technical replicate of T or B cell responses from 6 mice per group pooled together, ***p < 0.001, ****p < 0.0001, ns, not significant by unpaired t-test.
Prevnar-13 Vaccination Reduces TFH and Increases TFR Cells in MRL-lpr Mice
To understand the mechanisms responsible for amelioration of disease severity associated with Prevnar-13 administration, we measured percentages of total CD4+ and CD8+ T cells, CD4+CD25+FOXP3+ regulatory T cells (TREG), TFH, TFR, and germinal center B cells (GC B). We found that TFH were significantly lower, while TFR were significantly higher in Prevnar-13 treated mice compared to controls (Figures 4A–C), as was TFR/TFH (Figure 4D). No other T cell subsets were significantly different between groups (Supplementary Figure 1), suggesting that the dominant T cell effect responsible for the amelioration of disease severity in Prevnar-13 treated MRL-lpr mice is related to changes in TFH and TFR cells.
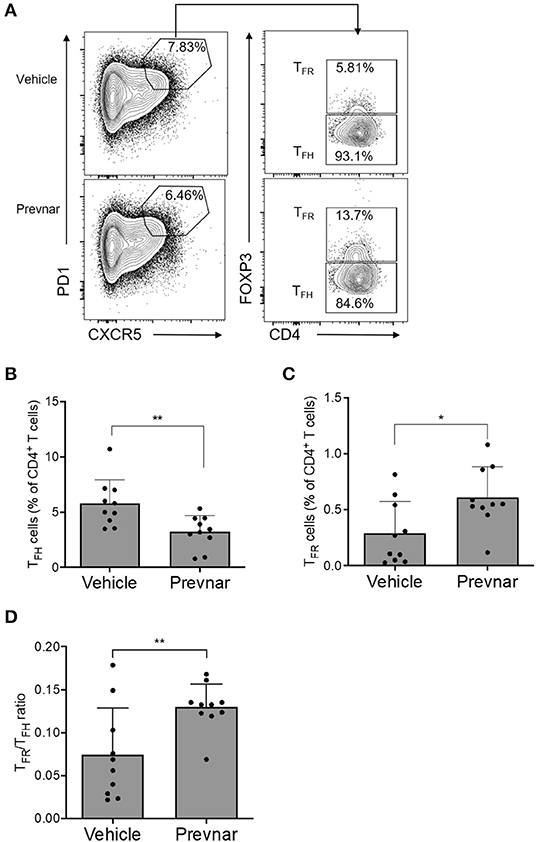
Figure 4. Prevnar-13 vaccination reduces TFH and increases TFR cells in MRL-lpr mice. Percentages of CD4+CXCR5+PD1+FOXP3+ TFH (A,B) and CD4+CXCR5+PD1+FOXP3− TFR (A,C) cells, and TFR/TFH ratio (D) in 6-month old MRL-lpr treated with Prevnar-13 or vehicle at 3 months of age. Data are presented as mean ± SD and they are the total of three independent experiments including each 3–4 mice per group, *p < 0.05, **p < 0.001 by unpaired t-test.
Discussion
Our data indicate that anti-pneumococcal vaccine Prevnar-13, effectively produces humoral PPS-specific immunity in lupus-prone MRL-lpr mice, while also ameliorating disease severity, a phenomenon associated with reduced TFH cells. Although SLE patients are at increased risk for Streptococcus pneumoniae infection (9, 11), vaccination coverage remains dramatically low (32). Limited data are available regarding efficacy of recommended prime-and-boost strategy using Prevnar-13 in SLE patients, but available evidence indicates that they can generate an effective response, albeit inferior compared to healthy controls (11, 33). Our data in MRL-lpr mice confirm that Prevnar-13 effectively promotes anti-pneumococcal response, even under MMF immunosuppression, supporting feasibility for vaccination use even in patients receiving immunosuppression.
The effect of Prevnar-13 vaccination on disease severity is poorly defined. Although overall data support its safety, cases of increased disease severity post-vaccination were reported (17, 18, 34). Our data in mice surprisingly showed significant amelioration of both renal and skin lesions in vaccinated mice. Our data were generated in 3-month-old mice, a phase when disease is only at its onset (35). This is rarely the case in humans with SLE whose vaccine indication is related to more advanced disease. It is possible that effects of Prevnar-13 would be diminished or absent in more established/ongoing disease. Another difference between our experiments in mice and human studies is that mice were not receiving immunosuppression, which is rarely the case for SLE patients. However, our data largely support safety of vaccination with Prevnar and support the concept that, if performed early during disease progression, it may be beneficial for disease management.
Generally, polysaccharides are considered to be classic T-cell-independent antigens that do not elicit cell-mediated immune responses. Most bacterial polysaccharides elicit humoral immune responses that result in the induction of low-affinity IgM and some IgG antibodies. Certain polysaccharides of microbial origin have been described to act as potent immunomodulators with specific activity for both T cells and antigen-presenting cells (36). These polysaccharides termed zwitterionic polysaccharides (ZPSs), have both positively and negatively charged moieties and share the same biologic function. These ZPSs include S. pneumoniae type 1 polysaccharide (Sp1), Staphylococcus aureus type 5 and 8 polysaccharides (CP5 and CP8), and PSA from B. fragilis. ZPSs are handled by the MHCII pathway in a manner similar to that documented for traditional protein antigens (8). The first step is uptake of antigen by antigen presenting cells (APCs). Sp1 is endocytosed by professional APCs such as dendritic cells (DCs), B cells, and macrophages.
In an animal model of intra-abdominal surgical adhesion formation, ZPSs (PSA and Sp1) induced formation of a distinct subpopulation of CD4+CD45RBlo T cells that produced IL-10 and have anti-inflammatory properties (37). Consistently, we found that Prevnar-13 was associated with increased IL-10 production by T cells which suppresses the IL-2 production required for T-cell clonal expansion (38, 39). Conversely, production of IL-4 and IL-17, two cytokines associated with active disease (40), were inhibited by Prevnar-13.
Polysaccharide-protein conjugates bind to the B cell receptor (BCR) of polysaccharide-specific pre-B cells and are taken into the endosome (41, 42). The protein portion is digested by proteases to release peptide epitopes, which bind to MHCII by replacing self-peptide. Our data show that B cells from Prevnar-13 treated mice display reduced production of TNF-α and IL-6, cytokines that are important for B cell maturation and have been shown to correlate with disease activity (43–45). Whether such effects are due to a direct effect of ZPSs on B cell or through affected T cells modifying B cell activity was not addressed by our studies.
Autoimmune disease in MRL-lpr mice is dependent on TLR7, TLR9, and toll-like receptor (TLR) signal transduction molecule Myeloid differentiation primary response 88 (MYD88). Mice lacking either the combined TLR7/9 or MYD88 do not develop autoimmune disease (46). Our ex vivo studies demonstrated that B cell from Prevnar-treated mice are hyporesponsive to stimulation with ligands for TLR 7/8 and TLR4, both of which utilize MYD88 for signal transduction. Although pneumococcal polysaccharides alone do not ligate TLRs, the formulation in Prevnar contains TLR2 agonists (47) dependent on MYD88 for signaling (48). While usually viewed in the context of macrophages, endotoxin tolerance causes immune cell changes, including increased regulatory T cells (49). We speculate that Prevnar antigens and duration of their signaling could be producing a relative “tolerant” state compared to untreated mouse, which would be consistent with observed impairments of cytokine production and increased TFR frequencies.
Previous studies indicate that polysaccharide vaccines may generate TFH cell responses in mice and humans, with a link to a functional association with the immunogenicity of the vaccine (50, 51). However, data on the effect of Prevnar-13 on TFH and TFR (a subset of regulatory T cells that controls TFH activation) are missing. Our data show that, in Prevnar-13 treated mice, the TFR/TFH ratio significantly increased. How Prevnar promoted these subpopulation changes and their role in modulating disease severity go beyond the scope of the present paper; it is possible to speculate that reduction of peripheral TFH cells is, at least in part, involved in the beneficial effect of Prevnar-13 on disease activity.
In conclusion, our studies showed that anti-pneumococcal vaccination with Prevnar-13 associates with amelioration of disease severity in murine lupus, a phenomenon associated with reduced TNF-α and IL-6 production by B cells and increased TFR/TFH ratio. These data provide further rationale and support to current guidelines recommending Prevnar-13 vaccination in SLE patients. Whether these mechanisms extend to other vaccines remains unknown.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Icahn School of Medicine.
Author Contributions
PC and IT designed the study, with the help from EF, GL, and GZ. CC, CG, SH, and VG performed all the experiments. FS scored kidney lesions. SA performed immunofluorescence staining. PC, JL, and IT wrote the initial draft of the paper. All the authors approved the paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.02695/full#supplementary-material
References
1. Tsokos GC. Systemic Lupus Erythematosus. N Engl J Med. (2011) 365:2110–21. doi: 10.1056/NEJMra1100359
2. Tsokos GC, Lo MS, Reis PC, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. (2016) 12:716. doi: 10.1038/nrrheum.2016.186
3. Bertsias G, Ioannidis JPA, Boletis J, Bombardieri S, Cervera R, Dostal C, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. (2008) 67:195. doi: 10.1136/ard.2007.070367
4. Singh JA, Hossain A, Kotb A, Wells G. Risk of serious infections with immunosuppressive drugs and glucocorticoids for lupus nephritis: a systematic review and network meta-analysis. BMC Med. (2016) 14:137. doi: 10.1186/s12916–016-0673–8
5. Rua-Figueroa I, Lopez-Longo J, Galindo-Izquierdo M, Calvo-Alen J, Del Campo V, Olive-Marques A, et al. Incidence, associated factors and clinical impact of severe infections in a large, multicentric cohort of patients with systemic lupus erythematosus. Semin Arthritis Rheum. (2017) 47:38–45. doi: 10.1016/j.semarthrit.2017.01.010
6. Feldman CH, Marty FM, Winkelmayer WC, Guan H, Franklin JM, Solomon DH, et al. Comparative Rates of Serious Infections Among Patients With Systemic Lupus Erythematosus Receiving Immunosuppressive Medications. Arthritis Rheumatol. (2017) 69:387–97. doi: 10.1002/art.39849
7. Feldman CH, Hiraki LT, Winkelmayer WC, Marty FM, Franklin JM, Kim SC, et al. Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol. (2015) 67:1577–85. doi: 10.1002/art.39070
8. Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. (2004) 117:677–87. doi: 10.1016/j.cell.2004.05.001
9. Schurder J, Goulenok T, Jouenne R, Dossier A, Van Gysel D, Papo T, et al. Pneumococcal infection in patients with systemic lupus erythematosus. Joint Bone Spine. (2018) 85:333–6. doi: 10.1016/j.jbspin.2017.05.012
10. Danza A, Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. (2013) 22:1286–94. doi: 10.1177/0961203313493032
11. Luijten RK, Cuppen BV, Bijlsma JW, Derksen RH. Serious infections in systemic lupus erythematosus with a focus on pneumococcal infections. Lupus. (2014) 23:1512–6. doi: 10.1177/0961203314543918
12. Furer V, Rondaan C, Heijstek MW, Agmon-Levin N, van Assen S, Bijl M, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. (2019) 2019:215882. doi: 10.1136/annrheumdis-2019–215882
13. Yazdany J, Tonner C, Trupin L, Panopalis P, Gillis JZ, Hersh AO, et al. Provision of preventive health care in systemic lupus erythematosus: data from a large observational cohort study. Arthritis Res Ther. (2010) 12:R84. doi: 10.1186/ar3011
14. Lawson EF, Trupin L, Yelin EH, Yazdany J. Reasons for failure to receive pneumococcal and influenza vaccinations among immunosuppressed patients with systemic lupus erythematosus. Semin Arthritis Rheum. (2015) 44:666–71. doi: 10.1016/j.semarthrit.2015.01.002
15. Chehab G, Richter JG, Brinks R, Fischer-Betz R, Winkler-Rohlfing B, Schneider M. Vaccination coverage in systemic lupus erythematosus-a cross-sectional analysis of the German long-term study (LuLa cohort). Rheumatology. (2018) 57:1439–47. doi: 10.1093/rheumatology/key120
16. Murdaca G, Orsi A, Spano F, Puppo F, Durando P, Icardi G, et al. Influenza and pneumococcal vaccinations of patients with systemic lupus erythematosus: current views upon safety and immunogenicity. Autoimmun Rev. (2014) 13:75–84. doi: 10.1016/j.autrev.2013.07.007
17. Elkayam O, Paran D, Burke M, Zakut V, Ben-Yitshak R, Litinsky I, et al. Pneumococcal vaccination of patients with systemic lupus erythematosus: Effects on generation of autoantibodies. Autoimmunity. (2005) 38:493–6. doi: 10.1080/08916930500285725
18. Shapiro E, Kopicky J. Comment on the article “can immunization precipitate connective tissue disease? Report of 5 cases of systemic lupus erythematosus and review of the literature”. Semin Arthritis Rheum. (2000) 30:215–6.
19. Kashef S, Ghazizadeh F, Derakhshan A, Farjadian S, Alyasin S. Antigen-specific antibody response in juvenile-onset SLE patients following routine immunization with tetanus toxoid. Iran J Immunol. (2008) 5:181–4.
20. Maillefert J, Sibilia J, Toussirot E, Vignon E, Eschard J, Lorcerie B, et al. Rheumatic disorders developed after hepatitis B vaccination. Rheumatology. (1999) 38:978–83. doi: 10.1093/rheumatology/38.10.978
21. Murdaca G, Orsi A, Spanò F, Faccio V, Puppo F, Durando P, et al. Vaccine-preventable infections in Systemic Lupus Erythematosus. Hum Vaccin Immunother. (2016) 12:632–43. doi: 10.1080/21645515.2015.1107685
22. Grayzel A, Solomon A, Aranow C, Diamond B. Antibodies elicited by pneumococcal antigens bear an anti-DNA–associated idiotype. J Clin Invest. (1991) 87:842–6. doi: 10.1172/JCI115088
23. Kowal C, Weinstein A, Diamond B. Molecular mimicry between bacterial and self antigen in a patient with systemic lupus erythematosus. Eur J Immunol. (1999) 29:1901–11.
24. McCool TL, Harding CV, Greenspan NS, Schreiber JR. B- and T-cell immune responses to pneumococcal conjugate vaccines: divergence between carrier- and polysaccharide-specific immunogenicity. Infect Immun. (1999) 67:4862–9.
25. Broker M, Berti F, Schneider J, Vojtek I. Polysaccharide conjugate vaccine protein carriers as a “neglected valency” - Potential and limitations. Vaccine. (2017) 35:3286–94. doi: 10.1016/j.vaccine.2017.04.078
26. Thorburn AN, Foster PS, Gibson PG, Hansbro PM. Components of Streptococcus pneumoniae Suppress Allergic Airways Disease and NKT cells by inducing regulatory T cells. J Immunol. (2012) 188:4611. doi: 10.4049/jimmunol.1101299
27. Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. (2010) 3:487–95. doi: 10.1038/mi.2010.29
28. Jonnalagadda M, Brown CE, Chang WC, Ostberg JR, Forman SJ, Jensen MC. Engineering human T cells for resistance to methotrexate and mycophenolate mofetil as an in vivo cell selection strategy. PLoS ONE. (2013) 8:e65519. doi: 10.1371/journal.pone.0065519
29. Saito K, Mori S, Date F, Ono M. Sjögren's syndrome-like autoimmune sialadenitis in MRL-Faslpr mice is associated with expression of glucocorticoid-induced TNF receptor-related protein (GITR) ligand and 4–1BB ligand. Autoimmunity. (2013) 46:231–7. doi: 10.3109/08916934.2012.757307
30. Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. (2018) 93:789–96. doi: 10.1016/j.kint.2017.11.023
31. Purroy C, Fairchild RL, Tanaka T, Baldwin WM III, Manrique J, Madsen JC, et al. Erythropoietin receptor-mediated molecular crosstalk promotes T cell immunoregulation and transplant survival. J Am Soc Nephrol. (2017) 28:2377–92. doi: 10.1681/ASN.2016101100
32. Serre J, Francois C, der Haegen MCV, Papo T, Goulenok T, Sacre K. Nurse-led vaccination program dramatically improves pneumococcal vaccination coverage among patients with autoimmune inflammatory disorders. Eur J Intern Med. (2017) 43:e43–5. doi: 10.1016/j.ejim.2017.05.023
33. Sacre K, Goulenok T, Bahuaud M, Francois C, Van der Haegen MC, Alexandra JF, et al. Impaired long-term immune protection following pneumococcal 13-valent/23-valent polysaccharide vaccine in systemic lupus erythematosus (SLE). Ann Rheum Dis. (2018) 77:1540–2. doi: 10.1136/annrheumdis-2017–212789
34. Older SA, Battafarano DF, Enzenauer RJ, Krieg AM editors. Can immunization precipitate connective tissue disease? Report of five cases of systemic lupus erythematosus and review of the literature. Semin Arthritis Rheum. (1999) 29:131–9. doi: 10.1016/S0049–0172(99)80024–9
35. Donadei C, Angeletti A, Cantarelli C, D'Agati VD, La Manna G, Fiaccadori E, et al. Erythropoietin inhibits SGK1-dependent TH17 induction and TH17-dependent kidney disease. JCI Insight. (2019) 5:127428. doi: 10.1172/jci.insight.127428
36. Jha V, Janoff EN. Complementary role of CD4+ T cells in response to pneumococcal polysaccharide vaccines in humans. Vaccines. (2019) 7:E18. doi: 10.3390/vaccines7010018
37. Stephen TL, Groneck L, Kalka-Moll WM. The modulation of adaptive immune responses by bacterial zwitterionic polysaccharides. Int J Microbiol. (2010) 2010:917075. doi: 10.1155/2010/917075
38. Ruiz-Perez B, Chung DR, Sharpe AH, Yagita H, Kalka-Moll WM, Sayegh MH, et al. Modulation of surgical fibrosis by microbial zwitterionic polysaccharides. Proc Natl Acad Sci USA. (2005) 102:16753–8. doi: 10.1073/pnas.0505688102
39. Stingele F, Corthesy B, Kusy N, Porcelli SA, Kasper DL, Tzianabos AO. Zwitterionic polysaccharides stimulate T cells with no preferential V beta usage and promote anergy, resulting in protection against experimental abscess formation. J Immunol. (2004) 172:1483–90. doi: 10.4049/jimmunol.172.3.1483
40. Zhou H, Hu B, Huang N, Mo X, Li W, Zhang B, et al. Aberrant T cell subsets and cytokines expression profile in systemic lupus erythematosus. Clin Rheumatol. (2018) 37:2405–13. doi: 10.1007/s10067–018-4124–0
41. Avci FY, Li X, Tsuji M, Kasper DL. Carbohydrates and T cells: a sweet twosome. Semin Immunol. (2013) 25:146–51. doi: 10.1016/j.smim.2013.05.005
42. Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med. (2011) 17:1602–9. doi: 10.1038/nm.2535
43. Ripley BJ, Goncalves B, Isenberg DA, Latchman DS, Rahman A. Raised levels of interleukin 6 in systemic lupus erythematosus correlate with anaemia. Ann Rheum Dis. (2005) 64:849–53. doi: 10.1136/ard.2004.022681
44. Aringer M, Smolen JS. The role of tumor necrosis factor-alpha in systemic lupus erythematosus. Arthritis Res Ther. (2008) 10:202. doi: 10.1186/ar2341
45. Godsell J, Rudloff I, Kandane-Rathnayake R, Hoi A, Nold MF, Morand EF, et al. Clinical associations of IL-10 and IL-37 in systemic lupus erythematosus. Sci Rep. (2016) 6:34604. doi: 10.1038/srep34604
46. Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. (2010) 184:1840–8. doi: 10.4049/jimmunol.0902592
47. Sen G, Khan AQ, Chen Q, Snapper CM. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J Immunol. (2005) 175:3084–91. doi: 10.4049/jimmunol.175.5.3084
48. Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. (2009) 30:475–87. doi: 10.1016/j.it.2009.07.009
49. Andrade MMC, Ariga SSK, Barbeiro DF, Barbeiro HV, Pimentel RN, Petroni RC, et al. Endotoxin tolerance modulates TREG and TH17 lymphocytes protecting septic mice. Oncotarget. (2019) 10:3451–61. doi: 10.18632/oncotarget.26919
50. Abudulai LN, Fernandez S, Corscadden K, Burrows SA, Hunter M, Tjiam MC, et al. Production of IgG antibodies to pneumococcal polysaccharides is associated with expansion of ICOS+ circulating memory T follicular-helper cells which is impaired by HIV infection. PLoS ONE. (2017) 12:e0176641. doi: 10.1371/journal.pone.0176641
Keywords: lupus, prevnar, vaccination, TFH, TFR
Citation: Cantarelli C, Guglielmo C, Hartzell S, Salem FE, Andrighetto S, Gazivoda VP, Fiaccadori E, La Manna G, Zaza G, Leventhal J, Tassiulas I and Cravedi P (2019) Pneumococcal Polysaccharide Vaccine Ameliorates Murine Lupus. Front. Immunol. 10:2695. doi: 10.3389/fimmu.2019.02695
Received: 17 September 2019; Accepted: 01 November 2019;
Published: 20 November 2019.
Edited by:
Stefania Gallucci, Temple University, United StatesReviewed by:
Xin M. Luo, Virginia Tech, United StatesTrine N. Jorgensen, Case Western Reserve University, United States
Copyright © 2019 Cantarelli, Guglielmo, Hartzell, Salem, Andrighetto, Gazivoda, Fiaccadori, La Manna, Zaza, Leventhal, Tassiulas and Cravedi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paolo Cravedi, cGFvbG8uY3JhdmVkaUBtc3NtLmVkdQ==
†These authors share first authorship
‡These authors share senior authorship
 Chiara Cantarelli
Chiara Cantarelli Chiara Guglielmo1,3†
Chiara Guglielmo1,3† Susan Hartzell
Susan Hartzell Fadi El Salem
Fadi El Salem Gaetano La Manna
Gaetano La Manna Gianluigi Zaza
Gianluigi Zaza Jeremy Leventhal
Jeremy Leventhal Ioannis Tassiulas
Ioannis Tassiulas Paolo Cravedi
Paolo Cravedi