
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 25 June 2019
Sec. Comparative Immunology
Volume 10 - 2019 | https://doi.org/10.3389/fimmu.2019.01432
 Guangqing Yu1,2,3,4†
Guangqing Yu1,2,3,4† Xing Liu1,2,3†
Xing Liu1,2,3† Jinhua Tang1,2,3,4
Jinhua Tang1,2,3,4 Chenxi Xu1,2,3
Chenxi Xu1,2,3 Gang Ouyang1,2,3
Gang Ouyang1,2,3 Wuhan Xiao1,2,3,4,5*
Wuhan Xiao1,2,3,4,5*Neddylation is a type of post-translational protein modifications, in which neural precursor cell expressed developmentally downregulated protein 8 (NEDD8) is covalently conjugated to the lysine residues of target substrates. The best characterized principal substrates of neddylation are the cullin-RING ligases (CRLs). In addition, neddylation also modifies non-cullin proteins to affect gene regulation, cell survival, organ development, and stress response. However, the role of neddylation in antiviral innate immunity remain largely unknown. Here, we found that when neddylation was blocked by the NEDD8 activating enzyme E1 (NAE) inhibitor, MLN4924, the cellular and organismal antiviral response was suppressed. Moreover, the disruption of nedd8 increased the sensitivity of zebrafish to SVCV infection. Further assays indicated that blocking or silencing neddylation significantly downregulated key antiviral genes after poly (I:C) stimulation or SVCV infection, but dramatically increased SVCV replication. Neddylation of Irf3 and Irf7 was readily detected, but not of Mda5, Mavs, and Tbk1. Thus, our results not only demonstrated that neddylation facilitated the antiviral response in vitro and in vivo, but also revealed a novel role of nedd8 in antiviral innate immunity.
Neddylation is a type of post-translational protein modifications in which neural precursor cell expressed developmentally downregulated protein 8 (NEDD8) is covalently conjugated to the lysine residues of target substrates (1). Like ubiquitination, neddylation is triggered by the sequential actions of NEDD8 activating enzyme E1 (NAE), NEDD8-conjugation enzyme E2 and NEDD8-E3 ligase (1). Neddylation is a reversible modifications; protein deneddylation is performed by deneddylases, such as DEN1/SENP8 (2, 3). The best characterized principal substrates of Neddylation are the cullin-RING ligases (CRLs), in the E3 ubiquitin ligase family (4). However, neddylation also modifies non-cullin targets, regulating substrate protein activity, stability, and subcellular localization (5–8). Functionally, neddylation is critical for gene regulation, cell survival, organ development, and the stress response (6, 7). Dysregulation of neddylation is associated with disease pathogenesis (9–11).
The role of protein neddylation in immunological regulation has received increasing attentions (12–14). The inhibition of neddylation leads to the suppression of LPS-induced pro-inflammatory cytokine production in macrophage cells (15). Neddylation regulates T-cell function by targeting Shc and Erk signaling (16), and is also required for HSV-1-induced early phase IFN-beta production (17). In addition, neddylation of Myd88 or BCA3 indirectly downregulates NF-κB signaling (18, 19). Recently, it has been shown that blocking the neddylation pathway suppresses influenza virus replication and the pro-inflammatory response (20). Finally, neddylation enhances CD4+ T cell-mediated protective immunity against–blood stage Plasmodium infection (12). Interestingly, MLN4924, an inhibitor of NAE, inhibits TLR3/4 and retinoic acid-inducible gene I-induced IFN-β expression by preventing IRF3 binding to the IFN-β promoter, with a neddylation-independent manner (21).
Although neddylation may be involved in multiple immune responses, it is still largely unclear whether neddylation in response to pathogenic infection benefits or damages the host. This uncertainty remains because suitable assays, particularly in vivo animal models are lacking. Zebrafish (Danio rerio) is a model organism that has been used for studies of the antiviral response in vivo (22, 23). Here, we used a cell culture system and a zebrafish model to show that blocking neddylation suppressed the antiviral immune response and that disruption of nedd8 reduced the ability of zebrafish to combat viral infection. Our results thus demonstrated that neddylation played an important role in facilitating the host antiviral response.
We cultured epithelioma papulosum cyprini (EPC) cells (originally obtained from the American Type Culture Collection, Manassas, VA, USA) in medium 199 (Biological Industries, Cromwell, CT, USA) supplemented with 10% fetal bovine serum (FBS). We cultured zebrafish liver (ZFL) cells (originally obtained from the American Type Culture Collection) in 50% L-15 (Invitrogen, Carlsbad, CA, USA), 35% DMEM-HG (Invitrogen), and 15% Ham's F12 medium (Invitrogen) supplemented with 0.15 g/l sodium bicarbonate (Sigma-Aldrich, St. Louis, MO, USA), 15 mM HEPES (Sigma-Aldrich), and 10% FBS. EPC cells and ZFL cells were maintained at 28°C in a humidified incubator containing 5% CO2. HEK293 T cells were maintained at 37°C in a humidified incubator containing 5% CO2.
We propagated Spring Viremia of Carp Virus (SVCV, an ssRNA virus that causes an important disease affecting cyprinids) in EPC cells until the cytopathic effect (CPE) was complete. We collected the culture medium and stored it at −80°C until use. Viral titers were determined by a 50% tissue culture-infective dose (TCID50) assay in EPC cells. The final virus titer was adjusted to ~2 × 108 TCID50/ml.
For viral challenge of zebrafish larvae, thirty 3-dpf zebrafish larvae per group in triplicate were challenged for 24 h at 25°C in disposable 60 mm cell culture dishes by immersion in ~2 × 108 TCID50/fish SVCV (24). Simultaneously, MLN4924 (1 mM-MLN4924 dissolved in DMSO; 5 μl) or vehicle (DMSO; 5 μl) were added to egg water. After challenge, the remaining fish in each group were transferred to fresh plates containing egg water and monitored every 8 h over a 48 h period to score mortality (25). In addition, for examining gene expression, the total RNA was extracted and quantitative real-time PCR (qPCR) assays were conducted.
For viral challenge of adult zebrafish, 3 mpf adult zebrafish (0.38 ± 0.02 g) were each intraperitoneally (i.p.) injected with 10 μl of SVCV (~2 × 108 TCID50/ml) using 10 μl Microliter syringes (Shanghai Gaoge Industry and Trade Co., Ltd., Shanghai, China). Zebrafish i.p. injected with PBS were used as the controls. After viral challenge for 48 h, zebrafish were anesthetized with tricaine methanesulfonate and dissected. The kidneys and spleens were collected and stored at −80°C for further qPCR assays.
Myc-nedd8 and GFP were subcloned into Psp64 poly (A) vector (Promega). AmpliCap SP6 High Yield message maker kit (Epicenter) was used for capped mRNA synthesis. Myc-nedd8 and GFP mRNA were synthesized and injected into zebrafish embryos at one-cell stage (400 pg/per embryo). To confirm expression of injected mRNAs, the embryos injected with Myc-nedd8 mRNA for 3 days were harvested and the expression of Myc-nedd8 was confirmed by Western blot using anti-Myc antibody (9E10, Santa Cruz).
We disrupt nedd8 in zebrafish using CRISPR/Cas9 techniques. The primers for detecting mutation are: 5′- AATGTGAATCTCGTTCAGGTGG-3′ and 5′-AGATGTACAGGAACACAACGTG−3′. The nedd8 mutant was named (nedd8 ihb1227/ihb1227) (https://zfin.org/ZDB-ALT-180718-1), following zebrafish nomenclature guidelines. To exclude off-targeting effects, we back-crossed nedd8-null zebrafish with wild-type (WT) zebrafish (strain AB; no siblings of the heterozygous zebrafish were included). After repeating this back-crossing for five generations, the F6 adult zebrafish carrying the same mutation were used for breeding. Due to the low fecundity of the nedd8-null females, we mated nedd8 +/− (♀) with nedd8 −/− (♂) to obtain nedd8 +/− and nedd8 −/− larvae for viral infection. To generate WT larvae, we mated the nedd8 +/+ (♀and ♂) siblings of the nedd8 −/− mutant.
Zebrafish were maintained in a re-circulating water system following standard protocols. All experiments with zebrafish were approved by the Institutional Animal Care and Use Committee of Institute of Hydrobiology, Chinese Academy of Sciences (protocol number 2016-018).
We dissolved MLN4924 (1 mM, in DMSO) (Selleckchem., Houston, TX, USA) to medium or egg water and yielded a final MLN4924 concentration of 1 μM. Controls were treated equivalent volumes of DMSO.
EPC cells were seeded in 12-well plates overnight and treated with either vehicle (DMSO) or MLN4924 (1 μM) for 24 h, then infected with SVCV at MOI of 1, 10, 100, 1,000 for 2 days. Subsequently, the cells were washed three times with 1 × PBS and fixed with 4% paraformaldehyde for 20 min. The fixed cells were stained with 1% crystal violet.
Cell viability was determined by the Cell Counting Kits (CCK-8) (Yeasen, HB171114) following the manufacturer's instructions. Briefly, EPC cells or ZFL cells were seeded into 96-well cell culture plates (approximately 1 × 104 cells/per well) and cultured for 24 h at 28°C. The medium was replaced with fresh medium supplemented with either vehicle (DMSO) or MLN4924 (1 μM), and the cells were inoculated with SVCV (MOI of 10) for 20 h, then added CCK-8 solution. At the time points: 24, 48, 72, 96, 120 h, we measured the cell viability, respectively. The optical density was determined at 450 nm using a microplate reader (Spectra Max® MiniMaxTM 300 Imaging Cytometer). All standards and samples were measured by three independent experiments performed in triplicate.
The open reading frame (ORF) of zebrafish nedd8 (Gene ID: 368667) was amplified by PCR and then cloned into pCI (Clontech). The cDNAs encoding mavs (Gene ID: 562867), mda5 (Gene ID: 565759), tbk1(Gene ID: 692289), irf3 (Gene ID: 564854), irf7 (Gene ID: 562867) were subcloned into pCMV-Myc (Clontech).
Neddylation assays were performed as reported previously with some modifications (26). Briefly, HEK293T cells were transfected with the indicated constructs for 16–22 h, and then harvested the cells. The cells were lysed using the lysis buffer (6 M guanidine hydrochloride, 0.1 M Na2HPO4/NaH2PO4, 10 mM imidazole and 10 mM mercaptoethanol). Subsequently, the lysates were mixed with Ni2-NTA-agarose beads (Qiagen, Valencia, CA) pre-washed with lysis buffer, and rotated at 4°C overnight. The beads were washed three times using washing buffer I (1/5 lysis buffer plus 4/5 washing buffer II (25 mM Tris/HCl (pH 6.8) plus 20 mM imidazole) and washed another 3 times using washing buffer II. The beads were eluted with the sample-loading buffer and analyzed by Western blot assays.
The following antibodies were used for Western blot assays: anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz), anti-β-actin (Santa Cruz), anti-Histone H3 (cell signaling technology), anti-nedd8 (ABclone).
HEK293T cells were transfected with different combinations for 24 h, then the cells were lysed in RIPA buffer containing 50 mM Tris (pH 7.4), 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mM EDTA (pH 8.0), 150 mM NaCl, 1 mM NaF, 1 mM PMSF, 1 mM Na3VO4, and a 1:100 dilution of protease inhibitor mixture (Sigma-Aldrich), After incubation on ice for 1 h, the lysates were centrifuged at 10,000 × g at 4°C for 15 min. The total cell lysate were boiled with 1xSDS sample loading buffer, separated on SDS-PAGE, and transferred to a polyvinylidene difluoride membrane (Millipore). Western blot assay was performed as described previously (22). The Fujifilm LAS4000 mini-luminescent image analyzer was used to image the blots.
Total RNA was extracted from cells, embryos, and tissues (kidney and spleen) using RNAiso Plus (TaKaRa Bio., Beijing, China), following the manufacturer's instruction. cDNAs were synthesized using the Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA). MonAmpTM SYBR® Green qPCR Mix (high Rox) (Monad Bio., Shanghai, China) was used for quantitative RT-PCR (qPCR) assays. The primers for qPCR assays are listed in Supplemental Table S1. Actb1 (β-actin) was used as an internal control.
Quantitative real-time PCR, and virus titer data are reported as means ± SEM of three independent experiments, each performed in triplicate. The statistical analysis was performed using GraphPad Prism 5 software using unpaired t-test (GraphPad Software). The log-rank test was used to calculate the differences in survival of the different experimental groups. Differences with P < 0.05 were considered statistically significant (25).
As a pharmacological inhibitor of NAE, MLN4924 specifically blocks NEDD8 activation and, consequently, the neddylation pathway (27). To date, most studies examining the role of neddylation in various cellular processes have used MLN4924 (12, 15, 17, 20, 21, 28). To elucidate the role of neddylation in cellular antiviral immunity, we treated cells with MLN4924 and then quantified the expression levels of the key antiviral genes after poly (I:C) treatment, the procedure mimicked a double-strand RNA (dsRNA) virus (22). Compared to the control treatment (DMSO), MLN4924 treatment suppressed the expression of several key antiviral genes (e.g., ifn1, ifn2, rsad, mxb, mxc, pkz, mavs, rig1, and tlr3) in ZFL cells after poly (I:C) stimulation (Figures 1A–I) (22). MLN4924 treatment also suppressed the expression of key antiviral genes (e.g., ifn, isg15, viperin, and β2m) in EPC cells after poly (I:C) stimulation, as compared to the control treatment (DMSO) (Figures 2A–D). In addition, MLN4924 treatment suppressed the expression of key antiviral genes (ifn and β2m) in EPC cells after SVCV infection compared to the control treatment (DMSO) (Figures 2E,F).
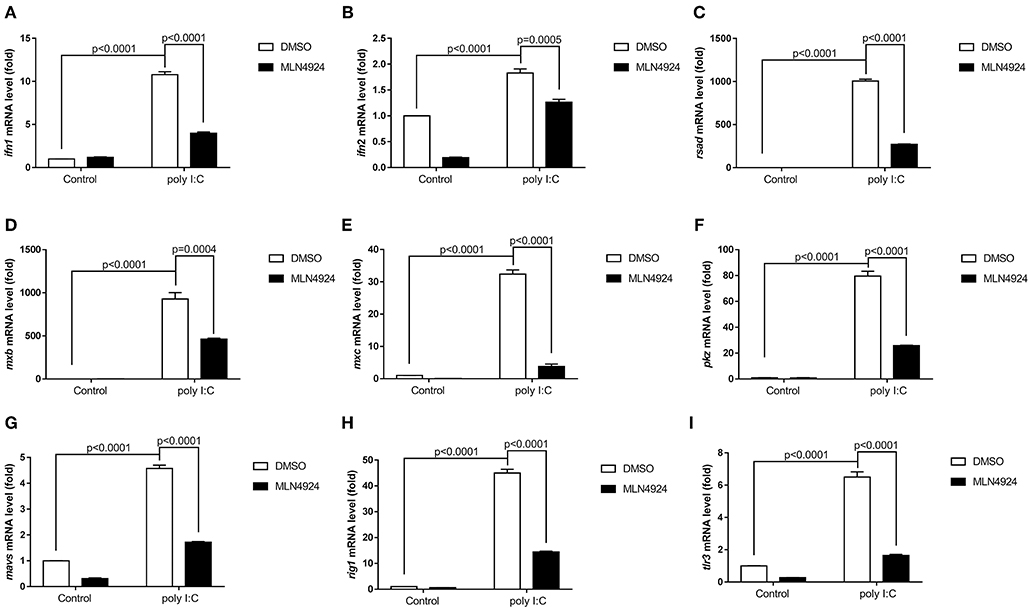
Figure 1. MLN4924 suppresses the expression of IFN and ISGs in ZFL cells after poly (I:C) stimulation. (A–I) Treatment of ZFL cells with 1 μM MLN4924 after poly(I:C) stimulation downregulated ifn1 (A), ifn2 (B), rsad (C), mxb (D), mxc (E), pkz (F), mavs (G), rig1 (H), tlr3 (I). Additions of the same amount of DMSO were used as controls. Data are shown as mean ± SEM of three independent experiments, each performed in triplicate; the statistical analysis was performed using GraphPad Prism 5 (unpaired t-test).
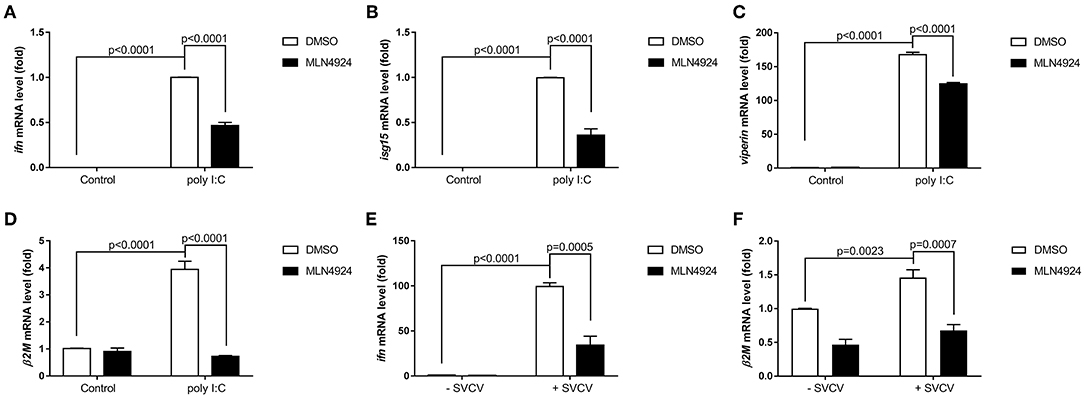
Figure 2. MLN4924 suppresses the expression of key antiviral genes in EPC cells after poly(I:C) stimulation or SVCV infection. (A–D) Treatment of EPC cells with 1 μM MLN4924 after poly(I:C) stimulation downregulated ifn (A), isg15 (B), and viperin (C), β2m (D). (E,F) Treatment of EPC cells with 1 μM MLN4924 after SVCV infection downregulated ifn (E) and β2m (F). Data are shown as mean ± SEM of three independent experiments, each performed in triplicate; the statistical analysis was performed using GraphPad Prism 5 (unpaired t-test).
Consistently, MLN4924 treatment increased SVCV replication in EPC cells, based on the increased expression of the SVCV P, G, and N genes, as compared to the control treatment (DMSO) (Figures 3A–C). As expected, CPE assays showed MLN4924 treatment reduced EPC cell survival after SVCV infection, as compared to the control treatment (DMSO) (Figure 3D). In consistent with the CPE assays, MLN4924 treatment inhibited cell proliferation of both EPC cells and ZFL cells after SVCV infection (Figures 3E,F).
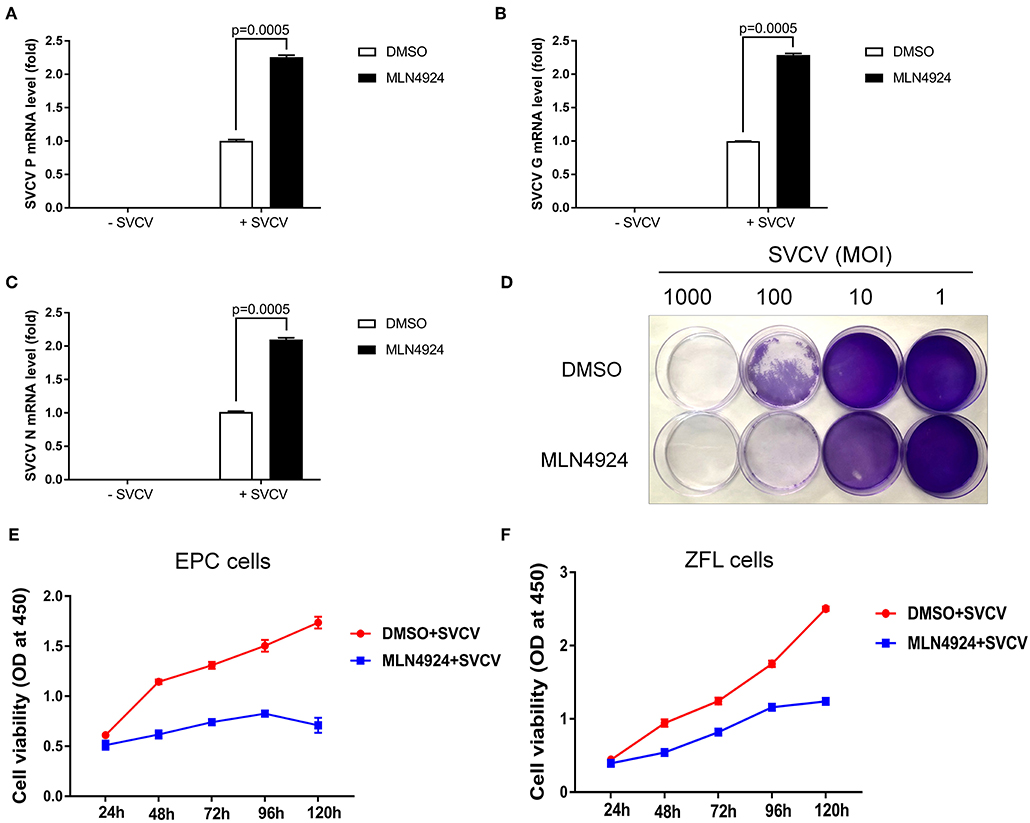
Figure 3. MLN4924 increases SVCV replication in EPC cells. (A–C) In EPC cells, MLN4924 treatment after SVCV infection increased the copy number of SVCV-related genes, as compared to vehicle (DMSO)-treated cells. We treated EPC cells with either vehicle (DMSO) or MLN4924 (1 μM) for 24 h, and then infected the cells with SVCV (MOI of 10). After SVCV infection for 24 h, we extracted total RNA and used quantitative real-time PCR (qPCR) assays to determine the mRNA expression levels of the SVCV P, G, and N genes. (D) Treatment with 1 μM MLN4924 after SVCV infection reduced survival in EPC cells. (E,F) In EPC cells (E) and ZFL cells (F), MLN4924 treatment after SVCV infection inhibited cell proliferation. We treated EPC cells or ZFL cells with either vehicle (DMSO) or MLN4924 (1 μM) for 24 h, and then infected the cells with SVCV (MOI of 10) for 24 h. Cell viability was determined using the Cell Counting Kits at the indicated time points. Data are shown as mean ± SEM of three independent experiments, each performed in triplicate; the statistical analysis was performed using GraphPad Prism 5 (unpaired t-test).
Our data thus suggested that the inhibition of neddylation suppressed the cellular antiviral response.
We examined the role of neddylation during the in vivo antiviral response using zebrafish as the model organism. Initially, we checked expression of inf1 and pkz in zebrafish larvae (3 dpf) treated with different dosage of MLN4924 for 24 h and subsequently infected with SVCV for 24 h. 1 μM MLN4924 could suppress both inf1 and pkz expression dramatically (Supplemental Figure S1). Subsequently, we chose 1 μM MLN4924 for treatment of zebrafish larvae. Similar to the results obtained for ZFL and EPC cells, MLN4924 treatment suppressed the expression of key antiviral genes (e.g., ifn1, mxc, pkz, and lta) after SVCV infection, as compared to the control treatment (DMSO) (Figures 4A–D). Furthermore, MLN4924 treatment decreased the survival rate of zebrafish larvae after SVCV infection, as compared to the control treatment (DMSO) (Figures 5A,B). Dead zebrafish larvae were recognized by lack of movement, absence of blood circulation, and bodily degeneration (Figure 5A). Consistently, SVCV replication, as indicated by the expression levels of the SVCV P, G, and N genes was significantly higher in MLN4924-treated larvae as compared to the DMSO-treated larvae (Figures 5C–E). Thus, our data suggested that blocking neddylation suppressed antiviral response in vivo.
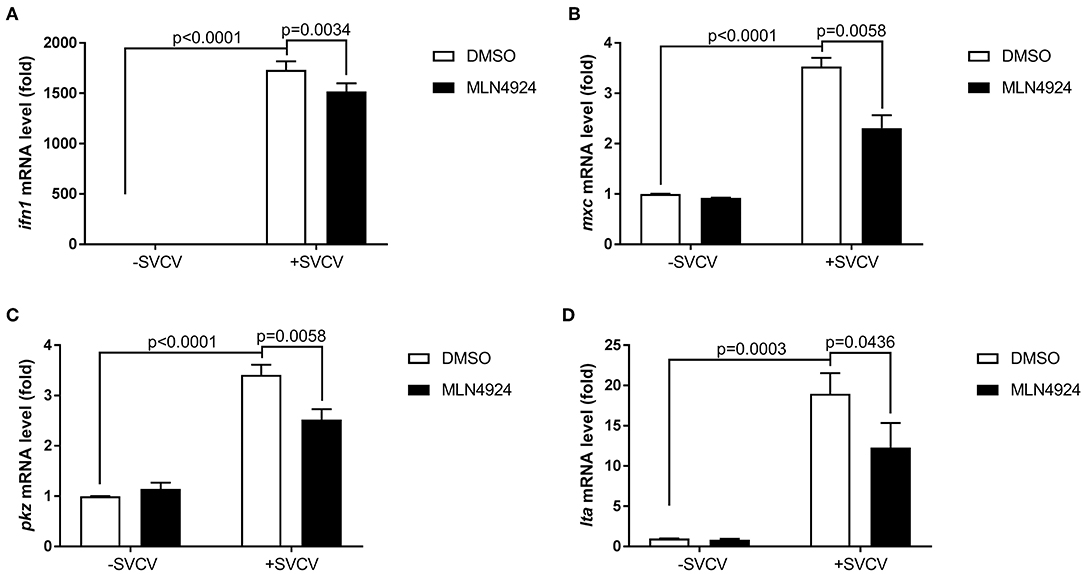
Figure 4. MLN4924 suppresses SVCV-induced activation of key antiviral genes in zebrafish larvae. (A–D) The expression levels of ifn1 (A), mxc (B), pkz (C), and lta (D) after SVCV infection were lower in zebrafish larvae treated with MLN4924 as compared to larvae treated with DMSO (control). Zebrafish larvae (three days post-fertilization, dpf) were infected with SVCV (~2 × 108 TCID50/ml) after pretreatment with either vehicle (DMSO) or MLN4924 (1 μM) for 24 h. After 24 h incubation, we extracted total RNA from all larvae and used qPCR assays to detect the expression levels of inf1, mxc, pkz, and lta. Data are shown as mean ± SEM of three independent experiments, each performed in triplicate; the statistical analysis was performed using GraphPad Prism 5 (unpaired t-test).
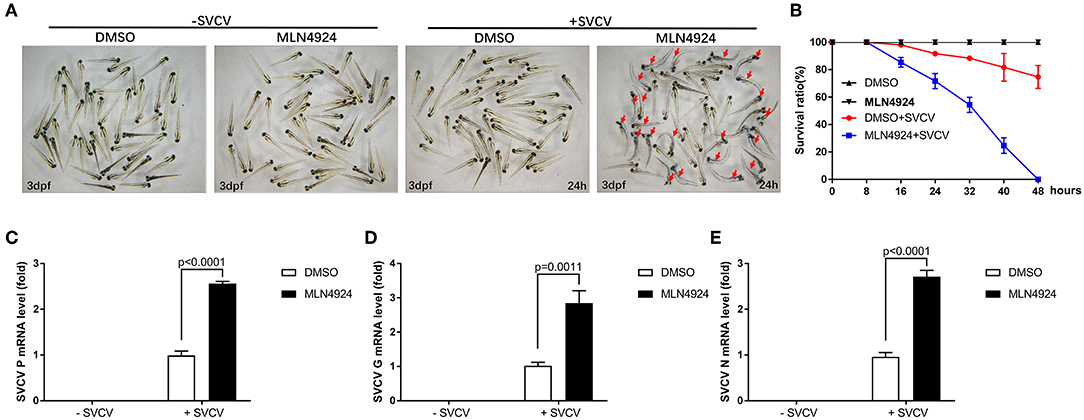
Figure 5. Zebrafish larvae treated with MLN4924 are more sensitive to SVCV infection. (A) Representative images of zebrafish larvae (3 dpf), both uninfected and infected with SVCV for 24 h, after treatment with the vehicle (DMSO; the control) or MLN4924 (1 μM). Dead larvae (indicated with red arrows) were characterized by a lack of movement, absence of blood circulation, and bodily degeneration. (B) Survival ratios indicated that zebrafish larvae treated with MLN4924 were more sensitive to SVCV infection than were larvae treated with vehicle (DMSO). Zebrafish larvae (3 dpf; n = 90 in total) were infected with SVCV (2 × 108 TCID50/ml) after pretreatment with either vehicle (DMSO) or MLN4924 (1 μM); this experiment was repeated three times (n = 30 for each). We counted the numbers of dead larvae at 8, 16, 24, 32, 40, and 48 h post-infection. (C–E) Viral replication was much greater in SVCV-infected zebrafish larvae treated with MLN4924 (1 μM), as compared with the control. Zebrafish larvae were infected with SVCV after pretreatment with either vehicle (DMSO; the control) or MLN4924 (1 μM). After incubation for 24 h, we used qRT-PCR assays to determine the expression levels of the SVCV genes P (C), G (D), and N genes (E). Data are shown as mean ± SEM of three independent experiments, each performed in triplicate; the statistical analysis was performed using GraphPad Prism 5 (unpaired t-test).
Nedd8 is a vital component of neddylation pathway (1, 29). Therefore, it is important to characterize the physiological functions of nedd8, in order to clarify the function of neddylation in various biological processes. To determine if neddylation was indeed involved in the antiviral response, we directly examined the importance of nedd8 after viral infection. After SVCV infection, the ectopic expression of nedd8 in zebrafish embryos via micro-injection of nedd8 mRNA upregulated the key antiviral genes, ifn1, mxc, pkz, and lta, as compared to the embryos injected with the control (GPF) mRNA (Figures 6A–D). As expected, SVCV replication, as reflected by the expression levels of the SVCV P, G, and N genes, was suppressed in embryos injected with nedd8 mRNA, as compared to embryos injected with GFP mRNA (Figures 6E–G). The expression levels of injected Myc-nedd8 mRNA or GFP mRNA was confirmed by Western blot assay or fluorescent imaging (Figures 6H,I).
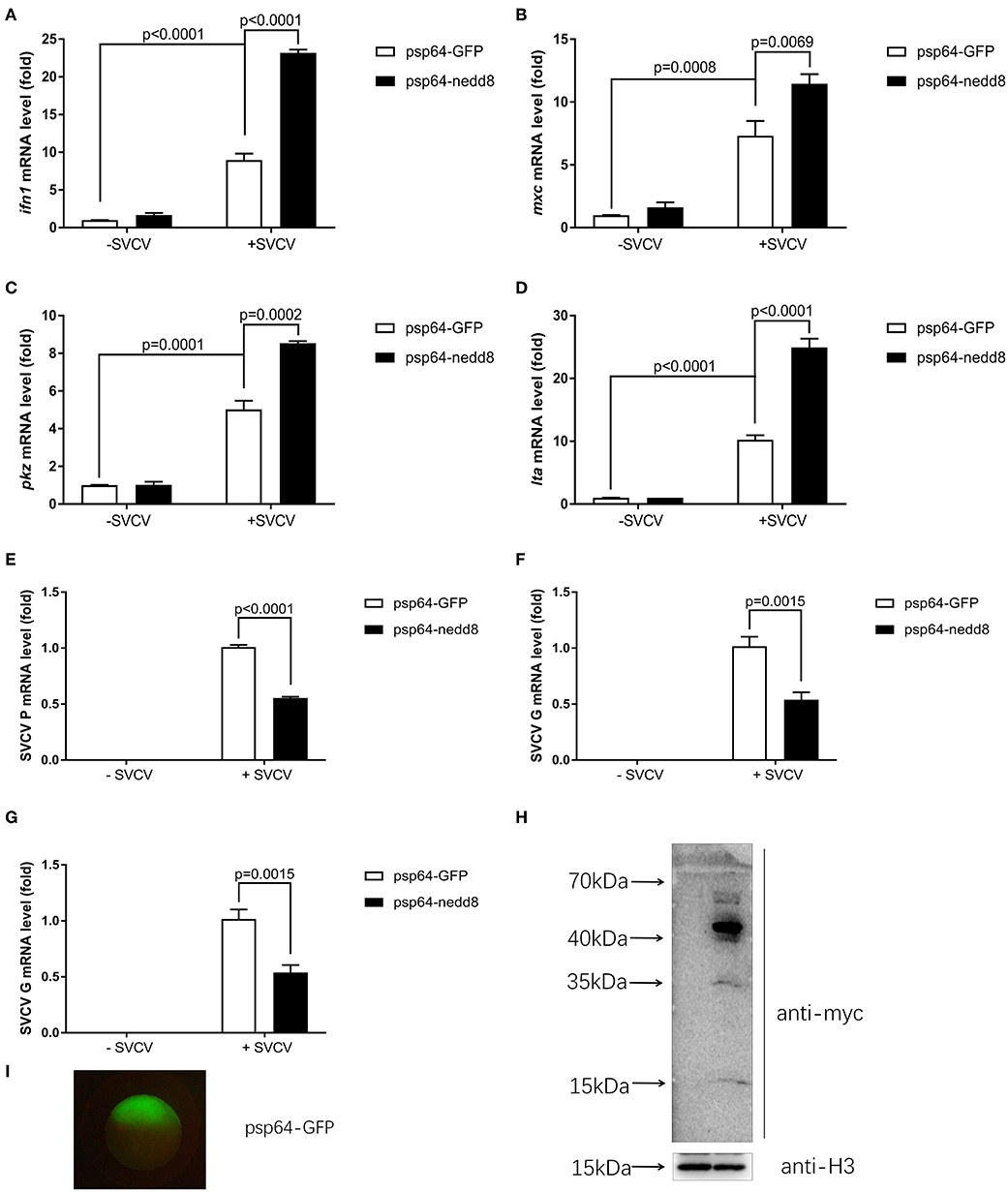
Figure 6. Overexpression of nedd8 upregulates key antiviral genes after SVCV infection and suppresses viral replication in vivo. (A–D) Ectopic expression of nedd8, induced by mRNA injection upregulated key antiviral genes in SVCV infected zebrafish larvae. We injected zebrafish embryos at the one-cell stage with either GFP mRNA (400 pg/per embryo) or Myc-tagged nedd8 mRNA (400 pg/per embryo). At 3 dpf, we added SVCV viruses (2 × 108 TCID50/ml) into the water containing zebrafish larvae. After incubation for 24 h, we extracted total RNA from all larvae and performed qPCR assays to detect the expression levels of ifn1 (A), mxc (B), and pkz (C) and lta (D). (E–G) Ectopic expression of nedd8 by mRNA injection suppressed SVCV replication in embryos. We performed qRT-PCR assays to detect the expression levels of the SVCV genes P (E), G (F), and N (G) genes of SVCV. (H,I) Western blot assay and Fluorescence micrographs of zebrafish embryos showed the expression levels of injected GFP mRNA or Myc-nedd8 mRNA. Data are shown as mean ± SEM of three independent experiments, each performed in triplicate; the statistical analysis was performed using GraphPad Prism 5 (unpaired t-test).
We knocked out nedd8 in zebrafish via CRISPR/Cas9 (Supplemental Figure S2). Subsequently, we used nedd8-knockout zebrafish larvae to determine the role played by nedd8 in the antiviral response. Due to the low fecundity of nedd8-null females, it was difficult to obtain pure nedd8 −/− larvae by directly mating nedd8 −/− (♀) with nedd8 −/− (♂). Therefore, we mated nedd8 +/− (♀) with nedd8 −/− (♂) to obtain nedd8+/−nedd8−/− larvae; we assumed nedd8 was at least partially silenced in these larvae. Compared to the WT larvae (nedd8+/+), SVCV replication was increased in nedd8+/−nedd8−/− larvae after SVCV infection (as reflected by the expression levels of the SVCV P, G, and N genes) (Figures 7A–C). Several key antiviral genes (i.e., ifn1, mxc, pkz, and lta) were downregulated in nedd8+/−nedd8−/− larvae, as compared to the WT larvae, after SVCV infection (Figures 7D–G).
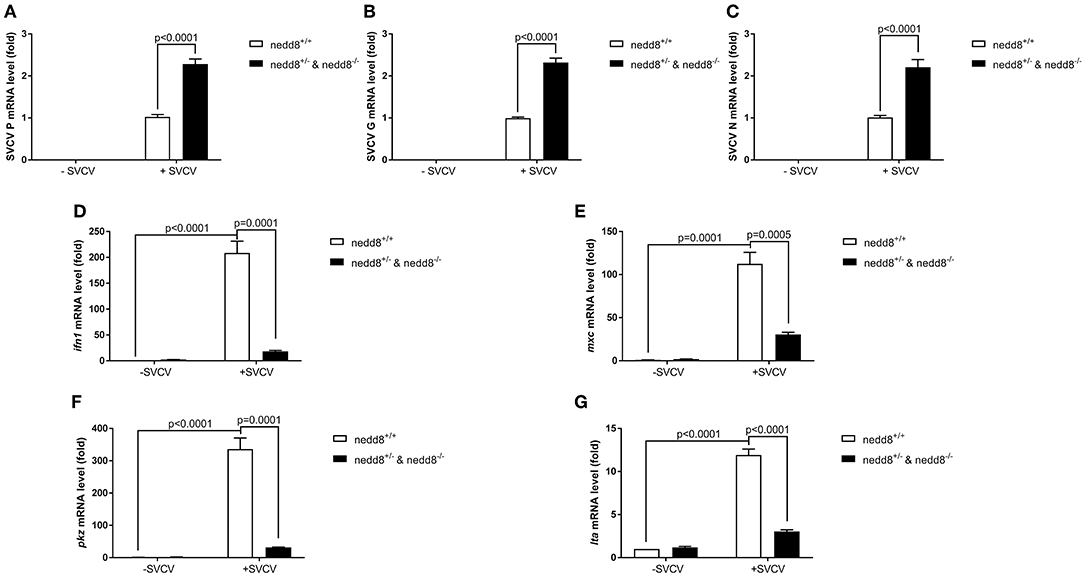
Figure 7. Disruption of nedd8 in zebrafish after SVCV infection increases viral replication and downregulates key antiviral genes. (A–C) Viral replication increased in nedd8-disrupted zebrafish larvae (nedd8+/−&nedd8−/−) larvae after SVCV infection, as compared to WT larvae. (D–G) The expression levels of key antiviral genes after SVCV infection were lower in nedd8-disrupted zebrafish larvae than in WT larvae. At 3 dpf, we added viruses (2 × 108 TCID50/ml) into the water containing zebrafish larvae. After incubation for 24 h, we extracted total RNA from all larvae and performed qPCR assays to detect the expression levels of ifn1 (D), mxc (E), and pkz (F), and ita (G). Data are shown as mean ± SEM of three independent experiments, each performed in triplicate; the statistical analysis was performed using GraphPad Prism 5 (unpaired t-test).
To determine whether nedd8 silencing also suppressed the antiviral response in adult zebrafish, we injected SVCV into nedd8 +/+ or nedd8 −/− adult zebrafish (3 mpf; months post fertilization). Nedd8-null adult zebrafish were more sensitive to SVCV infection than WT adult zebrafish, as indicated by swelling or hemorrhage in the abdomen; and early death (Figures 8A–F). In addition, the key antiviral genes, ifn1, mxc, pkz, and lta, were downregulated in the kidneys and spleens of nedd8-null zebrafish, as compared to WT zebrafish (Figures 9A–H).

Figure 8. nedd8-null adult zebrafish are more sensitive to SVCV infection than WT zebrafish. (A,B) nedd8-null zebrafish (3 mpf; 0.38 ± 0.02 g) and the WT (3 mpf; 0.38 ± 0.02 g) were each i.p. injected with 10 μL SVCV (~2 × 108 TCID50/ml) at 0 day. (C,D) At 1 day post-injection (dpi), there were no obvious differences between the WT (nedd8+/+) and nedd8-null zebrafish (nedd8−/−). (E,F) At 2 dpi, the WT zebrafish appeared normal, but the nedd8-null zebrafish had more swelling and hemorrhagic symptoms in the abdomen (indicated by red arrows).
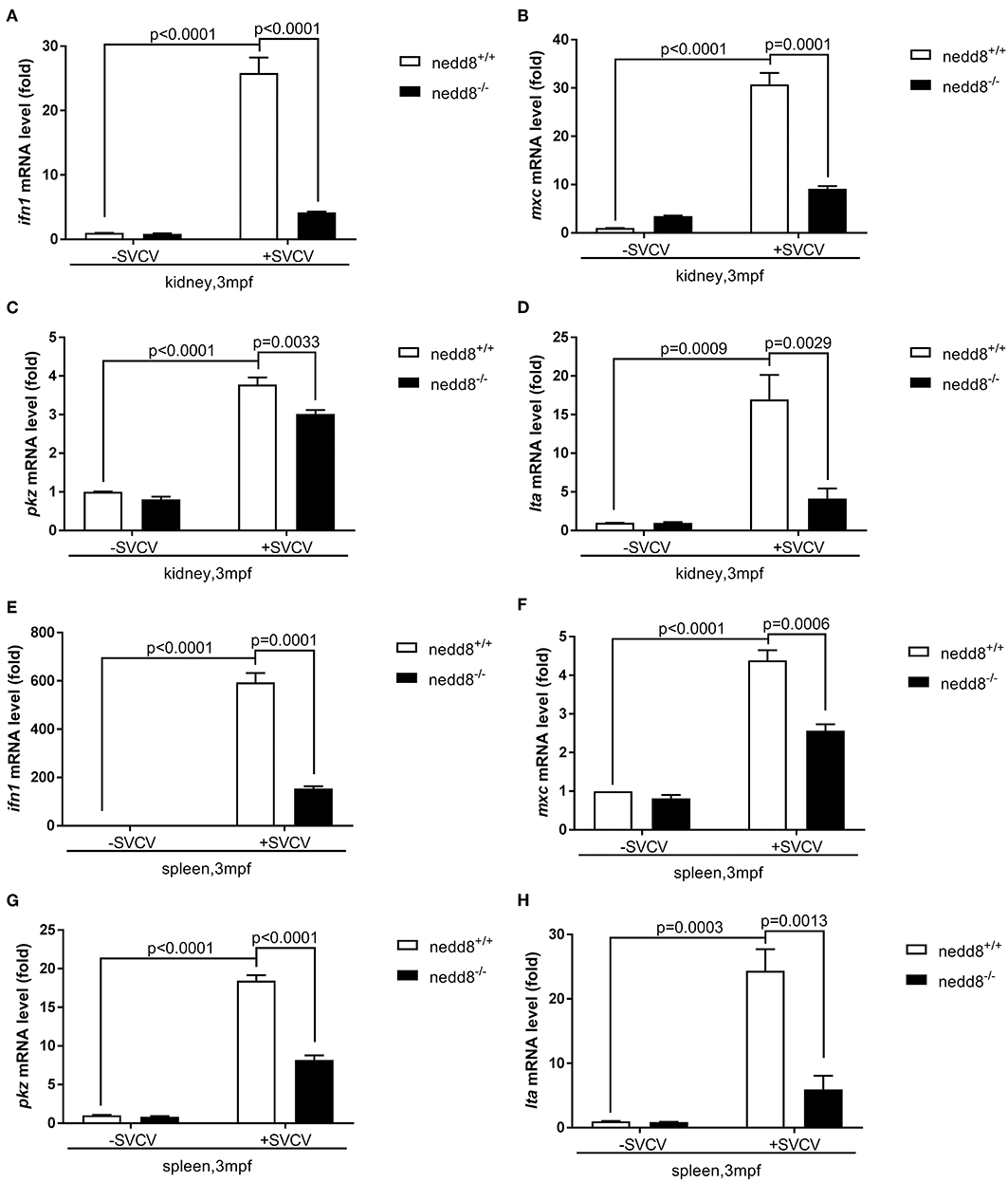
Figure 9. The antiviral response of nedd8-null adult zebrafish after SVCV infection is weaker than that of the WT zebrafish. (A–D) The key antiviral genes, ifn1 (A), mxc (B), pkz (C), and lta (D), downregulated in the kidneys of nedd8-null adult zebrafish (3 mpf; 0.38 ± 0.02 g), as compared to WT zebrafish (3 mpf; 0.38 ± 0.02 g). (E–H) The key antiviral genes, ifn1 (E), mxc (F), pkz (G), and lta (H), were downregulated in the spleens of nedd8−/− adult zebrafish (3 mpf; 0.38 ± 0.02 g), as compared to WT zebrafish (3 mpf; 0.38 ± 0.02 g). WT (nedd8+/+) and nedd8 −/− zebrafish were each i.p. injected with 10 μL SVCV (2 × 108 TCID50/ml). At 2 days post-injection (dpi), we extracted total RNA from the kidneys and spleens of all zebrafish and performed qPCR assays to determine the expression levels of ifn1, mxc, pkz, and lta. Data are shown as mean ± SEM of three independent experiments, each performed in triplicate; the statistical analysis was performed using GraphPad Prism 5 (unpaired t-test).
Thus, our results suggested that nedd8 was essential for the antiviral response in zebrafish.
To figure out the mechanisms of neddylation in zebrafish antiviral innate immunity, we conducted neddylation assays for the key factors of RLR signaling in response to viral infection (30). Neddylation of both Irf3 and Irf7 was readily detected (Figures 10A,B), but neddylation could not be detected in zebrafish Mda5, Mavs, and Tbk1 (Supplemental Figure S3). These data suggested that neddylation might facilitate antiviral response through modifying Irf3 and Irf7.
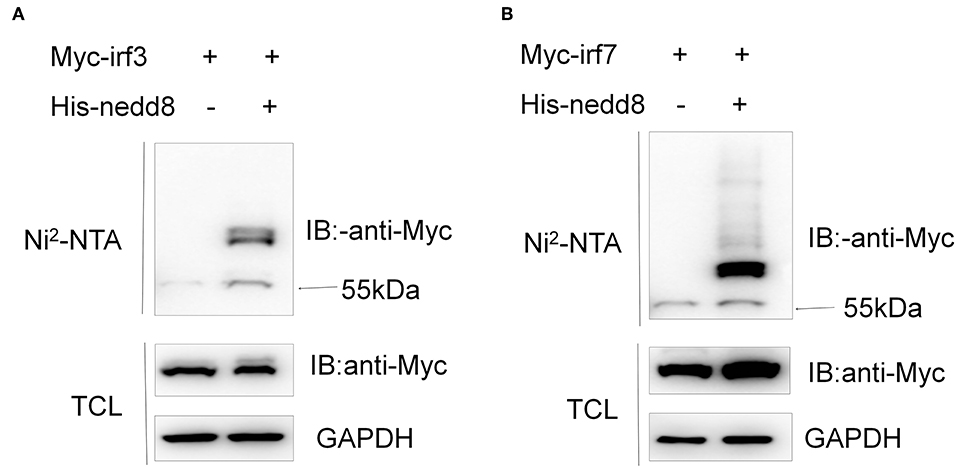
Figure 10. Neddylation of zebrafish Irf3 and Irf7 exists. HEK293T cells were transfected with Myc-irf3 (5 μg) (A) or Myc-irf7 (5 μg) (B) together with His-nedd8 (5 μg). After 36 h, cells were lysed in guanidinium chloride, and His-nedd8 was purified with Ni2-NTA agarose. TCL, total cell lysates; IP, immunoprecipitation.
The role of neddylation in the host immune response to pathogenic infection has received substantial research attention (12–14, 20). However, it remains unclear whether this process negatively or positively affects the host anti-pathogen response (15, 17, 20). In this study, using both cell cultures and a zebrafish model, we showed that neddylation benefits the host during viral infection. Given that the innate immune response to viral infection is similar in zebrafish and mammals, the antiviral neddylation process might be evolutionarily conserved (22).
The innate immune system acts as the first line of defense, protecting the host from viral infection (30). Host PRRs recognize viral nucleic acids and trigger innate immune signaling cascades (30–34). These signaling cascades activate the transcription factors IRF3/IRF7 and NF-kB, inducing the antiviral response and producing IFN-1, pro-inflammatory cytokines, and other important antiviral proteins (30, 35, 36). Here, we focused on the critical genes downstream of the innate immune signaling response to viral infection in zebrafish. The inhibition of neddylation via the addition of MLN4924 or via the disruption of nedd8 significantly downregulated these critical genes, suggesting that protein neddylation might be vital for the innate immune signaling pathway. Indeed, multiple lines of evidence indicate that post-translation modifications control innate immunity by targeting different components of the innate immune signaling pathway in various ways, including phosphorylation, ubiquitination, methylation, SUMOlation, and acetylation (37). Therefore, as an important post-translational modification, neddylation may also target components of the innate immune signaling pathway, modulating target function either directly or indirectly. Here, we identified that zebrafish Irf3 and Irf7 are possible neddylation targets. Therefore, neddylation might facilitate antiviral response through modifying Irf3 and Irf7. To further confirm neddylation Irf3 and Irf7 in vivo will re-enforce the importance of neddylation in antiviral innate immunity.
Based on our results, we cannot exclude the possibility that the beneficial antiviral effects conferred by neddylation were not due to modifications of innate immune signaling pathway components but instead due to modification of other molecules, such as the cullin-RING ligases.
Of note, MLN4924 can activate p53 through ribosomal-Mdm2 pathway (38). Here, we found that nedd8-deficient adult zebrafish were viable except the low fecundity displayed in nedd8-null females. It appears that activation of p53 resulted from deletion of nedd8 cannot affect general development other than female gonadogenesis in zebrafish. To further determine whether the defects exhibited in nedd8-null females are caused by p53 activation will expand our knowledge about the regulation of p53 by neddylation in vivo.
Increasing evidence indicates that neddylation is associated with the multiple cancer initiation and progression (11, 28, 39–41). As a potent and specific NAE inhibitor, MLN4924 has been widely used in clinical trials for cancer therapies (11, 42, 43). Here, we showed that neddylation benefits host during the antiviral response. Thus, before MLN4924; or other neddylation inhibitors are used in cancer treatments, the antiviral capabilities of patient should be carefully considered.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
GY and XL performed the experiments. GY, XL, and WX conceived and designed the experiments, analyzed the results, and oversaw the project. JT, CX, and GO contributed the reagents. WX wrote the main text of the manuscript. All authors reviewed and contributed to the preliminary and final draft of the manuscript.
This work was supported by National Natural Science Foundation of China Grant 31721005, 31830101, 31631102, 31671315, 31772872; and National Key R&D Program of China 2018YFD0900602.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Dawei Zhang for technique support and Jing Wang for valuable discussion.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.01432/full#supplementary-material
1. Rabut G, Peter M. Function and regulation of protein neddylation - ‘protein modifications: beyond the usual suspects’ review series. EMBO Rep. (2008) 9:969–76. doi: 10.1038/embor.2008.183
2. Wu K, Yamoah K, Dolios G, Gan-Erdene T, Tan PL, Chen A, et al. DEN1 is a dual function protease capable of processing the C terminus of Nedd8 and deconjugating hyper-neddylated CUL1. J Biol Chem. (2003) 278:28882–91. doi: 10.1074/jbc.M302888200
3. Gan-Erdene T, Nagamalleswari K, Yin LM, Wu K, Pan ZQ, Wilkinson KD. Identification and characterization of DEN1, a deneddylase of the ULP family. J Biol Chem. (2003) 278:28892–900. doi: 10.1074/jbc.M302890200
4. Enchev RI, Schulman BA, Peter M. Protein neddylation: beyond cullin-RING ligases. Nat Rev Mol Cell Biol. (2015) 16:30–44. doi: 10.1038/nrm3919
5. Zuo W, Huang F, Chiang YJ, Li M, Du J, Ding Y, et al. c-Cbl-mediated neddylation antagonizes ubiquitination and degradation of the TGF-beta Type II receptor. Mol Cell. (2013) 49:499–510. doi: 10.1016/j.molcel.2012.12.002
6. Vogl AM, Brockmann MM, Giusti SA, Maccarrone G, Vercelli CA, Bauder CA, et al. Neddylation inhibition impairs spine development, destabilizes synapses and deteriorates cognition. Nat Neurosci. (2015) 18:239–51. doi: 10.1038/nn.3912
7. Xirodimas DP, Saville MK, Bourdon JC, Hay RT, Lane DP. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell. (2004) 118:83–97. doi: 10.1016/j.cell.2004.06.016
8. Watson IR, Blanch AD, Lin CC, Ohh M, Irwin MS. Mdm2-mediated NEDD8 modification of TAp73 regulates its transactivation function. J Biol Chem. (2006) 281:34096–103. doi: 10.1074/jbc.M603654200
9. Watson IR, Irwin MS, Ohh M. NEDD8 pathways in cancer, Sine Quibus Non. Cancer Cell. (2011) 19:168–76. doi: 10.1016/j.ccr.2011.01.002
10. Abidi N, Xirodimas DP. Regulation of cancer-related pathways by protein NEDDylation and strategies for the use of NEDD8 inhibitors in the clinic. Endocr Relat Cancer. (2015) 22:T55–70. doi: 10.1530/ERC-14-0315
11. Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. (2009) 458:732–67. doi: 10.1038/nature07884
12. Cheng QQ, Liu J, Pei YJ, Zhang YL, Zhou DW, Pan WQ, et al. Neddylation contributes to CD4(+) T cell-mediated protective immunity against blood-stage Plasmodium infection. Plos Pathog. (2018) 14:e1007440. doi: 10.1371/journal.ppat.1007440
13. Mathewson ND, Fujiwara H, Wu SR, Toubai T, Oravecz-Wilson K, Sun YP, et al. SAG/Rbx2-dependent neddylation regulates T-cell responses. Am J Pathol. (2016) 186:2679–91. doi: 10.1016/j.ajpath.2016.06.014
14. Mathewson N, Toubai T, Kapeles S, Sun YP, Oravecz-Wilson K, Tamaki H, et al. Neddylation plays an important role in the regulation of murine and human dendritic cell function. Blood. (2013) 122:2062–73. doi: 10.1182/blood-2013-02-486373
15. Chang FM, Reyna SM, Granados JC, Wei SJ, Innis-Whitehouse W, Maffi SK, et al. Inhibition of neddylation represses lipopolysaccharide-induced proinflammatory cytokine production in macrophage cells. J Biol Chem. (2012) 287:35756–67. doi: 10.1074/jbc.M112.397703
16. Jin HS, Liao LJ, Park Y, Liu YC. Neddylation pathway regulates T-cell function by targeting an adaptor protein Shc and a protein kinase Erk signaling. Proc Natl Acad Sci USA. (2013) 110:624–9. doi: 10.1073/pnas.1213819110
17. Zhang XY, Ye ZJ, Pei YJ, Qiu GH, Wang QY, Xu YL, et al. Neddylation is required for herpes simplex virus type I (HSV-1)-induced early phase interferon-beta production. Cell Mol Immunol. (2016) 13:577–83. doi: 10.1038/cmi.2015.35
18. Yan FX, Guan JH, Peng YY, Zheng XF. MyD88 NEDDylation negatively regulates MyD88-dependent NF-kappa B signaling through antagonizing its ubiquitination. Biochem Biophys Res Commun. (2017) 482:632–7. doi: 10.1016/j.bbrc.2016.11.084
19. Gao F, Cheng JK, Shi TE, Yeh TH. Neddylation of a breast cancer-associated protein recruits a class III histone deacetylase that represses NF kappa B-dependent transcription. Nat Cell Biol. (2006) 8:1171–236. doi: 10.1038/ncb1483
20. Sun HW, Yao W, Wang K, Qian YJ, Chen HJ, Jung YS. Inhibition of neddylation pathway represses influenza virus replication and pro-inflammatory responses. Virology. (2018) 514:230–9. doi: 10.1016/j.virol.2017.11.004
21. Song H, Huai W, Yu Z, Wang W, Zhao J, Zhang L, et al. MLN4924, a first-in-class NEDD8-activating enzyme inhibitor, attenuates IFN-beta production. J Immunol. (2016) 196:3117–23. doi: 10.4049/jimmunol.1501752
22. Liu X, Cai X, Zhang D, Xu C, Xiao W. Zebrafish foxo3b negatively regulates antiviral response through suppressing the transactivity of irf3 and irf7. J Immunol. (2016) 197:4736–49. doi: 10.4049/jimmunol.1601187
23. Du J, Zhang DW, Zhang W, Ouyang G, Wang J, Liu X, et al. pVHL negatively regulates antiviral signaling by targeting MAVS for proteasomal degradation. J Immunol. (2015) 195:1782–90. doi: 10.4049/jimmunol.1500588
24. Sepahi A, Kraus A, Casadei E, Johnston CA, Galindo-Villegas J, Kelly C, et al. Olfactory sensory neurons mediate ultrarapid antiviral immune responses in a TrKA-dependent manner. Proc Natl Acad Sci USA. (2019) 116:12428–36. doi: 10.1073/pnas.1900083116
25. Galindo-Villegas J, Garcia-Moreno D, de Oliveira S, Meseguer J, Mulero V. Regulation of immunity and disease resistance by commensal microbes and chromatin modifications during zebrafish development. Proc Natl Acad Sci USA. (2012) 109:E2605–14. doi: 10.1073/pnas.1209920109
26. Ryu JH, Li SH, Park HS, Park JW, Lee B, Chun YS. Hypoxia-inducible factor alpha subunit stabilization by NEDD8 conjugation is reactive oxygen species-dependent. J Biol Chem. (2011) 286:6963–70. doi: 10.1074/jbc.M110.188706
27. Brownell JE, Sintchak MD, Gavin JM, Liao H, Bruzzese FJ, Bump NJ, et al. Substrate-assisted inhibition of ubiquitin-likeprotein-activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Mol Cell. (2010) 37:102–11. doi: 10.1016/j.molcel.2009.12.024
28. Zhou LS, Zhang WJ, Sun Y, Jia LJ. Protein neddylation and its alterations in human cancers for targeted therapy. Cell Signal. (2018) 44:92–102. doi: 10.1016/j.cellsig.2018.01.009
29. Merlet J, Burger J, Gomes JE, Pintard L. Regulation of cullin-RING E3 ubiquitin-ligases by neddylation and dimerization. Cell Mol Life Sci. (2009) 66:1924–38. doi: 10.1007/s00018-009-8712-7
30. Tan X, Sun L, Chen J, Chen ZJ. Detection of microbial infections through innate immune sensing of nucleic acids. Ann Rev Microbiol. (2018) 72:447–78. doi: 10.1146/annurev-micro-102215-095605
31. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. (2010) 140:805–20. doi: 10.1016/j.cell.2010.01.022
32. Cao XT. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol. (2016) 16:35–50. doi: 10.1038/nri.2015.8
33. Goubau D, Deddouche S, Reis e Sousa C. Cytosolic sensing of viruses. Immunity. (2013) 38:855–69. doi: 10.1016/j.immuni.2013.05.007
34. Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. (2013) 38:870–80. doi: 10.1016/j.immuni.2013.05.004
35. Loo YM, Gale M. Immune signaling by RIG-I-like receptors. Immunity. (2011) 34:680–92. doi: 10.1016/j.immuni.2011.05.003
36. Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell. (2014) 54:289–96. doi: 10.1016/j.molcel.2014.03.040
37. Liu J, Qian C, Cao XT. Post-translational modification control of innate immunity. Immunity. (2016) 45:15–30. doi: 10.1016/j.immuni.2016.06.020
38. Bailly A, Perrin A, Bou Malhab LJ, Pion E, Larance M, Nagala M, et al. The NEDD8 inhibitor MLN4924 increases the size of the nucleolus and activates p53 through the ribosomal-Mdm2 pathway. Oncogene. (2016) 35:415–26. doi: 10.1038/onc.2015.104
39. Delgado TC, Barbier-Torres L, Zubiete-Franco I, Lopitz-Otsoa F, Varela-Rey M, Fernandez-Ramos D, et al. Neddylation, a novel paradigm in liver cancer. Transl Gastroenterol Hepatol. (2018) 3:37. doi: 10.21037/tgh.2018.06.05
40. Vanderdys V, Allak A, Guessous F, Benamar M, Read PW, Jameson MJ, et al. The neddylation inhibitor pevonedistat (MLN4924) suppresses and radiosensitizes head and neck squamous carcinoma cells and tumors. Mol Cancer Ther. (2018) 17:368–80. doi: 10.1158/1535-7163.MCT-17-0083
41. Tong S, Si Y, Yu H, Zhang L, Xie P, Jiang W. MLN4924 (Pevonedistat), a protein neddylation inhibitor, suppresses proliferation and migration of human clear cell renal cell carcinoma. Sci Rep. (2017) 7:5599. doi: 10.1038/s41598-017-06098-y
42. G.Salavaggione NO, Duggan MC, Carson WE. Analysis of MLN4924 (pevonedistat) as a potential therapeutic agent in malignant melanoma. Melanoma Res. (2018) 28:390–7. doi: 10.1097/CMR.0000000000000474
43. Lockhart AC, Bauer TM, Aggarwal C, Lee CB, Harvey RD, Cohen RB, et al. Phase Ib study of pevonedistat, a NEDD8-activating enzyme inhibitor, in combination with docetaxel, carboplatin and paclitaxel, or gemcitabine, in patients with advanced solid tumors. Invest New Drugs. (2018) 37:87–97. doi: 10.1007/s10637-018-0610-0
Keywords: nedd8, zebrafish, MLN4924, antiviral response, innate immunity, neddylation, SVCV
Citation: Yu G, Liu X, Tang J, Xu C, Ouyang G and Xiao W (2019) Neddylation Facilitates the Antiviral Response in Zebrafish. Front. Immunol. 10:1432. doi: 10.3389/fimmu.2019.01432
Received: 14 March 2019; Accepted: 06 June 2019;
Published: 25 June 2019.
Edited by:
Gerardo R. Vasta, University of Maryland, Baltimore, United StatesReviewed by:
Jorge Galindo-Villegas, Nord University, NorwayCopyright © 2019 Yu, Liu, Tang, Xu, Ouyang and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wuhan Xiao, dy14aWFvQGloYi5hYy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.