- 1Department of Neurology, Hannover Medical School, Hanover, Germany
- 2Hannover Medical School, Institute of Neuroradiology, Hanover, Germany
- 3Department of Nuclear Medicine, Hannover Medical School, Hanover, Germany
- 4Clinic for Immunology and Rheumatology, Unit for Infectious Diseases, Hannover Medical School, Hanover, Germany
Background: Progressive multifocal leukoencephalopathy (PML) is an opportunistic infection with JC-virus (JCV), a papova-virus, affecting mostly oligodendrocytes and the white matter of the central nervous system. Progressive Multifocal Leukoencephalopathy (PML) almost exclusively occurs in immunocompromised patients based on different underlying conditions of severe cellular immunodeficiency such as HIV/AIDS, secondary to neoplastic and autoimmune diseases, or during immunosuppressive therapy.
Case presentation: We present the case of an otherwise healthy and immunocompetent patient without immunosuppressive therapy who was admitted with hemianopsia to the right side, sensory aphasia and changes of behavior. Magnet resonance imaging (MRI) and laboratory testing confirmed the diagnosis of PML, although functional tests did not show any evidence for cellular immunodeficiency. Extensive immunological tests did not reveal an apparent immunodeficiency. During symptomatic therapy the patient developed seizures which were assumed to be caused by a spontaneous immune reconstitution inflammatory syndrome (IRIS) demonstrated by MRI. We added a high dose of intravenous corticosteroids to the antiepileptic treatment and seizures ended shortly thereafter. However, the impairments of vision, behavior and language persisted.
Conclusions: Our case report highlights that an apparently immunocompetent patient can develop PML and IRIS spontaneously. Therefore, MRI should be applied immediately whenever a rapid progression of PML symptoms occurs as treatment of IRIS with corticosteroids can result in a marked clinical improvement.
Introduction
Progressive Multifocal Leukoencephalopathy (PML) is an opportunistic infection of white and gray matter cells caused by JCV-reactivation within the brain almost exclusively occurring in immunocompromised patients. Hence, PML has been explained as an opportunistic infection secondary to underlying predominantly cellular forms of immunodeficiencies (e.g., HIV/AIDS, neoplastic and autoimmune diseases or immunosuppressive treatments such as rituximab, natalizumab, and others) (1, 2). JCV latency in healthy individuals is common with 50–90% of the population being positive for JCV-antibodies (2). The incidence of PML is comparably low (4.4 of 100,000), as a severe impairment of specific parts of the immune system (especially CD4+-T-lymphocytes) is necessary for intracerebral virus reactivation (2). The prognosis of PML is often poor. There is no specific therapy with proven efficacy except for a sufficient treatment of immunodeficiency (e.g., antiviral treatment of HIV) (3). But on the other hand also the reconstitution of the deficient cellular immune system can lead to an excessive immune activation against the JCV called IRIS (4, 5). A PML-IRIS treatment with high doses of corticosteroids is recommended (5, 6). Nevertheless, the course of PML frequently remains unfavorable leading to prolonged neurological impairment or death of affected patients.
We present a case of PML in a patient with no detectable immunodeficiency at time of PML diagnosis. As far as neuroimaging by cMRI suggested a subsequent development of an IRIS, a corticosteroid-treatment was initiated resulting in a marked benefit of the PML.
Case Presentation
A 68-year-old male patient was transferred to our neurological department after he had been admitted to another hospital twice before. The first admission had taken place 3 months before in July 2018 due to visual impairments (hemianopsia to the right) and uncommon behavior. The MRI had shown a T2-weighted (T2w)-FLAIR hyperintensity in the left temporooccipital lobe that had been interpreted as an ischemic stroke. The results of cardiovascular investigations had been normal and the patient had been discharged. In August 2018 he had been admitted again with a progression of symptoms consisting of severe sensory aphasia, psychomotoric deficits and progressive visual impairments. The MRI had displayed a massive enlargement of parietooccipital lesions in both hemispheres predominantly affecting the left white matter (Figure 1). The polymerase chain reaction (PCR) for JCV in the cerebrospinal fluid (CSF) had been positive whereas routine analysis of the CSF had shown normal results (cell count and distribution, lactate, protein). PCR for Varizella zoster virus had been negative. Serologic tests for Borrelia burgdorferi, Treponema pallidum, Herpes simplex virus, measles, Varizella zoster virus, HIV and hepatitis B and C had also been negative.
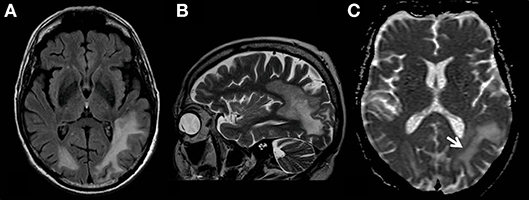
Figure 1. MR examination showing typical findings of PML with white matter FLAIR- (A) and T2 weighted- (B) hyperintensities without mass effect. T2-signal elevations (B) are in part spot-like and resemble the milky-way-appearance typical for PML. The area affected by the JC-virus infection does have a typical rim with reduced ADC values (C, white arrow) indicating the inflammatory border zone with acute demyelination combined with swelling of astrocytes and oligodendrocytes.
The patient was then transferred to our department for further diagnostics. His general physician and relatives revealed that the medical history contained no record of frequent or severe infections. He had never received any kind of immunosuppressive treatment. In 1999 the patient had suffered from a myocardial infarction. The medical history was unremarkable apart from arterial hypertension and a helicobacter-positive gastritis. In 2017 he had got a viral infection with mild influenza-like-symptoms. Neither the patient nor his family knew of any recent vaccinations.
Upon admission to our neurologic department he had developed a right sided homonymous hemianopsia, severe sensory aphasia and disorientation. Our CSF analysis showed a pleocytosis (22 cells/μl) and an elevated protein concentration (0.72 g/l). The cell-distribution was mostly lymphocytic, but monocytes and granulocytes were found as well. CSF specific oligoclonal bands (type 2) were present and there was an intrathecal production of IgG and IgM. The JCV-PCR (1E3 c/ml) from CSF and the JCV-antibody-index (24.000 AU/ml serum) were positive. All additional tests were negative (HSV, VZV, and Ebstein-barr-virus (EBV) in the CSF by PCR, as well as tickborn encephalitis (TBE), enterovirus, and cytomegalovirus serologically; CSF-antibody-indices for HSV, EBV, VZV and CMV). All investigated autoimmune encephalitis antibodies (anti-NMDAR, -AMPAR1/2, -CASPR2, -LGI1, -GABAR-b1/b2, -Yu, -Hu, -Ri, -Amphiphysin, -CV2/CRMP-5, -Ma1/2, -GAD, -SOX1, -Tr(DNER), -Zic4) were negative in serum and CSF.
Further investigations concerning an immunodeficiency were inconspicuous. An HIV-test was negative. The blood tests including peripheral blood cell count and inflammatory parameters showed mild eosinophilia (10,3%), elevated rheumatoid factor (60.5 IU/ml), Cardiolipin-IgM-antibodies (14 MPL-U/ml), and antinuclear antibodies (ANA, 1:320) of unspecific speckled pattern in the immunofluorescence and without specificity against ds-DNA or extractable nuclear antigens. An abdominal sonography showed a splenomegaly and prominent lymph nodes but no sign of neoplasia. We performed a whole-body PET/CT (including the brain) with 296 MBq 18F-Fluorodeoxyglucose (18F-FDG) 60 min p.i. to search for a malignancy or an inflammatory disease. The PET scan showed prominent mediastinal lymph nodes with moderately high metabolism, however, it did not reveal any profound hypermetabolic lesions. 18F-FDG uptake in the brain was further evaluated using statistical parametric mapping (SPM) for comparison with a reference group. The cerebral glucose metabolism was lowered bilaterally in parietotemporal and occipital areas with a clear predominance on the left side. The metabolism in the frontal cortex, basal ganglia, and cerebellar regions appeared to be normal (Figure 2).
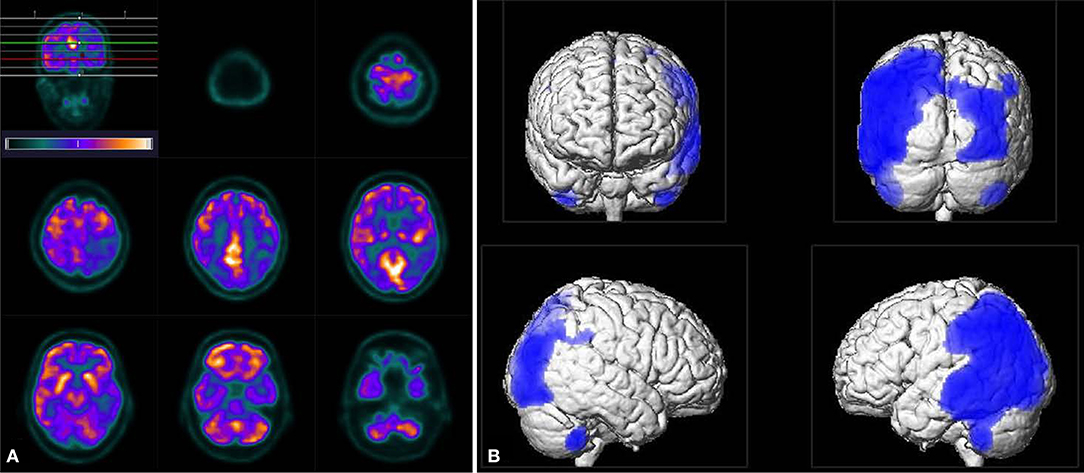
Figure 2. Tomograms of 18F-FDG uptake (A) as well as the result of SPM analysis (B, using an uncorrected p < 0.001 for statistical inferences) revealed distinct bilateral hypo-metabolism pronounced on the left side in parietotemporal and occipital areas. Note, unlike in tomograms the left side appears left in the SPM images.
A biopsy of the prominent mediastinal lymph nodes identified in the PET investigation showed no signs of malignancy or granuloma. Additionally ACE and the soluble IL-2-receptor in the blood were normal, so sarcoidosis seemed unlikely. The HLA-DR-expression and phenotyping of lymphocytes showed normal results [amount and distribution of CD3+, CD4+, CD8+, B-cells (CD20+), and natural killer cells (CD16+)]. In addition to extensive phenotyping of peripheral blood mononuclear cells (PBMC) even the functional analyses by proliferation assays of PBMC by plant lectins (PHA, Con A, PWM), tuberculin (PPD) and anti-CD3, spontaneous and antibody-dependent NK-cell activity (ADCC) were within normal range.
During inpatient treatment the patient developed a cough and had impaired breathing. The tracheal exudate was positive for HSV using PCR so we started a therapy with intravenous aciclovir which improved the respiratory symptoms markedly. In September 2018 a progression of the patient's symptoms with focal and generalized seizures occurred. We started a medication with levetiracetam and had to add valproate and lacosamide to the antiepileptic treatment due to recurring seizures. The MRI showed a further expansion of the lesions with a new peripheral contrast enhancement (Figure 3) as a sign of a PML-related immune reconstitution inflammatory syndrome (PML-IRIS). We treated the patient with a high dose of corticosteroids (1 g/d methylprednisolone for 5 days intravenously). After this therapy the seizures decreased and the anticonvulsive treatment could be reduced to levetiracetam and valproate. Finally, we applied a treatment with mirtazapine to our patient, which is able to block the specific serotonin-receptors used by JCV, since this was beneficial in a previous report of a PML in an immunocompetent patient (7). Mefloquine, another drug with potentially antiviral effects against JCV, was not included to the treatment as seizures are a strict contraindication (7).
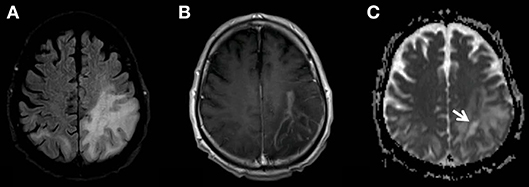
Figure 3. MR examination showing the progress of the PML-related FLAIR-hyperintensities (A). The new multifocal and strong contrast enhancement (B) indicates the PML-IRIS. The zone of active inflammation and demyelination is again indicated also by the low ADC-rim surrounding the area affected by the JC-virus (C, white arrow).
After nearly 2 months the patient was transferred to a rehabilitation facility. Visual impairments, disorientation and a sensory aphasia were still persistent. During the following weeks the patient developed severe dysphagia until oral intake of food and fluids was no longer possible. The patient died 2 months after the transfer to a nursery home due to a respiratory infection and pulmonary insufficiency.
Discussion and Conclusions
The presented case shows evidence for the rare development of severe PML and spontaneous IRIS in a patient with no apparent immunodeficiency. A neoplastic disease could not be detected by abdominal ultrasound, thoracic X-ray and a whole-body 18F-FDG-PET/CT scan. HIV-results were repeatedly negative. A chronic autoimmune disease was also not detected and a biopsy of mediastinal lymph nodes showed no sign of sarcoidosis. Blood tests revealed a normal distribution and function of PBMC, especially the amount of CD4+-cells was normal. However, we cannot exclude a stage of transient immunodeficiency before PML developed, e.g., secondary to an intercurrent viral infection which had presented “flu-like” and clinically self-limiting in 2017. However, an episode of symptomatic tracheobronchitis with HSV-reactivation shown in the BAL in 09/2018 might indicate persisting immunodefiency, although we did not find further evidence for a capable immunological impairment at the same time. Hence, a hypothetical explanation of the course of PML in the presented case may be a (a) transient cellular immunodeficiency potentially due to the suspected viral infection in 2017 as the predisposition for development of PML and JCV-reactivation; (b) clinical development of PML as a demasking IRIS since summer 2018 during spontaneous immunoreconstitution; (c) further progression of PML-IRIS after antiviral treatment of HSV-tracheobronchitis in 09/2018 and (d) clinical improvement by corticosteroid-pulse subsequently. The HSV reactivation in the respiratory tract would be in accordance to this hypothetical course because it is known to be caused by immunodeficiency and as well by IRIS (8, 9).
Few comparable cases with PML in apparently immunocompetent patients have been published (10–15) but a spontaneous PML-IRIS has not been demonstrated in those cases. In one case the development of a PML-IRIS was suggested but no evidence (MRI) of IRIS was demonstrated (16). Our case shows MRI-evidence of a new onset PML-IRIS that occurred spontaneously and caused rapidly progressive neurological symptoms. Treatment with high doses of corticosteroids led to marked clinical improvement. Nevertheless, severe neurological impairments persisted due to the PML. Hypometabolism in 18F-FDG PET/CT might indicate mild infiltration by inflammatory cells (17) or even a residual state of neuronal damage.
It has been demonstrated that JCV-genome can be found in brain tissue of patients suffering from other neurological diseases than PML and even in neurologically healthy individuals (18). However, the combined results of our neurological examination, MRI and CSF (19) analyses strongly indicate the diagnosis of PML. This case shows the relevance of close clinical monitoring of apparently immunocompetent patients with PML as a progression of symptoms may be due to the development of a spontaneous, steroid-responsive PML-IRIS.
Consent for Publication
All authors gave their consent for publication.
Data Availability
Please contact corresponding author for data requests. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
Written informed consent was obtained from the patient and his legal guardian for the participation as well as the publication of the case report and any accompanying images. A copy of the written consent is available upon request from the corresponding author.
Author Contributions
LK wrote the manuscript and was involved in the diagnostic and therapeutic clinical progress. PR contributed MR-images and legends (Figures 1, 3). RS analyzed PET-data and contributed images for Figure 2. GB contributed to the images for Figure 2 and critically revised the manuscript. MSto analyzed the patient data regarding the immunological aspects and helped in the diagnostic process as well as critically revising the manuscript. MS contributed to the diagnostic and therapeutic progress. FW wrote the manuscript and was responsible for diagnostics and treatment of the patient. All authors read and approved the final manuscript. MS is supported by the Deutsche Forschungsgemeinschaft through the Cluster of Excellence RESIST (EXC 2155).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AIDS, acquired immunodeficiency syndrome; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IRIS, immune reconstitution inflammatory syndrome; JCV, John-Cunningham-Virus; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; PML, progressive multifocal leukoencephalopathy.
References
1. Carson KR, Focosi D, Major EO, Petrini M, Richey EA, West DP, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. (2009) 10:816–24. doi: 10.1016/S1470-2045(09)70161-5
2. Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. (2010) 9:425–37. doi: 10.1016/S1474-4422(10)70040-5
3. Cinque P, Pierotti C, Vigano MG, Bestetti A, Fausti C, Bertelli D, et al. The good and evil of HAART in HIV-related progressive multifocal leukoencephalopathy. J Neurovirol. (2001) 7:358–63. doi: 10.1080/13550280152537247
4. Bowen L, Nath A, Smith B. CNS immune reconstitution inflammatory syndrome. Handb Clin Neurol. (2018) 152:167–76. doi: 10.1016/B978-0-444-63849-6.00013-X
5. Clifford DB. Neurological immune reconstitution inflammatory response: riding the tide of immune recovery. Curr Opin Neurol. (2015) 28:295–301. doi: 10.1097/WCO.0000000000000200
6. Scarpazza C, Prosperini L, De Rossi N, Moiola L, Sormani MP, Gerevini S, et al. To do or not to do? Plasma exchange and timing of steroid administration in progressive multifocal leukoencephalopathy. Ann Neurol. (2017) 82:697–705. doi: 10.1002/ana.25070
7. Christakis PG, Okin D, Huttner AJ, Baehring JM. Progressive multifocal leukoencephalopathy in an immunocompetent patient. J Neurol Sci. (2013) 326:107–10. doi: 10.1016/j.jns.2013.01.010
8. Mutnal MB, Schachtele SJ, Hu S, Lokensgard JR. T-cell reconstitution during murine acquired immunodeficiency syndrome (MAIDS) produces neuroinflammation and mortality in animals harboring opportunistic viral brain infection. J Neuroinflammation. (2013) 10:98. doi: 10.1186/1742-2094-10-98
9. Fife KH, Mugwanya K, Thomas KK, Baeten JM, Celum C, Bukusi E, et al. Transient Increase in Herpes Simplex Virus Type 2 (HSV-2)-Associated Genital Ulcers Following Initiation of Antiretroviral Therapy in HIV/HSV-2-Coinfected Individuals. J Infect Dis. (2016) 213:1573–8. doi: 10.1093/infdis/jiv765
10. Nanda T. Progressive multifocal leukoencephalopathy in a HIV negative, immunocompetent patient. A case report. Neurol Med. (2016) 2016:7050613. doi: 10.1155/2016/7050613
11. Garcia-Carretero R, San Jose Montano B. Cerebellar involvement in an immunocompetent patient presenting with progressive multifocal leukoencephalopathy. A case report. Neurol Med. (2017) 2396068. doi: 10.1155/2017/2396068
12. Van der Kolk NM, Arts P, Van Uden IWM, Hoischen A, Van de Veerdon FL, Netea MG, et al. Progressive multifocal leukoencephalopathy in an immunocompetent patient. Ann Clin Transl Neurol. (2016) 3:226–232. doi: 10.1002/acn3.279
13. Zucker BE, Stacpoole SRL. Progressive multifocal leukoencephalopathy in the absence of immunosuppression. J Neurovirol. (2018) 1:119–22. doi: 10.1007/s13365-017-0592-2
14. Grewal J, Dalal P, Bowman M, Kaya B, Otero JJ, Imitola J. Progressive multifocal leukoencephalopathy in a patient without apparent immunosuppression. J Neurovirol. (2016) 5:683–7. doi: 10.1007/s13365-016-0459-y
15. Chang YY, Lan MY, Peng CH, Wu HS, Chang D, Liu JS. Progressive multifocal leukoencephalopathy in an immunocompetent Taiwanese patient. J Formos Med Assoc. (2007) 106:S60–4. doi: 10.1016/S0929-6646(09)60355-7
16. Naess H, Glad S, Storstein A, Rinaldo CH, Mørk SJ, Myhr KM, et al. Progressive multifocal leucoencephalopathy in an immunocompetent patient with favourable outcome. A case report. BMC Neurol. (2010) 10:32. doi: 10.1186/1471-2377-10-32
17. Ishibashi K, Miura Y, Matsumura K, Kanemasa Y, Nakamichi K, Saijo M, et al. PET Imaging of 18F-FDG, 11C-methionine, 11C-flumazenil, and 11C-4DST in Progressive Multifocal Leukoencephalopathy. Intern Med. (2017) 56:1219–23. doi: 10.2169/internalmedicine.56.8080
18. Delbue S, Branchetti E, Boldorini R, Vago L, Zerbi P, Veggiani C, et al. Presence and expression of JCV early gene large T antigen in the brains of immunocompromised and immunocompetent individuals. J Med Virol. (2008) 12:2147–52. doi: 10.1002/jmv.21313
Keywords: JC-virus, immunocompetence, progressive multifocal leukoencephalopathy (PML), spontaneous immune reconstitution inflammatory syndrome (IRIS), corticosteroid
Citation: Krey L, Raab P, Sherzay R, Berding G, Stoll M, Stangel M and Wegner F (2019) Severe Progressive Multifocal Leukoencephalopathy (PML) and Spontaneous Immune Reconstitution Inflammatory Syndrome (IRIS) in an Immunocompetent Patient. Front. Immunol. 10:1188. doi: 10.3389/fimmu.2019.01188
Received: 08 March 2019; Accepted: 10 May 2019;
Published: 28 May 2019.
Edited by:
Zsolt Illes, University of Southern Denmark, DenmarkReviewed by:
Johann Sellner, University Hospital Salzburg, AustriaCarlos Rodrigo Camara-Lemarroy, University of Calgary, Canada
Copyright © 2019 Krey, Raab, Sherzay, Berding, Stoll, Stangel and Wegner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florian Wegner, d2VnbmVyLmZsb3JpYW5AbWgtaGFubm92ZXIuZGU=
 Lea Krey1
Lea Krey1 Georg Berding
Georg Berding Martin Stangel
Martin Stangel Florian Wegner
Florian Wegner