- 1Laboratory of Cellular and Molecular Mechanisms of Histogenesis, Koltsov Institute of Developmental Biology, Moscow, Russia
- 2Laboratory of Biotechnology, Department of Immunology, Central Tuberculosis Research Institute, Moscow, Russia
Efficient tuberculosis (TB) control depends on early TB prediction and prevention. Solution to these tasks requires knowledge of TB protection correlates (TB CoPs), i.e., laboratory markers that are mechanistically involved in the protection and which allow to determine how well an individual is protected against TB or how efficient the candidate TB vaccine is. The search for TB CoPs has been largely focused on different T-helper populations, however, the data are controversial, and no reliable CoPs are still known. Here we discuss the role of different T-helper populations in TB protection focusing predominantly on Th17, “non-classical” Th1 (Th1*) and “classical” Th1 (cTh1) populations. We analyze how these populations differ besides their effector activity and suggest the hypothesis that: (i) links the protective potential of Th17, Th1*, and cTh1 to their differentiation degree and plasticity; (ii) implies different roles of these populations in response to vaccination, latent TB infection (LTBI), and active TB. One of the clinically relevant outcomes of this hypothesis is that over-stimulating T cells during vaccination and biasing T cell response toward the preferential generation of Th1 are not beneficial. The review sheds new light on the problem of TB CoPs and will help develop better strategies for TB control.
Introduction
Tremendous efforts have been made to improve tuberculosis (TB) control. Nevertheless, TB remains a global public health threat. Multi-drug resistance of M. tuberculosis (Mtb), HIV infection, malnutrition, aging and increased usage of immune suppressant drugs contribute to TB spread (1–4). Most of these factors operate by altering host immune resistance to various infections, including TB. In these settings, evaluating the individual level of TB protection and TB risk and developing effective vaccines to increase population resistance are important tasks. Their solutions require knowledge of TB protection correlates (TB CoPs) (5).
Multiple cellular populations and molecular pathways mediate antimycobacterial immunity (6–14). Among them, IFN-γ-producing CD4 lymphocytes play the major role (6–9). Consequently, Th1 lymphocytes have long been regarded as TB CoPs. However, recent data do not fully support this concept. Other populations of Th lymphocytes have appeared as candidate TB CoPs. Yet, the data are controversial, and no reliable TB CoPs are still known. Here we analyze recent data and discuss protective potential of “classical” Th1 (cTh1), “non-classical” Th1 (Th1*), and Th17 lymphocytes during latent TB infection (LTBI), active TB and following vaccination.
The Multifaceted Face of TB Protection and Its Correlates
Mechanistic CoPs are defined as immune parameters directly responsible for host protection. In contrast, non-mechanistic CoPs (or biomarkers) are determined as markers that correlate with the protection but are not causally responsible for it (15, 16). Protection can be determined in different ways, i.e., as host ability to (i) prevent the acquisition of the infection; (ii) clear the pathogen after the initial onset of the infection; (iii) limit pathogen replication and maintain the infection in inactive (latent) form; or (iv) limit disease progression and severity. CoPs against Mtb infection, TB disease and TB disease progression/severity may differ.
Knowledge of TB CoPs is important for estimating the individual level of protection and developing and testing new vaccines. “Individual” and vaccine CoPs may differ.
The most common approaches to identify individual TB CoPs rely on: (i) the assessment of Mtb infection severity in immunologically-manipulated mice; (ii) the analysis of immune response and TB pathology in other animal models, including non-human primates (NHP) and bovine models [reviewed in (17–20)]; (iii) the comparison of immune responses in poorly and well-protected individuals, primarily in TB patients and LTBI subjects, and in TB patients with diverse TB severity. At the bottom, these approaches address CoPs against TB disease and TB severity (or their experimental surrogates), but do not measure CoPs against Mtb infection. The latter is difficult to address due to the lack of adequate animal models and methods to evaluate Mtb persistence and clearance in humans (19).
In the vaccination field, experimental studies allow us to directly compare vaccine immunogenicity and protectivity (21–24), whereas clinical evaluation of vaccine protectivity is difficult, limiting many studies to the analysis of vaccine immunogenicity only.
In the review we mainly focus on mechanistic CoPs paying attention to delineate individual and vaccine CoPs, and models used for their detection. We focus predominantly on blood CoPs due to their clinical relevance and limited number of studies addressing tissue-associated CoPs in humans.
Th1 Lymphocytes
Th1-response magnitude is often used as a measure of TB protection and vaccine immunogenicity (7–9, 11, 13, 19, 25–28). The concept relies on observations showing that failure to develop Th1 response increases severity of experimental Mtb infection in mice and TB risk in humans. Mice deficient in CD4 lymphocytes, IFN-γ or other type 1 response genes develop extremely severe TB (29–32). Patients with AIDS and patients receiving anti-TNF therapy have increased TB risk (4, 33–35). Children bearing mutations in the genes of IL-12/IFN-γ axis exhibit Mendelian susceptibility to mycobacterial diseases (44–46). Nevertheless, the fact that Th1 are needed for Mtb control does not signify that their magnitude reflects the degree of protection (11, 16, 47–49). Reports on the lack of correlation between Th1 and protection have been accumulated.
Experimental Studies
Mouse IFN-γ−/− CD4+ cells provided protection against Mtb in vitro (50) and following adaptive transfer in vivo (51, 52); in vivo, hyper-production of IFN-γ was deleterious (53). Vaccination of mice with BCG stimulated Th1, however the response did not reflect the strength of protection (21, 54). To enhance BCG-induced antimycobacterial immunity, prime-boost strategies were suggested. In mice and NHP, boosting strengthened Th1 response (55–59), but in most studies this did not correlate with protection (55–57).
Clinical Studies
In humans, LTBI and active TB serve as surrogates of effective and ineffective protection, respectively. Comparative analyses of Th1/IFN-γ during LTBI and TB have given inconsistent results on whether the responses are higher during LTBI or TB (60–66). Interferon-gamma release assays (IGRA) do not discriminate between LTBI and active TB, indicating that most TB patients do not exhibit Th1 deficiency (67, 68). In TB patients, disease severity does not correlate with diminished Th1/IFN-γ; patients with active disease develop higher Mtb-specific IFN-γ responses compared to patients with residual TB lesions (64, 65, 69).
While the magnitude of Th1 lymphocytes does not correlate with protection and does not differentiate between LTBI and TB, Th1 activation and differentiation allow distinguishing LTBI and TB. Specifically, Mtb-specific Th1 persisting during LTBI are significantly less activated, less differentiated and contain fewer cycling lymphocytes compared to Th1 circulating during TB (evaluation based on the expression of CD27, HLA-DR/CD38, and Ki67) (70–74). It remains, however, unclear whether low-activated/differentiated Th1 are mechanistically involved in LTBI maintenance or whether their predominance during LTBI simply reflects low infection activity.
In vaccine clinical studies, BCG and new candidate vaccines appeared as potent inducers of Th1. However, in most studies, Th1 response did not coincide with vaccine efficacy. In one recent study, BCG-induced IFN-γ-secreting T cells associated with reduced TB risk in South Africa infants (75). However, a previous study in the same population found no association of BCG-specific Th1 with TB risk (76). Similarly, BCG-boost strategies and attenuated vaccines enhanced Th1 response (77–80), but did not show satisfactory protection in clinical testing [(77, 78, 81), reviewed in (19, 27, 82)].
Overall, the levels of Th1 immunity reflect the strength of Mtb infection rather than the degree of protection and do not correlate reliably with vaccine efficacy.
Th17 Lymphocytes
Experimental Studies
Th17 are pleiotropic cells with neutrophil-stimulating and pro-inflammatory activities (49, 83, 84). As neutrophils had been implicated in TB pathology (47, 85, 86), Th17 were initially thought to promote TB progression. However, in most experimental studies they conferred protection (87–95).
In mice infected with Mtb, Th17 promoted granulomatous response, participated in the formation of B-cell follicles and activated macrophages for Mtb control (87–90). Vaccination of mice and NHP with BCG or subunit vaccines induced both Th17 and Th1 (56, 96, 97). However, in a mouse model, only Th17 were essential for vaccine efficacy (56). Of note, protection provided by Th17 was mediated through the enhanced generation of Th1 and their higher accumulation in the lung tissue (91–94). The data point to Th17 as mediators of vaccine-induced protection but raise a question on why Th1 themselves do not mark vaccine efficacy if they are needed to mediate Th17-dependent protection.
Clinical Studies
The results of clinical Th17/IL-17 analyses are ambiguous. Several, but not all studies reported associations between TB susceptibility and polymorphisms in genes encoding IL-17 (98, 99). Transcriptional and clinical analyses of healthy adolescents in South Africa revealed an association between inhibited Th17 responses and TB development (100). However, comparison of Th17/IL-17 levels in LTBI subjects and TB patients yielded inconsistent results: some studies reported heightened Th17/IL-17 levels in LTBI subjects (101–103), others demonstrated increased Th17/IL17 in TB patients (104–107), some did not reveal differences between LTBI and TB groups (108) or found extremely low frequencies of Mtb-specific Th17 in both groups (109, 110). In our study, Mtb-specific Th17 were rare in the blood, but readily identifiable in the lungs of TB patients (110). However, it remained unclear whether lung-residing Th17 contributed to TB protection or pathology, since there was no possibility to measure lung Th17 in well-protected individuals.
In vaccine clinical studies, BCG and new candidate vaccines induced IL-17 producing cells. Yet, the cells either did not correlate with the risk of TB [BCG (76)] or their role remained uncertain [MVA85A (77)].
In summary, Th17 are involved in vaccine-induced protection in mice. Their role in vaccine-induced immunity in humans as well as in immune protection and pathology during Mtb infection (in both humans and experimental animals) remains uncertain.
Polyfunctional Th1 and Th17 Lymphocytes
Th1 and Th17 populations are heterogeneous and contain subpopulations with diverse cytokine profiles.
Polyfunctional Th1 Lymphocytes
In experimental studies, polyfunctional IFN-γ+TNF-α+IL-2+ Th1 lymphocytes (PFL) were analyzed using murine, bovine and NHP models. In all of them, BCG and novel TB vaccine candidates elicited PFL, however, not in all studies correlative relationships between vaccine-induced PFL and protection were found [reviewed in details in (15)].
In clinical studies, many groups associated LTBI maintenance with the persistence of PFL (15, 65, 111–114), which was attributed to their low-differentiation degree and central memory characteristics (15). Yet, some groups found more PFL in TB patients (115–117) or did not find differences between LTBI and TB (106). Within the group of TB patients, PFL did not correlate with TB severity (65). In humans, BCG, subunit, recombinant protein and viral vector vaccines induced PFL; data on their correlation with vaccine efficacy are limited to BCG and BCG-prime/MVA85A-boost; both did not correlate with decreased TB risk [68, 69].
Recently, Orlando and co-authors described a new population of polyfunctional Mtb-responding CD4 human lymphocytes, TCNP (118, 119). TCNP produced IFN-γ, TNF-α, and IL-2 but expressed CD45RA+CCR7+ naïve-like phenotype. The magnitude of TCNP reflected TB activity. It was suggested that the cells present potential target for vaccination and immunotherapeutic strategies. However, more studies are needed to understand TCNP differentiation, function and link to Mtb infection activity.
Th17.1 Lymphocytes
Th17.1 co-produce IFN-γ/TNF-α and IL-17, co-express T-bet and RORγt and differentiate from Th17 in the presence of IL-12 and inflammatory cytokines, primarily IL-1β (120–122). During autoimmune diseases, Th17.1 are hyperpathogenic (49). During TB, Th17.1 are detected in the brochoalveolar fluid (123) and lungs (110), but are rare in blood (110, 124), leaving their role in TB uncertain.
Regulatory Th17
Regulatory Th17 co-produce IL-17 and IL-10. Recently, the enrichment of IL10+Th17 lymphocytes during LTBI has been reported (123). The data correspond to the beneficial, inflammation-limiting role of Treg demonstrated in NHP model of TB (125) and suggest IL10+Th17 as a new CoP (123). Yet, more studies are needed to support this conclusion.
Overall, the induction of Th1 PFL is not sufficient or even necessary for TB protection (15). The role of other polyfunctional populations remains to be established.
Non-classical Th1 Lymphocytes
T-helper populations are categorized based on cytokine profiles, transcriptional regulation and priming requirements (36, 37, 126, 127). Another categorization principle relies on the expression of chemokine receptors. In humans, the expression of CXCR3, CCR4, and CCR6 divides T cells into CXCR3+CCR4−CCR6−, CXCR3−CCR4+CCR6−, and CXCR3−CCR4−CCR6+ subsets that correspond to Th1, Th2, and Th17, respectively (128, 129). CXCR3 is a marker of Th1, CCR6 is a marker of Th17 and their progeny. Recently, a population of non-classical Th1 (Th1* or ex-Th17) has been described. Th1* produce IFN-γ in the absence of IL-17, but express CXCR3+CCR6+ phenotype (128). A few studies have demonstrated Th1* enrichment during LTBI and have suggested them as a new TB CoP (130–133). Nevertheless, it remains unclear why protection should correlate specifically with Th1* if functionally Th1* and cTh1 are similar.
Differences and Relationships Between cTh1, Th1* and Th17 During TB
Memory and Effector Populations of CD4+ Lymphocytes
Besides being divided into different Th populations based on cytokine profiles, CD4+ lymphocytes are divided into several clusters based on cell phenotype, differentiation, and homing properties. CD4+ lymphocytes circulating in human blood in the quiescent state are classified into TN, TSCM, TCM, TTM, TEM, and TTE/TEMRA clusters [(Table 1); (40, 119, 134–140)]. According to the lineage differentiation model, CD4+ cells progressively differentiate along these clusters, with less differentiated cells being longer-lived, having higher multipotency and higher self-renewal and protective capacities compared to more differentiated cells (138, 141–143).
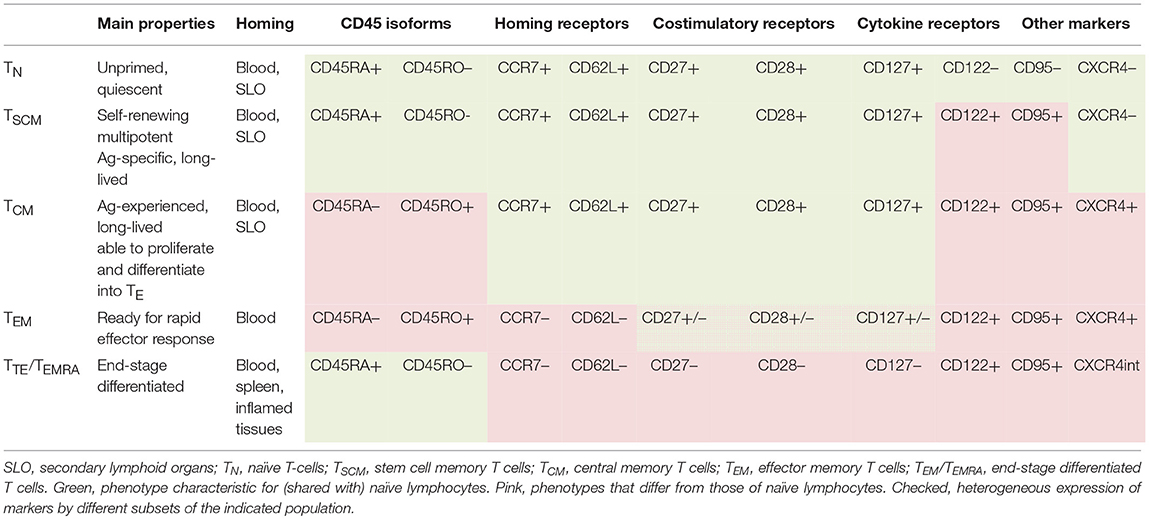
Table 1. Functional and phenotypic characteristics of naïve and memory populations of CD4+ human lymphocytes (40, 118, 134–139).
Following the interaction with the cognate antigen, naïve and memory cells differentiate into effector lymphocytes (140). Depending on the strength and duration of antigenic stimulation, effector cells reach different differentiation states, which can be delineated by cell phenotype (137, 140, 144–148). Particularly, early-differentiated CD4+ effectors are CD27+CD28+, late-differentiated effectors are CD27−CD28+, exhausted effectors are CD27−CD28−; terminally-differentiated effectors are CD27−CD28− and express CD57, other NK-cell and inhibitory receptors (140, 145). Along with their differentiation, effector CD4+ cells progressively decrease proliferative and survival potentials and increase cytokine secreting activity, with the exception of the exhausted and terminally-differentiated populations that decrease/lose cytokine production (137, 140, 145). Following antigen/pathogen clearance, most effectors die, whereas some give rise to memory populations. The less differentiated an effector cell is, the higher is its capacity to acquire memory state.
Overall, there is a link between T-cell stemness and memory potential and there are reciprocal relationships between these parameters and the strength of cell effector activity.
Th1* and cTh1 Display Phenotypic and Differentiation Differences
Considering that T-cell protective potential depends on cell differentiation, we have recently compared the differentiation states of Th1* and cTh1 persistent during TB (110). Th1* were less-differentiated: they contained more CD27+ cells, did not contain CD27−CD28− cells and expressed T-bet at a lower level than cTh1 [a sign of memory cell precursors (134)]. We also found out that CXCR3+CCR6+ Th1* cells stimulated in vitro with anti-CD3/CD28 antibodies, differentiated into CXCR3+CCR6− cTh1-like lymphocytes, but not vice versa (110). Other authors reported a higher expression of anti-apoptotic protein Bcl-2 by Th1* cells (41) and an overlap between the gene signatures of Th1* and memory CD4+ cells persistent during LTBI (132). There are also overlaps between Th1* and Mtb-specific TSCM, as both are CD27+, express CXCR3 and CCR6 and produce type 1 cytokines (149). Thus, compared to cTh1, Th1* are more memory-biased, which, as we suppose, determines their role in LTBI maintenance. Of note, most TB studies identified Th1 based on intracellular IFN-γ, i.e., they did not distinguish between cTh1 and Th1*. This could account for a poor correlation between Th1 and TB protection.
Additional mechanism providing Th1* with increased protective potential may lie in their expression of CCR6, as CCR6 participates in cell homing to the inflamed tissues (150, 151). Peripheral localization is critical for TB protection (152). Interestingly, tissue-resident memory cells (TRM) exhibit superior protective potential (153) and co-express CXCR3 and CCR6 (154).
Overall, although Th1* and cTh1 exhibit similar functional activity in terms of the secretion of type 1 cytokines, Th1* are less-differentiated, have higher survival and mucosal tissue homing capacities and higher protective potential.
Lineage Relationships Between Th17, Th1*, and cTh1 Populations: Data and Hypotheses
Some authors suggest that Th1* may originate from naïve precursors (40), yet multiple observations speak in favor of Th1* generation from Th17 (40, 41, 155, 156). The Th17 → Th1 differentiation provides a mechanism to maintain long-lasting Th1 response in vivo. Indeed, cTh1 are highly differentiated and have poor persistent capacity (38). In contrast, Th17 have self-renewal properties similar to those of TSCM (157), they are long-lived, plastic (38, 40) and acquire IFN-γ production in the presence of IL-12 and pro-inflammatory cytokines, primarily IL-1β (39, 42, 43, 158). Mtb have evolved multiple mechanisms to avoid host protective immunity, including the inhibition of IL-12 (159, 160). Yet, innate immune cells produce IL-12 and IL-1β during Mtb infection (161, 162). This creates conditions necessary for the differentiation of vaccine-induced Th17 into Th1*. Indeed, in mice vaccine-induced Th17 adapted Th1 characteristics following Mtb challenge (158). In a mouse model of autoimmune disease, Th17 comprised two subsets, CD27+ stemness-associated and CD27−/T-bet+ able to trans-differentiate into Th1-like cells (163). The data support Th17 → Th1 differentiation and the dichotomy between stemness and Th1-like properties.
Our data on a lower differentiation degree of Th1* and their ability to transform into CXCR3+CCR6− cells suggest Th1* → cTh1 transition and the existence of Th17 → Th1* → cTh1 differentiation pathway. Because T-cell differentiation is driven by antigenic and cytokine stimulation, the hypothesis implies that different populations dominate and mediate protection following vaccination, during LTBI and active TB (Figure 1). We suppose that Th17 persist in low-inflammatory conditions, maintain vaccine-induced protection and early response to Mtb; Th1* are generated and support long-lasting protection during LTBI; cTh1 originate from naïve and/or Th1* precursors during active TB. Certainly, more studies are needed to prove the hypothesis. However, it explains some existing data and controversies, such as: (i) the preferential link of vaccine-induced protection to Th17; (ii) a need for Th1 for the protection against TB along with the lack of correlation between post-vaccination Th1 and vaccine-induced protection; (iii) the predominance of Th1* during LTBI and their contraction during active TB (discussed above). Important outcomes of the hypothesis, if it is supported, are: (i) in different conditions, protection is associated with different Th populations, i.e., there is no single T-cell-associated TB CoP; (ii) over-stimulating T cells during vaccination and biasing host response toward the preferential generation of Th1 (the aim of many current vaccination-boost strategies) are not beneficial.
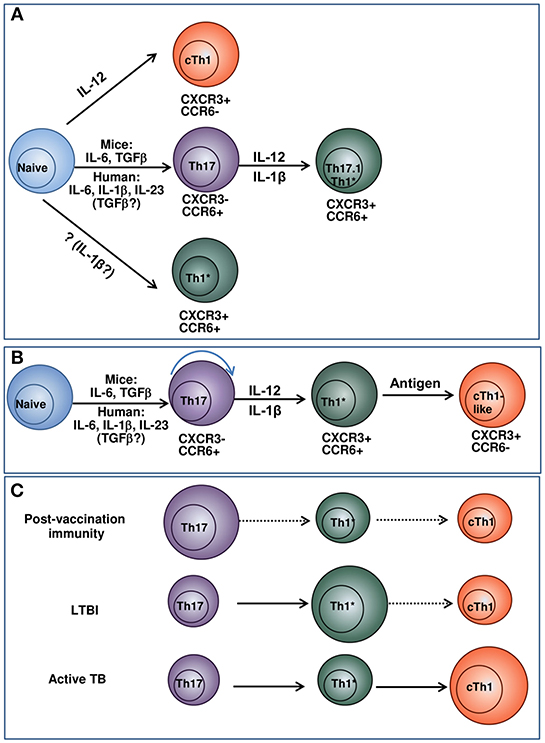
Figure 1. Model suggesting the relationships between Th17, Th1* and cTh1 populations and their potential roles in post-vaccination immunity, LTBI and active TB. (A) Current concepts of cTh1, Th17, and Th1* differentiation. cTh1 differentiate from naïve lymphocytes in the presence of IL-12 (36, 37). Th17 differentiate from naïve lymphocytes in the presence of cytokine mixture. In general, the differentiation of mouse Th17 cells depends on IL-6/TGF-β; the generation of human Th17 is driven by IL-23/IL-1β/IL-6. The involvement of TGF-β in the generation of human Th17 has been suggested by some authors, especially at low cytokine doses (36, 38, 39). When exposed to IL-12, IL-1β, or/and TNF-α, Th17 convert into IFN-γ producing Th17.1 and Th1* lymphocytes (38, 40–43). Alternatively, Th1* may derive directly from naïve lymphocytes under the action of cytokines that have not yet been identified (40). The concept considers Th1* and cTh1 as independent lineages of CD4+ T-cell differentiation. (B) Suggested pathway of cTh1, Th17, and Th1* differentiation. Naive cells progressively differentiate into Th17, Th1* and cTh1/cTh1-like lymphocytes. The depth of the differentiation depends on the strength of antigenic stimulation and cytokine milieu and is different in response to vaccination, LTBI and active TB. (C) Suggested pathway of cTh1, Th17, and Th1* differentiation and the predominance of different Th populations following vaccination, during LTBI and active TB. In response to vaccination, different populations of Th cells generate. Of them, Th17 have higher survival capacity and persist longer. Following Mtb infection, Th17 are exposed to antigen, IL-12 and pro-inflammatory cytokines and differentiate into IFN-γ producing Th1* CXCR3+CCR6+ cells. The cells persist during LTBI and maintain protection against TB disease. cTh1 are generated in response to vaccination, LTBI and TB disease, but do not persist for a long time. During active TB, their magnitude increases due to their permanent generation from naïve lymphocytes and/or Th1*. The size of the circles indicates the relative prevalence and protective roles of the corresponding subsets in the indicated conditions. For each condition, prevalent pathways of T-cell differentiation are indicated in solid arrows, otherwise dashed arrows are used.
Closing Summary
Multiple studies have searched for TB CoPs, but the data are contradictory, many questions remain unanswered, and no reliable CoPs have been identified. Particularly, it is not clear why vaccine-induced pre-challenge Th1 do not correlate with protection, why Th17 better correlate with protection compared to Th1, and why LTBI maintenance is associated with Th1* but not cTh1. The review addresses these questions by analyzing Th17, Th1*, and cTh1 properties and differentiation pathways. We suggest a hypothesis that links Th protective potential to cell differentiation degree, longevity and plasticity. We suppose that following the exposure to Mtb-antigens, Th cells transit along the Th17 → Th1* → cTh1 pathway. The differentiation depth depends on the strength of antigenic stimulation, which is different following vaccination, during LTBI and active TB. Thus, under different conditions, immunotherapy and vaccination strategies should target different populations, and over-stimulating T cells during vaccination is not beneficial. Although more studies are needed to confirm the suggested assumptions, the hypothesis sheds new light on TB CoPs and should help develop better TB control strategies.
Author Contributions
IL analyzed the literature and wrote the manuscript. IN contributed to manuscript writing.
Funding
The work of IL was conducted under the IDB RAS Government basic research program, N0108-2019-0004 (general concept and all aspects related to cell stemness, multipotency, and memory potential). The of work IN was conducted under the RSF research grant, N17-75-10197 (aspects related to Th1 effector activity).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. T. Radaeva for critically reading the manuscript.
References
1. Global Tuberculosis Report 2018. World Health Organization. Available online at: http://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?ua=1. (accessed Dec 23, 2018).
2. Kumar Nathella P, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology. (2017) 152:13–24. doi: 10.1111/imm.12762
3. Natori Y, Ferreira VH, Nellimarla S, Husain S, Rotstein C, Humar A, et al. Incidence, outcomes, and long-term immune response to tuberculosis in organ transplant recipients. Transplantation. (2018) 103:210–15. doi: 10.1097/TP.0000000000002340
4. Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. (2001) 345:1098–104. doi: 10.1056/NEJMoa011110
5. Brennan MJ, Stone MR, Evans T. A rational vaccine pipeline for tuberculosis. Int J Tuberc Lung Dis. (2012) 16:1566–73. doi: 10.5588/ijtld.12.0569
6. Ehlers S. Immunity to tuberculosis: a delicate balance between protection and pathology. FEMS Immunol Med Microbiol. (1999) 23:149–58. doi: 10.1111/j.1574-695X.1999.tb01234.x
7. Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. (2011) 4:288–93. doi: 10.1038/mi.2011.10
8. O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. (2013) 31:475–527. doi: 10.1146/annurev-immunol-032712-095939
9. Kaufmann SH. EFIS lecture. Immune response to tuberculosis: How to control the most successful pathogen on earth. Immunol Lett. (2016) 175:50–7. doi: 10.1016/j.imlet.2016.05.006
10. Schorey JS, Schlesinger LS. Innate immune responses to tuberculosis. Microbiol Spectr. (2016) 4:6. doi: 10.1128/microbiolspec.TBTB2-0010-2016
11. Cardona PJ. What we have learned and what we have missed in tuberculosis pathophysiology for a new vaccine design: searching for the “Pink Swan.” Front Immunol. (2017) 8:556. doi: 10.3389/fimmu.2017.00556
12. Scriba TJ, Coussens AK, Fletcher HA. Human immunology of tuberculosis. Microbiol Spectr. (2017) 5:1–2. . doi: 10.1128/microbiolspec.TBTB2-0016-2016
13. Orme IM, Henao-Tamayo MI. Trying to see the forest through the trees: deciphering the nature of memory immunity to mycobacterium tuberculosis. Front Immunol. (2018) 9:461. doi: 10.3389/fimmu.2018.00461
14. Stutz MD, Clark MP, Doerflinger M, Pellegrini M. Mycobacterium tuberculosis: rewiring host cell signaling to promote infection. J Leukoc Biol. (2018) 103:259–68. doi: 10.1002/JLB.4MR0717-277R
15. Lewinsohn DA, Lewinsohn DM, Scriba TJ. Polyfunctional CD4+ T cells as targets for tuberculosis vaccination. Front Immunol. (2017) 8:1262. doi: 10.3389/fimmu.2017.01262
16. Huang L, Russel DG Protective immunity against tuberculosis: what does it look like and how do we find it? Curr. Opin. Immunol. (2017) 48:44–50. doi: 10.1016/j.coi.2017.08.001
17. Capuano SV, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, et al. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. (2003) 71:5831–44. doi: 10.1128/IAI.71.10.5831-5844.2003
18. Scanga CA, Flynn JL. Modeling tuberculosis in nonhuman primates. Cold Spring Harb Perspect Med. (2014) 4:a018564. doi: 10.1101/cshperspect.a018564
19. Lalvani A, Sridhar S, von Reyn F. Tuberculosis vaccines: time to reset the paradigm? Thorax. (2013) 68:1092–4. doi: 10.1136/thoraxjnl-2013-203456
20. Sharpe SA, White AD, Sibley L, Gleeson F, Hall GA, Basaraba RJ, et al. An aerosol challenge model of tuberculosis in Mauritian cynomolgus. macaques. PLoS ONE. (2017) 12:e0171906. doi: 10.1371/journal.pone.0171906
21. Orme IM. Tuberculosis vaccine types and timings. Clin Vaccine Immunol. (2015) 22:249–57. doi: 10.1128/CVI.00718-14
22. Mothé BR, Lindestam Arlehamn CS, Dow C, Dillon MBC, Wiseman RW, Bohr P, et al. The TB-specific CD4(+) T cell immune repertoire in both cynomolgus and rhesus macaques largely overlap with humans. Tuberculosis. (2015) 95:722–35. doi: 10.1016/j.tube.2015.07.005
23. Laddy DJ, Bonavia A, Hanekom WA, Kaushal D, Williams A, Roederer M, et al. Toward tuberculosis vaccine development: recommendations for nonhuman primate study design. Infect Immun. (2018) 86:e00776–17. doi: 10.1128/IAI.00776-17
24. Voss G, Casimiro D, Neyrolles O, Williams A, Kaufmann S, McShane H, et al. Progress and challenges in TB vaccine development. F1000Research. (2018) 7:199. doi: 10.12688/f1000research.13588
25. Cooper AM. T cells in mycobacterial infection and disease. Curr Opin Immunol. (2009) 21:378–84. doi: 10.1016/j.coi.2009.06.004
26. Bold TD, Banaei N, Wolf AJ, Ernst JD. Suboptimal activation of antigen-specific CD4 + effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog. (2011) 7:e1002063. doi: 10.1371/journal.ppat.1002063
27. Lewinsohn DA, Gold MC, Lewinsohn DM. Views of immunology: effector T cells. Immunol Rev. (2011) 240:25–39. doi: 10.1111/j.1600-065X.2010.00997.x
28. Jasenosky LD, Scriba TJ, Hanekom WA, Goldfeld AE. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev. (2015) 264:74–87. doi: 10.1111/imr.12274
29. Cooper AM, Dalton DK, Stewart NA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. (1993) 178:2243–7.
30. Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. (1995) 2:561–72.
31. Ehlers S, Kutsch S, Ehlers EM, Benini J, Pfeffer K. Lethal granuloma disintegration in mycobacteria-infected TNFRp55-/- mice is dependent on T cells and IL-12. J Immunol. (2000) 65:483–92. doi: 10.4049/jimmunol.165.1.483
32. Cooper AM, Solache A, Khader SA. Interleukin-12 and tuberculosis: an old story revisited. Curr Opin Immunol. (2007) 19:441–7. doi: 10.1016/j.coi.2007.07.004
33. Harris J, Keane J. How tumor necrosis factor blockers interfere with tuberculosis immunity. Clin Exp Immunol. (2010) 161:1–9. doi: 10.1111/j.1365-2249.2010.04146.x
34. Geldmacher C, Zumla A, Hoelscher M. Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis. Curr Opin HIV. (2012) 7:268–75. doi: 10.1097/COH.0b013e3283524e32
35. Suthar AB, Lawn SD, del Amo J, Getahun H, Dye C, Sculier D, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLOS Med. (2012) 9:e1001270. doi: 10.1371/journal.pmed.1001270
36. Evans CM, Jenner RG. Transcription factor interplay in T helper cell differentiation. Briefings Funct Genomics. (2013) 12:499–511. doi: 10.1093/bfgp/elt025
37. Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, et al. Signaling and transcription in T helper development. Annu Rev Immunol. (2000) 18:451–94. doi: 10.1146/annurev.immunol.18.1.451
38. Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. (2013) 121:2402–14. doi: 10.1182/blood-2012-09-378653
39. McAleer JP, Kolls JK. Mechanisms controlling Th17 cytokine expression and host defense. J Leukoc Biol. (2011) 90:263–70. doi: 10.1189/jlb.0211099
40. Sallusto F. Heterogeneity of human CD4(+) T cells against microbes. Annu Rev Immunol. (2016) 34:317–34. doi: 10.1146/annurev-immunol-032414-112056
41. Basdeo SA, Cluxton D, Sulaimani J, Moran B, Canavan M, Orr C, et al. Ex-Th17 (Nonclassical Th1) cells are functionally distinct from classical Th1 and Th17 cells and are not constrained by regulatory T cells. J Immunol. (2017) 198:2249–59. doi: 10.4049/jimmunol.1600737
42. Nistala K, Adams S, Cambrook H, Ursu S, Olivito B, de Jager W, et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci USA. (2010) 107:14751–6. doi: 10.1073/pnas.1003852107
43. Cosmi L, Cimaz R, Maggi L, Santarlasci V, Capone M, Borriello F, et al. Evidence of the transient nature of the Th17 phenotype of CD4+CD161+ T cells in the synovial fluid of patients with juvenile idiopathic arthritis. Arthritis Rheum. (2011) 63:2504–15. doi: 10.1002/art.30332
44. Casanova JL, Abel L. Genetic dissection of immunity to mycobacteris: thehuman model. Annu Rev Immunol. (2002) 20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851
45. Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN- immunity. Semin Immunol. (2014) 26:454–70. doi: 10.1016/j.smim.2014.09.008
46. Abel L, El-Baghdadi J, Bousfiha AA, Casanova JL, Schurr E. Human genetics of tuberculosis: a long and winding road. Pjil Trans R Soc B. (2014) 369:20130428. doi: 10.1098/rstb.2013.0428
47. Lyadova IV. Inflammation and immunopathogenesis of tuberculosis progression. In: Cardona PJ, editor. Understanding Tuberculosis: Analyzing the Origin of Mycobacterium tuberculosis Pathogenicity. IntechOpen (2012) p. 19–42. Available online at: http://www.intechopen.com/books/understanding-tuberculosis-analyzing-the-origin-of-mycobacterium-tuberculosis-pathogenicity. doi: 10.5772/32060
48. Fletcher HA, Schrager L. TB vaccine development and the End TB strategy: importance and current status. Trans R Soc Trop Med Hyg. (2016) 110:212–8. doi: 10.1093/trstmh/trw016
49. Lyadova IV, Panteleev AV. Th1 and Th17 cells in tuberculosis: protection, pathology, and biomarkers. Mediat Inflamm. (2015) 2015:854507. doi: 10.1155/2015/854507
50. Cowley C, Elkins KL. CD4+ T cells mediate IFN-γ-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. J Immunol. (2003) 171:4689–99. doi: 10.4049/jimmunol.171.9.4689
51. Gallegos FM, van Heijst JW, Samstein M, Su X, Pamer EG, Glickman MS. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. (2011) 7:e1002052. doi: 10.1371/journal.ppat.1002052
52. Nandi B, Behar SM. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J Exp Med. (2011) 208:2251–62. doi: 10.1084/jem.20110919
53. Sakai S, Kauffman KD, Sallin MA, Sharpe AH, Young HA, Ganusov VV, et al. CD4 T cell-derived IFN-γ plays a minimal role in control of pulmonary Mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog. (2016) 12:e1005667. doi: 10.1371/journal.ppat.1005667
54. Mittrucker HW, Steinhoff U, Kohler A, Krause M, Lazar D, Mex P, et al. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci USA. (2007) 104:12434–9. doi: 10.1073/pnas.0703510104
55. Majlessi L, Simsova M, Jarvis Z, Brodin P, Rojas MJ, Bauche C, et al. An increase in antimycobacterial Th1-cell responses by prime-boost protocols of immunization does not enhance protection against tuberculosis. Infect Immun. (2006) 74:2128–37. doi: 10.1128/IAI.74.4.2128-2137.2006
56. Van Dis E, Sogi KM, Rae CS, Van Dis E, Sogi KM, Rae CS, et al. STING-activating adjuvants elicit a Th17 immune response and protect against Mycobacterium tuberculosis infection. Cell Rep. (2018) 23:1435–47. doi: 10.1016/j.celrep.2018.04.003
57. Sharpe SA, McShane H, Dennis MJ, Basaraba RJ, Gleeson F, Hall G, et al. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques, and an evaluation of endpoints for vaccine testing. Clin Vaccine Immunol. (2010) 17:1170–82. doi: 10.1128/CVI.00079-10
58. Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, et al. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J Clin Invest. (2011) 122:303–14. doi: 10.1172/JCI46252
59. Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ, et al. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc Nat Acad Sci USA. (2009) 106:2301–6. doi: 10.1073/pnas.0712077106
60. Hirsch S, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, et al. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. (1999) 180:2069–73. doi: 10.1086/315114
61. Sahiratmadja E, Alisjahbana B, de Boer T, Adnan I, Maya A, Danusantoso H, et al. Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect Immun. (2007) 75:820–9. doi: 10.1128/IAI.00602-06
62. Sutherland S, Lalor MK, Black GF, Ambrose LR, Loxton AG, Chegou NN, et al. GCGH biomarkers for TB consortium. analysis of host responses to Mycobacterium tuberculosis antigens in a multi-site study of subjects with different TB and HIV infection states in sub-Saharan Africa. PLoS ONE. (2013) 8:e74080. doi: 10.1371/journal.pone.0074080
63. Sahiratmadja E, Alisjahbana B, Buccheri S, Di Liberto D, de Boer T, Adnan I, et al. Plasma granulysin levels and cellular interferon-gamma production correlate with curative host responses in tuberculosis, while plasma interferon-gamma levels correlate with tuberculosis disease activity in adults. Tuberculosis. (2007) 87:312–21. doi: 10.1016/j.tube.2007.01.002
64. Nikitina IY, Panteleev AV, Sosunova EV, Karpina NL, Bagdasarian TR, Burmistrova IA, et al. Antigen-specific IFN-g responses correlate with the activity of M. tuberculosis infection but are not associated with the severity of tuberculosis disease. J Immunol Res. (2016) 2016:7249369. doi: 10.1155/2016/7249369
65. Panteleev A, Nikitina I, Burmistrova I, Kosmiadi GA, Radaeva TV, Amansahedov RB, et al. Severe tuberculosis in humans correlates best with neutrophil abundance and lymphocyte deficiency and does not correlate with antigen-specific CD4 T cell response. Front Immunol. (2017) 8:963. doi: 10.3389/fimmu.2017.00963
66. Kumar P. IFNγ-producing CD4+ T lymphocytes: the double-edged swords in tuberculosis. Clin Transl Med. (2017) 6:21. doi: 10.1186/s40169-017-0151-8
67. Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, et al. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. (2011) 37:100–11. doi: 10.1183/09031936.00114810
68. Pai M, Denkinger CM, Kik CV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. (2014) 1:3–20. doi: 10.1128/CMR.00034-13
69. Amelio P, Portevin D, Reither K, Mhimbira F, Mpina M, Tumbo A, et al. Mixed Th1 and Th2 Mycobacterium tuberculosis-specific CD4 T cell responses in patients with active pulmonary tuberculosis from Tanzania. PLoS Negl Trop Dis. (2017) 11:e0005817. doi: 10.1371/journal.pntd.0005817
70. Streitz M, Tesfa L, Yildirim V, Yahyazadeh A, Ulrichs T, Lenkei R, et al. Loss of receptor on tuberculin-reactive T-cells marks active pulmonary tuberculosis. PLoS ONE. (2007) 2:e735. doi: 10.1371/journal.pone.0000735
71. Nikitina IY, Kondratuk NA, Kosmiadi GA, Amansahedov RB, Vasilyeva IA, Ganusov VV, et al. Mtb-specific CD27low CD4 T cells as markers of lung tissue destruction during pulmonary tuberculosis in humans. PLoS ONE. (2012) 7:e43733. doi: 10.1371/journal.pone.0043733
72. Adekambi T, Ibegbu CC, Cagle S, Kalokhe AS, Wang YF, Hu Y, et al. Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response. J Clin Invest. (2015) 125:1827–38. doi: 10.1172/JCI77990
73. Portevin D, Moukambi F, Clowes P, Bauer A, Chachage M, Ntinginya NE, et al. Assessment of the novel T-cell activation marker-tuberculosis assay for diagnosis of active tuberculosis in children: a prospective proof-of-concept study. Lancet Infect Dis. (2014) 14:931–8. doi: 10.1016/S1473-3099(14)70884-9
74. Goletti D, Lee M-R, Wang J-Y, Walter N, Ottenhoff THM. Update on tuberculosis biomarkers: From correlates of risk, to correlates of active disease and of cure from disease. Respirology. (2018) 213:455–66. doi: 10.1111/resp.13272
75. Fletcher HA, Snowden MA, Landry B, Rida W, Satti I, Harris SA, et al. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun. (2016) 7:11290. doi: 10.1038/ncomms11290
76. Kagina B, Abel B, Scriba, Hughes EJ, Keyser A, Soares A, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Resp Crit Care Med. (2010) 182:1073–9. doi: 10.1164/rccm.201003-0334OC
77. Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. (2013) 381:1021–8. doi: 10.1016/S0140-6736(13)60177-4
78. Ndiaye BP, Thienemann F, Ota M, Landry BS, Camara M, Dièye S, et al. MVA85A 030 trial investigators. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. (2015) 3:190–200. doi: 10.1016/S2213-2600(15)00037-5
79. Spertini F, Audran R, Chakour R, Karoui O, Steiner-Monard V, Thierry AC, et al. Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: a randomised, double-blind, controlled phase I trial. Lancet Respir Med. (2015) 3:953–62. doi: 10.1016/S2213-2600(15)00435-X
80. Von Reyn CF, Lahey T, Arbeit RD, Landry B, Kailani L, Adams LV, et al. Safety and immunogenicity of an inactivated whole cell tuberculosis vaccine booster in adults primed with BCG: a randomized, controlled trial of DAR-901. PLoS ONE. (2017) 12:e0175215. doi: 10.1371/journal.pone.0175215
81. Gupta N, Garg S, Vedi S, Kunimoto DY, Kumar R, Agrawal B. Future path toward TB vaccine development: boosting BCG or re-educating by a new subunit vaccine. Front Immunol. (2018) 9:2371. doi: 10.3389/fimmu.2018.02371
82. Dockrell HM, Smith SG. What have we learnt about BCG vaccination in the last 20 years? Front Immunol. (2017) 8:1134. doi: 10.3389/fimmu.2017.01134
83. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. (2009) 27:485–517. doi: 10.1146/annurev.immunol.021908.132710
84. Mourik BC, Lubberts E, de Steenwinkel JEM, Ottenhoff THM, Leenen PJM. Interactions between type 1 interferons and the Th17 response in tuberculosis: lessons learned from autoimmune diseases. Front Immunol. (2017) 8:294. doi: 10.3389/fimmu.2017.00294
85. Lyadova IV. Neutrophils in tuberculosis: heterogeneity shapes the way? Mediat Inflamm. (2017) 2017:8619307. doi: 10.1155/2017/8619307
86. Dallenga T, Repnik U, Corleis B, Eich J, Reimer R, Griffiths GW, et al. Tuberculosis-induced necrosis of infected neutrophils promotes bacterial growth following phagocytosis by macrophages. Cell Host Microbe. (2017) 22:519–530.e3. doi: 10.1016/j.chom.2017.09.003
87. Gopal R, Monin L, Slight S, Uche U, Blanchard E, Fallert Junecko BA, et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. (2014) 10:e1004099. doi: 10.1371/journal.ppat.1004099
88. Monin L, Griffiths KL, Slight S, Lin Y, Rangel-Moreno J, Khader SA. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol. (2015) 8:1099–109. doi: 10.1038/mi.2014.136
89. Cavalcanti-Neto MP, Prado RQ, Piñeros AR, Sérgio CA, Bertolini TB, Gembre AD, et al. Improvement of the resistance against early Mycobacterium tuberculosis-infection in the absence of PI3Kγ enzyme is associated with increase of CD4+IL-17+ cells and neutrophils. Tuberculosis. (2018) 113:1–9. doi: 10.1016/j.tube.2018.08.009
90. Erdmann H, Behrends J, Ritter K, Hölscher A, Volz A, Rosenkrands I, et al. The increased protection and pathology in Mycobacterium tuberculosis-infected IL-27R-alpha-deficient mice is supported by IL-17A and is associated with the IL-17A-induced expansion of multifunctional T cells. Mucosal Immunol. (2018) 11:1168–80. doi: 10.1038/s41385-018-0026-3
91. Gopal R, Lin Y, Obermajer N, Nuthalapati N, Ahmed M, Kalinski P, et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol. (2012) 42:364–73. doi: 10.1002/eji.201141569
92. Trentini MM, de Oliveira FM, Kipnis A, Junqueira-Kipnis AP. The role of neutrophils in the induction of specific th1 and th17 during vaccination against tuberculosis. Front Microbiol. (2016) 7:898. doi: 10.3389/fmicb.2016.00898
93. Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. (2007) 8:369–77. doi: 10.1038/ni1449
94. Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. (2007) 178:3786–96. doi: 10.4049/jimmunol.178.6.3786
95. Cortes EA, Kaveh D, Nunez-Garcia J, Hogarth PJ, Vordermeier HM. Mycobacterium bovis-BCG Vaccination induces specific pulmonary transcriptome biosignatures in mice. PLoS ONE. (2010) 5:e11319. doi: 10.1371/journal.pone.0011319
96. Wareham AS, Tree JA, Marsh PD, Butcher PD, Dennis M, Sharpe SA. Evidence for a role for Interleukin-17, Th17 cells and iron homeostasis in protective immunity against tuberculosis in cynomolgus macaques. PLoS ONE. (2014) 9:e88149. doi: 10.1371/journal.pone.0088149
97. Chatterjee S, Dwivedi VP, Singh Y, Siddiqui I, Sharma P, Van Kaer L, et al. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog. (2011) 7:e1002378. doi: 10.1371/journal.ppat.1002378
98. Peng R, Yue J, Han M, Zhao Y, Liu L, Liang L. The IL-17F sequence variant is associated with susceptibility to tuberculosis. Gene. (2013) 515:229–32. doi: 10.1016/j.gene.2012.11.017
99. Abhimanyu, Bose M, Komal, Varma-Basil M. Lack of association between IL17A and IL17F polymorphisms and related serum levels in north Indians with tuberculosis. Gene. (2013) 529:195–8. doi: 10.1016/j.gene.2013.06.090
100. Scriba TJ, Penn-Nicholson A, Shankar S, Hraha T, Thompson EG, Sterling D, et al. Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog. (2017) 13:e1006687. doi: 10.1371/journal.ppat.1006687
101. Chen X, Zhang M, Liao M, Graner MW, Wu C, Yang Q, et al. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am J Respir Crit Care Med. (2010) 181:734–42. doi: 10.1164/rccm.200909-1463OC
102. Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. (2008) 180:1962–70. doi: 10.4049/jimmunol.180.3.1962
103. Li Q, Li J, Tian J, Zhu B, Zhang Y, Yang K, et al. IL-17 and IFN-γ production in peripheral blood following BCG vaccination and Mycobacterium tuberculosis infection in human. Eur Rev Med Pharmacol Sci. (2012) 16:2029–36.
104. Basile JI, Geffner LJ, Romero MM, Balboa L, Sabio Y, García C, et al. Outbreaks of Mycobacterium tuberculosis MDR strains induce high IL-17 T-cell response in patients with MDR tuberculosis that is closely associated with high antigen load. J Infect Dis. (2011) 204:1054–64. doi: 10.1093/infdis/jir460
105. Jurado JO, Pasquinelli V, Alvarez IB, Peña D, Rovetta AI, Tateosian NL, et al. IL-17 and IFN-γ expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J Leukoc Biol. (2012) 91:991–1009. doi: 10.1189/jlb.1211619
106. Marín ND, París SC, Rojas M, García LF. Reduced frequency of memory T cells and increased Th17 responses in patients with active tuberculosis. Clin Vaccine Immunol. (2012) 19:1667–76. doi: 10.1128/CVI.00390-12
107. He X, Huang X, Xiao L, Hao J, Li J, Chen H, et al. IFN-γ-, IL-4-, IL-17-, PD-1-expressing T cells and B cells in peripheral blood from tuberculosis patients. Adv Microbiol. (2012) 2:426–35. doi: 10.4236/aim.2012.24054
108. Dheda K, Chang J-S, Lala S, Hugget JF, Zumla A, Rook GAW, et al. Gene expression of IL17 and IL23 in the lungs of patients with active tuberculosis. Thorax. (2008) 63:566–8. doi: 10.1136/thx.2007.092205
109. Perreau M, Rozot V, Welles HC, Belluti-Enders F, Vigano S, Maillard M, et al. Lack of Mycobacterium tuberculosis-specific interleukin-17A-producing CD4+ T cells in active disease. Eur J Immunol. (2013) 43:939–48. doi: 10.1002/eji.201243090
110. Nikitina IY, Panteleev AV, Kosmiadi GA, Serdyuk YV, Nenasheva TA, Nikolaev AA, et al. Th1, Th17, and Th1Th17 lymphocytes during tuberculosis: Th1 lymphocytes predominate and appear as low-differentiated CXCR3+CCR6+ cells in the blood and highly differentiated CXCR3+/-CCR6- cells in the lungs. J Immunol. (2018) 200:2090–2103. doi: 10.4049/jimmunol.1701424
111. Harari A, Rozot V, Bellutti Enders F, Perreau M, Stalder JM, Nicod LP, et al. Dominant TNF-α+ Mycobacterium tuberculosis–specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med. (2011) 17:372–6. doi: 10.1038/nm.2299
112. Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'rie T, et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol. (2011) 187:2222–32. doi: 10.4049/jimmunol.1101122
113. Pollock KM, Whitworth HS, Montamat-Sicotte DJ, Grass L, Cooke GS, Kapembwa MS, et al. T-cell immunophenotyping distinguishes active from latent tuberculosis. J Infect Dis. (2013) 208:952–68. doi: 10.1093/infdis/jit265
114. Petruccioli E, Petrone L, Vanini V, Sampaolesi A, Gualano G, Girardi E, et al. IFNγ/TNFα specific-cells and effector memory phenotype associate with active tuberculosis. J Infect. (2013) 66:475–86. doi: 10.1016/j.jinf.2013.02.004
115. Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol. (2009) 39: 723–9. doi: 10.1002/eji.200838693
116. Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, et al. Multifunctional CD4+ T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. (2010) 40:2211–20. doi: 10.1002/eji.201040455
117. Prezzemolo T, Guggino G, La Manna MP, Di Liberto D, Dieli F, Caccamo N. Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis. Front Immunol. (2014) 5:180. doi: 10.3389/fimmu.2014.00180
118. Orlando V, La Manna MP, Goletti D, Palmieri F, Lo Presti E, Joosten SA, et al. Human CD4 T-cells with a naive phenotype produce multiple cytokines during Mycobacterium tuberculosis infection and correlate with active disease. Front Immunol. (2018) 9:1119. doi: 10.3389/fimmu.2018.01119
119. Caccamo N, Joosten SA, Ottenhoff THM, Dieli F. Atypical human effector/memory CD4+ T cells with a naive-like phenotype. Front Immunol. (2018) 9:2832. doi: 10.3389/fimmu.2018.02832
120. Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. (2007) 204:1849–61. doi: 10.1084/jem.20070663
121. Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, et al. Induction and molecular signature of pathogenic Th17 cells. Nat Immunol. (2012) 13:991–9. doi: 10.1038/ni.2416
122. Duhen T, Campbell DJ. IL-1β promotes the differentiation of polyfunctional human CCR6+CXCR3+ Th1/17 cells that are specific for pathogenic and commensal microbes. J Immunol. (2014) 193:120–9. doi: 10.4049/jimmunol.1302734
123. Rakshit S, Adiga V, Nayak S, Sahoo PN, Sharma PK, van Meijgaarden KE, et al. Circulating Mycobacterium tuberculosis DosR latency antigen-specific, polyfunctional, regulatory IL10+ Th17 CD4 T-cells differentiate latent from active tuberculosis. Sci Rep. (2017) 7:11948. doi: 10.1038/s41598-017-10773-5
124. Coulter F, Parrish A, Manning D, Kampmann B, Mendy J, Garand M, et al. IL-17 production from T helper 17, mucosal-associated invariant T, and γδ cells in tuberculosis infection and disease. Front Immunol. (2017) 8:1252. doi: 10.3389/fimmu.2017.01252
125. Green AM, Mattila JT, Bigbee CL, Bongers KS, Lin PL, Flynn JL. CD4(+) regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis infection. J Infect Dis. (2010) 202:533–41. doi: 10.1086/654896
126. Mosmann TR, CoffmanRL. Th1 and Th2 cells: different patterns of lymphockine secretion lead to different functional properties. Annu Rev Immunol. (1989) 7:145–73.
127. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. (2006) 441:235–8. doi: 10.1038/nature04753
128. Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17–producing T helper memory cells. Nat Immunol. (2007) 8:639–46. doi: 10.1038/ni1467
129. Eyerich S, Zielinski CE. Defining Th-cell subsets in a classical and tissue-specific manner: examples from the skin. Eur J Immunol. (2014) 44:3475–83. doi: 10.1002/eji.201444891
130. Lindestam Arlehamn CS, Gerasimova A, Mele F, Henderson R, Swann J, Greenbaum JA, et al. Memory T cells in latent Mycobacterium tuberculosis infection are directed against three antigenic islands and largely contained in a CXCR3+CCR6+ Th1 subset. PLoS Pathog. (2013) 9:e1003130. doi: 10.1371/journal.ppat.1003130
131. Arlehamn CL, Seumois G, Gerasimova A, Huang C, Fu Z, Yue X, et al. Transcriptional profile of tuberculosis antigen-specific T cells reveals novel multifunctional features. J Immunol. (2014) 193:2931–40. doi: 10.4049/jimmunol.1401151
132. Burel JG, Lindestam Arlehamn CS, Khan N, Seumois G, Greenbaum JA, Taplitz R, et al. Transcriptomic analysis of CD4+ T cells reveals novel immune signatures of latent tuberculosis. J Immunol. (2018) 200:3283–90. doi: 10.4049/jimmunol.1800118
133. Strickland N, Müller TL, Berkowitz N, Goliath R, Carrington MN, Wilkinson RJ, et al. Characterization of Mycobacterium tuberculosis-specific cells using MHC class II tetramers reveals phenotypic differences related to HIV infection and tuberculosis disease. J Immunol. (2017) 199:2440–50. doi: 10.4049/jimmunol.1700849
134. Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. (2002) 2:251–62. doi: 10.1038/nri778
135. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. (1999) 401:708–12.
136. Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. (2002) 2:982–7. doi: 10.1038/nri959
137. Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. (2013) 43:2797–809. doi: 10.1002/eji.201343751
138. Gasper DJ, Tejera MM, Suresh M. CD4 T-cell memory generation and maintenance. Crit Rev Immunol. (2014) 34:121–46. doi: 10.1615/critrevimmunol.2014010373
139. Sallusto F, Cassotta A, Hoces D, Foglierini M, Lanzavecchia A. Do memory CD4 T cells keep their cell-type programming: plasticity versus fate commitment? T-cell heterogeneity, plasticity, and selection in humans. Cold Spring Harb Perspect Biol. (2018) 10:a029421. doi: 10.1101/cshperspect.a029421
140. Brummelman J, Pilipow K, Lugli E. Single-cell phenotypic identity of human CD8+ and CD4+ T cells. Int Rev Cell Mol Biol. (2018) 341:63–124. doi: 10.1016/bs.ircmb.2018.05.007
141. Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. (2013) 123:594–9. doi: 10.1172/JCI66327
142. Gattinoni L, Lugli EJIY, Pos Z, Paulos CM, Quigley MF, Almeida J, et al. A human memory T cell subset with stem cell-like properties. Nat Med. (2011) 17:1290–7. doi: 10.1038/nm.2446
143. Riou C, Yassine-Diab B, Van Grevenynghe J, Somogyi R, Greller LD, Gagnon D, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. (2007) 204:79–91. doi: 10.1084/jem.20061681
144. Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. (1997) 186:1407–18.
145. Appay V, Bosio A, Lokan S, Wiencek Y, Biervert C, Küsters D, et al. Sensitive gene expression profiling of human T cell subsets reveals parallel post-thymic differentiation for CD4+ and CD8+ lineages. J Immunol. (2007) 179:7406–14. doi: 10.4049/jimmunol.179.11.7406
146. Appay V. The physiological role of cytotoxic CD4(+) T-cells: the holy grail? Clin Exp Immunol. (2004) 138:10–3. doi: 10.1111/j.1365-2249.2004.02605.x
147. Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27is required for generation and long-term maintenance of T cell immunity. Nat Immunol. (2000) 1:433–40. doi: 10.1038/80877
148. Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. (2003) 198:1369–80. doi: 10.1084/jem.20030916
149. Mpande CAM, Dintwe OB, Musvosvi M, Mabwe S1, Bilek N1, Hatherill M, et al. Functional, antigen-specific stem cell memory (TSCM) CD4+ T cells are induced by human Mycobacterium tuberculosis infection. Front Immunol. (2018) 9:324. doi: 10.3389/fimmu.2018.00324
150. Thomas SY, Banerji A, Medoff BD, Lilly CN, Luster AD. Multiple chemokine receptors, including CCR6 and CXCR3, regulate antigen-induced T cell homing to the human asthmatic airway. J Immunol. (2007) 179:1901–12. doi: 10.4049/jimmunol.179.3.1901
151. Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. (2009) 2:173–83. doi: 10.1038/mi.2008.84
152. Sakai S, Mayer-Barber KD, Barber DL. Defining features of protective CD4 T cell responses to Mycobacterium tuberculosis. Curr Opin Immunol. (2014) 29:137–42. doi: 10.1016/j.coi.2014.06.003
153. Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. (2013) 31:137–61. doi: 10.1146/annurev-immunol-032712-095954
154. Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS ONE. (2011) 6:e16245. doi: 10.1371/journal.pone.0016245
155. Lexberg MH, Taubner A, Albrecht I, Lepenies I, Richter A, Kamradt T, et al. IFN-γ and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur J Immunol. (2010) 40:3017–27. doi: 10.1002/eji.201040539
156. Kurschus FC, Croxford AL, Heinen AP, Wörtge S, Ielo D, Waisman A. Genetic proof for the transient nature of the Th17 phenotype. Eur J Immunol. (2010) 40:3336–46. doi: 10.1002/eji.201040755
157. Wacleche VS, Landay A, Routy J-P, Ancuta P. The Th17 lineage: from barrier surfaces homeostasis to autoimmunity, cancer, and HIV-1 pathogenesis. Viruses. (2017) 9:E303. doi: 10.3390/v9100303
158. Lindenstrøm T, Woodworth J, Dietrich J, Aagaard C, Andersen P, Agger EM. Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect Immun. (2012) 80:3533–44. doi: 10.1128/IAI.00550-12
159. Redford PS, Murray PJ, O'Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. (2011) 4:261–70. doi: 10.1038/mi.2011.7
160. Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. (2015) 264:182–203. doi: 10.1111/imr.12266
161. Ladel CH, Szalay G, Riedel D, Kaufmann SHE. Interleukin-12 secretion by Mycobacterium tuberculosis-infected macrophages. Infect Immun. (1997) 65:1936–8.
162. Sutherland JS, de Jong BC, Jeffries DJ, Adetifa IM, Ota MOC. Production of TNF-α, IL-12(p40) and IL-17 can discriminate between active TB Disease and latent infection in a West African cohort. PLoS ONE. (2010) 5:e12365. doi: 10.1371/journal.pone.0012365
Keywords: tuberculosis, Th1, Th17, non-classical Th1, correlates of protection
Citation: Lyadova I and Nikitina I (2019) Cell Differentiation Degree as a Factor Determining the Role for Different T-Helper Populations in Tuberculosis Protection. Front. Immunol. 10:972. doi: 10.3389/fimmu.2019.00972
Received: 24 December 2018; Accepted: 16 April 2019;
Published: 08 May 2019.
Edited by:
Christoph Hölscher, Forschungszentrum Borstel (LG), GermanyReviewed by:
Cheryl L. Day, Emory University, United StatesNadia Caccamo, University of Palermo, Italy
Copyright © 2019 Lyadova and Nikitina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Irina Lyadova, aXZseWFkb3ZhQG1haWwucnU=
 Irina Lyadova
Irina Lyadova Irina Nikitina
Irina Nikitina