- 1Departments of Medicine and Cellular and Molecular Medicine, University of California, San Diego, La Jolla, CA, United States
- 2Glycobiology Research and Training Center, University of California, San Diego, La Jolla, CA, United States
The description of “serum sickness” more than a century ago in humans transfused with animal sera eventually led to identification of a class of human antibodies directed against glycans terminating in the common mammalian sialic acid N-Glycolylneuraminic acid (Neu5Gc), hereafter called “Neu5Gc-glycans.” The detection of such glycans in malignant and fetal human tissues initially raised the possibility that it was an oncofetal antigen. However, “serum sickness” antibodies were also noted in various human disease states. These findings spurred further research on Neu5Gc, and the discovery that it is not synthesized in the human body due to a human-lineage specific genetic mutation in the enzyme CMAH. However, with more sensitive techniques Neu5Gc-glycans were detected in smaller quantities on certain human cell types, particularly epithelia and endothelia. The likely explanation is metabolic incorporation of Neu5Gc from dietary sources, especially red meat of mammalian origin. This incorporated Neu5Gc on glycans appears to be the first example of a “xeno-autoantigen,” against which varying levels of “xeno-autoantibodies” are present in all humans. The resulting chronic inflammation or “xenosialitis” may have important implications in human health and disease, especially in conditions known to be aggravated by consumption of red meat. In this review, we will cover the early history of the discovery of “serum sickness” antibodies, the subsequent recognition that they were partly directed against Neu5Gc-glycans, the discovery of the genetic defect eliminating Neu5Gc production in humans, and the later recognition that this was not an oncofetal antigen but the first example of a “xeno-autoantigen.” Further, we will present comments about implications for disease risks associated with red meat consumption such as cancer and atherosclerosis. We will also mention the potential utility of these anti-Neu5Gc-glycan antibodies in cancer immunotherapy and provide some suggestions and perspectives for the future. Other reviews in this special issue cover many other aspects of this unusual pathological process, for which there appears to be no other described precedent.
First Reports of “Serum Sickness” in Humans Infused With Animal Serum
Following the discovery of the effectiveness of tetanus and diphtheria antitoxins by Emil von Behring and Shibasaburo Kitasato, the popularity of serotherapy soared in the 1880s and 1890s (1). However, reports of reactions to the diphtheria antitoxin also started to appear. In 1899, Bolton reported 100 cases of reactions to the diphtheria antitoxin (2). Pirquet and Schick suggested the use of the phrase “serum sickness” in their book Die Serumkrankheit (3) recognizing that the reactions were against animal serum components present in the antitoxin preparations.
“Serum Sickness” Patients Have “H-D” Antibodies, Some of Which Recognize Neu5Gc-Containing Glycans Found in Human Cancers
The Initial Definition of “H-D” Antibodies
Two decades later, Hanganutziu and Deicher independently described human antibodies that agglutinated animal erythrocytes (4, 5). These Hanganutziu-Deicher antibodies (H-D antibodies) were prominent in subjects with serum sickness who had received therapeutic animal antisera. Subsequently, similar antibodies were reported in patients with no prior exposure to animal sera but instead suffering from other diseases (6).
A Portion of H-D Antibodies Are Directed Against Neu5Gc-Containing Glycans, but HD Antigens Can Also Be Present in Diseased Human Tissues
About 50 years later, two groups independently showed that a portion of these heterophile H-D antibodies recognized gangliosides containing the sialic acid N-Glycolylneuraminic acid (Neu5Gc) (7, 8). This sialic acid was later shown to be derived from the common mammalian sialic acid N-Acetylneuraminic acid (Neu5Ac) by the addition of a single oxygen atom that is added to CMP-Neu5Ac in a complex cytosolic reaction catalyzed by the enzyme cytidine monophosphate N-acetylneuraminic acid hydroxylase (Cmah) (9–13). The definition of H-D antibodies sparked further research and these were then detected in the sera of patients with multiple pathological conditions, including rheumatoid arthritis, infectious mononucleosis, leprosy, syphilis, leukemia, Kawasaki disease (a disease that causes inflamed blood vessels), and various cancers (14–24).
Generation of H-D Antibodies in Chickens, Confirming H-D Antigens in Human Cancers
Early on, it was also noted that anti-H-D serum of high titer could be generated in chickens immunized with H-D antigen-active glycosphingolipid, N-Glycolylneuraminyl-lactosylceramide (purified from equine erythrocytes) (18, 25). Immunohistochemistry or thin-layer chromatography using these polyclonal antibodies as well as indirect methods such as inhibition of bovine erythrocyte agglutination by human H-D antiserum were then used to confirm the presence of Neu5Gc-glycans in meconium and multiple human tumors (14–24). Paradoxically, the H-D antigens or Neu5Gc-glycans were also found on human tissue gangliosides and glycoproteins (18, 25–35). Much later, work from our group resulted in further affinity purification of such chicken polyclonal antibodies (36) (during the process we have noted that the bovine serum albumin preparation originally used as a “carrier” for the immunogen is contaminated with bovine serum glycoproteins bearing Neu5Gc-glycans, which also contribute importantly to the immune response in chickens). These preparations were used as a valuable tool for the detection of smaller amounts of Neu5Gc-glycans present even in normal human tissues (36, 37), particularly on epithelia lining hollow organs (the origin of carcinomas), and on endothelia (where atherosclerotic cardiovascular disease occurs).
Humans Cannot Synthesize Neu5Gc
Humans Are Genetically Deficient in CMAH, the Primary Enzyme That Generates Neu5Gc
These findings inspired further work on CMAH, and the discovery of an inactivating mutation that likely got fixed in the human lineage >2 million years ago. All humans were found to be homozygous for a deletion of exon 6 in the CMAH gene (38, 39) and this deletion was later shown to have been mediated by a single Alu-Alu fusion event (40). While the first published report incorrectly claimed that the mutation resulted in an altered reading frame and a large non-functional fusion protein (38), the second report the same year (41) showed that it actually results in a greatly truncated form of the enzyme. Comparisons with our closest living evolutionary relatives (42) indicated that this mutation occurred after our common ancestry with these “great apes” (Figure 1).
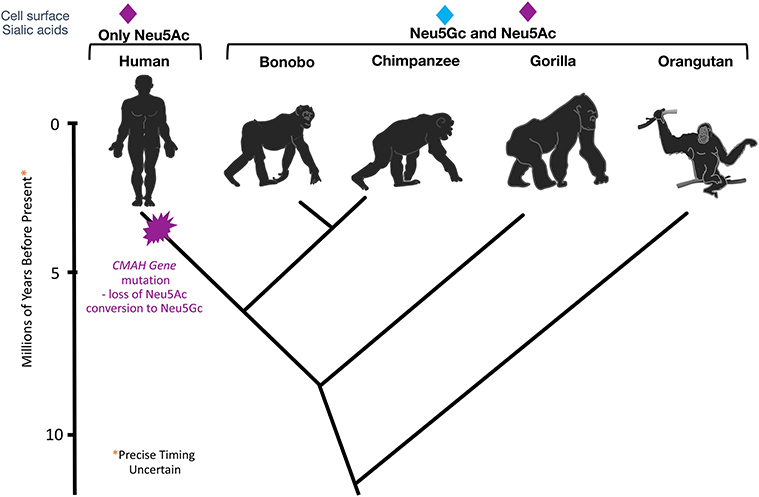
Figure 1. Evolutionary Loss of CMAH. Multiple methods of analysis indicate that the CMAH mutation occurred about 2–3 mya after the divergence from the Pan group.
Possible Selection Mechanisms for the Initial Hominin Mutation in CMAH
Whether this mutation got fixed in the human lineage as a result of positive or negative selection is still a matter of speculation. A pandemic caused by a lethal infectious pathogen that preferred to bind to Neu5Gc leading to negative selection is one possible explanation (43). Another mutually non-exclusive possibility is selective fertility of Neu5Gc-deficient females with Neu5Gc-deficient males, leading to positive selection of this genotype (44). This so-called “cryptic female choice” theory (44) is pictorially depicted in Figure 2 (The figure legend details this theory) (45).
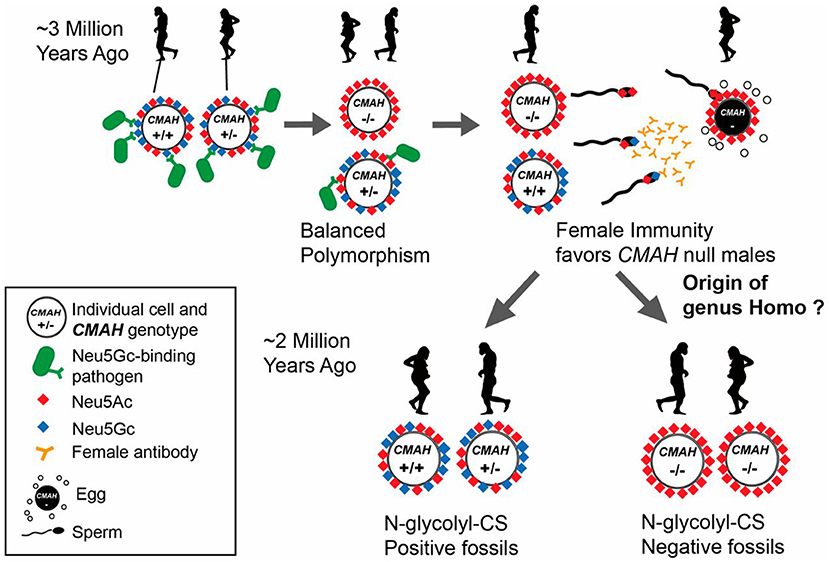
Figure 2. Potential scenario for the role of Neu5Gc loss and female anti-Neu5Gc immunity in the origin of the genus Homo via interplay of natural and sexual selection acting on cell-surface Sias. There are many known pathogens that recognize and exploit Neu5Gc (blue diamond) as a receptor on host target cells. Natural selection by such pathogens may have selected for rare CMAH null alleles that abolish Neu5Gc expression in homozygote individuals. Such individuals have only Neu5Ac and its derivatives on their cells (red diamonds) allowing an escape from pathogens, but at higher frequencies would be targeted by adapting pathogens, resulting in maintenance of a balanced polymorphism. CMAH−/− females with anti-Neu5Gc antibodies also present in their reproductive tract would favor sperm from CMAH−/− males due to anti-Neu5Gc antibody-mediated cryptic selection against CMAH+/− or CMAH+/+ males expressing Neu5Gc on their sperm. Once the frequency of the CMAH null allele reaches a critical level, this process can drive fixation of the loss-of-function allele in a population by directional selection. Figure and figure legend reproduced from Bergfeld et al. (45).
This mechanism was demonstrated in human-like Cmah null mice (44, 46). On the other hand, a random CMAH mutation may simply have become fixed in a small group of individuals who eventually gave rise to modern humans. Regardless, this inactivation of CMAH lead to drastic changes in the sialoglycome that likely pre-dated the origin of the genus Homo (44). Given that Neu5Gc has been found in multiple species of the deuterostome lineage ranging from sea urchins to non-human primates, CMAH is at least 500 million years old (47). Interestingly, Neu5Gc was independently lost in multiple lineages including sauropsids (birds and reptiles), monotremes (platypus) and certain other lineages (47, 48). More details about the evolutionary implications of Neu5Gc and anti-Neu5Gc glycan antibodies have been covered by P. Gagneux in another review in this special issue.
Humans Express Dietary-Derived Neu5Gc on Their Cell Surfaces
Neu5Gc-Glycans Are Present in Smaller Amounts in Normal Human Epithelia and Endothelia
Apart from onco-fetal human tissue, very small amounts of Neu5Gc-glycans were surprisingly also found to be incorporated in normal human secretory epithelia and small and large vessel endothelia (36, 37, 49) (Figure 3). Concurrent mass-spectrometric studies of purified sialic acids confirmed the presence of Neu5Gc (49) and in N-glycans released from tumor samples (50).
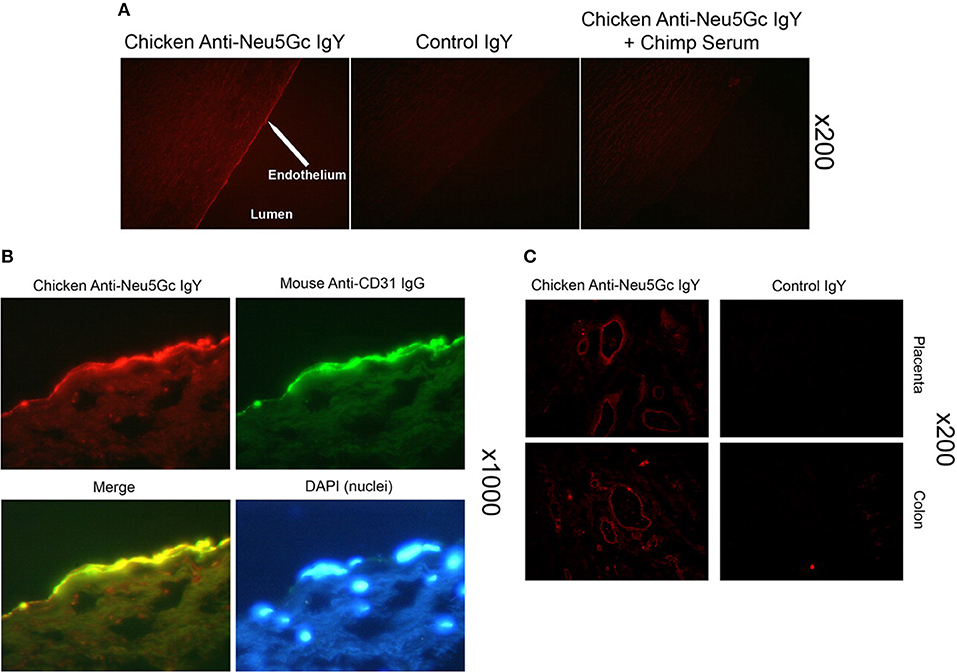
Figure 3. Detection of Neu5Gc in aortic endothelium of human autopsy samples and microvasculature of colon and placenta. The chicken anti-Neu5Gc antibody (cGcAb) was used to detect the presence of Neu5Gc on the endothelium of autopsy samples of normal-appearing human aorta. Typical representatives of 8 autopsy samples studied are shown. The red Cy3 fluorescence represents labeling of endothelial cells of the aorta. (A) Specificity of the antibody was demonstrated by the lack of signal with the non-immunized control chicken IgY (middle) and the abrogation of signal by adsorption with Neu5Gc-rich glycoproteins of chimpanzee serum (right). Magnification ×200. (B) Sections were double-stained with anti-CD31 for endothelial cells and counterstained with DAPI to visualize nuclei (magnification ×1000). (C) Sections of placenta (top) and colon (bottom) stain for Neu5Gc along microvasculature endothelial lining with the use of cGcAb. Control IgY (right) demonstrates specificity of signal (magnification ×200). Figure and figure legend reproduced from Pham et al. (37).
Neu5Gc-Glycans in CMAH Null Humans and Mice Are Exclusively Derived From Food Sources
Although human cells cultured in FCS have been reported to express Neu5Gc-glycans (42, 51) this appears to be due to metabolic incorporation or passive adsorption of glycoconjugates. So far it seems that the only source of exogenous Neu5Gc in human and humanized Cmah null mice is via dietary intake (49, 50, 52, 53) Sialic acids have never been detected in plants and are found in large amounts primarily in vertebrates and a few “higher” invertebrates as well as in some insects (54–58). The occurrence of Neu5Gc in poultry and fish is rare but common in some milk products and greatly enriched in red meats (49, 53, 59, 60).
Red Meat as the Primary Dietary Source of Neu5Gc–The First Example of a “Xenoautoantigen”
With no other explanation for the presence of Neu5Gc-glycans in human tissues as confirmed in the mouse model, it was concluded that humans incorporate Neu5Gc from dietary sources. Studies using a DMB-HPLC assay to detect Neu5Gc showed its enrichment in beef, pork and lamb (53). Additionally, all humans produce anti-Neu5Gc glycan antibodies in varying titers (61). In light of these antibodies that likely bind to any incorporated Neu5Gc-glycans, this is the first example of a “xenoautoantigen.” This state, with both the presence of Neu5Gc-glycans as well as the corresponding anti-Neu5Gc glycan antibodies has been called “Xenosialitis” and likely plays a role in multiple human pathologies, as elaborated in later sections of this review.
Mechanisms of Neu5Gc Uptake and Incorporation Into Human Tissues and Cells
When human volunteers ingested free Neu5Gc, it was shown to be largely excreted in the urine (49). Extended feeding of Cmah null mice with free Neu5Gc in drinking water also did not result in efficient tissue incorporation except in a malignant tumor (52). In contrast, feeding of glycosidically-bound Neu5Gc attached to porcine mucins gave low-level incorporation into normal tissues over a period of weeks (62). While it has been previously shown that N-glycolylmannosamine a degradation product of Neu5Gc which may more easily be taken up than the parental sialic acid (63), the exact mechanism by which bound Neu5Gc from the diet results in metabolic incorporation is not known and requires further investigation.
In contrast, human epithelial cells in culture can metabolically incorporate free or bound Neu5Gc and express it into endogenous glycoproteins (64) (Figure 4). The mechanism of uptake and incorporation of the Neu5Gc into human epithelial cells (derived from a primary colon carcinoma), fibroblast, and neuroblastoma cells was shown to be dependent on non-clathrin-mediated pinocytic pathways (64). Free Neu5Gc taken up by pinocytosis, or bound Neu5Gc released by a lysosomal sialidase, can then be exported to the cytosol by the lysosomal sialic acid transporter. Activation of the resulting cytosolic free Neu5Gc by the CMP-sialic acid synthase then generates the donor for incorporation into glycoconjugates in the Golgi apparatus, on newly synthesized glcoconjugates. The reason why free Neu5Gc gives incorporation in cultured cells but not in the intact organism is because of the rapid clearance by the kidney in the latter situation. The difference between free and bound Neu5Gc is also relevant to recognition by antibodies which can only interact with the latter. Moreover, the typical antibody binding site can accommodate glycan chains of 4–6 monosacharride (66). Antibodies typically cannot efficiently recognize just a terminal Neu5Gc even when glycosidically bound. For this reason, many studies that have utilized simple alpha-linked Neu5Gc as a target in ELISA assays grossly underestimate the amount and complexity of anti-Neu5Gc glycan antibody response (67). Hereafter, we therefore refer to antibodies against glycosidically-bound Neu5Gc as “anti-Neu5Gc-glycan antibodies” which are diverse and complex because of the underlying glycans.
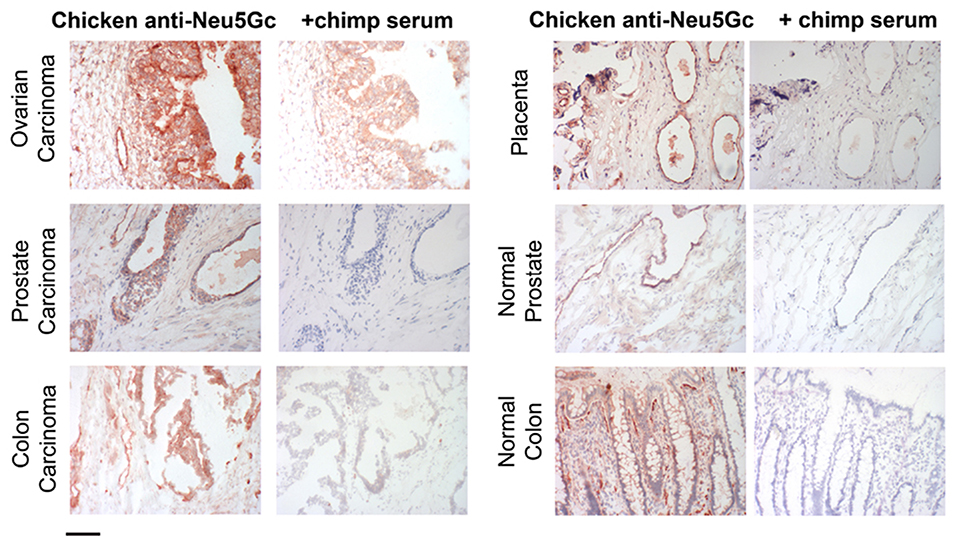
Figure 4. Examples of incorporation of Neu5Gc in malignant and healthy human tissue. Expression of Neu5Gc is observed to be enhanced in malignant epithelia as seen here in carcinomas of the ovary, prostate and colon (left panel). In contrast, expression of Neu5Gc in normal tissue is seen in the ducts of the prostate gland and in the epithelial lining of the colon (Right panel). Endothelial cells of the normal placenta are used here as a positive control for Neu5Gc immunostaining. As a negative control, the binding is blocked competitively with Neu5Gc-containing chimpanzee serum. Magnification used was 200× and scale bar is 100 μm. Figure and figure legend reproduced from Samraj et al. (65).
Metabolic Fate of Neu5Gc
As the reaction catalyzed by Cmah is irreversible, all mammalian cells must have pathways to adjust cellular Neu5Gc levels to their needs to avoid continued accumulation. We discovered a metabolic pathway for the turnover of exogenous Neu5Gc in human cells (68). It was shown that cytosolic extracts harbor the enzymatic machinery to sequentially convert Neu5Gc into N-glycolylmannosamine, N-glycolylglucosamine, and N-glycolylglucosamine 6-phosphate, whereupon irreversible de-N-glycolylation of the latter results in the ubiquitous metabolites glycolate, and glucosamine 6-phosphate. Later, it was shown that metabolic turnover of the dietary Neu5Gc in humans and Cmah null mice modifies chondroitin sulfate and this stable N-Glycolyl chondroitin sulfate (Gc-CS) survives even in ancient fossils (45). This discovery opened a door for “ancient glycomics” and could help in tracking early human lineages and their food habits. Additionally, we are working on developing a simplified assay to measure levels of Gc-CS in serum to predict red meat-related incorporation.
Parallel studies of the P. falciparum malarial protein VAR2CSA that mediates parasite attachment to the placental trophoblast led to discovery of the target “oncofetal chondroitin sulfate” (ofCS) which is not detected in normal tissues, but is shared by many types of cancers and can be detected using recombinant VAR2CSA(rVAR2) (69–72). As this pattern is similar to that of Neu5Gc-glycans in placental and tumor tissue, it was natural to suspect that it might be related to Gc-CS. However, this matter requires further investigation.
Humans Also Have Anti-Neu5Gc Antibodies
All Humans Have Circulating Anti-Neu5Gc-Glycan Antibodies
All human adults have varying levels of circulating IgM, IgG, and IgA antibodies against Neu5Gc-glycans (49, 61, 73–75). Human anti-Neu5Gc glycan antibodies interact with metabolically incorporated Neu5Gc to promote chronic inflammation, likely contributing to tumor inflammation and cancer progression (50, 53) and vascular inflammation (37).
Origin of Human Anti-Neu5Gc-Glycan Antibodies
Our group later showed that human anti-Neu5Gc glycan antibodies appear during the first year of life and correlate with the introduction of Neu5Gc in the diet (76). Sera from infants aged 0–12 months were analyzed, and anti-Neu5Gc IgM and IgG antibodies against Neu5Gcα2-6Lac started to appear at the time these infants were weaned on to cow's milk-based formula. Interestingly, anti-Neu5Gc IgM antibodies were absent at birth and at 3 months, appeared at 6 months and the levels stabilized at 12 months. There was no difference in anti-Neu5Gc IgM and IgG titers between male and female subjects. The absence of anti-Neu5Gc IgM antibodies in cord blood sera suggests that anti-Neu5Gc antibodies are not germ-line encoded “natural” antibodies (77) that occur naturally in human and other mammals, but instead require a postnatal antigenic stimulus. Anti-Neu5Gc antibodies are likely to be affinity matured antibodies as has been shown earlier (78). However, spontaneous generation of anti-Neu5Gc IgM or IgG antibodies in Cmah null mice did not occur even when large quantities of Neu5Gc were fed to them. This is despite the presence of relatively hyper-reactive B cells, apparently caused by the loss of Neu5Gc-containing Siglec ligands (79, 80). On the other hand, deliberate immunization with an artificial immunogen rich in Neu5Gc, such as chimpanzee RBCs, and complete Freund's adjuvant, did elicit anti-Neu5Gc IgM, and IgG antibodies in Cmah null, but not in wild type mice (50, 75).
N-Glycolyl Groups Are Rare in Nature, Increasing the Likelihood of Antigenicity
N-acetyl groups are common in nature (PubMed search of “N-Acetyl” gives >30,000 citations), often originating from the donor acetyl-CoA. In contrast, a search of “N-Glycolyl” gives ~270 citations, which are either about Neu5Gc or about N-Glycolylmuramic acid, found in certain bacterial peptidoglycans (81–86). The CMAH gene is a distant homolog of prokaryotic genes generating UDP-N-glycolylmuramic for peptidoglycan biosynthesis (82, 83). In both instances, a mono-oxygenase reaction is involved. It is unclear why glycolyl-CoA formed during fatty acid beta-oxidation (87, 88) is never utilized to make N-glycolyl groups. Regardless, the rarity of this modification makes it more likely to be antigenic. N-glycolylmuramic acid occurs in Freund's adjuvant (which has mycobacterial products), which we use to immunize Cmah null mice against Neu5Gc-glycans, but we do not observe anti-Neu5Gc Abs in mice given only adjuvant.
Markedly Different Antigenicity of Glycosidically-Bound vs. Free Neu5Gc and Impact of Underlying Glycans
As was touched upon earlier, the difference between free and bound Neu5Gc is also relevant to Ab recognition, which can only interact with the latter. Moreover, since the typical Ab binding site accommodates 4 to 6 monosaccharides (66, 89, 90), Neu5Gc-dependent Abs cannot efficiently recognize a terminal glycosidically-bound Neu5Gc by itself. Thus, studies that utilized simple alpha-linked Neu5Gc as a target in assays (67, 91–95) grossly underestimate the complexity of the human anti-Neu5Gc Abs, which are diverse and complex, because of variations in underlying glycans (61, 96, 97). Recently, it has also been shown that the presentation mode of Neu5Gc-containing glycans in various assays affects recognition by anti-Neu5Gc glycan IgGs (98).
Possible Mechanism of “Xenoauto-Immunization” by Microbes Like Haemophilus influenzae
While humans develop antibodies against Neu5Gc-containing glycans during infancy, the mechanism of immunization is still unclear. One possible explanation is “xeno-autoimmunization” by microbes such as H. influenzae, that normally colonize humans. Non-typeable H. influenzae (NTHi) like all other known microbes cannot synthesize Neu5Gc but has been shown to be able to incorporate trace amounts of free sialic acids into its cell-wall LPS (99). Also, anti-Neu5Gc antibodies appear in infants around the same time as antibodies against NTHi (76). One likely source of Neu5Gc for these microbes is foods of mammalian origin used for weaning. Indeed, NTHi was shown to be able to incorporate Neu5Gc from baby foods (76).
A Parallel but Inconsistent Literature About Anti-tumor MAbs Against (Neu5Gc)GM3
An extensive literature originating primarily from one group (100–113) claims that a Neu5Gc-version of ganglioside GM3 (Siaα2-3Galβ1-4Glcβ1-1'-Ceramide) is tumor-specific, and cancer vaccines and MAbs (idiotypic and anti-idiotypic) targeted against it are even in clinical trials (114, 115). Until recently this group assumed that expression was unrelated to dietary intake, and that the antigen is absent from normal cells. Moreover, a collaborating group recently suggested that hypoxia induces de novo synthesis of (Neu5Gc)GM3 in human cells through a poorly defined “CMAH domain substitute” (116). However, hypoxia also increases uptake and incorporation of Neu5Gc, and fetal calf serum contains Neu5Gc. Once human cancer cells are placed in Neu5Gc-free human serum, for several passages, we find that all traces of Neu5Gc disappear. Moreover, our broad-spectrum polyclonal monospecific chicken anti-Neu5Gc Ab cannot detect any Neu5Gc in Cmah null mice on a Neu5Gc-free diet. Further confusion arises because the original group also uses these antibodies to treat tumors in Cmah wild type mice (107), which already have a large amount of endogenously synthesized Neu5Gc-GM3. This is also true of preclinical toxicity studies done in CMAH-positive monkeys (117). We may be misunderstanding something about this body of work, but our present assumption is that the tumor-associated (Neu5Gc)GM3 being targeted arises from dietary Neu5Gc. Alternatively, the actual epitope may be different. Regardless of the final resolution, it does not change the basic underlying hypothesis driving our current work, on red meat-derived Neu5Gc-induced “xenosialitis.”
Anti-Neu5Gc Antibodies in Disease States
As alluded to earlier, anti-Neu5Gc antibodies have been described in a multitude of diseases. Anti-Neu5Gc antibodies have broad implications in transplantation (93, 118–125) which will be covered in a separate review in this special issue. While transplantation can be associated with high levels of anti-Neu5Gc-glycan antibodies due to ATG serum therapy and/or the xenotransplant itself, these are very unusual clinical states with associated immunosuppression and other pathologies. Also of note, the phenomenon of “hormesis” has been documented with these antibodies, with very highly levels having the opposite effects e.g., killing of tumors (126, 127). In this review, we will focus on a possible role of moderate levels of the antibodies in two diseases that otherwise normal humans are particularly prone to develop: epithelial cancers (carcinomas) and atherosclerosis (Figure 5).
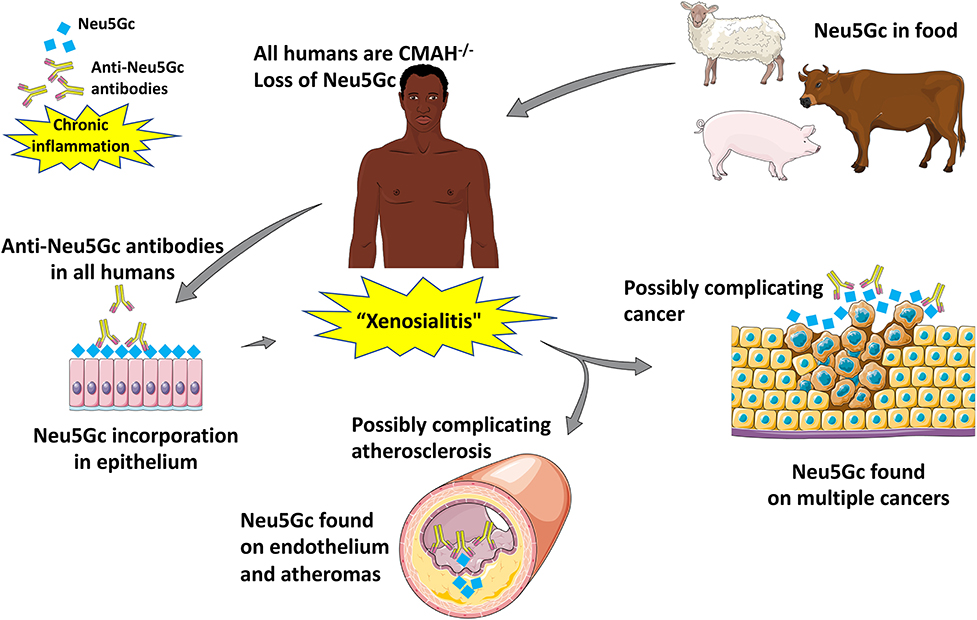
Figure 5. Suggested pathological implications of Neu5Gc consumption, accumulation, and subsequent inflammation in atherosclerosis and cancer [Image created with objects sourced from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 3.0 Generic License].
Carcinomas
Accumulation of Neu5Gc-glycans has been detected in human tumors such as breast, colon, ovary, and prostate carcinomas (49, 65, 128, 129). Distinctly, red meat is enriched with bound forms of the Neu5Gc. Numerous epidemiological studies concluded that consumption of red meat is associated with atherosclerotic cardiovascular diseases and an increased risk of cancer (130, 131). Recent findings involving the Health Professionals Follow-up Study and the Nurses' Health Study cohorts confirmed that a higher intake of red meat (specifically processed red meat products) was associated with a significantly elevated risk of cancer, prominently colorectal cancer (132). The epidemiological data ruled out alternate factors such as (a) high-fat intake (133); (b) the production of heterocyclic amines and polycyclic aromatic hydrocarbons (134); (c) the presence of mutagenic N-nitroso compounds (135), that were once believed to be the major promoter of carcinogenesis. Our laboratory has shown that the Neu5Gc and anti-Neu5Gc-glycan antibody interaction induced “xenosialitis” may promote chronic inflammation leading to cancer progression (53).
Another possibly related carcinogenic mechanism arising from red meat was revealed by the isolation of a number of small DNAs obviously derived from specific plasmids of Acinetobacter bacteria from commercially available cow milk samples by de Villiers and zur Hausen (136–138). These authors suggest that such infections with autonomously replicating plasmids early in life are risk factors for human colon and breast cancers several decades later (139), that incorporated Neu5Gc from dietary sources might provide receptors for the viruses, and that antibodies against these viral proteins may work in concert with Neu5Gc-induced “xenosialitis.”
As has been shown earlier, inflammation and associated activation of the immune system can promote carcinogenesis (inflammation-induced cancer) and cancer progression (140–142). The seminal review on the hallmarks of cancer by Hanahan and Weinberg also mentions tumor-promoting inflammation as one of the enabling factors of cancer (143). Moreover, growing tumors induce an inflammatory response that can support cancer progression (cancer-related inflammation) (140, 144). Chronic inflammation in auto-inflammatory diseases and diet-induced metabolic syndrome is also an important etiological factor for the development of cancer (142, 145). Hence it is not surprising that red meat consumption and the “Western diet” have often been associated with increased circulating markers of inflammation in human population studies (146). Cell surface glycosylation is heavily altered in cancer cells, as seen in malignant tissue that incorporate Neu5Gc (62, 64, 147). Thus, anti-Neu5Gc antibodies likely support cancer progression by enhancing tumor-related inflammation via induction of “xenosialitis” in the humanized mouse model (Cmah−/−) (53, 148, 149). A recent study showed that there is no increase in colon cancer risk following anti-Neu5Gc antibody induction with Neu5Gc-bearing rabbit anti-T cell IgG (ATG) in recipients of kidney (150). However, there was no estimation regarding red meat intake in this study and patients with renal failure are typically advised to reduce meat intake. Furthermore, some such patients are also under immunosuppression, which would alter outcomes*.
Sialoglycan microarray studies enabled us to differentiate between controls and patients with various carcinomas including prostate, ovary, endometrium, colon, lung, and pancreas with regard to antibodies against Neu5Gc-Sialyl-Tn (96). A recent nested case-control study from our laboratory assessed the association between total anti-Neu5Gc antibodies and the risk of colorectal cancer (CRC) in the Nurses' Health Study cohort. This study showed that the sum total of polyclonal anti-Neu5Gc glycan antibodies were associated with CRC risk (97).
Atherosclerosis
Myocardial infarctions (MIs), ischemic heart disease, strokes and peripheral vascular disease in humans are primarily caused by atherosclerotic cardiovascular disease (CVD) (151). Chimpanzees, our closest evolutionary cousins, on the other hand suffer from “heart attacks” as a result of idiopathic interstitial myocardial fibrosis (152). Additionally, captive chimps do not get human-like MIs despite major risk factors such as dyslipidemia and hypertension (152). There is a clear association between consumption of red meats and processed meats with increased risk of CVD in humans (131, 153). While multiple theories for this association have been put forward including cholesterol and saturated fat (154), conversion of choline and carnitine into proatherogenic Trimethylamine N-oxide (TMAO) (155–157), and oxidative damage due to heme iron (158–161), these mechanisms appear not to be specific for red meats as explained in an earlier review from our laboratory (162). “Xenosialitis,” unlike these theories, is specific to red meats and may contribute to the uniquely human severity of complications of atherosclerosis. Earlier studies from our lab have shown that Neu5Gc can be detected in the endothelium overlying the atherosclerotic plaque as well as the sub-endothelium (37). Further, human endothelial cells fed with Neu5Gc and subsequently exposed to serum containing anti-Neu5Gc glycan antibodies led to IgG and complement deposition which in turn led to increased endothelial activation, increased cytokine production, and selectin expression, events associated with early atherogenesis. These effects were inhibited by Neu5Gc-alpha-methyl glycoside, a specific competitor to anti-Neu5Gc antibodies. Cmah−/− mice also showed Neu5Gc accumulation in their endothelium when fed with Neu5Gc (62). We are currently studying Cmah−/− mice bred into a low-density lipoprotein knockout (Ldlr−/−) background fed with Neu5Gc and immunized with Neu5Gc bearing antigens to see if they have a higher risk of developing atherosclerosis as compared to controls fed Neu5Ac. Large human cohort studies are also necessary to confirm the role of anti-Neu5Gc antibodies in CVD.
Clinical Application of Anti-Neu5Gc Glycan Antibodies
Possible Therapeutic Role of Neu5Gc-Antigens and Anti-Neu5Gc Antibodies
Despite the possible pathogenic effects of these antibodies as described above, anti-Neu5Gc antibodies may also be potentially utilized as anti-cancer immunotherapeutic agents. Tumor cells are aberrantly sialylated and the content of sialic acid on these cells goes up markedly when compared to cells of healthy tissue (163, 164). This upregulation may explain why ingested Neu5Gc preferentially accumulates in cancer tissue (49, 62). There is also an upregulation of sialyl-Tn antigen (165–169), an epitope not commonly found (165, 170, 171) or “hidden” by O-acetylation of sialic acid (166) in healthy human tissue. Recent findings also show the presence of Sialyl-Tn in stem-like cells in cancer cell lines (172) and therapeutic benefits of antibodies that target these epitopes in patient-derived xenograft models of Ovarian carcinoma (173). If Neu5Gc-Sialyl-Tn is found to be relatively cancer specific, it may be used to image or even treat cancers. Indeed, in vitro assays have shown that human antibodies against Neu5Gc-Tn antigen purified from IVIG activate antibody-dependent cellular and complement-dependent cytotoxicity (ADCC and CDC) (96).
Another approach that has been tried is vaccination with (Neu5Gc)GM3 along with outer membrane protein complex of Neisseria meningitidis in proteoliposomes leading to antibody production in advanced stage breast cancer patients in a phase I study (174). A mouse-monoclonal antibody directed against (Neu5Gc)GM3, 14F7 was isolated (129) and further, has been humanized (175). 1E10, the corresponding anti-idiotype to 14F7, named racotumomab has also been tried in humans (176) and also shown to have non-apoptototic cytoxic effects in vitro (177). This antibody is able to bind to multiple malignant tissues including skin cancers, neuroectodermal tumors, genitourinary cancer, non-small cell lung cancer, and gastrointestinal tumors (178–182) and multiple human trials have also been conducted (e.g., NCT01598454, NCT01460472, NCT02998983, NCT01240447). However, as mentioned earlier, these studies do not make any direct link to dietary Neu5Gc, and the antibodies are reported to work even in Cmah wild-type mice, which have a vast excess of Neu5Gc antigens on normal tissues.
Despite all these efforts to develop effective immunotherapeutics, no efforts have been taken to control Neu5Gc consumption in cancer patients. Notably, if cancer patients are encouraged to reduce Neu5Gc consumption, a “washout” of Neu5Gc may occur in normal tissue. Following this, IV Neu5Gc may be used to “feed” tumors followed by an antibody that recognizes Neu5Gc-containing epitopes to now “find” the tumor. “Feeding” tumors is possible as Neu5Gc preferentially accumulates in malignant tissue due to increased micropinocytosis (64), rapid growth rates and hypoxic upregulation of the sialin transporter (147). This “feed-and-find” approach may turn out to be more effective than the present approaches. Additionally, monoclonal antibodies targeting Neu5Gc-containing glycans may be tested on an advanced sialoglycan microarray (183) and coupled with a newly developed computational methods (184) to confirm specificity.
Importantly, Neu5Gc has also been found in cancer therapeutic agents. Monoclonal antibodies such as trastuzumab, cetuximab and rituximab are integrated in today's cancer therapies (185). Glycosylation of these antibodies may involve Neu5Gc-rich media and/or mammalian cells that express Neu5Gc (186). Our laboratory has previously shown that incorporation of Neu5Gc in cetuximab enhanced the formation of immune complexes promoting drug clearance (187). Avoidance of Neu5Gc during production of glycoproteins may improve half-life of these antibodies while also reducing their immunogenicity.
Biomarkers in Pathological States
Anti-Neu5Gc glycan antibodies could serve as potential biomarkers for diseases associated with red meat consumption including carcinomas, atherosclerosis, and type 2 diabetes (188–192). Current biomarkers for cancer lack sufficient sensitivity and importantly the specificity for early diagnosis (193, 194). Although antibodies against tumor-associated antigens are commonly found in cancer patients at an early stage and could potentially be sensitive detectors for malignant transformation (195, 196), none of the previously described autoantibodies show sufficient specificity in screening. Given the incorporation and display of Neu5Gc by tumor cells, the detection of Neu5Gc body-burden and antibody response together might serve as a potential biomarker for early carcinoma detection. It has been demonstrated that comparison of anti-Neu5Gc antibody levels can be used to differentiate between controls and patients with various carcinomas (96, 97). Increased anti-Neu5Gc antibody levels were also found in patients with Kawasaki disease (197).
Conclusions and Perspectives
In this review, we have discussed important milestones from the early description of “Serum-sickness” as being due to antibodies directed against Neu5Gc epitopes all the way to the present-day therapeutic implications of these antibodies in cancer therapy. Some of these milestones have been represented in a concise timeline (Figure 6). While the “Xenosialitis” hypothesis is well-supported in the human-like mouse models, it has yet to be conclusively proven in humans. It remains to be seen if “Xenosialitis” plays a role in other uniquely-human diseases.
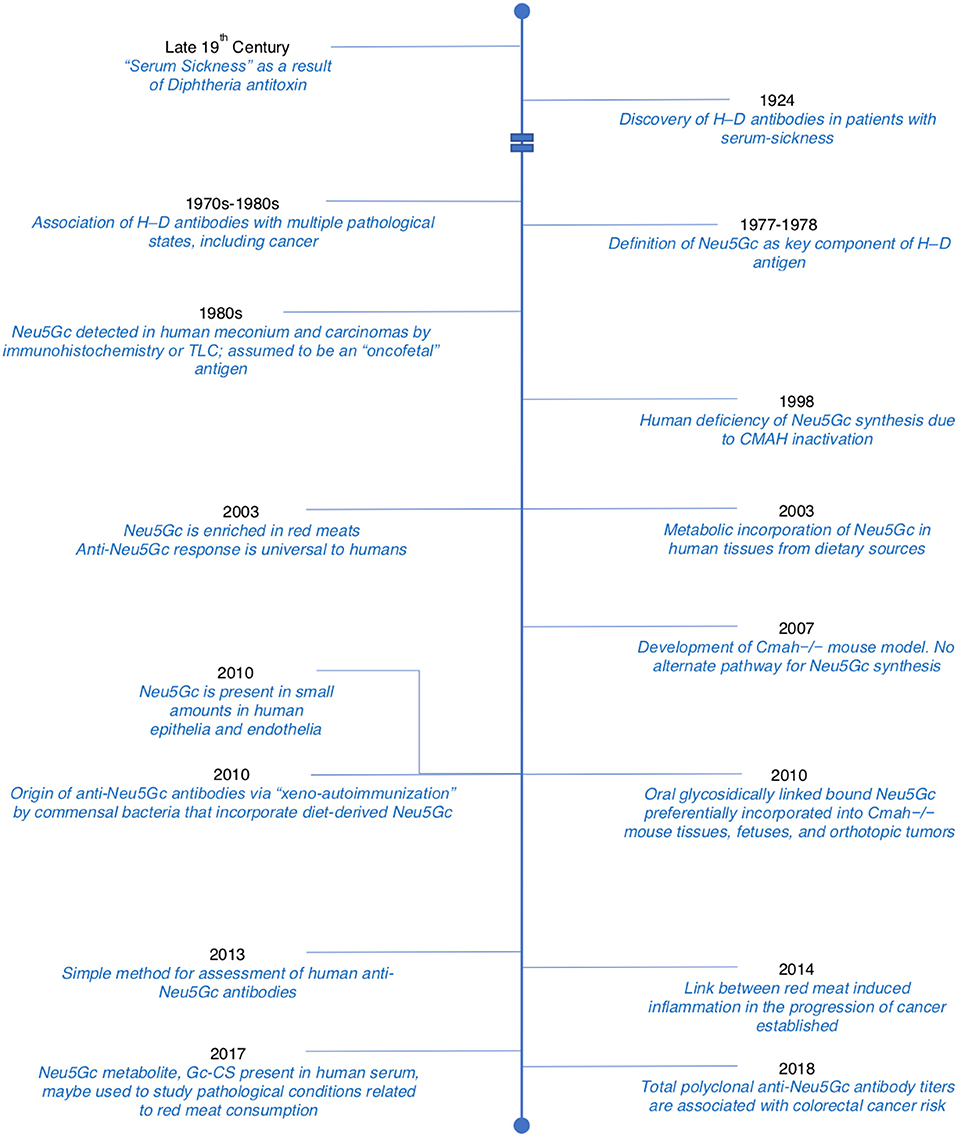
Figure 6. Timeline detailing important discoveries related to Neu5Gc and anti-Neu5Gc antibodies. Adopted and modified from Samraj et al. (65).
There also remain certain unresolved complexities of food sources of Neu5Gc and their propensity for metabolic incorporation. It is noteworthy that processed red meat is much more closely associated with disease risk than red meat per se. This is usually explained on the basis of preservatives added to process red meat. However, the same preservatives are added to other foods but are not associated with the same disease risks. One possible explanation is that the predigested nature of the processed food enhances absorption and incorporation of Neu5Gc. In this regard, there is currently no assessment of the relative impact of different foods and food processing on absorption in general. What is needed is that the equivalent of a glycemic index for the impact of glucose uptake (198, 199), i.e., “a GCemic index.” Along the same lines we are also missing an equivalent of the HbA1c (198, 199) as an index of long-term metabolic incorporation. We are currently studying the novel metabolite N-Glycolyl-chondroitin sulfate as a candidate.
It is also important to emphasize that there are other dietary sources of Neu5Gc besides red meat. While poultry is completely free of Neu5Gc, low levels are found in “fish” (which typically refers to the fish muscle). However, it is well-known that other food sources such as fish eggs, sea urchins, goat milk etc. can be high in Neu5Gc, and antibody development and xenosialitis in societies that consume large amounts of such foods needs to be studied further. Of course, the presence of bound Neu5Gc does not automatically equate to metabolic incorporation.
One other important perspective from these studies on Neu5Gc and anti-Neu5Gc antibodies is the consumption of red meat. With red meat being the richest source of Neu5Gc, abstaining may be the best way to prevent any “xenosialitis” induced pathologies though this would be largely improbable to sustain in the general population. Another possible way to prevent Neu5Gc uptake is to breed genetically-modified CMAH null livestock. Like humans, these animals will be unable to synthesize Neu5Gc and thereby prevent human dietary incorporation. But besides worries about “GMOs,” one dangerous implication of rearing such livestock is their increased susceptibility to pathogens that bind Neu5Ac which also likely affect humans. This may be combated by growing GMO modified CMAH−/− “cultured meat” that does not synthesize Neu5Gc under strict aseptic conditions. Other alternatives include competing with an excess of the human sialic acid Neu5Ac.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by NIH grant R01GM32373 to AV.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors, AV.
Acknowledgments
We would like to acknowledge Sandra Diaz, Sudeshna Saha, and Professor Harald Zur Hausen for their helpful comments.
References
2. Bolton C. The complications of the serum treatment of diptheria. Lancet. (1899) 153:891–3. doi: 10.1016/S0140-6736(01)68368-5
4. Hanganutziu M. Hémagglutinines hétérogénétiques après injection de sérum de cheval. CR Séances Soc Biol. (1924) 91:1457–9.
5. Deicher H. Über die Erzeugung heterospezifischer Hämagglutinine durch Injektion artfremden. Serums Z Hyg. (1926) 106:561–79.
6. Kasukawa R, Kano K, Bloom ML, Milgrom F. Heterophile antibodies in pathologic human sera resembling antibodies stimulated by foreign species sera. Clin Exp Immunol. (1976) 25:122–32.
7. Higashi H, Naiki M, Matuo S, Okouchi K. Antigen of “serum sickness” type of heterophile antibodies in human sera: indentification as gangliosides with N-glycolylneuraminic acid. Biochem Biophys Res Commun. (1977) 79:388–95.
8. Merrick JM, Zadarlik K, Milgrom F. Characterization of the hanganutziu-deicher (serum-sickness) antigen as gangliosides containing n-glycolylneuraminic acid. Int Arch Allergy Appl Immunol. (1978) 57:477–80. doi: 10.1159/000232140
9. Schauer R, Schoop HJ, Faillard H. On biosynthesis of the glycolyl groups of N-glycolylneuraminic acid Oxidative conversion of N-acetyl groups to glycolyl groups. Hoppe Seylers Z Physiol Chem. (1968) 349:645–52.
10. Shaw L, Schauer R. The biosynthesis of N-glycoloylneuraminic acid occurs by hydroxylation of the CMP-glycoside of N-acetylneuraminic acid. Biol Chem Hoppe Seyler. (1988) 369:477–86. doi: 10.1515/bchm3.1988.369.1.477
11. Muchmore EA, Milewski M, Varki A, Diaz S. Biosynthesis of N-glycolyneuraminic acid. The primary site of hydroxylation of N-acetylneuraminic acid is the cytosolic sugar nucleotide pool. J Biol Chem. (1989) 264:20216–23.
12. Kozutsumi Y, Kawano T, Yamakawa T, Suzuki A. Participation of cytochrome b5 in CMP-N-acetylneuraminic acid hydroxylation in mouse liver cytosol. J Biochem. (1990) 108:704–6. doi: 10.1093/oxfordjournals.jbchem.a123268
13. Kozutsumi Y, Kawano T, Kawasaki H, Suzuki K, Yamakawa T, Suzuki A. Reconstitution of CMP-N-acetylneuraminic acid hydroxylation activity using a mouse liver cytosol fraction and soluble cytochrome b5 purified from horse erythrocytes. J Biochem. (1991) 110:429–35. doi: 10.1093/oxfordjournals.jbchem.a123598
14. Nishimaki T, Kano K, Milgrom F. Studies on heterophile antibodies in rheumatoid arthritis. Arthritis Rheum. (1978) 21:634–8. doi: 10.1002/art.1780210604
15. Nishimaki T, Kano K, Milgrom F. Studies on immune complexes in rheumatoid arthritis. Arthritis Rheum. (1978) 21:639–44. doi: 10.1002/art.1780210605
16. Nishimaki T, Kano K, Milgrom F. Hanganutziu-Deicher antigen and antibody in pathologic sera and tissues. J Immunol. (1979) 122:2314–8.
17. Morito T, Kano K, Milgrom F. Hanganutziu-deicher antibodies in infectious mononucleosis and other diseases. J Immunol. (1982) 129:2524–8.
18. Ikuta K, Nishi Y, Shimizu Y, Higashi H, Kitamoto N, Kato S, et al. Hanganutziu-Deicher type-heterophile antigen-positive cells in human cancer tissues demonstrated by membrane immunofluorescence. Biken J. (1982) 25:47–50.
19. Arita K, Ikuta K, Nishi Y, Kato S, Yamauchi E, Maki S, et al. Heterophile Hanganutziu-deicher antibodies in sera of patients with kawasaki diseases. Biken J. (1982) 25:157–62.
20. Takiguchi M, Tamura T, Goto M, Kusakawa S, Milgrom F, Kano K. Immunological studies on Kawasaki disease. I. Appearance of Hanganutziu-Deicher antibodies. Clin Exp Immunol. (1984) 56:345–52.
21. Mukuria JC, Naiki M, Hashimoto M, Kato S. A specific enzyme-linked immunosorbent assay (ELISA) procedure for detection of heterophile Hanganutziu and Deicher (HD) antibodies. J Immunol Methods. (1986) 86:179–85. doi: 10.1016/0022-1759(86)90450-3
22. Morito T, Nishimaki T, Masaki M, Yoshida H, Kasukawa R, Nakarai H, et al. Studies on Hanganutziu-Deicher antigens-antibodies. I. Hanganutziu-Deicher antibodies of IgG class in liver diseases. Int Arch Allergy Appl Immunol. (1986) 81:204–8. doi: 10.1159/000234135
23. Nakarai H, Saida T, Shibata Y, Irie RF, Kano K. Expression of heterophile, Paul-Bunnell and Hanganutziu-Deicher antigens on human melanoma cell lines. Int Arch Allergy Appl Immunol. (1987) 83:160–6. doi: 10.1159/000234349
24. Higashihara T, Takeshima T, Anzai M, Tomioka M, Matsumoto K, Nishida K, et al. Survey of Hanganutziu and Deicher antibodies in operated patients. Int Arch Allergy Appl Immunol. (1991) 95:231–5. doi: 10.1159/000235434
25. Higashi H, Nishi Y, Fukui Y, Ikuta K, Ueda S, Kato S, et al. Tumor-associated expression of glycosphingolipid Hanganutziu-Deicher antigen in human cancers. Gann. (1984) 75:1025–9.
26. Ohashi Y, Sasabe T, Nishida T, Nishi Y, Higashi H. Hanganutziu-Deicher heterophile antigen in human retinoblastoma cells. Am J Ophthalmol. (1983) 96:321–5. doi: 10.1016/S0002-9394(14)77822-5
27. Higashi H, Fukui Y, Ueda S, Kato S, Hirabayashi Y, Matsumoto M, et al. Sensitive enzyme-immunostaining and densitometric determination on thin-layer chromatography of N-glycolylneuraminic acid-containing glycosphingolipids, Hanganutziu-Deicher antigens. J Biochem. (1984) 95:1517–20. doi: 10.1093/oxfordjournals.jbchem.a134760
28. Mukuria JC, Naiki M, Hashimoto M, Nishiura K, Okabe M, Kato S. A potential radioimmunoassay system for detection of Hanganutziu-Deicher type heterophile antigen(s) and antibodies in tissues and fluids. J Immunol Methods. (1985) 80:97–106. doi: 10.1016/0022-1759(85)90168-1
29. Higashi H, Hirabayashi Y, Fukui Y, Naiki M, Matsumoto M, Ueda S, et al. Characterization of N-glycolylneuraminic acid-containing gangliosides as tumor-associated Hanganutziu-Deicher antigen in human colon cancer. Cancer Res. (1985) 45:3796–802.
30. Nowak JA, Jain NK, Stinson MW, Merrick JM. Interaction of bovine erythrocyte N-glycolylneuraminic acid-containing gangliosides and glycoproteins with a human Hanganutziu-Deicher serum. Mol Immunol. (1986) 23:693–700. doi: 10.1016/0161-5890(86)90079-9
31. Mukuria JC, Naiki M, Kato S. Microstructure of the sialic acid moiety of N-glycolylneuraminyllactosylceramide and the elucidation of Hanganutziu and Deicher (HD) antigenicity. Immunol Lett. (1986) 12:165–9. doi: 10.1016/0165-2478(86)90100-8
32. Kawachi S, Saida T, Uhara H, Uemura K, Taketomi T, Kano K. Heterophile Hanganutziu-Deicher antigen in ganglioside fractions of human melanoma tissues. Int Arch Allergy Appl Immunol. (1988) 85:381–3.
33. Fukui Y, Maru M, Ohkawara K, Miyake T, Osada Y, Wang DQ, et al. Detection of glycoproteins as tumor-associated Hanganutziu-Deicher antigen in human gastric cancer cell line, NUGC4. Biochem Biophys Res Commun. (1989) 160:1149–54. doi: 10.1016/S0006-291X(89)80123-8
34. Nakarai H, Chandler PJ, Kano K, Morton DL, Irie RF. Hanganutziu-Deicher antigen as a possible target for immunotherapy of melanoma. Int Arch Allergy Appl Immunol. (1990) 91:323–8. doi: 10.1159/000235135
35. Saida T, Ikegawa S, Takizawa Y, Kawachi S. Immunohistochemical detection of heterophile Hanganutziu-Deicher antigen in human malignant melanoma. Arch Dermatol Res. (1990) 282:179–82. doi: 10.1007/BF00372619
36. Diaz SL, Padler-Karavani V, Ghaderi D, Hurtado-Ziola N, Yu H, Chen X, et al. Sensitive and specific detection of the non-human sialic acid N-glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS ONE. (2009) 4:e4241. doi: 10.1371/journal.pone.0004241
37. Pham T, Gregg CJ, Karp F, Chow R, Padler-Karavani V, Cao H, et al. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood. (2009) 114:5225–35. doi: 10.1182/blood-2009-05-220400
38. Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. (1998) 273:15866–71. doi: 10.1074/jbc.273.25.15866
39. Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. (1998) 95:11751–6. doi: 10.1073/pnas.95.20.11751
40. Hayakawa T, Satta Y, Gagneux P, Varki A, Takahata N. Alu-mediated inactivation of the human CMP- N-acetylneuraminic acid hydroxylase gene. Proc Natl Acad Sci USA. (2001) 98:11399–404. doi: 10.1073/pnas.191268198
41. Chou HH, Hayakawa T, Diaz S, Krings M, Indriati E, Leakey M, et al. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci USA. (2002) 99:11736–41. doi: 10.1073/pnas.182257399
42. Muchmore EA, Diaz S, Varki A. A structural difference between the cell surfaces of humans and the great apes. Am J Phys Anthropol. (1998) 107:187–98. doi: 10.1002/(SICI)1096-8644(199810)107:2<187::AID-AJPA5>3.0.CO;2-S
43. Varki A, Gagneux P. Human-specific evolution of sialic acid targets: explaining the malignant malaria mystery. Proc Natl Acad Sci USA. (2009) 106:14739–40. doi: 10.1073/pnas.0908196106
44. Ghaderi D, Springer SA, Ma F, Cohen M, Secrest P, Taylor RE, et al. Sexual selection by female immunity against paternal antigens can fix loss of function alleles. Proc Natl Acad Sci USA. (2011) 108:17743–8. doi: 10.1073/pnas.1102302108
45. Bergfeld AK, Lawrence R, Diaz SL, Pearce OMT, Ghaderi D, Gagneux P, et al. N-glycolyl groups of nonhuman chondroitin sulfates survive in ancient fossils. Proc Natl Acad Sci USA. (2017) 114:E8155–64. doi: 10.1073/pnas.1706306114
46. Ma F, Deng L, Secrest P, Shi L, Zhao J, Gagneux P. A mouse model for dietary xenosialitis: antibodies to xenoglycan can reduce fertility. J Biol Chem. (2016) 291:18222–31. doi: 10.1074/jbc.M116.739169
47. Schauer R, Srinivasan GV, Coddeville B, Zanetta JP, Guerardel Y. Low incidence of N-glycolylneuraminic acid in birds and reptiles and its absence in the platypus. Carbohydr Res. (2009) 344:1494–500. doi: 10.1016/j.carres.2009.05.020
48. Peri S, Kulkarni A, Feyertag F, Berninsone PM, Alvarez-Ponce D. Phylogenetic distribution of CMP-Neu5Ac hydroxylase (CMAH), the enzyme synthetizing the proinflammatory human xenoantigen Neu5Gc. Genome Biol Evol. (2018) 10:207–19. doi: 10.1093/gbe/evx251
49. Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. (2003) 100:12045–50. doi: 10.1073/pnas.2131556100
50. Hedlund M, Padler-Karavani V, Varki NM, Varki A. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci USA. (2008) 105:18936–41. doi: 10.1073/pnas.0803943105
51. Oetke C, Hinderlich S, Brossmer R, Reutter W, Pawlita M, Keppler OT. Evidence for efficient uptake and incorporation of sialic acid by eukaryotic cells. Eur J Biochem. (2001) 268:4553–61. doi: 10.1046/j.1432-1327.2001.02379.x
52. Hedlund M, Tangvoranuntakul P, Takematsu H, Long JM, Housley GD, Kozutsumi Y, et al. N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol Cell Biol. (2007) 27:4340–6. doi: 10.1128/MCB.00379-07
53. Samraj AN, Pearce OM, Läubli H, Crittenden AN, Bergfeld AK, Banda K, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA. (2015) 112:542–7. doi: 10.1073/pnas.1417508112
54. Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. (1982) 40:131–234. doi: 10.1016/S0065-2318(08)60109-2
55. Warren L. The distribution of sialic acids in nature. Comp Biochem Physiol. (1963) 10:153–71. doi: 10.1016/0010-406X(63)90238-X
56. Malykh YN, Krisch B, Gerardy-Schahn R, Lapina EB, Shaw L, Schauer R. The presence of N-acetylneuraminic acid in Malpighian tubules of larvae of the cicada Philaenus spumarius. Glycoconjugate J. (1999) 16:731–9. doi: 10.1023/A:1007115627708
57. Roth J, Kempf A, Reuter G, Schauer R, Gehring WJ. Occurrence of sialic acids in Drosophila melanogaster. Science. (1992) 256:673–5. doi: 10.1126/science.1585182
58. Schauer R, Kamerling JP. Exploration of the sialic acid world. Adv Carbohydr Chem Biochem. (2018) 75:1–213. doi: 10.1016/bs.accb.2018.09.001
59. Wang B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv Nutr. (2012) 3:465S−72S. doi: 10.3945/an.112.001875
60. Chen Y, Pan L, Liu N, Troy FA, Wang B. LC-MS/MS quantification of N-acetylneuraminic acid, N-glycolylneuraminic acid and ketodeoxynonulosonic acid levels in the urine and potential relationship with dietary sialic acid intake and disease in 3- to 5-year-old children. Br J Nutr. (2013) 111:1–10. doi: 10.1017/S0007114513002468
61. Padler-Karavani V, Yu H, Cao H, Chokhawala H, Karp F, Varki N, et al. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology. (2008) 18:818–30. doi: 10.1093/glycob/cwn072
62. Banda K, Gregg CJ, Chow R, Varki NM, Varki A. Metabolism of vertebrate amino sugars with N-glycolyl groups: mechanisms underlying gastrointestinal incorporation of the non-human sialic acid xeno-autoantigen N-glycolylneuraminic acid. J Biol Chem. (2012) 287:28852–64. doi: 10.1074/jbc.M112.364182
63. Nöhle U, Schauer R. Metabolism of sialic acids from exogenously administered sialyllactose and mucin in mouse and rat. Hoppe Seylers Z Physiol Chem. (1984) 365:1457–67.
64. Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. (2005) 280:4228–37. doi: 10.1074/jbc.M412040200
65. Samraj AN, Läubli H, Varki N, Varki A. Involvement of a non-human sialic Acid in human cancer. Front Oncol. (2014) 4:33. doi: 10.3389/fonc.2014.00033
66. Kabat EA, Liao J, Osserman EF, Gamian A, Michon F, Jennings HJ. The epitope associated with the binding of the capsular polysaccharide of the group B meningococcus and of Escherichia coli K1 to a human monoclonal macroglobulin, IgMNOV. J Exp Med. (1988) 168:699–711. doi: 10.1084/jem.168.2.699
67. Frei R, Ferstl R, Roduit C, Ziegler M, Schiavi E, Barcik W, et al. Exposure to nonmicrobial N-glycolylneuraminic acid protects farmers' children against airway inflammation and colitis. J Allergy Clin Immunol. (2018) 141:382–90.e7. doi: 10.1016/j.jaci.2017.04.051
68. Bergfeld AK, Pearce OM, Diaz SL, Pham T, Varki A. Metabolism of vertebrate amino sugars with N-glycolyl groups: elucidating the intracellular fate of the non-human sialic acid N-glycolylneuraminic acid. J Biol Chem. (2012) 287:28865–81. doi: 10.1074/jbc.M112.363549
69. Clausen TM, Pereira MA, Al Nakouzi N, Oo HZ, Agerbæk MØ, Lee S, et al. Oncofetal chondroitin sulfate glycosaminoglycans are key players in integrin signaling and tumor cell motility. Mol Cancer Res. (2016) 14:1288–99. doi: 10.1158/1541-7786.MCR-16-0103
70. Agerbaek MØ, Pereira MA, Clausen TM, Pehrson C, Oo HZ, Spliid C, et al. Burkitt lymphoma expresses oncofetal chondroitin sulfate without being a reservoir for placental malaria sequestration. Int J Cancer. (2017) 140:1597–608. doi: 10.1002/ijc.30575
71. Agerbæk MØ, Bang-Christensen S, Salanti A. Fighting cancer using an oncofetal glycosaminoglycan-binding protein from malaria parasites. Trends Parasitol. (2018) 35:178–81. doi: 10.1016/j.pt.2018.11.004
72. Agerbæk MØ, Bang-Christensen SR, Yang MH, Clausen TM, Pereira MA, Sharma S, et al. The VAR2CSA malaria protein efficiently retrieves circulating tumor cells in an EpCAM-independent manner. Nat Commun. (2018) 9:3279. doi: 10.1038/s41467-018-05793-2
73. Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. (2002) 9:376–81. doi: 10.1034/j.1399-3089.2002.02138.x
74. Nguyen DH, Tangvoranuntakul P, Varki A. Effects of natural human antibodies against a nonhuman sialic acid that metabolically incorporates into activated and malignant immune cells. J Immunol. (2005) 175:228–36. doi: 10.4049/jimmunol.175.1.228
75. Tahara H, Ide K, Basnet NB, Tanaka Y, Matsuda H, Takematsu H, et al. Immunological property of antibodies against N-glycolylneuraminic acid epitopes in cytidine monophospho-N-acetylneuraminic acid hydroxylase-deficient mice. J Immunol. (2010) 184:3269–75. doi: 10.4049/jimmunol.0902857
76. Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, et al. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med. (2010) 207:1637–46. doi: 10.1084/jem.20100575
77. Ochsenbein AF, Zinkernagel RM. Natural antibodies and complement link innate and acquired immunity. Immunol Today. (2000) 21:624–30. doi: 10.1016/S0167-5699(00)01754-0
78. Lu Q, Padler-Karavani V, Yu H, Chen X, Wu SL, Varki A, et al. LC-MS analysis of polyclonal human anti-Neu5Gc xeno-autoantibodies immunoglobulin G Subclass and partial sequence using multistep intravenous immunoglobulin affinity purification and multienzymatic digestion. Anal Chem. (2012) 84:2761–8. doi: 10.1021/ac2030893
79. Naito Y, Takematsu H, Koyama S, Miyake S, Yamamoto H, Fujinawa R, et al. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol Cell Biol. (2007) 27:3008–22. doi: 10.1128/MCB.02047-06
80. Cariappa A, Takematsu H, Liu H, Diaz S, Haider K, Boboila C, et al. B cell antigen receptor signal strength and peripheral B cell development are regulated by a 9-O-acetyl sialic acid esterase. J Exp Med. (2009) 206:125–38. doi: 10.1084/jem.20081399
81. Azuma I, Thomas DW, Adam A, Ghuysen JM, Bonaly R, Petit JF, et al. Occurrence of N-glycolylmuramic acid in bacterial cell walls. A preliminary survey. Biochim Biophys Acta. (1970) 208:444–51. doi: 10.1016/0304-4165(70)90217-5
82. Essers L, Schoop HJ. Evidence for the incorporation of molecular oxygen, a pathway in biosynthesis of N-glycolylmuramic acid in Mycobacterium phlei. Biochim Biophys Acta. (1978) 544:180–4. doi: 10.1016/0304-4165(78)90221-0
83. Raymond JB, Mahapatra S, Crick DC, Pavelka MSJ. Identification of the namH gene, encoding the hydroxylase responsible for the N-glycolylation of the mycobacterial peptidoglycan. J Biol Chem. (2005) 280:326–33. doi: 10.1074/jbc.M411006200
84. Mahapatra S, Crick DC, McNeil MR, Brennan PJ. Unique structural features of the peptidoglycan of Mycobacterium leprae. J Bacteriol. (2008) 190:655–61. doi: 10.1128/JB.00982-07
85. Coulombe F, Divangahi M, Veyrier F, de Léséleuc L, Gleason JL, Yang Y, et al. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med. (2009) 206:1709–16. doi: 10.1084/jem.20081779
86. Daryaee F, Kobarfard F, Khalaj A, Farnia P. Synthesis and evaluation of in vitro anti-tuberculosis activity of N-substituted glycolamides. Eur J Med Chem. (2009) 44:289–95. doi: 10.1016/j.ejmech.2008.02.022
87. Vamecq J, Draye JP, Poupaert JH. Studies on the metabolism of glycolyl-CoA. Biochem Cell Biol. (1990) 68:846–51. doi: 10.1139/o90-125
88. Vamecq J, Mestdagh N, Henichart JP, Poupaert J. Subcellular distribution of glycolyltransferases in rodent liver and their significance in special reference to the synthesis of N-glycolyneuraminic acid. J Biochem. (1992) 111:579–83. doi: 10.1093/oxfordjournals.jbchem.a123800
89. Wang D, Hubbard JM, Kabat EA. Modeling study of antibody combining sites to (α1-6)dextrans. Predictions of the conformational contribution of VL-CDR3 and Jkappa segments to groove-type combining sites. J Biol Chem. (1993) 268:20584–9.
90. Klebert S, Kratzin HD, Zimmermann B, Vaesen M, Frosch M, Weisgerber C, et al. Primary structure of the murine monoclonal IgG2a antibody mAb735 against (2-8) polysialic acid. Biol Chem Hoppe Seyler. (1993) 374:993–1000. doi: 10.1515/bchm3.1993.374.7-12.993
91. Eleftheriou P, Kynigopoulos S, Giovou A, Mazmanidi A, Yovos J, Skepastianos P, et al. Prevalence of anti-Neu5Gc antibodies in patients with hypothyroidism. Biomed Res Int. (2014) 2014:963230. doi: 10.1155/2014/963230
92. Shilova N, Huflejt ME, Vuskovic M, Obukhova P, Navakouski M, Khasbiullina N, et al. Natural antibodies against sialoglycans. Top Curr Chem. (2015) 366:169–81. doi: 10.1007/128_2013_469
93. Hurh S, Kang B, Choi I, Cho B, Lee EM, Kim H, et al. Human antibody reactivity against xenogeneic N-glycolylneuraminic acid and galactose-α-1,3-galactose antigen. Xenotransplantation. (2016) 23:279–92. doi: 10.1111/xen.12239
94. Gao B, Long C, Lee W, Zhang Z, Gao X, Landsittel D, et al. Anti-Neu5Gc and anti-non-Neu5Gc antibodies in healthy humans. PLoS ONE. (2017) 12:e0180768. doi: 10.1371/journal.pone.0180768
95. Gong S, Ren H, Lin C, Hu P, Tian R, Liu Z, et al. Immunochromatographic strip biosensor for the rapid detection of N-glycolylneuraminic acid based on aptamer-conjugated nanoparticle. Anal Biochem. (2018) 561–2:52–8. doi: 10.1016/j.ab.2018.07.017
96. Padler-Karavani V, Hurtado-Ziola N, Pu M, Yu H, Huang S, Muthana S, et al. Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res. (2011) 71:3352–63. doi: 10.1158/0008-5472.CAN-10-4102
97. Samraj AN, Bertrand KA, Luben R, Khedri Z, Yu H, Nguyen D, et al. Polyclonal human antibodies against glycans bearing red meat-derived non-human sialic acid N-glycolylneuraminic acid are stable, reproducible, complex and vary between individuals: Total antibody levels are associated with colorectal cancer risk. PLoS ONE. (2018) 13:e0197464. doi: 10.1371/journal.pone.0197464
98. Bashir S, Leviatan Ben Arye S, Reuven EM, Yu H, Costa C, Galiñanes M, et al. Presentation mode of glycans affect recognition of human serum anti-Neu5Gc IgG antibodies. Bioconjug Chem. (2018) 30:161–68. doi: 10.1021/acs.bioconjchem.8b00817
99. Apicella MA. Nontypeable Haemophilus influenzae: the role of N-acetyl-5-neuraminic acid in biology. Front Cell Infect Microbiol. (2012) 2:19. doi: 10.3389/fcimb.2012.00019
100. Carr A, Mesa C, del Carmen Arango M, Vázquez AM, Fernández LE. In vivo and in vitro anti-tumor effect of 14F7 monoclonal antibody. Hybrid Hybridomics. (2002) 21:463–8. doi: 10.1089/153685902321043990
101. Rodríguez M, Llanes L, Pérez A, Pérez R, Vázquez AM. Generation and characterization of an anti-idiotype monoclonal antibody related to GM3(NeuGc) ganglioside. Hybrid Hybridomics. (2003) 22:307–14. doi: 10.1089/153685903322538836
102. Díaz A, Alfonso M, Alonso R, Saurez G, Troche M, Catalá M, et al. Immune responses in breast cancer patients immunized with an anti-idiotype antibody mimicking NeuGc-containing gangliosides. Clin Immunol. (2003) 107:80–9. doi: 10.1016/S1521-6616(03)00036-6
103. Hernández AM, Rodríguez M, López-Requena A, Beausoleil I, Pérez R, Vázquez AM. Generation of anti-Neu-glycolyl-ganglioside antibodies by immunization with an anti-idiotype monoclonal antibody: a self versus non-self-matter. Immunobiology. (2005) 210:11–21. doi: 10.1089/153685903322328947
104. Leon JD, Fernandez A, Mesa C, Clavel M, Fernandez LE. Role of tumour-associated N-glycolylated variant of GM3 ganglioside in cancer progression: effect over CD4 expression on T cells. Cancer Immunol Immunother. (2005) 55:443–50. doi: 10.1007/s00262-005-0041-6
105. Oliva JP, Valdés Z, Casacó A, Pimentel G, González J, Alvarez I, et al. Clinical evidences of GM3 (NeuGc) ganglioside expression in human breast cancer using the 14F7 monoclonal antibody labelled with (99m)Tc. Breast Cancer Res Treat. (2006) 96:115–21. doi: 10.1007/s10549-005-9064-0
106. Neninger E, Díaz RM, de la Torre A, Rives R, Díaz A, Saurez G, et al. Active immunotherapy with 1E10 anti-idiotype vaccine in patients with small cell lung cancer: report of a phase I trial. Cancer Biol Ther. (2007) 6:145–50. doi: 10.4161/cbt.6.2.3574
107. Labrada M, Clavell M, Bebelagua Y, León Jd, Alonso DF, Gabri MR, et al. Direct validation of NGcGM3 ganglioside as a new target for cancer immunotherapy. Expert Opin Biol Ther. (2010) 10:153–62. doi: 10.1517/14712590903443084
108. Fernandez LE, Gabri MR, Guthmann MD, Gomez RE, Gold S, Fainboim L, et al. NGcGM3 ganglioside: a privileged target for cancer vaccines. Clin Dev Immunol. (2010) 2010:814397. doi: 10.1155/2010/814397
109. Vázquez AM, Hernández AM, Macías A, Montero E, Gómez DE, Alonso DF, et al. Racotumomab: an anti-idiotype vaccine related to N-glycolyl-containing gangliosides - preclinical and clinical data. Front Oncol. (2012) 2:150. doi: 10.3389/fonc.2012.00150
110. Blanco R, Blanco D, Quintana Y, Escobar X, Rengifo CE, Osorio M, et al. Immunoreactivity of the 14F7 Mab raised against N-glycolyl GM3 ganglioside in primary lymphoid tumors and lymph node metastasis. Patholog Res Int. (2013) 2013:920972. doi: 10.1155/2013/920972
111. Blanco R, Quintana Y, Blanco D, Cedeño M, Rengifo CE, Frómeta M, et al. Tissue Reactivity of the 14F7 mab raised against n-glycolyl GM3 ganglioside in tumors of neuroectodermal, mesodermal, and epithelial origin. J Biomark. (2013) 2013:602417. doi: 10.1155/2013/602417
112. Palomo AG, Santana RB, Pérez XE, Santana DB, Gabri MR, Monzon KL, et al. Frequent co-expression of EGFR and NeuGcGM3 ganglioside in cancer: it's potential therapeutic implications. Clin Exp Metastasis. (2016) 33:717–25. doi: 10.1007/s10585-016-9811-0
113. Labrada M, Dorvignit D, Hevia G, Rodríguez-Zhurbenko N, Hernández AM, Vázquez AM, et al. GM3(Neu5Gc) ganglioside: an evolution fixed neoantigen for cancer immunotherapy. Semin Oncol. (2018) 45:41–51. doi: 10.1053/j.seminoncol.2018.04.003
114. de la Luz-Hernández K, Rabasa Y, Montesinos R, Fuentes D, Santo-Tomás JF, Morales O, et al. Cancer vaccine characterization: from bench to clinic. Vaccine. (2014) 32:2851–8. doi: 10.1016/j.vaccine.2014.02.017
115. Gajdosik Z. Racotumomab - a novel anti-idiotype monoclonal antibody vaccine for the treatment of cancer. Drugs Today. (2014) 50:301–7. doi: 10.1358/dot.2014.50.4.2116670
116. Bousquet PA, Sandvik JA, Jeppesen Edin NF, Krengel U. Hypothesis: hypoxia induces de novo synthesis of NeuGc gangliosides in humans through CMAH domain substitute. Biochem Biophys Res Commun. (2018) 495:1562–6. doi: 10.1016/j.bbrc.2017.11.183
117. Bada A, Casacó Parada A, Arteaga M, Martínez J, León A, Santana E, et al. Toxicity of a GM3 cancer vaccine in Macaca fascicularis monkey: a 12-month study. Hum Exp Toxicol. (2002) 21:263–7. doi: 10.1191/0960327102ht248oa
118. Amon R, Ben-Arye SL, Engler L, Yu H, Lim N, Berre LL, et al. Glycan microarray reveal induced IgGs repertoire shift against a dietary carbohydrate in response to rabbit anti-human thymocyte therapy. Oncotarget. (2017) 8:112236–44. doi: 10.18632/oncotarget.23096
119. Burlak C, Paris LL, Lutz AJ, Sidner RA, Estrada J, Li P, et al. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am J Transplant. (2014) 14:1895–900. doi: 10.1111/ajt.12744
120. Couvrat-Desvergnes G, Salama A, Le Berre L, Evanno G, Viklicky O, Hruba P, et al. Rabbit antithymocyte globulin-induced serum sickness disease and human kidney graft survival. J Clin Invest. (2015) 125:4655–65. doi: 10.1172/JCI82267
121. Okamura RM, Lebkowski J, Au M, Priest CA, Denham J, Majumdar AS. Immunological properties of human embryonic stem cell-derived oligodendrocyte progenitor cells. J Neuroimmunol. (2007) 192:134–44. doi: 10.1016/j.jneuroim.2007.09.030
122. Reuven EM, Leviatan Ben-Arye S, Marshanski T, Breimer ME, Yu H, Fellah-Hebia I, et al. Characterization of immunogenic Neu5Gc in bioprosthetic heart valves. Xenotransplantation. (2016) 23:381–92. doi: 10.1111/xen.12260
123. Salama A, Evanno G, Harb J, Soulillou JP. Potential deleterious role of anti-Neu5Gc antibodies in xenotransplantation. Xenotransplantation. (2015) 22:85–94. doi: 10.1111/xen.12142
124. Salama A, Evanno G, Lim N, Rousse J, Le Berre L, Nicot A, et al. Anti-Gal and Anti-Neu5Gc responses in nonimmunosuppressed patients after treatment with rabbit antithymocyte polyclonal IgGs. Transplantation. (2017) 101:2501–7. doi: 10.1097/TP.0000000000001686
125. Wang ZY, Burlak C, Estrada JL, Li P, Tector MF, Tector AJ. Erythrocytes from GGTA1/CMAH knockout pigs: implications for xenotransfusion and testing in non-human primates. Xenotransplantation. (2014) 21:376–84. doi: 10.1111/xen.12106
126. Pearce OM, Läubli H, Bui J, Varki A. Hormesis in cancer immunology: does the quantity of an immune reactant matter. Oncoimmunology. (2014) 3:e29312. doi: 10.4161/onci.29312
127. Pearce OM, Läubli H, Verhagen A, Secrest P, Zhang J, Varki NM, et al. Inverse hormesis of cancer growth mediated by narrow ranges of tumor-directed antibodies. Proc Natl Acad Sci USA. (2014) 111:5998–6003. doi: 10.1073/pnas.1209067111
128. Marquina G, Waki H, Fernandez LE, Kon K, Carr A, Valiente O, et al. Gangliosides expressed in human breast cancer. Cancer Res. (1996) 56:5165–71.
129. Carr A, Mullet A, Mazorra Z, Vázquez AM, Alfonso M, Mesa C, et al. A mouse IgG1 monoclonal antibody specific for N-glycolyl GM3 ganglioside recognized breast and melanoma tumors. Hybridoma. (2000) 19:241–7. doi: 10.1089/02724570050109639
130. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. (2011) 94:1088–96. doi: 10.3945/ajcn.111.018978
131. Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. (2010) 121:2271–83. doi: 10.1161/CIRCULATIONAHA.109.924977
132. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. (2012) 172:555–63. doi: 10.1001/archinternmed.2011.2287
133. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. (1994) 54:2390–7.
134. Stavric B. Biological significance of trace levels of mutagenic heterocyclic aromatic amines in human diet: a critical review. Food Chem Toxicol. (1994) 32:977–94. doi: 10.1016/0278-6915(94)90093-0
135. Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer. (2008) 60:131–44. doi: 10.1080/01635580701684872
136. Lamberto I, Gunst K, Müller H, Zur Hausen H, de Villiers EM. Mycovirus-like DNA virus sequences from cattle serum and human brain and serum samples from multiple sclerosis patients. Genome Announc. (2014) 2:e00848–14. doi: 10.1128/genomeA.00848-14
137. Zur Hausen H, Bund T, de Villiers EM. Infectious agents in bovine red meat and milk and their potential role in cancer and other chronic diseases. Curr Top Microbiol Immunol. (2017) 407:83–116. doi: 10.1007/82_2017_3
138. Falida K, Eilebrecht S, Gunst K, Zur Hausen H, de Villiers EM. Isolation of two virus-like circular DNAs from commercially available milk samples. Genome Announc. (2017) 5:e00266–17. doi: 10.1128/genomeA.00266-17
139. Zur Hausen H, Bund T, de Villiers EM. Specific nutritional infections early in life as risk factors for human colon and breast cancers several decades later. Int J Cancer. (2018) 144:1574–83. doi: 10.1002/ijc.31882
140. Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. (2013) 339:286–91. doi: 10.1126/science.1232227
141. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. (2001) 357:539–45. doi: 10.1016/S0140-6736(00)04046-0
142. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
143. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
144. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
145. Wang D, DuBois RN. Inflammatory mediators and nuclear receptor signaling in colorectal cancer. Cell Cycle. (2007) 6:682–5. doi: 10.4161/cc.6.6.4030
146. Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. (2004) 80:1029–35. doi: 10.1093/ajcn/80.4.1029
147. Yin J, Hashimoto A, Izawa M, Miyazaki K, Chen GY, Takematsu H, et al. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res. (2006) 66:2937–45. doi: 10.1158/0008-5472.CAN-05-2615
148. Hedlund M, Ng E, Varki A, Varki NM. alpha 2-6-Linked sialic acids on N-glycans modulate carcinoma differentiation in vivo. Cancer Res. (2008) 68:388–94. doi: 10.1158/0008-5472.CAN-07-1340
149. Padler-Karavani V, Varki A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. (2011) 18:1–5. doi: 10.1111/j.1399-3089.2011.00622.x
150. Soulillou JP, Süsal C, Döhler B, Opelz G. No increase in Colon cancer risk following induction with Neu5Gc-bearing rabbit Anti-T Cell IgG (ATG) in recipients of kidney transplants. Cancers. (2018) 10:E324. doi: 10.3390/cancers10090324
151. Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American heart association. Circulation. (1999) 100:1134–46. doi: 10.1161/01.CIR.100.10.1134
152. Varki N, Anderson D, Herndon JG, Pham T, Gregg CJ, Cheriyan M, et al. Heart disease is common in humans and chimpanzees, but is caused by different pathological processes. Evol Appl. (2009) 2:101–12. doi: 10.1111/j.1752-4571.2008.00064.x
153. Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes–an updated review of the evidence. Curr Atheroscler Rep. (2012) 14:515–24. doi: 10.1007/s11883-012-0282-8
154. Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. (2013) 339:161–6. doi: 10.1126/science.1230719
155. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. (2013) 19:576–85. doi: 10.1038/nm.3145
156. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. (2013) 368:1575–84. doi: 10.1056/NEJMoa1109400
157. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. (2011) 472:57–63. doi: 10.1038/nature09922
158. Balla G, Jacob HS, Eaton JW, Belcher JD, Vercellotti GM. Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler Thromb. (1991) 11:1700–11. doi: 10.1161/01.ATV.11.6.1700
159. Balla J, Belcher JD, Balla G, Jacob HS, Vercellotti GM. Oxidized low-density lipoproteins and endothelium: oral vitamin E supplementation prevents oxidized low-density lipoprotein-mediated vascular injury. Trans Assoc Am Phys. (1993) 106:128–33.
160. Belcher JD, Balla J, Balla G, Jacobs DR, Gross M, Jacob HS, et al. Vitamin E, LDL, and endothelium. Brief oral vitamin supplementation prevents oxidized LDL-mediated vascular injury in vitro. Arterioscler Thromb. (1993) 13:1779–89.
161. Nagy E, Eaton JW, Jeney V, Soares MP, Varga Z, Galajda Z, et al. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler Thromb Vasc Biol. (2010) 30:1347–53. doi: 10.1161/ATVBAHA.110.206433
162. Alisson-Silva F, Kawanishi K, Varki A. Human risk of diseases associated with red meat intake: Analysis of current theories and proposed role for metabolic incorporation of a non-human sialic acid. Mol Aspects Med. (2016) 51:16–30. doi: 10.1016/j.mam.2016.07.002
163. Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity. (2012) 36:322–35. doi: 10.1016/j.immuni.2012.03.004
164. Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. (2005) 5:526–42. doi: 10.1038/nrc1649
165. Yonezawa S, Tachikawa T, Shin S, Sato E. Sialosyl-Tn antigen: Its distribution in normal human tissues and expression in adenocarcinomas. Am J Clin Pathol. (1992) 98:167–74. doi: 10.1093/ajcp/98.2.167
166. Ogata S, Koganty R, Reddish M, Longenecker BM, Chen A, Perez C, et al. Different modes of sialyl-Tn expression during malignant transformation of human colonic mucosa. Glycoconj J. (1998) 15:29–35. doi: 10.1023/A:1006935331756
167. Kobayashi H, Terao T, Kawashima Y. Serum sialyl Tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J Clin Oncol. (1992) 10:95–101. doi: 10.1200/JCO.1992.10.1.95
168. Imai J, Ghazizadeh M, Naito Z, Asano G. Immunohistochemical expression of T, Tn and sialyl-Tn antigens and clinical outcome in human breast carcinoma. Anticancer Res. (2001) 21:1327–34.
169. Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, Ho JJ, et al. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. (2002) 123:1052–60. doi: 10.1053/gast.2002.36018
170. Conze T, Carvalho AS, Landegren U, Almeida R, Reis CA, David L, et al. MUC2 mucin is a major carrier of the cancer-associated sialyl-Tn antigen in intestinal metaplasia and gastric carcinomas. Glycobiology. (2010) 20:199–206. doi: 10.1093/glycob/cwp161
171. Cao Y, Stosiek P, Springer GF, Karsten U. Thomsen-Friedenreich-related carbohydrate antigens in normal adult human tissues: a systematic and comparative study. Histochem Cell Biol. (1996) 106:197–207. doi: 10.1007/BF02484401
172. Starbuck K, Al-Alem L, Eavarone DA, Hernandez SF, Bellio C, Prendergast JM, et al. Treatment of ovarian cancer by targeting the tumor stem cell-associated carbohydrate antigen, Sialyl-Thomsen-nouveau. Oncotarget. (2018) 9:23289–305. doi: 10.18632/oncotarget.25289
173. Eavarone DA, Al-Alem L, Lugovskoy A, Prendergast JM, Nazer RI, Stein JN, et al. Humanized anti-Sialyl-Tn antibodies for the treatment of ovarian carcinoma. PLoS ONE. (2018) 13:e0201314. doi: 10.1371/journal.pone.0201314
174. Carr A, Rodríguez E, Arango Mdel C, Camacho R, Osorio M, Gabri M, et al. Immunotherapy of advanced breast cancer with a heterophilic ganglioside (NeuGcGM3) cancer vaccine. J Clin Oncol. (2003) 21:1015–21. doi: 10.1200/JCO.2003.02.124
175. Fernández-Marrero Y, Roque-Navarro L, Hernández T, Dorvignit D, Molina-Pérez M, González A, et al. Fernandez- A cytotoxic humanized anti-ganglioside antibody produced in a murine cell line defective of N-glycolylated-glycoconjugates. Immunobiology. (2011) 216:1239–47. doi: 10.1016/j.imbio.2011.07.004
176. Hernández AM, Toledo D, Martínez D, Griñán T, Brito V, Macías A, et al. Characterization of the antibody response against NeuGcGM3 ganglioside elicited in non-small cell lung cancer patients immunized with an anti-idiotype antibody. J Immunol. (2008) 181:6625–34. doi: 10.4049/jimmunol.181.9.6625
177. Segatori VI, Cuello HA, Gulino CA, Albertó M, Venier C, Guthmann MD, et al. Antibody-dependent cell-mediated cytotoxicity induced by active immunotherapy based on racotumomab in non-small cell lung cancer patients. Cancer Immunol Immunother. (2018) 67:1285–96. doi: 10.1007/s00262-018-2188-y
178. Scursoni AM, Galluzzo L, Camarero S, Pozzo N, Gabri MR, de Acosta CM, et al. Detection and characterization of N-glycolyated gangliosides in wilms tumor by immunohistochemistry. Pediatr Dev Pathol. (2010) 13:18–23. doi: 10.2350/08-10-0544.1
179. Scursoni AM, Galluzzo L, Camarero S, Lopez J, Lubieniecki F, Sampor C, et al. Detection of N-glycolyl GM3 ganglioside in neuroectodermal tumors by immunohistochemistry: an attractive vaccine target for aggressive pediatric cancer. Clin Dev Immunol. (2011) 2011:245181. doi: 10.1155/2011/245181
180. Blanco R, Rengifo E, Rengifo CE, Cedeno M, Frometa M, Carr A. Immunohistochemical reactivity of the 14F7 monoclonal antibody raised against N-Glycolyl GM3 ganglioside in some benign and malignant skin neoplasms. ISRN Dermatol. (2011) 2011:848909. doi: 10.5402/2011/848909
181. Blanco R, Rengifo E, Cedeno M, Rengifo CE, Alonso DF, Carr A. Immunoreactivity of the 14F7 mab raised against N-Glycolyl GM3 ganglioside in epithelial malignant tumors from digestive system. ISRN Gastroenterol. (2011) 2011:645641. doi: 10.5402/2011/645641
182. Blanco R, Rengifo CE, Cedeno M, Frometa M, Rengifo E, Carr A. Immunoreactivity of the 14F7 Mab (raised AGAINST N-Glycolyl Gm3 ganglioside) as a positive prognostic factor in non-small-cell lung cancer. Patholog Res Int. (2012) 2012:235418. doi: 10.1155/2012/235418
183. Leviatan Ben-Arye S, Yu H, Chen X, Padler-Karavani V. Profiling anti-Neu5Gc IgG in human sera with a sialoglycan microarray assay. J Vis Exp. (2017) 125:e56094. doi: 10.3791/56094
184. Amon R, Grant OC, Leviatan Ben-Arye S, Makeneni S, Nivedha AK, Marshanski T, et al. A combined computational-experimental approach to define the structural origin of antibody recognition of sialyl-Tn, a tumor-associated carbohydrate antigen. Sci Rep. (2018) 8:10786. doi: 10.1038/s41598-018-29209-9
185. Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. (2013) 341:1192–8. doi: 10.1126/science.1241145
186. Ghaderi D, Zhang M, Hurtado-Ziola N, Varki A. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev. (2012) 28:147–75. doi: 10.5661/bger-28-147
187. Ghaderi D, Taylor RE, Padler-Karavani V, Diaz S, Varki A. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotechnol. (2010) 28:863–7. doi: 10.1038/nbt.1651
188. Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Consumption of animal foods and endometrial cancer risk: a systematic literature review and meta-analysis. Cancer Causes Control. (2007) 18:967–88. doi: 10.1007/s10552-007-9038-0
189. Wallace K, Grau MV, Ahnen D, Snover DC, Robertson DJ, Mahnke D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev. (2009) 18:2310–7. doi: 10.1158/1055-9965.EPI-09-0211
190. McCullough ML, Gapstur SM, Shah R, Jacobs EJ, Campbell PT. Association between red and processed meat intake and mortality among colorectal cancer survivors. J Clin Oncol. (2013) 31:2773–82. doi: 10.1200/JCO.2013.49.1126
191. Kim Y, Je Y. Meat consumption and risk of metabolic syndrome: results from the Korean population and a meta-analysis of observational studies. Nutrients. (2018) 10 doi: 10.3390/nu10040390
192. Zelber-Sagi S, Ivancovsky-Wajcman D, Fliss Isakov N, Webb M, Orenstein D, Shibolet O, et al. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol. (2018) 68:1239–46. doi: 10.1016/j.jhep.2018.01.015
193. Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. (2005) 5:845–56. doi: 10.1038/nrc1739
194. Gupta D, Lis CG. Role of CA125 in predicting ovarian cancer survival - a review of the epidemiological literature. J Ovarian Res. (2009) 2:13. doi: 10.1186/1757-2215-2-13
195. Tan HT, Low J, Lim SG, Chung MC. Serum autoantibodies as biomarkers for early cancer detection. FEBS J. (2009) 276:6880–904. doi: 10.1111/j.1742-4658.2009.07396.x
196. Soussi T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. (2000) 60:1777–88.
197. Padler-Karavani V, Tremoulet AH, Yu H, Chen X, Burns JC, Varki A. A simple method for assessment of human anti-Neu5Gc antibodies applied to Kawasaki disease. PLoS ONE. (2013) 8:e58443. doi: 10.1371/journal.pone.0058443
198. Choo VL, Viguiliouk E, Blanco Mejia S, Cozma AI, Khan TA, Ha V, et al. Food sources of fructose-containing sugars and glycaemic control: systematic review and meta-analysis of controlled intervention studies. BMJ. (2018) 363:k4644. doi: 10.1136/bmj.k4644
Keywords: Neu5Gc, anti-Neu5Gc, serum sickness, xenosialitis, red meat, sialic acid, inflammation, antibodies
Citation: Dhar C, Sasmal A and Varki A (2019) From “Serum Sickness” to “Xenosialitis”: Past, Present, and Future Significance of the Non-human Sialic Acid Neu5Gc. Front. Immunol. 10:807. doi: 10.3389/fimmu.2019.00807
Received: 10 January 2019; Accepted: 26 March 2019;
Published: 17 April 2019.
Edited by:
Vered Padler-Karavani, Tel Aviv University, IsraelReviewed by:
Paula Alexandra Quintela Videira, Faculdade de Ciências e Tecnologia da Universidade Nova De Lisboa, PortugalPhilippe Delannoy, Université de Lille, France
Roland Schauer, University of Kiel, Germany
Bernard Vanhove, Centre National de la Recherche Scientifique (CNRS), France
Copyright © 2019 Dhar, Sasmal and Varki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ajit Varki, a1varki@ucsd.edu
 Chirag Dhar1,2
Chirag Dhar1,2 Ajit Varki
Ajit Varki