- 1Department of Zoology, Dr. Harisingh Gour Central University, Sagar, India
- 2Metagenomics and Secretomics Research Laboratory, Department of Botany, Dr. Harisingh Gour Central University, Sagar, India
- 3Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, Saudi Arabia
- 4Mycology and Plant Disease Survey Department, Plant Pathology Research Institute, Agriculture Research Center, Giza, Egypt
- 5Department of Plant Production, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia
The interaction between the human microbiome and immune system has an effect on several human metabolic functions and impacts our well-being. Additionally, the interaction between humans and microbes can also play a key role in determining the wellness or disease status of the human body. Dysbiosis is related to a plethora of diseases, including skin, inflammatory, metabolic, and neurological disorders. A better understanding of the host-microbe interaction is essential for determining the diagnosis and appropriate treatment of these ailments. The significance of the microbiome on host health has led to the emergence of new therapeutic approaches focused on the prescribed manipulation of the host microbiome, either by removing harmful taxa or reinstating missing beneficial taxa and the functional roles they perform. Culturing large numbers of microbial taxa in the laboratory is problematic at best, if not impossible. Consequently, this makes it very difficult to comprehensively catalog the individual members comprising a specific microbiome, as well as understanding how microbial communities function and influence host-pathogen interactions. Recent advances in sequencing technologies and computational tools have allowed an increasing number of metagenomic studies to be performed. These studies have provided key insights into the human microbiome and a host of other microbial communities in other environments. In the present review, the role of the microbiome as a therapeutic agent and its significance in human health and disease is discussed. Advances in high-throughput sequencing technologies for surveying host-microbe interactions are also discussed. Additionally, the correlation between the composition of the microbiome and infectious diseases as described in previously reported studies is covered as well. Lastly, recent advances in state-of-the-art bioinformatics software, workflows, and applications for analysing metagenomic data are summarized.
Introduction
Microbes are ubiquitous in nature, inhabiting almost every conceivable environment, and play an important role in human life. Microbes, though generally invisible, play an essential role in ecosystem functioning (1, 2), modulating key ecosystem processes such as plant growth, soil nutrient cycling, and marine biogeochemical cycling (3–6). An innumerable number of symbiotic, pathogenic, and commensal microbes colonized the human body; collectively constituting the human microbiota. Interactions between the human body and gut-microbiota are widely recognized as influencing several aspects of human health (7). A functioning microbiome is obligatory for host organisms, as it contributes to the smooth functioning of important physiological processes. In fact, host organisms have co-evolved with their microbiota; with some commensals having evolved as pathobionts while others as symbionts (8, 9). The presence of certain commensals in the human gut induces signals that drive proper functioning and maturation of the immune system. Microbial communities take on a specific structure within different hosts and physical environments (10). Consequently, identification and characterization of the microbes inhabiting a host, their distinct host phenotypes, and the biochemical pathways by which microbes impact their hosts are the major focus of host-microbiome research.
Analyses of host-microbe interactions can reveal the core characteristics of the interaction, including their identification, classification, profile prediction, and mechanisms of interaction. Although the structure, function, dynamics, and interactions of these microorganisms play an essential role in human metabolism; their identification, quantification and characterization can be problematic. The majority of microbial communities are extremely diverse and most of the individual organisms have not yet been cultured (11). Secondly, their interaction with each other and tendency to form intricate networks makes it difficult to predict their behavior (3). Establishing mechanistic connections between gut-microbiota and its functioning adds an extra challenge especially in understanding the biology of intricate microbial consortia (12). Classic approaches to microbial ecology have relied on cultivation-dependent techniques to study host-microbe interactions. Although these culture-dependent techniques have generated interesting data sets, they have also resulted in a spurious view of microbiota. Recently, however, a number of culture-independent techniques, mainly PCR-based methods, have evolved for the qualitative and quantitative identification of microbes. These techniques have entirely changed the perception of the human microbiome and have paved the way for the establishment of metagenomics. Metagenomic studies are increasing our knowledge of host-pathogenic interactions by revealing the genes that potentially allow microbes to influence their hosts in unexpected ways. Metagenomic studies of host-microbe interactions can provide useful information applicable to a wide array of disciplines; including pathogen surveillance, biotechnology, host-microbe interactions, functional dysbiosis, and evolutionary biology (13). Recent studies of host microbiomes using metagenomic approaches have offered key insights into host-microbe interactions.
In addition to allowing researchers to characterize the composition of microbial communities, metagenomic studies have also provided novel information on other aspects of the biological sciences. For example, metagenomic studies on the human microbiome have revealed possible links between the gut microbiome and human diseases such as depression (14), rheumatoid arthritis (15) and diabetes (16). Several studies have utilized materials from ancient communities to trace changes in the microbiome. These studies have conducted metagenomic studies of coprolites (17), teeth (18), and other tissues (19). Provided that nucleic acids can be extracted from the sample, almost any material from an environment can be used in metagenomic analyses. One of the largest metagenomic studies to date is the Global Ocean Sampling. Metagenomics is also being applied to the field of medicine. Figure 1 illustrates the timeline of sequence-based metagenomic studies and shows the range of environments that have been sampled and analyzed between 2003 and 2017. Several articles have been published that have focused on metagenomic methodology and analysis software (20–27). The present review attempts to provide an overview of the high-throughput sequencing technologies and analytical software currently available for studying host-microbe interactions. Moreover, there is an attempt to also highlight the advancement of sequencing techniques over time and provide information regarding the appropriateness for applications in exploring the human microbiome and the metagenomes of other diverse environments. Lastly, a discussion is provided of the various bioinformatic options that are available to successfully meet both de novo sequencing and sequence alignment challenges.
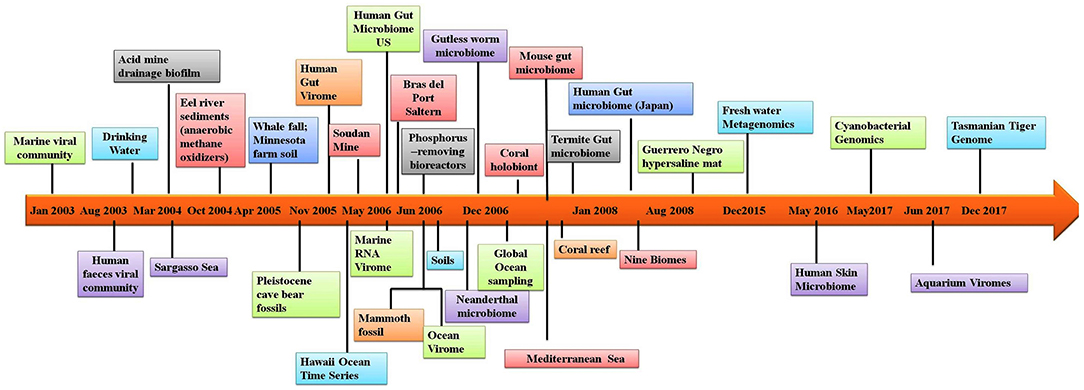
Figure 1. Timeline of the sequence-based metagenomic projects showing the variety of the environmental samples.
Unseen Microbial Diversity And Its Global Implications
Microbes conduct significant functions that greatly benefit the health of planet, as well as its inhabitants. Microbes help to regulate, modulate, and maintain earth's atmosphere (28), support the growth of plants and help to suppress plant diseases (29), contribute to human health (30), breakdown harmful chemicals present in contaminated environments (31, 32), support sustainable farming (33), modulate greenhouse gases (34), are primary components of various biogeochemical cycles (35) and greatly contribute to ecological processes, including climate change (36). In addition to remediating contaminated environments and modulating the atmosphere, the combined activity of these invisible microbial communities shape the face of the biosphere and represent untapped reservoirs of novel biomolecules; including pharmacological drugs and industrial enzymes (37). Microbes coexisting in the human body offer a variety of benefits by modulating fundamental metabolic processes, immunity, and signal transduction. Increasing evidence suggests that there is a significant association between the human gut microbiome and the development of human diseases (38).
Previously, it was difficult to study microbes in their natural environment and thus microbiologists were limited to studying individual species in the laboratory. This approach, however, has limited the data that can be obtained on microbial communities inhabiting diverse ecosystems. Metagenomics has helped to resolve this limitation and has greatly increased our understanding of entire microbial communities, thus significantly advancing our knowledge of microbial ecology and microbiology in general. Metagenomics, supported by next-generation-sequencing (NGS) has literally removed the limitations and boundaries associated with classic culture-based approaches (39–41). NGS technology has enabled the comprehensive study of diverse microbiomes in their native environments, including the ocean microbiome (42), human skin microbiome (43), human microbiome (44) and the Saragossa Sea microbiome (45). Some of the novel findings enabled by metagenomics involve the identification of endosymbiotic bacterial phyla (46), nitrification processes (47, 48), human disease pathogens associated with epidemics (49), bacteria (50), and viruses (51) associated with inflammatory bowel diseases, and the identification of commensal gut bacteria (52).
Microbiome in Human Health And Disease: a Mechanistic Link
The human body serves as a host to a networked community of microorganisms (microbiome) that outnumber the body's own cells. Research on the human microbiome has been the area of immense interest over the past few years due to intimate linkage of the microbiome with human health. The human microbiome “our second genome” has intimately co-evolved with humans for millions of years and plays a critical role in human health. Deciphering the composition and function of the human microbiome can provide a deeper understanding of its' structural and functional properties. In the future, our understanding of the human microbiome and the application of metagenomic analyses will greatly enhance our understanding of human health and disease in specific individuals. The exploration of human microbiome and metagenome is considered to represent a frontier in human genetics.
The majority of research on the human microbiome has focused on the microbes colonizing the human digestive system, as these microorganisms are believed to influence human health in a number of ways. The digestive system microbiome is extremely diverse, with significant variations in its constituents across individuals (44). Modulation of the microbiome by extraneous factors, such as fecal transplantation and dietary intervention, has been shown to be a potential therapeutic approach to addressing a number of health-related problems (53). The gastrointestinal tract (GIT) harbors a vast diversity of microbes, comprising the intrinsic networks of both microbe-microbe and host-microbe interactions (54). Microbial guilds (species that exploit the same resources) have been found to provide an interesting feature that can be used to help understand processes taking place at both a single cell and community level. Microbes under normal physiological conditions are commensal and mediate digestion, strengthen the immune system and inhibit or prevent pathogens from invading the body. The linkage between the human microbiome and human health remains largely unknown and unexplored, however, a number of epidemiological studies have found that the overall reduction in the diversity of digestive system microbiota is linked to diseases such as eczema (55), asthma and inflammatory diseases (56), diabetes and obesity (57), allergies (58), digestive tract disorders such as IBD (inflammatory bowel disease) (59) and IBS (irritable bowel syndrome) (60). Dysbiosis (microbial imbalance) has also been linked with the genesis and evolution of a plethora of other diseases, including chronic fatigue syndrome (61), cancer (62), colitis (63) bacterial vaginosis (64), and anxiety and depression (65). Several recent studies have highlighted the critical role that the gut microbiome plays in modulating different immune responses, including immune tolerance, via Treg (T regulatory) cell modulation. Studies carried out by Geuking et al. (66), indicated that short-chain fatty acids (SCFA) can promote the development of Treg cells in the gut. Gut-inhabiting microbes facilitate the breakdown of complex carbohydrate (67) and help in the utilization of polysaccharides (68). Other examples of the health-supporting functions of the gut-microbiome are protection against diseases via immune modulation (69), fecal microbiome transplantation (70), metabolism, xenobiotic toxicity and pharmacokinetics (71).
The Microbiome as a Therapeutic Agent
As mentioned, the human body is teeming with trillions of microbes, collectively called the “human microbiome.” Microbiome studies have now become a prominent field of research by offering potential and novel methods of disease diagnosis, prognosis, and treatment. Microbial ecology within an ecosystem involves a cross-talk among its inhabitants. The growth and survival of microbes in any ecosystem are largely governed by their chemical environments, and microbes have evolved the ability to adapt and utilize different chemicals through specific genes (72, 73). Alterations (good and bad) in the microbial equilibrium of the gut microbiome do occur. Science has developed medications that have a significant impact on the microbial equilibrium. Beneficial microbes colonizing the gut produce a variety of chemicals, including analgesics, vitamins, antioxidants and anti-inflammatory factors that protect and support the normal functioning of the human body. Dysbiosis (disruptions in microbiota) has been associated with different diseases. Therefore, maintaining a beneficial gut microbiome, in terms of both composition and function, is important for human health (74, 75). The gut microbiome has an active relationship with its human host and exhibits a regulatory role in cognition, mood, pain, and anxiety, exerted through a gut-brain axis. Drastic changes in the maternal microbiome that occur during pregnancy influence the maturation and immunity of neonates. Studies carried out by Ng et al. (76) indicated that increased levels of salicylic acid in the intestines contribute to the proliferation of pathogenic bacteria in the GIT when patients are treated with antibiotics. Roberfroid et al. (77) reported that the consumption of prebiotics (indigestible plant fiber) induces specific changes in the gut microbiome, elevating levels of SCFA (short chain fatty acid). Studies reported by Cani et al. (78) stated that fermentation activity carried out by the gut microbiome results in reduced hunger and increased satiety levels, which as a result, decreases total energy inputs. Similarly, studies carried out by Archer et al. (79) and Whelan et al. (80) confirmed that fermentation of non-digestible carbohydrates by the gut microbiome controls food intake activity and reduces energy intake. According to Parnell et al. (81), prebiotic-induced changes in the gut microbiome of obese patients decreases the circulation of lenomorelin or ghrelin (a hunger hormone) and increases the peptide, tyrosine or PYY. In contrast, however, studies carried out by Peters et al. (82) and Hess et al. (83) indicated that prebiotic treatments do not influence the appetite. A recent study by Tarini et al. (84) demonstrated that a single dose of insulin significantly decreases levels of lenomorelin blood plasma and augments post-prandial plasma levels of Glucagon-like peptide-1. In short, there is a growing body of evidence on the contribution of the microbiome on human health and increased understanding that the microbiome can serve as a potential therapeutic agent.
Dissecting the Host-Pathogen Microbiome
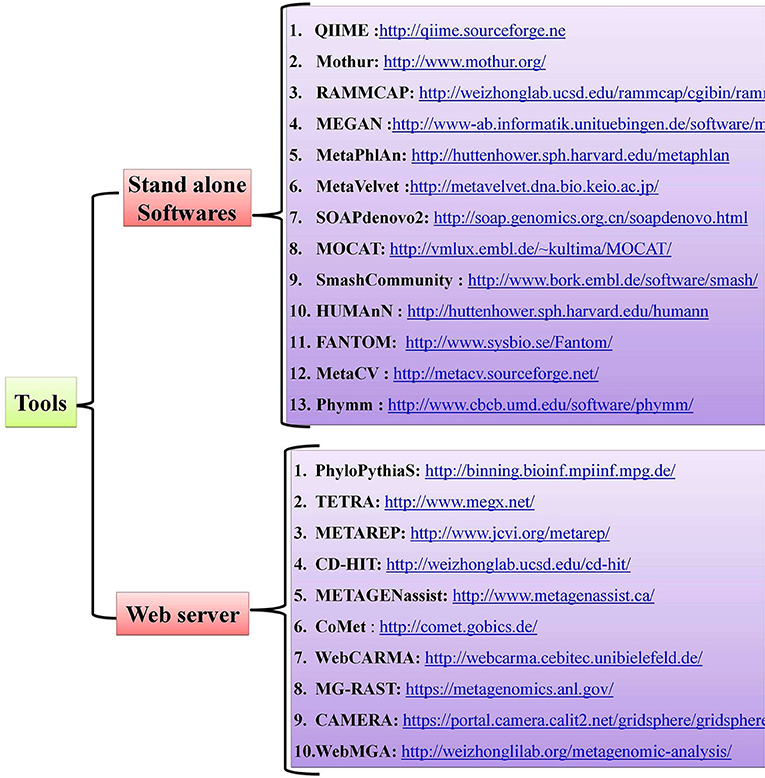
Host-pathogen interactions have profound consequences in human biology and can be viewed as a battle between two systems. Pathogens, which are the invaders, can seize host cells and use them for their own advantage (8), and they can evolve so quickly that they overpower the human immune system, as with HIV infection (85). The conflict between the interacting partners results in phenotypic changes and is believed to be the main driving force for a number of phenomena, such as speciation and the evolution of sex (86). Detailed mechanistic analyses of host-pathogen interactions are varied with most still in need of further study. Notably, little is known about the molecular level dynamics of host-pathogen interactions and the need for more studies on this topic are critical, especially those dealing with the molecular events that regulate phenotypic changes in the host. Advancements in Next Generation Sequencing (NGS) technologies and bioinformatic tools have offered new approaches for studying host-pathogen systems. Researchers are now able to construct the genomes of both model and non-model organisms. The use of these newly-developed tools allows researchers to not only study the behavior of a single gene under different conditions but also study the extensive impact of these host-pathogen interactions on molecular environments (global gene expression). Several open-source, standalone R packages and web-based software programs have been developed to help and acquire key insights in understanding the host-pathogen microbiome (Figure 2). A more detailed account of metagenomic software and resources are given in a separate section of this review wherein we mentioned some of the standard software used for quality control, taxonomic classification, diversity metrics, annotation and functional information, sequence classification, metabolic pathway reconstruction, and statistical analyses.
Microbial Census
Culture-independent methods are the most appropriate for ascertaining the abundance of microbes that are present within a community. DNA re-association kinetics provide information on both community structure and diversity (87). 16S rRNA gene sequencing is one of the main methods used for identifying the microbial taxa present in a community (88). The utility of this approach is based on the fact that the DNA sequence of regions between conserved areas of 16S rRNA vary among different bacterial species and can be species specific. Two different sequencing approaches used for studying microbial communities are (i) the targeted sequencing (16Sr RNA) and (ii) shotgun sequencing of the metagenome. Each of these methods can provide strikingly different results when used in metagenomic analyses. Shotgun sequencing methods are generally considered superior for the identification and characterization of microbial communities, as they typically provide a greater level of diversity compared to amplicon sequencing (89). Amplicon-based sequencing matches the DNA sequence amplified using a set of universal primers based on the highly-conserved 16S rRNA to sequences of known bacterial taxa. In contrast to amplicon sequencing, shotgun sequencing engages a genome-wide approach, utilizing random strings of genomic DNA sequences obtained by breaking total genomic DNA and matching the obtained sequences to an annotated database of known DNA sequences using clade-specific marker genes or common sequences. Shotgun metagenomics is often used for gene cataloging and functional inference (10). Deep sequencing of metagenomic samples, as was used in the Human Microbiome Project and Metagenomics of the Human Intestinal Tract, provides extensive sequence information even of minor components (taxa) present in the metagenome. This allows for the identification and characterization of the genes present within a given microbial community. The obtained sequences reads can either be used directly or first assembled into contigs, which are then compared to an available database for the identification of specific genes. De novo gene prediction is also possible (90), which may identify motifs with functional inference. Gene catalogs can also be compared with databases such as KEGG (the Kyoto Encyclopedia of Genes and Genomes) (91), which arranges the gene products into biological processes and pathways (Figure 3).
Metagenomics and Microbial Studies
Metagenomics is expected to play a major role in advancing our understanding of microbes and microbial communities. It is tempting to suggest that metagenomics can serve as a “universal test” for pathogens, eliminating the need to perform lengthy serial testing involving specific assays. Recent advances in sequencing techniques allow almost the entire genome of individual microorganisms to be assembled directly from environmental samples. Metagenomic analyses are playing a decisive role in the characterization of human microbial communities, as well as in determining the relationship between the resident microbiome and invasive pathogens. The accumulation of sequencing data has enhanced our recognition and understanding of the changing nature of microbial populations and their impact on the environment (92) and on human health (93). Metagenomics is not only helping to identify and characterize the human gut microbiome but is also identifying novel genes and microbial pathways, as well as functional dysbiosis. Clearly, metagenomics has become an indispensable and fast-growing discipline in modern science. Advances in NGS has led to a substantial increase in the number of metagenomic studies listed in the Genomes Online Database (GOLD) (https://gold.jgi.doe.gov). These studies span a broad environmental spectrum, including natural communities; as well as engineered and clinical environments (94, 95).
Study of Microbiome Prior To NGS
Prior to the advent of NGS technologies, the accurate profiling of microbial communities was challenging. The same was true for characterizing the human gut microbiome, a highly dense and diverse community containing only a small proportion of microbes that could be cultured (96). Early studies of the human gut microbiome involved the culturing of the microbes present in samples (97) and studying the interactions between co-cultured microbial taxa (98). These techniques, however, provided information on only a limited set of microbial taxa and microbial interactions. They failed to provide information about the composition of the entire community and the dynamics occurring between the taxa comprising the total community. The emergence of NGS technologies has overcome the limitations characteristic of studies based on culturing techniques.
Deciphering Host-Pathogen Interactions In The Era Of NGS Technologies
The advent of NGS technologies have greatly enhanced the ability to identify and characterize metabolic and regulatory mechanisms through which hosts and microbes interact with each other to define a healthy or diseased state in the host organism. NGS technologies are invaluable for the exploration of the composition of the microbiome and exploring the genetic, functional, and metabolic properties of the microbial community. Sanger sequencing (99), the first generation of DNA sequencing technology, was one of the widely used sequencing method for more than three decades and is still used today for low-throughput DNA sequencing or sequencing of single DNA entities. Sanger DNA sequencing is based on the principle of the selective incorporation of chain-terminating dideoxynucleotides by DNA polymerase. This technique was the major approach used in the Human Genome Project in 2001. The high cost of Sanger sequencing and volume (number of sequences) limitations reduced its potential for high-throughput sequencing.
Exploring Host-Pathogen Interactions
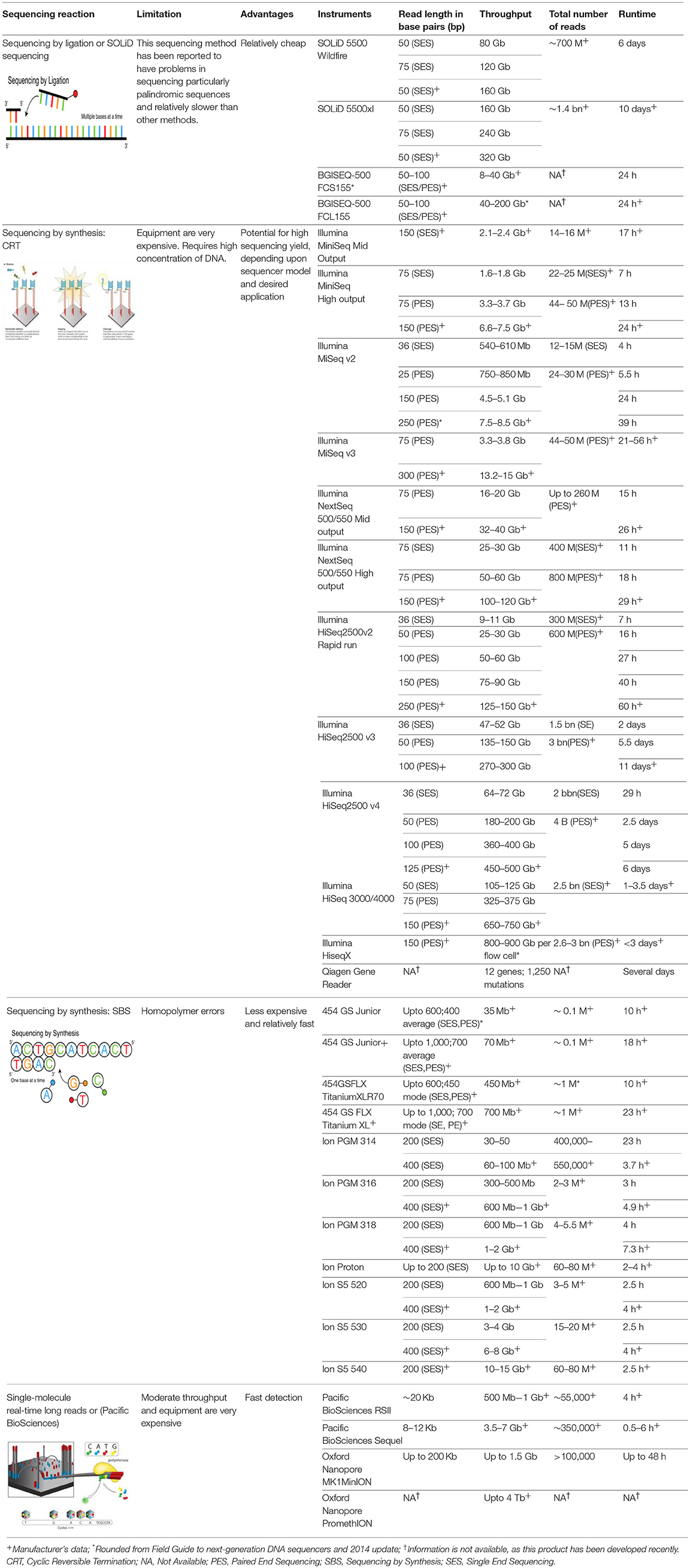
Advances in NGS technologies now provide a fast, cost-effective approach to delivering large volumes of highly-accurate data that has resulted in a major paradigm shift over the past few decades (100, 101). Time and cost were originally the main stumbling blocks associated with sequencing technology. The advantages of NGS over classic Sanger sequencing are that it is cost-effective, devoid of a cloning step, offers highthroughput, and requires minimal technical expertise. A major challenge with NGS data, however, is the analysis of millions of sequences that allows one to achieve statistically and scientifically meaningful conclusions (Table 1).
Several different NGS platforms have been developed (Figure 4) and are commonly used. These include the Roche 454 GS FLX, Illumina (MiSeq and HiSeq), Ion Torrent/IonProton/Ion Proton, SOLiD 5500 series, and Oxford Nanopore. At present, the majority of microbial studies using high-throughput sequencing have focused on either functional metagenomics (103) or amplicon sequencing (104).
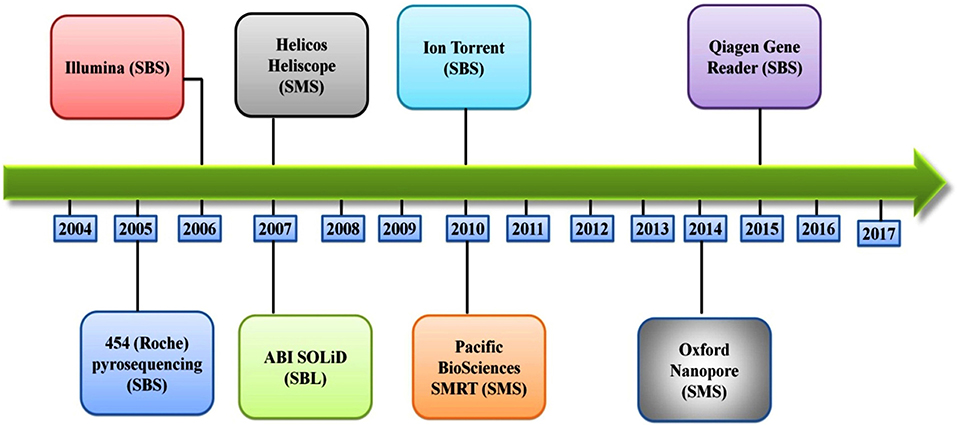
Figure 4. Timeline of the introduction of the next-generation DNA sequencing technologies and platforms.
Roche 454 Genome Sequencer
This sequencing platform is based on the principle of pyrophosphate release, which was originally described by Nyrén et al. (105) in 1985 and reported by Hyman (106) in 1988. Roche 454 was produced and made commercially available in 2005, and advertised as the first available high-throughput sequencing system. The system utilizes sequencing by synthesis (SBS), in which adapters are ligated to DNA fragments that cause the binding of the fragments to microbeads in a Pico Titer Plate (https://www.roche.com/). Amplification of the DNA fragments is carried out by Emulsion PCR, in which water droplets containing a single bead and PCR reagents are immersed in oil. The long read length (400–500 bases with paired-ends), along with its high efficiency, were more advantageous than what other NGS platforms could provide at that time; and thus was used for genome sequencing. The system generates 20 Mb of sequences per run with an average read length of approximately 100 bp (107). One of the notable applications of the Roche 454 system was the identification of the agents responsible for the epidemic disease of honeybees (108). Additional information about Roche 454 Genome Sequencer can be obtained at http://www.roche.com.
Illumina Genome Sequencer
The Illumina sequencing platform first emerged in 2006, and was followed by the acquisition of Solexa by Illumina in 2007. Illumina possesses an array of the most commonly sequencing systems and has rapidly been adopted by many researchers throughout the world. This is due to its' cost-effectiveness, and longer read length (although a limitation in the earlier version of the Illumina, which was subsequently improved in the newer version, MiSeq 2 × 300 bp). This led to a major shift by the scientific community from using the Roche 454 platform to Illumina technology (109). Illumina follows the principle of SBS chemistry, by incorporating reversible chain terminator nucleotides for all four bases, the labeling of each base with a different fluorescent dye, and the use of a DNA polymerase (110). Sequencing involves the ligation of specific adapters to both ends of short DNA fragments, and the immobilization of one of the adapters by binding to a solid support. The adapters hybridize with specific oligonucleotides bound to a proprietary substrate within a micro fluid flow cell. Fluorophore-bearing nucleotides are then introduced one by one and incorporated into the growing complementary strand by a DNA polymerase. Sequential images are captured and analyzed to identify the nucleotide that is incorporated in the growing strand and the cycle is repeated with different nucleotide species. The resulting reads have a final length of 35 nucleotides (111).
Illumina, however, introduced an upgraded version of their technology, the Genome Analyser II, which tripled the output relative to earlier versions of the Genome Analyser. Presently, the IlluminaMiSeq offers one of the longest (300 bp) read lengths of all of the Illumina products; facilitating the sequencing of paired-end reads (104). Another Illumina platform, the Illumina HiSeq, however, is able to generate approximately 200 Gbp of sequences with a single read of 2 × 100 bp (paired-end) per run (112). Additional information about the various Illumina sequencers can be obtained at http://solexaqa.sourceforge.net/ (113).
Qiagen Gene Reader
In 2012, Qiagen introduced the Intelligent BioSystems cyclic reversible termination platform, which was commercialized in 2015 under the name Gene Reader (114). In contrast to other next-generation platforms, the Qiagen Gene Reader is the first all-in-one platform that can execute all of the steps required for sequencing DNA, from sample preparation to analysis. To achieve this goal, the Gene Reader sequencer was combined with the QIA cube sample preparation system and the Qiagen Clinical Insight platform for variant analysis. Gene Reader virtually utilizes the same approach as Illumina, apart from the fact that only a small fraction of the added nucleotides incorporate fluorophore-labeled dNTP (115). Qiagen's Gene Reader usually runs up to four flow cells at a time, with each flow cell running up to ten samples. The flow cells can be added in mid-run via a “turntable” within the instrument. Additional information on the Qiagen Gene reader can be obtained at http://www.qiagen.com.
ABI SOLiD (Sequencing by Oligonucleotide Ligation and Detection) System
Applied Biosystems, through the Life Technology subsidiary, introduced the SOLiD platform in 2007. The system employs a unique chemistry for sequencing by ligating oligonucleotide adapters to DNA fragments and immobilizing the ligation products on beads, which are then placed on a water-oil emulsion (116). The beads on which DNA amplification occurs are deposited on glass slides and subjected to sequential hybridization with a universal PCR primer complementary to the adapters. The ligation step is then followed by fluorescence detection.
Ion Torrent Sequencing Technology (PGM, Proton, S5 Series)
Ion Torrent introduced the personal genome machine (PGM) in 2010 as a cost effective platform for DNA sequencing (117). Unlike other sequencing technology, Ion Torrent does not make use of optical signals (118) but rather utilizes an enzymatic cascade to generate a signal. The Ion Torrent system utilizes high-density micro-machined wells to carry out nucleotide additions in a massively parallel approach. Each micro-well contains a different DNA template. There is an ion-sensitive layer and an ion-sensor located under each well. The technology works on the principle of detecting the proton (H+) released during the incorporation of each dNTP in a growing DNA template. The release of H+ ion results in a change in pH that is detected by an integrated ion-sensitive, field-effect transistor (ISFET) (117). In the case of two identical bases, the output voltage is doubled. Ion torrent platform can generate upto 10 Gb of sequence data in a single run, with a maximum of 50 million reads having an average read length of 200 bases. The PGM can also provide 5.5 million reads having an average read length of 400 bp, producing a maximum of 2 Gb of sequence data from 318 V2 chip. A notable aspect of this technology is the size-selection step in which sequencing of longer fragments is omitted (https://www.thermofisher.com/in/en/home/brands/ion-torrent.html). Additional information about Ion Torrent technology can be obtained from https://www.thermofisher.com/in/en/home/brands/ion-torrent.html.
The Third Generation of Sequencing Technology
At present, the described sequencing technologies are the most commonly used for metagenome projects, however, sequencing technologies have undergone rapid advances during the past few years to attempt to resolve the biases associated with the current methods and to obtain a better balance between data yield, read length, and cost. These efforts have resulted in third generation sequencing technologies, such as Oxford Nanopore (119), and PacBiosequencing platforms (120) which are single-molecule and real-time technologies that reduce amplification bias, as well as short read length problems. The reduction in the cost and time presented by these sequencing methods are valuable asset. Although the error rate with the newer technologies is much higher relative to the other described sequencing technologies, this problem can be addressed by increasing the sequencing depth.
Pacific Biosciences
Pacific Biosciences established the first DNA sequencer that utilizes a single-molecule, real-time sequencing (SMRT) approach. This sequencing platform has become one of the most widely used third-generation sequencing technologies (121). The platform is based on the sequencing by synthesis principle. Pacific Biosciences makes use of the same fluorescent dyes as other NGS technologies, however, instead of carrying out the cycles of nucleotide amplification in the same manner as other sequencing technologies, the signals emitted upon the incorporation of the nucleotides are detected in real time. Sequencing is carried out on a chip (SMRT cell) that contains several zero mode wave (ZMW) guides. A single DNA polymerase is immobilized to the bottom of each ZMW guide with a molecule of single stranded DNA template (122). Four phospholinked nucleotides, each labeled with a different fluorescent dye producing a distinct emission spectrum, are also added to SMRT cells. Once the nucleotide is incorporated by the DNA polymerase, a light signal is produced and a base call is made and recorded (122).
Helicos Biosciences
Heliscope was released by Helicos Biosciences in 2007. It is also a single-molecule sequencing device. Sequencing is carried out in a glass flow cell with 25 channels for samples. The samples can either be replicates of the same sample or different samples. The Heliscope platform utilizes emulsion PCR amplification of DNA fragments in order to obtain significantly higher signals for reliable base detection by multiple charge-coupled device cameras. Single-molecule sequencing methods have the potential to deliver consistently low error rates by eliminating amplification-related bias, intensity averaging, and synchronization problems (123, 124). In the Heliscope platform, 100–200 oligonucleotide fragments are initially immobilized on a proprietary substrate within a microfluidics flow cell. Fluorescence-labeled nucleotides are then introduced individually and are incorporated by DNA polymerase into the growing complementary strand. The fluorophore-bearing nucleotide increases detectability and eliminates the need for amplification of the DNA template. Images are recorded and analyzed to identify the nucleotide that has been incorporated into the growing strand before the cycle begins with a different fluorescently-labeled nucleotide. At present, the Heliscope can only provide a read length of 35 nucleotides (111). Additional information can be obtained at http://www.helicosbio.com.
Oxford Nanopore Sequencing
Oxford Nanopore Technologies (ONT) is at the forefront of developing nanopore sequencing technology (http://www.nanoporetech.com/). The Nanopore platform does not require an amplification step as a part of library preparation. The novelty of this approach is that the DNA strand to be sequenced can be directly analyzed. Oxford Nanopore Technologies introduced the MinION (125) device in 2014. It has the potential to provide longer reads with better resolution of repeated sequence elements and structural genomic variants (126). MinION is a mobile, single-molecule Nanopore sequencer measuring four inches in length and is connected to a laptop with USB 3.0. Nanopore sequencing technology is based on the principle of modulation of the ionic current as a DNA molecule traverses through the nanopore, revealing characteristics of the molecule such as conformation, length and diameter. The pore consists of a protein within a conductive electrolytic solution which creates a small potential gradient across the protein pore (127). MinION mk1B is a pocket-sized portable sequencing device, containing 512 nanopore channels, and can be directly linked to a computer for data collection. More recently, a more advanced device, “PromethION,” has been commercialized (127). PromethION is a benchtop sequencer possessing 48 individual flow cells, each consisting of 3,000 pores that are equivalent to 48 MinIONs processing 500 bp/s (128). The capabilities of this instrument provide sequencing power that is sufficient to conduct sequencing of large genomes, such as the human genome. Additional information on Oxford Nanopore sequencing technologies can be obtained at https://www.nanoporetech.com.
So far, the present review has provided an overview of the first through third generations of sequencing technology that have provided significant improvements in the ability to conduct microbiome research. Metagenomic and other omic approaches are the most effective methods that can be used to characterize microbial communities, as well as their metabolic activity. It is now feasible to obtain information on the composition (taxa), diversity, pathogenesis, evolution, and drug resistance of microbes. The selection of any of the above mentioned platforms, however, should be mainly dependent on the aim, design, and purpose of the study. Illumina sequencing technology has made tremendous advances in data output and cost efficiency over the past few years and as a result, presently dominates the NGS market (129, 130). Illumina sequencing technology has been used extensively in numerous microbiome research projects (131–133), including the Human Microbiome Project (44). While both Ion Torrent and Illumina sequencers provide a number of advantages in terms of their cost and efficiency, the short read lengths they provide make them less appropriate for addressing a number of scientific questions, including detection of gene isoforms, methylation detection, and genome assembly (118). SMRT (single-molecule real-time) sequencing platforms offer approaches that are more suited for these research objectives. Since PacBiosequencing generates longer reads that provide longer scaffolds (134–136), it is well suited for denovo genome assembly. The commercial availability of MinION sequencers by Oxford Nanopore Technologies, which resemble a USB flash drive in appearance, has also enabled applications that require long-read sequencing (137, 138). The efficiency, long read lengths, and single-base sensitivity make nanopore sequencing technology a promising approach for high-throughput sequencing. The MinION system has been used for sequencing the genomes of infectious agents, including the analysis of bacterial antibiotic resistance islands (137), the influenza virus (139) and genome surveillance of the Ebola virus (140). The advancements in high-throughput sequencing technologies now provide the opportunity to choose different sequencing platforms to conduct microbiome research. In a comparative analysis of the Illumina MiSeq, Ion Torrent PGM, and 454 GS Junior sequencing platforms, Loman et al. (141) reported that Illumina provided the highest output per run (1.6Gb/run, 60Mb/h) and the lowest error rates. In a study comparing different sequencing platforms (Ion Torrent PGM, Illumina MiSeq and HiSeq) for the shotgun sequencing of six human stool samples, Clooney et al. (142) concluded that the best assembly values were obtained using the Illumina HiSeq platform, in which 10 million reads per sample were produced. In contrast, the Illumina MiSeq and Ion Torrent PGM did not produce a sufficient number of reads to produce an adequate genome assembly (143).
Correlation Between the Microbiome and Infectious Diseases
Human gut microbiome signatures exhibit individual specificity. There is a high degree of inter individual variation that is based on both host genetics and environmental factors (144, 145). The high degree of individual specificity, however, has hampered our understanding of function of the gut microbiome and its importance in health and disease. The human gut microbiome exhibits a high degree of plasticity, mainly in response to dietary changes that support a healthy gut ecosystem and minimize disease risk (146). The onset of new methodologies, including NGS and bioinformatic pipelines, have resulted in a paradigm shift in the fields of clinical microbiology and infectious diseases due to the realization of the complex interactions that occur within the microbiome. The relationship between human pathogens, infectious diseases, and the gut microbiome are slowly being revealed. Several studies have examined the correlation between the human gut microbiome and health status (141, 142). Reports have indicated that while the gut microbiome appears to be relatively stable under healthy conditions, any qualitative or quantitative changes in the gut microbiome can result in functional modifications and disease as reported (144, 147–149). A rich level of bacterial diversity is considered to be an indicator of a healthy status, while a low level of bacterial diversity is correlated with inflammatory, immune, and obesity-related diseases (58, 144, 147–153). Several studies have indicated that the human microbiota plays a crucial role in human health and disease (68, 154–168). Studies have also revealed that microbial symbiosis plays a central role in the development of a number of diseases, including liver diseases (156), metabolic disorders (154), gastrointestinal (GI) malignancy (157), respiratory diseases (158), autoimmune diseases (160), and mental or psychological diseases (160). Johnson et al. (169) discussed the Bacteroidetes, one of main components of the microbiome, their genetic variability and contrasting effect on metabolic diseases such as obesity and type II diabetes (169). Yiu et al. (170) proposed that body weight, metabolism, and diseases such as obesity are affected by the interplay between the immune system, metabolism and microbiome (170). In discussing chronic IBD, Frick and Wehkamp (171) outlined some of the available therapeutic interventions that can be used to alter mucosal immunity and the composition of the microbiome. While studying the molecular aspects of human gut-brain interactions, Lee et al. (172) demonstrated how the microbiota influences host physiology and neurodegenerative and neurological developmental diseases (172).
Bioinformatic Pipelines for Metagenomic Data Analysis
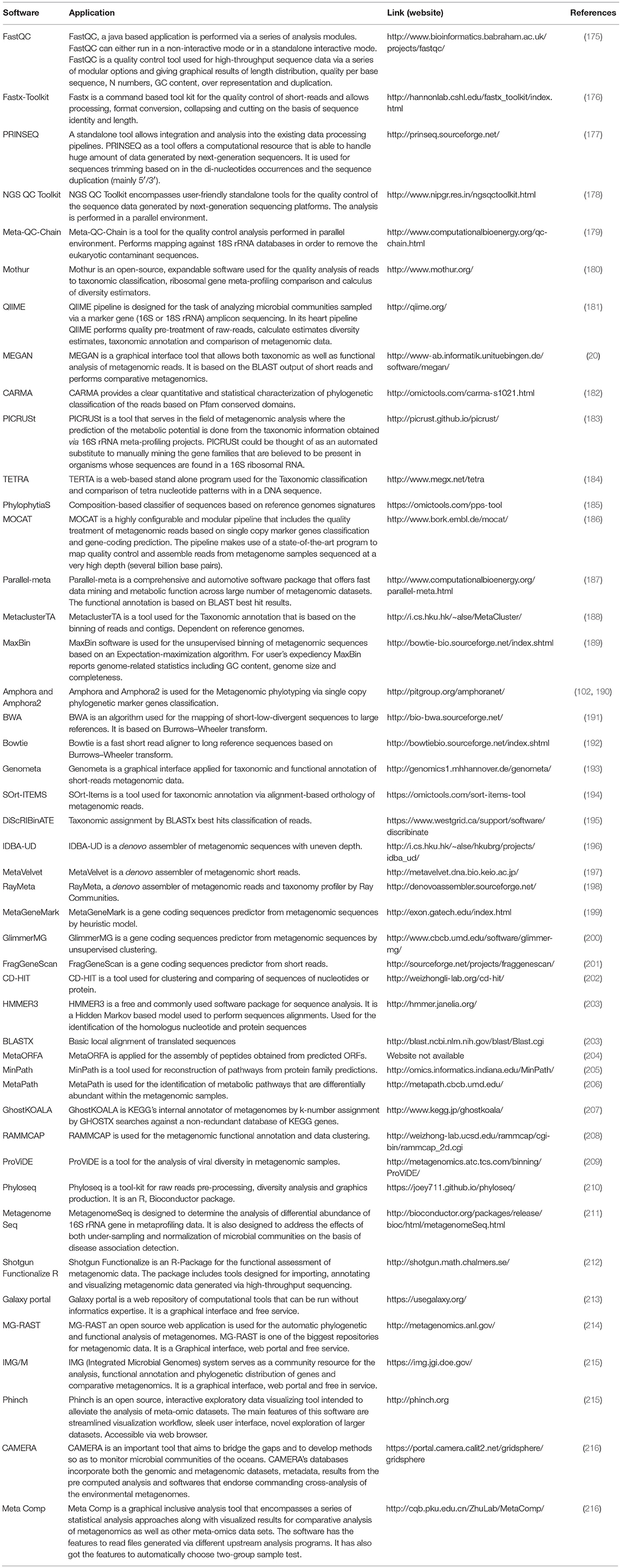
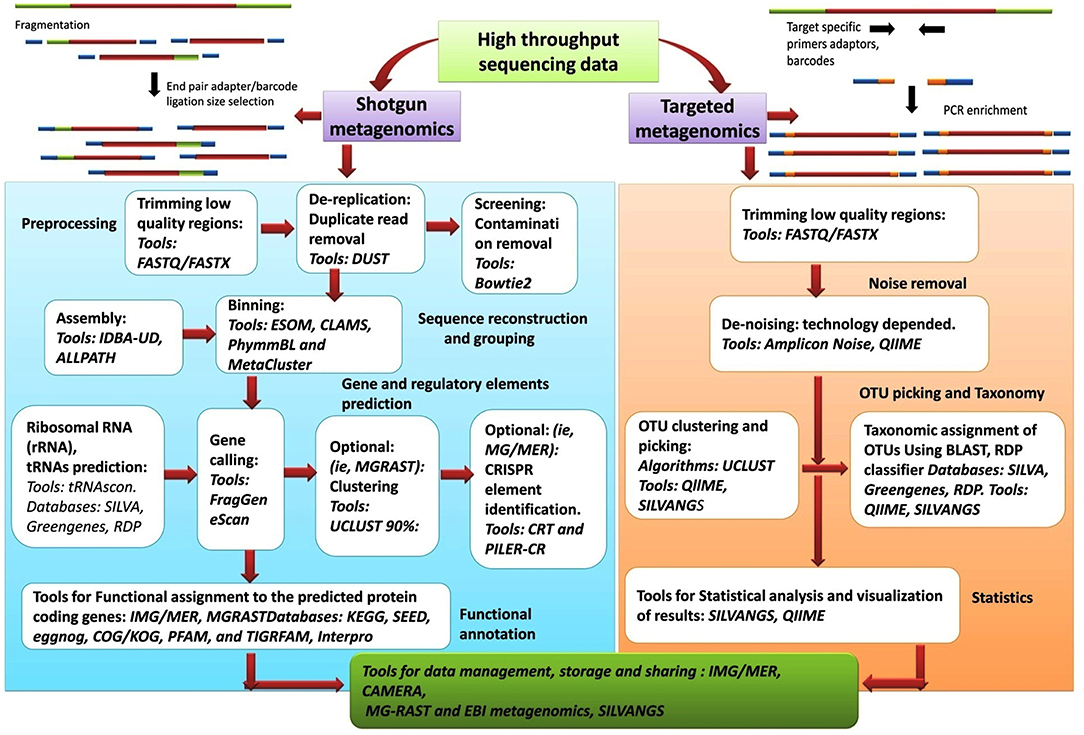
The advances in NGS have resulted in the production of massive datasets that are increasingly difficult to analyse (128). As larger datasets are generated, more sophisticated computational resources and bioinformatic tools are required. The interpretation and understanding of metagenomic studies depend on the computational tools that can be used to analyse enormous data sets and mine valuable, useful, and valid information regarding the microbial communities being studied. Bioinformatic tools used for metagenomic analysis, especially for translating raw sequences into meaningful data, are continually developing with the aim of providing the ability to examine both the taxonomic and the functional composition of diverse metagenomes (173, 174). A number of the specialized software programs available for analysing the metagenomic data are listed (Table 2). Based on the list provided, an example of a comparative analysis pipeline is presented in the present review that takes into consideration user friendliness, ease of access, open source availability, ability to analyse metagenomic datasets, and ability to provide graphical representations of the analyzed data (Figure 5). A description of the software (MG-RAST, EBI, QIIME and Mothur) used in the different pipelines is described in Table 3, which provides a detailed summary of the functionality and features of the mentioned software programs. The four pipelines share several steps during the analysis such as quality control, clustering, and annotation (Figure 5).
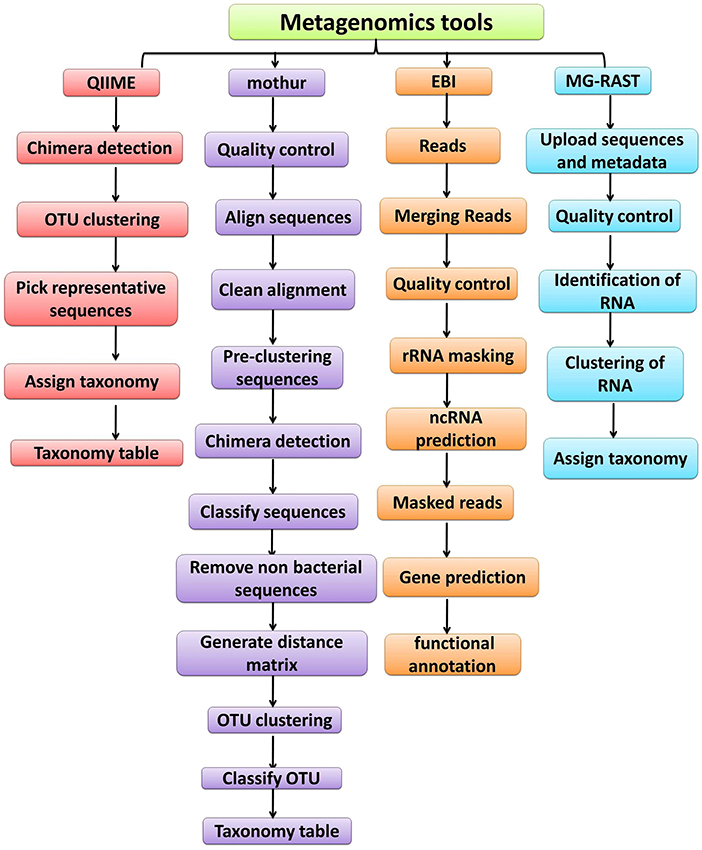
Figure 5. Overview of the workflow used by metagenomic analysis tools (QIIME, Mothur, EBI and MG-RAST).
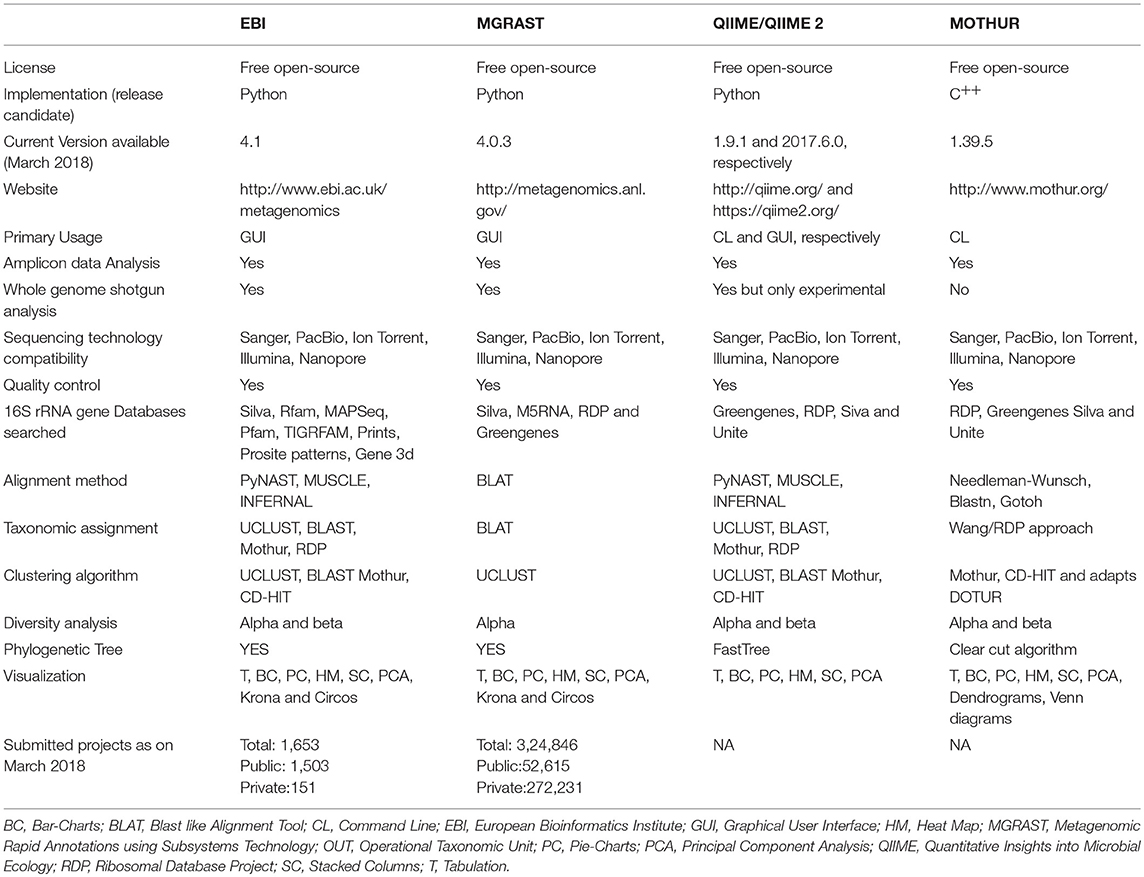
Table 3. Comparative workflow of the four most commonly used bioinformatics pipeline for analyzing metagenomic datasets.
Metagenomic Data Analysis Software: Command Based Vs. Graphical User Interface
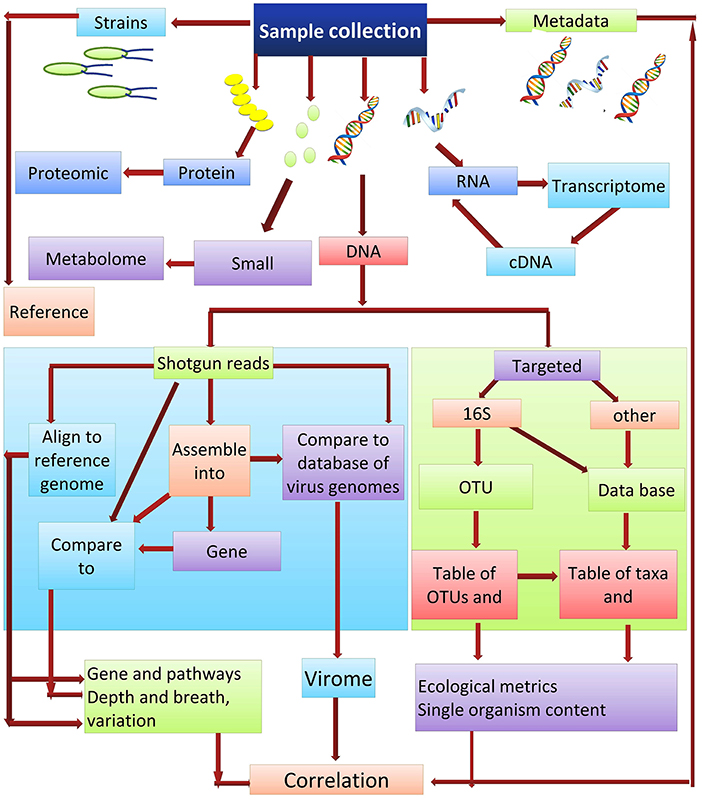
As comprehensive metagenomic studies are becoming more common, they are yielding novel and important insights into the microbial communities in diverse environments; from terrestrial to aquatic ecosystems and from human skin to the human gastrointestinal tract. Advances in NGS have made it more possible than ever for researchers to conduct whole genome sequencing. The analysis of the datasets obtained from NGS is complex and require an intelligent and systematic approach to process the data efficiently. The results obtained from any metagenomic study relies on in silico computational tools that can analyse large data sets and can mine and highlight various aspects about the community being examined. Although the tools and databases developed to investigate the taxonomic composition of a microbial community and provide information on the functional aspects of the community are becoming more elaborate and complex, though CLC microbial genomic package offered by Qiagen are good for these analysis. Nanopore sequencing technology has presented an option for an analysis pipeline, with novel options for assembly and annotation. Figure 6, presents the workflow involved in metagenomic analysis, and indicates all the steps and tools used for analyzing the data generated from metagenomic sequencing. The metagenomic pipeline can utilize any of the presented approaches, based on type of sequencing data (targeted metagenomics or shotgun metagenomics). The flowchart summarizes the basic steps that are followed in the analysis pipeline starting from preprocessing of the sequencing data to the final extraction, storage, and presentation of the data. The most popular tools, along with the databases and algorithms employed for the analysis, are indicated.
Technology and the Changing Landscape of Metagenomic Research
Over the past decade, advancements in NGS have led to a significant reduction in the cost of genome sequencing. These technological advances have enabled the sequencing of several genomes in a day at a cost of approximately $1,000 per genome (Figure 7). The cost estimates presented in Figure 7 represent (A) cost in U.S, dollars per Mb of sequence data from 2001 to 2009, (B) cost in U.S, dollars per Mb from 2009 to 2017, (C) cost in U.S, dollars per Genome from 2001 to 2009, and (D) cost in U.S, dollars per Genome from 2009 to 2017. Although sequencing is now relatively easy and straight forward, NGS technology is not perfect and errors in the data do occur. Moreover, some regions of the DNA have not been successfully sequenced. The underlying costs associated with different approaches to sequencing genomes are of great importance because they impact the scope and scale of genomic projects. Decreases in sequencing costs have led to the establishment of large collaborative projects with broad goals and individual laboratories targeting more specific questions.
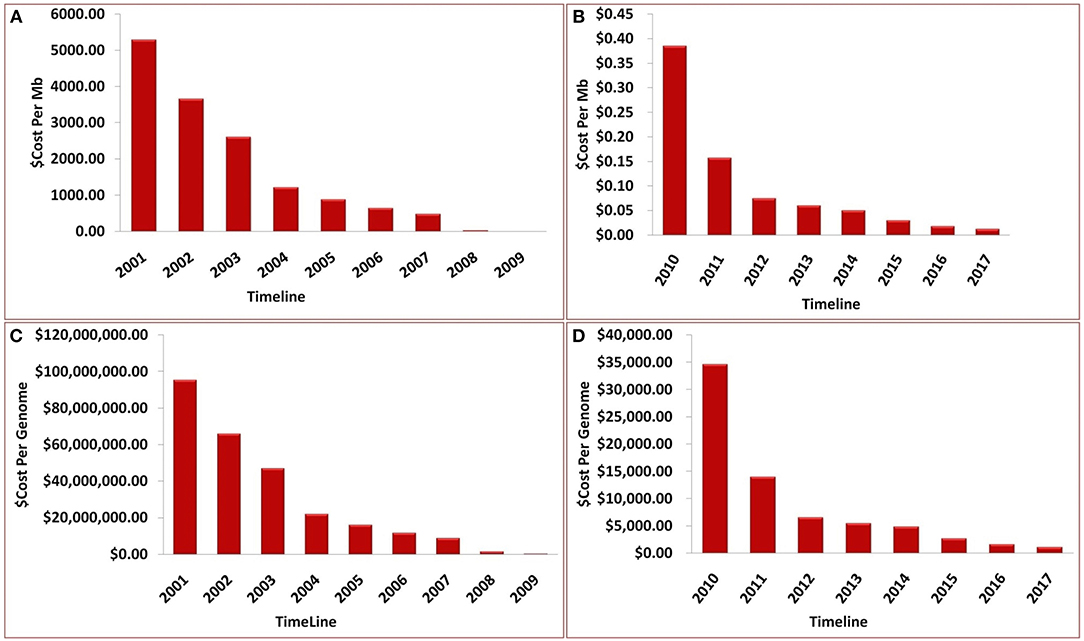
Figure 7. Timeline showing the sequencing cost (A) per Mb until year 2009, (B) per Mb between year 2009 and 2017, (C) per genome until year 2009, (D) per genome between year 2009 and 2017.
The decreasing cost structure of DNA sequencing has had and will continue to have an impact on genomics and bio-computing. With the size of databases expanding continuously, the translation of data into biological insight is becoming more and more important. As a result, data analysis a more prominent aspect in obtaining information and value from the data (217). Significant analytical efforts are needed to gain useful insights from the generated data. The fields of microbiology, biotechnology, and medicine are already benefiting from genome sequencing efforts, and as costs continue to decrease, the practice of genome sequencing is expected to become almost routine. For example, the Sanger Institute is sequencing the genomes of patients suffering from cancer and rare diseases as part of the 100,000 Genomes Project organized by Genomics England.
Some patients have already benefitted from metagenomic-based diagnoses and treatments, and researchers are continuing to gain more knowledge about the genetic variations that cause a variety of diseases. Sequencing, however, is not the only option for genetic analysis. An important part of the Precision Medicine Initiative, organized by the US National Institute of Health, is to develop a more predictable and possibly less technically complex method of genetic analysis. Sequencing, however, appears to be the only way to comprehensively explore the complex features of DNA that guide the initiation and progression of a number of diseases. Additionally, comprehensive sequencing also helps determine how our DNA keeps us healthy (218).
Future Perspectives of Metagenomics and Human Health
Though the field of metagenomics pre-dates NGS, modern high-throughput sequencing technologies have greatly transformed this promising field by enabling a comprehensive characterization of all microorganisms present in a sample. As metagenomic approaches become more developed and clinically corroborated, it is expected that metagenomics will be at the forefront as a method for diagnosing infectious diseases. When a complex or unknown infectious disease is encountered, the use of multiple conventional diagnostic tests can potentially lead to unnecessary expenses; more importantly, this can also result in the delay of a diagnosis. Metagenomics can be used to identify potential pathogens, both known and novel, and can also be used to assess the state of an individual's microbiome. As sequencing become easier, faster, and more cost-effective, it will be possible to serially characterize the human microbiota to explore changes that occur in the human microbiome over time. This knowledge could lead to the development of novel medicines and approaches for treating infectious diseases. Indeed, metagenomic studies may become so standard that DNA sequencers could be used in homes to monitor changes in the stool microbiome of an individual to guide the maintenance of health.
Conclusions
All forms of life on this planet are dependent on microbes. They define an environment and are in turn defined by it. Our understanding of host-pathogen systems, however, is only in its infancy. Over the past two decades, sequencing technology, along with bioinformatic tools, have improved significantly; making it feasible to explore microbial communities residing within diverse hosts. There is a strong recognition that the microbial diversity existing in extreme habitats has largely been unexplored. To gain insight into this “latent” microbial flora, novel methodologies are required. NGS technologies have provided a rapid, cost-efficient means of generating sequencing data and provided sequencing platforms that can be used in large genome-sequencing centers, as well as individual laboratories. Illumina, PacBio, and Applied Biosystems, have all announced upgraded versions of their respective DNA-sequencing platforms. These upgrades will increase high-throughput ability and read length, while at the same time significantly reduce the cost of sequencing per base. These developments will significantly contribute to and provide exciting new opportunities to microbiologists. The integration of several approaches to biological studies will be necessary to answer questions about the diversity and ecology of microbial flora. It is the opinion of the authors of the present review that the development of better bioinformatic tools for analysing metagenomic data is urgently needed. The vast amounts of metagenomic data that will be forthcoming will bring new challenges for analysing, storing, and transferring data. Genome-sequencing centers and laboratories are going to become more dependent on information technology and bioinformatics. Bioinformatic expertise will increasingly be necessary to analyse large amounts of data and to mine the data for useful information about microbial diversity. Metagenomics will play an increasing role in the fields of medicine, biotechnology, and environmental science. The authors hope that this review provides a clear overview of the sequencing platforms and bioinformatic analysis of software that are available, including their high value and limitations.
Author Contributions
MM prepared the draft of the manuscript under the guidance of AK and SY. AD prepared the illustrations. EA and AH edited the manuscript.
Funding
MM (Y15465008) was supported by the University Ph.D. Fellowship for this study. AD had financial support through the DST Inspire Ph.D. Fellowship (IF160797) from the Department of Science and Technology, New Delhi, India. AH and EA would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the Research Group No. (RGP-271).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
PCR, Polymerase chain reaction; NGS, Next generation sequencing; GIT, Gastrointestinal tract; IBD, Inflammatory bowel disease; IBS, Irritable bowel syndrome; SCFA, Short-chain fatty acids; PYY, Peptide YY; HIV, Human immunodeficiency virus; KEGG, Kyoto Encyclopaedia of genes and genomes; GOLD, Genomes Online Database; SBS, Sequencing by synthesis; dNTP, Deoxyribonucleotide triphosphate; PGM, Personal genome machine; ISFET, Ion-sensitive, field-effect transistor; SMRT, Single-molecule, real-time sequencing; ZMW, Zero mode wave; ONT, Oxford Nanopore Technologies; MG-RAST, Metagenomics Rapid Annotation using Subsystem Technology; EBI, European Bioinformatics Institute; QIIME, Quantitative Insights Into Microbial Ecology.
References
1. Fierer N. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol. (2017) 15:579. doi: 10.1038/nrmicro.2017.87
2. Bardgett RD, van der Putten WH. Belowground biodiversity and ecosystem functioning. Nature (2014) 515:505. doi: 10.1038/nature13855
3. Fuhrman JA. Microbial community structure and its functional implications. Nature (2009) 459:193. doi: 10.1038/nature08058
4. Van Der Heijden MGA, Bardgett, RD, Van Straalen NM. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett. (2008) 11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x
5. Graham EB, Knelman JE, Schindlbacher A, Siciliano S, Breulmann M, Yannarell A, et al. Microbes as engines of ecosystem function: when does community structure enhance predictions of ecosystem processes? Front Microbiol. (2016) 7:214. doi: 10.3389/fmicb.2016.00214
6. Hamady M, Knight R. Microbial community profiling for human microbiome projects: tools, techniques, and challenges. Genome Res. (2009) 19:1141–52. doi: 10.1101/gr.085464.108
7. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature (2010) 464:59–65. doi: 10.1038/nature08821
8. Kahn RA, Fu H, Roy CR. Cellular hijacking: a common strategy for microbial infection. Trends Biochem Sci. (2002) 27:308–14. doi: 10.1016/S0968-0004(02)02108-4
9. Vieira SM, Pagovich OE, Kriegel MA. Diet, microbiota and autoimmune diseases. Lupus (2014) 23:518–526. doi: 10.1177/0961203313501401
10. Weinstock GM. Genomic approaches to studying the human microbiota. Nature (2012) 489:250–6 doi: 10.1038/nature11553
11. Kallmeyer J, Pockalny R, Adhikari RR, Smith DC, D'Hondt S. Global distribution of microbial abundance and biomass in subseafloor sediment. Proc Natl Acad Sci USA. (2012) 109:16213–6. doi: 10.1073/pnas.1203849109
12. Zhao S, Wang J, Bu D, Liu K, Zhu Y, Dong Z, et al. Novel glycoside hydrolases identified by screening a Chinese Holstein dairy cow rumen-derived metagenome library. Appl Environ Microbiol. (2010) 76:6701–5. doi: 10.1128/AEM.00361-10
13. Rosario K, Breitbart M. Exploring the viral world through metagenomics. Curr Opin Virol. (2011) 1:289–97. doi: 10.1016/j.coviro.2011.06.004
14. Foster JA, Neufeld K-AM. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. (2013) 36:305–12. doi: 10.1016/j.tins.2013.01.005
15. Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. (2011) 7:569. doi: 10.1038/nrrheum.2011.121
16. Devaraj S, Hemarajata P, Versalovic J. The human gut microbiome and body metabolism: implications for obesity and diabetes. Clin Chem. (2013) 59:617–28. doi: 10.1373/clinchem.2012.187617
17. Tito RY, Knights D, Metcalf J, Obregon-Tito AJ, Cleeland L, Najar F, et al. Insights from characterizing extinct human gut microbiomes. PLoS ONE (2012) 7:e51146. doi: 10.1371/journal.pone.0051146
18. Adler CJ, Dobney K, Weyrich LS, Kaidonis J, Walker AW, Haak W, et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat Genet. (2013) 45:450. doi: 10.1038/ng.2536
19. D'Costa VM, King CE, Kalan L, Morar M, Sung WWL, Schwarz C, et al. Antibiotic resistance is ancient. Nature (2011) 477:457–61. doi: 10.1038/nature10388
20. Huson DH, Weber N. Microbial community analysis using MEGAN. Met. Enzymol. (2013) 531:465–85. doi: 10.1016/B978-0-12-407863-5.00021-6
21. Markowitz VM, Chen I-MA, Palaniappan K, Chu K, Szeto E, Pillay M, et al. IMG 4 version of the integrated microbial genomes comparative analysis system. Nucleic Acids Res. (2013) 42:D560–67. doi: 10.1093/nar/gkt963
22. Metzker ML. Sequencing technologies—the next generation. Nat Rev Genet. (2010) 11:31. doi: 10.1038/nrg2626
23. Glass EM, Meyer F. The metagenomics RAST server: a public resource for the automatic phylogenetic and functional analysis of metagenomes. In: FJ de Bruijn (editor). Handbook of Molecular Microbial Ecology I: Metagenomics and Complementary Approaches. New York, NY. (2011). p. 325–31. doi: 10.1002/9781118010518.ch37
24. Oulas A, Pavloudi C, Polymenakou P, Pavlopoulos GA, Papanikolaou N, Kotoulas G, et al. Iliopoulos loannis. Metagenomics: tools and insights for analyzing next-generation sequencing data derived from biodiversity studies. Bioinform Biol Insights (2015) 9:BBI-S12462. doi: 10.4137/BBI.S12462
25. Pandey RV, Nolte V, Schlötterer C. CANGS: a user-friendly utility for processing and analyzing 454 GS-FLX data in biodiversity studies. BMC Res Notes (2010) 3:3. doi: 10.1186/1756-0500-3-3
26. Rothberg JM, Leamon JH. The development and impact of 454 sequencing. Nat Biotechnol. (2008) 26:1117. doi: 10.1038/nbt1485
27. Thomas T, Gilbert J, Meyer F. Metagenomics-a guide from sampling to data analysis. Microb Inform Exp. (2012) 2:3. doi: 10.1186/2042-5783-2-3
28. Roux A, Payne SM, Gilmore MS. Microbial telesensing: probing the environment for friends, foes, and food. Cell Host Microbe (2009) 6:115–24. doi: 10.1016/j.chom.2009.07.004
29. Dubey, A, Kumar A, Abd_Allah EF, Hashem A, Khan ML. Growing more with less: Breeding and developing drought resilient soybean to improve food security. Ecol Indic. (2018). doi: 10.1016/j.ecolind.2018.03.003
30. Kumar A, Chordia N. Role of microbes in human health. Appl Microbiol. (2017) 3:2–4. doi: 10.4172/2471-9315.1000131
31. Ahmad I, Khan MSA, Aqil F, Singh M. Microbial applications in agriculture and the environment: a broad perspective. In: I Ahmad, F Ahmad, Pichtel J (editors). Microbes and Microbial Technology: Agricultural and Environmental Applications. New York, NY: Springer (2011). p. 1–27. doi: 10.1007/978-1-4419-7931-5_1
32. Malla MA, Dubey A, Yadav S, Kumar A, Hashem A, Abd-Allah EF. Understanding and designing the strategies for the microbe-mediated remediation of environmental contaminants using omics approaches. Front Microbiol. (2018) 9:1132. doi: 10.3389/fmicb.2018.01132
33. Hartman K, van der Heijden MGA, Wittwer RA, Banerjee S, Walser J-C, Schlaeppi K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome (2018) 6:14. doi: 10.1186/s40168-017-0389-9
34. Singh RR. Next generation sequencing technologies. ComprehMed Chem III. 354–61. doi: 10.1016/B978-0-12-409547-2.12327-3
35. Madsen EL. Microorganisms and their roles in fundamental biogeochemical cycles. Curr Opin Biotechnol. (2011) 22:456–64. doi: 10.1016/j.copbio.2011.01.008
36. Zhou J, He Z, Yang Y, Deng Y, Tringe SG, Alvarez-Cohen L. High-throughput metagenomic technologies for complex microbial community analysis: open and closed formats. MBio (2015) 6:e02288-14. doi: 10.1128/mBio.02288-14
37. Tripathi M, Singh D, Vikram S, Singh V, Kumar S. Metagenomic approach towards bioprospection of novel biomolecule (s) and environmental bioremediation. Annu Res Rev Biol. (2018) 22:1–12. doi: 10.9734/ARRB/2018/38385
38. Wu GD, Lewis JD. Analysis of the human gut microbiome and association with disease. Clin Gastroenterol Hepatol. (2013) 11:774–7. doi: 10.1016/j.cgh.2013.03.038
39. Cocolin L, Dolci P, Rantsiou K. Biodiversity and dynamics of meat fermentations: the contribution of molecular methods for a better comprehension of a complex ecosystem. Meat Sci. (2011) 89:296–302. doi: 10.1016/j.meatsci.2011.04.011
40. Ercolini D. PCR-DGGE fingerprinting: novel strategies for detection of microbes in food. J Microbiol Methods (2004) 56:297–314. doi: 10.1016/j.mimet.2003.11.006
41. Quigley L, O'Sullivan O, Beresford TP, Ross RP, Fitzgerald GF, Cotter PD. Molecular approaches to analysing the microbial composition of raw milk and raw milk cheese. Int J Food Microbiol. (2011) 150:81–94. doi: 10.1016/j.ijfoodmicro.2011.08.001
42. Sunagawa S, Coelho LP, Chaffron S, Kultima JR, Labadie K, Salazar G, et al. Structure and function of the global ocean microbiome. Science (2015) 348:1261359. doi: 10.1126/science.1261359
43. Oh J, Byrd AL, Deming C, Conlan S, Barnabas B, Blakesley R, et al. Biogeography and individuality shape function in the human skin metagenome. Nature (2014) 514:59. doi: 10.1038/nature13786
44. Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, et al. Structure, function and diversityof the healthy human microbiome. Nature (2012) 486:207–14. doi: 10.1038/nature11234
45. Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, et al. Environmental genome shotgun sequencing of the Sargasso Sea. Science (2004) 304:66–74. doi: 10.1126/science.1093857
46. Brown CT, Hug LA, Thomas BC, Sharon I, Castelle CJ, Singh A, et al. Unusual biology across a group comprising more than 15% of domain Bacteria. Nature (2015) 523:208. doi: 10.1038/nature14486
47. Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, et al. Complete nitrification by Nitrospira bacteria. Nature (2015) 528:504. doi: 10.1038/nature16461
48. van Kessel MAHJ, Speth DR, Albertsen M, Nielsen PH, den Camp HJMO, Kartal B, et al. Complete nitrification by a single microorganism. Nature (2015) 528:555. doi: 10.1038/nature16459
49. Loman NJ, Constantinidou C, Christner M, Rohde H, Chan JZ-M, Quick J, et al. A culture-independent sequence-based metagenomics approach to the investigation of an outbreak of Shiga-toxigenic Escherichia coli O104: H4. JAMA (2013) 309:1502–10. doi: 10.1001/jama.2013.3231
50. Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, et al. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe (2014) 15:382–92. doi: 10.1016/j.chom.2014.02.005
51. Norman JM, Handley SA, Baldridge MT, Droit L, Liu CY, Keller BC, et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell (2015) 160:447–60. doi: 10.1016/j.cell.2015.01.002
52. Donia MS, Cimermancic P, Schulze CJ, Brown LCW, Martin J, Mitreva M, et al. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell (2014) 158:1402–14. doi: 10.1016/j.cell.2014.08.032
53. Larsen OFA, Claassen E. The mechanistic link between health and gut microbiota diversity. Sci Rep. (2018) 8:6–10. doi: 10.1038/s41598-018-20141-6
54. Sung J, Kim S, Cabatbat JJT, Jang S, Jin YS, Jung GY, et al. Global metabolic interaction network of the human gut microbiota for context-specific community-scale analysis. Nat Commun. (2017) 8:15393. doi: 10.1038/ncomms15393
55. Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. (2008) 121:129–34. doi: 10.1016/j.jaci.2007.09.011
56. Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy (2014) 44:842–50. doi: 10.1111/cea.12253
57. Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes (2013) 62:3341–9. doi: 10.2337/db13-0844
58. Bisgaard H, Li N, Bonnelykke K, Chawes BLK, Skov T, Paludan-Müller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. (2011) 128:646–52. doi: 10.1016/j.jaci.2011.04.060
59. Ferreira CM, Vieira AT, Vinolo MAR, Oliveira FA, Curi R, Martins F. dos S The central role of the gut microbiota in chronic inflammatory diseases. J Immunol Res. (2014) 2014:689492. doi: 10.1155/2014/689492
60. Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol. (2014) 20:14105. doi: 10.3748/wjg.v20.i39.14105
61. Lakhan SE, Kirchgessner A. Gut inflammation in chronic fatigue syndrome. Nutr Metab (Lond) (2010) 7:79. doi: 10.1186/1743-7075-7-79
62. Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. (2012) 22:299–306. doi: 10.1101/gr.126516.111
63. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature (2011) 474:327. doi: 10.1038/nature10213
64. Africa CWJ, Nel J, Stemmet M. Anaerobes and bacterial vaginosis in pregnancy: virulence factors contributing to vaginal colonisation. Int J Environ Res Public Health (2014) 11:6979–7000. doi: 10.3390/ijerph110706979
65. Lach G, Schellekens H, Dinan TG, Cryan JF. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics (2018) 15:36–59. doi: 10.1007/s13311-017-0585-0
66. Geuking MB, McCoy KD, Macpherson AJ. Metabolites from intestinal microbes shape Treg. Cell Res. (2013) 23:1339–40. doi: 10.1038/cr.2013.125
67. Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature (2012) 489:242–9. doi: 10.1038/nature11552
68. Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science (2006) 312:1355–9. doi: 10.1126/science.1124234
69. Medina M, Izquierdo E, Ennahar S, Sanz Y. Differential immunomodulatory properties of Bifidobacterium logum strains: relevance to probiotic selection and clinical applications. Clin Exp Immunol. (2007) 150:531–8. doi: 10.1111/j.1365-2249.2007.03522.x
70. Gupta S, Allen-Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Therap Adv Gastroenterol. (2016) 9:229–39. doi: 10.1177/1756283X15607414
71. Spanogiannopoulos P, Bess EN, Carmody RN, Turnbaugh PJ. The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol. (2016) 14:273–87. doi: 10.1038/nrmicro.2016.17
72. Shank EA, Kolter R. New developments in microbial interspecies signaling. Curr Opin Microbiol. (2009) 12:205–14. doi: 10.1016/j.mib.2009.01.003
73. Cornforth DM, Foster KR. Antibiotics and the art of bacterial war. Proc Natl Acad Sci USA (2015) 112:10827–8. doi: 10.1073/pnas.1513608112
74. Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE (2011) 6:e16393. doi: 10.1371/journal.pone.0016393
75. Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Heal Dis. (2015) 26:26191. doi: 10.3402/mehd.v26.26191
76. Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, et al. post-antibiotic expansion of enteric pathogens. Nature (2013) 502:96–9. doi: 10.1038/nature12503
77. Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. (2010) 104(Suppl. 2):S1–63. doi: 10.1017/S0007114510003363
78. Cani PD, Possemiers S, Van De Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut (2009) 58:1091–103. doi: 10.1136/gut.2008.165886
79. Archer BJ, Johnson SK, Devereux HM, Baxter AL. Effect of fat replacement by inulin or lupin-kernel fibre on sausage patty acceptability, post-meal perceptions of satiety and food intake in men. Br J Nutr. (2004) 91:591–9. doi: 10.1079/BJN20031088
80. Whelan K, Efthymiou L, Judd PA, Preedy VR, Taylor MA. Appetite during consumption of enteral formula as a sole source of nutrition: the effect of supplementing pea-fibre and fructo-oligosaccharides. Br J Nutr (2006) 96:350–6. doi: 10.1079/BJN20061791
81. Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. (2009) 89:1751–9. doi: 10.3945/ajcn.2009.27465
82. Peters HPF, Boers HM, Haddeman E, Melnikov SM, Qvyjt F. No effect of added β-glucan or of fructooligosaccharide on appetite or energy intake. Am J Clin Nutr. (2009) 89:58–63. doi: 10.3945/ajcn.2008.26701
83. Hess JR, Birkett AM, Thomas W, Slavin JL. Effects of short-chain fructooligosaccharides on satiety responses in healthy men and women. Appetite (2011) 56:128–34. doi: 10.1016/j.appet.2010.12.005
84. Tarini J, Wolever TMS. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab. (2010) 35:9–16. doi: 10.1139/H09-119
85. Frost SD. Dynamics and evolution of HIV-1 during structured treatment interruptions. AIDS Rev. (2002) 4:119–27.
86. Poulin R, Thomas F. Epigenetic effects of infection on the phenotype of host offspring: parasites reaching across host generations. Oikos (2008) 117:331–5. doi: 10.1111/j.2007.0030-1299.16435.x
87. Gans J, Wolinsky M, Dunbar J. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science (2005) 309:1387–90. doi: 10.1126/science.1112665
88. Bent SJ, Pierson JD, Forney LJ. Measuring species richness based on microbial community fingerprints: the emperor has no clothes. Appl Environ Microbiol. (2007) 73:2399–401. doi: 10.1128/AEM.02383-06
89. Tessler M, Neumann JS, Afshinnekoo E, Pineda M, Hersch R, Velho LFM, et al. Large-scale differences in microbial biodiversity discovery between 16S amplicon and shotgun sequencing. Sci Rep. (2017) 7:1–14. doi: 10.1038/s41598-017-06665-3
90. Methé BA, Nelson KE, Pop M, Creasy HH, Giglio MG, Huttenhower C, et al. A framework for human microbiome research. Nature (2012) 486:215. doi: 10.1038/nature11209
91. Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. (2009) 38:D355–60. doi: 10.1093/nar/gkp896
92. Neal AL. Metagenomics: Current Advances and Emerging Concepts. Poole: Caister Academic Press (2017).
93. Lasken RS. Genomic sequencing of uncultured microorganisms from single cells. Nat Rev Microbiol. (2012) 10:631. doi: 10.1038/nrmicro2857
94. Mardis ER. Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet. (2008) 9:387–402. doi: 10.1146/annurev.genom.9.081307.164359
95. Roossinck MJ. Plant virus metagenomics: biodiversity and ecology. Annu Rev Genet. (2012) 46:359–69. doi: 10.1146/annurev-genet-110711-155600
96. Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science (2005) 308:1635–8. doi: 10.1126/science.1110591
97. Gibbons R, Socransky S, de Araujo W, van Houte J. Studies of the predominant cultivable microbiota of dental plaque. Arch Oral Biol. (1964) 9:365–70. doi: 10.1016/0003-9969(64)90069-X
98. Parker RB, Snyder ML. Interactions of the oral microbiota I. A system for the defined study of mixed cultures. Exp Biol Med. (1961) 108:749–52. doi: 10.3181/00379727-108-27055
99. Sanger F, Coulson AR. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. (1975) 94:441-6. doi: 10.1016/0022-2836(75)90213-2
100. Hutchison CA. DNA sequencing: bench to bedside and beyond. Nucleic Acids Res. (2007) 35:6227–37. doi: 10.1093/nar/gkm688
101. Sanger F. Sequences, sequences, and sequences. Annu Rev Biochem. (1988) 57:1–29. doi: 10.1146/annurev.bi.57.070188.000245
102. Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet. (2016) 17:333–51. doi: 10.1038/nrg.2016.49
103. Pester M, Rattei T, Flechl S, Gröngröft A, Richter A, Overmann J, et al. amoA-based consensus phylogeny of ammonia-oxidizing archaea and deep sequencing of amoA genes from soils of four different geographic regions. Environ Microbiol. (2012) 14:525–39. doi: 10.1111/j.1462-2920.2011.02666.x
104. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J (2012) 6:1621–4. doi: 10.1038/ismej.2012.8
105. Nyrén P, Lundin A. Enzymatic method for continuous monitoring of inorganic pyrophosphate synthesis. Anal Biochem. (1985) 151:504–9. doi: 10.1016/0003-2697(85)90211-8
106. Hyman ED. A new method of sequencing DNA. Anal Biochem. (1988) 174:423–36. doi: 10.1016/0003-2697(88)90041-3
107. Thakkar JR, Sabara PH, Koringa PG. Exploring metagenomes using next-generation sequencing. In: R Singh, R Kothari, P Koringa, S Singh (editors). Understanding Host-Microbiome Interactions–An Omics Approach: Omics of Host-Microbiome Association. Singapore: Springer (2017). p. 29–40. doi: 10.1007/978-981-10-5050-3_3
108. Granberg F, Vicente-Rubiano M, Rubio-Guerri C, Karlsson OE, Kukielka D, Belák S, et al. Metagenomic detection of viral pathogens in Spanish honeybees: co-infection by aphid lethal paralysis, Israel acute paralysis and Lake Sinai viruses. PLoS ONE (2013) 8:e57459. doi: 10.1371/journal.pone.0057459
109. Quail MA, Smith M, Coupland P, Otto TD, Harris SR, Connor TR, et al. A tale of three next generation sequencing platforms: comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genomics (2012) 13:341. doi: 10.1186/1471-2164-13-341
110. Ansorge WJ. Next-generation DNA sequencing techniques. N Biotechnol. (2009) 25:195–203. doi: 10.1016/j.nbt.2008.12.009
111. MacLean D, Jones JDG, Studholme DJ. Application of'next-generation'sequencing technologies to microbial genetics. Nat Rev Microbiol. (2009) 7:287. doi: 10.1038/nrmicro2122
112. Zhang J, Chiodini R, Badr A, Zhang G. The impact of next-generation sequencing on genomics. J Genet Genom. (2011) 38:95–109. doi: 10.1016/j.jgg.2011.02.003
113. Schuster SC, Chi KR, Rusk N, Kiermer V, Wold B, Myers RM. Method of the year, next-generation DNA sequencing. Funct Genomics Med Appl Nat Methods (2008) 5:11–21.
114. Karow J. Qiagen Launches GeneReader NGS System at AMP; Presents Performance Evaluation by Broad. GenomeWeb (2015). Available online at: https://www.genomeweb.com/molecular-diagnostics/qiagen-launches-genereader-ngs-system-amp-presents-performance-evaluation
115. Douglas R. Smith Kevin McKernan. Methods of producing and sequencing modified polynucleotides. Appl Biosyst. (2010) 2.
116. Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, et al. Corrigendum: genome sequencing in microfabricated high-density picolitre reactors. Nature (2006) 441:120. doi: 10.1038/nature04726
117. Merriman B, Torrent I, Rothberg JM, Team R. Progress in ion torrent semiconductor chip based sequencing. Electrophoresis (2012) 33:3397–417. doi: 10.1002/elps.201200424
118. Rothberg JM, Hinz W, Rearick TM, Schultz J, Mileski W, Davey M, et al. An integrated semiconductor device enabling non-optical genome sequencing. Nature (2011) 475:348. doi: 10.1038/nature10242
119. Kasianowicz JJ, Brandin E, Branton D, Deamer DW. Characterization of individual polynucleotide molecules using a membrane channel. Proc Natl Acad Sci U.S.A. (1996) 93:13770–3. doi: 10.1073/pnas.93.24.13770
120. Fichot EB, Norman RS. Microbial phylogenetic profiling with the Pacific Biosciences sequencing platform. Microbiome (2013) 1:10. doi: 10.1186/2049-2618-1-10
121. Kchouk M, Gibrat JF, Elloumi M. Generations of sequencing technologies: from first to next generation. Biol Med. (2017) 9:395. doi: 10.4172/0974-8369.1000395
122. Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, et al. Real-time DNA sequencing from single polymerase molecules. Science (2009) 323:133–8. doi: 10.1016/j.yofte.2016.04.005
123. Braslavsky I, Hebert B, Kartalov E, Quake SR. Sequence information can be obtained from single DNA molecules. Proc Natl Acad Sci USA (2003) 100:3960–4. doi: 10.1073/pnas.0230489100
124. Harris TD, Buzby PR, Babcock H, Beer E, Bowers J, Braslavsky I, et al. Single-molecule DNA sequencing of a viral genome. Science (2008) 320:106–9. doi: 10.1126/science.1150427
125. Mikheyev AS, Tin MMY. A first look at the Oxford Nanopore MinION sequencer. Mol Ecol Resour. (2014) 14:1097–102. doi: 10.1111/1755-0998.12324
126. Laehnemann D, Borkhardt A, McHardy AC. Denoising DNA deep sequencing data—high-throughput sequencing errors and their correction. Brief Bioinform. (2015) 17:154–79. doi: 10.1093/bib/bbv029
127. Carter JM, Hussain S. Robust long-read native DNA sequencing using the ONT CsgG N1. Wellcome Open Res. (2017) 2:23. doi: 10.12688/wellcomeopenres.11246.1
128. Lu H, Giordano F, Ning Z. Oxford Nanopore MinION sequencing and genome assembly. Genomics Proteomics Bioinformatics (2016) 14:265–79. doi: 10.1016/j.gpb.2016.05.004
129. Dohm JC, Lottaz C, Borodina T, Himmelbauer H. Substantial biases in ultra-short read data sets from high-throughput DNA sequencing. Nucleic Acids Res. (2008) 36:e105. doi: 10.1093/nar/gkn425
130. Reuter JA, Spacek DV, Snyder MP. High-throughput sequencing technologies. Mol Cell (2015) 58:586–97. doi: 10.1016/j.molcel.2015.05.004
131. Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE (2014) 9:e92193. doi: 10.1371/journal.pone.0092193
132. Lambeth SM, Carson T, Lowe J, Ramaraj T, Leff JW, Luo L, et al. Composition, diversity and abundance of gut microbiome in prediabetes and type 2 diabetes. J Diabetes Obes. (2015) 2:1. doi: 10.15436/2376-0949.15.031
133. Yasir M, Angelakis E, Bibi F, Azhar EI, Bachar D, Lagier JC, et al. Comparison of the gut microbiota of people in France and Saudi Arabia. Nutr Diabetes (2015) 5:e153. doi: 10.1038/nutd.2015.3
134. Travers KJ, Chin C-S, Rank DR, Eid JS, Turner SW. A flexible and efficient template format for circular consensus sequencing and SNP detection. Nucleic Acids Res. (2010) 38:e159. doi: 10.1093/nar/gkq543
135. Carneiro MO, Russ C, Ross MG, Gabriel SB, Nusbaum C, DePristo MA. Pacific biosciences sequencing technology for genotyping and variation discovery in human data. BMC Genomics (2012) 13:375. doi: 10.1186/1471-2164-13-375
136. Rhoads A, Au KF. PacBio sequencing and its applications. Genomics Proteomics Bioinformatics (2015) 13:278–89. doi: 10.1016/j.gpb.2015.08.002
137. Ashton PM, Nair S, Dallman T, Rubino S, Rabsch W, Mwaigwisya S, et al. MinION nanopore sequencing identifies the position and structure of a bacterial antibiotic resistance island. Nat Biotechnol. (2015) 33:296. doi: 10.1038/nbt.3103
138. Jain M, Fiddes IT, Miga KH, Olsen HE, Paten B, Akeson M. Improved data analysis for the MinION nanopore sequencer. Nat Methods (2015) 12:351–6. doi: 10.1038/nmeth.3290
139. Wang J, Moore NE, Deng Y-M, Eccles DA, Hall RJ. MinION nanopore sequencing of an influenza genome. Front Microbiol. (2015) 6:766. doi: 10.3389/fmicb.2015.00766
140. Quick J, Loman NJ, Duraffour S, Simpson JT, Severi E, Cowley L, et al. Real-time, portable genome sequencing for Ebola surveillance. Nature (2016) 530:228. doi: 10.1038/nature16996
141. Bäckhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe (2012) 12:611–22. doi: 10.1016/j.chom.2012.10.012
142. Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology (2014) 146:1449–58. doi: 10.1053/j.gastro.2014.01.052
143. Clooney AG, Fouhy F, Sleator RD, O'Driscoll A, Stanton C, Cotter PD, et al. Comparing apples and oranges? Next generation sequencing and its impact on microbiome analysis. PLoS ONE (2016) 11:e0148028. doi: 10.1371/journal.pone.0148028
144. Olivares M, Neef A, Castillejo G, De Palma G, Varea V, Capilla A, et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut (2014) 64:406–17. doi: 10.1136/gutjnl-2014-306931
145. Salonen A, Lahti L, Salojärvi J, Holtrop G, Korpela K, Duncan SH, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. (2014) 8:2218. doi: 10.1038/ismej.2014.63
146. Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. (2010) 12:5. doi: 10.1038/ni0111-5
147. Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology (2014) 146:1489–99. doi: 10.1053/j.gastro.2014.02.009
148. Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology (2014) 146:1513–24. doi: 10.1053/j.gastro.2014.01.020
149. Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: more than a gut feeling. Schizophr Res. (2016) 176:23–35. doi: 10.1016/j.schres.2014.06.027
150. Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. (2009) 3:944. doi: 10.1038/ismej.2009.37
151. Cani PD. Gut microbiota and obesity: lessons from the microbiome. Brief Funct Genom. (2013) 12:381–7. doi: 10.1093/bfgp/elt014
152. Clarke SF, Murphy EF, Nilaweera K, Ross PR, Shanahan F, Cotter PW, et al. The gut microbiota and its relationship to diet and obesity: new insights. Gut Microbes (2012) 3:1–17. doi: 10.4161/gmic.20168
153. Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. (2015) 3:207–15. doi: 10.1016/S2213-8587(14)70134-2
154. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature (2006) 444:1022. doi: 10.1038/4441022a
155. Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology (2008) 134:577–94. doi: 10.1053/j.gastro.2007.11.059
156. Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology (2004) 39:1441–9. doi: 10.1002/hep.20194
157. Scanlan PD, Shanahan F, Clune Y, Collins JK, O'sullivan GC, O'riordan M, et al. Culture-independent analysis of the gut microbiota in colorectal cancer and polyposis. Environ Microbiol. (2008) 10:789–98. doi: 10.1111/j.1462-2920.2007.01503.x
158. Verhulst SL, Vael C, Beunckens C, Nelen V, Goossens H, Desager K. A longitudinal analysis on the association between antibiotic use, intestinal microflora, and wheezing during the first year of life. J Asthma (2008) 45:828–32. doi: 10.1080/02770900802339734
159. Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen M-L, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. (2002) 35:S6–S16. doi: 10.1086/341914
160. Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature (2008) 455:1109. doi: 10.1038/nature07336
161. Roberfroid MB, Bornet F, Bouley C, Cummings JH. Colonic microflora: nutrition and health0. summary and conclusions of an International Life Sciences Institute (ILSI)[Europe] Workshop held in Barcelona, Spain. Nutr Rev. (1995) 53:127–30. doi: 10.1111/j.1753-4887.1995.tb01535.x
162. Cash HL, Whitham C V, Behrendt CL, Hooper L V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science (2006) 313:1126–30. doi: 10.1126/science.1127119
163. Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. (2003) 4:269. doi: 10.1038/ni888
164. Schauber J, Svanholm C, Termen S, Iffland K, Menzel T, Scheppach W, et al. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut (2003) 52:735–41. doi: 10.1136/gut.52.5.735
165. Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature (2008) 456:507. doi: 10.1038/nature07450
166. Rakoff-Nahoum S, Medzhitov R. Innate immune recognition of the indigenous microbial flora. Mucosal Immunol. (2008) 1:S10. doi: 10.1038/mi.2008.49
167. Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol (2004) 4:478. doi: 10.1038/nri1373
168. Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. (2010) 90:859–904. doi: 10.1152/physrev.00045.2009
169. Johnson EL, Heaver SL, Walters WA, Ley RE. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. J Mol Med. (2017) 95:1–8. doi: 10.1007/s00109-016-1492-2
170. Yiu JHC, Dorweiler B, Woo CW. Interaction between gut microbiota and toll-like receptor: from immunity to metabolism. J Mol Med. (2017) 95:13–20. doi: 10.1007/s00109-016-1474-4
171. Wehkamp J, Frick J-S. Microbiome and chronic inflammatory bowel diseases. J Mol Med. (2017) 95:21–8. doi: 10.1007/s00109-016-1495-z
172. Lee HU, McPherson ZE, Tan B, Korecka A, Pettersson S. Host-microbiome interactions: the aryl hydrocarbon receptor and the central nervous system. J Mol Med. (2017) 95:29–39. doi: 10.1007/s00109-016-1486-0
173. Branton D, Deamer DW, Marziali A, Bayley H, Benner SA, Butler T, et al. The potential and challenges of nanopore sequencing. Nat Biotechnol. (2008) 26:1146. doi: 10.1038/nbt.1495
174. Ladoukakis E, Kolisis FN, Chatziioannou AA. Integrative workflows for metagenomic analysis. Front Cell Dev Biol. (2014) 2:70. doi: 10.3389/fcell.2014.00070
175. Andrews S. FastQC: a Quality Control Tool for High Throughput Sequence Data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc
176. Gordon A, Hannon GJ. Fastx-Toolkit. FASTQ/A Short-Reads Pre-processing Tools (2010). Available online at: http//hannonlabcshledu/fastx_toolkit
177. Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics (2011) 27:863–4. doi: 10.1093/bioinformatics/btr026
178. Patel RK, Jain M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS ONE (2012) 7:e30619. doi: 10.1371/journal.pone.0030619
179. Zhou Q, Su X, Jing G, Ning K. Meta-QC-Chain: comprehensive and fast quality control method for metagenomic data. Genomics Proteomics Bioinformatics (2014) 12:52–6. doi: 10.1016/j.gpb.2014.01.002
180. Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. (2009) 75:7537–41. doi: 10.1128/AEM.01541-09
181. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods (2010) 7:335. doi: 10.1038/nmeth.f.303
182. Krause L, Diaz NN, Goesmann A, Kelley S, Nattkemper TW, Rohwer F, et al. Phylogenetic classification of short environmental DNA fragments. Nucleic Acids Res. (2008) 36:2230–9. doi: 10.1093/nar/gkn038
183. Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. (2013) 31:814. doi: 10.1038/nbt.2676
184. Teeling H, Waldmann J, Lombardot T, Bauer M, Glöckner FO. TETRA: a web-service and a stand-alone program for the analysis and comparison of tetranucleotide usage patterns in DNA sequences. BMC Bioinformatics (2004) 5:163. doi: 10.1186/1471-2105-5-163
185. McHardy AC, Martín HG, Tsirigos A, Hugenholtz P, Rigoutsos I. Accurate phylogenetic classification of variable-length DNA fragments. Nat Methods (2007) 4:63. doi: 10.1038/nmeth976
186. Kultima JR, Sunagawa S, Li J, Chen W, Chen H, Mende DR, et al. MOCAT: a metagenomics assembly and gene prediction toolkit. PLoS ONE (2012) 7:e47656. doi: 10.1371/journal.pone.0047656
187. Su X, Pan W, Song B, Xu J, Ning K. Parallel-META 2.0: enhanced metagenomic data analysis with functional annotation, high performance computing and advanced visualization. PLoS ONE (2014) 9:e89323. doi: 10.1371/journal.pone.0089323
188. Wang Y, Leung HCM, Yiu SM, Chin FYL. MetaCluster-TA: taxonomic annotation for metagenomic data based on assembly-assisted binning. in BMC Genomics 15(Suppl. 1):S12. doi: 10.1186/1471-2164-15-S1-S12
189. Wu Y-W, Tang Y-H, Tringe SG, Simmons BA, Singer SW. MaxBin: an automated binning method to recover individual genomes from metagenomes using an expectation-maximization algorithm. Microbiome (2014) 2:26. doi: 10.1186/2049-2618-2-26
190. Wu M, Eisen JA. A simple, fast, and accurate method of phylogenomic inference. Genome Biol. (2008) 9:R151. doi: 10.1186/gb-2008-9-10-r151
191. Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics (2009) 25:1754–60. doi: 10.1093/bioinformatics/btp324
192. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods (2012) 9:357. doi: 10.1038/nmeth.1923
193. Davenport CF, Tümmler B. Advances in computational analysis of metagenome sequences. Environ Microbiol. (2013) 15:1–5. doi: 10.1111/j.1462-2920.2012.02843.x
194. Monzoorul Haque M, Ghosh TS, Komanduri D, Mande SS. SOrt-ITEMS: sequence orthology based approach for improved taxonomic estimation of metagenomic sequences. Bioinformatics (2009) 25:1722–30. doi: 10.1093/bioinformatics/btp317
195. Ghosh TS, Haque M, Mande SS. DiScRIBinATE: a rapid method for accurate taxonomic classification of metagenomic sequences. BMC Bioinformatics 11(Suppl. 7):S14. doi: 10.1186/1471-2105-11-S7-S14
196. Peng Y, Leung HCM, Yiu S-M, Chin FYL. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics (2012) 28:1420–8. doi: 10.1093/bioinformatics/bts174
197. Namiki T, Hachiya T, Tanaka H, Sakakibara Y. MetaVelvet: an extension of Velvet assembler to de novo metagenome assembly from short sequence reads. Nucleic Acids Res. (2012) 40:e155. doi: 10.1093/nar/gks678
198. Boisvert S, Raymond F, Godzaridis É, Laviolette F, Corbeil J. Ray Meta: scalable de novo metagenome assembly and profiling. Genome Biol. (2012) 13:R122. doi: 10.1186/gb-2012-13-12-r122
199. Zhu W, Lomsadze A, Borodovsky M. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. (2010) 38:e132. doi: 10.1093/nar/gkq275
200. Kelley DR, Liu B, Delcher AL, Pop M, Salzberg SL. Gene prediction with Glimmer for metagenomic sequences augmented by classification and clustering. Nucleic Acids Res. (2011) 40:e9. doi: 10.1093/nar/gkr1067
201. Rho M, Tang H, Ye Y. FragGeneScan: predicting genes in short and error-prone reads. Nucleic Acids Res. (2010) 38:e191. doi: 10.1093/nar/gkq747
202. Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics (2006) 22:1658–9. doi: 10.1093/bioinformatics/btl158
203. Eddy SR. Accelerated profile HMM searches. PLoS Comput Biol. (2011) 7:e1002195. doi: 10.1371/journal.pcbi.1002195
204. Ye Y, Tang H. An ORFome assembly approach to metagenomics sequences analysis. J Bioinform Comput Biol. (2009) 7:455–71. doi: 10.1142/S0219720009004151
205. Ye Y, Doak TG. A parsimony approach to biological pathway reconstruction/inference for genomes and metagenomes. PLoS Comput Biol. (2009) 5:e1000465. doi: 10.1371/journal.pcbi.1000465
206. Liu B, Pop M. MetaPath: identifying differentially abundant metabolic pathways in metagenomic datasets. BMC Proc. 5(Suppl. 2):S9. doi: 10.1186/1753-6561-5-S2-S9
207. Kanehisa M, Sato Y, Morishima K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J Mol Biol. (2016) 428:726–31. doi: 10.1016/j.jmb.2015.11.006
208. Li W. Analysis and comparison of very large metagenomes with fast clustering and functional annotation. BMC Bioinformatics (2009) 10:359. doi: 10.1186/1471-2105-10-359
209. Ghosh TS, Mohammed MH, Komanduri D, Mande SS. ProViDE: a software tool for accurate estimation of viral diversity in metagenomic samples. Bioinformation (2011) 6:91. doi: 10.6026/97320630006091
210. McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE (2013) 8:e61217. doi: 10.1371/journal.pone.0061217
211. Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat Methods (2013) 10:1200. doi: 10.1038/nmeth.2658
212. Kristiansson E, Hugenholtz P, Dalevi D. ShotgunFunctionalizeR: an R-package for functional comparison of metagenomes. Bioinformatics (2009) 25:2737–8. doi: 10.1093/bioinformatics/btp508
213. Goecks J, Nekrutenko A, Taylor J. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. (2010) 11:R86. doi: 10.1186/gb-2010-11-8-r86
214. Meyer F, Paarmann D, D'Souza M, Olson R, Glass EM, Kubal M, et al. The metagenomics RAST server–a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics (2008) 9:386. doi: 10.1186/1471-2105-9-386
215. Markowitz VM, Chen I-MA, Chu K, Szeto E, Palaniappan K, Grechkin Y, et al. IMG/M: the integrated metagenome data management and comparative analysis system. Nucleic Acids Res. (2011) 40:D123–9. doi: 10.1093/nar/gkr975
216. Seshadri R, Kravitz SA, Smarr L, Gilna P, Frazier M. CAMERA: a community resource for metagenomics. PLoS Biol. (2007) 5:e75. doi: 10.1371/journal.pbio.0050075
217. Truong DT, Tett A, Pasolli E, Huttenhower C, Segata N. Microbial strain-level population structure and genetic diversity from metagenomes. Genome Res. (2017) 27:626–38. doi: 10.1101/gr.216242.116
Keywords: microbes, human microbiome, host-microbe interactions, metagenomics, next generation sequencing, bioinformatics, dysbiosis, diseases
Citation: Malla MA, Dubey A, Kumar A, Yadav S, Hashem A and Abd_Allah EF (2019) Exploring the Human Microbiome: The Potential Future Role of Next-Generation Sequencing in Disease Diagnosis and Treatment. Front. Immunol. 9:2868. doi: 10.3389/fimmu.2018.02868
Received: 04 July 2018; Accepted: 21 November 2018;
Published: 07 January 2019.
Edited by:
Ashutosh K. Mangalam, University of Iowa, United StatesReviewed by:
Dimitry N. Krementsov, University of Vermont, United StatesRajesh Kumar Mondal, National Institute of Research in Tuberculosis (ICMR), India
Copyright © 2019 Malla, Dubey, Kumar, Yadav, Hashem and Abd_Allah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashwani Kumar, YXNod2FuaWlpdGRAaG90bWFpbC5jb20=
 Muneer Ahmad Malla
Muneer Ahmad Malla Anamika Dubey
Anamika Dubey Ashwani Kumar
Ashwani Kumar Shweta Yadav1
Shweta Yadav1 Abeer Hashem
Abeer Hashem Elsayed Fathi Abd_Allah
Elsayed Fathi Abd_Allah