- 1Centre of Excellence for Biomedical Research and Department of Internal Medicine, University of Genoa, Genoa, Italy
- 2Biotherapy Unit, Ospedale Policlinico San Martino, Genoa, Italy
- 3Infectious Disease Unit, Ospedale Policlinico San Martino, Genoa, Italy
The pathogenesis of HIV immunodeficiency is mainly dependent on the cytopatic effects exerted by the virus against infected CD4+ T cells. However, CD4+ T cell loss cannot be the only pathogenic factor since severe opportunistic infections may develop in HIV infected patients with normal CD4+ T cell counts and since the recent START study indicated that absolute CD4+ T cell counts are not predictive for AIDS and non-AIDS events. Recently our group demonstrated that CD8+CD28-CD127lowCD39+ regulatory T lymphocytes, previously found highly concentrated within tumor microenvironment, circulate with elevated frequency in the peripheral blood of HIV infected patients. Here, we show that these cells, that at least in part are HIV specific, express the PD1 immune checkpoint. Based on these evidences and considerations, in this Perspective article we speculate on the opportunity to treat HIV infected patients with anti-PD1 immune checkpoint inhibitors as a way to counteract the T regulatory cell compartment and to unleash virus-specific immune responses. In order to potentiate the immune responses against HIV we also propose the potential utility to associate immune checkpoint inhibition with HIV-specific therapeutic vaccination, reminiscent of what currently applied in oncologic protocols. We suggest that such an innovative strategy could permit drug-sparing regimens and, perhaps, lead to eradication of the infection in some patients.
The pathogenesis of HIV immunodeficiency is dependent on the cytopatic effects exerted by the virus against infected CD4+ T cells and to subsequent CD4+ T cell loss (1, 2). Some aspects of the disease remain unexplained as the case of HIV infected patients with normal CD4+ T cell counts after anti-retroviral therapy (ART) initiation who develop severe opportunistic infections (3, 4), or that of HIV infected patients with reduced CD4+ T cell counts who do not show immunodeficiency manifestations (5). Accordingly, the results of the recent START study indicated that absolute CD4+ T cell counts are not predictive for AIDS and non-AIDS events since these events may occur in ART treated patients with absolute CD4 counts >500 cells/μl (6). Searching for other pathogenic mechanisms, we focused our attention on regulatory T lymphocytes (Treg). The role of these cells in HIV immunodeficiency pathogenesis is still unclear since studies on alterations of CD4+ Treg in HIV infected patients led to controversial results (7–17). Hence, we took into consideration a different subset of Treg constituted by CD8+ Treg (18). CD8+ Treg, in particular those expressing the CD8+CD28-CD127lowCD39+ phenotype, are regulatory T lymphocytes found highly concentrated within tumor microenvironment, where they can exert remarkable immunosuppressive activity due to their capacity to target T cell proliferation and cytotoxicity (19–21). We investigated on the presence of these cells in the circulation of HIV-infected patients, and on possible correlations between their frequency and markers of disease activity. The results of this study demonstrated that HIV-infected patients have elevated circulating levels of functional CD8+CD28-CD127lowCD39+ Treg, the majority of which is antigen-specific for HIV proteins. This observation is remarkable since these cells are virtually absent from the circulation of healthy subjects (19, 21). In HIV patients, their frequency post-ART correlates with HIV-RNA, CD4+ T cell count, and immune activation markers, suggesting their pathogenic involvement in AIDS or non-AIDS related complications. Moreover, their increase after initiation of ART heralds a lack of virological or clinical response (i.e., appearance of co-morbidity): hence their monitoring is clinically relevant (22).
Further studies from our group show that CD8+CD28-CD127lowCD39+ Treg stably and consistently express PD1 (Figure 1 and Supplemental Material). PD1 is a member of immune checkpoints (23–26). These are molecules expressed by immune cells in order to control the immune responses. In particular, PD1 is mainly expressed by T lymphocytes at advanced stage of maturation, i.e., effector memory and terminally-differentiated effector memory cells. Hence, it is involved in the control of the effector phase of the immune response. Indeed, PD1+ T cells are present within tumor infiltrating lymphocytes, suggesting that PD1 expression contributes to tumor immune evasion (24, 27). In HIV-infected patients CD4+PD1+ T cells constitute the major HIV cell reservoir (28, 29). Interestingly, PD1+ T cell frequency is elevated in HIV-infected patients and reduces after beginning of the treatment, a behavior reminiscent of what occurs to circulating CD8+CD28-CD127loCD39+ Treg frequency (30, 31). In HIV-infected patients, the presence of increased frequency of PD1+ T cells, including abnormally expanded CD8+CD28-CD127loCD39+PD1+ Treg, could be involved in generating immunodeficiency and hampering anti-virus immune responses. Hence, the fact that expansion of CD8+ Treg and of PD1+ T lymphocytes co-exist in both tumors and HIV infection envisages a pathogenic crossing between the two pathologic conditions. This suggests that targeting PD1+ T cells through a specific checkpoint inhibitor could be a useful therapeutic strategy for HIV infection borrowed from anti-cancer protocols, based that recent trials support the safety of this approach (32, 33). The potential efficacy of this strategy is further supported by previous studies in which a PD1 inhibitor was administered to non-human primates. In these studies, anti-PD1 treatment of uninfected animals co-immunized with a SIV-gag adenovirus vector vaccine enhanced the frequency of gag-specific T cells (34), while treatment of SIV infected macaques increased SIV-specific immune response, decreased viral load and prolonged survival (35). Accordingly, administration of anti-PD-L1 monoclonal antibodies to ART-treated SIV infected macaques allowed the maintenance of a lower viral load after ART suspension than in non-administered animals (36).
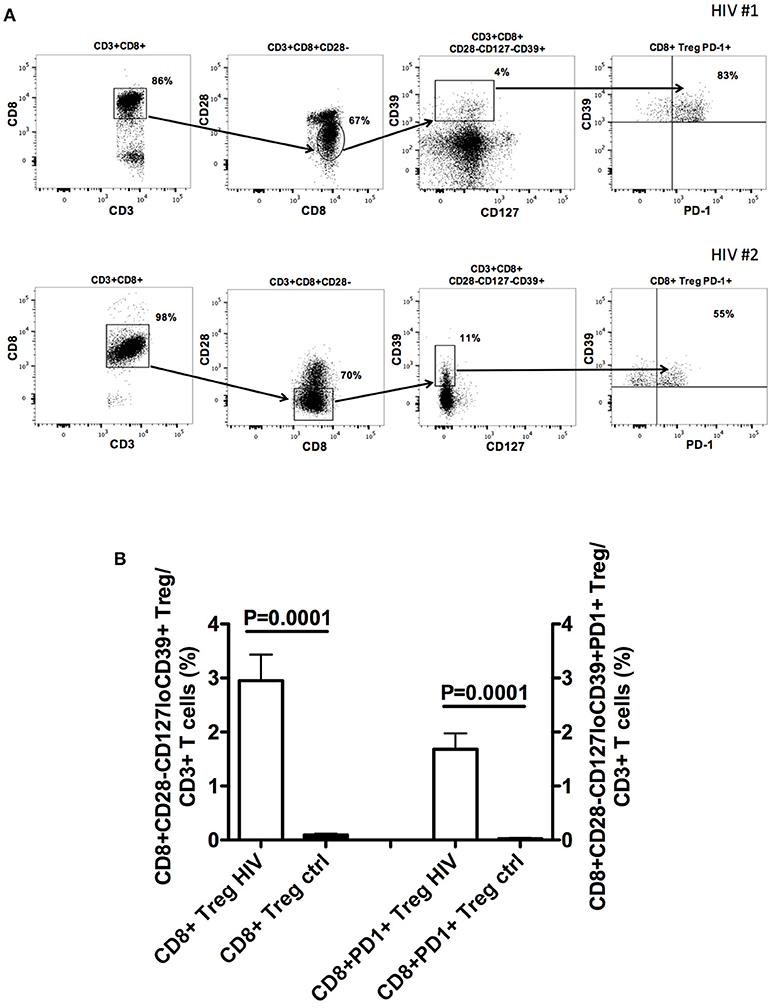
Figure 1. CD8+CD28−CD127loCD39+PD1+ Treg frequency in the peripheral blood of naïve HIV-infected patients. (A) The gating strategy for the analysis of CD8+CD28−CD127loCD39+PD1+ Treg frequency in the circulation of two representative naïve HIV-infected patients is shown. (B) Comparison between the mean frequency of CD8+CD28−CD127loCD39+ Treg as well as that of CD8+CD28−CD127loCD39+PD1+ Treg in the circulation of 22 naïve HIV-infected patients and those of 22 healthy controls. Detailed information on patient population and methods is provided in the Supplementary File—Patients and Methods.
However, in the majority of cancer patients the activity of the sole checkpoint inhibitor is not sufficient to provide a robust therapeutic effect. Hence, association of checkpoint inhibitors with therapeutic vaccination is currently proposed as optimal way for inducing/reinforcing anti-tumor immune responses in the absence of abnormal and detrimental regulatory mechanisms (37, 38). Interestingly, the development of a vaccine against HIV has been recently evoked as urgent medical need, notwithstanding the efficacy of ART. In fact, HIV pandemia is so wide (more than 36 million infected people, about 1.8 million people newly infected each year) that the costs for life-long ART are huge for government health care systems. Hence, therapeutic vaccination has been proposed as a preferential therapeutic tool for corroborating ART activity possibly through the eradication of HIV-1 latent reservoirs (39, 40). Indeed, an optimal vaccine against HIV should elicit both virus-specific cytotoxic CD8+ T cells and neutralizing antibodies in order to kill virus-infected cells, that constitute the viral reservoir, and to avoid spreading of infection by viral particles released by already infected cells. Among HIV antigens, gag has been considered a useful immunogen for vaccine preparation since the presence of elevated titers of antibodies against gag, but not against other HIV antigens, correlated with reduced viremia in HIV infected patients (41). Disappointingly, all vaccination trials so far performed in prophylactic or therapeutic settings, including those using gag as immunogen, did not achieve brilliant clinical results (42). Now, the finding of an abnormal expansion of CD8+CD28-CD127loCD39+PD1+ Treg in these patients suggests that such defective activity of HIV vaccines could have a bi-faceted origin, related to inner deficiency of vaccine immunogenicity and/or to the generalized immunosuppressive effect exerted by CD8+ Treg. Accordingly, a DNA vaccine based on a chimeric gene product fusing PD1 and gag moieties induced high frequency of gag-specific cytotoxic CD8+ T cells associated with high titers of virus-specific antibodies, conferring remarkable protection against mucosal challenge with vaccinia gag viruses in experimental animals (43).
These considerations may constitute a robust rationale for a combination therapy associating anti-PD1 checkpoint inhibition and therapeutic vaccination in ART treated HIV-infected patients. Our hypothesis is that early co-administration of ART, checkpoint inhibition, and vaccination in recently HIV-infected patients could allow to take advantage of the synergic effect of the three-faceted approach when the HIV latent reservoir is not yet consolidated. We expect that such an innovative strategy could lead to the onset of HIV specific immune responses more effective than those spontaneously developed in the absence of Treg inhibition, since unleashed by the regulatory control of PD1+ Treg. Hopefully, this strategy could permit drug-sparing regimens and, perhaps, lead to eradicate the infection in some patients.
Ethics Statement
The study was carried out in compliance with the Helsinki Declaration and was approved by the Ethics Committee of the San Martino Hospital in Genoa, Italy (P.R.251REG2014). All enrolled patients provided written informed consent.
Author Contributions
GF, FI, and AD wrote the manuscript; DF performed the phenotypic analyses; LT enrolled and clinically managed the patients.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2018.02447/full#supplementary-material
References
1. Phetsouphanh C, Xu Y, Zaunders J. CD4 T cells mediate both positive and negative regulation of the immune response to HIV infection: complex role of T follicular helper cells and regulatory T cells in pathogenesis. Front Immunol. (2015) 5:681. doi: 10.3389/fimmu.2014.00681
2. Haase AT. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. (1999) 17:625–56. doi: 10.1146/annurev.immunol.17.1.625
3. Rey D, de Mautort E, Saussine C, Hansmann Y, Waller J, Herbrecht R, et al. Isolated renal Aspergillus abscess in an AIDS patient with a normal CD4+ cell count on highly active antiretroviral therapy. Eur J Clin Microbiol Infect Dis. (1999) 18:137–41. doi: 10.1007/s100960050242
4. Mori S, Polatino S, Estrada-Y-Martin RM. Pneumocystis-associated organizing pneumonia as a manifestation of immune reconstitution inflammatory syndrome in an HIV-infected individual with a normal CD4+ T-cell count following antiretroviral therapy. Int J STD AIDS (2009) 20:662–5. doi: 10.1258/ijsa.2008.008428
5. Mandalia S, Westrop SJ, Beck EJ, Nelson M, Gazzard BG, Imami N. Are long-term non-progressors very slow progressors? Insights from the Chelsea and Westminster HIV cohort, 1988–2010. PLoS ONE (2012) 7:e29844. doi: 10.1371/journal.pone.0029844
6. INSIGHT START Study Group Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. (2015) 373:795–807. doi: 10.1056/NEJMoa1506816
7. Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood (2013) 121:29–37. doi: 10.1182/blood-2012-07-409755
8. Veiga-Parga T, Sehrawat S, Rouse BT. Role of regulatory T cells during virus infection. Immunol Rev. (2013) 255:182–96. doi: 10.1111/imr.12085
9. Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood (2004) 104:3249–56. doi: 10.1182/blood-2004-01-0365
10. Kinter AL, Horak R, Sion M, Riggin L, McNally J, Lin Y, et al. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8+ T cells in vitro. AIDS Res Hum Retroviruses (2007) 23:438–50. doi: 10.1089/aid.2006.0162
11. Jiao Y, Fu J, Xing S, Fu B, Zhang Z, Shi M, et al. The decrease of regulatory T cells correlates with excessive activation and apoptosis of CD8+ T cells in HIV-1-infected typical progressors, but not in long-term non-progressors. Immunology (2009) 128:e366–75. doi: 10.1111/j.1365-2567.2008.02978.x
12. Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. (2005) 174:4407–14. doi: 10.4049/jimmunol.174.7.4407
13. Oswald-Richter K, Grill SM, Shariat N, Leelawong M, Sundrud MS, Haas DW, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. (2004) 2:E198. doi: 10.1371/journal.pbio.0020198
14. Angin M, Sharma S, King M, Murooka TT, Ghebremichael M, Mempel TR, et al. HIV-1 infection impairs regulatory T-cell suppressive capacity on a per-cell basis. J Infect Dis. (2014) 210:899–903. doi: 10.1093/infdis/jiu188
15. Schulze Zur Wiesch J, Thomssen A, Hartjen P, Tóth I, Lehmann C, Meyer-Olson D, et al. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J Virol. (2011) 85:1287–97. doi: 10.1128/JVI.01758-10
16. Ndhlovu LC, Loo CP, Spotts G, Nixon DF, Hecht FM. FOXP3 expressing CD127lo CD4+ T cells inversely correlate with CD38+ CD8+ T cell activation levels in primary HIV-1 infection. J Leukoc Biol. (2008) 83:254–62. doi: 10.1189/jlb.0507281
17. Whiteside TL. Clinical impact of regulatory T cells (Treg) in cancer and HIV. Cancer Microenviron. (2015) 8:201–7. doi: 10.1007/s12307-014-0159-1
18. Filaci G, Fenoglio D, Indiveri F. CD8+ T regulatory/suppressor cells and their relationships with autoreactivity and autoimmunity. Autoimmunity (2011) 44:51–7. doi: 10.3109/08916931003782171
19. Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, et al. CD8+ CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. (2007) 179:4323–34. doi: 10.4049/jimmunol.179.7.4323
20. Filaci G, Fravega M, Negrini S, Procopio F, Fenoglio D, Rizzi M, et al. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28− T cells and inhibit both T-cell proliferation and CTL function. Hum Immunol. (2004) 65:142–56. doi: 10.1016/j.humimm.2003.12.001
21. Parodi A, Battaglia F, Kalli F, Ferrera F, Conteduca G, Tardito S, et al. CD39 is highly involved in mediating the suppression activity of tumor-infiltrating CD8+ T regulatory lymphocytes. Cancer Immunol Immunother. (2013) 62:851–62. doi: 10.1007/s00262-013-1392-z
22. Fenoglio D, Dentone C, Signori A, Di Biagio A, Parodi A, Kalli F, et al. CD8+CD28−CD127loCD39+ regulatory T-cell expansion: a new possible pathogenic mechanism for HIV infection? J Allergy Clin Immunol. (2018) 141:2220–2233.e4. doi: 10.1016/j.jaci.2017.08.021
23. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12:252–64. doi: 10.1038/nrc3239
24. Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett. (2014) 588:368–76. doi: 10.1016/j.febslet.2013.10.015
25. Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Br J Cancer (2014) 111:2214–9. doi: 10.1038/bjc.2014.348
26. Klocke K, Sakaguchi S, Holmdahl R, Wing K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc Natl Acad Sci USA. (2016) 113:E2383–92. doi: 10.1073/pnas.1603892113
27. Khagi Y, Kurzrock R, Patel SP. Next generation predictive biomarkers for immune checkpoint inhibition. Cancer Metastasis Rev. (2017) 36:179–90. doi: 10.1007/s10555-016-9652-y
28. Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. (2013) 210:143–56. doi: 10.1084/jem.20121932
29. Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, et al. PD-1+ and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med. (2016) 22:754–61. doi: 10.1038/nm.4113
30. Yamamoto T, Price DA, Casazza JP, Ferrari G, Nason M, Chattopadhyay PK, et al. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood (2011) 117:4805–15. doi: 10.1182/blood-2010-11-317297
31. Cockerham LR, Jain V, Sinclair E, Glidden DV, Hartogenesis W, Hunt PW, et al. Programmed death-1 expression on CD4+ and CD8+ T cells in treated and untreated HIV disease. AIDS (2014) 28:1749–58. doi: 10.1097/QAD.0000000000000314
32. Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL, et al. Clinical trial of the anti-PD-L1 antibody BMS-936559 in HIV-1 infected participants on suppressive antiretroviral therapy. J Infect Dis. (2017) 215:1725–33. doi: 10.1093/infdis/jix191
33. Ostios-Garcia L, Faig J, Leonardi GC, Adeni AE, Subegdjo SJ, Lydon CA, et al. Safety and efficacy of PD-1 inhibitors among HIV-positive patients with non-small-cell lung cancer. J Thorac Oncol. (2018) 13:1037–42. doi: 10.1016/j.jtho.2018.03.031
34. Finnefrock AC, Tang A, Li F, Freed DC, Feng M, Cox KS, et al. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J Immunol. (2009) 182:980–7. doi: 10.4049/jimmunol.182.2.980
35. Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature (2009) 458:206–10. doi: 10.1038/nature07662
36. Gill AL, Green SA, Abdullah S, Le Saout C, Pittaluga S, Chen H, et al. Programed death-1/programed death-ligand 1 expression in lymph nodes of HIV infected patients: results of a pilot safety study in rhesus macaques using anti-programed death-ligand 1 (Avelumab). AIDS (2016) 30:2487–93. doi: 10.1097/QAD.0000000000001217
37. Koster BD, de Gruijl TD, van den Eertwegh AJ. Recent developments and future challenges in immune checkpoint inhibitory cancer treatment. Curr Opin Oncol. (2015) 27:482–8. doi: 10.1097/CCO.0000000000000221
38. Zanetti M. A second chance for telomerase reverse transcriptase in anticancer immunotherapy. Nat Rev Clin Oncol. (2017) 14:115–28. doi: 10.1038/nrclinonc.2016.67
39. Fauci AS. An HIV vaccine is essential for ending the HIV/AIDS pandemic. JAMA (2017) 318:1535–6. doi: 10.1001/jama.2017.13505
40. Perreau M, Banga R, Pantaleo G. Targeted immune interventions for an HIV-1 cure. Trends Mol Med. (2017) 23:945–61. doi: 10.1016/j.molmed.2017.08.006
41. Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. (2007) 13:46–53. doi: 10.1038/nm1520
42. Pantaleo G, Levy Y. Therapeutic vaccines and immunological intervention in HIV infection: a paradigm change. Curr Opin HIV AIDS (2016) 11:576–84. doi: 10.1097/COH.0000000000000324
Keywords: HIV, Treg, immune checkpoints, HIV vaccine, PD1
Citation: Filaci G, Fenoglio D, Taramasso L, Indiveri F and Di Biagio A (2018) Rationale for an Association Between PD1 Checkpoint Inhibition and Therapeutic Vaccination Against HIV. Front. Immunol. 9:2447. doi: 10.3389/fimmu.2018.02447
Received: 18 June 2018; Accepted: 03 October 2018;
Published: 23 October 2018.
Edited by:
Martin Hoenigl, University of California, San Diego, United StatesReviewed by:
Johannes S. Gach, University of California, Irvine, United StatesSaid Dermime, National Center for Cancer Care and Research, Qatar
Copyright © 2018 Filaci, Fenoglio, Taramasso, Indiveri and Di Biagio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gilberto Filaci, Z2ZpbGFjaUB1bmlnZS5pdA==
 Gilberto Filaci
Gilberto Filaci Daniela Fenoglio
Daniela Fenoglio Lucia Taramasso
Lucia Taramasso Francesco Indiveri
Francesco Indiveri Antonio Di Biagio
Antonio Di Biagio