
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 19 September 2018
Sec. Immunological Memory
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.02093
Tissue-resident memory T (Trm) cells are a subset of recently identified memory T cells that mainly reside and serve as sentinels in non-lymphoid peripheral tissues. Unlike the well-characterized circulating central memory T (Tcm) cells and effector memory T (Tem) cells, Trm cells persist in the tissues, do not recirculate into blood, and offer immediate protection against pathogens upon reinfection. In this review, we focus on CD8+ Trm cells and briefly introduce their characteristics, development, maintenance, and function during viral infection. We also discuss some unresolved problems, such as how CD8+ Trm cells adapt to the local tissue microenvironment, how Trm cells interact with other immune cells during their development and maintenance, and the mechanisms by which CD8+ Trm cells confer immune protection. We believe that a better understanding of these problems is of great clinical and therapeutic value and may contribute to more effective vaccination and treatments against viral infection.
Upon infection, the host immune system initiates immune responses against invading pathogens, a process in which both innate and adaptive immune cells participate sequentially and synergistically. Pathogen-specific memory T cells and B cells persist long after the infection has been cleared (1–3). Until recently, memory T cells had been categorized into central memory T (Tcm) cells and effector memory T (Tem) cells. Tcm cells are a small population of memory T cells that circulate between the secondary lymphoid organs (SLOs) and the blood. They are long-lived and can be activated rapidly upon reencountering their cognate antigen in SLOs. Tem cells have been proposed to migrate through the blood, lymphoid and non-lymphoid tissues (NLT). They kill pathogens via a variety of effector mechanisms and disappear gradually after the pathogens have been eliminated (4–8).
Resident memory T (Trm) cells, a third population of memory T cells, have been identified recently, especially in barrier tissues and SLOs (6, 9–12). They persist permanently in these tissues and do not recirculate into the blood. They can mount a rapid immune response upon reencountering the same pathogen and restrict infection within the local tissue sites (11–14). In addition, emerging data indicate that Trm cells are also involved in tumor immunosurveillance (15). CD103 and CD69 have been considered as two common surface markers in distinguishing Trm cells from other memory T cells (16–20). However, some studies have demonstrated that CD69− or CD103− Trm cells also exist in non-lymphoid peripheral tissues (21–23). This indicates that the development and maintenance of Trm cells, including their phenotypic characteristics, are tightly regulated by local microenvironment (24, 25).
While both CD4+ and CD8+ Trm cells have been identified, CD8+ Trm cells are more extensively investigated in viral infection (26). CD8+ Trm cells that arise through infections due to a variety of pathogens have been identified and characterized in many studies. For example, after acute herpes simplex virus (HSV) infection, CD8+ Trm cells are generated and retained in the skin to protect against reinfection of HSV (27). Skin CD8+ Trm cells produce abundant interferon (IFN)-γ and tumor necrosis factor (TNF)-α following cognate antigen stimulation and are responsible for efficient control of vaccinia virus (VACV) re-infection (12). Using intraglandular infection, Thom et al. demonstrated that CD8+ Trm cells immediately defend the host against local murine cytomegalovirus (MCMV) infection, despite active viral immune evasion (28). Influenza virus-specific CD8+ Trm cells in the nasal epithelia prevent the transmission of influenza virus from the upper respiratory tract to the lung (29). These cells are also sequestered in the walls of the large airways and are crucial for ideal cross-protection against pulmonary influenza virus infection (30–32). Intranasal vaccination of live-attenuated influenza virus generates virus-specific CD8+ Trm cells as well (33). Moreover, both mouse and human respiratory syncytial virus (RSV) specific CD8+ Trm cells are associated with control of lung RSV infection (34, 35). Immune responses of human immunodeficiency virus (HIV)-1-specific CD8+ Trm cells are the strongest in patients whose immune systems are able to naturally control HIV-1 infection, suggesting the involvement of these cells in local anti-HIV immunity (36). In immunosuppressed renal transplant recipients (RTRs), impaired effector differentiation of polyomavirus BK (BKPyV) major capsid protein (VP1)-specific CD8+ Trm cells is associated with BKPyV-induced interstitial nephritis (BKVN), which is caused by BKPyV reactivation after initial control of the virus (37). In addition to non-lymphoid peripheral tissues, CD8+ Trm cells are also embedded in thymus and mediate local immunity against lymphocytic choriomeningitis virus (LCMV) reinfection through degranulation and cytokine (IFN-γ and TNF-α) production (38). Together, all these data indicate that CD8+ Trm cells play an important role in anti-viral immunity not only in non-lymphoid peripheral tissues but also in lymphoid tissues.
In this review, we mainly focus on CD8+ Trm cells and briefly introduce their characteristics, development, maintenance and functions in viral infection. We also discuss the impact of local tissue microenvironment on determining phenotypes of CD8+ Trm cells, the mutual conversion of Trm, Tem, and Tcm cells, the mechanisms of long-term maintenance of Trm cells, and crucial steps in initiating CD8+ Trm cell immune responses. To understand these fundamental questions and further illustrate the underlying mechanisms will help find better strategies for control of viral infection.
Unlike circulating CD8+ Tcm and Tem cells, CD8+ Trm cells locate permanently in the tissues and do not recirculate into the blood (39). More importantly, CD8+ Trm cells are distributed widely in non-lymphoid peripheral tissues including the skin, lung, gastrointestinal tract, female reproductive tract (FRT), brain, liver, kidney, salivary glands, etc (16, 18, 40–44). Recently, some studies reported that CD8+ Trm cells also persist in lymphoid tissues including SLOs and thymus (11, 38, 45). The broad distribution of Trm cells indicates their importance in local immunity.
CD8+ Trm cells in non-lymphoid tissues were initially defined as CD103+ CD69+ (16–20). But later CD69− and CD103− Trm cells were also identified, suggesting that CD69 and CD103 may not be the definite markers of Trm cells (21–23). Interestingly, to some extent these CD103 or CD69 negative CD8+ Trm cells are different from those positive populations. For example, both CD69+ and CD69− CD8+ Trm cells were identified in the pancreas, salivary gland (SG) and FRT, but they have different population sizes (21). In Yersinia pseudotuberculosis (Yptb) oral infection model, CD103+ CD8+ Trm cells are mainly localized in the intestinal epithelium (IEL) and lamina propria (LP) while CD103− CD8+ Trm cells mainly reside in LP and are close to the crypts (46). CD103+ CD8+ and CD103− CD8+ Trm cells are found preferentially in epidermis and in dermis, respectively (18). After murine polyomavirus (MuPyV) infection, brain CD103+ CD8+ Trm cells uniformly express programmed cell death protein 1 (PD-1), in contrast to CD103+ CD8+ Trm cells in the spleen, which are PD-1 negative (23). In addition, CD8+ Trm cells within intestinal mucosa express a variety of distinct molecules that distinguish themselves from memory T cells in SLOs: up-regulate CD28 and CD11c and rapidly produce IFN-γ after reactivation by antigen (47).
Like circulating Tcm and Tem cells, CD8+ Trm cells in different tissues also have distinct transcriptional programs. Lung, skin or gut CD8+ Trm cells have a unique core transcriptional profile with 25–127 specific transcripts, which are progressively engaged during differentiation (18). Liver, known as an immune tolerance organ, retains large numbers of CD8+ Trm cells that express low levels of sphingosine 1-phosphate receptor-1 (S1PR1) and Krüppel-like Factor 2 (KLF2); interestingly, most of these CD8+ Trm cells in the liver are CXCR6 and granzyme positive, and are localized in portal fields, central veins, and parenchymal zones in CHB patients (48). CD8+ Trm cells isolated from the brain possess altered molecular signatures including chemokines and chemokine receptors (up-regulation of CCL3, CXCL10, and CCL4 and down-regulation of CX3CR1 and CCL9), transcription factors (down-regulation of eomes, Tcf-1, lef1, and T-bet and up-regulation of IFITM3, Irf4, and Isg20) and several inhibitory receptors (CTLA-4 and PD-1) after recombinant vesicular stomatitis virus (VSV) infection (49). Similar to mouse CD8+ Trm cells, human CD8+ Trm cells up-regulate ITGA1 (CD49a), ICOS, and the transcription factor IRF4 but down-regulate eomes (43, 50).
CD8+ Trm cells can mount a rapid and robust immune response against reinfection, which is thought to be critical for the efficacy of vaccination. Some functional differences between Trm populations among children, adults, and the elderly have been observed (51). Compared to adults, fewer lung CD8+ and CD4+ Trm cells are established after influenza infection during infancy, which may be associated with more serious or frequent respiratory infections and reduced vaccine responses. The difference between adult and infant Trm cell establishment can be attributed to increased T-bet expression in infant T cells after activation, as is demonstrated in both murine and human models (52).
Taken together, current studies indicate that CD8+ Trm cells in different tissues share some common characteristics in phenotype and functions. However, they also have distinct properties in phenotypes, transcriptional profiling and function as well. The differences among them may be caused by the regulation of their unique tissue microenvironment, which affects their developmental fates.
How memory T cells are generated is a fundamental question in the research field of immunological memory. For classical Tcm and Tem cell development, there are several differentiation hypotheses including linear differentiation model and asymmetric division model (53–55). CD127+ killer cell lectin-like receptor G1 (KLRG1)− CD8+ T cells have been demonstrated to be memory precursor effector cells (MPECs) (56). Whether CD8+ Trm cells also have precursors and what the underlying transcriptional mechanisms in CD8+ Trm cell development are critical questions in the research field of Trm cells.
Mackay et al. (18) recently found that KLRG1−, not KLRG1+, activated CD8+ T cells can develop into skin epithelium-infiltrating CD103+ CD8+ Trm cells. CD127+ KLRG1− CD8+ T cells have been demonstrated to be the intestinal CD8+ Trm precursors in an oral Listeria monocytogenes infection model (57). However, CD127+ KLRG1+ effector CD8+ T cells may lose KLRG1 and differentiate into all memory T cell lineages including CX3CR1− Trm cells (58, 59). Gerlach et al. recently demonstrated that CX3CR1 is a critical chemokine receptor correlated with CD8+ T cell differentiation and further suggested that CD8+ Trm cells are derived from CX3CR1− activated CD8+ T cells (59). It was reported that DC NK lectin group receptor-1 (DNGR-1)+ dendritic cells (DCs) may prime naïve CD8+ T cells to become Trm cell precursors in draining lymph nodes (dLNs), but are not required for Trm differentiation in the skin. Expression of interleukin (IL)-12, IL-15, and CD24 is essential for optimal formation of Trm cells (60). To date, how DC subsets play an important role in generating CD8+ Trm cell precursor is still unclear. In addition, it is known that CD4+ T cell help is required for DCs to induce a robust effector CD8+ T cell response (61). In the absence of CD4+ T cells, fewer CD103+ CD8+ Trm cells are developed in the lungs. Reduced expression of CD103 results from increased expression of the transcription factor T-bet in “unhelped” lung Trm cells. Generation of CD103+ CD8+ Trm cells also requires CD4+ T cell-derived IFN-γ (62). However, in acute VACV skin infection mouse model we did not see a reduction of skin CD8+ Trm cells in the absence of CD4+ T cells, though the function of skin CD8+ Trm cells was found to be partially impaired (12). Moreover, we also did not see any significant reduction of CD8+ Trm cells in the absence of IFNγ (our unpublished data).
Several distinct transcription factors or proteins are involved in the development and homeostasis of CD8+ Trm cells. For instance, CD8+ Trm cells can utilize the transcription factor AhR to maintain residency in the epidermis and compete with dendritic epidermal γδ T cells for space within the epidermal niche (63). In mice, development of CD8+ Trm cells in the skin, gut, liver, and kidney requires cooperation of transcription factors Hobit and Blimp1 (64). Moreover, the function and development of Trm cells can be influenced by nuclear receptor subfamily 4 group A member 1 (NR4A1) and ATP-binding cassette (ABC) transporters (65). Using computational and pooled in vivo RNA interference screens, Milner et al. showed that the transcription factor Runx3 also plays a crucial role in the differentiation and homeostasis of CD8+ Trm cells (66). Purinergic receptor P2RX7 has recently been found to be involved in the generation of CD8+ Trm cells in various non-lymphoid sites (67). In addition to local antigen presentation, intrinsic 4-1BB signals are essential in mediating the generation of CD8+ Trm cells in the lung during influenza infection (31, 68, 69).
Furthermore, peripheral tissue microenvironment is crucial in shaping the development of CD8+ Trm cells. Hair follicle derived cytokines such as IL-7 and IL-15 play critical roles in skin Trm cell homeostasis (70), while transforming growth factor (TGF)-β promotes the formation of kidney CD8+ Trm cells by enhancing expression of E- and P-selectin and chemokine receptor CXCR3, which mediate the extravasation of effector T cells (71). Despite the involvement of TGF-β in Trm development, Smad4, which is required for normal differentiation of circulating memory T cells, is not necessary for Trm cell differentiation (72). Adhesion- and degranulation-promoting adapter protein (ADAP) integrin facilitates CD8+ Trm cells formation in non-lymphoid tissues (73). Formalin-inactivated RSV combined with CpG (an agonist of TLR9) and L685,458 (an inhibitor of Notch signaling) promote protective CD8+ Trm cells in the lungs (74). Additionally, brain TGF-β producing regulatory T cells (Tregs) are found to be involved in CD8+ Trm cell accumulation and granzyme B production after West Nile virus (WNV) and MCMV infection (75, 76). Besides non-specific stimulation, specific stimulation such as local antigen in skin is also required for the formation of functional CD8+ Trm cells and amplifies their generation. Although recruitment of activated CD8+ T cells to VACV infected skin is antigen independent, significant increase in Trm formation is observed when local antigen is present (77). In skin that has been previously infected, antigen-dependent cross-competition is involved in shaping the repertoire of polyclonal antiviral Trm cells (78). Secondary Trm cells form from both pre-existing Trm cells and Trm precursors recruited from the blood in response to local antigen presence (79). Transient introduction of antigen results in the generation of Trm in the brain via an intracranial dendritic cell immunization regimen (80). However, local inflammation in the skin and mucosa alone can drive recruitment of effector populations and direct their conversion to CD8+ Trm cells (24). Similarly, differentiation and maintenance of CD8+ Trm cells are antigen-independent in small intestine, kidney, pancreas, stomach, heart, and FRT of mice (81, 82).
CD8+ Trm cells have long been thought to reside exclusively in non-lymphoid tissues. However, in SLOs such as the splenic marginal zone, red pulp, and lymph node sinuses, CD8+ Trm cells are also present. These Trm cells can be derived from the skin or mucosa after restimulation (11, 45). Although great progress has been made in characterizing CD8+ Trm cell development, the exact mechanisms are still unclear. Both intrinsic and extrinsic factors are involved in the development of CD8+ Trm cells. More details need to be known before therapeutically manipulating CD8+ Trm cell development, which is important for the control of viral infection and vaccine design. A developmental scheme for CD8+ Trm cells is shown in Figure 1.
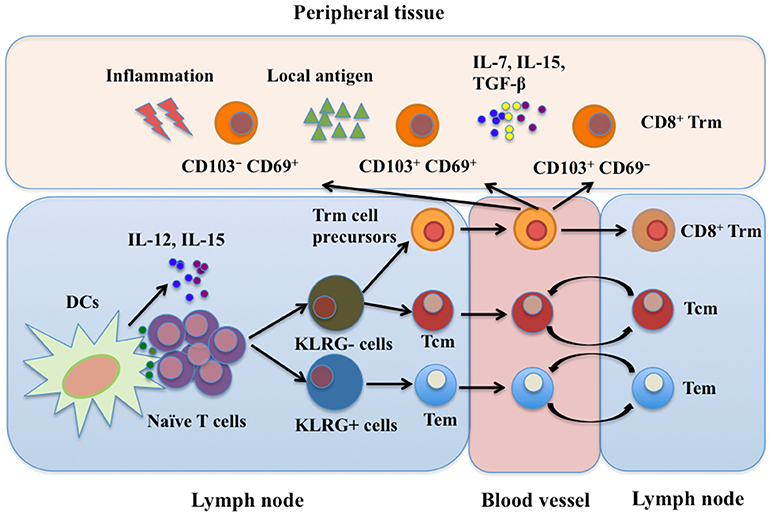
Figure 1. CD8+ Trm cell development. Trm, Tem, and Tcm cells are derived from the same naïve T cell clone upon activation in SLOs. CD8+ Trm cell precursors migrate into peripheral tissues as well as SLOs where they differentiate into Trm cells. Currently, at least three subtypes of CD8+ Trm cells have been identified: CD103+ CD69+, CD103+ CD69−, and CD103− CD69+ Trm cells. Local microenvironment, including cytokines, local antigens and inflammatory mediators, is important for development of CD8+ Trm cells. SLOs, secondary lymphoid organs; Tcm, central memory T cells; Tem, effector memory T cells; Trm, resident memory T cells; DCs, dendritic cells; LN, lymph node; IL-7, interleukin-7; IL-12, interleukin-12; IL-15, interleukin-15; TGF-β, transforming growth factor-β; KLRG, killer cell lectin-like receptor G.
IL-7 and IL-15 are two critical cytokines required for the survival and homeostasis of classical memory CD8+ T cells (17, 83). In some but not all peripheral tissues, IL-15 is required for the survival of CD8+ Trm cells (18, 84). This suggests that other factors may also be involved in the maintenance of Trm cells. TGF-β is not only critical for Trm formation but also required for skin and gut Trm maintenance (18, 25). Expression of TGF-β and IL-15 are controlled by T-box transcription factors (TFs) Eomes and T-bet (85). Retention of intestinal CD8+ Trm cells is partly associated with integrins αEβ7 and α1 as well as CD69, whose expression is induced by TGF-β (25). CD69 may retain Trm cells in the skin by blocking sphingosine-1-phosphate (S1P)-regulated tissue egress (86). Downregulation of S1PR1 is controlled by the transcription factor Kruppel-like factor 2 (KLF2) (87). However, CD69 is not required for Trm cell retention in the lung when they have entered the Trm cell niches (88). In addition, E-cadherin and integrin α4β1 promote CD8+ Trm cells accumulation in salivary glands (89, 90).
Some other factors also play important roles in maintenance of Trm cells. For example, exogenous free fatty acid (FFA) can be used by skin CD8+ Trm cells via fatty-acid-binding proteins (FABP4) 4 and FABP5 for their maintenance (91). Deletion of CCR2+ IL-12-producing cells, most of which are macrophages, reduces the size of the CD103− CD8+ Trm population during infection, suggesting that macrophages or the mediators they produce may be involved in Trm cell persistence (22). Although local antigen contributes to in situ proliferation of Trm cells (77, 78), it is not indispensable for local Trm cell persistence, indicating a dependence on the local microenvironment for Trm function and survival (80). Moreover, CD8+ Trm and lymph node Tcm cell clones are generated from the same naïve T cell precursor after skin immunization (92). Therefore, CD8+ Tcm could be a potential reservoir for CD8+ Trm cells upon reinfection. In addition, reactivation of Trm cells recruit recirculating memory T cells that undergo antigen-independent Trm cell differentiation in situ (82). In brain, B7-H1 has a critical role in the maintenance of CD8+ Trm cells (93). Understanding how Trm cells maintain long-term residency within barrier tissues will enable the manipulation of these cells in vitro. A scheme for possible mechanisms of CD8+ Trm cell maintenance is shown in Figure 2.
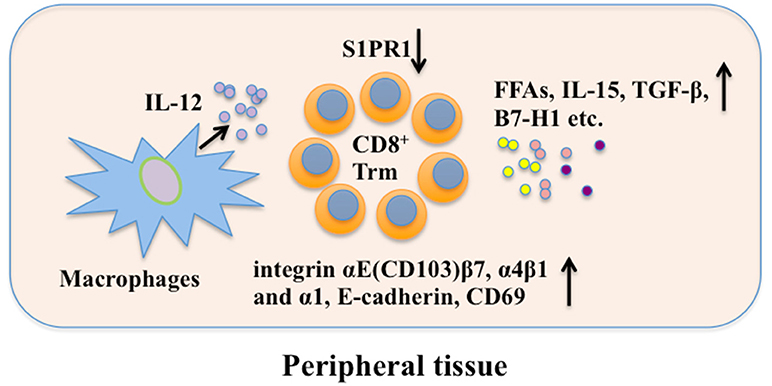
Figure 2. CD8+ Trm cell maintenance. Local factors in peripheral tissue are crucial in long-term maintaining CD8+ Trm cells. CD69, E-cadherin, and integrin promote retention of CD8+ Trm while S1PR1 can mediate CD8+ Trm cell tissue egress. FFAs, IL-15, TGF-β, and B7-H1 are involved in CD8+ Trm cell maintenance. Besides, macrophages may promote CD8+ Trm cell maintenance through secreting IL-12. FFAs, free fatty acids; S1PR1, sphingosine 1-phosphate receptor-1; IL-12, interleukin-12; IL-15, interleukin-15; TGF-β, transforming growth factor-β; B7-H1, B7 homolog 1; Trm, resident memory T cells.
The importance of CD8+ Trm cells in peripheral tissue protection has been widely recognized. Skin CD8+ Trm cells serve as sentinels and continuously migrate through the epidermis. They change size, length, and direction of dendrites, which are independent of skin inflammatory state. They quickly identify antigen-expressing cells in vivo and initiate in situ immune responses (94). The CXCL17/CXCR8 and CXCL10/CXCR3 chemokine pathways are involved in CD8+ Trm cell mobilization to infected barrier tissues (95, 96). The rapid control of viral infection is related to abundant IFN-γ and TNF-α produced by CD8+ Trm cells following cognate antigen stimulation (12, 97–100). After LCMV reinfection in mice, brain CD8+ Trm cells rapidly produce IFN-γ and perforin and prevent fatal brain infection in a manner independent of circulating CD8+ memory T cells. Presentation of cognate antigen on major histocompatibility complex (MHC)-I is required for brain Trm cell protective immunity (100). Control of the female mice genital HSV-2 infection by CD8+ Trm cells requires expression of MHC-I on CD301b+ DCs in the lamina propria (99). However, infected epidermal cells may directly present viral antigen to CD8+ T cells to induce cytokine production, which may also be involved in the activation of CD8+ Trm cells (94, 101). It is well-known that activated T cells express inhibitory molecules (102). For example, activated intrahepatic CD8+ Trm cells express both PD-1 and CD39 after sequential IL-15 or antigen exposure. These inhibitory molecules combined with IL-2 and IFN-γ promote liver CD8+ Trm cell survival while contribute to local non-cytolytic hepatic immunosurveillance (103).
Reactivation of CD8+ Trm cells by peptide challenge can trigger strong antiviral immunity against antigenically unrelated pathogens. In addition to inducing a number of broadly active antiviral and antibacterial genes, reactivated Trm cells orchestrate both innate and adaptive immune components including recruitment of recirculating CD4+ T cells, CD8+ T cells and B cells, maturation of DCs, and activation of natural killer (NK) cells to develop a “pathogen alert” state. Achievement of these functions relies on IFN-γ, TNF-α, and IL-2Rβ-dependent cytokines (104–106). IFN-γ secreted by activated CD8+ Trm cells enhances expression of vascular cell adhesion molecule-1 (VCAM-1) on vascular endothelium, which contributes to recruitment of CD4+ T cells, CD8+ T cells, and B cells to local tissues. In addition, TNF-α and IL-2Rβ-dependent cytokines are essential for DC maturation and granzyme B upregulation in both NK cells and bystander memory CD8+ T cells, respectively (105, 107). However, the crucial steps for the initiation of CD8+ Trm cell immune responses are still obscure. Further exploration should be focused on how to optimize their antiviral functions. A scheme for possible mechanisms of CD8+ Trm cells in viral protection is shown in Figure 3.
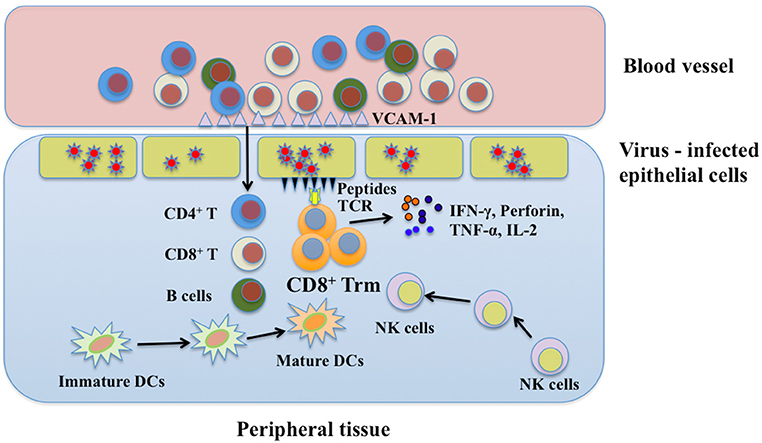
Figure 3. The protective function of CD8+ Trm cells in viral infection. Upon encountering the same pathogen, CD8+ Trm cells can be reactivated immediately and secrete cytokines in which IFN-γ may help recruit immune cells from blood stream via enhancing expression of endothelial vessel addressin. These immune cells include CD4+ T cells, CD8+ T cells, and B cells. Besides, NK cells and immature DCs in local tissue can also be recruited to the place where CD8+ Trm cells are reactivated. CD8+ Trm cells cooperate with these immune cells to synergistically combat with viruses by secreting perforin, IFN-γ, and TNF-α. Trm, resident memory T cells; DCs, dendritic cells; NK, natural killer; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; IL-2, interleukin-2; VCAM-1, vascular cell adhesion molecule-1.
It is now clear that CD8+ Trm cells play an important role in peripheral immune surveillance and protection against invading pathogens, especially in viral infection. The diversities of CD8+ Trm cells may be caused by different tissue microenvironments. The exact roles of different components involved in the process of CD8+ Trm cell mediated immunity are still obscure. Although great progress has been made in CD8+ Trm cell research, several problems need to be further explored: What is the origin of CD8+ T cell precursor in dLNs? What are the tissue-specific adaptations of CD8+ Trm cell development? What is the role of local tissue antigen-presenting cells in CD8+ Trm cell differentiation vs. recall reaction? How are CD8+ Trm cells regulated during reactivation? We believe that the strategies that modulate the functions of CD8+ Trm cells will be helpful for the control of viral infection if more details about CD8+ Trm cells are unraveled.
XW and PW drafted the primary manuscript and figures. FX, YS, and XJ designed and corrected the manuscript. All the authors have read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was supported by grants from the National Natural Science Foundation of China (81400621, 81370176, and 81572800) and the Major Science and Technology Special Project of Zhejiang Province (2014C03033).
1. Zinkernagel RM. Immunology taught by viruses. Science (1996) 271:173–8. doi: 10.1126/science.271.5246.173
2. Gourley TS, Wherry EJ, Masopust D, Ahmed R. Generation and maintenance of immunological memory. Semin Immunol. (2004) 16:323–33. doi: 10.1016/j.smim.2004.08.013
3. Dorner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity (2007) 27:384–92. doi: 10.1016/j.immuni.2007.09.002
4. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature (1999) 401: 708–12. doi: 10.1038/44385
5. Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science (2001) 291: 2413–7. doi: 10.1126/science.1058867
6. Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. (2013) 13:309–20. doi: 10.1038/nri3442
7. Klonowski KD, Marzo AL, Williams KJ, Lee SJ, Pham QM, Lefrançois L. CD8 T cell recall responses are regulated by the tissue tropism of the memory cell and pathogen. J Immunol. (2006) 177:6738–46. doi: 10.4049/jimmunol.177.10.6738
8. Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity (2007) 27:393–405. doi: 10.1016/j.immuni.2007.08.007
9. Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature (2011) 477:216–9. doi: 10.1038/nature10339
10. Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. (2011) 187:5510–4. doi: 10.4049/jimmunol.1102243
11. Schenkel JM, Fraser KA, Masopust D. Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J Immunol. (2014) 192:2961–4. doi: 10.4049/jimmunol.1400003
12. Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature (2012) 483:227–31. doi: 10.1038/nature10851
13. Marriott CL, Dutton EE, Tomura M, Withers DR. Retention of Ag-specific memory CD4+ T cells in the draining lymph node indicates lymphoid tissue resident memory populations. Eur J Immunol. (2017) 47:860–71. doi: 10.1002/eji.201646681
14. Wilk MM, Misiak A, McManus RM, Allen AC, Lynch MA, Mills KHG. Lung CD4 tissue-resident memory T cells mediate adaptive immunity induced by previous infection of mice with Bordetella pertussis. J Immunol. (2017) 199:233–43. doi: 10.4049/jimmunol.1602051
15. Gebhardt T, Palendira U, Tscharke DC, Bedoui S. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol Rev. (2018) 283:54–76. doi: 10.1111/imr.12650
16. Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. (2015) 21:688–97. doi: 10.1038/nm.3883
17. Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. (2014) 14:24–35. doi: 10.1038/nri3567
18. Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. (2013) 14:1294–301. doi: 10.1038/ni.2744
19. Pichyangkul S, Yongvanitchit K, Limsalakpetch A, Kum-Arb U, Im-Erbsin R, Boonnak K, et al. Tissue distribution of memory T and B cells in rhesus monkeys following influenza A infection. J Immunol. (2015) 195:4378–86. doi: 10.4049/jimmunol.1501702
20. Reilly EC, Lambert-Emo K, Topham DJ. The effects of acute neutrophil depletion on resolution of acute influenza infection, establishment of tissue resident memory (TRM), and heterosubtypic immunity. PLoS ONE (2016) 11:e0164247. doi: 10.1371/journal.pone.0164247
21. Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell (2015) 161:737–49. doi: 10.1016/j.cell.2015.03.031
22. Bergsbaken T, Bevan MJ, Fink PJ. Local inflammatory cues regulate differentiation and persistence of CD8(+) tissue-resident memory T cells. Cell Rep. (2017) 19:114–24. doi: 10.1016/j.celrep.2017.03.031
23. Shwetank Abdelsamed HA, Frost EL, Schmitz HM, Mockus TE, Youngblood BA, et al. Maintenance of PD-1 on brain-resident memory CD8 T cells is antigen independent. Immunol Cell Biol. (2017) 95:953–9. doi: 10.1038/icb.2017.62
24. Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA. (2012) 109:7037–42. doi: 10.1073/pnas.1202288109
25. Zhang N, Bevan MJ. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity (2013) 39:687–96. doi: 10.1016/j.immuni.2013.08.019
26. Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. (2013) 31:137–161. doi: 10.1146/annurev-immunol-032712-095954
27. Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. (2009) 10:524–30. doi: 10.1038/ni.1718
28. Thom JT, Weber TC, Walton SM, Torti N, Oxenius A. The salivary gland acts as a sink for tissue-resident memory CD8(+) T Cells, facilitating protection from local cytomegalovirus infection. Cell Rep. (2015) 13:1125–36. doi: 10.1016/j.celrep.2015.09.082
29. Pizzolla A, Nguyen THO, Smith JM, Brooks AG, Kedzieska K, Heath WR, et al. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol. (2017) 2:eaam6970. doi: 10.1126/sciimmunol.aam6970
30. Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. (2014) 95:215–24. doi: 10.1189/jlb.0313180
31. Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, et al. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol. (2011) 85:4085–94. doi: 10.1128/JVI.02493-10
32. Zarnitsyna VI, Handel A, McMaster SR, Hayward SL, Kohlmeier JE, Antia R. Mathematical model reveals the role of memory CD8 T cell populations in recall responses to influenza. Front Immunol. (2016) 7:165. doi: 10.3389/fimmu.2016.00165
33. Zens KD, Chen JK, Farber DL. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight (2016) 1:e85832. doi: 10.1172/jci.insight.85832
34. Kinnear E, Lambert L, McDonald JU, Cheeseman HM, Caproni LJ, Tregoning JS. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol. (2018) 11:249–56. doi: 10.1038/mi.2017.46
35. Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun. (2015) 6:10224. doi: 10.1038/ncomms10224
36. Kiniry BE, Li S, Ganesh A, Hunt PW, Somsouk M, Skinner PJ, et al. Detection of HIV-1-specific gastrointestinal tissue resident CD8(+) T-cells in chronic infection. Mucosal Immunol. (2018) 11:909–20. doi: 10.1038/mi.2017.96
37. van Aalderen MC, Remmerswaal EB, Heutinck KM, Ten Brinke A, Feltkamp MC, van der Weerd NC, et al. Clinically relevant reactivation of polyomavirus BK (BKPyV) in HLA-A02-positive renal transplant recipients is associated with impaired effector-memory differentiation of BKPyV-specific CD8+ T cells. PLoS Pathog. (2016) 12:e1005903. doi: 10.1371/journal.ppat.1005903
38. Hofmann M, Oschowitzer A, Kurzhals SR, Kruger CC, Pircher H. Thymus-resident memory CD8+ T cells mediate local immunity. Eur J Immunol. (2013) 43:2295–304. doi: 10.1002/eji.201343519
39. Shane HL, Klonowski KD. Every breath you take: the impact of environment on resident memory CD8 T cells in the lung. Front Immunol. (2014) 5:320. doi: 10.3389/fimmu.2014.00320
40. Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity (2013) 38:187–97. doi: 10.1016/j.immuni.2012.09.020
41. Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. (2015) 7:279ra39. doi: 10.1126/scitranslmed.3010302
42. Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell (2014) 159:814–28. doi: 10.1016/j.cell.2014.10.026
43. Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. (2017) 20:2921–34. doi: 10.1016/j.celrep.2017.08.078
44. Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. (2006) 176:2079–83. doi: 10.4049/jimmunol.176.4.2079
45. Beura LK, Wijeyesinghe S, Thompson EA, Macchietto MG, Rosato PC, Pierson MJ, et al. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity (2018) 48:327–38. doi: 10.1016/j.immuni.2018.01.015
46. Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat Immunol. (2015) 16:406–14. doi: 10.1038/ni.3108
47. Kim SK, Schluns KS, Lefrancois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J Immunol. (1999) 163:4125–32.
48. Stelma F, de Niet A, Sinnige MJ, van Dort KA, van Gisbergen K, Verheij J, et al. Human intrahepatic CD69+ CD8+ T cells have a tissue resident memory T cell phenotype with reduced cytolytic capacity. Sci Rep. (2017) 7:6172. doi: 10.1038/s41598-017-06352-3
49. Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol. (2012) 189:3462–71. doi: 10.4049/jimmunol.1201305
50. McCully ML, Ladell K, Andrews R, Jones RE, Miners KL, Roger L, et al. CCR8 expression defines tissue-resident memory T cells in human skin. J Immunol. (2018) 200:1639–50. doi: 10.4049/jimmunol.1701377
51. Booth JS, Toapanta FR, Salerno-Goncalves R, Patil S, Kader HA, Safta AM, et al. Characterization and functional properties of gastric tissue-resident memory T cells from children, adults, and the elderly. Front Immunol. (2014) 5:294. doi: 10.3389/fimmu.2014.00294
52. Zens KD, Chen JK, Guyer RS, Wu FL, Cvetkovski F, Miron M, et al. Reduced generation of lung tissue-resident memory T cells during infancy. J Exp Med. (2017) 214:2915–32. doi: 10.1084/jem.20170521
53. Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science (2007) 315:1687–91. doi: 10.1126/science.1139393
54. Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity (2007) 27:985–97. doi: 10.1016/j.immuni.2007.10.012
55. Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity (2009) 31:859–71. doi: 10.1016/j.immuni.2009.11.007
56. Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. (2003) 4:1191–8. doi: 10.1038/ni1009
57. Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrancois L. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity (2014) 40:747–57. doi: 10.1016/j.immuni.2014.03.007
58. Herndler-Brandstetter D, Ishigame H, Shinnakasu R, Plajer V, Stecher C, Zhao J, et al. KLRG1+ effector CD8+ T cells lose KLRG1, differentiate into all memory T cell lineages, and convey enhanced protective immunity. Immunity (2018) 48:716–29. doi: 10.1016/j.immuni.2018.03.015
59. Gerlach C, Moseman EA, Loughhead SM, Alvarez D, Zwijnenburg AJ, Waanders L, et al. The chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity (2016) 45:1270–84. doi: 10.1016/j.immuni.2016.10.018
60. Iborra S, Martinez-Lopez M, Khouili SC, Enamorado M, Cueto FJ, Conde-Garrosa R, et al. Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires crosspriming by DNGR-1(+) dendritic cells. Immunity (2016) 45:847–60. doi: 10.1016/j.immuni.2016.08.019
61. Laidlaw BJ, Craft JE, Kaech SM. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat Rev Immunol. (2016) 16:102–11. doi: 10.1038/nri.2015.10
62. Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity (2014) 41:633–45. doi: 10.1016/j.immuni.2014.09.007
63. Zaid A, Mackay LK, Rahimpour A, Braun A, Veldhoen M, Carbone FR, et al. Persistence of skin-resident memory T cells within an epidermal niche. Proc Natl Acad Sci USA. (2014) 111:5307–12. doi: 10.1073/pnas.1322292111
64. Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science (2016) 352:459–63. doi: 10.1126/science.aad2035
65. Boddupalli CS, Nair S, Gray SM, Nowyhed HN, Verma R, Gibson JA, et al. ABC transporters and NR4A1 identify a quiescent subset of tissue-resident memory T cells. J Clin Invest. (2016) 126:3905–16. doi: 10.1172/JCI85329
66. Milner JJ, Toma C, Yu B, Zhang K, Omilusik K, Phan AT, et al. Runx3 programs CD8(+) T cell residency in non-lymphoid tissues and tumours. Nature (2017) 552:253–7. doi: 10.1038/nature24993
67. Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, et al. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8+ T cells. Nature (2018) 559:264–8. doi: 10.1038/s41586-018-0282-0
68. Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity (2006) 24:439–49. doi: 10.1016/j.immuni.2006.01.015
69. Zhou AC, Wagar LE, Wortzman ME, Watts TH. Intrinsic 4-1BB signals are indispensable for the establishment of an influenza-specific tissue-resident memory CD8 T-cell population in the lung. Mucosal Immunol. (2017) 10:1294–309. doi: 10.1038/mi.2016.124
70. Adachi T, Kobayashi T, Sugihara E, Yamada T, Ikuta K, Pittaluga S, et al. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat Med. (2015) 21:1272–9. doi: 10.1038/nm.3962
71. Ma C, Mishra S, Demel EL, Liu Y, Zhang N. TGF-beta controls the formation of kidney-resident T cells via promoting effector T cell extravasation. J Immunol. (2017) 198:749–56. doi: 10.4049/jimmunol.1601500
72. Hu Y, Lee YT, Kaech SM, Garvy B, Cauley LS. Smad4 promotes differentiation of effector and circulating memory CD8 T cells but is dispensable for tissue-resident memory CD8 T cells. J Immunol. (2015) 194:2407–14. doi: 10.4049/jimmunol.1402369
73. Fiege JK, Beura LK, Burbach BJ, Shimizu Y. Adhesion- and degranulation-promoting adapter protein promotes CD8 T cell differentiation and resident memory formation and function during an acute infection. J Immunol. (2016) 197:2079–89. doi: 10.4049/jimmunol.1501805
74. Zhang L, Li H, Hai Y, Yin W, Li W, Zheng B, et al. CpG in Combination with an inhibitor of notch signaling suppresses formalin-inactivated respiratory syncytial virus-enhanced airway hyperresponsiveness and inflammation by inhibiting Th17 memory responses and promoting tissue-resident memory cells in lungs. J Virol. (2017) 91:e02111–16. doi: 10.1128/JVI.02111-16
75. Graham JB, Da Costa A, Lund JM. Regulatory T cells shape the resident memory T cell response to virus infection in the tissues. J Immunol. (2014) 192:683–90. doi: 10.4049/jimmunol.1202153
76. Prasad S, Hu S, Sheng WS, Singh A, Lokensgard JR. Tregs modulate lymphocyte proliferation, activation, and resident-memory T-cell accumulation within the brain during MCMV infection. PLoS ONE (2015) 10:e0145457. doi: 10.1371/journal.pone.0145457
77. Khan TN, Mooster JL, Kilgore AM, Osborn JF, Nolz JC. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J Exp Med. (2016) 213:951–66. doi: 10.1084/jem.20151855
78. Muschaweckh A, Buchholz VR, Fellenzer A, Hessel C, Konig PA, Tao S, et al. Antigen-dependent competition shapes the local repertoire of tissue-resident memory CD8+ T cells. J Exp Med. (2016) 213:3075–86. doi: 10.1084/jem.20160888
79. Park SL, Zaid A, Hor JL, Christo SN, Prier JE, Davies B, et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat Immunol. (2018) 19:183–91. doi: 10.1038/s41590-017-0027-5
80. Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. (2010) 107:17872–9. doi: 10.1073/pnas.1010201107
81. Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. (2012) 188:4866–75. doi: 10.4049/jimmunol.1200402
82. Beura LK, Mitchell JS, Thompson EA, Schenkel JM, Mohammed J, Wijeyesinghe S, et al. Intravital mucosal imaging of CD8(+) resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat Immunol. (2018) 19:173–82. doi: 10.1038/s41590-017-0029-3
83. Kaneko S, Mastaglio S, Bondanza A, Ponzoni M, Sanvito F, Aldrighetti L, et al. IL-7 and IL-15 allow the generation of suicide gene-modified alloreactive self-renewing central memory human T lymphocytes. Blood (2009) 113:1006–15. doi: 10.1182/blood-2008-05-156059
84. Schenkel JM, Fraser KA, Casey KA, Beura LK, Pauken KE, Vezys V, et al. IL-15-independent maintenance of tissue-resident and boosted effector memory CD8 T cells. J Immunol. (2016) 196:3920–6. doi: 10.4049/jimmunol.1502337
85. Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, et al. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity (2015) 43:1101–11. doi: 10.1016/j.immuni.2015.11.008
86. Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol. (2015) 194:2059–63. doi: 10.4049/jimmunol.1402256
87. Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. (2013) 14:1285–93. doi: 10.1038/ni.2745
88. Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T, et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med. (2016) 213:3057–73. doi: 10.1084/jem.20160938
89. Woyciechowski S, Hofmann M, Pircher H. α4 β1 integrin promotes accumulation of tissue-resident memory CD8(+) T cells in salivary glands. Eur J Immunol. (2017) 47:244–50. doi: 10.1002/eji.201646722
90. Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci USA. (2011) 108:16741–6. doi: 10.1073/pnas.1107200108
91. Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature (2017) 543:252–6. doi: 10.1038/nature21379
92. Gaide O, Emerson RO, Jiang X, Gulati N, Nizza S, Desmarais C, et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med. (2015) 21:647–53. doi: 10.1038/nm.3860
93. Pavelko KD, Bell MP, Harrington SM, Dong H. B7-H1 influences the accumulation of virus-specific tissue resident memory T cells in the central nervous system. Front Immunol. (2017) 8:1532. doi: 10.3389/fimmu.2017.01532
94. Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci USA. (2012) 109:19739–44. doi: 10.1073/pnas.1208927109
95. Srivastava R, Hernandez-Ruiz M, Khan AA, Fouladi MA, Kim GJ, Ly VT, et al. CXCL17 chemokine-dependent mobilization of CXCR8(+)CD8(+) effector memory and tissue-resident memory T cells in the vaginal mucosa is associated with protection against genital herpes. J Immunol. (2018) 200:2915–26. doi: 10.4049/jimmunol.1701474
96. Srivastava R, Khan AA, Chilukuri S, Syed SA, Tran TT, Furness J, et al. CXCL10/CXCR3-dependent mobilization of herpes simplex virus-specific CD8(+) TEM and CD8(+) TRM cells within infected tissues allows efficient protection against recurrent herpesvirus infection and disease. J Virol. (2017) 91:e00278-17. doi: 10.1128/JVI.00278-17
97. Aguilar-Valenzuela R, Netland J, Seo YJ, Bevan MJ, Grakoui A, Suthar MS. Dynamics of tissue-specific CD8+ T cell responses during West Nile virus infection. J Virol. (2018) 92:e00014–18. doi: 10.1128/JVI.00014-18
98. McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-gamma production. J Immunol. (2015) 195:203–9. doi: 10.4049/jimmunol.1402975
99. Shin H, Kumamoto Y, Gopinath S, Iwasaki A. CD301b+ dendritic cells stimulate tissue-resident memory CD8+ T cells to protect against genital HSV-2. Nat Commun. (2016) 7:13346. doi: 10.1038/ncomms13346
100. Steinbach K, Vincenti I, Kreutzfeldt M, Page N, Muschaweckh A, Wagner I, et al. Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J Exp Med. (2016) 213:1571–87. doi: 10.1084/jem.20151916
101. Macleod BL, Bedoui S, Hor JL, Mueller SN, Russell TA, Hollett NA, et al. Distinct APC subtypes drive spatially segregated CD4+ and CD8+ T-cell effector activity during skin infection with HSV-1. PLoS Pathog. (2014) 10:e1004303. doi: 10.1371/journal.ppat.1004303
102. Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. (2015) 36:265–76. doi: 10.1016/j.it.2015.02.008
103. Pallett LJ, Davies J, Colbeck EJ, Robertson F, Hansi N, Easom NJW, et al. IL-2(high) tissue-resident T cells in the human liver: Sentinels for hepatotropic infection. J Exp Med. (2017) 214:1567–80. doi: 10.1084/jem.20162115
104. Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, et al. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science (2014) 346:101–5. doi: 10.1126/science.1254803
105. Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science (2014) 346:98–101. doi: 10.1126/science.1254536
106. Tan HX, Wheatley AK, Esterbauer R, Jegaskanda S, Glass JJ, Masopust D. Induction of vaginal-resident HIV-specific CD8 T cells with mucosal prime-boost immunization. Mucosal Immunol. (2018) 11:994–1007. doi: 10.1038/mi.2017.89
Keywords: memory T cells, tissue-resident memory T cells, microenvironment, viral infection, immune response
Citation: Wu X, Wu P, Shen Y, Jiang X and Xu F (2018) CD8+ Resident Memory T Cells and Viral Infection. Front. Immunol. 9:2093. doi: 10.3389/fimmu.2018.02093
Received: 01 April 2018; Accepted: 24 August 2018;
Published: 19 September 2018.
Edited by:
Kamal M. Khanna, New York University, United StatesReviewed by:
Brian S. Sheridan, Stony Brook University, United StatesCopyright © 2018 Wu, Wu, Shen, Jiang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Xu, eHVmZW5nOTlAemp1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.