- 1Center for Experimental and Molecular Medicine, Amsterdam UMC, Amsterdam Infection & Immunity Institute, University of Amsterdam, Amsterdam, Netherlands
- 2Department of Medicine, University of Michigan, Ann Arbor, MI, United States
- 3VA Center for Clinical Management Research, Ann Arbor, MI, United States
- 4Division of Infectious Diseases, Department of Medicine, Amsterdam UMC, Amsterdam Infection & Immunity Institute, University of Amsterdam, Amsterdam, Netherlands
Alongside advances in understanding the pathophysiology of sepsis, there have been tremendous strides in understanding the pervasive role of the gut microbiota in systemic host resistance. In pre-clinical models, a diverse and balanced gut microbiota enhances host immunity to both enteric and systemic pathogens. Disturbance of this balance increases susceptibility to sepsis and sepsis-related organ dysfunction, while restoration of the gut microbiome is protective. Patients with sepsis have a profoundly distorted composition of the intestinal microbiota, but the impact and therapeutic potential of the microbiome is not well-established in human sepsis. Modulation of the microbiota consists of either resupplying the pool of beneficial microbes by administration of probiotics, improving the intestinal microenvironment to enhance the growth of beneficial species by dietary interventions and prebiotics, or by totally recolonizing the gut with a fecal microbiota transplantation (FMT). We propose that there are three potential opportunities to utilize these treatment modalities over the course of sepsis: to decrease sepsis incidence, to improve sepsis outcome, and to decrease late mortality after sepsis. Exploring these three avenues will provide insight into how disturbances of the microbiota can predispose to, or even perpetuate the dysregulated immune response associated with this syndrome, which in turn could be associated with improved sepsis management.
Introduction
Sepsis is a highly heterogeneous and multifaceted syndrome that is caused by a dysregulated host response to an infection (1, 2). A disproportionate pro-inflammatory response to invasive infection was once believed to be the main pathophysiological paradigm of sepsis syndrome. However, recent studies have shown that patients with sepsis suffer from both sustained excessive inflammation and immune suppression, with a failure to return to normal homeostasis (3). Despite our increased understanding of the underlying pathophysiology of the syndrome, targeted therapies aimed at modifying the disrupted host response in patients with sepsis have been unsuccessful to date (4). Treating physicians are limited to supportive care and antibiotics as the disorder continues to drain billions of dollars and contributes to an estimated 5 million deaths worldwide each year (5, 6). Therefore, new tools in the management of sepsis are highly warranted.
In tandem with the advances of our understanding on the pathophysiology of sepsis, tremendous strides have been made in understanding the pervasive role of the gut microbiota in both health and disease states. Pre-clinical work has shown that a diverse and balanced gut microbiota is able to enhance host immunity to both enteric and systemic pathogens, and that disturbance of this balance potentially leads to increased susceptibility of sepsis (7, 8). Of interest, a handful of pioneering studies have shown that the composition of the intestinal microbiota is severely affected by sepsis, but the short- and long-term clinical consequences of these disturbances remain largely unknown (9–12). In this Review, we aim to provide an overview of the mechanisms through which the microbiota contributes to host defense in sepsis, and how these insights could be of relevance to this multi-facetted syndrome. Better insights into the drivers of microbiota-mediated host resistance could provide novel preventive and therapeutic strategies against sepsis.
Microbiota-Mediated Host Resistance
The intestinal microbiota is a complex ecosystem that consists of trillions of bacterial cells that have evolved with its hosts over millions of years (13). In the last decade, it has been revealed that these microbial communities are involved in a wide of array of functions, such as the digestion of food, production of hormones, and the development and maturation of the immune system (14–16). In addition, it has been shown that a state of disturbance of the intestinal microbiota, otherwise known as dysbiosis, plays an important role in increasing susceptibility to infectious diseases (17, 18). Several mechanisms have been uncovered on how the intestinal microbiota contributes to protection against enteric pathogens, such as Clostridium difficile (19). For example, intestinal microbes directly outcompete pathogens for nutrients, possess the capacity of producing antibacterial peptides, modify bile salts to render them harmful to other microorganisms, as well as drive increased mucus production and intestinal epithelial integrity. In addition, gut bacteria contribute to resistance to enteric pathogens by inducing the production of antibacterial factors by epithelial cells, and enhancing humoral responses against invading pathogens (19).
Recent insights have revealed that the microbiota also modulate systemic immunity (Figure 1). A 2016 hallmark study involving 500 healthy human volunteers linked the gut microbiota to inflammatory cytokine production capacity using ex vivo stimulation of whole blood and peripheral blood mononuclear cells: the observed inter-individual variation in cytokine responses was significantly correlated with the composition and function of the microbiota (20). Moreover, in healthy subjects in which the microbiota is disrupted by broad-spectrum antibiotics, systemic mononuclear cells produced lower levels of tumor necrosis factor (TNF)-α after ex vivo stimulation with lipopolysaccharide (LPS) (21). Of interest however, microbiota disruption by broad-spectrum antibiotics did not affect systemic innate immune responses in a human endotoxemia model, perhaps due to redundancies in the human immune response (22).
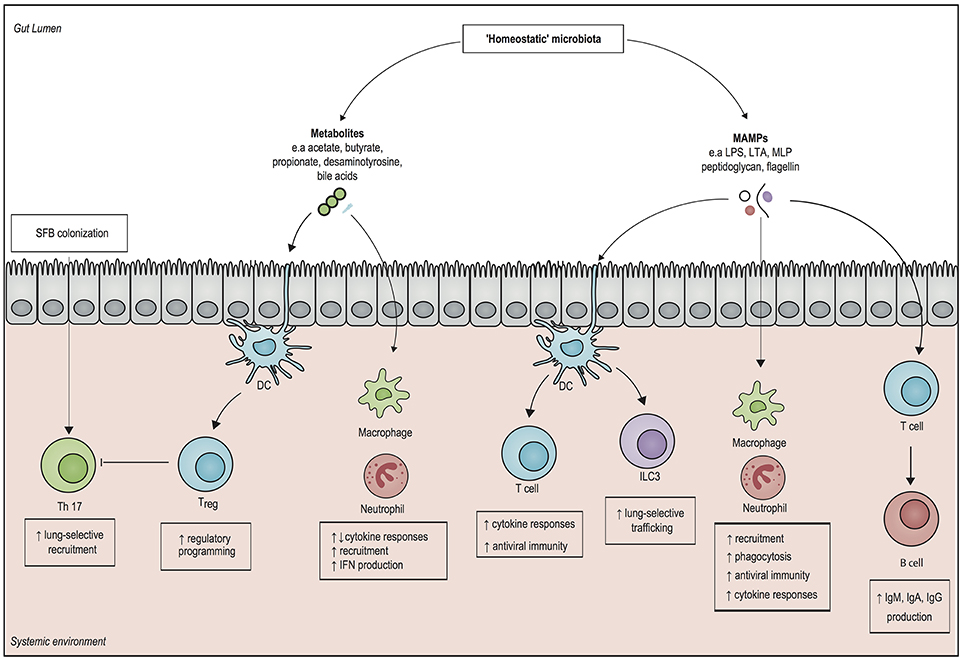
Figure 1. Overview of systemic immunomodulatory mechanisms associated with the microbiota. Structural components of gut microbiota, otherwise known as microbe-associated molecular patterns (MAMPs), can elicit a systemic pro-inflammatory response by activating pattern recognition receptors of both the innate and the adaptive immune system. Microbial metabolites, such as short chain fatty acids (SCFAs) modulate epigenetic changes in host leukocytes, which can induce both pro- and anti-inflammatory responses. The presence of the SCFAs butyrate and propionate drives the generation of regulatory T cells (Treg), which dampen inflammation. In addition, the gut metabolite desaminotyrosine enhances clearance of respiratory viruses by inducing type 1 interferon (IFN) responses. Direct interactions with epithelial cells by segmented filamentous bacteria (SFB) can enhance mucosal immunity by upregulating T helper 17 (Th 17) cells in both in the gut and in the lung. It is important to realize that our knowledge on microbiota-derived host-resistance is fragmented and it remains to be determined how these individual mechanisms fit in an overarching framework of systemic immunity. DC, dendritic cell; ILC3, type 3 innate lymphoid cell; Treg cell, regulatory T cell; IgA, Immunoglobulin A; IgG, Immunoglobulin G; IgM, immunoglobulin M; LPS, lipopolysaccharide; LTA, lipoteichoic acid; MLP, murein lipoprotein.
Numerous murine models have shown the existence of so called “gut-organ axes” such as the “gut-lung axis”, and the “gut-brain axis.” Besides cytokines, communication in these axes is probably mediated by microbe-associated molecular patterns (MAMPs), such as LPS, peptidoglycan and flagellin, as well as by microbiota-derived metabolites that are able to translocate from the gut into the systemic circulation, where they have the potential to modulate immune cells to enhance regulatory or pro-inflammatory responses (23, 24). In this way, intestinal bacteria can even direct the influx of immune effector cells into distant organs. For example, in newborn mice, exposure to gut bacteria increased homing of group 3 innate lymphoid cells (ILC3) from the gut mucosa toward the lung, which increased resistance against pneumonia (25). Other studies have shown that systemic exposure of microbiota-derived ligands increases the activity of alveolar macrophages and bone marrow derived neutrophils, which enhances the killing of Gram-positive and Gram-negative pathogens in the lung (26–28).
Similar priming mechanisms by toll-like receptor (TLR) ligands have shown to induce protection against respiratory viruses and fungi (29, 30). For example, neomycin-sensitive commensal bacteria induce the expression of messenger RNA (mRNA) for pro–IL-1β and pro–IL-18, which enhances the clearance of influenza virus in a T helper cell type 1, Cytotoxic T cell, and Immunoglobulin A (IgA) dependent manner (31). These findings were recently confirmed by Rosshart and colleagues, who developed a model of microbiota reconstitution from mice that were caught in the wild into genetically identical laboratory mice (32). Recolonization with a “natural” microbiota increased survival following influenza virus infection, which was attributed to a decreased pro-inflammatory response and a pronounced IL-10- and IL-13-mediated anti-inflammatory phenotype (32).
It has been shown that short-chain fatty acids (SCFAs), products of fiber fermentation by anaerobic bacteria, possess strong immunomodulatory properties through activation of certain G-protein-coupled receptors (GPRs), such as GPR41 and GPR43 (14, 33). In addition, the SCFAs butyrate and propionate facilitate the generation of extrathymic regulatory T cells, which have a key role in limiting inflammatory processes (34). Administration of butyrate also has the potential of restoring interleukin (IL)-10 levels in the lung by inhibiting histone deacetylase in myeloid-derived suppressor cells (MDSCs), which in turn reduces persistent lung inflammation during murine K. pneumoniae infection (35). Similar findings were observed in a cohort of allogeneic stem cell recipients, as a higher representation of butyrate-producing bacteria in the gut was associated with ameliorated respiratory viral infections (36). Besides short-chain fatty acids, it has also been found that desamonityrosine (DAT), a degradation product of flavonoids that is produced by Flavonifractor plautii, increases survival in mice infected with influenza through augmentation of type I interferon (IFN) signaling (37).
The immunomodulatory role of the microbiota extends beyond the lung, as presence of microbiota-derived LPS leads to increased recruitment of bone marrow-derived neutrophils, which enhances clearance of blood-borne pathogens, such as Escherichia coli and K. pneumoniae (38). Additionally, outer membrane components of Gram-negative bacteria in the gut, such as murein lipoprotein (MLP) and LPS, protect against experimental sepsis by modulating serum levels of Immunoglobulin G (IgG) and IgM, in a T cells and Toll-like receptor 4 on B cells-dependent manner (39, 40). These findings have recently been supplemented by Wilmore and colleagues (41), who have shown the that enriching the microbiota with members of the Proteobacteria phylum led to T cell-dependent increases in serum IgA levels by IgA-secreting plasma cells in the bone marrow. The resulting serum IgA bound specifically targeted a restricted number of pathogens that translocate from the gut into the systemic circulation, which increased resistance to sepsis (41). Despite the abovementioned indications that the microbiota is involved in the systemic host-defense against a wide variety of pathogens, further mechanistic studies are needed to provide overarching pathways that can be fitted into the framework of host-defense against sepsis.
Causes and Consequences of Dysbiosis in Sepsis
It has been shown that patients with sepsis have a profoundly distorted composition of the intestinal microbiota (9–12). In general, the diversity of the microbiota in patients with sepsis decreases rapidly upon hospital admission, a finding that becomes even more pronounced in later stages of their hospitalization (11). An extensive overview of the potential effects of sepsis on the intestinal microbiota has recently been published elsewhere (42). In short, these shifts in microbiota composition can be partially explained by clinical interventions, such as (par)enteral feeding, mechanical ventilation, as well as the omnipresent administration of proton pump inhibitors, opioids, vasopressors and—above all—antibiotics (42–44). Moreover, patients with sepsis have impaired gastrointestinal motility and diminished intestinal epithelial integrity, leading to a loss of “beneficial” anaerobic bacterial families, such as Lachnospiraceae and Ruminococcaceae, which further impairs intestinal epithelium function and allows for the expansion and potential translocation of aerobic opportunistic pathogens (45–47). The effects of sepsis on the microbiota extend beyond the gastrointestinal tract and can also influence the composition of for instance the lung and skin microbiota (42).
Several researchers have hypothesized that these shifts in microbiota composition potentially predispose patients to a state of immunosuppression (21, 48). There has also been renewed attention to the theory that systemic translocation of opportunistic gut bacteria increases the risk of organ failure (49). For example, the severity of acute kidney injury during sepsis can be ameliorated by administration of the three SCFAs acetate, propionate, and butyrate, which epigenetically attenuate the inflammatory process and reduce subsequent damage (50, 51). In addition, reconstitution of mice with the commensal E. coli O21:H+ induces signaling of the IGF1/PI3K/Akt pathway, which in turn prevents muscle wasting triggered by sepsis (52). Studies from the previous century showed that translocation of intestinal bacteria seems to be also associated with the development of acute respiratory distress syndrome (ARDS) (53, 54). Dickson and colleagues have recently found support for these findings by revealing that during murine sepsis and in human patients with ARDS, lung communities are enriched by bacteria that have translocated from the intestine. The presence of these communities, such as Bacteroides spp, is correlated with the intensity of systemic inflammation (55). Finally, preliminary research in mice and patients who died of sepsis suggests that bacterial translocation could also be associated with acute neuro-inflammation in sepsis (56), which aligns with the notion that gut microbes play a role in regulating the central nervous system (57). These studies provide clues that the extreme shifts observed in sepsis patients are not merely markers of illness severity, but also potentially contribute to worse outcome. However, human translational studies are lacking, so our current understanding of the short- and long-term consequences of ICU-related dysbiosis in clinical practice is limited (42). Further studies are needed to confirm that disturbances of the microbiota truly contribute to organ failure, and that dysbiosis is not merely a reflection of the profound dysregulation that is present during sepsis.
Potential for Modulation of the Microbiota in the Management of Sepsis
Harnessing the immunomodulatory properties of the microbiota could provide an attractive preventive and therapeutic opportunity for sepsis. Modulation of the microbiota has been studied extensively outside of the sepsis arena, and predominantly consists of either resupplying the pool of beneficial microbes by administration of probiotics, improving the intestinal microenvironment to enhance the growth of beneficial species by dietary interventions and prebiotics, or by totally recolonizing the gut with a fecal microbiota transplantation (FMT). We propose that, within the course of sepsis, three therapeutic approaches exist that, when exploited, could potentially improve prevention and management of the disorder.
Avenue 1: Targeted Microbiota Modulation for At-Risk Patients, Prior to Sepsis Development
The abovementioned pre-clinical observations demonstrating a link between dysbiosis and increased risk of sepsis have now gained some preliminary footing in human studies. For example, patients undergoing allo-HCT who develop antibiotic-induced dysbiosis have a 5- to 9-fold increased risk of bloodstream infection and sepsis (58). In line with these findings, a retrospective cohort of over 10.000 elderly patients in the United States showed that hypothesized instances of dysbiosis were associated with a more than 3-fold increased incidence of a subsequent hospitalization for sepsis (7). And, expanding on these findings, Baggs et al. recently showed that exposure to longer durations of antibiotics, additional classes of antibiotics, and broader-spectrum antibiotics during hospitalization are each associated with dose-dependent increases in the risk of subsequent sepsis (59). This association was not found for other causes of hospital readmissions, suggesting that the association between antibiotic exposure and subsequent sepsis is related to microbiome depletion, not merely illness severity (59).
Probiotics have been evaluated for preventing nosocomial infection in many smaller studies, and meta-analyses suggest that probiotics are safe and effective at preventing infection in both post-operative and mechanically ventilated patients (60, 61). However, the small size of the individual studies, as well as the variable type and dose of probiotic therapy limit strong conclusions. A large-scale study of probiotics is underway in Canada to test the benefit of probiotics for preventing ventilator-associated pneumonia (clinicaltrials.gov: NCT02462590). Excitingly, in a recent randomized, double blind, placebo-controlled trial of 4,556 healthy, term infants in rural India, administration of an oral synbiotic preparation (Lactobacillus plantarum plus a fructooligosaccharide) resulted in a 40% relative risk reduction for lower respiratory tract infections, sepsis, and death (62). The study was stopped early due to overwhelming benefit, and the authors have asserted that with a single investment of only 27 dollars, one case of neonatal sepsis could be prevented, which would be an unparalleled breakthrough in decreasing the worldwide incidence of sepsis. However, other studies that assessed the role of probiotics in other populations, such as pre-term and underweight children, showed no differences in sepsis incidence and mortality, indicating that the potential effects of microbiota restoration are not uniformly conserved across populations and settings (63, 64). Therefore, large stratified cohorts that collect data before the potential onset of sepsis are warranted to help pinpoint in which context gut commensals are able drive protection against sepsis. Recognizing these drivers of protection could help develop means of targeted microbiota restoration in order to decrease the incidence of sepsis.
Avenue 2: Addressing Dysbiosis During the Course of Sepsis
Based on the abovementioned findings that probiotics and synbiotics decrease the incidence of infections complications at the ICU, a strong interest exists to investigate the use of these agents to improve outcome in patients with sepsis (60, 61). However, implementation of probiotic treatment in sepsis has been limited due to a lack of consensus with regard to the optimal choice of probiotic species and dosages, as well as lingering concerns for patient safety (65, 66). Recently, FMT has been used successfully in four cases of therapy-resistant sepsis and diarrhea (67–69). These initial studies describe that recolonization of the intestinal microbiota via FMT during sepsis could counterbalance dysbiosis, induce recovery of gut microbial barrier, which in turn could potentially improves the outcome of sepsis on the ICU. However, the first clinical studies investigating this modality on a larger scale have yet to commence. Other studies propose that treatment with microbiota-targeted metabolites, such as butyrate or other SCFAs, could be used as more stable immunomodulators than their bacterial counterparts (70). However, at the moment, surprisingly few studies have investigated the use of microbiota-targeted therapies during sepsis (45). These studies are essential to define the efficacy, safety and potential benefit of these therapies in the management of the disease.
Avenue 3: Rapid Microbiota Restoration in Sepsis Survivors
Pre-clinical models have shown that a state of immunosuppression can be maintained long after recovery from sepsis (71, 72), which is thought to predispose sepsis survivors to infections and increased mortality up to 2 years following sepsis discharge (73–75). Indeed, recent studies have shown that 20% of the patients who survive sepsis has a late death—predominantly caused by infections, respiratory failure, and aspiration pneumonitis—that cannot be attributed to prior health status and comorbidities (76). These findings have two implications. First, the general dysregulation that occurs during sepsis is profound and its consequences are long lasting. Second, as late death after sepsis cannot be attributed to previous health status, it suggests that targeted interventions can be designed to reverse this hypothesized state of immunosuppression and decrease mortality after sepsis (76). Given the potential role of dysbiosis in maintaining immunosuppression, restoration of gut commensals after sepsis discharge by probiotic administration in order to reduce late infections and subsequent mortality warrants further research. Another concept within the scope of microbiota restoration after sepsis discharge consists of microbiota auto banking, which encompasses the storage of feces of patients in an ambulatory setting (77). These samples could subsequently be used in an autologous FMT in order to restore the microbiota after periods of dysbiosis, in this case after sepsis discharge. Of interest, the efficacy of auto-FMT for the prevention of infections after a period of dysbiosis is currently studied within a cohort of patients receiving allo-HCT (clinicaltrials.gov: NCT02269150). A schematic overview of the proposed therapeutic avenues in the prevention and management of sepsis is depicted in Figure 2.
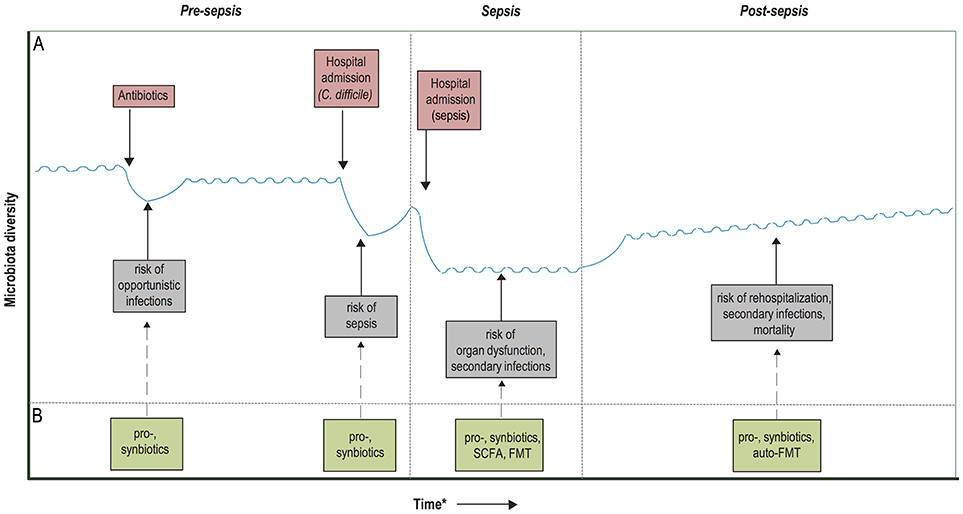
Figure 2. Timeline of potential microbiota-associated interventions prior to, during and post-sepsis. (A) Hypothetical diagram of the associations between the risk of dysbiosis and sepsis development and outcome. Dysbiosis occurs due to the administration of antibiotics and/or hospitalizations, recover quickly upon hospital discharge or cessation of antibiotic treatment, but it often takes long for the microbiota to recover entirely. These periods of dysbiosis predispose to the development of opportunistic infections, which in turn further worsens the state of dysbiosis and predispose to sepsis development. During sepsis, the microbiota composition is extremely hampered, which has been associated with an increased risk of secondary infections, immunosuppression and potentially even organ dysfunction. Recolonization of a homeostatic microbiota is slow upon hospital discharge and sepsis recovery, which might contribute to prolonged immunosuppression, rehospitalization due to infections and increased mortality. (B) Probiotics and synbiotics have the potential to enhance microbiota colonization in neonates, as well as accelerate the recovery of the microbiota after periods of dysbiosis, which in turn could provide protection against the development of sepsis. During the course of sepsis, microbiota disruption could be ameliorated by the administration of pro- and synbiotics, which potentially reduces the occurrence of secondary infections. In addition, future treatment with fecal microbiota transplantation (FMT) or targeted restoration with microbiota-associated metabolites, such as short-chain fatty acids (SCFAs), could reduce the risk of prolonged immunosuppression and organ dysfunction. Finally, microbiota restoration after sepsis recovery could be accelerated with pro- and synbiotics, or by an autologous FMT. *Time depiction is schematic.
The Road Ahead
Our understanding on how disturbances of the microbiota can predispose to, or even perpetuate the dysregulated immune response associated with sepsis is fragmented. The more we know about these collections of micro-organisms that inhabit our body compartments, the more we realize how delicate the mechanisms are through which these microbes contribute to homeostasis. Moreover, micro-organisms that reside in other body compartments than the gut, such as the skin and the lung, are likely to play an equally important but underexplored role in baseline immune function (42, 78, 79). To add an additional layer of complexity; emerging data indicate that eukaryotic viruses regulate, and are in turn regulated by the host and other microbial constituents that inhabit the intestine (80, 81). These co-inhabitants consist not only of bacteria, but also bacteriophages, helminths, and fungi, which influence each other through a series of processes termed “transkingdom interactions.” This holistic view of the human microbiota represents an uncharted paradigm that will need to be further evaluated in the context of sepsis.
In general, large human cohort studies that document microbiota composition, prior to, during and after an episode of sepsis are needed to identify those commensals that protect against sepsis vs. those that are potentially associated with increased susceptibility and worse outcome. These insights could allow us to identify bacterial groups that are associated with immunological resilience, which could be harnessed as potential biomarkers of susceptibility to sepsis or adverse outcome. In parallel, mechanistic animal studies—that should include the use of older and “dirtier” mice (32, 82, 83) and better mimic the clinical scenario by using antibiotics and other treatments often given in sepsis (84)—are needed to disentangle potential mechanisms that drive the phenotypes seen in the human situation. These combined efforts have recently lead to the successful identification and development of new next-generation probiotics that are able to selectively treat specific pathogens, such as C. difficile and vancomycin resistant Enterococcus (VRE) (85, 86). Despite the significant challenges ahead, we foresee that further implementation of microbiota-targeted therapies could improve sepsis management and prevention.
Disclosures
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Author Contributions
BH, WW, and HP equally contributed to the write-up of the manuscript. BH drafted the figures and performed the literature search.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
WW is financially supported by the Netherlands Organization for Scientific Research (NWO; VIDI grant) and the European Union (H2020 MC-ITN; the European Sepsis Academy). HP is supported by grant K08 GM115859.
References
1. Singer M, Deutschman CS, Seymour C, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. (2017) 17:407–20. doi: 10.1038/nri.2017.36
3. Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. (2013) 13:862–74. doi: 10.1038/nri3552
4. Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ (2016) 353:i1585. doi: 10.1136/bmj.i1585
5. Arefian H, Heublein S, Scherag A, Brunkhorst FM, Younis MZ, Moerer O, et al. Hospital-related cost of sepsis: a systematic review. J Infect. (2017) 74:107–17. doi: 10.1016/j.jinf.2016.11.006
6. Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. (2016) 193:259–72. doi: 10.1164/rccm.201504-0781OC
7. Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ. Hospitalization type and subsequent severe sepsis. Am J Respir Crit Care Med. (2015) 192:581–88. doi: 10.1164/rccm.201503-0483OC
8. Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol. (2017) 2:135–43. doi: 10.1016/S2468-1253(16)30119-4
9. Ojima M, Motooka D, Shimizu K, Gotoh K, Shintani A, Yoshiya K, et al. Metagenomic analysis reveals dynamic changes of whole gut microbiota in the acute phase of intensive care unit patients. Dig Dis Sci. (2016) 61:1628–34. doi: 10.1007/s10620-015-4011-3
10. Zaborin A, Smith D, Garfield K, Quensen J, Shakhsheer B, Kade M, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. MBio (2014) 5:e01361-14. doi: 10.1128/mBio.01361-14
11. McDonald D, Ackerman G, Khailova L, Baird C, Heyland D, Kozar R, et al. Extreme dysbiosis of the microbiome in critical illness. mSphere (2016) 1:e00199-16. doi: 10.1128/mSphere.00199-16
12. Lankelma JM, van Vught LA, Belzer C, Schultz MJ, van der Poll T, de Vos WM, et al. Critically ill patients demonstrate large interpersonal variation in intestinal microbiota dysregulation: a pilot study. Intensive Care Med. (2017) 43:59–68. doi: 10.1007/s00134-016-4613-z
13. Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell (2016) 164:337–40. doi: 10.1016/j.cell.2016.01.013
14. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. (2017) 8:172–84. doi: 10.1080/19490976.2017.1290756
15. Li X, Watanabe K, Kimura I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front Immunol. (2017) 8:1882. doi: 10.3389/fimmu.2017.01882
16. Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell (2012)149:1578–93. doi: 10.1016/j.cell.2012.04.037
17. Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. (2008) 197:435–8. doi: 10.1086/525047
18. Harris VC, Haak BW, Boele van Hensbroek M, Wiersinga WJ. The intestinal microbiome in infectious diseases: the clinical relevance of a rapidly emerging field. Open Forum Infect Dis. (2017) 4:ofx144. doi: 10.1093/ofid/ofx144
19. Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. (2017) 279:90–105. doi: 10.1111/imr.12563
20. Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell (2016) 167:1125–36. doi: 10.1016/j.cell.2016.10.020
21. Lankelma JM, Belzer C, Hoogendijk AJ, de Vos AF, de Vos WM, van der Poll T, et al. Antibiotic-induced gut microbiota disruption decreases TNF-α release by mononuclear cells in healthy adults. Clin Transl Gastroenterol. (2016) 7:e186. doi: 10.1038/ctg.2016.43
22. Lankelma JM, Cranendonk DR, Belzer C, de Vos AF, de Vos WM, van der Poll T, et al. Antibiotic-induced gut microbiota disruption during human endotoxemia: a randomised controlled study. Antibiotic-induced gut microbiota disruption during human endotoxemia: a randomised controlled study. Gut (2017) 66:1623–30. doi: 10.1136/gutjnl-2016-312132
23. Schuijt TJ, van der Poll T, de Vos WM, Wiersinga WJ. The intestinal microbiota and host immune interactions in the critically ill. Trends Microbiol. (2013) 21:221–29. doi: 10.1016/j.tim.2013.02.001
24. Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. (2017) 15:55–63. doi: 10.1038/nrmicro.2016.142
25. Gray J, Oehrle K, Worthen G, Alenghat T, Whitsett J, Deshmukh H. Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection. Sci Transl Med. (2017) 9:aaf9412. doi: 10.1126/scitranslmed.aaf9412
26. Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nature Med. (2010) 16:228–31. doi: 10.1038/nm.2087
27. Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut (2016) 65:575–83. doi: 10.1136/gutjnl-2015-309728
28. Lankelma JM, Birnie E, Weehuizen TAF, Scicluna BP, Belzer C, Houtkooper RH, et al. The gut microbiota as a modulator of innate immunity during melioidosis. PLoS Negl Trop Dis. (2017) 11:e0005548. doi: 10.1371/journal.pntd.0005548
29. Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity (2012) 37:158–70. doi: 10.1016/j.immuni.2012.04.011
30. McAleer JP, Nguyen NLH, Chen K, Kumar P, Ricks DM, Binnie M, et al. Pulmonary Th17 anti-fungal immunity is regulated by the gut microbiome. J Immunol. (2016) 197:97–107. doi: 10.4049/jimmunol.1502566
31. Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. (2011) 108:5354–9. doi: 10.1073/pnas.1019378108
32. Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K, et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell (2017) 171:1015–28.e13. doi: 10.1016/j.cell.2017.09.016
33. Galvão I, Tavares LP, Corrêa RO, Fachi JL, Rocha VM, Rungue M, et al. The metabolic sensor GPR43 receptor plays a role in the control of Klebsiella pneumoniae infection in the lung. Front Immunol. (2018) 9:142. doi: 10.3389/fimmu.2018.00142
34. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature (2013) 504:451–5. doi: 10.1038/nature12726
35. Chakraborty K, Raundhal M, Chen BB, Morse C1, Tyurina YY, Khare A, et al. The mito-DAMP cardiolipin blocks IL-10 production causing persistent inflammation during bacterial pneumonia. Nat Commun. (2017) 8:13944. doi: 10.1038/ncomms13944
36. Haak BW, Littmann ER, Chaubard J-L, Pickard AJ, Fontana E, Adhi F, et al. Impact of gut colonization with butyrate producing microbiota on respiratory viral infection following Allo-HCT. Blood (2018) 131:2978–86. doi: 10.1182/blood-2018-01-828996
37. Steed AL, Christophi GP, Kaiko GE, Sun L, Goodwin VM, Jain U, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science (2017) 357:498–502. doi: 10.1126/science.aam5336
38. Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O'Leary CE, et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. (2014) 20:524–30. doi: 10.1038/nm.3542
39. Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, et al. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity (2016) 44:647–58. doi: 10.1016/j.immuni.2016.02.006
40. Proietti M, Cornacchione V, Rezzonico Jost T, Romagnani A, Faliti CE, Perruzza L, et al. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer's patches to promote host-microbiota mutualism. Immunity (2014) 41:789–801. doi: 10.1016/j.immuni.2014.10.010
41. Wilmore JR, Gaudette BT, Gomez Atria D, Hashemi T1, Jones DD1, Gardner CA, et al. Commensal microbes induce serum IgA responses that protect against polymicrobial sepsis. Cell Host Microbe. (2018) 23:302–11. doi: 10.1016/j.chom.2018.01.005
42. Dickson RP. The microbiome and critical illness. Lancet Respir Med. (2016) 4:59–72. doi: 10.1016/S2213-2600(15)00427-0
43. Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA (2009) 302:2323–9. doi: 10.1001/jama.2009.1754
44. Benus RF, Harmsen HJ, Welling GW, Spanjersberg R, Zijlstra JG, Degener JE, et al. Impact of digestive and oropharyngeal decontamination on the intestinal microbiota in ICU patients. Intensive Care Med. (2010) 36:1394–402. doi: 10.1007/s00134-010-1826-4
45. Haak BW, Levi M, Wiersinga WJ. Microbiota-targeted therapies on the intensive care unit. Curr Opin Crit Care. (2017) 23:167–74. doi: 10.1097/MCC.0000000000000389
46. Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis. (2004) 39:219–26. doi: 10.1086/422002
47. Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nat Immunol. (2018) 18:327–41. doi: 10.1038/s41590-018-0064-8
48. Krezalek MA, DeFazio J, Zaborina O, Zaborin A, Alverdy JC. The shift of an intestinal “Microbiome” to a “Pathobiome” governs the course and outcome of sepsis following surgical injury. Shock (2016) 45:475–82. doi: 10.1097/SHK.0000000000000534
49. Klingensmith NJ, Coopersmith CM. The gut as the motor of multiple organ dysfunction in critical illness. Crit Care Clin. (2016) 32:203–12. doi: 10.1016/j.ccc.2015.11.004
50. Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med. (2009) 87:859–64. doi: 10.1007/s00109-009-0491-y
51. Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol. (2015) 26:1877–88. doi: 10.1681/ASN.2014030288
52. Schieber AM, Lee YM, Chang MW, Leblanc M, Collins B, Downes M, et al. Disease tolerance mediated by microbiome E. coli involves inflammasome and IGF-1 signaling. Science (2015) 350:558–63. doi: 10.1126/science.aac6468
53. Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon (2012) 10:350–6. doi: 10.1016/j.surge.2012.03.003
54. Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg. (1986) 121:196–208. doi: 10.1001/archsurg.1986.01400020082010
55. Dickson RP, Singer BH, Newstead MW, Falkowski NR, Erb-Downward JR, Standiford TJ, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. (2016) 1:16113. doi: 10.1038/nmicrobiol.2016.113
56. Singer BH, Dickson RP, Denstaedt SJ, Newstead MW, Kim K, Falkowski NR, et al. Bacterial dissemination to the brain in sepsis. Am J Respir Crit Care Med. (2018) 197:747–56. doi: 10.1164/rccm.201708-1559OC
57. Sundman MH, Chen NK, Subbian V, Chou YH. The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun. (2017) 66:31–44. doi: 10.1016/j.bbi.2017.05.009
58. Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. (2012) 55:905–14. doi: 10.1093/cid/cis580
59. Baggs J, Jernigan JA, Halpin AL, Epstein L, Hatfield KM, McDonald LC. Risk of subsequent sepsis within 90 days after a hospital stay by type of antibiotic exposure. Clin Infect Dis. (2018) 66:1004–12. doi: 10.1093/cid/cix947
60. Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Critical Care (2016) 20:262. doi: 10.1186/s13054-016-1434-y
61. Kasatpibal N, Whitney JD, Saokaew S, Kengkla K, Heitkemper MM, Apisarnthanarak A. Effectiveness of probiotic, prebiotic, and synbiotic therapies in reducing postoperative complications: a systematic review and network meta-analysis. Clin Infect Dis. (2017) 64:S153–60. doi: 10.1093/cid/cix114
62. Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature (2017) 548:407–12. doi: 10.1038/nature23480
63. Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, Pirotta M, et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics (2013) 132:1055–62. doi: 10.1542/peds.2013-1339
64. Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet (2016) 387:649–60. doi: 10.1016/S0140-6736(15)01027-2
65. Morrow LE, Wischmeyer P. Blurred lines: dysbiosis and probiotics in the intensive care unit. Chest (2016) 151:492–9. doi: 10.1016/j.chest.2016.10.006.35
66. Bongaerts GP, Severijnen RS. A reassessment of the PROPATRIA study and its implications for probiotic therapy. Nat Biotechnol. (2016) 34:55–63. doi: 10.1038/nbt.3436
67. Li Q, Wang C, Tang C, He Q, Zhao X, Li N, et al. Successful treatment of severe sepsis and diarrhea after vagotomy utilizing fecal microbiota transplantation: a case report. Crit Care (2015) 19:37. doi: 10.1186/s13054-015-0738-7
68. Li Q, Wang C, Tang C, He Q, Zhao X, Li N, et al. Therapeutic modulation and reestablishment of the intestinal microbiota with fecal microbiota transplantation resolves sepsis and diarrhea in a patient. Am J Gastroenterol. (2014) 109:1832–4. doi: 10.1038/ajg.2014.299
69. Wei Y, Yang J, Wang J, Yang Y, Huang J, Gong H, et al. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit Care (2016) 20:332. doi: 10.1186/s13054-016-1491-2
70. Dessein R, Gironella M, Vignal C, Peyrin-Biroulet L, Sokol H, Secher T, et al. Toll-like receptor 2 is critical for induction of Reg3 beta expression and intestinal clearance of Yersinia pseudotuberculosis. Gut (2009) 58:771–6. doi: 10.1136/gut.2008.168443
71. Nascimento DC, Melo PH, Piñeros AR, Ferreira RG, Colón DF, Donate PB, et al. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat Commun. (2017) 8:14919. doi: 10.1038/ncomms14919
72. Nascimento DC, Alves-Filho JC, Sônego F, Fukada SY, Pereira MS, Benjamim C, et al. Role of regulatory T cells in long-term immune dysfunction associated with severe sepsis. Crit Care Med. (2010) 38:1718–25. doi: 10.1097/CCM.0b013e3181e78ad0
73. Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. (2012) 60:1070–7. doi: 10.1111/j.1532-5415.2012.03989.x
74. Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA (2015) 313:1055–7. doi: 10.1001/jama.2015.1410
75. Shen HN, Lu CL, Yang HH. Risk of recurrence after surviving severe sepsis: a matched cohort study. Crit Care Med. (2016) 44:1833–41. doi: 10.1097/CCM.0000000000001824
76. Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ (2016) 353:i2735. doi: 10.1136/bmj.i2375
77. Tosh PK, McDonald LC. Infection control in the multidrug-resistant era: tending the human microbiome. Clin Infect Dis. (2012) 54:707–13. doi: 10.1093/cid/cir899
78. Dickson RP, Erb-Downward JR, Falkowski NR, Hunter EM, Ashley SL, Huffnagle GB. The lung microbiota of healthy mice are highly variable, cluster by environment, and reflect variation in baseline lung innate immunity. Am J Respir Crit Care Med. (2018) 198:497–508. doi: 10.1164/rccm.201711-2180OC
79. Chen YE, Fischbach MA, Belkaid Y. Skin microbiota-host interactions. Nature (2018) 553:427–36. doi: 10.1038/nature25177
80. Pfeiffer JK, Virgin HW. Viral immunity. Transkingdom control of viral infection and immunity in the mammalian intestine. Science (2016) 351:aad5872. doi: 10.1126/science.aad5872
81. Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol. (2013) 21:334–41. doi: 10.1016/j.tim.2013.04.002
82. Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature (2016) 532:512–6. doi: 10.1038/nature17655
83. Abolins S, King EC, Lazarou L, Weldon L, Hughes L, Drescher P, et al. The comparative immunology of wild and laboratory mice, Mus musculus domesticus. Nat Commun. (2017) 8:14811. doi: 10.1038/ncomms14811
84. Rivard AL, Simura KJ, Mohammed S, Magembe AJ, Pearson HM, Hallman MR, et al. Rat intubation and ventilation for surgical research. J Invest Surg. (2006) 19:267–74. doi: 10.1080/08941930600778297
85. Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature (2015) 517:205–8. doi: 10.1038/nature13828
Keywords: microbiota, sepsis, pathogenesis, therapeutics, probiotics and synbiotics, fecal microbiota transplantation
Citation: Haak BW, Prescott HC and Wiersinga WJ (2018) Therapeutic Potential of the Gut Microbiota in the Prevention and Treatment of Sepsis. Front. Immunol. 9:2042. doi: 10.3389/fimmu.2018.02042
Received: 25 April 2018; Accepted: 20 August 2018;
Published: 10 September 2018.
Edited by:
Luregn J. Schlapbach, The University of Queensland, AustraliaReviewed by:
Maryam Dadar, Razi Vaccine and Serum Research Institute, IranSunil Joshi, University of Miami, United States
Copyright © 2018 Haak, Prescott and Wiersinga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bastiaan W. Haak, Yi53LmhhYWtAYW1jLm5s
 Bastiaan W. Haak
Bastiaan W. Haak Hallie C. Prescott2,3
Hallie C. Prescott2,3