
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 13 August 2018
Sec. Molecular Innate Immunity
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.01873
This article is part of the Research Topic Mast Cell and Basophil-Driven Diseases View all 8 articles
 Elden Berla Thangam1
Elden Berla Thangam1 Ebenezer Angel Jemima1
Ebenezer Angel Jemima1 Himadri Singh2
Himadri Singh2 Mirza Saqib Baig3
Mirza Saqib Baig3 Mahejibin Khan4
Mahejibin Khan4 Clinton B. Mathias5
Clinton B. Mathias5 Martin K. Church6
Martin K. Church6 Rohit Saluja2,7*
Rohit Saluja2,7*
Histamine and its receptors (H1R–H4R) play a crucial and significant role in the development of various allergic diseases. Mast cells are multifunctional bone marrow-derived tissue-dwelling cells that are the major producer of histamine in the body. H1R are expressed in many cells, including mast cells, and are involved in Type 1 hypersensitivity reactions. H2R are involved in Th1 lymphocyte cytokine production. H3R are mainly involved in blood–brain barrier function. H4R are highly expressed on mast cells where their stimulation exacerbates histamine and cytokine generation. Both H1R and H4R have important roles in the progression and modulation of histamine-mediated allergic diseases. Antihistamines that target H1R alone are not entirely effective in the treatment of acute pruritus, atopic dermatitis, allergic asthma, and other allergic diseases. However, antagonists that target H4R have shown promising effects in preclinical and clinical studies in the treatment of several allergic diseases. In the present review, we examine the accumulating evidence suggesting novel therapeutic approaches that explore both H1R and H4R as therapeutic targets for histamine-mediated allergic diseases.
Allergic diseases, for example, allergic asthma, pruritus, atopic dermatitis, and allergic rhinitis are due to a complex interaction between several inflammatory cells, including basophils, mast cells, lymphocytes, dendritic cells, neutrophils, and eosinophils in response to various environmental/allergic stimuli (1, 2). These cells produce a plethora of inflammatory mediators, such as histamine, eicosanoids, chemokines, cytokines, and reactive oxygen species (3, 4). Among these, mast cell histamine is an axial player in stimulating the development of allergic-related inflammatory diseases by regulating the maturation and activation of leukocytes and directing their migration to target sites where they cause chronic inflammation (5–8). Histamine also exerts a various other immune regulatory functions by modulating the functions of monocytes (9), T cells (10, 11), macrophages (12), neutrophils (13), eosinophils (14), B cells, and dendritic cells (15). The biological impact of histamine follow their interaction with four types histamine receptors, H1R, H2R, H3R, and H4R, all of which belong to the G protein coupled receptor family (8, 16–20).
In this review, we focus on the importance and present knowledge about the histamine and histamine receptor-mediated activation in mast cell-mediated allergic disorders.
Mast cells are the major producer of histamine and express a vast array of receptors on their surface such as FcεR1, FcγRI, and receptors for complement components (C3aR and C5aR), nerve growth factor (NGF) (Trk A), substance P, vasoactive intestinal peptide (MrgX2), adenosine phosphate, etc. (21–24). Activation through these receptors by their respective stimulants, such as allergens, complement peptides C3a, C5a (25, 26), NGF (27), neuropeptides, adenosine mono-phosphate activate human cord blood-derived mast cells to release various inflammatory mediators including histamine. Histamine can also be produced by basophils and other immune cells (28) but much higher concentrations of histamine may be found in intestinal mucosa, skin, and bronchial tissues. Histamine regulates a plethora of pathophysiological and physiological processes, such as secretion of gastric acid, inflammation, and the regulation of vasodilatation and bronchoconstriction (29, 30). In addition, it can also serve as a neurotransmitter (31).
Histamine plays a central role in the pathogenesis of several allergic diseases, such as atopic dermatitis, allergic rhinitis, and allergic asthma through differential regulation of T helper lymphocytes. Enhancement of Th2 cytokine secretion [such as interleukin (IL)-5, IL-4, IL-10, and IL-13] and inhibition of Th1 cytokine production [interferon-γ (IFN-γ), monokine IL-12, and IL-2] are mediated by histamine. Thereby, histamine regulates the effective balance between Th1 and Th2 cells by assisting a shift toward Th2 (32). Histamine-mediated mast cell activation plays a critical role in various allergic diseases. Histamine may induce the release of leukotrienes, cytokines, and chemokines via H4R in CD34+ cord blood-derived human mast cells (33). In mouse mast cells, both histamine and 4-methylhistamine can induce IL-6 production individually, an effect that is potentiated by LPS stimulation. This effect can be blocked by H4R antagonists and does not occur in H4R-deficient allergic mice (34). Recent findings have shown that activation of H4 receptors by histamine stimulates the synthesis of IL-4 and IL-5 in human cord blood mast cells and tumor necrosis factor (TNF)-α in bone marrow-derived murine mast cells (BMMCs), both of which have a potential role in inducing allergic inflammation (33, 35).
Histamine receptors (H1R–H4R) are characterized by their function, structure, distribution, and their affinity to histamine (36, 37). Histamine has diverse effects, both pro-inflammatory and anti-inflammatory, which are determined by both the histamine receptor subtype and the cells stimulated types (38). The H1-receptor drives cellular migration, nociception, vasodilatation, and bronchoconstriction (39), whereas the H2-receptor modifies gastric acid secretion, airway mucus production, and vascular permeability (40). The H3-receptor plays an important role in neuro-inflammatory diseases (37). The H4-receptor has also been shown to be involved in allergy and inflammation (38, 41). H4R-mediated mast cell activation can regulate a powerful inflammatory cascade by releasing several inflammatory mediators; these mediators may stimulate the migration of different inflammatory cells into the inflammatory site (33). Likewise, the activation of H1R also regulates allergic responses by enhancing the migration of Th2 cells toward the allergen during lung inflammation (42). A more detailed summary of histamine receptor expression is shown in Table 1.
The H1R is ubiquitously expressed and is involved in allergy and inflammation. H1R is expressed in many tissues and cells, including nerves, respiratory epithelium, endothelial cells, hepatic cells, vascular smooth muscle cells, dendritic cells, and lymphocytes (8, 19). Histamine activates H1R through Gαq/11, which then activates phospholipase C and increases intracellular Ca++ levels. As a consequence, histamine elicits the contraction of smooth muscle of the respiratory tract, increases vascular permeability, and induces the production of prostacyclin and platelet activating factor by activating H1R (Figure 1) (58). Thus, almost all immediate hypersensitivity reactions, including symptoms observed in the skin, such as erythema, pruritus, and edema, may be elicited by the activation of H1R (59).
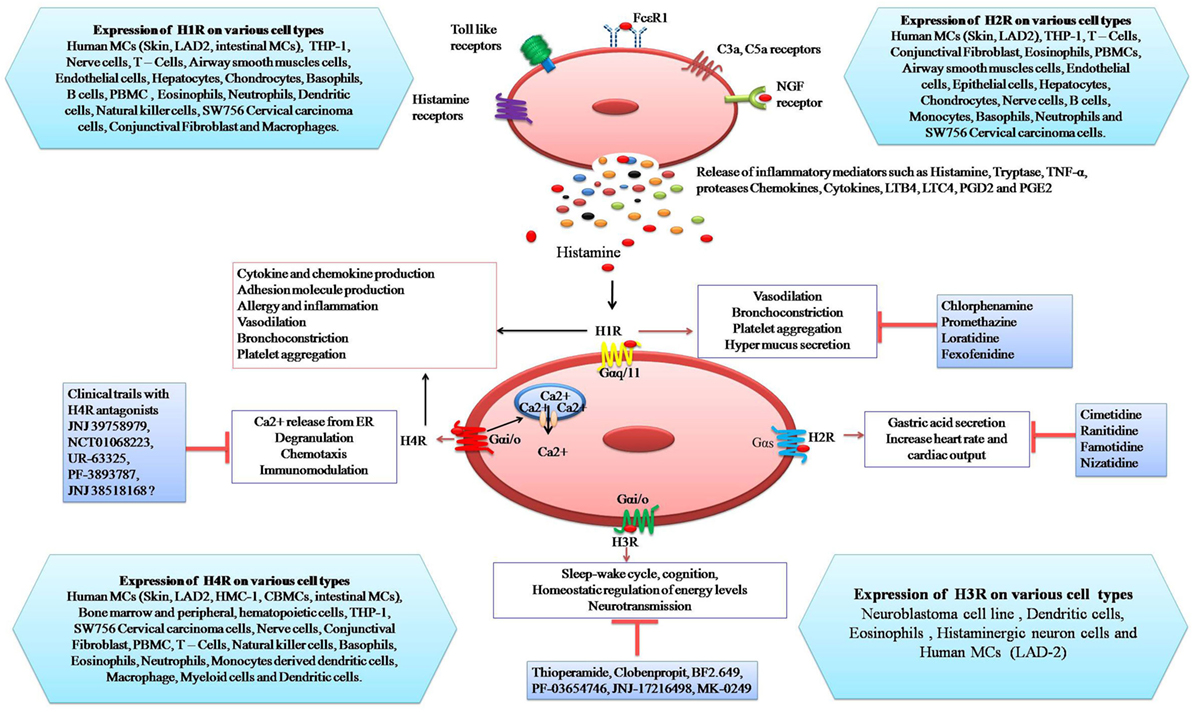
Figure 1. Schematic representation of the expression of histamine receptors on mast cells and their potential response to histamine: binding of histamine to H1R induces vasodilatation, bronchoconstriction, platelet aggregation, and mucus hyper-secretion. Stimulation of H2R by histamine causes gastric acid secretion, increase heart rate, and cardiac output. Activation of H3R is involved in sleep-wake cycle, cognition, homeostatic regulation of energy levels, and neurotransmission. H4R activation leads to Ca++ release from endoplasmic reticulum, degranulation, chemotaxis, and immuno-modulation whereas inhibitors of histamine receptors (H1R–H4R) inhibit specific responses.
Activation via H1R may also enhance both Th1- and Th2-type immune responses (11). In mice, deletion of H1R leads to the release of Th2 cytokines (IL-4 and IL-13) and inhibition of IFN-γ (60). Similarly, Bryce et al. (42) demonstrated that allergen-challenged H1R-deficient mice had attenuated lung allergic responses. They also demonstrated that histamine may act as a chemotactic factor for Th2 cells, stimulating their migration into lung tissues (42).
In addition, IL-3 activation can increase H1R expression on Th1 cells (61, 62), and histamine can enhance B cell proliferation, which is absent in H1R-deficient mice (63). The role of H1R activation in asthma may be further corroborated by observations showing that use of H1R-antagonists can significantly decrease asthma symptoms and improve pulmonary function in persistent asthma (58, 64, 65).
Histamine H1 receptor is also expressed in dermal dendritic cells and keratinocytes in the skin tissue, and histamine increases the NGF production via H1R in human keratinocytes (66). The secretion of NGF is caused by the phosphorylation of protein kinase C, extracellular signal-regulated kinases (ERK), and the activation of AP-1 resulting from H1R stimulation. Similarly, histamine, acting via H1R, has also been shown to enhance the production of chemokines, such as granulocyte macrophage colony stimulating factor, regulated on activation T cell expressed and secreted (RANTES), and monocyte chemotactic protein-1 (MCP-1) in IFN-γ-stimulated keratinocytes. It also upregulates the antigen-presenting capability of dendritic cells, and leads to Th1 polarization through H1R (67).
Histamine induces IL-31 production, which plays an important and crucial role in pruritus and skin barrier function in allergic dermatitis (54). Administration of an H1R antagonist decreased IL-31 levels in the serum of atopic dermatitis patients (68). These data therefore suggest that H1R activation by histamine has the ability to induce various symptoms related with allergic skin diseases such as pruritus and atopic dermatitis.
The Gαs-coupled H2R is highly expressed in various cells and tissues, such as B cells, T cells, dendritic cells, gastricparietal cells, smooth muscle cells, and the brain and cardiac tissues (Table 1). Activation of the receptor can induce airway mucus production, vascular permeability, and secretion of gastric acid (69). The role of the H2R is well studied in histidine decarboxylase knockout mice (HDC−/−) models which suggest that the lack of histamine can enhance downregulation of H2R expression in a tissue-specific manner (70). Furthermore, the H2R is importantly accountable for the relaxation of the airways, uterus, and smooth muscle cells in the blood vessels. Moreover, the H2R is involved in the activation of the immune system, such as Th1 cytokine production, reduction of basophil degranulation, T-cell proliferation, and antibody synthesis (71, 72). Knockdown of H2R−/− mice show impaired immune functions, gastric acid secretion, and cognitive function associated with hippocampal potentiation impairments (73, 74) and nociception abnormalities (75).
The H3R is coupled to Gαi/o and exclusively expressed in neurones. It is important for homeostatic regulation of energy levels, sleep-wake cycle, cognition, and inflammation (76) (Figure 1). H3R-deficient mice exhibit altered behavior and locomotion (77) and display a metabolic syndrome characterized by obesity, hyperphagia, and increased leptin and insulin levels (78, 79). Similarly, several studies suggest that H3R knockout can also lead to an increase in severity of neuro-inflammatory diseases and can enhance the expression of IFN-inducible protein 10, MIP 2, and CXCR3 in T cells (80). These investigators also showed that H3R can be involved in blood–brain barrier function.
The H3R has also been associated with rhinitis (81). This is likely because it is expressed on presynaptic nerves in the peripheral sympathetic adrenergic system and also on nasal sub-mucosal glands. Stimulation of H3R suppressed norepinephrine release at presynaptic nerve endings and stimulated nasal sub-mucosal gland secretion (82).
Currently, several H3R ligands are available, but not in clinical use. H3R antagonists, such as clobenpropit and thioperamide, were extensively used as a research tools and few early stage clinical trial reports are also available for H3R antagonists (83). However, these antagonists are used to treat obesity, myocardial ischemic arrhythmias, cognition disorders, and insomnia (84).
The histamine H4R is coupled to Gα/io proteins (85) and is expressed on a variety of immune cells as well as on other cells such as spleen, intestinal epithelia, lung, synovial tissue, central nervous system, sensory neurons, and cancer cells (86–94). Stimulation of H4R reduces forskolin-induced cyclic AMP formation, which leads to the activation of MAPK and enhanced Ca++ release (6, 95). H4R mediates the pro-inflammatory responses of histamine in both autocrine and paracrine manners. Histamine enhances adhesion molecule expression, cell shape change, and cytoskeletal rearrangement via H4R, leading to the increased migration of eosinophils (5).
In various allergic diseases allergen cross linkage of FcεRI is the primary driver of mast cell activation. However, H4R is constitutively expressed on human mast cells such as LAD-2 and HMC-1 (33, 43). H4R-mediated activation of mast cells leads to the expression of various pro-inflammatory cytokine and chemokines, such as IL-6, TNF-α, TGF-β1, RANTES, IL-8, MIP-1α, and MCP-1 (33). Histamine H4R stimulation of mast cells may have three positive effects. First, it increases chemotaxis of mast cells thus encouraging their accumulation at the site of an allergic response (6). Second, it upregulates the expression of FcεRI on mast cells, thereby priming them for allergen-induced activation (96). Third, it mobilizes intracellular calcium to either prime mast cells for activation or, indeed, induce degranulation. These effects have been studied using histamine, the H4R-agonist 4-methylhistamine, the H4R-antagonists thioperamide or JNJ 7777120 and mast cells from H4R-deficient mice.
Basophils also express H4R on their surface and release histamine following antigen stimulation (55). However, basophils and mast cells differ in several important aspects, such as anatomical localization, the production of cytokines, and antigen-presenting activity. Histamine, acting via H4R, induces chemotaxis of bone marrow-derived basophils. H4R may play significant roles in basophil regulation in allergic dermatitis (97).
Among the Th subsets, the mRNA and protein of H4R are preferentially expressed in Th2 cells over naive T cells and Th1 cells. H4R may be involved in the pathogenesis of allergy and inflammation by activating Th2 as well as Th17 cells (68, 98). In human Th17 cells, H4R antagonists inhibit the IL-17 production, induced by H4R agonist (68).
Stimulation of H4R can also enhance the migration of eosinophils and the recruitment of mast cells leading to the amplification of immune responses and chronic inflammation. Similarly, H4R are involved in T cell differentiation and dendritic cell activation and its immunomodulatory function (6). Histamine and selective H4R agonists were shown to induce the shape change of eosinophils, an effect that maybe blocked by selective H4R antagonists (5). Treatment with JNJ 39758979 (H4R antagonist) resulted in a statistically significant inhibition of eosinophil shape change. These results showed that administration of H4R antagonists may have an impact on eosinophil function (38).
Finally, the activation of H4R involves several signaling cascades for the release of various allergic inflammatory mediators. ERK is a member of MAPK family and mediates the proliferation, differentiation, anti-apoptosis, regulation, and cytokine expression at gene level. There are reports showing that histamine can induce phosphorylation of ERK through H4R in peripheral blood derived CD34+ human mast cells as well as in mouse BMMCs (34) and HEK-293 cells (99). H4R-involved ERK and PI3 kinase pathways have been shown to be involved in the release of IL-6 in mouse BMMCs (34) and JAK/STAT signaling pathways for the release of TNF-α in a rat model (100). Recent studies (101) demonstrated that the activation of NFκB through H4R has followed the JAK/STAT signaling pathway.
In addition to H1R, H4R is considered as a novel drug target for the treatment of allergy and inflammation. Recently, the H4R antagonists such as JNJ 7777120 and JNJ 39758979 have been extensively used as a tools to understand the pathophysiological involvement of H4R and have been studied extensively in both cell culture and in vivo animal models (102, 103). Furthermore, H4R antagonists have been used to explore the role of H4R in allergic inflammatory disorders, such as allergic asthma, allergic rhinitis, and chronic pruritus (31).
Mast cells play an active role in various allergic diseases such as acute pruritus, atopic dermatitis, allergic asthma, allergic rhinitis, and pulmonary fibrosis (104, 105). H1-antihistamines, such as azatadine, cetirizine, and mizolastine are used for the treatment mast cell activated diseases (106). Cimetidine, ranitidine, famotidine, and nizatidine are H2R selective antihistamines that reduce gastric acid secretion (107). H3R antihistamines include thioperamide, clobenpropit, BF2. 649, PF-03654746, JNJ-17216498, and MK 0249.
JNJ 7777120 is a selective H4R antihistamine that is widely used in inflammation and pruritus (108). There are some H4R antihistamines which are under clinical trial, such as JNJ 39758979, NCT 01068223, UK-63325, PF-3893787, and JNJ 38518168 (Figure 1) (108, 109). H1-antihistamines are a standard treatment for mast cell-mediated allergic diseases. There is increasing evidence that histamine binding to H4 receptors exacerbates allergy and inflammation. Indeed, mast cells themselves have H4 receptors which when stimulated increased degranulation and cytokine production. Therefore, antihistamines targeting both the H1 and H4 receptor could be an effective treatment for mast cell-mediated allergic diseases (110).
Pharmacological properties of H4R have been exhibited by various H4R transfected cells (87, 89, 99, 111, 112). It was observed that H1R and H2R specific agonist/antagonists cannot bind to the H4R. However, some H3R ligands such as imetit, clobenpropit, thioperamide, and R-methylhistamine are also able to bind to the H4R with different affinities. Currently, a number of H4R antagonists have been developed but only a few are undergoing clinical trials. JNJ 39758979, a potent and selective H4R antagonist, has shown impressive results in different allergic inflammatory diseases such as dermatitis, asthma, pruritus, and arthritis (102, 103).
Recent clinical trials (NCT01068223) with the H4R antagonist JNJ 39758979 help to demonstrate a significant role of the H4 receptor in pruritus in humans. Interestingly, the combination therapy of this H4R antagonist and the H1R antihistamine, cetirizine, showed a more beneficial effect in the treatment of pruritus as compared with H1R alone (113–116). Furthermore, a study was carried out by using JNJ 39758979 to treat persistent asthma (NCT00946569), but no results have yet been reported. There are some H4R antagonists under the clinical trial including toreforant (JNJ 38518168), PF-3893787, and UR-63325. Toreforant (JNJ 38518168) has been used for the treatment of rheumatoid arthritis (clinical trial numbers: NCT01679951, NCT00941707, and NCT01862224). However, a study in rheumatoid arthritis (NCT01679951) was terminated due to issues related to efficacy. Even though, studies are still going on with the H4R antagonist toreforant (JNJ 38518168) in patients with asthma and psoriasis (clinical trial numbers NCT01823016 and NCT02295865, respectively) (38).
The recent developments in research on histamine pathway underscore the importance of histamine in allergic inflammation through its effects on the H1R and H4R. Although, drugs targeting H1R are being explored for the treatment of various mast cell-associated allergic disorders, they are not always clinically effective. Several H4R antagonists have entered the later stages of clinical trials for a different range of allergic and inflammatory diseases. However, their clinical efficacy reports are not yet published. Furthermore, there appears to be some overlap in function between H1R and H4R, opening up the possibility for using synergistic strategies for therapeutic approaches. As such, we suggest the combination therapies by using both H4R together with H1R antagonists may provide a potential benefit in the treatment of various allergic and inflammatory diseases.
ET, EJ, HS, MB, MK, CM, MC, and RS designed the manuscript; were involved in drafting/revising the manuscript; and read and approved the final manuscript. ET, EJ, and RS wrote the first draft.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was primarily supported by DBT, Government of India, Ref. No: BT/PR3230/BRB/10/965/2011. ET and EJ thank SRM Institute of Science and Technology for the constant support and institutional facilities throughout the study. RS (BT/RLF/Re-entry/53/2013) and MB (BT/RLF/Re-entry/26/2013) acknowledge support from the Department of Biotechnology, India for Ramalingaswami Re-entry Fellowship.
BMMCs, bone marrow-derived murine mast cells; ERK, extracellular signal-regulated kinases; GM-CSF, granulocyte macrophage colony stimulating factor; H1R, histamine H1 receptor; H2R, histamine H2 receptor; H3R, histamine H3 receptor; H4R, histamine H4 receptor; IFN-γ, interferon-γ; IL, interleukin; MCP-1, monocyte chemotactic protein-1; NGF, nerve growth factor; PKC, protein kinase C; TNF, tumor necrosis factor; RANTES, regulated on activation T cell expressed and secreted; PBMC, peripheral blood mononuclear cells; THP-1, Tamm–Horsfall protein 1.
2. Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med (2001) 344:30–7. doi:10.1056/NEJM200101043440106
3. Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature (1990) 346:274–6. doi:10.1038/346274a0
4. Young JD, Liu CC, Butler G, Cohn ZA, Galli SJ. Identification, purification, and characterization of a mast cell-associated cytolytic factor related to tumor necrosis factor. Proc Natl Acad Sci U S A (1987) 84:9175–9. doi:10.1073/pnas.84.24.9175
5. Buckland KF, Williams TJ, Conroy DM. Histamine induces cytoskeletal changes in human eosinophils via the H(4) receptor. Br J Pharmacol (2003) 140:1117–27. doi:10.1038/sj.bjp.0705530
6. Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther (2003) 305:1212–21. doi:10.1124/jpet.102.046581
7. O’Reilly M, Alpert R, Jenkinson S, Gladue RP, Foo S, Trim S, et al. Identification of a histamine H4 receptor on human eosinophils – role in eosinophil chemotaxis. J Recept Signal Transduct Res (2002) 22:431–48. doi:10.1081/RRS-120014612
8. Thurmond RL, Desai PJ, Dunford PJ, Fung-Leung WP, Hofstra CL, Jiang W, et al. A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. J Pharmacol Exp Ther (2004) 309:404–13. doi:10.1124/jpet.103.061754
9. Laszlo V, Rothe G, Hegyesi H, Szeberenyi JB, Orso E, Schmitz G, et al. Increased histidine decarboxylase expression during in vitro monocyte maturation; a possible role of endogenously synthesised histamine in monocyte/macrophage differentiation. Inflamm Res (2001) 50:428–34. doi:10.1007/PL00000266
10. Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature (2001) 413:420–5. doi:10.1038/35096564
11. Jutel M, Klunker S, Akdis M, Malolepszy J, Thomet OA, Zak-Nejmark T, et al. Histamine upregulates Th1 and downregulates Th2 responses due to different patterns of surface histamine 1 and 2 receptor expression. Int Arch Allergy Immunol (2001) 124:190–2. doi:10.1159/000053707
12. Triggiani M, Gentile M, Secondo A, Granata F, Oriente A, Taglialatela M, et al. Histamine induces exocytosis and IL-6 production from human lung macrophages through interaction with H1 receptors. J Immunol (2001) 166:4083–91. doi:10.4049/jimmunol.166.6.4083
13. Hirasawa N, Ohtsu H, Watanabe T, Ohuchi K. Enhancement of neutrophil infiltration in histidine decarboxylase-deficient mice. Immunology (2002) 107:217–21. doi:10.1046/j.1365-2567.2002.01482.x
14. Ling P, Ngo K, Nguyen S, Thurmond RL, Edwards JP, Karlsson L, et al. Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br J Pharmacol (2004) 142:161–71. doi:10.1038/sj.bjp.0705899
15. Damaj BB, Becerra CB, Esber HJ, Wen Y, Maghazachi AA. Functional expression of H4 histamine receptor in human natural killer cells, monocytes, and dendritic cells. J Immunol (2007) 179:7907–15. doi:10.4049/jimmunol.179.11.7907
16. Parsons ME, Ganellin CR. Histamine and its receptors. Br J Pharmacol (2006) 147(Suppl 1):S127–35. doi:10.1038/sj.bjp.0706440
17. O’Sullivan MP, Tyner JW, Holtzman MJ. Apoptosis in the airways: another balancing act in the epithelial program. Am J Respir Cell Mol Biol (2003) 29:3–7. doi:10.1165/rcmb.F273
18. Jutel M, Blaser K, Akdis CA. Histamine in allergic inflammation and immune modulation. Int Arch Allergy Immunol (2005) 137:82–92. doi:10.1159/000085108
19. Akdis CA, Simons FE. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol (2006) 533:69–76. doi:10.1016/j.ejphar.2005.12.044
20. Packard KA, Khan MM. Effects of histamine on Th1/Th2 cytokine balance. Int Immunopharmacol (2003) 3:909–20. doi:10.1016/S1567-5769(02)00235-7
21. Metz M, Siebenhaar F, Maurer M. Mast cell functions in the innate skin immune system. Immunobiology (2008) 213:251–60. doi:10.1016/j.imbio.2007.10.017
23. Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev (1997) 77:1033–79. doi:10.1152/physrev.1997.77.4.1033
24. Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun (2006) 349:1322–8. doi:10.1016/j.bbrc.2006.08.177
25. Theoharides TC. The mast cell: a neuroimmunoendocrine master player. Int J Tissue React (1996) 18:1–21.
26. Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol (2004) 146:1–12. doi:10.1016/j.jneuroim.2003.10.041
27. Tal M, Liberman R. Local injection of nerve growth factor (NGF) triggers degranulation of mast cells in rat paw. Neurosci Lett (1997) 221:129–32. doi:10.1016/S0304-3940(96)13318-8
28. Saluja R, Ketelaar ME, Hawro T, Church MK, Maurer M, Nawijn MC. The role of the IL-33/IL-1RL1 axis in mast cell and basophil activation in allergic disorders. Mol Immunol (2015) 63:80–5. doi:10.1016/j.molimm.2014.06.018
30. Hill SJ. Multiple histamine receptors: properties and functional characteristics. Biochem Soc Trans (1992) 20:122–5. doi:10.1042/bst0200122
31. Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. Br J Pharmacol (2009) 157:24–33. doi:10.1111/j.1476-5381.2009.00151.x
32. Jutel M, Akdis CA. Histamine as an immune modulator in chronic inflammatory responses. Clin Exp Allergy (2007) 37:308–10. doi:10.1111/j.1365-2222.2007.02666.x
33. Jemima EA, Prema A, Thangam EB. Functional characterization of histamine H4 receptor on human mast cells. Mol Immunol (2014) 62:19–28. doi:10.1016/j.molimm.2014.05.007
34. Desai P, Thurmond RL. Histamine H(4) receptor activation enhances LPS-induced IL-6 production in mast cells via ERK and PI3K activation. Eur J Immunol (2011) 41:1764–73. doi:10.1002/eji.201040932
35. Zhang B, Alysandratos KD, Angelidou A, Asadi S, Sismanopoulos N, Delivanis DA, et al. Human mast cell degranulation and preformed TNF secretion require mitochondrial translocation to exocytosis sites: relevance to atopic dermatitis. J Allergy Clin Immunol (2011) 127:1522–31.e8. doi:10.1016/j.jaci.2011.02.005
36. Leurs R, Chazot PL, Shenton FC, Lim HD, De Esch IJ. Molecular and biochemical pharmacology of the histamine H4 receptor. Br J Pharmacol (2009) 157:14–23. doi:10.1111/j.1476-5381.2009.00250.x
37. Singh M, Jadhav HR. Histamine H3 receptor function and ligands: recent developments. Mini Rev Med Chem (2013) 13:47–57. doi:10.2174/138955713804484695
38. Thurmond RL. The histamine H4 receptor: from orphan to the clinic. Front Pharmacol (2015) 6:65. doi:10.3389/fphar.2015.00065
39. Bakker RA, Schoonus SB, Smit MJ, Timmerman H, Leurs R. Histamine H(1)-receptor activation of nuclear factor-kappa B: roles for G beta gamma- and G alpha(q/11)-subunits in constitutive and agonist-mediated signaling. Mol Pharmacol (2001) 60:1133–42. doi:10.1124/mol.60.5.1133
40. Seifert R, Strasser A, Schneider EH, Neumann D, Dove S, Buschauer A. Molecular and cellular analysis of human histamine receptor subtypes. Trends Pharmacol Sci (2013) 34:33–58. doi:10.1016/j.tips.2012.11.001
41. Tiligada E. Editorial: is histamine the missing link in chronic inflammation? J Leukoc Biol (2012) 92:4–6. doi:10.1189/jlb.0212093
42. Bryce PJ, Mathias CB, Harrison KL, Watanabe T, Geha RS, Oettgen HC. The H1 histamine receptor regulates allergic lung responses. J Clin Invest (2006) 116:1624–32. doi:10.1172/JCI26150
43. Lippert U, Artuc M, Grutzkau A, Babina M, Guhl S, Haase I, et al. Human skin mast cells express H2 and H4, but not H3 receptors. J Invest Dermatol (2004) 123:116–23. doi:10.1111/j.0022-202X.2004.22721.x
44. Rudolph MI, Boza Y, Yefi R, Luza S, Andrews E, Penissi A, et al. The influence of mast cell mediators on migration of SW756 cervical carcinoma cells. J Pharmacol Sci (2008) 106:208–18. doi:10.1254/jphs.FP0070736
45. Sander LE, Lorentz A, Sellge G, Coeffier M, Neipp M, Veres T, et al. Selective expression of histamine receptors H1R, H2R, and H4R, but not H3R, in the human intestinal tract. Gut (2006) 55:498–504. doi:10.1136/gut.2004.061762
46. Nagai Y, Tanaka Y, Kuroishi T, Sato R, Endo Y, Sugawara S. Histamine reduces susceptibility to natural killer cells via down-regulation of NKG2D ligands on human monocytic leukaemia THP-1 cells. Immunology (2012) 136:103–14. doi:10.1111/j.1365-2567.2012.03565.x
47. Kawabata M, Ohori J, Kurono Y. Effects of benzalkonium chloride on histamine H1 receptor mRNA expression in nasal epithelial cells. Auris Nasus Larynx (2016) 43:685–8. doi:10.1016/j.anl.2016.02.003
48. Leonardi A, Di Stefano A, Motterle L, Zavan B, Abatangelo G, Brun P. Transforming growth factor-beta/Smad – signalling pathway and conjunctival remodelling in vernal keratoconjunctivitis. Clin Exp Allergy (2011) 41:52–60. doi:10.1111/j.1365-2222.2010.03626.x
49. Gantner F, Sakai K, Tusche MW, Cruikshank WW, Center DM, Bacon KB. Histamine h(4) and h(2) receptors control histamine-induced interleukin-16 release from human CD8(+) T cells. J Pharmacol Exp Ther (2002) 303:300–7. doi:10.1124/jpet.102.036939
50. Reher TM, Brunskole I, Neumann D, Seifert R. Evidence for ligand-specific conformations of the histamine H(2)-receptor in human eosinophils and neutrophils. Biochem Pharmacol (2012) 84:1174–85. doi:10.1016/j.bcp.2012.08.014
51. Vannier E, Dinarello CA. Histamine enhances interleukin (IL)-1-induced IL-1 gene expression and protein synthesis via H2 receptors in peripheral blood mononuclear cells. Comparison with IL-1 receptor antagonist. J Clin Invest (1993) 92:281–7. doi:10.1172/JCI116562
52. Vannier E, Dinarello CA. Histamine enhances interleukin (IL)-1-induced IL-6 gene expression and protein synthesis via H2 receptors in peripheral blood mononuclear cells. J Biol Chem (1994) 269:9952–6.
53. Burde R, Seifert R. Stimulation of histamine H2- (and H1)-receptors activates Ca2+ influx in all-trans-retinoic acid-differentiated HL-60 cells independently of phospholipase C or adenylyl cyclase. Naunyn Schmiedebergs Arch Pharmacol (1996) 353:123–9. doi:10.1007/BF00168748
54. Gutzmer R, Mommert S, Gschwandtner M, Zwingmann K, Stark H, Werfel T. The histamine H4 receptor is functionally expressed on T(H)2 cells. J Allergy Clin Immunol (2009) 123:619–25. doi:10.1016/j.jaci.2008.12.1110
55. Mommert S, Kleiner S, Gehring M, Eiz-Vesper B, Stark H, Gutzmer R, et al. Human basophil chemotaxis and activation are regulated via the histamine H4 receptor. Allergy (2016) 71:1264–73. doi:10.1111/all.12875
56. Gutzmer R, Diestel C, Mommert S, Kother B, Stark H, Wittmann M, et al. Histamine H4 receptor stimulation suppresses Il-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. J Immunol (2005) 174:5224–32. doi:10.4049/jimmunol.174.9.5224
57. Capelo R, Lehmann C, Ahmad K, Snodgrass R, Diehl O, Ringleb J, et al. Cellular analysis of the histamine H4 receptor in human myeloid cells. Biochem Pharmacol (2016) 103:74–84. doi:10.1016/j.bcp.2016.01.007
58. Simons FE. Advances in H1-antihistamines. N Engl J Med (2004) 351:2203–17. doi:10.1056/NEJMra033121
59. Schaefer U, Schmitz V, Schneider A, Neugebauer E. Histamine induced homologous and heterologous regulation of histamine receptor subtype mRNA expression in cultured endothelial cells. Shock (1999) 12:309–15. doi:10.1097/00024382-199910000-00010
60. Ma RZ, Gao J, Meeker ND, Fillmore PD, Tung KS, Watanabe T, et al. Identification of Bphs, an autoimmune disease locus, as histamine receptor H1. Science (2002) 297:620–3. doi:10.1126/science.1072810
61. Horio S, Fujimoto K, Mizuguchi H, Fukui H. Interleukin-4 up-regulates histamine H1 receptors by activation of H1 receptor gene transcription. Naunyn Schmiedebergs Arch Pharmacol (2010) 381:305–13. doi:10.1007/s00210-010-0491-z
62. Osna N, Elliott K, Khan MM. Regulation of interleukin-10 secretion by histamine in TH2 cells and splenocytes. Int Immunopharmacol (2001) 1:85–96. doi:10.1016/S0162-3109(00)00268-X
63. Banu Y, Watanabe T. Augmentation of antigen receptor-mediated responses by histamine H1 receptor signaling. J Exp Med (1999) 189:673–82. doi:10.1084/jem.189.4.673
64. Baena-Cagnani CE, Berger WE, Dubuske LM, Gurne SE, Stryszak P, Lorber R, et al. Comparative effects of desloratadine versus montelukast on asthma symptoms and use of beta 2-agonists in patients with seasonal allergic rhinitis and asthma. Int Arch Allergy Immunol (2003) 130:307–13. doi:10.1159/000070218
65. Simons FE. Is antihistamine (H1-receptor antagonist) therapy useful in clinical asthma? Clin Exp Allergy (1999) 29(Suppl 3):98–104. doi:10.1046/j.1365-2222.1999.0290s3098.x
66. Kanda N, Watanabe S. Histamine enhances the production of nerve growth factor in human keratinocytes. J Invest Dermatol (2003) 121:570–7. doi:10.1046/j.1523-1747.2003.12428.x
67. Giustizieri ML, Albanesi C, Fluhr J, Gisondi P, Norgauer J, Girolomoni G. H1 histamine receptor mediates inflammatory responses in human keratinocytes. J Allergy Clin Immunol (2004) 114:1176–82. doi:10.1016/j.jaci.2004.07.054
68. Mommert S, Gschwandtner M, Koether B, Gutzmer R, Werfel T. Human memory Th17 cells express a functional histamine H4 receptor. Am J Pathol (2012) 180:177–85. doi:10.1016/j.ajpath.2011.09.028
69. Smit MJ, Leurs R, Alewijnse AE, Blauw J, Van Nieuw Amerongen GP, Van De Vrede Y, et al. Inverse agonism of histamine H2 antagonist accounts for upregulation of spontaneously active histamine H2 receptors. Proc Natl Acad Sci U S A (1996) 93:6802–7. doi:10.1073/pnas.93.13.6802
70. Fitzsimons CP, Lazar-Molnar E, Tomoskozi Z, Buzas E, Rivera ES, Falus A. Histamine deficiency induces tissue-specific down-regulation of histamine H2 receptor expression in histidine decarboxylase knockout mice. FEBS Lett (2001) 508:245–8. doi:10.1016/S0014-5793(01)03070-8
71. Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to Il-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med (2008) 205:2887–98. doi:10.1084/jem.20080193
72. Lichtenstein LM, Gillespie E. The effects of the H1 and H2 antihistamines on “allergic” histamine release and its inhibition by histamine. J Pharmacol Exp Ther (1975) 192:441–50.
73. Teuscher C, Poynter ME, Offner H, Zamora A, Watanabe T, Fillmore PD, et al. Attenuation of Th1 effector cell responses and susceptibility to experimental allergic encephalomyelitis in histamine H2 receptor knockout mice is due to dysregulation of cytokine production by antigen-presenting cells. Am J Pathol (2004) 164:883–92. doi:10.1016/S0002-9440(10)63176-8
74. Dai H, Kaneko K, Kato H, Fujii S, Jing Y, Xu A, et al. Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci Res (2007) 57:306–13. doi:10.1016/j.neures.2006.10.020
75. Mobarakeh JI, Takahashi K, Sakurada S, Kuramasu A, Yanai K. Enhanced antinociceptive effects of morphine in histamine H2 receptor gene knockout mice. Neuropharmacology (2006) 51:612–22. doi:10.1016/j.neuropharm.2006.05.003
76. Dimitriadou V, Rouleau A, Dam Trung Tuong M, Newlands GJ, Miller HR, Luffau G, et al. Functional relationship between mast cells and C-sensitive nerve fibres evidenced by histamine H3-receptor modulation in rat lung and spleen. Clin Sci (Lond) (1994) 87:151–63. doi:10.1042/cs0870151
77. Toyota H, Dugovic C, Koehl M, Laposky AD, Weber C, Ngo K, et al. Behavioral characterization of mice lacking histamine H(3) receptors. Mol Pharmacol (2002) 62:389–97. doi:10.1124/mol.62.2.389
78. Tokita S, Takahashi K, Kotani H. Recent advances in molecular pharmacology of the histamine systems: physiology and pharmacology of histamine H3 receptor: roles in feeding regulation and therapeutic potential for metabolic disorders. J Pharmacol Sci (2006) 101:12–8. doi:10.1254/jphs.FMJ06001X4
79. Yoshimoto R, Miyamoto Y, Shimamura K, Ishihara A, Takahashi K, Kotani H, et al. Therapeutic potential of histamine H3 receptor agonist for the treatment of obesity and diabetes mellitus. Proc Natl Acad Sci U S A (2006) 103:13866–71. doi:10.1073/pnas.0506104103
80. Teuscher C, Subramanian M, Noubade R, Gao JF, Offner H, Zachary JF, et al. Central histamine H3 receptor signaling negatively regulates susceptibility to autoimmune inflammatory disease of the CNS. Proc Natl Acad Sci U S A (2007) 104:10146–51. doi:10.1073/pnas.0702291104
81. Lieberman P. The basics of histamine biology. Ann Allergy Asthma Immunol (2011) 106:S2–5. doi:10.1016/j.anai.2010.08.005
82. Suzuki S, Takeuchi K, Majima Y. Localization and function of histamine H3 receptor in the nasal mucosa. Clin Exp Allergy (2008) 38:1476–82. doi:10.1111/j.1365-2222.2008.03041.x
83. Wijtmans M, Leurs R, De Esch I. Histamine H3 receptor ligands break ground in a remarkable plethora of therapeutic areas. Expert Opin Investig Drugs (2007) 16:967–85. doi:10.1517/13543784.16.7.967
84. Leurs R, Bakker RA, Timmerman H, De Esch IJ. The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov (2005) 4:107–20. doi:10.1038/nrd1631
85. de Esch IJ, Thurmond RL, Jongejan A, Leurs R. The histamine H4 receptor as a new therapeutic target for inflammation. Trends Pharmacol Sci (2005) 26:462–9. doi:10.1016/j.tips.2005.07.002
86. Nakamura T, Itadani H, Hidaka Y, Ohta M, Tanaka K. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem Biophys Res Commun (2000) 279:615–20. doi:10.1006/bbrc.2000.4008
87. Liu C, Ma X, Jiang X, Wilson SJ, Hofstra CL, Blevitt J, et al. Cloning and pharmacological characterization of a fourth histamine receptor (H(4)) expressed in bone marrow. Mol Pharmacol (2001) 59:420–6. doi:10.1124/mol.59.3.420
88. Yamaura K, Shigemori A, Suwa E, Ueno K. Expression of the histamine H4 receptor in dermal and articular tissues. Life Sci (2013) 92:108–13. doi:10.1016/j.lfs.2012.10.030
89. Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem (2000) 275:36781–6. doi:10.1074/jbc.M006480200
90. Horr B, Borck H, Thurmond R, Grosch S, Diel F. STAT1 phosphorylation and cleavage is regulated by the histamine (H4) receptor in human atopic and non-atopic lymphocytes. Int Immunopharmacol (2006) 6:1577–85. doi:10.1016/j.intimp.2006.06.005
91. Connelly WM, Shenton FC, Lethbridge N, Leurs R, Waldvogel HJ, Faull RL, et al. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br J Pharmacol (2009) 157:55–63. doi:10.1111/j.1476-5381.2009.00227.x
92. Medina VA, Rivera ES. Histamine receptors and cancer pharmacology. Br J Pharmacol (2010) 161:755–67. doi:10.1111/j.1476-5381.2010.00961.x
93. Breunig E, Michel K, Zeller F, Seidl S, Weyhern CW, Schemann M. Histamine excites neurones in the human submucous plexus through activation of H1, H2, H3 and H4 receptors. J Physiol (2007) 583:731–42. doi:10.1113/jphysiol.2007.139352
94. Morgan RK, Mcallister B, Cross L, Green DS, Kornfeld H, Center DM, et al. Histamine 4 receptor activation induces recruitment of FoxP3+ T cells and inhibits allergic asthma in a murine model. J Immunol (2007) 178:8081–9. doi:10.4049/jimmunol.178.12.8081
95. Lim HD, Van Rijn RM, Ling P, Bakker RA, Thurmond RL, Leurs R. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. J Pharmacol Exp Ther (2005) 314:1310–21. doi:10.1124/jpet.105.087965
96. Mirzahosseini A, Dalmadi B, Csutora P. Histamine receptor H4 regulates mast cell degranulation and IgE induced FcepsilonRI upregulation in murine bone marrow-derived mast cells. Cell Immunol (2013) 283:38–44. doi:10.1016/j.cellimm.2013.05.006
97. Ohsawa Y, Hirasawa N. The role of histamine H1 and H4 receptors in atopic dermatitis: from basic research to clinical study. Allergol Int (2014) 63:533–42. doi:10.2332/allergolint.13-RA-0675
98. Cowden JM, Yu F, Banie H, Farahani M, Ling P, Nguyen S, et al. The histamine H4 receptor mediates inflammation and Th17 responses in preclinical models of arthritis. Ann Rheum Dis (2014) 73:600–8. doi:10.1136/annrheumdis-2013-203832
99. Morse KL, Behan J, Laz TM, West RE Jr, Greenfeder SA, Anthes JC, et al. Cloning and characterization of a novel human histamine receptor. J Pharmacol Exp Ther (2001) 296:1058–66.
100. Hinz M, Arslan SC, Scheidereit C. It takes two to tango: IkappaBs, the multifunctional partners of NF-kappaB. Immunol Rev (2012) 246:59–76. doi:10.1111/j.1600-065X.2012.01102.x
101. Ahmad SF, Ansari MA, Zoheir KM, Bakheet SA, Korashy HM, Nadeem A, et al. Regulation of TNF-alpha and NF-kappaB activation through the Jak/Stat signaling pathway downstream of histamine 4 receptor in a rat model of LPS-induced joint inflammation. Immunobiology (2015) 220:889–98. doi:10.1016/j.imbio.2015.01.008
102. Thurmond RL, Chen B, Dunford PJ, Greenspan AJ, Karlsson L, La D, et al. Clinical and preclinical characterization of the histamine H(4) receptor antagonist JNJ-39758979. J Pharmacol Exp Ther (2014) 349:176–84. doi:10.1124/jpet.113.211714
103. Savall BM, Chavez F, Tays K, Dunford PJ, Cowden JM, Hack MD, et al. Discovery and Sar of 6-alkyl-2,4-diaminopyrimidines as histamine H(4) receptor antagonists. J Med Chem (2014) 57:2429–39. doi:10.1021/jm401727m
104. Amin K. The role of mast cells in allergic inflammation. Respir Med (2012) 106:9–14. doi:10.1016/j.rmed.2011.09.007
105. Cruse G, Bradding P. Mast cells in airway diseases and interstitial lung disease. Eur J Pharmacol (2016) 778:125–38. doi:10.1016/j.ejphar.2015.04.046
106. Church MK, Church DS. Pharmacology of antihistamines. Indian J Dermatol (2013) 58:219–24. doi:10.4103/0019-5154.110832
107. Shim YK, Kim N. The effect of H2 receptor antagonist in acid inhibition and its clinical efficacy. Korean J Gastroenterol (2017) 70:4–12. doi:10.4166/kjg.2017.70.1.4
108. Thurmond RL, Venable J, Savall B, La D, Snook S, Dunford PJ, et al. Clinical development of histamine H4 receptor antagonists. Handb Exp Pharmacol (2017) 241:301–20. doi:10.1007/164_2016_130
109. Schwartz JC. The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol (2011) 163:713–21. doi:10.1111/j.1476-5381.2011.01286.x
110. Mishra GP, Tamboli V, Jwala J, Mitra AK. Recent patents and emerging therapeutics in the treatment of allergic conjunctivitis. Recent Pat Inflamm Allergy Drug Discov (2011) 5:26–36. doi:10.2174/187221311794474883
111. Nguyen T, Shapiro DA, George SR, Setola V, Lee DK, Cheng R, et al. Discovery of a novel member of the histamine receptor family. Mol Pharmacol (2001) 59:427–33. doi:10.1124/mol.59.3.427
112. Zhu Y, Michalovich D, Wu H, Tan KB, Dytko GM, Mannan IJ, et al. Cloning, expression, and pharmacological characterization of a novel human histamine receptor. Mol Pharmacol (2001) 59:434–41. doi:10.1124/mol.59.3.434
113. Dunford PJ, Williams KN, Desai PJ, Karlsson L, Mcqueen D, Thurmond RL. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol (2007) 119:176–83. doi:10.1016/j.jaci.2006.12.606
114. Nakano Y, Takahashi Y, Ono R, Kurata Y, Kagawa Y, Kamei C. Role of histamine H(4) receptor in allergic conjunctivitis in mice. Eur J Pharmacol (2009) 608:71–5. doi:10.1016/j.ejphar.2009.02.035
115. Rossbach K, Wendorff S, Sander K, Stark H, Gutzmer R, Werfel T, et al. Histamine H4 receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Exp Dermatol (2009) 18:57–63. doi:10.1111/j.1600-0625.2008.00762.x
Keywords: histamine, histamine receptors, mast cells, allergy, inflammation, antihistamines
Citation: Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, Church MK and Saluja R (2018) The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 9:1873. doi: 10.3389/fimmu.2018.01873
Received: 14 March 2018; Accepted: 30 July 2018;
Published: 13 August 2018
Edited by:
Carlo Pucillo, Università degli Studi di Udine, ItalyReviewed by:
Axel Lorentz, University of Hohenheim, GermanyCopyright: © 2018 Thangam, Jemima, Singh, Baig, Khan, Mathias, Church and Saluja. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rohit Saluja, ZHJyb2hpdHNhbHVqYUBnbWFpbC5jb20=, cm9oaXQuYmlvY2hlbWlzdHJ5QGFpaW1zYmhvcGFsLmVkdS5pbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.