
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 31 July 2018
Sec. Vaccines and Molecular Therapeutics
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.01783
Proinflammatory cytokines have the capacity to increase inflammatory reaction and play a central role in first line of defence against invading pathogens. Proinflammatory inducing peptides (PIPs) have been used as an antineoplastic agent, an antibacterial agent and a vaccine in immunization therapies. Due to the advancement in sequence technologies that resulted an avalanche of protein sequence data. Therefore, it is necessary to develop an automated computational method to enable fast and accurate identification of novel PIPs within the vast number of candidate proteins and peptides. To address this, we proposed a new predictor, PIP-EL, for predicting PIPs using the strategy of ensemble learning (EL). Our benchmarking dataset is imbalanced. Thus, we applied a random under-sampling technique to generate 10 balanced models for each composition. Technically, PIP-EL is the fusion of 50 independent random forest (RF) models, where each of the five different compositions, including amino acid, dipeptide, composition–transition–distribution, physicochemical properties, and amino acid index contains 10 RF models. PIP-EL achieves the Matthews’ correlation coefficient (MCC) of 0.435 in a 5-fold cross-validation test, which is ~2–5% higher than that of the individual classifiers and hybrid feature-based classifier. Furthermore, we evaluate the performance of PIP-EL on the independent dataset, showing that our method outperforms the existing method and two different machine learning methods developed in this study, with an MCC of 0.454. These results indicate that PIP-EL will be a useful tool for predicting PIPs and for researchers working in the field of peptide therapeutics and immunotherapy. The user-friendly web server, PIP-EL, is freely accessible.1
Inflammation is modulated by a host of molecular regulators, such as cytokines, complement eicosanoids, growth factors, and peptides (1). The key modulators of inflammation are cytokines, which participate in both acute and chronic inflammation. Cytokines can be classified based on the nature of immune response, cell type, location, and receptor type, used for signalling. Critical proinflammatory cytokines include interleukin (IL)-1, IL-6, IL-8, IL-12, IL-18, interferon (IFN)-γ, and tumour necrosis factor (TNF)-α (2, 3).
Peptides are gaining momentum in pharmaceutical research and development because of their improved selectivity, high efficacy, tolerability, and biosafety. More than 150 peptide therapeutics are currently being evaluated in clinical trials (4). Besides their attractive pharmacological profile and intrinsic properties, peptides provide an excellent starting point for novel therapeutics. The role of peptides in inflammation can be proven via pathophysiological events, such as the release of tachykinins from sensory nerves for mediation of neurogenic inflammation and bradykinin from local and systemic inflammation (5). Peptides that induce proinflammatory cytokines are known as proinflammatory inducing peptides (PIPs), which can be utilized as therapeutic candidates to alleviate and cure various diseases (6, 7). For example, Helicobacter pylori produces a cecropin-like peptide [i.e., Hp(2–20)] that induces a proinflammatory response in human neutrophils, thereby acting as a potent antineoplastic agent (8). Prostate-specific antigen peptides have also been used in immunotherapies (9). Human cathelicidin LL-37 proinflammatory peptide has a role in the pathogenesis of rheumatoid disease, atherosclerosis, and antibacterial activities (10, 11). The gG-2p20 peptide induces a proinflammatory response by recruiting and activating phagocytic cells, thus reducing the function of NK cells (12).
Identification of PIPs is one of the hot topics in immunoinformatics and computational biology. An increasing number of PIPs have been experimentally identified and validated (13), it is expected that the number of PIPs will grow rapidly. As for the discovery of PIPs from protein primary sequence, experimental methods are time consuming, expensive, and difficult to be applied in a high-throughput manner. Therefore, development of a sequence-based computational method is needed to identify the possible potential candidates prior to the experimental procedure. To this end, several computational studies have focused on the prediction of different types of immune epitopes, including IL-4-inducing peptides (14), IL-10-inducing peptides (15), anti-inflammatory cytokine-inducing peptides (16), MHC binders (17), T-cell epitopes (17, 18), B-cell epitopes (19), and allergenicity (20–22). However, a few methods focused on predicting specific proinflammatory cytokine (i.e., IL17 and IFN-γ) inducing peptides (7, 23). Only one method (i.e., ProInflam) is available to predict general proinflammatory responses (i.e., IL1α, IL1β, TNFα, IL12, IL18, and IL23) that induce peptides (6).
Although ProInflam has contributed to stimulating development in this area, more work is needed for the following reasons. (i) With the rapidly increasing number of pro-inflammatory inducing epitopes in the Immune Epitope Database (IEDB), it remains an important and urgent task to develop more accurate prediction methods with a larger benchmarking dataset. (ii) The feature space used by the existing method is incomplete. Thus, additional potent features are needed for characterization. Owing to these deficiencies, other methods are necessary to accurately predict PIPs by taking advantage of machine learning (ML) algorithms, based on high-quality benchmarking datasets.
In this study, we constructed a nonredundant (nr) dataset of experimentally validated PIPs and non-PIPs extracted from the IEDB, sharing relatively low sequence similarities (i.e., no more than 80%) to avoid performance bias. From the nr dataset, we randomly select 80% of the data as the benchmarking dataset and 20% as the independent dataset. Various features extracted from the benchmarking dataset, including amino acid composition (AAC), dipeptide composition (DPC), composition–transition–distribution (CTD), amino acid index (AAI), and physicochemical properties (PCP), an input to the random forest (RF) algorithm to develop classification models. Because our benchmarking dataset is imbalanced, we applied a random under-sampling technique and generated 10 balanced models for each composition. Technically, PIP-EL is the fusion of 50 models from five different compositions (Figure 1). In addition to PIP-EL, we also develop extremely randomized trees (ERT) and support vector machine (SVM) methods using the same protocol as PIP-EL. Note that when objectively evaluated using an independent dataset, PIP-EL displays superior performance compared to the currently available method (i.e., ProInflam) and two other methods (i.e., ERT and SVM) developed in this study.
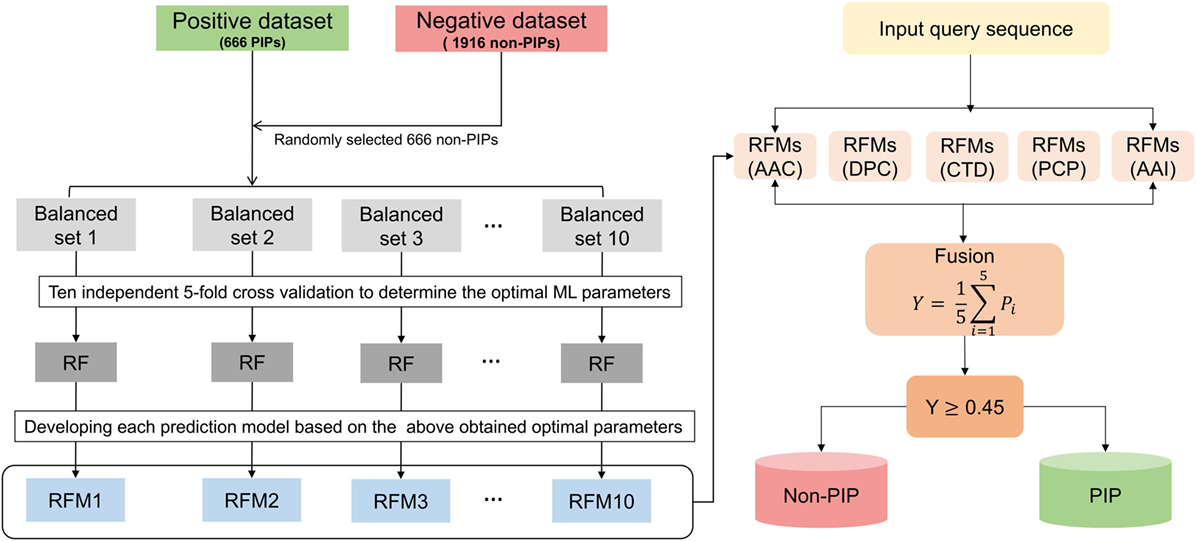
Figure 1. Overall framework of PIP-EL. The random under-sampling technique was applied to handle the imbalanced benchmarking dataset, generating 10 balanced RFMs. In total, 50 prediction models (i.e., 10 RFMs × 5 compositions = 50 RFMs) were obtained for five different compositions, including amino acid composition, dipeptide composition, composition–transition–distribution, amino acid index, and physicochemical properties. All these models were utilized to generate the final ensemble model.
To build an ML model, a well-curated and clear-cut dataset is required. Therefore, we extracted experimentally validated positive (i.e., 1,502 PIPs) and negative (i.e., 3,335 non-PIPs) linear peptides or epitopes from the IEDB (13, 24, 25). A peptide was considered positive if it induced any one of the proinflammatory cytokines (i.e., IL1α, IL1β, TNFα, IL6, IL8, IL12, IL17, IL18, and IL23) in T-cell assays of human and mouse. Similarly, linear peptides tested negative in inducing proinflammatory cytokines were considered negative. Due to their lower frequency, we excluded the peptides that have a length lower than 5 or greater than 25 amino acid residues from our dataset, since such inclusions may form an outlier during prediction model development. To generate an nr dataset, we eliminate redundant peptides using CD-HIT by applying a sequence identity threshold of 0.8, indicating that sequence identity between any two sequences greater than 80% is discarded. Using a more stringent criterion, such as 30 or 40%, as imposed in Ref. (19, 26, 27), could improve the credibility reliable of the model. However, in this study, we do not use such a stringent criterion, because our currently available data does not allow it. Otherwise, the number of samples for some subsets would be insufficient for showing statistical significance.
Finally, we obtained an nr dataset of 833 PIPs and 2,395 non-PIPs, whose size is ~4-fold bigger than the dataset used in the previous method (i.e., ProInflam). Our dataset contained nine proinflammatory cytokines, including six of them (i.e., IL1α, IL1β, TNFα, IL12, IL18, and IL23) used by ProInflam. From this nr dataset, 80% of the data was randomly selected as the benchmarking dataset (i.e., 666 PIPs and 1,916 non-PIPs) to develop a prediction model, whereas the remaining 20% was considered the independent dataset (i.e., 167 PIPs and 479 non-PIPs).
For the computational approach, each peptide sequence is represented as a numerical vector (i.e., features) input to ML algorithms for binary classification (i.e., PIP or non-PIP). Here, we used five different compositions, as follows.
Amino acid composition is the percentage of natural amino acids in a given peptide sequence, having a fixed length of 20 features. It was calculated using the following equation:
where i can be one of 20 possible amino acids.
Dipeptide composition represents the frequency of dipeptides normalized by all possible dipeptide combinations, having a fixed length of 400 features. It is calculated as follows:
where i can be one of 400 possible dipeptides.
The CTD feature was introduced by Dubchak et al. (28) for predicting protein-folding classes. Thereafter, it was successfully applied in various sequence-based classification algorithms (29–33). CTD represents the distribution of amino acid patterns along the primary sequence, based on their physicochemical or structural properties. There are seven physiochemical properties, including hydrophobicity, polarizability, normalized van der Waals volume, secondary structure, polarity, charge, and solvent accessibility.
All amino acids are divided into three groups: polar, neutral, and hydrophobic. C consists of three percentage composition values for a given peptide: polar, neutral, and hydrophobic. T consists of the percentage frequency of a polar followed by a neutral residue or of a neutral by a polar residue. It may also consist of a polar, followed by a hydrophobic residue or a hydrophobic followed by a polar residue. It may also consist of a neutral, followed by a hydrophobic or a hydrophobic, followed by a neutral residue. D consists of five values for each of the three groups. It measures the chain length, within which the first, 25, 50, 75, and 100% of the amino acids of a specific property are located. There are three descriptors and 3(C) + 3(T) + 5 × 3(D) = 21 descriptor values for a single amino acid attribute. Consequently, seven different amino acid attributes produce a total of 7 × 21 = 147 features.
The AAIndex database contains amino acid indices of various physicochemical and biochemical properties (34). Saha et al. classified these amino acid indices into eight clusters, and the central indices of each cluster were named as high-quality amino acid indices (35): BLAM930101, BIOV880101, MAXF760101, TSAJ990101, NAKH920108, CEDJ970104, LIFS790101, and MIYS990104. We utilize this information, which encodes as a 160 (20 × 8 = 160)−dimensional vectors from the peptide sequence.
Additionally, we averaged eight high-quality amino acid indices (i.e., a 20-dimensional vector) as an input feature. Our preliminary analysis showed that these two feature sets (i.e., 160 and 20) produce similar results. Thus, we use the 20-dimensional vector as the final one to save computational time.
Frequencies of the following features are directly computed from the sequence consisting of: (1) hydrophobic (i.e., F, I, W, L, V, M, Y, C, and A); (2) hydrophilic (i.e., R, K, N, D, E, and P); (3) neutral (i.e., T, H, G, S, and Q); (4) positively charged (i.e., K, H, and R); (5) negative-charged (i.e., D and E); (6) turn-forming residues fraction [i.e., (N + G + P + S)/n, where n = sequence length]; (7) absolute charge per residue (i.e., ); (8) molecular weight; and (9) aliphatic index [i.e., (A + 2.9V + 3.9I + 3.9L)/n].
All of the above feature vectors are normalized in the range of 0–1, according to the formula described in our previous study (36).
In this study, three ensemble models are proposed using three different ML algorithms, including RF (37), SVM (38), and ERT (39), implemented per the Scikit-Learn package (v0.18) (40). A brief description of each algorithm and how it is used in this study follows.
Support vector machine is used to develop both classification and regression models based on the principle of structural risk minimization, which has been successfully applied in many bioinformatics fields (41–44). SVM maps the input features into a high-dimensional feature space and then determines the optimal separating hyperplane between two classes. In our study, a Gaussian radial-basis function (RBF) is used to obtain the classification hyperplane. An RBF-SVM requires the optimization of two critical parameters: C and γ. C controls the trade-off between correct classification and a large margin and γ controls how fast RBF similarity vanishes with growing Euclidean distance between vectors. Therefore, a grid search is conducted in the following ranges: C from 2−15 to 210 and γ from 2−10 to 210 in log2-scale, conducted to tune the SVM parameters (i.e., C and γ).
Random forest is one of the most successful ML method that utilizes hundreds or thousands of independent decision trees to perform classification and regression (37), which has been widely used in bioinformatics (36, 45, 46). RF combined the concept of bagging and random feature selection. For a given training data set (D), generate a new training data set (Di) by drawing N bootstrapped samples from D uniformly and with replacement, which is called as bootstrap sample. Grow a tree using Di repeat the following steps at each node of the tree until its fully grown (i) select mtry random features from the original features and select the best variable by optimizing the impurity criteria, and (ii) split the node into two child nodes. The tree grows until the number of data in the node smaller than the given threshold (nsplit). Repeating the above-mentioned steps to build a large number (ntree) of classification trees. To classify a test data, input features are passed through from the root to end node of each tree based on the predetermined splits. The majority of the class from the forest is considered as the final one. The three most influential parameters are ntree, mtry, and nsplit. A grid search range is given in Table S1 in Supplementary Material, optimized using a 5-fold cross-validation.
Extremely randomized trees belong to another class of ensemble methods widely used for developing classification and regression models (39). ERT aim to further reduce the variance of the prediction model by adding stronger randomization technique. The ERT algorithm is similar to the RF method. Specifically, ERT uses the whole dataset instead of bootstrap sample used in RF, but the trees are generated randomly. The random selection at each node reduces the tree construction time as fewer tests are performed to search for the best split. Furthermore, the parameter optimization procedure in ERT is the same as that in the RF method.
Predictions were classified into four groups: true positive (TP) is the number of PIPs correctly predicted as PIPs; true negative is the number of non-PIPs correctly predicted as non-PIPs; false positive (FP) is the number of non-PIPs wrongly predicted as PIPs; and false negative is the number of PIPs wrongly predicted as non-PIPs. To measure prediction quality, we used the following five metrics: sensitivity, specificity, accuracy, the Matthews’ correlation coefficient (MCC), and the area under receiver operating characteristics (ROC) curve. All these metrics are commonly used in the literature to measure the binary classification (47–50):
AUC is the area under the ROC curve, representing the relationship between TP rate and FP rate of the model. The AUC is an indicator of the performance quality of the binary classifier. The AUC value of 0.5 is equivalent to random prediction, but, an AUC value of 1 represents perfection.
There are three kinds of cross-validations (CVs): k-fold CV, jackknife CV, and independent dataset (51) are often used to evaluate the anticipated success rate of a predictor. Among these three approaches, jackknife test is deemed the least arbitrary and most objective one as demonstrated by Eqs 28–32 of Ref. (52), and hence has been widely used in bioinformatics because it could produce unique outcome (43, 53–62). However, it is time- and source-consuming. Thus, in this paper, we used 5-fold CV to examine the proposed models, where benchmarking data set is randomly divided into five parts, from which four parts were used for training, and the fifth part was used for testing. This process was repeated until all the parts were used at least once as a test set, and the overall performance with all five parts was evaluated.
We performed compositional analysis using the combined dataset (i.e., benchmarking and independent). AAC analysis revealed that average composition of certain residues, including Arg and Leu, were dominant in PIPs. However, Gly, Asp, and Pro were dominant in non-PIPs (Welch’s t-test; P ≤ 0.01) (Figure 2A). Furthermore, DPC analysis revealed that 21% of dipeptides differed significantly between PIPs and non-PIPs (Welch’s t-test; P ≤ 0.01). Of these, the top-10 most abundant dipeptides in PIPs and non-PIPs were FF, SL, SR, SF, SV, LL, LI, RT, RA, and RM and GP, GE, GD, YK, YY, KG, DG, DD, DV, and PG, respectively (Figure 2B). These results suggest that the most abundant dipeptides in PIPs consist primarily of pairs of aliphatic–aliphatic, positively charged-aliphatic, and hydroxyl group-aliphatic or -aromatic amino acids. However, the most abundant dipeptides in the non-PIPs were negatively charged-negatively charged, small-positive or -negatively charged, and aromatic-aromatic or -positively charged amino acids. Overall, significant differences observed in compositional analysis could be integrated into ML algorithms to improve prediction performances. Thus, we considered them as input features.
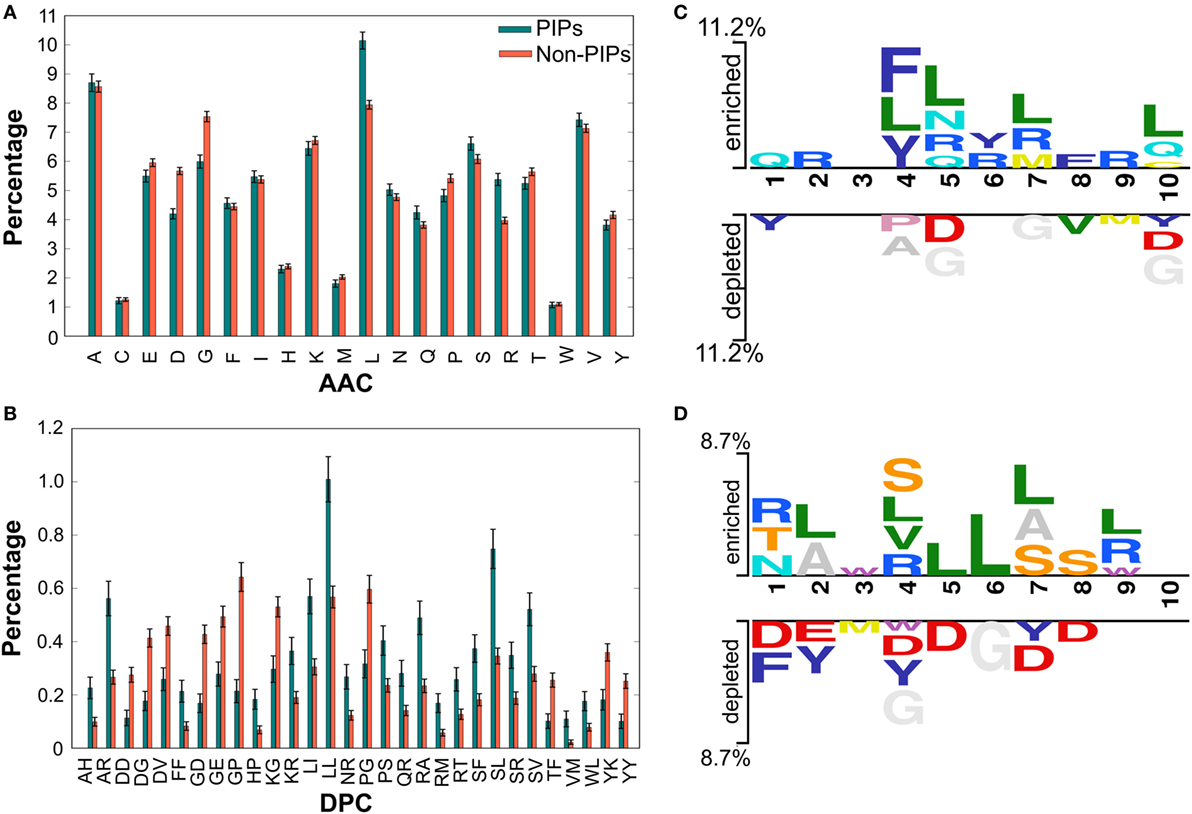
Figure 2. Compositional and positional preference analysis. (A,B) Respectively represent the amino acid and dipeptide preferences between proinflammatory inducing peptides (PIPs) and non-PIPs. In (B) significantly different top-30 dipeptides are shown. (C,D) Represent positional conservation of 10 residues at N- and C-terminal between PIPs and non-PIPs, respectively, generated using two sample logos.
To understand the positional information of each residue, a sequence logo of the first 10 residues from the N- and the C-terminal of PIPs and non-PIPs were generated using two sample logos.2 To test their statistical significance, the height of the peptide logos were scaled (t-test by P < 0.05). At the N-terminal, we found that, compared to other amino acids, R, at positions 2, 5, 6, 7, and 9; L, at positions 4, 5, 7, and 10; and Q, at positions 1, 5, and 10 were significantly overrepresented. Alternatively, negatively charged residue D, at positions 5 and 10 and G, at positions 5, 7, and 10 were significantly underrepresented (Figure 2C). No significant amino acids were found at enriched position 3 or the depleted positions 2, 3, and 6. C-terminal R, at positions 1, 4, and 9; L, at positions 2, 4, 5, 6, 7, and 9; and S/T, at positions 1, 4, 7, and 8 were significantly overrepresented. Alternatively, negatively charged residues D/E, at positions 1, 2, 4, 5, 7, and 8 and Y, at positions 2, 4, and 7 were significantly underrepresented (Figure 2D). No significant amino acids were found at enriched position 10 or the depleted positions 9 and 10. These results suggest that comparatively residues, R and L, are preferred in PIPs. This is consistent with the AAC analysis observation. Furthermore, positional preference analysis will be helpful for experimenters who design de novo PIPs and substitute amino acids at particular positions to make the peptides more effective.
We employed the RF method to construct an ensemble predictor, called PIP-EL. A framework for the construction of PIP-EL is shown in Figure 1. Note that our benchmarking dataset was imbalanced (i.e., 666 PIPs and 1,916 non-PIPs). Thus, it needed special treatment while developing the prediction models. Although several solutions for the imbalanced problem has been proposed in the literature (63, 64), we considered the most straightforward random under-sampling technique, where the majority class was subjected to random sampling, that was equal to the minority class in each subset. Here, we generated 10 different balanced datasets (i.e., B1–10) with the ratio of 1:1, or 663 PIPs:663 non-PIPs, randomly selected from the original. This step ensured that each sample from the majority class was used at least once. For a given feature set (e.g., AAC), we carried out a 5-fold CV grid search to optimize parameters (see Table S1 in Supplementary Material). However, other hyper-parameters remained at their default value. Considering that one-time 5-fold CV with random portioning might produce biased ML parameters, we repeated 5-fold CV 10 more times and considered median ML parameters as the optimized value. This was utilized to develop a final prediction model. This CV procedure applied to B1–10 and resulted in 10 models (i.e., RF1-10) for each composition.
Ensemble learning can be formed by fusing an array of independent models via voting or averaging the outcome of independent predictions. Whereas this approach is computationally expensive, it has been shown to produce more accurate and robust results than constituent models. This approach has been successfully applied in various bioinformatics applications (65–68). In this study, we generated an ensemble predictor for PIPs, as follows:
RFM refers to the RF model. The ensemble predictor, RFE, contained 5 composition-based × 10 balanced models = 50 models. ∀ denotes the fusing operator. After fusion, we optimized the average probability cut-off value with respect to MCC using grid search to define the class (PIPs or non-PIPs). The cut-off of 0.45 produced the best performance, hence we fixed this as an optimal cut-off value. Thus,
Finally, PIP-EL was composed of 50 prediction models, and each classifier used their own optimal parameters.
In addition to PIP-EL, we also developed five different ensemble models by fusing various combinations of five composition-based models. PIP-EL produced the best performance among them (data not shown). Thus, we considered it as the final model. To demonstrate the performance of PIP-EL, we compared it with five composition-based models (i.e., AAC, DPC, CTD, PCP, and AAI) and the hybrid classifier (i.e., H: combination of five composition as the input feature to the RF) with the benchmarking dataset. Figure 3 shows that PIP-EL performed consistently better than other models, both in terms of MCC and accuracy. The average values of these metrics, with SD, are shown in Table 1, showing that PIP-EL achieved values of 0.435 and 0.717 for MCC and accuracy, respectively. Indeed, the corresponding metrics were ~2–11 and ~1–6% higher than those achieved by individual compositions, indicating superiority of PIP-EL. According to P-value <0.05, PIP-EL performed better than AAC, DPC, AAI, and H. It performed significantly better than CTD- and PCP-based models. Moreover, PIP-EL has an advantage over other composition-based models because it covers various angles of sequence information.
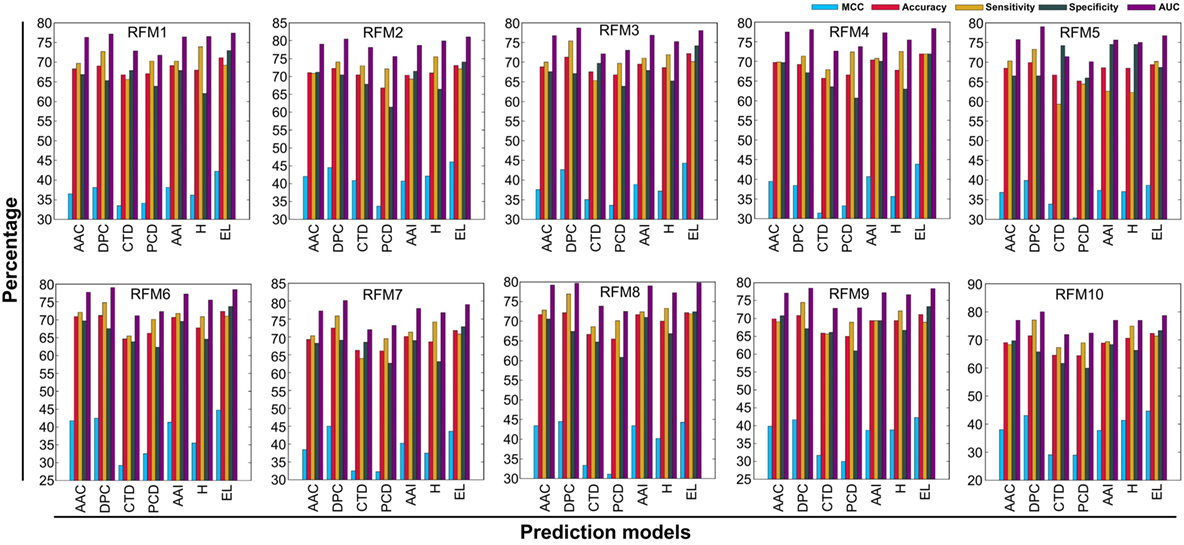
Figure 3. Performance of five composition-based models, hybrid model, and ensemble model on 10 alternatively balanced datasets. X- and Y-axes, respectively, correspond to prediction models and performance (%). RFMX (i.e., X = 1–10) represents RFM from balanced dataset X.
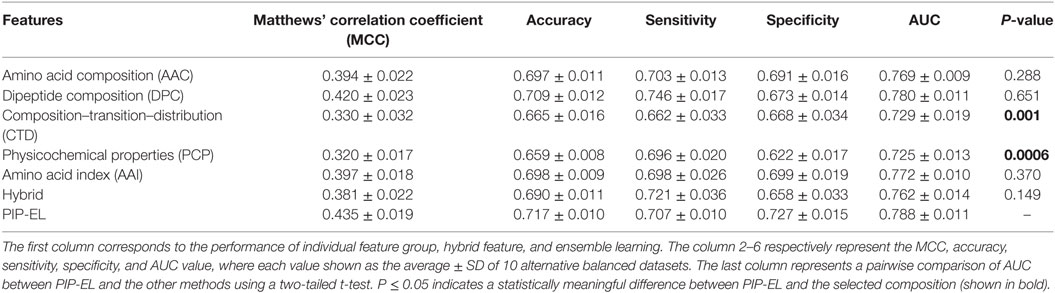
Table 1. Performance comparison of random forest (RF)-based ensemble method with RF-based other classifiers on benchmarking dataset.
Generally, it is quite difficult to choose a suitable ML method for a given problem because of the problem-specific nature of the ML algorithms. Hence, it is essential to explore the performance of different ML methods while using the same benchmarking dataset and selecting the best one, instead of selecting method arbitrarily. In addition to PIP-EL, we developed an ensemble model using ERT and SVM. Here, the procedure of ML parameter optimization for the other two methods and the construction of ensemble models were the same as PIP-EL. Surprisingly, ERT and SVM exhibited their best performances using the ensemble model (Tables 2 and 3) when compared to their individual composition and hybrid models.
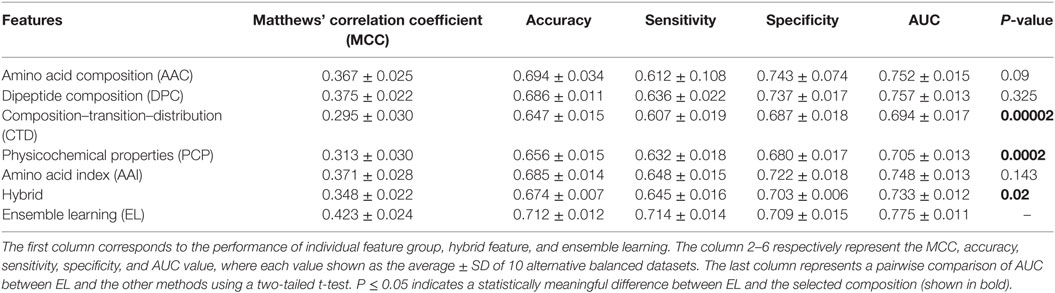
Table 2. Performance comparison of extremely randomized trees (ERT)-based ensemble method with ERT-based other classifiers on benchmarking dataset.
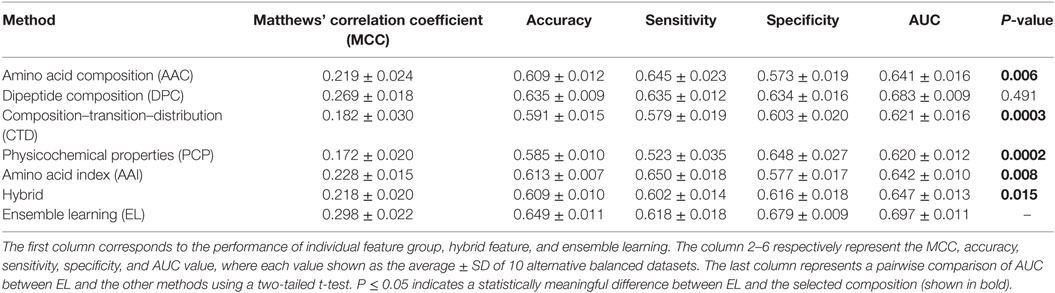
Table 3. Performance comparison of support vector machine (SVM)-based ensemble method with SVM-based other classifiers on benchmarking dataset.
Next, we compared the performance PIP-EL with other methods; results are shown in Table 4, where methods are ranked per MCC. This is regarded as one of the best measures in the classification. From Table 4, it is difficult to discriminate the best performance between PIP-EL and ERT, both in terms of accuracy and MCC. However, according to the P-value threshold of <0.05, PIP-EL was marginally better than ERT and significantly better than the SVM method, demonstrating that decision tree-based algorithms are more suitable for PIP prediction.
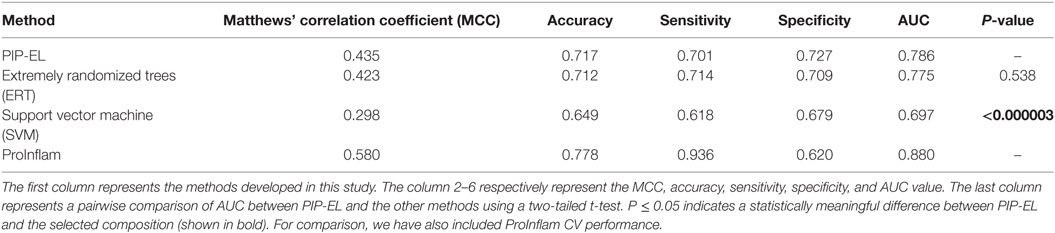
Table 4. Performance comparison of PIP-EL with other machine learning-based methods on the same benchmarking dataset.
For comparison, we also included ProInflam CV performance, using an imbalanced dataset, reported in Ref. (6). Although it is not intuitive to compare the performance between ProInflam and other methods developed in this study, owing to the variation in the benchmarking dataset, between the sensitivity and specificity [i.e., ΔS = absolute (Sensitivity − Specificity)] between these methods. Here, a smaller value of S is considered more balanced performance. Results show that PIP-EL prediction was more balanced with a ΔS value of 3%, whereas the corresponding value of ProInflam was 26%. This clearly indicates that our approach resulted in balanced performance.
In addition to PIP-EL, SVM and ERT, we also generated their corresponding models using an imbalanced dataset. The balanced dataset contained 50 models for each predictor, whereas the imbalanced dataset contained only five models for each predictor. As expected, the performances of the imbalanced dataset-based models were marginally better than the balanced dataset-based models, in terms of MCC and accuracy (Figure 4). However, in terms of more balanced performance (i.e., ΔS), the balanced dataset-based models (i.e., PIP-EL, SVM, and ERT) produced an average ΔS value of 3%, whereas the corresponding metrics in the unbalanced dataset-based models was 32%, indicating that the unbalanced dataset-based models produced biased predictions and misleading accuracies. This analysis clearly shows the importance of handling an imbalanced dataset during prediction model development, regardless of the ML algorithms.
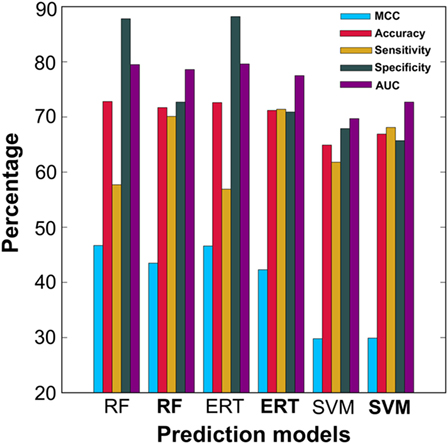
Figure 4. Performance comparison of ensemble models developed using balanced dataset and unbalanced dataset. In the x-axis, normal font and bold respectively represent the ensemble model developed using unbalanced and balanced datasets.
To assess the generalization of the models and their ability to perform with unseen data, we evaluated the performances of our three methods with that of the state-of-the-art method (i.e., ProInflam) with an independent dataset. Table 5 shows that PIP-EL achieved values of 0.454 and 0.748 for MCC and accuracy, respectively. Indeed, the corresponding metrics were ~2–35 and ~1–21%, higher than those achieved by other methods, indicating superiority of PIP-EL. Interestingly, PIP-EL performed consistently well, both with benchmarking and on an independent dataset, suggesting its ability to do well with unseen peptides when compared to other ML-based models developed during this study. According to the P-value <0.05, PIP-EL performed better than ERT and significantly better than SVM and ProInflam (Figure 5).
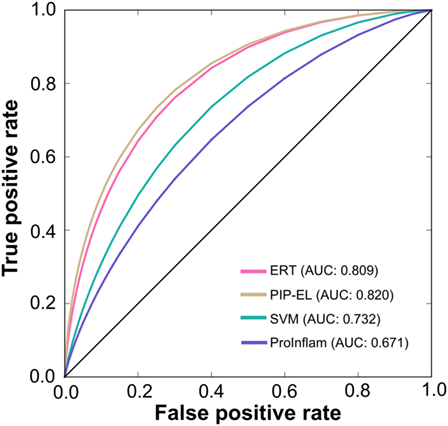
Figure 5. A graphical illustration to show the performance of the method by which receiver operating characteristics curves are obtained from the independent test. X- and Y-axes, respectively, correspond to the false positive and true positive rates.
Establishing free webservers (69–74) or database (75–77) will provide more convenience for most of the wet-experiment scholars. Several instances of bioinformatics web servers utilized for protein function prediction have been reported (3, 78–83) and are of great practical use to the scientific community. Therefore, the online prediction server for PIP-EL was developed.3 All datasets used in this study can be downloaded from our web server. PIP-EL represents the second publicly available method for PIP prediction and delivers a higher level of accuracy than ProInflam.
Identifying the epitopes or peptides that induce proinflammatory responses is one of the most challenging tasks of vaccine design; it is of great importance in immunology and peptide therapeutics. The computational identification of PIPs from a given primary sequence remains one of the most challenging problems for immunoinformaticians and computational biologists. In this study, we presented novel software, PIP-EL, which allowed us to predict whether a given peptide induced proinflammatory cytokines, based on the features derived from a set of experimentally validated PIPs and non-PIPs.
First, we constructed an nr dataset of experimentally validated PIPs and non-PIPs extracted from the IEDB, whose size was ~4-fold bigger than the dataset used in the state-of-the-art method (i.e., ProInflam). Interestingly, our nr dataset contained nine proinflammatory cytokines (i.e., IL1α, IL1β, TNFα, IL6, IL8, IL12, IL17, IL18, and IL23), including six used in ProInflam. Compositional and positional preference analyses revealed that Leu and Arg is highly abundant in PIPs, compared to non-PIPs. Previous studies showed that Leu-rich and Arg-rich peptides play an important role in inducing pro-inflammatory cytokines in different autoimmune diseases (84–87) and collage-induced arthritis (88), respectively. Furthermore, determining the biological significance of various dipeptides in proinflammatory induction, observed in our study, requires further studies and experimental validation.
We explored various ML algorithms (i.e., RF, ERT, and SVM) to build models for predicting PIPs. Furthermore, we used a wide range of compositional features for discriminating PIPs and non-PIPs. Note that all five compositions and ML algorithms were used in various sequence-based classification techniques (30, 42, 43, 66, 67, 89–95). However, only two compositions (i.e., AAC and DPC) and SVM were used with previous PIP prediction (6). Generally, ML algorithms produce bias predictions and misleading accuracies when dealing with an imbalanced dataset (63). Although several solutions for the imbalanced problem have been proposed in the literature (63, 64), we chose the most straightforward random under-sampling technique. Finally, an EL approach, called PIP-EL, was developed by fusing an array of 50 RFMs (see Results), which is computationally expensive and has been shown to produce more accurate and robust results, compared to individual composition-based or hybrid models. Although this approach has been successfully applied in various bioinformatics applications (65–67), this is the first instance that this approach has been applied to PIP predictions. Interestingly, PIP-EL performances, both on benchmarking and independent datasets, were more balanced, with an average ΔS of 4%, whose difference is ~9-fold bigger (i.e., 36%) in ProInflam. This is because the authors used an imbalanced dataset for prediction model development, indicating the importance of special handling for the imbalanced dataset during prediction model development.
PIP-EL performed better than the other two methods developed in this study. It performed significantly better than the existing method when objectively evaluated on an independent dataset. The improved performance of PIP-EL was primarily caused by the larger size of benchmarking dataset, random sampling technique followed by EL, rigorous optimisation procedure to select final ML parameters, and the choice of ML method. In future work, it will be beneficial to identify novel contributions that can be used in combination with the current feature set to further improve prediction performance.
Our proposed method is very promising for PIP prediction. Thus, a user-friendly web interface was made available, allowing researchers access to our prediction method. Although, PIP-EL represents the second publicly available method for predicting PIPs, the delivery of higher accuracy is remarkable. Compared to experimental approaches, bioinformatics methods (e.g., PIP-EL) represent a powerful and cost-effective approach to the proteome-wide prediction of PIPs. Therefore, PIP-EL should be useful for large-scale PIP prediction, facilitating hypothesis-driven experimental design.
The authors declare that there are no ethics problem.
Conceived and designed the experiments: BM and GL. Performed the experiments: BM. Analyzed the data: BM and TS. Contributed reagents/materials/software tools: GL and MK. Wrote paper: BM and GL.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Da Yeon Lee for assistance in manuscript preparation. We would also like to thank Dr. Jin Young Kim and Dr. Ju Yeon Lee (Korea Basic Science Institute, Ochang Headquarter, Division of Bioconvergence Analysis) for the nano LC-LTQ-Orbitrap analysis.
This work was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science, and Technology (2018R1D1A1B07049572 and 2009-0093826) and ICT & Future Planning (2016M3C7A1904392).
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01783/full#supplementary-material.
1. Ansar W, Ghosh S. C-reactive protein and the biology of disease. Immunol Res (2013) 56:131–42. doi:10.1007/s12026-013-8384-0
2. Manavalan B, Basith S, Choi S. Similar structures but different roles–an updated perspective on TLR structures. Front Physiol (2011) 2:41. doi:10.3389/fphys.2011.00041
3. Basith S, Manavalan B, Govindaraj RG, Choi S. In silico approach to inhibition of signaling pathways of toll-like receptors 2 and 4 by ST2L. PLoS One (2011) 6:e23989. doi:10.1371/journal.pone.0023989
4. Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today (2015) 20:122–8. doi:10.1016/j.drudis.2014.10.003
5. Holzer P. Proinflammatory and antiinflammatory peptides. Trends Pharmacol Sci (1998) 19:516–7. doi:10.1016/S0165-6147(98)01256-5
6. Gupta S, Madhu MK, Sharma AK, Sharma VK. ProInflam: a webserver for the prediction of proinflammatory antigenicity of peptides and proteins. J Transl Med (2016) 14:178. doi:10.1186/s12967-016-0928-3
7. Gupta S, Mittal P, Madhu MK, Sharma VK. IL17eScan: a tool for the identification of peptides inducing IL-17 response. Front Immunol (2017) 8:1430. doi:10.3389/fimmu.2017.01430
8. Bylund J, Christophe T, Boulay F, Nystrom T, Karlsson A, Dahlgren C. Proinflammatory activity of a cecropin-like antibacterial peptide from Helicobacter pylori. Antimicrob Agents Chemother (2001) 45:1700–4. doi:10.1128/AAC.45.6.1700-1704.2001
9. Maurer T, Pournaras C, Aguilar-Pimentel JA, Thalgott M, Horn T, Heck M, et al. Immunostimulatory CpG-DNA and PSA-peptide vaccination elicits profound cytotoxic T cell responses. Urol Oncol (2013) 31:1395–401. doi:10.1016/j.urolonc.2011.09.002
10. Bjorstad A, Fu H, Karlsson A, Dahlgren C, Bylund J. Interleukin-8-derived peptide has antibacterial activity. Antimicrob Agents Chemother (2005) 49:3889–95. doi:10.1128/AAC.49.9.3889-3895.2005
11. Chen X, Takai T, Xie Y, Niyonsaba F, Okumura K, Ogawa H. Human antimicrobial peptide LL-37 modulates proinflammatory responses induced by cytokine milieus and double-stranded RNA in human keratinocytes. Biochem Biophys Res Commun (2013) 433:532–7. doi:10.1016/j.bbrc.2013.03.024
12. Bellner L, Thorén F, Nygren E, Liljeqvist J-Å, Karlsson A, Eriksson K. A proinflammatory peptide from herpes simplex virus type 2 glycoprotein G affects neutrophil, monocyte, and NK cell functions. J Immunol (2005) 174:2235–41. doi:10.4049/jimmunol.174.4.2235
13. Fleri W, Paul S, Dhanda SK, Mahajan S, Xu X, Peters B, et al. The immune epitope database and analysis resource in epitope discovery and synthetic vaccine design. Front Immunol (2017) 8:278. doi:10.3389/fimmu.2017.00278
14. Dhanda SK, Gupta S, Vir P, Raghava GP. Prediction of IL4 inducing peptides. Clin Dev Immunol (2013) 2013:263952. doi:10.1155/2013/263952
15. Nagpal G, Usmani SS, Dhanda SK, Kaur H, Singh S, Sharma M, et al. Computer-aided designing of immunosuppressive peptides based on IL-10 inducing potential. Sci Rep (2017) 7:42851. doi:10.1038/srep42851
16. Gupta S, Sharma AK, Shastri V, Madhu MK, Sharma VK. Prediction of anti-inflammatory proteins/peptides: an insilico approach. J Transl Med (2017) 15:7. doi:10.1186/s12967-016-1103-6
17. Bhasin M, Raghava GP. A hybrid approach for predicting promiscuous MHC class I restricted T cell epitopes. J Biosci (2007) 32:31–42. doi:10.1007/s12038-007-0004-5
18. Bhasin M, Raghava GP. Prediction of CTL epitopes using QM, SVM and ANN techniques. Vaccine (2004) 22:3195–204. doi:10.1016/j.vaccine.2004.02.005
19. Gupta S, Ansari HR, Gautam A; Open Source Drug Discovery Consortium, Raghava GP. Identification of B-cell epitopes in an antigen for inducing specific class of antibodies. Biol Direct (2013) 8:27. doi:10.1186/1745-6150-8-27
20. Saha S, Raghava GP. AlgPred: prediction of allergenic proteins and mapping of IgE epitopes. Nucleic Acids Res (2006) 34:W202–9. doi:10.1093/nar/gkl343
21. Dimitrov I, Bangov I, Flower DR, Doytchinova I. AllerTOP v.2 – a server for in silico prediction of allergens. J Mol Model (2014) 20:2278. doi:10.1007/s00894-014-2278-5
22. Dimitrov I, Flower DR, Doytchinova I. AllerTOP – a server for in silico prediction of allergens. BMC Bioinformatics (2013) 14(Suppl 6):S4. doi:10.1186/1471-2105-14-S6-S4
23. Dhanda SK, Vir P, Raghava GP. Designing of interferon-gamma inducing MHC class-II binders. Biol Direct (2013) 8:30. doi:10.1186/1745-6150-8-30
24. Fleri W, Vaughan K, Salimi N, Vita R, Peters B, Sette A. The immune epitope database: how data are entered and retrieved. J Immunol Res (2017) 2017:5974574. doi:10.1155/2017/5974574
25. Vita R, Overton JA, Greenbaum JA, Ponomarenko J, Clark JD, Cantrell JR, et al. The immune epitope database (IEDB) 3.0. Nucleic Acids Res (2015) 43:D405–12. doi:10.1093/nar/gku938
26. Chen X-X, Tang H, Li W-C, Wu H, Chen W, Ding H, et al. Identification of bacterial cell wall lyases via pseudo amino acid composition. Biomed Res Int (2016) 2016:1654623. doi:10.1155/2016/1654623
27. Ding H, Feng P-M, Chen W, Lin H. Identification of bacteriophage virion proteins by the ANOVA feature selection and analysis. Mol Biosyst (2014) 10:2229–35. doi:10.1039/c4mb00316k
28. Dubchak I, Muchnik I, Holbrook SR, Kim SH. Prediction of protein folding class using global description of amino acid sequence. Proc Natl Acad Sci U S A (1995) 92:8700–4. doi:10.1073/pnas.92.19.8700
29. Hasan MM, Guo D, Kurata H. Computational identification of protein S-sulfenylation sites by incorporating the multiple sequence features information. Mol Biosyst (2017) 13:2545–50. doi:10.1039/c7mb00491e
30. Wang X, Yan R, Li J, Song J. SOHPRED: a new bioinformatics tool for the characterization and prediction of human S-sulfenylation sites. Mol Biosyst (2016) 12:2849–58. doi:10.1039/c6mb00314a
31. Magnan CN, Randall A, Baldi P. SOLpro: accurate sequence-based prediction of protein solubility. Bioinformatics (2009) 25:2200–7. doi:10.1093/bioinformatics/btp386
32. Wang H, Feng L, Zhang Z, Webb GI, Lin D, Song J. Crysalis: an integrated server for computational analysis and design of protein crystallization. Sci Rep (2016) 6:21383. doi:10.1038/srep21383
33. Cai CZ, Han LY, Ji ZL, Chen X, Chen YZ. SVM-Prot: web-based support vector machine software for functional classification of a protein from its primary sequence. Nucleic Acids Res (2003) 31:3692–7. doi:10.1093/nar/gkg600
34. Kawashima S, Pokarowski P, Pokarowska M, Kolinski A, Katayama T, Kanehisa M. AAindex: amino acid index database, progress report 2008. Nucleic Acids Res (2008) 36:D202–5. doi:10.1093/nar/gkm998
35. Saha I, Maulik U, Bandyopadhyay S, Plewczynski D. Fuzzy clustering of physicochemical and biochemical properties of amino acids. Amino Acids (2012) 43:583–94. doi:10.1007/s00726-011-1106-9
36. Manavalan B, Lee J, Lee J. Random forest-based protein model quality assessment (RFMQA) using structural features and potential energy terms. PLoS One (2014) 9:e106542. doi:10.1371/journal.pone.0106542
38. Chang C-C, Lin C-J. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol (2011) 2:27. doi:10.1145/1961189.1961199
39. Geurts P, Ernst D, Wehenkel L. Extremely randomized trees. Mach Learn (2006) 63:3–42. doi:10.1007/s10994-006-6226-1
40. Abraham A, Pedregosa F, Eickenberg M, Gervais P, Mueller A, Kossaifi J, et al. Machine learning for neuroimaging with scikit-learn. Front Neuroinform (2014) 8:14. doi:10.3389/fninf.2014.00014
41. Manavalan B, Kuwajima K, Joung I, Lee J. Structure-based protein folding type classification and folding rate prediction. 2015 IEEE International Conference on Bioinformatics and Biomedicine (BIBM). Washington: IEEE (2015). p. 1759–61.
42. Manavalan B, Lee J. SVMQA: support-vector-machine-based protein single-model quality assessment. Bioinformatics (2017) 33(16):2496–503. doi:10.1093/bioinformatics/btx222
43. Chen W, Yang H, Feng P, Ding H, Lin H. iDNA4mC: identifying DNA N4-methylcytosine sites based on nucleotide chemical properties. Bioinformatics (2017) 33:3518–23. doi:10.1093/bioinformatics/btx479
44. Cao R, Wang Z, Wang Y, Cheng J. SMOQ: a tool for predicting the absolute residue-specific quality of a single protein model with support vector machines. BMC Bioinformatics (2014) 15:120. doi:10.1186/1471-2105-15-120
45. Lee J, Gross SP, Lee J. Improved network community structure improves function prediction. Sci Rep (2013) 3:2197. doi:10.1038/srep02197
46. Lee J, Lee K, Joung I, Joo K, Brooks BR, Lee J. Sigma-RF: prediction of the variability of spatial restraints in template-based modeling by random forest. BMC Bioinformatics (2015) 16:94. doi:10.1186/s12859-015-0526-z
47. Chen W, Feng PM, Deng EZ, Lin H, Chou KC. iTIS-PseTNC: a sequence-based predictor for identifying translation initiation site in human genes using pseudo trinucleotide composition. Anal Biochem (2014) 462:76–83. doi:10.1016/j.ab.2014.06.022
48. Chen W, Feng PM, Lin H, Chou KC. iSS-PseDNC: identifying splicing sites using pseudo dinucleotide composition. Biomed Res Int (2014) 2014:623149. doi:10.1155/2014/623149
49. Chen W, Feng P, Ding H, Lin H, Chou KC. iRNA-methyl: identifying N(6)-methyladenosine sites using pseudo nucleotide composition. Anal Biochem (2015) 490:26–33. doi:10.1016/j.ab.2015.08.021
50. Chen W, Feng P, Yang H, Ding H, Lin H, Chou KC. iRNA-3typeA: identifying three types of modification at RNA’s adenosine sites. Mol Ther Nucleic Acids (2018) 11:468–74. doi:10.1016/j.omtn.2018.03.012
51. Dao FY, Yang H, Su ZD, Yang W, Wu Y, Hui D, et al. Recent advances in conotoxin classification by using machine learning methods. Molecules (2017) 22(7):E1057. doi:10.3390/molecules22071057
52. Chou K-C. Some remarks on protein attribute prediction and pseudo amino acid composition. J Theor Biol (2011) 273:236–47. doi:10.1016/j.jtbi.2010
53. Lin H, Ding C, Song Q, Yang P, Ding H, Deng KJ, et al. The prediction of protein structural class using averaged chemical shifts. J Biomol Struct Dyn (2012) 29:643–9. doi:10.1080/07391102.2011.672628
54. Chen W, Feng PM, Lin H, Chou KC. iRSpot-PseDNC: identify recombination spots with pseudo dinucleotide composition. Nucleic Acids Res (2013) 41:e68. doi:10.1093/nar/gks1450
55. Feng PM, Chen W, Lin H, Chou KC. iHSP-PseRAAAC: identifying the heat shock protein families using pseudo reduced amino acid alphabet composition. Anal Biochem (2013) 442:118–25. doi:10.1016/j.ab.2013.05.024
56. Chen W, Feng P, Tang H, Ding H, Lin H. Identifying 2’-O-methylationation sites by integrating nucleotide chemical properties and nucleotide compositions. Genomics (2016) 107:255–8. doi:10.1016/j.ygeno.2016.05.003
57. Chen W, Tang H, Ye J, Lin H, Chou KC. iRNA-PseU: identifying RNA pseudouridine sites. Mol Ther Nucleic Acids (2016) 5:e332. doi:10.1038/mtna.2016.37
58. Yang H, Tang H, Chen XX, Zhang CJ, Zhu PP, Ding H, et al. Identification of secretory proteins in Mycobacterium tuberculosis using pseudo amino acid composition. Biomed Res Int (2016) 2016:5413903. doi:10.1155/2016/5413903
59. Chen W, Xing P, Zou Q. Detecting N 6-methyladenosine sites from RNA transcriptomes using ensemble support vector machines. Sci Rep (2017) 7:40242. doi:10.1038/srep40242
60. Lai HY, Chen XX, Chen W, Tang H, Lin H. Sequence-based predictive modeling to identify cancerlectins. Oncotarget (2017) 8:28169–75. doi:10.18632/oncotarget.15963
61. Lin H, Liang ZY, Tang H, Chen W. Identifying sigma70 promoters with novel pseudo nucleotide composition. IEEE/ACM Trans Comput Biol Bioinform (2017). doi:10.1109/TCBB.2017.2666141
62. Zhao YW, Su ZD, Yang W, Lin H, Chen W, Tang H. IonchanPred 2.0: a tool to predict ion channels and their types. Int J Mol Sci (2017) 18(9):E1838. doi:10.3390/ijms18091838
63. Libbrecht MW, Noble WS. Machine learning applications in genetics and genomics. Nat Rev Genet (2015) 16:321–32. doi:10.1038/nrg3920
64. Kotsiantis S, Kanellopoulos D, Pintelas P. Handling imbalanced datasets: a review. GESTS Int Trans Comput Sci Eng (2006) 30:25–36.
65. Jia J, Liu Z, Xiao X, Liu B, Chou KC. iPPI-Esml: an ensemble classifier for identifying the interactions of proteins by incorporating their physicochemical properties and wavelet transforms into PseAAC. J Theor Biol (2015) 377:47–56. doi:10.1016/j.jtbi.2015.04.011
66. Liu B, Long R, Chou KC. iDHS-EL: identifying DNase I hypersensitive sites by fusing three different modes of pseudo nucleotide composition into an ensemble learning framework. Bioinformatics (2016) 32:2411–8. doi:10.1093/bioinformatics/btw186
67. Zhang L, Ai H, Chen W, Yin Z, Hu H, Zhu J, et al. CarcinoPred-EL: novel models for predicting the carcinogenicity of chemicals using molecular fingerprints and ensemble learning methods. Sci Rep (2017) 7:2118. doi:10.1038/s41598-017-02365-0
68. Cao R, Bhattacharya D, Adhikari B, Li J, Cheng J. Large-scale model quality assessment for improving protein tertiary structure prediction. Bioinformatics (2015) 31:i116–23. doi:10.1093/bioinformatics/btv235
69. Tang H, Zhao Y-W, Zou P, Zhang C-M, Chen R, Huang P, et al. HBPred: a tool to identify growth hormone-binding proteins. Int J Biol Sci (2018) 14:957–64. doi:10.7150/ijbs.24174
70. Yang H, Qiu W-R, Liu G, Guo F-B, Chen W, Chou K-C, et al. iRSpot-Pse6NC: identifying recombination spots in Saccharomyces cerevisiae by incorporating hexamer composition into general PseKNC. Int J Biol Sci (2018) 14(8):883–91. doi:10.7150/ijbs.24616
71. Adhikari B, Bhattacharya D, Cao R, Cheng J. CONFOLD: residue-residue contact-guided ab initio protein folding. Proteins (2015) 83:1436–49. doi:10.1002/prot.24829
72. Bhattacharya D, Nowotny J, Cao R, Cheng J. 3Drefine: an interactive web server for efficient protein structure refinement. Nucleic Acids Res (2016) 44:W406–9. doi:10.1093/nar/gkw336
73. Cao R, Adhikari B, Bhattacharya D, Sun M, Hou J, Cheng J. QAcon: single model quality assessment using protein structural and contact information with machine learning techniques. Bioinformatics (2017) 33:586–8. doi:10.1093/bioinformatics/btw694
74. Cao R, Cheng J. Protein single-model quality assessment by feature-based probability density functions. Sci Rep (2016) 6:23990. doi:10.1038/srep23990
75. Feng P, Ding H, Lin H, Chen W. AOD: the antioxidant protein database. Sci Rep (2017) 7:7449. doi:10.1038/s41598-017-08115-6
76. Liang ZY, Lai HY, Yang H, Zhang CJ, Yang H, Wei HH, et al. Pro54DB: a database for experimentally verified sigma-54 promoters. Bioinformatics (2017) 33:467–9. doi:10.1093/bioinformatics/btw630
77. Zhang T, Tan P, Wang L, Jin N, Li Y, Zhang L, et al. RNALocate: a resource for RNA subcellular localizations. Nucleic Acids Res (2017) 45:D135–8. doi:10.1093/nar/gkw728
78. Basith S, Manavalan B, Gosu V, Choi S. Evolutionary, structural and functional interplay of the IkappaB family members. PLoS One (2013) 8:e54178. doi:10.1371/journal.pone.0054178
79. Govindaraj RG, Manavalan B, Basith S, Choi S. Comparative analysis of species-specific ligand recognition in toll-like receptor 8 signaling: a hypothesis. PLoS One (2011) 6:e25118. doi:10.1371/journal.pone.0025118
80. Govindaraj RG, Manavalan B, Lee G, Choi S. Molecular modeling-based evaluation of hTLR10 and identification of potential ligands in toll-like receptor signaling. PLoS One (2010) 5:e12713. doi:10.1371/journal.pone.0012713
81. Manavalan B, Basith S, Choi YM, Lee G, Choi S. Structure-function relationship of cytoplasmic and nuclear IkappaB proteins: an in silico analysis. PLoS One (2010) 5:e15782. doi:10.1371/journal.pone.0015782
82. Manavalan B, Govindaraj R, Lee G, Choi S. Molecular modeling-based evaluation of dual function of IkappaBzeta ankyrin repeat domain in toll-like receptor signaling. J Mol Recognit (2011) 24:597–607. doi:10.1002/jmr.1085
83. Manavalan B, Murugapiran SK, Lee G, Choi S. Molecular modeling of the reductase domain to elucidate the reaction mechanism of reduction of peptidyl thioester into its corresponding alcohol in non-ribosomal peptide synthetases. BMC Struct Biol (2010) 10:1. doi:10.1186/1472-6807-10-1
84. Lee J-J, Lee S-T, Jung K-H, Chu K, Lee SK. Anti-lGI1 Limbic encephalitis presented with atypical manifestations. Exp Neurobiol (2013) 22:337–40. doi:10.5607/en.2013.22.4.337
85. Zandi MS. Defining and treating leucine-rich glioma inactivated 1 antibody associated autoimmunity. Brain (2013) 136:2933–5. doi:10.1093/brain/awt256
86. Nalbandian A, Crispin J, Tsokos G. Interleukin-17 and systemic lupus erythematosus: current concepts. Clin Exp Immunol (2009) 157:209–15. doi:10.1111/j.1365-2249.2009.03944.x
87. Gris D, Ye Z, Iocca HA, Wen H, Craven RR, Gris P, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol (2010) 185:974–81. doi:10.4049/jimmunol.0904145
88. Yoo S-A, Bae D-G, Ryoo J-W, Kim H-R, Park G-S, Cho C-S, et al. Arginine-rich anti-vascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-α and IL-6 by human monocytes. J Immunol (2005) 174:5846–55. doi:10.4049/jimmunol.174.9.5846
89. Li F, Li C, Wang M, Webb GI, Zhang Y, Whisstock JC, et al. GlycoMine: a machine learning-based approach for predicting N-, C- and O-linked glycosylation in the human proteome. Bioinformatics (2015) 31:1411–9. doi:10.1093/bioinformatics/btu852
90. Manavalan B, Basith S, Shin TH, Choi S, Kim MO, Lee G. MLACP: machine-learning-based prediction of anticancer peptides. Oncotarget (2017) 8:77121–36. doi:10.18632/oncotarget.20365
91. Manavalan B, Shin TH, Lee G. DHSpred: support-vector-machine-based human DNase I hypersensitive sites prediction using the optimal features selected by random forest. Oncotarget (2018) 9(2):1944–56. doi:10.18632/oncotarget.23099
92. Manavalan B, Subramaniyam S, Shin TH, Kim MO, Lee G. Machine-learning-based prediction of cell-penetrating peptides and their uptake efficiency with improved accuracy. J Proteome Res (2018). doi:10.1021/acs.jproteome.8b00148
93. Manavalan B, Shin TH, Lee G. PVP-SVM: sequence-based prediction of phage virion proteins using a support vector machine. Front Microbiol (2018) 9:476. doi:10.3389/fmicb.2018.00476
94. Manavalan B, Shin TH, Kim MO, Lee G. AIPpred: sequence-based prediction of anti-in ammatory peptides using random forest. Front Pharmacol (2018) 9:276. doi:10.3389/fphar.2018.00276
Keywords: proinflammatory peptide, ensemble learning, random forest, machine learning, immunotherapy
Citation: Manavalan B, Shin TH, Kim MO and Lee G (2018) PIP-EL: A New Ensemble Learning Method for Improved Proinflammatory Peptide Predictions. Front. Immunol. 9:1783. doi: 10.3389/fimmu.2018.01783
Received: 08 March 2018; Accepted: 19 July 2018;
Published: 31 July 2018
Edited by:
Fabio Bagnoli, GlaxoSmithKline (Italy), ItalyReviewed by:
Renzhi Cao, Pacific Lutheran University, United StatesCopyright: © 2018 Manavalan, Shin, Kim and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Balachandran Manavalan, YmFsYUBham91LmFjLmty;
Gwang Lee, Z2xlZUBham91LmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.