
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 13 August 2018
Sec. Inflammation
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.01668
This article is part of the Research Topic Immunology of Psoriatic Disease View all 18 articles
 Giuseppina Caiazzo1
Giuseppina Caiazzo1 Gabriella Fabbrocini1
Gabriella Fabbrocini1 Roberta Di Caprio1
Roberta Di Caprio1 Annunziata Raimondo1
Annunziata Raimondo1 Emanuele Scala1
Emanuele Scala1 Nicola Balato1
Nicola Balato1 Anna Balato2*
Anna Balato2*
Nowadays, it is well established a link between psoriasis and cardiovascular (CV) diseases. A series of different overlapping mechanisms including inflammation, homeostasis dysregulation, and genetic susceptibility are thought to underlie this association. Advances in understanding the molecular patterns involved in the complex scenario of psoriasis have highlighted a tight correlation with atherosclerosis. Indeed, common profiles are shared in term of inflammatory cytokines and cell types. In the last decade, the management of psoriasis patients has been revolutionized with the introduction of biological therapies, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-12/23, and IL-17 inhibitors. In clinical setting, the effectiveness of these therapies as well as the incidence of CV events is related to the type of biologics. In particular, anti-TNF-α agents seem to reduce these events in psoriasis patients whereas anti-IL-12/23 agents related CV events reduction still remain to clarify. It has to be taken into account that IL-12/23 inhibitors have a shorter post-marketing surveillance period. An even more restricted observational time is available for anti-IL-17 agents. IL-17 is associated with psoriasis, vascular disease, and inflammation. However, IL-17 role in atherosclerosis is still debated, exerting both pro-atherogenic and anti-atherogenic effects depending on the specific context. In this review, we will discuss the differences between the onset of CV events in psoriasis patients, referred to specific biological therapy and the underlying immunological mechanism. Given the development of new therapeutic strategies, the investigation of these inhibitors impact on heart failure outcome is extremely important.
The relationship between psoriasis (Pso) and an increased incidence of major adverse cardiovascular events (MACEs) has been observed for decades, since McDonald and Calabresi first demonstrated that the risk associated with arterial and vascular diseases was 2.2 times higher in more of 300 hospitalized patients with Pso compared with controls with other dermatological conditions (1). Since then, several studies have confirmed these findings, convincingly proving that patients with Pso have an effective higher risk of developing severe CV events, such as myocardial infarction (MI) and stroke (2). In 2006, using the General Practice Research Database (GPRD) source, Gelfand et al. suggested that Pso is an independent risk factor for acute MI and cardiovascular disease (CVD), particularly in young patients, and that this risk is most significant in patients with severe disease (3). In 2007, Ludwig and colleagues also identified Pso as a possible independent risk factor for CVD development founding a significantly increased prevalence and severity of the CVD indicator coronary artery calcification factor in these patients (4). In addition, increases in the prevalence of other independent “traditional” risk factors for CVD, including smoking, excess alcohol intake, as well as obesity, hypertension, dyslipidemia, and insulin resistance (the common underlying factors of metabolic syndrome), have been also reported in psoriatic patients (5–7). However, despite the evidences, some studies failed to find a significant independent association between Pso and CVD (8, 9). In 2015, using the same population-based GPRD, Parisi et al. conducted a series of multivariable analyses on patients with incident Pso concluding that neither Pso nor severe Pso are associated with a risk of MACE even after adjustment for traditional CVD risk factors (10). To date, the debate is whether or not this link represents a causal relationship or is a predisposition due to the underlying risk factors exhibited by patients with severe Pso (9, 10). The main hypothesis is that chronic inflammation which occurs in Pso is more than skin deep and results in a “psoriatic march” driving systemic mechanisms that are shared with other chronic inflammatory diseases, including atherosclerosis (Figure 1) (11–14). This concept was introduced for the first time in 2011 by Boehncke and colleagues to describe how systemic psoriatic inflammation may lead to insulin resistance as well as endothelial cell dysfunction, causing atherosclerosis, the major pathological change preceding MI and stroke development (15). Indeed, psoriatic patients with altered glucose metabolism and insulin resistance showed an increased arterial stiffness compared with healthy subjects, with a positive correlation between arterial stiffness and Pso disease duration (16, 17). Understanding why Pso may be a risk factor for atherosclerosis requires a basic understanding of their shared pathogenic features. In 2012, Flammer and Ruschitzka proposed the theory of “two plaques for one syndrome” since molecular mechanisms as well as pro-inflammatory cytokine profile of psoriatic lesions are remarkably similar to that of atherosclerosis ones, with a comparable inflammatory infiltrate of T cells, macrophages, and monocytes (18, 19). In addition, both diseases display a common pattern of T-cell activation, with T helper (Th)1 and Th17 cytokine upregulation, as well as increased local and systemic expression of adhesion molecules and endothelins (19). Thus, there are displacements of inflammatory cells between lesional psoriatic skin, peripheral circulation, and atheromatous plaques of coronary vasculature caused by the releasing cytokines derived from the skin and inflammatory mediators derived from Pso lesions into the circulation, together with an upregulation of cell adhesion molecules (20). Moreover, Pso-associated pro-inflammatory cytokines, such as interferon (IFN)-γ, tumor necrosis factor-alpha (TNF-α), and interleukin (IL)-17, have been found increased in atherosclerotic plaques and sera of patients with unstable CVD (21, 22). Similarly, increased expression of well known CV biomarkers, including monocyte chemoattractant protein (MCP)-1 and macrophage-derived chemokines, have been measured in the lesional skin and serum of psoriatic patients suggesting shared inflammatory pathways linking Pso and CVD (23). Recently, Kolliker Frers et al. have demonstrated that pro-atherogenic inflammation marker C-reactive protein (CRP) and soluble intercellular adhesion molecule-1 levels are increased also in psoriatic patients with no CV history or traditional CV risk factors, compared with healthy subjects, as well as in patients with recent-onset PsA, even in the absence of CV risks. These data reinforce the concept that that the degree of atherosclerosis tendency might be related to the amount of the inflammatory psoriatic burden and highlight the importance of primary prevention in Pso also in those psoriatic patients with no history of CV events (24).
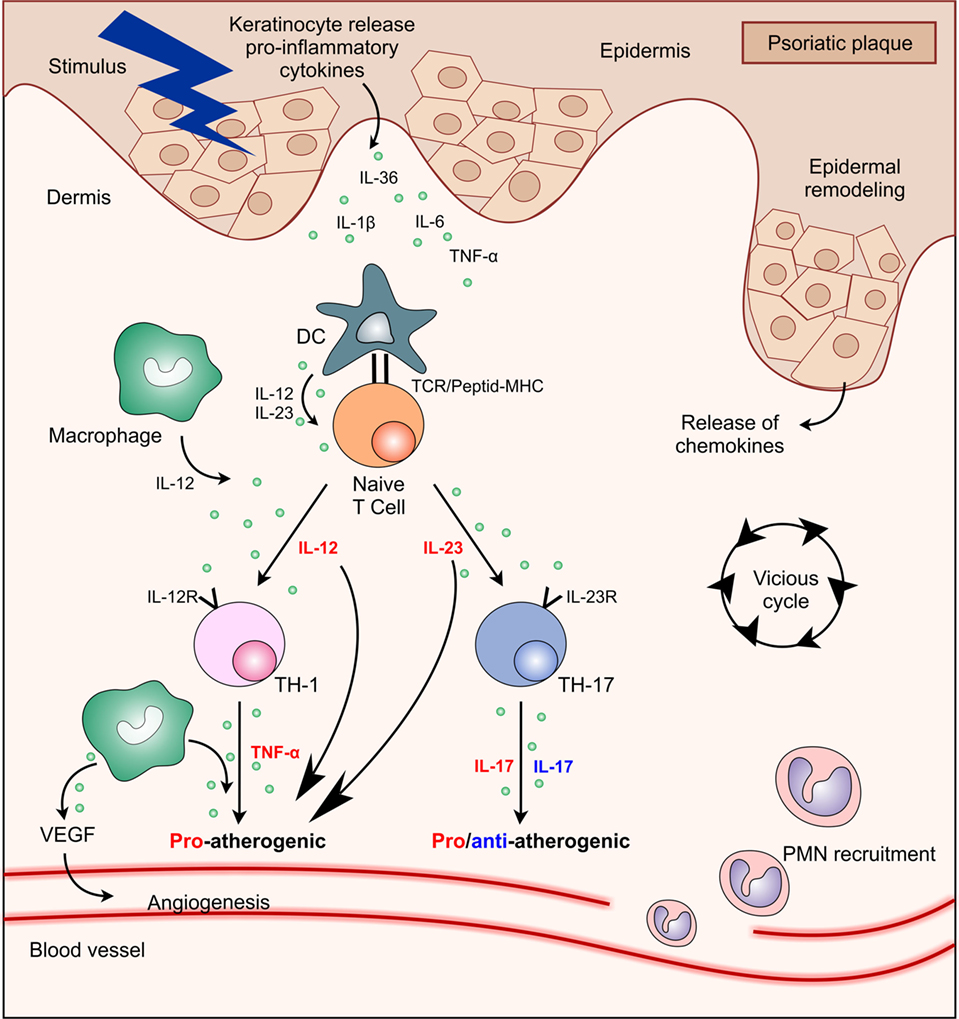
Figure 1. Interactions between main cell types and cytokines present in psoriasis plaque showing their functional significance in atherosclerosis process. Abbreviations: DC, dendritic cell; IL, interleukin; MHC, major histocompatibility complex; PMN, polymorphonuclear; TCR, T cell receptor; Th, T helper; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
The growing body of evidences pointing to increases in MI and CV events in psoriatic patients has raised the question whether treating cutaneous disease might also prevent heart attacks and decrease CVD risk development. Moreover, the postulated hypothesis that inflammatory cascade activated in Pso contributes to the atherosclerotic process has laid the groundwork for supposing that the anti-inflammatory Pso therapy could theoretically improve also atherosclerosis and reduce the risk of MACE (25). Prodanovich et al. first reported that treatment with methotrexate (MTX), especially when used at low doses and in combination with folic acid supplementation, was able to reduce the rate of vascular disease in patients with Pso or rheumatoid arthritis (RA) (26). A subsequent meta-analysis of 10 studies confirmed these data concluding that the use of MTX resulted in 21% lower risk of CVD and 18% lower risk of MI (27). Moreover, data from a large health-care system in USA proved that decreased MI risk in psoriatic patients treated with MTX was greater than those receiving topical therapy (28). In 2013, using a population-based Danish cohort, an observational study found that the risk of MACE development decreased in psoriatic patients in treatment with MTX compared with other non-biological agents, including oral retinoids, cyclosporine, and phototherapy (29). Indeed, treatment with cyclosporine, leading to impaired renal function and hypertension, may negatively affect the MACE profile in these patients by increasing the risk of hypertension and dyslipidemia (30). For that reason, in psoriatic patients, cyclosporine should only be used for a limited period and should be substituted with another systemic agent once the skin condition is improved (20). Similarly, acitretin, the most commonly agent used to treat generalized pustular Pso, it has been associated with an increased risk of hypertension, hyperlipidemia, and CVD even if several investigations have shown that retinoids also improve and ameliorate the formation of atherosclerotic plaques (31, 32). Moreover, Boehncke et al. found that also treatment with fumaric acid esters resulted in an improvement of CV risk biomarkers in patients with moderate-to-severe plaque-type Pso. In particular, the authors demonstrated that a continuous systemic therapy with fumaric acid esters, behind reducing Pso severity, was able to improve the endothelial vasodilator function, reduce serum levels of CRP, and increase the potentially cardioprotective adiponectin (33). However, it has been with the introduction of biological therapies that Pso treatment expectations and long-term control have greatly improved and the idea that these therapies, more than the systemic ones, might reduce the risk of CVD reinforced (34). To date, although several biological therapies have been approved and licensed for the treatment of Pso, their CV safety profile is not yet well established (35). Currently, it is unclear whether any of these therapies, which include TNF-α inhibitors (TNFi) (infliximab, etanercept, and adalimumab); inhibitors of the p40 subunit common to IL-12 and IL-23 (ustekinumab and briakinumab); IL-17A inhibitors (secukinumab and ixekizumab), and its receptor antagonist (brodalumab) could alter the risk of CVD development (35). Thus, the aim of this review has been to evaluate whether possible associations exist between currently licensed biological therapies and risk of MACEs in adult patients with Pso.
Psoriasis is an immune-mediated inflammatory skin disorder that affects 2.5% of the population worldwide. While the exact etiology of psoriasis is unknown, genetic and environmental factors are important in disease development (36, 37). Moreover, the immune system plays a crucial role in the overall disease pathogenesis, with various innate and adaptive immune cells and pro-inflammatory mediators involved at different stages of the disease (38). T cells are known to be the main actors in the pathogenesis, in particular Th1 and Th17 lymphocytes contribute through the inflammatory cytokines release that promote further recruitment of immune cells, keratinocyte proliferation, and inflammation (39). More specifically, CD4+ and CD8+ T cells with an IL-17-secretory phenotype are important contributors owing to their production of the pro-inflammatory cytokines IL-17, IL-22, and TNFα (40). For over 30 years, Pso was thought to involve a Th1 response, driven by the cytokines IFN-γ and IL-12; however, the discovery of the Th17 cell population has revolutionized the complex scenario of Pso. Indeed, the IL-23/Th17 cell signaling axis effects on keratinocytes and infiltrating immune cells in the skin has shaped the current disease model of Pso. Therefore, psoriatic disease is understood as a patterned response to chronic activation of the IL-23/Th17 axis (36). Recently, it has been identified T-regulatory cells expressing IL-17A in which, Foxp3 expression is progressively lost, whereas RORγ expression is increased (39, 40). This process is upregulated by IL-23 and concurring to the chronic inflammation seen in Pso. In particular, IL-23 regulates the maintenance of Th17 cells, whereas IL-17 and TNF mediate effector functions of innate (TNF) and adaptive (TNF, IL-17) immune cells.
Pso is considered a T cell-mediated immune disease with a mixed Th1/Th17 cytokines environment. The interplay of these cytokines has a central role in the disease process by causing the spread of local inflammation at systemic levels. Specifically, elevated levels of TNF-α and soluble TNF receptors have been found in lesional psoriatic skin and in the serum of patients with severe Pso, which similarly reflect those detected in congestive heart failure (CHF) patients (41–43). TNF-α activation system can lead to different outcomes: (i) the development of atherosclerosis, (ii) the deterioration of cardiac functions, and (iii) the vascular smooth muscle cell remodeling (44–46). Since 2004, US Food and Drug Administration (FDA) has approved TNFi, such as etanercept, infliximab, and adalimumab for Pso treatment. Most recently, five TNFi biosimilars have been approved by FDA for Pso: infliximab-dyyb, infliximab-abda, etanercept-szzs, adalimumab-atto, and adalimumab-adbm (47, 48). The effectiveness of these biologics in the management of psoriatic patients has been highlighted since the earliest literature (49–54). Anti-TNF-α agents can reduce the CRP, the vascular endothelial growth factor (VEGF), and the chemotactic factors (e.g., VCAM-1, E-selectin, IL-8, and MCP-1) (55, 56) as well as the Th17 cell count in the peripheral blood of psoriatic patients (49, 57). In fact, TNFi stop CD4+ T cells differentiation into Th1, Th17, and Th22 cells and the consequent release of IL-17A, IL-17F, and IL-22 (47, 58). Although the evidence indicate that TNFi are effective in reducing the systemic inflammation, it is still debated whether anti-TNF-α agents can decrease CV risk in these patients or they only represent a random association. It has been reported in literature that psoriatic patients who received TNFi showed an improvement of psoriasis area severity index (PASI) score characterized by a reduction of CV risk biomarkers (e.g., CRP, VEGF, and resistin serum levels) after 24 weeks of therapy (59). Additional studies have highlighted that the vascular function was somehow restored after 10 weeks of etanercept treatment, leading to a significant reduction in CRP levels and improvements of insulin sensitivity (60, 61). The same authors also proved that TNFi statistically led to a significant reduction (P = 0.0001) of retinol-binding protein 4 (RBP4) circulating levels in psoriatic patients (62). RBP4 is highly linked to subclinical atherosclerosis, due to its positive correlation to carotid intima-media thickness (IMT) indicator (61, 62). Recently, it has been assumed that psoriatic patients treated with TNFi showed IMT indicator decrease associated with appropriate therapeutic responses (63). Another experimental study has reported the aortic inflammation reduction by 18F-fluoro-deoxyglucose (FDG)-positron emission tomography/computed tomography (CT) in 30 psoriatic patients who received adalimumab (64). Similarly, the TNFi effects on the development of atherosclerosis has been studied in 58 psoriatic patients during a 13-month period by CT imaging (65). In addition, the patients who did not received TNFi showed a significant progression of the coronary calcification, while the TNFi-treated group did not. Another research based on echocardiographic data confirmed the improvement of the myocardial function in 18 psoriatic subjects treated with TNFi and IL12/23 inhibitors (66). Likewise, right ventricular systolic function was improved in a 30-month study of 44 psoriatic patients treated with TNFi (67). Therefore, anti-TNF-α agents might be identified as CV protective factors together with statin drug, female gender, and age (68). However, no therapeutic effect of TNFi has been demonstrated in CHF patients classified as grade III–IV by the New York Heart Association. Initially, etanercept seemed to have beneficial effects in heart failure patients (69). Subsequently, a large study consisting of 1,500 patients with symptomatic heart failure has shown no relation to mortality or hospitalizations after treatment with etanercept (70). Other clinical trials have reported a higher percentage of mortality in CHF patients who received infliximab at 10 mg/kg (higher dose than that used in Pso and RA) than the controls (71, 72). These preliminary discovers have provided the evidence for the contraindication against the use of TNFi in CHF patients. Nowadays, anti-TNF-α drugs are not contraindicated in patients with heart diseases other than CHF (34). Random control trials (RCTs) including severe psoriatic patients who received at least one of TNFi, resulted in a number of MACEs (35, 73–76). Overall, the pooled analysis confirmed that no statistically significant difference exists between patients treated with biologics or placebo (35) (Table 1). Considering TNFi separately, there was also no statistically significant difference in patients receiving etanercept, infliximab, or adalimumab (35). In addition, the incidence rates (IRs) of MACEs were reduced by the use of anti-TNF-α agents respect to conventional therapies (28, 29, 77, 78). The use of TNFi for patients with Pso or psoriatic arthritis was associated with a 55% reduction in the incidence of MI compared with those patients who were only treated with topical therapy (28). Similarly, psoriatic patients treated with TNFi displayed a significantly lower risk of MI when compared with MTX (79). Furthermore, treatment with TNFi is associated with an 11% reduced CV risk every 6 months of additional treatment (79). On the other hand, some authors suggested that these therapies do not seem to reduce the risk of MI, because they increase patients’ weight and cholesterol levels after 3 or 6 months of therapy (66, 79, 80). These results could be related to a subsequently biological effect of TNFi due to neutralization of TNF-α cachetic properties (81). On the contrary, stable lipids levels were found in psoriatic patients treated with adalimumab, etanercept, or infliximab for 13 months (65). Psoriatic patients randomized to adalimumab or non-systemic treatment (topical therapies or phototherapy) showed no changes in total cholesterol, high-density lipoprotein, low-density lipoprotein, or triglycerides after 15 weeks of treatment (64). Despite some scientific reports overshadows TNFi role on CV risk, current studies support TNFi benefic effects on cardio metabolic parameters. To conclude, although literature remains heterogeneous, in part due to methodological differences, anti-TNF-α agents exert a protective effect on CV risk.
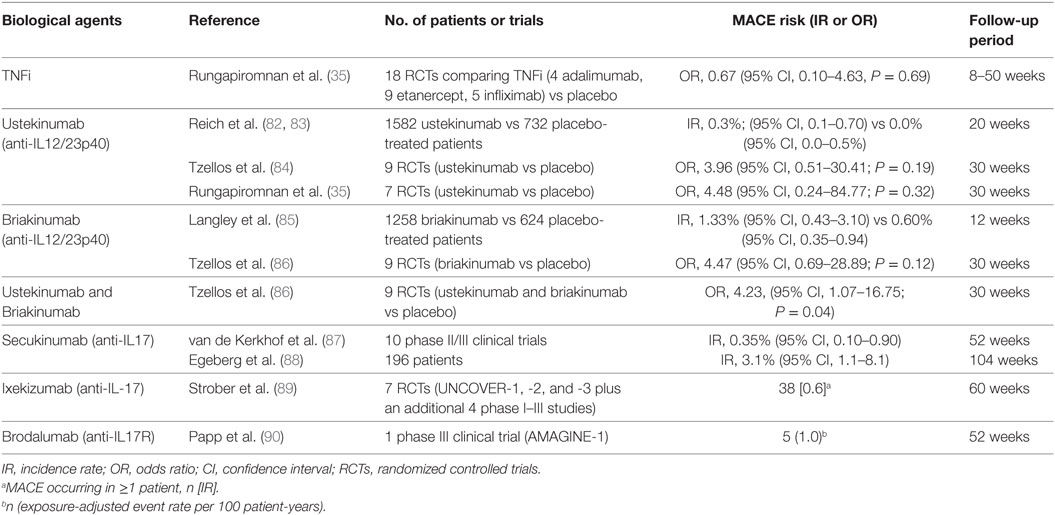
Table 1. Main randomized controlled trials and meta-analysis studies on the rates of major adverse cardiovascular events (MACEs) in psoriatic patients treated with biological agents.
The basis for developing anti-IL-12/23 biological drugs was encouraged by evidences that mice deficient in the subunit p40, shared by both IL-12 and IL-23, are resistant to experimentally induced autoimmune conditions, such as psoriasis (91, 92). Levels of IL-12/23p40 mRNA are more elevated in psoriatic than in healthy skin (93, 94). Cytokines induced by IL-12 (such as IFN-γ) and by IL-23 (such as IL-17A, IL-17F, and IL-22) are increased in psoriatic plaques (94). Moreover, in the last two decades, these inflammatory mediators have also been shown to contribute in CVD development. Indeed, it has been shown that serum levels of IL-12 and IL-23 are augmented in patients with CVD (95, 96). IL-12 and IL-23 are presented in atherosclerotic plaque and thereby affecting the pro-inflammatory status in these patients (96–98). Taken together, these evidences suggest that targeting IL-12/23 could represent a valid therapeutic option for psoriatic disease, with benefits on cutaneous involvement and on CV comorbidities. The efficacy of anti-IL-12/23p40 biological drugs (ustekinumab and briakinumab) for psoriasis treatment (Table 2) has been evaluated in two phase III RCTs and in four phase III RCTs, respectively (82, 85, 99–103). The hypothesis that these agents could possibly increase CV risk while improving cutaneous disease is unexpected. Nevertheless, safety concerns have been raised regarding the possibility of an increased risk of MACEs with the utilize of anti-IL-12/23 biological agents. We will discuss on lights and shadows of this hot question. In 2011, Reich et al. (83) evaluated this point due to the fact that in the ustekinumab clinical studies target population reported a higher rate of MACEs than placebo one. In the 1,582 ustekinumab-treated patients enrolled in the phase II and III placebo-controlled psoriasis studies, five MACE events occurred [0.3%; 95% confidence interval (CI), 0.1–0.7%] respect to no events in 732 placebo-treated patients (0.0%; 95% CI, 0.0–0.5%) (83). In particular, an important numerical imbalance in MACE rate was observed in the phase II trial with a 4:1 randomization ratio (risk difference 1.2%; 95% CI, 3.9–3.7%). However, the two phase III trials did not replicate a same high risk difference (0.2%; 95% CI, 1.2–1.2 and 0.1%; 95% CI, 0.7–0.7% for PHOENIX 1 and 2, respectively) (83) (Table 1). During the 12/20-week follow-up periods, MACE events did not show tendency to cluster. Indeed, the five MACEs occurred at weeks 2, 6, 10, 14, and 17 (83). The standardized IR in these studies ranged between 0.34 and 0.52, a rate lower than that estimated for the general population in the USA or in psoriatic patients (83). Moreover, all patients who experienced MACEs had at least three established CV risk factors. Longer term analyses demonstrated that rates of CV events remained low with up to 3 years. However, the absence of a control group precludes definitive assessment of the effect of ustekinumab on MACE risk (83). Thus, data from short-term, controlled trials give only partial information regarding the possible impact of ustekinumab on CV risk: the results suggest neither harmful nor beneficial effects. However, a smaller risk increase cannot be totally ruled out. Concerning another anti-IL-12/23p40 monoclonal antibody, briakinumab, the question is even more burning. Results from one of the four clinical trials have reported more MACE events in briakinumab-treated patients compared with placebo ones over a 12-week period (101). In details, 18 MACEs had been recorded with 4 CV deaths compared with no events in placebo group. The frequency of MACE was spread in an apparently uniform manner over time. The standardized IRs were 1.33/100 patient-years (95% CI, 0.43–3.10) respect to 0.60 (95% CI, 0.35–0.94) in the placebo-controlled phase (Table 1). In patients with two or more CV risk factors, the IR was more elevated (2.15 events/100 patient-years). For all these reasons, the study protocol underwent an amendment (85). Interestingly, no MACEs were reported during other two published clinical trials (briakinumab vs placebo or etanercept) as well as in another trial in which briakinumab was compared with MTX with a 52-week follow-up (82, 102, 103). However, the application for authorization to market briakinumab has been withdrawn.1 Overall, definitive conclusions did not could be performed. For this reason, two industry-independent meta-analyses of nine randomized, double-blind, placebo-controlled, monotherapy trials have been conducted to deeper examine the possible association of MACEs with anti-IL-12/23 agents (84, 104) (Table 1). The meta-analysis by Ryan et al. found no increased risk of MACEs in treated patients compared with placebo ones, using the Mantel–Haenszel fixed-statistical effects model (104). On the other hand, Tzellos et al. analysis showed that the odds ratio (OR) for briakinumab- and ustekinumab-treated patients was not statistically significant (OR 4.47, 95% CI, 0.69–28.89, P = 0.12; OR 3.96, 95% CI, 0.51–30.41, P = 0.19, respectively). When combined, the OR for MACEs between patients treated with biological agents and those receiving placebo was found to be statistically significant (OR = 4.23, 95% CI, 1.07–16.75, P = 0.04) (84). In this case, the statistical effects model used was the Peto One-Step OR method. The divergence in these results has been attributable to the use of two different methods to estimate the risk. The Mantel–Haenszel fixed-statistical model using zero-cell corrections provides estimates for all studies, including zero event ones. This make it less suitable for meta-analysis of rare events (105–108) resulting in low statistical power. MACE event rates were estimated to be 0.28, 0.35, and 0.31% for ustekinumab, briakinumab, and both, respectively (108). It has been established that in case of an event IR of 1% or less, the best statistical approach is Peto method (105). However, the Peto OR method excluding trials with zero events from the analysis can lead to an overestimation of true relative risk. Moreover, neither the analysis by Tzellos et al. nor that by Ryan et al. have been adjusted for dropouts; this likely resulted in an overestimation of true risk (106). A large 5-year post-marketing study on the use of ustekinumab for psoriasis did not support the hypothesis of an augmented risk of MACEs (109). Moreover, an imbalance in MACE event rate has not been reported in other therapeutic indications of ustekinumab, such as psoriatic arthritis and inflammatory bowel disease (110–112). In addition, to date, the FDA has not communicated any changes regarding the prescribing for ustekinumab related to CV risk. More recently, a new meta-analysis has been performed by Rungapiromnan et al. (35) to evaluate the impact of biological therapies on risk of MACEs (Table 1). In this meta-analysis, they have selected RCTs in which patients received only licensed dose regimens of biological therapies. For this reason, briakinumab has been excluded from the analysis. Peto ORs with 95% CIs has been applied for statistical analysis. Regarding ustekinumab, no statistically significant difference in risk of MACEs has been reported respect to placebo (OR 4.48, 95% CI, 0.24–84.77, P = 0.32); similarly, comparing ustekinumab 45 mg with 90 mg the OR was not statistically significant (OR 1.00, 95% CI, 0.06–16.03, P = 1.00) (35). The most important limitation is that these findings were mainly based on small sample sizes and short-term follow-up (ranging from 12 to 30 weeks). This is an important aspect to considering since it has been demonstrated that during the initial stage of therapy with ustekinumab inflammatory pro-atherogenic mediators such as IL-12/23 p40 temporarily increased and then dramatically decreased at week 32 (113, 114). Thus, other post-marketing studies and novel larger randomized controlled trials will be needed to continue the surveillance to assess the potential association connecting the use of anti-IL-12/23p40 biological drugs and increased CV risk.
In the past decade, the research has been focused on Th17 cells, which during differentiation secrete IL-17. IL-17 cytokine family consists of six members (IL-17A, B, C, D, E, and F). IL-17A (referred to here as IL-17) is among them, the major isoform (115). IL-17 is produced by Th17 cells and also by other cell subtypes, including γδ T, natural killer, etc. (116). However, interest in the Th17 population in relationship to Pso increased when numbers of circulating Th17 cells and IL-17 expression levels were found upregulated in the Pso lesional skin compared with non-lesional skin (49, 117). Recently, several anti-IL-17 agents have been developed. These primarily include anti-IL-17A monoclonal antibodies, secukinumab and ixekizumab, and brodalumab, an anti-IL-17 receptor monoclonal antibody. These biological agents that target the IL-17 signaling pathway have currently been evaluated and approved for the treatment of moderate-to-severe plaque Pso. The results reported by studies on efficacy and safety of IL-17 inhibitors are very promising. They showed the superiority of these new biological agents respect to both placebo and other biologics, such as ustekinumab and etanercept. For the first time, these trials include as end point the percentage of patient that achieving 90 or 100% of clinical improvement (PASI-90 or PASI-100, respectively) (47). In addition, although IL-17 antagonists had a higher rate of any adverse events than placebo, there was no significant difference in severe adverse events. This suggests that IL-17 antagonists are well tolerated (118). Even if the effects of IL-17 therapy on CVD in psoriatic patients is yet to be fully elucidated. Indeed, the interest on MACE is a crucial and much debated point. Likewise, Th17 pathway has a crucial role in CVD (22). Indeed, IL-17 is also involved in angiogenesis process, and on the synthesis of MMPs and CRP. Thus, in theory, Th17 cells could also play a crucial role in atherosclerotic and in CVD. Reports in atherosclerosis showed a conflicting result on IL-17 and Th17 cells in disease onset and plaque stability. IL-17 may exhibit both pro- and anti-atherogenic effects, depending on the inflammatory context (115). IL-17 pro-atherogenic effects may result from the induction of pro-inflammatory cytokines or chemokines (IL-6, GM-CSF, CCL2, and CXCL1) by endothelial cells or macrophages. The IL-17 atheroprotective effects may be dued by IFN-γ decreased and to its inhibitory effect on the expression of vascular cell adhesion molecule, this molecule is important to mediating the accumulation of monocytes and T cells within the lesions. Indeed, it was showed that IL-17, IL-21, and IL-23 were found in atherosclerotic plaques and associated with increased inflammation and plaque vulnerability (119). By contrast, Taleb et al. have reported a role for IL-17 in promoting plaque stability. Indeed, IL-17 expression in human carotid lesions was related to a fibrous phenotype with a lower macrophage and higher smooth muscle cell content (120). Despite some data suggesting increased levels of Th17 cells and IL-17 in patients with acute coronary syndromes (121, 122), the bulk of evidence indicate that circulating IL-17 levels are similar in patients with or without coronary artery disease (123). Indeed, some authors have reported that the patients with IL-17 low levels were more susceptible to the risk of death and recurrent MI (124). On the other hand, several experimental evidences and biomarker studies, which indicate a link between IL17 and instability in atherosclerotic plaques, supporting the hypothesis that in part explain the high risk of MI in Pso patients (22). Indeed, Pso patients have significantly elevated IL-17 serum levels (49) and they have a high risk to developing CV comorbidities. The conception that Th17 cytokines may provide a link between Pso and CVD comes from literature data that reporting a pathogenic role of IL-17 in Pso and examining the Th17 axis contribution into atherosclerosis (125).
As regarding secukinumab, data reported that there is a favorable safety profile in patients with moderate-to-severe plaque Pso over a total follow-up period of 52 weeks in a pooled safety analysis of 10 studies. However, at baseline, more subjects in the secukinumab groups had CV risk than comparator groups. Even if overall exposure-adjusted IRs of adjudicated MACE in secukinumab-treated subjects was comparable to etanercept-treated subjects (87). During the first 12 weeks of secukinumab studies, MACEs were reported in three patients receiving secukinumab 300 mg (0.26%), in no patients receiving etanercept, and in one patient treated with placebo. Over 52 weeks, exposure-adjusted IRs of MACEs were comparable in patients receiving secukinumab 300 mg (0.42/100 subject-years), secukinumab 150 mg (0.35/100 subject-years), and etanercept (0.34/100 subject-years), despite the presence of higher baseline CV risk factors in the two secukinumab groups (Table 1). All patients with a MACE had CVD risks, such as hypertension, dyslipidemia, etc. (87). In summary, from this comprehensive analysis of pooled safety data from 10 studies reported that the incidences of MACE in secukinumab-treated patients was low. A very recent real-life observational study of 195 patients (Table 1) treated with secukinumab for up to 2 years reported that 2% of patients suffered a CV event yielding a conspicuously elevated IR compared with findings from the secukinumab phase III clinical trial program, although the absolute numbers were very low (n = 4) (88).
The other antibody that inhibits IL-17 is ixekizumab; recently, safety data from a 12-week induction period, a 12- to 60-week maintenance period, and from all ixekizumab-treated patients were presented. The analysis of these data essentially reported similar rates of MACE between ixekizumab and etanercept and low rates of MACE with continued exposure for ixekizumab until week 60 (Table 1) (89). In particular, during the induction period, the between-group rates of adjudicated MACE were similar. The MACE individual components did not change substantially with longer exposure to ixekizumab. 7 of 4,035 ixekizumab-treated patients experienced vascular death. At baseline, patients subsequently developing MACE had a higher prevalence of risk factors for acute atherothrombotic events than patients without MACE. In conclusion, ixekizumab use was not associated with an increased risk of MACE. The safety profile reported in this analysis study is consistent with previous reports for ixekizumab (76, 126).
As regarding Brodalumab that selectively targets human IL-17RA and antagonizes the IL-17 pathway, it was considered safety from analysis of a prospective study, indeed incidences of serious adverse events in the induction phase were low among groups. There were no reports of MACEs during the induction phase, and only five reports (exposure-adjusted event rate of 1.0/100 patient-years) through week 52 (Table 1) (90). Conversely, in another phase III study, brodalumab treatment was compared with ustekinumab treatment in psoriasis, and the adverse effects were more recurrent in the brodalumab group than in the ustekinumab one. Indeed, in the AMAGINE-2 study, one death by stroke happened during the induction phase, and five deaths occurred through week 52; whereas in the AMAGINE-3 study, two deaths occurred: one from cardiac arrest and one from accidental death (127). However, the sizes of study populations, which were adequate for efficacy and common adverse event assessments, may have been inadequate to assess rare adverse events, which would require longer follow-up of large numbers of patients to better understand the safety profile of brodalumab.
Taken together, results from short-term safety and efficacy trials exploring anti-IL-17 therapy in psoriatic patients suggest no increased CVD risk compared with placebo or other classes of biologics used in Pso. Indeed, we may infer that IL-17 antagonists could possibly play an outstanding role in reducing CV morbidity. Even if there are several limitations in these clinical trials, e.g., patients highly selected, short duration of treatment with placebo or TNFi in relative few number of patients compared with the number of patients receiving IL-17 inhibitors. The advent of IL-17 biologics has improved significantly Pso disease (Table 2), but time will tell whether this IL-17 inhibition will also improve CVD outcomes in Pso.
Since systemic inflammation is currently considered the main cause of CVD risk in Pso, it has been thought that biological therapies might be helpful in treating not only cutaneous manifestations but have also positive or negative CV effects depending on the specific cytokine target and molecular mode of action. However, despite the recognized efficacy of biological agents in psoriasis treatment, little is known about their impact on risk of MACE in these patients. Data from meta-analyses have suggested that biological therapies including TNFi, anti-IL-12/23p40, and IL-17A agents did not have a significant impact on the risk of MACE in psoriatic patients over the short term. In particular, the use of TNFi is currently associated with a minor CVD risk in these patients, whereas the role of anti-IL-12/23p40 agents remains conflicting. Nevertheless ustekinumab, the only IL-12/23p40 inhibitor currently approved for Pso, results neutral on CV parameters. Regarding newer IL-17 inhibitors, early data have suggested no increased CVD risk respect to placebo or other classes of biologics used in psoriasis. However, because of follow-up short duration, many of these results should be interpreted with caution. Moreover, more studies, involving recruitment of a larger numbers of patients as well as a longer duration of treatment exposure, are needed to better evaluate the impact of biological therapies on the risk of MACEs in patients with Pso and to develop effective strategies for CVD prevention in these patients.
AB designed the manuscript structure, reviewed the scientific literature, and supervised the final version of the manuscript. GC designed the review structure and contributed to the writing of the manuscript. ES contributed to the writing of the manuscript and created graphical illustration. RC and AR reviewed the scientific literature and contributed to the writing of the manuscript. GF and NB reviewed the scientific literature and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
CI, confidence interval; CRP, C-reactive protein; CT, computed tomography; CV, cardiovascular; CVD, cardiovascular disease; FDG, 18F-fluoro-deoxyglucose; GPRD, General Practice Research Database; HDL, high-density lipoprotein; IFN, interferon; IL, interleukin; IMT, intima-media thickness; IRs, incidence rates; LDL, low-density lipoprotein; MACEs, major adverse cardiovascular events; MCP, monocyte chemoattractant protein; MI, myocardial infarction; MTX, methotrexate; OR, odds ratio; PASI, psoriasis area severity index; PET, positron emission tomography; RBP4, retinol-binding protein 4; RCTs, random control trials; Th, T helper; TNF, tumor necrosis factor; TNFi, TNF-α inhibitors; V-CAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor.
1. McDonald CJ, Calabresi P. Occlusive vascular disease in psoriatic patients. N Engl J Med (1973) 288:912. doi:10.1056/NEJM197304262881715
2. Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol (2013) 133:2340–6. doi:10.1038/jid.2013.149
3. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA (2006) 296:1735–41. doi:10.1001/jama.296.14.1735
4. Ludwig RJ, Herzog C, Rostock A, Ochsendorf FR, Zollner TM, Thaci D, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol (2007) 156:271–6. doi:10.1111/j.1365-2133.2006.07562.x
5. Naldi L, Chatenoud L, Linder D, Belloni Fortina A, Peserico A, Virgili AR, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol (2005) 125:61–7. doi:10.1111/j.0022-202X.2005.23681.x
6. Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res (2006) 298:321–8. doi:10.1007/s00403-006-0703-z
7. Padhi T, Garima. Metabolic syndrome and skin: psoriasis and beyond. Indian J Dermatol (2013) 58:299–305. doi:10.4103/0019-5154.113950
8. Wakkee M, Herings RM, Nijsten T. Psoriasis may not be an independent risk factor for acute ischemic heart disease hospitalizations: results of a large population-based Dutch cohort. J Invest Dermatol (2010) 130:962–7. doi:10.1038/jid.2009.321
9. Stern RS. Psoriasis is not a useful independent risk factor for cardiovascular disease. J Invest Dermatol (2010) 130:917–9. doi:10.1038/jid.2009.446
10. Parisi R, Rutter MK, Lunt M, Young HS, Symmons DPM, Griffiths CEM, et al. Psoriasis and the risk of major cardiovascular events: cohort study using the clinical practice research datalink. J Invest Dermatol (2015) 135:2189–97. doi:10.1038/jid.2015.87
11. Eder L, Gladman DD. Atherosclerosis in psoriatic disease: latest evidence and clinical implications. Ther Adv Musculoskelet Dis (2015) 7:187–95. doi:10.1177/1759720X15591801
12. Chiricozzi A, Raimondo A, Lembo S, Fausti F, Dini V, Costanzo A, et al. Crosstalk between skin inflammation and adipose tissue-derived products: pathogenic evidence linking psoriasis to increased adiposity. Expert Rev Clin Immunol (2016) 12:1299–308. doi:10.1080/1744666X.2016.1201423
13. Raimondo A, Lembo S, Di Caprio R, Donnarumma G, Monfrecola G, Balato N, et al. Psoriatic cutaneous inflammation promotes human monocyte differentiation into active osteoclasts, facilitating bone damage. Eur J Immunol (2017) 47:1062–74. doi:10.1002/eji.201646774
14. Boehncke WH. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front Immunol (2018) 9:579. doi:10.3389/fimmu.2018.00579
15. Boehncke WH, Boehncke S, Tobin AM, Kirby B. The ‘psoriatic march’: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol (2011) 20:303–7. doi:10.1111/j.1600-0625.2011.01261.x
16. Gisondi P, Fantin F, Del Giglio M, Valbusa F, Marino F, Zamboni M, et al. Chronic plaque psoriasis is associated with increased arterial stiffness. Dermatology (2009) 218:110–3. doi:10.1159/000182256
17. Fang N, Jiang M, Fan Y. Association between psoriasis and subclinical atherosclerosis: a meta-analysis. Medicine (Baltimore) (2016) 95:e3576. doi:10.1097/MD.0000000000003576
18. Flammer AJ, Ruschitzka F. Psoriasis and atherosclerosis: two plaques, one syndrome? Eur Heart J (2012) 33:1989–91. doi:10.1093/eurheartj/ehr425
19. Davidovici BB, Sattar N, Prinz J, Puig L, Emery P, Barker JN, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol (2010) 130:1785–96. doi:10.1038/jid.2010.103
20. Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin (2015) 33:41–55. doi:10.1016/j.det.2014.09.004
21. Ghazizadeh R, Shimizu H, Tosa M, Ghazizadeh M. Pathogenic mechanisms shared between psoriasis and cardiovascular disease. Int J Med Sci (2010) 7:284–9. doi:10.7150/ijms.7.284
22. Chen S, Crother TR, Arditi M. Emerging role of IL-17 in atherosclerosis. J Innate Immun (2010) 2:325–33. doi:10.1159/000314626
23. Mehta NN, Li K, Szapary P, Krueger J, Brodmerkel C. Modulation of cardiometabolic pathways in skin and serum from patients with psoriasis. J Transl Med (2013) 11:194. doi:10.1186/1479-5876-11-194
24. Kolliker Frers RA, Cosentino V, Tau J, Kerzberg EM, Urdapilleta A, Chiocconi M, et al. Immune-mediated inflammation promotes subclinical atherosclerosis in recent-onset psoriatic arthritis patients without conventional cardiovascular risk factors. Front Immunol (2018) 9:139. doi:10.3389/fimmu.2018.00139
25. Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol (2009) 54:2129–38. doi:10.1016/j.jacc.2009.09.009
26. Prodanovich S, Ma F, Taylor JR, Pezon C, Fasihi T, Kirsner RS. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol (2005) 52:262–7. doi:10.1016/j.jaad.2004.06.017
27. Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernán MA, Ridker PM, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol (2011) 108:1362–70. doi:10.1016/j.amjcard.2011.06.054
28. Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol (2012) 148:1244–50. doi:10.1001/archdermatol.2012.2502
29. Ahlehoff O, Skov L, Gislason G, Lindhardsen J, Kristensen SL, Iversen L, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med (2013) 273:197–204. doi:10.1111/j.1365-2796.2012.02593.x
30. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci (2017) 18:E2211. doi:10.3390/ijms18102211
31. Mottaghi A, Ebrahimof S, Angoorani P, Saboor-Yaraghi AA. Vitamin A supplementation reduces IL-17 and RORc gene expression in atherosclerotic patients. Scand J Immunol (2014) 80:151–7. doi:10.1111/sji.12190
32. Mahmoudi MJ, Saboor-Yaraghi AA, Zabetian-Targhi F, Siassi F, Zarnani AH, Eshraghian MR, et al. Vitamin A decreases cytotoxicity of oxidized low-density lipoprotein in patients with atherosclerosis. Immunol Invest (2016) 45:52–62. doi:10.3109/08820139.2015.1095208
33. Boehncke S, Fichtlscherer S, Salgo R, Garbaraviciene J, Beschmann H, Diehl S, et al. Systemic therapy of plaque-type psoriasis ameliorates endothelial cell function: results of a prospective longitudinal pilot trial. Arch Dermatol Res (2011) 303:381–8. doi:10.1007/s00403-010-1108-6
34. Puig L. Cardiovascular risk and psoriasis: the role of biologic therapy. Actas Dermosifiliogr (2012) 103:853–62. doi:10.1016/j.ad.2012.02.003
35. Rungapiromnan W, Yiu ZZN, Warren RB, Griffiths CEM, Ashcroft DM. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol (2017) 176:890–901. doi:10.1111/bjd.14964
36. Raimondo A, Balato A, Megna M, Balato N. Limitations of current monoclonal antibodies for plaque-type psoriasis and an outlook for the future. Expert Opin Biol Ther (2018) 18(6):605–7. doi:10.1080/14712598.2018.1479738
37. Balato A, Mattii M, Caiazzo G, Raimondo A, Patruno C, Balato N, et al. IL-36γ is involved in psoriasis and allergic contact dermatitis. J Invest Dermatol (2016) 136:1520–3. doi:10.1016/j.jid.2016.03.020
38. Schiattarella M, Caiazzo G, Di Caprio R, Lembo S, Raimondo A, Ayala F, et al. Paraoxonases and psoriasis: negative imbalance of anti-oxidant endogenous mechanisms. G Ital Dermatol Venereol (2017). doi:10.23736/S0392-0488.17.05537-7
39. Furue M, Tsuji G, Chiba T, Kadono T. Cardiovascular and metabolic diseases comorbid with psoriasis: beyond the skin. Intern Med (2017) 56:1613–9. doi:10.2169/internalmedicine.56.8209
40. Karczewski J, Dobrowolska A, Rychlewska-Hańczewska A, Adamski Z. New insights into the role of T cells in pathogenesis of psoriasis and psoriatic arthritis. Autoimmunity (2016) 49:435–50. doi:10.3109/08916934.2016.1166214
41. Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine (2015) 73:342–50. doi:10.1016/j.cyto.2014.12.014
42. Testa M, Yeh M, Lee P, Fanelli R, Loperfido F, Berman JW, et al. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J Am Coll Cardiol (1996) 28:964–71. doi:10.1016/S0735-1097(96)00268-9
43. Karbach S, Croxford AL, Oelze M, Schüler R, Minwegen D, Wegner J, et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol (2014) 34:2658–68. doi:10.1161/ATVBAHA.114.304108
44. Bozkurt B, Kribbs SB, Clubb FJ Jr, Michael LH, Didenko VV, Hornsby PJ, et al. Pathophysiologically relevant concentrations of tumor necrosis factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation (1997) 97:1382–91. doi:10.1161/01.CIR.97.14.1382
45. Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-α. Circ Res (1997) 81:627–35. doi:10.1161/01.RES.81.4.627
46. Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and cardiovascular disease: the health, aging and body composition [health ABC] study. Am J Cardiol (2003) 92:522–8. doi:10.1016/S0002-9149(03)00718-5
47. Balato A, Scala E, Balato N, Caiazzo G, Di Caprio R, Monfrecola G, et al. Biologics that inhibit the Th17 pathway and related cytokines to treat inflammatory disorders. Expert Opin Biol Ther (2017) 17:1363–74. doi:10.1080/14712598.2017.1363884
48. FDA Approves Biosimilar Products. Silver Spring, MD: US Food and Drug Administration (2018). Available from: https://www.fda.gov/Drugs/Development Approval Process/How Drugsare Developed and Approved/Approval Applications/Therapeutic Biologic Applications/Biosimilars/ucm580432.htm (Accessed: May 29, 2018).
49. Balato A, Schiattarella M, Di Caprio R, Lembo S, Mattii M, Balato N, et al. Effects of adalimumab therapy in adult subjects with moderate-to-severe psoriasis on Th17 pathway. J Eur Acad Dermatol Venereol (2014) 28:1016–24. doi:10.1111/jdv.12240
50. Caldarola G, De Simone C, Carbone A, Tulli A, Amerio P, Feliciani C. TNF and its receptors in psoriatic skin, before and after treatment with etanercept. Int J Immunopathol Pharmacol (2009) 22:961–6. doi:10.1177/039463200902200411
51. Cordiali-Fei P, Trento E, D’Agosto G, Bordignon V, Mussi A, Ardigò M, et al. Decreased levels of metalloproteinase-9 and angiogenic factors in skin lesions of patients with psoriatic arthritis after therapy with anti-TNF-α. J Autoimmune Dis (2006) 5:3–5. doi:10.1186/1740-2557-3-5
52. Strober B, Foley P, Philipp S, Zhang N, Kaur P. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol (2016) 74(5, Suppl 1):AB249. doi:10.1016/j.jaad.2016.02.972
53. Momenta Pharmaceuticals Announces Positive Top-Line Phase 3 Results for M923, a Proposed Humira (Adalimumab) Biosimilar [News Release]. Cambridge, MA: Momenta Pharmaceuticals, Inc (2016). Available from: http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255 (Accessed: January 25, 2017).
55. Campanati A, Orciani M, Ganzetti G, Consales V, Di Primio R, Offidani A. The effect of etanercept on vascular endothelial growth factor production by cutaneous mesenchymal stem cells from patients with psoriasis. J Int Med Res (2016) 44:6–9. doi:10.1177/0300060515593229
56. Henning M, Göcker Tekin H, Skov L, Egeberg A. Effects of biologic therapy on cardiovascular disease in psoriasis. Curr Derm Rep (2018) 7:37–42. doi:10.1007/s13671-018-0210-4
57. Gkalpakiotis S, Arenbergerova M, Gkalpakioti P, Potockova J, Arenberger P, Kraml P. Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study. J Dermatol (2017) 44:363–9. doi:10.1111/1346-8138.13661
58. Goldminz AM, Suárez-Fariñas M, Wang AC, Dumont N, Krueger JG, Gottlieb AB. CCL20 and IL22 messenger RNA expression after adalimumab vs methotrexate treatment of psoriasis: a randomized clinical trial. JAMA Dermatol (2015) 151:837–46. doi:10.1001/jamadermatol.2015.0452
59. Boehncke S, Salgo R, Garbaraviciene J, Beschmann H, Hardt K, Diehl S, et al. Effective continuous systemic therapy of severe plaque-type psoriasis is accompanied by amelioration of biomarkers of cardiovascular risk: results of a prospective longitudinal observational study. J Eur Acad Dermatol Venereol (2011) 25:1187–93. doi:10.1111/j.1468-3083.2010.03947.x
60. Marra M, Campanati A, Testa R, Sirolla C, Bonfigli AR, Franceschi C, et al. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol (2007) 20:731–6. doi:10.1177/039463200702000408
61. Pina T, Armesto S, Lopez-Mejias R, Genre F, Ubilla B, Gonzalez-Lopez MA, et al. Anti-TNF-α therapy improves insulin sensitivity in non-diabetic patients with psoriasis: a 6-month prospective study. J Eur Acad Dermatol Venereol (2015) 29:1325–30. doi:10.1111/jdv.12814
62. Pina T, Genre F, Lopez-Mejias R, Armesto S, Ubilla B, Mijares V, et al. Anti-TNF-a therapy reduces retinol-binding protein 4 serum levels in non-diabetic patients with psoriasis: a 6-month prospective study. J Eur Acad Dermatol Venereol (2015) 30:92–5. doi:10.1111/jdv.13005
63. Jókai H, Szakonyi J, Kontár O, Marschalkó M, Szalai K, Kárpáti S, et al. Impact of effective tumor necrosis factor-alfa inhibitor treatment on arterial intima-media thickness in psoriasis: results of a pilot study. J Am Acad Dermatol (2013) 69:523–9. doi:10.1016/j.jaad.2013.06.019
64. Bissonnette R, Tardif JC, Harel F, Pressacco J, Bolduc C, Guertin MC. Effects of the tumor necrosis factor-α antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging (2013) 6:83–90. doi:10.1161/CIRCIMAGING.112.975730
65. Hjuler KF, Bøttcher M, Vestergaard C, Bøtker HE, Iversen L, Kragballe K. Association between changes in coronary artery disease progression and treatment with biologic agents for severe psoriasis. JAMA Dermatol (2016) 152:1114–21. doi:10.1001/jamadermatol.2016.1984
66. Ahlehoff O, Hansen PR, Gislason GH, Frydland M, Bryld LE, Elming H, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study. J Eur Acad Dermatol Venereol (2016) 30:819–23. doi:10.1111/jdv.13152
67. Herédi E, Végh J, Pogacsas L, Gaspar K, Varga J, Kincse G. Subclinical cardiovascular disease and it’s improvement after longterm TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol (2016) 30:1531–6. doi:10.1111/jdv.13649
68. Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol (2013) 69(1):167–8. doi:10.1016/j.jaad.2013.02.019
69. Deswal A, Bozkurt B, Seta Y, Parilti-Eiswirth S, Hayes FA, Blosch C, et al. Safety and efficacy of a soluble P75 tumor necrosis factor receptor (Enbrel, etanercept) in patients with advanced heart failure. Circulation (1999) 99:3224–6. doi:10.1161/01.CIR.99.25.3224
70. Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation (2004) 109:1594–602. doi:10.1161/01.CIR.0000124490.27666.B2
71. Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation (2003) 107:3133–40.
72. Pham T, Bachelez H, Berthelot JM, Blacher J, Bouhnik Y, Claudepierre P, et al. TNF alpha antagonist therapy and safety monitoring. Joint Bone Spine (2011) 78(Suppl 1):15–185. doi:10.1016/S1297-319X(11)70001-X
73. AbbVie. Safety and Efficacy Study of Adalimumab in the Treatment of Plaque Psoriasis. (2017). Available from: https://clinicaltrials.gov/show/NCT01646073 (Accessed: January 17, 2017).
74. Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol (2003) 139:1627–32. doi:10.1001/archderm.139.12.1627
75. Bachelez H, van de Kerkhof PC, Strohal R, Kubanov A, Valenzuela F, Lee JH, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet (2015) 386:552–61. doi:10.1016/S0140-6736(14)62113-9
76. Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet (2015) 386:541–51. doi:10.1016/S0140-6736(15)60125-8
77. Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA (2011) 305:2525–31. doi:10.1001/jama.2011.878
78. Abuabara K, Lee H, Kimball AB. The effect of systemic psoriasis therapies on the incidence of myocardial infarction: a cohort study. Br J Dermatol (2011) 165:1066–73. doi:10.1111/j.1365-2133.2011.10525.x
79. Wu JJ, Guérin A, Sundaram M, Dea K, Cloutier M, Mulani P. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol (2017) 76:81–90. doi:10.1016/j.jaad.2016.07.042
80. Gisondi P, Cotena C, Tessari G, Girolomoni G. Anti-tumour necrosis factor-alpha therapy increases body weight in patients with chronic plaque psoriasis: a retrospective cohort study. J Eur Acad Dermatol Venereol (2008) 22:341–4. doi:10.1111/j.1468-3083.2007.02429.x
81. Cawthorn WP, Sethi JK. TNF-α and adipocyte biology. FEBS Lett (2007) 582:117–31. doi:10.1016/j.febslet.2007.11.051
82. Reich K, Langley RG, Papp KA, Ortonne JP, Unnebrink K, Kaul M, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med (2011) 365:1586–96. doi:10.1056/NEJMoa1010858
83. Reich K, Langley RG, Lebwohl M, Szapary P, Guzzo C, Yeilding N, et al. Cardiovascular safety of ustekinumab in patients with moderate to severe psoriasis: results of integrated analyses of data from phase II and III clinical studies. Br J Dermatol (2011) 164:862–72. doi:10.1111/j.1365-2133.2011.10257.x
84. Tzellos T, Kyrgidis A, Zouboulis CC. Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti-IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol (2013) 27:622–7. doi:10.1111/j.1468-3083.2012.04500.x
85. Langley R, Valdes J, Gordon K, Papp K. P3312. Long-term safety and efficacy of ABT-874 for the treatment of moderate to severe psoriasis: interim analysis from an open-label extension study. J Am Acad Dermatol (2011) 64:AB148.
86. Tzellos T, Kyrgidis A, Trigoni A, Zouboulis CC. Point: major adverse cardiovascular events and anti-IL 12/23 agents. J Am Acad Dermatol (2014) 70(2):380–1. doi:10.1016/j.jaad.2013.07.050
87. van de Kerkhof PC, Griffiths CE, Reich K, Leonardi CL, Blauvelt A, Tsai TF, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol (2016) 75:83–98.e4. doi:10.1016/j.jaad.2016.03.024
88. Egeberg A, Ottosen MB, Gniadecki R, Broesby-Olsen S, Dam TN, Bryld LE, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol (2018) 178:509–19. doi:10.1111/bjd.16102
89. Strober B, Leonardi C, Papp KA, Mrowietz U, Ohtsuki M, Bissonnette R, et al. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: etanercept comparisons and integrated data. J Am Acad Dermatol (2017) 76:432–40. doi:10.1016/j.jaad.2016.09.026
90. Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol (2016) 175:273–86. doi:10.1111/bjd.14493
91. Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med (2008) 205:799–810. doi:10.1084/jem.20071258
92. Mitra A, Fallen RS, Lima HC. Cytokine-based therapy in psoriasis. Clin Rev Allergy Immunol (2013) 44:173–82. doi:10.1007/s12016-012-8306-2
93. Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med (2004) 199:125–30. doi:10.1084/jem.20030451
94. Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med (2006) 203:2577–87. doi:10.1084/jem.20060244
95. Correia LCL, Andrade BB, Borges VM, Clarêncio J, Bittencourt AP, Freitas R, et al. Prognostic value of cytokines and chemokines in addition to the GRACE score in non-ST-elevation acute coronary syndromes. Clin Chim Acta (2010) 411:540–5. doi:10.1016/j.cca.2010.01.011
96. Abbas A, Gregersen I, Holm S, Daissormont I, Bjerkeli V, Krohg-Sørensen K, et al. Interleukin 23 levels are increased in carotid atherosclerosis. Stroke (2015) 46:793–9. doi:10.1161/STROKEAHA.114.006516
97. Uyemura LL, Demer SC, Castle D, Jullien JA, Berliner MK, Gately RR, et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest (1996) 97:2130–8. doi:10.1172/JCI118650
98. Yong K, Dogra G, Boudville N, Chan D, Adams L, Ching H, et al. Interleukin-12 is associated with arterial stiffness in healthy individuals. Am J Hypertens (2013) 26:159–62. doi:10.1093/ajh/hps032
99. Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet (2008) 371:1675–84. doi:10.1016/S0140-6736(08)60726-6
100. Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet (2008) 371:1665–74. doi:10.1016/S0140-6736(08)60725-4
101. Gordon KB, Langley RG, Gottlieb AB, Papp KA, Krueger GG, Strober BE, et al. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol (2012) 132:304–14. doi:10.1038/jid.2011.304
102. Gottlieb AB, Leonardi C, Kerdel F, Mehlis S, Olds M, Williams DA. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol (2011) 165:652–60. doi:10.1111/j.1365-2133.2011.10418.x
103. Strober BE, Crowley JJ, Yamauchi PS, Olds M, Williams DA. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol (2011) 165:661–8. doi:10.1111/j.1365-2133.2011.10419.x
104. Ryan C, Leonardi CL, Krueger JG, Kimball AB, Strober BE, Gordon KB, et al. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA (2011) 306:864–71. doi:10.1001/jama.2011.1211
105. Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med (2007) 26:53–77. doi:10.1002/sim.2528
106. Dommasch ED, Troxel AB, Gelfand JM. Major cardiovascular events associated with anti-IL 12/23 agents: a tale of two meta-analyses. J Am Acad Dermatol (2013) 68:863–5. doi:10.1016/j.jaad.2013.01.011
107. Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med (2002) 21:1575–600. doi:10.1002/sim.1188
108. Tzellos T, Kyrgidis A, Toulis K. Biologic therapies for chronic plaque psoriasis and cardiovascular events. JAMA (2011) 306:2095. doi:10.1001/jama.2011.1660
109. Kimball AB, Papp KA, Wasfi Y, Chan D, Bissonnette R, Sofen H, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol (2012) 27:1535–45. doi:10.1111/jdv.12046
110. Gottlieb A, Menter A, Mendelsohn A, Shen Y-K, Li S, Guzzo C, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet (2009) 373:633–40. doi:10.1016/S0140-6736(09)60140-9
111. Sandborn WJ, Feagan BG, Fedorak RN, Scherl E, Fleisher MR, Katz S, et al. A randomized trial of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology (2008) 135:1130–41. doi:10.1053/j.gastro.2008.07.014
112. Sandborn WJ, Gasink C, Gao L-L, Blank MA, Johanns J, Guzzo C, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med (2012) 367:1519–28. doi:10.1056/NEJMoa1203572
113. Callen JP. Are Major Adverse Cardiovascular Events Associated With Anti–IL-12/23 Therapies? (2017). Available from: http://www.jwatch.org/jd201109160000001/2011/09/16/are-major-adverse-cardiovascular-events (Accessed: January 17, 2017).
114. Reddy M, Torres G, McCormick T, Marano C, Cooper K, Yeilding N, et al. Positive treatment effects of ustekinumab in psoriasis: analysis of lesional and systemic parameters. J Dermatol (2010) 37:413–25. doi:10.1111/j.1346-8138.2010.00802.x
115. Taleb S, Tedgui A, Mallat Z. IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler Thromb Vasc Biol (2015) 35:258–64. doi:10.1161/ATVBAHA.114.303567
116. Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med (2009) 206:35–41. doi:10.1084/jem.20072713
117. Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol (2010) 130:1373–83. doi:10.1038/jid.2009.399
118. Wu D, Hou SY, Zhao S, Hou LX, Jiao T, Xu NN, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol (2017) 31:992–1003. doi:10.1111/jdv.14125
119. Erbel C, Dengler TJ, Wangler S, Lasitschka F, Bea F, Wambsganss N, et al. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res Cardiol (2011) 106:125–34. doi:10.1007/s00395-010-0135-y
120. Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med (2009) 206:2067–77. doi:10.1084/jem.20090545
121. Hashmi S, Zeng QT. Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron Artery Dis (2006) 17:699–706. doi:10.1097/01.mca.0000236288.94553.b4
122. Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol (2008) 127:89–97. doi:10.1016/j.clim.2008.01.009
123. Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation (2009) 119:1424–32. doi:10.1161/CIRCULATIONAHA.108.827618
124. Simon T, Taleb S, Danchin N, Laurans L, Rousseau B, Cattan S, et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur Heart J (2013) 34:570–7. doi:10.1093/eurheartj/ehs263
125. Golden JB, McCormick TS, Ward NL. IL-17 in psoriasis: implications for therapy and cardiovascular co-morbidities. Cytokine (2013) 62:195–201. doi:10.1016/j.cyto.2013.03.013
126. Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med (2016) 375:345–56. doi:10.1056/NEJMoa1512711
Keywords: anti-IL12/23, anti-IL17, anti-tumor necrosis factor-alpha, atherosclerosis, cardiovascular risk, psoriasis
Citation: Caiazzo G, Fabbrocini G, Di Caprio R, Raimondo A, Scala E, Balato N and Balato A (2018) Psoriasis, Cardiovascular Events, and Biologics: Lights and Shadows. Front. Immunol. 9:1668. doi: 10.3389/fimmu.2018.01668
Received: 28 March 2018; Accepted: 04 July 2018;
Published: 13 August 2018
Edited by:
Nicolò Costantino Brembilla, Université de Genève, SwitzerlandReviewed by:
Mausita Karmakar, Case Western Reserve University, United StatesCopyright: © 2018 Caiazzo, Fabbrocini, Di Caprio, Raimondo, Scala, Balato and Balato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Balato, YW5uYWJhbGF0b0B5YWhvby5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.