- 1Centre for Inflammatory Diseases, Department of Medicine, Monash Medical Centre, Monash University, Clayton, VIC, Australia
- 2Department of Immunology, Monash University, Prahran, VIC, Australia
Immune cell recruitment and migration is central to the normal functioning of the immune system in health and disease. Numerous adhesion molecules on immune cells and the parenchymal cells they interact with are well recognized for their roles in facilitating the movements of immune cells throughout the body. A growing body of evidence now indicates that tetraspanins, proteins known for their capacity to organize partner molecules within the cell membrane, also have significant impacts on the ability of immune cells to migrate around the body. In this review, we examine the tetraspanins expressed by immune cells and endothelial cells that influence leukocyte recruitment and motility and describe their impacts on the function of adhesion molecules and other partner molecules that modulate the movements of leukocytes. In particular, we examine the functional roles of CD9, CD37, CD63, CD81, CD82, and CD151. This reveals the diversity of the functions of the tetraspanin family in this setting, both in the nature of adhesive and migratory interactions that they regulate, and the positive or inhibitory effects mediated by the individual tetraspanin proteins.
Introduction
The ability of leukocytes to migrate from the circulation to sites of inflammation is essential for effective host defense. To undertake this journey, leukocytes undergo a series of interactions in the bloodstream with endothelial cells lining the vasculature (1, 2). The critical roles of cell surface-expressed adhesion molecules on leukocytes and vascular endothelial cells in mediating these interactions are well established. Less appreciated is the emerging evidence indicating important contributions for members of the tetraspanin family of cell membrane proteins in this process. Tetraspanins work differently to classical adhesion molecules; they do not have ligands on other cells, but regulate the actions of target molecules in cis, i.e., expressed in the same cell. In this review, we will analyze the developing knowledge on the role of tetraspanins in controlling the movements of immune cells.
Leukocyte Recruitment is a Sequential, Multistep Process
Recruitment of leukocytes from the circulation is essential both to homeostatic immune surveillance and the response to infection and injury. In innate inflammation, neutrophils and monocytes undergo rapid recruitment to the affected site to mediate the appropriate response (1). Similarly, in adaptive immunity, the recirculation and trafficking of B and T lymphocytes is crucial to ongoing surveillance against potential invading pathogens (2). In both cases, leukocytes leave the bloodstream via a sequence of steps, collectively known as the leukocyte recruitment cascade. This involves interactions mediated by an array of adhesion molecules that function cooperatively to arrest the cell on the endothelial surface and facilitate its transmigration into the surrounding tissue (3). The main sequential steps in leukocyte recruitment are rolling, adhesion, crawling, and transmigration (1, 3), and tetraspanin family members have been shown to contribute to each of these steps (Figure 1).
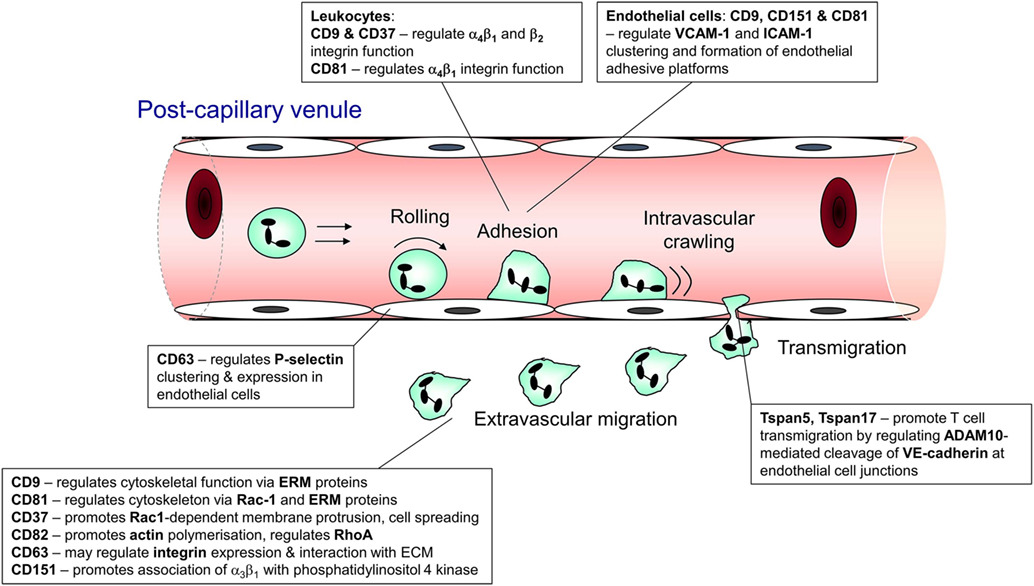
Figure 1. Steps in leukocyte trafficking influenced by tetraspanins. Image shows the sequence of interactions undergone by leukocytes during their recruitment from the bloodstream and after they exit the vasculature, with the tetraspanins that influence these interactions shown adjacent to the interaction. This information is taken from the following publications: CD63 (4, 5); CD9 (6, 7); CD37 (8, 9); CD81 (10, 11); Tspan 5 and 17 (12); CD82 (9); and CD151 (13, 14).
Selectins Mediate Early Interactions During Leukocyte Recruitment
The initial interactions between leukocytes and the activated vascular endothelium are mediated by the selectins. The selectin family consists of three members; L-selectin, which is constitutively expressed on leukocytes (15), and E- and P-selectin, found on activated endothelial cells (16–18). The selectins show overlapping properties and are able to interact with ligands such as P-selectin glycoprotein ligand 1 via recognition of the crucial SLex carbohydrate motif (19–21). The rapid on–off interactions mediated by selectins and their ligands allow for the initial capture of rapidly moving leukocytes in the bloodstream and their subsequent rolling along the vessel wall (22–26).
Integrins Mediate Arrest of Leukocytes on the Endothelium
Leukocyte integrins are the main adhesion molecules responsible for mediating leukocyte firm adhesion to the endothelium. G protein-coupled chemoattractant receptors expressed on the surface of rolling leukocytes are able to detect and respond to chemoattractants present within the microvasculature (27, 28). These signals rapidly (sub-second) induce integrins to undergo a conformational change from a low affinity to high affinity form, leading to integrin-dependent arrest of the leukocyte (27, 29, 30). The key integrins on circulating leukocytes are the β2 integrins LFA-1 (αLβ2) and Mac-1 (αMβ2), which interact with their ligands on endothelial cells including ICAM-1 and ICAM-2, and the α4 integrins VLA-4 (α4β1) and α4β7 which interact with VCAM-1 and MAdCAM-1, respectively (3, 31). After arrest, integrin-mediated outside-in signaling promotes the strengthening of adhesion to the endothelium (3, 32).
Intravascular Crawling and Transmigration
Integrins also contribute to processes downstream of leukocyte adhesion, particularly intraluminal crawling and transmigration. Upon integrin binding, signal transduction results in the alteration of the internal dynamics of the cell; cytoskeletal changes allow for pseudopodia formation and intraluminal crawling along the endothelium. Crawling allows leukocytes to scan the endothelium for suitable locations for transmigration (33). Transmigration occurs predominantly at inter-endothelial cell junctions (paracellular transmigration), where leukocytes initiate transmigration via extension of uropods into the junction before migrating through. While paracellular migration is the predominant mode of transendothelial migration, under some circumstances leukocytes cross the endothelial barrier by migrating directly through endothelial cells, in what is termed transcellular migration (34, 35). Various adhesion molecules have roles in transmigration, including PECAM-1, CD99, JAM-A, β2 and β1 integrins, and L-selectin (36–39). Leukocytes then migrate through the interstitium by following a chemotactic gradient to the source of inflammation, a process involving further interactions of leukocyte integrins with extracellular matrix (ECM) ligands (3).
Immune Cell Migration and the Cytoskeleton
Immune cell motility and directional migration requires the formation of lamellipodia at the leading edge with adhesion to ECM matrix proteins, while simultaneously there is a requirement for detachment at the trailing edge (40). These tightly regulated events require coordinated assembly and disassembly of actin and myosin filaments, processes heavily influenced by members of the Rho family of GTPases. Here, Rac1 regulates actin polymerization at the lamellipodia, while RhoA influences the contraction of actin at the rear of the cell, allowing for forward movement. Meanwhile, evidence indicates Cdc42 is involved in controlling the direction of migration (40).
Dendritic cell (DC) migration is essential for the initiation of the adaptive immune response and exemplifies the importance of cytoskeletal rearrangement in immune cell migration. Here, migration is driven by chemotactic gradients that guide DCs in the interstitium to the lymphatic microvasculature en route to local draining lymph nodes (41). The role of adhesion molecules in DC migration is less clear, with evidence supporting both adhesion-dependent and -independent modes of migration (42). For the latter mode, it is apparent that actin polymerization and cytoskeletal rearrangement are of critical importance (43).
Tetraspanins: Organizers of the Surface Membrane
Successful interactions between a receptor–ligand pair result in the generation of intracellular signals that alter the environmental dynamics of the cell. However, for receptors to productively interact with their ligands and efficiently transduce signals, they must be organized at the cell surface. Tetraspanins are a family of 33 membrane proteins (in humans) which are central to this membrane organization (44). Tetraspanins have the ability to interact and cluster with an array of tetraspanin and non-tetraspanin partners within the cell membrane, forming organized networks of signal transducing complexes termed tetraspanin-enriched microdomains (TEMs) (45–48).
Tetraspanins are distinguished from other four transmembrane proteins by the presence of key conserved amino acid residues located in the transmembrane regions, as well as in the large extracellular loop (LEL) (48). At least in the recently solved X-ray structure of the tetraspanin CD81, the four transmembrane domains form alpha helices that create an intramembrane cholesterol-binding pocket (49). Historically, the LEL of tetraspanins has been the predominant structure studied as it contains the sites responsible for generating protein–protein interactions (45). In addition, much attention has focused on the cytoplasmic domains which can interact with signaling molecules and contain conserved membrane-proximal cysteine residues that are palmitoylation sites (48) which aid in the stabilization of tetraspanin–tetraspanin interactions (50), and contribute to the formation of the TEMs (48).
The Diversity of Tetraspanin Interactions
Although tetraspanins lack conventional ligands, they interact with a diverse assortment of molecules within the TEM (51). Recent super-resolution microscopy studies indicate a considerable heterogeneity among TEMs, in that tetraspanins such as CD53 form nanoclusters in the plasma membrane, and are more likely to be directly associated with non-tetraspanin partners than with other tetraspanin family members (52). This diversity of molecular interactions and heterogeneity of microdomains explain the pleiotropic functions a single tetraspanin may play. CD81 is an excellent example: in macrophages CD81 suppresses cell growth (53), while in B cells, CD81 regulates CD19 expression, lowering the threshold for activation, and promotes adhesiveness of the α4β1 integrin (10, 54). In T cells, CD81 interacts with CD3ζ of the TCR, regulating T cell activation in response to antigen recognition (55) as well as controlling sustained T cell activation following antigen presentation through interactions with both CD3ζ and ICAM-1 (56).
Role of Tetraspanins in Immune Cell Migration and Recruitment
The role of tetraspanins in cellular migration has been examined in detail in regards to tumor cells. However, a series of more recent studies now implicate an important role of tetraspanins including CD9, CD37, CD63, CD81, CD82, CD151, and Tspan5 and Tspan17 in immune cell migration (Figure 1) (57). Here, there appears to be two mechanisms at play that are not mutually exclusive. First, many cell membrane-expressed adhesion molecules are tetraspanin-partner proteins, and their adhesive function and downstream intracellular signaling are regulated by tetraspanins (Figures 2 and 3). Second, extracellular signals stimulating migration have to be communicated to the cytoskeleton for cytoskeletal reorganization to occur. Here, tetraspanins may play a key role through their communication with Rho GTPases and other cytoskeleton-associated proteins (Figure 2).
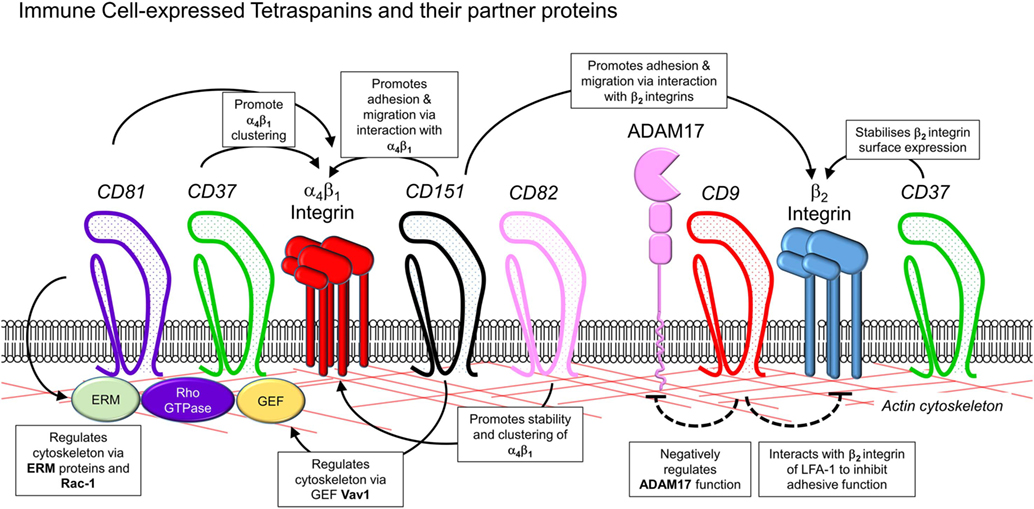
Figure 2. Interactions of leukocyte-expressed tetraspanins and co-expressed molecules involved in leukocyte trafficking. Interactions of tetraspanins expressed in immune cells can occur with other tetraspanins, along with members of several other families of molecules involved in control of adhesion and cytoskeletal function. These include β1 and β2 integrins, metalloproteases such as ADAM17, adhesion molecules of the immunoglobulin superfamily such as ICAM-1, the actin cytoskeleton, and intracellular signaling molecules such as guanosine exchange factors (Vav1, SLP76), Rho GTPases, and ezrin/radixin/moesin proteins.
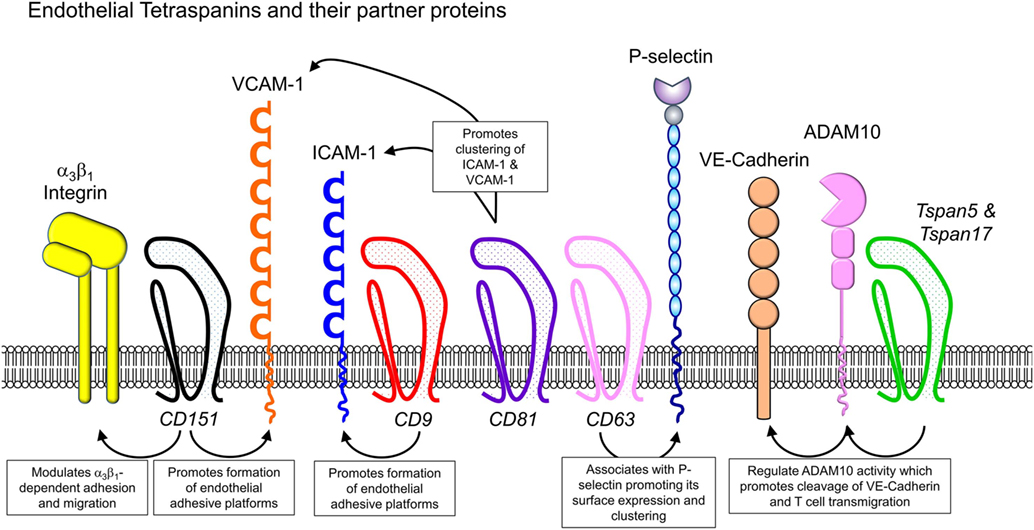
Figure 3. Endothelial cell-expressed tetraspanins and co-expressed proteins relevant to leukocyte trafficking. Endothelial cells play critical roles in directing immune cells from the bloodstream into sites of inflammation or secondary lymphoid organs. Tetraspanins expressed in endothelial cells, including CD9, CD63, CD81, CD82, CD151, and Tspan5 and Tspan17, have been shown to impact on endothelial cell adhesive function, by regulating the function of various adhesion molecules (integrins, ICAM-1, VCAM-1, and P-selectin) and MMPs such as ADAM10.
The tetraspanins that influence leukocyte migration and their mechanisms of action are summarized in Table 1. This review will discuss how tetraspanins expressed in immune cells influence adhesion molecule function and immune cell recruitment, and also examine the functions of tetraspanins expressed in endothelial cells, which play an essential role in directing leukocytes as they migrate through the body.
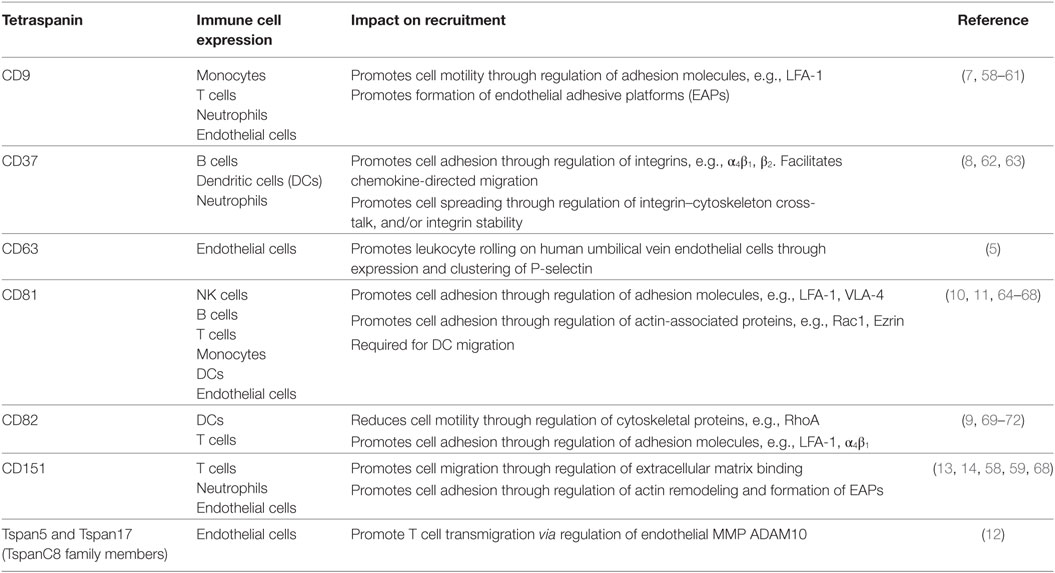
Table 1. Roles of tetraspanin family members in leukocyte–endothelial cell interactions, recruitment, and migration.
Tetraspanins as Regulators of the Leukocyte Integrins
β1 Integrins
The first publication that provided evidence of a role for tetraspanins in immune cell adhesion and migration was the report that the ectopic expression of the tetraspanin CD9 in B cell lines promoted adhesion and haptotactic migration in fibronectin-coated transwells (6). Since then, no less than four tetraspanins have been convincingly reported to regulate α4β1 integrin function in various immune or hemopoietic cells: CD37, CD81, CD82, and CD151 (6, 73–77).
In B cells, there are similarities in the way CD37 and CD81 regulate this key integrin. Tetraspanin CD37 is expressed on most lymphoid cells and has particularly high expression on B cells. Under shear flow conditions in vitro, CD37 was required for optimal B cell rolling and adhesion on fibronectin and VCAM-1. CD37 was found to colocalize with α4β1 in clusters within the B cell membrane and to be essential in the formation of high avidity α4β1 clusters upon ligand binding to VCAM-1 and the subsequent transduction of survival signals through the Akt pathway (62). Similarly, the ubiquitously expressed CD81, which has been shown using biochemical approaches to preferentially interact with α4 integrins, is also essential for α4β1 function (69). CD81 strengthened α4β1-dependent adhesion of B cells and monocytes to VCAM-1 under flow and promoted multivalent integrin interactions (10). Despite these studies, the in vivo implications of CD81-mediated regulation of α4β1 adhesiveness are yet to be determined—neither CD81 nor CD37 has been reported to have a role in B cell migration. By contrast, the many B cell impairments caused by CD81 deficiency have been attributed to impaired CD19 expression (78, 79).
Tetraspanin CD151 is best known for its regulation of the laminin and fibrinogen-binding integrins α3β1, α6β1, and α6β4 in non-immune cells [reviewed in Ref. (73)]. Nonetheless, there is some evidence for a role of CD151 within immune cells, where the principal β1 integrin is α4β1 which mediates leukocyte adhesion to the ECM protein fibronectin and the endothelial cell adhesion molecule VCAM-1. Early studies of the role of CD151 in the immune system focused on CD151 expressed by neutrophils. Inhibition of CD151 via function-blocking antibodies abolished neutrophil migration on the ECM protein fibronectin (13). However, as tetraspanins exist in supramolecular complexes, it is not clear what physiological processes monoclonal antibody (mAb) cross-linking may be mimicking. As such, we have long urged caution in attributing functions to tetraspanins based solely on the use of mAbs (80).
Nonetheless, recent molecular analyses of CD151 function in T cells suggest that CD151 does indeed regulate α4 integrin adhesiveness and immune cell migration (14). This paper, by Zelman-Toister and colleagues, eloquently described CD151-integrin interactions in T cells and showed that low-dose CCL2 modulated CD151 expression and cell migration (14). Immunoprecipitation analyses of T cells exposed to CCL2 revealed CCL2-dependent dissociation of CD151/α4β1 complexes. In addition, ligation of CD151 on the surface of mouse T cells induced actin polymerization through Vav1 phosphorylation and elevated CCL21-induced T cell migration (14). Finally, CD151 ablation on T cells was shown to protect mice from experimental colitis, a result confirmed by interruption of CD151:CD151 associations using an antagonistic peptide to the CD151 LEL (14). The latter reagent resulted in impaired T cell actin remodeling and chemotactic migration in vitro. As CD151 has been shown to directly interact with integrin α but not β chains (81), and as α4β7 is known to be critical for immune cell recruitment to the gut, it is tempting to interpret these data as an effect of CD151 on α4β7 rather than α4β1 function. However, a biochemical interaction between any tetraspanin and α4β7 integrin has not been reported. Nonetheless, these findings illustrate that the CD151 tetraspanin directly affects leukocyte migration and importantly that this role extends to the capacity to influence inflammatory responses.
Finally, the tetraspanin CD82 has also been reported to interact with several integrins including α4β1 (69, 82). In hemopoietic stem cells (HSC), the CD82/α4β1 axis has functional relevance. These two molecules colocalize to a polarized membrane domain that has been implicated in mediating HSC adhesion to osteoblasts. Treating HSCs with mAbs against CD82 impairs both their homing to the bone marrow and adhesion to osteoblasts (83). Elegant analyses by super-resolution microscopy in a transfection system using a leukemic progenitor cell line demonstrated that CD82 expression promoted adhesion to fibronectin by promoting both the stability of α4β1 at the cell surface and the formation of high avidity α4β1 clusters (84).
Certainly, CD37, CD81, CD82, and to a lesser extent, CD151 are co-expressed in many immune cell types. Why there appears to be functional overlap where all four tetraspanins can promote α4β1 clustering and adhesiveness and whether there is interplay between the tetraspanins in regulating this integrin is not known. It will be interesting to determine whether these tetraspanins exist within the same microdomain together with α4β1, or whether distinct tetraspanin/α4β1 complexes exist.
β2 Integrins
In contrast to the well-described interactions between tetraspanins and the β1 integrins, the literature implicating molecular and functional interaction between tetraspanins and β2 integrins is less extensive. Nonetheless, CD63, CD82, and CD9 have all been reported to interact with β2 integrins (4, 70, 85–87). The functional consequences of the interactions of CD63 and CD82 with β2 integrins remain to be defined, although CD82 overexpression has been implicated in LFA-1-dependent homotypic and heterotypic cell–cell adhesion (88). However, the CD9/LFA-1 interaction on monocyte and T cell lines has been confirmed using various techniques including co-precipitation with chemical cross-linking and proximity ligation assays. This association is mediated via interaction of the β2 subunit of LFA-1 with the LEL of CD9 and is of functional significance, as CD9 negatively regulates LFA-1 adhesive function. However, the mechanism is not fully elucidated, as CD9 did not affect inside-out signaling or display of high affinity integrin, although it was suggested that CD9 modulates LFA-1 clustering (7). Whether this interaction has a functional impact on leukocyte migration in vivo remains unknown. Deletion of CD9 was observed to restrict neutrophil and macrophage migration in experimental colitis, an effect consistent with dysregulated LFA-1 function. However, further analyses using bone marrow chimeric mice demonstrated that CD9 expressed on non-hematopoietic cells, rather than leukocytes, was required for disease attenuation (89). Given this observation, the importance of leukocyte-expressed CD9 in immune cell migration remains to be determined.
Perhaps the best evidence for the functional regulation of β2 integrins comes from analysis of the CD37−/− mouse. CD37−/− neutrophils display reduced capacity to adhere to β2 integrin ligands in vitro. In vivo, CD37−/− neutrophils also displayed reduced chemokine-induced adhesion in postcapillary venules, as well as dysregulated directional migration in response to chemotactic stimuli. Deletion of CD37 reduced the stability of integrin expression on the surface of activated neutrophils, by promoting an increase in the rate of β2 integrin internalization (8). Thus, CD37 constitutively acts to retain β2 integrins on the cell surface, a function that would act to stabilize leukocyte adhesion. However, despite these findings of a functional link between CD37 and the β2 integrins, super-resolution microscopy analyses failed to reveal significant co-clustering of CD37 and the β2 integrin. Furthermore, the absence of CD37 did not affect β2 integrin clustering (8). Further experiments will be required to understand the molecular basis of this functional interaction.
Tetraspanins as Regulators of the Cytoskeleton and DC Migration
How then can a tetraspanin like CD37, which does not colocalize with the β2 integrin and does not regulate β2 integrin clustering, control integrin adhesiveness, and internalization? A recurring theme in the literature is the concept that the cytoskeletal rearrangements required for cellular polarization, spreading, adhesion, and migration are under the influence of CD37. Indeed, both neutrophils (8) and DCs (63) from CD37−/− mice have been found to be impaired in their capacity to spread and form membrane protrusions on adhesive substrates, processes which are actin-dependent. CD37−/− DCs also displayed impaired adhesion to fibronectin, as well as impairments in migratory function (63). Together, these findings raise the possibility that CD37 functions as a molecular link between integrins and the cytoskeleton, possibly by regulating signaling through the Rho GTPase Rac1 (9). However, the observation that CD37−/− DCs display reduced migration to lymph nodes (63), behavior that can occur in the absence of integrins (43), indicates that CD37 may also regulate integrin-independent migration. The CD81−/− DC phenotype is strikingly similar to that of CD37−/− DCs, in that CD81−/− DCs are also unable to form Rac1-dependent membrane protrusions and show impaired motility and reduced Rac1 activation (11).
However, while migration of CD81−/− DCs on two-dimensional substrates was impeded, their migration in three-dimensional collagen gels was equivalent to that of wild-type DCs (11), indicating that the contribution of CD81 to DC migration is variable, according to the migratory field being encountered. Moreover, these observations are reminiscent of studies showing that DC migration in three-dimensional substrates is unimpeded in the absence of integrins, being dependent instead on actin-mediated cellular contraction and protrusion (43). One possible explanation for this unexpected finding is that in the absence of integrins, actin polymerization and retrograde flow are increased, compensating for reduced capacity to attach to the ECM (90). Interestingly, Quast et al. also observed increased retrograde actin flow in CD81−/− DCs (11). Together, these similar observations seen in the absence of CD81 and integrins provide further evidence of an intimate functional association between these molecular pathways.
By contrast, DCs lacking expression of CD82 are hypermigratory, as shown in both in vitro chemotactic assays and in vivo lymph node homing assays (9). Like CD37−/− and CD81−/− DCs, CD82−/− DCs lack membrane protrusions, but in contrast, spread to a greater extent than wild-type DCs upon adhesion to fibronectin. Thus, the CD82−/− phenotype is associated with a defect in actin polymerization, likely brought about by dysregulation of signaling through another Rho GTPase, RhoA (9). This integrated relationship between CD82 and the cytoskeleton is reminiscent of previous investigations in T cells. Here, CD82 was found to colocalize with F-actin in lipid rafts (71), and cross-linking CD82 induced dynamic morphological changes such as pseudopodia formation, that are dependent on Rho GTPase activity (72, 91).
The precise molecular interactions by which tetraspanins regulate the cytoskeleton are not understood for CD37 and CD82. On the other hand, in T cells, CD81, via its cytoplasmic tail, can interact with Rac1 (92). A further key mechanism bridging membrane proteins to cytoskeletal actin is the ezrin/radixin/moesin (ERM) proteins (93, 94). Tetraspanins CD9 and CD81 have been shown to interact directly with ERM proteins in immune cells (95). In NK cells, cross-linking of CD81 induces phosphorylation of ERM proteins and colocalization of CD81 with phosphorylated ERM at uropods. This is associated with increased cell polarization and migration toward chemoattractants (64). Similarly in B cells and human PBMCs, CD81 cross-linking induces Syk-dependent ezrin phosphorylation and CD81 colocalization with ezrin and polymerized actin (65). Deletion of the C-terminal tail of CD81 resulted in reduced ezrin phosphorylation, providing clear evidence that the association between these molecules impacts on ERM function.
Influence of Tetraspanins on Endothelial Cell Adhesive Function
As leukocytes are required to undergo extensive interactions with, and eventually transmigrate through, the endothelium during the process of recruitment, adhesive function of endothelial cells is equally as important as that of leukocytes in facilitating an appropriate inflammatory response. In addition to their effects in leukocytes, multiple members of the tetraspanin family have been shown to act in endothelial cells to modulate their capacity to support interactions with leukocytes (Figure 3). For instance, CD9 silencing in human umbilical vein endothelial cells (HUVECs) led to decreased ICAM-1 expression and abrogated leukocyte adhesion and transendothelial migration under flow conditions (58). Subsequent analysis of this phenomenon revealed that both CD9 and CD151 are integral to the formation of membrane structures termed endothelial adhesive platforms (EAPs) in which ICAM-1 and VCAM-1 cluster in the endothelial cell membrane at contact sites with adherent leukocytes (59). This leads to increased avidity for leukocyte integrin ligands and tetraspanin-dependent promotion of leukocyte adhesion and transmigration. The role of CD9 in promoting endothelial cell adhesive function was further examined in a study that used atomic force microscopy to examine the morphology of the endothelial surface at high resolution (60). In response to TNF stimulation, F-actin-containing microvilli decorated with ICAM-1 formed on the endothelium. While these structures developed in the absence of adherent leukocytes, they were thought to serve as precursors for EAPs that form around adherent immune cells (58). CD9 was also incorporated in these structures, and CD9-siRNA studies demonstrated that their formation required CD9. These studies provide further evidence for a role for CD9 in endothelial cells in supporting leukocyte adhesion and recruitment (60).
Endothelial cell-expressed CD81 has also been shown to contribute to leukocyte–endothelial cell interactions. In early atherosclerotic lesions, where monocyte–endothelial interactions are increased, endothelial cells express CD81 at elevated levels (66). Via confocal microscopy of TNF-treated endothelial cells, CD81 was found to colocalize with both VCAM-1 and ICAM-1 at contact sites with monocytes. Furthermore, forced expression of CD81 in endothelial cells was sufficient to increase monocyte adhesion to endothelial monolayers, notably without a requirement for stimulation of the endothelium with inflammatory mediators. This increased adhesion was dependent on endothelial ICAM-1 and VCAM-1 but occurred in the absence of upregulation of these adhesion molecules (66). By contrast, overexpression of CD81 increased clustering of ICAM-1 and VCAM-1, with this increased avidity likely to facilitate monocyte adhesion. These findings indicate a role for CD81 in the redistribution of ICAM-1 and VCAM-1 into adhesion-supporting clusters within the endothelial cell membrane (66). This is paralleled by studies in T cells which demonstrate that CD81 influences leukocyte recruitment by promoting integrin avidity (10, 67).
Weibel–Palade bodies in endothelial cells are secretory vesicles that contain von Willebrand factor and P-selectin. These vesicles are released upon endothelial cell activation and aid in promotion of leukocyte rolling in response to acute inflammatory stimuli and hemostasis (96, 97). It has been long established that the tetraspanin CD63 is an additional major component of these structures, although its function was not clear. This was addressed in studies in which CD63 expression was silenced in HUVECs using siRNA (5). CD63-deficient HUVECs showed a reduced capacity to support leukocyte rolling, findings supported by in vivo analyses of leukocyte rolling in postcapillary venules of CD63−/− mice. The nature of the association between CD63 and P-selectin was examined using immunogold scanning electron microscopy in HUVECs, revealing that CD63 clustered with P-selectin on the endothelial cell surface. In addition, proximity ligation assays in HEK293 cells showed that surface CD63 and P-selectin colocalized within 20–30 nm of each other (5). Finally, in the absence of CD63, both P-selectin clustering and the level of P-selectin surface expression were reduced relative to non-silenced cells. This indicated that CD63 is a molecular partner of P-selectin and supports its clustering with this being essential for the capacity of P-selectin to mediate rolling.
Metalloproteases (MP) are also tetraspanin-partner proteins. For example, CD9 is a molecular partner and negative regulator of MP ADAM17 (61), substrates of which include ICAM-1 and L-selectin (98). Similarly, the TspanC8 subfamily of tetraspanins (consisting of six members: Tspan5, 10, 14, 15, 17, and 33) have been reported to regulate ADAM10 (99), the targets of which include Notch proteins, amyloid precursor protein associated with Alzheimer’s disease (100), and adhesion molecules. In regards to leukocyte recruitment, TspanC8 members Tspan5 and Tspan17 promote transmigration of T cells by regulating cleavage of VE-cadherin on endothelial cells (12). VE-cadherin is an ADAM10 substrate, and its cleavage is a necessary step toward the completion of T cell transmigration (101). Reyat et al. demonstrated that endothelial ADAM10 function is regulated by Tspan5 and Tspan17, and that silencing of these tetraspanins in HUVECs resulted in inhibition of T lymphocyte transmigration (12). The mechanisms whereby TspanC8 subgroup tetraspanins regulate ADAM10 activity are thought to include effects on intracellular trafficking, promoting ADAM10 exit from the endoplasmic reticulum and enzymatic processing, promoting cleavage of ADAM10 into its mature form (102).
Tetraspanins are also known to be highly expressed on extracellular vesicles (EVs) (103). EVs are membrane-bound subcellular particles released by a wide range of cells, including immune cells such as DCs, macrophages, B cells and endothelial cells (104, 105). Exosomes, EVs in the ~50–100 nm diameter range, are enriched in CD9, CD37, CD53 CD63, CD81, and CD82, with the relative abundance of different tetraspanins varying according to the cell of origin. In many cases, these particles also carry adhesion molecules (103). Furthermore, in some circumstances, EVs have been shown capable of modulating immune cell migration (106–110). However, whether the tetraspanins contained within EVs contribute to this effect on immune cell migration requires further investigation.
Future Directions
Other tetraspanins may also be worthy of investigation for their actions in leukocyte recruitment. For example, a small case study of individuals deficient in the leukocyte-restricted tetraspanin CD53 revealed that they were affected by recurrent bacterial infections (111, 112). This observation is consistent with this genetic defect resulting in a form of immune deficiency. As effective combat of bacteria by neutrophils is heavily reliant upon their migratory capacity, these observations raise the yet to be investigated possibility of a role for CD53 in these activities.
There is no question that the tetraspanin family of transmembrane molecules is instrumental in ensuring the correct functioning of proteins and processes involved during immune cell migration. Of particular interest is the ability of tetraspanins to functionally associate with the cytoskeleton and influence the remodeling of actin filaments to produce extensions such as lamellipodia and filopodia, structures which are required for leukocyte recruitment and migration. As detailed, some tetraspanins, such as CD82, can elicit inhibitory effects on cell migration, while others, including CD37 and CD151, are able to enhance recruitment events. Though research in this field has documented numerous tetraspanin partners and downstream signaling pathways modulated by these interactions, there is still much to be learnt about the significance of these interactions during immune cell migration. Further investigation into tetraspanin regulation of localization and clustering of integrins and other adhesion molecules in the cell membrane are warranted. In addition, an important distinction that needs to be made is how applicable these mechanisms of regulation are to different immune cell subsets, as tetraspanins have been repeatedly demonstrated to mediate different functions in different cell types. Finally, and most importantly, the influence of these functions on leukocyte recruitment and behavior in vivo during inflammatory responses must be examined, to determine if these molecules have potential as therapeutic targets in inflammatory disease.
Author Contributions
LY wrote the initial draft of the manuscript and was involved in its editing. MH is an expert in leukocyte recruitment and had particular responsibility for the parts of the manuscript dealing with this topic. He had a major role in the editing of the manuscript and made the figures. MW is an expert in tetraspanin immunology and had particular responsibility for the parts of the manuscript dealing with this topic. He also made suggestions to amend the figures and edited the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol (2013) 13(3):159–75. doi:10.1038/nri3399
2. Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol (2005) 6(12):1182–90. doi:10.1038/ni1275
3. Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol (2007) 7(9):678–89. doi:10.1038/nri2156
4. Mantegazza AR, Barrio MM, Moutel S, Bover L, Weck M, Brossart P, et al. CD63 tetraspanin slows down cell migration and translocates to the endosomal-lysosomal-MIICs route after extracellular stimuli in human immature dendritic cells. Blood (2004) 104(4):1183–90. doi:10.1182/blood-2004-01-0104
5. Doyle EL, Ridger V, Ferraro F, Turmaine M, Saftig P, Cutler DF. CD63 is an essential cofactor to leukocyte recruitment by endothelial P-selectin. Blood (2011) 118(15):4265–73. doi:10.1182/blood-2010-11-321489
6. Shaw AR, Domanska A, Mak A, Gilchrist A, Dobler K, Visser L, et al. Ectopic expression of human and feline CD9 in a human B cell line confers beta 1 integrin-dependent motility on fibronectin and laminin substrates and enhanced tyrosine phosphorylation. J Biol Chem (1995) 270(41):24092–9. doi:10.1074/jbc.270.41.24092
7. Reyes R, Monjas A, Yánez-Mó M, Cardeñes B, Morlino G, Gilsanz A, et al. Different states of integrin LFA-1 aggregation are controlled through its association with tetraspanin CD9. Biochim Biophys Acta (2015) 1853(10 Pt A):2464–80. doi:10.1016/j.bbamcr.2015.05.018
8. Wee JL, Schulze KE, Jones EL, Yeung L, Cheng Q, Pereira CF, et al. Tetraspanin CD37 regulates β2 integrin-mediated adhesion and migration in neutrophils. J Immunol (2015) 195(12):5770–9. doi:10.4049/jimmunol.1402414
9. Jones EL, Wee JL, Demaria MC, Blakeley J, Ho PK, Vega-Ramos J, et al. Dendritic cell migration and antigen presentation are coordinated by the opposing functions of the tetraspanins CD82 and CD37. J Immunol (2016) 196(3):978–87. doi:10.4049/jimmunol.1500357
10. Feigelson SW, Grabovsky V, Shamri R, Levy S, Alon R. The CD81 tetraspanin facilitates instantaneous leukocyte VLA-4 adhesion strengthening to vascular cell adhesion molecule 1 (VCAM-1) under shear flow. J Biol Chem (2003) 278(51):51203–12. doi:10.1074/jbc.M303601200
11. Quast T, Eppler F, Semmling V, Schild C, Homsi Y, Levy S, et al. CD81 is essential for the formation of membrane protrusions and regulates Rac1-activation in adhesion-dependent immune cell migration. Blood (2011) 118(7):1818–27. doi:10.1182/blood-2010-12-326595
12. Reyat JS, Chimen M, Noy PJ, Szyroka J, Rainger GE, Tomlinson MG. ADAM10-interacting tetraspanins Tspan5 and Tspan17 regulate VE-cadherin expression and promote T lymphocyte transmigration. J Immunol (2017) 199(2):666–76. doi:10.4049/jimmunol.1600713
13. Yauch RL, Berditchevski F, Harler MB, Reichner J, Hemler ME. Highly stoichiometric, stable, and specific association of integrin alpha3beta1 with CD151 provides a major link to phosphatidylinositol 4-kinase, and may regulate cell migration. Mol Biol Cell (1998) 9(10):2751–65. doi:10.1091/mbc.9.10.2751
14. Zelman-Toister E, Bakos E, Cohen S, Zigmond E, Shezen E, Grabovsky V, et al. CD151 regulates T-cell migration in health and inflammatory bowel disease. Inflamm Bowel Dis (2016) 22(2):257–67. doi:10.1097/MIB.0000000000000621
15. Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature (1982) 304(5921):30–4. doi:10.1038/304030a0
16. Bevilacqua MP, Stengelin S, Gimbrone MA Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science (1989) 243(4895):1160–5. doi:10.1126/science.2466335
17. Geng JG, Bevilacqua MP, Moore KL, McIntyre TM, Prescott SM, Kim JM, et al. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature (1990) 343(6260):757–60. doi:10.1038/343757a0
18. Johnston GI, Cook RG, McEver RP. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell (1989) 56(6):1033–44. doi:10.1016/0092-8674(89)90636-3
19. Moore KL, Patel KD, Bruehl RE, Li F, Johnson DA, Lichenstein HS, et al. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol (1995) 128(4):661–71. doi:10.1083/jcb.128.4.661
20. Asa D, Raycroft L, Ma L, Aeed PA, Kaytes PS, Elhammer AP, et al. The P-selectin glycoprotein ligand functions as a common human leukocyte ligand for P- and E-selectins. J Biol Chem (1995) 270(19):11662–70. doi:10.1074/jbc.270.19.11662
21. Spertini O, Cordey AS, Monai N, Giuffre L, Schapira M. P-selectin glycoprotein ligand 1 is a ligand for L-selectin on neutrophils, monocytes, and CD34+ hematopoietic progenitor cells. J Cell Biol (1996) 135(2):523–31. doi:10.1083/jcb.135.2.523
22. Tedder TF, Steeber DA, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J (1995) 9(10):866–73. doi:10.1096/fasebj.9.10.7542213
23. Arbonés ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity (1994) 1(4):247–60. doi:10.1016/1074-7613(94)90076-0
24. Tedder TF, Steeber DA, Pizcueta P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med (1995) 181(6):2259–64. doi:10.1084/jem.181.6.2259
25. Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell (1993) 74(3):541–54. doi:10.1016/0092-8674(93)80055-J
26. Yang J, Hirata T, Croce K, Merrill-Skoloff G, Tchernychev B, Williams E, et al. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med (1999) 190(12):1769–82. doi:10.1084/jem.190.12.1769
27. Mocsai A, Walzog B, Lowell CA. Intracellular signalling during neutrophil recruitment. Cardiovasc Res (2015) 107(3):373–85. doi:10.1093/cvr/cvv159
28. Ley K. Integration of inflammatory signals by rolling neutrophils. Immunol Rev (2002) 186:8–18. doi:10.1034/j.1600-065X.2002.18602.x
29. Smith DF, Deem TL, Bruce AC, Reutershan J, Wu D, Ley K. Leukocyte phosphoinositide-3 kinase {gamma} is required for chemokine-induced, sustained adhesion under flow in vivo. J Leukoc Biol (2006) 80(6):1491–9. doi:10.1189/jlb.0306227
30. Hyduk SJ, Chan JR, Duffy ST, Chen M, Peterson MD, Waddell TK, et al. Phospholipase C, calcium, and calmodulin are critical for alpha4beta1 integrin affinity up-regulation and monocyte arrest triggered by chemoattractants. Blood (2007) 109(1):176–84. doi:10.1182/blood-2006-01-029199
31. Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol (1999) 163(9):5029–38.
32. Gakidis MA, Cullere X, Olson T, Wilsbacher JL, Zhang B, Moores SL, et al. Vav GEFs are required for beta2 integrin-dependent functions of neutrophils. J Cell Biol (2004) 166(2):273–82. doi:10.1083/jcb.200404166
33. Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med (2006) 203(12):2569–75. doi:10.1084/jem.20060925
34. Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol (2006) 8(2):156–62. doi:10.1038/ncb1355
35. Mamdouh Z, Mikhailov A, Muller WA. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J Exp Med (2009) 206(12):2795–808. doi:10.1084/jem.20082745
36. Muller WA. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol (2014) 184(4):886–96. doi:10.1016/j.ajpath.2013.12.033
37. Wang S, Dangerfield JP, Young RE, Nourshargh S. PECAM-1, alpha6 integrins and neutrophil elastase cooperate in mediating neutrophil transmigration. J Cell Sci (2005) 118(Pt 9):2067–76. doi:10.1242/jcs.02340
38. Hyun YM, Sumagin R, Sarangi PP, Lomakina E, Overstreet MG, Baker CM, et al. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J Exp Med (2012) 209(7):1349–62. doi:10.1084/jem.20111426
39. Hickey MJ, Forster M, Mitchell D, Kaur J, De Caigny C, Kubes P. L-selectin facilitates emigration and extravascular locomotion of leukocytes during acute inflammatory responses in vivo. J Immunol (2000) 165(12):7164–70. doi:10.4049/jimmunol.165.12.7164
40. Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol (2004) 265(1):23–32. doi:10.1016/j.ydbio.2003.06.003
41. Tiberio L, Del Prete A, Schioppa T, Sozio F, Bosisio D, Sozzani S. Chemokine and chemotactic signals in dendritic cell migration. Cell Mol Immunol (2018). doi:10.1038/s41423-018-0005-3
42. Worbs T, Hammerschmidt SI, Forster R. Dendritic cell migration in health and disease. Nat Rev Immunol (2017) 17(1):30–48. doi:10.1038/nri.2016.116
43. Lämmermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Söldner R, Hirsch K, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature (2008) 453(7191):51–5. doi:10.1038/nature06887
44. Singethan K, Schneider-Schaulies J. Tetraspanins: small transmembrane proteins with big impact on membrane microdomain structures. Commun Integr Biol (2008) 1(1):11–3. doi:10.4161/cib.1.1.6406
45. Kovalenko OV, Metcalf DG, DeGrado WF, Hemler ME. Structural organization and interactions of transmembrane domains in tetraspanin proteins. BMC Struct Biol (2005) 5:11. doi:10.1186/1472-6807-5-11
46. Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J (1997) 11(6):428–42. doi:10.1096/fasebj.11.6.9194523
47. Hemler ME. Specific tetraspanin functions. J Cell Biol (2001) 155(7):1103–7. doi:10.1083/jcb.200108061
48. Levy S, Shoham T. Protein-protein interactions in the tetraspanin web. Physiology (Bethesda) (2005) 20:218–24. doi:10.1152/physiol.00015.2005
49. Zimmerman B, Kelly B, McMillan BJ, Seegar TCM, Dror RO, Kruse AC, et al. Crystal structure of a full-length human tetraspanin reveals a cholesterol-binding pocket. Cell (2016) 167(4):1041e–51.e11. doi:10.1016/j.cell.2016.09.056
50. Yang X, Kovalenko OV, Tang W, Claas C, Stipp CS, Hemler ME. Palmitoylation supports assembly and function of integrin-tetraspanin complexes. J Cell Biol (2004) 167(6):1231–40. doi:10.1083/jcb.200404100
51. Rubinstein E, Charrin S, Tomlinson MG. Organisation of the tetraspanin web. In: Berditchevski F, Rubinstein E, editors. Tetraspanins. (Vol. 9), Dordrecht: Springer (2013). p. 47–90.
52. Zuidscherwoude M, Gottfert F, Dunlock VM, Figdor CG, van den Bogaart G, van Spriel AB. The tetraspanin web revisited by super-resolution microscopy. Sci Rep (2015) 5:12201. doi:10.1038/srep12201
53. Mordica WJ, Woods KM, Clem RJ, Passarelli AL, Chapes SK. Macrophage cell lines use CD81 in cell growth regulation. In Vitro Cell Dev Biol Anim (2009) 45(5–6):213–25. doi:10.1007/s11626-008-9167-0
54. Luo RF, Zhao S, Tibshirani R, Myklebust JH, Sanyal M, Fernandez R, et al. CD81 protein is expressed at high levels in normal germinal center B cells and in subtypes of human lymphomas. Hum Pathol (2010) 41(2):271–80. doi:10.1016/j.humpath.2009.07.022
55. Cevik SI, Keskin N, Belkaya S, Ozlu MI, Deniz E, Tazebay UH, et al. CD81 interacts with the T cell receptor to suppress signaling. PLoS One (2012) 7(11):e50396. doi:10.1371/journal.pone.0050396
56. Rocha-Perugini V, Zamai M, González-Granado JM, Barreiro O, Tejera E, Yañez-Mó M, et al. CD81 controls sustained T cell activation signaling and defines the maturation stages of cognate immunological synapses. Mol Cell Biol (2013) 33(18):3644–58. doi:10.1128/MCB.00302-13
57. Jiang X, Zhang J, Huang Y. Tetraspanins in cell migration. Cell Adh Migr (2015) 9(5):406–15. doi:10.1080/19336918.2015.1005465
58. Barreiro O, Yáñez-Mó M, Sala-Valdés M, Gutiérrez-López MD, Ovalle S, Higginbottom A, et al. Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood (2005) 105(7):2852–61. doi:10.1182/blood-2004-09-3606
59. Barreiro O, Zamai M, Yáñez-Mó M, Tejera E, López-Romero P, Monk PN, et al. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol (2008) 183(3):527–42. doi:10.1083/jcb.200805076
60. Franz J, Brinkmann BF, König M, Hüve J, Stock C, Ebnet K, et al. Nanoscale imaging reveals a tetraspanin-CD9 coordinated elevation of endothelial ICAM-1 clusters. PLoS One (2016) 11(1):e0146598. doi:10.1371/journal.pone.0146598
61. Gutiérrez-López MD, Gilsanz A, Yáñez-Mó M, Ovalle S, Lafuente EM, Domínguez C, et al. The sheddase activity of ADAM17/TACE is regulated by the tetraspanin CD9. Cell Mol Life Sci (2011) 68(19):3275–92. doi:10.1007/s00018-011-0639-0
62. van Spriel AB, de Keijzer S, van der Schaaf A, Gartlan KH, Sofi M, Light A, et al. The tetraspanin CD37 orchestrates the alpha(4)beta(1) integrin-Akt signaling axis and supports long-lived plasma cell survival. Sci Signal (2012) 5(250):ra82. doi:10.1126/scisignal.2003113
63. Gartlan KH, Wee JL, Demaria MC, Nastovska R, Chang TM, Jones EL, et al. Tetraspanin CD37 contributes to the initiation of cellular immunity by promoting dendritic cell migration. Eur J Immunol (2013) 43(5):1208–19. doi:10.1002/eji.201242730
64. Krämer B, Schulte D, Körner C, Zwank C, Hartmann A, Michalk M, et al. Regulation of NK cell trafficking by CD81. Eur J Immunol (2009) 39(12):3447–58. doi:10.1002/eji.200939234
65. Coffey GP, Rajapaksa R, Liu R, Sharpe O, Kuo CC, Krauss SW, et al. Engagement of CD81 induces ezrin tyrosine phosphorylation and its cellular redistribution with filamentous actin. J Cell Sci (2009) 122(Pt 17):3137–44. doi:10.1242/jcs.045658
66. Rohlena J, Volger OL, van Buul JD, Hekking LH, van Gils JM, Bonta PI, et al. Endothelial CD81 is a marker of early human atherosclerotic plaques and facilitates monocyte adhesion. Cardiovasc Res (2009) 81(1):187–96. doi:10.1093/cvr/cvn256
67. VanCompernolle SE, Levy S, Todd SC. Anti-CD81 activates LFA-1 on T cells and promotes T cell-B cell collaboration. Eur J Immunol (2001) 31(3):823–31. doi:10.1002/1521-4141(200103)31:3<823::AID-IMMU823>3.0.CO;2-D
68. Yáñez-Mó M, Alfranca A, Cabañas C, Marazuela M, Tejedor R, Ursa MA, et al. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with alpha3 beta1 integrin localized at endothelial lateral junctions. J Cell Biol (1998) 141(3):791–804. doi:10.1083/jcb.141.3.791
69. Mannion BA, Berditchevski F, Kraeft SK, Chen LB, Hemler ME. Transmembrane-4 superfamily proteins CD81 (TAPA-1), CD82, CD63, and CD53 specifically associated with integrin alpha 4 beta 1 (CD49d/CD29). J Immunol (1996) 157(5):2039–47.
70. Shibagaki N, Hanada K, Yamashita H, Shimada S, Hamada H. Overexpression of CD82 on human T cells enhances LFA-1/ICAM-1-mediated cell-cell adhesion: functional association between CD82 and LFA-1 in T cell activation. Eur J Immunol (1999) 29(12):4081–91. doi:10.1002/(SICI)1521-4141(199912)29:12<4081::AID-IMMU4081>3.0.CO;2-I
71. Delaguillaumie A, Harriague J, Kohanna S, Bismuth G, Rubinstein E, Seigneuret M, et al. Tetraspanin CD82 controls the association of cholesterol-dependent microdomains with the actin cytoskeleton in T lymphocytes: relevance to co-stimulation. J Cell Sci (2004) 117(Pt 22):5269–82. doi:10.1242/jcs.01380
72. Delaguillaumie A, Lagaudriere-Gesbert C, Popoff MR, Conjeaud H. Rho GTPases link cytoskeletal rearrangements and activation processes induced via the tetraspanin CD82 in T lymphocytes. J Cell Sci (2002) 115:433–43.
73. Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol (2005) 6(10):801–11. doi:10.1038/nrm1736
74. Karamatic Crew V, Burton N, Kagan A, Green CA, Levene C, Flinter F, et al. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood (2004) 104(8):2217–23. doi:10.1182/blood-2004-04-1512
75. Cowin AJ, Adams D, Geary SM, Wright MD, Jones JC, Ashman LK. Wound healing is defective in mice lacking tetraspanin CD151. J Invest Dermatol (2006) 126(3):680–9. doi:10.1038/sj.jid.5700142
76. Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, et al. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol (2006) 175(1):33–9. doi:10.1083/jcb.200603073
77. Sachs N, Claessen N, Aten J, Kreft M, Teske GJ, Koeman A, et al. Blood pressure influences end-stage renal disease of Cd151 knockout mice. J Clin Invest (2012) 122(1):348–58. doi:10.1172/JCI58878
78. Tsitsikov EN, Gutierrez-Ramos JC, Geha RS. Impaired CD19 expression and signaling, enhanced antibody response to type II T independent antigen and reduction of B-1 cells in CD81-deficient mice. Proc Natl Acad Sci U S A (1997) 94(20):10844–9. doi:10.1073/pnas.94.20.10844
79. van Zelm MC, Smet J, Adams B, Mascart F, Schandené L, Janssen F, et al. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J Clin Invest (2010) 120(4):1265–74. doi:10.1172/JCI39748
80. Wright MD, Moseley GW, van Spriel AB. Tetraspanin microdomains in immune cell signalling and malignant disease. Tissue Antigens (2004) 64(5):533–42. doi:10.1111/j.1399-0039.2004.00321.x
81. Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci (2001) 114:4143–51.
82. Miranti CK. Controlling cell surface dynamics and signaling: how CD82/KAI1 suppresses metastasis. Cell Signal (2009) 21(2):196–211. doi:10.1016/j.cellsig.2008.08.023
83. Larochelle A, Gillette JM, Desmond R, Ichwan B, Cantilena A, Cerf A, et al. Bone marrow homing and engraftment of human hematopoietic stem and progenitor cells is mediated by a polarized membrane domain. Blood (2012) 119(8):1848–55. doi:10.1182/blood-2011-08-371583
84. Termini CM, Cotter ML, Marjon KD, Buranda T, Lidke KA, Gillette JM. The membrane scaffold CD82 regulates cell adhesion by altering alpha4 integrin stability and molecular density. Mol Biol Cell (2014) 25(10):1560–73. doi:10.1091/mbc.e13-11-0660
85. Skubitz KM, Campbell KD, Iida J, Skubitz AP. CD63 associates with tyrosine kinase activity and CD11/CD18, and transmits an activation signal in neutrophils. J Immunol (1996) 157(8):3617–26.
86. Skubitz KM, Campbell KD, Skubitz AP. CD63 associates with CD11/CD18 in large detergent-resistant complexes after translocation to the cell surface in human neutrophils. FEBS Lett (2000) 469(1):52–6. doi:10.1016/S0014-5793(00)01240-0
87. Takeda Y, Tachibana I, Miyado K, Kobayashi M, Miyazaki T, Funakoshi T, et al. Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J Cell Biol (2003) 161(5):945–56. doi:10.1083/jcb.200212031
88. Shibagaki N, Hanada K, Yamaguchi S, Yamashita H, Shimada S, Hamada H. Functional analysis of CD82 in the early phase of T cell activation: roles in cell adhesion and signal transduction. Eur J Immunol (1998) 28(4):1125–33. doi:10.1002/(SICI)1521-4141(199804)28:04<1125::AID-IMMU1125>3.0.CO;2-C
89. Saiz ML, Cibrian D, Ramirez-Huesca M, Torralba D, Moreno-Gonzalo O, Sanchez-Madrid F. Tetraspanin CD9 limits mucosal healing in experimental colitis. Front Immunol (2017) 8:1854. doi:10.3389/fimmu.2017.01854
90. Renkawitz J, Schumann K, Weber M, Lämmermann T, Pflicke H, Piel M, et al. Adaptive force transmission in amoeboid cell migration. Nat Cell Biol (2009) 11(12):1438–43. doi:10.1038/ncb1992
91. Lagaudrière-Gesbert C, Le Naour F, Lebel-Binay S, Billard M, Lemichez E, Boquet P, et al. Functional analysis of four tetraspans, CD9, CD53, CD81, and CD82, suggests a common role in costimulation, cell adhesion, and migration: only CD9 upregulates HB-EGF activity. Cell Immunol (1997) 182(2):105–12. doi:10.1006/cimm.1997.1223
92. Tejera E, Rocha-Perugini V, López-Martín S, Pérez-Hernández D, Bachir AI, Horwitz AR, et al. CD81 regulates cell migration through its association with Rac GTPase. Mol Biol Cell (2013) 24(3):261–73. doi:10.1091/mbc.E12-09-0642
93. Tsukita S, Yonemura S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol (1997) 9(1):70–5. doi:10.1016/S0955-0674(97)80154-8
94. Ivetic A, Ridley AJ. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology (2004) 112(2):165–76. doi:10.1111/j.1365-2567.2004.01882.x
95. Sala-Valdés M, Ursa A, Charrin S, Rubinstein E, Hemler ME, Sánchez-Madrid F, et al. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J Biol Chem (2006) 281(28):19665–75. doi:10.1074/jbc.M602116200
96. Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol (1982) 95(1):355–60. doi:10.1083/jcb.95.1.355
97. Vischer UM, Wagner DD. CD63 is a component of Weibel-Palade bodies of human endothelial cells. Blood (1993) 82(4):1184–91.
98. Tsakadze NL, Sithu SD, Sen U, English WR, Murphy G, D’Souza SE. Tumor necrosis factor-alpha-converting enzyme (TACE/ADAM-17) mediates the ectodomain cleavage of intercellular adhesion molecule-1 (ICAM-1). J Biol Chem (2006) 281(6):3157–64. doi:10.1074/jbc.M510797200
99. Haining EJ, Yang J, Bailey RL, Khan K, Collier R, Tsai S, et al. The TspanC8 subgroup of tetraspanins interacts with A disintegrin and metalloprotease 10 (ADAM10) and regulates its maturation and cell surface expression. J Biol Chem (2012) 287(47):39753–65. doi:10.1074/jbc.M112.416503
100. Matthews AL, Szyroka J, Collier R, Noy PJ, Tomlinson MG. Scissor sisters: regulation of ADAM10 by the TspanC8 tetraspanins. Biochem Soc Trans (2017) 45(3):719–30. doi:10.1042/BST20160290
101. Schulz B, Pruessmeyer J, Maretzky T, Ludwig A, Blobel CP, Saftig P, et al. ADAM10 regulates endothelial permeability and T-Cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res (2008) 102(10):1192–201. doi:10.1161/CIRCRESAHA.107.169805
102. Matthews AL, Noy PJ, Reyat JS, Tomlinson MG. Regulation of A disintegrin and metalloproteinase (ADAM) family sheddases ADAM10 and ADAM17: the emerging role of tetraspanins and rhomboids. Platelets (2017) 28(4):333–41. doi:10.1080/09537104.2016.1184751
103. Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol (2014) 5:442. doi:10.3389/fimmu.2014.00442
104. Tkach M, Kowal J, Zucchetti AE, Enserink L, Jouve M, Lankar D, et al. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J (2017) 36(20):3012–28. doi:10.15252/embj.201696003
105. Groot Kormelink T, Mol S, de Jong EC, Wauben MHM. The role of extracellular vesicles when innate meets adaptive. Semin Immunopathol (2018). doi:10.1007/s00281-018-0681-1
106. Esser J, Gehrmann U, D’Alexandri FL, Hidalgo-Estévez AM, Wheelock CE, Scheynius A, et al. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J Allergy Clin Immunol (2010) 126(5):.e1031–4. doi:10.1016/j.jaci.2010.06.039
107. Walters SB, Kieckbusch J, Nagalingam G, Swain A, Latham SL, Grau GE, et al. Microparticles from mycobacteria-infected macrophages promote inflammation and cellular migration. J Immunol (2013) 190(2):669–77. doi:10.4049/jimmunol.1201856
108. Lee HD, Kim YH, Kim DS. Exosomes derived from human macrophages suppress endothelial cell migration by controlling integrin trafficking. Eur J Immunol (2014) 44(4):1156–69. doi:10.1002/eji.201343660
109. Lee H, Zhang D, Wu J, Otterbein LE, Jin Y. Lung epithelial cell-derived microvesicles regulate macrophage migration via microRNA-17/221-induced integrin beta1 recycling. J Immunol (2017) 199(4):1453–64. doi:10.4049/jimmunol.1700165
110. Nguyen MA, Karunakaran D, Geoffrion M, Cheng HS, Tandoc K, Perisic Matic L, et al. Extracellular vesicles secreted by atherogenic macrophages transfer microRNA to inhibit cell migration. Arterioscler Thromb Vasc Biol (2018) 38(1):49–63. doi:10.1161/ATVBAHA.117.309795
111. Mollinedo F, Fontan G, Barasoain I, Lazo PA. Recurrent infectious diseases in human CD53 deficiency. Clin Diagn Lab Immunol (1997) 4(2):229–31.
Keywords: tetraspanin, leukocyte migration, adhesion molecules, inflammation, integrins
Citation: Yeung L, Hickey MJ and Wright MD (2018) The Many and Varied Roles of Tetraspanins in Immune Cell Recruitment and Migration. Front. Immunol. 9:1644. doi: 10.3389/fimmu.2018.01644
Received: 14 April 2018; Accepted: 04 July 2018;
Published: 18 July 2018
Edited by:
Carlos Cabañas, Consejo Superior de Investigaciones Científicas (CSIC), SpainReviewed by:
Francisco Sanchez-Madrid, Universidad Autonoma de Madrid, SpainMartin Johannes Hoogduijn, Erasmus University Rotterdam, Netherlands
Eric Rubinstein, INSERM U935 Modèles de Cellules Souches Malignes et Thérapeutiques, France
Copyright: © 2018 Yeung, Hickey and Wright. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark D. Wright, bWFyay53cmlnaHRAbW9uYXNoLmVkdQ==
 Louisa Yeung
Louisa Yeung Michael J. Hickey
Michael J. Hickey Mark D. Wright
Mark D. Wright