- Department of Preventive Veterinary Medicine, College of Veterinary Medicine, Nanjing Agricultural University, Nanjing, China
Serine/threonine-protein phosphatases (STPs), as integral constituents of parasitic excretory/secretory proteins, are assumed to be released during the host–parasite interactions. However, knowledge about these phosphatases and their immunoregulatory and immune protective efficiencies with host peripheral blood mononuclear cells (PBMCs) is scant. In this study, an open reading frame of STP from Haemonchus contortus designated as HcSTP-1 was amplified and cloned using reverse-transcription-polymerase chain reaction (RT-PCR) method. The 951-bp nucleotides sequence was encoded to a protein of 316 amino acid residues, conserved in characteristics motifs GDXHG, GDYVDRG, GNHE, HGG, RG, and H. The HcSTP-1 protein was detected at approximately 35 kDa as recombinant protein fused in an expression vector system and resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunohistochemically, HcSTP-1 was found to be localized in both male and female adult worm sections. Using immunofluorescence assay, the binding activity of rHcSTP-1 was confirmed on surface of goat PBMCs, which resulted in expression of multiple cytokines and various immunoregulatory activities in vitro. The RT-PCR results showed that mRNA level of interleukin-2, TGF-β1, IFN-γ, and IL-17 (with 10 µg/ml) was upregulated and IL-10 was decreased. However, IL-6 showed no change after PBMCs incubated with rHcSTP-1 protein. Further functional analysis showed that migratory activity of cells, intracellular nitrite production (NO), and apoptotic efficiency of PBMCs were elevated at significant level, whereas the proliferation of goat PBMCs and monocytes-associated major histocompatibility complex (MHC)-I and MHC-II expressions were decreased significantly at concentration-dependent fashion. Our results showed that the HcSTP-1 protein engaged in vital suppressive regulatory roles on host immune cells, which might represent a potential molecular target for controlling H. contortus infection in future.
Introduction
Among gastrointestinal nematodes, Haemonchus contortus (barber pole worm) is most important parasite, responsible for health and economic problems in livestock industry worldwide (1). In severely infected animals like sheep and goats, the adult parasite penetrates in abomasal mucosa to feed on blood, results in anemia, loss of body weight and growth, and even death of the animals (2). Existing approaches for control of nematode parasites including H. contortus, mainly through widespread antihelmintics have resulted in stern drug resistance in domestic livestock (3, 4). Hence, the emergence of drug resistance in H. contortus demands for discoveries of novel anti-parasite drugs, vaccines, and development of immunological control strategies against nematode infections. In this regard, a comprehensive insight into the developmental biology of H. contortus at the molecular level might pinpoint some key antigens as new drug targets for parasite control (5).
The serine/threonine-protein phosphatase 1 (STP-1) belong to phosphoprotein phosphatase (PPP) family, and protein phosphatases (PPs) is largest class which is divided functionally into two major groups: first serine/threonine phosphatases (commonly cytoplasmic structural component, concerned with transcriptional activity and signal transduction) and second tyrosine phosphatases (typically bound with membrane and implicated in receptor-arbitrated signal transduction) (6). PPs can also be divided into four subclasses, such as PP1, PP2A, PP2B, and PP2C, based on substrate and inhibitory specificities (7). Furthermore, it was reported that PPs have been implicated in range of biological functions, such as exocytosis and apoptosis, neuronal activity, ion channel electrophysiology, and cell cycle (8–12).
Previously, various STPs from number of parasites have been identified and characterized, including those of Oesophagostomum dentatum, Trichostrongylus vitrinus, Toxoplasma gondii, Toxocara canis, and Plasmodium falciparum (13–17), and also suggested that STP-1 from H. contortus play a key role in some biological processes, such as spermatogenesis (18). However, information regarding the functions of STPs in parasitic nematode H. contortus is scant.
In our previous comparative proteomics analysis of excretory/secretory proteins (ESPs) from H. contortus (HcESPs), 59 proteins were identified at early and late adult developmental stages of parasite and amongst all, serine/threonine-protein phosphatase (STPs) were recognized as vital interacting proteins bind to the goat peripheral blood mononuclear cells (PBMCs) in vivo (19). The main objectives of current study were to clone and characterize a stage-specific gene from H. contortus designated as HcSTP-1, to express its recombinant protein in an expression vector system, and to evaluate potential immune regulatory roles of this protein (rHcSTP-1) with goat PBMCs in vitro. Our findings will provide valuable bases to better understand the biology of this parasite and to develop effective drugs and useful vaccines, which could be helpful in the prevention and control of H. contortus infection.
Materials and Methods
Ethics Statement
All animals and laboratory experiments were strictly performed in accordance with the recommendations of the Animal Ethics Committee, Nanjing Agricultural University, China. All experimental procedures used in this study were permitted by the science and Technology Agency of Jiangsu Province [ID: SYXK (SU) 2010-0005].
Animals, Parasites, and PBMC Isolation
The native crossbred goats (3–6 months old) were housed indoor, provided with hay and corn (whole shelled) and water ad libitum. The anti-parasitic drug, levamisole (8 mg/kg body weight) was used with 2 weeks interval to keep goats free from naturally acquired helminth infection. The fecal samples were checked twice per week, using standard parasitological techniques and goats with no sign of helminths infection were used in subsequent experiments.
Sprague-Dawley (SD) rats with average body weight of 150 g, were bought at Experimental Animal Center of Jiangsu, PR China (Certified: SCXK 2008-0004). The rats were raised in microbe free condition.
The H. contortus strain was maintained by serial passage in helminth-free goats, at laboratory of immunology and molecular parasitology, Nanjing Agricultural University. The parasite eggs, larvae, and adults worms were collected and preserved according to the methods stated previously (20). The blood samples were taken from jugular vein of dewormed goats and PBMCs were collected by gradient centrifugation method (21). The PBMCs were cultured as the procedure stated previously (22).
Cloning and Sequence Analysis H. contortus STP-1 Gene
The freshly adults H. contortus parasites were collected from abomasum of the infected goats and the extraction of RNA was carried out using Trizol method according to the previously described procedure (23), followed by cDNA synthesis as per the manufacturer’s guidelines of cDNA synthesis Kit (TaKaRa Biotech), and was preserved at −20°C for down-stream applications.
The open reading frame of HcSTP-1 was amplified by reverse-transcription-polymerase chain reaction (RT-PCR), from conserved domain sequences of H. contortus STP-1 gene (GenBank: GQ280010.1). For the subsequent cloning, underlined BamH I and Xho I restriction enzyme were inserted at the 5′-end of forward primer: (5′-GGATCCATGGACCCTACTCAAT-3′), and reverse primer: (5′-CTCGAGTTATTGACAAGGTGGAGC-3′). The amplified PCR fragment was electrophoresed and eluted with Gel purification Kit (Omega, USA) in accordance with kit protocol. The eluted product was then inserted into pMD19-T cloning vector (TaKaRa Biotechnology, China). The recombinant plasmid pMD19-T/HcSTP-1 was inserted into DH5α competent strain of Escherichia coli and cultured in Luria–Bertini medium with ampicillin (100 µg/ml). The positive clones followed by sequencing (Invitrogen Biotech, Shanghai, China) were validated by sequence analysis online using Blast program.1 The obtained nucleotide sequence was translated into amino acids sequence and aligned and compared with different nematode species using GENETYX Version 7.0.9 software. In addition, a number of online approaches/programs were used to detect N-terminal signal peptides/http://www.cbs.dtu.dk/services/SignalP/, Transmembrane protein prediction/http://www.cbs.dtu.dk/services/TMHMM/, T cell motifs/DNAstar: EditSeq, Protean, B cell epitopes/http://tools.immuneepitope.org/tools/bcell/iedb_input, and GPI modification Site Prediction/http://mendel.imp.ac.at/sat/gpi/gpi_server.html.
Expression and Purification of Recombinant HcSTP-1 Protein
The recognized recombinant pMD19-T/HcSTP-1 plasmid was digested with dual restriction enzymes BamH I and Xho I and ligated into prokaryotic expression vector pET32a (+) (Novagen, USA). Finally, the successful cloned STP-1 gene in a recombinant expression vector was sequenced to confirm its placement in the accurate reading frame. The recombinant plasmid pET32a (+)-HcSTP-1 was transferred into E. coli strain (BL21) and induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma-Aldrich) after the OD600 of the culture reached 0.6 at 37°C. The cell pellet after centrifugation was lysed using 10 µg/ml of lysozyme (Sigma-Aldrich) followed by sonication, and was resolved on 12% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The purification of recombinant protein was carried out according to the manufacturer’s instructions of Ni2+-nitrilotriacetic acid (Ni-NTA) column (GE Healthcare, USA). The histidine-tagged protein (empty pET32a) used as control protein in multiple assays in this study was purified and expressed similar to the procedure described for rHcSTP-1 protein and determined at 12% SDS-PAGE after Coomassie blue staining and quantified by Bradford method (24).
Immuno-Blot Analysis
Polyclonal antibodies against recombinant protein were generated from SD rats by subcutaneous injection of 300 µg of rHcSTP-1 protein mixed equally with Freund’s complete. After 2 weeks of first immunization, rats were injected three times at 1 week interval with same protein and Freund’s incomplete mixture. The sera were collected after 10 days of last injection and preserved at −80°C for later use. The sera against H. contortus parasites were collected from naturally infected goats (25), and sera collected from normal goats or rats were used as negative control.
The SDS-PAGE products of recombinant HcSTP-1 and soluble/ES products from adult parasites, were shifted nitrocellulose membrane (Millipore, USA) for western blot analysis. After blocked with 5% (w/v) skimmed milk powder in TBST (TBS with 0.5% Tween-20) at 37°C for 2 h, the strips were incubated with anti-H. contortus sera from goats or rat anti-sera against rHcSTP-1 (1:300 dilutions) as first antibody for treatment groups and normal goat serum or normal rat serum for control groups at 4°C overnight. Then, the membranes were incubated with the secondary antibody, HRP-conjugated goat anti-rat IgG (Santa Cruz, USA) in TBST (1:3,000 dilutions) at 37°C for 2 h. At end, the immunoreactions were visualized within 3–5 min, as per defined protocol of DAB Horseradish Peroxidase Color Development Kit (Beyotime Biotechnology).
Localization Assay
The mature parasites were suspended in TISSUE-TEK® O.C.T. compound (SAKURA, Torrance, CA, USA) and snap-frozen in liquid nitrogen. By using cryotome (CM1950, Wetzlar, Germany), worms were cut into sections with 10-µm thickness and fixed on poly-l-lysine hydrobromide glass slides. Non-specific bindings were confiscated by treating the slides with 10% normal goat serum, followed by incubation with rat-anti-STP-1 antiserum (1:300 dilutions) or normal rat serum as first antibody and Cy3-labeled Goat Anti-Rat IgG (1:3,000 dilutions) as second antibody (Beyotime, Shanghai, China) at 37°C for 2 h in each step. For DNA staining, the sections were marked with 1.5 µM 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI: Sigma, MO, USA) for 5 min. Finally, Anti-Fade Fluoromount Medium (Beyotime, Shanghai, China) was used and sections were examined under confocal microscope.
Binding of rHcSTP-1 on the Surface of Goat PBMCs
Freshly collected PBMCs, after washed with phosphate buffer saline (PBS: Ca2+/Mg2+-free, pH 7.4), were maintained at density of 1 × 105 cells/ml in cell culture medium (RPMI 1640), containing 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin (Gibco, Life Technology), and incubated with rHcSTP-1 or control protein pET32a or control buffer (PBS) at constant temperature (37°C) and humidity (5% CO2) for 4 h. Protein binding at surface of goat PBMCs were confirmed by immunofluorescence assay (IFA). Briefly, PBMCs after being washed and stabled with 4% paraformaldehyde on poly-l-lysine-treated slides were then permeabilized using 1% TritonX-100 in PBS at normal temperature. The PBMCs were subjected to primary antibodies rat anti-rHcSTP-1-IgG (1:300 dilutions) or normal rat serum followed by Cy3-coupled goat anti-rat IgG (Beyotime, China) (1:3,000 dilutions) as second antibody for 1 h at 37°C temperature. At last, the slides were stained in darkness with DAPI (Sigma, USA) for 5 min, and subjected to Anti-Fade Fluoromount solution (Beyotime, China) before laser scanning confocal microscopic examination (LSM710, Zeiss, Jena, Germany) at 100× magnifications.
Detection of Cytokine Transcripts by RT-PCR
The PBMCs were stimulated with Concanavalin A (ConA/10 μg/ml), and treated with rHcSTP-1 (10, 20, and 40 µg/ml) or pET32a protein (10 µg/ml) and control buffer (PBS) in 24-well plate containing RPMI 1640 medium at 5% CO2 and 37°C. The PBMCs were collected after 48 h of culture, by centrifugation and PrimeScript™ RT reagent kit (TaKaRa, CA, USA) was utilized to extract RNA from cells sediment as per the manufacturer’s guidelines. The cDNA-based quantifications of cytokine transcriptions were evaluated as previously stated protocol of RT-PCR. The reaction conditions and set of primers used for cytokines [interleukin-2 (IL-2), IL-10, TGF-β1, IL-6, IFN-γ, and IL-17] and endogenous reference gene (β-actin), as well as their amplification efficiencies are mentioned in Tables S1–S3 in Supplementary Material. The data were analyzed based on Raw cycle thresholds (Ct), obtained from the ABI Prism 7500 software (Applied Biosystems, USA) by comparative Ct (2−ΔΔ Ct) method (26).
Analysis of Major Histocompatibility Complex (MHC)-I and -II Molecule Expression
After separation of PBMCs by standard Ficoll-hypaque (GE Healthcare, USA) gradient centrifugation and two times washing with the PBS (Ca2+/Mg2+-free, pH 7.4), were poured into flat-bottom six well culture plates (Corning Inc., USA) containing RPMI 1640 medium (Invitrogen, USA) supplemented with 10% FBS and penicillin + streptomycin (Invitrogen, USA). The monocytes stick to the bottom of the plate, were collected (27) and adjusted at density of 1 × 106 cells/ml, whereas, Non-sticky cells were discarded by multiple washing steps. The purified monocytes were incubated in 24-well culture plates with different concentrations of rHcSTP-1 (treatment group) or pET32a protein and PBS (control group) at 37°C for 24 h. Subsequently, the monocytes were marked with monoclonal antibodies MHC-I (MCA2189A647, AbDserotec, Bio-Rad, USA) and MHC-II (MCA2225PE, AbDserotec), followed by flow cytometric analysis at FACS Calibur cytometer (BD Biosciences, San Jose, CA, USA) (Figure 7A).
Cell Proliferation Assay
The 100 µl of freshly isolated PBMCs at density of 1 × 106 cells/ml was poured into 96-well culture plate and stimulated with ConA (10 µg/ml). The PBMCs were incubated with serial concentrations of rHcSTP-1 protein (10, 20, and 40 µg/ml) in treatment group and pET32a empty protein or control vehicle (PBS) were added into control groups at 5% humidity and 37°C temperature for 72 h. Detection of PBMC proliferation was carried out according to the previously mentioned procedure of cell proliferation assay kit (28). 10 µl of CCK-8 solution (Beyotime China) was mixed in every well of plate 6 h prior to harvest and absorbance were checked by using spectrophotometer (Bio-Rad, USA) at wavelength 450 nm (OD450). Result presented here are from three independent experiments.
Cell Migration Assay
The migration activity of cultured PBMCs was measured with 8.0-µm pores Millicell® insert (Merck-Millipore, USA) as stated previously. Briefly, in treatment group 200 µl of freshly separated cells were poured into the upper chamber with various concentrations of rHcSTP-1 (10, 20, and 40 µg/ml) and pET32a control protein or equal volume of control buffer (PBS) were added in control groups. Subsequently, 1,300 µl cell culture medium was filled in the lower chamber for 2 h incubation at 37°C and 5% CO2. After that, columns were removed, and PBMC passed through polycarbonate layer were figure out by a Neubauer counting chamber. Data were resulted as percentage of seeded PBMCs. Three independent experiments were conducted.
Intracellular Nitrite Production by PBMCs
The goat PBMCs were separated and washed with Ca2+/Mg2+-free PBS (pH 7.4). 100 µl of cells containing DMEM medium was poured in 96-well plate with varying concentrations of rHcSTP-1 (10, 20, and 40 µg/ml), similarly the control groups were incubated with recombinant protein of pET32a or PBS (control buffer). NO production by cells was determined by Griess assay (29), by using Total Nitric Oxide Assay Kit (Beyotime, China) according to the manufacturer’s instructions. The values in the reaction mixture were measured at microplate spectrophotometer (Bio-Rad Laboratories, USA) at 540 nm (OD540) wavelength. Data presented here are results of triplicate experiments.
Apoptotic Efficiency of rHcSTP-1 on PBMC
Efficiency of rHcSTP-1 to induce apoptosis of PBMC was assessed by flow cytometric analysis (BD Biosciences, USA). The PBMCs (1 × 107 cells/ml) treated with different rHcSTP-1 protein concentrations (5, 10, 20, and 40 µg/ml) and empty pET32a protein, were stained with Annexin V-FITC kit (Miltenyi Biotec, Germany) according to the manufacturer’s protocols. The apoptosis rate was calculated by the percentage of early (Annexin V+ PI−) and late (Annexin V+ PI+) PBMC apoptosis. Cells without any treatment were set as control. Data were analyzed using Flow Jo 7.6 software (Tree Star, USA).
Statistical Analysis
Data analysis regarding all experiments was performed by using the statistical package, GraphPad Premier 6.0 (GraphPad Prism, San Diego, CA, USA). Data are presented as mean ± SEM. The differences between groups were compared by one-way ANOVA, followed by a Tukey test and were considered statistically significant at p < 0.05.
Results
Cloning and Protein Expression of H. contortus STP-1
The RT-PCR generated fragment of STP-1 gene with molecular size of 951 bp, was obtained from H. contortus cDNA and successfully cloned into an expression vector system, confirmed by enzymatic digestion with BamH I and Xho I (Figure 1). After that, cloned HcSTP-1 sequence was translated into 316 amino acids with predicted 35 kDa molecular mass and calculated pI of 6.328. The nucleotide and amino acid sequence identity HcSTP-1 using BLASTx and BLASTp revealed highly sequence resemblance with STPs from other parasite species, existing at NCBI database (see text footnote 1). In this study, characterization of HcSTP-1 cDNA showed 98% significant sequence identity to H. contortus (ADJ96628.1), 80% with C. briggsae (XM_002631635.1) (data not shown). Multiple sequence alignment showed that HcSTP-1 polypeptide shares a significant similarity of 91% with T. vitrinus (GenBank: CAM84506.1), 62% with T. canis (GenBank: KHN86897.1), 62% with Ascaris suum (GenBank: CAJ98743.1), and 58% with Caenorhabditis elegans (GenBank: NP_505086.2). The BLAST analysis predicted number of conserved metal ion-binding sites and histidine residue predicted to be involved in proton donation. The amino acids expressing STP protein motif LRGNHE was found during sequence analysis. The aligned STPs sequences were highly similar in central regions, particularly in term of characteristics sequence motifs, such as GDXHG, GDYVDRG, GNHE, HGG, RG, and H (30), whereas there was a higher variation occurred regarding to N- and C-terminal regions among all align sequences (Figure 1). Further sequence analysis indicated lack of signal peptide, transmembrane domain, and GPI anchor site, whereas T and B cell motifs were predicted in deduced protein structure (Figures S1–S4 in Supplementary Material).
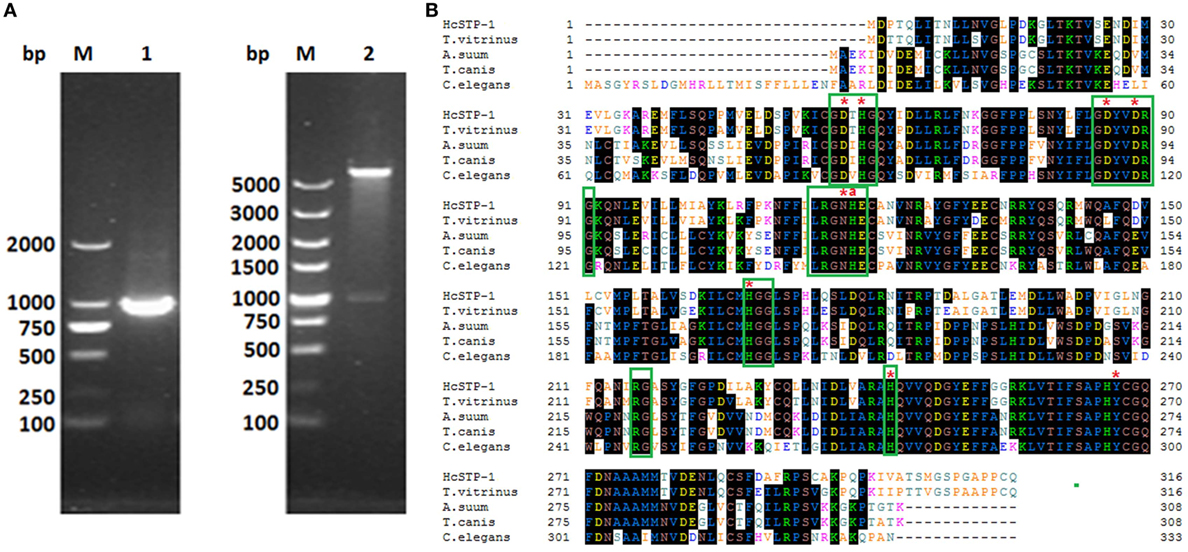
Figure 1. Cloning and sequence analysis of HcSTP-1 gene. (A) Electrophoresis of PCR product of HcSTP-1 open reading frame (Lane 1). Restriction enzyme digestion of pET32a-HcSTP-1 with BamH I and Xho I (Lane 2) and molecular weight DNA marker (Lane M). (B) The comparison of amino acid sequence of Haemonchus contortus serine/threonine-protein phosphatase 1 (STP-1) and with predicted STPs from Trichostrongylus vitrinus (CAM84506.1), A. suum (CAJ98743.1), Toxocara canis (KHN86897.1), and C. elegans (NP_505086.2). Amino acids predicted to be involved in the catalytic metal binding are indicated by asterisks “*,” and the histidine residue proposed for proton donation is indicated with “a.” The conserved motifs found in all sequences including Prosite motif PS00125 (LRGNHE) are boxed in green color.
The recombinant product of HcSTP-1 gene inserted into E. coli was expressed after IPTG induction and detected after staining with Coomassie brilliant blue R-250 on SDS-PAGE. The rHcSTP-1 protein was expressed with molecular weight of about 53 kDa (Figure 2, Lane P) along with 18 kDa calculated mass of vector protein (pET32a). The target protein was confirmed at its original size of 35 kDa after reduction from vector protein. Polyclonal antibodies against rHcSTP-1 were identified by immuno-blot analysis, which revealed that rHcSTP-1 could be recognized by sera from goats infected with H. contortus and native STP-1 protein from H. contortus could be recognized by antibodies generated against rHcSTP-1 protein (Figures 2C,D, Lane 1). However, no protein was detected with normal sera taken from un-immunized goats/rats (Figures 2C,D, Lane 2).
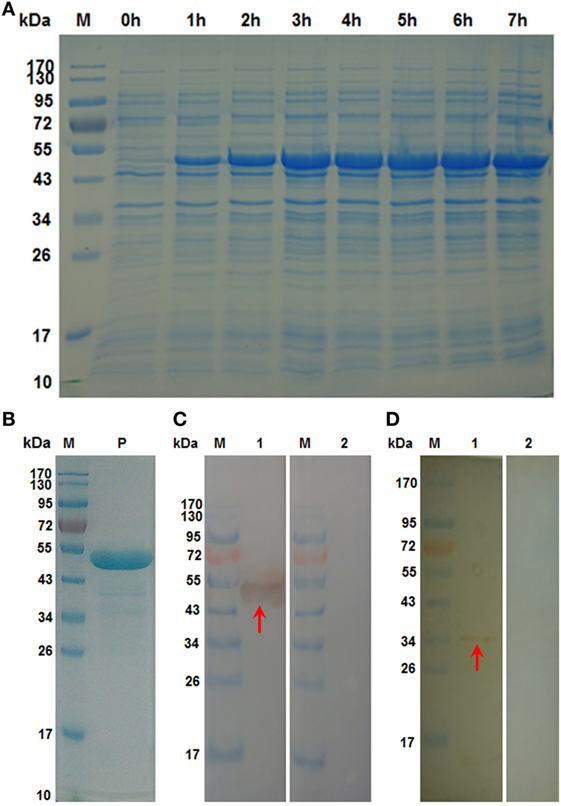
Figure 2. Expression, purification and immuno reactivity of rHcSTP-1. (A) Recombinant expression vector before (Lane 0 h) and after 1 mM IPTG induction (Lane 1–7 h). Lane M: standard protein molecular weight marker. (B) Purified rHcSTP-1 protein resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Lane P). (C) Western blot of rHcSTP-1 protein. (Lane 1) Purified recombinant protein was electrophoresed and transferred to a membrane probed with serum from goat infected with Haemonchus contortus and (Lane 2) with normal goat serum as negative control. (D) Western blot of total ES proteins of H. contortus. (Lane 1) ES proteins from H. contortus parasites were detected by rat antibodies against serine/threonine-protein phosphatase 1 (STP-1) protein and (Lane 2) with serum from normal rat as control.
HcSTP-1 Localized Outer and Inner Lining of H. contortus Worm Sections
The partial body sections of male and female adult worms are shown in Figure 3. Immunohistochemical analysis was performed to detect distribution of native STP-1 protein within the worms. The red lining at outer and inner layer of membranes and in gut region strongly indicated the presence of this protein within worms sections. However, no fluorescence was detected in the control section (Figure 3).
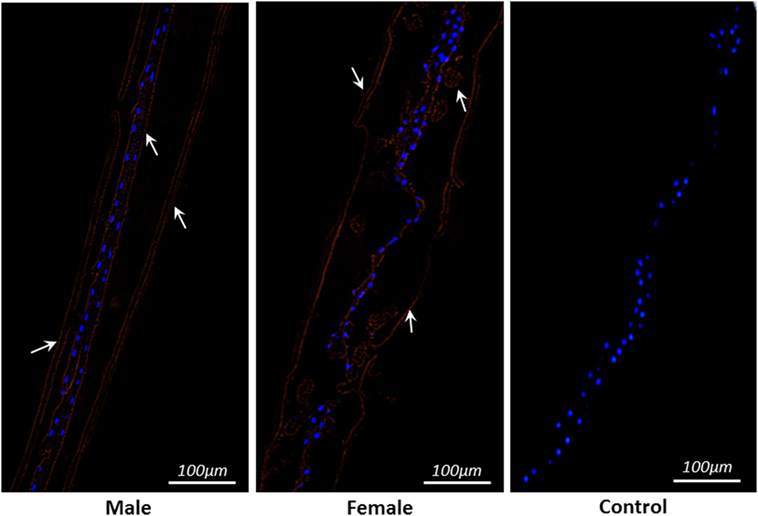
Figure 3. Localization of serine/threonine-protein phosphatase 1 protein within parasite structure. Immunohistochemically STP-1 protein localizes inner and outer structure of Haemonchus contortus male and female adult worms. The red color indicates target protein stained with Cy3 and blue dots are localization of nuclei stained with DAPI. No red fluorescence was detected in control panel. Scale-bars: 100 µm.
rHcSTP-1 Binding Confirmation on Surface of Goat PBMCs
The interaction of HcSTP-1 with PBMCs, which are key elements for immune responses, was investigated by using IFA. As shown in Figure 4, the Cy3-labeled target protein and the DAPI labeled nuclei displayed red and blue fluorescence, respectively. The red visualization on the surface of cells in treatment group (rHcSTP-1) indicated the binding of the protein whereas, no red labeling was detected in the control groups (pET32a or PBS), which clearly specified that rHcSTP-1 could bind on the surface of goat PBMCs and exerts its immune effects in a multiple manners (Figure 4).
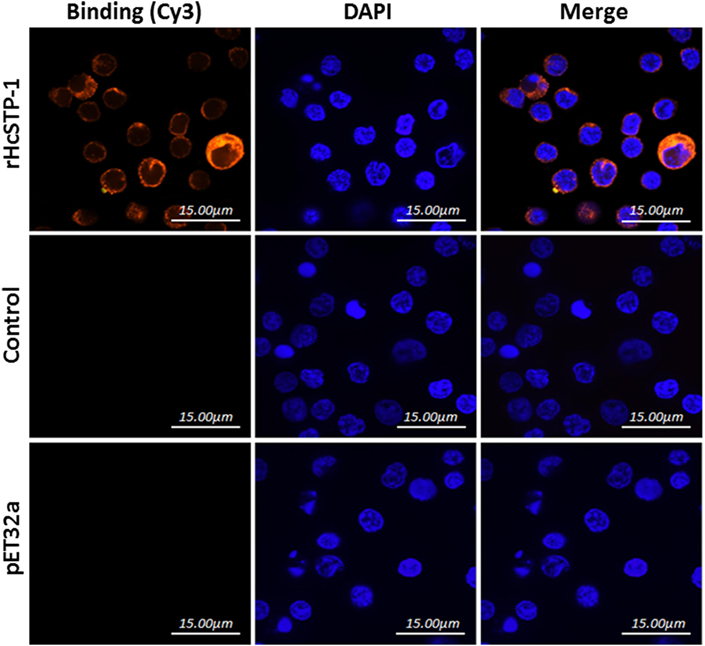
Figure 4. Binding of rHcSTP-1 protein with goat peripheral blood mononuclear cells in vitro. The cells were incubated with rat sera anti-rHcSTP-1-O IgG, anti-HisTag protein (pET32a) or negative rat IgG as primary antibody followed by Cy3-labeled goat anti-rat IgG (red) as secondary antibody. Red fluorescence on surface of cells showed target protein staining (Cy3) and nuclei of cells were visualized by DAPI (blue). No red fluorescence was observed in control groups. The results were analyzed by using confocal laser scanning microscopy.
rHcSTP-1 Decreased Anti-Inflammatory and Increased Pro-Inflammatory Cytokines Expression in a Balanced Th1-Th2 State In Vitro
The mRNA levels of cytokines produced by cultured goat immune cells (PBMCs) in response to STP-1 protein were tested at RT-PCR machine. As depicted in results of Figure 5, transcriptions level of IL-2 [ANOVA, F(5,12) = 198.9, p = 0.0001] and transforming growth factor-β1 (TGF-β1) [ANOVA, F(5,12) = 108.0, p = 0.0001] were increased significantly at dose-dependent manner to that of pET32a protein and PBS control group. However, the IL-10 transcription was decreased [ANOVA, F(5,12) = 19.04, p = 0.0001] dose dependently to that of control groups. Here, we also noted that interferon gamma (IFN-γ) at protein concentration of 10 µg/ml showed no difference (p = 0.337); however, at 20 µg/ml (p = 0.002) and 40 µg/ml [ANOVA, F(5,12) = 105.6, p = 0.0001] the transcription level was augmented significantly. After incubation with rHcSTP-1 protein, the expressions of IL-17 at 10 µg/ml reached at its significant level [ANOVA, F(5,12) = 11.76, p = 0.0003], while at 20 and 40 µg/ml remained non-significant. However, IL-6 was not significantly different [ANOVA, F(5,12) = 0.604, p = 0.699] when compared with control groups (Figure 5).
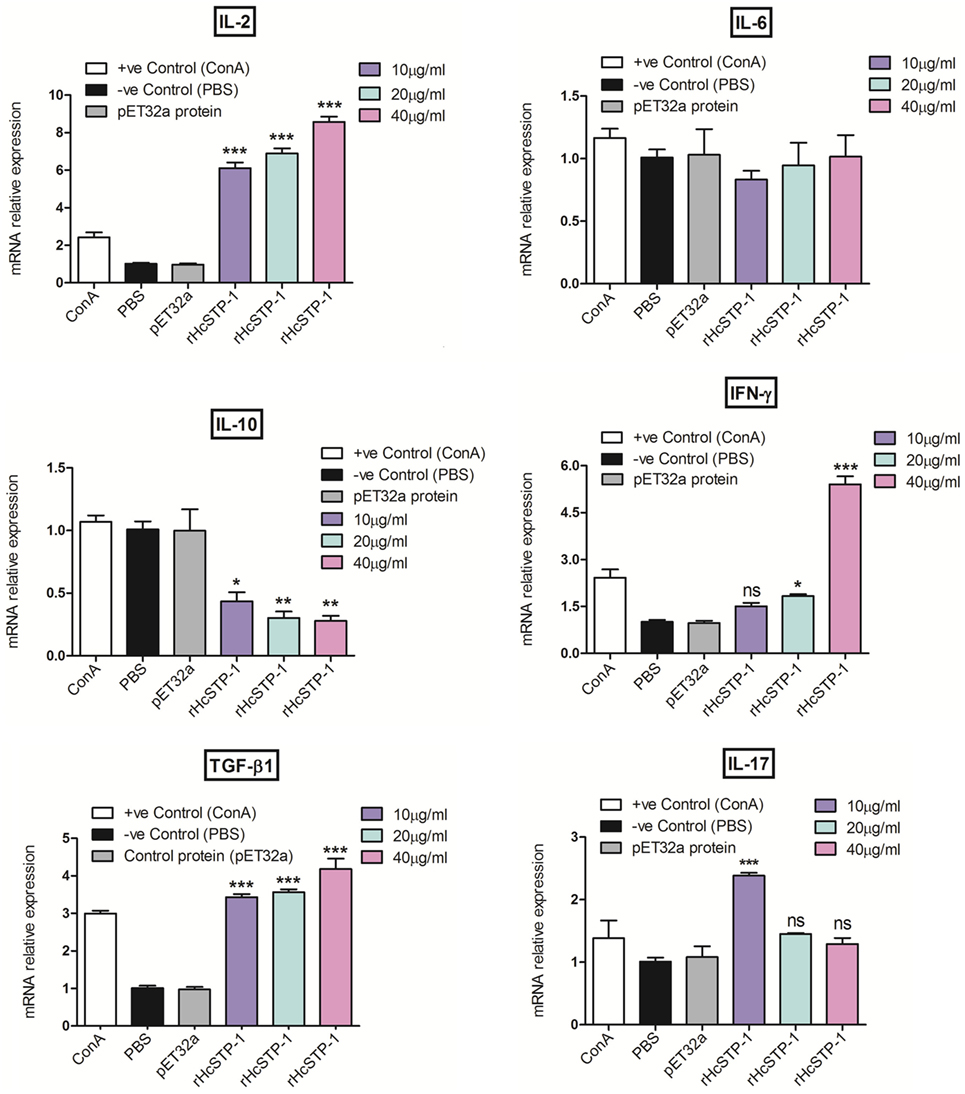
Figure 5. Relative expression of multiple cytokines in goat peripheral blood mononuclear cells stimulated by the recombinant HcSTP-1. Cells were incubated with the recombinant serine/threonine-protein phosphatase 1 (STP-1) for 48 h, the mRNAs encoding interleukin-2 (IL-2), IL-6, IL-10, IFN-γ, TGF-β1, and IL-17 were quantified by reverse-transcription-polymerase chain reaction. The significant level was set at *p < 0.05, **p < 0.01, ***p < 0.001, and ns non-significant compared with the untreated group (control). Data are representative of three independent experiments.
rHcSTP-1 Protein Affected PBMCs Proliferation
Antiproliferative effects of rHcSTP-1, compared to the negative control and control protein treated groups were evaluated by using cell counting kit (CCK8). The results demonstrated that, cells stimulated with ConA and incubated with rHcSTP-1 protein downregulated [ANOVA, F(5,12) = 67.38, p = 0.0001] the multiplication efficiency dose dependently (Figure 6). However, no significant change was observed between control groups [ANOVA, F(5,12) = 67.38, p = 0.557].
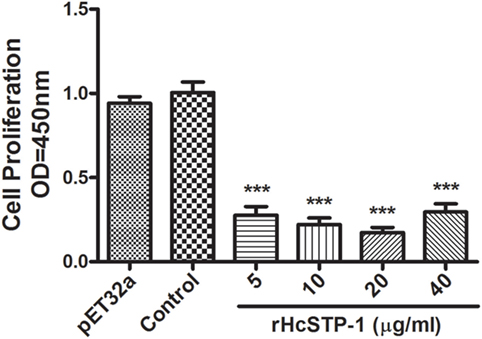
Figure 6. Influences of different concentrations of rHcSTP-1 on peripheral blood mononuclear cells proliferation in vitro. Cells were simulated with ConA and treated with control buffer, His-Tag protein (pET32a), and various concentrations of recombinant serine/threonine-protein phosphatase 1 (STP-1) protein for 72 h. Proliferation test was conducted by CCK-8 and OD450 value was measured using microplate spectrophotometer. Cell proliferation index was calculated by considering the OD450 values in controls as 100%. The data are expressed as mean ± SEM of three independent experiments (***p < 0.001).
rHcSTP-1 Inhibited MHC-I and -II Expression on Goat Monocytes
In response to the rHcSTP-1, the expression of MHC-I and -II surface markers on monocytes were evaluated, and results showed that as compared to expression in control groups, rHcSTP-1 significantly decreased MHC-I (Figures 7B,D) molecule expression [F(5,12) = 780.3] as well as MHC-II [F(5,12) = 157.2] on the surface of the goat monocytes in a dose-dependent manner p < 0.0001 (Figures 7C,E).
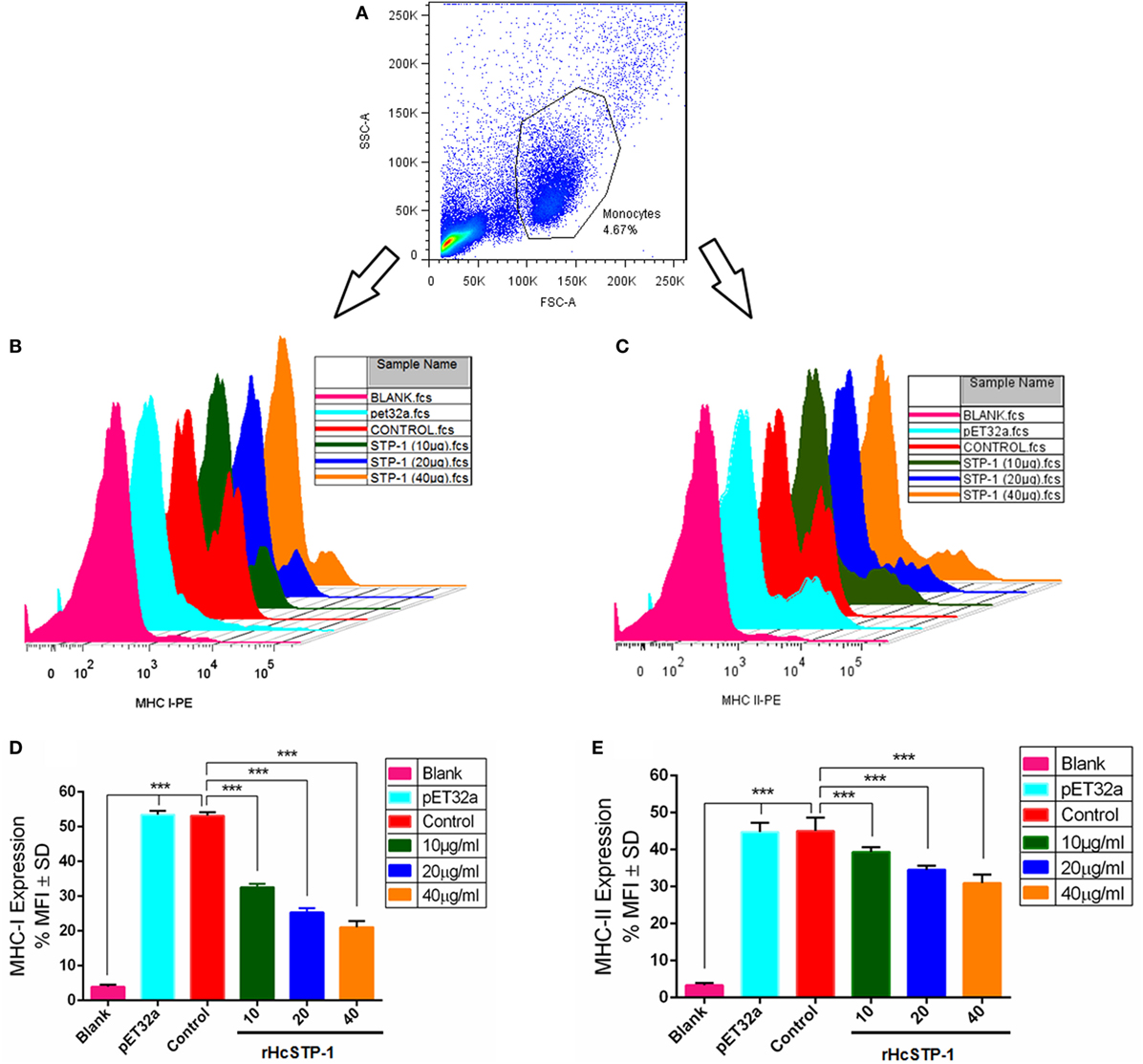
Figure 7. rHcSTP-1 suppressed both major histocompatibility complex (MHC) class-I and II on goat monocytes. Monocytes were cultured in the presence of control buffer, HisTag protein (pET32a), and multiple concentrations of rHcSTP-1 for 24 h. MHC-II expression was measured by flow cytometric analysis and calculated as the percentage of mean fluorescence intensity (MFI). (A) Histogram corresponds to one representative of three independent experiments. (B,C) Stagger offset showing expression level of MHCs on monocytes. (D,E) Bar graphs represent the MFI ± SD of controls. The data are representative of three independent experiments (***p < 0.001).
PBMC Migration Assay
To investigate impact of the rHcSTP-1 on immune cell migration, the percentage of migrated cells through Millipore polycarbonate membrane into the lower chamber was calculated. Results showed that 16.67 ± 0.8819% cells with control and 16.33 ± 1.764% cells with control protein group (pET32a) were migrated into lower chamber (p = 0.087). At 10 µg/ml (27.00 ± 1.528%), 20 µg/ml (32.67 ± 2.603%), and 40 µg/ml (35.00 ± 2.309%) rHcSTP-1 protein concentrations, significant amount of cells were passed through membrane (Figure 8). However, no difference was found between control and 5 µg/ml (20.00 ± 1.155%) protein concentration (p = 0.084).
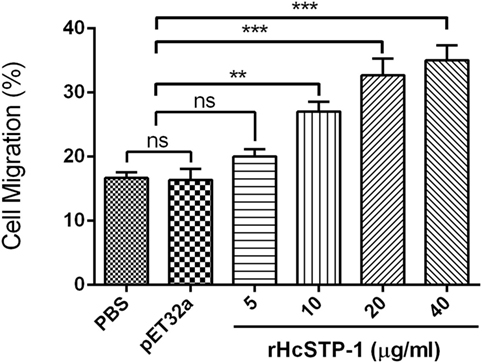
Figure 8. Effects of the various concentrations of rHcSTP-1 on peripheral blood mononuclear cell migration in vitro. Cells were treated with control buffer, HisTag protein (pET32a), and multiple concentrations of rHcSTP-1. The migration percentage was determined randomly. The difference between the mean values was calculated using ANOVA. Data are representative of three independent experiments (**p < 0.01, ***p < 0.001, and ns non-significant versus the control).
rHcSTP-1 Involved in Intra-PBMCs NO Production
Nitric oxide plays a crucial role in the host defense mechanism by preventing growth or killing the parasite directly during infection. By using total nitric oxide kit, we found that no significant production was occurred [ANOVA, F(5,12) = 25.92, p = 0.504] at 5 and 10 µg/ml compared with the groups treated as control. Here, we also observed that at 20 and 40 µg/ml, a significant level of NO was produced [ANOVA, F(5,12) = 25.92, p = 0.0001]. However, production of NO between control (PBS) and pET32a group was also non-significant [ANOVA, F(5,12) = 25.92, p = 0.473] (Figure 9).
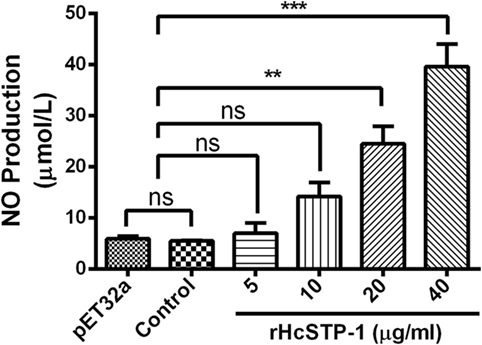
Figure 9. Measurement of the intracellular nitric oxide production by goat peripheral blood mononuclear cells in vitro. Nitric oxide concentration in the supernatant of cell cultures was performed according to the instructions of Griess assay by Total Nitric Oxide Assay Kit. The data were presented here as mean ± SEM of three independent experiments (**p < 0.01 and ***p < 0.001).
rHcSTP-1 Dramatically Modulated Early and Late Apoptosis of PBMCs
It has been suggested that STPs obviously involved in various cellular processes including cell viability and cell death (10). The apoptotic activity of rHcSTP-1 on PBMCs was evaluated by using the externalization of membrane phospholipid phosphatidylserine (PS) as a marker of cell apoptosis. Flow cytometry assay revealed that cells incubated with different protein concentrations (5, 10, 20, and 40 µg/ml) showed significantly increased frequency of apoptotic percentage in both early [ANOVA, F(5,12) = 69.08, p = 0.0001] and late stage apoptosis [ANOVA, F(5,12) = 68.30, p = 0.0001] compared with the control groups. Meanwhile, no significant change was observed [ANOVA, F(5,12) = 200.8, p = 0.0986] between control and pET32a group (Figure 10).
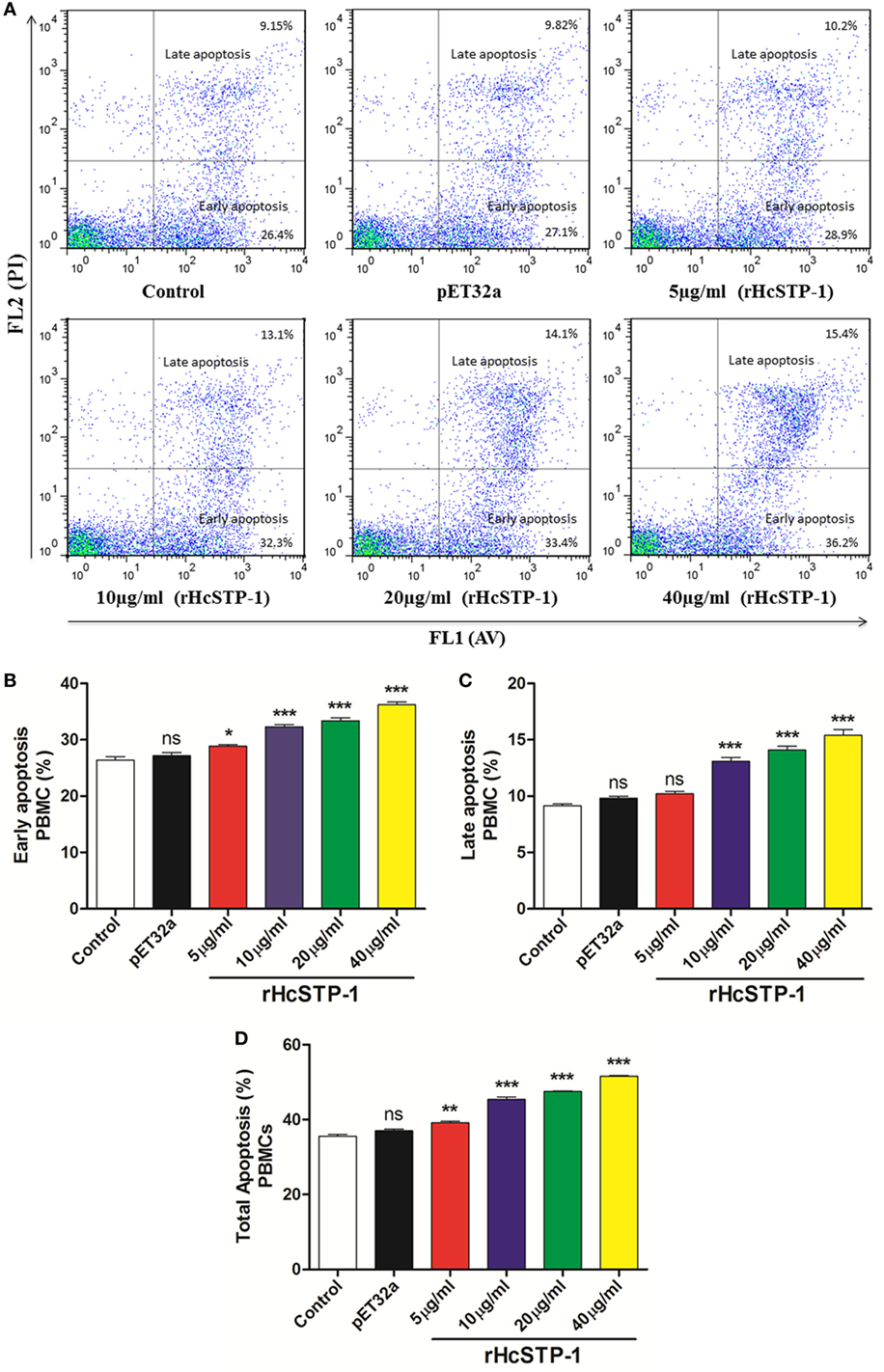
Figure 10. Apoptotic analysis of peripheral blood mononuclear cells (PBMCs) by flow cytometry. (A) The dot plot showing percentages of PBMCs undergoing early and late stage apoptosis dually stained with Annexin V-FITC/PI. (B) Bar graph showing percentage of early stage apoptosis of PBMCs after rHcSTP-1 treatment. (C) Bar graph showing percentage of late stage apoptosis of PBMCs after rHcSTP-1 treatment. (D) The effect of the different protein treatments on total % (early + late) of apoptotic cells. Data are presented as the mean ± SEM (n = 3) from triplicate experiments. Asterisks designate treatment groups are significantly differ in respect to control one (*p < 0.05, **p < 0.01, ***p < 0.001, and ns non-significant).
Discussion
Identification and development of novel drug targets are always a primacy in the biological study of socio-economically important parasites. Helminths ESPs including some antigens with unknown biological functions were characterized (31–33), and due to their sequences homology, these molecules have been proven as promising vaccine candidate against nematodes infections (34, 35). Among PPs, PP1 elaborate numerous biological activities, such as membrane receptors/channels regulations, protein synthesis, glycogen metabolism, transcription, cellular division, and apoptosis (36). In current investigation, an ES protein of H. contortus (STP-1) was characterized and localization of this protein through IFA confirmed its attachment with host immune cells for the first time, in shape of surface ligand complex (37), which is characteristic feature of ES proteins to suppress or modulate the immune functions of host PBMCs.
The PP1 is a major ubiquitously expressed STP, which shares a conserved catalytic domain with all other isotypes (PP2A, PP2B, PP4, PP5, PP6, and PP7) (38) and provides extensive information regarding their functions involved in numerous vital cellular mechanisms in all organisms (30, 39). In this study, highly significant amino acid identity of HcSTP-1 with presence of a conserved motif (LRGNHE) for STP protein pattern at positions 115–120 (18), involvement of amino acids for catalytic activity like metal ion-binding and proton donation were found (7). In the central regions of polypeptides, the amino acid sequences were highly conserved, particularly with the signature sequence motifs of the PPP family (GDXHG, GDXVDRG, GNHE, HGG, RG, and H) (30). However, the N- and C-terminal amino acids were variable, which suggested that it might be involved in protein binding and regulatory activity.
Previous studies suggested that STPs were associated with male reproductive processes of parasites, including sperm maturation, motility, and viability (40). In our previous proteomic analysis, STP-1 was found at adult and late adult developmental stages of H. contortus in vivo (19). In this study, immunohistochemically HcSTP-1 was localized in both male and female adult worms of H. contortus. Somehow, most PPs considered to be conserved during free-living as well as parasitic developmental stages; however, the biological pathways involved in the growth, development, survival, and/or reproduction reflects between invertebrates and vertebrates. Based on current information, a deep insight into the biochemical localization of STPs in H. contortus is required to understand the gender dependent immune stimulation by STPs during host–parasite interface.
The immune (Th1 and Th2) and inflammatory responses are deeply allied with secretion of various chemokines and cytokines by number of immune cell types (lymphocytes and monocytes), which perform crucial immunoregulatory functions (41), against parasitic infections like H. contortus (42). In this study, the levels of mRNA transcripts for IL-2, interleukin-10, transforming growth factor beta 1, interleukin-6, interferon-γ, and interleukin-17 in cultured PBMCs after treatment with rHcSTP-1 were detected for the first time. Schallig (43) suggested that sheep challenged with H. contortus infection responded cell mediate immune reaction (Th1), categorized by upregulation of IFN-γ and IL-2 mRNA expression. Similarly, in current research, goat PBMCs in response to rHcSTP-1 protein showed higher expression levels of IL-2 and IFN-γ, suggesting that these cytokines might be in favor of non-protective Th responses against hemonchosis. The immunosuppressive cytokine IL-10 was responsible for inhibition of Th2 immune responses (44, 45). In this research, rHcSTP-1 protein showed a significant Th2 type immune response by decreased IL-10 cytokine in culture PBMCs, and thus exerts suppressive potential on differentiation of Treg cells, which might be immune modulatory role of HcSTP-1 against parasite infection.
Previously, some researches demonstrated that IL-17 cytokine were involved in pathogenesis and inflammatory responses by numerous parasites (46–48). It was also elucidated that decreased level of IL-17 resulted favorable host protective responses (49). In our previous study, HcESPs were found to induce IL-17 cytokine by stimulation of the Th17 cells and resulted in inflammatory reaction favorable for worm survival in host. In this study, we found that rHcSTP-1 with concentration of 10 µg/ml significantly upregulated IL-17 cytokine. Our findings indicated that HcSTP-1 protein of H. contortus was adept in Th17 cells induction, which lead to inflammatory response. However, the resulted inflammatory reactions might be in favor of parasitic infection. TGF-β1 is functionally multidimensional cytokine that controls various immune system activities differentially on different cell types and potentially regulate various biological processes (50). It also plays vital roles to inhibit proliferation and to stimulate the apoptosis of various immune cells subset (51). In this investigation, the binding of recombinant protein HcSTP-1 to the goat PBMCs increased the production of TGF-β1, which could be a one of mechanism of rHcSTP-1 protein to facilitate immune evasion during host–parasite interactions.
Major histocompatibility complex is associated with many important immunological activities like: stimulation of antibody production and immune responses against microbial and parasitic pathogens (52, 53), although some cytokines especially IFN-γ and TGF-β (54) have been proven to upregulate MHC-I and -II gene expression in different immune cells. Here, we noted that STP-1 protein of H. contortus potentially suppress the MHC-I and -II molecules expression in goat monocytes. This indicated that STP-1 protein interferes with the MHC-I and -II antigen presentation pathway, which might be a key mechanism for evasion from the host’s immune response.
The cellular processes such as cell migration part play an importance role in immune surveillance and tissue regeneration, by recruiting of immune cells into the surrounding tissue to destroy invading microorganisms. In addition, Type-1 as precise inhibitor of protein serine/threonine phosphatase (PHI-1) participated in regulatory events by modulating the migration of human endothelial and epithelial cells (55). Moreover, it was suggested that helminths actively induce migration of immune cell to infected sites (56). In this research, migration experiment concluded that rHcSTP-1 is an important binding protein to the goat PBMCs, which regulate the migration of immune cells to combat with invading H. contortus parasite and might be a contributor of HcESPs in the PBMCs migration.
Many proteins including ser/thr phosphatases were thought to be implicated in regulation of apoptosis (57). Till now, studies on cell death regulation by PPP subfamilies like PP2A and PP2B were well documented (10, 58); however, few studies highlighted the direct association and involvement of PP1, PP4, PP5, PP6, and PP7 members of phosphatase family in apoptosis (59–62). This study showed that rHcSTP-1 was involved in apoptosis as well as cell necrotic activity at dose-dependent manner.
In addition, cell proliferation is an indispensable process which is regulated or suppressed by both antigen-presenting cells (APCs) and T cells, thus modulate the host immune responses (63). In our earlier work, HcESPs significantly suppressed PBMCs multiplication at dose-dependent manner. In this study, PBMCs proliferation was decreased at significant level, which ultimately increased the pre- and post-apoptotic percentage in response to rHcSTP-1 treatment. Our study demonstrated that rHcSTP-1 as a constituent of HcESPs might interfere with modulatory activity on PBMCs proliferation and cell death regulation; however, the exact mechanism and pathways involved during host–parasite interaction upon HcSTP-1 protein involvement remains to be determined.
The immune cell-derived molecule, nitric oxide (NO) has been concerned with host non-specific defense against variety of infections such as hemonchosis, leishmaniasis, trypanosomiasis, malaria, toxoplasmosis, and schistosomiasis (64, 65). Previous studies provoked that PPs including P1 and P2A are involved in iNOS assertion in murine macrophages (66). Another study also witnessed that serine-threonine phosphatases post-transcriptionally regulate iNOS translation in rat hepatocytes (67). Furthermore, IFN-γ and TNF-α activated release of NO by macrophages was usually associated with killing functions on the helminths (68). In our previous study, the generation of NO was significantly suppressed by HcESPs due to decreased level of IFN-γ (69). In this study, intracellular nitrite production of goat PBMCs was significantly upregulated in a concentration-dependent fashion. These findings suggested that H. contortus STP-1 protein could induce the production of NO through increasing IFN-γ level which might mediate immunomodulatory functions on NO production and thus intensified the harmful effects of some chemical factors produced by the host cells on the helminths during host–parasite interface.
Conclusion
The current observations highlighted that H. contortus STP-1 as an important constituents of HcESPs localized in both gender, play pivotal stage-specific regulatory roles in interactions with host immune cells. This protein after being bind to host immune cells, regulate cytokines expression, cell proliferation, MHC expression, cell migration, NO production, and cell death regulation differentially. This study might be a powerful clue and would have major fundamental significant contribution in the discovery of novel therapeutic approaches to control hemonchosis. However, immune cells specific role of rHcSTP-1 and other related phosphatases subunits with PBMC sub-populations like T cell and B cell, NK cell, APCs, and macrophages involved in host–parasite relationship in activating immune and cellular response are worthy for future research.
Ethics Statement
All experiments were conducted in accordance with the guidelines of the Animal Ethics Committee, Nanjing Agricultural University, China. All experimental protocols were approved by the science and Technology Agency of Jiangsu Province. The approval ID is SYXK (SU) 2010-0005.
Author Contributions
XLi concerned with project designing, management, and coordination of this study. ME analyzed data and wrote the manuscript. WW, JG, ML, and YW provided technical support during experiments. MWH, MH, and XLiu participated in data analysis. LX, RY, and XS helped for providing all necessary materials during experiments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This research work was financially supported by the “National Key Basic Research Program (973 Program) of P.R. China” (Grant No. 2015CB150300) and by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01627/full#supplementary-material.
Footnote
References
1. Coles GC. The future of veterinary parasitology. Vet Parasitol (2001) 98:31–9. doi:10.1016/S0304-4017(01)00421-6
2. Jasmer DP, Lahmers KK, Brown WC. Haemonchus contortus intestine: a prominent source of mucosal antigens. Parasite Immunol (2007) 29:139–51. doi:10.1111/j.1365-3024.2006.00928.x
3. Kaplan RM, Vidyashankar AN. An inconvenient truth: global worming and anthelmintic resistance. Vet Parasitol (2012) 186:70–8. doi:10.1016/j.vetpar.2011.11.048
4. Kotze AC, Hunt PW, Skuce P, Von Samson-Himmelstjerna G, Martin RJ, Sager H, et al. Recent advances in candidate-gene and whole-genome approaches to the discovery of anthelmintic resistance markers and the description of drug/receptor interactions. Int J Parasitol Drugs Drug Resist (2014) 4:164–84. doi:10.1016/j.ijpddr.2014.07.007
5. Britton C, Roberts B, Marks ND. Functional genomics tools for Haemonchus contortus and lessons from other helminths. Adv Parasitol (2016) 93:599–623. doi:10.1016/bs.apar.2016.02.017
6. Sarmiento M, Zhao Y, Gordon SJ, Zhang ZY. Molecular basis for substrate specificity of protein-tyrosine phosphatase 1B. J Biol Chem (1998) 273:26368–74. doi:10.1074/jbc.273.41.26368
7. Barford D, Das AK, Egloff MP. The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu Rev Biophys Biomol Struct (1998) 27:133–64. doi:10.1146/annurev.biophys.27.1.133
8. Cohen PT. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Biochem Sci (1997) 22:245–51. doi:10.1016/S0968-0004(97)01060-8
9. Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev (2000) 80:173–210. doi:10.1152/physrev.2000.80.1.173
10. Klumpp S, Krieglstein J. Serine/threonine protein phosphatases in apoptosis. Curr Opin Pharmacol (2002) 2:458–62. doi:10.1016/S1471-4892(02)00176-5
11. Berndt N. Roles and regulation of serine/threonine-specific protein phosphatases in the cell cycle. Prog Cell Cycle Res (2003) 5:497–510.
12. Sim AT, Baldwin ML, Rostas JA, Holst J, Ludowyke RI. The role of serine/threonine protein phosphatases in exocytosis. Biochem J (2003) 373:641–59. doi:10.1042/bj20030484
13. Delorme V, Garcia A, Cayla X, Tardieux I. A role for Toxoplasma gondii type 1 ser/thr protein phosphatase in host cell invasion. Microbes Infect (2002) 4:271–8. doi:10.1016/S1286-4579(02)01538-1
14. Boag PR, Ren P, Newton SE, Gasser RB. Molecular characterisation of a male-specific serine/threonine phosphatase from Oesophagostomum dentatum (Nematoda: Strongylida), and functional analysis of homologues in Caenorhabditis elegans. Int J Parasitol (2003) 33:313–25. doi:10.1016/S0020-7519(02)00263-1
15. Hu M, Abs EL-Osta YG, Campbell BE, Boag PR, Nisbet AJ, Beveridge I, et al. Trichostrongylus vitrinus (Nematoda: Strongylida): molecular characterization and transcriptional analysis of Tv-stp-1, a serine/threonine phosphatase gene. Exp Parasitol (2007) 117:22–34. doi:10.1016/j.exppara.2007.03.008
16. Kutuzov MA, Andreeva AV. Protein Ser/Thr phosphatases of parasitic protozoa. Mol Biochem Parasitol (2008) 161:81–90. doi:10.1016/j.molbiopara.2008.06.008
17. Ma GX, Zhou RQ, Hu SJ, Huang HC, Zhu T, Xia QY. Molecular characterization and functional analysis of serine/threonine protein phosphatase of Toxocara canis. Exp Parasitol (2014) 141:55–61. doi:10.1016/j.exppara.2014.03.019
18. Campbell BE, Rabelo EM, Hofmann A, Hu M, Gasser RB. Characterization of a Caenorhabditis elegans glc seven-like phosphatase (gsp) orthologue from Haemonchus contortus (Nematoda). Mol Cell Probes (2010) 24:178–89. doi:10.1016/j.mcp.2010.02.001
19. Gadahi JA, Wang S, Bo G, Ehsan M, Yan R, Song X, et al. Proteomic analysis of the excretory and secretory proteins of Haemonchus contortus (HcESP) binding to goat PBMCs In vivo revealed stage-specific binding profiles. PLoS One (2016) 11:e0159796. doi:10.1371/journal.pone.0159796
20. Ehsan M, Gao W, Gadahi JA, Lu M, Liu X, Wang Y, et al. Arginine kinase from Haemonchus contortus decreased the proliferation and increased the apoptosis of goat PBMCs in vitro. Parasit Vectors (2017) 10:311. doi:10.1186/s13071-017-2244-z
21. Nicholson IC, Mavrangelos C, Fung K, Ayhan M, Levichkin I, Johnston A, et al. Characterisation of the protein composition of peripheral blood mononuclear cell microsomes by SDS-PAGE and mass spectrometry. J Immunol Methods (2005) 305:84–93. doi:10.1016/j.jim.2005.07.005
22. Yuan C, Zhang H, Wang W, Li Y, Yan R, Xu L, et al. Transmembrane protein 63A is a partner protein of Haemonchus contortus galectin in the regulation of goat peripheral blood mononuclear cells. Parasit Vectors (2015) 8:211. doi:10.1186/s13071-015-0816-3
23. Gadahi JA, Ehsan M, Wang S, Zhang Z, Wang Y, Yan R, et al. Recombinant protein of Haemonchus contortus 14-3-3 isoform 2 (rHcftt-2) decreased the production of IL-4 and suppressed the proliferation of goat PBMCs in vitro. Exp Parasitol (2016) 171:57–66. doi:10.1016/j.exppara.2016.10.014
24. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem (1976) 72:248–54. doi:10.1016/0003-2697(76)90527-3
25. Yi D, Xu L, Yan R, Li X. Haemonchus contortus: cloning and characterization of serpin. Exp Parasitol (2010) 125:363–70. doi:10.1016/j.exppara.2010.03.002
26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (2001) 25:402–8. doi:10.1006/meth.2001.1262
27. Kaleab B, Ottenoff T, Converse P, Halapi E, Tadesse G, Rottenberg M, et al. Mycobacterial-induced cytotoxic T cells as well as nonspecific killer cells derived from healthy individuals and leprosy patients. Eur J Immunol (1990) 20:2651–9. doi:10.1002/eji.1830201219
28. Wang W, Wang S, Zhang H, Yuan C, Yan R, Song X, et al. Galectin Hco-gal-m from Haemonchus contortus modulates goat monocytes and T cell function in different patterns. Parasit Vectors (2014) 7:342. doi:10.1186/1756-3305-7-342
29. Sun J, Zhang X, Broderick M, Fein H. Measurement of nitric oxide production in biological systems by using griess reaction assay. Sensors (2003) 3:276. doi:10.3390/s30800276
30. Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell (2009) 139:468–84. doi:10.1016/j.cell.2009.10.006
31. García-Coiradas L, Angulo-Cubillán F, Méndez S, Larraga V, De La Fuente C, Cuquerella M, et al. Isolation and immunolocalization of a putative protective antigen (p26/23) from adult Haemonchus contortus. Parasitol Res (2008) 104:363. doi:10.1007/s00436-008-1204-0
32. Lejambre LF, Windon RG, Smith WD. Vaccination against Haemonchus contortus: performance of native parasite gut membrane glycoproteins in Merino lambs grazing contaminated pasture. Vet Parasitol (2008) 153:302–12. doi:10.1016/j.vetpar.2008.01.032
33. García-Coiradas L, Angulo-Cubillán F, Valladares B, Martínez E, De La Fuente C, Alunda JM, et al. Immunization against lamb haemonchosis with a recombinant somatic antigen of Haemonchus contortus (rHcp26/23). Vet Med Int (2010) 2010:8. doi:10.4061/2010/852146
34. Knox DP, Redmond DL, Skuce PJ, Newlands GFJ. The contribution of molecular biology to the development of vaccines against nematode and trematode parasites of domestic ruminants. Vet Parasitol (2001) 101:311–35. doi:10.1016/S0304-4017(01)00558-1
35. Knox DP, Smith WD. Vaccination against gastrointestinal nematode parasites of ruminants using gut-expressed antigens. Vet Parasitol (2001) 100:21–32. doi:10.1016/S0304-4017(01)00480-0
36. Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev (2004) 84:1–39. doi:10.1152/physrev.00013.2003
37. Sun Y, Yan R, Muleke CI, Zhao G, Xu L, Li X. Recombinant galectins of Haemonchus contortus parasite induces apoptosis in the peripheral blood lymphocytes of goat. Int J Pept Res Ther (2007) 13:387–92. doi:10.1007/s10989-006-9045-0
38. Li BW, Rush AC, Crosby SD, Warren WC, Williams SA, Mitreva M, et al. Profiling of gender-regulated gene transcripts in the filarial nematode Brugia malayi by cDNA oligonucleotide array analysis. Mol Biochem Parasitol (2005) 143:49–57. doi:10.1016/j.molbiopara.2005.05.005
39. Ingebritsen TS, Cohen P. The protein phosphatases involved in cellular regulation. 1. Classification and substrate specificities. Eur J Biochem (1983) 132:255–61. doi:10.1111/j.1432-1033.1983.tb07357.x
40. Smith GD, Wolf DP, Trautman KC, Da Cruz E, Silva EF, Greengard P, et al. Primate sperm contain protein phosphatase 1, a biochemical mediator of motility. Biol Reprod (1996) 54:719–27. doi:10.1095/biolreprod54.3.719
41. Grencis RK. Cytokine regulation of resistance and susceptibility to intestinal nematode infection – from host to parasite. Vet Parasitol (2001) 100:45–50. doi:10.1016/S0304-4017(01)00482-4
42. Shakya KP, Miller JE, Horohov DW. A Th2 type of immune response is associated with increased resistance to Haemonchus contortus in naturally infected Gulf coast native lambs. Vet Parasitol (2009) 163:57–66. doi:10.1016/j.vetpar.2009.03.052
43. Schallig HD. Immunological responses of sheep to Haemonchus contortus. Parasitology (2000) 120(Suppl):S63–72. doi:10.1017/S003118209900579X
44. Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology (2006) 117:433–42. doi:10.1111/j.1365-2567.2006.02321.x
45. Grencis RK, Humphreys NE, Bancroft AJ. Immunity to gastrointestinal nematodes: mechanisms and myths. Immunol Rev (2014) 260:183–205. doi:10.1111/imr.12188
46. Larkin BM, Smith PM, Ponichtera HE, Shainheit MG, Rutitzky LI, Stadecker MJ. Induction and regulation of pathogenic Th17 cell responses in schistosomiasis. Semin Immunopathol (2012) 34:873–88. doi:10.1007/s00281-012-0341-9
47. Song X, Gao H, Qian Y. Th17 differentiation and their pro-inflammation function. Adv Exp Med Biol (2014) 841:99–151. doi:10.1007/978-94-017-9487-9_5
48. Katawa G, Layland LE, Debrah AY, Von Horn C, Batsa L, Kwarteng A, et al. Hyperreactive onchocerciasis is characterized by a combination of Th17-Th2 immune responses and reduced regulatory T cells. PLoS Negl Trop Dis (2015) 9:e3414. doi:10.1371/journal.pntd.0003414
49. Wen X, He L, Chi Y, Zhou S, Hoellwarth J, Zhang C, et al. Dynamics of Th17 cells and their role in Schistosoma japonicum infection in C57BL/6 Mice. PLoS Negl Trop Dis (2011) 5:e1399. doi:10.1371/journal.pntd.0001399
50. Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol (2006) 24:99–146. doi:10.1146/annurev.immunol.24.021605.090737
51. Lebman DA, Edmiston JS. The role of TGF-beta in growth, differentiation, and maturation of B lymphocytes. Microbes Infect (1999) 1:1297–304. doi:10.1016/S1286-4579(99)00254-3
52. Keskinen P, Ronni T, Matikainen S, Lehtonen A, Julkunen I. Regulation of HLA class I and II expression by interferons and influenza A virus in human peripheral blood mononuclear cells. Immunology (1997) 91:421–9. doi:10.1046/j.1365-2567.1997.00258.x
53. Lüder CGK, Lang T, Beuerle B, Gross U. Down-regulation of MHC class II molecules and inability to up-regulate class I molecules in murine macrophages after infection with Toxoplasma gondii. Clin Exp Immunol (1998) 112:308–16. doi:10.1046/j.1365-2249.1998.00594.x
54. Panek RB, Benveniste EN. Class II MHC gene expression in microglia. Regulation by the cytokines IFN-gamma, TNF-alpha, and TGF-beta. J Immunol (1995) 154:2846–54.
55. Tountas NA, Brautigan DL. Migration and retraction of endothelial and epithelial cells require PHI-1, a specific protein-phosphatase-1 inhibitor protein. J Cell Sci (2004) 117:5905–12. doi:10.1242/jcs.01506
56. Mcgovern KE, Wilson EH. Role of chemokines and trafficking of immune cells in parasitic infections. Curr Immunol Rev (2013) 9:157–68. doi:10.2174/1573395509666131217000000
57. Sun H, Wang Y. Novel Ser/Thr protein phosphatases in cell death regulation. Physiology (Bethesda) (2012) 27:43–52. doi:10.1152/physiol.00034.2011
58. Liu Q, Wilkins BJ, Lee YJ, Ichijo H, Molkentin JD. Direct interaction and reciprocal regulation between ASK1 and calcineurin-NFAT control cardiomyocyte death and growth. Mol Cell Biol (2006) 26:3785–97. doi:10.1128/MCB.26.10.3785-3797.2006
59. Cohen PT, Philp A, Vazquez-Martin C. Protein phosphatase 4 – from obscurity to vital functions. FEBS Lett (2005) 579:3278–86. doi:10.1016/j.febslet.2005.04.070
60. Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ (2007) 14:400–10. doi:10.1038/sj.cdd.4402085
61. Huang H-S, Lee EYC. Protein phosphatase-1 inhibitor-3 is an in vivo target of caspase-3 and participates in the apoptotic response. J Biol Chem (2008) 283:18135–46. doi:10.1074/jbc.M709735200
62. Kang Y, Lee JH, Hoan NN, Sohn HM, Chang IY, You HJ. Protein phosphatase 5 regulates the function of 53BP1 after neocarzinostatin-induced DNA damage. J Biol Chem (2009) 284:9845–53. doi:10.1074/jbc.M809272200
63. Loke PN, Macdonald AS, Robb A, Maizels RM, Allen JE. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur J Immunol (2000) 30:2669–78. doi:10.1002/1521-4141(200009)30:9<2669::AID-IMMU2669>3.0.CO;2-1
64. Kotze AC, Mcclure SJ. Haemonchus contortus utilises catalase in defence against exogenous hydrogen peroxide in vitro. Int J Parasitol (2001) 31:1563–71. doi:10.1016/S0020-7519(01)00303-4
65. Wandurska-Nowak E. [The role of nitric oxide (NO) in parasitic infections]. Wiad Parazytol (2004) 50:665–78.
66. Dong Z, Yang X, Xie K, Juang SH, Llansa N, Fidler IJ. Activation of inducible nitric oxide synthase gene in murine macrophages requires protein phosphatases 1 and 2A activities. J Leukoc Biol (1995) 58:725–32. doi:10.1002/jlb.58.6.725
67. Taylor BS, Liu S, Villavicencio RT, Ganster RW, Geller DA. The role of protein phosphatases in the expression of inducible nitric oxide synthase in the rat hepatocyte. Hepatology (1999) 29:1199–207. doi:10.1002/hep.510290419
68. Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol (1992) 148:1792–6.
Keywords: Haemonchus contortus, serine/threonine phosphatase, peripheral blood mononuclear cells, cytokines, immune responses
Citation: Ehsan M, Wang W, Gadahi JA, Hasan MW, Lu M, Wang Y, Liu X, Haseeb M, Yan R, Xu L, Song X and Li X (2018) The Serine/Threonine-Protein Phosphatase 1 From Haemonchus contortus Is Actively Involved in Suppressive Regulatory Roles on Immune Functions of Goat Peripheral Blood Mononuclear Cells. Front. Immunol. 9:1627. doi: 10.3389/fimmu.2018.01627
Received: 25 January 2018; Accepted: 02 July 2018;
Published: 16 July 2018
Edited by:
Xun Suo, China Agricultural University, ChinaReviewed by:
Phileno Pinge-Filho, Universidade Estadual de Londrina, BrazilLaura Noelia Cariddi, National University of Río Cuarto, Argentina
Copyright: © 2018 Ehsan, Wang, Gadahi, Hasan, Lu, Wang, Liu, Haseeb, Yan, Xu, Song and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: XiangRui Li, bGl4aWFuZ3J1aUBuamF1LmVkdS5jbg==
 Muhammad Ehsan
Muhammad Ehsan Javaid Ali Gadahi
Javaid Ali Gadahi Muhammad Waqqas Hasan
Muhammad Waqqas Hasan XinChao Liu
XinChao Liu RuoFeng Yan
RuoFeng Yan XiaoKai Song
XiaoKai Song XiangRui Li
XiangRui Li