- Department of Chemotherapy and Mycoses, National Institute of Infectious Diseases, Tokyo, Japan
CD1d-restricted invariant natural killer T (iNKT) cells are innate-type lymphocytes that express a T-cell receptor (TCR) containing an invariant α chain encoded by the Vα14 gene in mice and Vα24 gene in humans. These iNKT cells recognize endogenous, microbial, and synthetic glycolipid antigens presented by the major histocompatibility complex (MHC) class I-like molecule CD1d. Upon TCR stimulation by glycolipid antigens, iNKT cells rapidly produce large amounts of cytokines, including interferon-γ (IFNγ) and interleukin-4 (IL-4). Activated iNKT cells contribute to host protection against a broad spectrum of microbial pathogens, and glycolipid-mediated stimulation of iNKT cells ameliorates many microbial infections by augmenting innate and acquired immunity. In some cases, however, antigen-activated iNKT cells exacerbate microbial infections by promoting pathogenic inflammation. Therefore, it is important to identify appropriate microbial targets for the application of iNKT cell activation as a treatment or vaccine adjuvant. Many studies have found that iNKT cell activation induces potent adjuvant activities promoting protective vaccine effects. In this review, we summarize the functions of CD1d-restricted iNKT cells in immune responses against microbial pathogens and describe the potential applications of glycolipid-mediated iNKT cell activation for preventing and controlling microbial infections.
Introduction
Natural killer T (NKT) cells are innate-type lymphocytes that recognize glycolipid antigens presented by the MHC class I-like molecule CD1d (1–7). The CD1d molecule is critical for both thymic development and the effector functions of NKT cells (1–7). These CD1d-restricted NKT cells are classified into two subpopulations, invariant NKT (iNKT) cells or type I NKT cells and type II NKT cells. In this review, we focus on iNKT cell functions in immunity and potential therapeutic applications, while the features and functions of type II NKT cells are summarized in several recent reviews (8, 9). The iNKT cell subtype expresses an invariant T-cell receptor (TCR) α chain encoded by the Vα14-Jα18 gene in mice and Vα24-Jα18 gene in humans. This TCRα chain is paired with a restricted repertoire of TCRα chains, such as Vβ8, 7, and 2 in mice and Vβ11 in humans (1–7). Upon TCR stimulation by microbe-derived glycolipid antigens or the potent synthetic lipid antigen α-galactosylceramide (α-GalCer), iNKT cells rapidly activate and produce large amounts of cytokines, including interferon-γ (IFNγ), interleukin-2, IL-4, IL-13, and IL-17A, and stimulate innate immune responses by activating antigen-presenting cells (APCs) and NK cells (1–6). iNKT cells activated by glycolipid antigens not only produce cytokines but also express CD40 ligand (CD40L), which stimulates the maturation of APCs, such as dendritic cells (DCs), leading to the augmentation of acquired immune responses (1–6). Through these unique signaling functions, CD1d-restricted iNKT cells participate in both innate and acquired immune responses against a variety of microbial pathogens, including bacteria, fungi, viruses, and protozoan parasites (10–15). In this review, we summarize the contributions of CD1d-restricted iNKT cells to immune responses against microbial pathogens by focusing on selected microbial infections. We also describe the potential applications of glycolipid-mediated iNKT cell activation for the development of new therapies and vaccines against infectious diseases.
iNKT Cells Contribute to Innate Immune Responses Against Microbial Pathogens
Invariant natural killer T cells participate in the early phase of the immune response against various microbes through recognition of microbial components and stimulation of innate immune cells (10–15). Following infection by Aspergillus fumigatus, a major cause of invasive fungal infection in immunocompromised patients, iNKT cells produce IFNγ in response to recognition of endogenous antigens presented by CD1d, while APCs such as DCs release IL-12 in response to stimulation by β-glucan, resulting in the promotion of fungal clearance (16). Conversely, CD1d-deficient mice that lack iNKT cells exhibit delayed fungal clearance following infection by A. fumigatus (16).
Streptococcus pneumoniae (Pneumococcus) is the major cause of community-acquired pneumonia and meningitis, and is responsible for more than one million deaths annually. In the early phase of pneumococcal infection, iNKT cells contribute to neutrophil recruitment and bacterial clearance in the lungs through the release of neutrophil-recruiting cytokines such as tumor necrosis factor (TNF) and macrophage inflammatory protein-2 (17). Cell transfer experiments suggest that IFNγ produced by iNKT cells is essential for neutrophil recruitment (18). Other studies have reported that the iNKT cell response to Pneumococcus involves recognition of pneumococcal glycolipids (19, 20) and production of cytokines including IFNγ. The production of these cytokines is enhanced by IL-12 released from APCs stimulated by toll-like receptor (TLR) ligands of Pneumococcus (21).
CXCR6+ NKT cells, which consist mainly of iNKT cells, patrol liver sinusoids for signs of bacterial infection (22). When the TCR is stimulated by αGalCer, an anti-CD3 antibody, or bacterial glycosphingolipid, these CXCR6+ NKT cells stop crawling (22, 23). These observations suggest that iNKT cells play a major role in liver surveillance and arrest when stimulated. It has been demonstrated that Kupffer cells in liver sinusoids capture intravenously injected Borrelia burgdorferi, a causative bacterium of the inflammatory disorder Lyme disease (24, 25). iNKT cells then accumulate around Borrelia-ingested Kupffer cells and form clusters, a response dependent on the stimulation of the cytokine receptor CXCR3 (26). Borrelia-ingested Kupffer cells express CD1d and can, therefore, activate iNKT cells (26). iNKT cells have been shown to recognize a B. burgdorferi glycolipid presented by CD1d (27, 28), and the ensuing activation contributes to bacterial clearance and prevention of joint and heart inflammation (24, 25). Consistent with these observations, mice deficient in iNKT cells or depleted of Kupffer cells exhibited bacterial dissemination to bladder, joints, and heart (26). These results indicate that iNKT cells contribute to the immune response against B. burgdorferi during the early phase of infection by recognizing bacterial glycolipids presented by Kupffer cells in liver sinusoids, thereby preventing bacterial dissemination to other tissues.
Invariant natural killer T cells also participate in host protection against post-stroke bacterial infection, a major cause of stroke-related death. In mice, the number of crawling or CD69-expressing iNKT cells rapidly declined following transient middle cerebral artery occlusion, while the number of iNKT cells producing IL-10 (but not IFNγ or IL-4) increased (29). At 24 h after stroke, mice exhibited systemic infection as evidenced by the detection of endogenous bacteria in multiple organs and tissues (29). In contrast, the activation of iNKT cells by α-GalCer induced bacterial clearance from these organs and tissues. Furthermore, stroke-induced bacterial infection was prevented by the administration of propranolol, a nonspecific β-adrenergic receptor blocker, or by 6-hidroxydopamine, a neurotoxin that depletes peripheral neuronal terminals of noradrenaline, through recovery of iNKT cell functions such as crawling and IFNγ production and by shifting to a Th1-dominant response (29). Intriguingly, protection from bacterial infection by propranolol has not been observed in CD1d-deficient mice that lack iNKT cells. These results imply that stroke-associated infection is mediated by the suppression of iNKT cell function.
Collectively, these results indicate that iNKT cells play an important role in host defense against the early phase of microbial infection through the recognition of microbial glycolipids and stimulation of innate immune cells.
Mechanisms of iNKT Cell Responses Against Microbial Pathogens
Previous studies have identified at least three mechanisms that trigger iNKT cell response to microbial pathogens: microbial glycolipid-mediated TCR activation, endogenous antigen-mediated weak TCR stimulation with concomitant inflammatory cytokine-mediated stimulation, and activation solely by inflammatory cytokines (2, 10, 12, 15).
Several microbial lipid antigens have been identified that activate iNKT cells through CD1d presentation to the TCR. For example, mouse and human iNKT cells recognize α-linked glycosphingolipids (GSLs) containing either a galacturonic acid or a glucuronic acid derived from commensal Sphingomonas species of the intestine (30–32). The structures of these glycolipids are very similar to α-GalCer, but with subtle differences such as the carbohydrate moiety and a shorter C14 acyl chain replacing the C26 acyl chain of α-GalCer (30, 31, 33). In addition to GSLs, iNKT cells also recognize glycerol-containing glycolipids. B. burgdorferi expresses a diacylglycerol containing α-linked galactose called B. burgdorferi glycolipid-II (BbGL-II). A BbGL-II isoform containing a palmitic acid (C16:0) and an oleic acid (C18:1) potently stimulated mouse iNKT cells (27, 28). Human iNKT cells respond more strongly to BbGL-II isoforms containing fatty acids with greater unsaturation, such as oleic acid (C18:1) and linoleic acid (C18:2) (27, 28). Streptococcus pneumoniae express an α-linked diacylglycerol containing a glucose (Glc-DAG). The Glc-DAG containing a palmitic acid (C16:0) and a vaccenic acid (C18:1) is recognized by mouse and human iNKT cells (19). These Sphingomonas, B. burgdorferi, and S. pneumoniae glycolipids act as antigens that stimulate mouse and human iNKT cell TCRs and induce cytokine release. One intriguing question is how the iNKT cell TCR with an invariant α chain recognize different antigens. Recent structural analyses of the iNKT cell TCR−glycolipid−CD1d ternary complex revealed that iNKT cell TCR induces conformational changes to both the bacterial glycolipid antigen and CD1d, thereby allowing the recognition of different glycolipid antigens by a conserved binding orientation (20, 33).
Activation of iNKT cells by combined endogenous antigen-mediated weak TCR stimulation and inflammatory cytokine-mediated stimulation (2, 10, 12) is exemplified by Salmonella typhimurium, a Gram-negative bacterium expressing lipopolysaccharide (LPS). S. typhimurium has been shown to stimulate IFNγ release from iNKT cells despite the absence of a recognized glycolipid antigen (34). The activation of iNKT cells by S. typhimurium is mediated by IL-12 released from APCs stimulated by LPS through TLR4 and myeloid differentiation primary response 88 signaling (34). In addition, iNKT cell activation is partially dependent on CD1d (34), suggesting that iNKT cell activation during S. typhimurium infection requires a combination of weak TCR stimulation by an endogenous antigen and stimulation by inflammatory cytokines released by APCs in response to S. typhimurium.
In other cases, iNKT cells are activated solely by inflammatory cytokines (10, 12, 15). In the early phase of murine cytomegalovirus (MCMV) infection, a substantial number of iNKT cells produce IFNγ (35). However, this MCMV-associated cytokine production is independent of CD1d, but highly dependent on IL-12 and partially dependent on type I IFN (35). iNKT cells also amplify IFNγ release from NK cells and contribute to host protection against MCMV infection (35). In Nur77gfp reporter mice harboring T cells that express green fluorescent protein (GFP) upon antigen-mediated TCR stimulation, but not inflammatory cytokines, MCMV infection induced IFNγ production by iNKT cells without GFP expression (36). Collectively, these results show that the iNKT cell response to MCMV is independent of TCR stimulation but dependent on inflammatory cytokines. In contrast to MCMV, S. pneumoniae and Sphingomonas paucimobilis induced the expression of GFP and IFNγ in iNKT cells, indicating that these species activate iNKT cells through TCR stimulation (36). Alternatively, S. typhimurium and LPS did not induce GFP expression by iNKT cells, although these cells did produce IFNγ (36). These results suggest that inflammatory signals play an important role in iNKT cell activation in response to microbes that do not possess glycolipid antigens, greatly expanding the spectrum of iNKT cell-activating pathogens.
iNKT Cells Contribute to Acquired Immune Responses Against Microbial Pathogens
Cryptococcus neoformans is a fungal pathogen that causes pulmonary infection and can also disseminate to the central nervous system and cause meningitis, especially in immunocompromised individuals such as those with acquired immune deficiency syndrome. Following pulmonary infection of mice with C. neoformans, iNKT cells accumulated in the lungs, a response dependent on monocyte chemoattractant protein-1 (37). Jα18-deficient mice lacking iNKT cells exhibited delayed fungal clearance due to a weak Th1 response, normally a key immune response against C. neoformans infection (37). These results suggest that iNKT cells contribute to protection against cryptococcal infection through the stimulation of Th1 response. It was subsequently reported, however, that Jα18-deficient mice also exhibit defects in the rearrangement of Jα segments upstream of Jα18 (38). Therefore, the results obtained in Jα18-deficient mice may not be solely due to iNKT cell deficiency, especially under conditions involving the adaptive immune response.
A recent study has revealed an important role of iNKT cells in the initial formation of germinal centers during influenza infection. iNKT cells are a major source of early IL-4 release during infection, which is essential for the induction of germinal center B cells and ensuing IgG1 production (39). This enhanced IL-4 release is triggered by CD1d and IL-18 stimulation from CD169+ macrophages (39). Furthermore, the transcriptomic analysis of lymph nodes in Zika virus-infected macaque monkeys revealed that IL-4 and NKT cell signatures, but not the Tfh cell signature, was strongly correlated with neutralizing antibody titer in the early phase of infection (39). These results suggest that iNKT cells promote initial germinal center formation and IgG production during the early stage of viral infection through macrophage-induced IL-4 release.
Taken together, these results highlight the importance of iNKT cells in host defense against various microbial infections through the stimulation of both innate and acquired immunity.
Glycolipid-Mediated Activation of iNKT Cells Enhances Antimicrobial Immunity
As discussed, iNKT cells contribute to neutrophil recruitment during pneumococcal infection (17, 18). For instance, the activation of iNKT cells by α-GalCer promoted bacterial clearance through the recruitment of neutrophils and protected mice from lethal pneumococcal infection (17). Respiratory DCs, especially CD103+ DCs, promote iNKT cell activation through the release of IFNγ and IL-17A, key cytokines conferring protection against pneumococcal infection (40). Stimulation of iNKT cells by α-GalCer also induces macrophage activation. During lung infection by Pseudomonas aeruginosa, α-GalCer treatment increased IFNγ and TNF in bronchoalveolar lavage fluid and the phagocytosis of bacteria by alveolar macrophages, resulting in rapid recovery from pneumonia (41). It has also been shown that α-GalCer treatment significantly inhibits malaria infection at the liver stage, but not at the blood stage, in an IFNγ-dependent manner (42). Alpha-C-galactosylceramide (α-C-GalCer), a C-glycoside analog of α-GalCer, induces longer IFNγ production and lower IL-4 production than α-GalCer (43), and α-C-GalCer has been shown to exhibit superior antimicrobial efficacy during the liver stage of malaria infection compared with α-GalCer (43). This superior effect of α-C-GalCer is dependent on IL-12, which is necessary for IFNγ production by NK cells, a major source of IFNγ. These results indicate that glycolipid-mediated iNKT cell activation enhances innate immune responses, resulting in a greater control of microbial infections at the early phase.
Glycolipid-activated iNKT cells augment the induction of effector CD4T cells and CD8T cells through the activation of APCs such as DCs. During Chlamydophila pneumoniae infection, α-GalCer-activated iNKT cells upregulated CD40 expression and IL-12 production by DCs, leading to the expansion of IFNγ-producing CD4T cells and IFNγ-producing CD8T cells and ultimately decreasing the bacterial burden in lungs (44, 45). It has also been shown that α-GalCer treatment enhances the Th1 response and fungal clearance during C. neoformans infection in an IFNγ-dependent manner (46). In the absence of IL-18, the increased IFNγ production and inhibition of fungal growth induced by α-GalCer were further enhanced through a greater production of IL-12 and IL-4 (47). Alpha-GalCer treatment also increases the memory CD4T cell pool size and alters the function of memory Th2 cells for increased IFNγ production (48). Further, α-GalCer treatment promotes the differentiation of central memory CD8T cells. During MCMV infection, α-GalCer treatment rapidly induced IFNγ and IL-4 production and decreased viral titers in spleen and liver (49). These α-GalCer-treated mice also exhibited greater numbers of MCMV antigen-specific central memory CD8T cells (49). These results suggest that glycolipid-mediated iNKT cell activation may be an effective strategy to augment the induction of effector and memory CD4T cells and CD8T cells that contribute to host protection against microbial infections.
iNKT Cells Contribute to the Pathogenesis of Some Microbial Infections
In contrast to these documented benefits, iNKT cells play a detrimental role against the host during certain microbial infections by the induction or augmentation of inflammation, which results in the exacerbation of infection or causes severe acute or chronic inflammatory diseases (12, 13). Candida species colonize the skin and gastrointestinal and genitourinary mucosal surfaces and are a major cause of bloodstream infections among inpatients, with mortality rates from candidemia and invasive candida infections as high as 30−40% (50, 51). iNKT cells contribute to the pathogenesis of C. albicans infection, the most frequent Candida species. Following systemic C. albicans infection, Jα18-deficient mice lacking iNKT cells exhibited a higher survival rate and a lower fungal burden in various organs than wild-type (WT) mice because of the increased accumulation of macrophages and neutrophils in the peritoneal cavity (52). Consistent with the amelioration of infection by iNKT cell depletion, IL-10 levels were lower and IL-12p40 levels were higher in the serum of C. albicans-infected Jα18-deficient mice than infected WT mice. Conversely, NKT cell transfer exacerbated C. albicans infection in Jα18-deficient mice concomitant with reduced accumulation of macrophages and neutrophils (52). Furthermore, IL-10 treatment exacerbated C. albicans infection in Jα18-deficient mice, and transfer of IL-10-deficient NKT cells into Jα18-deficient mice significantly increased survival following C. albicans infection compared to the transfer of WT NKT cells (52). However, another study found no difference in susceptibility to C. albicans infection between Jα18-deficient and WT mice (53). This discrepancy is probably because of the different C. albicans strains employed and distinct routes of infection. It should also be reiterated that the difference in infection response by Jα18-deficient mice may not be due to iNKT cell deficiency alone, as these mice also show deficits in the rearrangement of Jα segments upstream of Jα18 (38).
Alpha-GalCer-mediated iNKT cell activation also exacerbates C. albicans infection. Alpha-GalCer-treated mice exhibited higher fungal burden in kidneys, higher IL-6 levels in serum and kidneys, wider dissemination of fungi, and shorter survival than control-infected mice (54). The number of neutrophils, the main effector cells controlling C. albicans infection, was significantly decreased in C. albicans infected and α-GalCer-treated mice, and this difference was IFNγ-dependent (54). It is thought that some bacterial species can disseminate to blood from the intestine in immunocompromised patients and activate iNKT cells. Furthermore, this mode of iNKT cell activation may exacerbate certain infections. Mice pre-infected with Sphingomonas bacteria, which are commensal and possess glycolipid antigens for iNKT cells (30–32), prior to C. albicans exposure exhibited enhanced IFNγ-dependent iNKT cell activation, increased production of inflammatory cytokines, and greater fungal burden (54). Collectively, these results indicate that iNKT cells participate in the pathogenesis of C. albicans infection and that iNKT cell activation by glycolipid antigens or bacterial infection can exacerbate C. albicans infection.
Glycolipid-Activated iNKT Cells Exhibit Effective Adjuvant Activities to Prevent Microbial Infections
Many studies have demonstrated the potential adjuvant activities of glycolipid-activated iNKT cells for protection against microbial infections (11, 12, 14). For instance, immunization with malarial antigens and α-GalCer inhibited the liver stage of malaria and prevented parasitemia more effectively than malarial antigen alone. Immunization with malarial antigens and α-GalCer also increased the number of IFNγ-producing antigen-specific CD8T cells, major effector cells controlling the liver stage of malaria infection (55). Mice sublingually immunized with the Mycobacterium tuberculosis antigens Ag85B and ESAT-6 together with α-GalCer exhibited stronger antigen-specific CD4T- and CD8T-cell responses than mice immunized with Ag85B and ESAT-6 alone, and resulted in a significantly lower organ bacterial burden (56). Immunization with bacillus Calmette–Guérin (BCG)-incorporated α-GalCer or α-C-GalCer, an analog with a C-glycoside, induced a greater number of antigen-specific IFNγ-producing CD8T cells than unmodified BCG through increased maturation of DCs by iNKT cells (57). Mice immunized with glycolipid-incorporated BCG also exhibited reduced bacterial loads in lungs and spleen compared with mice receiving unmodified BCG immunization (57). These vaccine effects were more evident with α-C-GalCer than α-GalCer, probably due to the lower IL-4 production and prolonged IL-12 production induced by α-C-GalCer compared to α-GalCer (43).
Glycolipid-mediated iNKT cell activation also augments antibody production by B cells (58, 59). Intranasal administration of α-GalCer and influenza hemagglutinin (HA) vaccine or formalin-inactivated whole-virion vaccine induced higher titers of mucosal IgA and systemic IgG compared to influenza vaccine alone (60–62). Co-administration of α-GalCer and influenza vaccine also protected mice from lethal influenza virus infection, including H5N1 influenza virus infection (62), through enhanced viral clearance (60–62). Glycolipid-mediated iNKT cell activation also has adjuvant activity in swine. Indeed, α-GalCer showed excellent adjuvant activity with UV-inactivated influenza virus for increasing virus-specific antibody titers and IFNγ-producing cells in swine, and this immunization strategy protected against pandemic H1N1 influenza infection (63).
Although it is well known that follicular helper T (TFH) cells play a critical role in the stimulation of germinal center B cells for high-affinity antibody production, as well as differentiation of memory B cells and long-lived plasma cells, recent studies have demonstrated that follicular helper NKT (NKTFH) cells contribute to augmented IgG antibody production by vaccines containing an iNKT cell glycolipid antigen (59, 64–67). Immunization of mice with liposomes containing pneumococcal capsular polysaccharide (CPS) and PBS57, an α-GalCer analog, or CPS-α-GalCer conjugate vaccine induced NKTFH cells expressing PD-1 and CXCR5 or PD-1 and ICOS (66, 67). Mice treated with these vaccines showed an enhanced IgG1 production, indicating that cognate B cells are activated by T cells. Intriguingly, IgG1 production was dependent on CD1d expression by B cells and DCs, indicating that IgG1 production is induced by the cognate interaction of iNKT cells and B cells (66). These vaccines containing pneumococcal CPS and a glycolipid induced germinal center formation and CPS-specific memory B cells and long-lived plasma cells, which provided long-term protection against pneumococcal infection (67).
Collectively, glycolipid-mediated iNKT cell activation provides excellent adjuvant activities for inducing effector T-cell responses, promoting high-affinity antibody production by B cells, and for augmenting memory T- and B-cell responses (Figure 1).
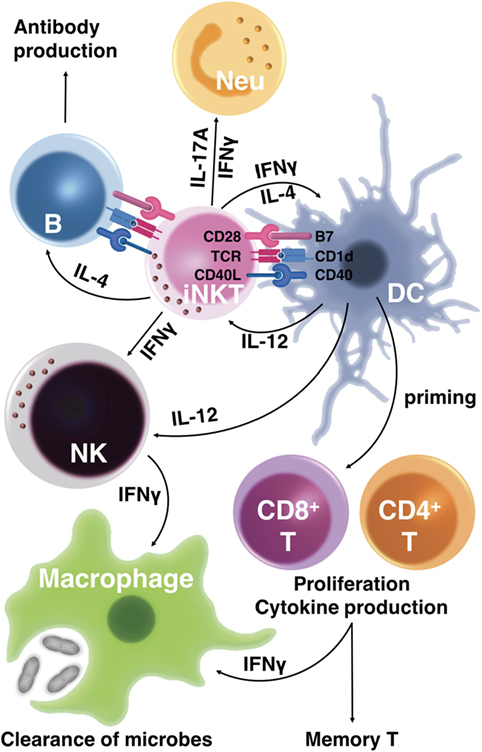
Figure 1. Activation of CD1d-restricted invariant natural killer T (iNKT) cells augments both innate and acquired immunity to control microbial infection. The T-cell receptor (TCR) of iNKT cells recognizes glycolipid antigens presented by CD1d on antigen-presenting cells (APCs). In response, activated iNKT cells produce cytokines, including interferon-γ (IFNγ), interleukin-4 (IL-4), and IL-17A, that stimulate innate immune responses such as neutrophil (Neu) recruitment. Glycolipid-activated iNKT cells also express CD40 ligand (CD40L), which promotes APC maturation. iNKT cells provide cognate help to B cells to promote antibody production when glycolipid-conjugated antigens are presented by B cells. Through cytokine release and CD40L–CD40 interaction, iNKT cells stimulate dendritic cells (DCs), triggering DC production of cytokines such as IL-12. These DC-derived cytokines stimulate IFNγ production by iNKT cells, which in turn enhances microbial clearance by stimulating macrophages. Activated iNKT cells also induce maturation of DCs that prime IFNγ-producing effector CD4T and CD8T cells, resulting in the clearance of microbes. The mature DCs induced by activated iNKT cells enhance the differentiation not only of effector T cells but also of memory T cells, conferring long-term protection against microbial infection.
Concluding Remarks
Due to space limitations, this review focused on only a few selected studies of iNKT cell responses to microbes. However, numerous studies have demonstrated that CD1d-resticted iNKT cells contribute to immune responses against a broad spectrum of pathogenic microbes despite constituting only a small fraction of the leukocyte population. Due to the capacity for rapidly producing large quantities of cytokines in response to TCR stimulation, glycolipid-activated iNKT cells can augment both innate and acquired immunity, thereby providing protection against disparate microbial pathogens. However, in some cases, glycolipid-mediated iNKT cell activation may contribute to the pathogenesis of infection by exacerbating inflammation. Therefore, it is critical to distinguish which microbial targets are suppressed by iNKT cell activation for treatment or vaccine development. Considering the accumulating evidence for excellent adjuvant activities of glycolipid-mediated iNKT cell activation and the strong evolutionary conservation of iNKT cell responses to glycolipid antigens among experimental animals (usually mice) and humans (10–12), the inclusion of glycolipids inducing iNKT cell activation in vaccination regimens may be an effective strategy to prevent and control microbial infections in humans. It should be noted, however, that human and mouse iNKT cell responses to some glycolipids may differ as previously demonstrated by two C-glycoside analogs of α-GalCer (68) and Borrelia glycolipids (27). Therefore, careful consideration is needed when choosing a glycolipid antigen for clinical application of glycolipid-mediated iNKT cell activation.
Author Contributions
All authors contributed to this work and approved submission for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Yasuko Takatsuka for the preparation of the figure. The authors thank the NIH tetramer core facility for providing the CD1d tetramer.
Funding
This study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (KAKENHI 16H05349); the Japan Agency for Medical Research and Development, AMED (18im0210107j0002); the Ministry of Health, Labour and Welfare of Japan (H28 Shinko-Gyosei-005); the Yakult Bio-Science Foundation; the Takeda Science Foundation; the Life Science Foundation of Japan; and the Astellas Foundation for Research on Metabolic Disorders.
References
1. Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol (2003) 21:483–513. doi:10.1146/annurev.immunol.21.120601.141057
2. Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol (2004) 22:817–90. doi:10.1146/annurev.immunol.22.012703.104608
3. Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol (2005) 23:877–900. doi:10.1146/annurev.immunol.23.021704.115742
4. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol (2007) 25:297–336. doi:10.1146/annurev.immunol.25.022106.141711
5. Godfrey DI, Pellicci DG, Patel O, Kjer-Nielsen L, McCluskey J, Rossjohn J. Antigen recognition by CD1d-restricted NKT T cell receptors. Semin Immunol (2010) 22:61–7. doi:10.1016/j.smim.2009.10.004
6. Drennan MB, Aspeslagh S, Elewaut D. Invariant natural killer T cells in rheumatic disease: a joint dilemma. Nat Rev Rheumatol (2010) 6:90–8. doi:10.1038/nrrheum.2009.261
7. Gapin L. Development of invariant natural killer T cells. Curr Opin Immunol (2016) 39:68–74. doi:10.1016/j.coi.2016.01.001
8. Dhodapkar MV, Kumar V. Type II NKT cells and their emerging role in health and disease. J Immunol (2017) 198:1015–21. doi:10.4049/jimmunol.1601399
9. Kato S, Berzofsky JA, Terabe M. Possible therapeutic application of targeting type II natural killer T cell-mediated suppression of tumor immunity. Front Immunol (2018) 9:314. doi:10.3389/fimmu.2018.00314
10. Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol (2007) 5:405–17. doi:10.1038/nrmicro1657
11. Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol (2009) 9:28–38. doi:10.1038/nri2451
12. Kinjo Y, Kitano N, Kronenberg M. The role of invariant natural killer T cells in microbial immunity. J Infect Chemother (2013) 19:560–70. doi:10.1007/s10156-013-0638-1
13. Van Kaer L, Parekh VV, Wu L. The response of CD1d-restricted invariant NKT cells to microbial pathogens and their products. Front Immunol (2015) 6:226. doi:10.3389/fimmu.2015.00226
14. Kohlgruber AC, Donado CA, LaMarche NM, Brenner MB, Brennan PJ. Activation strategies for invariant natural killer T cells. Immunogenetics (2016) 68:649–63. doi:10.1007/s00251-016-0944-8
15. Tessmer MS, Fatima A, Paget C, Trottein F, Brossay L. NKT cell immune responses to viral infection. Expert Opin Ther Targets (2009) 13:153–62. doi:10.1517/14712590802653601
16. Cohen NR, Tatituri RVV, Rivera A, Watts GFM, Kim EY, Chiba A, et al. Innate recognition of cell wall & β-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe (2011) 10:437–50. doi:10.1016/j.chom.2011.09.011
17. Kawakami K, Yamamoto N, Kinjo Y, Miyagi K, Nakasone C, Uezu K, et al. Critical role of Valpha14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol (2003) 33:3322–30. doi:10.1002/eji.200324254
18. Nakamatsu M, Yamamoto N, Hatta M, Nakasone C, Kinjo T, Miyagi K, et al. Role of interferon-gamma in Valpha14+ natural killer T cell-mediated host defense against Streptococcus pneumoniae infection in murine lungs. Microbes Infect (2007) 9:364–74. doi:10.1016/j.micinf.2006.12.003
19. Kinjo Y, Illarionov P, Vela JL, Pei B, Girardi E, Li X, et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol (2011) 12:966–74. doi:10.1038/ni.2096
20. Girardi E, Yu ED, Li Y, Tarumoto N, Pei B, Wang J, et al. Unique interplay between sugar and lipid in determining the antigenic potency of bacterial antigens for NKT cells. PLoS Biol (2011) 9:e1001189. doi:10.1371/journal.pbio.1001189
21. Brigl M, Tatituri RVV, Watts GFM, Bhowruth V, Leadbetter EA, Barton N, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med (2011) 208:1163–77. doi:10.1084/jem.20102555
22. Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol (2005) 3:e113. doi:10.1371/journal.pbio.0030113
23. Velázquez P, Cameron TO, Kinjo Y, Nagarajan N, Kronenberg M, Dustin ML. Cutting edge: activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J Immunol (2008) 180:2024–8. doi:10.4049/jimmunol.180.4.2024
24. Tupin E, Benhnia MR-E-I, Kinjo Y, Patsey R, Lena CJ, Haller MC, et al. NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi. Proc Natl Acad Sci U S A (2008) 105:19863–8. doi:10.1073/pnas.0810519105
25. Olson CM, Bates TC, Izadi H, Radolf JD, Huber SA, Boyson JE, et al. Local production of IFN-gamma by invariant NKT cells modulates acute Lyme carditis. J Immunol (2009) 182:3728–34. doi:10.4049/jimmunol.0804111
26. Lee W-Y, Moriarty TJ, Wong CHY, Zhou H, Strieter RM, van Rooijen N, et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol (2010) 11:295–302. doi:10.1038/ni.1855
27. Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR-E-I, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol (2006) 7:978–86. doi:10.1038/ni1380
28. Wang J, Li Y, Kinjo Y, Mac T-T, Gibson D, Painter GF, et al. Lipid binding orientation within CD1d affects recognition of Borrelia burgorferi antigens by NKT cells. Proc Natl Acad Sci U S A (2010) 107:1535–40. doi:10.1073/pnas.0909479107
29. Wong CHY, Jenne CN, Lee W-Y, Léger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science (2011) 334:101–5. doi:10.1126/science.1210301
30. Kinjo Y, Wu D, Kim G, Xing G-W, Poles MA, Ho DD, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature (2005) 434:520–5. doi:10.1038/nature03407
31. Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, Zhou D, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature (2005) 434:525–9. doi:10.1038/nature03408
32. Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol (2005) 35:1692–701. doi:10.1002/eji.200526157
33. Zajonc DM, Girardi E. Recognition of microbial glycolipids by natural killer T cells. Front Immunol (2015) 6:400. doi:10.3389/fimmu.2015.00400
34. Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol (2003) 4:1230–7. doi:10.1038/ni1002
35. Wesley JD, Tessmer MS, Chaukos D, Brossay L. NK cell-like behavior of Valpha14i NK T cells during MCMV infection. PLoS Pathog (2008) 4:e1000106. doi:10.1371/journal.ppat.1000106
36. Holzapfel KL, Tyznik AJ, Kronenberg M, Hogquist KA. Antigen-dependent versus -independent activation of invariant NKT cells during infection. J Immunol (2014) 192:5490–8. doi:10.4049/jimmunol.1400722
37. Kawakami K, Kinjo Y, Uezu K, Yara S, Miyagi K, Koguchi Y, et al. Monocyte chemoattractant protein-1-dependent increase of V alpha 14 NKT cells in lungs and their roles in Th1 response and host defense in cryptococcal infection. J Immunol (2001) 167:6525–32. doi:10.4049/jimmunol.167.11.6525
38. Bedel R, Matsuda JL, Brigl M, White J, Kappler J, Marrack P, et al. Lower TCR repertoire diversity in Traj18-deficient mice. Nat Immunol (2012) 13:705–6. doi:10.1038/ni.2347
39. Gaya M, Barral P, Burbage M, Aggarwal S, Montaner B, Navia AW, et al. Initiation of antiviral B cell immunity relies on innate signals from spatially positioned NKT cells. Cell (2018) 172:517–33.e20. doi:10.1016/j.cell.2017.11.036
40. Ivanov S, Fontaine J, Paget C, Macho Fernandez E, Van Maele L, Renneson J, et al. Key role for respiratory CD103(+) dendritic cells, IFN-γ, and IL-17 in protection against Streptococcus pneumoniae infection in response to α-galactosylceramide. J Infect Dis (2012) 206:723–34. doi:10.1093/infdis/jis413
41. Nieuwenhuis EES, Matsumoto T, Exley M, Schleipman RA, Glickman J, Bailey DT, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med (2002) 8:588–93. doi:10.1038/nm0602-588
42. Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, Hong S, Bruna-Romero O, Nakayama T, et al. alpha-galactosylceramide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci U S A (2000) 97:8461–6. doi:10.1073/pnas.97.15.8461
43. Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand α-galactosylceramide. J Exp Med (2003) 198:1631–41. doi:10.1084/jem.20031192
44. Joyee AG, Qiu H, Wang S, Fan Y, Bilenki L, Yang X. Distinct NKT cell subsets are induced by different chlamydia species leading to differential adaptive immunity and host resistance to the infections. J Immunol (2007) 178:1048–58. doi:10.4049/jimmunol.178.2.1048
45. Joyee AG, Qiu H, Fan Y, Wang S, Yang X. Natural killer T cells are critical for dendritic cells to induce immunity in chlamydial pneumonia. Am J Respir Crit Care Med (2008) 178:745–56. doi:10.1164/rccm.200804-517OC
46. Kawakami K, Kinjo Y, Yara S, Koguchi Y, Uezu K, Nakayama T, et al. Activation of Valpha14(+) natural killer T cells by alpha-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect Immun (2001) 69:213–20. doi:10.1128/IAI.69.1.213-220.2001
47. Kawakami K, Kinjo Y, Yara S, Uezu K, Koguchi Y, Tohyama M, et al. Enhanced gamma interferon production through activation of Valpha 14+ natural killer T cells by alpha-galactosylceramide in interleukin-18-deficient mice with systemic cryptococcosis. Infect Immun (2001) 69:6643–50. doi:10.1128/IAI.69.11.6643-6650.2001
48. Iwamura C, Shinoda K, Endo Y, Watanabe Y, Tumes DJ, Motohashi S, et al. Regulation of memory CD4 T-cell pool size and function by natural killer T cells in vivo. Proc Natl Acad Sci U S A (2012) 109:16992–7. doi:10.1073/pnas.1203494109
49. Reilly EC, Thompson EA, Aspeslagh S, Wands JR, Elewaut D, Brossay L. Activated iNKT cells promote memory CD8+ T cell differentiation during viral infection. PLoS One (2012) 7:e37991. doi:10.1371/journal.pone.0037991
50. Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev (2007) 20:133–63. doi:10.1128/CMR.00029-06
51. Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis (2009) 48:1695–703. doi:10.1086/599039
52. Haraguchi N, Kikuchi N, Morishima Y, Matsuyama M, Sakurai H, Shibuya A, et al. Activation of murine invariant NKT cells promotes susceptibility to candidiasis by IL-10 induced modulation of phagocyte antifungal activity. Eur J Immunol (2016) 46:1691–703. doi:10.1002/eji.201545987
53. Tarumoto N, Kinjo Y, Ueno K, Okawara A, Watarai H, Taniguchi M, et al. A limited role of iNKT cells in controlling systemic Candida albicans infections. Jpn J Infect Dis (2012) 65:522–6. doi:10.7883/yoken.65.522
54. Tarumoto N, Kinjo Y, Kitano N, Sasai D, Ueno K, Okawara A, et al. Exacerbation of invasive Candida albicans infection by commensal bacteria or a glycolipid through IFN-γ produced in part by iNKT cells. J Infect Dis (2014) 209:799–810. doi:10.1093/infdis/jit534
55. Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med (2002) 195:617–24. doi:10.1084/jem.20011889
56. Khan A, Singh S, Galvan G, Jagannath C, Sastry K. Prophylactic sublingual immunization with Mycobacterium tuberculosis subunit vaccine incorporating the natural killer T cell agonist alpha-galactosylceramide enhances protective immunity to limit pulmonary and extra-pulmonary bacterial burden in mice. Vaccines (Basel) (2017) 5:E47. doi:10.3390/vaccines5040047
57. Venkataswamy MM, Baena A, Goldberg MF, Bricard G, Im JS, Chan J, et al. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis Bacillus Calmette-Guerin. J Immunol (2009) 183:1644–56. doi:10.4049/jimmunol.0900858
58. Vomhof-DeKrey EE, Yates J, Leadbetter EA. ScienceDirect invariant NKT cells provide innate and adaptive help for B cells. Curr Opin Immunol (2014) 28:12–7. doi:10.1016/j.coi.2014.01.007
59. Lang ML. The influence of invariant natural killer T cells on humoral immunity to T-dependent and -independent antigens. Front Immunol (2018) 9:305. doi:10.3389/fimmu.2018.00305
60. Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol (2005) 175:3309–17. doi:10.4049/jimmunol.175.5.3309
61. Youn H-J, Ko S-Y, Lee K-A, Ko H-J, Lee Y-S, Fujihashi K, et al. A single intranasal immunization with inactivated influenza virus and α-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine (2007) 25:5189–98. doi:10.1016/j.vaccine.2007.04.081
62. Kamijuku H, Nagata Y, Jiang X, Ichinohe T, Tashiro T, Mori K, et al. Mechanism of NKT cell activation by intranasal coadministration of α-galactosylceramide, which can induce cross-protection against influenza viruses. Mucosal Immunol (2008) 1:208–18. doi:10.1038/mi.2008.2
63. Artiaga BL, Yang G, Hackmann TJ, Liu Q, Richt JA, Salek-Ardakani S, et al. α-Galactosylceramide protects swine against influenza infection when administered as a vaccine adjuvant. Sci Rep (2016) 6:23593. doi:10.1038/srep23593
64. Chang P-P, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol (2011) 13:35–43. doi:10.1038/ni.2166
65. King IL, Fortier A, Tighe M, Dibble J, Watts GFM, Veerapen N, et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol (2011) 13:44–50. doi:10.1038/ni.2172
66. Bai L, Deng S, Reboulet R, Mathew R, Teyton L, Savage PB, et al. Natural killer T (NKT)-B-cell interactions promote prolonged antibody responses and long-term memory to pneumococcal capsular polysaccharides. Proc Natl Acad Sci U S A (2013) 110:16097–102. doi:10.1073/pnas.1303218110
67. Cavallari M, Stallforth P, Kalinichenko A, Rathwell DCK, Gronewold TMA, Adibekian A, et al. A semisynthetic carbohydrate-lipid vaccine that protects against S. pneumoniae in mice. Nat Chem Biol (2014) 10(11):950–6. doi:10.1038/nchembio.1650
Keywords: invariant natural killer T cell, CD1d, glycolipid, adjuvant activity, microbial infection
Citation: Kinjo Y, Takatsuka S, Kitano N, Kawakubo S, Abe M, Ueno K and Miyazaki Y (2018) Functions of CD1d-Restricted Invariant Natural Killer T Cells in Antimicrobial Immunity and Potential Applications for Infection Control. Front. Immunol. 9:1266. doi: 10.3389/fimmu.2018.01266
Received: 02 April 2018; Accepted: 22 May 2018;
Published: 06 June 2018
Edited by:
Kazuya Iwabuchi, Kitasato University School of Medicine, JapanReviewed by:
Moriya Tsuji, Aaron Diamond AIDS Research Center, United StatesLaurent Brossay, Brown University, United States
Copyright: © 2018 Kinjo, Takatsuka, Kitano, Kawakubo, Abe, Ueno and Miyazaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuki Kinjo, eWtpbmpvQG5paWQuZ28uanA=
 Yuki Kinjo
Yuki Kinjo Shogo Takatsuka
Shogo Takatsuka