- 1Department of Medical Microbiology, University Medical Centre Utrecht, Utrecht University, Utrecht, Netherlands
- 2Centre for Infectious Disease Control, National Institute for Public Health and the Environment (RIVM), Bilthoven, Netherlands
- 3Department of Clinical Chemistry and Haematology, University Medical Centre Utrecht, Utrecht University, Utrecht, Netherlands
- 4Sir William Dunn School of Pathology, University of Oxford, Oxford, United Kingdom
Bordetella pertussis is a Gram-negative bacterium and the causative agent of whooping cough. Whooping cough is currently re-emerging worldwide and, therefore, still poses a continuous global health threat. B. pertussis expresses several virulence factors that play a role in evading the human immune response. One of these virulence factors is virulence associated gene 8 (Vag8). Vag8 is a complement evasion molecule that mediates its effects by binding to the complement regulator C1 inhibitor (C1-INH). This regulatory protein is a fluid phase serine protease that controls proenzyme activation and enzyme activity of not only the complement system but also the contact system. Activation of the contact system results in the generation of bradykinin, a pro-inflammatory peptide. Here, the activation of the contact system by B. pertussis was explored. We demonstrate that recombinant as well as endogenous Vag8 enhanced contact system activity by binding C1-INH and attenuating its inhibitory function. Moreover, we show that B. pertussis itself is able to activate the contact system. This activation was dependent on Vag8 production as a Vag8 knockout B. pertussis strain was unable to activate the contact system. These findings show a previously overlooked interaction between the contact system and the respiratory pathogen B. pertussis. Activation of the contact system by B. pertussis may contribute to its pathogenicity and virulence.
Introduction
Bordetella pertussis is the causative agent of whooping cough, also known as pertussis, a contagious disease of the respiratory tract that is re-emerging worldwide despite high vaccination coverage. To date, pertussis is still ranked in the top 10 most deadly childhood diseases posing a serious health problem (1). The acellular pertussis vaccine (ACV), used in many industrialized countries, protects against disease for up to 7 years while natural infection confers protection for up to 20 years (2, 3). Alarmingly, the ACV does not prevent transmission of the pathogen (4). For this reason, it is widely accepted that an improved pertussis vaccine is needed (5). In order to improve the pertussis vaccine, it is of great importance to better understand the interactions between the respiratory pathogen B. pertussis and the immune system.
The contact system is a key player in innate immunity and is part of the coagulation system (6, 7). The contact system consists of the two proenzymes factor XII (FXII) and plasma prekallikrein and the cofactor high-molecular weight kininogen (HK). In vitro, the contact system is activated when FXII binds to a negatively charged surface and is autocleaved forming FXIIa that is further processed to βFXIIa (8). Lessons from human pathology imply that analogous processes may take place on the surface of vascular endothelial cells (9) or platelets (10). FXIIa cleaves plasma prekallikrein forming active plasma kallikrein (PK). Activation of this protease subsequently mediates the cleavage of HK and formation of the pro-inflammatory peptide bradykinin (BK) (11). BK release triggers endothelial permeability resulting in vasodilation and infiltration of leukocytes (7). Activation and activity of the contact system is regulated by the 105 kDa complement regulator C1 inhibitor (C1-INH) (12), which inhibits the activity of β-FXIIa, α-FXIIa, and PK, by forming covalent complexes with its target proteases (13). C1-INH consists of a C-terminal protease inhibiting serpin domain and an N-terminal domain that is predicted to be heavily O- and N-linked glycosylated. Besides being involved in contact system regulation, C1-INH is also the main inhibitor of the classical and lectin pathways of the complement system where it inactivates the respective proteases necessary for activation of the complement cascade (14). Interestingly, the interplay between B. pertussis and the contact system remains unexplored even though Escherichia coli and Salmonella (15), Streptococcus pyogenes, Bacillus stearothermophilus, Bacillus subtilis, Porphyromonas gingivalis, Pseudomonas aeruginosa, Serratia maracescens, as well as several Vibrio species (16) have been shown to activate this system.
B. pertussis produces multiple virulence factors involved in immune evasion (17). It was recently shown that virulence associated gene 8 (Vag8) of B. pertussis binds to C1-INH (18, 19). Vag8 is a 95 kDa autotransporter. Autotransporters are typically processed into a channel and a passenger domain (20). The passenger domain will pass through the channel and can either remain attached to the bacterial membrane or be secreted into the bacterial surrounding (21). Autotransporter proteins, including Vag8, are also present on the surface of outer membrane vesicles (OMVs) that are secreted by Gram-negative bacteria (18, 22, 23). We have recently shown that secreted Vag8 binding to C1-INH away from the bacterial surface leads to complement evasion. This binding result in consumption of complement components C2 and C4 via uncontrolled cleavage by the proteases C1r, C1s, and MASP-2, away from the bacterial surface (18).
Since C1-INH controls both the complement and the contact system, we here investigated whether Vag8 influences contact system activity. We demonstrate that both recombinant and endogenously secreted Vag8 enhanced contact system activity by attenuation of the inhibitory function of C1-INH. Moreover, we show that B. pertussis effectively activated the contact system by producing Vag8.
Materials and Methods
Bacterial Strains and Growth Conditions
B. pertussis wild type B1917 strain (isolated in 2000), the isogenic Vag8 knockout strain B1917ΔVag8 (18), the B0442 strain producing a mutated lipooligosaccharide (LOS) that was isolated in 1954 (24) and the pertactin-deficient B4418 and B4374 strains as well as the pertactin-producing B4430 and B4393 strains isolated in 2016 were grown at 35°C, 5% CO2 on Bordet Gengou plates containing glycerol and 15% defibrinated sheep blood (BD Biosciences, Franklin Lakes, NJ, USA). After 3–5 days of culture, the bacteria were collected in buffer containing 50 mM HEPES, 2 mM CaCl2, 50 µM ZnCl2, 0.02% NaN3, and 0.05% Tween-20 (pH 7.35) further referred to as buffer A, the optical density was measured at 600 nm and bacteria were washed in buffer A. OMVs of both strains were prepared by ultracentrifugation as described previously (18, 25, 26).
Recombinant Production of Histidine-Tagged (his-tag) Vag8 and the Negative Control Bordetella Resistance to Killing A (BrkA) Passenger Domain
Recombinant his-tag passenger domain of Vag8 was produced as previously described (18). BrkA was cloned using primers 5′-ATATGGATCCCAGGAAGGAGAGTTCGAC-3′ and 5′-ATATGCGGCCGCCTACTGCAAGCTCCAGACATG-3′ (restriction sites underlined) and ligated into a modified pRSET-B vector containing a non-cleavable six residue his-tag (MHHHHHHGS) at the N-terminus of the protein as described previously (18, 27). BrkA was expressed and purified as Vag8 (18).
Surface Plasmon Resonance (SPR)
SPR was performed using a Biacore T200 (GE Healthcare, Little Chalfont, UK). Recombinant passenger domain of Vag8 was dissolved in 50 mM sodium acetate pH 5.0 and immobilized using primary amine coupling onto a CM5 sensor chip (GE Healthcare). All binding experiments were performed at 25°C in 10 mM HEPES pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.005% (v/v) surfactant P20. Increasing concentrations (2.5–160 nM) of either full length C1-INH (C1-INHFL, Complement Technology) or C1-INH containing only the serpin domain (C1-INHNT98) (28) were injected over the flow channels at 30 µL/min. Dissociation was allowed for 300 s followed by surface regeneration with 10 mM glycine pH 2.5. BIAevaluation software (GE Healthcare) was used to analyze the data.
Size Exclusion Chromatography With Multi-Angle Light Scattering Analysis (SEC-MALS)
100 µL of protein samples were injected onto an S200 increase 10/300 column (GE Healthcare) equilibrated in 50 mM Tris pH 7.5, 150 mM NaCl and eluted with a flow rate of 0.4 mL/min. Light scattering and refractive index changes were measured using a Dawn Heleos-II light scattering detector and an Optilab-TrEX refractive index monitor respectively. Analysis was carried out using ASTRA 6.1.1.175.3.4.14 software assuming a dn/dc value of 0.186 mL/g.
Ethics
The study was conducted using blood donation from ±50 healthy adults for plasma collection and according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all blood donors before collection and samples were used anonymously. Approval was obtained from the medical ethics committee of the University Medical Centre Utrecht.
Plasma
Blood was collected in blood tubes containing sodium citrate (Vacuette tube, Greiner Bio-one, Kremsmünster, Austria) from ±50 healthy volunteers after written informed consent. Following collection, samples were centrifuged twice at 2,000 g for 10 min and plasma of all donors was pooled. The pooled plasma was stored in aliquots at −80°C until use.
Activation of the Contact System: Chromogenic Substrate Assay
To determine whether Vag8 could interfere with the inhibitory function of C1-INH on βFXIIa and PK, we made use of the chromogenic substrate H-d-Pro-Phe-Arg-pNA (L-2120, Sigma (Merck), Darmstadt, Germany) that can be cleaved by both proteases (29). All experiments were performed in 96-well PVC flat-bottom microplate (Corning GmbH, Wiesbaden, Germany). Activation of the contact system in the presence of Vag8 and BrkA was first analyzed in a purified system. βFXIIa (33.3 nM corresponding to 1 µg/mL, Enzyme Research Laboratories, South Bend, Ind, USA) or PK (5.81 nM corresponding to 0.5 µg/mL, Enzyme Research Laboratories) was pre-incubated with or without serum-derived C1-INH (95.2 nM corresponding to 10 µg/mL, Complement Technologies, Tyler, TX, USA) that was pre-incubated for 10 min at 37°C with, Vag8, BrkA, buffer A (for βFXIIa), or phosphate buffered saline (PBS) (for PK). Activity was measured following the addition of 0.5 mM L-2120 (Bachem, Bubendorf, Switzerland) (29). Activation of the contact system in a more complex system was studied in citrated human plasma. For these experiments, 60% plasma was activated with βFXIIa (16.7 nM corresponding to 0.5 µg/mL) in the presence of PBS (buffer control), Vag8, BrkA (concentrations indicated in the figures), or OMVs obtained from the B. pertussis wild type strain B1917 or knockout strain B1917ΔVag8 (60 µg/mL) with 10 min pre-incubation at 37°C before addition of the chromogenic substrate L-2120. For maximum contact system activity, referred to as control, βFXIIa was added to the plasma at the same time as the addition of L-2120 ensuring that C1-INH did not have the chance to inhibit the contact proteases. The substrate conversion, referred to as kallikrein-like activity, was measured in a kinetic fashion up until 30 min (Figure S1 in Supplementary Material) and reported at the 10 min time point. Substrate conversion was measured with a microplate reader at 405 nm at 37°C over time (VersaMax microplate reader, Molecular Devices, Sunnyvale, CA, USA).
Activation of the contact system by B. pertussis was assessed by incubating B. pertussis wild type strain B1917 or knockout strain B1917ΔVag8 (3 × 107 CFU each) with 60% plasma or buffer A. Plasma incubated with βFXIIa (16.7 nM corresponding to 0.5 µg/mL, Enzyme Research Laboratories) or only buffer A was taken along as positive and negative controls, respectively. These experiments were performed with 5 min pre-incubation at 37°C before addition of the substrate L-2120. Substrate conversion was measured at 37°C every 30 s with a microplate reader at 405 nm over time (PowerWave XS Microplate Spectrophotometer, BioTek, Winooski, VT, USA).
Cleavage of HK: Immunoblotting
To determine cleavage of HK in plasma in the presence of Vag8, βFXIIa (16.7 nM corresponding to 0.5 µg/mL) was added to 90% plasma and incubated with either PBS or Vag8 (1,017 nM corresponding to 60 µg/mL) for 10 min at 37°C. Samples were diluted 40 times in reducing sample buffer (15.5% glycerol, 96.8 mM Tris–HCl, 3.1% SDS, 0.003% bromophenol blue, and 25 mM DTT), incubated for 10 min at 100°C and separated on a 10% SDS-PAGE gel. Proteins were blotted onto PVDF membranes. Membranes were blocked with 4% skimmed milk in PBS containing 0.05% Tween (PBS-T) and washed with PBS-T three times for 10 min at 37°C between each incubation step. The immunoblot was subsequently incubated with a primary goat anti-human HK antibody (3 µg/mL final concentration, Affinity Biologicals, Ancaster, ON, Canada) overnight at 4°C and a secondary donkey-anti-goat-HRP antibody (0.5 µg/mL, Southern Biotech, Birmingham, AL, USA) mainly reacting with the HK light chain (30) for 2 h at 37°C. All antibodies were diluted in 1% skimmed milk in PBS-T. For detection, the Pierce ECL Western Blotting Substrate (Thermofisher Scientific, Waltham, MA, USA) was used and visualized using the ImageQuant (GE Life Sciences, Chicago, IL, USA).
For assessment of HK cleavage upon incubation of plasma with B. pertussis, several bacterial strains (2 × 109 CFU) were incubated with 50% plasma either alone or in the presence of the contact protease inhibitors aprotinin (31) (100 units/mL, Sigma), which inhibits PK and d-phenylalanyl-prolyl-arginyl chloromethyl ketone (32) (PPACK; 200 µM, Hematologic Technologies, Essex Junction, VT, USA), a multi-target serine protease inhibitor which restricts auto activation and self-digestion of FXIIa, and sampled after 30 min at 37°C shaking at 300 rpm. Plasma samples incubated with βFXIIa (16.7 nM corresponding to 0.5 µg/mL) or only buffer A were taken along as positive and negative controls, respectively. Samples were analyzed as described above.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 6.02 and the differences between groups were analyzed for significance using the two-tailed Student’s t-test. A p-value of ≤0.05 was considered statistically significant.
Results
Vag8 Attenuates the Role of C1-INH as Inhibitor of βFXIIa and PK
It was recently shown that Vag8 binds to C1-INH using both ELISA and gel filtration chromatography (18, 19). In order to further characterize this interaction, we performed the combination of SEC-MALS on the individual proteins and the complex (Figure 1A). Both Vag8 and C1-INH behaved as monomers on the column. Addition of Vag8 to C1-INH shifted the elution position and calculation of the mass across the peak revealed that a 1:1 complex had been formed. We next performed SPR analysis to attempt to assess the affinity of the interaction (Figures 1B,C). Flowing increasing concentrations of C1-INHFL over Vag8 on the surface demonstrated clear binding. The almost non-existent off-rate of the interaction, combined with a slow enough on-rate that precluded reaching equilibrium, meant that we were unable to robustly calculate the KD of the interaction. However, attempts to fit the kinetics using a variety of binding models always produced KD values of 1 nM or lower, consistent with a tight interaction. In order to confirm that the interaction between Vag8 and C1-INH involved the serpin domain, we repeated the SPR with C1-INH lacking the N-terminal O-linked glycosylation domain. This construct interacted with Vag8 with very similar kinetics to the full length protein (Figure 1C).
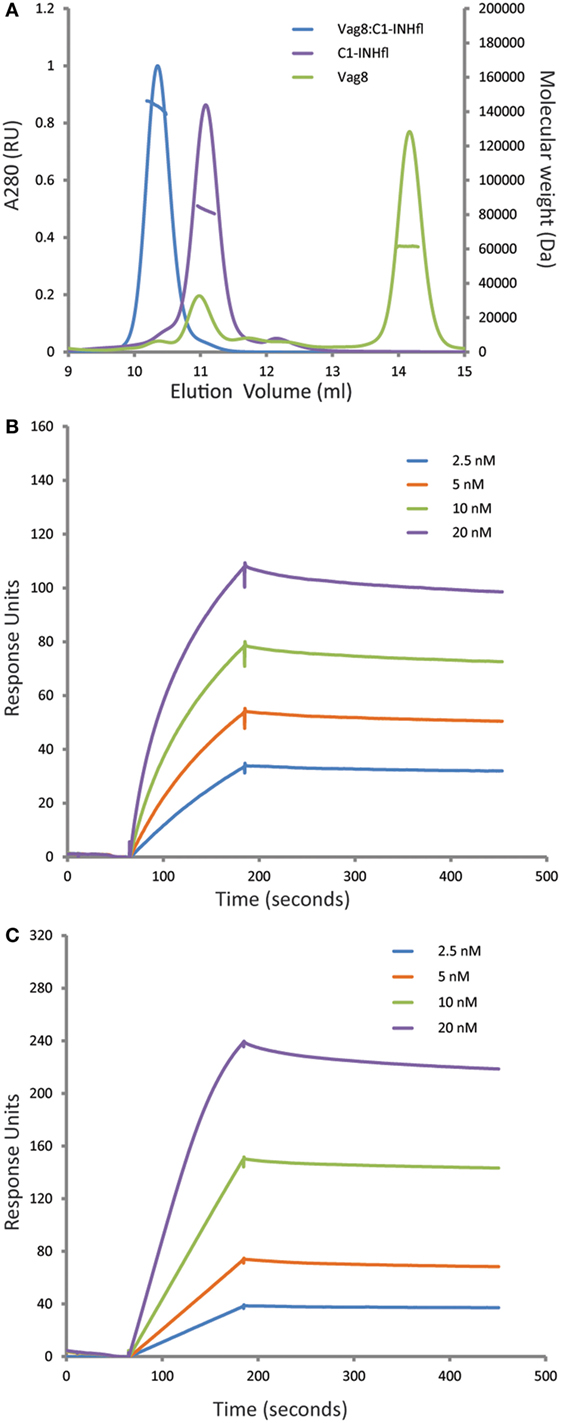
Figure 1. Virulence associated gene 8 (Vag8) and complement regulator C1 inhibitor (C1-INH) interact to form a tight 1:1 complex. (A) Size exclusion chromatography with multi-angle light scattering analysis (SEC-MALS) of Vag8 (green), C1-INH (purple), and Vag8_C1-INH complex (blue). The masses calculated from the scattering are shown as lines across the peaks. Protein conjugate analysis demonstrates that the mass of complex (146 kDa) corresponds to 118 kDa of protein and 28 kDa of sugar. This is consistent with a 1:1 complex of Vag8 (60 kDa) and glycosylated C1-INH (58 kDa protein/28 kDa sugar). (B,C) SPR analyses of the binding between Vag8 on the chip surface and increasing concentrations of C1-INHFL (B) and C1-INHNT98 (C) in solution.
We have demonstrated that the binding of secreted Vag8 to C1-INH results in attenuation of the inhibitory effect of C1-INH leading to the cleavage of essential complement proteins away from the bacterial surface (18). Since C1-INH is also one of the main inhibitors of the contact system, we hypothesized that the interaction between C1-INH and Vag8 would have a similar effect on the activation of the contact system. To investigate the effect of Vag8 on the contact system, we first studied this in a purified system. Addition of Vag8 to purified βFXIIa and C1-INH resulted in a dose-dependent enhanced conversion of the chromogenic substrate. Addition of 10 µg/mL Vag8 (169.5 nM) is sufficient to completely neutralize C1-INH activity as comparable levels of activation as that of βFXIIa alone were reached (Figure 2A). This dose-dependent attenuation of inhibition by C1-INH was also observed upon the addition of Vag8 to a purified system containing PK and C1-INH (Figure 2B). A concentration of 12.5 µg/mL Vag8 (203.4 nM) was sufficient to completely block the inhibitory capacity of C1-INH on PK activation. As a negative control we used BrkA. BrkA is another autotransporter protein of B. pertussis involved in complement evasion. BkrA has a similar structure and size as Vag8 and was produced in a similar way as Vag8 (33, 34). The inhibitory properties of C1-INH on βFXIIa and PK were not disturbed by the addition of BrkA at equimolar concentrations (Figure S2 in Supplementary Material). Taken together, Vag8 dose dependently attenuates the inhibitory effect of C1-INH on βFXIIa and PK in a purified system.
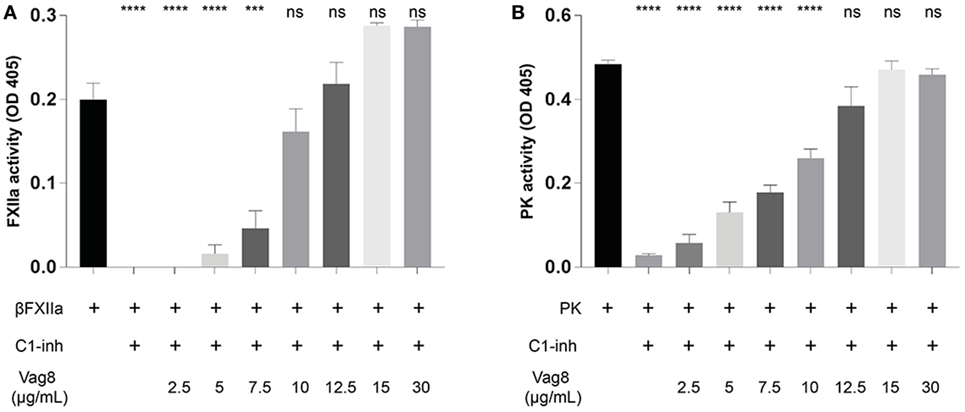
Figure 2. Virulence associated gene 8 (Vag8) interferes with the inhibition of βFXIIa and plasma kallikrein (PK) by complement regulator C1 inhibitor (C1-INH). (A) Vag8 (2.5, 5, 7.5, 10, 12.5, 15, and 30 µg/mL) dose-dependently enhances the activity of 1 µg/mL βFXIIa in the presence of 10 µg/mL C1-INH reaching similar activity as βFXIIa alone upon using 10 µg/mL of Vag8. (B) Vag8 (2.5, 5, 7.5, 10, 12.5, 15, and 30 µg/mL) dose-dependently enhances the activity of 0.5 µg/mL PK in the presence of 10 µg/mL C1-INH reaching similar activity as PK alone upon using 12.5 µg/mL of Vag8. Data represent the mean ± SEM of three separate experiments. ***p ≤ 0.001, ****p ≤ 0.0001, ns = non-significant compared to the black bar.
Vag8 Enhances Contact System Activity in Plasma
Since we have shown that Vag8 can interfere with the regulatory activity of C1-INH on βFXIIa and PK in a purified system, we next assessed the effect of Vag8 in a more physiological setting. To this end, we incubated plasma either with buffer (pre-incubated plasma) or increasing concentrations of Vag8 in the presence of the contact system activator βFXIIa for 10 min before addition of the chromogenic substrate. This pre-incubation step is needed for C1-INH to inhibit the contact proteases βFXIIa and PK. Moreover, the chromogenic substrate was added immediately following βFXIIa addition (control plasma) indicating the maximum kallikrein-like activity. Figure 3A shows that Vag8 dose-dependently attenuates the inhibitory function of C1-INH thus enhancing the activation of the contact system, referred to as kallikrein-like activity. Comparable levels as control plasma are reached upon using 7.5 µg/mL of Vag8 (127.1 nM), with no significant difference between the control plasma and addition of 30 µg/mL of Vag8 (508.5 nM). The negative control, BrkA, did not show any effect on contact system activity (data not shown). To determine whether endogenously secreted Vag8 is also capable of mediating this effect, OMVs derived from B. pertussis wild type strain B1917 or the knockout strain B1917ΔVag8 (18) were incubated with plasma and βFXIIa. We show that the OMVs derived from B. pertussis wild type strain B1917 were capable of attenuating the inhibitory function of C1-INH resulting in enhanced contact system activity to levels observed in control plasma conditions, whereas the OMVs obtained from the knockout strain B1917ΔVag8 were not (Figure 3B).
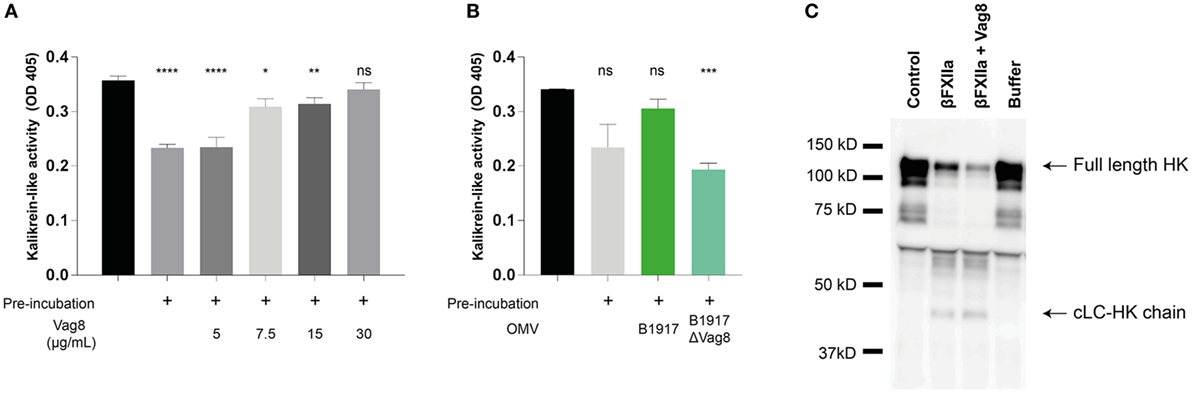
Figure 3. Virulence associated gene 8 (Vag8) induces enhanced activation of the contact system in plasma. (A) 60% plasma was incubated with Vag8 (5, 7.5, 15, and 30 µg/mL) following 10 min pre-incubation with 0.5 µg/mL βFXIIa. Vag8 dose-dependently enhances the induced kallikrein-like activity reaching similar levels upon using 7.5 µg/mL of Vag8 as the maximum kallikrein-like activity observed by βFXIIa alone without pre-incubation. (B) Moreover, the kallikrein-like activity in 60% plasma was also enhanced in the presence of endogenous Vag8 on outer membrane vesicles (OMVs) (60 µg/mL) derived from the wild type Bordetella pertussis strain B1917 but not when using OMVs of the knockout strain B1917ΔVag8. (C) Enhanced contact system activation was also shown by immunoblot. 90% plasma was incubated with 0.5 µg/mL βFXIIa either in the presence of 60 µg/mL Vag8 or buffer for 10 min and analyzed using anti-high-molecular-weight-kininogen (HK). The presence of Vag8 results in increased cleavage of the ~120 kDa full length HK compared to βFXIIa alone and the detection of the ~46 kDa cLC-HK chain (note the second and third lane). Plasma alone at t = 0 (control) and without βFXIIa at t = 10 (buffer) showed no cleavage of HK. Data shown in panel (A) represent the mean ± SEM of three separate experiments, while panels B and C are representative of three separate experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001, ns, non-significant compared to the black bar.
As previously mentioned, activation of the contact system ultimately results in the cleavage of HK and the subsequent release of BK. Also clinically, cleavage of HK is related to BK production (30). To investigate the effect of recombinant Vag8 binding to C1-INH on HK cleavage in plasma, βFXIIa was incubated with plasma in the presence or absence of Vag8 and was assessed by immunoblotting to visualize HK cleavage. Figure 3C shows increased HK cleavage as indicated by the decreased intensity of the ~120 kDa full length HK band (30) in the presence of Vag8 compared to incubation with βFXIIa alone as well as by the detection of the ~46 kDa cLC-HK chain. This is the cleaved L-chain which is often used as a marker of extensive contact system activation in plasma (35). In conclusion, we show enhanced contact system activity in the presence of recombinant and endogenous Vag8, which is most likely due to Vag8 binding to C1-INH and hence hampering the inhibitory properties of C1-INH on βFXIIa and PK.
B. pertussis Activates the Contact System Predominantly Through Vag8 Production
Although several bacteria are known to activate the contact system (15, 36–42), the interaction between B. pertussis and the contact system has not been investigated. As shown above, Vag8 of B. pertussis hampers the inhibition of the contact proteases by binding C1-INH and hence enhances contact system activity (Figures 2 and 3). Next, we investigated whether B. pertussis itself can effectively activate the contact system. We show that B. pertussis wild type strain B1917 successfully activates the contact system in plasma as an increase in kallikrein-like activity was observed over time (Figure 4A). This activation was further examined by assessment of HK cleavage using immunoblot. Figure 4B shows a representative immunoblot in which degradation of the ~120 kDa full length HK and appearance of a ~46 kDa cLC-HK chain (30) was observed when B. pertussis strain B1917 was added to the plasma. To verify that the observed HK cleavage was the result of contact system activation, aprotinin (31) and PPACK (32) were added to the B1917 samples to prevent FXIIa and PK activity. Figure 4B shows a lack of HK cleavage upon addition of both these inhibitors to plasma incubated with B. pertussis wild type strain B1917 indicating that the HK cleavage observed in the presence of this bacterium can be attributed to the activation of the contact system. Next, we assessed whether Vag8 production was responsible for the activation of the contact system by B. pertussis. Figure 4C shows the lack of HK cleavage in the presence of the B. pertussis knockout strain B1917ΔVag8 by immunoblot. Even after 180 min of incubation, no activation of the contact system by the B. pertussis knockout strain B1917ΔVag8 was observed (data not shown). This is further supported by the decreased kallikrein-like activity shown upon incubating plasma with the B. pertussis knockout strain B1917ΔVag8 when compared to incubation with wild type strain B1917 (Figure 4D). Moreover, we show that contact system activation is not restricted to B. pertussis strain B1917. Incubation of plasma with the LOS-mutant B0442 as well as clinical strains either producing (B4430 and B4393) or not producing pertactin (B4418 and B4374) also shows contact system activation as indicated by the detection of the ~46 kDa cLC-HK chain (Figure 5). In summary, B. pertussis is capable of activating the contact system as demonstrated by the observed kallikrein-like activity as well as by the cleavage of HK detected by immunoblot. We show that this is mainly dependent on the production of the autotransporter Vag8, which will bind to C1-INH and thus attenuate its inhibitory function, as the B. pertussis knockout strain B1917ΔVag8 showed no HK cleavage and decreased kallikrein-like activity compared to its isogenic wild type strain B1917.
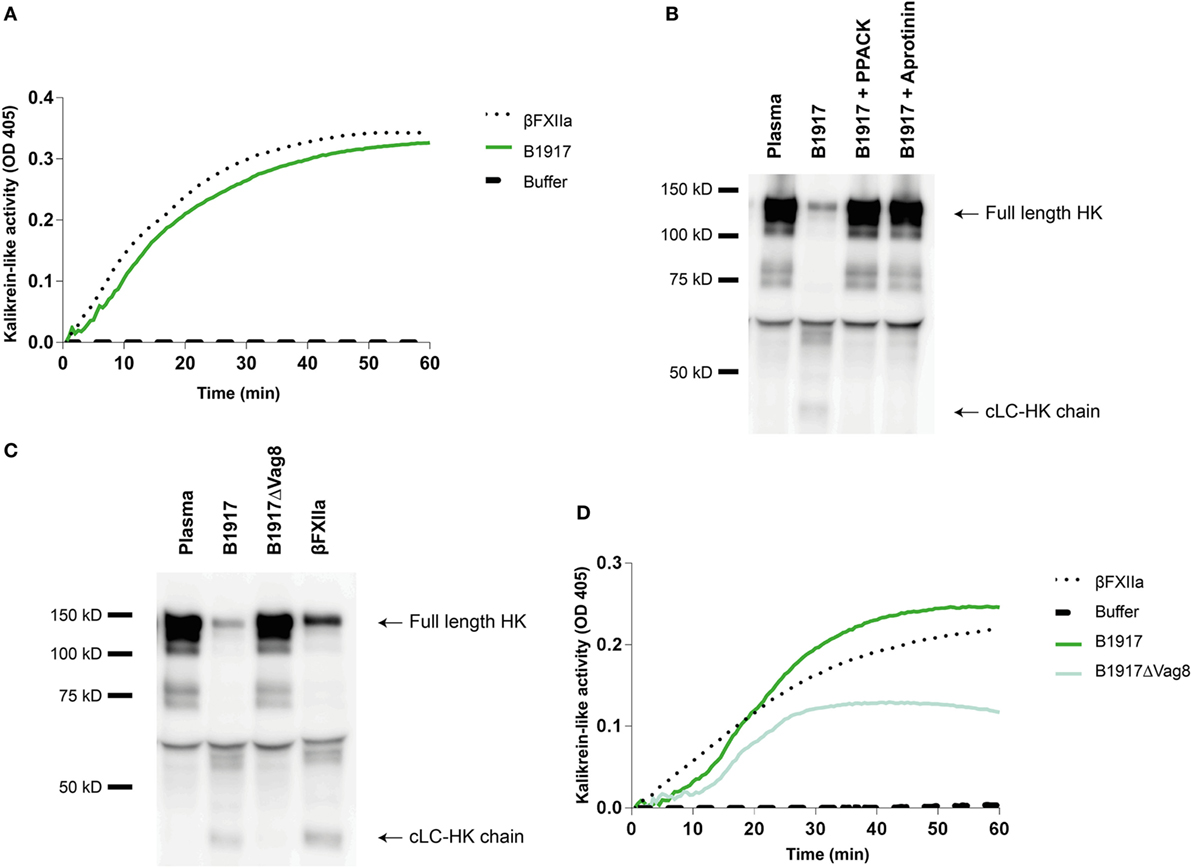
Figure 4. Bordetella pertussis activates the contact system mainly by Virulence associated gene 8 (Vag8) production. (A) B. pertussis wild type strain B1917 (3 × 107 CFU) induces similar kallikrein-like activity in 60% plasma compared to addition of 0.5 µg/mL βFXIIa. (B) Contact system activation by B. pertussis wild type strain B1917 was also shown by immunoblot. 50% plasma was incubated for 60 min with B. pertussis wild type strain B1917 (2 × 109 CFU) alone or in combination with the contact system inhibitors d-phenylalanyl-prolyl-arginyl chloromethyl ketone (PPACK) or aprotinin and analyzed using anti-high-molecular-weight-kininogen (HK). Incubation with B. pertussis wild type strain B1917 resulted in almost complete cleavage of the ~120 kDa full length HK as indicated by the appearance of the ~46 kDa cLC-HK chain. This feature was not observed in the presence of the inhibitors. (C) To determine whether Vag8 production was involved in contact system activation by this pathogen, 50% plasma was incubated either with B. pertussis wild type strain B1917 or with the knockout strain B1917ΔVag8 (2 × 109 CFU) and analyzed using anti-HK. The B. pertussis knockout strain B1917ΔVag8 was unable to activate the contact system as no cleavage of the ~120 kDa full length HK was detected. (D) This was also shown by the decreased kallikrein-like activity in 60% plasma upon incubation with the knockout strain B1917ΔVag8 (3 × 107 CFU) compared to the B. pertussis wild type strain B1917. Panels A–D are representative of three separate experiments.
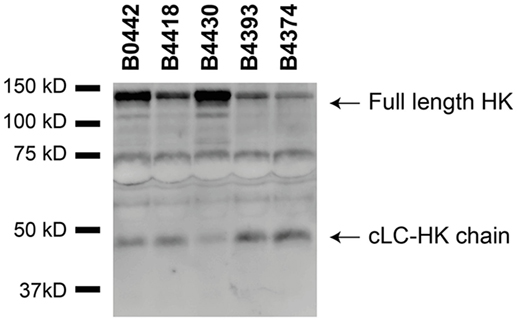
Figure 5. Various Bordetella pertussis strains activate the contact system. Contact system activation by B. pertussis strains B0442, B4418, B4430, B4393, and B4374 was shown by immunoblot. 50% plasma was incubated for 60 min with the B. pertussis strains (2 × 109 CFU) and analyzed using anti-high-molecular-weight-kininogen (HK). Incubation with all the B. pertussis strains resulted in cleavage of the ~120 kDa full length HK as indicated by the appearance of the ~46 kDa cLC-HK chain.
Discussion
Recently, we unraveled the mechanism responsible for secreted Vag8-mediated complement evasion (18). We showed that binding of secreted Vag8 to C1-INH resulted in the release of the active proteases C1s, C1r, and MASP-2 since C1-INH could no longer bind and thus inhibit their proteolytic activity. The presence of active C1s, C1r, and MASP-2 proteases in the serum resulted in the degradation of the complement proteins C4 and C2 away from the bacterial surface (18). We suggest that B. pertussis uses this complement evasion strategy to aid in the prevention of opsonization and complement-mediated lysis. Vag8 is not only secreted but also present on the bacterial surface where it binds to C1-INH (19, 43). Binding of C1-INH to the bacterial surface could also result in the inhibition of complement activation which has been shown for other bacteria such as Borrelia recurrentis (44). The contact system is another innate immune component consisting of proteases (11). Here, we show that in addition to interacting with the complement system (18), Vag8 of B. pertussis induced enhanced activation of the contact system as demonstrated by increased contact system activity and HK cleavage. We propose that Vag8 mediates activation of the contact pathway by binding to C1-INH and attenuating its inhibitory function as we have previously shown for its effect on the complement system (18). C1-INH is the predominant fluid phase inactivator of FXIIa and PK. This serpin inactivates these proteases by irreversibly binding to them resulting in conformational changes that disrupt the active site of the target proteases (13). This process is hampered in the presence of Vag8, which we expect to bind to C1-INH, and consequently interfere with the protease inhibition allowing for enhanced activation of the contact system (illustrated in Figure 6). As activation of the contact system by bacteria is often induced by membrane-bound components (16), Figure 6 depicts the activation of the contact system on the bacterial surface. However, we cannot exclude that activation occurs both on the surface as well as in fluid phase. Our results indicate that blockage of protease inhibition is essential for B. pertussis wild type strain B1917 induced activation of the contact system as in the absence of Vag8, C1-INH is free to inhibit the contact system proteases and, therefore, activation of the contact system is not induced (Figure 3). Whether B. pertussis can also interact with the PK inhibitor alpha-2-macroglobulin (45) remains to be investigated.
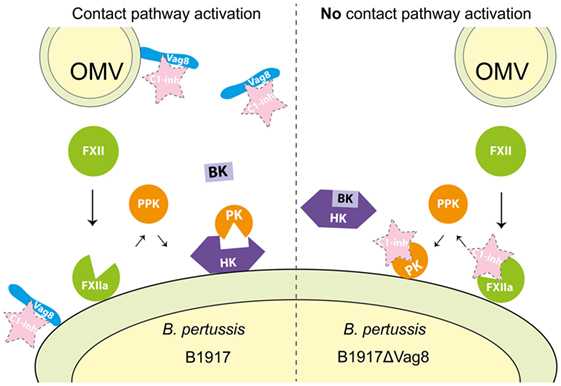
Figure 6. Proposed mechanism for Virulence associated gene 8 (Vag8) mediated activation of the contact system. Vag8, either on the bacterial surface as part of an outer membrane vesicles (OMVs) or as the secreted passenger, binds to complement regulator C1 inhibitor (C1-INH) (left panel). This results in the lack of inhibition of the contact system proteases FXIIa and plasma kallikrein (PK) by C1-INH. The lipooligosaccharide and polyphosphates present on the outer membrane of Bordetella pertussis are most likely responsible for FXII activation on the bacterial membrane as has been shown for other bacteria (15, 36–42). This activation, which cannot be inhibited by C1-INH as it is bound to Vag8, will result in the release of bradykinin (BK). In the absence of Vag8, C1-INH will inhibit FXIIa and PK when formed and high-molecular-weight-kininogen (HK) will remain intact away from the bacterial surface (right panel).
Here we show for the first time that B. pertussis can activate the contact system, which as a bacterium is not unique (15, 36–42). Other bacteria have been shown to activate this system via polyphosphates, which are present on E. coli, Vibrio cholerae, Corynebacterium diphtheria, and Haemophilus influenzae but also on B. pertussis (46, 47). Moreover, LPS present on Gram-negative bacteria have been implicated in the activation of the contact system in vitro (48, 49). Contact system proteins were furthermore shown to assemble on the bacterial surfaces of Salmonella typhimurium and E. coli resulting in the release of BK (15). An increase in BK at the site of infection may cause leakage of plasma and be beneficial for the bacteria as this will provide bacteria with nutrients (16). Nonetheless, BK triggers endothelial permeability resulting in infiltration of leukocytes (11). Moreover, cleavage of HK results in the generation of the antimicrobial peptide NAT-26 which could drive bacterial killing (38). Hence, whether activation of the contact system is beneficial or detrimental for the bacteria remains unclear. Contact system protein assembly on E. coli occurs on curli pili (42). As we do not observe HK cleavage in the presence of the B. pertussis knockout strain B1917ΔVag8, we expect that although the B. pertussis membrane-associated polyphosphates or LOS may trigger the contact system, attenuation of C1-INH via sequestration by Vag8 is essential for full activation of this system. Alternatively, bacteria can express proteases that actively cleave contact system proteins such as staphopains of Staphylococcus aureus or streptokinase of S. pyogenes, which both cleave HK releasing BK (38, 50). B. pertussis is unique in enhancing the activation of the contact system by producing a protein, Vag8, which inhibits the inhibitory function of the contact system regulator C1-INH.
Infection with B. pertussis results in the disease whooping cough, which is typically associated with fits of coughs (or paroxysms) followed by a typical high-pitched whoop. These coughing fits generally persist weeks after the bacterium has been cleared and contribute greatly to the morbidity caused by this disease (51). To date, it is not completely understood what causes this type of cough. The persistence of a chronic cough in the absence of a stimulus is not unique to pertussis but has also been observed in patients on angiotensin converting enzyme inhibitors (ACEi) that are being treated for hypertension (32). ACE, which is produced by lung endothelial cells, breaks down BK (52). The cough associated with ACEi often remains for several days or weeks after the patients have withdrawn from taking the drug. Although the mechanism of ACEi-induced cough remains unresolved, there are indications that BK, of which the levels are increased during ACEi treatment, might be involved (53). The contact system is not only present in plasma but also in the lungs (54) and respiratory administration of BK to guinea pigs but also humans evokes a paroxysmal cough much like the cough associated with pertussis (55). In guinea pigs, this cough was shown to be induced by BK activating the B2 receptors on the bronchopulmonary C-fibers (56). These receptors are also expressed in humans on epithelial cells, fibroblasts, and endothelial cells of the bronchial lamina propria (57). It is likely that B. pertussis infection results in increased levels of BK in the lungs as we have shown HK cleavage in the presence of B. pertussis wild type strain B1917 and BK is a cleavage product of HK. Consequently, we speculate that B. pertussis-induced activation of the contact system may be involved in the induction of pertussis-specific cough and thus transmission. Moreover, the activation of the contact system may also play a role in lung pathology. Lung lesions caused by an infection with S. typhimurium were shown to be prevented upon inhibition of the contact system in a rat model (40). Fatal B. pertussis infection is also characterized by lung lesions (58) and hence the increased BK levels in the lungs following an infection with B. pertussis may contribute to lung pathology. Further research needs to be conducted to really understand the role of the activation of the contact system on pertussis pathology.
In light of the re-emergence of pertussis, it has become evident that the development of a novel pertussis vaccine is necessary (5). One of the potential proteins that could be included in such a vaccine is Vag8. Vaccination with Vag8, which was previously only known as a complement evasion molecule of B. pertussis, has been shown to give rise to antibodies which protect mice from infection following a B. pertussis challenge (59). Moreover, Vag8-specific antibodies have been detected in pertussis patients indicating that Vag8 is produced by B. pertussis during human infection (60). Next to the proposed possibility of including Vag8 in a novel acellular pertussis vaccine, this protein is also a component of the OMV-based pertussis vaccine and the live attenuated BPZE1 vaccine that are currently being investigated (61, 62). Vag8 is highly present on OMVs of B. pertussis making up 34–50% of the total OMV proteins and can also be found on the bacterial membrane (18, 20, 23). Due to Vag8’s high abundance on OMVs, presence on the outer membrane of B. pertussis and the protective effect of this protein as a potential vaccine antigen, the overactivation of the contact system described here, together with the degradation of essential complement protein described earlier (18) may have implications for the inclusion of Vag8 in novel pertussis vaccines. These side effects could include local C1-INH deficiency with consequences for complement and contact system mediated adverse reactions (63). Inhibition of C1-INH could result in increased BK formation which may mediate increased inflammation at the site of vaccination. Moreover, consumption of complement proteins resulting in decreased complement activation following vaccination could have a negative effect on the induction of memory B-cells which are normally induced via interactions between C3d-tagged microorganisms or immune-complex antigens and complement receptor 2 (64, 65). It may be advisable to modify Vag8 before the potential inclusion of this antigen in novel pertussis vaccines in order to avoid side effects that could be induced by binding of Vag8 to C1-INH.
In conclusion, we show that Vag8 enhances contact system activity and is mainly responsible for the observed activation of the contact system induced by B. pertussis. We propose that this is the result of C1-INH binding by Vag8. This potent C1-INH inhibitor secreted by B. pertussis not only mediates complement evasion but also an overlooked interaction between the contact system and the respiratory pathogen B. pertussis that may contribute to its pathogenicity and virulence.
Ethics Statement
The study was conducted using blood donation from healthy adults for plasma collection and according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all blood donors before collection and samples were used anonymously. Approval was obtained from the medical ethics committee of the University Medical Centre Utrecht.
Author Contributions
EH, SM, AC, SJ, and IJ performed the experiments. EH and SJ drafted the figures. IJ, SM, and CM were involved in the study design. EP and IJ were responsible for funding. EH, SJ, and IJ wrote the manuscript. All authors approved the manuscript’s final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
We thank Dorina Roem (Sanquin, The Netherlands) for C1-INHNT98. This work is part of the research program VENI with project number 016.156.051 awarded to IJ, which is (partly) financed by the Netherlands Organization for Scientific Research (NWO). In addition, this project was supported by the Dutch Ministry for Public Health and the Environment (RIVM) in the framework of the S/112001 grant. CM gratefully acknowledges financial support from the Landsteiner Foundation for Blood Transfusion Research and the Netherlands Thrombosis Foundation. Finally, SJ was supported by the Welcome Trust Investigator Award (100298) awarded to Prof. S.M. Lea.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01172/full#supplementary-material.
Figure S1. Substrate assay kinetics. Substrate conversion, referred to as kallikrein-like activity, was measured in a kinetic fashion up until 30 min. (A) 1 µg/mL βFXIIa was incubated alone or with 10 µg/mL complement regulator C1 inhibitor (C1-INH) alone or in combination with different concentrations of virulence-associated gene 8 (Vag8) (2.5, 5, 7.5, 10, 12.5, 15, and 30 µg/mL). (B) 0.5 µg/mL plasma kallikrein (PK) was incubated alone or with 10 µg/mL C1-INH alone or in combination with different concentrations of Vag8 (2.5, 5, 7.5, 10, 12.5, 15, and 30 µg/mL). (C) 60% plasma was incubated with Vag8 (5, 7.5, 15, and 30 µg/mL) following 10 min pre-incubation with 0.5 µg/mL βFXIIa. Incubation of plasma with βFXIIa alone either without or with pre-incubation served as a control. In the main text, substrate conversion is reported at the 10 min time point. Data represent the mean of three separate experiments.
Figure S2. The negative control BrkA does not interfere with the inhibition of βFXIIa and plasma kallikrein (PK) by complement regulator C1 inhibitor (C1-INH). (A) Bordetella resistance to killing A (BrkA) (30 µg/mL) has no effect on the kallikrein-like activity of 1 µg/mL βFXIIa in the presence of 10 µg/mL C1-INH when compared to 1 µg/mL βFXIIa in the presence of 10 µg/mL C1-INH alone. (B) BrkA (30 µg/mL) has no effect on the kallikrein-like activity of 0.5 µg/mL PK in the presence of 10 µg/mL C1-INH when compared to 0.5 µg/mL PK in the presence of 10 µg/mL C1-INH alone. Data represent the mean ± SEM of three separate experiments.
References
1. Crowcroft NS, Stein C, Duclos P, Birmingham M. How best to estimate the global burden of pertussis? Lancet Infect Dis (2003) 3(7):413–8. doi:10.1016/S1473-3099(03)00669-8
2. Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J (2005) 24(5 Suppl):S58–61. doi:10.1097/01.inf.0000160914.59160.41
3. Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med (2012) 367(11):1012–9. doi:10.1056/NEJMoa1200850
4. Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A (2014) 111(2):787–92. doi:10.1073/pnas.1314688110
5. Locht C. Pertussis: acellular, whole-cell, new vaccines, what to choose? Expert Rev Vaccines (2016) 15(6):671–3. doi:10.1586/14760584.2016.1161511
6. Frick IM, Akesson P, Herwald H, Morgelin M, Malmsten M, Nagler DK, et al. The contact system – a novel branch of innate immunity generating antibacterial peptides. EMBO J (2006) 25(23):5569–78. doi:10.1038/sj.emboj.7601422
7. Hofman Z, de Maat S, Hack CE, Maas C. Bradykinin: inflammatory product of the coagulation system. Clin Rev Allergy Immunol (2016) 51(2):152–61. doi:10.1007/s12016-016-8540-0
8. de Maat S, Maas C. Factor XII: form determines function. J Thromb Haemost (2016) 14(8):1498–506. doi:10.1111/jth.13383
9. de Maat S, Bjorkqvist J, Suffritti C, Wiesenekker CP, Nagtegaal W, Koekman A, et al. Plasmin is a natural trigger for bradykinin production in patients with hereditary angioedema with factor XII mutations. J Allergy Clin Immunol (2016) 138(5):1414–23.e9. doi:10.1016/j.jaci.2016.02.021
10. Verhoef JJ, Barendrecht AD, Nickel KF, Dijkxhoorn K, Kenne E, Labberton L, et al. Polyphosphate nanoparticles on the platelet surface trigger contact system activation. Blood (2017) 129(12):1707–17. doi:10.1182/blood-2016-08-734988
11. Maas C, Oschatz C, Renne T. The plasma contact system 2.0. Semin Thromb Hemost (2011) 37(4):375–81. doi:10.1055/s-0031-1276586
12. Silverberg M, Longo J, Kaplan AP. Study of the effect of high molecular weight kininogen upon the fluid-phase inactivation of kallikrein by C1 inhibitor. J Biol Chem (1986) 261(32):14965–8.
13. Maas C, Renne T. Regulatory mechanisms of the plasma contact system. Thromb Res (2012) 129(Suppl 2):S73–6. doi:10.1016/j.thromres.2012.02.039
14. Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol (2009) 9(10):729–40. doi:10.1038/nri2620
15. Herwald H, Morgelin M, Olsen A, Rhen M, Dahlback B, Muller-Esterl W, et al. Activation of the contact-phase system on bacterial surfaces – a clue to serious complications in infectious diseases. Nat Med (1998) 4(3):298–302. doi:10.1038/nm0398-298
16. Frick IM, Bjorck L, Herwald H. The dual role of the contact system in bacterial infectious disease. Thromb Haemost (2007) 98(3):497–502. doi:10.1160/TH07-01-0051
17. Higgs R, Higgins SC, Ross PJ, Mills KH. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol (2012) 5(5):485–500. doi:10.1038/mi.2012.54
18. Hovingh ES, van den Broek B, Kuipers B, Pinelli E, Rooijakkers SHM, Jongerius I. Acquisition of C1 inhibitor by Bordetella pertussis virulence associated gene 8 results in C2 and C4 consumption away from the bacterial surface. PLoS Pathog (2017) 13(7):e1006531. doi:10.1371/journal.ppat.1006531
19. Marr N, Shah NR, Lee R, Kim EJ, Fernandez RC. Bordetella pertussis autotransporter Vag8 binds human C1 esterase inhibitor and confers serum resistance. PLoS One (2011) 6(6):e20585. doi:10.1371/journal.pone.0020585
20. Finn TM, Amsbaugh DF. Vag8, a Bordetella pertussis bvg-regulated protein. Infect Immun (1998) 66(8):3985–9.
21. Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala’Aldeen D. Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev (2004) 68(4):692–744. doi:10.1128/MMBR.68.4.692-744.2004
22. Luu LDW, Octavia S, Zhong L, Raftery M, Sintchenko V, Lan R. Characterisation of the Bordetella pertussis secretome under different media. J Proteomics (2017) 158:43–51. doi:10.1016/j.jprot.2017.02.010
23. Raeven RH, Brummelman J, Pennings JL, van der Maas L, Tilstra W, Helm K, et al. Bordetella pertussis outer membrane vesicle vaccine confers equal efficacy in mice with milder inflammatory responses compared to a whole-cell vaccine. Sci Rep (2016) 6:38240. doi:10.1038/srep38240
24. Brummelman J, Veerman RE, Hamstra HJ, Deuss AJ, Schuijt TJ, Sloots A, et al. Bordetella pertussis naturally occurring isolates with altered lipooligosaccharide structure fail to fully mature human dendritic cells. Infect Immun (2015) 83(1):227–38. doi:10.1128/IAI.02197-14
25. Saunders NB, Shoemaker DR, Brandt BL, Moran EE, Larsen T, Zollinger WD. Immunogenicity of intranasally administered meningococcal native outer membrane vesicles in mice. Infect Immun (1999) 67(1):113–9.
26. Zollinger WD, Donets MA, Schmiel DH, Pinto VB, Labrie JE III, Moran EE, et al. Design and evaluation in mice of a broadly protective meningococcal group B native outer membrane vesicle vaccine. Vaccine (2010) 28(31):5057–67. doi:10.1016/j.vaccine.2010.05.006
27. Oliver DC, Huang G, Fernandez RC. Identification of secretion determinants of the Bordetella pertussis BrkA autotransporter. J Bacteriol (2003) 185(2):489–95. doi:10.1128/JB.185.2.489-495.2003
28. Bos IG, Lubbers YT, Roem D, Abrahams JP, Hack CE, Eldering E. The functional integrity of the serpin domain of C1-inhibitor depends on the unique N-terminal domain, as revealed by a pathological mutant. J Biol Chem (2003) 278(32):29463–70. doi:10.1074/jbc.M302977200
29. de Maat S, Tersteeg C, Herczenik E, Maas C. Tracking down contact activation – from coagulation in vitro to inflammation in vivo. Int J Lab Hematol (2014) 36(3):374–81. doi:10.1111/ijlh.12222
30. Hofman ZLM, de Maat S, Suffritti C, Zanichelli A, van Doorn C, Sebastian SAE, et al. Cleaved kininogen as a biomarker for bradykinin release in hereditary angioedema. J Allergy Clin Immunol (2017) 140(6):1700–3.e8. doi:10.1016/j.jaci.2017.07.012
31. Hoffmann H, Siebeck M, Thetter O, Jochum M, Fritz H. Aprotinin concentrations effective for the inhibition of tissue kallikrein and plasma kallikrein in vitro and in vivo. Adv Exp Med Biol (1989) 247B:35–42. doi:10.1007/978-1-4615-9546-5_6
32. de Maat S, van Dooremalen S, de Groot PG, Maas C. A nanobody-based method for tracking factor XII activation in plasma. Thromb Haemost (2013) 110(3):458–68. doi:10.1160/TH12-11-0792
33. Barnes MG, Weiss AA. BrkA protein of Bordetella pertussis inhibits the classical pathway of complement after C1 deposition. Infect Immun (2001) 69(5):3067–72. doi:10.1128/IAI.69.5.3067-3072.2001
34. Oliver DC, Huang G, Nodel E, Pleasance S, Fernandez RC. A conserved region within the Bordetella pertussis autotransporter BrkA is necessary for folding of its passenger domain. Mol Microbiol (2003) 47(5):1367–83. doi:10.1046/j.1365-2958.2003.03377.x
35. Baroso R, Sellier P, Defendi F, Charignon D, Ghannam A, Habib M, et al. Kininogen cleavage assay: diagnostic assistance for kinin-mediated angioedema conditions. PLoS One (2016) 11(9):e0163958. doi:10.1371/journal.pone.0163958
36. Nickel KF, Renne T. Crosstalk of the plasma contact system with bacteria. Thromb Res (2012) 130(Suppl 1):S78–83. doi:10.1016/j.thromres.2012.08.284
37. Nitzsche R, Rosenheinrich M, Kreikemeyer B, Oehmcke-Hecht S. Streptococcus pyogenes triggers activation of the human contact system by streptokinase. Infect Immun (2015) 83(8):3035–42. doi:10.1128/IAI.00180-15
38. Wollein Waldetoft K, Svensson L, Morgelin M, Olin AI, Nitsche-Schmitz DP, Bjorck L, et al. Streptococcal surface proteins activate the contact system and control its antibacterial activity. J Biol Chem (2012) 287(30):25010–8. doi:10.1074/jbc.M112.373217
39. Mattsson E, Herwald H, Cramer H, Persson K, Sjobring U, Bjorck L. Staphylococcus aureus induces release of bradykinin in human plasma. Infect Immun (2001) 69(6):3877–82. doi:10.1128/IAI.69.6.3877-3882.2001
40. Persson K, Morgelin M, Lindbom L, Alm P, Bjorck L, Herwald H. Severe lung lesions caused by Salmonella are prevented by inhibition of the contact system. J Exp Med (2000) 192(10):1415–24. doi:10.1084/jem.192.10.1415
41. Rapala-Kozik M, Bras G, Chruscicka B, Karkowska-Kuleta J, Sroka A, Herwald H, et al. Adsorption of components of the plasma kinin-forming system on the surface of Porphyromonas gingivalis involves gingipains as the major docking platforms. Infect Immun (2011) 79(2):797–805. doi:10.1128/IAI.00966-10
42. Ben Nasr A, Olsen A, Sjobring U, Muller-Esterl W, Bjorck L. Assembly of human contact phase proteins and release of bradykinin at the surface of curli-expressing Escherichia coli. Mol Microbiol (1996) 20(5):927–35. doi:10.1111/j.1365-2958.1996.tb02534.x
43. Brookes C, Irene F-M, Breeze C, Frances A, Stephen T, Ruby P, et al. Bordetella pertussis isolates vary in their interactions with human complement components. Emerg Microbes Infect (2018) 7:81. doi:10.1038/s41426-018-0084-3
44. Grosskinsky S, Schott M, Brenner C, Cutler SJ, Simon MM, Wallich R. Human complement regulators C4b-binding protein and C1 esterase inhibitor interact with a novel outer surface protein of Borrelia recurrentis. PLoS Negl Trop Dis (2010) 4(6):e698. doi:10.1371/journal.pntd.0000698
45. van der Graaf F, Keus JF, Koedam JA, Rietveld A, Bouma BN. Prekallikrein activation and kallikrein inactivation in human plasma. Adv Exp Med Biol (1983) 156:143–8.
46. Rao NN, Gomez-Garcia MR, Kornberg A. Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem (2009) 78:605–47. doi:10.1146/annurev.biochem.77.083007.093039
47. Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A (2006) 103(4):903–8. doi:10.1073/pnas.0507195103
48. Kalter ES, van Dijk WC, Timmerman A, Verhoef J, Bouma BN. Activation of purified human plasma prekallikrein triggered by cell wall fractions of Escherichia coli and Staphylococcus aureus. J Infect Dis (1983) 148(4):682–91. doi:10.1093/infdis/148.4.682
49. Roeise O, Bouma BN, Stadaas JO, Aasen AO. Dose dependence of endotoxin-induced activation of the plasma contact system: an in vitro study. Circ Shock (1988) 26(4):419–30.
50. Imamura T, Tanase S, Szmyd G, Kozik A, Travis J, Potempa J. Induction of vascular leakage through release of bradykinin and a novel kinin by cysteine proteinases from Staphylococcus aureus. J Exp Med (2005) 201(10):1669–76. doi:10.1084/jem.20042041
51. Hewlett EL, Burns DL, Cotter PA, Harvill ET, Merkel TJ, Quinn CP, et al. Pertussis pathogenesis – what we know and what we don’t know. J Infect Dis (2014) 209(7):982–5. doi:10.1093/infdis/jit639
52. Dusser DJ, Nadel JA, Sekizawa K, Graf PD, Borson DB. Neutral endopeptidase and angiotensin converting enzyme inhibitors potentiate kinin-induced contraction of ferret trachea. J Pharmacol Exp Ther (1988) 244(2):531–6.
53. Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ. Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough. Nat Med (1996) 2(7):814–7. doi:10.1038/nm0796-814
54. Hess R, Wujak L, Hesse C, Sewald K, Jonigk D, Warnecke G, et al. Coagulation factor XII regulates inflammatory responses in human lungs. Thromb Haemost (2017) 117(10):1896–907. doi:10.1160/TH16-12-0904
55. Hewitt M, Canning BJ. Coughing precipitated by Bordetella pertussis infection. Lung (2010) 188(Suppl 1):S73–9. doi:10.1007/s00408-009-9196-9
56. Hewitt MM, Adams G Jr, Mazzone SB, Mori N, Yu L, Canning BJ. Pharmacology of bradykinin-evoked coughing in guinea pigs. J Pharmacol Exp Ther (2016) 357(3):620–8. doi:10.1124/jpet.115.230383
57. Ricciardolo FL, Sabatini F, Sorbello V, Benedetto S, Defilippi I, Petecchia L, et al. Expression of vascular remodelling markers in relation to bradykinin receptors in asthma and COPD. Thorax (2013) 68(9):803–11. doi:10.1136/thoraxjnl-2012-202741
58. Paddock CD, Sanden GN, Cherry JD, Gal AA, Langston C, Tatti KM, et al. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis (2008) 47(3):328–38. doi:10.1086/589753
59. de Gouw D, de Jonge MI, Hermans PW, Wessels HJ, Zomer A, Berends A, et al. Proteomics-identified Bvg-activated autotransporters protect against Bordetella pertussis in a mouse model. PLoS One (2014) 9(8):e105011. doi:10.1371/journal.pone.0105011
60. Otsuka N, Gotoh K, Nishimura N, Ozaki T, Nakamura Y, Haga K, et al. A novel IgM-capture enzyme-linked immunosorbent assay using recombinant Vag8 fusion protein for the accurate and early diagnosis of Bordetella pertussis infection. Microbiol Immunol (2016) 60(5):326–33. doi:10.1111/1348-0421.12378
61. Roberts R, Moreno G, Bottero D, Gaillard ME, Fingermann M, Graieb A, et al. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine (2008) 26(36):4639–46. doi:10.1016/j.vaccine.2008.07.004
62. Thorstensson R, Trollfors B, Al-Tawil N, Jahnmatz M, Bergstrom J, Ljungman M, et al. A phase I clinical study of a live attenuated Bordetella pertussis vaccine – BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS One (2014) 9(1):e83449. doi:10.1371/journal.pone.0083449
63. Conway EM. Reincarnation of ancient links between coagulation and complement. J Thromb Haemost (2015) 13(Suppl 1):S121–32. doi:10.1111/jth.12950
64. Toapanta FR, Ross TM. Complement-mediated activation of the adaptive immune responses: role of C3d in linking the innate and adaptive immunity. Immunol Res (2006) 36(1–3):197–210. doi:10.1385/IR:36:1:197
Keywords: whooping cough, contact system, virulence associated gene 8, Bordetella pertussis, C1 inhibitor
Citation: Hovingh ES, de Maat S, Cloherty APM, Johnson S, Pinelli E, Maas C and Jongerius I (2018) Virulence Associated Gene 8 of Bordetella pertussis Enhances Contact System Activity by Inhibiting the Regulatory Function of Complement Regulator C1 Inhibitor. Front. Immunol. 9:1172. doi: 10.3389/fimmu.2018.01172
Received: 14 March 2018; Accepted: 11 May 2018;
Published: 04 June 2018
Edited by:
Tom E. Mollnes, University of Oslo, NorwayReviewed by:
Erik Waage Nielsen, Nord University, NorwayChristian Drouet, Université Grenoble Alpes, France
Copyright: © 2018 Hovingh, de Maat, Cloherty, Johnson, Pinelli, Maas and Jongerius. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ilse Jongerius, aS5qb25nZXJpdXNAc2FucXVpbi5ubA==
†Present address: Ilse Jongerius, Department of Immunopathology, Sanquin Research and Landsteiner Laboratory of the Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands
 Elise S. Hovingh
Elise S. Hovingh Steven de Maat3
Steven de Maat3 Alexandra P. M. Cloherty
Alexandra P. M. Cloherty Steven Johnson
Steven Johnson Elena Pinelli
Elena Pinelli Coen Maas
Coen Maas Ilse Jongerius
Ilse Jongerius