
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 17 June 2020
Sec. Genomic Medicine
Volume 11 - 2020 | https://doi.org/10.3389/fgene.2020.00553
This article is part of the Research Topic Advances in Genomic and Genetic Tools, and Their Applications for Understanding Embryonic Development and Human Diseases View all 33 articles
 Halima El Omri1*
Halima El Omri1* Ruba Yasin Taha1
Ruba Yasin Taha1 Adel Elomri2
Adel Elomri2 Nancy Kacem3
Nancy Kacem3 Hesham Elsabah1
Hesham Elsabah1 Anil Yousaf Ellahie1
Anil Yousaf Ellahie1 Amna Gamil1
Amna Gamil1 Firyal Ibrahim4
Firyal Ibrahim4 Dina Sameh Abdelrahman Soliman4
Dina Sameh Abdelrahman Soliman4 Susanna Jane Lawson El Akiki5
Susanna Jane Lawson El Akiki5 Zafar Nawaz5
Zafar Nawaz5 Ahmad Al Sabbagh4
Ahmad Al Sabbagh4 Abdelfatteh El Omri6
Abdelfatteh El Omri6The current study retrospectively evaluated cytogenetic profiles, various prognostic factors, and survival outcomes in 128 acute myeloid leukemia (AML) patients (14 ≤ age ≤ 70 years) admitted to the National Center for Cancer Care and Research (NCCCR), Hamad Medical Corporation, Doha, Qatar, between January 2010 and December 2016. The median age at diagnosis was 43 years, and 80% were less than 60 years old; 75% of patients were male. Cytogenetic analysis was integrated into the World Health Organization 2008 classification and showed that the percentages of normal and abnormal karyotypes were similar, accounting for 48.4% of each group of patients. The AML risk stratification based on cytogenetic analysis resulted in the following distribution: 18% in the favorable risk group, 57% in the intermediate-risk group, 24% in the unfavorable risk group, and 1% unknown. Only 88 patients received therapy with curative intent; 67% achieved complete remission, increasing to 81% after inductions 1 and 2. The median overall survival (OS) and disease-free survival (DFS) in AML patients were 26.6 and 19.5 months, respectively. The 3-year OS and DFS were 40 and 36%, respectively. Prognostic factors including age, gender, white blood cell count, and risk stratification were not significantly associated with treatment outcomes, whereas response to treatment vs. failure was significantly associated with the outcome (p = 0.01). The current study supports the importance of cytogenetics as a useful tool in diagnosis, prognosis, and risk assessment in AML treatment.
Abnormal growth of white blood cells (WBCs) in the bone marrow, typically known as acute myeloid leukemia (AML), usually hampers normal blood cell production. Intensity of AML is higher in the white population (3.8 per 100,000) than in Asian people (3.2 per 100,000) (Howlader et al., 2012). AML is a common type of blood cancer, accounting for 80% of all leukemias. Males are more likely to be affected than females. The risk of AML increases with age, particularly in those above 60 years old. In the United States, the incidence of AML increases by around 10 times in patients above 65 years old, with a rate of 12.2 per 100,000 people (De Kouchkovsky and Abdul-Hay, 2016).
Acute myeloid leukemia is a heterogeneous group of diseases with several morphological, immunophenotypic, cytogenetic, and molecular genetic features (Döhner et al., 2010). Cytogenetic examination is considered the most important prognostic factor to predict clinical outcomes in AML patients (Döhner et al., 2010; De Kouchkovsky and Abdul-Hay, 2016). Cytogenetic results have been integrated into the World Health Organization (WHO) classification of AML. Three important multicenter clinical trials conducted by Cancer and Leukemia Group B (Byrd et al., 2002), the United Kingdom Medical Research Council (MRC) (Grimwade et al., 1998), and the Southwest Oncology Group (SWOG) (Slovak et al., 2000) demonstrated the importance of cytogenetic analysis and its significant impact on AML patients outcomes, leading to the stratification of AML risk into three groups: favorable, intermediate, and unfavorable.
The favorable risk group included balanced translocation t(8;21), t(15;17), inversion inv(16), and t(16;16); the intermediate risk group included normal karyotype (CN-AML), t(9;11), −Y (loss of the Y chromosome), +8 (trisomy of chromosome 8), +11, +13, +21, del(7q) (removal of the long arm of chromosome 7), del(9q), and del(20q); and the unfavorable risk group included complex karyotype, inv(3) or t(3;3), t(6;9), t(6;11), t(11;19), del(5q), −5 (monosomy of chromosome 5), and −7 (Grimwade et al., 1998; Slovak et al., 2000; Byrd et al., 2002; Marchesi et al., 2011). In the favorable risk group, the presence of additional chromosomal abnormalities has no significant effect on prognosis (Appelbaum et al., 2006).
An appropriate and accurate assessment of prognosis is fundamental to the management of AML. This involves stratifying patients according to their risk of treatment resistance or treatment-related mortality. Several prognostic factors, including cytogenetic analysis (Grimwade et al., 2010), age (Appelbaum et al., 2006), WBC count, de novo or secondary AML, presence of any antecedent hematological disease (Grimwade et al., 1998; Slovak et al., 2000), and performance status are used by physicians to choose the best treatment procedure—standard or increased treatment intensity, consolidated chemotherapy or allogeneic hematopoietic stem cell transplant (HSCT) (Byrd et al., 2002)—or, more crucially, to choose between established and investigational therapies (Grimwade et al., 1998, 2010; Slovak et al., 2000; Byrd et al., 2002; Appelbaum et al., 2006). High cytogenic risk AML patients are potential candidates for allogeneic HSCT, whereas low cytogenetic risk patients are candidates for intensive chemotherapy. In intermediate-risk AML, the most suitable treatment remains to be defined (Grimwade et al., 1998; Byrd et al., 2002; Döhner et al., 2010).
In newly identified AML patients with abnormal karyotype, cytogenetic analysis is also recommended for documenting complete remission (CR) (Grimwade et al., 1998; Slovak et al., 2000; Marcucci et al., 2004; Hirsch et al., 2014). Several studies have found that persistence of cytogenetic abnormalities found in leukemic blast cells at diagnosis, following chemotherapy induction, may predict a high relapse rate of leukemia and a poorer clinical outcome with lower disease-free survival (DFS) and overall survival (OS) rates (Grimwade et al., 1998, 2010; Slovak et al., 2000; Marcucci et al., 2004; Hirsch et al., 2014).
Despite many advances in diagnosis, prognosis and risk stratification, and treatment of AML, the cure rate remains modest, at 60–80% at first induction in young adult patients (age ≤60 years) and 30–40% in older individuals (Appelbaum et al., 2006; Odenike et al., 2011).
This study aimed to determine the cytogenetic profile of AML in adults, to correlate cytogenetic abnormalities to the WHO 2008 classification, to evaluate the risk stratification, and to study the response to treatment of AML patients in Qatar from 2010 to 2016.
The current study was an observational investigation that was conducted retrospectively based on AML patients’ records, including those aged less than 70 years and more than 14 years, diagnosed and treated at the National Center for Cancer Care and Research (NCCCR), Hamad Medical Corporation, Doha, Qatar, between January 2010 and December 2016, in relation to WHO 2008 guidelines. The follow-up was a minimum 2 years from inclusion so the results were considered until 2018 onward. The study was approved by the Medical Research Center Institutional Review Board (MRC-IRB) for the research proposal number “17287/17, 15/5/20”, and was exempted from ethical approval.
Among 208 patients diagnosed with AML in the department of clinical hematology at NCCCR, only 128 were included in this study after the exclusion of patients over 70 years and those with acute promyelocytic leukemia. Patient data regarding sex, age, nationality, hematological features, diagnosis date, WHO classification, cytogenetic abnormalities, risk stratification, first and further line of treatment, response to treatment, consolidation, date of relapse, bone marrow transplantation, date of last follow-up, and date and cause of death were collected (Döhner et al., 2010).
Peripheral blood smears and bone marrow aspirations were stained with Wright’s stain. Differential counts of at least 100 cells in the peripheral blood smear and of at least 500 cells in the bone marrow smear were performed. AML was defined by the presence of at least 20% blasts in bone marrow and/or peripheral blood samples, except for AML with t(15;17), t(8;21), inv(16), or t(16;16), and some cases of erythroleukemia. AML was classified according to the 2008 WHO classification (Döhner et al., 2010).
Immunophenotyping was performed using multicolor flow cytometry on bone marrow aspirate/peripheral blood using a CD45-gating strategy to identify the immunophenotype of the blasts. An acute leukemia panel of 28 antibodies in a four-color combination (FITC/PE/ECD/PC5 fluorescent conjugates) was used: (1) CD34/CD117/CD45/CD19, (2) CD14/CD13/CD45/CD64, (3) HLADR/CD7/CD45/CD5, (4) CD34/CD33/CD45/CD56, (5) CD19/CD10/CD45/CD3, (6) CD15/CD33/CD45/CD2, (7) CD9/CD19/CD45/CD4, (8) CD20/CD10/CD19/CD45, (9) cMPO/cCD79a/cCD3/sCD45, (10) TdT/sCD19/sCD3/sCD45, (11) CD36/CD11c/CD45/CD11b, and (12) CD41/glycophorin A/CD45/CD61(PC7).
Data acquisition and analysis were performed using a Navios flow cytometer (Beckman Coulter) and Novio software.
Cytogenetic analysis was performed in bone marrow or blood cells during metaphase. Karyotypes were identified following the rules of the International System for Human Cytogenetic Nomenclature (Gonzalez Garcia and Meza-Espinoza, 2006). Clonal abnormalities were considered when at least two metaphases showed the same aberration either in the structure or in the extra chromosome. Monosomy was considered significant if a minimum of three metaphases showed the same abnormality. Cytogenetic risk groups were assessed using the SWOG/ECOG (Southwest Oncology Group/Eastern Cooperative Oncology Group) criteria (Slovak et al., 2000). Fluorescence in situ hybridization (FISH) analysis was performed using specific probes for inv(16)(p13;q22), t(15;17)(q22;q21), t(8;21)(q22;q22), and 11q2.3 abnormalities, for mixed lineage leukemia (MLL) involving translocations, and for abnormalities (deletions or trisomy) of chromosome 5, 7, 8, 9, 11, 13, or Y. For all FISH analyses, at least 200 interphase nuclei were examined.
Gene mutation analyses were performed for FLT3-ITD, NPM1, and c-Kit.
Treatments were administered based on NCCCR’s AML guidelines. Treatment in patients 14 ≤ age ≤ 70 years old consists of double induction therapy and consolidation therapy based on cytogenetic stratification (high-dose cytarabine and/or allogeneic transplant). The induction chemotherapy (3 + 7) regimen consists of standard-dose cytarabine (200 mg/m2/d) continuous intravenous (IV) infusion on days 1–7 and anthracycline (idarubicin 12 mg/m2/d or daunorubicin 60 mg/m2/d or mitoxantrone 10–12 mg/m2/d IV on days 1–3). Consolidation consists of high-dose cytarabine (3 g/m2) IV every 12 h on days 1, 3, and 5. Patients aged between 60 and 70 years old, with a good performance status (less than 2), no comorbidities, and no adverse cytogenetics are treated with one induction (3 + 7 regimen) followed by 2–3 consolidations with intermediate-dose cytarabine (1000–1500 mg/m2) IV over 3 h every 12 h on days 1, 3, and 5. Finally, non-fit patients and those older than 70 years are treated with low-dose cytarabine (20–40 mg subcutaneously on days 1–10 for 4–5 weeks) or with hypomethylating agents (azacitidine 75 mg/m2 subcutaneously on days 1–7 or days 1–5, 8, and 9 every 28 days until progression).
Data were analyzed using SPSS statistical software 23.0. Differences in proportions were evaluated by Chi-square test. A value of p < 0.05 was considered to indicate a statistically significant difference. OS was determined based on the time between diagnosis and death or the time of the final clinical evaluation. DFS was defined as the time from CR to relapse or death or last follow-up. The Kaplan–Meier method was used to estimate OS and DFS, and survival curves were compared using the log-rank test. Cox proportional regression was used for the multivariate analysis. Odds ratios were calculated and reported with 95% confidence intervals. CR was confirmed when all the following conditions were fulfilled: less than 5% of blasts in the bone marrow, no leukemic blasts in the peripheral blood or extramedullary sites, and recovery of blood counts.
Of the 128 AML patients diagnosed and treated at NCCCR, Qatar, from January 2010 to December 2016, 97 (76%) were male and 31 (24%) were female, constituting a male to female ratio of 3.12:0.32. AML was more common in males than females in this sample, consistent with previous estimates (Howlader et al., 2012). Patient age ranged between 14 and 70 years with a median of 43.3 years; 103 patients, accounting for 80%, were younger than 60 years old (Table 1 and Figure 1). Qatari patients (n = 11) represented 8.5% of the total, while non-Qataris (n = 117) represented 91.5%; the latter were mainly from South Asia (36%) and the Middle East/North Africa region (26%) (Table 1 and Figure 1).
At the time of diagnosis, WBC ranged between 0.7 and 307 × 103/mm3, with a median of 20 × 103/mm3. Hemoglobin distribution was in a range of 2.9–14.9 g/dL with a median of 8.1 g/dL. The platelet count median was 54.5 × 103/mm3 in the range 3–1306. The median peripheral blast was 40% in the range 0–70, and the median marrow blast percentage was 54%, distributed between 20 and 99% (Table 2).
Karyotype analysis was performed in all patients and was considered to have failed in four patients (3.1%) because of inadequate metaphases. CN-AML and abnormal karyotype were each observed in 62 patients, accounting for 48.4% of each group of patients (Table 3). Molecular analysis was performed for 16 patients, focusing on FLT3-ITD and NPM1 mutations. Mutant FLT3-ITD/mutant NPM1 was found in one case, mutant FLT3-ITD/wild-type NPM1 in five cases, wild-type FLT3-ITD/mutant NPM1 in four cases, and wild-type FLT3-ITD/wild-type NPM1 in six cases. The cohort’s results according to the WHO 2008 AML classification are summarized in Table 4.
Using the SWOG pretreatment risk criteria system, patients were divided into four groups: 73 patients representing the majority (57%) were in the intermediate group, 23 patients (18%) were in the favorable risk group, 31 patients (24%) were in the unfavorable risk group, and one patient (0.8%) was classified as unknown.
Of the 128 patients, 88 (68.8%) received curative-intent treatment, 20 patients (15.6%) traveled back to their countries, 13 patients (10.2%) received palliative treatment, and seven patients (5.5%) died before treatment (Figure 2 and Supplementary Material). Fifty-nine patients (67%) were in CR, partial remission in 7 cases (7.95%), and refractory disease was present in 11 patients (12.5%). After the first induction (at day 30), 10 (11.3%) patients died and one patient traveled before evaluation. Seventy-seven patients received the second induction and/or salvage therapy; among them, CR was achieved in 50 (81%) of cases and 7 (9%) patients died by day 60. Ten (12.9%) patients traveled and another nine did not receive the second induction.
Following the first and second inductions, a total of 17 patients died with microbial infection in 15 cases (88.2%) and cerebral bleeding in 2 cases (11.8%). The 15 deaths caused by microbial infection are summarized as follows: (1) Gram-negative bacilli (GNB) septicemia in eight cases caused by Pseudomonas aeruginosa and Stenotrophomonas maltophilia in two cases each; Acinetobacter baumannii, Klebsiella oxytoca, Escherichia coli, and Burkholderia cepacia in the other four cases, (2) Gram-positive cocci (GPC) septicemia in two cases caused by Staphylococcus aureus and Enterococcus faecium, and (3) invasive fungal infection in five cases caused by Candida glabrata in two cases, and Candida krusei, Candida tropicalis, and Trichosporon asahii in the remaining three cases.
Cytarabine was given to 67 patients in a larger dose (one cycle in 13 cases, two cycles in 21 cases, and 3 cycles in 18 cases). Allogeneic HSCT was administered to 19 patients (15 patients following the first CR and 4 following the second CR) (Supplementary Material).
Two patients relapsed after chemotherapy and were alive in complete remission 2 (CR-2) following salvage therapy and allogeneic HSCT. Four patients relapsed after allogeneic HSCT and died (Supplementary Material).
The median OS for the 88 patients who received curative-intent treatment was around 26.6 months, and the median DFS was about 19.5 months (Figures 3, 4). Prognostic factors including age, gender, WBC, risk stratification, and response to treatment showed no significant differences for OS and DFS (Figures 3A,B). We compared the age effect below and above 40 years old. Our results showed no significant difference for either OS (p = 0.9) or DFS (p = 0.17). In the current cohort study, the young patient group (<40 years of age) are presenting better OS and DFS than older patients. However, the difference was not significant enough to consider age as a risk factor (Figures 3C,D). Similarly, we studied the gender effect on OS and DFS; the results showed no significant difference for either OS (p = 0.57) or DFS (p = 0.37). Nevertheless, female patients showed better OS and DFS (Figures 3E,F). WBC findings for response-related survival showed that the threshold value (≥50 × 109/L vs. <50 × 109/L) had no significant effect on either OS (p = 0.21) or DFS (p = 0.30), although patients with WBC (<50 × 109/L) had better OS and DFS (Figures 3G,H and Supplementary Material).
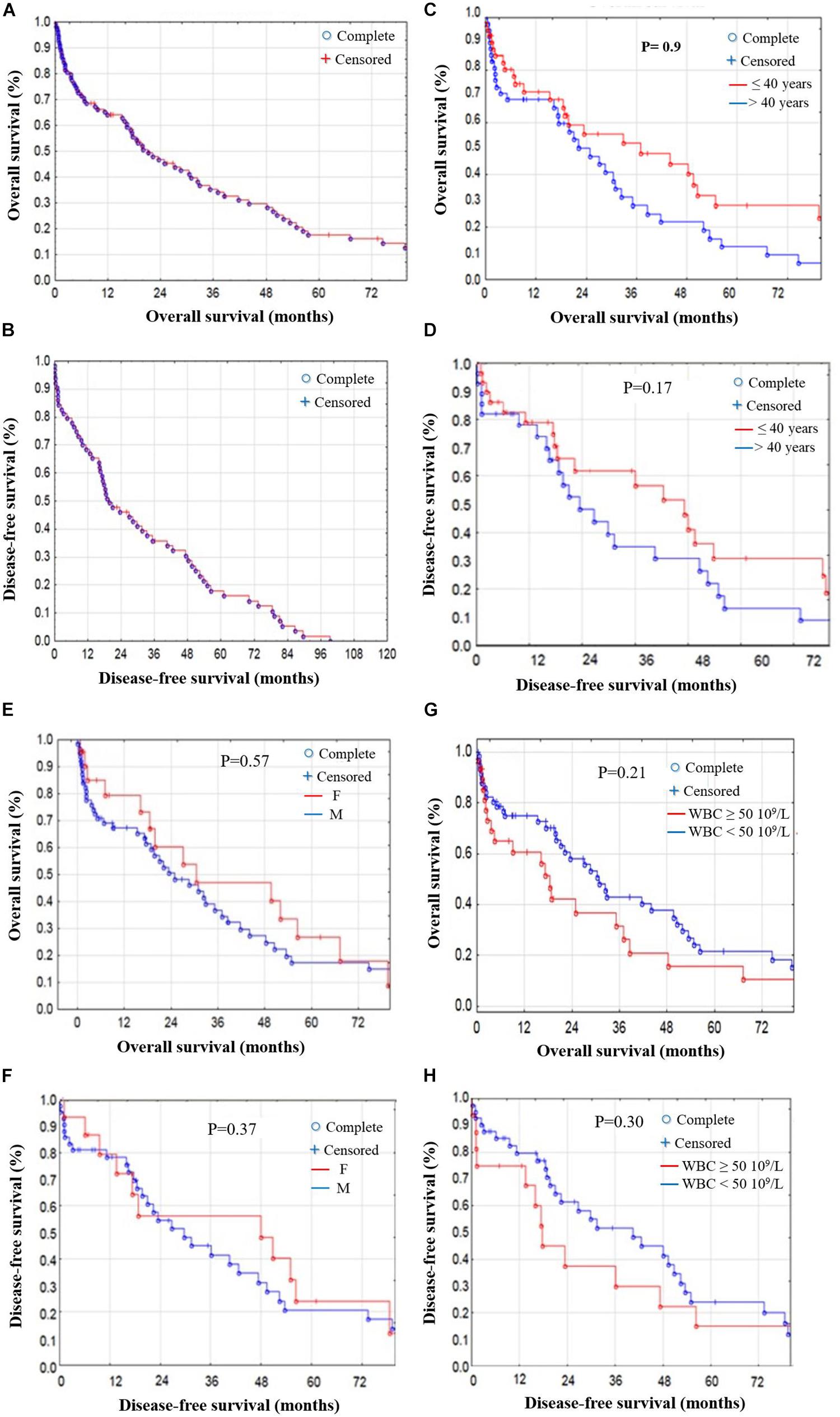
Figure 3. OS and DFS stratified by prognostic factors in AML patients. (A) OS and (B) DFS stratified in AML patients. (C) OS and (D) DFS stratified by age; no significant difference in OS (p = 0.9) or DFS (p = 0.17) for patients <40 or >40 years old. (E) OS and (F) DFS stratified by gender; no significant difference in OS (p = 0.57) or DFS (p = 0.37) between male and female patients. (G) OS and (H) DFS stratified by WBC findings; threshold values (≥50 × 109/L) vs. (<50 × 109/L) showed no significant difference in OS (p = 0.21) or DFS (p = 0.30).
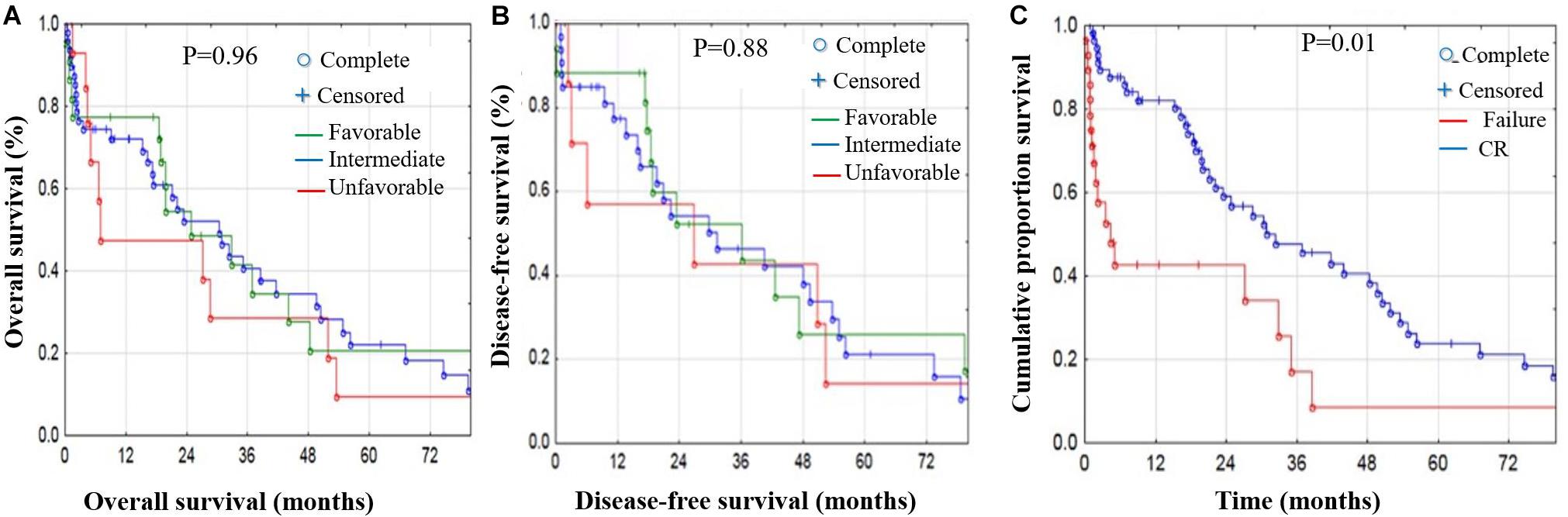
Figure 4. OS and DFS stratified by risk factors in AML patients. Risk stratification effect on OS (A) and DFS (B) showed no significant difference in OS or DFS; however, the unfavorable group showed poorer OS and DFS. (C) Cumulative proportion for treatment response-related survival; the CR patient group had significantly better OS and DFS compared with the non-CR group (p < 1%).
Risk stratification had no significant effect on either OS or DFS. However, the unfavorable group showed poorer OS and DFS (Figures 4A,B). Finally, we studied treatment response-related survival by comparing the CR vs. non-CR patient groups. Our results showed that the CR group had significantly better OS and DFS compared with the non-CR group (p < 0.01) (Figure 4C and Supplementary Material).
The current study is the first to be conducted on AML in Qatar from January 2010 until December 2016 for patients aged between 14 and 70 years old. Moreover, it is the first of its kind to determine the cytogenetic abnormalities in AML in adults and to evaluate the risk stratification according to the WHO 2008 classification, and to report the clinical outcomes in Qatar. The median OS rate was around 26.6 months and the median DFS was about 19.5 months. There were no significant differences associated with the prognostic factors (age, gender, WBC, risk stratification, and response to treatment) considered in this study. However, subjects younger than 40 years old, females, and patients with WBC below 50 × 109/L showed better OS and DFS. Age and performance status are the most powerful patient-related risk factors in adult patients with AML (Grimwade et al., 1998; Slovak et al., 2000; Byrd et al., 2002; Döhner et al., 2010; Marchesi et al., 2011). The results showing reduced significance in this study may have been confounded by ethnic variation, a low number of subjects, and the high proportion of patients traveling at different phases of the treatments. Cytogenetics and molecular genetics are considered the most powerful prognostic factors to predict clinical outcomes in AML patients. Furthermore, when they are integrated with WHO classification of AML, they may have a significant impact on patients’ outcomes, which led to the AML risk stratification (Grimwade et al., 1998; Slovak et al., 2000; Byrd et al., 2002; Döhner et al., 2010; De Kouchkovsky and Abdul-Hay, 2016). The most common abnormalities are dominated by t(8;21) in 8.6% of cases and inversion 16/t (16;16) in 7.8% of cases, based on some Western studies (Grimwade et al., 19982010; Slovak et al., 2000; Byrd et al., 2002). These abnormalities are seen in AML de novo and are correlated with good prognosis. Trisomy 8, the third abnormality, representing 5.5%, was the most common numerical aberration in our cohort. The rate is 6% in Western countries (Grimwade et al., 1998; Slovak et al., 2000; Byrd et al., 2002), 3% in Malaysia (Meng et al., 2013) and 3.8% in China (Cheng et al., 2009).
The fourth type of abnormality, related to 11q23, was associated with de novo AML in our study (Grimwade et al., 1998; Slovak et al., 2000; Byrd et al., 2002). It occurs in no more than 4% of adult AML patients and is correlated with a poor prognosis (Grimwade et al., 1998, 2010; Slovak et al., 2000; Byrd et al., 2002). The frequency of partial and/or complete deletion of chromosomes 5 and 7, which is associated with poor prognosis, ranged from 0 to 2.3% (Grimwade et al., 1998; Slovak et al., 2000; Byrd et al., 2002; Marchesi et al., 2011), significantly lower than the previously reported range of 6–10% in de novo AML (Grimwade et al., 1998; Byrd et al., 2002; Cheng et al., 2009). A complex karyotype is found in about 10–12% of AML patients (Marchesi et al., 2011). The incidence of second AML with a poor prognosis increases with age (Appelbaum et al., 2006; Juliusson et al., 2009; Qatar Social Statistics, 2007-2016; Shysh et al., 2018), and the use of leukemogenic drugs is common in such cases (Grimwade et al., 2001; Sanderson et al., 2006; Mrózek, 2008; Haferlach et al., 2012). In routine diagnosis of AML, cytogenetic analysis and WHO 2008 classifications are highly recommended procedures for their central role in the management of the disease (Bacher et al., 2005). Thus, our cohort’s data were analyzed accordingly (Table 4). In fact, 30 AML patients were identified with recurrent genetic abnormalities, 18 with myelodysplasia-related changes, 3 with therapy-related myeloid neoplasms, and 33 not otherwise specified (Table 4).
The results reflect limitations due to the low sensitivity of conventional cytogenetics and the high prevalence of normal cytogenetic AML, as well as a shortage of molecular studies. Failed karyotype and normal karyotype represented 3.2 and 48.4%, respectively.
The missed chromosome aberrations may have been due to technical problems. Trisomy 8 and trisomy 11 have been reported in interphase cells of AML with normal karyotype, probably owing to the inability of the abnormal clone with aneuploidy to proliferate in vitro (Frohling et al., 2002). It is difficult to determine the quality of chromosome morphology in the G-banding resolution by a conventional cytogenetic method (Cox et al., 2003). The difficulties also occur in cryptic gene fusions, for example, NUP98-NSD1, CBFA2T3–GLIS2, and MNX1–ETV6, which predict poor outcomes in pediatric and young adult AML (Grimwade et al., 2016). Moreover, t(8;21), carrying a mutation of the KIT gene, has a negative impact on outcome, with a significantly lower OS compared with wild-type KIT (Klein et al., 2015). Detection of these abnormalities is important to determine the appropriate treatment and decrease the risk of death. Cytogenetic analysis may encounter problems where breakpoints occur in close proximity; for example, at least five different genes that can potentially recombine with the MLL locus fall within the 19p13.1;13.3 regions (Grimwade et al., 2016). Furthermore, cytogenetics provides no clear information regarding the molecular mechanisms underlying AMLs with numerical or other structural changes, or, importantly, those with CN-AML, which account for 40% of adult AML and are highly heterogeneous in terms of clinical outcome (Grimwade et al., 2016).
The ELN classification (2010), recognized by the WHO, divides patients on the basis of CN-AML molecular alterations, namely NPM1, CEBPA, and FLT3 mutations (Döhner et al., 2010). Later, in 2016, a new revised version was released. The WHO classification of AML defines six major disease entities based on genetic information together with morphology, immunophenotype, and clinical presentation (De Kouchkovsky and Abdul-Hay, 2016).
Here, we conducted a FISH study of the five most common abnormalities (see section “Patients and Methods”). Such molecular genetic studies have been performed since 2015 and have been applied only to FLT3 and NPM1 in CN-AML. The presence of FLT3-ITD with wild-type NPM1 predicted a poor prognosis, whereas NPM1 mutation in the absence of FL3-ITD was associated with reduced risk of relapse and improved OS (Döhner et al., 2010; De Kouchkovsky and Abdul-Hay, 2016; Grimwade et al., 2016). Cytogenetic analysis was used to stratify our AML cohort into three groups—low risk (18%), intermediate (57%), and high risk (24%)—concordant with previous reports (Appelbaum et al., 2006; Espirito Santo et al., 2017).
Of the total of 128 patients, 67.7% were in complete remission, 20.5% were resistant to disease, and 11.3% had died by day 30. The CR rate after induction 2 and/or salvage therapy was 81%, and the death rate at day 60 was 9%. The death rate was high owing to infectious disease. The CR rate after inductions 1 and 2 and the resistant disease rate were comparable to those reported by previous studies (Büchner et al., 2012; Burnett et al., 2013; Willemze et al., 2014). The MRC AML15 trial (Burnett et al., 2013) reported a CR rate of 78%, and death rates at days 30 and 60 of 6 and 8%, respectively. The 8-year survival rate was around 72% (favorable 95% and intermediate 63%) in the FLAG–idarubicine arm (two inductions and two consolidations). Moreover, the German AML intergroup study (Büchner et al., 2012) reported a CR rate of 70% in the standard treatment arm; the death rate was 5% in cases of aplasia and 25% in patients with resistant disease. According to the same study, the 5-year survival rate was 44.3% and the 5-year relapse-free survival rate was 44.8%. CR rates were 68.2% after induction 1 and 72% after induction 2. In the EORTC-GIMEMA AML-12 trial (standard treatment arm), the death rate during induction 1 was 9%, the resistant disease rate was 18.9%, DFS at 6 years was 41.6%, and relapse incidence was 47.9% (Willemze et al., 2014). The median survival rate of our cohort was 26.6 months, and the OS at 3 years and 5 years was 40 and 18.3%, respectively. The only predictive prognostic factor affecting survival was response to treatment. The other factors, including age, gender, WBC, and risk stratification, were not statistically significant. However, we noticed a better survival rate in female patients below 40 years old, in patients with WBC less than 50 × 109/L, and in the favorable and intermediate groups.
In our study, the mortality rates at day 30 and day 60 were 11.3 and 9%, respectively, and mainly associated with bacteremia and fungemia. This rate was relatively high when compared to previous studies (Büchner et al., 2012; Burnett et al., 2013; Willemze et al., 2014). However, it falls within acceptable ranges when it is compared to some febrile neutropenia studies (Wisplinghoff et al., 2003; Ruhnke et al., 2014), where the mortality rate is around 36% due to blood stream infections: 18% due to GNB, 13% due to polymicrobial infections, and 5% to GPC.
The high infection incidence in our patients can be attributed to the following reasons. First, the majority of patients are in expatriate services, coming from low-income countries where poor hygienic conditions and GNB invasions are common. Second, in our institution guidelines, the use of antibiotic prophylaxis in AML neutropenic patients is not recommended because it may increase the selection of resistant microbes.
To overcome these problems, since 2017, hematology, Medical Intensive Care Unit (MICU), and the infection disease and infection control teams have been closely collaborating to reduce the incidence of infection in patients with hematological malignancies. In this respect, the following aspects were carefully implemented: (1) national guidelines for febrile neutropenia based on hospital microbial and antibiogram data; (2) antimicrobial stewardship program (ASP) to promote the appropriate use of antimicrobials and help clinicians improve clinical outcomes and minimize harms due to the spread of infections caused by multidrug-resistant organisms; (3) sepsis bundle established and frequently monitored and reviewed to set the best evidence base for maximum care and outcomes for patients; and (4) compulsory detection of carbapenem-resistant organisms through a rectal swab before any chemotherapy for acute leukemia and transplant patients.
In the current study, the stratification of AML was based on conventional cytogenetic analysis as per the WHO 2008 guidelines. The patients received treatment with conventional chemotherapy and/or allogenic transplant. Molecular tools such as reverse transcription-polymerase chain reaction (RT-PCR) and next generation sequencing (NGS) have been included in AML diagnosis since 2017 (Döhner et al., 2017). These techniques are becoming integral part in the initial work-up and follow-up in AML in several hospitals, resulting in target and personalized therapy protocols. However, these techniques are not affordable in many countries, still lack standardization of data analysis, and rely on highly skilled personnel. In the past few years, treatment decisions in AML have become more and more dependent on target therapy. Unfortunately, in NCCCR at Hamad Medical Corporation, molecular testing based on NGS and novel therapies based on FLT3, BCL-2, and JAK inhibitors are not yet available in our setting at the clinical level. Our treatment protocols are still based on conventional cytogenetics and FISH studies.
Novel therapies are showing some promising improvements in AML outcomes (Tamamyan et al., 2017). Target therapy in AML can be categorized in different groups such as: (1) protein kinase inhibitors (PI3K/AKT/mTOR, Aurora and polo-like kinase, CDK4/6, CHK1, WEE1, MPS1 inhibitors, SRC and HCK inhibitors); (2) epigenetic modulators (SGI-110, HDAC, IDH1, IDH2, DOT1L, and BET-bromodomain inhibitors); (3) new chemotherapeutic agents (CPX-351, vosaroxin, nucleoside analogs); (4) mitochondrial inhibitors (Bcl-2, Bcl-xL, Mcl-1, and caseinolytic protease inhibitors); (5) therapies targeting specific oncogenic proteins (fusion transcripts targeting EVI1, NPM1 targeting, and Hedgehog inhibitors); (6) therapeutic and immune checkpoint antibodies [mAbs against CD33, CD44, CD47, CD123, CLEC12A, immunoconjugates (e.g., GO, SGN33A), BiTEs and DARTs, CAR T cells or genetically engineered TCR T cells, immune checkpoint inhibitors (PD-1/PD-L1, CTLA-4), anti-KIR antibody, vaccines (e.g., WT1)]; and (7) cellular immunotherapies and therapies targeting the AML microenvironment (Döhner et al., 2017; Tamamyan et al., 2017). Recent research reported modest achievement in targeted immunotherapies along with curative-intent allogeneic hematopoietic stem cell transplantation in AML (Knaus et al., 2018). The two best-known checkpoints are cytotoxic T-lymphocyte antigen-4 (CTLA-4) (Knaus et al., 2018) and the programmed cell death protein 1 receptor (PD-1) (Giannopoulos, 2019). In AML, increased PD-1 expression on CD8+ T lymphocytes may be a leading factor to immune suppression during the progression course of the disease. CD8+ T cell dysfunction was in part reversible on PD-1 blockade or OX40 costimulation in vitro (Daassi et al., 2020). The PD-1 inhibitor nivolumab with HMAs and CTLA-4 inhibitor ipilimumab are still in early phases of clinical trials, and they are commonly associated with immune-related adverse events (irAEs), which can be fatal for patients (Liao et al., 2019). Moreover, it is difficult to consider PD-1 as a prognostic factor in hematological malignancies unless considering how to distinguish between the several forms of soluble and extracellular PDL1 secreted in blood when analyzing responses to immunotherapy (Giannopoulos, 2019).
The main limitations of the study that affected the survival rate and the results in general were the high ethnic diversity among patients, the small number of subjects included in the study, and the missing data due to missing follow-up because many patients traveled during various phases of the treatments. Moreover, postinduction therapy was given to transplanted and non-transplanted patients in the same arm. Implementation of NGS in AML patients’ diagnosis in NCCCR at Hamad Medical Corporation together with protocols for target therapy will be our main focus for better improvement of the quality of care in our institution.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher with respect to institutions standards.
The study was approved by the Medical Research Center Institutional Review Board (MRC-IRB) for the research proposal number “17287/17, 15/5/20”, and was exempted from ethical approval.
HEO, RT, AE, AEO, and NK: conceptualization. HEO, AE, AEO, NK, and SE: data curation. HEO, FI, DS, HE, AG, AYE, ZN, and AA: formal analysis. HEO and AEO: funding acquisition and project administration. HEO, RT, AE, NK, HE, AG, FI, DS, SE, ZN, and AA: methodology. HEO, NK, and DS: resources. AEO: software. HEO, RT, and AE: supervision. AEO, ZN, AA, AYE, and AG: validation. AE and AEO: visualization. HEO, AE, RT, and AEO: writing – original draft. HEO, RT, AE, NK, HE, AYE, AG, FI, DS, SE, ZN, AA, and AEO: writing – review and editing.
AE contribution was made possible by Grant NPRP12S-0219-190108 from Qatar National Research Fund (a member of Qatar Foundation). The contents herein are solely the responsibility of the authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are thankful to Medical Research Center (MRC) at Hamad Medical Corporation, Qatar, for providing services and assistance during the course of the project.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.00553/full#supplementary-material
Appelbaum, F. R., Gundacker, H., Head, D. R., Slovak, M. L., Willman, C. L., Godwin, J. E., et al. (2006). Age and acute myeloid leukemia. Blood 107, 3481–3485.
Bacher, U., Kern, W., Schnittger, S., Hiddemann, W., Schoch, C., and Haferlach, T. (2005). Further correlations of morphology according to FAB and WHO classification to cytogenetics in de novo acute myeloid leukemia: a study on 2,235 patients. Ann. Hematol. 84, 785–791. doi: 10.1007/s00277-005-1099-0
Büchner, T., Schlenk, R. F., Schaich, M., Döhner, K., Krahl, R., Krauter, J., et al. (2012). Acute myeloid leukemia (AML): different treatment strategies versus a common standard arm—combined prospective analysis by the German AML intergroup. J. Clin. Oncol. 30, 3604–3610. doi: 10.1200/JCO.2012.42.2907
Burnett, A. K., Russell, N. H., Hills, R. K., Hunter, A. E., Kjeldsen, L., Yin, J., et al. (2013). Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the Medical Research Council AML15 trial. J. Clin. Oncol. 31, 3360–3368. doi: 10.1200/JCO.2012.47.4874
Byrd, J. C., Mrózek, K., Dodge, R. K., Carroll, A. J., Edwards, C. G., Arthur, D. C., et al. (2002). Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood 100, 4325–4336. doi: 10.1182/blood-2002-03-0772
Cheng, Y., Wang, Y., Wang, H., Chen, Z., Lou, J., Xu, H., et al. (2009). Cytogenetic profile of de novo acute myeloid leukemia: a study based on 1432 patients in a single institution of China. Leukemia 23, 1801–1806. doi: 10.1038/leu.2009.107
Cox, M. C., Panetta, P., Venditti, A., Del Poeta, G., Franchi, A., Buccisano, F., et al. (2003). Comparison between conventional banding analysis and FISH screening with an AML–specific set of probes in 260 patients. Hematol. J. 4, 263–270. doi: 10.1038/sj.thj.6200262
Daassi, D., Mahoney, K. M., and Freeman, G. J. (2020). The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 20, 209–215. doi: 10.1038/s41577-019-0264-y
De Kouchkovsky, I., and Abdul-Hay, M. (2016). Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 6:e441. doi: 10.1038/bcj.2016.50
Döhner, H., Estey, E., Grimwade, D., Amadori, S., Appelbaum, F. R., Büchner, T., et al. (2017). Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129, 424–447. doi: 10.1182/blood-2016-08-733196
Döhner, H., Estey, E. H., Amadori, S., Appelbaum, F. R., Büchner, T., Burnett, A. K., et al. (2010). Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115, 453–474. doi: 10.1182/blood-2009-07-235358
Espirito Santo, A., Chacim, S., Ferreira, I., Leite, L., Moreira, C., Pereira, D., et al. (2017). Southwestern Oncology Group pretreatment risk criteria as predictive or prognostic factors in acute myeloid leukemia. Mol. Clin. Oncol. 6, 384–388. doi: 10.3892/mco.2017.1134
Frohling, S., Skelin, C., Liebisch, C., Scholl, C., Schlenk, R, F., Döhner, H., et al. (2002). Comparison of cytogenetic and molecular cytogenetic detection of chromosome abnormalities in 240 consecutive adult patients with acute myeloid leukemia. J. Clin. Oncol. 20, 2480–2485. doi: 10.1200/jco.2002.08.155
Giannopoulos, K. (2019). Targeting immune signaling checkpoints in acute Myeloid Leukemia. J. Clin. Med. 8:236. doi: 10.3390/jcm8020236
Gonzalez Garcia, J. R., and Meza-Espinoza, J. P. (2006). Use of the international system for human Cytogenetic Nomenclature (ISCN). Blood 108, 3952–3953. doi: 10.1182/blood-2006-06-031351
Grimwade, D., Hills, R. K., Moorman, A. V., Walker, H., Chatters, S., Goldstone, A. H., et al. (2010). Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 116, 354–365. doi: 10.1182/blood-2009-11-254441
Grimwade, D., Ivey, A., and Huntly, B. J. P. (2016). Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance. Blood 127, 29–41. doi: 10.1182/blood-2015-07-604496
Grimwade, D., Walker, H., Harrison, G., Oliver, F., Chatters, S., Harrison, C. J., et al. (2001). The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML 11 trial. Blood 98, 1312–1320. doi: 10.1182/blood.v98.5.1312
Grimwade, D., Walker, H., Oliver, F., Wheatley, K., Harrison, C., Harrison, G., et al. (1998). The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood 92, 2322–2333.
Haferlach, C., Alpermann, T., Schnittger, S., Kern, W., Chromik, J., Schmid, C., et al. (2012). Prognostic value of monosomal karyotype in comparison to complex aberrant karyotype in acute myeloid leukemia: a study on 824 cases with aberrant karyotype. Blood 119, 2122–2125. doi: 10.1182/blood-2011-10-385781
Hirsch, P., Labopin, M., Viguié, F., Perot, C., Isnard, F., Mamez, A. C., et al. (2014). Interest of cytogenetic and FISH evaluation for prognosis evaluation in 198 patients with acute myeloid leukemia in first complete remission in a single institution. Leuk. Res. 38, 907–912. doi: 10.1016/j.leukres.2014.05.021
Howlader, N., Noone, A. M., Krapcho, M., Neyman, N., Aminou, R., Waldron, W., et al. (2012). SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). Bethesda, MD: National Cancer Institute.
Juliusson, G., Antunovic, P., Derolf, A., Lehmann, S., Möllgård, L., Stockelberg, D., et al. (2009). Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 113, 4179–4187. doi: 10.1182/blood-2008-07-172007
Klein, K., Kaspers, G., Harrison, C. J., Berna Beverloo, H., Reedijk, A., Bongers, M., et al. (2015). Clinical impact of additional cytogenetic aberrations, cKIT and RAS mutations, and treatment elements in pediatric t(8;21)-AML: results from an international retrospective study by the International Berlin-Frankfurt-Münster Study Group. J. Clin. Oncol. 33, 4247–4258. doi: 10.1200/JCO.2015.61.1947
Knaus, H. A., Berglund, S., Hackl, H., Blackford, A. L., Zeidner, J. F., Montiel-Esparza, R., et al. (2018). Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight. 3:e120974. doi: 10.1172/jci.insight.120974
Liao, D., Wang, M., Liao, Y., Li, J., and Niu, T. (2019). A review of efficacy and safety of checkpoint inhibitor for the treatment of acute Myeloid Leukemia. Front. Pharmacol. 10:609. doi: 10.3389/fphar.2019.00609
Marchesi, F., Annibali, O., Cerchiara, E., Tirindelli, M. C., and Avvisati, G. (2011). Cytogenetic abnormalities in adult non-promyelocytic acute myeloid leukemia: a concise review. Crit. Rev. Oncol. Hematol. 80, 331–346. doi: 10.1016/j.critrevonc.2010.11.006
Marcucci, G., Mrózek, K., Ruppert, A. S., Archer, K. J., Pettenati, M. J., Heerema, N. A., et al. (2004). Abnormal cytogenetics at date of morphologic complete remission predicts short overall and disease-free survival, and higher relapse rate in adult acute myeloid leukemia: results from cancer and leukemia group B study 8461. J. Clin. Oncol. 22, 2410–2418. doi: 10.1200/JCO.2004.03.023
Meng, C. Y., Noor, P. J., Ismail, A., Ahid, M. F., and Zakaria, Z. (2013). Cytogenetic profile of de novo acute myeloid leukemia patients in Malaysia. Int. J. Biomed. Sci. 9, 26–32.
Mrózek, K. (2008). Cytogenetic, molecular genetic, and clinical characteristics of acute myeloid leukemia with a complex karyotype. Semin. Oncol. 35, 365–377. doi: 10.1053/j.seminoncol.2008.04.007
Odenike, O., Thirman, M. J., Artz, A. S., Godley, L. A., Larson, R. A., and Stock, W. (2011). Gene mutations, epigenetic dysregulation and personalized therapy in myeloid neoplasia: are we there yet? Semin. Oncol. 38, 196–214. doi: 10.1053/j.seminoncol.2011.01.010
Statistics Qatar Social Qatar Social Statistics (2007–2016). Available online at: https://www.psa.gov.qa/en/statistics/Statistical%20Releases/Social/GenrealSocialStatistics/QatarSocialStatistics/Qatar_Social_Statistics_2007_2016_En.pdf (accessed December, 2019).
Ruhnke, M., Arnold, R., and Gastmeier, P. (2014). Infection control issues in patients with haematological malignancies in the era of multidrug-resistant bacteria. Lancet Oncol. 15, e606–e619. doi: 10.1016/S1470-2045(14)70344-4
Sanderson, R. N., Johnson, P. R. E., Moorman, A. V., Roman, E., Willett, E., Taylor, P. R., et al. (2006). Population-based demographic study of karyotypes in 1709 patients with adult acute myeloid leukemia. Leukemia 20, 444–450. doi: 10.1038/sj.leu.2404055
Shysh, A. C., Nguyen, L. T., Guo, M., Vaska, M., Naugler, C., and Rashid-Kolvear, F. (2018). The incidence of acute myeloid leukemia in Calgary, Alberta, Canada: a retrospective cohort study. BMC Public Health 18:94. doi: 10.1186/s12889-017-4644-6
Slovak, M. L., Kopecky, K. J., Cassileth, P. A., Harrington, D. H., Theil, K. S., Mohamed, A., et al. (2000). Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood 96, 4075–4083.
Tamamyan, G., Kadia, T., Ravandi, F., Borthakur, G., Cortes, J., Jabbour, E., et al. (2017). Frontline treatment of acute myeloid leukemia in adults. Crit. Rev. Oncol. Hematol. 110, 20–34. doi: 10.1016/j.critrevonc.2016.12.004
Willemze, R., Suciu, S., Meloni, G., Labar, B., Marie, J. P., Halkes, C. J., et al. (2014). High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMA AML-12 trial. J. Clin. Oncol. 32, 219–228. doi: 10.1200/JCO.2013.51.8571
Keywords: acute myeloid leukemia, cancer epidemiology, cytogenetic, Qatar, remission, survival, WHO classification of AML
Citation: El Omri H, Taha RY, Elomri A, Kacem N, Elsabah H, Ellahie AY, Gamil A, Ibrahim F, Soliman DSA, El Akiki SJL, Nawaz Z, Al Sabbagh A and El Omri A (2020) Acute Myeloid Leukemia in Qatar (2010–2016): Clinical, Biological, and Prognostic Factors and Treatment Outcomes. Front. Genet. 11:553. doi: 10.3389/fgene.2020.00553
Received: 05 March 2020; Accepted: 07 May 2020;
Published: 17 June 2020.
Edited by:
Ahmed Rebai, Centre of Biotechnology of Sfax, TunisiaReviewed by:
Dhouha Daassi, Harvard Medical School, United StatesCopyright © 2020 El Omri, Taha, Elomri, Kacem, Elsabah, Ellahie, Gamil, Ibrahim, Soliman, El Akiki, Nawaz, Al Sabbagh and El Omri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Halima El Omri, SEVMT01SSUBoYW1hZC5xYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.