- 1Institute of Clinical Pharmacy & Pharmacology, Jining First People’s Hospital, Jining Medical University, Jining, China
- 2Department of Cardiology, Jining First People’s Hospital, Jining Medical University, Jining, China
- 3Department of Mental Health, Jining Medical University, Jining, China
- 4Research Center of Basic Medical Sciences, Tianjin Medical University, Tianjin, China
- 5Department of Pharmacy, Shandong Provincial Hospital affiliated to Shandong University, Jinan, China
There is a strong link between heart disease and depression, both of which are closely related to lifetime stress exposure. Serum/glucocorticoid-regulated kinase 1 (SGK1) is a stress-responsive gene with a pivotal role in both the heart and brain. To determine the role of SGK1 polymorphisms (rs2758151, rs1743963, rs9493857, rs1763509, rs9376026, and rs9389154) in susceptibility to comorbid coronary heart disease (CHD) and depression, we conducted a hospital-based case–control study involving 257 CHD cases (including 69 cases with depression and 188 cases without depression) and 107 controls in a Chinese Han population. Six single-nucleotide polymorphisms (SNPs) in the SGK1 gene were successfully genotyped by polymerase chain reaction–ligase detection reaction (PCR-LDR) assay. Our results showed no significant differences in SGK1 genetic polymorphisms between CHD patients and controls, whereas significant associations were observed between SGK1 SNPs (rs1743963 and rs1763509) and the development of depression in CHD patients (P = 0.018 by genotype, P = 0.032 by allele; P = 0.017 by genotype, P = 0.003 by allele, respectively). However, none of these associations remained significant after Bonferroni correction (P = 0.054 for rs1743963; P = 0.051 for rs1763509). Interestingly, both the GG genotype of SGK1 rs1743963 and AA genotype of SGK1 rs1763509 were associated with a higher risk of depression in CHD patients; for rs1763509, the Patient Health Questionnaire-9 (PHQ-9) scores in the carriers of the risk genotype for comorbid depression, AA, were significantly higher than in GG and AG carriers (P = 0.008). Notably, haplotype analysis indicated that haplotype GGA significantly increased the risk of depression in CHD patients (P = 0.011, odds ratio (OR) = 1.717, 95% confidence interval (CI) = 1.132–2.605), whereas haplotype AAG may be a protective factor for CHD patients with comorbid depression (P = 0.038, OR = 0.546, 95% CI = 0.307–0.972). It should be noted that only the significance of haplotype GGA survived after Bonferroni adjustment (P = 0.044) and that no significant differences were found for other SGK1 SNPs (rs2758151, rs9493857, rs9376026, and rs9389154) between CHD patients with and without depression. These findings, for the first time, elucidate the important role of SGK1 variants in the comorbidity of CHD and depression.
Introduction
Coronary heart disease (CHD) is among the most common chronic diseases, with a severe impact on human health and quality of life. It is also considered to be a psychosomatic disease. The poor prognosis and high sudden death rate associated with CHD may also result in the development of comorbid psychological complications such as anxiety and depression. Accumulating evidence shows that CHD patients suffer from depression to some extent and that the prevalence of depression in CHD patients is twice as high as in the general population (Follath, 2003). As a result, CHD with comorbid depression has become a concern worldwide. An increasing number of studies show that CHD and depression share common risk mechanisms, including inflammation (Shimohina et al., 2015), autonomic dysfunction (Drago et al., 2007), hypothalamus–pituitary–adrenocortical axis dysfunction (Lederbogen and Strohle, 2012), and enhancement of platelet aggregation activity (Tseng et al., 2010). Multiple genetic factors have also become the new focus of scientific studies. Emerging data suggest that genetic defects in 5-hydroxytryptamine (5-HT) (Golimbet et al., 2012), apolipoprotein E (ApoE) (Fritze et al., 2011), endothelial NOS (eNOS) (Salimi et al., 2012; Talarowska et al., 2012), and plasminogen-activator inhibitor-1 (PAI-1) (Lahlou-Laforet et al., 2006) may be related to the risk of CHD with comorbid depression.
A member of the serum/glucocorticoid-regulated kinase (SGK) family, serum/glucocorticoid-regulated kinase 1 (SGK1) regulates several ion channels and participates in many cellular reactions, including cell growth, proliferation, migration, survival, and apoptosis (Talarico et al., 2016). A recent study showed that SGK1 contributes to the regulation of renal Na+ reabsorption, K+ secretion, and blood pressure (Valinsky et al., 2018). Given the close association between high blood pressure levels and risk of CHD, it is reasonable to speculate that SGK1 is related to the risk of CHD. Additionally, SGK1 plays a vital part in the regulation of neuronal activity, proliferation, and apoptosis and thus is a key determinant of susceptibility to mental illness. As the downstream target molecule of the glucocorticoid receptor (GR), SGK1 is involved in the development of depression via the glucocorticoid signaling pathway. There is also growing evidence indicating that SGK1 stimulates the production of pro-inflammatory cytokines and oxidants (Lang et al., 2010), which are also closely related to depression. Taking into consideration the complex relationships among SGK1, CHD, and depression, we hypothesize that SGK1 may be a co-pathogenic gene underlying the comorbid mechanisms of CHD and depression. Thus, to further evaluate the role of SGK1 single-nucleotide polymorphisms (SNPs) in susceptibility to CHD with comorbid depression, we carried out a case–control study involving 257 CHD patients with or without depression and 107 controls.
Materials and Methods
Subjects
A total of 257 CHD patients were recruited at the outpatient clinic of the Jining First People’s Hospital in Shandong Province, China. For all patients, the diagnosis of CHD was made by at least two experienced cardiologists and confirmed using coronary angiography results (significant coronary artery stenosis ≥ 50% in at least one of the three major coronary arteries or major branches). Those with valvular heart disease, severe autoimmunity disease, cancer, or severe liver and/or kidney disease were excluded. In addition, all CHD patients with or without depression were assessed by at least two experienced psychiatrists according to DSM-5 (5th edition of the Diagnostic and Statistical Manual of Mental Disorders) criteria for major depressive disorder, which is characterized by significant depressed mood and anhedonia. Then, the severity of depressive symptoms was scored by Patient Health Questionnaire-9 (PHQ-9), a nine-item questionnaire that is commonly used in outpatients. The scale uses a cutoff score for depression analysis of greater than or equal to 5 (Duko et al., 2018). The 107 age- and sex-matched healthy controls were adults without CHD who had undergone a series of assessments including clinical physical examination, radiographic chest examination, electrocardiogram, and evaluation of medical history. Our study received approval from the medical ethics committee of the Jining First People’s Hospital, and informed consents were obtained from all participants.
Genetic Studies (DNA Isolation and Genotyping)
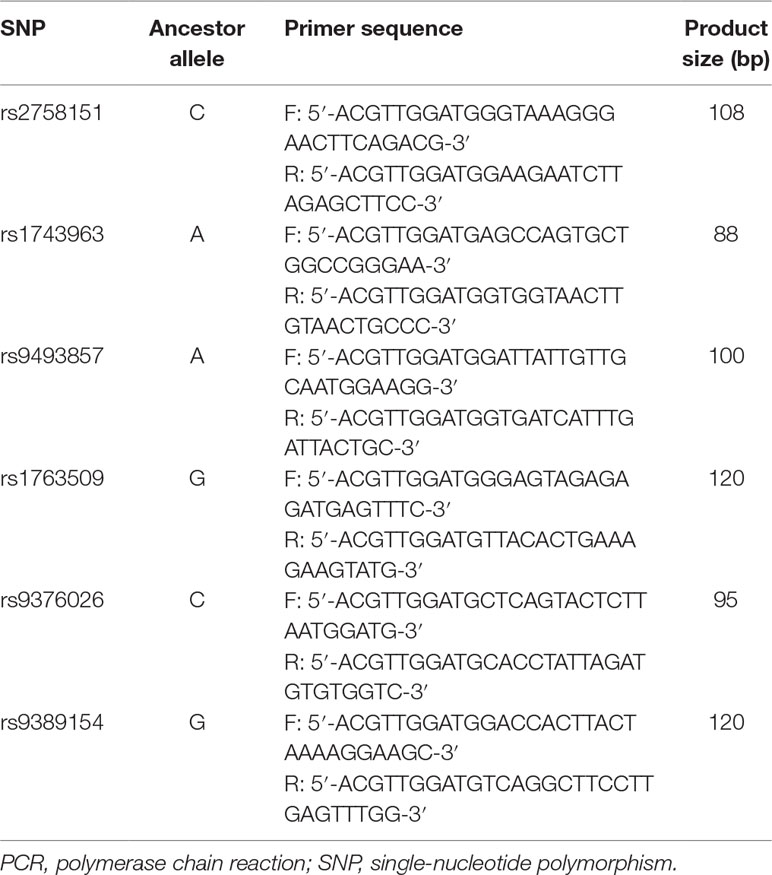
Genomic DNA was isolated from whole blood using a TIANamp Blood DNA Kit (TIANGEN, China) following the manufacturer’s instructions. The genotypes of polymorphisms were identified by polymerase chain reaction–ligase detection reaction (PCR-LDR) assay. All primer sequences for both PCR and LDR are shown in Table 1. After identification using 1.5% agarose gel and a multiplex ligase detection reaction with an LDR probe, products were determined by direct sequencing with a DNA sequencer. To ensure the quality of genotyping, random DNA samples accounting for not less than 10% of the total subjects were genotyped twice. Genotyping quality assessment of the SNPs tested is presented in Supplementary Table 1.
Statistical Analysis
Demographic and clinical characteristics of the study population were evaluated by t-test (for continuous variables) and Pearson’s χ2-test (for categorical variables). Genotype distributions and allele frequencies of CHD patients and controls were analyzed by Pearson’s χ2-test. To evaluate the quality of the genotyping data, the SHEsis online haplotype analysis software (http://analysis.bio-x.cn/myAnalysis.php) was applied to calculate the linkage disequilibrium and check Hardy–Weinberg equilibrium in controls based on Pearson’s χ2-test. Additionally, the SHEsis online haplotype analysis software was also performed for calculating the probability of obtaining a difference in the haplotype frequencies observed between patients and controls and for analyzing the haplotype frequencies and probabilities. Bonferroni adjustment was applied to correct for multiple comparisons. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were also calculated. Differences in PHQ-9 scores among different genotypic individuals were assessed using one-way analysis of variance (ANOVA) or Student’s t-test, when appropriate. All analyses were carried out using SPSS (version 17.0), and P < 0.05 was defined as statistically significant.
Results
Basic Characteristics of Study Participants
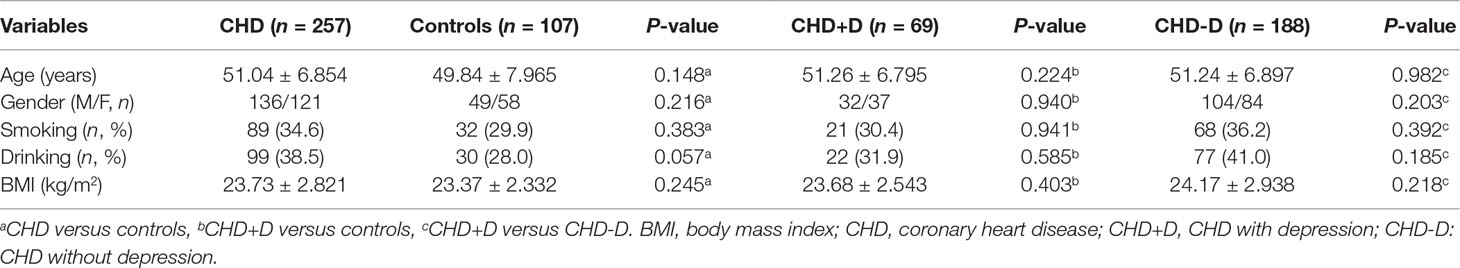
The demographic and clinical characteristics of the study participants are described in Table 2. There were no significant differences between the CHD and control groups in terms of age, gender, body mass index (BMI), and smoking or drinking (P > 0.05). Then, CHD patients were further divided into CHD+D and CHD-D groups according to whether comorbid depression was present. There were still no significant differences concerning the basic characteristics between groups (P > 0.05).
Hardy–Weinberg Equilibrium Analysis
The locations of the SGK1 gene and six SNPs are presented in Supplementary Table 2. The genotypes of all SGK1 SNPs in control groups were in Hardy–Weinberg equilibrium based on the χ2-test results (rs2758151: χ2 = 0.020, P = 0.887; rs1743963: χ2 = 0.115, P = 0.734; rs9493857: χ2 = 0.472, P = 0.492; rs1763509: χ2 = 0.080, P = 0.778; rs9376026: χ2 = 2.909, P = 0.088; rs9389154: χ2 = 0.072, P = 0.789), suggesting that the groups are representative of the population.
SGK1 Polymorphisms
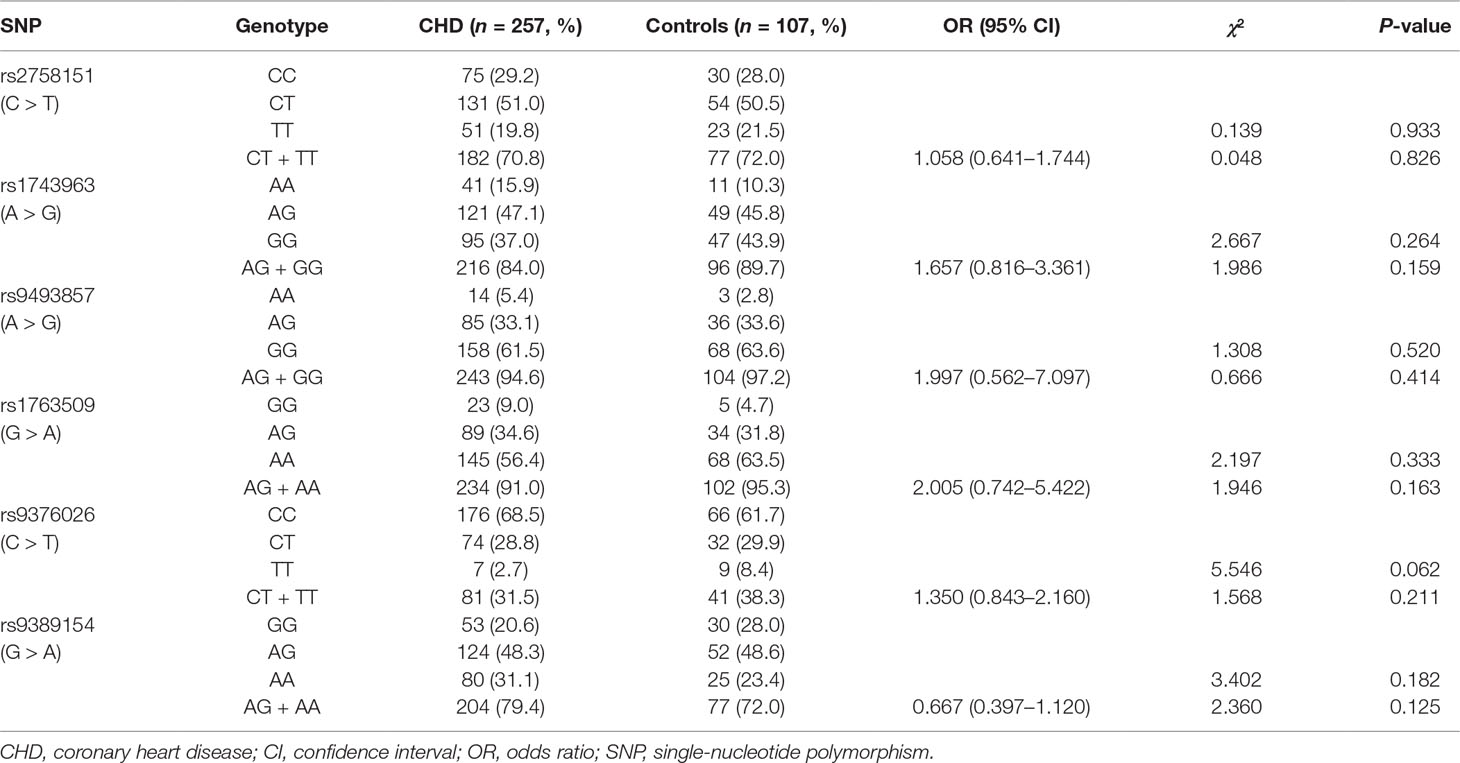
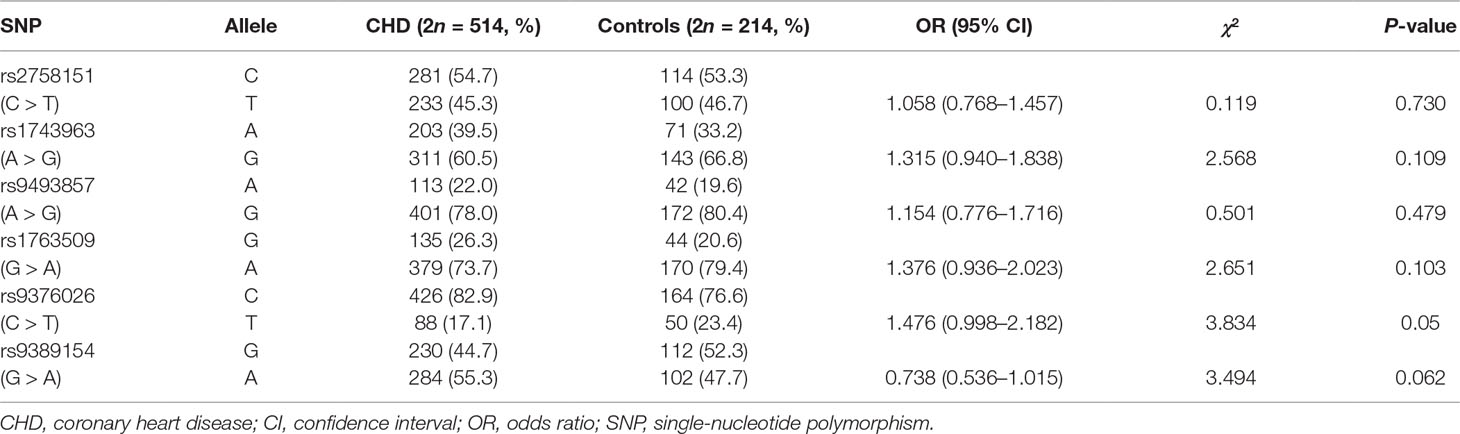
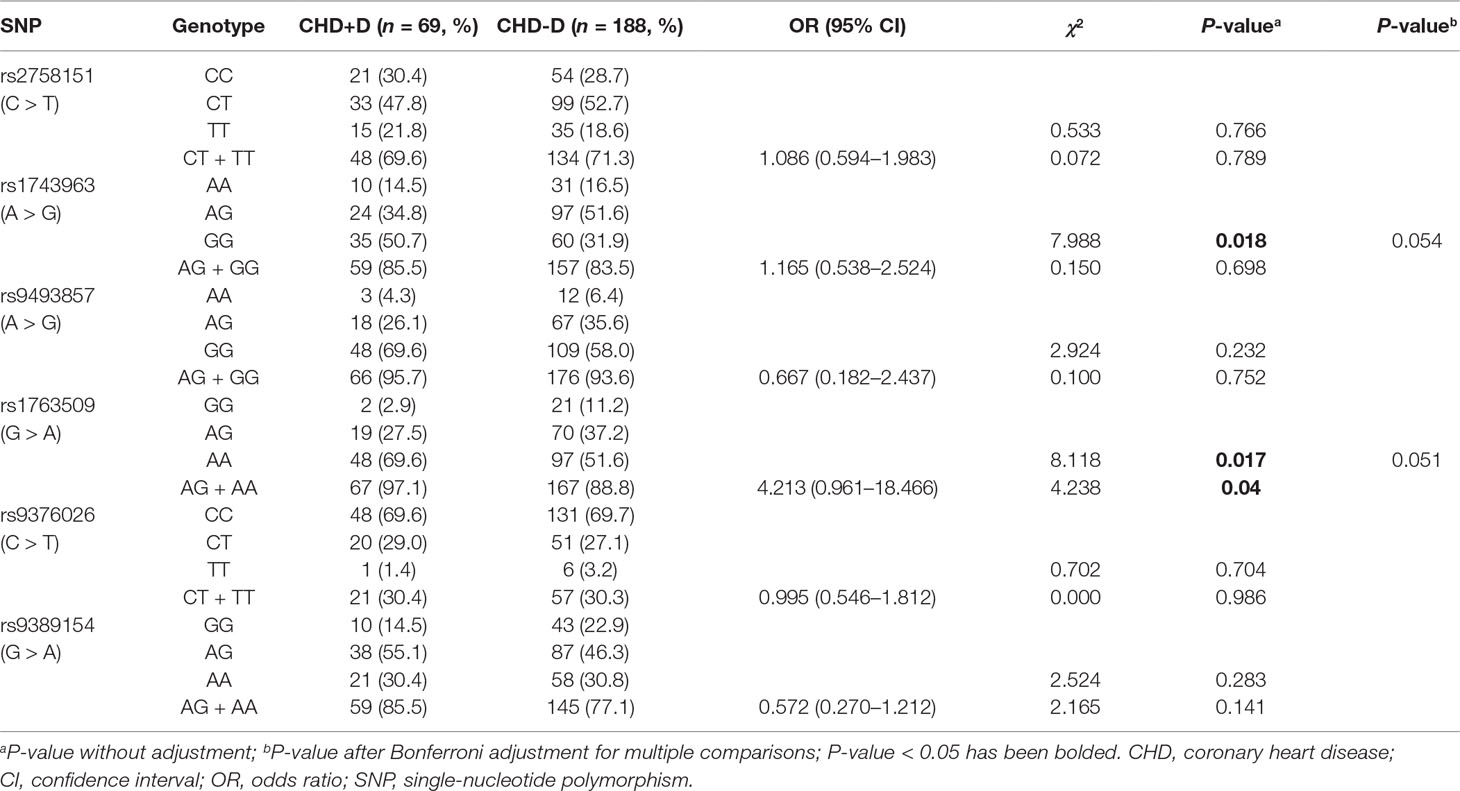
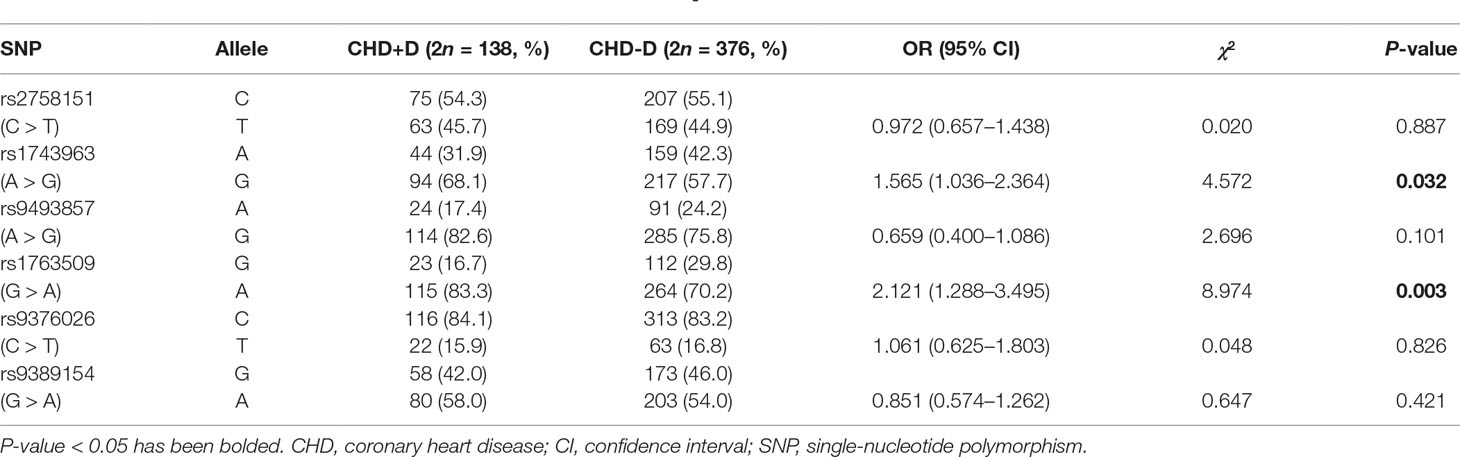
Frequency distributions of genotypes and alleles of six SNPs in CHD patients and controls are shown in Tables 3 and 4. Our results suggest the absence of a significant relationship between SGK1 SNPs and CHD risk. However, significant differences were found between CHD patients with and without depression in the genotype distribution and allele frequency of rs1743963 (A > G) and rs1763509 (G > A). For rs1743963, CHD patients with the GG genotype showed a significant susceptibility to depression (χ2 = 7.988, P = 0.018). Thus, the G allele may be a risk factor in the development of depression in CHD patients (χ2 = 4.572, P = 0.032). For rs1763509, the AA genotype and A allele were associated with a higher risk of depression in CHD patients (χ2 = 8.118, P = 0.017 by genotype; χ2 = 8.974, P = 0.003 by allele). However, the significance of the genotype distribution frequency could not be confirmed after strict Bonferroni adjustment (P = 0.054 for rs1743963; P = 0.051 for rs1763509). Interestingly, when subdividing these samples into GG and AA + AG groups, statistical analysis showed that carriers with allele A were more likely to have comorbid depression (χ2 = 4.238, P = 0.04, OR = 4.213, 95% CI = 0.961–18.466). However, the other four SNPs, rs2758151, rs9493857, rs9376026, and rs9389154, were not significantly related to the risk of depression in CHD patients (as shown in Tables 5 and 6).
Association of SGK1 Polymorphisms With Severity of Depressive Symptoms
As shown in Figure 1A, no significant differences in PHQ-9 scores were observed among the three genotypes of rs1743963 (P > 0.05). For rs1763509 (Figure 1B), GG and AG carriers were combined because of the few GG carriers. The PHQ-9 scores in the AA carriers, the risk genotype for comorbid depression, were significantly higher than GG and AG carriers (10.63 ± 2.900 versus 8.62 ± 2.500, P = 0.008).
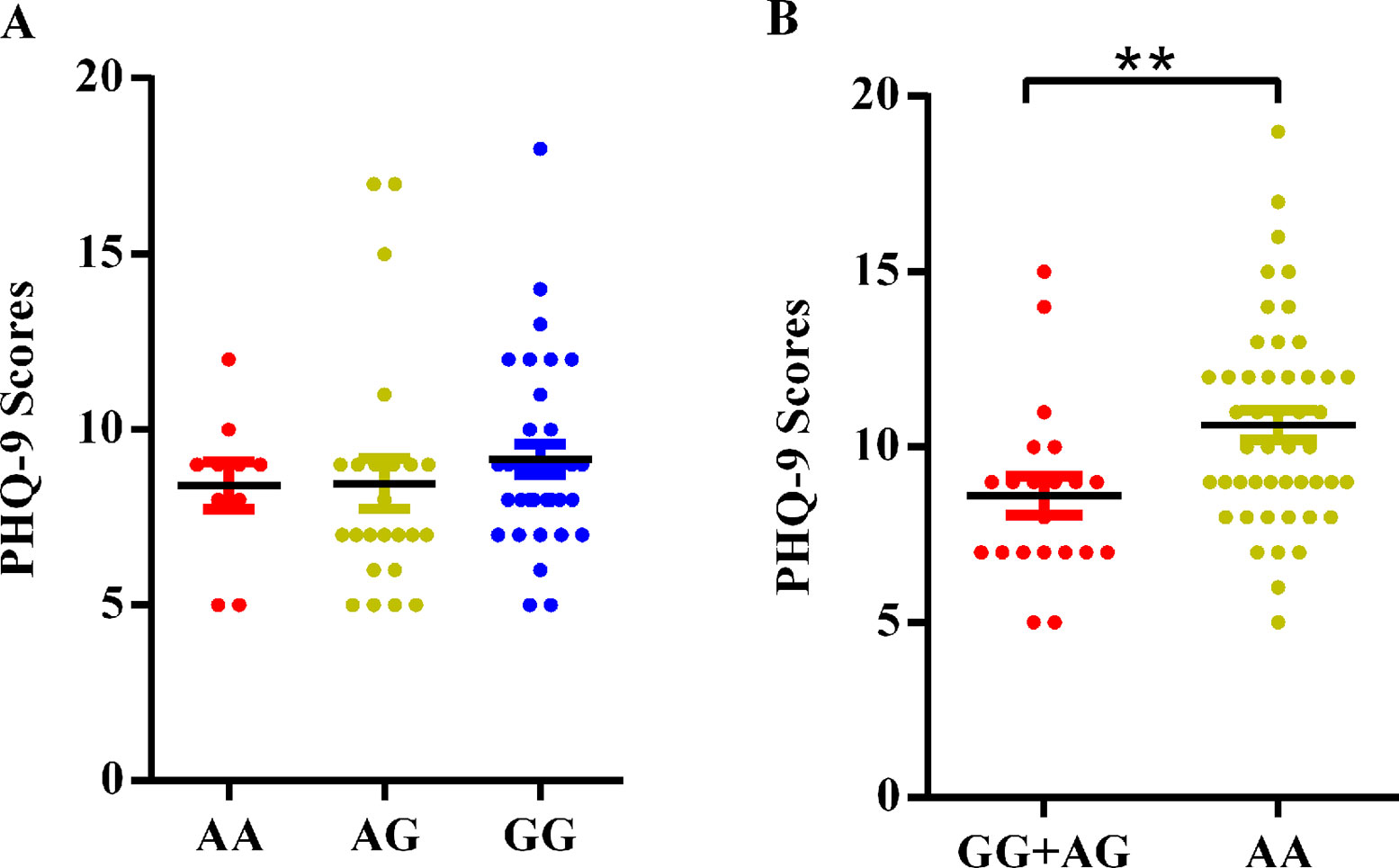
Figure 1 Association of SGK1 polymorphisms and PHQ-9 scores in CHD patients with comorbid depression. (A) rs1743963 and (B) rs1763509. **P < 0.01. CHD, coronary heart disease; PHQ-9, Patient Health Questionnaire-9.
Haplotype Analysis
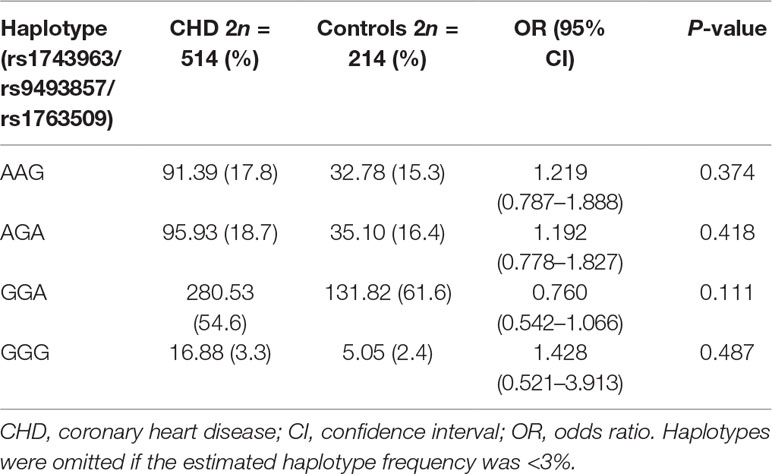
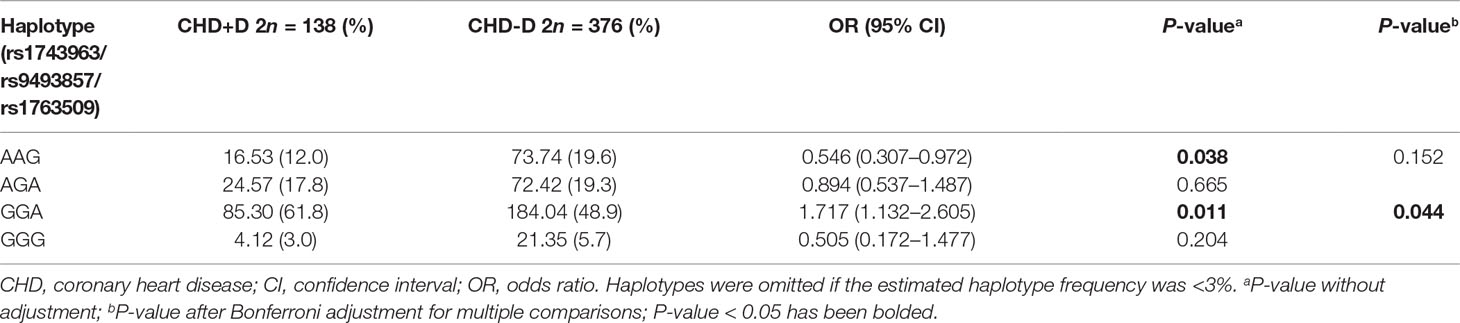
As shown in Figure 2, the LD block in the SGK1 gene on chromosome 6 comprised rs1743963, rs9493857, and rs1763509, with a strong linkage (rs1743963/rs9493857: D′ = 0.793, r2 = 0.282; rs9493857/rs1763509: D′ = 0.869, r2 = 0.675; rs1743963/rs1763509: D′ = 0.633, r2 = 0.201). Haplotype frequencies indicated that there were no significant differences of haplotype distribution between CHD patients and healthy controls (as shown in Table 7). Interestingly, haplotype analysis of the CHD+D and CHD-D groups revealed that haplotype GGA significantly increased the risk of depression in CHD patients (P = 0.011, OR = 1.717, 95% CI = 1.132–2.605), while haplotype AAG may be a protective factor against comorbid depression in CHD patients (P = 0.038, OR = 0.546, 95% CI = 0.307–0.972). After Bonferroni adjustment, only the haplotype GGA remained significantly associated with the susceptibility to depression in CHD patients (P = 0.044) (Table 8).
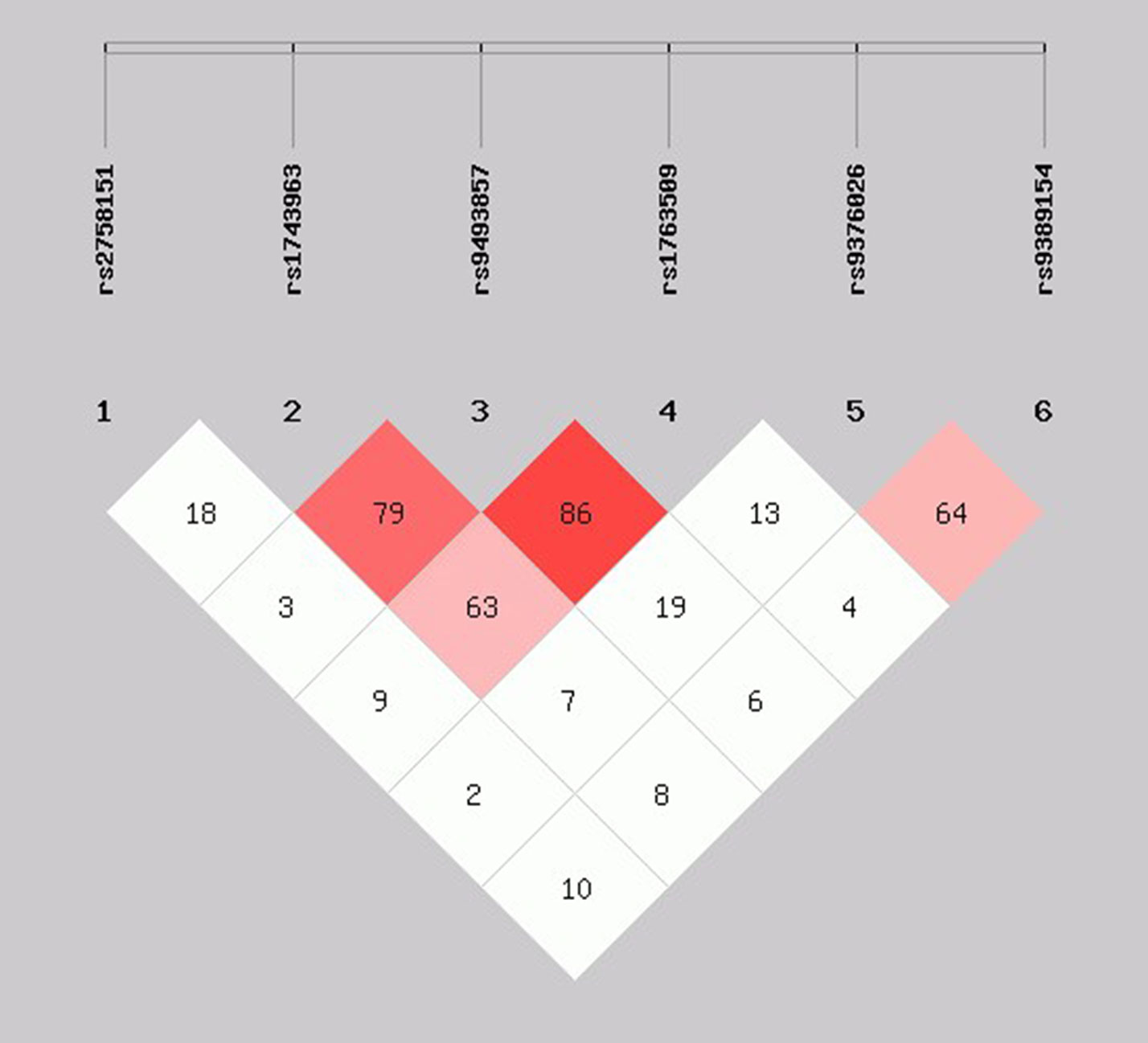
Figure 2 Linkage disequilibrium pattern between three SNPs, rs1743963, rs9493857, and rs1763509, in CHD patients and healthy controls. CHD, coronary heart disease; SNP, single-nucleotide polymorphism.
Discussion
The gene encoding human SGK1 is located in chromosome 6q23.2. SGK1 transcripts have been found in virtually all tissues tested (Raikwar et al., 2008). A key regulatory enzyme, SGK1 was originally described as being involved in the hormonal regulation of several ion channels (Lang et al., 2011; Chraibi and Renauld, 2014). SGK1 is linked to the regulation of Na+ and K+ transport in epithelial cells (Valinsky et al., 2018). Studies have shown that dysregulation of SGK1 causes renal Na+ retention and enhancement of cardiac output, followed by elevated blood pressure (Kawarazaki et al., 2012; Nakano et al., 2013). Several SGK1 gene variants have been shown to affect blood pressure (Busjahn et al., 2002; Rao et al., 2013). Accumulating strong evidence indicates a direct connection between SGK1 and cardiovascular development via involvement in the phosphatidylinositol 3-kinase (Catela et al., 2010) and ALK1 (Araki et al., 2018) signaling pathways. Notably, SGK1 has been shown to contribute to cardiac remodeling and fibrosis, and development of heart failure. These findings suggest that SGK1 regulates blood pressure and participates in cardiovascular development and occurrence of heart failure, indicating a potential link to CHD. In this regard, we consider that SGK1 polymorphisms may be related to the occurrence of CHD.
Furthermore, SGK1 participates in the regulation of dendrite growth (Lang et al., 2006) and long-term memory formation (Ma et al., 2006) and contributes to the pathophysiology of several neuronal diseases including Parkinson’s disease, Alzheimer’s disease, schizophrenia, and depression (Lang et al., 2010; Miyata et al., 2015). Animal experiments have shown that the mRNA level of SGK1 in the hippocampus of mice increased significantly under acute cold water swimming stress (Bohacek et al., 2015), suggesting that SGK1 is closely related to stress-related mental disorders. Accumulating evidence also suggests that SGK1 participates in the occurrence of depression via the glucocorticoid (Sato et al., 2008) and brain-derived neurotrophic factor (BDNF) (Lang et al., 2007) signaling pathway. Similarly, decreased hippocampal neurogenesis and structural abnormalities have been reported to occur in depressed patients owing to the up-regulation of SGK1 (Cattaneo and Riva, 2016). SGK1 additionally contributes to the regulation of neuroexcitability, inflammation, and oxidative stress reactions (Lang et al., 2010), which may be involved in the pathogenesis of depression. In view of the complex relationships between SGK1, CHD, and depression, SGK1 is likely to be a potential co-pathogenic gene underlying susceptibility to CHD with depression.
To test this hypothesis, a case–control study was carried out to identify the role of SGK1 variants in susceptibility to comorbidity of CHD and depression. Six candidate intron variants located in the upstream of SGK1 gene were selected. These SNPs were reported to have a tight link with multiple disorders, including blood pressure and renin response to dietary salt intake, and type 2 diabetes (Schwab et al., 2008; Luca et al., 2009; Rao et al., 2013; Chu et al., 2015), with the possibility to affect the process of splicing, processing, and editing of mRNA. Our study of 69 CHD cases with depression and 188 cases without depression found significant differences in the genotype distribution and allele frequency of rs1743963 (A > G) and rs1763509 (G > A). For rs1743963, CHD patients with the GG genotype showed a modest but non-significant susceptibility to depression (P = 0.054), and the G allele was found to be a risk factor for depression in patients with CHD (P = 0.032). Similarly, for rs1763509, the allele A was associated with a higher risk of depression in CHD patients (P = 0.003). Interestingly, when patients were divided into GG and AA + AG groups according to whether they carried allele A, CHD patients in AA + AG group are more likely to have comorbidity with depression. The PHQ-9 scores in the carriers of the risk genotype for comorbid depression, AA, were significantly higher than in GG and AG carriers. In support, Chu reported that SNP rs1763509 of SGK1 was significantly associated with blood pressure response to the intervention of dietary sodium (Chu et al., 2015). Notably, single marker association analysis is sometimes not sufficient in complex diseases, whereas haplotype-based linkage disequilibrium mapping has been considered a more informative approach for genetic association studies. In the present study, strong linkage disequilibrium was observed between the three SNPs rs1743963, rs9493857, and rs1763509 in the intron region of SGK1 gene, and haplotype analysis suggests that the haplotype GGA is likely to be involved in the development of depression in CHD patients after Bonferroni adjustment, which may affect RNA splicing, processing, and editing. Overall, our study demonstrates for the first time the importance of SGK1 variants in the development of depression in CHD patients. Although many genome-wide association studies (GWASs) on depression or CHD (Li et al., 2019; Liu et al., 2019; Wong et al., 2019) have been published, none of these have identified SGK1 as a risk factor.
The remaining three SNPs, rs2758151 (Rao et al., 2013), rs9376026, and rs9389154 (Chu et al., 2015), have been reported to be associated with blood pressure response to dietary salt intake, and rs9493857 was found to regulate SGK1 expression in response to stress (Luca et al., 2009). However, no significant differences were found between the genotypic and allelic frequencies of polymorphic sites of any of these four SNPs in our study. These negative results can be explained by the relatively small sample size, regional and racial biases, and no correction for potential population stratification, which are major limitations of the present study. Moreover, our study is also limited by the lack of a comparison group of subjects with depression but no CHD for the replication of positive results. Considering that the interactions between various genes and/or environmental factors play a part in the effects of SGK1, the association between SGK1 polymorphisms and depression in CHD patients is likely to be confounded by various potential gene–gene and/or gene–environment interactions. Thus, additional association studies investigating SGK1 diversity and susceptibility to depression in CHD patients are also required to replicate the associations. We are additionally unable to analyze the expression of SGK1 and the functional consequence of these genetic variations. Thus, future studies are needed to further examine the effects of these SNPs on the expression of key components of SGK1 signaling in the peripheral blood mononuclear cells of CHD patients with comorbid depression and thus confirm the relationship between SGK1 and susceptibility to depression in CHD patients.
Conclusion
In conclusion, the present study supports the hypothesis that SGK1 polymorphisms contribute to the susceptibility to depression in CHD patients of the Chinese Han population. To exclude the many environmental and geographical influences on study outcomes, replication studies with large samples are needed to verify the role of these SGK1 polymorphisms in CHD patients with comorbid depression.
Ethics Statement
This study was carried out in accordance with the recommendations of medical ethics committee of Jining First People’s Hospital guidelines with written informed consents from all subjects. All subjects gave written informed consents and the protocol was approved by the medical ethics committee of the Jining First People’s Hospital.
Author Contributions
PJ conceived and designed the study; HaixZ, XZ, XG, and YG were responsible for the sample collection; MY and HailZ conducted the experiments and had access to all the data in the study; WH analyzed the data and led the drafting of the manuscript; HaixZ, GL, YL, and GY provided critical revisions of the manuscript. WH and HaixZ contributed equally to the work. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (81602846, 81602724, and 81571334) and Shandong Medical and Health Science and Technology Development Program Project (2016WS0155).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to particularly acknowledge Dr. Pei Jiang for his input on the study, and we wish to thank all participants for their contributions to the present study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00921/full#supplementary-material
Supplementary Table 1 | Genotyping quality assessment of the SNPs tested.
Supplementary Table 2 | The information and location of SGK1 gene and these SNPs.
References
Araki, M., Hisamitsu, T., Kinugasa-Katayama, Y., Tanaka, T., Harada, Y., Nakao, S., et al. (2018). Serum/glucocorticoid-regulated kinase 1 as a novel transcriptional target of bone morphogenetic protein-ALK1 receptor signaling in vascular endothelial cells. Angiogenesis 21, 415–423. doi: 10.1007/s10456-018-9605-x
Bohacek, J., Manuella, F., Roszkowski, M., Mansuy, I. M. (2015). Hippocampal gene expression induced by cold swim stress depends on sex and handling. Psychoneuroendocrinology 52, 1–12. doi: 10.1016/j.psyneuen.2014.10.026
Busjahn, A., Aydin, A., Uhlmann, R., Krasko, C., Bahring, S., Szelestei, T., et al. (2002). Serum- and glucocorticoid-regulated kinase (SGK1) gene and blood pressure. Hypertension 40, 256–260. doi: 10.1161/01.HYP.0000030153.19366.26
Catela, C., Kratsios, P., Hede, M., Lang, F., Rosenthal, N. (2010). Serum and glucocorticoid-inducible kinase 1 (SGK1) is necessary for vascular remodeling during angiogenesis. Dev. Dyn. 239, 2149–2160. doi: 10.1002/dvdy.22345
Cattaneo, A., Riva, M. A. (2016). Stress-induced mechanisms in mental illness: a role for glucocorticoid signalling. J. Steroid Biochem. Mol. Biol. 160, 169–174. doi: 10.1016/j.jsbmb.2015.07.021
Chraibi, A., Renauld, S. (2014). PPARgamma-induced stimulation of amiloride-sensitive sodium current in renal collecting duct principal cells is serum and insulin dependent. Cell Physiol. Biochem. 33, 581–593. doi: 10.1159/000358636
Chu, C., Wang, Y., Wang, M., Mu, J.-J., Liu, F.-Q., Wang, L., et al. (2015). Common variants in serum/glucocorticoid regulated kinase 1 (SGK1) and blood pressure responses to dietary sodium or potassium interventions: a family-based association study. Kidney and Blood Press. Res. 40, 424–434. doi: 10.1159/000368518
Drago, S., Bergerone, S., Anselmino, M., Varalda, P. G., Cascio, B., Palumbo, L., et al. (2007). Depression in patients with acute myocardial infarction: influence on autonomic nervous system and prognostic role. Results of a five-year follow-up study. Int. J. Cardiol. 115, 46–51. doi: 10.1016/j.ijcard.2006.04.029
Duko, B., Geja, E., Zewude, M., Mekonen, S. (2018). Prevalence and associated factors of depression among patients with HIV/AIDS in Hawassa, Ethiopia, cross-sectional study. Ann. Gen. Psychiatry 17, 45. doi: 10.1186/s12991-018-0215-1
Follath, F. (2003). Depression, stress and coronary heart disease—epidemiology, prognosis and therapeutic sequelae. Ther. Umsch. 60, 697–701. doi: 10.1024/0040-5930.60.11.697
Fritze, F., Ehrt, U., Sonnesyn, H., Kurz, M., Hortobagyi, T., Nore, S. P., et al. (2011). Depression in mild dementia: associations with diagnosis, APOE genotype and clinical features. Int. J. Geriatr. Psychiatry 26, 1054–1061. doi: 10.1002/gps.2643
Golimbet, V. E., Volel, B. A., Dolzhikov, A. V., Isaeva, M. I. (2012). The role of the 5-HTTLPR polymorphism of the serotonin transporter gene in the development of depression in patients with coronary heart disease. Zh. Nevrol. Psikhiatr. Im. S. S. 112, 63–69
Kawarazaki, H., Ando, K., Shibata, S., Muraoka, K., Fujita, M., Kawarasaki, C., et al. (2012). Mineralocorticoid receptor–Rac1 activation and oxidative stress play major roles in salt-induced hypertension and kidney injury in prepubertal rats. J. Hypertens. 30, 1977–1985. doi: 10.1097/HJH.0b013e3283576904
Lahlou-Laforet, K., Alhenc-Gelas, M., Pornin, M., Bydlowski, S., Seigneur, E., Benetos, A., et al. (2006). Relation of depressive mood to plasminogen activator inhibitor, tissue plasminogen activator, and fibrinogen levels in patients with versus without coronary heart disease. Am. J. Cardiol. 97, 1287–1291. doi: 10.1016/j.amjcard.2005.11.062
Lang, F., Bohmer, C., Palmada, M., Seebohm, G., Strutz-Seebohm, N., Vallon, V. (2006). (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 86, 1151–1178. doi: 10.1152/physrev.00050.2005
Lang, F., Strutz-Seebohm, N., Seebohm, G., Lang, U. E. (2010). Significance of SGK1 in the regulation of neuronal function. J. Physiol. 588, 3349–3354. doi: 10.1113/jphysiol.2010.190926
Lang, P. A., Graf, D., Boini, K. M., Lang, K. S., Klingel, K., Kandolf, R., et al. (2011). Cell volume, the serum and glucocorticoid inducible kinase 1 and the liver. Z. Gastroenterol. 49, 713–719. doi: 10.1055/s-0031-1273425
Lang, U. E., Puls, I., Muller, D. J., Strutz-Seebohm, N., Gallinat, J. (2007). Molecular mechanisms of schizophrenia. Cell Physiol. Biochem. 20, 687–702. doi: 10.1159/000110430
Lederbogen, F., Strohle, A. (2012). [Stress, mental disorders and coronary heart disease]. Nervenarzt 83, 1448–1457. doi: 10.1007/s00115-012-3666-7
Li, H., Chang, H., Song, X., Liu, W., Li, L., Wang, L., et al. (2019). Integrative analyses of major histocompatibility complex loci in the genome-wide association studies of major depressive disorder. Neuropsychopharmacology 44, 1552–1561. doi: 10.1038/s41386-019-0346-3
Liu, Y., Ma, H., Zhu, Q., Zhang, B., Yan, H., Li, H., et al. (2019). A genome wide association study on lipoprotein(a) levels and coronary artery disease severity in a Chinese population. J. Lipid Res. 60, 1440–1448. doi: 10.1194/jlr.P091009
Luca, F., Kashyap, S., Southard, C., Zou, M., Witonsky, D., Di Rienzo, A., et al. (2009). Adaptive variation regulates the expression of the human SGK1 gene in response to stress. PLoS Genet. 5, e1000489. doi: 10.1371/journal.pgen.1000489
Ma, Y. L., Tsai, M. C., Hsu, W. L., Lee, E. H. (2006). SGK protein kinase facilitates the expression of long-term potentiation in hippocampal neurons. Learn. Mem. 13, 114–118. doi: 10.1101/lm.179206
Miyata, S., Hattori, T., Shimizu, S., Ito, A., Tohyama, M. (2015). Disturbance of oligodendrocyte function plays a key role in the pathogenesis of schizophrenia and major depressive disorder. Biomed Res. Int. 2015, 1–26. doi: 10.1155/2015/492367
Nakano, M., Hirooka, Y., Matsukawa, R., Ito, K., Sunagawa, K. (2013). Mineralocorticoid receptors/epithelial Na(+) channels in the choroid plexus are involved in hypertensive mechanisms in stroke-prone spontaneously hypertensive rats. Hypertens. Res. 36, 277–284. doi: 10.1038/hr.2012.174
Raikwar, N. S., Snyder, P. M., Thomas, C. P. (2008). An evolutionarily conserved N-terminal Sgk1 variant with enhanced stability and improved function. Am. J. Physiol. Renal Physiol. 295, F1440–F1448. doi: 10.1152/ajprenal.90239.2008
Rao, A. D., Sun, B., Saxena, A., Hopkins, P. N., Jeunemaitre, X., Brown, N. J., et al. (2013). Polymorphisms in the serum- and glucocorticoid-inducible kinase 1 gene are associated with blood pressure and renin response to dietary salt intake. J. Hum. Hypertens. 27, 176–180. doi: 10.1038/jhh.2012.22
Salimi, S., Naghavi, A., Firoozrai, M., Zand, H., Tavilani, H., Nakhaee, A., et al. (2012). Association of plasma nitric oxide concentration and endothelial nitric oxide synthase T-786C gene polymorphism in coronary artery disease. Pathophysiology 19, 157–162. doi: 10.1016/j.pathophys.2012.04.003
Sato, H., Horikawa, Y., Iizuka, K., Sakurai, N., Tanaka, T., Shihara, N., et al. (2008). Large-scale analysis of glucocorticoid target genes in rat hypothalamus. J. Neurochem. 106, 805–814. doi: 10.1111/j.1471-4159.2008.05489.x
Schwab, M., Lupescu, A., Mota, M., Mota, E., Frey, A., Simon, P., et al. (2008). Association of SGK1 gene polymorphisms with type 2 diabetes. Cell Physiol. Biochem. 21, 151–160. doi: 10.1159/000113757
Shimohina, N. Y., Savchenko, A. A., Petrova, M. M., Chernyaeva, M. S. (2015). The state of hemostasis and immune system in patients’ with acute coronary syndrome combined with anxiety-depressive disorder. Kardiologiia 55, 12–20. doi: 10.18565/cardio.2015.8.12-20
Talarico, C., Dattilo, V., D’antona, L., Menniti, M., Bianco, C., Ortuso, F., et al. (2016). SGK1, the new player in the game of resistance: chemo-radio molecular target and strategy for inhibition. Cell Physiol. Biochem. 39, 1863–1876. doi: 10.1159/000447885
Talarowska, M., Galecki, P., Maes, M., Orzechowska, A., Chamielec, M., Bartosz, G., et al. (2012). Nitric oxide plasma concentration associated with cognitive impairment in patients with recurrent depressive disorder. Neurosci. Lett. 510, 127–131. doi: 10.1016/j.neulet.2012.01.018
Tseng, Y. L., Chiang, M. L., Huang, T. F., Su, K. P., Lane, H. Y., Lai, Y. C. (2010). A selective serotonin reuptake inhibitor, citalopram, inhibits collagen-induced platelet aggregation and activation. Thromb. Res. 126, 517–523. doi: 10.1016/j.thromres.2010.09.017
Valinsky, W. C., Touyz, R. M., Shrier, A. (2018). Aldosterone, SGK1, and ion channels in the kidney. Clin. Sci. (Lond) 132, 173–183. doi: 10.1042/CS20171525
Keywords: serum/glucocorticoid-regulated kinase 1, coronary heart disease, depression, polymorphism, stress
Citation: Han W, Zhang H, Gong X, Guo Y, Yang M, Zhang H, Zhou X, Li G, Liu Y, Jiang P and Yan G (2019) Association of SGK1 Polymorphisms With Susceptibility to Coronary Heart Disease in Chinese Han Patients With Comorbid Depression. Front. Genet. 10:921. doi: 10.3389/fgene.2019.00921
Received: 25 July 2018; Accepted: 30 August 2019;
Published: 01 October 2019.
Edited by:
Yu-Qiang Ding, Tongji University, ChinaReviewed by:
Toni Clarke, University of Edinburgh, United KingdomWeihua Yue, Peking University Sixth Hospital, China
Copyright © 2019 Han, Zhang, Gong, Guo, Yang, Zhang, Zhou, Li, Liu, Jiang and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei Jiang, amlhbmdwZWljc3VAc2luYS5jb20=
†These authors have contributed equally to this work
 Wenxiu Han
Wenxiu Han Haixia Zhang2†
Haixia Zhang2† Gongying Li
Gongying Li Pei Jiang
Pei Jiang