- 1Department of Rheumatism and Immunology, Peking University Shenzhen Hospital, Shenzhen, China
- 2Department of Rheumatology and Immunology, Peking University People’s Hospital, Beijing, China
- 3Health Management Center, Peking University Shenzhen Hospital, Shenzhen, China
Introduction: Leukocyte immunoglobulin-like receptor A3 (LILRA3) belongs to the LILR family with unique feature of a 6.7-kb deletion variation among individuals. Frequencies of the 6.7-kb deletion vary widely across populations, but so far it has not been carefully investigated among Han Chinese subpopulations. Furthermore, we previously identified the non-deleted (functional) LILRA3 as a novel genetic risk for multiple autoimmune diseases. The current study aimed to investigate (i) whether frequencies of the LILRA3 6.7-kb deletion differ within Han Chinese subpopulations and (ii) whether the functional LILRA3 is a novel genetic risk for ankylosing spondylitis (AS).
Methods: The LILRA3 6.7-kb deletion was genotyped in two independent cohorts, including 1,567 subjects from Shenzhen Hospital and 2,507 subjects from People’s Hospital of Peking University. Frequencies of the 6.7-kb deletion were first investigated in combined healthy cohort according to the Chinese administrative district divisions. Association analyses were performed on whole dataset and subsets according to the geographic regions. Impact of the functional LILRA3 on AS disease activity was evaluated.
Results: Frequencies of LILRA3 6.7-kb deletion were highly differentiated within Han Chinese subpopulations, being gradually decreased from Northeast (80.6%) to South (47.4%). Functional LILRA3 seemed to be a strong genetic risk in susceptibility to AS under almost all the alternative genetic models, if the study subjects were not geographically stratified. However, stratification analysis revealed that the functional LILRA3 was consistently associated with AS susceptibility mainly in Northern Han subgroup under the alternative genetic models, but not in Central and Southern Hans. Functional LILRA3 conferred an increased disease activity in AS patients (P < 0.0001 both for CRP and ESR, and P = 0.003 for BASDAI).
Conclusions: The present study is the first to report that the frequencies of LILRA3 6.7-kb deletion vary among Chinese Hans across geographic regions. The functional LILRA3 is associated with AS susceptibility mainly in Northern Han, but not in Central and Southern Han subgroups. Our finding provides new evidence that LILRA3 is a common genetic risk for multiple autoimmune diseases and highlights the genetic differentiation among different ethnicities, even within the subpopulations of an ethnic group.
Introduction
Ankylosing spondylitis (AS) is a chronic autoimmune disease characterized by new bone formation, progressively leading to ankylosis of the axial skeleton and functional disability. The disease predominantly affects young men. The etiology of AS is not completely understood, but it is believed that genetic factors play a major role in AS pathogenesis, particularly the MHC class I allele HLA-B27, which has been recognized as the best genetic marker for AS susceptibility (reviewed in (Brown et al., 2016)). However, despite over 80% of AS patients are HLA-B27 carriers, only a small proportion of HLA-B27 positive individuals ever develop AS (reviewed in (Reveille, 2012)). Furthermore, the genome-wide association studies (GWAS) have revealed that more than 60 additional genetic risk factors contributed to the disease, indicating a polygenic nature of AS. To date, only approximately 30% of AS heritability has been explained by the known genetic loci; many remain unidentified (reviewed in (Li and Brown, 2017; Ranganathan et al., 2017)).
The leukocyte immunoglobulin-like receptor genes (LILRs) is a highly homologous multigene family located on human chromosome 19q13.4 (Samaridis and Colonna, 1997; Kelley et al., 2005). One of characteristics of LILR family is their specific recognition of MHC class I molecules (Cosman et al., 1997). According to the signaling pathways through immunoreceptor tyrosine-based activating or inhibitory motifs, two subgroups of the LILRs have been defined: activating (LILRA1-6) and inhibitory (LILRB1-5) receptors (Samaridis and Colonna, 1997; Nakajima et al., 1999). Of which, LILRA3 (OMIM 604818) is unique, due to a premature stop codon in the extracellular stalk region, leading to a loss of transmembrane domain and therefore expressed only as a soluble receptor (Arm et al., 1997; Borges et al., 1997; Colonna et al., 1997). Furthermore, LILRA3 exhibits a presence or absence of 6.7-kb variation among individuals. The 6.7-kb deletion comprises of the first six of total seven exons and removes all of four Ig-like domains, leading to a truncated protein (Torkar et al., 2000; Wilson et al., 2000; Norman et al., 2003). Interestingly, the frequencies of LILRA3 6.7-kb deletion vary widely among ethnic groups, being much higher in Northeast Asians such as Japanese (71%), Chinese Han (76%), Chinese Manchu (79%), and Koreans (84%), compared to Europeans (15–26%), South Asians (10%), or Africans (7%) (Hirayasu et al., 2006; Hirayasu et al., 2008; Du et al., 2014). However, so far, the frequencies of the LILRA3 6.7-kb deletion have not been carefully investigated among the Han Chinese subpopulations across the geographic regions.
To date the function of LILRA3 remains obscure, but LILRA3 could bind to HLA class I molecules HLA-G and HLA-C (Jones et al., 2011; Ryu et al., 2011) and may act as an antagonist on other LILRs or a soluble ligand to other receptors (Torkar et al., 2000; Burshtyn and Morcos, 2016). In Caucasian populations, the LILRA3 6.7-kb deletion has been reported as a genetic risk for primary Sjogren’s syndrome (pSS) (Kabalak et al., 2009) and multiple sclerosis (MS) (Koch et al., 2005; Ordonez et al., 2009; Wisniewski et al., 2013; Ortiz et al., 2015; An et al., 2016). Nevertheless, our previous studies have demonstrated that, in Han Chinese population, the non-deleted (functional) LILRA3 allele, rather than the 6.7-kb deleted LILRA3, was the genetic risk for pSS, systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA) (Du et al., 2014; Du et al., 2015). A GWAS study has also reported the functional LILRA3 was a risk factor for susceptibility to prostate cancer in Han population (Xu et al., 2012). These reports have provided strong evidence that the functional LILRA3 is a genetic risk for multiple chronic diseases. However, whether the functional LILRA3 is a novel susceptibility factor for AS has not been investigated. We undertook the present study (i) to investigate the frequencies of the LILRA36.7-kb deletion among Han Chinese subpopulations across the geographic regions, (ii) to examine the possible genetic association between LILRA3 and AS, and (iii) to examine whether LILRA3 influences the disease activity in AS.
Material and Methods
Study Subjects
Two independent cohorts were enrolled, including 1,567 subjects (821 cases and 746 healthy controls) from Peking University Shenzhen Hospital (SZH) and 2,507 subjects (300 cases and 995 selected healthy subjects for case-control analysis by taking account of gender and age matching, and 2,207 healthy subjects for subpopulation stratification analysis, respectively) from Peking University People’s Hospital (PH). All patients with AS fulfilled the 1984 Modified New York Criteria for the diagnosis of AS (van der Linden et al., 1984). All cases and healthy controls are Han Chinese.
In the SZH cohort, the patients were recruited from the Department of Rheumatology of Shenzhen Hospital and from both out-patient and in-patient departments between Jan 2012 and May 2019. The healthy controls were from the Health Care Center affiliated to Shenzhen Hospital.
In the PH cohort, the patients were recruited from the Department of Rheumatology and Immunology of People’s Hospital and from in-patient department between Jan 2015 and May 2019. The healthy controls were recruited from the Health Care Center of PKU People’s Hospital, the First Affiliated Hospital of Anhui Medical University, and Gulou Hospital Affiliated to Medical College of Nanjing University, respectively (Du et al., 2014; Du et al., 2015). The baseline demographic characteristics of patients and controls are shown in Table 1. The geographic characteristics of the patients and controls from the two independent cohorts, according to the the Chinese administrative district divisions and the latitudes, are shown in the Supplementary Table 4 and 5, respectively.
The study was approved by the Medical Ethics Committee of Peking University Shenzhen Hospital. Written informed consent was obtained from all participants.
Subpopulation Stratifications
In SZH cohort, majority of the patients and healthy controls came from Guangdong, Hubei, Hunan, Jiangxi, Sichuan, and Fujian. In the PH cohort, majority of cases and healthy individuals were from Beijing, Tianjin, Hebei, Shandong, Liaoning, Inner Mongolia, Jilin, and Heilongjiang. In addition, a small proportion of healthy individuals in PH cohort were from Jiangsu and Anhui.
For the subpopulation stratification analysis, the two independent healthy cohorts were merged, and then, the total healthy subjects were classified into subgroups according to the Chinese administrative district divisions, i.e., Northeastern China, Northern China, Eastern China, Central China, Western China, and Southern China (He et al., 2017). For the case-control stratification analysis, the two independent case-control cohorts were respectively pooled, and the samples were then stratified into the three case-control sub-cohorts according to the latitudes, i.e., (i) latitude ≥ 35° (roughly corresponding to Northeastern and Northern regions), (ii) latitude 25–35° (roughly corresponding to Western, Central, and Eastern regions), and (iii) latitude ≤ 25° (roughly corresponding to Southern region). Of note, there were a number of cases and healthy controls from the metropolises of Shenzhen and Beijing, where the geographical location is no longer a good indicator of ancestral origin due to the impact of modern immigration (He et al., 2017). These individuals were self-identified their ancestral origins in the stratification analysis.
Determination of LILRA3-del by Sequence-Specific Primer-Polymerase Chain Reaction (PCR-SSP)
Genotypes of the LILRA3 6.7-kb deletion polymorphism were obtained by PCR amplification with modified sequence-specific primers (PCR-SSP) (Du et al., 2014; Du et al., 2015). The cases and controls from the SZH cohort were genotyped at Shenzhen Hospital, and the AS cases from the PH cohort were genotyped at People’s Hospital. The genotyping success and confirmation rates were 99.1 and 100%, respectively. The genotyping dataset has been deposited in the figshare database (DOI: 10.6084/m9.figshare.9685619, https://figshare.com/s/0eab58f90f3b1b20a181).
In the PH cohort, the genotyping data for healthy controls were cited from our previous publications (Du et al., 2014; Du et al., 2015).
Serum Dkk-1 Measurements
A total of 384 AS patients were measured for serum levels of Dkk-1. Serum samples were collected and stored immediately at −80°C prior to be used. All cases were genotyped for the LILRA3 6.7-kb deletion polymorphism. Quantification of serum Dkk-1 concentration was performed by using commercially available ELISA kits, according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). The detection range for Dkk-1 is from 31.3 to 2,000 pg/ml with an assay sensitivity around 15.6 pg/ml (catalogue number: DKK100).
Power Analysis
The power analyses were performed retrospectively for the available samples (cases and controls), using a fixed minor allele frequency of 31.0% (the MAF in healthy controls from the combined cohort), a type I error P of 0.05, and an OR of 1.4. The PS software (version 3.0.14) was used for power calculation (available at http://www.mc.vanderbilt.edu/prevmed/ps).
Statistical Analyses
The Hardy–Weinberg equilibrium (HWE) test was performed for the polymorphism, using Pearson’s goodness-of-fit chi-square test. The Pearson chi-square test was performed for the comparisons of allelic frequency differences between cases and controls. Odds ratios (OR) and 95% confidence intervals (CI) for genetic model analysis were calculated using logistic regression, adjusting for age and sex. The independent T-test was applied for analysis of serum levels of CRP, ESR, and Dkk-1, and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) between two genotypic groups. All statistical analyses were conducted using program SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The P-value < 0.05 was considered statistically significant.
Results
The 6.7-kb deletion variant was in Hardy–Weinberg equilibrium (HWE) (P > 0.05) in healthy controls (data not shown). The study had a statistical power of 0.987 to detect the modest effect size of OR = 1.40, and a fixed minor allele frequency (MAF) of 31.0% (the MAF in healthy controls from the combined cohort) between LILRA3 and AS. However, the single-subpopulation study power was generally low (study power = 0.769 in Northern Han, 0.708 in Central Han, and 0.520 in Southern Han, respectively).
Frequencies of LILRA3 6.7-kb Deletion Are Highly Differentiated Within Han Chinese Subpopulations
As the frequencies of LILRA3 6.7-kb deletion were highly different in different populations worldwide and the study subjects were came from multiple geographical regions across China in present study, we hypothesize that the frequencies of LILRA3 6.7-kb deletion may also vary among Chinese Hans. To this end, we first pooled the two healthy cohorts and roughly classified the subjects into six subgroups according to the Chinese administrative district divisions (He et al., 2017) (Figure 1A). Interestingly, we found that allele frequencies of the 6.7-kb deletion varied remarkably according to the administrative district divisions. As shown in Figure 1B and Table 2, allele distribution of the 6.7-kb deletion were gradually decreased from Northeast (80.6%) to South (47.4%). Accordingly, frequencies of the functional LILRA3 allele were gradually increased from Northeast (19.4%) to South (52.6%). Next, we investigated the geographical distribution of the LILRA3 variants according to the latitude. As shown in Figure 1C, the homozygous of the functional LILRA3 was about 4.5% at latitude ≥ 35°(roughly corresponding to Northeastern and Northern regions), 10.4% at latitude 25–35° (roughly corresponding to Western, Central, and Eastern regions), and up to 30.6% at latitude ≤ 25°(corresponding to Southern region). Collectively, the frequencies of LILRA3 6.7-kb deletion was highly differentiated within Han Chinese subpopulations. Frequencies of the functional LILRA3 were reversely correlated with the latitude in Han Chinese, with the highest frequency seen in Southern Hans (52.6%).
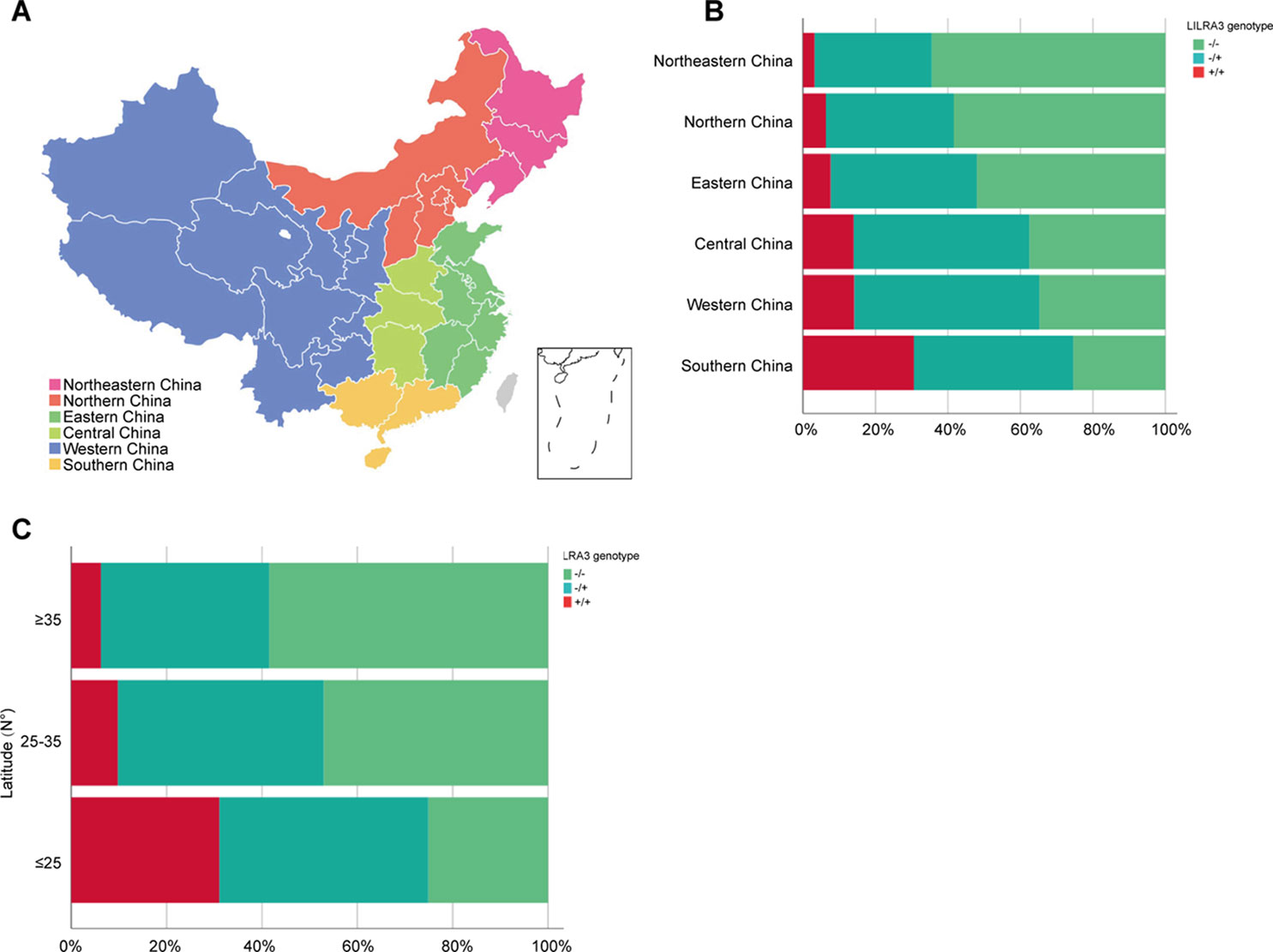
Figure 1 Geographical distribution of the LILRA3 6.7-kb deletion frequencies in the Han Chinese healthy individuals. (A) The combined healthy individuals cohorts were classified into subpopulations according to the Chinese administrative district divisions, i.e., Northeastern China (pink), Northern China (orange), Eastern China (green), Central China (light green), Western China (blue), and Southern China (yellow). (B) Genotypic frequencies of the LILRA3 variations in the administrative geographic regions. (C) Genotypic frequencies of the LILRA3 variations according to the latitude.
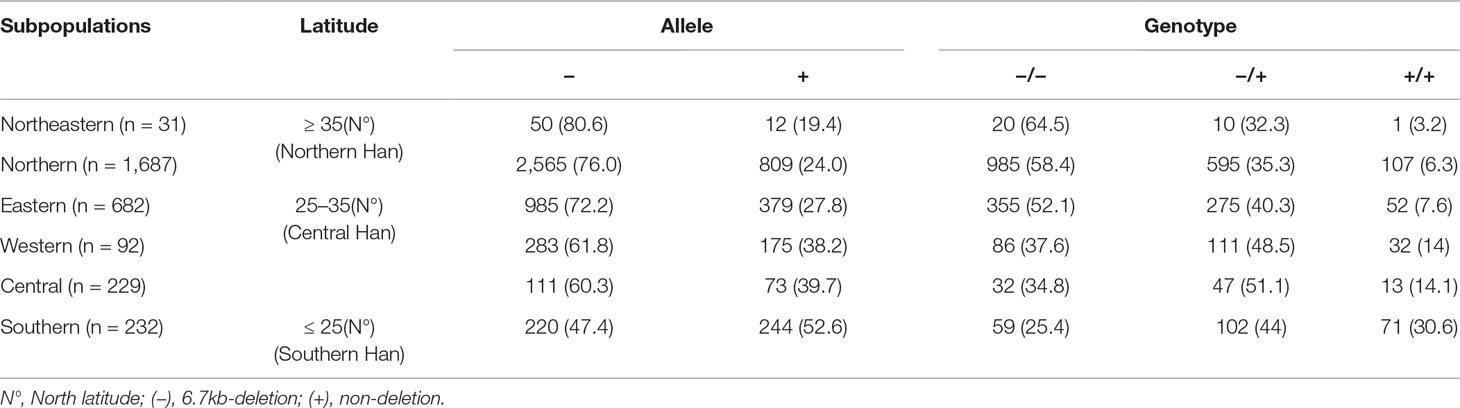
Table 2 Geographical distribution of the LILRA3 variations in Han Chinese healthy individuals, according to the Chinese administrative district divisions (n = 3,343).
Functional LILRA3 Seems to Be a Strong Genetic Risk for Development of AS, If the Study Subjects Were Not Geographically Stratified
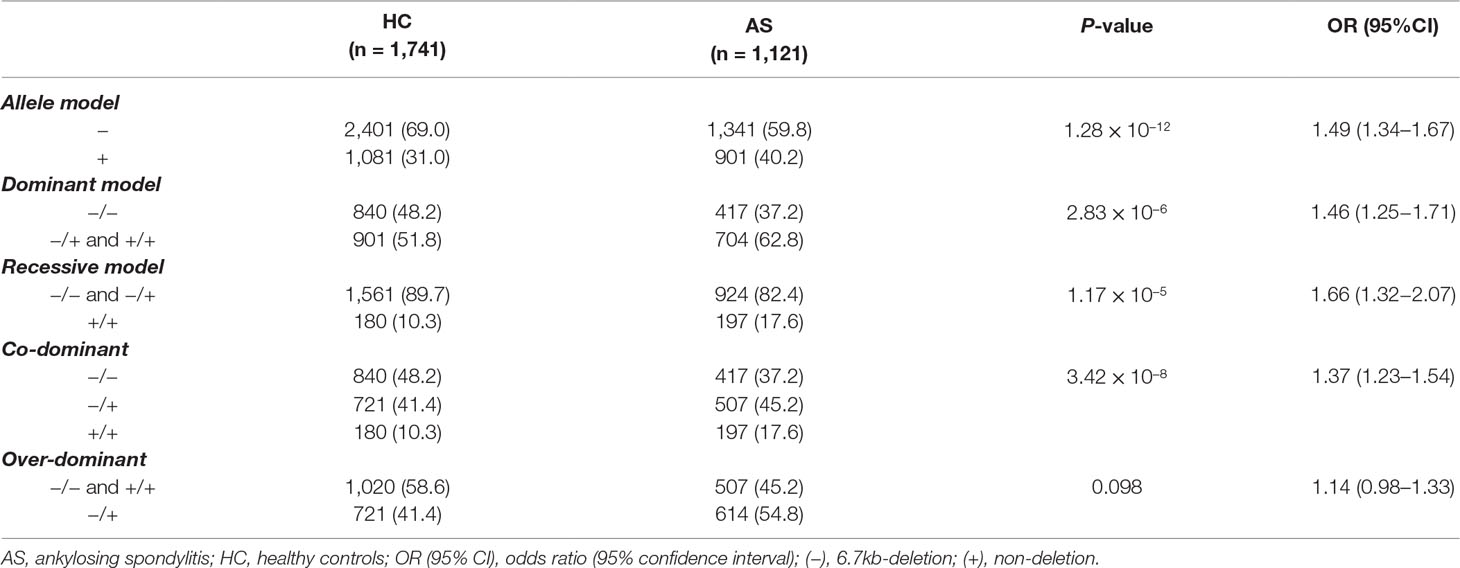
To investigate the possible genetic association between the functional LILRA3 and AS, we first assessed the impact of LILRA3 on AS susceptibility in whole study subjects. Interestingly, frequencies of the functional LILRA3 were remarkably increased in AS patients compared with healthy controls, either in allele model (40.2% vs. 31.0%, P = 1.28´10−12, OR = 1.49, Table 3) or in almost all the alternative genotypic models (e.g., recessive model [+/+ versus -/- and +/-]: 17.6% vs. 10.3%, P = 1.17´10−5, OR = 1.66; Figure 2 and Table 3). It seems that the functional LILRA3 is a strong genetic risk factor for AS susceptibility in Han Chinese, if the study subjects were not geographically stratified.
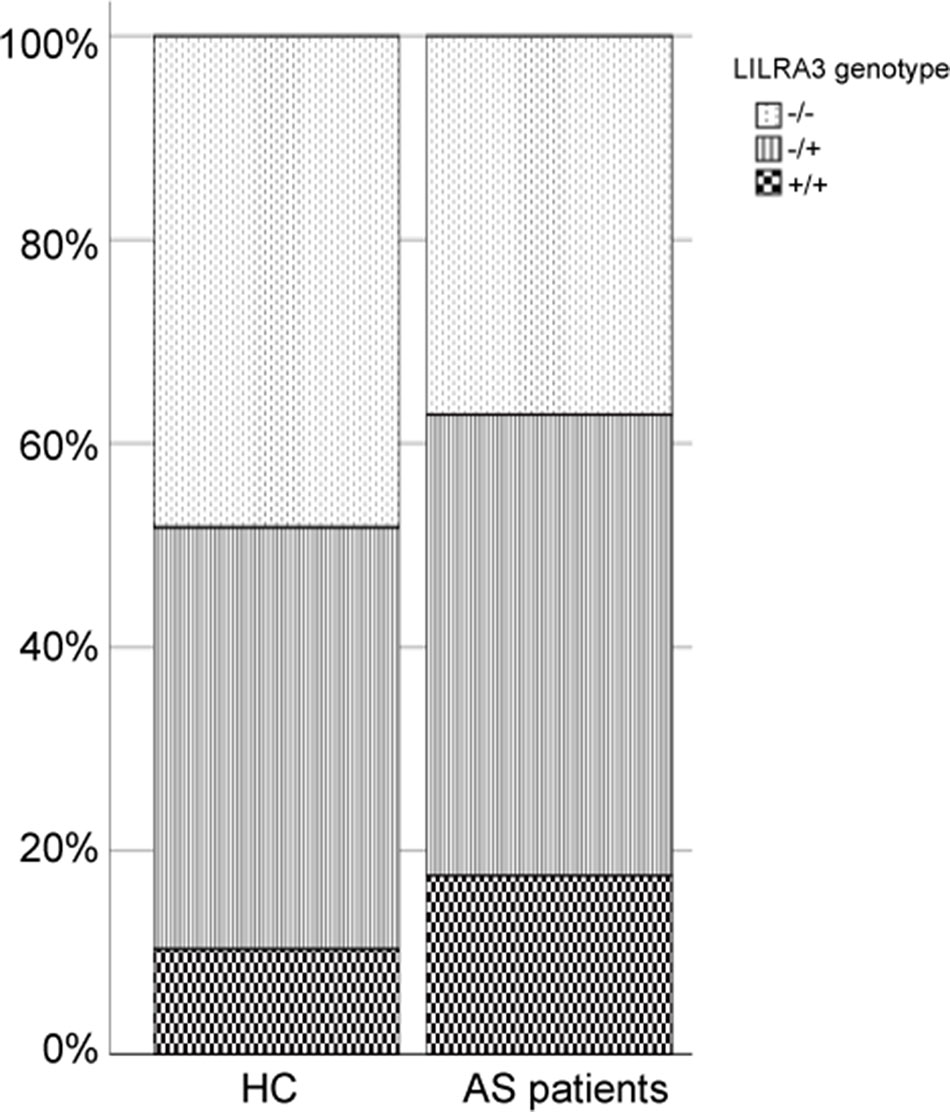
Figure 2 Comparison of the functional LILRA3 frequencies between healthy controls and AS patients in whole study subjects without geographic stratification.
Stratification Analysis Reveals That Functional LILRA3 Is Associated With AS Susceptibility Only in North Han, but Not in Central and South Hans
Previous studies have shown that the population stratification is a potential issue for genetic association studies and may confound results and cause spurious associations (Chen et al., 2009; Xu et al., 2009). As allele frequencies of LILRA3 were highly differentiated in our healthy cohort, we next stratified the cases and healthy individuals into subgroups corresponding to the three ranges of latitude. The three subgroups were then renamed as: (i) Northern Han (corresponding to latitude ≥35°), (ii) Central Han (corresponding to latitude 25–35°), and (iii) Southern Han (corresponding to latitude ≤ 25°). As shown in Figure 3A, frequencies of the functional LILRA3 (homozygous) were gradually increased in both healthy controls and AS patients from North to South. However, the associations between the functional LILRA3 and AS susceptibility were different among the three subgroups. In the Northern Han subgroup, the functional LILRA3 showed consistent association with AS susceptibility under almost all the alternative genetic models (allele model: P = 3.55 ´ 10−3, OR = 1.33; recessive model: P = 0.076, OR = 1.64; dominant model: P = 0.013, OR = 1.36; co-dominant model: 6.25 ´ 10−3, OR = 1.32; over-dominant model: 0.078, OR = 1.25), but not in Central and Southern Han subgroups (Figure 3B, Tables 4 and 5 and Supplementary Table 1). Our results indicate that the functional LILRA3 maybe a genetic risk for AS susceptibility in Northern Han subpopulation but not in Central and Southern Hans.
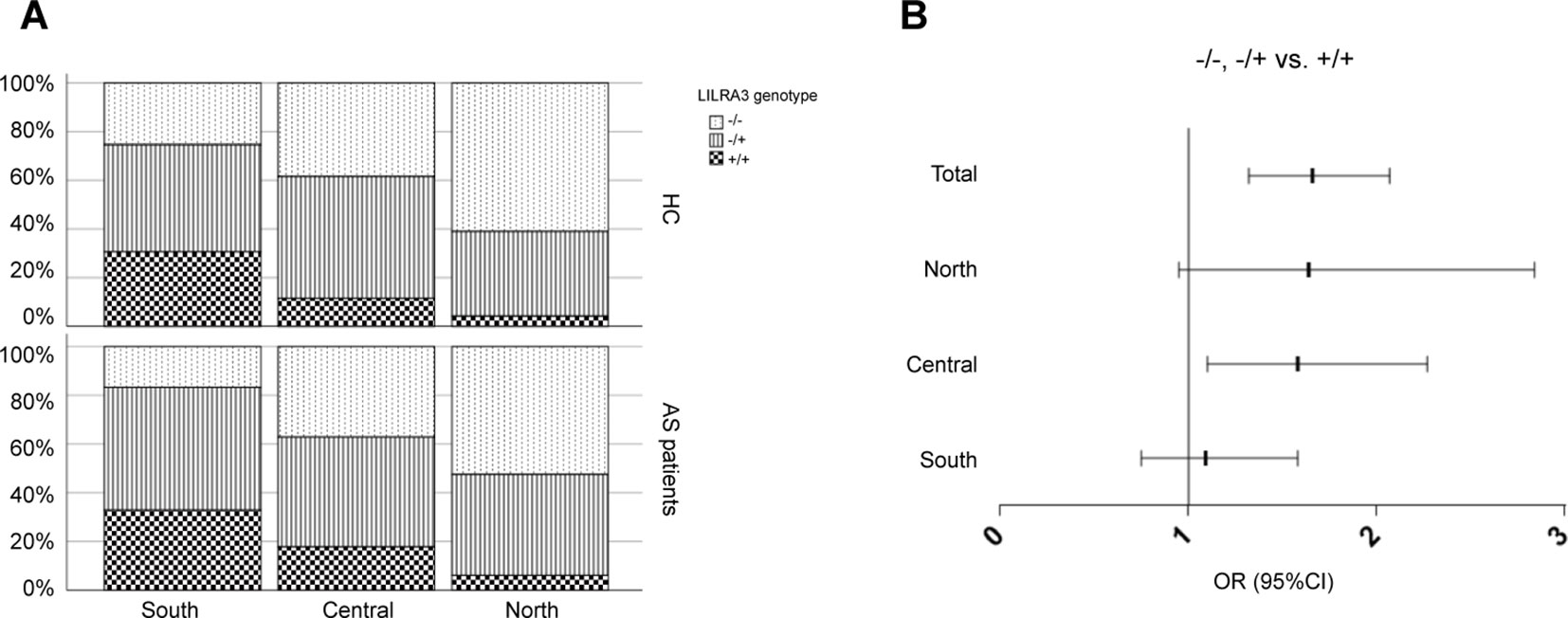
Figure 3 Comparison of the functional LILRA3 frequencies between healthy controls and AS patients in Han Chinese subpopulations. (A) Either of healthy controls or AS patients were stratified into three subgroups, i.e., Southern Han, Central Han, and Northern Han, corresponding to the latitude ≥ 35, 25–35, and ≤ 25°, respectively. Frequencies of the functional LILRA3 in each subgroup were compared between healthy controls and AS patients. (B) The forest plots of the association analysis between functional LILRA3 and AS susceptibility with or without geographic stratifications (recessive model).
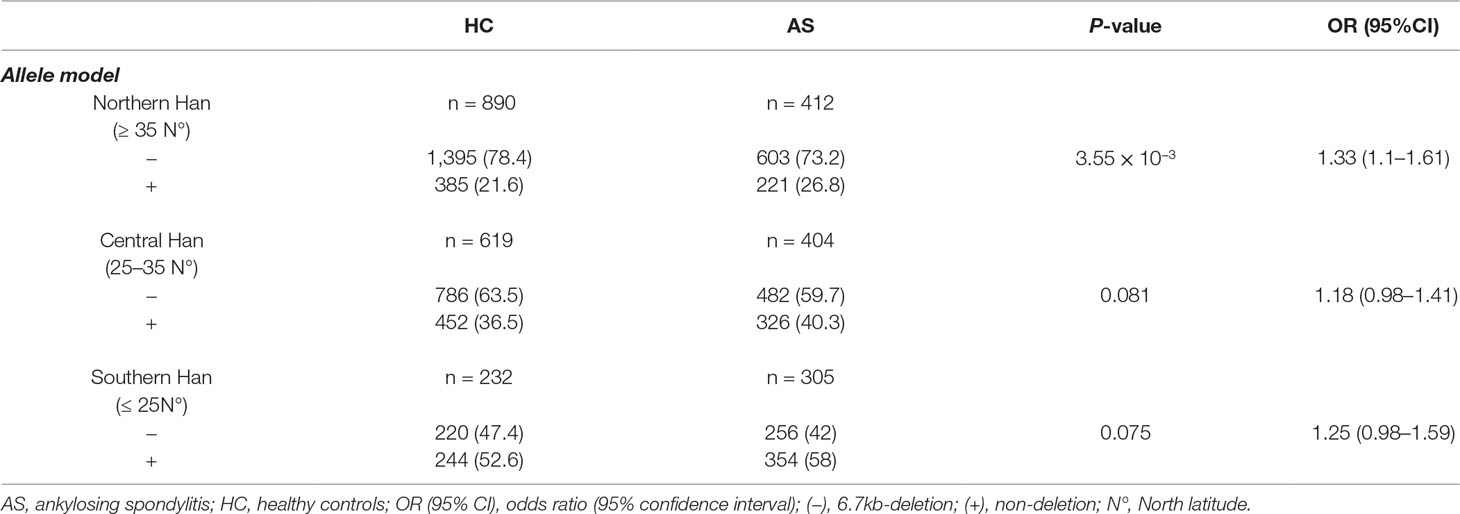
Table 4 Association analysis of LILRA3 with AS, according to the latitudes and adjusting for sex and age (allele model).
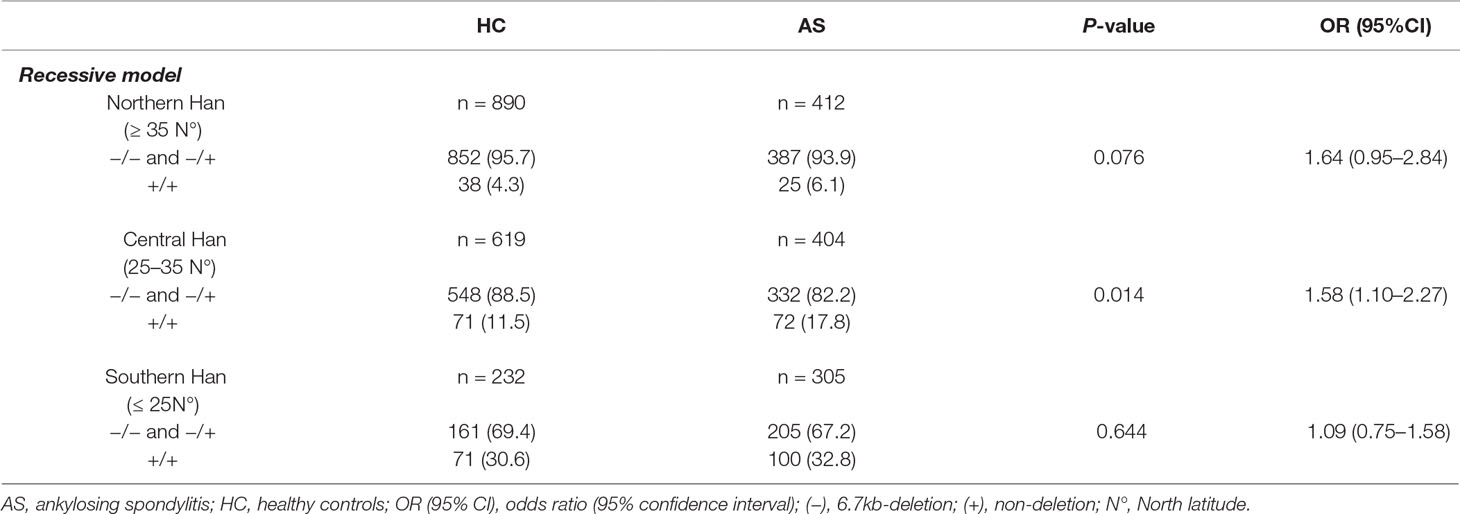
Table 5 Association analysis of LILRA3 with AS, according to the latitudes and adjusting for sex and age (recessive model).
Functional LILRA3 Confers an Increased Disease Activity in AS Patients
We next examined whether the functional LILRA3 had an impact on disease activity in AS patients. As CRP and ESR are the two biomarkers most commonly utilized for evaluating AS disease activity, we evaluated the impact of LILRA3 genotypes on serum levels of CRP and ESR in AS patients. As shown in Figure 4, the patients homozygous for the functional LILRA3 had a significant higher levels of CRP and ESR than the nonfunctional LILRA3 carriers (P < 0.0001 both for CRP and ESR, Figures 4A, B). Interestingly, we further observed that the BASDAI (a validated diagnostic test and gold standard for measuring disease activity in AS) was also significantly increased in AS patients homozygous for the functional LILRA3 (P = 0.003, Figure 4C). We also evaluated the impact of LILRA3 genotypes on serum Dkk-1, a molecule related to AS disease activity. As shown in Figure 4D, no significant differences were observed for serum levels of Dkk-1 between different LILRA3 genotypes (P = 0.764).
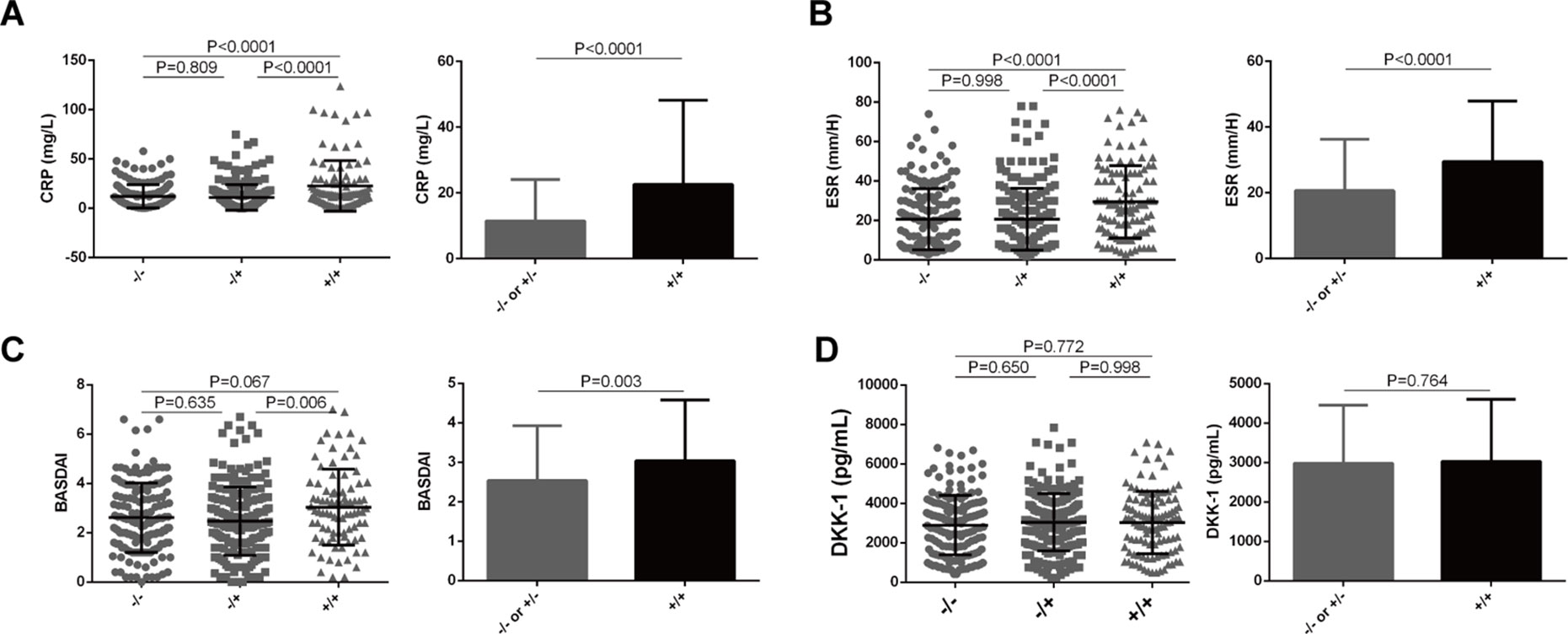
Figure 4 Association of LILRA3 genotypes with disease activity in AS patients. The AS patients homozygous for the functional LILRA3 had a significant higher levels of CRP, ESR, and BASDAI than nonfunctional LILRA3 carriers (A), (B), and (C), but not for Dkk-1 (D).
Discussion
Previous several studies have reported that frequencies of LILRA3 6.7-kb deletion vary widely across populations (Hirayasu et al., 2006; Hirayasu et al., 2008). Here, we demonstrate that allele frequencies of the 6.7-kb deletion also differ remarkably among the Han Chinese subpopulations across geographic regions, being the highest in Northeast China (80.6%) and the lowest in South China (47.4%), and positively correlated with the latitude. Conversely, frequencies of the functional LILRA3 were reversely correlated with the latitude, being the highest in South (52.6%) and the lowest in Northeast (19.4%). If the study subjects were not carefully geographically stratified, the functional LILRA3 seemed to be a strong susceptibility factor for AS. However, after stratifying the cases and healthy individuals according to the geographical regions, we find the functional LILRA3 is mainly associated with AS susceptibility in North and Han, but not in Central and South Hans.
Genetic differentiations among Han Chinese subpopulations have been reported previously. Xu et al. (2009) reported that Chinese Han population is complicatedly substructured, with the main clusters corresponding roughly to Northern Han, Central Han, and Southern Han. By simulated case-control analysis, the study showed that the genetic differentiations among these clusters were sufficient to lead spurious associations in GWAS, if Han population was not properly stratified. Thus, any association studies should be carefully explained in Han Chinese population, especially when sample sources are diverse. Chen et al. (2009) reported that the structure of Han population is onedimensional and clearly characterized by a continuous genetic gradient along the north-south geographical axis, rather than the east-west pattern. Interestingly, the study further showed that the Cantonese is the most differentiated subpopulation from the Northern Hans. Our data are consistent with these findings; that is, the allele frequencies of LILRA3 are mainly differentiated alongside the north-south gradient, being a much higher frequency in Southern Han. The mechanism for this gene selection within Han Chinese population is unknown. We speculate that the gene flow, environmental factors such as exposure to ultraviolet light, diet, life style, and immune systems suffering from different pressures might account for the differentiation between Han Chinese subpopulations.
Despite the well-established genetic association between LILRA3 and autoimmune diseases, the molecular function of LILRA3 remains undefined. However, LILRA3 is highly homologous to LILRB1 and LILRB2 in the extracellular domains, suggesting it may act as a soluble antagonist to these inhibitory receptors via shared ligands (Torkar et al., 2000; Burshtyn and Morcos, 2016). Previously, we and others have reported that the expression of LILRA3 was significantly increased in RA and SLE patients. LILRA3 had an impact on disease activity in RA and SLE (An et al., 2010; Du et al., 2015). Functional LILRA3 conferred a risk to disease severity in RA patients with early disease (Du et al., 2014). Serum LILRA3 was one of the strongest independent markers for disease severity in patients with MS (An et al., 2016). In present study, we also find the functional LILRA3 has an impact on disease activity in AS patients.
Dkk-1 is a key inhibitory molecule in the Wnt pathway and is critically important in bone homeostasis. Therefore, Dkk-1 may play an important role in AS pathogenesis (Heiland et al., 2012). Increased Dkk-1 levels have been linked to bone resorption, whereas decreased levels are linked to new bone formation (Li et al., 2006; MacDonald et al., 2007). However, there are inconsistent findings regarding the relationship between serum Dkk-1 levels and the occurrence of AS. For instance, several studies have reported that serum Dkk-1 levels were significantly increased in patients with AS compared with normal subjects or bone-related disease controls (Daoussis et al., 2010; Zhang et al., 2016). The inhibitory effect of Dkk-1 in sera from AS patients on Wnt pathway activation was negligible and may be functionally impaired (Daoussis et al., 2010). In present study, we didn’t find any differences between LILRA3 genotypes and Dkk-1 production.
One of limitations in current study is that the single-subpopulation study power was generally low due to the geographical stratification and the rare allele frequency of the functional LILRA3 in Northern Han subgroup. Thus, it may lead to an increased chance of type II errors, i.e., a false negative result. Additional studies with larger sample sizes are desired to confirm our findings.
In summary, the present study provides the first evidence that the frequencies of LILRA3 6.7-kb deletion vary widely among the Chinese Hans across geographic regions and positively correlated to the latitude. The functional LILRA3 is associated with AS susceptibility in North Han, but not in Central and South Han subpopulations. LILRA3 has an impact on disease activity in AS patients. These findings suggest LILRA3 is a common genetic risk for multiple autoimmune diseases and provide clues for further functional studies. Our study further highlights the importance of genetic differentiations among ethnicities, even within the subpopulations of an ethnic group.
Data Availability
This manuscript contains previously unpublished data. The name of the repository and accession number are not available.
Ethics Statement
This study was performed in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee, Peking University Shenzhen Hospital. All patients provided informed consent to participate in the study in accordance with the Declaration of Helsinki. The protocol was approved by the Medical Ethics Committee, Peking University Shenzhen Hospital.
Author Contributions
HW contributed to the collection of DNA samples and clinical data from SZH cohort, participated in genotyping, ELISA experiments, statistical analysis and manuscript drafting. YW contributed to the collection of DNA samples from PH cohort, participated in genotyping, data analysis and manuscript drafting. YT, HY and XZ participated in the collection and interpretation of clinical data from PH cohort. GZ, YC and JL participated in the collection and interpretation of clinical data from SZH cohort. ZL participated in the study design and revised the manuscript. JG contributed to the study design and data interpretation, supervision of the data analysis, manuscript drafting and revision. QW participated in the study design, data interpretation, and manuscript revision. All authors read and approved the final manuscript.
Funding
This work was supported in part by the National Natural Science Foundation of China (No. 31470875, No. 31670915, No. 31870913, No. 31711530023, No. 31530020, No.81871289, No. 81771678), Beijing Natural Science Foundation (No. 7162192), the Shenzhen Science and Technology Program for Basic Research (No. JCYJ20170307112009204), Traditional Chinese Medicine Bureau of Guangdong Province (No.20183011), and Sanming Project of Medicine in Shenzhen (No.SZSM201612009).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the staff from two Departments of Rheumatology and Immunology, Peking University Shenzhen Hospital and Peking University People’s Hospital for recruiting healthy control and AS patient and managing the DNA samples. We are also grateful for the consent and cooperation to participate in this study from all the healthy people and AS patient volunteers.
Abbreviations
AS, ankylosing spondylitis; LILRA3, leukocyte immunoglobulin-like receptor A3; MHC, major histocompatibility complex; GWAS, genome-wide association study; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; MS, multiple sclerosis; BASDAI, the Bath Ankylosing Spondylitis Disease Activity Index.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00869/full#supplementary-material
References
An, H., Chandra, V., Piraino, B., Borges, L., Geczy, C., McNeil, H. P., et al. (2010). Soluble LILRA3, a potential natural antiinflammatory protein, is increased in patients with rheumatoid arthritis and is tightly regulated by interleukin 10, tumor necrosis factor-alpha, and interferon-gamma. J. Rheumatol. 37, 1596–1606. doi: 10.3899/jrheum.091119
An, H., Lim, C., Guillemin, G. J., Vollmer-Conna, U., Rawlinson, W., Bryant, K., et al. (2016). Serum Leukocyte Immunoglobulin-like receptor A3 (LILRA3) is increased in patients with multiple sclerosis and is a strong independent indicator of disease severity; 6.7kbp LILRA3 gene deletion is not associated with diseases susceptibility. PLoS One 11, e0149200. doi: 10.1371/journal.pone.0149200
Arm, J. P., Nwankwo, C., Austen, K. F. (1997). Molecular identification of a novel family of human Ig superfamily members that possess immunoreceptor tyrosine-based inhibition motifs and homology to the mouse gp49B1 inhibitory receptor. J. Immunol. 159, 2342–2349.
Borges, L., Hsu, M. L., Fanger, N., Kubin, M., Cosman, D. (1997). A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J. Immunol. 159, 5192–5196.
Brown, M. A., Kenna, T., Wordsworth, B. P. (2016). Genetics of ankylosing spondylitis—insights into pathogenesis. Nat. Rev. Rheumatol. 12, 81–91. doi: 10.1038/nrrheum.2015.133
Burshtyn, D. N., Morcos, C. (2016). The expanding spectrum of ligands for leukocyte Ig-like receptors. J. Immunol. 196, 947–955. doi: 10.4049/jimmunol.1501937
Chen, J., Zheng, H., Bei, J. X., Sun, L., Jia, W. H., Li, T., et al. (2009). Genetic structure of the Han Chinese population revealed by genome-wide SNP variation. Am. J. Hum. Genet. 85, 775–785. doi: 10.1016/j.ajhg.2009.10.016
Colonna, M., Navarro, F., Bellon, T., Llano, M., Garcia, P., Samaridis, J., et al. (1997). A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 186, 1809–1818. doi: 10.1084/jem.186.11.1809
Cosman, D., Fanger, N., Borges, L., Kubin, M., Chin, W., Peterson, L., et al. (1997). A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity 7, 273–282. doi: 10.1016/S1074-7613(00)80529-4
Daoussis, D., Liossis, S. N., Solomou, E. E., Tsanaktsi, A., Bounia, K., Karampetsou, M., et al. (2010). Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 62, 150–158. doi: 10.1002/art.27231
Du, Y., Cui, Y., Liu, X., Hu, F., Yang, Y., Wu, X., et al. (2014). Contribution of functional LILRA3, but not nonfunctional LILRA3, to sex bias in susceptibility and severity of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 66, 822–830. doi: 10.1002/art.38308
Du, Y., Su, Y., He, J., Yang, Y., Shi, Y., Cui, Y., et al. (2015). Impact of the leucocyte immunoglobulin-like receptor A3 (LILRA3) on susceptibility and subphenotypes of systemic lupus erythematosus and Sjogren’s syndrome. Ann. Rheum. Dis. 74, 2070–2075. doi: 10.1136/annrheumdis-2013-204441
He, M. Z., Zeng, X., Zhang, K., Kinney, P. L. (2017). Fine particulate matter concentrations in Urban Chinese Cities, 2005-2016: a systematic review. Int. J. Environ. Res. Public Health 14, 191. doi: 10.3390/ijerph14020191
Heiland, G. R., Appel, H., Poddubnyy, D., Zwerina, J., Hueber, A., Haibel, H., et al. (2012). High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann. Rheum. Dis. 71, 572–574. doi: 10.1136/annrheumdis-2011-200216
Hirayasu, K., Ohashi, J., Kashiwase, K., Takanashi, M., Satake, M., Tokunaga, K., et al. (2006). Long-term persistence of both functional and non-functional alleles at the leukocyte immunoglobulin-like receptor A3 (LILRA3) locus suggests balancing selection. Hum. Genet. 119, 436–443. doi: 10.1007/s00439-006-0152-y
Hirayasu, K., Ohashi, J., Tanaka, H., Kashiwase, K., Ogawa, A., Takanashi, M., et al. (2008). Evidence for natural selection on leukocyte immunoglobulin-like receptors for HLA class I in Northeast Asians. Am. J. Hum. Genet. 82, 1075–1083. doi: 10.1016/j.ajhg.2008.03.012
Jones, D. C., Kosmoliaptsis, V., Apps, R., Lapaque, N., Smith, I., Kono, A., et al. (2011). HLA class I allelic sequence and conformation regulate leukocyte Ig-like receptor binding. J. Immunol. 186, 2990–2997. doi: 10.4049/jimmunol.1003078
Kabalak, G., Dobberstein, S. B., Matthias, T., Reuter, S., The, Y. H., Dorner, T., et al. (2009). Association of immunoglobulin-like transcript 6 deficiency with Sjogren’s syndrome. Arthritis Rheum. 60, 2923–2925. doi: 10.1002/art.24804
Kelley, J., Walter, L., Trowsdale, J. (2005). Comparative genomics of natural killer cell receptor gene clusters. PLoS Genet. 1, 129–139. doi: 10.1371/journal.pgen.0010027
Koch, S., Goedde, R., Nigmatova, V., Epplen, J. T., Muller, N., de Seze, J., et al. (2005). Association of multiple sclerosis with ILT6 deficiency. Genes Immun. 6, 445–447. doi: 10.1038/sj.gene.6364187
Li, Z., Brown, M. A. (2017). Progress of genome-wide association studies of ankylosing spondylitis. Clin. Transl. Immunol. 6, e163. doi: 10.1038/cti.2017.49
Li, J., Sarosi, I., Cattley, R. C., Pretorius, J., Asuncion, F., Grisanti, M., et al. (2006). Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39, 754–766. doi: 10.1016/j.bone.2006.03.017
MacDonald, B. T., Joiner, D. M., Oyserman, S. M., Sharma, P., Goldstein, S. A., He, X., et al. (2007). Bone mass is inversely proportional to Dkk1 levels in mice. Bone 41, 331–339. doi: 10.1016/j.bone.2007.05.009
Nakajima, H., Samaridis, J., Angman, L., Colonna, M. (1999). Human myeloid cells express an activating ILT receptor (ILT1) that associates with Fc receptor gamma-chain. J. Immunol. 162, 5–8.
Norman, P. J., Carey, B. S., Stephens, H. A. F., Vaughan, R. W. (2003). DNA sequence variation and molecular genotyping of natural killer leukocyte immunoglobulin-like receptor, LILRA3. Immunogenetics 55, 165–171. doi: 10.1007/s00251-003-0561-1
Ordonez, D., Sanchez, A. J., Martinez-Rodriguez, J. E., Cisneros, E., Ramil, E., Romo, N., et al. (2009). Multiple sclerosis associates with LILRA3 deletion in Spanish patients. Genes Immun. 10, 579–585. doi: 10.1038/gene.2009.34
Ortiz, M. A., Nunez, C., Ordonez, D., Alvarez-Cermeno, J. C., Martinez-Rodriguez, J. E., Sanchez, A. J., et al. (2015). Influence of the LILRA3 deletion on multiple sclerosis risk: original data and meta-analysis. PLoS One 10, e0134414. doi: 10.1371/journal.pone.0134414
Ranganathan, V., Gracey, E., Brown, M. A., Inman, R. D., Haroon, N. (2017). Pathogenesis of ankylosing spondylitis—recent advances and future directions. Nat. Rev. Rheumatol. 13, 359–367. doi: 10.1038/nrrheum.2017.56
Reveille, J. D. (2012). Genetics of spondyloarthritis—beyond the MHC. Nat. Rev. Rheumatol. 8, 296–304. doi: 10.1038/nrrheum.2012.41
Ryu, M., Chen, Y., Qi, J., Liu, J., Fan, Z., Nam, G., et al. (2011). LILRA3 binds both classical and non-classical HLA class I molecules but with reduced affinities compared to LILRB1/LILRB2: structural evidence. PLoS One 6, e19245. doi: 10.1371/journal.pone.0019245
Samaridis, J., Colonna, M. (1997). Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cells: structural evidence for new stimulatory and inhibitory pathways. Eur. J. Immunol. 27, 660–665. doi: 10.1002/eji.1830270313
Torkar, M., Haude, A., Milne, S., Beck, S., Trowsdale, J., Wilson, M. J. (2000). Arrangement of the ILT gene cluster: a common null allele of the ILT6 gene results from a 6.7-kbp deletion. Eur. J. Immunol. 30, 3655–3662. doi: 10.1002/1521-4141(200012)30:12<3655::AID-IMMU3655>3.0.CO;2-Y
van der Linden, S., Valkenburg, H. A., Cats, A. (1984). Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368. doi: 10.1002/art.1780270401
Wilson, M. J., Torkar, M., Haude, A., Milne, S., Jones, T., Sheer, D., et al. (2000). Plasticity in the organization and sequences of human KIR/ILT gene families. Proc. Natl. Acad. Sci. U. S. A. 97, 4778–4783. doi: 10.1073/pnas.080588597
Wisniewski, A., Wagner, M., Nowak, I., Bilinska, M., Pokryszko-Dragan, A., Jasek, M., et al. (2013). 6.7-kbp deletion in LILRA3 (ILT6) gene is associated with later onset of the multiple sclerosis in a Polish population. Hum. Immunol. 74, 353–357. doi: 10.1016/j.humimm.2012.12.006
Xu, J., Mo, Z., Ye, D., Wang, M., Liu, F., Jin, G., et al. (2012). Genome-wide association study in Chinese men identifies two new prostate cancer risk loci at 9q31.2 and 19q13.4. Nat. Genet. 44, 1231–1235. doi: 10.1038/ng.2424
Xu, S., Yin, X., Li, S., Jin, W., Lou, H., Yang, L., et al. (2009). Genomic dissection of population substructure of Han Chinese and its implication in association studies. Am. J. Hum. Genet. 85, 762–774. doi: 10.1016/j.ajhg.2009.10.015
Keywords: LILRA3, genetic differentiation, genetic susceptibility, ankylosing spondylitis, Han subpopulations
Citation: Wang H, Wang Y, Tang Y, Ye H, Zhang X, Zhou G, Lv J, Cai Y, Li Z, Guo J and Wang Q (2019) Frequencies of the LILRA3 6.7-kb Deletion Are Highly Differentiated Among Han Chinese Subpopulations and Involved in Ankylosing Spondylitis Predisposition. Front. Genet. 10:869. doi: 10.3389/fgene.2019.00869
Received: 15 February 2019; Accepted: 20 August 2019;
Published: 18 September 2019.
Edited by:
Zané Lombard, University of the Witwatersrand, South AfricaReviewed by:
Guanglin He, Sichuan University, ChinaJacqueline Michelle Frost, Mount Sinai Medical Center, United States
Copyright © 2019 Wang, Wang, Tang, Ye, Zhang, Zhou, Lv, Cai, Li, Guo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanguo Li, emdsaTk5QGFsaXl1bi5jb20=; Jianping Guo, amlhbnBpbmcuZ3VvQGJqbXUuZWR1LmNu; Qingwen Wang, d3F3X3N3QDE2My5jb20=
†These authors have contributed equally to this work
 Han Wang
Han Wang Yuxuan Wang2†
Yuxuan Wang2† Gengmin Zhou
Gengmin Zhou Jiyang Lv
Jiyang Lv Jianping Guo
Jianping Guo Qingwen Wang
Qingwen Wang