
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 12 April 2019
Sec. Genomic Assay Technology
Volume 10 - 2019 | https://doi.org/10.3389/fgene.2019.00309
This article is part of the Research Topic Transcript Profiling Using Single Molecule, Long-Read Sequencing Technologies View all 6 articles
 Simon A. Hardwick1,2,3†
Simon A. Hardwick1,2,3† Samuel D. Bassett1,2†
Samuel D. Bassett1,2† Dominik Kaczorowski1
Dominik Kaczorowski1 James Blackburn1,2
James Blackburn1,2 Kirston Barton1
Kirston Barton1 Nenad Bartonicek1,2
Nenad Bartonicek1,2 Shaun L. Carswell1
Shaun L. Carswell1 Hagen U. Tilgner3
Hagen U. Tilgner3 Clement Loy1,4
Clement Loy1,4 Glenda Halliday4
Glenda Halliday4 Tim R. Mercer1,2,5
Tim R. Mercer1,2,5 Martin A. Smith1,2*
Martin A. Smith1,2* John S. Mattick1,2,6*
John S. Mattick1,2,6*The human brain is one of the last frontiers of biomedical research. Genome-wide association studies (GWAS) have succeeded in identifying thousands of haplotype blocks associated with a range of neuropsychiatric traits, including disorders such as schizophrenia, Alzheimer’s and Parkinson’s disease. However, the majority of single nucleotide polymorphisms (SNPs) that mark these haplotype blocks fall within non-coding regions of the genome, hindering their functional validation. While some of these GWAS loci may contain cis-acting regulatory DNA elements such as enhancers, we hypothesized that many are also transcribed into non-coding RNAs that are missing from publicly available transcriptome annotations. Here, we use targeted RNA capture (‘RNA CaptureSeq’) in combination with nanopore long-read cDNA sequencing to transcriptionally profile 1,023 haplotype blocks across the genome containing non-coding GWAS SNPs associated with neuropsychiatric traits, using post-mortem human brain tissue from three neurologically healthy donors. We find that the majority (62%) of targeted haplotype blocks, including 13% of intergenic blocks, are transcribed into novel, multi-exonic RNAs, most of which are not yet recorded in GENCODE annotations. We validated our findings with short-read RNA-seq, providing orthogonal confirmation of novel splice junctions and enabling a quantitative assessment of the long-read assemblies. Many novel transcripts are supported by independent evidence of transcription including cap analysis of gene expression (CAGE) data and epigenetic marks, and some show signs of potential functional roles. We present these transcriptomes as a preliminary atlas of non-coding transcription in human brain that can be used to connect neurological phenotypes with gene expression.
Over the past decade, genome-wide association studies (GWAS) have facilitated a multitude of discoveries in human genetics, identifying variants associated with a number of complex phenotypes and diseases (Visscher et al., 2017; Visscher Peter, 2012). This includes many neuropsychiatric traits, such as schizophrenia, for which over 100 genetic risk loci have been discovered through GWAS studies to date (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). However, the journey from GWAS hit to biological function has proven challenging, for several reasons. Firstly, the sentinel SNP identified by GWAS is rarely the causal variant for the associated trait, but is instead a marker for a co-inherited genomic region known as the haplotype block (Bartonicek et al., 2017). The causal variant could lie anywhere within the haplotype block, which extends outward to all SNPs that are in linkage disequilibrium (LD) with the GWAS SNP. This is further complicated by pleiotropy, whereby one locus can influence multiple seemingly unrelated phenotypic traits. Second, even if GWAS accurately pinpoints a variant, the mechanism by which that variant is causally associated with the trait in question can be uncertain (Visscher et al., 2017). A third and related challenge is that the majority of SNPs identified by GWAS fall outside of protein-coding parts of the genome, which has hindered their functional characterization (Hindorff et al., 2009; Welter et al., 2013; Schierding et al., 2014; Xiao et al., 2017).
Several hypotheses exist to explain the preponderance of GWAS-identified SNPs in non-coding genomic regions. The most obvious is that these regions contain regulatory elements that have an influence on the trait in question. These may be cis-regulatory DNA sequences such as enhancers or silencers (Albert and Kruglyak, 2015). Alternatively, non-coding variants could affect chromatin looping, interfere with the binding of proteins or RNAs to DNA, or disrupt epigenetic marks (Edwards et al., 2013). These are all plausible, but another, not mutually exclusive, possibility is that these regions are transcribed into cis- and trans-acting non-coding (regulatory) RNAs, many of which may not yet be documented in public databases. Indeed, recent studies have indicated that human transcriptome annotations are far from complete, particularly in relation to long non-coding RNAs (lncRNAs) (Cao et al., 2018; Uszczynska-Ratajczak et al., 2018).
lncRNAs are a diverse class of gene products that qualitatively constitute the major portion of the mammalian transcriptome, but which are still poorly cataloged and characterized (Derrien et al., 2012). The number of annotated lncRNAs has grown enormously in recent years, with almost 10,000 new lncRNA loci being added to GENCODE since 2009 (Uszczynska-Ratajczak et al., 2018), and many more likely to exist (Carninci et al., 2005; Katayama et al., 2005; Mercer et al., 2012; Deveson et al., 2018). While only a small subset of annotated lncRNAs have been functionally characterized (e.g., XIST, NEAT1, HOTAIR), many have been shown to play unexpectedly diverse roles in epigenetic signaling and gene regulation including genetic imprinting, shaping chromosome conformation, forming subcellular organelles (Bond and Fox, 2009; Lin et al., 2015), allosterically regulating enzymatic activity, and acting as scaffolds, guides, decoys or signals (Mercer and Mattick, 2013; Quinn and Chang, 2015). On average, lncRNAs are less abundant than protein-coding mRNAs, which can cause them to be missed (or dismissed as noise) by traditional RNA-seq assays (Clark et al., 2015). This is due to the expression-dependent bias of RNA-seq, in which lowly expressed transcripts are less frequently sampled (Jiang et al., 2011; Hardwick et al., 2016). However, accumulating evidence shows that lncRNAs are not simply weakly expressed but rather are precisely expressed in highly specific patterns (Mercer et al., 2008; Deveson et al., 2017). For example, approximately 40% of known lncRNAs are exclusively expressed in brain (Briggs et al., 2015).
In order to address this problem, a technique known as RNA CaptureSeq has recently been developed. RNA CaptureSeq works by targeting specific genomic regions of interest for capture using oligonucleotide probes as baits, providing enhanced sequencing coverage of those regions (Mercer et al., 2012, 2014; Clark et al., 2015). RNA CaptureSeq has facilitated the identification of novel transcript isoforms within even well-studied loci such as TP53 (Mercer et al., 2012), has provided the first genome-wide map of human splicing branchpoints (Mercer et al., 2015), and has proven particularly useful for the detection and quantification of lncRNAs and their many isoforms (Clark et al., 2015; Bussotti et al., 2016; Lagarde et al., 2017; Deveson et al., 2018). The increased sensitivity and resolution of RNA CaptureSeq make it a logical choice for the profiling of unannotated transcripts arising from non-coding GWAS haplotype blocks. To date, CaptureSeq has typically been performed using short-read RNA-seq, which relies on computational assembly of reads (∼100 bp) into transcript models. This process is notoriously difficult and error-prone, and does not provide certainty around isoform structures (Steijger et al., 2013). In contrast, long-read sequencing can sequence full-length transcripts ‘in one go,’ and is able to resolve splicing events between distant exons (Tilgner et al., 2015). However, long-read sequencing typically suffers from much lower throughput than short-read RNA-seq, and thus has almost exclusively been limited to profiling highly expressed, protein-coding genes.
Here, we have used RNA CaptureSeq in conjunction with Oxford Nanopore Technologies (ONT) long-read cDNA sequencing in order to achieve an unprecedented level of sensitivity and resolution. While two recent studies have coupled CaptureSeq with Pacific Biosciences (PacBio) sequencing (Lagarde et al., 2017; Deveson et al., 2018), the present study represents the first use of CaptureSeq in conjunction with ONT sequencing to profile brain tissue. To investigate the transcriptional landscape of GWAS-identified genomic loci in human brain, we performed RNA CaptureSeq targeting 1,023 discrete haplotype blocks containing 1,352 non-coding GWAS SNPs associated with neuropsychiatric phenotypes. Transcripts were captured and sequenced from four regions of post-mortem brain tissue from three neurologically healthy donors. CaptureSeq was independently performed with both long-read and short-read RNA-seq, which provided orthogonal validation of our results. We used our recently developed set of spliced RNA spike-ins (‘sequins’) (Hardwick et al., 2016) as internal controls to assess the efficiency of RNA capture, and also to benchmark the performance of ONT’s new PromethION instrument. We find that the majority (62.4%) of targeted haplotype blocks contain novel, multi-exonic transcripts, including 13% of targeted intergenic blocks. We uncover a wealth of unannotated transcripts, many of which are supported by independent evidence of transcription and show signs of potential functional roles. We present these transcriptomes as the foundation of an atlas of non-coding transcription in human brain that can be used to connect neuropsychiatric phenotypes with gene expression.
Using the NHGRI GWAS database (Welter et al., 2013; Macarthur et al., 2017), we identified 1,023 discrete haplotype blocks across the genome containing 1,352 non-coding (intronic and intergenic) SNPs associated with neuropsychiatric phenotypes (see Materials and Methods). These phenotypes include behavioral traits, predisposition to addiction, mental illness and neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease (see Supplementary Table 1). We designed tiling oligonucleotide probes targeting the selected GWAS-defined haplotype blocks, with annotated protein-coding exons (GENCODE v24, Harrow et al., 2012) and repeat elements (RepeatMasker) omitted (Figure 1). This resulted in a target territory of 96.2 Mb (∼3% of hg38). Of the 1,023 blocks targeted, 162 were intergenic (i.e., had no overlap on either strand with any GENCODE transcript). We employed targeted RNA capture (‘RNA CaptureSeq’) (Mercer et al., 2014) on RNA samples extracted from post-mortem brain tissue obtained from three neurologically healthy middle-aged males of European ancestry (see Materials and Methods). Brains were dissected into four different regions: caudate, prefrontal cortex (PFC), primary visual cortex (VCx) and superior colliculus (SupCol). Samples were spiked with RNA sequins (Hardwick et al., 2016) (Mix A), a subset of which were also targeted for capture (25/78 genes; 49/164 isoforms) as part of our experimental design.
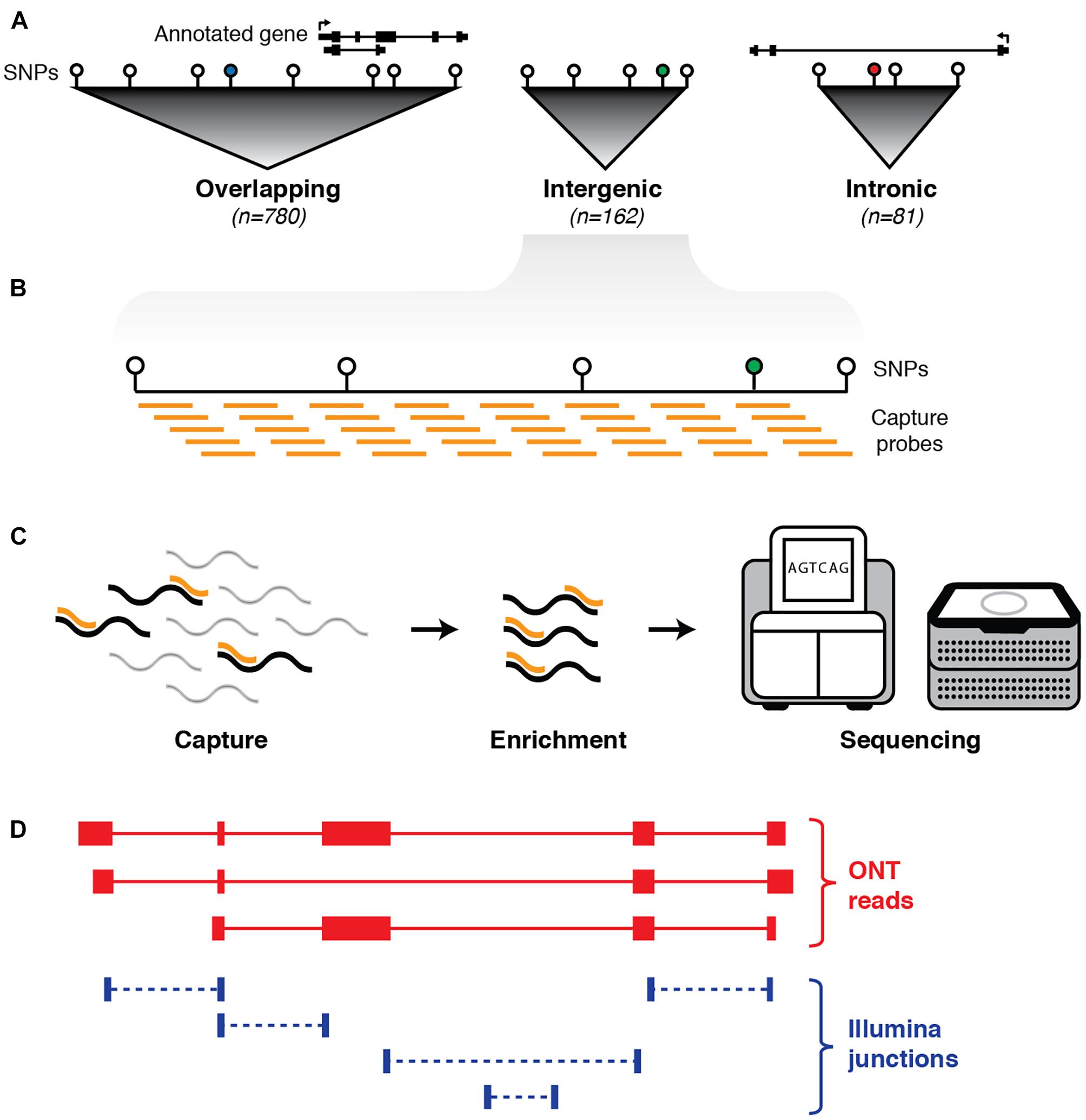
Figure 1. Schematic outline of experimental design. (A) First, haplotype blocks were predicted around 1,352 non-coding GWAS SNPs (colored circles) associated with neurological phenotypes. Blocks were defined by identifying all SNPs in linkage disequilibrium (LD) with the GWAS SNP (white circles). Of the 1,023 blocks, 780 overlap an annotated exon (GENCODE v24), 81 are located entirely within an annotated intron, and 162 are intergenic. (B) Biotinylated oligonucleotide probes (orange bars) were designed to tile across haplotype blocks (with annotated protein-coding exons and repeat elements omitted). (C) Probes are used as baits to capture any transcripts generated from targeted regions, followed by pull-down enrichment and subsequent transcriptional profiling with both long- and short-read RNA sequencing. (D) Sequence reads are aligned to the genome (hg38) and a hybrid transcriptome is assembled by leveraging the advantages of both long- and short-read RNA-seq.
RNA CaptureSeq was performed as previously described (Mercer et al., 2014), with some minor modifications for the ONT libraries (see Materials and Methods). Following capture, we carried out single-molecule, long-read cDNA sequencing using ONT’s PromethION platform. This yielded a total of 62,553,766 base-called reads (58.7 Gbp of sequence) with a mean length of 1,035 nt and a median quality score of 8.9 (Supplementary Figure 1). Reads were aligned using minimap2 (Li, 2018) to a combined index comprising the human genome (hg38) and in silico chromosome (chrIS) (Hardwick et al., 2016). Overall, we observed an alignment rate of 82.1%, with 56.9% of reads aligning to hg38 and 25.1% to chrIS (Supplementary Figure 2a). We retained only reads with a perfect mapping score (mapQ = 60). Of 22,967,553 reads that aligned to hg38 (mapQ = 60), 13,187,936 mapped to regions targeted for capture, corresponding to an average on-target rate of 57.4% (Supplementary Figure 2c). In parallel, we sequenced the same four brain cDNA samples using Illumina’s HiSeq 2500 instrument, yielding a total of 299,140,569 read pairs. Reads were aligned using STAR (Dobin et al., 2013) to the hg38 + chrIS reference. We observed an overall alignment rate of 78.4%, with 64.1% of reads aligning to hg38 and 14.3% to chrIS (Supplementary Figure 2b). For the Illumina samples, we observed an average on-target rate of 83.4% (Supplementary Figure 2c). By analyzing reads aligned to the chrIS reference sequence, we calculated an overall error rate for the PromethION of 10.8733% (mismatch rate 5.11%; indel rate 5.7633%) (Supplementary Figure 2d). This was ∼50-fold higher than the rate observed for Illumina reads, which had an overall error rate of 0.2341% (mismatch 0.2241%; indel 0.01%).
We used RNA sequins to assess the efficiency of RNA capture and the quantitative accuracy of ONT sequencing using the PromethION device. We carried out isoform-level quantification using Salmon (Patro et al., 2017), finding a strong correlation between measured abundance (transcripts per million; TPM) and input concentration for captured (R2 = 0.851, slope = 0.822) and non-captured (R2 = 0.864, slope = 1.07) sequins alike (Figure 2A). By calculating the average difference between captured and non-captured sequins at each matched concentration point, we observed a ∼230-fold enrichment of CaptureSeq (Figure 2A). At higher concentrations, we observed diminishing capture efficiency, which corresponds to saturation of capture probes but is unlikely to affect transcripts within the physiological range of gene expression (Mercer et al., 2014; Clark et al., 2015). While we successfully detected all captured sequins, we failed to detect the 10 sequin isoforms of lowest input concentration that were not targeted for capture, equating to a lower limit of detection (LoD) of 0.059 attomoles/μL. Further, to assess the variation of expression measurements at different concentrations, we plotted the coefficient of variation (CV; SD divided by mean) for each sequin against its input concentration, observing that CV declined with increasing concentration for both captured and non-captured standards (Figure 2B). This illustrates the expression dependent bias of RNA-seq, whereby lowly expressed transcripts are less accurately quantified. We also quantified sequins at the gene-level (reads per gene per 10k reads; RPG10K, Byrne et al., 2017), observing similar results (Supplementary Figure 3).
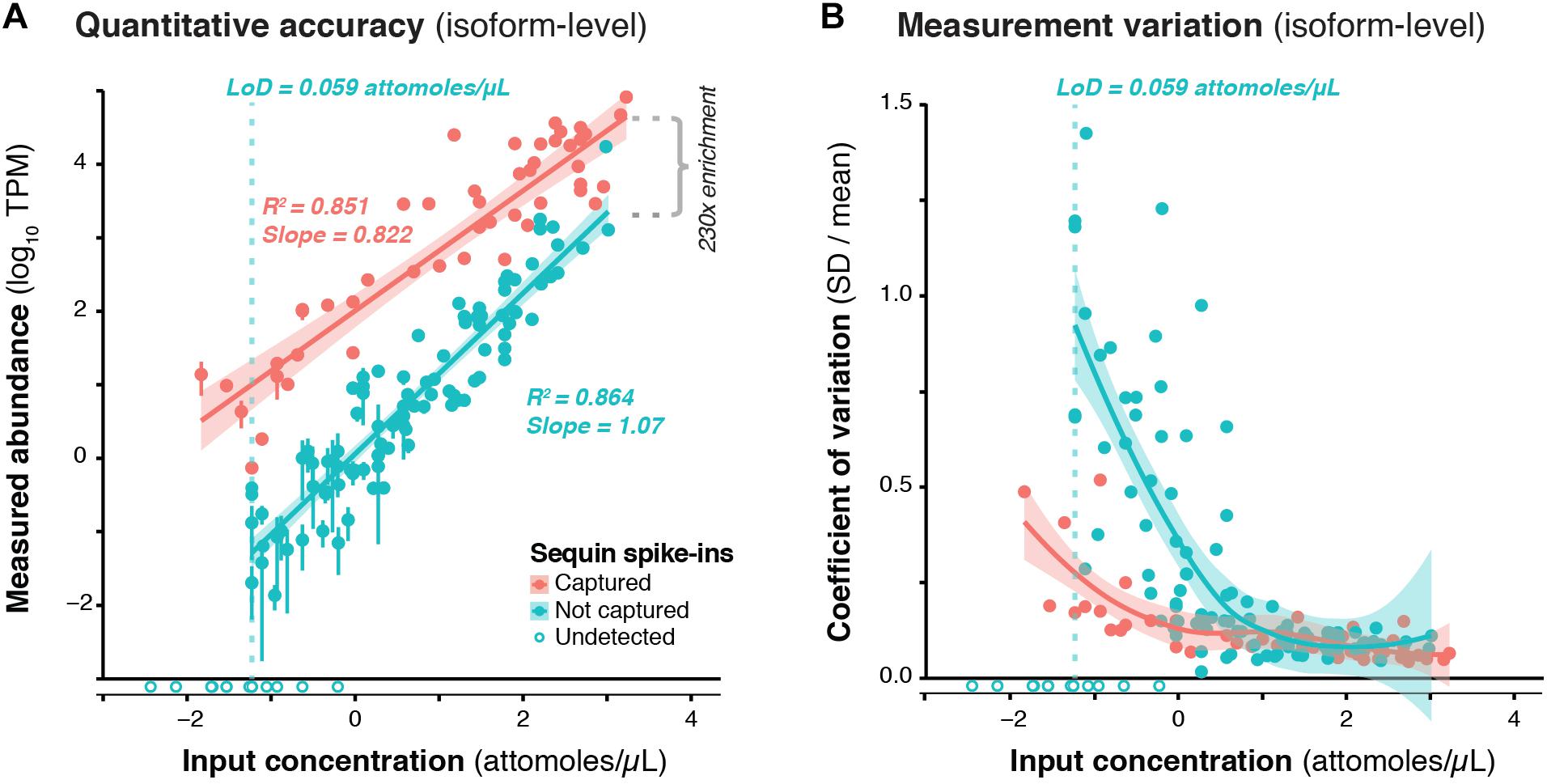
Figure 2. Validation of CaptureSeq design and ONT sequencing. (A) A subset of RNA sequins were targeted for capture as part of our CaptureSeq design (n = 25/78 genes; 49/164 isoforms). By plotting the measured abundance (TPM; y-axis) against the known input concentration (x-axis) for captured (red) and non-captured (green) sequin isoforms, we can compare the quantitative accuracy of captured vs. non-captured transcripts. Open circles indicate sequin isoforms that were not detected. Vertical dotted green line indicates the limit of detection (LoD) for non-captured transcripts. By comparing the difference between the measured abundance of captured and non-captured transcripts, we observe a ∼230-fold enrichment of CaptureSeq. Error bars represent standard deviation (SD) between the four replicate ONT samples. (B) Scatter plot shows the coefficient of variation (SD divided by mean) of each spike-in plotted against its respective input concentration, indicating the expression dependent bias of RNA-seq.
Our spliced ONT reads aligned to hg38 (mapQ = 60) contained a total of 939,558 splice junctions. In comparison, we detected a total of 234,138 uniquely mapped junctions in the matched Illumina data. We found that 164,183 of these junctions were shared between the two technologies; that is, 17.5% of junctions detected using ONT were validated by Illumina, and 70.1% of junctions detected by Illumina were validated by ONT (Supplementary Figure 4a). Of the ‘shared’ junctions, 144,227 (87.8%) were previously annotated in GENCODE (v29), while the remaining 19,956 junctions were novel (12.2%) (Supplementary Figure 4b). ONT detected an additional 32,777 GENCODE junctions that Illumina missed, while Illumina detected an additional 18,860 GENCODE junctions that ONT missed (Supplementary Figure 4c). Of the 19,956 ‘shared novel’ junctions, we found that 2,036 (10.2%) were validated by two recent studies which coupled RNA CaptureSeq with Pacific Biosciences (PacBio) long-read sequencing (Lagarde et al., 2017; Deveson et al., 2018), providing independent evidence for their credibility.
We next used RNA sequins to assess the performance of the PromethION instrument in accurately sequencing full-length transcripts. In contrast to the ERCC spike-ins, sequins have a more realistic range of transcript sizes, with 15 isoforms ≥ 2.5 kb in length. Of these 15, only six were fully sequenced ‘in one go’ with a single ONT read. The longest sequin isoform that was fully sequenced with single reads was R2_26_1, with a 4,375 nt read that covered all 18 of its exons (Supplementary Figure 5a). The longest read that mapped to chrIS was 5,213 nt, which represented the majority of the R2_19_2 isoform (6.9 kb in total). Another notable example was a 3,473 nt read which mapped to R1_24_1 (4.6 kb in total), spanning 33 out of 36 exons. Most synthetic isoforms < 2.5 kb were fully sequenced with single ONT reads, allowing complex, alternatively spliced synthetic loci to be unambiguously deconvoluted (Supplementary Figure 5b). We observed a similar distribution of mapped reads lengths aligning to chrIS (mean = 762 nt) and hg38 (mean = 937 nt) (Supplementary Figure 2e).
We used the gffcompare tool (Pertea et al., 2016) to assess the sensitivity and precision of our synthetic transcriptome in relation to the chrIS annotation, at the base-, exon-, intron-, transcript- and gene-levels. When considering the raw read alignments, we observed high sensitivity across these features, but large numbers of false-positive events led to poor precision scores (Supplementary Figure 6a). This is due to the higher error rate of ONT sequencing, which leads to spurious alignments around the boundaries of exon junctions (Supplementary Figure 5c). We compared the number of reads spanning annotated chrIS junctions (true-positives) and unannotated chrIS junctions (false-positives), finding that ONT performed poorly in comparison to Illumina (Supplementary Figure 6b). We used the ‘Pinfish’ suite of tools1 to cluster together ONT reads (mapQ = 60) having similar exon/intron structures, and then create a consensus of the clusters by calculating the median of exon boundaries from all transcripts in the cluster (see Materials and Methods). After clustering, we observed marked improvements in precision with only minor drops in sensitivity, validating that the approach was effective in processing raw ONT reads (Supplementary Figure 6c). After benchmarking the ‘Pinfish’ clustering approach using RNA sequins, we went on to apply this to ONT reads mapping to hg38 (mapQ = 60). This generated a set of 10,528 consensus multi-exonic transcripts that overlapped targeted regions, comprising 12,369 unique splice junctions. We found that the rate of ONT junction validation by Illumina data increased from only 17.5% before clustering to 83.0% after clustering (Supplementary Figure 6d).
Using sequins, we observed that ONT sequencing had a slight length bias, with synthetic transcripts at around the ∼1 kb size over-represented and transcripts at either end of the size spectrum under-represented. This effect was seen for both captured (Supplementary Figure 7a) and non-captured (Supplementary Figure 7c) sequins, suggesting that the length bias was caused by ONT sequencing rather than by the process of capture. Similarly, we observed a slight GC content bias for captured sequins (Supplementary Figure 7b), but this effect largely disappeared for non-captured sequins (Supplementary Figure 7d), implying that GC bias arose due to the process of capture rather than ONT sequencing. In most cases, ONT sequencing had more uniform coverage of exons compared to Illumina (Supplementary Figures 8a,b). With Illumina sequencing, many exons exhibited highly uneven coverage profiles that were reproducible between replicates (Supplementary Figures 8a,b). We calculated the coverage of each base in every sequin exon targeted for capture (n = 279), finding that there was significantly less variation between bases for ONT (median CV = 0.0339) than Illumina sequencing (median CV = 0.172) (Supplementary Figure 8c). This difference was statistically significant (paired t-test; p-val < 0.0001).
Long-read sequencing provides accurate full-length isoform structures, but suffers from a relatively high sequencing error rate that leads to spurious splice junction detection (Tang et al., 2018). Conversely, short-read sequencing relies on the error-prone computational reconstruction of short reads into transcript models, but has highly accurate splice junction detection (Steijger et al., 2013). We sought to leverage the advantages of both technologies by defining a hybrid transcriptome that incorporates ONT reads with splice junctions corrected using matched Illumina short-read data. To do so we used the FLAIR tool (Tang et al., 2018), which corrects misaligned ONT splice junctions using genome annotations and accompanying Illumina junctions (see Materials and Methods). Of 3,586,593 spliced ONT reads that mapped to hg38, we were able to successfully correct 2,422,358 of these (67.5%) using GENCODE annotations (v29) and our matched Illumina splice junction data. These corrected reads were then collapsed into a non-redundant transcriptome.
Next, we developed a comprehensive pipeline to retain only high-confidence transcripts that overlapped targeted haplotype blocks (see Materials and Methods). We filtered out all transcripts that met any of the following criteria: (i) no overlap with any capture probe, (ii) contained < 3 exons; (iii) contained an intron > 1 Mb, (iv) contained a non-canonical splice junction (i.e., not GT-AG, GC-AG or AT-AC); (v) had any of the following gffcompare (Pertea et al., 2016) classification codes: ‘e’, ‘p’, ‘r’, ‘s’; (vi) was < 200 nt in length; or (vii) was redundant or contained wholly within another transcript. These filtering steps produced a set of 22,114 multi-exonic transcripts overlapping GWAS haplotype blocks associated with neuropsychiatric functions. These transcripts collectively comprised 27,181 unique introns (splice junctions) and 19,288 discrete internal exons (Figure 3A). This represents a comparable number of features as are contained in the GENCODE (v29) annotation across the genomic regions we targeted for capture (Figure 3B). While our transcriptome was less comprehensive than the MiTranscriptome annotation (v2) (Iyer et al., 2015), the latter was generated by merging ∼6,500 independent RNA-seq datasets into a consensus set, while our dataset was generated in just two experiments using four brain samples.
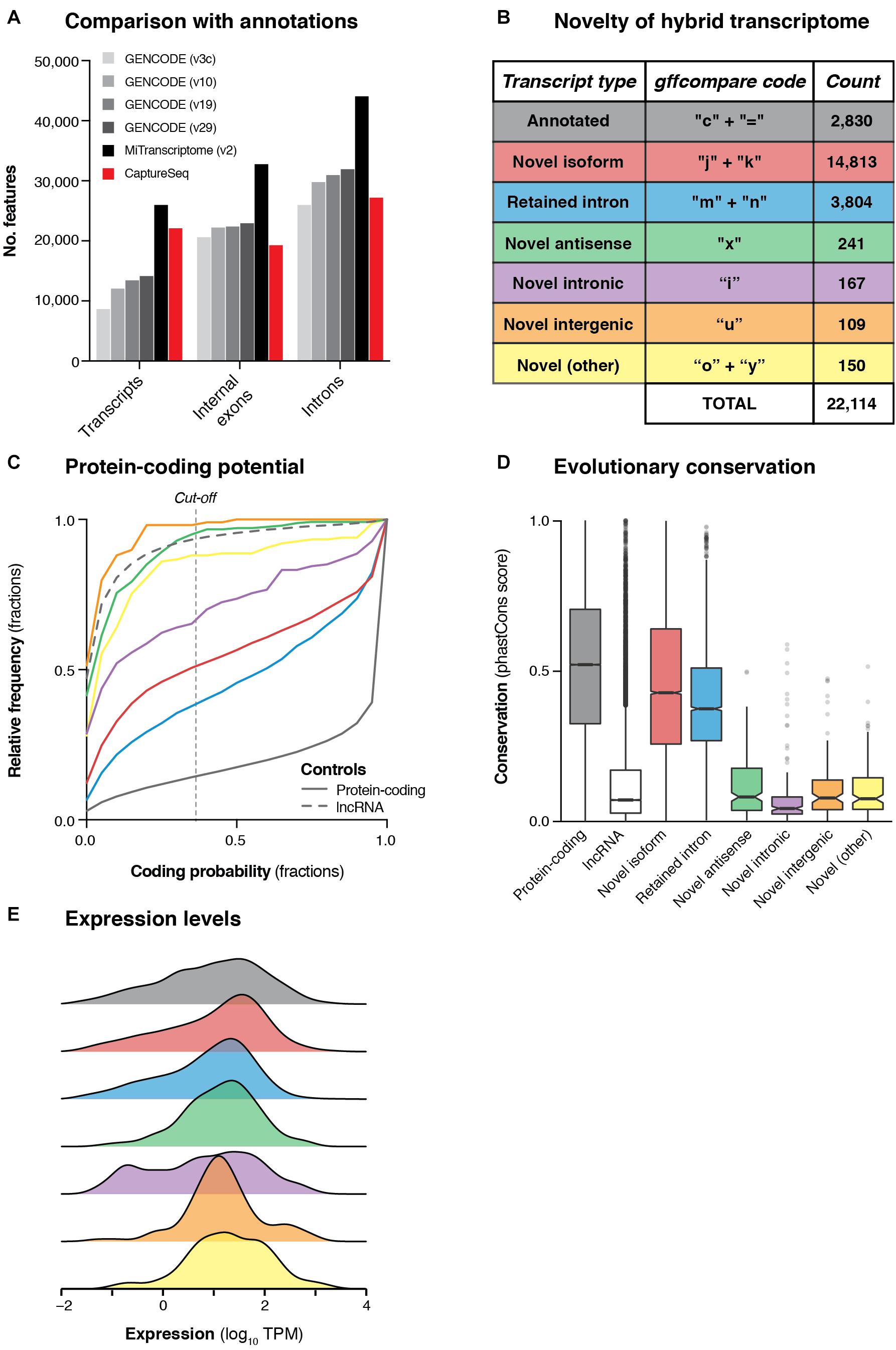
Figure 3. Transcriptional landscape of haplotype blocks associated with neuropsychiatric functions. (A) Bar charts show the number of transcripts, introns and internal exons contained in our filtered hybrid transcriptome (red) compared to existing annotations (four versions of GENCODE and MiTranscriptome v2). Only multi-exonic transcripts that overlap with targeted haplotype blocks are considered in this analysis. (B) Table shows the classification of transcripts in our hybrid transcriptome in relation to the latest GENCODE annotation (v29). (C) Cumulative frequency histograms show the coding potential of our transcripts, as assessed by CPAT. Colors refer to the categories defined in part (B). Vertical dotted line indicates the commonly used cut-off for human transcripts (0.364). GENCODE (v29) annotated lncRNAs (gray dotted line) and protein-coding genes (gray solid line) are also plotted for reference. (D) Box plots show the distribution of phastCons scores (Siepel et al., 2005) (vertebrate 100-way alignment) of our transcripts, colored by type. Box edges indicate lower and upper quartiles, center lines indicate median, notches indicate the 95% confidence interval around the median. (E) Density plots show the mean expression (log10 TPM) of transcripts across all four samples, as measured by Illumina short-read sequencing.
We compared our hybrid transcriptome to the GENCODE (v29) annotation using gffcompare (Pertea et al., 2016). Only 2,830 transcripts (12.8%) were exact matches of (or contained within) GENCODE transcripts, while the remainder were categorized as putative novel transcripts (Figure 3B). Most of the novel transcripts represented unannotated splice isoforms of known genes, but we also detected 241 novel antisense transcripts (no GENCODE overlap on the same strand) and 109 novel intergenic transcripts (no GENCODE overlap on either strand) (Figure 3B). We assessed the protein-coding capacity (Figure 3C) and evolutionary conservation (Figure 3D) of our transcriptome, finding that the novel antisense and novel intergenic transcripts closely resembled annotated lncRNAs. Overall, we found that 638/1,023 (62.4%) targeted haplotype blocks contained at least one novel transcript, including 21/162 (13.0%) targeted intergenic blocks. We used the matched short-read RNA-seq data to quantify the expression of our hybrid transcriptome (Figure 3E). We also assessed the concordance between ONT and Illumina quantitative expression measurements for our transcriptome. Using Salmon (Patro et al., 2017) with our hybrid transcriptome as a reference, we quantified the expression of transcripts in each sample individually, observing reasonably strong concordance between the two orthogonal technologies [Spearman correlation coefficient (ρ) for SupCol: 0.612; PFC: 0.616; VCx: 0.650; caudate: 0.615] (Supplementary Figure 9).
The combined resolution of CaptureSeq with ONT long-read sequencing enabled us to identify 109 novel intergenic transcripts overlapping GWAS SNPs associated with neuropsychiatric traits. Only two of these transcripts were predicted to have protein-coding potential; the remaining 107 are therefore classed as putative lncRNAs (Figure 3C). We investigated whether any of the novel intergenic transcripts were supported by independent signatures of transcription, such as cap analysis of gene expression (CAGE) peaks or epigenetic marks. We found that 21 transcripts (19.3%) had an annotated FANTOM CAGE robust peak (Forrest et al., 2014) in the vicinity of their TSS (within 500 bp on the same strand) (Figure 4A). This represented a significant enrichment (odds ratio = 1.95) compared to randomized regions, but did not reach the enrichment of CAGE peaks in the TSSs of annotated lncRNAs (odds ratio = 2.65) or protein-coding transcripts (odds ratio = 4.55) (Figure 4B). Notably, of our intergenic transcripts that were not supported by CAGE peaks, 8/88 (9.1%) were independently validated by a recent study that coupled CaptureSeq with PacBio long-read sequencing(Lagarde et al., 2017), thus providing orthogonal evidence for their veracity (Supplementary Figure 10). In several of these examples, our hybrid assembly expanded upon the PacBio assembly, identifying additional exons and splicing events. Further, the three epigenetic marks that are typically associated with actively transcribed promoters (H3K4me1, H3K4me3, and H3K27ac) were present near the TSS of 80 (73.4%), 94 (86.2%) and 69 (63.3%) of the novel intergenic transcripts, respectively (Roadmap Epigenomics Consortium, Kundaje et al., 2015) (Figure 4A). Finally, we overlapped our novel intergenic transcripts with a recent dataset of conserved RNA structures (CRSs) (Seemann et al., 2017). We found that 9/109 transcripts (9.2%) contained one or more CRSs overlapping their exons, which may be indicative of RNA-mediated functionality.
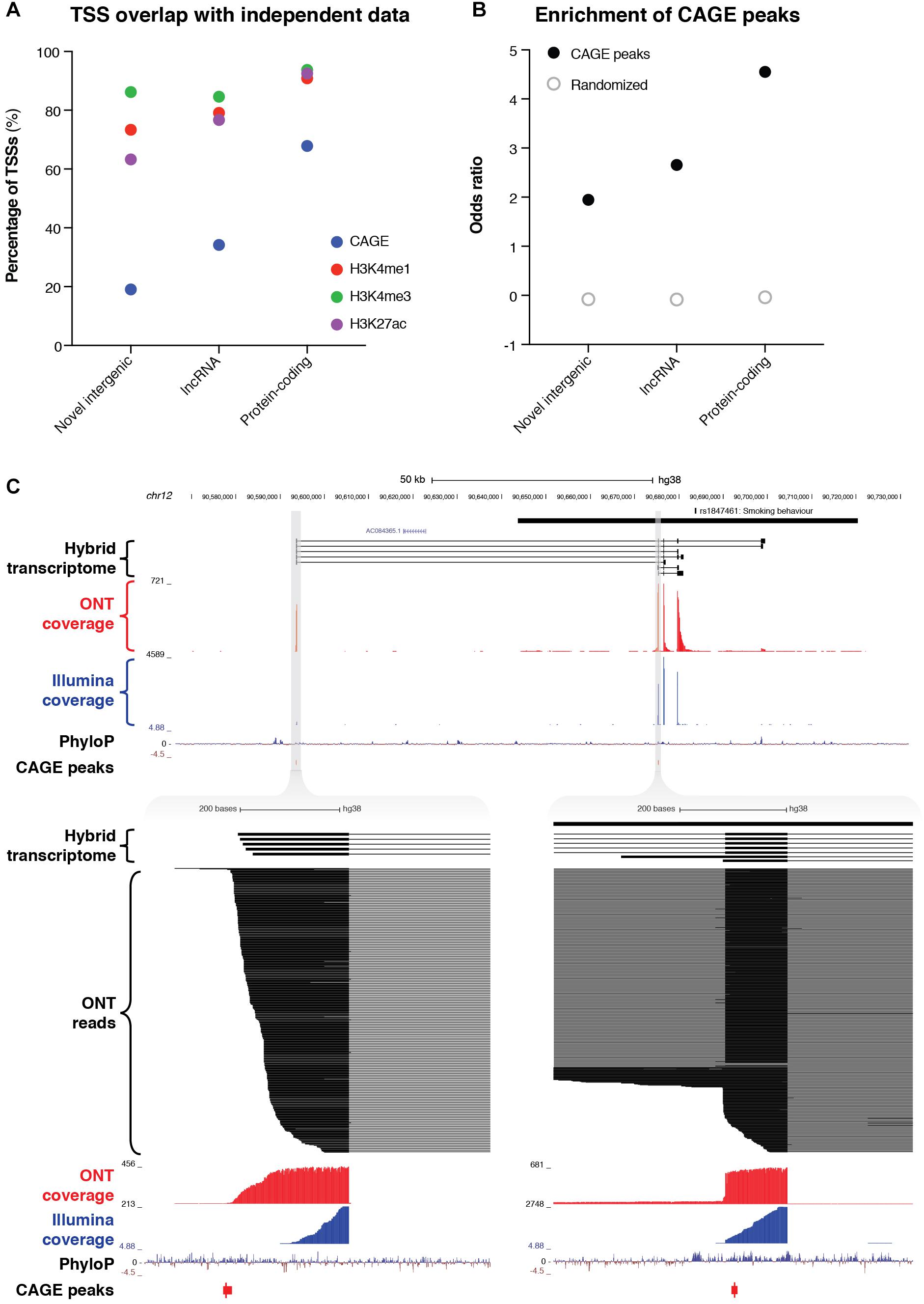
Figure 4. Identification of novel intergenic transcripts. (A) Bar charts indicate the fraction of transcription start sites (TSSs) occupied by cap analysis of gene expression (CAGE) peaks (blue), as well as the three epigenetic marks that are typically associated with actively transcribed promoters: H3K4me1 (red), H3K4me3 (green), and H3K27ac (purple). GENCODE (v29) lncRNAs and protein-coding genes are also plotted for reference. (B) Bar charts show the enrichment of the promoter regions of transcripts for CAGE peaks. Odds ratio of enrichment is plotted for novel intergenic transcripts compared to lncRNAs and protein-coding genes. (C, upper) Genome browser view shows a novel intergenic locus identified overlapping a GWAS haplotype block (solid black bar, top) associated with smoking behavior (rs1847461) on chromosome 12. Transcripts from our filtered hybrid transcriptome are shown below, followed by spliced ONT sequencing coverage (red), spliced Illumina sequencing coverage (blue), PhyloP conservation track, and CAGE robust peaks. (C, lower) Two separate magnified views show novel TSSs supported by CAGE peaks and highly conserved promoter regions. ONT read alignments are also shown.
For example, we discovered a novel intergenic locus overlapping a GWAS haplotype block associated with ‘smoking behavior’ (rs1847461) (Caporaso et al., 2009) on chromosome 12 (Figure 4C, upper). We identified 7 multi-exonic transcripts at this locus, with all splice junctions confirmed by Illumina short-read sequencing. Many exons were highly conserved, and the two TSSs were supported by CAGE peaks (Figure 4C, lower). Another example included a novel intergenic locus overlapping a GWAS SNP associated with multiple sclerosis (rs354033) (The International Multiple Sclerosis Genetics Consortium, 2011) on chromosome 7 (Supplementary Figure 11). Once again, splice junctions were validated with Illumina sequencing, several exons were highly conserved, and most transcripts overlapped a CAGE peak at the 5′ end.
As well as discovering novel gene loci in the vicinity of GWAS neuropsychiatric SNPs, CaptureSeq enabled us to identify hundreds of novel splicing events in annotated genes with established roles in neuropsychiatric function. For example, we captured a ∼570 kb haplotype block on chromosome 8 that includes a SNP associated with cocaine dependence (rs75686122) (Gelernter et al., 2014) (Figure 5A). This SNP is located within the first intron of the RIMS2 gene, which encodes a protein that interacts with various synaptic proteins that are important for normal neurotransmitter release (Kaeser et al., 2012). We identified three novel internal exons in the first intron of RIMS2 that are highly conserved and are predicted to encode novel ORFs (Figure 5B). The first novel exon encodes a start codon and the first 16 amino acids of the novel ORF, while the second and third exons are in-frame and encode another 11 and 29 amino acids, respectively. Collectively, these novel exons are predicted to add 56 amino acids to the start of the RIMS2 protein. Whilst interesting, this finding would require further proteomic validation.
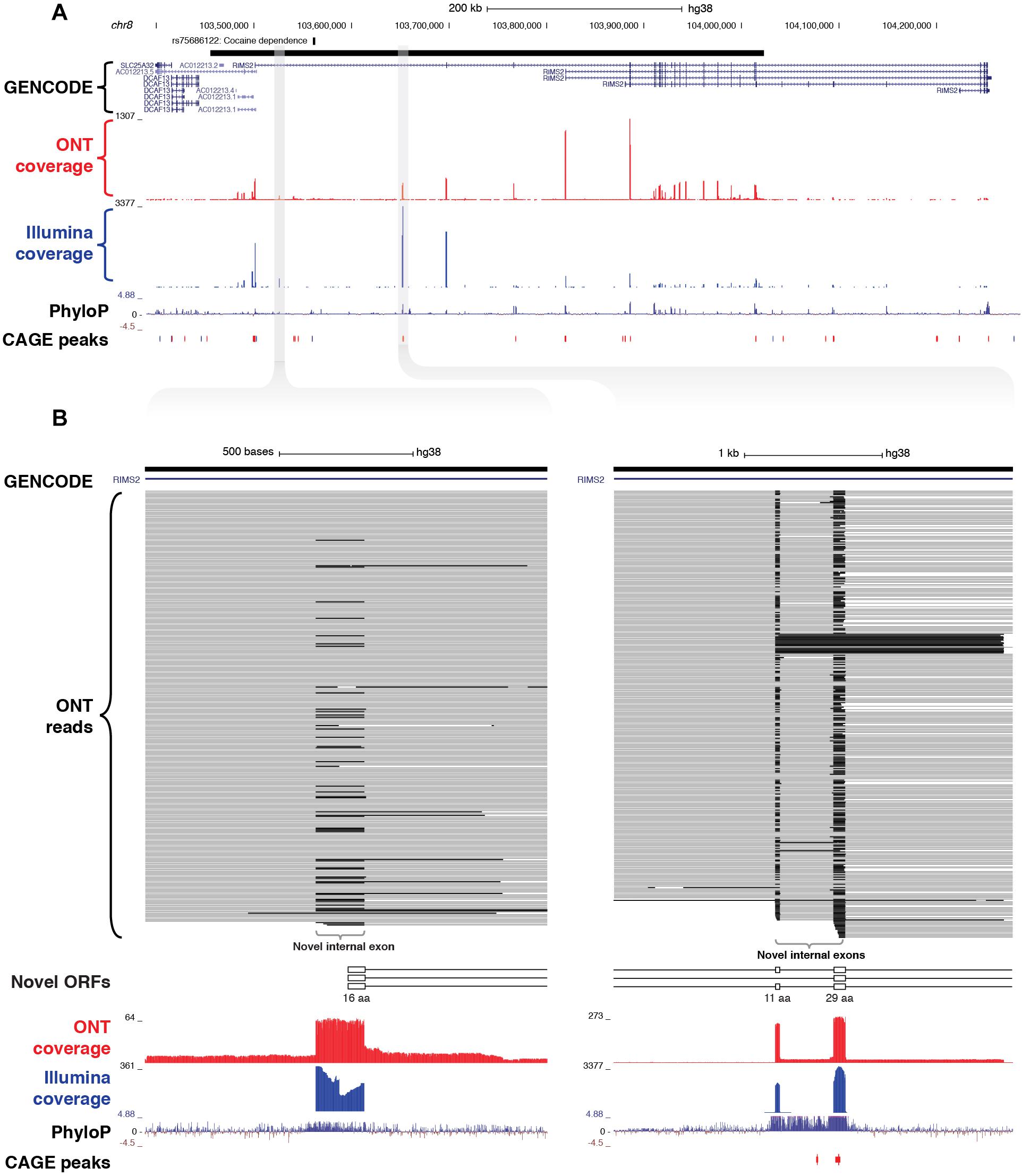
Figure 5. Identification of novel internal coding exons in RIMS2 gene. (A) Genome browser view shows a ∼570 kb GWAS haplotype block (solid black bar, top) on chromosome 8 associated with cocaine dependence (rs75688122). CaptureSeq detected multiple novel splice isoforms of RIMS2, a gene involved in neurotransmitter release. (B) Magnified views show three novel, highly conserved exons detected in the first intron of RIMS2, which are predicted to collectively add 56 amino acids to the start of the RIMS2 protein. The first novel internal exon includes a start codon (left), while the second and third exons (right) are in-frame (33 and 87 bp, respectively).
In another example, we captured a ∼4 kb haplotype block containing a GWAS SNP associated with schizophrenia (rs12807809) (Stefansson et al., 2009), located immediately upstream of the NRGN gene on chromosome 11 (Supplementary Figure 12). NRGN encodes a postsynaptic protein that is thought to be a direct target for thyroid hormone in human brain (Coloma et al., 1999). We identified a novel TSS located ∼20 kb upstream of the annotated TSS for NRGN that was supported by a CAGE peak and had a highly conserved promoter region. The novel transcripts incorporate annotated exons of NRGN, such that the novel introns span SNP rs12807809.
One of the promised advantages of long-read sequencing is that it can deconvolute coordinated alternative splicing events between distant pairs of exons (Bolisetty et al., 2015; Tilgner et al., 2015). This type of information cannot be gleaned using short-read RNA-seq because distant exon pairs are never sequenced on the same fragment. Since several RNA sequin loci provide known examples of coordinated splicing between distant exon pairs, we first used chrIS as a proof-of-principle to verify that ONT sequencing could reliably detect such events. For example, the sequin locus R1_22 comprises two alternatively spliced isoforms that contain a distant mutually associated pair (dMAP) of exons (Supplementary Figure 13a). With ONT sequencing, we were able to accurately resolve the long-range connectivity between these exons. However, with short-read Illumina sequencing, computational assembly (with StringTie, Pertea et al., 2015) produced a false-positive transcript structure in which one exon in the pair was included and the other excluded. Likewise, the R1_103 sequin locus comprises two alternative isoforms that contain a distant mutually exclusive pair (dMEP) of exons (Supplementary Figure 13b). Again, ONT sequencing enabled their long-range relationship to be resolved, while short-read sequencing produced a false-positive transcript structure in which both exons were present. We used our previously published methods (Tilgner et al., 2015, 2018) to search for similar examples of coordinated alternative exon pairing in the human genome. For example, we detected a dMEP of exons in MBNL2, a gene implicated in the development of myotonic dystrophy that overlaps a targeted haplotype block associated with alcoholism (rs9556711) (Heath et al., 2011) on chromosome 13 (Supplementary Figure 13c). This exon pair (exons number 7 and 9 of transcript ENST00000469707.5) was never simultaneously present on the same molecule, with no reads containing both exons, 19 reads containing only exon 7 but not exon 9, 37 reads containing exon 9 but not exon 7, and 50 reads which skipped both exons (Supplementary Figure 13c). This coordination was highly significant, with a Fisher’s exact test p-value of 1.23 × 10-4.
This study has successfully employed RNA CaptureSeq to reveal the rich transcriptional diversity hidden within non-coding regions of the genome that have previously been associated by GWAS with neuropsychiatric functions. In doing so, we have vastly expanded existing transcriptome annotations of these regions, creating an expression atlas of thousands of novel isoforms in human brain. Many of these assembled isoforms show preliminary evidence of functional roles, including highly conserved exons and promoter regions, TSSs supported by CAGE peaks and epigenetic marks, and novel predicted ORFs.
The improved sensitivity of CaptureSeq allowed us to assemble 109 novel intergenic transcripts in regions of the genome that were previously thought to be transcriptionally silent. As a class, these transcripts resemble annotated lncRNAs, as judged by their protein-coding probability scores, relatively low overall expression and evolutionary conservation compared to protein-coding genes, which is typical of regulatory sequences (Pheasant and Mattick, 2007). While only a minority (∼19%) of these were supported by CAGE peaks, their TSSs were nonetheless enriched for CAGE peaks compared to the genomic background. It is also worth pointing out that CAGE data is itself expression-dependent, and the fact that ∼9% of our novel intergenic transcripts lacking CAGE support were independently validated by a recent study (Lagarde et al., 2017) implies that the sensitivity of CaptureSeq surpasses that of CAGE over targeted regions.
To date, a poor understanding of the sequence–function relationship of lncRNAs (as opposed to protein-coding genes) has hindered their functional characterization. Promisingly, several technologies for probing lncRNA functions and mechanisms have begun to emerge, including ChIRP-seq (for assaying DNA/protein binding partners) and SHAPE-seq (for RNA structure) (Chu et al., 2015). Further, two landmark studies have recently employed CRISPR-Cas9 genome editing to perturb lncRNA loci in vivo, leading to the identification of hundreds of lncRNAs that have an effect on cell growth (Liu et al., 2016a; Zhu et al., 2016). Mechanistically, non-coding GWAS SNPs could be located within the promoters of lncRNAs, hence influencing their expression. Alternatively, they could fall within lncRNA exons, thereby potentially affecting RNA secondary structure (so-called ‘riboSNitches’) (Wan et al., 2014; Corley et al., 2015). Indeed, we found that ∼9% of our novel intergenic transcripts had exonic overlap with one or more CRSs (Seemann et al., 2017), providing potential evidence for RNA-mediated functionality. GWAS SNPs located in proximity to exon boundaries can potentially alter splicing patterns in these loci, which display complex transcriptional activity.
It is conceivable that some of our novel intergenic transcripts represent the output of enhancers. Indeed, recent evidence indicates that most – if not all – enhancers are transcribed into non-coding RNAs that have been termed ‘eRNAs’ (Li et al., 2016). However, there is still significant controversy around whether eRNA transcripts are functional per se, or whether it is merely the act of their transcription that is indicative of some underlying function (Li et al., 2016). Of the intergenic haplotype blocks we targeted for capture, we failed to detect transcription in the majority of cases. However, it must be borne in mind that we only polled four brain regions and that many non-coding RNAs are only expressed in very specific cell populations (Mercer et al., 2008; Liu et al., 2016b).
This study also demonstrated the utility of spike-in controls in analyzing NGS data (Hardwick et al., 2017). The use of RNA sequins (Hardwick et al., 2016) provided a faithful set of internal controls that helped to validate our experimental design, including an assessment of capture efficiency, sensitivity and quantitative accuracy. Of the 15 sequin isoforms ≥ 2.5 kb in length, six were fully sequenced ‘in one go,’ including a ∼4.4 kb synthetic transcript comprising 18 exons. Furthermore, we showed how the in silico chromosome (chrIS) can act as a comprehensive ‘ground truth’ reference against which FP and FN findings with RNA-seq can be evaluated. Because chrIS emulates the features of a real human chromosome, we would expect similar rates of TP and FP events for human transcripts. Notably, this type of analysis is not possible with previous spike-in controls (e.g., the ERCC spike-ins, Jiang et al., 2011), because they are mono-exonic and do not recapitulate the complexity of eukaryotic gene splicing. Despite these advantages, this study also illustrates some of the limitations of spike-in controls. While sequins were added to samples at a low fractional concentration (2%), we found that a disproportionally high fraction of reads aligned to chrIS, thereby sacrificing reads that could otherwise have come from endogenous transcripts. While we added sequins in proportion to each sample, the process of capture positively selects on the targeted genomic regions in a way that is not entirely predictable, thereby inadvertently changing the ratio of sample to spike-ins.
In conclusion, this study substantially expands the transcriptome annotations for regions of the genome associated with important neuropsychiatric traits, including diseases like Alzheimer’s, Parkinson’s and schizophrenia. These transcriptomes collectively comprise a valuable atlas that can be used to connect gene expression with neuropsychiatric traits. Ultimately, novel transcripts identified herein could act as biomarkers for disease or potential therapeutic targets.
The complete GWAS database was downloaded from the NHGRI catalog (Welter et al., 2013; Macarthur et al., 2017), then filtered to include only studies with a sample size of n ≥ 1000 that focused on traits associated with the brain (including behavioral traits, mental illness, as well as neuropsychiatric disorders like Alzheimer’s, Parkinson’s and schizophrenia). Since GWAS simply associates traits with regions of the genome that are in LD, the causative SNP could be anywhere within the region of LD. In order to ensure the causative SNP is included, it is necessary to capture the full haplotype block associated with the SNP reported by GWAS. As such, we used SNAP (Johnson et al., 2008) to identify all SNPs in LD, with an LD threshold of R > 0.5 and a maximum distance between SNPs of 500 kb. All SNPs in LD were then assumed to comprise a single haplotype block. This resulted in 1,323 haplotype blocks comprising a total of 1,352 GWAS SNPs associated with neuropsychiatric phenotypes (see Supplementary Table 1). Since some of these haplotype blocks overlapped, we merged them into a set of 1,023 discrete, non-overlapping blocks.
Biotinylated oligonucleotide probes were designed to tile across the abovementioned 1,023 blocks, excluding any protein-coding exons (GENCODE v24) and repeat elements (RepeatMasker). Probes were designed in accordance with previous guidelines (Mercer et al., 2014). This resulted in a final capture space of 96,234,476 bp. In addition, a subset of our RNA sequins spike-ins (Hardwick et al., 2016) were targeted for capture (25/78 genes; 49/164 isoforms). In selecting spike-ins to target, we chose transcripts that spanned across the range of concentrations in the staggered mixture (Mix A). Probe designs were submitted to Roche NimbleGen for synthesis.
Brain tissue from three de-identified, neurologically healthy males of European ancestry was supplied by the NSW Brain Tissue Resource Centre (BTRC) under Project ID 0379 and ethics approval number HC15878. Donors were aged between 61 and 64, had cardiac causes of death, with post-mortem intervals of brain collection between 17 and 41 h. From each brain, 3 mg of tissue was dissected by the BTRC from four regions: prefrontal cortex (PFC), primary visual cortex (VCx), caudate, and superior colliculus (SupCol). RNA was extracted from samples using the protocol for QIAGEN’s miRNeasy kit: 700 μL QIAzol was added per sample on ice, which were then transferred to 2 mL tubes containing a single 5 mm ball bearing and lysed on the Tissue Lyser II for 2 min at 20 Hz. Upon completion of the cycle, adapters were rotated and shaken for an additional 2 min at 20 Hz. Once lysed, the samples were purified according to the miRNeasy mini kit protocol instructions, including the on-column DNAse treatment. Elution was in 15 μL nuclease-free H2O, with the eluate being placed back onto the columns for an additional run in order to ensure maximum recovery and concentration.
RNA CaptureSeq was performed as previously described (Mercer et al., 2014), with the following modifications for the ONT platform. One μg of each of 4 human brain samples (SupCol, PFC, VCx, and caudate) was independently combined with 10 ng of RNA sequins (Hardwick et al., 2016) (Mix A; 2% fractional abundance). Volumes were adjusted to 11.5 μl per sample with nuclease-free water, followed by the addition of 0.5 μl of 250 mM Random Primer 6 (S1230S, NEB) and 1 μl Deoxynucleotide Solution Mix (N0447, NEB). Samples were mixed by tapping, briefly spun by microfuge, incubated at 65°C for 5 min and immediately cooled on ice. Four μl of First Strand buffer and 2 μl 100 mM DTT (SuperScript II Reverse Transcriptase: 18064014, Thermo Fisher) were added to the samples, which were mixed by tapping, briefly spun by microfuge and incubated at 42°C for 2 min. One μl of SuperScript II Reverse Transcriptase was added to the 19 μl reaction mixes, which were mixed by tapping, briefly spun by microfuge and incubated with the following thermocycler conditions: RT reaction at 50°C for 50 min; denaturation at 70°C for 15 min; and holding indefinitely at 4°C. The second strand synthesis reaction mix was assembled as follows (NEBNext Ultra II Non-Directional RNA Second Strand Synthesis Module: E6111S, NEB): 20 μl of first strand cDNA, 10 μl buffer, 5 μl enzyme mix, and 45 μl nuclease-free water. Reactions were incubated with the following thermocycler conditions: second strand synthesis at 16°C for 1 h; and holding indefinitely at 4°C. Resulting cDNA was purified by a 10 min incubation with 144 μl (1.8X) Agencourt AMPure XP beads (A63880, Beckman Coulter), 2 × 200 μl washes with 80% ethanol for 30 s each, 5 min air drying, and elution in 52 μl nuclease-free water.
Following reverse transcription, the four samples had an average fragment length of 1500 bp with the following concentrations: 6.99 ng/μl (SupCol), 5.56 ng/μl (PFC), 5.5 ng/μl (VCx), and 6.5 ng/μl (caudate) according to a genomic DNA screentape analysis (Agilent 5067-5365, 5067-5366). The samples were each end-prepped and dA-tailed using the NEBNext Ultra II End Repair/dA-Tailing Module (E7595) as follows: 45 μl DNA, 7 μl Ultra II End-prep reaction buffer, 3 μl Ultra end-prep enzyme mix, and 5 μl nuclease-free water. Each reaction was incubated at 20°C for 30 min followed by 65°C for 30 min. Following end-prep, the samples were purified using AMPure beads (A63882) at a 1:1 ratio with incubation at room temperature for 5 min, two 140 μl washes with 70% ethanol, and elution for 2 min in 31 μl nuclease-free water. The PCA adapters from the ONT ligation sequencing kit 1D (SQK-LSK108) were ligated onto the end-prepped samples as follows: 30 μl of the end-prepped DNA, 20 μl PCR adapters (PCA), and 50 μl Blunt/TA ligase master mix (NEB) were combined and incubated at room temperature for 10 min. The adapted DNA was purified using Agencourt AMPure Beads as described above. The DNA was then eluted in nuclease-free water. Next, each sample was amplified in a 100 μl reaction using NEB Long-Amp Taq (M0323) as follows: 1X LongAmp Taq reaction buffer, 300 μM dNTPs, 2 μl PRM primers, and 48 μl DNA from the previous step. The reaction for each sample was divided into two 50 μl reactions and amplified with the following thermocycler conditions: an initial denaturation at 95°C for 1 min; 18 cycles of 95°C for 15 s, 62°C for 15 s, and 65°C for 2 min; a final extension at 65°C for 10 min; and a 4°C hold. Following amplification, the samples were purified using a 1:1 dilution of AMPure beads as described above.
Following ligation of the PCR adapters, the 4 samples had the following concentrations: 36.0 ng/μl (SupCol), 25.2 ng/μl (PFC), 30.2 ng/μl (VCx), and 24.6 ng/μl (caudate) according to genomic DNA screentape analysis (5067–5365/5067–5365, Agilent). To prepare the hybridization reaction, 1 μg of each of the samples was combined with 5 μg Cot-1 DNA (15279011, Thermo Fisher) and 1 μl of 1mM blocking oligo (5′ AGGTTAAACACCCAAGCAGACGCCGCAATATCAGCACCAACAGAA 3′) and dried down in a vacuum concentrator for 1 h. The dry contents were resuspended by addition of 7.5 μl hybridization buffer and 3 μl hybridization component A (SeqCap EZ Hybridization and Wash Kit: 05 634 261 001, Roche), mixed by tapping, briefly spun by microfuge, and denatured at 95°C for 10 min. The 10.5 μl contents were briefly spun by microfuge and immediately transferred to a pre-warmed 4.5 μl aliquot of the NimbleGen SeqCap EZ Capture probe in a 0.2 ml PCR tube housed in a thermocycler set to 47°C (lid 57°C). Following overnight incubation (∼18 h), 100 μl M-270 Streptavidin Dynabeads (65305, Thermo Fisher) per sample were washed twice with 200 μl 1x wash buffer (SeqCap EZ Hybridization and Wash Kit: 05 634 261 001, Roche), once with 100 μl, and then placed in the thermocycler set to 47°C. Incubated samples (15 μl) were transferred immediately to the beads for 45 min. Non-target DNA was removed through washing the beads with buffers of the SeqCap EZ Hybridization and Wash Kit (05 634 261 001, Roche) as per manufacturer’s instructions with the following modifications: pipette-mixing was replaced by inversion-mixing and brief centrifugation. Beads were finally resuspended in 48 μl nuclease-free water. The following PCR reaction was set up: 48 μl sample; 2 μl PRM primers; 50 μl LongAmp Taq 2x master mix (M0287S, NEB). The reaction mixes were split into 50 μl aliquots and amplified with the following thermocycler conditions: initial denaturation at 95°C for 3 min; 22 cycles of 95°C for 15 s, 62°C for 15 s, and 65°C for 10 min; a final extension at 65°C for 10 min; and holding indefinitely at 4°C. Resulting DNA was purified by a 10 min incubation with 70 μl (0.7X) Agencourt AMPure XP beads (A63880, Beckman Coulter), 2 × 200 μl washes with 80% ethanol for 30 s each, 3 min air drying, and elution in 52 μl nuclease-free water. Sample concentrations were determined by Qubit as: 43.5 ng/μl (SupCol), 48.6 ng/μl (PFC), 30.5 ng/μl (VCx), 38.2 ng/μl (caudate).
The captured samples were barcoded using ONT 1D native barcoding genomic DNA kit (EXP-NBD103), and the library prep was performed using the 1D genomic DNA by ligation kit and protocol for the PromethION sequencer (SQK-LSK109). The preparation was performed according to manufacturer recommendations with some modifications. Briefly, each sample underwent end-prep in a 60 μl reaction containing the product from capture, 7 μl Ultra II End-prep reaction buffer, and 3 μl Ultra end-prep enzyme mix. The resulting DNA samples were purified with AMPure beads at a ratio of 1:1 as above. After end-prep, 120 ng of each sample was barcoded by ligation of a barcoding adaptor in a 50 μl reaction containing 2.5 μl of the respective barcode and 25 μl NEB Blunt/TA ligase master mix (M0367). The reaction was incubated at room temperature for 10 min and the resulting product was purified with AMPure beads at t a 1:1 dilution as above. Then, 100 ng of each sample was combined into a final reaction to ligate on the sequencing adaptor in a 100 μl reaction containing 400 ng total combined DNA, 25 μl ligation buffer, 10 μl NEBNext Quick T4 DNA ligase, and 5 μl adapter mix. The reaction was incubated for 10 min at room temperature and then purified with AMPure beads at a.55 ratio, to preserve the smaller cDNA fragments, at room temperature for 5 min. The DNA bound to the beads were then washed with and resuspended in 250 μl short fragment buffer and rebound to the magnets twice before being eluted from the beads for 10 min in 25 μl of elution buffer. A PRO002 PromethION flow cell was primed and loaded with the resulting 41.04 ng of captured and barcoded cDNA according to the manufacturer’s recommendation. The LSK109 sequencing protocol was executed, and the sequencing ran for 52 h.
Oxford Nanopore Technologies reads were base-called using Guppy base-calling software (ONT) (v1.8.5). Quality control of reads was undertaken using ONT ‘fastq deconcatenate’ (v0.6.2) and POREquality2. Demultiplexing base-called reads was performed using Porechop (v0.2.3)3 with the enforced barcode detection parameter. Demultiplexed ONT reads were mapped to a combined index comprising the human genome (hg38) and in silico chromosome (chrIS) (Hardwick et al., 2016) using minimap2 (v2.14-r883) (Li, 2018). The following parameters were used: –ax splice –secondary = no. After retaining only uniquely mapped reads (mapQ = 60), reads were processed using the Pinfish suite of tools4. First, BAM files were converted into GFF files using the ‘spliced_bam2gff’ tool. We then used the ‘cluster_gff’ tool, which clusters together reads having similar exon/intron structure and creates a rough consensus of the clusters by taking the median of exon boundaries from all transcripts in the cluster. The following parameters were used: –c 3 –d 10 –e 30 –p 1. To assess the performance of ONT in sequencing RNA sequins, we isolated all clustered transcripts aligned to chrIS and compared them against the chrIS synthetic gene annotations using gffcompare (v0.10.6) (Pertea et al., 2016), with the following non-default parameters: –M –C –K. A graphical summary of the bioinformatics pipeline is presented in Supplementary Figure 1.
Samples were spiked with RNA sequins (Hardwick et al., 2016) (Mix A; 2% fractional abundance) before library preparation. RNA CaptureSeq was performed as previously described (Mercer et al., 2014), using the KAPA Stranded RNA-Seq Library Preparation Kit (Roche). Pre-sequencing quality control qPCR and LabChip GX results were normal. The samples were partitioned into capture pools by RNA yield similarity in order to minimize variation in sample dilution. Sequencing was carried out on an Illumina HiSeq 2500 instrument in high output mode, with 2 × 125 bp paired-end reads.
Adapters were trimmed from reads using CutAdapt (Martin, 2011) (v1.8.1) and basic quality control undertaken using FastQC5. Reads were aligned using STAR (v2.5.3a) (Dobin et al., 2013) to a combined index comprising hg38 and chrIS. STAR was run with the following parameters: –alignMatesGapMax 1000000 –alignIntronMax 1000000 –outFilterIntronMotifs RemoveNoncanonicalUnannotated. Alignments were then assembled into transcript models using Stringtie (v 1.3.3) (Pertea et al., 2015), without providing any reference annotations to guide the assembly process. The minimum isoform abundance of predicted transcripts for each locus was required to be at least 1% (–f 0.01). Library type was set as –rf.
We compared quantitative gene expression measurements for ONT and Illumina using Salmon (v0.11.3) (Patro et al., 2017), providing the chrIS annotation and our hybrid transcriptome as a reference. For ONT samples, we used the following non-default parameters: –fldMean 1000 –fldSD 100 –libtype U. For Illumina samples, we used –libtype ISR. Salmon outputs expression measurements in TPM.
Uniquely mapped reads were isolated from BAM files using samtools (v1.6) (Li et al., 2009) ‘view’ (–q 60 for ONT or –q 255 for Illumina). BAM alignments were converted to BED12 files using BEDtools (v2.25.0) (Quinlan and Hall, 2010) ‘bamtobed’ tool (using the –split flag). We retrieved reads that mapped to targeted regions using BEDtools ‘intersect’; the on-target rate was calculated as the number of reads mapping to targeted regions as a fraction of all reads mapping to hg38.
To assess the performance of CaptureSeq, we plotted the observed abundance (TPM) of each sequin isoform against its input concentration (log10 scale). We then carried out simple linear regression of captured and non-captured sequins separately. The LoD was defined as the spike-in transcript of lowest abundance that was detected in more than one sample. To estimate the enrichment provided by capture, we compared the measured abundance (TPM) of captured vs. non-captured standards at each of the shared concentration points. At each shared point, we divided the average TPM for captured standards by the average TPM for non-captured standards, and then averaged all of these to obtain an overall enrichment. CV was calculated by dividing the standard deviation (SD) of each spike-in by its mean TPM across all four samples. To assess quantitative accuracy at the gene-level, we first counted the number of reads mapping uniquely to annotated sequin loci with featureCounts (v1.6.3) (Liao et al., 2013) (using the –L option for ONT data). We then quantified gene expression by calculating the number of reads per gene per 10k reads (RPG10K) (Byrne et al., 2017) for each sequin locus.
To assess the uniformity of sequencing coverage, we calculated the read coverage of every base in every sequin exon targeted for capture (n = 279) using BEDtools (v2.25.0) (Quinlan and Hall, 2010) ‘coverage’ (with –d and –split options). We then computed the mean and SD for each exon using BEDtools ‘groupby,’ then calculated coefficient of variation (SD/mean) from these metrics. We compared the difference between ONT and Illumina using a paired t-test (two-tailed).
To generate a hybrid transcriptome that leverages both long- and short-read data, we used the FLAIR tool (v1.2) (Tang et al., 2018). First, we used FLAIR ‘correct’ by inputting spliced ONT read alignments (from minimap2) and correcting misaligned ONT splice junctions using genome annotations (GENCODE v29; with the –f option) and our accompanying Illumina splice junctions (with the –j option). We used the default window size of 10 bp for correcting splice sites. We then collapsed corrected reads into a non-redundant transcriptome using FLAIR ‘collapse’ with default parameters. This produced a PSL file containing corrected ONT reads, which we converted to BED and GTF files using the UCSC Table Browser6.
We then undertook a number of steps in order to produce a high-confidence set of multi-exonic transcripts overlapping GWAS haplotype blocks associated with neuropsychiatric functions. First, we filtered our transcripts using gffread (Pertea et al., 2016) with the following options: –i 1000000 –U –N –M –K –T. We then annotated our transcripts using gffcompare (Pertea et al., 2016) (v0.10.6), providing GENCODE v29 as a reference annotation (and using the options –C –K). Any transcript with any the following gffcompare classification codes was removed: ‘e’, ‘p’, ‘r’ or ‘s’. Finally, we removed any transcript that met any of the following criteria: (i) was < 200 nt in length, (ii) had < 3 exons, or (iii) had no overlap with any capture probe.
Our set of filtered transcripts was split into unique introns and internal exons using in-house Perl scripts (we disregarded terminal exons because their boundaries are imprecise). We compared these feature counts with the current version of GENCODE (v29), three previous GENCODE releases (v3c, v10 and v19) and MiTranscriptome (v2) (Iyer et al., 2015). Only multi-exonic transcripts that overlapped targeted haplotype blocks were considered in this analysis. Hg19 annotations were converted to hg38 coordinates using LiftOver (Kuhn et al., 2013).
We assessed protein-coding capacity of all assembled transcripts with CPAT (v1.2.3) (Wang et al., 2013), using the prebuilt hexamer frequency table and training model (human) obtained from https://sourceforge.net/projects/rna-cpat/files/v1.2.2/prebuilt_model/. For putative coding transcripts (coding probability ≥ 0.364), open reading frames (ORFs) were predicted and annotated using TransDecoder (v5.3.0) (Haas et al., 2013). Protein homology searches were carried out on selected transcripts using the BlastP (Altschul et al., 1997) and UniProt (The UniProt Consortium, 2008) databases. Evolutionary conservation of transcripts was investigated using the phastCons 100-way vertebrate alignment (Siepel et al., 2005); the average score of all nucleotides in each transcript was computed using UCSC’s ‘bigWigAverageOverBed’ script (Kuhn et al., 2013). For both analyses, previously annotated protein-coding transcripts and lncRNAs (GENCODE v29) were included as controls.
We retrieved promoter coordinates from our transcripts using BEDtools ‘flank’ (with –l 1 –r 0 –s options). We downloaded the latest dataset of cap analysis of gene expression (CAGE) robust peaks (hg38) from the FANTOM5 consortium (Forrest et al., 2014). We used the BEDTools (Quinlan and Hall, 2010) ‘window’ feature to identify transcripts whose TSS was within 500 bp of a CAGE robust peak on the same strand. We calculated odds ratios of enrichment using the script from Bartonicek et al. (2017). We also overlapped our transcript TSSs with the epigenetic marks that are typically associated with actively transcribed promoters (H3K4me1, H3K4me3, and H3K27ac). We downloaded broad peak calls for these marks (human brain) from the Roadmap Epigenomics Consortium (Kundaje et al., 2015), again lifting them over to hg38 coordinates. Again, previously annotated protein-coding transcripts and lncRNAs (GENCODE v29) were included as controls. Finally, we overlapped our transcripts with a recently published dataset of conserved RNA structures (CRSs) which may be indicative of RNA-mediated functionality (Seemann et al., 2017).
We used our previously developed scripts (Tilgner et al., 2015, 2018) to look for coordination between distant pairs of exons.
Genomic alignments were visualized using the UCSC genome browser (Kuhn et al., 2013) and IGV (v2.4.10) (Robinson et al., 2011).
Statistical analyses and plotting were carried out using Prism (v7) and R (v3.5.0).
This study was carried out in accordance with the recommendations of the Australian NHMRC National Statement on Ethical Conduct in Human Research and the NSW Health Sydney Local Health District Human Research Ethics Committee, with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the NSW Health Sydney Local Health District Human Research Ethics Committee.
JM, SB, GH, and CL conceived the study and experimental design. SB, SH, NB, and TM designed the RNA capture panel. SH and SB carried out bioinformatics analysis. MS supervised ONT sequencing experiments, bioinformatics analyses, and manuscript preparation. JB and KB carried out RNA capture and library preparation for ONT long-read sequencing. DK carried out RNA capture and library preparation for Illumina short-read sequencing. SC processed ONT sequencing data. HT carried out long-range exon coordination analysis. SH and JM wrote the manuscript, with advice from all authors.
This work was supported by National Health and Medical Research Council (NHMRC) Program Grant 1132524 to GH, JM, and CL. SH is supported by a NHMRC Early Career Fellowship (APP1156531). CL is supported by a NHMRC Dementia Research Development Fellowship (APP1107657). GH is supported by a NHMRC Senior Principal Research Fellowship (1079679). TM is supported by a NHMRC Australia Fellowship (1062470) and also by the Paramor Family Fellowship. MS, KB, and SC are supported by a philanthropic program grant from The Kinghorn Foundation. HT is a Leon Levy Research Fellow in Neuroscience and is furthermore grateful for a generous gift by Anita Garoppolo. Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R28AA012725. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
MS has received travel and accommodation expenses to speak at Oxford Nanopore Technologies conferences. TM is an inventor on a patent (PCT/AU2015/050797) relating to sequin spike-in controls.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Mr. James Ferguson and Mr. Ted Wong (Garvan Institute) for providing computational support during data analysis. We would also like to thank Dr. Ira Deveson (Garvan Institute) for providing access to chromosome 21 CaptureSeq datasets. Brain tissue was received from the New South Wales Brain Tissue Resource Centre at the University of Sydney, which is supported by the Schizophrenia Research Institute.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00309/full#supplementary-material
TABLE S1 | Description of GWAS haplotype blocks that were targeted for capture in this study, including the GWAS SNPs contained therein. Chromosome coordinates refer to hg38 reference.
Albert, F. W., and Kruglyak, L. (2015). The role of regulatory variation in complex traits and disease. Nat. Rev. Genet. 16, 197–212. doi: 10.1038/nrg3891
Altschul, S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. doi: 10.1093/nar/25.17.3389
Bartonicek, N., Clark, M. B., Quek, X. C., Torpy, J. R., Pritchard, A. L., Maag, J. L. V., et al. (2017). Intergenic disease-associated regions are abundant in novel transcripts. Genome Biol. 18:241. doi: 10.1186/s13059-017-1363-3
Bolisetty, M. T., Rajadinakaran, G., and Graveley, B. R. (2015). Determining exon connectivity in complex mRNAs by nanopore sequencing. Genome Biol. 16:204. doi: 10.1186/s13059-015-0777-z
Bond, C. S., and Fox, A. H. (2009). Paraspeckles: nuclear bodies built on long noncoding RNA. J. Cell Biol. 186, 637–644. doi: 10.1083/jcb.200906113
Briggs, J A., Wolvetang, E J., Mattick, John S., Rinn, John L., and Barry, G. (2015). Mechanisms of long non-coding RNAs in mammalian nervous system development, plasticity, disease, and evolution. Neuron 88, 861–877. doi: 10.1016/j.neuron.2015.09.045
Bussotti, G., Leonardi, T., Clark, M. B., Mercer, T. R., Crawford, J., Malquori, L., et al. (2016). Improved definition of the mouse transcriptome via targeted RNA sequencing. Genome Res. 26, 705–716. doi: 10.1101/gr.199760.115
Byrne, A., Beaudin, A. E., Olsen, H. E., Jain, M., Cole, C., Palmer, T., et al. (2017). Nanopore long-read RNAseq reveals widespread transcriptional variation among the surface receptors of individual B cells. Nat. Commun. 8:16027. doi: 10.1038/ncomms16027
Cao, H., Wahlestedt, C., and Kapranov, P. (2018). Strategies to annotate and characterize long noncoding RNAs: advantages and pitfalls. Trends Genet. 34, 704–721. doi: 10.1016/j.tig.2018.06.002
Caporaso, N., Gu, F., Chatterjee, N., Sheng-Chih, J., Yu, K., Yeager, M., et al. (2009). Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS One 4:e4653. doi: 10.1371/journal.pone.0004653
Carninci, P., Kasukawa, T., Katayama, S., Gough, J., Frith, M. C., Maeda, N., et al. (2005). The transcriptional landscape of the mammalian genome. Science 309, 1559–1563. doi: 10.1126/science.1112014
Chu, C., Spitale, R. C., and Chang, H. Y. (2015). Technologies to probe functions and mechanisms of long noncoding RNAs. Nat. Struct. Mol. Biol. 22, 29–35. doi: 10.1038/nsmb.2921
Clark, M. B., Mercer, T. R., Bussotti, G., Leonardi, T., Haynes, K. R., Crawford, J., et al. (2015). Quantitative gene profiling of long noncoding RNAs with targeted RNA sequencing. Nat. Methods 12, 339–342. doi: 10.1038/nmeth.3321
Coloma, A., Morte, B., de Arrieta, C. M. N., and Bernal, J. (1999). The human RC3 gene homolog, NRGN contains a thyroid hormone-responsive element located in the first intron. Endocrinology 140, 335–343. doi: 10.1210/endo.140.1.6461
Corley, M., Solem, A., Qu, K., Chang, H. Y., and Laederach, A. (2015). Detecting riboSNitches with RNA folding algorithms: a genome-wide benchmark. Nucleic Acids Res. 43, 1859–1868. doi: 10.1093/nar/gkv010
Derrien, T., Derrien, T., Johnson, R., Johnson, R., Bussotti, G., and Bussotti, G. (2012). The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 22, 1775–1789. doi: 10.1101/gr.132159.111
Deveson, I. W., Brunck, M. E., Blackburn, J., Tseng, E., Hon, T., Clark, T. A., et al. (2018). Universal alternative splicing of noncoding exons. Cell Syst. 6:245-255.e5. doi: 10.1016/j.cels.2017.12.005
Deveson, I. W., Hardwick, S. A., Mercer, T. R., and Mattick, J. S. (2017). The dimensions, dynamics, and relevance of the mammalian noncoding transcriptome. Trends Genet. 33, 464–478. doi: 10.1016/j.tig.2017.04.004
Dobin, A., Davis, C. A., Schlesinger, F., Drenkow, J., Zaleski, C., Jha, S., et al. (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. doi: 10.1093/bioinformatics/bts635
Edwards, S. L., Beesley, J., French, J. D., and Dunning, A. M. (2013). Beyond GWASs: illuminating the dark road from association to function. Am. J. Hum. Genet. 93, 779–797. doi: 10.1016/j.ajhg.2013.10.012
Forrest, A. R. R., Kawaji, H., Rehli, M., Baillie, J. K., Kenneth Baillie, J., and Hoon, M. J. L. (2014). A promoter-level mammalian expression atlas. Nature 507, 462–470. doi: 10.1038/nature13182
Gelernter, J., Sherva, R., Koesterer, R., Almasy, L., Zhao, H., Kranzler, H. R., et al. (2014). Genome-wide association study of cocaine dependence and related traits: FAM53B identified as a risk gene. Mol. Psychiatry 19, 717–723. doi: 10.1038/mp.2013.99
Haas, B. J., Papanicolaou, A., Yassour, M., Grabherr, M., Blood, P. D., Bowden, J., et al. (2013). De novo transcript sequence reconstruction from RNA-seq using the trinity platform for reference generation and analysis. Nat. Protoc. 8, 1494–1512. doi: 10.1038/nprot.2013.084
Hardwick, S. A., Chen, W. Y., Wong, T., Deveson, I. W., Blackburn, J., Andersen, S. B., et al. (2016). Spliced synthetic genes as internal controls in RNA sequencing experiments. Nat. Methods 13, 792–798. doi: 10.1038/nmeth.3958
Hardwick, S. A., Deveson, I. W., and Mercer, T. R. (2017). Reference standards for next-generation sequencing. Nat. Rev. Genet. 18, 473–484. doi: 10.1038/nrg.2017.44
Harrow, J., Frankish, A., Gonzalez, J. M., Tapanari, E., Diekhans, M., Kokocinski, F., et al. (2012). GENCODE: the reference human genome annotation for the ENCODE Project. Genome Res. 22, 1760–1774. doi: 10.1101/gr.135350.111
Heath, A. C., Whitfield, J. B., Martin, N. G., Pergadia, M. L., Goate, A. M., Lind, P. A., et al. (2011). A quantitative-trait genome-wide association study of alcoholism risk in the community: findings and implications. Biol. Psychiatry 70, 513–518. doi: 10.1016/j.biopsych.2011.02.028
Hindorff, L. A., Sethupathy, P., Junkins, H. A., Ramos, E. M., Mehta, J. P., Collins, F. S., et al. (2009). Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc. Natl. Acad. Sci. U.S.A. 106, 9362–9367. doi: 10.1073/pnas.0903103106
Iyer, M. K., Niknafs, Y. S., Malik, R., Singhal, U., Sahu, A., Hosono, Y., et al. (2015). The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 47, 199–208. doi: 10.1038/ng.3192
Jiang, L., Schlesinger, F., Davis, C. A., Zhang, Y., Li, R., Salit, M., et al. (2011). Synthetic spike-in standards for RNA-seq experiments. Genome Res. 21, 1543–1551. doi: 10.1101/gr.121095.111
Johnson, A. D., Handsaker, R. E., Pulit, S. L., Nizzari, M. M., O’donnell, C. J., and De Bakker, P. I. W. (2008). SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 24, 2938–2939. doi: 10.1093/bioinformatics/btn564
Kaeser, P. S., Deng, L., Fan, M., and Südhof, T. C. (2012). RIM genes differentially contribute to organizing presynaptic release sites. Proc. Natl. Acad. Sci. U.S.A. 109, 11830–11835. doi: 10.1073/pnas.1209318109
Katayama, S., Tomaru, Y., Kasukawa, T., Waki, K., Nakanishi, M., Nakamura, M., et al. (2005). Antisense transcription in the mammalian transcriptome. Science 309, 1564–1566. doi: 10.1126/science.1112009
Kuhn, R. M., Haussler, D., and Kent, W. J. (2013). The UCSC genome browser and associated tools. Brief. Bioinform. 14, 144–161. doi: 10.1093/bib/bbs038
Kundaje, A., Meuleman, W., Ernst, J., Yen, A., Heravi-Moussavi, A., and Zhang, Z. (2015). Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330. doi: 10.1038/nature14248
Lagarde, J., Uszczynska-Ratajczak, B., Carbonell, S., Pérez-Lluch, S., Abad, A., Davis, C., et al. (2017). High-throughput annotation of full-length long noncoding RNAs with capture long-read sequencing. Nat. Genet. 49, 1731–1740. doi: 10.1038/ng.3988
Li, H. (2018). Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100. doi: 10.1093/bioinformatics/bty191
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Li, W., Notani, D., and Rosenfeld, M. G. (2016). Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nat. Rev. Genet. 17, 207–223. doi: 10.1038/nrg.2016.4
Liao, Y., Smyth, G. K., and Shi, W. (2013). The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 41:e108. doi: 10.1093/nar/gkt214
Lin, Y., Protter, D. S., Rosen, M. K., and Parker, R. (2015). Formation and maturation of phase-separated liquid droplets by RNA-Binding proteins. Mol. Cell 60, 208–219. doi: 10.1016/j.molcel.2015.08.018
Liu, S. J., Horlbeck, M. A., Cho, S. W., Birk, H. S., Malatesta, M., He, D., et al. (2016a). CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science 355:aah7111. doi: 10.1126/science.aah7111
Liu, S. J., Nowakowski, T. J., Pollen, A. A., Lui, J. H., Horlbeck, M. A., Attenello, F. J., et al. (2016b). Single-cell analysis of long non-coding RNAs in the developing human neocortex. Genome Biol. 17:67. doi: 10.1186/s13059-016-0932-1
Macarthur, J., Bowler, E., Cerezo, M., Gil, L., Hall, P., Hastings, E., et al. (2017). The new NHGRI-EBI catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 45, D896-D901. doi: 10.1093/nar/gkw1133
Martin, M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10–12. doi: 10.14806/ej.17.1.200
Mercer, T. R., Clark, M. B., Andersen, S. B., Brunck, M. E., Haerty, W., and Crawford, J. (2015). Genome-wide discovery of human splicing branchpoints. Genome Res. 25, 290–303. doi: 10.1101/gr.182899.114
Mercer, T. R., Clark, M. B., Crawford, J., Brunck, M. E., Gerhardt, D. J., Taft, R. J., et al. (2014). Targeted sequencing for gene discovery and quantification using RNA CaptureSeq. Nat. Protoc. 9, 989–1009. doi: 10.1038/nprot.2014.058
Mercer, T. R., Dinger, M. E., Sunkin, S. M., Mehler, M. F., and Mattick, J. S. (2008). Specific expression of long noncoding RNAs in the mouse brain. Proc. Natl. Acad. Sci. U.S.A. 105, 716–721. doi: 10.1073/pnas.0706729105
Mercer, T. R., Gerhardt, D. J., Dinger, M. E., Crawford, J., Trapnell, C., and Jeddeloh, J. A. (2012). Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat. Biotechnol. 30, 99–104. doi: 10.1038/nbt.2024
Mercer, T. R., and Mattick, J. S. (2013). Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 20, 300–307. doi: 10.1038/nsmb.2480
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A., and Kingsford, C. (2017). Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419. doi: 10.1038/nmeth.4197
Pertea, M., Kim, D., Pertea, G. M., Leek, J. T., and Salzberg, S. L. (2016). Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 11, 1650–1667. doi: 10.1038/nprot.2016.095
Pertea, M., Pertea, G. M., Antonescu, C. M., Chang, T. -C., Mendell, J. T., and Salzberg, S. L. (2015). StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295. doi: 10.1038/nbt.3122
Pheasant, M., and Mattick, J. S. (2007). Raising the estimate of functional human sequences. Genome Res. 17, 1245–1253. doi: 10.1101/gr.6406307
Quinlan, A. R., and Hall, I. M. (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. doi: 10.1093/bioinformatics/btq033
Quinn, J. J., and Chang, H. Y. (2015). Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 17, 47–62. doi: 10.1038/nrg.2015.10
Robinson, J. T., Thorvaldsdóttir, H., Winckler, W., Guttman, M., Lander, E. S., Getz, G., et al. (2011). Integrative genomics viewer. Nat. Biotechnol. 29, 24–26. doi: 10.1038/nbt.1754
Schierding, W., Cutfield, W. S., and O’Sullivan, J. M. (2014). The missing story behind genome wide association studies: single nucleotide polymorphisms in gene deserts have a story to tell. Front. Genet. 5:39. doi: 10.3389/fgene.2014.00039
Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. doi: 10.1038/nature13595
Seemann, S. E., Mirza, A. H., Hansen, C., Bang-Berthelsen, C. H., Garde, C., Christensen-Dalsgaard, M., et al. (2017). The identification and functional annotation of RNA structures conserved in vertebrates. Genome Res. 27, 1371–1383. doi: 10.1101/gr.208652.116
Siepel, A., Bejerano, G., Pedersen, J. S., Hinrichs, A. S., Hou, M., Rosenbloom, K., et al. (2005). Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 15, 1034–1050. doi: 10.1101/gr.3715005
Stefansson, H., Ophoff, R. A., Steinberg, S., Andreassen, O. A., Cichon, S., Rujescu, D., et al. (2009). Common variants conferring risk of schizophrenia. Nature 460, 744–747. doi: 10.1038/nature08186
Steijger, T., Abril, J. F., Engstrom, P. G., Kokocinski, F., The, R. C., Hubbard, T. J., et al. (2013). Assessment of transcript reconstruction methods for RNA-seq. Nat. Methods 10, 1177–1184. doi: 10.1038/nmeth.2714
Tang, A. D., Soulette, C. M., Van Baren, M. J., Hart, K., Hrabeta-Robinson, E., Wu, C. J., et al. (2018). Full-length transcript characterization of SF3B1 mutation in chronic lymphocytic leukemia reveals downregulation of retained introns. bioRxiv [Preprint]. doi: 10.1101/410183
The International Multiple Sclerosis Genetics Consortium (2011). Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476, 214–219. doi: 10.1038/nature10251
The UniProt Consortium (2008). The Universal Protein Resource (UniProt). Nucleic Acids Res. 36, D190-D195.
Tilgner, H., Jahanbani, F., Blauwkamp, T., Moshrefi, A., Jaeger, E., Chen, F., et al. (2015). Comprehensive transcriptome analysis using synthetic long-read sequencing reveals molecular co-association of distant splicing events. Nat. Biotechnol. 33, 736–742. doi: 10.1038/nbt.3242
Tilgner, H., Jahanbani, F., Gupta, I., Collier, P., Wei, E., Rasmussen, M., et al. (2018). Microfluidic isoform sequencing shows widespread splicing coordination in the human transcriptome. Genome Res. 28, 231–242. doi: 10.1101/gr.230516.117
Uszczynska-Ratajczak, B., Lagarde, J., Frankish, A., Guigó, R., and Johnson, R. (2018). Towards a complete map of the human long non-coding RNA transcriptome. Nat. Rev. Genet. 19, 535–548. doi: 10.1038/s41576-018-0017-y
Visscher, P. M., Wray, N. R., Zhang, Q., Sklar, P., Mccarthy, M. I., Brown, M. A., et al. (2017). 10 Years of GWAS discovery: biology, function, and translation. Am. J. Hum. Genet. 101, 5–22. doi: 10.1016/j.ajhg.2017.06.005
Visscher Peter. M., Brown, M A., McCarthy, M I., and Yang, J. (2012). Five years of GWAS discovery. Am. J. Hum. Genet. 90, 7–24. doi: 10.1016/j.ajhg.2011.11.029
Wan, Y., Qu, K., Zhang, Q. C., Flynn, R. A., Manor, O., Ouyang, Z., et al. (2014). Landscape and variation of RNA secondary structure across the human transcriptome. Nature 505, 706–709. doi: 10.1038/nature12946
Wang, L., Park, H. J., Dasari, S., Wang, S., Kocher, J. -P., and Li, W. (2013). CPAT: coding-potential assessment tool using an alignment-free logistic regression model. Nucleic Acids Res. 41:e74. doi: 10.1093/nar/gkt006
Welter, D., Macarthur, J., Morales, J., Burdett, T., Hall, P., and Junkins, H. (2013). The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 42, D1001-D1006. doi: 10.1093/nar/gkt1229
Xiao, X., Chang, H., and Li, M. (2017). Molecular mechanisms underlying noncoding risk variations in psychiatric genetic studies. Mol. Psychiatry 22, 497–511. doi: 10.1038/mp.2016.241
Keywords: GWAS, non-coding RNA, haplotype blocks, brain, long-read sequencing, RNA-seq, sequins
Citation: Hardwick SA, Bassett SD, Kaczorowski D, Blackburn J, Barton K, Bartonicek N, Carswell SL, Tilgner HU, Loy C, Halliday G, Mercer TR, Smith MA and Mattick JS (2019) Targeted, High-Resolution RNA Sequencing of Non-coding Genomic Regions Associated With Neuropsychiatric Functions. Front. Genet. 10:309. doi: 10.3389/fgene.2019.00309
Received: 03 February 2019; Accepted: 21 March 2019;
Published: 12 April 2019.
Edited by:
Ishaan Gupta, Cornell University, United StatesReviewed by:
Prashanth N. Suravajhala, Birla Institute of Scientific Research, IndiaCopyright © 2019 Hardwick, Bassett, Kaczorowski, Blackburn, Barton, Bartonicek, Carswell, Tilgner, Loy, Halliday, Mercer, Smith and Mattick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin A. Smith, bWFydGluYWxleGFuZGVyc21pdGhAZ21haWwuY29t John S. Mattick, am9obi5tYXR0aWNrQGd0Yy5veC5hYy51aw==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.