
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 05 March 2019
Sec. Evolutionary and Population Genetics
Volume 10 - 2019 | https://doi.org/10.3389/fgene.2019.00172
This article is part of the Research Topic Bioinformatics of Genome Regulation and Systems Biology View all 21 articles
Sex differences in gene expression have been extensively documented, but little is known about these differences in parasitoid species that are widely applied to control pests. Brachymeria lasus is a solitary parasitoid species and has been evaluated as a potential candidate for release to control Lymantria dispar. In this study, gender differences in B. lasus were investigated using Illumina-based transcriptomic analysis. The resulting 37,453 unigene annotations provided a large amount of useful data for molecular studies of B. lasus. A total of 1416 differentially expressed genes were identified between females and males, and the majority of the sex-biased genes were female biased. Gene Ontology (GO) and Pathway enrichment analyses showed that (1) the functional categories DNA replication, fatty acid biosynthesis, and metabolism were enhanced in females and that (2) the only pathway enriched in males was phototransduction, while the GO subcategories enriched in males were those involved in membrane and ion transport. In addition, thirteen genes involving transient receptor potential (TRP) channels were annotated in B. lasus. We further explored and discussed the functions of TRPs in sensory signaling of light and temperature. In general, this study provides new molecular insights into the biological and sexually dimorphic traits of parasitoids, which may improve the application of these insects to the biological control of pests.
Parasitoids are animals that parasitize other organisms (Godfray, 1994). All invertebrate life stages, such as egg, larva or nymph, pupa and adult, can be attacked by oviposition on or in the host or by depositing a larva on or near a host (Boulton et al., 2015). Based on the number of offspring reared in a host, parasitoid wasps are classified as solitary (one parasitoid per host), quasi-gregarious (one parasitoid per host, but hosts are spatially clumped, such as a clutch of eggs on a leaf), or gregarious (multiple parasitoids per host). The vast majority of parasitoids are solitary wasps (Mayhew, 1998). Parasitoids can also be classified as koinobionts (in which hosts continue to develop and grow to some extent) or idiobionts (in which hosts do not grow further). Parasitoid wasps are haplodiploid: males develop from unfertilized eggs and are haploid, while females develop from fertilized eggs and are diploid (Cook, 1993; Heimpel and de Boer, 2008). Parasitoid species (e.g., Sclerodermus harmandi, Trichogramma) are important insects and have been extensively applied to reduce the population size of pest species (Hassan, 1993; Li, 1994; Terayama, 1999; Zhishan et al., 2003; Parra and Zucchi, 2004; Lim et al., 2006). In addition to having important applications, parasitoid and mutualistic Chalcidoidea, such as jewel (Nasonia vitripennis) and fig (Pleistodontes froggatti) wasps, have been important study models of behavioral ecology and evolutionary biology for such traits as their sexual dimorphism in longevity, body size, and dispersal (Hamilton, 1967; Charnov, 1982; Yan et al., 1989; Godfray, 1994).
Animals from a broad range of taxa show sex differences, which include behavioral (Breedlove, 1992), physiological (Bardin and Catterall, 1981), and morphological dimorphisms (Darwin, 1871). It is often assumed that the majority of sexually dimorphic traits arise from differences in the expression of genes present in both sexes (Connallon and Knowles, 2005; Rinn and Snyder, 2005). Sex-biased gene expression has been documented in brown algae (Lipinska et al., 2015), birds (Pointer et al., 2013), nematodes (Albritton et al., 2014), Daphnia pulex (Eads et al., 2007), and multiple insect species, including Drosophila (Jin et al., 2001; Arbeitman et al., 2002; Ranz et al., 2003; Chang et al., 2011), Anopheles gambiae (Hahn and Lanzaro, 2005; Marinotti et al., 2006; Baker et al., 2011), Tribolium castaneum (Prince et al., 2010), vespid wasps (Hunt and Goodisman, 2010), and Bemisia tabaci (Wen et al., 2014). However, few studies of sex differences in gene expression have been done in Hymenoptera insects, and these studies have focussed mainly on social species (e.g., honeybee; Cameron et al., 2013) and model organisms of parasitoids, e.g., jewel wasp N. vitripennis (Wang et al., 2015), which is a classic gregarious species. Most species of parasitoid wasps are thought of as solitary species (Mayhew, 1998), but their sexual transcription differences have not been addressed.
Gypsy moth, Lymantria dispar is a worldwide pest, and its pupal stage can be parasitized by Brachymeria lasus. B. lasus is a solitary parasitoid species and has been evaluated as a potential candidate for release to control L. dispar (Simser and Coppel, 1980), Homona magnanima (Mao and Kunimi, 1991) and Sylepta derogate (Kang et al., 2006). In addition, B. lasus has a wide host range, including many Lepidoptera species (e.g., Mythimna separata, Hyphantria cunea, and Cnaphalocrocis medinalis) (Habu, 1960). Male and female B. lasus differ in many important biological traits, including longevity (Mao and Kunimi, 1994b); development time in the egg, larval and pupal stages (Mao and Kunimi, 1994a); secondary symbionts; and body size (Yan et al., 1989). As B. lasus is a classic solitary species with many documented sex differences, though not yet at the gene expression level, it was used as the experimental material in this study. To reveal B. lasus sex differences at the transcriptional level, we carried out an Illumina-based transcriptomic analysis. This study attempted to provide comprehensive insight into the sexually dimorphic traits of parasitoid wasps at the transcriptome level to improve our understanding of other biological traits with the aim of improving the application of parasitoids to the biological control of pest species.
In northern China, in addition to L. dispar, B. lasus is also an important pupal parasitoid of H. cunea, for which the parasitism ratio is approximately 1.06–3.39% in the field (Yang et al., 2001). To acquire B. lasus adults, we collected the pupae of H. cunea, which may be parasitized by B. lasus and other parasitoid species (e.g., Coccygomimus disparis Viereck; Chouioia cunea Yang) from a field in Xuzhou City, Jiangsu Province, China, in March 2016. After collection, we isolated the pupae individually in polyethylene tubes (height: 7.5 cm; diameter: 1 cm) whose openings were covered by a cotton ball and incubated them at a temperature of 28 ± 0.5°C, a relative humidity (RH) of 70 ± 5% and a photoperiod of 14 L:10 D. We observed and selected B. lasus after adult eclosion.
For the transcriptomic experiment, only 1-day-old B. lasus adults were selected, and the sex was determined under a microscope (Leica M205A, Germany). Then, five adults of the same sex were pooled into a plastic tube (1.5 ml), snap-frozen in liquid nitrogen, and transferred to a –80°C freezer for long-term storage. RNA from each sample group (whole bodies of female and male adults) was extracted with TRIzol reagent (Invitrogen; United States). Each group had three replicates. The quality of the isolated RNA was assessed using a NanoDrop (Thermo Fisher Scientific NanoDrop 2000, United States), and the A260/280 values were all above 2.0. A total of 3 μg total RNA from each sample was converted into cDNA using the NEBNext® UltraTM RNA Library Prep Kit for Illumina® (NEB, United States). In total, six cDNA libraries were constructed and subsequently sequenced with the Illumina HiSeq 2000 platform by Beijing Biomarker Technologies Co., Ltd, resulting in raw reads. Raw sequence data generated were deposited into Sequence Read Archive (SRA) database of NCBI with the accession no. PRJNA513855. Clean reads were obtained by removing reads containing adapter, poly-N reads and low-quality reads from the raw data using FASTX-Toolkit1, and these clean reads were used for further analysis. Then, transcriptome assembly was performed using Trinity (v2.5.1) with the default parameters (Grabherr et al., 2011). For functional annotation, pooled assembled unigenes were searched using BLASTX (v2.2.31) against five public databases, Clusters of Orthologous Groups (COG), Swiss-Prot, NCBI non-redundant protein sequences (nr), KEGG Ortholog database (KO) and GO, with an E-value cutoff of 10-5. Using our assembled transcriptome as a reference, we identified putative genes expressed in males and females by RSEM (Li and Dewey, 2011), using the reads per kb per million reads (RPKM) method. Genes with at least 2-fold changes (i.e., log2∣FC∣≥ 1) and a false discovery rate [FDR] < 0.01 as found by DESeq R package (1.10.1) were considered differentially expressed. The GOseq R package (Young et al., 2010) and KOBAS software (Mao et al., 2005) were used to implement the statistical enrichment of differentially expressed genes (DEGs) in the GO and KEGG pathways, respectively, and an adjusted Q-value <0.05 was chosen as the significance cutoff.
Based on transcriptomic data, a gene of transient receptor potential (trp) involved in the phototransduction pathway enriched only in males (ko: 04745; Supplementary Figure S1-d), trp (Leung et al., 2000), was down-regulated in females, which may lead to a reduction in light response (Leung et al., 2000; Popescu et al., 2006). Therefore, we checked this result at the mRNA expression and behavioral levels.
Total RNA was extracted from the whole bodies of five female or five male adults reared on the pupae of H. cunea using TRIzol (Invitrogen; United States) according to the manufacturer’s protocols, then resuspended in nuclease-free water. Finally, the RNA concentration was measured using a NanoDrop (Thermo Fisher Scientific NanoDrop 2000; United States). Each group have four replicates. Approximately 0.5 mg of total RNA was used as template to synthesize the first-strand cDNA using a PrimeScript RT Reagent Kit (TaKaRa; Japan) following the manufacturer’s protocols. The resultant cDNA was diluted to 0.1 mg/ml for further qRT-PCR analysis (ABI StepOne Plus; United States) using SYBR Green Real-Time PCR Master Mix (TaKaRa; Japan). Primers (Supplementary Table S1) for trp gene were designed using Primer Express 2.0 software. The cycling parameters were 95°C for 30 s followed by 40 cycles of 95°C for 5 s and 62°C for 34 s, ending with a melting curve analysis (65 to 95°C in increments of 0.5°C every 5 s) to check for nonspecific product amplification. Relative gene expression was calculated by the 2-ΔΔCt method using the housekeeping gene GAPDH as a reference to eliminate sample-to-sample variations in the initial cDNA samples.
A glass Y-maze (main arm: 12 cm; two side arms: 5 cm; inner diameter: 1.5 cm; angle between two side arms: 75°) was used for phototaxis assays in a completely dark room (<10 lux, measured by illuminometer, LX-9621, China) at a temperature of 22–26°C. One 1-day-old B. lasus adult (female or male) began the trial in a tube at the base of the apparatus and faced a choice between two tubes, one of which was dark and the other of which was lighted with a 40-watt bulb (approximately 600 lux). After 1 min, the choice was recorded. The sample sizes of the male and female groups were 18 and 24, respectively. After each test, the Y-maze was washed and dried, and the two side arms were changed for the new test.
Prior to analysis, the raw data were tested for normality and homogeneity of variances with the Kolmogorov-Smirnov test and Levene’s test, respectively, and the data were log-transformed if necessary. The qRT-PCR data comparing gene expression in females and males were analyzed with the independent t-test. In phototaxis assays, the preferences for light and dark were analyzed using sign tests, and the differences in female and male phototaxis were analyzed by the chi-square test. All analyses were performed using SPSS v.20 (IBM SPSS, Armonk, NY, United States).
Sexual dimorphism is the condition where the two sexes of the same species exhibit different characteristics (e.g., size, color, behavior) beyond the differences in their sexual organs (Bonduriansky, 2007). Most sexually dimorphic traits are often assumed to arise from differences in the expression of genes present in both sexes (Connallon and Knowles, 2005; Rinn and Snyder, 2005). To reveal B. lasus sex differences at the transcriptional level, we carried out an Illumina-based transcriptomic analysis.
All high-quality reads (101,945,678) from the six samples were pooled and assembled by using Trinity with the default parameters, and a total of 254,656 transcripts with lengths longer than 200 bp were generated. The N50 size was 2706 bp with 57,605 sequences longer than 1 kb. We chose the longest isoform of each gene to construct the unigene set. After isoforms were considered, these assembled transcripts were predicted to be produced from a total of 164,709 unigenes. The N50 size of the unigenes was approximately 814 bp, and their mean length was 572.08 bp (Supplementary Table S2). For annotation, the pooled assembled unigenes were searched using blastx against five public databases with an E-value cutoff of 10-5. A total of 37,453 unigenes were successfully annotated, as shown in Table 1, including 17,248 genes in GO, 13,491 genes in COG, 35,427 genes in nr, 18,195 genes in Swiss-Prot, and 15,133 genes in KEGG.
In the GO analysis, 17,248 unigenes were successfully annotated and classified into three major GO categories: molecular function (MF), cell component (CC), and biological processes (BP), then assigned to 56 subcategories based on GO level 2. The dominant subcategories for the classified genes were catalytic activity and binding for the MF category; cell and cell part for the CC category; and metabolic process, cellular process, and single-organism process for the BP category (Supplementary Table S3). A total of 15,133 KEGG-annotated unigenes were classified into 190 pathways (>10 associated unigenes). Among these pathways, the ten most highly represented were ribosome, carbon metabolism, protein processing in endoplasmic reticulum, oxidative phosphorylation, biosynthesis of amino acids, spliceosome, RNA transport, purine metabolism, peroxisome, and ubiquitin mediated proteolysis (Supplementary Table S4).
Although in most species the male and female genomes differ by a few genes located on sex-specific chromosomes (such as the Y chromosome of mammals), the vast majority of sexually dimorphic traits result from the differential expression of genes that are present in both sexes (Connallon and Knowles, 2005; Rinn and Snyder, 2005; Ellegren and Parsch, 2007), and this is especially true in hymenopteran insects. Because sex determination in hymenopteran species is haplodiploid, females and males are nearly identical genetically (Ellegren and Parsch, 2007). Such DEGs include those that are expressed exclusively in one sex (sex-specific expression) and those that are expressed in both sexes but at a higher level in one sex (sex-biased expression). These sex-biased genes can be further separated into male-biased and female-biased genes, depending on which sex shows higher expression. Genes with equal expression in the two sexes are referred to as unbiased (Ellegren and Parsch, 2007).
Using our assembled transcriptome as a reference, we identified putative genes expressed in males and females using the RPKM method, and genes with at least 2-fold changes and FDR < 0.01 were defined as DEGs. By comparing female and male transcriptomes, 1416 DEGs were found in B. lasus, of which 442 genes were annotated in GO, 420 in COG, 1024 in nr, 613 in Swiss-Prot, and 396 in KEGG (Table 1). Among these DEGs, 986 were up-regulated in females and 430 were up-regulated in males (Supplementary Table S5).
In the GO enrichment analyses, 12 and five subcategories were enriched in females and males, respectively. In females, the enriched subcategories were microtubule cytoskeleton, cytoskeletal part, MCM complex, nucleus, protein complex, kinesin complex, and nucleosome for the CC category; DNA replication initiation, cell division and protein phosphorylation for the BP category; and alpha-1,4-glucosidase activity and zinc ion binding for the MF category (Figure 1A). These results showed that, consistent with the results in flies, mosquitoes, and Daphnia (Ranz et al., 2003; Hahn and Lanzaro, 2005; Eads et al., 2007), including Hymenoptera insects of Nasonia (Wang et al., 2015), most categories were related to DNA replication, which are probably expressed to produce eggs in females (Spradling, 1993; Parisi et al., 2004). The over-representation of transcripts from genes required for DNA replication may be required for nurse cell polyploidization or for the rapid division of embryonic cells, which rely on maternally deposited gene products (Spradling, 1993; Parisi et al., 2004).
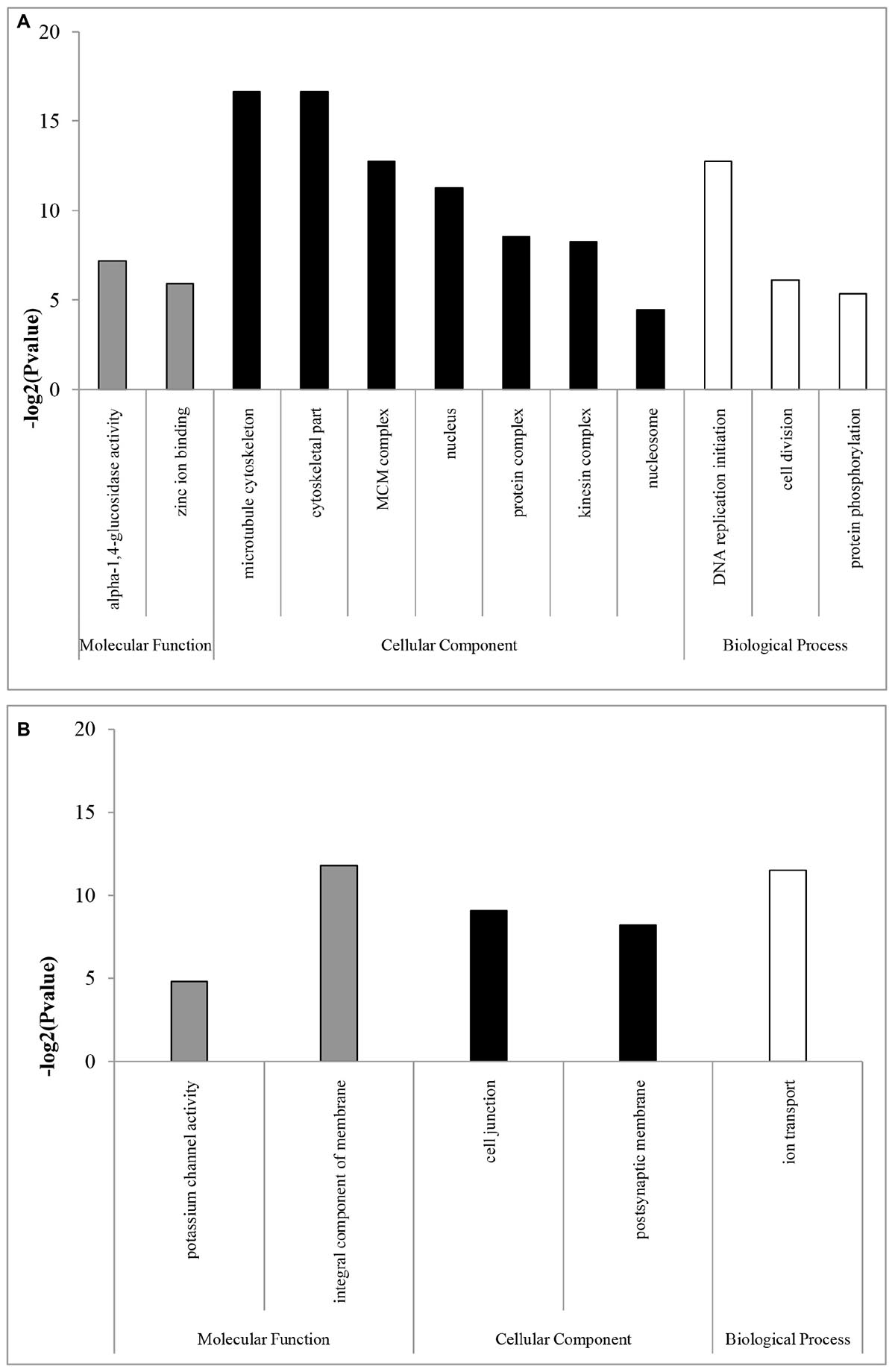
Figure 1. GO enrichment analysis of (A) female- and (B) male-biased genes. GOSeq explicitly takes into account gene selection bias due to differences in gene length and thus the numbers of overlapping sequencing reads. GOSeq was used for the GO enrichment analysis, and an adjusted Q-value <0.05 was chosen as the significance cutoff.
In males, the enriched subcategories were integral component of membrane, cell junction, and postsynaptic membrane for the CC category; ion transport for the BP category; and potassium channel activity for the MF category (Figure 1B), consistent with a study in D. melanogaster (Parisi et al., 2004), which may be mainly related to spermatogenesis (Fuller, 1993). For example, the enriched subcategories associated with membranes were likely due to the requirements of the sperm axoneme structure (Parisi et al., 2004). However, in parasitoids of N. vitripennis species, highly enriched subcategories in males are related to sex-pheromone synthetic enzymes (Wang et al., 2015). Those differences might be likely to contribute by their difference in sexual maturity period. Sexual maturity in many gregarious and quasi-gregarious males (e.g., N. vitripennis) happens before eclosion, and these males can immediately mate with females after eclosion and near the emergence site (Boulton et al., 2015), while solitary B. lasus have mating ability for some days after eclosion (Yan et al., 1989).
Consistent with the results of GO enrichment in females, pathway enrichment tests revealed that DNA replication (ko: 03030; Supplementary Figure S1-a) was enriched in B. lasus females. The functional categories enriched in females also included fatty acid biosynthesis (ko: 00061; Supplementary Figure S1-b) and metabolism (ko01212; Supplementary Figure S1-c). The fatty acid synthase gene (FASN), which encoded the enzyme catalyzing fatty acid synthesis (Jayakumar et al., 1994, 1995; Persson et al., 2008), was probably crucial for egg yolk production and thus female fecundity. In some insects, for example yellow fever mosquito Aedes aegypti, brown planthopper Nilaparvata lugens) (Alabaster et al., 2011; Li et al., 2016), when FAS expression decreases in females, the number of oviposited eggs significantly decreases.
We found that only the phototransduction-fly pathway (ko: 04745; Supplementary Figure S1-d) was enriched in males, which is associated with perception of light signals (Leung et al., 2000). Its potential functions are discussed below.
In terms of biological control, parasitoid species have been extensively applied for reducing pest species population sizes (Hassan, 1993; Li, 1994; Terayama, 1999; Zhishan et al., 2003; Parra and Zucchi, 2004; Lim et al., 2006) because parasitoids can propagate on or in other arthropods. The venom of parasitoid wasps, which is injected into a host by females before or at oviposition, is important for the successful development of the progeny. Parasitoid venoms have diverse physiological effects on hosts, including developmental arrest; alteration in growth and physiology; suppression of immune responses; induction of paralysis, oncosis, or apoptosis; and alteration of host behavior (Edwards et al., 2006; Price et al., 2009; Tian et al., 2010; Kryukova et al., 2011). In total, three female-biased genes (c100635.graph_c0, c101314.graph_c0, c101670.graph_c0; Supplementary Table S5) in this study were annotated for venom proteins, which were related to known insect venoms from N. vitripennis and belonged to previously known insect venom families, such as serine proteases (Graaf et al., 2010; Werren et al., 2010). Despite the large diversity of parasitoid wasp species, only a small number of venom proteins have been described from wasps. A wealth of unexplored biomolecules is present in parasitoid venoms; these proteins are of value in basic evolutionary studies, venom biology, host-parasite interactions, and the study of the evolution of life strategies, and they may potentially contain components that could be used in biological control and pharmacology (Moreau and Asgari, 2015).
Transient Receptor Potential channels are cation channels that are mainly considered as unique polymodal cell sensors; TRPs can be subdivided into six main subfamilies: the TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPP (polycystin), TRPML (mucolipin), and TRPA (ankyrin) groups (Gees et al., 2010). Functionally, TRP channels cause cell depolarization when activated, which may trigger many voltage-dependent ion channels. Upon stimulation, Ca2+-permeable TRP channels generate changes in the intracellular Ca2+ concentration, [Ca2+]i, due to Ca2+ entry via the plasma membrane. However, evidence is increasing that TRP channels are also located in intracellular organelles and serve as intracellular Ca2+ release channels (Berridge et al., 2000; Bootman et al., 2001; Gees et al., 2010). TRP channels in Drosophila are involved in the perception of sensory signals such as light, temperature, humidity, pheromones, sound, and touch (Lin et al., 2005). In our study, we found 13 TRP channel genes in B. lasus; Nasonia and honey bee contain 12 and 11 genes, respectively, indicating that the number of trp channels seems to be well conserved in Hymenoptera (Werren et al., 2010). Of the TRP channel genes in B. lasus, most belong to two subfamilies: TRPC and TRPA (Table 2).
In Drosophila, TRPC plays an important role in the perception of light signals, i.e., the phototransduction pathway (Leung et al., 2000) (ko: 04745; Supplementary Figure S1-d), which was enriched in B. lasus male adults. In Drosophila, a number of genes in the visual signal transduction pathway have been characterized, with functions including rhodopsin activation, phosphoinoside signaling, and the opening of TRP and TRPL channels (Wolff and Ready, 1993; Zuker, 1996; Leung et al., 2000; Wang and Montell, 2007). Our transcriptional analyses (Figure 2A: FDR < 0.01, log2 FC = 1.62) and q-PCR results (Figure 2B: t = -3.169, df = 6, p = 0.019), showed that the gene corresponding to trp (c103240.graph_c0) was more highly expressed in B. lasus males, consistent with the phototaxis test. Although both females and males tended to move toward light (Figure 2C: female, Z = -1.34, p < 0.05; male, Z = -1.6, p < 0.05), the tendency to prefer light was significantly influenced by sex in adults (Figure 2C. χ2 = 4.17, df = 1, p < 0.05), males more preferring to move to light. This result is similar to the results of research on trp mutants in Drosophila, which had altered phenotypes, including a reduction in light response (Leung et al., 2000; Popescu et al., 2006). Female reduction in light response might be due to their long periods living in the dark to search for hosts and lay offspring into them, as most host species (e.g., pupae of L. dispar or H. cunea) hide in dark environments, such as the litter horizon (Yan et al., 1989; Yang et al., 2001). Surprisingly, five members of the TRPA subfamily, which is involved in sensing environmental temperature, were annotated in our study. Animals must maintain thermal homeostasis and avoid prolonged contact with harmfully hot or cold objects (Caterina, 2007; Karashima et al., 2009). Unlike most parasitoid species, which overwinter in their hosts as eggs or larvae, B. lasus lives through the winter in its adult stage (Yan et al., 1989). Thus, TRPA may be essential for B. lasus adults, allowing them to sense harmful cold during winter. In addition, intraspecific aggregations in B. lasus have been observed in previous research, and an active component that elicited the aggregation response was isolated and identified as 3-hexanone (Mohamed and Coppel, 1987). The effects of aggregation behavior include mating, host attack, defense, and thermoregulation, and in this species, a previous study suggested that aggregation resulted from an increase in reproductive success by increasing the probability of mate location, as well as offering the possibility of mate choice (Mohamed and Coppel, 1987). However, combining the above results, adults may also aggregate at a site for purposes of thermoregulation, especially in winter, in response to cold. Further studies are required to elucidate the nature of this cue.
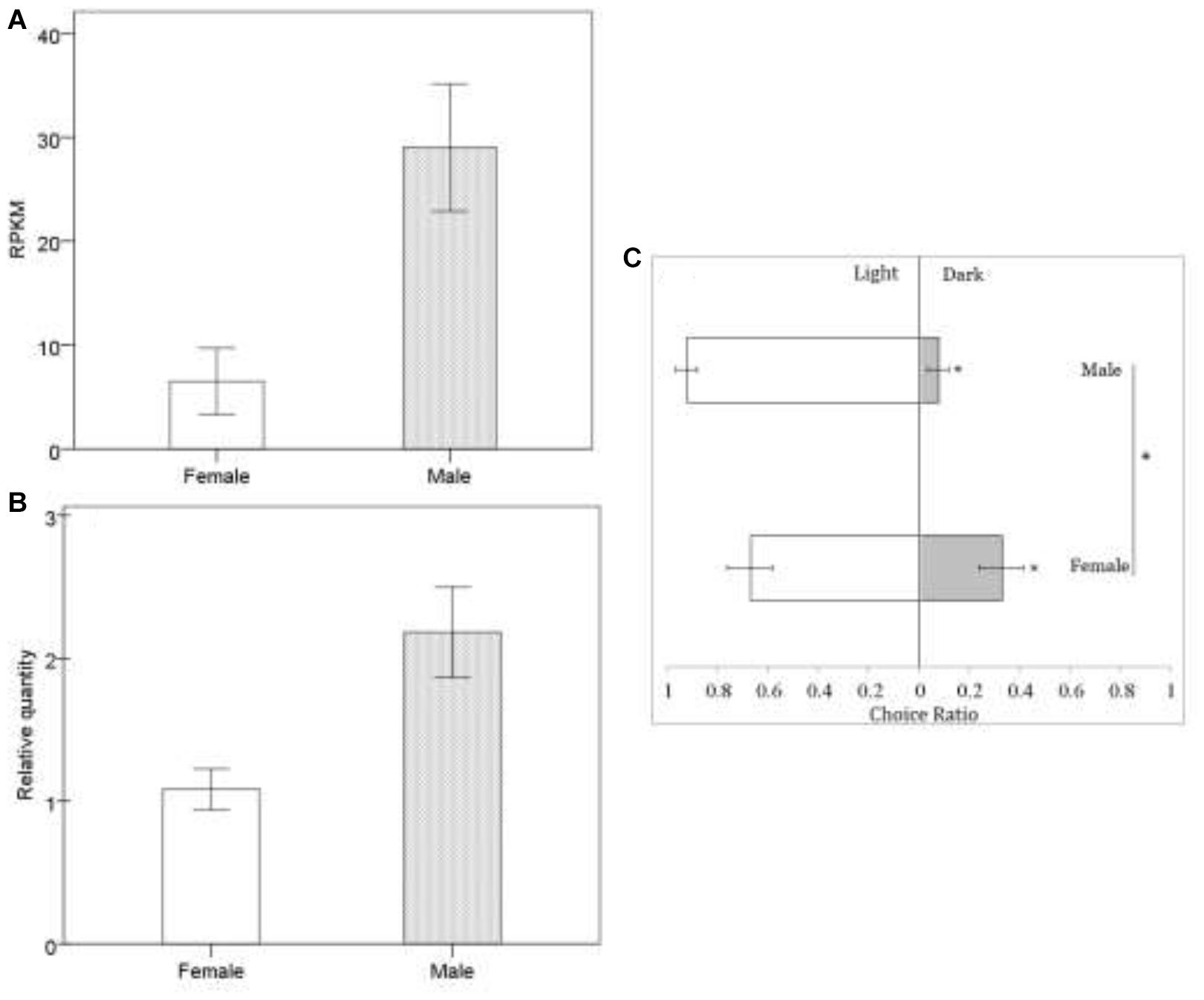
Figure 2. Sexual difference in response to light at mRNA level (A,B) and behavioral level (C). In transcriptomic data, we identified putative genes expressed using the reads per kb per million reads (RPKM) method. Quantitative real-time PCR (qRT-PCR) analysis was used to calculated the relative gene expression to further check the transcriptomic data, in which the differences in female and male were analyzed by the independent t-test. There was a highly significant correlation co-efficient of 0.885 between transcriptomic data and qRT-PCR data. Behavioral responses of Brachymeria lasus adults to dark or light were tested with phototaxis assays. The differences in female (n = 24) and male (n = 18) phototaxis were analyzed by the chi-square test. ∗indicates p < 0.05. The error bars indicate standard errors.
Brachymeria lasus is a solitary parasitoid species and has been evaluated as a potential candidate for release to control L. dispar. Whereas previous studies have focussed on the application of parasitoids and their sex differences in phenotypes, this study focussed mainly on sex differences in gene expression. Brachymeria lasus as a representative of solitary species was studied, which enriched our understanding of sexual transcription differences in parasitoid wasps, especially solitary species. Here, we performed transcriptome assembly using the Trinity program, which provided a large amount of useful information for molecular studies of B. lasus, including venom protein and perception of sensory signals. In addition to sex-biased genes, epigenetic processes, such as DNA methylation, are known to play important roles in differentiating phenotype and have been widely studied in Hymenopteran insects, for example, female morphs (queens and workers) in the honeybee, Apis mellifera (Kucharski et al., 2008; Lyko et al., 2010), although these processes do not appear to be in Nasonia (Wang et al., 2015). More future research will be conducted to better understand the molecular mechanisms underlying the biological traits of sex differences in B. lasus and to better apply this parasitoid to the biological control of pests.
Publicly available datasets were analyzed in this study. This data can be found here: https://dataview.ncbi.nlm.nih.gov/object/PRJNA513855.
There was no requirement to seek ethical approval to carry out the work described above. However, the use of insects in the above experiments was kept to a minimum.
P-CL conceived and designed the experiments. P-CL and ST performed the experiments. P-CL and D-JH wrote the manuscript. All the authors reviewed the manuscript.
A project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). This work was also supported by the Doctorate Fellowship Foundation of Nanjing Forestry University and the Natural Science Foundation of Jiangsu Province (BK20131421).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We gratefully acknowledge undergraduates Ju Luo, Min Li, and Chenxi Zhao of the Nanjing Forestry University for their assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00172/full#supplementary-material
Alabaster, A., Isoe, J., Zhou, G., Lee, A., Murphy, A., Day, W. A., et al. (2011). Deficiencies in acetyl-CoA carboxylase and fatty acid synthase 1 differentially affect eggshell formation and blood meal digestion in Aedes aegypti. Insect Biochem. Mol. Biol. 41, 946–955. doi: 10.1016/j.ibmb.2011.09.004
Albritton, S. E., Kranz, A. L., Rao, P., Kramer, M., Dieterich, C., and Ercan, S. (2014). Sex-biased gene expression and evolution of the x chromosome in nematodes. Genetics 197, 865–883. doi: 10.1534/genetics.114.163311
Arbeitman, M. N., Furlong, E. E., Imam, F., Johnson, E., Null, B. H., Baker, B. S., et al. (2002). Gene expression during the life cycle of Drosophila melanogaster. Science 297, 2270–2275. doi: 10.1126/science.1072152
Baker, D. A., Nolan, T., Fischer, B., Pinder, A., Crisanti, A., and Russell, S. (2011). A comprehensive gene expression atlas of sex- and tissue-specificity in the malaria vector. Anopheles gambiae. BMC Genomics 12:296. doi: 10.1186/1471-2164-12-296
Bardin, C. W., and Catterall, J. F. (1981). Testosterone: a major determinant of extragenital sexual dimorphism. Science 211, 1285–1294. doi: 10.1126/science.7010603
Berridge, M. J., Lipp, P., and Bootman, M. D. (2000). The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21. doi: 10.1038/35036035
Bonduriansky, R. (2007). The evolution of condition-dependent sexual dimorphism. Am. Nat. 169, 9–19.
Bootman, M. D., Collins, T. J., Peppiatt, C. M., Prothero, L. S., MacKenzie, L., De Smet, P., et al. (2001). Calcium signalling-an overview. Semin. Cell Dev. Biol. 12, 3–10. doi: 10.1006/scdb.2000.0211
Boulton, R. A., Collins, L. A., and Shuker, D. M. (2015). Beyond sex allocation: the role of mating systems in sexual selection in parasitoid wasps. Biol. Rev. 90, 599–627. doi: 10.1111/brv.12126
Breedlove, S. M. (1992). Sexual dimorphism in the vertebrate nervous-system. J. Neurosci. 12, 4133–4142. doi: 10.1523/JNEUROSCI.12-11-04133.1992
Cameron, R. C., Duncan, E. J., and Dearden, P. K. (2013). Biased gene expression in early honeybee larval development. BMC Genomics 14:903. doi: 10.1186/1471-2164-14-903
Caterina, M. J. (2007). Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R64–R76. doi: 10.1152/ajpregu.00446.2006
Chang, P. L., Dunham, J. P., Nuzhdin, S. V., and Arbeitman, M. N. (2011). Somatic sex specific transcriptome differences in Drosophila revealed by whole transcriptome sequencing. BMC Genomics 12:364. doi: 10.1186/1471-2164-12-364
Connallon, T., and Knowles, L. L. (2005). Intergenomic conflict revealed by patterns of sex-biased gene expression. Trends Genet. 21, 495–499. doi: 10.1016/j.tig.2005.07.006
Cook, J. M. (1993). Sex determination in the hymenoptera-a review of models and evidence. Heredity 71, 421–435. doi: 10.1038/hdy.1993.157
Darwin, C. R. (1871). The Descent of Man, and Selection in Relation to Sex, 2nd Edn. London: John Murray.
Eads, B. D., Colbourne, J. K., Bohuski, E., and Andrews, J. (2007). Profiling sex-biased gene expression during parthenogenetic reproduction in Daphnia pulex. BMC Genomics 8:464. doi: 10.1186/1471-2164-8-464
Edwards, J. P., Bell, H. A., Audsley, N., Marris, G. C., Kirkbride-Smith, A., Bryning, G., et al. (2006). The ectoparasitic wasp Eldophus pennicornis (Hymenoptera: Eulophiclae) uses instar-specific endocrine disruption strategies to suppress the development of its host Lacanobia oleracea (Lepidoptera: Noctuidae). J. Insect Physiol. 52, 1153–1162. doi: 10.1016/j.jinsphys.2006.08.003
Ellegren, H., and Parsch, J. (2007). The evolution of sex-biased genes and sex-biased gene expression. Nat. Rev. Genet. 8:689. doi: 10.1038/nrg2167
Fuller, M. T. (1993). “Spermatogenesis,” in The Development of Drosophila, eds M. Bate and A. Martinez-Arias (Cold Sping Harbor, NY: Cold Sping Harbor Laboratory Press), 71–148.
Gees, M., Colsoul, B., and Nilius, B. (2010). The role of transient receptor potential cation channels in Ca2+ Signaling. Cold Spring Harb. Perspect. Biol. 2:a003962. doi: 10.1101/cshperspect.a003962
Godfray, H. C. J. (1994). Parasitoids: Behavioural and Evolutionary Ecology. Princeton: Princeton University Press.
Graaf, D. C. D., Aerts, M., Brunain, M., Desjardins, C. A., Jacobs, F. J., Werren, J. H., et al. (2010). Insights into the venom composition of the ectoparasitoid wasp Nasonia vitripennis from bioinformatic and proteomic studies. (special issue: the Nasonia genome.). Insect Mol. Biol. 19, 11–26. doi: 10.1111/j.1365-2583.2009.00914.x
Grabherr, M. G., Haas, B. J., Yassour, M., Levin, J. Z., Thompson, D. A., Amit, I., et al. (2011). Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 29, 644–652. doi: 10.1038/nbt.1883
Habu, A. (1960). A revision of the Chalcididae (Hymenoptera) of Japan, with descriptions of sixteen new species. Bull. Natl. Inst. Agric. Sci. 11, 131–363.
Hahn, M. W., and Lanzaro, G. C. (2005). Female-biased gene expression in the malaria mosquito Anopheles gambiae. Curr. Biol. 15, 192–193. doi: 10.1016/j.cub.2005.03.005
Hamilton, W. D. (1967). Extraordinary sex ratios. Science 156, 477–488. doi: 10.1126/science.156.3774.477
Hassan, S. A. (1993). The mass rearing and utilization of Trichogramma to control lepidopterous pests: achievements and outlook. Pest Manage. Sci. 37, 387–391. doi: 10.1002/ps.2780370412
Heimpel, G. E., and de Boer, J. G. (2008). Sex determination in the Hymenoptera. Ann. Rev. Entomol. 53, 209–230. doi: 10.1146/annurev.ento.53.103106.093441
Hunt, B. G., and Goodisman, M. A. (2010). Evolutionary variation in gene expression is associated with dimorphism in eusocial vespid wasps. Insect Mol. Biol. 19, 641–652. doi: 10.1111/j.1365-2583.2010.01021.x
Jayakumar, A., Chirala, S. S., Chinault, A. C., Baldini, A., Abu-Elheiga, L., and Wakil, S. J. (1994). Isolation and chromosomal mapping of genomic clones encoding the human fatty acid synthase gene. Genomics 23, 420–424. doi: 10.1006/geno.1994.1518
Jayakumar, A., Tai, M. H., Huang, W. Y., Al-Feel, W., Hsu, M., Abu-Elheiga, L., et al. (1995). Human fatty acid synthase: properties and molecular cloning. Proc. Natl. Acad. Sci. U.S.A. 92, 8695–8699. doi: 10.1073/pnas.92.19.8695
Jin, W., Riley, R. M., Wolfinger, R. D., White, K. P., Passadorgurgel, G., and Gibson, G. (2001). The contributions of sex, genotype and age to transcriptional variance in Drosophila melanogaster. Nat. Genet. 29:389. doi: 10.1038/ng766
Kang, X. X., Chen, J., Wang, C. C., and Yang, Y. Z. (2006). Identification and behaviors of parasitoids of Sylepta derogata in the Yangtze River and Huihe Valley. Chin. Bull. Entomol. 35, 241–245.
Karashima, Y., Talavera, K., Everaerts, W., Janssens, A., Kwan, K. Y., Vennekens, R., et al. (2009). Trpa1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 106, 1273–1278. doi: 10.1073/pnas.0808487106
Kryukova, N., Dubovskiy, I., Chertkova, E., Vorontsova, Y., Slepneva, I., and Glupov, V. (2011). The effect of Habrobracon hebetor venom on the activity of the prophenoloxidase system, the generation of reactive oxygen species and encapsulation in the haemolymph of Galleria mellonella larvae. J. Insect Physiol. 57, 769–800. doi: 10.1016/j.jinsphys.2011.03.008
Kucharski, R., Maleszka, J., Foret, S., and Maleszka, R. (2008). Nutritional control of reproductive status in honeybees via DNA methylation. Science 319, 1827–1830. doi: 10.1126/science.1153069
Leung, H. T., Geng, C., and Pak, W. L. (2000). Phenotypes of trpl mutants and interactions between the transient receptor potential (TRP) and TRP-like channels in Drosophila. J. Neurosci. 20, 6797–6803. doi: 10.1523/JNEUROSCI.20-18-06797.2000
Li, B., and Dewey, C. N. (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12:323. doi: 10.1186/1471-2105-12-323
Li, L. (1994). “Worldwide use of Trichogramma for biological control on different crops: a survey,” in Biological Control with Egg Parasitoids, eds E. Wajnberg and S. A. Hassan (Wallingford: Cab International).
Li, L., Jiang, Y., Liu, Z., You, L., Wu, Y., Xu, B., et al. (2016). Jinggangmycin increases fecundity of the brown planthopper, Nilaparvata lugens (Stål) via fatty acid synthase gene expression. J. Proteomics 130, 140–149. doi: 10.1016/j.jprot.2015.09.022
Lim, J. O., Lyu, D. P., Choi, G. S., Jeong, Y. J., Shin, S. C., and Lee, S. H. (2006). A taxonomie note on Sclerodermas harmandi, ectoparasite of stem and wood boring insect larvae (Hymenoptera: Chrysidoidea’-Bethylidae) in South Korea. J. Asia Pac. Entomol. 9, 115–119. doi: 10.1016/S1226-8615(08)60282-4
Lin, H., Mann, K. J., Starostina, E., Kinser, R. D., and Pikielny, C. W. (2005). A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones. Proc. Natl. Acad. Sci. U.S.A. 102, 12831–12836. doi: 10.1073/pnas.0506420102
Lipinska, A., Cormier, A., Luthringer, R., Peters, A. F., Corre, E., Gachon, C. M., et al. (2015). Sexual dimorphism and the evolution of sex-biased gene expression in the brown alga ectocarpus. Mol. Biol. Evol. 32, 1581–1597. doi: 10.1093/molbev/msv049
Lyko, F., Foret, S., Kucharski, R., Wolf, S., Falckenhayn, C., and Maleszka, R. (2010). The honey bee epigenomes: differential methylation of brain DNA in queens and workers. PLoS Biol. 8:e1000506. doi: 10.1371/journal.pbio.1000506
Mao, H., and Kunimi, Y. (1991). Pupal mortality of the oriental tea tortrix, Homona magnanima Diakonoff (Lepidoptera: Tortricidae), caused by parasitoids and pathogens. Jpn. J. Appl. Entomol. Zool. 35, 241–245. doi: 10.1303/jjaez.35.241
Mao, H., and Kunimi, Y. (1994a). Effects of temperature on the development and parasitism of Brachymeria lasus, a pupal parasitoid of Homona magnanima. Entomol. Exp. Appl. 71, 87–90. doi: 10.1111/j.1570-7458.1994.tb01773.x
Mao, H., and Kunimi, Y. (1994b). Longevity and fecundity of Brachymeria lasus (Walker) (Hymenoptera: Chalcididae), a pupal parasitoid of the Oriental tea tortrix, Homona magnanima Diakonoff (Lepidoptera: Tortricidae) under laboratory conditions. Appl. Entomol. Zool. 29, 237–243. doi: 10.1303/aez.k29.237
Mao, X., Cai, T., Olyarchuk, J. G., and Wei, L. (2005). Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 21, 3787–3793. doi: 10.1093/bioinformatics/bti430
Marinotti, O., Calvo, E., Nguyen, Q. K., Dissanayake, S., Ribeiro, J. M., and James, A. A. (2006). Genome-wide analysis of gene expression in adult Anopheles gambiae. Insect Mol. Biol. 15, 1–12. doi: 10.1111/j.1365-2583.2006.00610.x
Mayhew, P. J. (1998). The life-histories of parasitoid wasps developing in small gregarious broods. Neth. J. Zool. 48, 225–240. doi: 10.1163/156854298X00084
Mohamed, M. A., and Coppel, H. C. (1987). Pheromonal basis for aggregation behavior of parasitoids of the gypsy moth: Brachymeria intermedia, (Nees) and Brachymeria lasus, (Walker) (Hymenoptera: Chalcididae). J. Chem. Ecol. 13, 1385–1393. doi: 10.1007/BF01012285
Moreau, S. J. M., and Asgari, S. (2015). Venom proteins from parasitoid wasps and their biological functions. Toxins 7, 2385–2412. doi: 10.3390/toxins7072385
Parisi, M., Nuttall, R., Edwards, P., Minor, J., Naiman, D., Lü, J., et al. (2004). A survey of ovary-, testis-, and soma-biased gene expression in Drosophila melanogaster adults. Genome Biol. 5:R40. doi: 10.1186/gb-2004-5-6-r40
Parra, J. R. P., and Zucchi, A. R. (2004). Trichogramma in Brazil: feasibility of use after twenty years of research. Neotrop. Entomol. 33, 271–281. doi: 10.1590/S1519-566X2004000300001
Persson, B., Bray, J. E., Bruford, E., Dellaporta, S. L., Favia, A. D., Duarte, R. G., et al. (2008). The sdr (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chem. Biol. Interact. 178, 94–98. doi: 10.1016/j.cbi.2008.10.040
Pointer, M. A., Harrison, P. W., Wright, A. E., and Mank, J. E. (2013). Masculinization of gene expression is associated with exaggeration of male sexual dimorphism. PLoS Genet. 9:e1003697. doi: 10.1371/journal.pgen.1003697
Popescu, D. C., Ham, A. J., and Shieh, B. H. (2006). Scaffolding protein INAD regulates deactivation of vision by promoting phosphorylation of transient receptor potential by eye protein kinase C in Drosophila. J. Neurosci. 26, 8570–8577. doi: 10.1523/JNEUROSCI.1478-06.2006
Price, D., Bell, H., Hinchliffe, G., Fitches, E., Weaver, R., and Gatehouse, J. A. (2009). Venom metalloproteinase from the parasitic wasp Eulophus pennicornis is toxic towards its host, tomato moth (Lacanobia oleracae). Insect Mol. Biol. 18, 195–202. doi: 10.1111/j.1365-2583.2009.00864.x
Prince, E. G., Kirkland, D., and Demuth, J. P. (2010). Hyperexpression of the X chromosome in both sexes results in extensive female bias of X-linked genes in the flour beetle. Genome Biol. Evol. 2, 336–346. doi: 10.1093/gbe/evq024
Ranz, J., Castillo-Davis, C., Meiklejohn, C., and Hartl, D. (2003). Sex-dependent gene expression and evolution of the Drosophila transcriptome. Science 300, 1742–1745. doi: 10.1126/science.1085881
Rinn, J. L., and Snyder, M. (2005). Sexual dimorphism in mammalian gene expression. Trends Genet. 21, 298–305. doi: 10.1016/j.tig.2005.03.005
Simser, D. H., and Coppel, H. C. (1980). Female-produced sex pheromone in Brachymeria lasus and B. intermedia (Hym.: Chalcididae). BioControl 25, 373–380.
Spradling, A. C. (1993). “Developmental genetics of oogenesis,” in The Development of Drosophila, eds M. Bate and A. Martinez-Arias (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), 1–70.
Terayama, M. (1999). “Description of new species of the family Bethylidae from the Ryukyus, and taxonomic notes on the Japanese species of the genus Sclerodermus,” in Identification guide to the Aculeata of the Nansei Islands, eds Y. Seiki, S. Ikudome, and M. Terayama (Sapporo: Hokkaido University Press).
Tian, C., Wang, L., Ye, G., and Zhu, S. (2010). Inhibition of melanization by a Nasonia defensin-like peptide: implications for host immune suppression. J. Insect Physiol. 56, 1857–1862. doi: 10.1016/j.jinsphys.2010.08.004
Wang, T., and Montell, C. (2007). Phototransduction and retinal degeneration in Drosophila. Pflügers Arch. Eur. J. Physiol. 454, 821–847. doi: 10.1007/s00424-007-0251-1
Wang, X., Werren, J. H., and Clark, A. G. (2015). Genetic and epigenetic architecture of sex-biased expression in the jewel wasps Nasonia vitripennis and giraulti. Proc. Natl. Acad. Sci. U.S.A. 112, E3545–E3554. doi: 10.1073/pnas.1510338112
Wen, X., Guo, L., Jiao, X., Yang, N., Xin, Y., Wu, Q., et al. (2014). Transcriptomic dissection of sexual differences in Bemisia tabaci, an invasive agricultural pest worldwide. Sci. Rep. 4:4088. doi: 10.1038/srep04088
Werren, J. H., Richards, S., Desjardins, C. A., Niehuis, O., Gadau, J., Colbourne, J. K., et al. (2010). Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science 327, 343–348. doi: 10.1126/science.1178028
Wolff, T., and Ready, D. (1993). “Pattern formation in the Drosophila retina,” in The Development of Drosophila melanogaster, eds M. Bate and A. M. Arias (Plainview, NY: Cold Spring Harbor Lab. Press), 1277.
Yan, J. J., Xu, C. H., Li, G. W., Zhang, P. Y., Gao, W. C., Yao, D. F., et al. (1989). Parasites and Predators of Forest Pest. Beijing: China Forestry Publishing House.
Yang, X. Q., Wei, J. R., and Yang, Z. Q. (2001). A survey on insect natural enemies of Hyphantriacunea in Dalian district, Liaoning Province. Chin. J. Biol. Control 17, 40–42.
Young, M. D., Wakefield, M. J., Smyth, G. K., and Oshlack, A. (2010). Gene ontology analysis for rna-seq: accounting for selection bias. Genome Biol. 11:R14. doi: 10.1186/gb-2010-11-2-r14
Zhishan, W., Hopper, K. R., Ode, P. J., Fuester, R. W., Jia-Hua, C., and Heimpel, G. E. (2003). Complementary sex determination in Hymenopteran parasitoids and its implications for biological control. Entomol. Sin. 10, 81–93. doi: 10.1111/j.1744-7917.2003.tb00369.x
Keywords: sexually dimorphic, Brachymeria lasus, transcriptomic analysis, sex determination, venom protein, transient receptor potential channels
Citation: Liu P-C, Tian S and Hao D-J (2019) Sexual Transcription Differences in Brachymeria lasus (Hymenoptera: Chalcididae), a Pupal Parasitoid Species of Lymantria dispar (Lepidoptera: Lymantriidae). Front. Genet. 10:172. doi: 10.3389/fgene.2019.00172
Received: 12 October 2018; Accepted: 18 February 2019;
Published: 05 March 2019.
Edited by:
Ancha Baranova, George Mason University, United StatesReviewed by:
Juan Pedro M. Camacho, University of Granada, SpainCopyright © 2019 Liu, Tian and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: De-Jun Hao, ZGpoYW9AbmpmdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.