
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Genet. , 27 November 2018
Sec. Genetics of Common and Rare Diseases
Volume 9 - 2018 | https://doi.org/10.3389/fgene.2018.00529
This article is part of the Research Topic Proceedings of the ‘Third International Conference of FMR1 Premutation: Basic Mechanisms and Clinical Involvement’ View all 20 articles
 Dorothy A. Fink1
Dorothy A. Fink1 Lawrence M. Nelson2
Lawrence M. Nelson2 Reed Pyeritz3
Reed Pyeritz3 Josh Johnson4
Josh Johnson4 Stephanie L. Sherman5
Stephanie L. Sherman5 Yoram Cohen6
Yoram Cohen6 Shai E. Elizur6*
Shai E. Elizur6*Abnormalities in the X-linked FMR1 gene are associated with a constellation of disorders, which have broad and profound implications for the person first diagnosed, and extended family members of all ages. The rare and pleiotropic nature of the associated disorders, both common and not, place great burdens on (1) the affected families, (2) their care providers and clinicians, and (3) investigators striving to conduct research on the conditions. Fragile X syndrome, occurring more severely in males, is the leading genetic cause of intellectual disability. Fragile X associated tremor and ataxia syndrome (FXTAS) is a neurodegenerative disorder seen more often in older men. Fragile X associated primary ovarian insufficiency (FXPOI) is a chronic disorder characterized by oligo/amenorrhea and hypergonadotropic hypogonadism before age 40 years. There may be significant morbidity due to: (1) depression and anxiety related to the loss of reproductive hormones and infertility; (2) reduced bone mineral density; and (3) increased risk of cardiovascular disease related to estrogen deficiency. Here we report the case of a young woman who never established regular menses and yet experienced a 5-year diagnostic odyssey before establishing a diagnosis of FXPOI despite a known family history of fragile X syndrome and early menopause. Also, despite having clearly documented FXPOI the woman conceived spontaneously and delivered two healthy children. We review the pathophysiology and management of FXPOI. As a rare disease, the diagnosis of FXPOI presents special challenges. Connecting patients and community health providers with investigators who have the requisite knowledge and expertise about the FMR1 gene and FXPOI would facilitate both patient care and research. There is a need for an international natural history study on FXPOI. The effort should be coordinated by a global virtual center, which takes full advantage of mobile device communication systems.
Primary ovarian insufficiency (POI) is part of the differential diagnosis for any woman of reproductive age who presents with irregular menstrual cycles or infertility. In women under the age of 40 years, POI is characterized by at least 4 months of unpredictable or absent menstrual periods and two serum follicle stimulating hormone (FSH) levels in the menopausal range at least 1 month apart (based on the particular laboratory reference range) (Nelson, 2009). In this case presentation, we describe a 20-year-old woman with fragile X associated primary ovarian insufficiency (FXPOI) who experienced a diagnostic odyssey of nearly 5 years despite: (1) genetic testing at an early age revealing a positive FMR1 premutation carrier status; (2) ongoing oligo-amenorrhea; (3) elevated FSH levels; and (4) a family history of POI. The result was a 5-year delay in starting appropriate hormone replacement therapy. She was also treated with thyroid and psychiatric medications that may have been avoided with an appropriate diagnosis and hormone replacement regimen. Despite the diagnosis of FXPOI, the young woman conceived two healthy pregnancies without medical intervention while on hormone replacement therapy. Many women with POI and their clinicians do not realize that it is possible to conceive without medical intervention and do not understand the need for appropriate hormone replacement (Hipp et al., 2016).
Progress in rare disease research presents special challenges due to small, geographically dispersed patient populations and underlying clinical heterogeneity. Evidence supports a need to move beyond methodological methods to address these challenges and to begin to understand the patient perspective at a deeper level in order to develop more pragmatic approaches (Tingley et al., 2018). The traditional approach fashions a clinical case history, which becomes progressively abstracted from the patient’s experience and the context of its original telling. The patient becomes increasingly incidental and takes on what might be best described as an anonymous shadow in the course of events. This prevents a full appreciation of the patient narrative sense, which is fundamental to the care, clinical management of individuals over time, as well as to effective clinical research (Greenhalgh and Meadows, 1999). Therefore, we include experiential quotes from the patient in our case report.
In May of 2006 a 20 year-old woman presented to the National Institutes of Health (NIH) Clinical Center for evaluation. Her chief complaint was “I am not feeling like myself.” She reported experiencing hot flashes, night sweats, insomnia, occasional crying episodes, sadness, and an unpleasant jittery feeling. She had experienced loss of interest in activities she normally enjoyed. She also complained of waking up in the middle of the night with intense hunger. At age 18, she developed symptoms of severe depression that required her to take medical leave from her freshman year of college. Since then she was on numerous psychotropic medications and at the time of admission was on an extensive and complex regimen. By report of the patient and her mother, her depression had been relentless and difficult to treat.
Here is how the patient described the situation:
“I left my university on medical leave and spent my freshman year in bed or at doctors’ offices. No one knew what was wrong with me, so they kept referring me to different doctors and prescribing more medicines to treat the symptoms. The psych docs sent me to the medical docs and the medical docs sent me to the psych! It was the most frustrating, upsetting, and debilitating year of my life.”
Cascade genetic testing at 4 years old had uncovered the patient carried an FMR1 premutation (100–110 CGG repeats). Her older brother was found to have fragile X syndrome by genetic testing at age 9 years. Her mother and aunt also carried an FMR1 premutation and both had experienced “premature menopause.” The patient reported menarche occurred at age 11. She never established regular menses. She began taking the oral contraceptive at age 13 due to debilitating dysmenorrhea and menorrhagia. She stopped the oral contraceptives at age 16. From age 16 to 18 she experienced only “spotting” every 3 to 4 months. Between age 17 and 18 she began having night sweats. Her endocrinologist began her on levothyroxine replacement despite normal free T4 and TSH levels. Subsequent endocrinologic evaluation at a referral center suggested possible Cushing’s syndrome. She had an elevated morning serum cortisol, an elevated urinary free cortisol (twice the upper limit of normal), and an abnormal overnight dexamethasone suppression test.
On admission to the NIH Clinical Center, the woman had normal vital signs. Her body mass index (BMI) was 28.3 kg/m2. Physical exam revealed no stigmata of Cushing’s syndrome. Initial psychiatric consultation at the NIH concluded the young woman had a mood disorder due to her general medical condition, possibly primary ovarian insufficiency. Diurnal serum cortisol levels and 24-h urine free cortisol were normal. Transvaginal ultrasound findings were consistent with a diagnosis of POI [a 2 mm endometrial stripe, a very small left ovary (1.2 × 1.6 × 1.0 cm) with no visible follicles, and the right ovary could not be convincingly demonstrated]. Serum gonadotropins were in the menopausal range (FSH 46 and LH 26 IU/L). Serum estradiol was also in the menopausal range (23.3 pg/ml). Serum 21-hydroxylase antibodies, thyroid peroxidase antibodies, and thyroglobulin antibodies were all negative. Serum prolactin and MRI of the pituitary were normal. TSH and free T4 were normal.
The NIH discharged the woman with the newly recognized diagnosis of FXPOI. She began taking hormone replacement therapy (a daily dose of 100 microgram of estradiol by transdermal patch and cyclic medroxyprogesterone acetate by mouth 10 mg per day for the first 12 days of each month). She kept a menstrual calendar as instructed. After discharge her psychotropic medications and thyroid replacement were tapered, she menstruated regularly on the hormone replacement regimen, and her symptoms of anxiety and depression resolved.
Here is how the patient described the situation:
“Therefore, when NIH diagnosed me with FXPOI, I was thrilled. It was the first time in 3 years somebody understood me and could help me. All I wanted to do was to go to college and experience a typical 20-year-old life. I did, and it was fabulous!”
The young woman married in August 2012. She conceived shortly thereafter without medical intervention while on hormone replacement therapy. She terminated the pregnancy as prenatal genetic testing showed a full mutation in FMR1 in the fetus. Thereafter, she conceived two more pregnancies without medical intervention while on hormone replacement therapy, one in 2013 and one in 2016. Shortly prior to the conception in 2016 a physician at an IVF clinic suggested she proceed to egg donation and quoted a current chance of conception of less than 0.1%. This is despite her history of having had two prior pregnancies subsequent to the diagnosis of FXPOI. Both pregnancies and deliveries were unremarkable with the birth of two healthy girls.
The FMR1 premutation is now an established cause of POI (Sherman, 2000). A premutation is defined as having 55–199 expanded CGG repeats located in the 5′ untranslated region (UTR) of the X-linked gene, FMR1. The frequency of women who carry a premutation is about 1/300 (Hunter et al., 2014). The diagnostic criteria required to confirm a diagnosis of POI are summarized in Supplementary Table 1. The indicated clinical assessments recommended after making the diagnosis are summarized in Supplementary Table 2.
The most immediate and significant consequence of FXPOI is reduced fertility (Allen et al., 2007; Streuli et al., 2009). POI occurs in about 20% of women with a premutation, making the risk of POI in this population about 20 times higher than the general population (Sherman, 2000; De Caro et al., 2008; Sullivan et al., 2011). Taking all women who carry a premutation, on average, they go through menopause about 5 years earlier than those without a premutation (Murray, 2000; Sullivan et al., 2005; Seltzer et al., 2012). Also, among women attending a reproductive endocrine clinic, about 11% with familial POI and about 3.2% with isolated POI are found to carry a premutation; thus, FXPOI is the most common genetic form of POI (Sherman et al., 2007).
There are at least three risk factors associated with the onset of FXPOI. The first is repeat size. Among premutation carriers, there is a non-linear relationship between CGG repeat length and risk for POI (Sullivan et al., 2005; Ennis et al., 2006; Allen et al., 2007; Tejada et al., 2008; Spath et al., 2011), irregular menses (Allen et al., 2007) and subfertility (Allen et al., 2007). For example, respectively, the risk for FXPOI is about 10, 32, and 16% for women with 55–79 repeats, 80–100 repeats, and greater than 100 repeats (Allen et al., 2007). The onset of POI is earliest among those with 80–100 repeats, sometimes as early as the adolescent years (De Caro et al., 2008). The risk for FXPOI is increased for women who report a family history of early menopause (Hunter et al., 2008; Spath et al., 2011), indicating background genetic variants as the second risk factor. Third, a woman who has ever smoked in her lifetime is subject to reduced age at menopause, although this effect among women with and without a premutation is similar (Allen et al., 2007).
Genetic counseling is essential once a woman is found to carry a premutation. In addition to the risk of FXPOI and its clinical consequences, each woman has a risk for having a child with fragile X syndrome. The magnitude of this risk is related to the number of CGG repeats identified in her FMR1 gene. The larger the number of repeats, the higher the risk for expansion from a premutation to a full mutation. There is also risk for developing another established premutation-associated disorder, fragile X-associated tremor/ataxia syndrome (FXTAS). FXTAS is a neurodegenerative disorder with an onset around age 60 (Hagerman et al., 2001). Although men who carry a premutation are more frequently affected by FXTAS, women have a lifetime prevalence of 6–18% (Wheeler et al., 2014).
The median age of menopause in the United States is ∼51 ± 1 years, with 1% of women experiencing menopause prematurely (referred to as primary ovarian insufficiency, POI) (Palacios et al., 2010). POI is diagnosed when a woman has (1) experienced at least 4 months of unpredictable or absent menstrual periods before age 40 and (2) has two serum FSH levels in the menopausal range (Nelson, 2009).
The overall methods to detect the expanded CGG repeat in the 5′ UTR of the FMR1 gene have not changed significantly over the years – all involve conducting PCR using probes that surround the repeat, followed by Southern blot analysis if required (Fu et al., 1991; Yu et al., 1991; Erster et al., 1992; Brown et al., 1993). Advances of PCR technologies have led to the ability to capture large premutation and full mutation repeats with accuracy, sometimes eliminating the need to follow the results with Southern blot analyses [e.g., triplet repeat-primed PCR (Tassone et al., 2008; Chen et al., 2010; Filipovic-Sadic et al., 2010; Hantash et al., 2011)]. All diagnostic methods are reviewed in Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics and Genomics, which were most recently updated by Monaghan et al. (2013).
The most recent advance in characterizing the FMR1 repeat expansion is inclusion of the AGG interruption pattern within the CGG repeat (reviewed in Latham et al., 2014). The finding that the CGG repeat was interrupted with periodic single AGG sequences in the 5′ region of the FMR1 repeat was first described by Eichler et al. (1994). Once an accurate PCR-based method to deduce the AGG interruption pattern was developed in 2010 (Chen et al., 2010), several large studies were conducted and showed clearly that the repeat structure, including repeat length and AGG interruption pattern, altered the risk for instability of the repeat during transmission from parent to child (Yrigollen et al., 2012, 2013; Nolin et al., 2013, 2015). Whether or not this added information about repeat structure alters the risk for FXPOI is not clear, as studies have shown conflicting results (Lekovich et al., 2017; Allen et al., 2018).
Evidence suggests the most common mechanism of FXPOI is one of abnormal follicle function rather than a depletion of primordial follicles. There is limited histologic evidence regarding the ovarian pathology in women with FXPOI. One small study showed no significance difference from normal control ovaries with regard to ovarian histology or follicle number (Chang et al., 2011). These data are consistent with the mechanism of FXPOI being mainly one of follicle dysfunction rather than follicle depletion. These human findings are consistent with histologic evidence in mouse models of FXPOI. As shown in Figure 1, mice carrying an FMR1 premutation had a normal number of primordial follicles. The significant differences from control mice were: (1) fewer large antral follicles, (2) fewer corpora lutea, and (3) a greater number of atretic large antral follicles. (Figure 1; Conca Dioguardi et al., 2016). Thus, available evidence suggests FXPOI is a primarily a disorder of impaired follicle function.
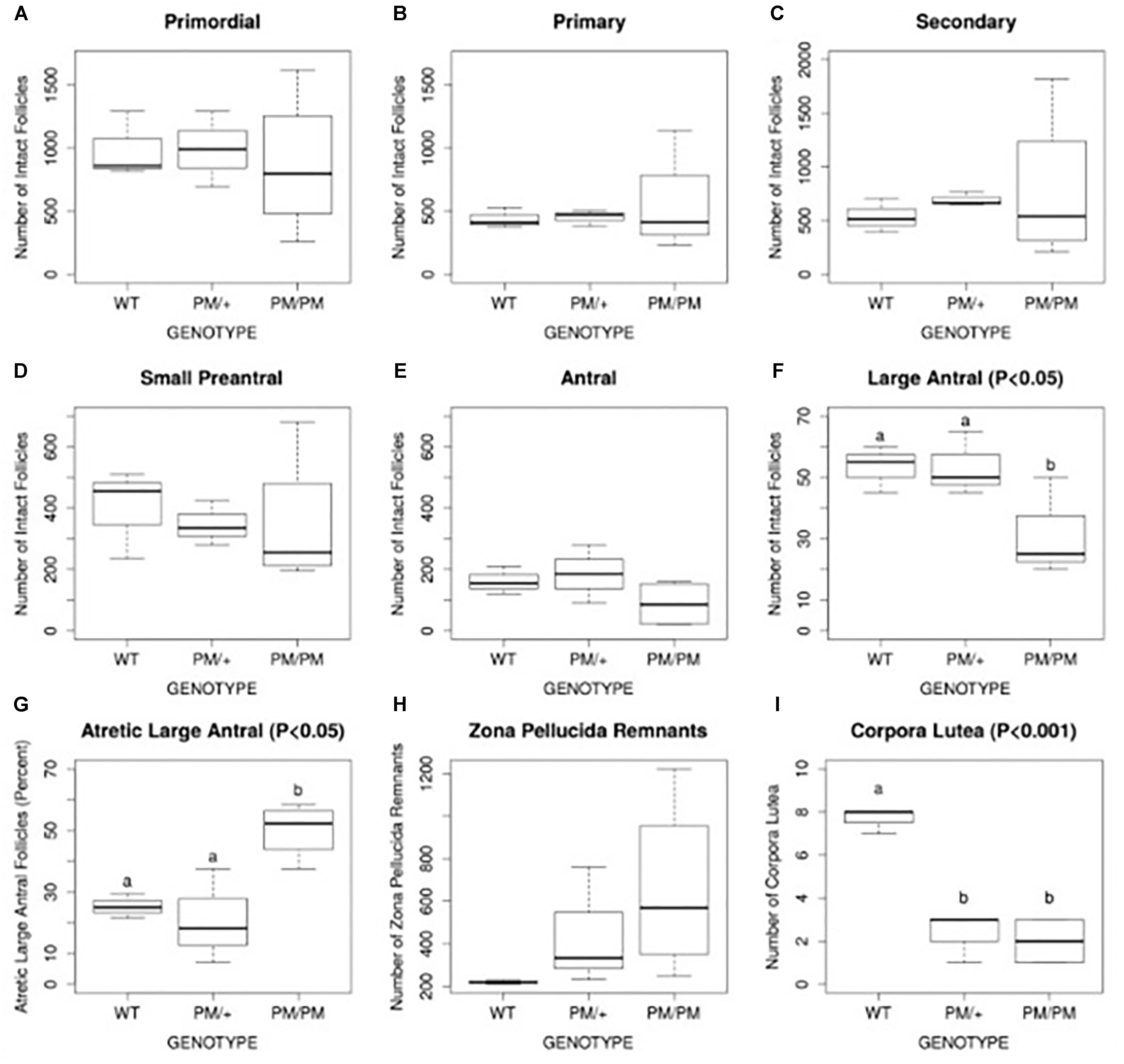
FIGURE 1. Histomorphometric analysis of follicle number, atresia, and corpus luteum number of 8 month old FXPM mutants and wild type control animals. Quantification of intact primordial, primary, secondary, pre-antral, and large antral/periovulatory follicles per ovary (n = 4 per genotype) is shown in A–F. Follicle atresia was evaluated by counting atretic large antral follicles (G) and zona pellucida remnants (ZPR, H). Corpus luteum (CL) number is shown in (I). Note increased variability in the number of PM/PM follicles, ZPR, and CL. Statistically different means, where applicable, are denoted by letters “a” and “b,” with P-values shown above each plot as calculated by ANOVA analysis. Conca Dioguardi et al. (2016).
Published data are available about FXPOI models in three separate mouse strains (Sherman et al., 2014). The first, the “NIH” mouse, harbors 130 CGG repeats (here, “130R”) in the Fmr1 5′ UTR (Entezam et al., 2007). The second, referred to here as the “90R” mouse, has 90 repeats (Peier and Nelson, 2002). Newer data from the third “Dutch” CGG knock-in strain are also discussed.
Reproductive and ovarian parameters have largely been consistent between the 130 and 90 R strains (Pastore and Johnson, 2014). This includes negative impacts upon ovarian follicle growth and survival that correspond to decreased litter size(Lu et al., 2012; Conca Dioguardi et al., 2016). Further, alterations in the levels of hormones estradiol and FSH have been detected in the 90 R mouse (Lu et al., 2012). Additionally, the growth of ovarian follicles is slower due to an elevation in the follicular apoptotic index (Uslu et al., 2017). Ultimately, the number of cumulus granulosa cells in mature follicles, and, those ovulated with the egg from mature ovulatory follicles, are decreased in number (Hoffman et al., 2012). Both models are also associated with increased follicle atresia (Hoffman et al., 2012; Lu et al., 2012).
All of the above features of compromised growth and function of mouse follicles were recently found to correspond to reduced mitochondrial (mt) DNA copy number, total mitochondrial mass, and altered expression of genes that control mt function (Conca Dioguardi et al., 2016). Causal links between the premutation, sub-functional mt, and follicle and ovary function remain to be elucidated. Interestingly, mt abnormalities have also been detected in other tissues (Alvarez-Mora et al., 2016; Giulivi et al., 2016), including those from women who carry a premutation.
In a separate study using ovaries from the “Dutch” exCGG-KI mice (100–199 CGGs), Buijsen et al. (2016) found evidence for translation of the CGG-bearing Fmr1 RNA, resulting in the production of Repeat-associated non-AUG (RAN)-translated poly-glycine species (Kearse and Todd, 2014). These aberrant translation products have been detected in neuronal intracellular inclusions that correspond to the pathology seen in FXTAS (Sellier et al., 2017). Buijsen et al. (2016) detected RAN inclusions within ovarian stromal cells in the “Dutch” mouse, as well as the ovarian stromal of a woman with FXPOI. Taken together, this suggests that RAN translation could contribute to ovarian pathophysiology as well.
The ovary develops a graafian follicle from a primordial follicle every month. After ovulation, a corpus luteum forms (Hawkins and Matzuk, 2008). These secretory structures produce a complex symphony of reproductive hormones, which create the menstrual cycle. After the diagnosis of POI, about 5–10% of women have a spontaneous conception (van Kasteren and Schoemaker, 1999; Nelson et al., 2005). A more recent study noted a spontaneous conception percent of 12.6 specifically in women with FXPOI (Hipp et al., 2016).
The goal in treating women with POI is to optimize their health in an integrated manner and to replace their missing reproductive hormones in the most physiologic manner possible (Sullivan et al., 2016). In women of reproductive age who are having regular menstrual cycles the average serum estradiol level across the menstrual cycle is about 100 pg/mL (Mishell et al., 1971). The 100 mcg per day estradiol patch and vaginal ring deliver an appropriate amount of estradiol to maintain this serum level. Not only does this approach normalize serum estradiol levels, but also the regimen frequently normalized serum LH levels. A study of 137 women with spontaneous POI demonstrated that the transdermal estrogen patch at a dose of 100 mcg/day normalized LH levels in approximately half of the women (Popat et al., 2008). Normalizing serum LH levels is an important consideration because the most common mechanism of follicle dysfunction in women with POI is inappropriate follicle luteinization (Popat et al., 2008; Nelson, 2009). Theoretically, normalizing serum LH levels would improve the chance of ovulation.
Studies focusing on POI and bone health have helped to define an optimal hormone replacement regimen. Fifty-nine women with spontaneous POI participated in a 2-year open randomized trial comparing physiologic hormone replacement therapy to oral contraceptive pills. The study found that women taking physiological hormone replacement therapy had significantly increased lumbar spine bone mineral density compared to women taking the oral contraceptive (Cartwright et al., 2016). Oral contraceptive pills are not as effective as physiologic hormone replacement therapy at improving or maintaining bone density. An NIH 3-year randomized controlled trial in women with spontaneous 46,XX (normal karyotype) POI demonstrated a 7.7% gain in femoral neck BMD with physiological transdermal estrogen and oral medroxyprogesterone replacement (Popat et al., 2014; Figure 2). The women in this study receiving the transdermal estrogen and oral medroxyprogesterone treatment had a mean age of 33 years. This study is striking because in normal women with regular menses, peak bone density is not reached until the early 30 s. The NIH study is good news for young women with POI. Even when women with POI develop an estrogen deficient state during the time of peak bone mass accrual, they can still regain lost BMD to normal over 3 years of replacement. On average, at the end of the NIH study period the BMD of women with POI did not differ from the control women with regular menses. Of note, a study evaluating hormone replacement regimens in ovariectomized rats showed the best outcome for vertebral BMD was when estrogen and progesterone were administered sequentially rather than in a continuous/combined approach as in oral contraceptive pills (Vanin et al., 1995). This is possibly the reason cyclic physiologic hormone replacement has a better effect on bone than oral contraceptive pills in women with POI.
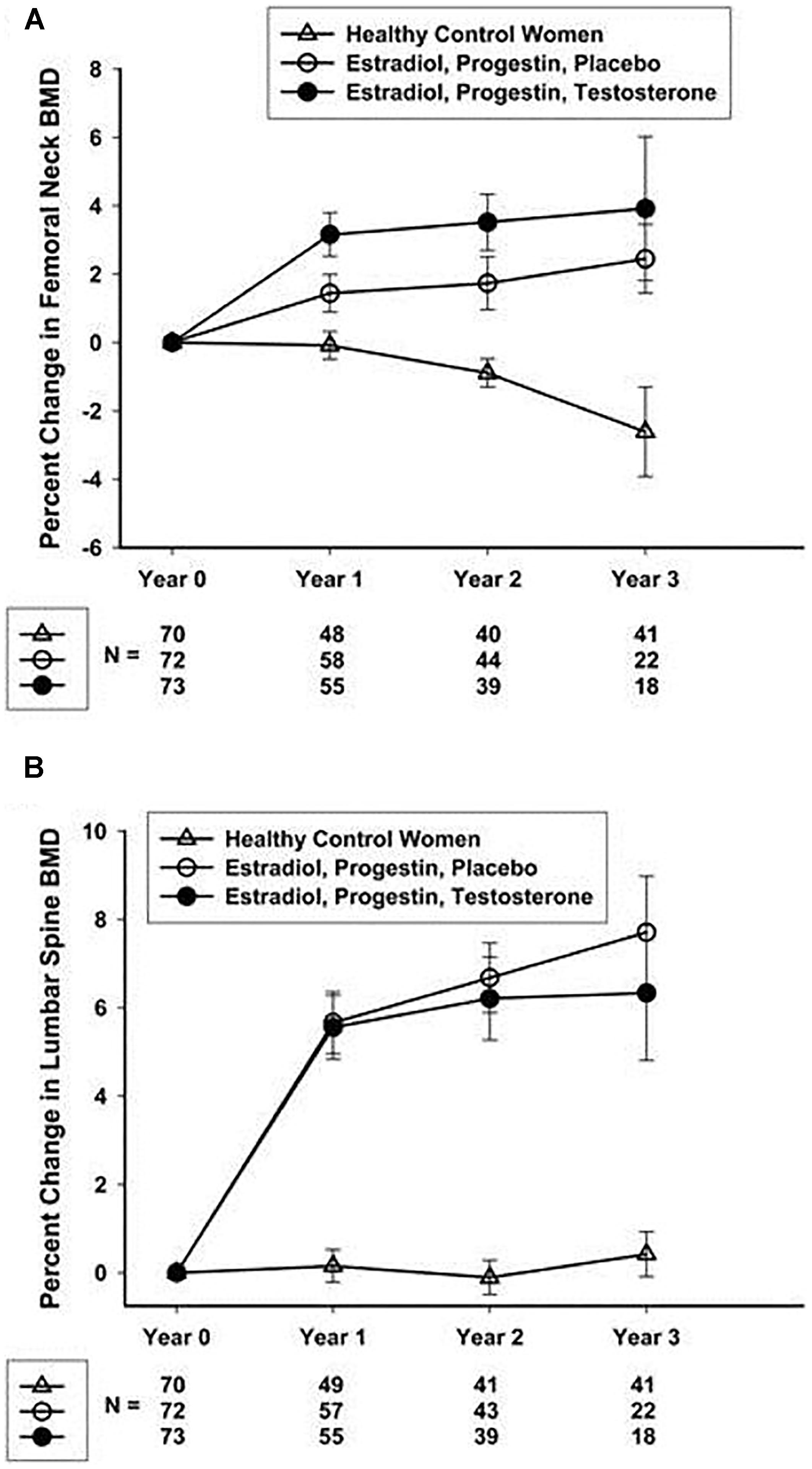
FIGURE 2. Percentage change over 3 years in femoral neck (A) and lumbar spine (B) BMD in healthy control women and women with 46,XX sPOI treated with E + P or E + P + T. Popat et al. (2014).
Oral contraceptives provide supraphysiologic doses of hormones and provide a continuous dose of estrogen and progestin, typically for 3 weeks followed by one week of placebo. They also induce unwanted physiologic changes as compared to physiologic hormone replacement (Langrish et al., 2009; Figure 3). Many women do not realize that oral contraceptive pills provide higher effective doses of estrogen and progestin compared to physiologic hormone replacement of estrogen and progestin. For women with POI who desire pregnancy, oral contraceptive pills are a problem as they induce hostile cervical mucus (Steward et al., 2012) as well as atrophic endometrium (ESHRE Capri Workshop Group, 2001).
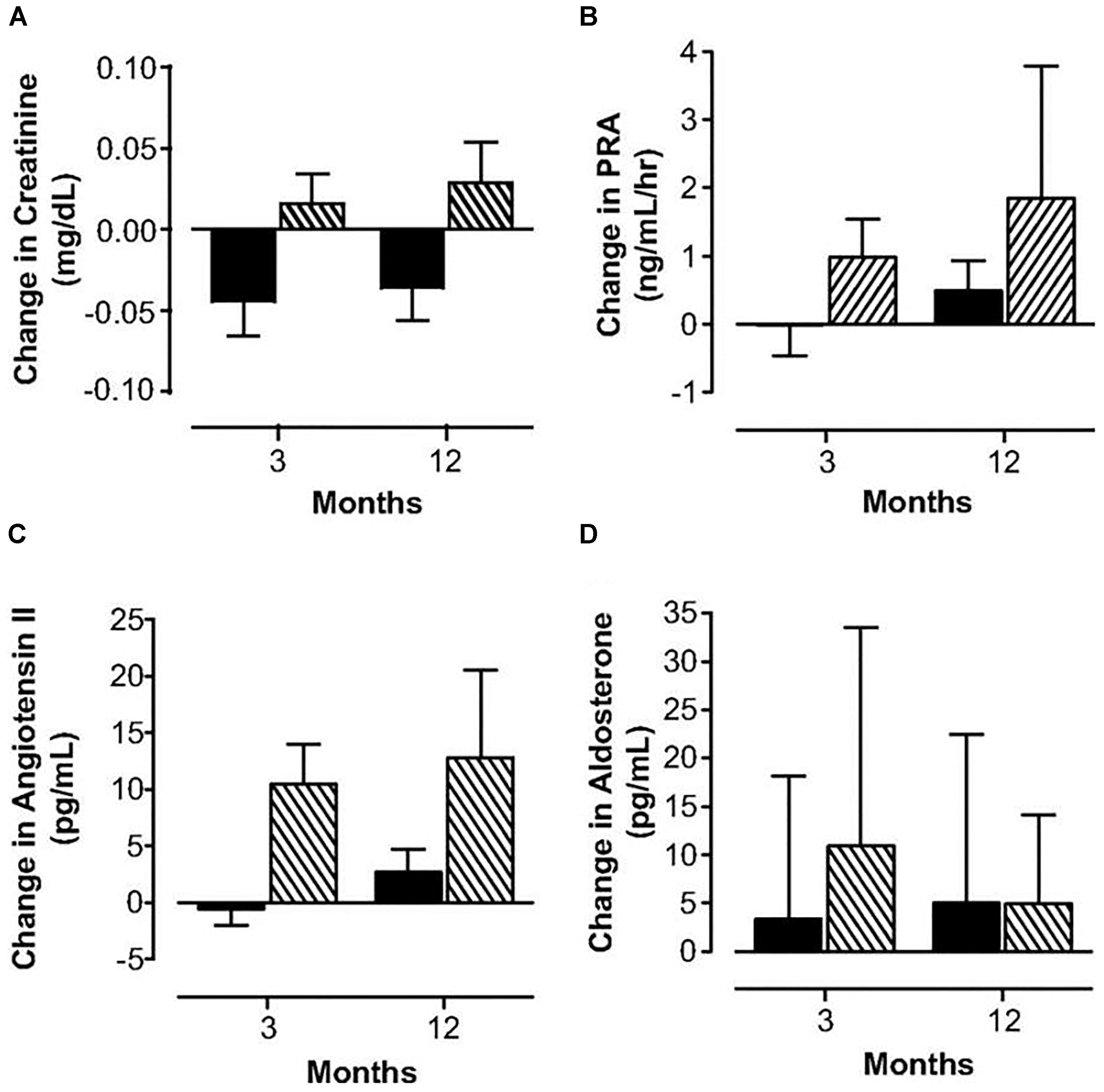
FIGURE 3. Changes in serum creatinine (A), plasma renin activity (B), angiotensin II (C) and aldosterone (D) concentrations in women with POI treated with physiologic HRT ( ) or standard OCPs (
) or standard OCPs ( ). Langrish et al. (2009).
). Langrish et al. (2009).
The normal ovary also produces testosterone (T). Free T levels are reduced in women with POI (Kalantaridou et al., 2006). In another domain, a meta-analysis of 42 studies demonstrated normal women who take oral contraceptive pills have reduced circulating levels of total T and free T as well as an increase in SBHG concentrations (Zimmerman et al., 2014). The use of oral contraceptives as hormone replacement therapy would thus be expected to further reduce free T levels, mediated by increased levels of SHBG.
The North American Menopause Society 2017 hormone therapy position statement recommends hormone therapy until the median age of menopause, which is 52-years-old in the United States (The NAMS 2017 Hormone Therapy Position Statement Advisory Panel, 2017). Unfortunately, hormone treatment is often delayed or inappropriately administered to women with POI. A study of 79 women with FXPOI found that (1) many women took hormones for less than a year or never received hormone replacement, (2) had a greater than 1 year delay in beginning hormone replacement after the POI diagnosis, or (3) discontinued hormone replacement therapy before age 45 years (Hipp et al., 2016).
Emotionally, POI can be a devastating diagnosis for women. Compared to controls women with POI score adversely on measures of anxiety, depression, as well as positive and negative affect. In controlled study, measures of illness uncertainty and purpose in life were significant independent factors associated with anxiety. Also, measures of stigma and purpose in life were significant independent factors associated with depression. Further, measures of goal reengagement and purpose in life were significant independent factors associated with positive affect. These findings suggest clinicians could help women with POI improve their quality of life by: (a) informing them better about their diagnosis, (b) helping them feel less stigmatized by the disorder, and (c) assisting them in developing alternative goals with regard to family planning as well as other life goals. In a study evaluating depression in women with POI, women with idiopathic POI had a much higher incidence of depression than women with Turner Syndrome who had POI (Schmidt et al., 2011). In a separate study, many women with idiopathic POI had depression that began during the time of menstrual cycle irregularity, which preceded the diagnosis of POI (Schmidt et al., 2011). In addition, the patient–physician interaction sometimes causes significant emotional distress. A study evaluating the emotional needs of women at the time of POI diagnosis found that most of them were not satisfied with how their physician informed them about the diagnosis (Groff et al., 2005). As a result of the emotional stressors that occur at the time of diagnosis with POI there is a need to address these issues in an integrated manner (Covington et al., 2011).
Fragile X syndrome (FXS; OMIM 300624) is a common form of X-linked intellectual and developmental disability (Crawford et al., 2001) with a prevalence of 1/4000 – 5000 in males and 1/6000 – 8000 in females (de Vries et al., 1997; Crawford et al., 2001; Coffee et al., 2009; Hill et al., 2010). It belongs to a family of more than 40 disorders characterized by repeat instability on transmission from parent to child (Pearson et al., 2005).
Most cases of the syndrome result from expansion of a CGG trinucleotide repeat located in the 5′ UTR of the FMR1 gene to more than 200 repeats (Oberlé et al., 1991; Verkerk et al., 1991; Yu et al., 1991). FMR1 alleles with this expanded repeat are referred to as the full mutation. In a response to the expanded repeat, the FMR1 gene undergoes locus-specific hypermethylation and chromatin remodeling that epigenetically silences the gene. Many alleles in the premutation range (55–199 CGG repeats) are remarkably unstable and at risk for full mutation expansions even in one generation. As many as 94% of alleles with more than 90 repeats expand to a full mutation (Nolin et al., 2011). Expansion to a full mutation occurs almost solely in transmission from mother to child and not from father to daughter although rare exceptions have occurred (Zeesman et al., 2004).
For this reason, in some countries, women who wish to avoid the risk of having a child affected with FXS are offered preconception genetic screening for FMR1 premutation. Currently, FMR1 premutation carriers who wish to conceive and avoid the risk of having an affected child have three options:
(1) To conceive using In Vitro Fertilization and preimplantation genetic testing for monogenic gene diseases (IVF-PGT-M).
(2) Spontaneous conception and prenatal genetic diagnosis during pregnancy by either chorionic villous sampling (CVS) or amniocentesis (AC).
(3) Using a donor oocyte.
Each approach has its advantages and disadvantages. Spontaneous conception carries a risk of bearing an affected child and the need to perform a termination of pregnancy. Termination of pregnancy involves medical, emotional and ethical issues. On the other hand, IVF using PGT-M avoids the need for a termination of pregnancy, and offers the opportunity to transfer only non-carrier embryos. However, this procedure arouses financial and emotional difficulties and is not the obvious choice for a fertile couple, especially considering the higher prevalence of ovarian dysfunction and reduced ovarian response observed in FMR1 premutation carriers undergoing IVF compared to non-carrier women (Elizur et al., 2014).
In addition to the risk of having a child affected with FXS women who carry an FMR1 premutation may suffer from ongoing deterioration of ovarian function. This can be demonstrated by various markers such as high serum follicular phase FSH levels (Murray et al., 1999) low serum Inhibin A, Inhibin B (Welt et al., 2004), Anti-Mullerian hormone levels (AMH) (Spath et al., 2011) and low antral follicle count (AFC) (Elizur et al., 2014).
In the western world, there has been an overall increase of mean maternal age as a result of delayed childbearing (Mathews and Hamilton, 2016). Thus, it is essential to identify in a timely manner women who carry a premutation and are at risk for developing diminished ovarian function. This knowledge will offer these women the opportunity to make an informed decision regarding their reproductive and family planning. Some might pursue childbearing earlier than first planned or choose fertility preservation options.
There is a need for an International FXPOI Natural History Study (Figure 4). Unfortunately, today, we do not have the ability to prevent or reverse the impaired ovarian function associated with FXPOI. However, we can take advantage of the latest developments in the rapidly evolving field of fertility preservation. Emphasis should be on early identification of women with a premutation and diminished ovarian function at the primary care level. Embryo and oocyte cryopreservation are currently the best available fertility preservation option for women who carry a premutation. Ovarian tissue cryopreservation is used today mainly as fertility preservation option for young women facing gonadotoxic treatment due to malignancy. It requires two surgeries: one to harvest the ovarian tissue and the second to implant it back when the woman heals from her primary disease. It is most successful in young women with normal functioning ovaries who face an acute and isolated potential damage to the ovaries. It is uncertain at this time whether women who carry a premutation will benefit from such a procedure.

FIGURE 4. Schematic flow diagram depicts the process to be followed by the proposed International FXPOI Natural History Study.
As mentioned previously, it should be noted that FXPOI should not be equated with menopause. Ovarian function can still be present in women with established FXPOI, albeit the function is intermittent and unpredictable. Hipp et al. (2016) reported that 12.6% of women diagnosed with FXPOI conceived spontaneously after diagnosis. The time to conception after diagnosis ranged up to 12 years.
The new site-specific genomic editing tool, the CRISPR (clustered regularly interspaced short palindromic repeats) system, has recently been developed and implemented to target and mutate specific genomic regions (Cong et al., 2013). Xie et al. (2016) utilized the CRISPR genome editing technology to excise the expanded CGG repeat from the full mutation allele in FXS cells resulting in an FMR1 allele without CGG repeats. The excision of the expanded CGG-repeat from the fragile X chromosome resulted in FMR1 reactivation thereby restoring FMRP production. Liu et al. (2018) applied recently developed DNA methylation editing tools and demethylated the CGG expansion by dCas9-Tet1/single guide RNA (sgRNA). This switched the heterochromatin status of the upstream FMR1 promoter to an active chromatin state, restoring a persistent expression of FMR1 in FXS iPSCs. These new developments in gene editing technology may offer us in the future the option to “cure” in the laboratory FXS affected embryos of FMR1 premutation carriers undergoing IVF.
Engaging a collaborative team is the most efficient and productive manner in which to conduct research on genetics and clinical care. This is true for virtually all disorders, and especially those which are relatively rare and pleiotropic (Harris et al., 2016). One effective way to stimulate and organize research at all levels is to develop centers of excellence (Martin et al., 2017). The National Fragile X Foundation has established a Fragile X Clinical and Research Consortium [FXCRC] (2018). Such centers can attract patients and their families for specialized care and research regarding all of the organ systems that might be affected. The patient described in this report initially required the services of psychiatry, endocrinology, reproductive endocrinology, medical genetics and genetic counseling. Later in life, neurology might become involved related to FXTAS.
All of these evaluations and management plans would best be coordinated by a clinician who understands the underlying mutation and its multiple effects (Rafique et al., 2012). Centers of excellence, typically at academic medical centers, are well positioned to serve all of the clinical needs and potential therapies for those with fragile X-associated disorders. Through regular meetings of the center’s health care professionals, individual patients can be discussed and new clinical management issues planned. Often such discussions lead to stimulation of clinical studies, even clinical trials (Falorni et al., 2014). Moreover, individual members of a center often interact with basic science colleagues, engage them with ideas, and sometimes stimulate them to undertake pertinent research.
Fragile X-associated primary ovarian insufficiency (FXPOI) is a chronic disorder characterized by oligo/amenorrhea and hypergonadotropic hypogonadism before age 40 years. There may be significant morbidity due to: (1) depression and anxiety related to the loss of reproductive hormones and infertility; (2) reduced bone mineral density; and (3) increased risk of cardiovascular disease related to estrogen deficiency. We report the case of a young woman who never established regular menses and yet experienced a 5-year diagnostic odyssey before establishing a diagnosis of FXPOI. This is despite a known family history of fragile X syndrome and early menopause in the family. Despite having clearly documented FXPOI, the woman spontaneously conceived and delivered two healthy children. This is consistent with the pathophysiology of FXPOI being primarily a situation of ovarian follicle dysfunction rather than ovarian follicle depletion. As a rare disease, the diagnosis of FXPOI presents special challenges. There is a need for increased awareness of this disorder among health care professions. International centers of excellence may be helpful to address the needs of families dealing with the sequelae of abnormalities in FMR1. Such centers should be coordinated by a global virtual center, which takes full advantage of mobile device communication systems.
Such an approach would put patients and community health providers in touch with investigators who have the requisite knowledge and expertise about the FMR1 gene and its various manifestations. This would facilitate patient care and research on an international level. One of the top priorities of centers would be to conduct a natural history study on FXPOI.
DF organized the abstract, endocrinology, behavioral health, and conclusions section. LN organized the abstract, case report, and conclusions. RP contributed to the reproduction and fertility preservation section. JJ organized the basic science section. SS organized the epidemiology and genetics section. YC contributed to the reproduction and fertility preservation section. SE contributed to the reproduction and fertility preservation section.
This work was supported, in part, from The Azrieli Foundation Canada-Israel (SE and YC); an award (NS091859) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Neurological Disorders and Stroke (NINDS) (SS); Mary Elizabeth Conover Foundation, Inc (LN); and University of Colorado Department of Obstetrics and Gynecology Research Funds (JJ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2018.00529/full#supplementary-material
Allen, E. G., Glicksman, A., Tortora, N., Charen, K., He, W., Amin, A., et al. (2018). FXPOI: pattern of AGG interruptions does not show an association with age at amenorrhea among women with a premutation. Front. Genet. 9:292. doi: 10.3389/fgene.2018.00292
Allen, E. G., Sullivan, A. K., Marcus, M., Small, C., Dominguez, C., Epstein, M. P., et al. (2007). Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum. Reprod. 228, 2142–2152. doi: 10.1093/humrep/dem148
Alvarez-Mora, M. I., Rodriguez-Revenga, L., Madrigal, I., Guitart-Mampel, M., Garrabou, G., and Mila, M. (2016). Impaired mitochondrial function and dynamics in the pathogenesis of FXTAS. Mol. Neurobiol. 54, 6896–6902. doi: 10.1007/s12035-016-0194-7
Brown, W. T., Houck, G. E. Jr., Jeziorowska, A., Levinson, F. N., Ding, X., Dobkin, C., et al. (1993). Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. JAMA 270, 1569–1575. doi: 10.1001/jama.1993.03510130075034
Buijsen, R. A., Visser, J. A., Kramer, P., Severijnen, E. A., Gearing, M., Charlet-Berguerand, N., et al. (2016). Presence of inclusions positive for polyglycine containing protein, FMRpolyG, indicates that repeat-associated non-AUG translation plays a role in fragile X-associated primary ovarian insufficiency. Hum. Reprod. 311, 158–168. doi: 10.1093/humrep/dev280
ESHRE Capri Workshop Group (2001). Ovarian and endometrial function during hormonal contraception. Hum. Reprod. 167, 1527–1535. doi: 10.1093/humrep/16.7.1527
Cartwright, B., Robinson, J., Seed, P. T., Fogelman, I., and Rymer, J. (2016). Hormone replacement therapy versus the combined oral contraceptive pill in premature ovarian failure: a randomized controlled trial of the effects on bone mineral density. J. Clin. Endocrinol. Metab. 1019, 3497–3505. doi: 10.1210/jc.2015-4063
Chang, M. C., DeCaro, J. J., Zheng, M., Gearing, M., Shubeck, L., Sherman, S. L., et al. (2011). Ovarian histopathological and ubiquitin-immunophenotypic features in fragile X-associated primary ovarian insufficiency: a study of five cases and selected controls histopathology. 595, 1018–1023. doi: 10.1111/j.1365-2559.2011.03959.x
Chen, L., Hadd, A., Sah, S., Filipovic-Sadic, S., Krosting, J., Sekinger, E., et al. (2010). An information-rich CGG repeat primed PCR that detects the full range of fragile X expanded alleles and minimizes the need for southern blot analysis. JMD 12, 589–600. doi: 10.2353/jmoldx.2010.090227
Coffee, B., Keith, K., Albizua, I., Malone, T., Mowrey, J., Sherman, S. L., et al. (2009). Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am. J. Hum. Genet. 85, 503–514. doi: 10.1016/j.ajhg.2009.09.007
Conca Dioguardi, C., Uslu, B., Haynes, M., Kurus, M., Gul, M., Miao, D. Q., et al. (2016). Granulosa cell and oocyte mitochondrial abnormalities in a mouse model of fragile X primary ovarian insufficiency. Mol. Hum. Reprod. 226, 384–396. doi: 10.1093/molehr/gaw023
Cong, L., Ran, F. A., Cox, D., Lin, S., Barretto, R., Habib, N., et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823. doi: 10.1126/science.1231143
Covington, S. N., Hillard, P. J., Sterling, E. W., Nelson, L. M., Primary Ovarian, Insufficiency Recovery, et al. (2011). A family systems approach to primary ovarian insufficiency. J. Pediatr. Adolesc. Gynecol. 243, 137–141. doi: 10.1016/j.jpag.2010.12.004
Crawford, D. C., Acuna, J. M., and Sherman, S. L. (2001). FMR1 and the fragile X syndrome: human genome epidemiology review. Genet. Med. 3, 359–371. doi: 10.1097/00125817-200109000-00006
de Vries, B. B., van den Ouweland, A. M., Mohkamsing, S., Duivenvoorden, H. J., Mol, E., Gelsema, K., et al. (1997). Screening and diagnosis for the fragile X syndrome among the mentally retarded: an epidemiological and psychological survey. collaborative fragile X study group. Am. J. Hum. Genet. 61, 660–667. doi: 10.1086/515496
De Caro, J. J., Dominguez, C., and Sherman, S. L. (2008). Reproductive health of adolescent girls who carry the FMR1 premutation: expected phenotype based on current knowledge of fragile x-associated primary ovarian insufficiency. Ann. N. Y. Acad. Sci. 1135, 99–111. doi: 10.1196/annals.1429.029
Eichler, E. E., Holden, J. A., Popovich, B. W., Reiss, A. L., Snow, K., Thibodeau, S. N., et al. (1994). Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat. Genet. 8, 88–94. doi: 10.1038/ng0994-88
Elizur, S. E., Lebovitz, O., Derech-Haim, S., Dratviman-Storobinsky, O., Feldman, B., Dor, J., et al. (2014). Elevated levels of FMR1 mRNA in granulosa cells are associated with low ovarian reserve in FMR1 premutation carriers. PLoS One 98:e105121. doi: 10.1371/journal.pone.0105121
Ennis, S., Ward, D., and Murray, A. (2006). Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur. J. Hum. Genet. 142, 253–255. doi: 10.1038/sj.ejhg.5201510
Entezam, A., Biacsi, R., Orrison, B., Saha, T., Hoffman, G. E., Grabczyk, E., et al. (2007). Regional FMRP deficits and large repeat expansions into the full mutation range in a new fragile X premutation mouse model. Gene 395, 125–134. doi: 10.1016/j.gene.2007.02.026
Erster, S. H., Brown, W. T., Goonewardena, P., Dobkin, C. S., Jenkins, E. C., and Pergolizzi, R. G. (1992). Polymerase chain reaction analysis of fragile X mutations. Hum. Genet. 90, 55–61. doi: 10.1007/BF00210744
Falorni, A., Minarelli, V., Eads, C. M., Joachim, C. M., Persani, L., Rossetti, R., et al. (2014). A clinical research integration special program (CRISP) for young women with primary ovarian insufficiency. Panminerva Med. 564, 245–261.
Filipovic-Sadic, S., Sah, S., Chen, L., Krosting, J., Sekinger, E., Zhang, W., et al. (2010). A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin. Chem. 56, 399–408. doi: 10.1373/clinchem.2009.136101
Fragile X Clinical and Research Consortium [FXCRC] (2018). Available at: https://fragilex.org/research/
Fu, Y. H., Kuhl, D. P. A., Pizzuti, A., Pieretti, M., Sutcliffe, J. S., Richards, S., et al. (1991). Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 67, 1047–1058. doi: 10.1016/0092-8674(91)90283-5
Giulivi, C., Napoli, E., Tassone, F., Halmai, J., and Hagerman, R. (2016). Plasma metabolic profile delineates roles for neurodegeneration, pro-inflammatory damage and mitochondrial dysfunction in the FMR1 premutation. Biochem. J. 47321, 3871–3888. doi: 10.1042/BCJ20160585
Greenhalgh, J., and Meadows, K. (1999). The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: a literature review. J. Eval. Clin. Pract. 5, 401–416.
Groff, A. A., Covington, S. N., Halverson, L. R., Fitzgerald, O. R., Vanderhoof, V., Calis, K., et al. (2005). Assessing the emotional needs of women with spontaneous premature ovarian failure. Fertil. Steril. 836, 1734–1741. doi: 10.1016/j.fertnstert.2004.11.067
Hagerman, R. J., Leehey, M., Heinrichs, W., Tassone, F., Wilson, R., Hills, J., et al. (2001). Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 571, 127–130. doi: 10.1212/WNL.57.1.127
Hantash, F. M., Goos, D. M., Crossley, B., Anderson, B., Zhang, K., Sun, W., et al. (2011). FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency in the United States. Genet. Med. 13, 39–45. doi: 10.1097/GIM.0b013e3181fa9fad
Harris, J. G., Bingham, C. A., and Morgan, E. M. (2016). Improving care delivery and outcomes in pediatric rheumatic diseases. Curr. Opin. Rheumatol. 282, 110–116. doi: 10.1097/BOR.0000000000000257
Hawkins, S. M., and Matzuk, M. M. (2008). The menstrual cycle: basic biology. Ann. N. Y. Acad. Sci. 1135, 10–18. doi: 10.1196/annals.1429.018
Hill, M. K., Archibald, A. D., Cohen, J., and Metcalfe, S. A. (2010). A systematic review of population screening for fragile X syndrome. Genet. Med. 12, 396–410. doi: 10.1097/GIM.0b013e3181e38fb6
Hipp, H. S., Charen, K. H., Spencer, J. B., Allen, E. G., and Sherman, S. L. (2016). Reproductive and gynecologic care of women with fragile X primary ovarian insufficiency (FXPOI). Menopause 239, 993–999. doi: 10.1097/GME.0000000000000658
Hoffman, G. E., Le, W. W., Entezam, A., Otsuka, N., Tong, Z. B., Nelson, L., et al. (2012). Ovarian abnormalities in a mouse model of fragile X primary ovarian insufficiency. J. Histochem. Cytochem. 606, 439–456. doi: 10.1369/0022155412441002
Hunter, J., Rivero-Arias, O., Angelov, A., Kim, E., Fotheringham, I., and Leal, J. (2014). Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am. J. Med. Genet. A 164A7, 1648–1658. doi: 10.1002/ajmg.a.36511
Hunter, J. E., Epstein, M. P., Tinker, S. W., Charen, K. H., and Sherman, S. L. (2008). Fragile X-associated primary ovarian insufficiency: evidence for additional genetic contributions to severity. Genet. Epidemiol. 326, 553–559. doi: 10.1002/gepi.20329
Mishell, D. R. Jr., Nakamura, R. M., Crosignani, P. G., Stone, S., Kharma, K., Nagata, Y., et al. (1971). Serum gonadotropin and steroid patterns during the normal menstrual cycle. Am. J. Obstet. Gynecol. 1111, 60–65.
Kalantaridou, S. N., Calis, K. A., Vanderhoof, V. H., Bakalov, V. K., Corrigan, E. C., Troendle, J. F., et al. (2006). Testosterone deficiency in young women with 46,XX spontaneous premature ovarian failure. Fertil. Steril. 865, 1475–1482. doi: 10.1016/j.fertnstert.2006.04.028
Kearse, M. G., and Todd, P. K. (2014). Repeat-associated non-AUG translation and its impact in neurodegenerative disease. Neurotherapeutics 114, 721–731. doi: 10.1007/s13311-014-0292-z
Langrish, J. P., Mills, N. L., Bath, L. E., Warner, P., Webb, D. J., Kelnar, C. J., et al. (2009). Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension 535, 805–811. doi: 10.1161/HYPERTENSIONAHA.108.126516
Latham, G. J., Coppinger, J., Hadd, A. G., and Nolin, S. L. (2014). The role of AGG interruptions in fragile X repeat expansions: a twenty-year perspective. Front. Genet. 5:244. doi: 10.3389/fgene.2014.00244
Lekovich, J., Man, L., Xu, K., Canon, C., Lilienthal, D., Stewart, J. D., et al. (2017). CGG repeat length and AGG interruptions as indicators of fragile X-associated diminished ovarian reserve. Genet. Med. doi: 10.1038/gim [Epub ahead of print].
Liu, X. S., Wu, H., Krzisch, M., Wu, X., Graef, J., Muffat, J., et al. (2018). Rescue of fragile X syndrome neurons by DNA methylation editing of the FMR1 gene. Cell 172, 979.e6–992.e6. doi: 10.1093/hmg/dds348
Lu, C., Lin, L., Tan, H., Wu, H., Sherman, S. L., Gao, F., et al. (2012). Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice. Hum. Mol. Genet. 2123, 5039–5047.
Martin, L. A., Porter, A. G., Pelligrini, V. A., Schnatz, P. F., Jiang, X., Kleinstreuer, N., et al. (2017). A design thinking approach to primary ovarian insufficiency. Panminerva Med. 59, 15–32.
Mathews, T. J., and Hamilton, B. E. (2016). Mean age of mothers is on the rise: United States, 2000–2014. NCHS Data Brief, 1–8.
Monaghan, K. G., Lyon, E., Spector, E. B., and American College of Medical Genetics and Genomics. (2013). ACMG standards and guidelines for fragile X testing: a revision to the disease-specific supplements to the standards and guidelines for clinical genetics laboratories of the american college of medical genetics and genomics. Genet. Med. 15, 575–586. doi: 10.1038/gim.2013.61
Murray, A. (2000). Premature ovarian failure and the FMR1 gene. Semin. Reprod. Med. 18, 59–66. doi: 10.1055/s-2000-13476
Murray, A., Webb, J., Mac Swiney, F., Shipley, E. L., Morton, N. E., and Conway, G. S. (1999). Serum concentrations of follicle stimulating hormone may predict premature ovarian failure in FRAXA premutation women. Hum. Reprod. 14, 1217–1218. doi: 10.1093/humrep/14.5.1217
Nelson, L. M. (2009). Clinical practice. Primary ovarian insufficiency. N. Engl. J. Med. 3606, 606–614. doi: 10.1056/NEJMcp0808697
Nelson, L. M., Covington, S. N., and Rebar, R. W. (2005). An update: spontaneous premature ovarian failure is not an early menopause. Fertil. Steril. 835, 1327–1332. doi: 10.1016/j.fertnstert.2004.11.059
Nolin, S. L., Glicksman, A., Ding, X., Ersalesi, N., Brown, W. T., Sherman, S. L., et al. (2011). Fragile X analysis of 1112 prenatal samples from 1991 to 2010. Prenat. Diagn. 31, 925–931. doi: 10.1002/pd.2815
Nolin, S. L., Glicksman, A., Ersalesi, N., Dobkin, C., Brown, W. T., Cao, R., et al. (2015). Fragile X full mutation expansions are inhibited by one or more AGG interruptions in premutation carriers. Genet. Med. 17, 358–364. doi: 10.1038/gim.2014.106
Nolin, S. L., Sha, S., Glicksman, A., Sherman, S. L., Allen, E., Berry-Kravis, E., et al. (2013). Fragile X AGG analysis provides new risk predictions for 45-69 repeat alleles. Am. J. Med. Gen. 161, 771–788. doi: 10.1002/ajmg.a.35833
Oberlé, I., Rousseau, F., Heitz, D., Kretz, C., Devys, D., Hanauer, A., et al. (1991). Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252, 1097–1102. doi: 10.1126/science.252.5009.1097
Palacios, S., Henderson, V. W., Siseles, N., Tan, D., and Villaseca, P. (2010). Age of menopause and impact of climacteric symptoms by geographical region. Climacteric 13, 419–428. doi: 10.3109/13697137.2010.507886
Pastore, L. M., and Johnson, J. (2014). The FMR1 gene, infertility, and reproductive decision-making: a review. Front. Genet. 5:195. doi: 10.3389/fgene.2014.00195
Pearson, C. E., Nichol Edamura, K., and Cleary, J. D. (2005). Repeat instability: mechanisms of dynamic mutations. Nat. Rev. Genet. 6, 729–742. doi: 10.1038/nrg1689
Peier, A. M., and Nelson, D. L. (2002). Instability of a premutation-sized CGG repeat in FMR1 YAC transgenic mice. Genomics 80, 423–432. doi: 10.1006/geno.2002.6849
Popat, V. B., Calis, K. A., Kalantaridou, S. N., Vanderhoof, V. H., Koziol, D., Troendle, J. F., et al. (2014). Bone mineral density in young women with primary ovarian insufficiency: results of a three-year randomized controlled trial of physiological transdermal estradiol and testosterone replacement. J. Clin. Endocrinol. Metab. 999, 728–753. doi: 10.1210/jc.2013-4145
Popat, V. B., Vanderhoof, V. H., Calis, K. A., Troendle, J. F., and Nelson, L. M. (2008). Normalization of serum luteinizing hormone levels in women with 46,XX spontaneous primary ovarian insufficiency. Fertil. Steril. 892, 429–433. doi: 10.1016/j.fertnstert.2007.02.032
Rafique, S., Sterling, E. W., and Nelson, L. M. (2012). A new approach to primary ovarian insufficiency. Obstet. Gynecol. Clin. North Am. 394, 567–586. doi: 10.1016/j.ogc.2012.09.007
Schmidt, P. J., Luff, J. A., Haq, N. A., Vanderhoof, V. H., Koziol, D. E., Calis, K. A., et al. (2011). Depression in women with spontaneous 46, XX primary ovarian insufficiency. J. Clin. Endocrinol. Metab. 962, E278–E287. doi: 10.1210/jc.2010-0613
Sellier, C., Buijsen, R. A. M., He, F., Natla, S., Jung, L., Tropel, P., et al. (2017). Translation of expanded CGG repeats into FMRpolyG is pathogenic and may contribute to fragile X tremor ataxia syndrome. Neuron 932, 331–347. doi: 10.1016/j.neuron.2016.12.016
Seltzer, M. M., Baker, M. W., Hong, J., Maenner, M., Greenberg, J., and Mandel, D. (2012). Prevalence of CGG expansions of the FMR1 gene in a US population-based sample. Am. J. Med. Genet. B Neuropsychiatr. Genet. 159B5, 589–597. doi: 10.1002/ajmg.b.32065
Sherman, S. L. (2000). Premature ovarian failure in the fragile X syndrome. Am. J. Med. Genet. 973, 189–194. doi: 10.1002/1096-8628(200023)97:3<189::AID-AJMG1036>3.0.CO;2-J
Sherman, S. L., Curnow, E. C., Easley, C. A., Jin, P., Hukema, R. K., Tejada, M. I., et al. (2014). Use of model systems to understand the etiology of fragile X-associated primary ovarian insufficiency (FXPOI). J Neurodev. Disord. 61:26. doi: 10.1186/1866-1955-6-26
Sherman, S. L., Taylor, K., and Allen, E. G. (2007). FMR1 Premutation: A Leading Cause of Inherited Ovarian Dysfunction. In Fragile Sites: New Discoveries and Changing Perspectives. Hauppauge, NY: Nova Science Publishers, Inc.
Spath, M. A., Feuth, T. B., Smits, A. P., Yntema, H. G., Braat, D. D., Thomas, C. M., et al. (2011). Predictors and risk model development for menopausal age in fragile X premutation carriers. Genet. Med. 137. 643–650. doi: 10.1097/GIM.0b013e31821705e5
Steward, R., Melamed, A., Granat, A., and Mishell, D. R. Jr. (2012). Comparison of cervical mucus of 24/4 vs. 21/7 combined oral contraceptives. Contraception 866, 710–715. doi: 10.1016/j.contraception.2012.05.004
Streuli, I., Fraisse, T., Ibecheole, V., Moix, I., Morris, M. A., and de Ziegler, D. (2009). Intermediate and premutation FMR1 alleles in women with occult primary ovarian insufficiency. Fertil. Steril. 922, 464–470. doi: 10.1016/j.fertnstert.2008.07.007
Sullivan, A. K., Marcus, M., Epstein, M. P., Allen, E. G., Anido, A. E., Paquin, J. J., et al. (2005). Association of FMR1 repeat size with ovarian dysfunction. Hum. Reprod. 202, 402–412. doi: 10.1093/humrep/deh635
Sullivan, S. D., Sarrel, P. M., and Nelson, L. M. (2016). Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil. Steril. 1067, 1588–1599. doi: 10.1016/j.fertnstert.2016.09.046
Sullivan, S. D., Welt, C., and Sherman, S. (2011). FMR1 and the continuum of primary ovarian insufficiency. Semin. Reprod. Med. 294, 299–307. doi: 10.1055/s-0031-1280915
Tassone, F., Pan, R., Amiri, K., Taylor, A. K., and Hagerman, P. J. (2008). A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J. Mol. Diagn. 10, 43–49. doi: 10.2353/jmoldx.2008.070073
Tejada, M. I., Garcia-Alegria, E., Bilbao, A., Martinez-Bouzas, C., Beristain, E., Poch, M., et al. (2008). Analysis of the molecular parameters that could predict the risk of manifesting premature ovarian failure in female premutation carriers of fragile X syndrome. Menopause 155, 945–949. doi: 10.1097/gme.0b013e3181647762
The NAMS 2017 Hormone Therapy Position Statement Advisory Panel (2017). The NAMS 2017 Hormone Therapy Position Statement Advisory Panel, 2017 hormone therapy position statement of The North American menopause society. Menopause 247, 728–753.
Tingley, K., Coyle, D., Graham, I. D., Sikora, L., Chakraborty, P., Wilson K, et al. (2018). Using a meta-narrative literature review and focus groups with key stakeholders to identify perceived challenges and solutions for generating robust evidence on the effectiveness of treatments for rare diseases. Orphanet J. Rare Dis. 28:104. doi: 10.1186/s13023-018-0851-1
Uslu, B., Dioguardi, C. C., Haynes, M., Miao, D., Kurus, M., Hoffman, G., et al. (2017). Quantifying growing versus non-growing ovarian follicles in the mouse. J. Ovarian. Res. 10:3. doi: 10.1186/s13048-016-0296-x
van Kasteren, Y. M., and Schoemaker, J. (1999). Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum. Reprod. Update 5, 483–492. doi: 10.1093/humupd/5.5.483
Vanin, C. M., MacLusky, N. J., Grynpas, M. D., and Casper, R. F. (1995). The effect of three hormone replacement regimens on bone density in the aged ovariectomized rat. Fertil. Steril. 633, 643–651. doi: 10.1016/S0015-0282(16)57439-1
Verkerk, A. J., Pieretti, M., Sutcliffe, J. S., Fu, Y. H., Duhl, D. P. A., Pizzuti, A., et al. (1991). Identification of a gene FMR-1 containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 6–15. doi: 10.1016/0092-8674(91)90397-H
Welt, C. K., Smith, P. C., and Taylor, A. E. (2004). Evidence of early ovarian aging in fragile X premutation carriers. J. Clin. Endocrinol. Metab. 899, 4569–4574. doi: 10.1210/jc.2004-0347
Wheeler, A. C., Jr, D. B., Berry-Kravis, E., Greenberg, J., Losh, M., Mailick, M., et al. (2014). Associated features in females with an FMR1 premutation. J. Neurodev. Disord. 61:30. doi: 10.1186/1866-1955-6-30
Xie, N., Gong, H., Suhl, J. A., Chopra, P., Wang, T., and Warren, S. T. (2016). Reactivation of FMR1 by CRISPR/Cas9- mediated deletion of the expanded CGG-repeat of the fragile X chromosome. PLoS One 11:e0165499. doi: 10.1371/journal.pone.0165499
Yrigollen, C. M., Durbin-Johnson, B., Gane, L., Nelson, D. L., Hagerman, R., Hagerman, P. J., et al. (2012). AGG interruptions within the maternal FMR1 gene reduce the risk of offspring with fragile X syndrome. Genet. Med. 14, 729–736. doi: 10.1038/gim.2012.34
Yrigollen, C. M., Mendoza-Morales, G., Hagerman, R., and Tassone, F. (2013). Transmission of an FMR1 premutation allele in a large family identified through newborn screening: the role of AGG interruptions. J. Hum. Genet. 58, 553–559. doi: 10.1038/jhg.2013.50
Yu, S., Pritchard, M., Kremer, E., Lynch, M., Nancarrow, J., Baker, E., et al. (1991). Fragile X genotype characterized by an unstable region of DNA. Science 252, 1179–1181. doi: 10.1126/science.252.5009.1179
Zeesman, S., Zwaigenbaum, L., Whelan, D. T., Hagerman, R. J., Tassone, F., and Taylor, S. A. (2004). Paternal transmission of fragile X syndrome. Am. J. Med. Genet. A 129A, 184–189. doi: 10.1002/ajmg.a.30191
Keywords: FXPOI, POI, fragile X syndrome (FXS), primary ovarian insufficiency, fertility, irregular periods
Citation: Fink DA, Nelson LM, Pyeritz R, Johnson J, Sherman SL, Cohen Y and Elizur SE (2018) Fragile X Associated Primary Ovarian Insufficiency (FXPOI): Case Report and Literature Review. Front. Genet. 9:529. doi: 10.3389/fgene.2018.00529
Received: 28 May 2018; Accepted: 22 October 2018;
Published: 27 November 2018.
Edited by:
M. Z. A. Bhuiyan, Lausanne University Hospital (CHUV), SwitzerlandReviewed by:
Peter Turnpenny, Royal Devon and Exeter Hospital, United KingdomCopyright © 2018 Fink, Nelson, Pyeritz, Johnson, Sherman, Cohen and Elizur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shai E. Elizur, c2hhaS5lbGl6dXJAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.