
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Genet. , 19 July 2018
Sec. Applied Genetic Epidemiology
Volume 9 - 2018 | https://doi.org/10.3389/fgene.2018.00261
This article is part of the Research Topic Host Genetics in Viral Pathogenesis and Controls View all 22 articles
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and the second leading cause of cancer deaths globally. The high prevalence of HCC is due in part to the high prevalence of chronic HBV infection and the high mortality rate is due to the lack of biomarkers for early detection and limited treatment options for late stage HCC. The observed individual variance in development of HCC is attributable to differences in HBV genotype and mutations, host predisposing germline genetic variations, the acquisition of tumor-specific somatic mutations, as well as environmental factors. HBV genotype C and mutations in the preS, basic core promoter (BCP) or HBx regions are associated with an increased risk of HCC. Genome-wide association studies have identified common polymorphisms in KIF1B, HLA-DQ, STAT4, and GRIK1 with altered risk of HBV-related HCC. HBV integration into growth control genes (such as TERT), pro-oncogenic genes, or tumor suppressor genes and the oncogenic activity of truncated HBx promote hepatocarcinogenesis. Somatic mutations in the TERT promoter and classic cancer signaling pathways, including Wnt (CTNNB1), cell cycle regulation (TP53), and epigenetic modification (ARID2 and MLL4) are frequently detected in hepatic tumor tissues. The identification of HBV and host variation associated with tumor initiation and progression has clinical utility for improving early diagnosis and prognosis; whereas the identification of somatic mutations driving tumorigenesis hold promise to inform precision treatment for HCC patients.
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and the second leading cause of cancer deaths worldwide (El-Serag, 2011; Torre et al., 2015). HCC prevalence is highest in East and Southeast Asia and sub-Saharan Africa, but the incidence rates of HCC have increased in the United States and Western Europe over the past few decades (Lee, 2015; Njei et al., 2015). Early diagnosis and surgical resection remain the key to potential curative treatment; however, most HCC patients present with late stage tumors and have poor prognosis. HCC surveillance is mainly based on sonography and alpha-fetoprotein (AFP) measurement, both of which lack sufficient sensitivity and specificity (Yim and Lok, 2006; Bruix et al., 2011).
Hepatitis B virus (HBV) is one of the leading risk factors for HCC, especially in HBV endemic areas. Despite the existence of an effective vaccine, about 257 million people, or 3.5% of the global population, were afflicted with chronic HBV infection in 2015, WHO (2017). The clinical spectrum of chronic HBV infection ranges from asymptomatic carrier status to chronic hepatitis B (CHB), which may evolve to liver cirrhosis and HCC (EASL, 2012). An estimated 8–20% of untreated adults with CHB will develop liver cirrhosis within 5 years (Terrault et al., 2016) and 2–8% of those with cirrhosis develop HCC annually (Yim and Lok, 2006; Bruix et al., 2011; Hung et al., 2017).
Environmental and host risk factors for HCC include childhood acquisition of HBV infection, cirrhosis, aflatoxin B1 exposure, heavy alcohol use, smoking, male sex, advancing age, obesity, type 2 diabetes and an impaired immune response (Bruix et al., 2016). In addition to HBV, hepatitis C virus (HCV) chronic infection is a major cause of HCC, but co-infection by HBV and HCV do not seem to confer greater HCC risk than HBV or HCV monoinfection in a meta-analysis (Cho et al., 2011). Co-infection of HBV and hepatitis delta virus (HDV), occurring in ~5% of those HBV infected, was associated with 3 to 6-fold higher incidence of HCC in longitudinal cohorts (Fattovich et al., 2000; Romeo et al., 2009; Ji et al., 2012; Kushner et al., 2015).
Family clustering and incidence differences among different ancestry groups suggests that inherited genetic factors may contribute to HCC risk (Shen et al., 1991). Common and rare single nucleotide polymorphisms (SNPs) and structural genomic changes may predispose or restrict HCC development. The identification of moderate to high penetrant genetic variants associated with HBV-related HCC might identify HCC susceptible patients that would lead to earlier intervention and better outcomes (Thomas et al., 2009; Brennan et al., 2011; Michailidou et al., 2015). For example, screening for BRCA1 and BRCA2 mutations is routinely used to identify women at risk for breast and ovarian cancer and is routinely used in precision treatment protocols (Brennan et al., 2011; Michailidou et al., 2015).
Somatic mutations occurring in hepatocytes as the consequence of exogenous and endogenous mutagenic factors, are likely involved in HCC initiation and progression, as they are in many other cancers. Identification of cancer diver mutations in HCC are essential for HCC prognostics and for precision therapy targeting perturbed pathways.
Here, we review recent advancements and identify knowledge gaps in HBV-related HCC genetics at three levels: the human host, the virus, and somatic mutations in liver tumors and their associations with clinical outcomes.
HBV has a partially double-stranded circular DNA genome of ~3.2 kilobase (kb) pairs, comprising four overlapping open reading frames (ORF): preS/S, P, preC/C, and X (Liang, 2009). The PreS/S gene region encodes large (preS1+preS2 +S), middle (preS2+S), and small (S) HBsAg envelope proteins (Figure 1). The P region encodes the polymerase/reverse transcriptase, which is involved in genome replication. The preC/C codes the nucleocapsid hepatitis B core antigen (HBcAg) or the hepatitis B e antigen (HBeAg) translated from initiated codons at the core or precore regions, respectively. HBcAg and HBeAg are biomarkers for HBV active infection or infectivity. The X ORF encodes a nonstructural protein (HBx) with multiple functions in viral replication and oncogenic activity (Liang, 2009).
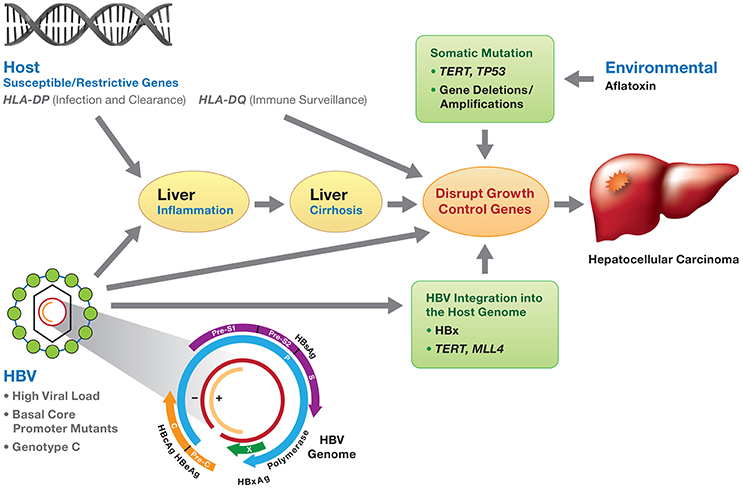
Figure 1. Viral and host genetic factors involved in the development of HBV-induced HCC. HBV infection, if not resolved, may develop to chronic hepatitis and progress to liver cirrhosis and subsequently HCC. Molecular mechanisms of HBV-related HCC involve (1) chronic inflammation and regeneration of hepatocytes; (2) accumulation of genetic alterations that confer cell growth advantage; (3) integration of HBV DNA into the host genome and activation of host genes controlling cell proliferation; (4) genomic instability; and (5) direct promotion of cell proliferation by viral proteins (mainly HBx). The development of HCC is the consequence of the interaction of environmental factors (e.g., aflatoxin), HBV viral factors (genotypes, HBV DNA levels and HBV mutants) and host genetic susceptible variants (e.g., HLA-DP variants affecting HBV clearance), along with somatic mutations (TP53, TERT, gene deletion/amplification) disrupting cell growth control. HBV genotype C and mutations in the preS, basic core promoter or HBx gene are associated with an increased risk of HCC.
There are at least three prevailing mechanisms proposed for the development of HBV-related HCC (Kremsdorf et al., 2006; Block et al., 2007; Hai et al., 2014; Figure 1). First, chronic inflammation and regeneration of hepatocytes during chronic HBV infection may lead to the accumulation of genetic alterations that confer cell growth advantage. Second, the integration of HBV DNA into the host genome may activate the host genes controlling cell proliferation and cause genomic instability. Finally, HBV proteins, mainly HBx, may promote cell proliferation (Kremsdorf et al., 2006; Block et al., 2007; Hai et al., 2014). It is also likely that all three mechanisms contribute to HCC development.
The general consensus is that persistent high-level HBV replication poses greater risk of developing liver cirrhosis and HCC (Sanchez-Tapias et al., 2002; Chen et al., 2006a,b, 2007; Fattovich et al., 2008). A large prospective study, which followed 3653 HBsAg positive participants enrolled in the Taiwanese Reveal-HBV cohort for over a decade, found that HBV DNA levels at study entry were positively correlated with incidence of HCC in a dose-dependent manner. Individuals with HBV levels greater than 1 million copies /mL were 10-fold more likely to develop HCC than those with less than 300 copies/mL (Chen et al., 2006b). Serum HBV DNA viral load is also associated HCC tumor recurrence (Hung et al., 2008; Wu et al., 2009). A viral load of greater than 10,000 copies/mL (2000 IU/mL) was independently associated with HBV-related HCC recurrence in patients who underwent liver resection (Hung et al., 2008). Antiviral therapy in these patients decreased tumor recurrence (Li et al., 2010).
Ten HBV genotypes (A to J) that diverge by >8% of their nucleotide sequences have been identified globally. HBV genotypes distribute within distinct geographic regions and ethnic populations(Lin and Kao, 2015). Phylogenetic analysis of HBV genotypes indicate that global distribution of HBV genotypes corresponds with the major prehistoric and modern human migration patterns, after HBV established infection in humans around 33,000 years ago (Paraskevis et al., 2013). Different genotypes prevail in the two regions with the highest HBV and HCC prevalence: genotypes B and C are prevalent in East Asia where vertical transmission is predominant, whereas genotypes A and D are prevalent in sub-Saharan Africa where horizontal transmission is more common. HBV genotypes A and D are the main genotypes in low HBV prevalence regions including Europe and North America (Lin and Kao, 2015).
The risk of HCC appears to differ by HBV subtypes. In East Asia, HBV genotype C is associated with higher risk of HCC compared to genotype B (Chan et al., 2004; Yu et al., 2005; Yang et al., 2008; Tseng et al., 2012; Vutien et al., 2013; Lin and Kao, 2015; Raffetti et al., 2016). Serum HBV viral load is significantly higher in patients with HBV genotype C than in those infected with genotype B (Chu et al., 2002; Chan et al., 2004; Yu et al., 2005; Lin and Kao, 2015). The increased risk of HCC might be the consequence of longer exposure to high levels of HBV in patients with genotype C (Kao et al., 2004; Ni et al., 2004). In contrast, HCC risk for genotype D and A appear to be similar, although there are reports that patients with HBV genotype A experience longer sustained remissions (Sanchez-Tapias et al., 2002) and are more responsive to interferon alpha (IFN- α) treatment compared to those infected with HBV genotype D (Erhardt et al., 2005). There are at least 5 co-circulating HBV genotypes in Alaska Native people, which offers rare opportunity for direct comparisons of HCC risk among genotypes. A longitudinal cohort of Alaska Natives followed for over 30 years reported that carriers of genotypes A (OR 4), C (OR 16), and F (OR 14) are at higher risk compared with genotypes B or D (Livingston et al., 2007; Ching et al., 2016). A study of 1,000 Alaska Native children and young adults followed for over 23 years found that HBV genotype F1 was associated with the highest risk for HCC (Gounder et al., 2016). These studies support a strong role for HBV genotypes in predicting HCC risk. In comparison, HCV genotype 3 was also associated with higher risk of HCC compared to other HCV genotypes (van der Meer et al., 2012; Kanwal et al., 2014).
HBV DNA replication errors occur at a much higher rate than for other DNA viruses because HBV reverse transcriptase lacks a proofreading function (Chotiyaputta and Lok, 2009). The estimated nucleotide substitution rate was 2.2 × 10−6 substitutions/site/year, which is higher than other DNA viruses but lower than RNA viruses (Paraskevis et al., 2013). Mutations in the HBV genome commonly associated with HCC are summarized in Table 1, with the preC/C and basic core promoter (BCP) regions harboring the most frequent mutations.
PreS mutations, especially the preS1 deletion, preS2 deletion, and preS2 start codon mutations, may induce an unbalanced production of envelope proteins that accumulate in the endoplasmic reticulum (ER) of the hepatocytes, leading to ER stress, oxidative DNA damage and genomic instability (Pollicino et al., 2014). These cytotoxic effects cause liver cell damage and regeneration (Pollicino et al., 2014). A meta-analysis of 9 cross-sectional studies including 388 HCC cases demonstrated that any one of the preS mutants (C1653T, T1753V, and A1762T/G1764A) was associated with a 3.8-fold increased risk of HCC (Liu et al., 2009), consistent with the result from a recent meta-analysis of prospective studies comprising 360 cases (RR 3.8) (Yang et al., 2015). PreS/S deletions and the Pre S2 start codon mutation had a RR 4.0 and 2.6, respectively (Yang et al., 2015). Specifically, C2964A, C3116T, and C7A were independently associated with an increased risk of HCC (Yin et al., 2010).
Mutations introducing stop codons in the S genomic region have been proposed to enhance tumor development by encoding truncated proteins with transcriptional transactivation activity (Pollicino et al., 2014). The premature stop codon mutations at position 172 or 182 in the S genomic region are associated with higher risk for liver cirrhosis and HCC (Lai et al., 2009; Pollicino et al., 2014).
Mounting evidence supports the importance of the naturally occurring basal core promoter (BCP) double mutation, A1762T/G1764A, as a risk factor for HCC (Fang et al., 2008; Yang et al., 2008, 2015; Yang Z. et al., 2016). In a meta-analysis of 15 prospective studies comprising 1336 HCC cases, individuals with the double mutation had a 3-fold higher risk of developing HCC (Yang et al., 2015). In a more recent meta-analysis that included 3729 HCC cases, double mutation carriers had a 5-fold higher HCC risk (Yang Z. et al., 2016). Other prospective long-term follow-up studies found that the incidence of HCC in double mutation carriers was 4 to 5-fold higher (Fang et al., 2008; Yang et al., 2008). The presence of the BCP double mutation reduces HBeAg production and viral load before anti-HBe seroconversion (Buckwold et al., 1996; Fang et al., 2009), suggesting that its oncogenic mechanism is not directly attributable to viral replication level. However, the preC stop codon G1896A, which prevents the production of HBeAg, does not appear to affect HCC risk as demonstrated in a recent meta-analysis with 600 HCC cases (Yang et al., 2015).
Anti-HBV nucleos(t)ide analogs (NAs) are effective in lowering HBV DNA levels through their inhibition of HBV polymerase/reverse transcriptase (Terrault et al., 2016). However, the emergence of and the selection for resistance mutations in the polymerase/reverse transcriptase region confer a virial survival advantage and is a major barrier to the success of NAs treatment. In a longitudinal study of lamivudine (LMV)-resistant CHB patients, emergence of the rtA181T/sW172X mutant in LMV-resistant patients increased the risk of HCC (Lai et al., 2009; Yeh et al., 2011). NIH3T3 cells expressing this mutant showed greater oncogenic potential in nude mice (Lai and Yeh, 2008; Lai et al., 2009). It is an open question whether resistance to other nucelos(t)ide analogs also increases the risk of HCC.
HBx protein is a transcriptional activator of various host cellular genes involved in growth control, DNA repair, and epigenetic modification (Xu et al., 2014). It activates the Ras/Raf/mitogen-activated protein (MAP) kinase pathway, which is involved in hepatocarcinogenesis, and interacts with the tumor protein 53 gene (TP53), interfering with its function as a tumor suppressor (Di Bisceglie, 2009).
Certain point mutations in the HBx gene, in particular, the K130M and V131I double substitutions, are more frequent in patients with liver cirrhosis and/or HCC than in patients with chronic hepatitis B (Baptista et al., 1999). Due to the partial overlap of BCP with Hbx, the same nucleotide substitutions result in both HBx K130M and V131I and pre-core BCP A1762T/G1764A changes. Increasing number of HBx mutations is correlated with increased risk of HCC. The double xK130M+xV131I and triple xK130M+xV131I+ xV5M mutations are associated with a 4-5-fold increased the risk of HCC (Lee et al., 2011). Substitutions at position 10, 30, 38, 88, 94, and 144 have also been reported to be associated with HCC (Muroyama et al., 2006; Lee et al., 2011; Wang et al., 2012; Shi et al., 2016; Table 1). Even though the precise mechanism remains to be elucidated, HBx plays a central role in hepatocarcinogenesis and could be an attractive therapeutic target for HCC suppression.
In summary, major HBV viral factors associated with increased risk of HCC include genotype C and F, higher HBV-DNA levels, mutations in the preS/S region, the double mutation A1762T/G1764A in the basic core promoter, and the double or triple mutation K130M, V131I xV5M in the HBx gene. Different genotypes of the virus (which are due to the accumulation of viral mutations over time) have differing propensities for the development of HCC. Epidemiological association data and some functional evidence suggest the likely causal role of viral mutations, particularly for genotype differences. However, the temporal and causal relationship of HBV mutations with HCC remains to be firmly established, as most results were obtained from case-control and cross-sectional studies and rarely from prospective longitudinal HBV cohorts. HBV mutations may also increase risk of HCC indirectly by the acquisition of mutations that leads to immune escape and increased viral replication. Higher HBV burden would contribute to chronic inflammation promoting the destruction and regeneration of HBV infected hepatocytes mediated by host cytotoxic T lymphocyte (CTL) immune response and increasing the opportunity for replication errors during cell mitosis (Block et al., 2007). The emergence and accumulation of genetic and epigenetic alterations with cell growth advantage eventually lead to hepatocarcinogenesis (Block et al., 2007). The exact molecular mechanisms by which HBV mutations promote hepatocarcinogenesis warrants further investigation.
Although the incidence of HCC among HBV carriers is much higher (223-fold) than among non-carriers (Beasley et al., 1981), only a fraction of patients with chronic HBV infection develop HCC. Results from twin studies, family clustering studies and incidence differences between continental ancestry groups suggest that host genetic factors contribute to HCC susceptibility (Shen et al., 1991). Genome-wide association studies (GWAS) provide an agnostic (hypothesis-free) method to identify susceptibility and resistance loci for HBV-related HCC. The ability to securely identify genetic susceptibility loci associated with HCC is critically dependent on sample size for sufficient power to detect associations with small to moderate effect sizes in GWAS. This is due to the need to correct for multiple testing that tends to inflate false positive signals; therefore, the human genetics community has determined that a genome-wide significance threshold of p < 5 × 10−8 and independent replication association is required to firmly establish a genotype-phenotype association. The top associations between host genetic variants with HCC are summarized in Table 2. Only associations in the HLA class II region have been found to replicate in at least two studies. These studies, performed primarily in East Asians, suggest that no common, moderate to high penetrant alleles contribute to HCC development.
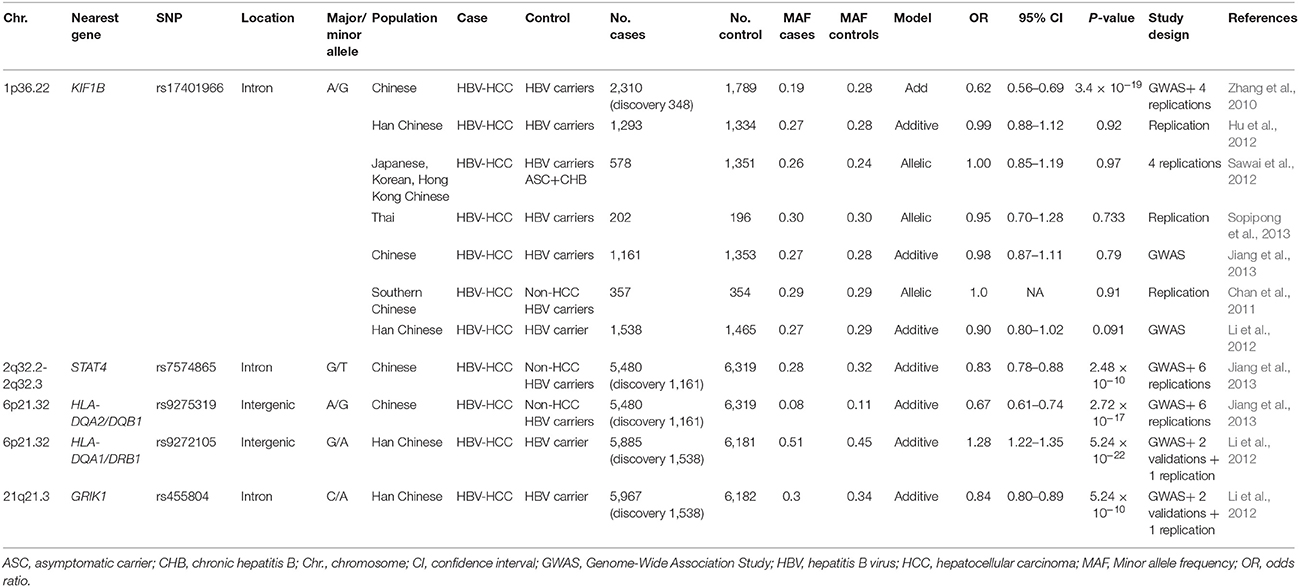
Table 2. Associations of host genetic variants with HBV-related HCC from GWASs and replication studies.
The HCC association of intergenic SNPs near HLA-DQA1 and HLA-DQA2, and HLA-DQB1, encoding the alpha and beta chains of the HLA-DQ protein, were identified by GWAS in two large studies (Li et al., 2012; Jiang et al., 2013). HLA class II proteins, comprising HLA-DP, DQ, and DR proteins, play a central role in extracellular antigen presentation to CD4 T cells stimulating B cell that lead to antibody production against foreign pathogens, including HBV and HCV.
Outside of the HLA region, genetic associations with HBV-related HCC have been reported for several genes. A two-stage GWAS composed of five cohorts from East Asian identified the intronic rs17401966 SNP in the KIF1B gene (OR = 0.61) with genome-wide significance in a meta-analysis (Zhang et al., 2010). KIF1B encodes a kinesin superfamily member involved in the transport of organelles and vesicles. Both germline and somatic loss-of-function mutations in the KIF1B isoform have been detected in multiple cancers (Zhang et al., 2010). However, this association failed to replicate in multiple independent studies, several of which were well-powered (Table 2; Hu et al., 2012; Li et al., 2012; Sawai et al., 2012; Jiang et al., 2013; Sopipong et al., 2013). The latest meta-analysis of 12 cohorts found statistical significance only when the original 5 cohorts reporting positive associations were included, but when these 5 cohorts were removed the association was abrogated (Su et al., 2017). Further validation and functional studies are warranted to support or not a role of KIF1b variation in HCC.
Other candidate genes implicated by GWAS include STAT4, and GRIK1 (Table 2; Li et al., 2012; Jiang et al., 2013). STAT4 is a transcription factor involved in development of Th1 cells and production of IFN-γ, a cytokine with antiviral and antitumor activities. STAT4 rs7574865 is an expression quantitative trait locus (eQTL) with dosage effect in HCC tissues (Jiang et al., 2013). Glutamate receptor GRIK1, is involved in cancer development (Li et al., 2012), though its functional role in HCC has not been experimentally demonstrated. These associations have not yet been replicated or validated by functional assessment. Recently, a functional genomic approach revealed a c-Myc binding SNP that regulates a putative tumor suppressor gene EPB41 and was associated with predisposition to HCC (Yang et al., 2016b).
A 2011 experts′ commentary highlighted the need for further GWAS studies of HCC with larger subject enrollment, clearly delineated phenotypes, and replication (Budhu and Wang, 2011). The lack of replication for HCC GWAS studies is most likely due to relatively small sample sizes of the study although genetic and phenotypic heterogeneity may have also contributed. True causal variants should have similar effects across all populations while marker SNPs may have different effects in different ancestry or ethnic populations. These differences in effect size or statistical significance in haplotype structure among different populations, the inclusion of trans-ethnic or continental racial populations can be used to refine position of the causal locus by fine-mapping (Franceschini et al., 2012). The newer population specific genotyping arrays, in combination with larger case-control groups representing diverse populations, should reveal additional germline variants that contribute to the observed variance in HBV-HCC susceptibility. Finally, whole genome or exome sequencing might also identify causal rare variants associated with HBV-HCC. These studies should identify critical pathophysiological pathways for HBV-related liver cirrhosis and HCC.
Environmental factors, HBV genotypes and genetic variation, and host genetic variation are involved in the development and progression of HCC. The interplay between these factors might lead to the initiation of HCC (Figure 1).
HBV DNA integration into the host genome is one of the proposed molecular mechanisms of hepatocarcinogenesis. HBV-DNA integration occurs during both the acute and chronic stages and integrated HBV-DNA is detectable in 75-90% of HCC tissues (Murakami et al., 2005; Zhao et al., 2016). HBV integration events may cause direct gene disruption, HBV promoter-driven transcription of host genes, viral-host transcript fusion and induce genome instability (Tao et al., 2011; Jiang et al., 2012; Sung et al., 2012), which may lead to activation of proto-oncogenes or inactivation of tumor suppressor genes. Oncogenic activity of the cellular and viral genes resulting from the HBV integration confers a selective growth advantage to cells with accumulation of genetic defects, leading to hepatocarcinogenesis (Tao et al., 2011; Jiang et al., 2012; Sung et al., 2012).
Advanced genome-wide sequencing technology has enabled non-biased genome scans of HBV integration sites in HCCs (Table 3; Murakami et al., 2005; Ding et al., 2012; Toh et al., 2013; Lau et al., 2014). HBV integration sites are randomly distributed across the whole genome with a handful of hotspots (Sung et al., 2012). Some investigators found preferential integration in chromosome 10 and 17 (Ding et al., 2012; Toh et al., 2013; Zhao et al., 2016). HBV appears to preferentially integrate near coding genes that are transcriptionally active (Ding et al., 2012; Sung et al., 2012; Toh et al., 2013), as was observed for HIV integration (Maldarelli et al., 2014).
Recurrent HBV integration sites have been detected in many cancer-related genes. The highest frequency of HBV integration is detected in the telomerase reverse transcriptase (TERT) gene (Paterlini-Brechot et al., 2003; Murakami et al., 2005; Ding et al., 2012; Fujimoto et al., 2012; Sung et al., 2012; Li et al., 2013; Toh et al., 2013; Lau et al., 2014; Kawai-Kitahata et al., 2016). Integration in the proximity of TERT is correlated with TERT gene expression (Sung et al., 2012; Zhao et al., 2016); reactivation of TERT likely confers early clonal advantage during chronic HBV infection. Whole-genome sequencing identified clonal expansion of HBV integration in the TERT locus in HCC tumors but not in adjacent non-tumor tissue DNA, suggesting its role in liver carcinogenesis (Fujimoto et al., 2012). Additional commonly identified recurrent target genes for HBV integration in HCC liver tissue are listed in Table 3, including MLL4 and CCNE1 (Saigo et al., 2008; Ding et al., 2012; Jiang et al., 2012; Sung et al., 2012; Li et al., 2013) among others (Murakami et al., 2005; Tao et al., 2011; Ding et al., 2012; Jiang et al., 2012; Sung et al., 2012; Toh et al., 2013). Individuals with a high number of integration sites have unfavorable HCC survival (Murakami et al., 2005; Ding et al., 2012; Toh et al., 2013; Lau et al., 2014; Zhao et al., 2016).
The integrated HBV genome itself may be oncogenic (Ding et al., 2012; Fujimoto et al., 2012; Jiang et al., 2012; Li et al., 2013; Toh et al., 2013). Upon integration, the 3′-end of the HBx is often deleted, resulting in C-terminal truncated HBx (ct-HBx) protein, which contributes to HCC initiation and progression (Tu et al., 2001; Ma et al., 2008; Sze et al., 2013; Yin et al., 2013; Wang et al., 2014; Zhu et al., 2015; Li et al., 2016). The truncated HBx protein is reported to promote the transforming ability of hepatocytes (Tu et al., 2001), induce C-Jun/MMP10 activation to increase cellular proliferation (Ma et al., 2008; Sze et al., 2013), as well as enhance hepatoma cell invasion and metastasis (Sze et al., 2013; Li et al., 2016). Ct- HBx is present significantly more in tumors compared to adjacent non-tumorous tissues in several studies (Yin et al., 2013; Wang et al., 2014; Zhu et al., 2015; Li et al., 2016) and Ct-HBx expression in HCC tissue correlates with decreased patient survival (Yin et al., 2013).
Quantification of HBV integration sites may have prognostic value in predicting HCC survival. In a genome-wide survey of 88 HCC patients, increasing number of HBV integrations was correlated with HCC occurrence at younger age and shorter survival (Sung et al., 2012). A viral-human chimeric HBx-LINE1 RNA hybrid, detected in 23% of HCC tumors, was also predictive of poorer survival (Lau et al., 2014).
Most somatic mutations are harmless passenger mutations accumulated in the process of tumor growth, which occur at random and confer no selective advantage for tumor cells. Driver mutations confer selective growth advantage and cause transformation of a normal cell to a cancer cell, which are clinically relevant. Recurrent coding changes in multiple tumor cases may identify putative cancer driver mutations (Bozic et al., 2010). Recent whole-genome and whole-exome deep sequencing of tumor and non-tumor tissues have revealed the importance of somatic mutations and structural variations in tumor development across cancer types and has led to more efficacious treatment modalities based on molecular changes or genomic classification rather than target organs of cancer (e.g., BRAF V600E, KIT, EGFR, ERBB2) (Swanton et al., 2016).
Recurrent somatic mutations in HCC have been identified in several known cancer-related genes and pathways. These include genes required for telomere maintenance, the Wnt signaling pathway, which regulates cell proliferation, cell cycle regulation, epigenetic modification, and the PI3K/Akt/mTOR, Ras/Raf/MAP, oxidative stress, and JAK/STAT pathways (Li et al., 2011; Woo et al., 2011; Fujimoto et al., 2012; Guichard et al., 2012; Huang et al., 2012; Cleary et al., 2013; Kan et al., 2013; Nault et al., 2013; Ahn et al., 2014; Meng et al., 2014; Totoki et al., 2014; Schulze et al., 2015; Kawai-Kitahata et al., 2016; Yao S. et al., 2016). The perturbed genes identified in HCC tumor genomes are listed in Table 4. Copy number alterations result from chromosomal focal amplifications of some oncogenes and less frequently from homozygous deletions of tumor suppressors (Sawey et al., 2011; Guichard et al., 2012; Wang et al., 2013; Totoki et al., 2014; Schulze et al., 2015). Recurrent focal amplifications have been observed for TERT, MET, and others (Sawey et al., 2011; Guichard et al., 2012; Wang et al., 2013; Totoki et al., 2014; Schulze et al., 2015), while recurrent homozygous deletions have been reported for CDKN2A, ARID1A, and others (Table 4; Guichard et al., 2012; Wang et al., 2013; Totoki et al., 2014; Schulze et al., 2015).
The TERT gene encodes a catalytic subunit of telomerase that maintains genomic integrity. TERT expression is repressed in somatic cells, but not in proliferative cells in self-renewing tissues and cancers. Somatic mutations in the TERT promoter are found across multiple cancer types. Immortality associated with cancer cells has been attributed to telomerase over-expression. TERT promoter mutations create a potential binding site for E-twenty-six/ternary complex factors (ETS/TCF) transcription factors and are associated with increased promoter activity, increased expression of TERT and increased telomerase activity (Heidenreich et al., 2014; Bell et al., 2015; Borah et al., 2015).
TERT promoter mutations are the most frequent somatic genetic alterations observed in HCC, with an overall prevalence of approximately 60%, with ranges from 30 to 40% for HBV-related HCC to 60 to 80% for HCV-related HCC (Nault et al., 2013; Totoki et al., 2014; Fujimoto et al., 2015; Schulze et al., 2015; Kawai-Kitahata et al., 2016; Yang et al., 2016a). HCC with HCV infection and alcohol intake more often harbor TERT promoter mutations than those with HBV infection (Nault et al., 2013). The lower rate of TERT promoter mutations in HBV-related HCC could be partially explained by the frequent insertion of HBV DNA in the TERT promoter serving as additional mechanism inducing telomerase transcription (Nault et al., 2013).
Somatic TERT promoter mutations may represent an early event in liver carcinogenesis in a setting of cirrhosis leading to malignant transformation (Nault et al., 2013, 2014). Mutations in the TERT promoter but not in classical liver cancer driver genes such as CTNNB1 and TP53 can be found in cirrhotic preneoplasia (Nault et al., 2013). TERT promoter mutations have been identified in 6% of low-grade dysplastic nodules, 19% of high-grade dysplastic nodules, 61% of early hepatocellular carcinomas, and 42% of small and progressed HCC, correlating with step-wise development of hepatocarcinogenesis. TERT promoter mutations may have utility as a marker for high risk of malignant transformation in cirrhotic tissue (Nault et al., 2014). TERT promoter mutations were more frequent in those with lower AFP serum levels, usually in small tumors (Nault et al., 2013; Yang et al., 2016a). Thus, detection of TERT promoter mutations may aid in diagnosis of atypical or early HCC cases with lower serum AFP levels.
Catenin beta 1 (CTNNB1), a key signaling transducer in the Wnt pathway, regulates cellular proliferation and differentiation. CTNNB1 is the most frequently mutated oncogene in HCCs (10–50%) (Satoh et al., 2000; Li et al., 2011; Fujimoto et al., 2012; Guichard et al., 2012; Cleary et al., 2013; Kan et al., 2013; Ahn et al., 2014; Totoki et al., 2014; Schulze et al., 2015; Kawai-Kitahata et al., 2016; Rebouissou et al., 2016; Yao S. et al., 2016). Most mutated residues are in or near phosphorylation sites and prevent phosphorylation-dependent ubiquitination, resulting in abnormal accumulation of β-catenin protein that in turn causes abnormal expression of cell proliferation genes (Klaus and Birchmeier, 2008). Like TERT promoter mutations, CTNNB1 mutation frequency varies in HCC cases by etiological factors, with ranges from 10 to 16% in HBV-related HCC, 20 to 40% in HCV-related HCC, and 30 to 50% in alcoholic HCC (Li et al., 2011; Ahn et al., 2014; Kawai-Kitahata et al., 2016; Rebouissou et al., 2016). CTNNB1 mutations were associated with lower AFP levels (Rebouissou et al., 2016), indicating detection of CTNNB1 mutations may also have diagnostic value for HCC with atypical presentation.
AXIN1 is the second most frequently mutated gene in the Wnt pathway (occurring in 2–20% of HCC cases) (Satoh et al., 2000; Guichard et al., 2012; Kan et al., 2013; Ahn et al., 2014; Totoki et al., 2014; Schulze et al., 2015; Kawai-Kitahata et al., 2016). Axin may be an effective therapeutic target for suppressing growth of HCC tumors (Satoh et al., 2000).
As a tumor suppressor and transcription factor, TP53 can both activate and repress gene expression to initiate cell-cycle arrest, apoptosis, and senescence in response to cellular stresses, including DNA damage, oncogene activation, and hypoxia, to maintain the integrity of the genome (Lee, 2015). TP53 mutations occurred in approximately 10% to 50% of HCC cases (Li et al., 2011; Woo et al., 2011; Fujimoto et al., 2012; Guichard et al., 2012; Huang et al., 2012; Cleary et al., 2013; Kan et al., 2013; Nault et al., 2013; Ahn et al., 2014; Meng et al., 2014; Totoki et al., 2014; Schulze et al., 2015; Kawai-Kitahata et al., 2016; Yao S. et al., 2016). The mutation spectrum of TP53 varies depending on etiological and environmental factors. The TP53 R249S hotspot mutation is particularly associated with aflatoxin B1 exposure, which interacts synergistically with HBV infection to promote hepatocarcinogenesis (Kew, 2003; Hussain et al., 2007). TP53 mutations occurred more frequently in HBV-related HCC (~30 to 70%) than in non-HBV HCC (Li et al., 2011; Ahn et al., 2014; Kawai-Kitahata et al., 2016). TP53 mutation is also associated with tumor histological grade. HCCs with a high histological grade have a higher TP53 mutation rate (40%) than those with a low histological grades (10%) (Ahn et al., 2014).
Abrogation of the IRF2 (encoding interferon regulatory factor 2), occurring in ~2–5% of HCCs, also leads to impaired TP53 function (Guichard et al., 2012).
Somatic mutations in HCC tumors have also been observed in other cell cycle genes, including the tumor suppressor genes RB1 (~3–10%) (Kan et al., 2013; Ahn et al., 2014; Totoki et al., 2014; Yao S. et al., 2016) and CDKN2A (2~8%) (Guichard et al., 2012; Ahn et al., 2014; Totoki et al., 2014; Schulze et al., 2015; Kawai-Kitahata et al., 2016).
Alterations of chromatin regulator genes are recurrently observed in HCC cases. Of 27 HCC tumors with WGS, 52% had somatic mutations or indels in at least one chromatin regulator genes (e.g., ARID1A, ARID1B, ARID2, MLL, and MLL3; Fujimoto et al., 2012). ARID1A and ARID2 are chromatin remodeling factors, which regulate DNA accessibility to transcription, DNA replication, and repair machineries. ARID2 mutations were significantly enriched in HCV-associated HCC (18%) compared with HBV-related HCC (2%) (Li et al., 2011). MLL, MLL2, MLL3, and MLL4 genes encode H3K4 methyltransferases that regulate methylation, acetylation and remodeling of nucleosomes. The mutation rates at these genes are presented in (Table 4; Li et al., 2011; Fujimoto et al., 2012, 2015; Guichard et al., 2012; Huang et al., 2012; Cleary et al., 2013; Ahn et al., 2014; Totoki et al., 2014; Schulze et al., 2015; Kawai-Kitahata et al., 2016; Yao S. et al., 2016). Less common mutations (< 10%) were detected in genes involved in PI3K/Akt/mTOR and Ras/Raf/MAP pathways, stress oxidative pathway (Guichard et al., 2012; Cleary et al., 2013; Totoki et al., 2014; Schulze et al., 2015; Kawai-Kitahata et al., 2016) and JAK/STAT pathway (Kan et al., 2013; Nault et al., 2013; Ahn et al., 2014; Totoki et al., 2014) (Table 4).
Certain subsets of altered genes share the same pathways or interact, contributing to the complexity and heterogeneity of hepatocarcinogenesis. Activated mutations of CTNNB1 are significantly associated with mutations in the TERT promoter (Nault et al., 2013; Totoki et al., 2014). It has been proposed that TERT might be a direct target of CTNNB1 (Hoffmeyer et al., 2012; Zhang et al., 2012). Alterations in RPS6KA3 are frequently associated with AXIN1 mutations, suggesting cooperation between RPS6KA3 inactivation and Wnt/β-catenin activation in tumorigenesis (Guichard et al., 2012). On the other hand, a number of mutations appear to be mutually exclusive and rarely appear together in the same tumor, such as CTNNB1 mutations with TP53 mutations (Guichard et al., 2012; Ahn et al., 2014) and AXIN1 mutations with CTNNB1 mutations (Satoh et al., 2000). ARID1A/ARID2 mutations are negatively associated with mutations in TP53 (Lee, 2015). The consequences of network interactions between driver mutations may offer deeper insight into tumorigenesis.
Targeted therapy based on genomic alterations is a core tenet of precision treatment. Exome sequencing analysis of 243 liver tumors found that 28% of patients harbor at least one alteration potentially targetable by an FDA-approved drug, and 86% harbored a mutation targetable by a drug studied in phase I to phase III clinical trials (Schulze et al., 2015). β-catenin reduction in CTNNB1-mutated HCCs in a murine model led to complete tumor response, showing a clear benefit of therapeutic targeting of this molecule (Delgado et al., 2015). Adenovirus mediated gene transfer of wild-type AXIN1 induced apoptosis in hepatocellular cancer cells that had accumulated β-catenin as a consequence of mutations in APC, CTNNB1, or AXIN1 genes, suggesting that AXIN1 may be an effective therapeutic molecule for suppressing HCC growth (Satoh et al., 2000).
Somatic mutations of driver genes may be predictive of HCC prognosis. In a study of over 300 HCC Chinese patients, TP53 hotspot mutations (R249S and V157F were strongly associated with decreased overall survival, indicating that these mutations can be used as prognostic markers in HCC in patients at risk for high aflatoxin exposures (Woo et al., 2011). Mechanistically, a synergistic interaction of aflatoxin B1 induced TP53 mutations together with HBV chronic inflammation may advance the development of HCC. In a multivariate analysis of 231 Korean HCC patients, the RB1 somatic mutation was the only independent prognostic factor for reduced cancer-specific survival and accelerated recurrence (Ahn et al., 2014). In a cohort of resected HCCs, CDKN2A inactivation was associated with poor prognosis (Schulze et al., 2015). These predictive and prognostic molecular markers may have clinical utility for personalized treatment plans.
The development of HCC is multifactorial with viral, host and environmental factors contributing to chronic inflammation, cirrhosis and ultimately, hepatocarcinogenesis (Figure 1). Interplay between host germline variants, persistent high HBV viral load, viral genotypes and mutations, HBV integration into host chromosomes, the oncogenic potential of the HBx protein, and the occurrence of somatic cancer driver mutations may contribute independently or jointly to the oncogenesis of HBV-related HCC (Figure 1). We hope that eventually genetic profiling of the virus and host will identify the individuals who are at higher risk of HCC and those who will benefit most from HCC treatment options. Since early HCC is largely asymptomatic and biopsies are rarely performed; there is a paucity of pre-tumor and early-stage tumor tissue available, a major impediment for genetic interrogation. This is in contrast to breast, colon, and prostate cancers where biopsies are standard of care for patients with abnormal findings during routine screening. Validated genetic biomarkers would have utility for early diagnosis, molecular classification of tumors, and prognostics as well as identify new targets for drug interventions. With the advent of ever larger HCC cohorts, denser, population-specific genotyping arrays, and next generation whole exome/genome sequencing of HCC family clusters, it may be possible to provide earlier diagnosis of HCC and to develop bespoke treatment for persons with HCC to improve outcomes.
PA and CW: conceived and wrote the paper; JX: wrote and revision of the manuscript; YY: revision of the manuscript.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of health, under contract HHSN26120080001E. This Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank George Nelson and Victor David for proofreading.
Ahn, S. M., Jang, S. J., Shim, J. H., Kim, D., Hong, S. M., Sung, C. O., et al. (2014). Genomic portrait of resectable hepatocellular carcinomas: implications of RB1 and FGF19 aberrations for patient stratification. Hepatology 60, 1972–1982. doi: 10.1002/hep.27198
Baptista, M., Kramvis, A., and Kew, M. C. (1999). High prevalence of 1762(T) 1764(A) mutations in the basic core promoter of hepatitis B virus isolated from black Africans with hepatocellular carcinoma compared with asymptomatic carriers. Hepatology 29, 946–953. doi: 10.1002/hep.510290336
Beasley, R. P., Hwang, L. Y., Lin, C. C., and Chien, C. S. (1981). Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet 2, 1129–1133.
Bell, R. J., Rube, H. T., Kreig, A., Mancini, A., Fouse, S. D., Nagarajan, R. P., et al. (2015). The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science 348, 1036–1039. doi: 10.1126/science.aab0015
Block, T. M., Guo, H., and Guo, J. T. (2007). Molecular virology of hepatitis B virus for clinicians. Clin. Liver Dis. 11, 685–706. doi: 10.1016/j.cld.2007.08.002
Borah, S., Xi, L., Zaug, A. J., Powell, N. M., Dancik, G. M., Cohen, S. B., et al. (2015). Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science 347, 1006–1010. doi: 10.1126/science.1260200
Bozic, I., Antal, T., Ohtsuki, H., Carter, H., Kim, D., Chen, S., et al. (2010). Accumulation of driver and passenger mutations during tumor progression. Proc. Natl. Acad. Sci. U.S.A. 107, 18545–18550. doi: 10.1073/pnas.1010978107
Brennan, P., Hainaut, P., and Boffetta, P. (2011). Genetics of lung-cancer susceptibility. Lancet Oncol. 12, 399–408. doi: 10.1016/S1470-2045(10)70126-1
Bruix, J., Reig, M., and Sherman, M. (2016). Evidence-based diagnosis, staging, and treatment of patients with Hepatocellular Carcinoma. Gastroenterology 150, 835–853. doi: 10.1053/j.gastro.2015.12.041
Bruix, J., Sherman, M., and American Association for the Study of Liver, D. (2011). Management of hepatocellular carcinoma: an update. Hepatology 53, 1020–1022. doi: 10.1002/hep.24199
Buckwold, V. E., Xu, Z., Chen, M., Yen, T. S., and Ou, J. H. (1996). Effects of a naturally occurring mutation in the hepatitis B virus basal core promoter on precore gene expression and viral replication. J. Virol. 70, 5845–5851.
Budhu, A., and Wang, X. W. (2011). Power play: scoring our goals for liver cancer with better GWAS study design. J. Hepatol. 54, 823–824. doi: 10.1016/j.jhep.2010.10.035
Chan, H. L., Hui, A. Y., Wong, M. L., Tse, A. M., Hung, L. C., Wong, V. W., et al. (2004). Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut 53, 1494–1498. doi: 10.1136/gut.2003.033324
Chan, K. Y., Wong, C. M., Kwan, J. S., Lee, J. M., Cheung, K. W., Yuen, M. F., et al. (2011). Genome-wide association study of hepatocellular carcinoma in Southern Chinese patients with chronic hepatitis B virus infection. PLoS ONE 6:e28798. doi: 10.1371/journal.pone.0028798
Chen, C. H., Hung, C. H., Lee, C. M., Hu, T. H., Wang, J. H., Wang, J. C., et al. (2007). Pre-S deletion and complex mutations of hepatitis B virus related to advanced liver disease in HBeAg-negative patients. Gastroenterology 133, 1466–1474. doi: 10.1053/j.gastro.2007.09.002
Chen, C. J., Yang, H. I., Su, J., Jen, C. L., You, S. L., Lu, S. N., et al. (2006a). Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295, 65–73. doi: 10.1001/jama.295.1.65
Chen, G., Lin, W., Shen, F., Iloeje, U. H., London, W. T., and Evans, A. A. (2006b). Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am. J. Gastroenterol. 101, 1797–1803. doi: 10.1111/j.1572-0241.2006.00647.x
Ching, L. K., Gounder, P. P., Bulkow, L., Spradling, P. R., Bruce, M. G., Negus, S., et al. (2016). Incidence of hepatocellular carcinoma according to hepatitis B virus genotype in Alaska Native people. Liver Int. 36, 1507–1515. doi: 10.1111/liv.13129
Cho, L. Y., Yang, J. J., Ko, K. P., Park, B., Shin, A., Lim, M. K., et al. (2011). Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: systematic review and meta-analysis. Int. J. Cancer 128, 176–184. doi: 10.1002/ijc.25321
Chotiyaputta, W., and Lok, A. S. (2009). Hepatitis B virus variants. Nat. Rev. Gastroenterol. Hepatol. 6, 453–462. doi: 10.1038/nrgastro.2009.107
Chu, C. J., Hussain, M., and Lok, A. S. (2002). Hepatitis B virus genotype B is associated with earlier HBeAg seroconversion compared with hepatitis B virus genotype C. Gastroenterology 122, 1756–1762. doi: 10.1053/gast.2002.33588
Cleary, S. P., Jeck, W. R., Zhao, X., Chen, K., Selitsky, S. R., Savich, G. L., et al. (2013). Identification of driver genes in hepatocellular carcinoma by exome sequencing. Hepatology 58, 1693–1702. doi: 10.1002/hep.26540
Delgado, E., Okabe, H., Preziosi, M., Russell, J. O., Alvarado, T. F., Oertel, M., et al. (2015). Complete response of Ctnnb1-mutated tumours to beta-catenin suppression by locked nucleic acid antisense in a mouse hepatocarcinogenesis model. J. Hepatol. 62, 380–387. doi: 10.1016/j.jhep.2014.10.021
Di Bisceglie, A. M. (2009). Hepatitis B and hepatocellular carcinoma. Hepatology 49, S56–60. doi: 10.1002/hep.22962
Ding, D., Lou, X., Hua, D., Yu, W., Li, L., Wang, J., et al. (2012). Recurrent targeted genes of hepatitis B virus in the liver cancer genomes identified by a next-generation sequencing-based approach. PLoS Genet. 8:e1003065. doi: 10.1371/journal.pgen.1003065
EASL (2012). EASLclinical practice guidelines: management of chronic hepatitis B virus infection. J. Hepatol. 57, 167–185. doi: 10.1016/j.jhep.2012.02.010
El-Serag, H. B. (2011). Hepatocellular carcinoma. N. Engl. J. Med. 365, 1118–1127. doi: 10.1056/NEJMra1001683
Erhardt, A., Blondin, D., Hauck, K., Sagir, A., Kohnle, T., Heintges, T., et al. (2005). Response to interferon alfa is hepatitis B virus genotype dependent: genotype A is more sensitive to interferon than genotype D. Gut 54, 1009–1013. doi: 10.1136/gut.2004.060327
Fang, Z. L., Sabin, C. A., Dong, B. Q., Ge, L. Y., Wei, S. C., Chen, Q. Y., et al. (2008). HBV A1762T, G1764A mutations are a valuable biomarker for identifying a subset of male HBsAg carriers at extremely high risk of hepatocellular carcinoma: a prospective study. Am. J. Gastroenterol. 103, 2254–2262. doi: 10.1111/j.1572-0241.2008.01974.x
Fang, Z. L., Sabin, C. A., Dong, B. Q., Wei, S. C., Chen, Q. Y., Fang, K. X., et al. (2009). The association of HBV core promoter double mutations (A1762T and G1764A) with viral load differs between HBeAg positive and anti-HBe positive individuals: a longitudinal analysis. J. Hepatol. 50, 273–280. doi: 10.1016/j.jhep.2008.09.014
Fattovich, G., Bortolotti, F., and Donato, F. (2008). Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J. Hepatol. 48, 335–352. doi: 10.1016/j.jhep.2007.11.011
Fattovich, G., Giustina, G., Christensen, E., Pantalena, M., Zagni, I., Realdi, G., et al. (2000). Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut 46, 420–426. doi: 10.1136/gut.46.3.420
Franceschini, N., van Rooij, F. J., Prins, B. P., Feitosa, M. F., Karakas, M., Eckfeldt, J. H., et al. (2012). Discovery and fine mapping of serum protein loci through transethnic meta-analysis. Am. J. Hum. Genet. 91, 744–753. doi: 10.1016/j.ajhg.2012.08.021
Fujimoto, A., Furuta, M., Shiraishi, Y., Gotoh, K., Kawakami, Y., Arihiro, K., et al. (2015). Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat. Commun. 6:6120. doi: 10.1038/ncomms7120
Fujimoto, A., Totoki, Y., Abe, T., Boroevich, K. A., Hosoda, F., Nguyen, H. H., et al. (2012). Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat. Genet. 44, 760–764. doi: 10.1038/ng.2291
Gounder, P. P., Bulkow, L. R., Snowball, M., Negus, S., Spradling, P. R., and McMahon, B. J. (2016). Hepatocellular Carcinoma Risk in Alaska Native Children and Young Adults with Hepatitis B Virus: Retrospective Cohort Analysis. J. Pediatr. 178, 206–213. doi: 10.1016/j.jpeds.2016.08.017
Guichard, C., Amaddeo, G., Imbeaud, S., Ladeiro, Y., Pelletier, L., Maad, I. B., et al. (2012). Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet. 44, 694–698. doi: 10.1038/ng.2256
Hai, H., Tamori, A., and Kawada, N. (2014). Role of hepatitis B virus DNA integration in human hepatocarcinogenesis. World J. Gastroenterol. 20, 6236–6243. doi: 10.3748/wjg.v20.i20.6236
Heidenreich, B., Rachakonda, P. S., Hemminki, K., and Kumar, R. (2014). TERT promoter mutations in cancer development. Curr. Opin. Genet. Dev. 24, 30–37. doi: 10.1016/j.gde.2013.11.005
Hoffmeyer, K., Raggioli, A., Rudloff, S., Anton, R., Hierholzer, A., Del Valle, I., et al. (2012). Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science 336, 1549–1554. doi: 10.1126/science.1218370
Hu, L., Zhai, X., Liu, J., Chu, M., Pan, S., Jiang, J., et al. (2012). Genetic variants in human leukocyte antigen/DP-DQ influence both hepatitis B virus clearance and hepatocellular carcinoma development. Hepatology 55, 1426–1431. doi: 10.1002/hep.24799
Huang, J., Deng, Q., Wang, Q., Li, K. Y., Dai, J. H., Li, N., et al. (2012). Exome sequencing of hepatitis B virus-associated hepatocellular carcinoma. Nat. Genet. 44, 1117–1121. doi: 10.1038/ng.2391
Hung, I. F., Poon, R. T., Lai, C. L., Fung, J., Fan, S. T., and Yuen, M. F. (2008). Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am. J. Gastroenterol. 103, 1663–1673. doi: 10.1111/j.1572-0241.2008.01872.x
Hung, T. H., Liang, C. M., Hsu, C. N., Tai, W. C., Tsai, K. L., Ku, M. K., et al. (2017). Association between complicated liver cirrhosis and the risk of hepatocellular carcinoma in Taiwan. PLoS ONE 12:e0181858. doi: 10.1371/journal.pone.0181858
Hussain, S. P., Schwank, J., Staib, F., Wang, X. W., and Harris, C. C. (2007). TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene 26, 2166–2176. doi: 10.1038/sj.onc.1210279
Ji, J., Sundquist, K., and Sundquist, J. (2012). A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J. Natl. Cancer Inst. 104, 790–792. doi: 10.1093/jnci/djs168
Jiang, D. K., Sun, J., Cao, G., Liu, Y., Lin, D., Gao, Y. Z., et al. (2013). Genetic variants in STAT4 and HLA-DQ genes confer risk of hepatitis B virus-related hepatocellular carcinoma. Nat. Genet. 45, 72–75. doi: 10.1038/ng.2483
Jiang, Z., Jhunjhunwala, S., Liu, J., Haverty, P. M., Kennemer, M. I., Guan, Y., et al. (2012). The effects of hepatitis B virus integration into the genomes of hepatocellular carcinoma patients. Genome Res. 22, 593–601. doi: 10.1101/gr.133926.111
Kan, Z., Zheng, H., Liu, X., Li, S., Barber, T. D., Gong, Z., et al. (2013). Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 23, 1422–1433. doi: 10.1101/gr.154492.113
Kanwal, F., Kramer, J. R., Ilyas, J., Duan, Z., and El-Serag, H. B. (2014). HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology 60, 98–105. doi: 10.1002/hep.27095
Kao, J. H., Chen, P. J., Lai, M. Y., and Chen, D. S. (2004). Hepatitis B virus genotypes and spontaneous hepatitis B e antigen seroconversion in Taiwanese hepatitis B carriers. J. Med. Virol. 72, 363–369. doi: 10.1002/jmv.10534
Kawai-Kitahata, F., Asahina, Y., Tanaka, S., Kakinuma, S., Murakawa, M., Nitta, S., et al. (2016). Comprehensive analyses of mutations and hepatitis B virus integration in hepatocellular carcinoma with clinicopathological features. J. Gastroenterol. 51, 473–486. doi: 10.1007/s00535-015-1126-4
Kew, M. C. (2003). Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 23, 405–409. doi: 10.1111/j.1478-3231.2003.00869.x
Klaus, A., and Birchmeier, W. (2008). Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 8, 387–398. doi: 10.1038/nrc2389
Kremsdorf, D., Soussan, P., Paterlini-Brechot, P., and Brechot, C. (2006). Hepatitis B virus-related hepatocellular carcinoma: paradigms for viral-related human carcinogenesis. Oncogene 25, 3823–3833. doi: 10.1038/sj.onc.1209559
Kushner, T., Serper, M., and Kaplan, D. E. (2015). Delta hepatitis within the Veterans Affairs medical system in the United States: prevalence, risk factors, and outcomes. J. Hepatol. 63, 586–592. doi: 10.1016/j.jhep.2015.04.025
Lai, M. W., and Yeh, C. T. (2008). The oncogenic potential of hepatitis B virus rtA181T/ surface truncation mutant. Antivir. Ther. (Lond). 13, 875–879.
Lai, M. W., Huang, S. F., Hsu, C. W., Chang, M. H., Liaw, Y. F., and Yeh, C. T. (2009). Identification of nonsense mutations in hepatitis B virus S gene in patients with hepatocellular carcinoma developed after lamivudine therapy. Antivir. Ther. (Lond). 14, 249–261.
Lau, C. C., Sun, T., Ching, A. K., He, M., Li, J. W., Wong, A. M., et al. (2014). Viral-human chimeric transcript predisposes risk to liver cancer development and progression. Cancer Cell 25, 335–349. doi: 10.1016/j.ccr.2014.01.030
Lee, J. H., Han, K. H., Lee, J. M., Park, J. H., and Kim, H. S. (2011). Impact of hepatitis B virus (HBV) x gene mutations on hepatocellular carcinoma development in chronic HBV infection. Clin. Vaccine Immunol. 18, 914–921. doi: 10.1128/CVI.00474-10
Lee, J. S. (2015). The mutational landscape of hepatocellular carcinoma. Clin. Mol. Hepatol. 21, 220–229. doi: 10.3350/cmh.2015.21.3.220
Li, M., Zhao, H., Zhang, X., Wood, L. D., Anders, R. A., Choti, M. A., et al. (2011). Inactivating mutations of the chromatin remodeling gene ARID2 in hepatocellular carcinoma. Nat. Genet. 43, 828–829. doi: 10.1038/ng.903
Li, N., Lai, E. C., Shi, J., Guo, W. X., Xue, J., Huang, B., et al. (2010). A comparative study of antiviral therapy after resection of hepatocellular carcinoma in the immune-active phase of hepatitis B virus infection. Ann. Surg. Oncol. 17, 179–185. doi: 10.1245/s10434-009-0694-z
Li, S., Qian, J., Yang, Y., Zhao, W., Dai, J., Bei, J. X., et al. (2012). GWAS identifies novel susceptibility loci on 6p21.32 and 21q21.3 for hepatocellular carcinoma in chronic hepatitis B virus carriers. PLoS Genet. 8:e1002791. doi: 10.1371/journal.pgen.1002791
Li, W., Li, M., Liao, D., Lu, X., Gu, X., Zhang, Q., et al. (2016). Carboxyl-terminal truncated HBx contributes to invasion and metastasis via deregulating metastasis suppressors in hepatocellular carcinoma. Oncotarget. 7, 55110–55127. doi: 10.18632/oncotarget.10399
Li, W., Zeng, X., Lee, N. P., Liu, X., Chen, S., Guo, B., et al. (2013). HIVID: an efficient method to detect HBV integration using low coverage sequencing. Genomics 102, 338–344. doi: 10.1016/j.ygeno.2013.07.002
Liang, T. J. (2009). Hepatitis B: the virus and disease. Hepatology 49, S13–S21. doi: 10.1002/hep.22881
Lin, C. L., and Kao, J. H. (2015). Hepatitis B virus genotypes and variants. Cold Spring Harb. Perspect. Med. 5:a021436. doi: 10.1101/cshperspect.a021436
Liu, S., Zhang, H., Gu, C., Yin, J., He, Y., Xie, J., et al. (2009). Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis. J. Natl. Cancer Inst. 101, 1066–1082. doi: 10.1093/jnci/djp180
Livingston, S. E., Simonetti, J. P., McMahon, B. J., Bulkow, L. R., Hurlburt, K. J., Homan, C. E., et al. (2007). Hepatitis B virus genotypes in Alaska Native people with hepatocellular carcinoma: preponderance of genotype F. J. Infect. Dis. 195, 5–11. doi: 10.1086/509894
Ma, N. F., Lau, S. H., Hu, L., Xie, D., Wu, J., Yang, J., et al. (2008). COOH-terminal truncated HBV X protein plays key role in hepatocarcinogenesis. Clin. Cancer Res. 14, 5061–5068. doi: 10.1158/1078-0432.CCR-07-5082
Maldarelli, F., Wu, X., Su, L., Simonetti, F. R., Shao, W., Hill, S., et al. (2014). HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345, 179–183. doi: 10.1126/science.1254194
Meng, X., Franklin, D. A., Dong, J., and Zhang, Y. (2014). MDM2-p53 pathway in hepatocellular carcinoma. Cancer Res. 74, 7161–7167. doi: 10.1158/0008-5472.CAN-14-1446
Michailidou, K., Beesley, J., Lindstrom, S., Canisius, S., Dennis, J., Lush, M. J., et al. (2015). Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat. Genet. 47, 373–380. doi: 10.1038/ng.3242
Murakami, Y., Saigo, K., Takashima, H., Minami, M., Okanoue, T., Bréchot, C., et al. (2005). Large scaled analysis of hepatitis B virus (HBV) DNA integration in HBV related hepatocellular carcinomas. Gut 54, 1162–1168. doi: 10.1136/gut.2004.054452
Muroyama, R., Kato, N., Yoshida, H., Otsuka, M., Moriyama, M., Wang, Y., et al. (2006). Nucleotide change of codon 38 in the X gene of hepatitis B virus genotype C is associated with an increased risk of hepatocellular carcinoma. J. Hepatol. 45, 805–812. doi: 10.1016/j.jhep.2006.07.025
Nault, J. C., Calderaro, J., Di Tommaso, L., Balabaud, C., Zafrani, E. S., Bioulac-Sage, P., et al. (2014). Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology 60, 1983–1992. doi: 10.1002/hep.27372
Nault, J. C., Mallet, M., Pilati, C., Calderaro, J., Bioulac-Sage, P., Laurent, C., et al. (2013). High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 4:2218. doi: 10.1038/ncomms3218
Ni, Y.-H., Chang, M.-H., Wang, K.-J., Hsu, H.-Y., Chen, H.-L., Kao, J.-H., et al. (2004). Clinical relevance of hepatitis B virus genotype in children with chronic infection and hepatocellular carcinoma. Gastroenterology 127, 1733–1738. doi: 10.1053/j.gastro.2004.09.048
Njei, B., Rotman, Y., Ditah, I., and Lim, J. K. (2015). Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 61, 191–199. doi: 10.1002/hep.27388
Paraskevis, D., Magiorkinis, G., Magiorkinis, E., Ho, S. Y., Belshaw, R., Allain, J. P., et al. (2013). Dating the origin and dispersal of hepatitis B virus infection in humans and primates. Hepatology 57, 908–916. doi: 10.1002/hep.26079
Paterlini-Brechot, P., Saigo, K., Murakami, Y., Chami, M., Gozuacik, D., Mugnier, C., et al. (2003). Hepatitis B virus-related insertional mutagenesis occurs frequently in human liver cancers and recurrently targets human telomerase gene. Oncogene 22, 3911–3916. doi: 10.1038/sj.onc.1206492
Pollicino, T., Cacciola, I., Saffioti, F., and Raimondo, G. (2014). Hepatitis B virus PreS/S gene variants: pathobiology and clinical implications. J. Hepatol. 61, 408–417. doi: 10.1016/j.jhep.2014.04.041
Raffetti, E., Fattovich, G., and Donato, F. (2016). Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: a systematic review and meta-analysis. Liver Int. 36, 1239–1251. doi: 10.1111/liv.13142
Rebouissou, S., Franconi, A., Calderaro, J., Letouze, E., Imbeaud, S., Pilati, C., et al. (2016). Genotype-phenotype correlation of CTNNB1 mutations reveals different ß-catenin activity associated with liver tumor progression. Hepatology 64, 2047–2061. doi: 10.1002/hep.28638
Romeo, R., Del Ninno, E., Rumi, M., Russo, A., Sangiovanni, A., de Franchis, R., et al. (2009). A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 136, 1629–1638. doi: 10.1053/j.gastro.2009.01.052
Saigo, K., Yoshida, K., Ikeda, R., Sakamoto, Y., Murakami, Y., Urashima, T., et al. (2008). Integration of hepatitis B virus DNA into the myeloid/lymphoid or mixed-lineage leukemia (MLL4) gene and rearrangements of MLL4 in human hepatocellular carcinoma. Hum. Mutat. 29, 703–708. doi: 10.1002/humu.20701
Sanchez-Tapias, J. M., Costa, J., Mas, A., Bruguera, M., and Rodes, J. (2002). Influence of hepatitis B virus genotype on the long-term outcome of chronic hepatitis B in western patients. Gastroenterology 123, 1848–1856. doi: 10.1053/gast.2002.37041
Satoh, S., Daigo, Y., Furukawa, Y., Kato, T., Miwa, N., Nishiwaki, T., et al. (2000). AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat. Genet. 24, 245–250. doi: 10.1038/73448
Sawai, H., Nishida, N., Mbarek, H., Matsuda, K., Mawatari, Y., Yamaoka, M., et al. (2012). No association for Chinese HBV-related hepatocellular carcinoma susceptibility SNP in other East Asian populations. BMC Med. Genet. 13:47. doi: 10.1186/1471-2350-13-47
Sawey, E. T., Chanrion, M., Cai, C., Wu, G., Zhang, J., Zender, L., et al. (2011). Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell 19, 347–358. doi: 10.1016/j.ccr.2011.01.040
Schulze, K., Imbeaud, S., Letouzé, E., Alexandrov, L. B., Calderaro, J., Rebouissou, S., et al. (2015). Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 47, 505–511. doi: 10.1038/ng.3252
Shen, F. M., Lee, M. K., Gong, H. M., Cai, X. Q., and King, M. C. (1991). Complex segregation analysis of primary hepatocellular carcinoma in Chinese families: interaction of inherited susceptibility and hepatitis B viral infection. Am. J. Hum. Genet. 49, 88–93.
Shi, Y., Wang, J., Wang, Y., Wang, A., Guo, H., Wei, F., et al. (2016). A novel mutant 10Ala/Arg together with mutant 144Ser/Arg of hepatitis B virus X protein involved in hepatitis B virus-related hepatocarcinogenesis in HepG2 cell lines. Cancer Lett 371, 285–291. doi: 10.1016/j.canlet.2015.12.008
Sopipong, W., Tangkijvanich, P., Payungporn, S., Posuwan, N., and Poovorawan, Y. (2013). The KIF1B (rs17401966) Single Nucleotide Polymorphism is not Associated with the Development of HBV-related Hepatocellular Carcinoma in Thai Patients. Asian Pacific J. Cancer Prevent. 14, 2865–2869. doi: 10.7314/apjcp.2013.14.5.2865
Su, M., Guo, J., and Huang, J. (2017). Meta-analysis of the correlation between the rs17401966 polymorphism in kinesin family member 1B and susceptibility to hepatitis B virus related hepatocellular carcinoma. Clin. Mol. Hepatol. 23, 138–146. doi: 10.3350/cmh.2016.0083
Sung, W. K., Zheng, H., Li, S., Chen, R., Liu, X., Li, Y., et al. (2012). Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat. Genet. 44, 765–769. doi: 10.1038/ng.2295
Swanton, C., Soria, J. C., Bardelli, A., Biankin, A., Caldas, C., Chandarlapaty, S., et al. (2016). Consensus on precision medicine for metastatic cancers: a report from the MAP conference. Ann. Oncol. 27, 1443–1448. doi: 10.1093/annonc/mdw192
Sze, K. M., Chu, G. K., Lee, J. M., and Ng, I. O. (2013). C-terminal truncated hepatitis B virus x protein is associated with metastasis and enhances invasiveness by C-Jun/matrix metalloproteinase protein 10 activation in hepatocellular carcinoma. Hepatology 57, 131–139. doi: 10.1002/hep.25979
Tao, Y., Ruan, J., Yeh, S. H., Lu, X., Wang, Y., Zhai, W., et al. (2011). Rapid growth of a hepatocellular carcinoma and the driving mutations revealed by cell-population genetic analysis of whole-genome data. Proc. Natl. Acad. Sci. U.S.A. 108, 12042–12047. doi: 10.1073/pnas.1108715108
Terrault, N. A., Bzowej, N. H., Chang, K. M., Hwang, J. P., Jonas, M. M., Murad, M. H., et al. (2016). AASLD guidelines for treatment of chronic hepatitis B. Hepatology 63, 261–283. doi: 10.1002/hep.28156
Thomas, D. L., Thio, C. L., Martin, M. P., Qi, Y., Ge, D., O'Huigin, C., et al. (2009). Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461, 798–801. doi: 10.1038/nature08463
Toh, S. T., Jin, Y., Liu, L., Wang, J., Babrzadeh, F., Gharizadeh, B., et al. (2013). Deep sequencing of the hepatitis B virus in hepatocellular carcinoma patients reveals enriched integration events, structural alterations and sequence variations. Carcinogenesis 34, 787–798. doi: 10.1093/carcin/bgs406
Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J., and Jemal, A. (2015). Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108. doi: 10.3322/caac.21262
Totoki, Y., Tatsuno, K., Covington, K. R., Ueda, H., Creighton, C. J., Kato, M., et al. (2014). Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat. Genet. 46, 1267–1273. doi: 10.1038/ng.3126
Tseng, T. C., Liu, C. J., Yang, H. C., Su, T. H., Wang, C. C., Chen, C. L., et al. (2012). High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology 142, 1140–1149 e1143; quiz e1113–1144. doi: 10.1053/j.gastro.2012.02.007
Tu, H., Bonura, C., Giannini, C., Mouly, H., Soussan, P., Kew, M., et al. (2001). Biological impact of natural COOH-terminal deletions of hepatitis B virus X protein in hepatocellular carcinoma tissues. Cancer Res. 61, 7803–7810.
van der Meer, A. J., Veldt, B. J., Feld, J. J., Wedemeyer, H., Dufour, J. F., Lammert, F., et al. (2012). Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 308, 2584–2593. doi: 10.1001/jama.2012.144878
Vutien, P., Trinh, H. N., Nguyen, K., Garcia, R. T., Nguyen, H. A., Levitt, B. S., et al. (2013). Precore and basal core promoter mutations in Asian American patients with hepatitis B e antigen-positive chronic hepatitis B. Aliment. Pharmacol. Ther. 37, 464–472. doi: 10.1111/apt.12193
Wang, D., Cai, H., Yu, W. B., and Yu, L. (2014). Identification of hepatitis B virus X gene variants between hepatocellular carcinoma tissues and pericarcinoma liver tissues in Eastern China. Int. J. Clin. Exp. Pathol. 7, 5988–5996.
Wang, K., Lim, H. Y., Shi, S., Lee, J., Deng, S., Xie, T., et al. (2013). Genomic landscape of copy number aberrations enables the identification of oncogenic drivers in hepatocellular carcinoma. Hepatology 58, 706–717. doi: 10.1002/hep.26402
Wang, Q., Zhang, T., Ye, L., Wang, W., and Zhang, X. (2012). Analysis of hepatitis B virus X gene (HBx) mutants in tissues of patients suffered from hepatocellular carcinoma in China. Cancer Epidemiol. 36, 369–374. doi: 10.1016/j.canep.2011.11.006
WHO. (2017). WHO Global Hepatitis Report 2017. Available online at: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ [Accessed July 22, 2017].
Woo, H. G., Wang, X. W., Budhu, A., Kim, Y. H., Kwon, S. M., Tang, Z. Y., et al. (2011). Association of TP53 mutations with stem cell-like gene expression and survival of patients with hepatocellular carcinoma. Gastroenterology 140, 1063–1070. doi: 10.1053/j.gastro.2010.11.034
Wu, J. C., Huang, Y. H., Chau, G. Y., Su, C. W., Lai, C. R., Lee, P. C., et al. (2009). Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J. Hepatol. 51, 890–897. doi: 10.1016/j.jhep.2009.07.009
Xu, C., Zhou, W., Wang, Y., and Qiao, L. (2014). Hepatitis B virus-induced hepatocellular carcinoma. Cancer Lett. 345, 216–222. doi: 10.1016/j.canlet.2013.08.035
Yang, H. I., Yeh, S. H., Chen, P. J., Iloeje, U. H., Jen, C. L., Su, J., et al. (2008). Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J. Natl. Cancer Inst. 100, 1134–1143. doi: 10.1093/jnci/djn243
Yang, X., Guo, X., Chen, Y., Chen, G., Ma, Y., Huang, K., et al. (2016a). Telomerase reverse transcriptase promoter mutations in hepatitis B virus-associated hepatocellular carcinoma. Oncotarget 7, 27838–27847. doi: 10.18632/oncotarget.8539
Yang, X., Yu, D., Ren, Y., Wei, J., Pan, W., Zhou, C., et al. (2016b). Integrative Functional Genomics Implicates EPB41 Dysregulation in Hepatocellular Carcinoma Risk. Am. J. Hum. Genet. 99, 275–286. doi: 10.1016/j.ajhg.2016.05.029
Yang, Y., Sun, J. W., Zhao, L. G., Bray, F., and Xiang, Y. B. (2015). Quantitative evaluation of hepatitis B virus mutations and hepatocellular carcinoma risk: a meta-analysis of prospective studies. Chin. J. Cancer Res. 27, 497–508. doi: 10.3978/j.issn.1000-9604.2015.10.05
Yang, Z., Zhuang, L., Lu, Y., Xu, Q., Tang, B., and Chen, X. (2016). Naturally occurring basal core promoter A1762T/G1764A dual mutations increase the risk of HBV-related hepatocellular carcinoma: a meta-analysis. Oncotarget 7, 12525–12536. doi: 10.18632/oncotarget.7123
Yao, S., Johnson, C., Hu, Q., Yan, L., Liu, B., Ambrosone, C. B., et al. (2016). Differences in somatic mutation landscape of hepatocellular carcinoma in Asian American and European American populations. Oncotarget. 7, 40491–40499. doi: 10.18632/oncotarget.9636
Yeh, C. T., Chen, T., Hsu, C. W., Chen, Y. C., Lai, M. W., Liang, K. H., et al. (2011). Emergence of the rtA181T/sW172* mutant increased the risk of hepatoma occurrence in patients with lamivudine-resistant chronic hepatitis B. BMC Cancer 11:398. doi: 10.1186/1471-2407-11-398
Yim, H. J., and Lok, A. S. (2006). Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology 43(2 Suppl. 1), S173–S181. doi: 10.1002/hep.20956
Yin, J., Li, N., Han, Y., Xue, J., Deng, Y., Shi, J., et al. (2013). Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J. Clin. Oncol. 31, 3647–3655. doi: 10.1200/JCO.2012.48.5896
Yin, J., Xie, J., Zhang, H., Shen, Q., Han, L., Lu, W., et al. (2010). Significant association of different preS mutations with hepatitis B-related cirrhosis or hepatocellular carcinoma. J. Gastroenterol. 45, 1063–1071. doi: 10.1007/s00535-010-0253-1
Yu, M. W., Yeh, S. H., Chen, P. J., Liaw, Y. F., Lin, C. L., Liu, C. J., et al. (2005). Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J. Natl. Cancer Inst. 97, 265–272. doi: 10.1093/jnci/dji043
Zhang, H., Zhai, Y., Hu, Z., Wu, C., Qian, J., Jia, W., et al. (2010). Genome-wide association study identifies 1p36.22 as a new susceptibility locus for hepatocellular carcinoma in chronic hepatitis B virus carriers. Nat. Genet. 42, 755–758. doi: 10.1038/ng.638
Zhang, Y., Toh, L., Lau, P., and Wang, X. (2012). Human telomerase reverse transcriptase (hTERT) is a novel target of the Wnt/beta-catenin pathway in human cancer. J. Biol. Chem. 287, 32494–32511. doi: 10.1074/jbc.M112.368282
Zhao, L. H., Liu, X., Yan, H. X., Li, W. Y., Zeng, X., Yang, Y., et al. (2016). Genomic and oncogenic preference of HBV integration in hepatocellular carcinoma. Nat. Commun. 7:12992. doi: 10.1038/ncomms12992
Keywords: genotype, hepatitis B virus, hepatocellular carcinoma, mutation, single nucleotide polymorphisms
Citation: An P, Xu J, Yu Y and Winkler CA (2018) Host and Viral Genetic Variation in HBV-Related Hepatocellular Carcinoma. Front. Genet. 9:261. doi: 10.3389/fgene.2018.00261
Received: 13 April 2018; Accepted: 27 June 2018;
Published: 19 July 2018.
Edited by:
William Scott Bush, Case Western Reserve University, United StatesReviewed by:
Michael Scheurer, Baylor College of Medicine, United StatesCopyright © 2018 An, Xu, Yu and Winkler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping An, cGluZy5hbkBuaWguZ292
Cheryl A. Winkler, d2lua2xlcmNAbWFpbC5uaWguZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.