- 1Natural Resources Canada, Canadian Forest Service-Atlantic Forestry Centre, Fredericton, NB, Canada
- 2Changbai Mountain Academy of Sciences, Changbai, China
- 3Department of Biology, Acadia University, Wolfville, NS, Canada
- 4Forest Protection Limited, Lincoln, NB, Canada
The beech leaf-mining weevil, Orchestes fagi (L.), is native to Europe where it commonly attacks European beech. The weevil was discovered infesting American beech in Halifax and Cape Breton Island, Nova Scotia, Canada in 2012, but anecdotal reports of defoliated beech in the Halifax area as early as 2006 suggest it established 5–10 years prior to its discovery. Our objectives were to estimate the impact of O. fagi on American beech in forested sites and urban areas, as well as its economic impact on owners of residential properties with mature American beech. In 2014, we established fifteen plots in forested sites containing a total of 260 American beech at Sandy Lake, Oakfield, and Mount Uniacke (n = 5 plots per site), where weevil infestation levels were moderate, low, and nil, respectively. At the same time we recorded the degree of cankering by beech bark disease on the main stems of each tree. Plots were visited annually to record tree mortality (2014–2019) and percentage of leaves with larval mines or adult feeding (2016–2019). Between 2016 and 2019, the percentage of leaves mined by weevil larvae increased from 6 to 59% at Mount Uniacke and from 48 to 83% at Oakfield. During the same period, cumulative beech mortality increased from 35 to 48% at Mount Uniacke and from 10 to 70% at Oakfield. At Sandy Lake in 2016, 88% of the beech trees had died and there were too few living beech to collect a leaf sample in our plots so estimates of weevil damage (87% of leaves with mines) were obtained from life table plots in the same area. Tree mortality was associated with severity of cankering by beech bark disease only at Mount Uniacke, the site with the fewest years of defoliation by the leaf-mining weevil. We also surveyed residents of Halifax in 2016 and 2018 to determine the rate of beech mortality and costs of tree removal in urban residential areas in the same region (within 40 km) of the forest areas. Relative to the forested sites at Sandy lake and Oakfield, mortality rates were lower in urban areas (32% in 2016, 44% in 2018), even though signs of weevil defoliation had been apparent to residents as early as 2011–2012. Direct costs ($CAN) to property owners who hired arborists to remove dead beech trees averaged $1934 ($300–$6600) per resident in 2018. Options for mitigating the impact of O. fagi on American beech are briefly discussed.
Introduction
There are about 10000 species of leafminers known worldwide (Connor and Taverner, 1997), including several native European species that have become invasive pests of woody plants in North America (Kirichenko et al., 2019). Most invasive leaf-mining species are Lepidopterans (e.g., Gracillaridae), Dipterans (Agromyzidae), Hymenopterans (Tenthridindae) or Coleopterans (Curculionidae) (Kirichenko et al., 2019). In general, outbreaks of invasive leafminers rarely cause tree mortality but can reduce fruit production in orchards (e.g., citrus leafminer, Phyllocnistis citrella Stainton) and damage the esthetic value of urban ornamentals [e.g., horse chestnut leafminer, Cameraria ohridella (Deschka and Dimic) (Reinhardt et al., 2003)].
In recent decades, several species of Palearctic leaf-mining weevils from the taxonomic tribe Rhamphini have invaded and established in North America hardwoods, including Orchestes steppensis (formerly confused with Orchestes alni (L.) (Anderson et al., 2007; Korotyaev, 2016; Looney et al., 2012), and Isochnus sequensi (Stierlin) (Sweeney et al., 2012). In this article, we discuss another invasive leaf miner, the beech leaf-mining weevil, Orchestes fagi (L.) (formerly Rhynchaenus fagi) (Coleoptera: Curculionidae). This weevil is native to Europe and a common pest of European beech, Fagus sylvatica L. Populations occasionally reach outbreak levels and may reduce growth rates and seed production, but do not kill trees (Verkaik et al., 2009; Rullán-Silva et al., 2015). In 2012, O. fagi was discovered infesting American beech, Fagus grandifolia Ehrh., in Halifax and Cape Breton, Nova Scotia. However, anecdotal reports of American beech with thin, defoliated crowns (Figure 1) in Halifax prior to the weevil’s discovery suggest it may have established in the area as early as 2006 (Sweeney et al., 2012). As of 2017, O. fagi had been detected in other areas of Nova Scotia, including Wolfville and Cornwallis, Nova Scotia, located about 100 and 200 km northwest and west of Halifax, respectively (Ron Neville, Canadian Food Inspection Agency, pers. comm).

Figure 1. American beech with thin crown as a result of beech weevil defoliation, Dartmouth, Nova Scotia, July 2012 (photo credit: J. Sweeney).
The weevil is univoltine and spends most of the year as an adult, overwintering under bark scales on the trunks of surrounding trees or in the leaf litter (Morrison et al., 2017; Nielsen, 1970). Adults emerge in spring to feed on and oviposit in young developing leaves just as buds begin to burst (Bale, 1984; Nielsen, 1966). Eggs are typically laid in the central leaf vein on the underside of developing leaves and larvae feed outwards to the leaf margin for three instars eventually forming a blotch mine. The mines of O. fagi are readily distinctive from those of the only native leaf miner of American beech reported in Nova Scotia, Phyllonorycter restrictella Braun (Lepidoptera: Gracillariidae) which makes an elongate, tentiform mine between two lateral veins on the underside of a leaf (Eiseman, 2018). By mid-summer the beech weevil larva forms a cocoon inside the mine. The total developmental time from egg to adult is 30–35 days (Bale and Luff, 1978). Prior to larval mining, adults will feed on leaves causing typical “shot-holes” in leaves. The collective result of adult and larval feeding is a characteristic browning and wilting at the leaf tips, giving the leaves a general “scorched” appearance (Sweeney et al., 2012). In contrast to reportedly mild impacts on European beech, feeding damage on American beech can be quite extensive and there have been anecdotal observations of tree mortality in areas suffering successive years of infestation and obvious defoliation by O. fagi. Due to the small size of adults (2.2–2.8 mm long) and their cryptic habit of overwintering under the tree bark and logs, there is high risk of its spread by human movement of firewood and saw logs (Morrison et al., 2017).
American beech is a shade-tolerant, slow-growing species that can live 300–400 years (Tubbs and Houston, 1990). Its nuts are a major food source for a large variety of birds and mammals, including ruffed grouse, blue jays, mice, and black bears, and its lumber can be used for flooring, furniture, and fuelwood (McLaughlin and Griefenhagen, 2012; Tubbs and Houston, 1990). Commercial importance of American beech has declined because of beech bark disease, caused by an invasive scale insect, Cryptococcus fagisuga Lind. (Hemiptera: Eriococcidae), introduced to Halifax, Nova Scotia from Europe around 1890, which predisposes beech bark to infection by the fungal pathogens, Neonectria faginata (Lohman, Watson, and Ayers) (Hypocreales: Nectriaceae), and Neonectria ditissima (Tulasne and C. Tulasne) Samuels and Rossman (Ehrlich, 1934; Houston et al., 1979). The fungal pathogens kill bark and cambium tissue, creating necrotic lesions or cankers on the main stem which can girdle and kill the tree (Ehrlich, 1934; Giencke et al., 2014). Shigo (1972) categorized the progression of beech bark disease in three stages: (1) the advance front, characterized by high populations of the beech scale and very low incidence of the fungal pathogens; (2) the killing front, with high levels of scale insect, fungal pathogens, and mortality of mature beech; and (3) the aftermath forest, where apparently resistant beech survive with various levels of cankers and deformities on the trunk, and densities of both the scale and pathogens are reduced (Giencke et al., 2014; Cale et al., 2017). Although radial growth rate of beech is significantly reduced in aftermath forests, tree survival is relatively high (Gavin and Peart, 1993). A native scale insect, Xylococculus betulae (Pergrande) also causes wounds on bark of beech trunks and has been found to be more prevalent than C. fagisuga in aftermath forests (Houston, 1975). Cale et al. (2015) also found X. betulae was more prevalent than C. fagisuga in aftermath forests and that it predisposed trees bark damage by the native scale insect predisposed trees to infection by N. distissima. Beech bark disease has spread about 15 km per year and often killed ≥50% of American beech >25 cm DBH in the first 10 years along the invasive front but many beech trees survive in the aftermath forest (Houston, 1994; Morin et al., 2007). Defoliation by insects increases the susceptibility of American beech to decay fungi (Tubbs and Houston, 1990), so foliar damage by O. fagi may increase mortality rates in aftermath forests.
Our objectives were to monitor the impact of the weevil defoliation on American beech in forested sites over a period of 5 years, and also estimate its impact in residential areas of Halifax Regional Municipality. We also estimated the economic impact of the weevil on residents in terms of costs of tree removal. We predicted that tree mortality would be associated with weevil infestation levels and would be greater in sites that had experienced more years of moderate to severe defoliation. We also predicted that tree mortality rates would be greater in trees with higher incidence of beech bark disease.
Materials and Methods
Forested Sites
In August 2014, we selected three sites in Nova Scotia, located at varying distances from Halifax, to provide a range of beech weevil infestation levels (Figure 2), from “nil,” at Mount Uniacke (44.904°N, 63.848°W), where no signs of O. fagi damage were apparent to low at Oakfield (44.922°N, 63.581°W) (with averages (±SE) of 13.3 ± 9.0% of leaves with larval mines and 38.1 ± 9.8% of leaves with adult feeding holes), to moderate at Sandy Lake Park (44.744°N, 63.669°W) (with averages of 24.2 ± 10.7% of leaves with larval mines and 62.7 ± 10.5% of leaves with adult feeding holes). Five 11.28 m radius plots were established at each site and within each plot, all trees ≥6 cm diameter at breast height (DBH), where breast height is defined as 1.3 m above the ground, were tagged and numbered, regardless of species. The 11.28 m radius plot is equivalent to 400 m2 or 0.04 ha and is a standard plot size used in timber cruising (Anonymous, 2014). Criteria for selection of plot centers were: (1) to include at least six American beech ≥ 6 cm DBH, and preferably more, per plot; and (2) have at least 50 m distance between plot centers at each site. American beech, balsam fir [Abies balsamea (L.) Mill.], and red spruce (Picea rubens Sarg.) were the most common tree species, comprising an average of 33, 24, and 18% of stems >6 cm DBH per plot, respectively (Table 1). Red maple (Acer rubrum L.), yellow birch (Betula alleghaniensis Britton. white birch (Betula papyrifera Marsh.), ash (Fraxinus spp. L.), eastern hemlock (Tsuga canadensis (L.) Carr.), and striped maple (Acer pensylvanicum L.) were less common (2–8% of stems per plot), and occasional species such as white pine (Pinus strobus L.) and sugar maple (Acer saccharum March.) accounted for <2% of stems per plot (Table 1).
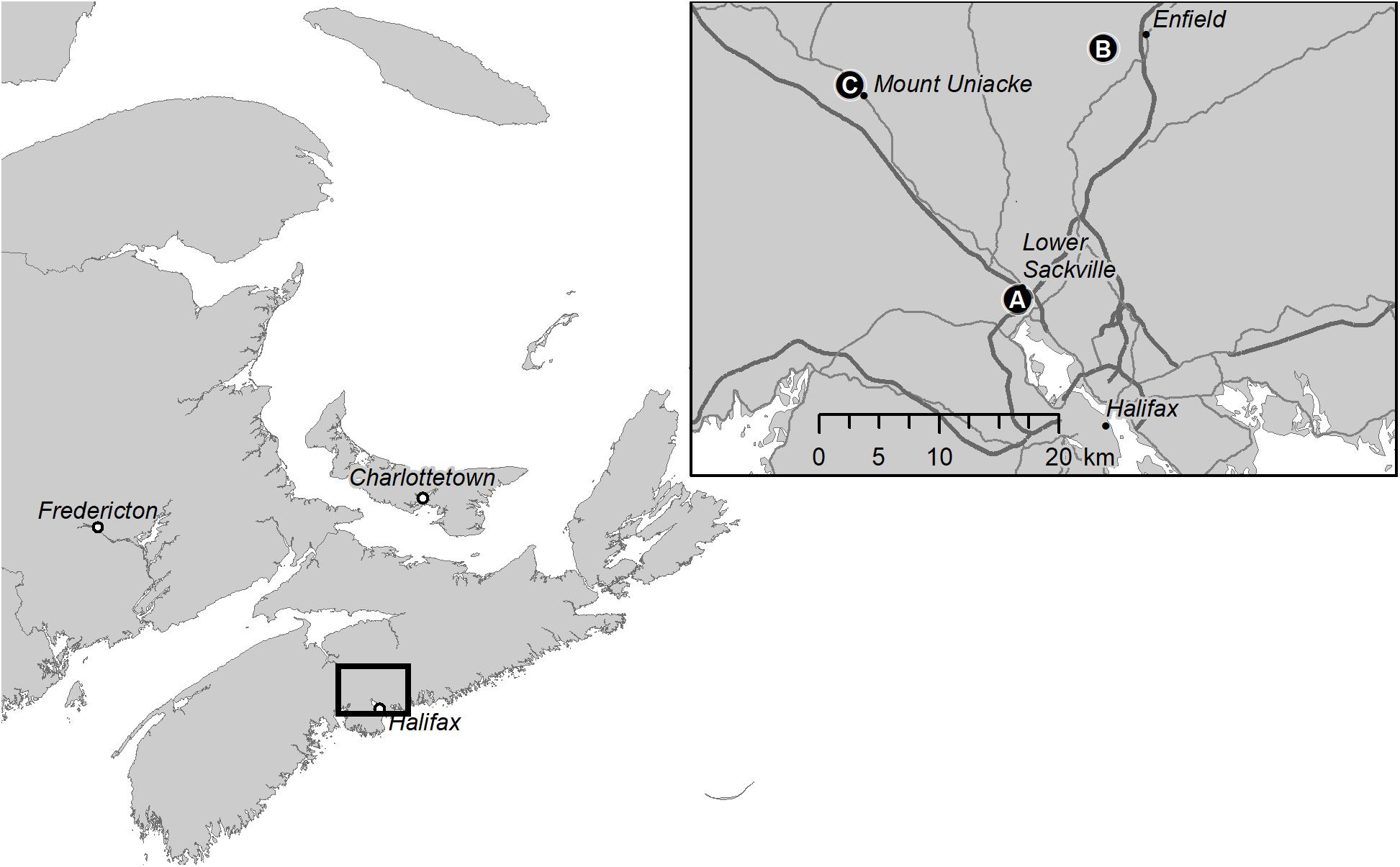
Figure 2. Map showing the location of beech weevil impact plots in Nova Scotia, Canada: (A) Sandy Lake; (B) Oakfield Provincial Park; and (C) Mount Uniacke.

Table 1. Mean (± SE) numbers of stems per plot of American beech and other tree species at three forested sites in Nova Scotia, Canada, in 2014.
For each American beech tree we recorded DBH, whether it was alive or dead, and visually assessed the basal 2 m of the trunk for percentage of area with cankers as Light (0–30%), Medium (31–60%), or High (61–100%) as an index of disease severity (Morrison et al., 2017). We did not attempt to record the relative incidence or species of scale insects and fungal pathogens present. However, in other northeastern aftermath forests, X. betulae and N. ditissima were the most prevalent species (Houston, 1975; Cale et al., 2015). Gavin and Peart (1993) showed that radial growth of beech declined with increasing severity of external bark defects (e.g., cankering) by beech bark disease. Therefore we are confident that our visual estimate of cankering severity is a reasonable index of disease severity. We re-surveyed plots in August of 2015–2019 to record tree mortality. From 2016 to 2019, we also estimated beech weevil infestation levels in each site in mid-August by using a pole-pruner to collect ten 60 cm-long branches per site (one mid-crown branch per tree from two randomly-selected beech trees per plot × five plots per site). On each branch we counted the: (1) total number of leaves; (2) number of leaves with evidence of larval mines (whether they had completed development to the blotch stage or not); and (3) number of leaves with signs of adult feeding (shot holes). We pooled counts from both trees per plot to estimate the percentage of leaves with larval mines and percentage of leaves with adult feeding in each plot. Total number of leaves examined per plot averaged 105 ± 6. By 2016, it was no longer possible to collect leaf samples from trees in our fixed radius plots at Sandy Lake plots because most of the trees were dead. However, additional estimates of larval mines and adult feeding were obtained for Oakfield (2014) and Sandy Lake (2014, 2016) from contemporaneous life table studies being conducted in the same areas (RJ and JS, unpublished data) using similar methods. There were five life table plots per site. For each plot, counts of total leaves, leaves with larval mines, and leaves with adult feeding were pooled from five mid-crown twigs (one twig per tree × five trees per plot). Each twig was the product of only one expanded bud and had an average of three leaves (2–5). The total number of leaves examined per plot averaged 15.8 ± 0.9.
To measure the effects of beech weevil defoliation on radial growth rate, we felled 12 live mature American beech at Sandy Lake (in a stand about 250 m from our fixed radius plots) in the fall of 2017 and collected a cross sectional disk at 1.3 m height. We also felled and collected disks from 12 live sugar maples of similar diameters to the beech trees at Sandy Lake, to provide an example of radial growth of a similarly shade-tolerant hardwood species without beech weevil defoliation. We predicted weevil defoliation would result in a decrease in annual radial increment of beech relative to sugar maple, which might indicate the approximate year when defoliation by O. fagi began. Each disk was run through an industrial planer (King Canada Industries, Dorval, QC, Canada), sanded smooth on one side using an industrial belt sander (King Canada Industries, Dorval, QC, Canada) with 100 grit sandpaper, followed by hand-sanding with 220 grit sand paper, and then coated with fast-drying semi-gloss polyurethane (Minwax, Cleveland, OH, United States). The width of each annual ring was measured along four radial transects (North, South, East, and West) from bark to pith using the WinDENDRO 2017a system for Tree-Ring Analysis (Regent Instruments Canada, Inc.)1. We avoided areas with cankers on the bark when positioning the radial transects. Diameter growth of American beech is influenced by soil moisture and in very dry years, annual rings may be missing entirely from basal sections of trees (Tubbs and Houston, 1990). However, close examination of full cross sections usually allows appropriate identification of problem rings that may be missed in increment cores (Speer, 2010). On three beech disks (and zero sugar maple disks), the annual ring count differed among the four radial transects of an individual cross-section, e.g., one transect with 100 annual rings and the other three transects with 101, indicating either the presence of false rings along three transects or more likely, a missing ring along one transect. When this happened, we zoomed in and examined the disk very carefully to determine the year of the missing or very thin ring. For each disk (tree) we averaged four measures per ring (year) to give mean radial increment for each year of growth, and then calculated the average of those means from the 12 trees to yield mean (±SE) increment per year for each species. Our methods would not have detected an annual ring that was missing along all four radial transects. If this occurred, actual mean radial increment would be lower than our estimate and there would be slight error in exact year of growth assigned to each ring. However, the fairly consistent patterns of radial growth that we observed in individual beech trees suggests that error due to missing rings was relatively small (Supplementary Material Figures S1A,B).
Urban Properties
The impact of beech weevil on American beech growing in urban Halifax was assessed by surveying properties in three residential areas of Halifax Regional Municipality (HRM) [Fairmount (44.644°N, 63.635°W), Kent Park (44.691°N, 63.662°W), and Fall River (44.827°N, 63.611°W)] as well as two HRM parks: Birch Cove [44.680°N, 63.561°W] and Graham’s Grove [44.682°N, 63.551°W]. Our objectives were to estimate the mortality of American beech growing on residential properties, as well as the direct costs of tree removal incurred by owners of those properties. We selected areas of the city where American beech was relatively common. The survey sample was intentionally biased to include only properties with American beech trees and therefore results are not applicable to HRM residential properties in general. In September 2016 and October 2018, we went door to door, surveying residents in person, using a one-page questionnaire plus a second page with images of beech weevil damage to leaves and trees (Supplementary Material Data Sheet S1). Our questions were prefaced with a statement of the survey’s purpose (“…to determine the impact of the beech leaf-mining weevil on American beech trees and on HRM residents who have beech trees on their property.”) and assurances that all responses were voluntary, that information on tree mortality and costs may be published, and that identity of all respondents would remain confidential. We asked the following five questions: “(Q1) Do you have any American beech trees (live, dead, or cut and removed within the last 5 years) on your property?; (Q2) If the answer to Q1 is yes, how many of your beech trees are: (a) still alive and appear healthy (lots of foliage, full green crown)?, (b) alive but the trees appear unhealthy, e.g., thin crown?, (c) dead and still standing (no green leaves visible this past spring or summer)?, or (d) dead and cut down and removed in the last 5 years?; (Q3) In what year did you first notice damage to the leaves on your beech trees? (Please see images of typical damage by the beech leaf-mining attached); (Q4) If you have had to have dead or dying beech trees cut and removed on your property, how much did that cost (to the nearest $100)?; and (Q5) Will you allow us to measure the diameter of your beech trees (live or stumps)?” If no one was home we left the questionnaire by the door with our contact information. We received responses from 54 residential property owners (38 from Fairmount, 9 from Kent Park, and 7 from Fall River) in 2016 and from 48 of these same properties in 2018. All residents to whom we spoke were informed of the study’s objectives and verbally consented to answer our questions with no reservations, so explicit written consent was considered unnecessary. Most (66%) answers were obtained in person in door-to-door interviews and 34% were received by email or regular mail. We visited all but one or two properties to inspect the beech and confirm assessments.
Data Analysis
Mean values of all parameters are reported with standard errors. To compare changes in tree mortality among sites and years, we tested the effects of site, year and site×year interaction on the cumulative percentage mortality of American beech in the fixed radius plots using multivariate ANOVAs (SAS PROC GLM for repeated measures) (Sas Institute, 2002–2012). The same method was used to compare changes in percentages of leaves with larval mines and percentage of leaves with adult feeding, but only for Oakfield and Mount Uniacke, 2016–2019, because there were no data on foliage damage at Sandy Lake after 2016 (no leaves to sample as most beech were dead). Percentage data were transformed by arcsine square root to improve fit to a normal distribution (Zar, 1999). Residuals from GLMs on percentage data fit the normal distribution, but the arcsine square root transformation improved fit, i.e., reduced departure from normality [% leaves with mines (W = 0.90, P = 0.20) vs. transformed data (W = 0.96, P = 0.80);% leaves with adult feeding (W = 0.94 P = 0.50) vs. transformed data (W = 0.98 P = 0.94); and % tree mortality (W = 0.94, P = 0.35) vs. transformed data (W = 0.94, P = 0.36); Shapiro-Wilks test, SAS PROC UNIARIATE]. We also used generalized mixed models (SAS PROC GLIMMIX) with binomial distributions and logit link to test for differences among sites in each year of the: (1) proportion of leaves with larval mines; (2) proportion of leaves with adult feeding damage; and (3) proportion of total beech that had died. We tested for correlations between mean values of percentage of leaves damaged at each site-year and cumulative percentage of beech trees that died per site-year using SAS PROC CORR. We used SAS GLM to test regressions of percentage beech mortality vs. percentage of leaves damaged, using means for site-years. Means for site-years were calculated from five plots per site in each year; life table plots were used in place of our fixed radius plots at Oakfield-2014 and Sandy Lake-2016. We report results from regressions on raw percentage data rather than percentages transformed by arcsine of the square root, because their residuals fit the normal distribution better (percentage data, P > 0.93; transformed data, P > 0.85).
To test whether cumulative beech tree mortality between 2014 and 2019 was independent of the severity of beech bark disease, we used 2 × 3 Chi-square contingency tables to compare the proportion of live vs. dead beech in 2019 in each of three categories of trunk canker severity (light, medium, high), as classified in 2014. We did this for each site separately, as well as for all three sites pooled and tested our prediction that the association between beech bark disease severity and tree mortality would differ among sites and be less evident at sites with greater weevil damage, by testing for heterogeneity among the three 2 × 3 contingency tables (Zar, 1999).
Results
Forested Sites
In 2014, there were 260 American beech >6 cm DBH (217 live, 43 dead) in the fifteen plots. Of the 217 beech that were alive in 2014, only 80 remained alive in 2019, an overall mortality rate of 64% in 5 years. Damage to foliage by the weevil and cumulative mortality increased at each site most years (Figures 3A–C). Between 2016 and 2019, the percentage of leaves mined by weevil larvae increased from 6 to 59% at Mount Uniacke and from 48 to 83% at Oakfield (Figure 3B). During the same period, cumulative beech mortality increased from 35% (in 2016) to 48% (in 2019) at Mount Uniacke and from 10% (in 2016) to 70% (in 2019) at Oakfield (Figure 3C). At Sandy Lake in 2016, 88% of the beech trees had died (Figure 3C) and there were too few living beech to collect a leaf sample in our impact plots, but in life table plots in the same area, 87% of leaves were mined by the weevil (Figure 3B). There were significant effects of site, year and site×year interaction on cumulative tree mortality (Table 2). When data were analyzed separately by year, leaf damage by O. fagi (adult feeding, larval mines) and tree mortality differed significantly among sites in most years (Figures 3A–C and Supplementary Material Table S1). Mean percentage of leaves mined per site-year were significantly correlated with mean percentage of leaves with adult feeding damage (r = 0.86, n = 11, P = 0.0007). Cumulative tree mortality per site-year was only weakly correlated with the mean percentage of leaves mined per site-year (r = 0.54, n = 11, P = 0.08) and was not correlated with percentage of leaves with adult feeding damage per site year (r = 0.34, n = 11, P = 0.30).
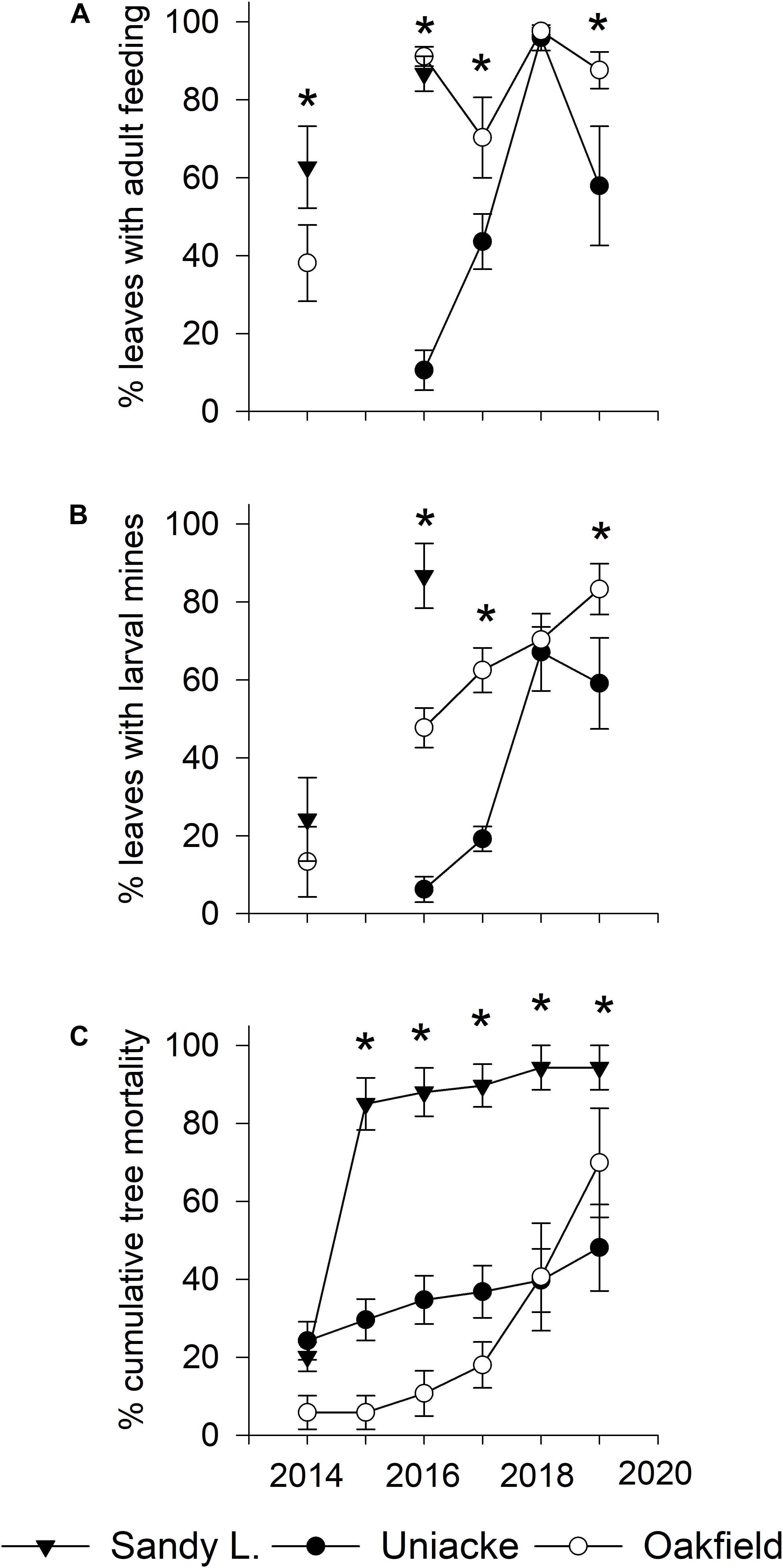
Figure 3. Average (±SE) values for: (A) percentage of leaves with Orchestes fagi adult feeding damage; (B) percentage of leaves with larval mines of O. fagi; and (C) percentage mortality of American beech trees (≥ 6 cm diameter at breast height) from 2014 to 2019 in five 11.28 m radius plots at each of three sites (Sandy Lake, Oakfield, Mount Uniacke) in Nova Scotia, Canada. Quantitative estimates of leaf damage were not made at Mount Uniacke in 2014 because visual surveys found no evidence of weevil damage. No estimates of leaf damage were made at any site in 2015 due to logistical constraints. Asterisks denote significant differences in mean values among sites within year (generalized linear models on binomial data, P < 0.05, see Supplementary Material Table S1 for F, df, and P values).
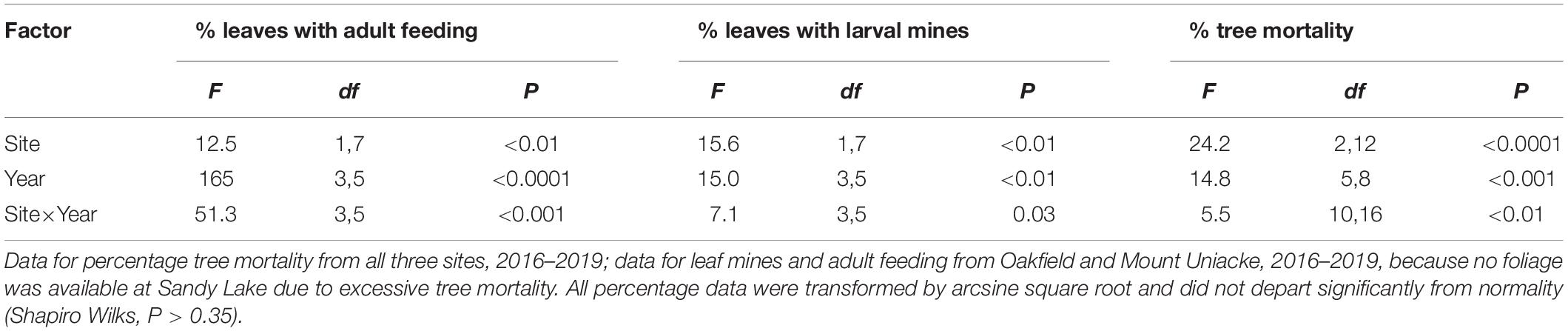
Table 2. Results of multivariate ANOVA (SAS GLM for repeated measures) testing the effect of site, year, and site × year interaction on the percentages of American beech: (1) leaves damaged by Orchestes fagi adult feeding; (2) leaves mined by O. fagi larvae; and (3) trees that were dead, in Nova Scotia, Canada.
The association between cumulative beech mortality in 2019 and severity of beech bark disease differed significantly among sites (heterogeneity Chi-square, χ2 = 19.73, df = 4, P < 0.001) and was significant only at Mount Uniacke (χ2 = 18.5, df = 2, P < 0.0001) (Table 3). Of the three sites, Mount Uniacke had the greatest percentage of beech rated with high severity of cankering from beech bark disease in 2014 (75%) and the lowest percentage mortality (44%) in 2019 (Table 3).

Table 3. Numbers of live and dead American beech in 2019 in each of three categories of beech bark disease (BBD) severity at three sites in Nova Scotia, Canada.
Annual radial increment of American beech at Sandy Lake showed a period of slow growth from 1930 to 1950 followed by increasing growth rates from 1950 to 1970 and decreasing growth rates from 1970–2000 (Supplementary Material Figure S1) with an overall mean (±SE) of 1.09 (0.05) mm, comparable to that reported for beech by others (Tubbs and Houston, 1990; Gavin and Peart, 1993). From 1957 to 2017, mean annual radial increment (±SE) of American beech peaked at 2.1 (0.26) mm in 1970 and then declined, leveling off at 0.3–0.4 (0.05–0.07) mm from 2007 to 2017, whereas growth rate of sugar maples showed a marked increase beginning in the mid-2000’s (Figure 4).
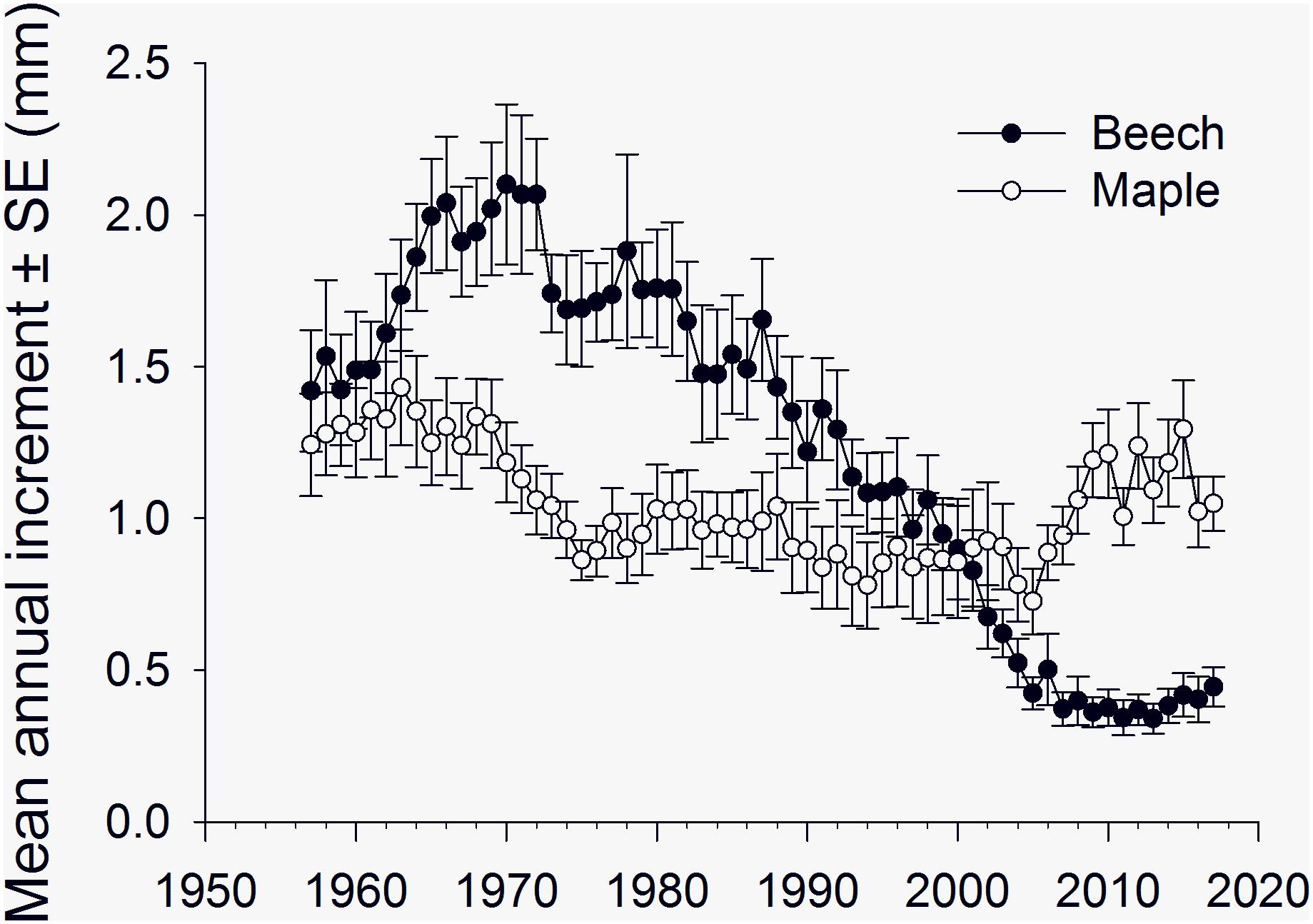
Figure 4. Mean annual increment (±SE, n = 12 trees per species) of American beech and sugar maple, 1952–2017, Sandy Lake, Nova Scotia, showing a general decline in growth rate of American beech from 1970 to 2010 and an increase in growth rates of sugar maple relative to American beech, around 2003–2004.
Residential Properties
An average of 32.2 ± 5.3% of American beech on residential properties in HRM were dead in the fall of 2016 and most of the trees that were still alive appeared unhealthy with very thin crowns and obvious signs of beech weevil damage (Table 4). Percentage mortality was greater in the neighborhoods of Fairmount (36.9 ± 6.8) and Kent (35.3 ± 10.2) than in Fall River (2.4 ± 2.4). Residents of Fairmount, Kent, and Fall River estimated they first noticed defoliation on their beech trees an average of 5.1 ± 0.5, 3.9 ± 0.4, and 3.6 ± 0.6 years prior to 2016, respectively; the overall mean was 4.6 ± 0.3 years, suggesting defoliation was obvious to residents in 2011–2012. In 2016, 15 property owners had paid arborists to remove dead beech from their properties at an average cost of $567 ± 91 per tree and $1260 ± 195 per resident (Table 4). The mean number of beech trees removed per property was 0.96 ± 0.24 (Table 4). The percentage of American beech that were dead or had already been removed in 2016 was greater in Graham’s Cove Park (53.5%) and Birch Cove Park (57.1%) than in the residential areas.
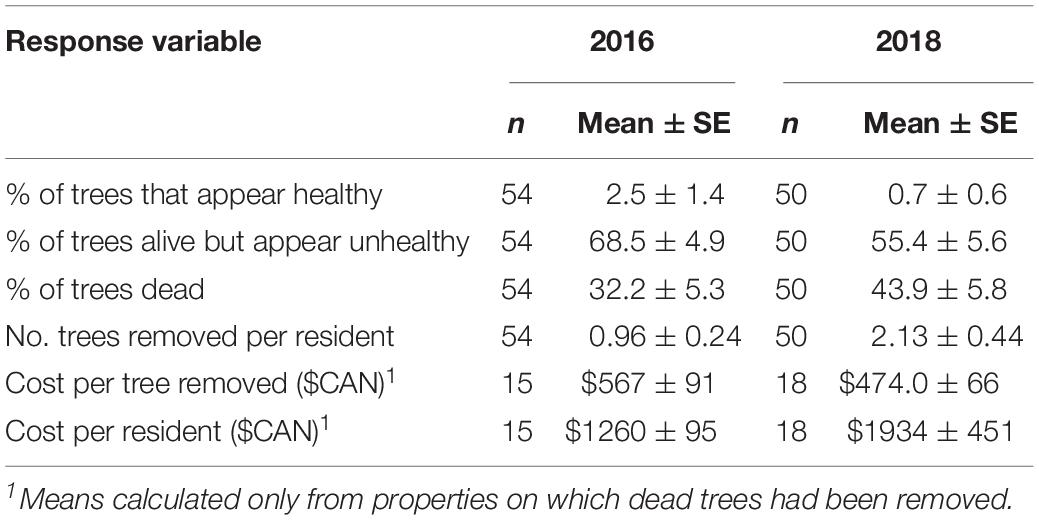
Table 4. Impact of Orchestes fagi on American beech on residential properties in Halifax Regional Municipality, as determined by resident surveys and site visits in fall of 2016 and 2018.
In the fall of 2018 the mean cumulative percentage of beech that had died had increased to 44% (Table 4), and as in 2016, was greater in Fairmount (47.0 ± 6.8) and Kent (53.1 ± 11.7) than at Fall River (4.4 ± 4.4). Cost per residents who paid arborists to remove dead trees had increased to $1934 ± 451 but cost per tree decreased to $474 ± 66 (Table 4). The average number of beech trees removed per property was 2.1 ± 0.44, about double the average in 2016 (Table 4). In Graham’s Cove Park and Birch Cove Park, the percentage of American beech that had died and/or had been removed was 57 and 64% respectively.
Discussion
Our data clearly indicate that American beech trees in Nova Scotia are dying after an estimated 5–7 consecutive years of defoliation by the beech leaf-mining weevil, O. fagi. Although positive correlations between cumulative percentage tree mortality and percentage of leaves mined by the weevil were only weakly significant (P < 0.10), tree mortality was significantly greater in stands that had experienced more consecutive years of moderate to high weevil infestation levels (Sandy Lake, Oakfield) than in a stand where infestation levels were relatively low until 2018 (Mount Uniacke). Beech bark disease was prevalent at all three sites and probably caused some tree mortality at each site but it is unlikely it was responsible for the 20 and 60% increases in cumulative beech mortality we observed at Oakfield in 2018 and Sandy Lake in 2015, respectively. Annual mortality from beech bark disease is greatest during the first 10 years of invasion (the “killing front”) and subsequently averages only about 2% of live biomass in the aftermath forest (Fei et al., 2019); diseased beech that survive the initial wave of mortality survive for many years (Gavin and Peart, 1993; Morin et al., 2007). Contrary to our prediction, our contingency table analysis showed that beech mortality was independent of the degree of cankering by beech bark disease, except at Mount Uniacke, where infestation by the weevil was relatively low until 2018. Rather than directly causing tree mortality, it is likely that successive years of defoliation by the weevil has weakened trees and made them more susceptible to mortality from root rot fungi. American beech is host to a number of decay fungi and trees weakened by defoliators are more susceptible to fungal invasion and mortality, especially Armillaria ostoyae (Romagnesi) Herink (Tubbs and Houston, 1990).
Relative to sugar maple, the radial growth of beech declined from 1970 to the early 2000’s, but we did not see an obvious decrease in growth rate of beech in the mid-2000’s to correspond with anecdotal reports of beech defoliation in the Halifax area. Beech defoliated by O. fagi in spring will often respond with a second flush of foliage in mid-summer and it is possible this may have compensated for the effects of spring defoliation on radial growth. The overall mean annual radial increment of sugar maples was 1.04 (0.06) mm, lower than the 1.1–1.3 mm reported for sugar maple on poor sites (Godman et al., 1990), and there appeared to be more variation in growth rate among the maples than the beech (Supplementary Material Figure S2). There was an increase in mean annual radial increment of sugar maples in the mid-2000s (Figure 4) that may have been due to increased sun exposure associated with defoliation of neighboring beech. Sugar maples survive long periods under heavy shade and respond strongly to release, with maximum photosynthetic activity at 25% full sunlight (Godman et al., 1990). Openings in the canopy created by beech bark disease-induced mortality may also have contributed to the increase in radial growth of sugar maples in our plots. DiGregario et al. (1999) reported significantly greater radial growth of sugar maples in the subcanopy of a northern hardwood forest during a period of beech bark disease-induced canopy decline in upstate New York. However, it is unlikely that mortality from beech bark disease was as prevalent in our plots at Sandy Lake in the mid-2000s as it was in the study plots of DiGregario et al. (1999) near Ithaca, New York in the mid-1980s. The “killing front” period of high beech mortality from beech bark disease passed through the Sandy Lake area of Nova Scotia (containing our radial growth plots) in the early 1900’s (Houston et al., 1979) whereas the disease arrived at the Ithaca area in the mid-1970’s with heavy beech mortality in the mid-1980’s (DiGregario et al., 1999).
Mortality rates of American beech on residential properties in HRM averaged about 44% and were lower than those in the closest forested site at Sandy Lake. This was most likely because urban trees were more open grown with less competition for light and other resources than trees in the forested sites, and not due to differences in beech weevil infestation levels. We did not directly measure infestation levels on the residential properties but data from a separate study (by JS, testing stem-injected insecticides for control of O. fagi) showed that infestation levels (% of leaves mined) in the Fairmount area averaged 72% between 2014 and 2018 (JS, unpublished data), comparable to infestation levels at Sandy Lake in 2016 (Figure 3). The proportion of American beech that were dead or had already been removed in 2016 was greater in Halifax’s Municipal parks (Graham’s Cove, Birch Cove) than in the residential areas, likely because a portion of unhealthy, nearly dead trees had been removed by Park staff for public safety reasons. Direct costs to property owners who paid arborists to remove dead beech were significant, averaging $1934 ($300–$6600) per resident in 2018. In addition to these direct costs is the loss in property values when mature trees are killed and removed due to an invasive pest like the beech leaf-mining weevil. A single mature tree can increase property values by 3–5% (Nowak and Dwyer, 2007) which translates into increased assessment values and revenue for municipalities. For high value urban street trees of American beech, it may be possible to protect them from beech weevil defoliation by treatment with stem-injected insecticides, as done for the emerald ash borer (McKenzie et al., 2010; McCullough et al., 2011). Efficacy trials using TreeAzin (active ingredient azadirachtin) have had promising results (Sweeney et al., 2015) but no insecticides are yet registered for control of O. fagi in Canada.
While our data indicate the weevil has had a significant impact on American beech, its overall impact on forests in Nova Scotia may be relatively small because American beech is relatively uncommon in the province’s urban areas and natural forests. In a preliminary survey along roadways in 53 urban centers in eastern Canada, Pedlar et al. (2013) estimated an average of only 0.49 ± 0.86 Fagus spp. trees per km compared to the far more common Acer and Betula with 31.5 ± 5.5 and 20.3 ± 9.1 trees per km, respectively. Using data from more than 3000 permanent sample plots, Keys et al. (2007) estimated that American beech comprised about 3% of total merchantable volume of hardwoods in Nova Scotia, compared to 14% for sugar maple and 44% for red maple. Furthermore, 22% of the merchantable volume of beech was considered commercially inaccessible due to slope constraints, and most was classified as low quality (Keys et al., 2007), likely due to damage caused by beech bark disease.
We can only speculate as to why O. fagi populations have remained at high levels for longer and caused more damage on American beech in Nova Scotia than they are reported to do on European beech in its native habitat. As European beech leaves sclerify with age, it becomes more difficult for early-instar O. fagi larvae to mine leaves, thus increasing mortality rates when oviposition occurs too long after budburst (Bale, 1984; Nielsen, 1966). It is possible that American beech may be more susceptible than European beech to damage from O. fagi, due to lower leaf toughness or a slower rate of sclerification, but this hypothesis remains to be investigated. There may be less competition for food and space in American beech leaves because they are larger and thicker than those on European beech and this may result in increased larval survival (Moise et al., 2015). The weevil may also suffer less mortality from parasitism in its new range than in its native Europe, where it is host to many species of parasitoids, both generalists and specialists (Péré et al., 2011). In contrast, evidence of parasitism of O. fagi in Nova Scotia has been very rare to date (RJ and JS, unpublished data). The phenomenon of longer outbreaks in populations of leafminers in invaded versus native habitats has previously been reported for the ambermarked birch leafminer, Profenusa thomsoni (Konow) (Hymenoptera: Tenthredinidae). Outbreaks of P. thomsoni have lasted for decades in parts of Canada whereas it is considered relatively rare in its native Palearctic range, possibly due to regulation by natural enemies or resistance of European birch (Digweed et al., 1997, 2003, 2009). Since the 1990’s, outbreaks of P. thomsoni in Canada have been suppressed by the parasitoid, Lathrolestes thomsoni Reshchikov (Hymenoptera: Ichneumonidae), believed to be native to North America (MacQuarrie et al., 2013).
Our study indicate that defoliation by the beech leaf-mining weevil has resulted in considerable mortality of American beech in Nova Scotia in the last 5–6 years. We predict the weevil will continue to spread throughout the range of American beech through both natural dispersal and human-assisted movement, and may have considerable impact on American beech and species highly dependent upon it. Stem-injected insecticides have good potential for protecting high value beech trees on residential properties, but classical biological control may be a more practical control option in forests. Studies are ongoing in Europe to identify parasitoid candidates that are sufficiently host-specific to O. fagi, and low risk to non-target species, to be considered for possible introduction into North America.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This research was approved by a committee of NRCan science directors. Approval by an ethics committee was not required for this research but all employees of Natural Resources Canada are required to follow the NRCan Values and Ethics Code as well as the Canadian Public Service Code of Ethics https://www.tbs-sct.gc.ca/pol/doc-eng.aspx?id=25049.
Author Contributions
JS and RJ contributed procurement of funds. JS, CH, and AM designed the experiment. CH and AM performed site selection and plot set-up. CH and HZ collected the data. JS analyzed the data. JS, RJ, and NH wrote the manuscript. All authors reviewed drafts and approved the final version of the manuscript.
Funding
Natural Resources Canada, Canadian Forest Service, A-base funding on forest invasive alien species.
Conflict of Interest
AM was employed by Canadian Forest Service at the time the research plots were set up and is now employed by the company Forest Protection Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Chantelle Kostanowicz, Colin Mackay, Troy Kimoto, Ron Neville, Emily Owens, Simon Pawlowski, and Vincent Webster for their technical support, Ian DeMerchant for preparing Figure 2, Natural Resources Canada-Canadian Forest Service for funding, Celia Boone and Michael Stastny for reviewing an earlier version of this manuscript, and John Simmons and Crispin Wood of Halifax Regional Municipality, and Nova Scotia Provincial Parks for giving us permission to work on their properties.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2020.00046/full#supplementary-material
Footnotes
References
Anderson, R. S., O’Brien, C. W., Salsbury, G. A., and Krauth, S. J. (2007). Orchestes alni (L.) newly discovered in North America (Coleoptera: Curculionidae). J. Kansas Entomol. Soc. 80, 78–79. doi: 10.2317/0022-8567(2007)80[78:oalndi]2.0.co;2
Anonymous (2014). Cruising Manual, British Columbia Ministry of Forests. Available online at: https://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/forestry/timber-pricing/cruising-manual/2014_cruise_master_amend_2.pdf (accessed March, 2020).
Bale, J. S. (1984). Budburst and success of the beech weevil, Rhynchaenus fagi L.: feeding and oviposition. Ecol. Entomol. 9, 139–148. doi: 10.1111/j.1365-2311.1984.tb00708.x
Bale, J. S., and Luff, M. L. (1978). The food plants and feeding preferences of the beech leaf mining weevil, Rhynchaenus fagi L. Ecol. Entomol. 3, 245–249. doi: 10.1111/j.1365-2311.1978.tb00925.x
Cale, J. A., Garrison-Johnston, M. T., Teale, S. A., and Castello, J. D. (2017). Beech bark disease in North America: over a century of research revisited. For. Ecol. Manage. 394, 86–103. doi: 10.1016/j.foreco.2017.03.031
Cale, J. A., Teale, S. A., Johnston, M. T., Boyer, G. L., Perri, K. A., and Castello, J. D. (2015). New ecological and physiological dimensions of beech bark disease development in aftermath forests. For. Ecol. Manage. 336, 99–108. doi: 10.1016/j.foreco.2014.10.019
Connor, E. F., and Taverner, M. (1997). The evolution and adaptive significance of the leaf-mining habit. Oikos 79, 6–25. doi: 10.2307/3546085
DiGregario, L. M., Krasny, M. E., and Fahey, T. J. (1999). Radial growth trends of sugar maple (Acer saccharum) in an Allegheny northern hardwood forest affected by beech bark disease. J. Torrey Bot. Soc. 126, 245–254.
Digweed, S. C., MacQuarrie, C. J. K., Langor, D. W., Williams, D. J. M., Spence, J. R., Nystrom, K. L., et al. (2009). Current status of invasive alien birch-leafmining sawflies (Hymenoptera: Tenthredinidae) in Canada, with keys to species. Can. Entomol. 141, 201–235. doi: 10.4039/n09-003
Digweed, S. C., McQueen, R. L., Spence, J. R., and Langor, D. W. (2003). Biological Control of the ambermarked birch leafminer, Profenusa thomsoni (Hymenoptera: Tenthredinidae). Alberta Information Report No. R-X-389. Edmonton: Natural Resources Canada, Edmonton.
Digweed, S. C., Spence, J. R., and Langor, D. W. (1997). Exotic birch-leafmining sawflies (Hymenoptera: Tenthredinidae) in Alberta: distributions, seasonal activities and the potential for competition. Can. Entomol. 129, 319–333. doi: 10.4039/Ent129319-2
Ehrlich, J. (1934). The beech bark disease. A Nectria disease of Fagus, following Cryptoccus fagi (Baer.). Can. J. Res. 10, 593–692. doi: 10.1139/cjr34-070
Eiseman, C. (2018). Leafminers of North America, 1st Edn. Available online at: http:/charleyeiseman.com/leafminers (accessed April 8, 2020).
Fei, S., Morin, R. S., Oswalt, C. M., and Liebhold, A. M. (2019). Biomass losses resulting from insect and disease invasions in US forests. Proc. Natl. Acad. Sci. U.S.A. 116, 17371–17376. doi: 10.1073/pnas.1820601116
Gavin, D. G., and Peart, D. R. (1993). Effects of beech bark disease on the growth of American beech (Fagus grandifolia). Can. J. For. Res. 23, 1566– 1575. doi: 10.1139/x93-197
Giencke, L. M., Dovèiak, M., Mountrakis, G., Cale, J. A., and Mitchell, M. J. (2014). Beech bark disease: spatial patterns of thicket formation and disease spread in an aftermath forest in the northeastern United States. Can. J. For. Res. 44, 1042–1050. doi: 10.1139/cjfr-2014-0038
Godman, R. M., Yawney, H. W., and Tubbs, C. H. (1990). “Acer saccharum Marsh. Sugar Maple,” in Silvics of North America: Hardwoods, Vol. 2, eds (coord.) R. M. Burns and B. H. Honkala (Washington, DC: USDA Forest Service), 194–215.
Houston, D. R. (1975). Beech bark disease–the aftermath forests are structured for a new outbreak. J. For. 73, 660–663. doi: 10.1093/jof/73.10.660
Houston, D. R. (1994). Major new tree disease epidemics: beech bark disease. Annu. Rev. Phytopathol. 32, 75–87. doi: 10.1146/annurev.py.32.090194.000451
Houston, D. R., Parker, E. J., and Lonsdale, D. (1979). Beech bark disease: patterns of spread and development of the initiating agent Cryptococcus fagisuga. Can. J. For. Res. 9, 336–343. doi: 10.1139/cjfr-2014-0038
Keys, K., Townsend, P., Morash, R., and McGrath, T. (2007). Nova Scotia’s Hardwood Resource: Estimating Sawlog Volumes by species, Quality, and Accessibility. Available online at: https://novascotia.ca/natr/library/publications/forest-research-hardwood.asp (accessed August 30, 2019).
Kirichenko, N., Augustin, S., and Kenis, M. (2019). Invasive leafminers on woody plants: a global review of pathways, impact, and management. J. Pest Sci. 92, 93–106. doi: 10.1007/s10340-018-1009-6
Korotyaev, B. A. (2016). New data on the changes in the abundance and distribution of several species of beetles (Coleoptera) in European Russia and the Caucasus. Entomol. Rev. 96, 620–630. doi: 10.1134/S0013873816050080
Looney, C., Humble, L. M., and Cranshaw, W. (2012). Orchestes alni (L.) (Coleoptera: Curculionidae): new records from western North America with notes on parasitoids. Coleopt. Bull. 66, 63–66. doi: 10.1649/072.066.0115
MacQuarrie, C. J. K., Langor, D. W., Digweed, S. C., and Spence, J. R. (2013). “Fenusa pumila Leach, Birch leaf miner, Profenusa thomsoni (Konow), ambermarked birch leaf miner (Hymenoptera: Tenthredinidae,” in Biological Control Programmes in Canada 2001–2012, eds P. G. Mason and D. R. Gillespie (Wallingford: CAB International), 175–181. doi: 10.1079/9781780642574.0175
McCullough, D. G., Poland, T. M., Anulewicz, A. C., Lewis, P., and Cappaert, D. (2011). Evaluation of Agrilus planipennis (Coleoptera: Buprestidae) control provided by emamectin benzoate and two neonicotinoid insecticides, one and two seasons after treatment. J. Econ. Entomol. 104, 1599–1612. doi: 10.1603/EC11101
McKenzie, N., Helson, B., Thompson, D., Otis, G., McFarlane, J., Buscarini, T., et al. (2010). Azadirachtin: an effective systemic insecticide for control of Agrilus planipennis (Coleoptera: Buprestidae). J. Econ. Entomol. 103, 708–717. doi: 10.1603/EC09305
McLaughlin, J., and Griefenhagen, S. (2012). Beech Bark Disease in Ontario: A Primer and Management Recommendations. Sault Ste Marie: Ontario Forest Research Institute, Sault Ste.
Moise, E. R. D., Forbes, G. B. H., Morrison, A., Sweeney, J. D., Hillier, N. K., and Johns, R. C. (2015). Evidence for a substantial host-use bottleneck following the invasion of an exotic, polyphagous weevil. Ecol. Entomol. 40, 796–804. doi: 10.1111/een.12268
Morin, R. S., Liebhold, A. M., Tobin, P. C., Gottschalk, K. W., and Luzader, E. (2007). Spread of beech bark disease in the eastern United States and its relationship to regional composition. Can J. For. Res. 37, 726–736. doi: 10.1139/X06-281
Morrison, A., Sweeney, J., Hughes, C., and Johns, R. C. (2017). Hitching a ride: firewood as a potential pathway for range expansion of an exotic beech leaf-mining weevil, Orchestes fagi (Coleoptera: Curculionidae). Can. Entomol. 149, 129–137. doi: 10.4039/tce.2016.42
Nielsen, B. O. (1966). Studies on the fauna of beech foliage. 1. Contributions to the biology of the early stages of the beech weevil. (Rhynchaenus [Orchestes) fagi L.), (Coleoptera. Curculionidae). Nat. Juttand. 12, 162–181.
Nielsen, B. O. (1970). Observations on the hibernation of the beech weevil (Rhynchaenus fagi L.) in Denmark. Entomol. Scand. 1, 223–226. doi: 10.1163/187631270x00069
Nowak, D. J., and Dwyer, J. F. (2007). “Understanding the benefits and costs of urban forest ecosystems,” in Urban and Community Forestry in the Northeast, 2nd Edn, ed. J. E. Kuser (Dordrecht: Springer), 444. doi: 10.1007/978-1-4020-4289-8_2
Pedlar, J. J., McKenney, D. W., Allen, D., Lawrence, K., Lawrence, G., and Campbell, K. (2013). A street tree survey for Canadian communities: Protocol and early results. For. Chron. 89, 753–758. doi: 10.5558/tfc2013-137
Péré, C., Bell, R., Turlings, T. C. J., and Kenis, M. (2011). Does the invasive horsechestnut leaf mining moth, Cameraria ohridella, affect the native beech leaf mining weevil, Orchestes fagi, through apparent competition? Biodivers. Conserv. 20, 3003–3016. doi: 10.1007/s10531-011-0134-9
Reinhardt, F., Herle, M., Bastiansen, F., and Streit, B. (2003). Economic Impact of the Spread of Alien species in Germany. Berlin: Umwelbundesamt.
Rullán-Silva, C., Olthoff, A. E., Pando, V., Pajares, J. A., and Delgado, J. A. (2015). Remote monitoring of defoliation by the beech leaf-mining weevil Rhychaenus fagi in northern Spain. For. Ecol. Manage. 347, 200–208. doi: 10.1016/j.foreco.2015.03.005
Sas Institute Inc (2002–2012). SAS/STAT software, Version 9.4 of the SAS System for Windows. Cary, NC: SAS Institute Inc. doi: 10.1016/j.foreco.2015.03.005
Shigo, A. L. (1972). The beech bark disease today in the northeastern U.S. J. For. 70, 286–289. doi: 10.1093/jof/70.5.286
Sweeney, J., Anderson, R. S., Webster, R. P., and Neville, R. (2012). First records of Orchestes fagi (L.) (Coleoptera: Curculionidae: Curculioninae) in North America, with a checklist of the North American Rhamphini. Coleopt. Bull. 66, 297–304. doi: 10.1649/072.066.040
Sweeney, J., Johns, R., Moise, E., Silk, P., Mayo, P., Hillier, K., et al. (2015). “European beech leaf mining weevil: a new invasive established in Nova Scotia,” in Proceedings of the 26th U.S. Department of Agriculture Interagency research forum on Invasive species, eds K. A. McManus and W. Kurt (Fort Collins, CO: U.S. Department of Agriculture), 42–43.
Tubbs, C. H., and Houston, D. R. (1990). “American beech (Fagus grandifolia Ehrh),” in Silvics of North America: Hardwoods, Vol. 2, eds (cord.) R. M. Burns and B. H. Honkala (Washington, DC: USDA), 654–667.
Verkaik, E., Moraal, L., and Nabuurs, G. (2009). Potential Impacts of Climate Change on Dutch Forests, Mapping the Risks. Wageningen: Wageningen Environmental Research.
Keywords: impact, invasive, Orchestes fagi, beech leaf-mining weevil, American beech
Citation: Sweeney JD, Hughes C, Zhang H, Hillier NK, Morrison A and Johns R (2020) Impact of the Invasive Beech Leaf-Mining Weevil, Orchestes fagi, on American Beech in Nova Scotia, Canada. Front. For. Glob. Change 3:46. doi: 10.3389/ffgc.2020.00046
Received: 26 November 2019; Accepted: 27 March 2020;
Published: 24 April 2020.
Edited by:
Davide Rassati, University of Padua, ItalyReviewed by:
Natalia Kirichenko, Sukachev Institute of Forest (RAS), RussiaBernd Panassiti, Independent Researcher, Munich, Germany
Copyright © 2020 Sweeney, Hughes, Zhang, Hillier, Morrison and Johns. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan D. Sweeney, am9uLnN3ZWVuZXlAY2FuYWRhLmNh
 Jonathan D. Sweeney
Jonathan D. Sweeney Cory Hughes1
Cory Hughes1 N. Kirk Hillier
N. Kirk Hillier