- 1Forest Industries Research Centre, University of the Sunshine Coast, Sippy Downs, QLD, Australia
- 2Forest Science, NSW Department of Primary Industries – Forestry, Parramatta, NSW, Australia
Geographic isolation, unique native flora, and a robust biosecurity system have resulted in Australia remaining free from many of the devastating exotic pests found in other countries. Nevertheless, at least 260 non-native arthropods and pathogens of forest hosts have established in Australia since 1885. Although the risk of invasive species arriving and establishing in Australia is increasing through raised levels of trade and travel, the rate of establishment of non-native forest pests has remained relatively constant over the last 130 years, accumulating at a rate of about two per year. The majority of these are arthropods and pathogens of tree host genera exotic to Australia, including the main plantation species, Pinus radiata; few are significant pests of tree host genera native to Australia. Eighteen percent of these pests have caused moderate to significant impact or resulted in ongoing management costs in commercial plantations, native forests, or amenity trees. Asian and European species accounted for two-thirds of Australia’s non-native forest pests, and were equivalently represented numerically, temporally, and compositionally. Asian species were more polyphagous and more frequently established in northern Australia, possibly reflecting climatic similarity, geographic proximity, and host plant suitability. Earlier-establishing species were more polyphagous and had broader Australian and global non-native distributions. We here provide the first comprehensive database of non-native arthropod and pathogen species of relevance to Australia’s plantation, amenity, and native forest trees in Australia. This knowledge will assist with identifying key traits of exotic pest threats to forests in Australia and globally to inform national and international biosecurity policy.
Introduction
Numerous studies have documented the steady rise in invasions of non-native plant pests, including forest insects and pathogens (Xu et al., 2006; Smith et al., 2007; Aukema et al., 2010; Santini et al., 2013; Lovett et al., 2016; Brockerhoff and Liebhold, 2017; Edney-Browne et al., 2018). The main invasion pathways for forest pests are live plant imports, solid wood packaging material, and log/timber imports (Smith et al., 2007; Liebhold et al., 2012; Lovett et al., 2016; Brockerhoff and Liebhold, 2017; Meurisse et al., 2018), or wilful introduction (e.g., Paine et al., 2010). A key factor associated with this increasing invasiveness is the substantial rise in global trade and travel (Levine and D’antonio, 2003; Hulme, 2009; Seebens et al., 2017; Sikes et al., 2018), with predictions that the spread of non-native plant pests will increase correspondingly (Bebber et al., 2014; Paini et al., 2016; Brockerhoff and Liebhold, 2017). The economic and environmental costs of non-native forest pests are substantial to native ecosystems, commercial plantations, and amenity forests (Holmes et al., 2009; Loo, 2009; Aukema et al., 2011; Liebhold et al., 2017; Cameron et al., 2018; Carnegie and Pegg, 2018). Economic impacts include eradication attempts, control and management costs borne by governments and local councils, loss of property values, costs associated with felling and replacing affected trees, and productivity losses for plantation growers. Ecological impacts include loss of biodiversity, knock-on effects on fauna reliant on impacted tree species, and ultimately species extinction in severe cases (Loo, 2009; Fisher et al., 2012; Tobin, 2015). Recognition of the ecological and economic cost of invasions and the apparent increase in invasion rates has led many countries to develop policies and management strategies to limit the risk of future invasions and to reduce the establishment, spread, and impact of non-native pests (Ruiz and Carlton, 2003; Eschen et al., 2015).
Forests cover about 17% (134 million hectares) of Australia’s land, and constitute a highly valued ecological, economic, and cultural resource (ABARES, 2018). Native forests and woodlands are predominately eucalypt and acacia (around 121 million hectares), 3.5 million hectares are rainforest, while roughly 2 million hectares comprise commercial softwood (Pinus) and hardwood (Eucalyptus) plantation forests (ABARES, 2018). Turnover from the Australian forest wood and products sector exceeds AUD$20 billion/year, and contributes over AUD$8 billion to Australia’s gross domestic product, while non-wood forest products have an estimated gross production value of almost AUD$200 million (ABARES, 2018). Trees constitute around 40% of the urban environment, and are highly valued as “amenity” trees—so-called for their contribution to aesthetics, carbon sequestration, air and water quality, and social, cultural, and property values (Jacobs et al., 2014). Urban trees also play an important role in the arrival and establishment of forest pest invasions (Paap et al., 2017; Branco et al., 2019).
Australia’s geographic isolation, strict biosecurity system, and unique flora have—so far—kept it free from many of the pests that have devastated Northern Hemisphere forests, including Dutch elm disease (caused by Ophiostoma ulmi and Ophiostoma nova-ulmi; Gibbs, 1978), pine wilt disease (caused by Bursaphalenchus xylophilus; Zhao et al., 2008), emerald ash borer (Agrilus planipennis; Herms and McCullough, 2014), sudden oak death (caused by Phytophthora ramorum; Brasier and Webber, 2010), and Asian longhorned beetle (Anoplophora glabripennis; Haack et al., 2009). As an island nation, Australia does not have the porous borders of Europe, America, Africa, and Asia that can allow unfettered movement of goods and people, and for invasive pests to evade border protection and phytosanitary barriers through natural spread (Eschen et al., 2015; Santini et al., 2018).
Australia also has a comprehensive biosecurity system, with the Biosecurity Act 2015 and the Quarantine Proclamation 1998 being the principle legislative tools to reduce the chance of entry, establishment and spread of non-native pests (Pheloung, 2003; Anderson et al., 2017). Pre-border activities include strict regulations on the entry of live plants, particularly known hosts of high-priority pests (Department of Agriculture and Water Resources, 2015); identifying high risk sites and seasons to optimize surveillance (Carnegie et al., 2017); and the Canadian Accreditation Timber Scheme that has significantly reduced the risk of pine bark beetles entering Australia on imported logs (Carnegie et al., 2016). Border activities include screening and surveillance of international vessels, mail, passengers and cargo, and quarantine of live plants (Department of Agriculture and Department of Environment, 2014). Post-border activities include surveillance for high priority pests at high-risk sites, and agreed government and industry preparedness and response procedures for exotic pest invasions (Plant Health Australia, 2016; Carnegie et al., 2017, 2018a). Australia has among the most stringent biosecurity processes globally (Ruiz and Carlton, 2003; Eschen et al., 2015; Edney-Browne et al., 2018), including for importation of live plants (e.g., Department of Agriculture and Water Resources, 2015), which is recognized as one of the major pathways of invasive species globally (Palm and Rossman, 2003; Liebhold et al., 2012).
Despite these advantages and safeguards, Australia continues to accumulate non-native pests on forest and amenity trees (Carnegie and Nahrung, 2019; Tovar and Carnegie, 2019). Sirex woodwasp in Australian softwood plantations is estimated to have cost around AUD$35M in losses and control (Cameron et al., 2018). A similar sum has been spent on attempted eradication and containment of European house borer since its detection in 2004 (Carnegie and Nahrung, 2019), while Phytophthora cinnamomi and Austropuccinia psidii have severely damaged native forest ecosystems through mortality and biodiversity loss (Cahill et al., 2008; Carnegie et al., 2016).
This study adds to the growing body of work examining the accumulation of plant pests—and forest insects and pathogens more specifically—in North America (Aukema et al., 2010; Lovett et al., 2016), New Zealand (Withers, 2001; Brockerhoff and Liebhold, 2017), Europe (Roques, 2010b; Santini et al., 2013), Asia (Xu et al., 2006), and Africa (Hurley et al., 2016; Graziosi et al., 2019), with the tenet that reviewing historical invasions enhances our ability to anticipate future events (Santini et al., 2018). We had three specific aims: first, to create a comprehensive database of non-native forest pests on exotic and indigenous trees (plantation, amenity, and native forest) in Australia; second, to identify temporal, biogeographical, and host association patterns connected with these establishments; and third, to identify high impact non-native forest pests in Australia. By synthesizing and presenting these data, we add to recent work from Australia (Tovar et al., 2016; Cameron et al., 2018; Carnegie et al., 2018a, b; Department of Agriculture and Water Resources, 2018b; Lawson et al., 2018; Wardlaw et al., 2018; Carnegie and Nahrung, 2019), and parallel studies overseas (Aukema et al., 2010; Santini et al., 2013; Brockerhoff and Liebhold, 2017) aimed at improving forest biosecurity.
Materials and Methods
Non-native Species Database
A comprehensive database of non-native arthropod pests (insects and mites) and pathogens (fungi, oomycetes and proteobacteria) of tree hosts in native forests, plantations, and amenity trees in Australia was compiled from records in literature and the Australian Plant Pest Database (APPD1). Here we use “arthropod” and “pathogen” to distinguish between the two types of organisms and “pest” to refer to both groups collectively. Key forest pest literature for Australia was searched (e.g., Froggatt, 1923, 1927; Browne, 1968; Neumann and Marks, 1976; Marks et al., 1982; Elliott and deLittle, 1985; Simpson, 1996; Elliott et al., 1998; Wylie and Speight, 2012), with more recent exotic pest detections recorded from Australia’s National Plant Biosecurity Status Reports (e.g., Plant Health Australia, 2014). We further searched the APPD for records of non-native insects, mites, and pathogens on 60 key arborescent genera; native and exotic tree genera were equivalently represented with about 30 of each (Host List: Supplementary Table S1). The APPD contains collection records from State and national fungal herbaria and insect collections and includes data on host, location, and date of collection. We focused on key plantation genera (Eucalyptus, Pinus, and Araucaria), the main exotic (e.g., Populus, Salix, Ulmus, Quercus, Platanus, Acer, Jacaranda, Shinus, and Fraxinus) and native (e.g., Casuarina, Syzygium, Acacia, Brachychiton) genera planted as amenity or farm woodlots in Australia, and native timber species (e.g., Flindersia, Eucalyptus, Syncarpia, Terminalia, Agathis2). Amenity genera that fit into horticultural industries (e.g., Persea, Mangifera, Corylus) and palms were not included, and nor were those planted primarily as shrubs (e.g., Melaleuca, Callistemon, Tristania), with the exception of Grevillea robusta, which is an important native timber species. We excluded pests that primarily colonize agricultural and horticultural crops unless (1) they had been identified in literature causing damage to forest taxa; (2) they were recorded from three or more of our key forest genera, or (3) >10% of their APPD records were from forest hosts. Pests whose association with forest hosts was nursery or glasshouse records only were excluded, as were stored product pests; timber-in-service pests were included. For species in Botryosphaeriaceae, we used Burgess et al. (2019), and for timber-decay fungi, we used Simpson (1996). Our final list comprised 260 established non-native forest pests: 143 arthropod species and 117 pathogens. Taxonomic names were checked for conformity to accepted names in the Global Biodiversity Information Facility (GBIF3) database.
The suite of non-native forest pests in Australia was considered in terms of each of the three post-arrival steps in the invasion process (establishment; spread; and impact—see Liebhold et al., 2016). For each pest, we determined the year of detection (as a proxy for establishment) in Australia. Where year of first detection was not detailed in the literature, we used the earliest date recorded in APPD. We acknowledge that this does not necessarily represent the actual year of establishment, due to lag times in detection (Kiritani and Yamamura, 2003; Aikio et al., 2010); we were unable to determine a first record year for 10 pest species (primarily timber decay fungi).
Accumulation of Non-native Species
The accumulation of non-native forest arthropods and pathogens was plotted according to year of detection, and used to test for differences in rate of establishments between taxa and time periods. The 1950s are often considered a turning point in invasion processes (Richardson, 2011), where air travel replaced sea travel (Hlasny and Livingston, 2008). We divided the dataset into “early” (≤1955) and “later” (>1955) invaders according to classification proposed by Paine et al. (2011) and Nahrung and Swain (2015). Chi-square tests were used to compare frequencies of insect and pathogen establishments between these time periods. Additionally, pre- and post-1990 accumulations were compared to test any impact of trade globalization, as the latter era showed acceleration in forest pests in Europe (Roques, 2007). Linear regressions were fitted to the data for each of these phases, and for pathogens and insects separately, and the difference between their slopes was compared (Soper, 2019).
Detections of non-native arthropods and pathogens were plotted separately by decade and fitted with a trendline whose fit was estimated using linear regression.
Native Range
The native range of 157 species and the broad global invasive distribution of each species (n = 260) [regions: New Zealand (=Oceania excluding Australia), South America, North America, Europe, Asia, Africa] was determined with assistance from literature and databases including CABI4, García Morales et al. (2016), and Farr and Rossman (2019). Higher level taxonomic composition was compared using Chi-square tests, and median Australian detection year was compared using Kruskall–Wallis test with post hoc pairwise comparisons (Bonferonni-adjusted for multiple comparisons).
Distribution in Australia
For the 248 species where information was available, the Australian state in which each species was first recorded was noted, and its current Australian distribution was given a count score representing the number of states recorded as present (1–7) (records from the Australian Capital Territory were included as New South Wales). These data were used to compare the arrival of species to each state to infer whether species represented inter-country or intra-country movement. For those that comprised a first Australian record, the native origin was plotted (although we acknowledge this does not necessarily represent the source of the invasive population), while those that represented species already present in Australia were considered as having spread. Frequencies were compared between states using Chi-square tests.
Global Distribution
Invasive global distribution for each species was scored between 0 (only non-native in Australia) and 5 (non-native in each global region); for species where the native range was unknown the invasive distribution score was determined by subtracting one from the global range score. Pair-wise chi-square tests were used to compare non-native species in common between Australia and other regions, and Spearman rank correlations were used to examine relationships between detection year and Australian and global distributions.
Host Range
Species were allocated to two groups: those for which > 50% of host genera were indigenous, and those for which > 50% of host genera were exotic. Each species was further given a rating on a 1–3 scale according to host range: (1) narrow (hosts restricted to 1–3 genera in the same family); (2) moderate (>four genera in one family or hosts in two to three families); (3) broad (hosts across > three families).
Impact
For each pest, we categorized their impact [1 (low), 2 (medium), 3 (high)], based on literature, unpublished data, and our own observations from > 25 years of forest health surveillance, and noted whether forestry was the primary industry affected. The impact rating was somewhat subjective, but considered economic, environmental, and social impact as well as management costs, based on our previous method (Carnegie and Nahrung, 2019) where low impact species were those where no intervention, management, or damage records were found; medium impact species had evidence of damage, management, or control but this was either short-term, localized, or minor; and high impact species were those that required ongoing management, and/or had significant economic or environmental effects recorded. Spearman rank correlations and Kruskal–Wallis test with pairwise comparisons were used to examine relationships between polyphagy scores and geographic distributions, establishment year, and impact scores.
Results
Non-native Species Database
Our list comprised 260 non-native species associated with forest genera in Australia since 1885: 143 arthropods and 117 pathogens (Pest List: Supplementary Table S2). Seventeen insects and two fungal species were detected prior to 1900 (Figure 1).
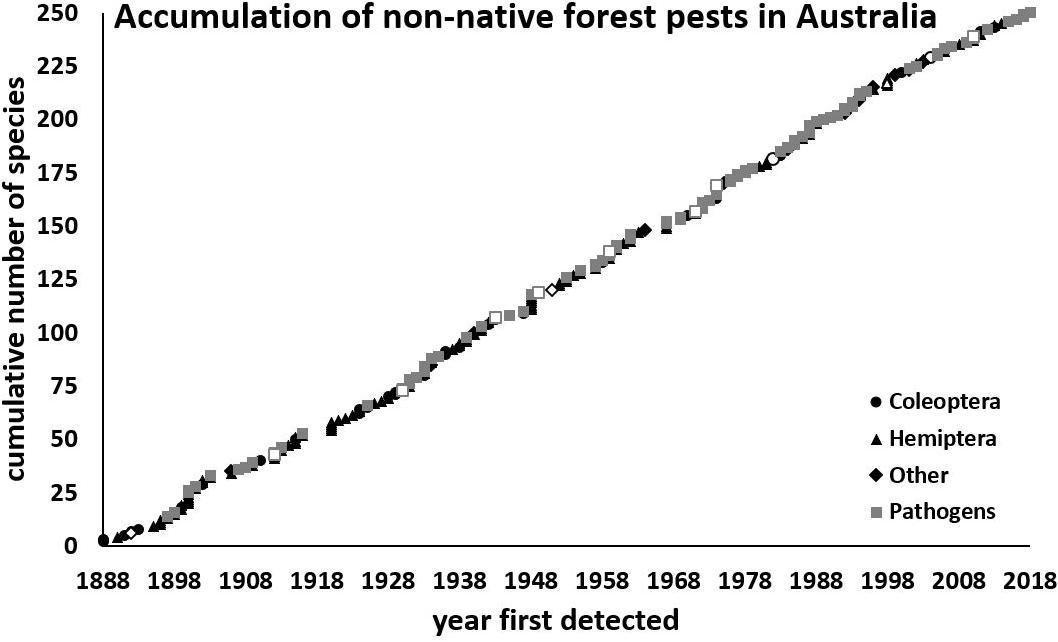
Figure 1. Accumulation of forest arthropods (black: Hemiptera = triangles; Coleoptera = circles; other = diamond) and pathogens (gray squares) established in Australia between 1885 and 2018. Those with high impact are in outline format.
Accumulation of Non-native Species
Overall, 1.9 ± SE 0.1 non-native species were detected per year, equally distributed among insects (1.1/year) and pathogens (0.9/year), and with no change in overall detection rates between early (1.9/year) (prior to 1955) and later (2.0/year) (from 1955 onward), or since 1990 (1.8/year) (t246 = 0.03, P = 0.98; t117 = 0.11, P = 0.91, respectively). Although an equivalent number of pests established before (52%) and after (48%) 1955 (χ21 = 0.26, P = 0.61), more arthropods established prior to 1955 and more pathogens after (χ21 = 26.7, P < 0.001) (Figure 2). Indeed, despite the overall accumulation (arthropods and pathogens combined) occurring at a statistically indistinguishable rate, fluctuations in detections per decade showed a decrease in the number of arthropods (linear regression, R2 = 0.53, P = 0.005), and an increase (R2 = 0.31, P = 0.048) in pathogens (Figure 2).
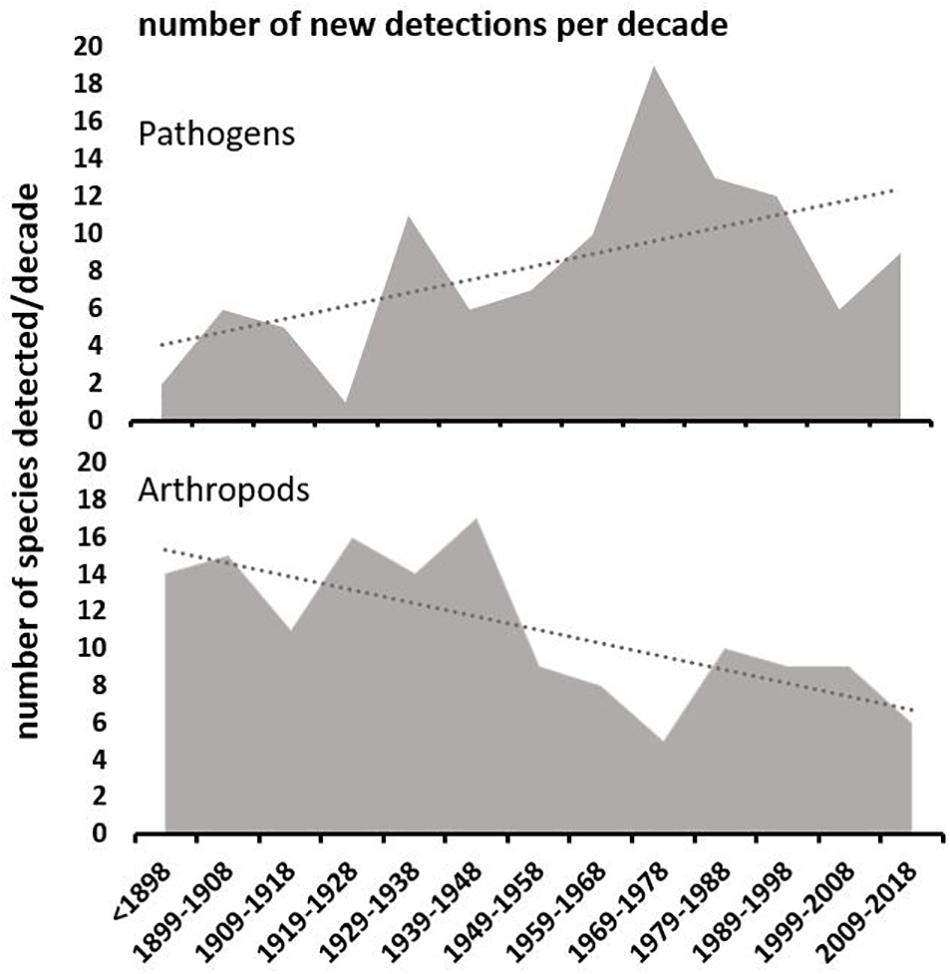
Figure 2. Number of new non-native forest pathogen and arthropod detections recorded each decade to 2018. The trendlines are significant at P < 0.05 (see text).
Native Range
The native range of 157 of Australia’s established non-native forest pests could be determined from literature and databases; 90 pathogens and 13 arthropods—largely cosmopolitan or holarctic species—were unable to be assigned a specific region of endemism so were excluded from this analysis. Europe (52) and Asia (52) each accounted for one-third of the regions of origin of non-native establishments in Australia (Supplementary Figure S1), with 44 arthropods and eight pathogens each.
The pests whose known native range was Europe and Asia (n = 104), while not differing in higher level taxonomic composition (χ21 = 0–2.2, P = 0.14–1) nor in median establishment year (1956 and 1939, respectively) (Kruskall–Wallis test, H6 = 21.2, P = 0.001—see post hoc results in Supplementary Figure S1), differed significantly in their host range, with a higher proportion of Asian-origin pests being highly polyphagous (73%) than European-origin pests (15%) (χ21 = 28.3, P < 0.001). European- and Asian-origin pests also differed in their geographic establishment range in Australia. Significantly more species (27) whose native range was Asia established in Queensland and the Northern Territory (subtropical to tropical climate) than species from Europe (3) (χ21 = 17.5, P < 0.006). Significantly more species (24) from Europe established in South Australia, Victoria, and Tasmania (Mediterranean to temperate) than species from Asia (4) (χ21 = 12.6, P < 0.001). There were no differences in native range for pests establishing in New South Wales (temperate to subtropical) (χ21 = 0.13, P = 0.73) or Western Australia (Mediterranean to tropical) (χ21 = 0.02, P = 0.88). While partially a reflection of first arrival [significantly more first detections in Queensland and Northern Territory were of pests native to Asia (χ21 = 4.2, P = 0.04) and significantly more first detections in Victoria, Tasmania, and South Australia were of pests native to Europe (χ21 = 12.6, P < 0.001)], for all states except New South Wales the majority of established pests arrived through interstate movement (or at least, were established elsewhere in Australia beforehand) (Figure 3).
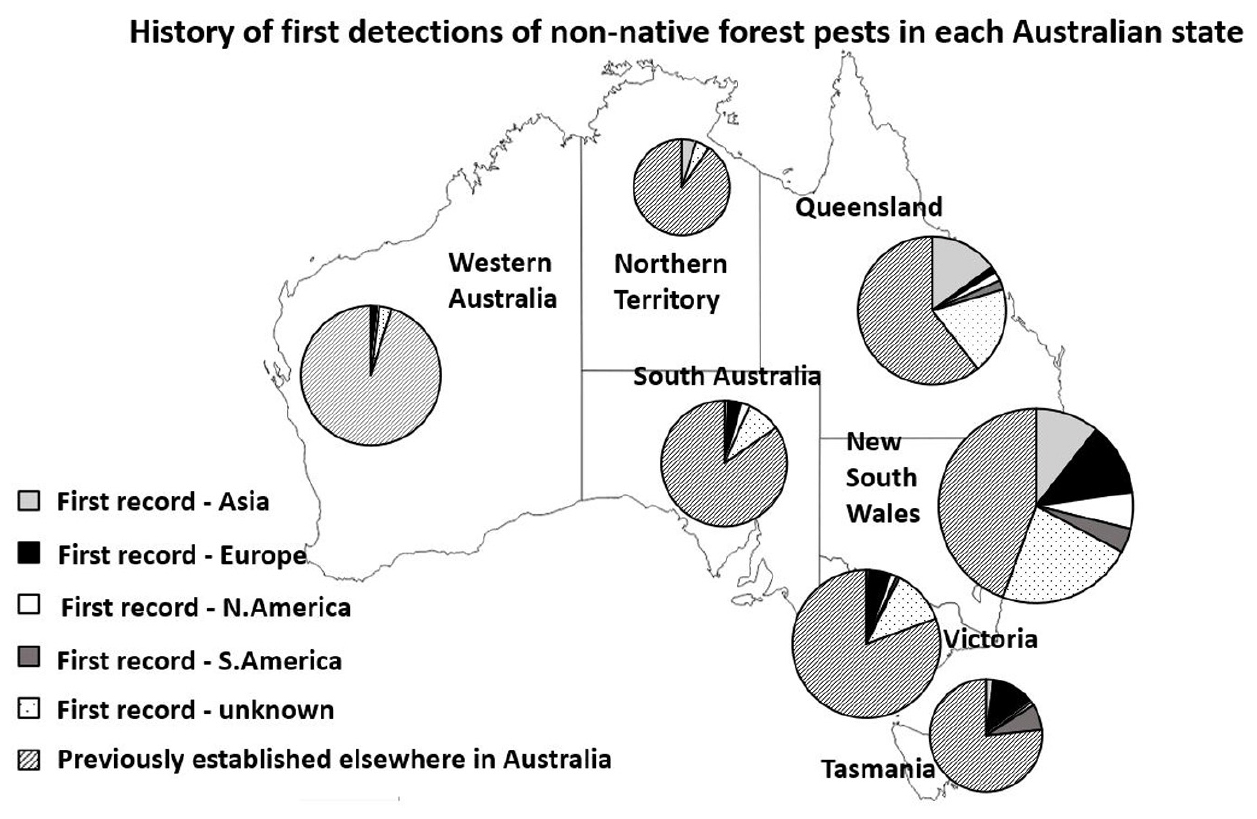
Figure 3. Non-native species established in each Australian state by first record status. Species’ first Australian establishment is designated according to native range: Asia (light gray), Europe (black), North America (white), South America (dark gray), and unknown/other origin (stippled). Species that established in each state following a first establishment elsewhere in Australia are striped.
Australian Distribution
Within Australia, ∼80% of established species have been recorded in more than one state, with 20% established in six to seven states countrywide, and 20% that have not been recorded outside the state they were first detected (Figure 4A). Almost half of all the non-native forest pests in Australia were first detected in New South Wales (Figure 4B). Pests detected in Queensland were significantly more likely to spread (88%) to at least one other state than pests detected in Tasmania (64%) (χ21 = 6.2, P = 0.01).
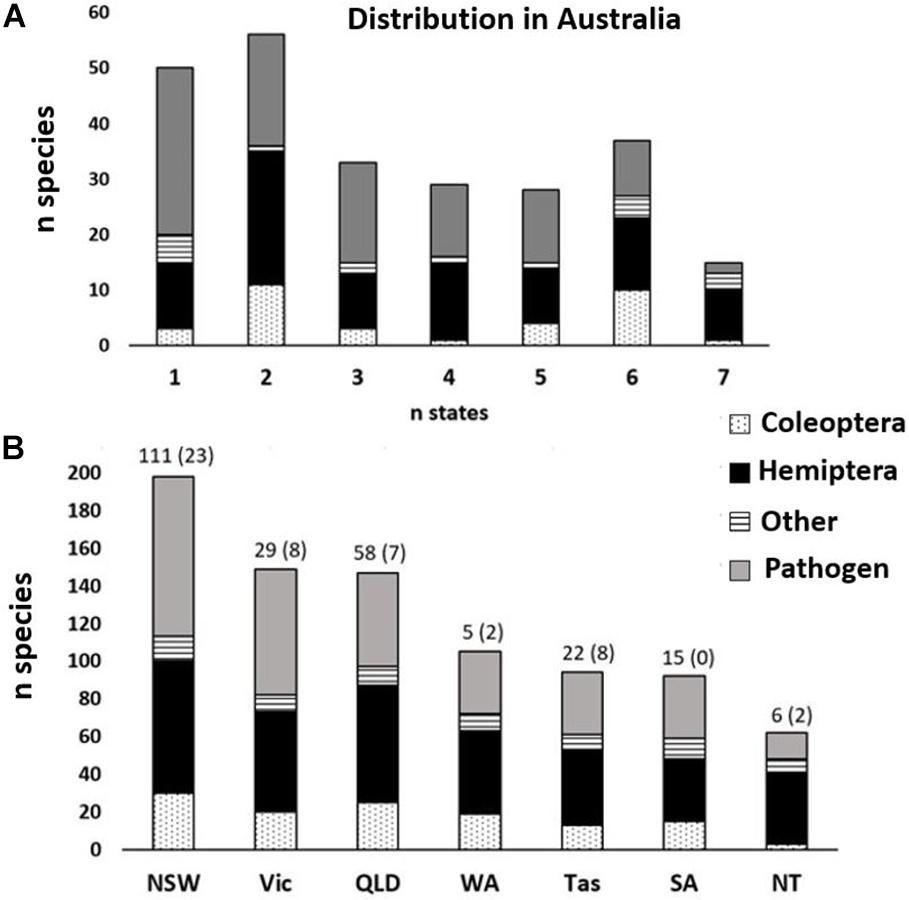
Figure 4. State-wide adventive distribution of non-native forest arthropod (Coleoptera = stippled; Hemiptera = black; other = striped) and pathogen (gray) species (n = 140) established in Australia by (A) number of states now present and (B) distribution within states. Numbers above the bars indicate the number of first detections in each state, with the number of species unique to that state (i.e., those that did not spread) in parentheses.
There was a significant negative correlation between detection year and the number of states a non-native species was present in (Spearman rank correlation, rho = −0.57, P < 0.001). Non-native pests with wider Australian geographic distributions had higher polyphagy scores (rho = 0.41, P < 0.001) and were of higher impact in forestry (rho = 0.29, P < 0.001).
Global Distribution
Overall, <5% of the 260 non-native forest species (12: six insects and six fungal species) in Australia are not recorded as invasive anywhere else (Figure 5A). Of these 12 species, five were detected in Australia only since 2000. There was a significant relationship between the number of global regions occupied and median detection year in Australia (linear regression, R2 = −0.93, P = 0.007) (Figure 5A). Pests with broader global distribution had higher polyphagy scores (Spearman rank correlation rho = 0.34, P < 0.001) and were detected earlier in Australia (Spearman correlation, rho = −0.38, P < 0.001). The largest numbers of forest species as non-natives in Australia were shared with North America (Figure 5B), with 75% of non-native species-in-common, significantly higher than the percentage of species-in-common between other regions (χ21 = 0.12–29.7, P < 0.001–0.73).
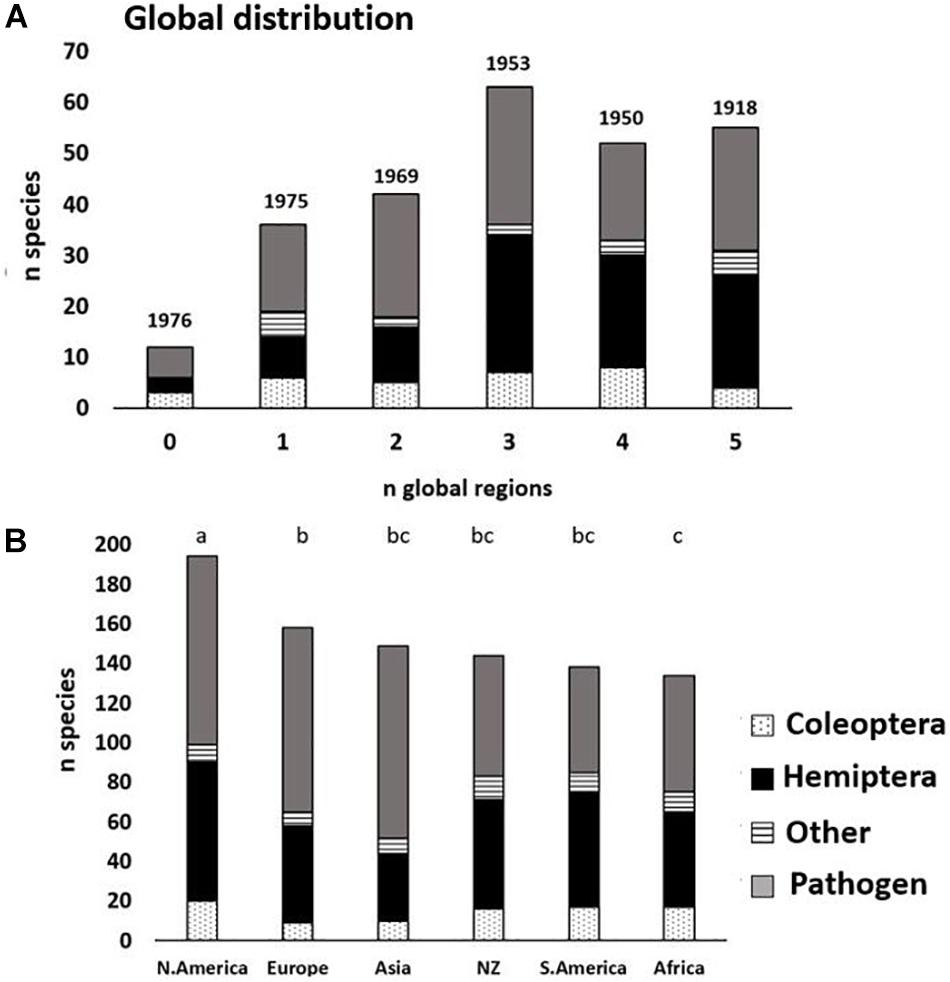
Figure 5. Invasive range score (number of global regions) for the non-native forest pests established in Australia (n = 260) (A); and their distribution as non-native species throughout these global regions (B) (Coleoptera = stippled; Hemiptera = black; other = striped) and pathogen (gray) species. Above bars is shown the median year of Australian establishment for the species in those global regions (A) and letters that designate significant differences in frequencies of species in common among global regions (B).
Host Range
Half (131/260) of the pests on our list were highly polyphagous, feeding on hosts across > three families, with 36 and 13% exhibiting narrow and moderate host range, respectively (Supplementary Figure S2A). Highly polyphagous species established earlier than oligophagous and specialist plant pests (Kruskall–Wallis test, H2 = 59.9, P < 0.001). Of the 260 non-native pests associated with indigenous and exotic trees, over 60% were exclusively forest pests, while less than 40% impacted other plant industries. Furthermore, all pest species with narrow host range were strictly forest related, opposed to polyphagous species, of which only 27% impacted forest systems. Over one-quarter of non-native pests in this study were associated with Pinus. The majority (87%) of non-native pests in Australia were associated with at least one species of non-native host plants, while about half were associated with at least one native host plant; those utilizing indigenous plants were more likely to be polyphagous than those feeding primarily on exotic hosts (Supplementary Figure S2B). There was no relationship between polyphagy and impact (rho = 0.006, P = 0.84), although 65% of high impact pests were restricted to confamilial hosts.
Impact
Overall, only 6.5% (17/260) of the non-native pests associated with trees in Australia are considered to cause high impact; of those that are primarily forest pest species (n = 159), 11% are high-impact. Overall, 18% of established non-native species (and 28% of those associated predominantly with forests) are considered of moderate to high impact.
Arthropods and pathogens were similarly represented among high-impact species (χ21 = 2.9, P = 0.09). Seventy-four percent of the 117 non-native forest pathogens are in the Ascomycota, of which 7% are considered high impact; Basidiomycota and Oomycota have a similar proportion of high impact species (Table 1).
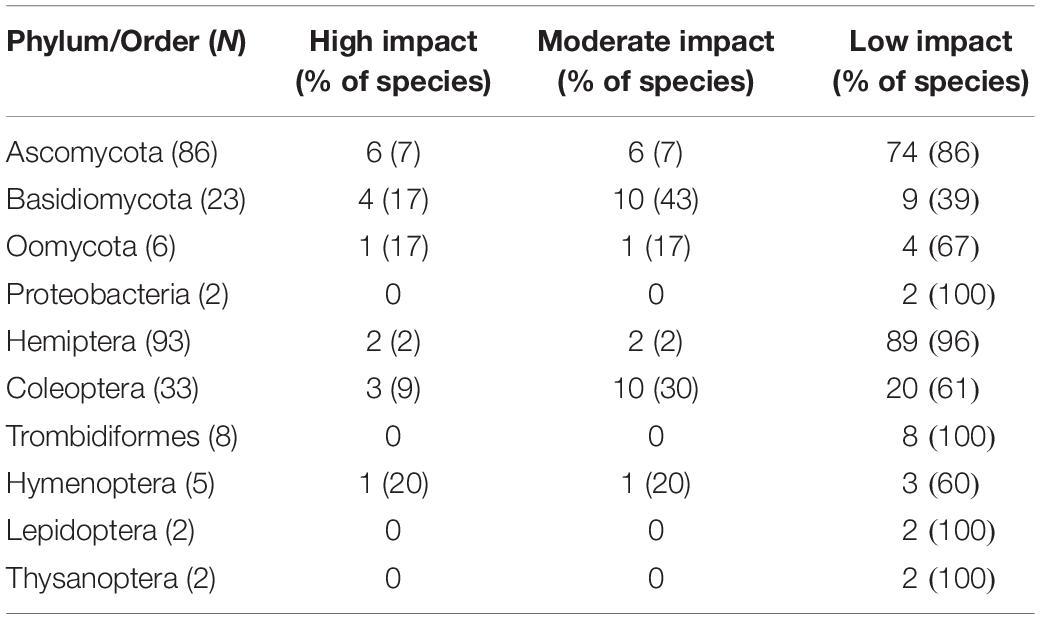
Table 1. Number (and %) of non-native forest pest species by Phylum (for pathogens) and Order (for arthropods) according to impact level in Australia.
Among arthropod pests, the majority (88%) are Hemiptera (93) and Coleoptera species (33), of which 13% cause high to moderate impact. All mites, Lepidoptera, and Thysanoptera are considered low impact (Table 1).
Two of the 17 high-impact pests are of timber-in-service, and with the exception of these and P. cinnamomi (Table 2), high impact pests were not highly polyphagous (i.e., host range > three families). Three high-impact pests in Australia established in the last 15 years.
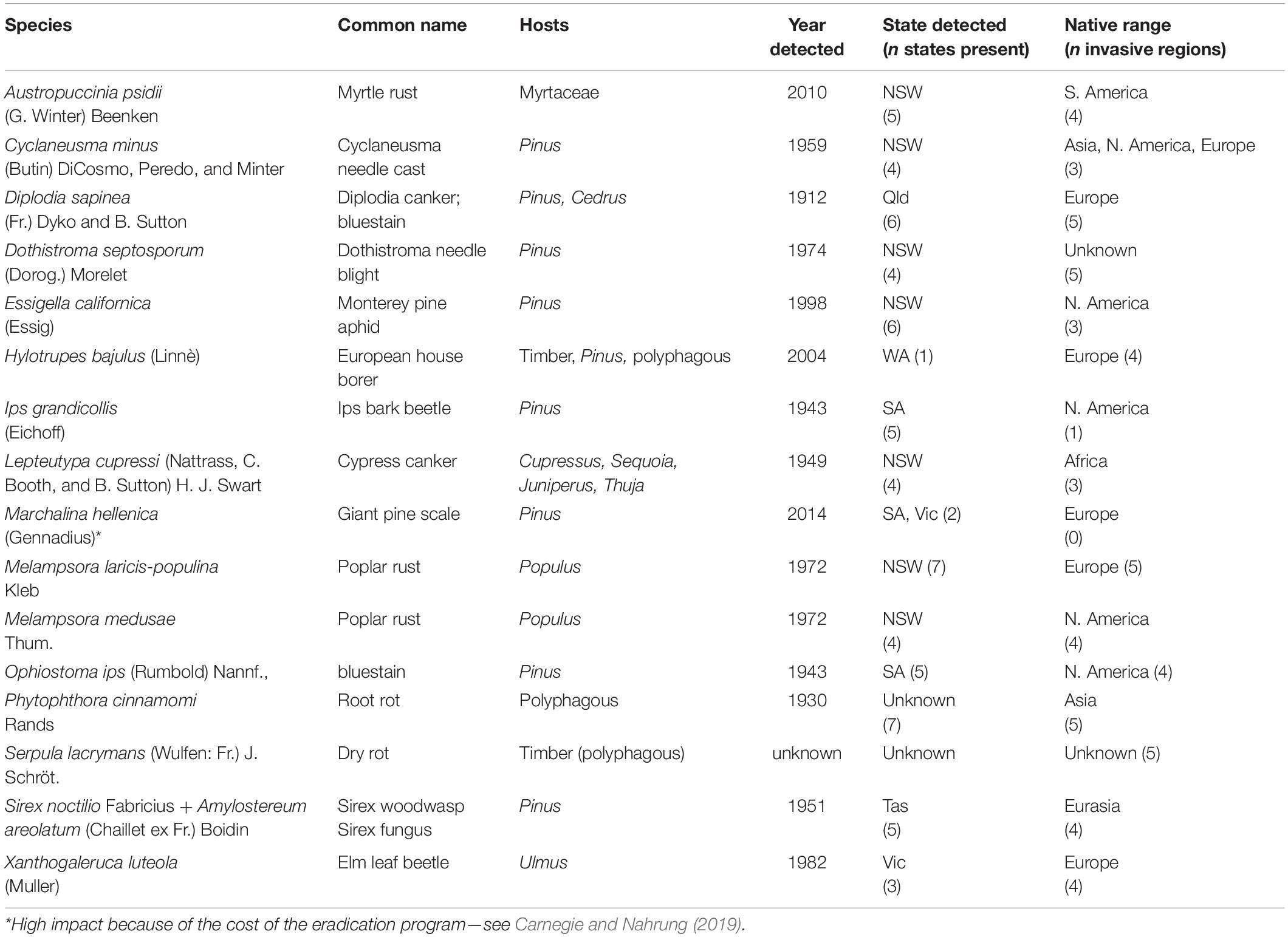
Table 2. High-impact non-native forest pests established in Australia, main hosts affected, year, and state (Tas = Tasmania, Qld = Queensland, NSW = New South Wales, SA = South Australia; Vic = Victoria) of first detection, number of Australian states now present, native range and number of global regions the pest is non-native (in addition to Australia).
Discussion
Australian forests accumulated approximately two non-native pest species per year since the 1880s, with almost one-fifth of them causing moderate to high impact. However, although the overall accumulation rates and numbers of arthropods and pathogens did not differ over the last 120 years, there was a downward trend in the number of arthropods detected over time, and an upward trend in the number of pathogens. For example, half of the arthropods were detected prior to 1940 and half of the pathogens after 1960.
New Zealand and United States also recorded linear establishment rates of exotic forest pests, with 0.5 and 2.5 exotic insect species establishing annually, respectively (calculated from Supplementary Data in Aukema et al., 2010; Brockerhoff and Liebhold, 2017). In contrast, Roques (2010a) and Santini et al. (2013) described exponential increases in accumulation of forest pathogens and phytophagous insects, respectively, in Europe.
A similar increase for New Zealand forestry pathogen arrivals was attributed in part to enhanced biosecurity (Sikes et al., 2018), as there is no evidence of saturation of non-native species accumulation worldwide (Bebber et al., 2014; Seebens et al., 2017). The linear trend of non-native introductions in Australia is surprising considering the exponential increases of trade and travel in the country5 and is contrary to the general perception of an upward trend in non-natives establishment in Australian forests (Stone and Carnegie, 2018). Within Australia, while significant biosecurity reviews (e.g., Nairn et al., 1996; Beale et al., 2008) and resultant policy changes may have dampened the effect of increased trade and travel, enhanced biosecurity awareness and surveillance (e.g., for forestry: Agriculture, Fisheries and Forestry Australia, 2000; Plant Health Australia, 2007, 2015; Carnegie et al., 2018a) may also have increased post-border detection rates. It is therefore difficult to measure the impact of these, although there has been a tendency toward lower detections, particularly of arthropods, in the last two decades (Figure 2). Further, the International Sanitary and Phytosanitary Measure 15 (ISPM 15) was designed to regulate the wood packaging material pathway, a major source of entry for forest pests, but showed uncertain benefit in the United States (Haack et al., 2014) and Australia (Lawson et al., 2018).
Native European and Asian species were equivalently represented numerically, temporally, and compositionally among Australia’s non-native forest pests, although the latter were more polyphagous and more frequently established in northern Australia. Similar origin-polyphagy patterns were described by Aukema et al. (2010), and possibly reflect the host range of species pools in the native range. The high number of species endemic to Europe probably reflects the deliberate or accidental introduction of their host plants to Australia since early European settlement (Pheloung, 2003), a notion supported by the higher degree of host plant specialization in pests originating there. However, although reflecting broad patterns of potential climatic and host suitability, the native range does not necessarily imply the direct origin of introduction to Australia: only 10 of the 157 species for which the native range is known are non-invasive in at least one other world region; thus, these invasive populations may themselves represent source populations for bridgehead introductions. For example, although native to Eurasia, Sirex woodwasp, Sirex noctilio, was accidentally introduced from New Zealand to Australia (Boissin et al., 2012).
Overall, >95% of the 260 non-native forest species in Australia are invasive species in at least one other global region. Of the 12 exceptions, five established in Australia only since 2000 so have a short and novel invasion history, with potential to become bridgehead populations. That almost all of Australia’s non-native forest species are invasive elsewhere supports the notion of “invasion begets invasion” (Bertelsmeier and Keller, 2018). Indeed, this may be further illustrated by the observation that the more global regions in which a non-native species was established, the earlier the species was detected in Australia, although whether Australia was the recipient or donor of bridgehead population invasion or colonized via the native range is unknown. Complex histories of introduction are increasingly recognized as contributing to global invasion processes (Garnas et al., 2016; Javal et al., 2019).
The species composition of Australia’s non-native forest pests were broadly reflected elsewhere: invasive pathogens in Europe were likewise predominantly Ascomycota (Santini et al., 2013), while Roques (2010b) identified the same top three arthropod Orders (Coleoptera, Hemiptera, and Trombidiformes) as invasive in Europe between 1995 and 2005. Aukema et al. (2010) and Brockerhoff and Liebhold (2017) reported similar compositional patterns in United States and New Zealand, respectively, with Hemiptera and Coleoptera comprising the highest invasive Orders—but with Lepidoptera much more highly represented among invasive forest species than in Australia. Lepidoptera comprise almost one-third of Australia’s high-priority exotic forestry insects (Department of Agriculture and Water Resources, 2018b), yet only two non-native forest species have established in Australia over the last 130 years—one of which is the broad agricultural pest Helicoverpa armigera, included here because it was recorded damaging pine and eucalypts by Elliott et al. (1998). The relative paucity of non-native Lepidoptera in Australia’s Mediterranean-climate forests was also noted by Nahrung et al. (2016) and may relate to host plant suitability, and phylogenetic similarity of Australian Lepidopteran species and their natural enemies to potential invaders (Nahrung et al., 2016).
New Zealand lists 39 non-native species of Lepidoptera of which 32 are native to Australia (Brockerhoff and Liebhold, 2017, Supplementary Material). New Zealand forests also accumulated ten non-native species of Cerambycidae (including a third Arhopalus species) over 175 years, eight of which originated from Australia (Sopow et al., 2015; Brockerhoff and Liebhold, 2017, Supplementary Material). Despite trans-Tasman trade, close geographic proximity, the high number of Australian native insects (Withers, 2001; Sopow et al., 2015; Brockerhoff and Liebhold, 2017) and pathogens (e.g., Dick, 1982, 1990) invasive in New Zealand forests, and the high number of shared non-native forest species (Figure 5), no pests native to New Zealand have established in Australian forests. Despite long-horned borers (Coleoptera: Cerambycidae) being the most highly intercepted forest pests in Australia (Lawson et al., 2018), with several species listed as high risk to Australian forests (Department of Agriculture and Water Resources, 2018b), only three forest-related species have established: European house borer (currently with restricted distribution and under containment), and two Arhopalus species (these latter among the 4% of non-natives that are not invasive elsewhere). All three of these are native to Europe, where one-third of all of Australia’s non-native forest pests originated.
Many invasive pests affecting native host plants in the Northern Hemisphere originated from related host plants in a similar biogeographical region (Brockerhoff and Liebhold, 2017). Much of Australia’s unique flora (e.g., Eucalyptus sensu lato, Brachychiton, Acacia) have few or no congeners on another continent. The biggest threat to eucalypt forests in Australia are pests that have host-shifted onto Australian eucalypts grown as exotics (Slippers et al., 2005; Paine et al., 2011), many of which have confamilial relatives where eucalypts are planted outside Australia (Burgess and Wingfield, 2017; Crous et al., 2017). Other potential threats to Australia are “new encounter” pathogens (Roux and Wingfield, 2009), and the potential for future new associations between insects, microorganisms, and tree hosts (Wingfield et al., 2010, 2017), as well as polyphagous insects that have host shifted onto eucalypts planted as exotics (Paine et al., 2011). Likewise, Australia’s Acacia forests are at risk from diseases that have either host shifted, or are new encounter diseases, onto Acacia grown as exotics (Wingfield et al., 2011).
One thing is certain: non-native forest pests will continue to establish in Australia, and some of these will severely impact the country’s forests. The database we produced, describing established non-native pests and their associated year of establishment, regions of endemism, forest hosts, and their broad Australian distribution provides the first comprehensive inventory of non-native forest pests in Australia. This expanded knowledge on biological invasions of Australian forests improves our understanding of pathways of forest invaders in the country thus allowing us to gauge upcoming risks related to accidental introductions. Alongside our earlier paper reviewing recent forest pest detections and responses (Carnegie and Nahrung, 2019), we add to recent work that has identified the risk of non-native pests entering Australia (Lawson et al., 2018), the economic impact of non-native pests establishing (Cameron et al., 2018; Carnegie et al., 2018b), and the strengths, weaknesses, and opportunities of Australia’s biosecurity system with respect to forestry (Tovar et al., 2016; Carnegie et al., 2018a; Carnegie and Nahrung, 2019), which builds on previous work (Mohammed et al., 2011) aimed at engaging the forest industry and illustrating the benefits of biosecurity. This has led to the development of the National Forest Biosecurity Surveillance Strategy (Department of Agriculture and Water Resources, 2018b) and its Implementation Plan (Department of Agriculture and Water Resources, 2018a), both of which if funded will significantly enhance Australia’s capacity to detect and respond to future pest threats. The National Forest Biosecurity Surveillance Strategy aims to increase capacity and capability, and combined with improved collaboration between government and industry, will ensure Australia is better able to respond to exotic pest incursions, thus increasing the chance of eradicating those deemed to be potential high impact pests.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
HN and AC jointly conceived the study and compiled arthropod and pathogen records, respectively. HN prepared figures and results. Both authors wrote and edited the manuscript.
Funding
HN was funded by an Advance Queensland Fellowship through the Queensland Department of Innovation and Tourism Industry Development supported by the University of the Sunshine Coast, Queensland Department of Agriculture and Fisheries, Forest and Wood Products Australia, HQPlantations Pty Ltd., Plant Health Australia, and the National Sirex Coordination Committee. AC acknowledges support from Forestry Corporation of NSW.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Treena Burgess (Murdoch University) for advice on Phytophthora and Bostryosphaeriaceae, and Simon Lawson (USC), Tim Wardlaw (University of Tasmania), and Francisco Tovar (Plant Health Australia) for helpful discussion and comments on an earlier draft.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2020.00037/full#supplementary-material
FIGURE S1 | Native range of non-native insect (Coleoptera = stippled; Hemiptera = black; other = striped) and fungal (gray) species established in Australia. Median year of detection from each region is also presented, with regions sharing same letters not differing significantly.
FIGURE S2 | (A) Polyphagy (narrow host range, moderate host range, and broad host range) of non-native forest insect (Coleoptera = stippled; Hemiptera = black; other = striped) and pathogen (gray) species established in Australia. Median establishment year for each group is above the bars. (B) distribution of polyphagy (narrow, moderate, broad host range) among species (n = 221) whose hosts are largely native (black) or largely exotic (gray).
TABLE S1 | The list of 60 plantation, native, and amenity tree species used to generate the pest list.
TABLE S2 | The list of non-native pest scientific and common names, and their year and state of first record, forest host genera, native range, and Australian and global invasive range distribution.
Footnotes
- ^ www.planthealthaustralia.com.au
- ^ https://au.fsc.org/en-au/buy-fsc-certified/timber/timber-species-grown-in-australia
- ^ www.gbif.org
- ^ https://www.cabi.org/isc
- ^ https://dfat.gov.au
References
ABARES (2018). Montreal Process Implementation Group for Australia and National Forest Inventory Steering Committee, 2018, Australia’s State of the Forests Report 2018. Canberra: ABARES.
Agriculture, Fisheries and Forestry Australia (2000). Forests and Timber: A Field Guide to Exotic Pests and Diseases. Canberra: Commonwealth of Australia.
Aikio, S., Duncan, R. P., and Hulme, P. E. (2010). Lag-phases in alien plant invasions: separating the facts from the artefacts. Oikos 119, 370–378. doi: 10.1111/j.1600-0706.2009.17963.x
Anderson, C., Low-Choy, S., Whittle, P., Taylor, S., Gambley, C., Smith, L., et al. (2017). Australian plant biosecurity surveillance systems. Crop Protect. 100, 8–20. doi: 10.1016/j.cropro.2017.05.023
Aukema, J. E., Leung, B., Kovacs, K., Chivers, C., Britton, K. O., Englin, J., et al. (2011). Economic impact of non-native forest insects in the continental United States. PLoS One 6:e24587. doi: 10.1371/journal.pone.0024587
Aukema, J. E., McCullough, D. G., von Holle, B., Liebhold, A. M., Britton, K., and Frankel, S. J. (2010). Historical accumulation of nonindigenous forest pests in the continental United States. Bioscience 60, 886–897. doi: 10.1525/bio.2010.60.11.5
Beale, R., Fairbrother, J., Inglis, A., and Trebeck, D. (2008). One Biosecurity: A Working Partnership. The Independent Review of Australia’s Quarantine and Biosecurity Arrangements. Canberra: Commonwealth of Australia.
Bebber, D. P., Holmes, T., and Gurr, S. J. (2014). The global spread of crop pests and pathogens. Glob. Ecol. Biogeogr. 23, 1398–1407. doi: 10.1111/geb.12214
Bertelsmeier, C., and Keller, L. (2018). Bridgehead effects and role of adaptive evolution in invasive populations. Trends Ecol. Evol. 33, 527–534. doi: 10.1016/j.tree.2018.04.014
Boissin, E., Hurley, B., Wingfield, M. J., Vasaitis, R., Stenlid, J., Davis, C., et al. (2012). Retracing the routes of introduction of invasive species: the case of the Sirex noctilio woodwasp. Mol. Ecol. 21, 5728–5744. doi: 10.1111/mec.12065
Branco, M., Nunes, P., Roques, A., Fernandes, M. R., Orazio, C., and Jactel, H. (2019). Urban trees facilitate the establishment of non-native forest insects. NeoBiota 52, 25–46. doi: 10.3897/neobiota.52.36358
Brockerhoff, E. G., and Liebhold, A. M. (2017). Ecology of forest insect invasions. Biol. Invasions 19, 3141–3159. doi: 10.1007/s10530-017-1514-1
Browne, F. G. (1968). Pests and Diseases of Forest Plantation Trees: An Annotated List of the Principal Species Occurring in the British Commonwealth. Oxford: Oxford University Press.
Burgess, T. I., Tan, Y. P., Garnas, J., Edwards, J., Scarlett, K. A., Shuttleworth, R. D., et al. (2019). Current status of the Botryosphaeriaceae in Australia. Austra. Plant Pathol. 48, 35–44. doi: 10.1007/s13313-018-0577-5
Burgess, T. I., and Wingfield, M. J. (2017). Pathogens on the move: a 100-year global experiment with planted eucalypts. BioScience 67, 14–25. doi: 10.1093/biosci/biw146
Cahill, D. M., Rookes, J. E., Wilson, B. A., Gibson, L., and McDougall, K. L. (2008). Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Austral. J. Bot. 56, 279–310.
Cameron, N. L., Carnegie, A. J., Wardlaw, T., Lawson, S. A., and Venn, T. (2018). An economic evaluation of sirex wood wasp (Sirex noctilio) control in Australian pine plantations. Austral. For. 81, 37–45. doi: 10.1080/00049158.2018.1430436
Carnegie, A. J., Kathuria, A., Pegg, G. S., Entwistle, P., Nagel, M., and Giblin, F. R. (2016). Impact of the invasive rust Puccinia psidii (myrtle rust) on native Myrtaceae in natural ecosystems in Australia. Biol. Invasions 18, 127–144. doi: 10.1007/s10530-015-0996-y
Carnegie, A. J., Lawson, S., Cameron, N., Wardlaw, T., and Venn, T. (2017). Evaluating the Costs and Benefits of Managing new and Existing Biosecurity Threats to Australia’s Plantation Industry. Melbourne: Forest and Wood Products Australia.
Carnegie, A. J., Lawson, S., Wardlaw, T., Cameron, N., and Venn, T. (2018a). Benchmarking forest health surveillance and biosecurity activities for managing Australia’s exotic forest pest and pathogen risks. Austral. For. 81, 14–23. doi: 10.1080/00049158.2018.1433271
Carnegie, A. J., and Nahrung, H. F. (2019). Post-Border forest biosecurity in Australia: response to recent exotic detections, current surveillance and ongoing needs. Forests 10:336. doi: 10.3390/f10040336
Carnegie, A. J., and Pegg, G. S. (2018). Lessons from the incursion of myrtle rust in Australia. Annu. Rev. Phytopathol. 56, 457–478. doi: 10.1146/annurev-phyto-080516-035256
Carnegie, A. J., Venn, T., Lawson, S. A., Nagel, M., Wardlaw, T., Cameron, N. L., et al. (2018b). An analysis of pest risk and potential economic impact of pine wilt disease to Pinus plantations in Australia. Austral. For. 81, 24–36. doi: 10.1080/00049158.2018.1440467
Crous, C. J., Burgess, T. I., Le Roux, J. J., Richardson, D. M., Slippers, B., and Wingfield, M. J. (2017). Ecological disequilibrium drives insect pest and pathogen accumulation in non-native trees. AoB Plants [Epub ahead of print].
Department of Agriculture and Department of Environment (2014). Available at: https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Environment_and_Communications/biosecurity/Submissions
Department of Agriculture and Water Resources (2015). Final Review of Policy: Importation of Phytophthora Ramorum Host Propagative Material into Australia. Canberra: Department of Agriculture and Water Resources.
Department of Agriculture and Water Resources (2018a). National Forest Biosecurity Surveillance Strategy: Implementation Plan. Canberra: Plant Health Australia.
Department of Agriculture and Water Resources (2018b). National Plant Biosecurity Surveillance Strategy 2018-2023. Canberra: Plant Health Australia.
Dick, M. (1982). Leaf-inhabiting fungi of eucalypts in New Zealand. New Zealand J. For. Sci. 12, 525–537.
Dick, M. (1990). Leaf-inhabiting fungi of eucalypts in New Zealand. II. New Zeal. J. For. Sci. 20, 65–74.
Edney-Browne, E., Brockerhoff, E. G., and Ward, D. E. (2018). Establishment patterns of non-native insects in New Zealand. Biol. Invasions 20, 1657–1669. doi: 10.1007/s10530-017-1652-5
Elliott, H. J., and deLittle, D. W. (1985). Insect Pests of Trees and TIMBER in Tasmania. Edinburgh: Forestry Commission.
Elliott, H. J., Ohmart, C. P., and Wylie, F. R. (1998). Insect Pests of Australian Forests: Ecology and Management. Melbourne: Inkata Press.
Eschen, R., Britton, K., Brockerhoff, E., Burgess, T., Dalley, V., Epanchin-Niell, R. S., et al. (2015). International variation in phytosanitary legislation and regulations governing importation of plants for planting. Environ. Sci. Policy 51, 228–237. doi: 10.1016/j.envsci.2015.04.021
Farr, D. F., and Rossman, A. Y. (2019). Fungal Databases, U.S. National Fungus Collections. Washington, DC: USDA.
Fisher, M. C., Henk, D. A., Briggs, C. J., Brownstein, J. S., Madoff, L. C., McCraw, S. L., et al. (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. doi: 10.1038/nature10947
García Morales, M., Denno, B. D., Miller, D. R., Miller, G. L., Ben-Dov, Y., and Hardy, N. B. (2016). ScaleNet: a literature-based model of scale insect biology and systematics. Database 2016:bav118. doi: 10.1093/database/bav118
Garnas, J. R., Auger-Rozenberg, M. A., Roques, A., Bertelsmeier, C., Wingfield, M. J., Saccaggi, D. L., et al. (2016). Complex patterns of global spread in invasive insects: eco-evolutionary and management consequences. Biol. Invasions 18, 935–952. doi: 10.1007/s10530-016-1082-9
Gibbs, J. N. (1978). Intercontinental epidemiology of Dutch elm disease. Annu. Rev. Phytopathol. 16, 287–307. doi: 10.1146/annurev.py.16.090178.001443
Graziosi, I., Tembo, M., Kuate, J., and Muchugi, A. (2019). Pests and diseases of trees in Africa: a growing continental emergency. Plants People Planet 2, 14–28. doi: 10.1002/ppp3.31
Haack, R. A., Britton, K. O., Brockerhoff, E. G., Cavey, J. F., Garrett, L. J., and Kimberley, et al. (2014). Effectiveness of the International phytosanitary standard ISPM No. 15 on reducing wood borer infestation rates in wood packaging material entering the United States. PLoS One 14:e96611. doi: 10.1371/journal.pone.0096611
Haack, R. A., Hérard, F., Sun, J., and Turgeon, J. J. (2009). Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: a worldwide perspective. Annu. Rev. Entomol. 55, 521–546. doi: 10.1146/annurev-ento-112408-085427
Herms, D. A., and McCullough, D. G. (2014). Emerald ash borer invasion in North America: history, biology, impacts, and management. Annu. Rev. Entomol. 59, 13–30. doi: 10.1146/annurev-ento-011613-162051
Hlasny, V., and Livingston, M. J. (2008). Economic determinants of invasion and discovery of nonindigenous insects. J. Agric. Appl. Econ. 40, 37–52. doi: 10.1017/s1074070800027966
Holmes, T. P., Aukema, J. E., Von Holle, B., Liebhold, A., and Sills, E. (2009). Economic impacts of invasive species in forest past, present, and future. Ann. NY Acad. Sci. 1162, 18–38. doi: 10.1111/j.1749-6632.2009.04446.x
Hulme, P. E. (2009). Trade, transport and trouble: managing invasive species pathways in an era of globalization. J. Appl. Ecol. 46, 10–18. doi: 10.1111/j.1365-2664.2008.01600.x
Hurley, B. P., Garnas, J., Wingfield, M. J., Branco, M., Richardson, D. M., and Slippers, B. (2016). Increasing numbers and intercontinental spread of invasive insects on eucalypts. Biol. Invasions 18, 921–933. doi: 10.1007/s10530-016-1081-x
Jacobs, B., Mikailovich, N., and Delaney, C. (2014). Benchmarking Australia’s urban tree canopy: an i-tree assessment, prepared for Horticulture Australia Limited. Sydney: Sydney University of Technology.
Javal, M., Lombaert, E., Tsykun, T., Courtin, C., Kerdelhué, C., Prospero, S., et al. (2019). Deciphering the worldwide invasion of the Asian long-horned beetle: a recurrent invasion process from the native area together with a bridgehead effect. Mol. Ecol. 28, 951–967. doi: 10.1111/mec.15030
Kiritani, K., and Yamamura, K. (2003). Exotic Insects and their Pathways for Invasion. Invasive Species: Vectors and Management Strategies. Washington, DC: Island Press.
Lawson, S. A., Carnegie, A. J., Cameron, N. L., Wardlaw, T., and Venn, T. (2018). Risk of exotic pests to the Australian forest industry. Austral. For. 81, 3–13. doi: 10.1080/00049158.2018.1433119
Levine, J. M., and D’antonio, C. M. (2003). Forecasting biological invasions with increasing international trade. Conserv. Biol. 17, 322–326. doi: 10.1046/j.1523-1739.2003.02038.x
Liebhold, A. M., Berec, L., Brockerhoff, E. G., Epanchin-Niell, R. S., Hastings, A., Herms, D. A., et al. (2016). Eradication of invading insect populations: from concepts to applications. Annu. Rev. Entomol. 61, 335–352. doi: 10.1146/annurev-ento-010715-023809
Liebhold, A. M., Brockerhoff, E. G., Garrett, L. J., Parke, J. L., and Britton, K. O. (2012). Live plant imports: the major pathway for forest insect and pathogen invasions of the US. Front. Ecol. Environ. 10, 135–143. doi: 10.1890/110198
Liebhold, A. M., Brockerhoff, E. G., Kalisz, S., Nuñez, M. A., Wardle, D. A., and Wingfield, M. J. (2017). Biological invasions in forest ecosystems. Biol. Invasions 11, 3437–3458. doi: 10.1007/s10530-017-1458-5
Loo, J. A. (2009). Ecological impacts of non-indigenous invasive fungi as forest pathogens. Biol. Invasions 11, 81–96. doi: 10.1007/978-1-4020-9680-8_6
Lovett, G. M., Weiss, M., Liebhold, A. M., Holmes, T. P., Leung, B., Lambbert, K. F., et al. (2016). Non-native forest insects and pathogens in the United States: impacts and policy options. Ecol. Appl. 26, 1437–1455. doi: 10.1890/15-1176
Marks, G. C., Fuhrer, B. A., and Walters, N. E. M. (1982). “Tree diseases in Victoria,” in Forests Commission Victoria, Handbook No. 1, ed. M. L. Huebner (Melbourne: Forests Commission).
Meurisse, N., Rassati, D., Hurley, B. P., Brockerhoff, E. G., and Haack, R. A. (2018). Common pathways by which non-native forest insects move internationally and domestically. J. Pest Sci. 92, 13–27. doi: 10.1007/s10340-018-0990-0
Mohammed, C., Glen, M., Walshe, T., Wardlaw, T., Stone, C., Beadle, C., et al. (2011). An Audit of Forest Biosecurity Arrangements and Preparedness in Australia. Melbourne: Forests and Wood Products Australia. FWPA Report PNC159-0910.
Nahrung, H. F., Loch, A. D., and Matsuki, M. (2016). “Invasive insects in mediterranean forest systems: Australia,” in Insects and Diseases of Mediterranean Forest Systems, eds T. Paine and F. Lieutier (Cham: Springer), 431–454.
Nahrung, H. F., and Swain, A. J. (2015). Strangers in a strange land: do coloniser traits in novel environments differ between aliens and natives? Biol. Invasions 17, 699–709. doi: 10.1007/s10530-014-0761-7
Nairn, M. E., Allen, P. G., Inglis, A. R., and Tanner, C. (1996). Australian Quarantine: A Shared Responsibility. Canberra: Department of Primary Industries and Energy.
Neumann, F. G., and Marks, G. C. (1976). A synopsis of important pests and diseases in Australian forests and forest nurseries. Austral. For. 39, 83–102. doi: 10.1080/00049158.1976.10675644
Paap, T., Burgess, T. I., and Wingfield, M. J. (2017). Urban trees: bridgeheads for forest pest invasions and sentinels for early detection. Biol. Invasions 19, 3515–3526. doi: 10.1007/s10530-017-1595-x
Paine, T. D., Millar, J. G., and Daane, K. M. (2010). Accumulation of pest insects on eucalyptus in California: random process or smoking gun. J. Econ. Entomol. 103, 1943–1949. doi: 10.1603/ec10214
Paine, T. D., Steinbauer, M. J., and Lawson, S. A. (2011). Native and exotic pests of Eucalyptus: a worldwide perspective. Annu. Rev. Entomol. 56, 181–201. doi: 10.1146/annurev-ento-120709-144817
Paini, D. R., Sheppard, A. W., Cook, D. C., De Barro, P. J., Worner, S. P., and Thomas, M. B. (2016). Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. U.S.A. 113, 7575–7579. doi: 10.1073/pnas.1602205113
Palm, M. E., and Rossman, A. Y. (2003). “Invasion pathways of terrestrial plant-inhabiting fungi,” in Invasive Species: Vectors and Management Strategies, eds G. M. Ruiz and J. T. Carlton (Washington, DC: Island Press), 31–43.
Pheloung, P. (2003). ““An Australian perspective on the management of pathways for invasive species”,” in Invasive Species: Vectors and Management Strategies, eds G. M. Ruiz and J. T. Carlton (Washington, DC: Island Press), 249–269.
Plant Health Australia (2007). Plantation Forest Biosecurity Plan, Version 1.0. Canberra: Plant Health Australia.
Plant Health Australia (2014). National Plant Biosecurity Status Report 2013. Canberra: Plant Health Australia.
Plant Health Australia (2015). Biosecurity Manual for the Plantation Timber Industry. Canberra: Plant Health Australia.
Plant Health Australia (2016). PLANTPLAN: Australian Emergency Plant Pest Response Plan – Version 3.0. Canberra: Plant Health Australia.
Richardson, D. M. (2011). Fifty Years of Invasion Ecology: the Legacy of Charles Elton. Hoboken, NJ: Wiley-Blackwell, West Sussex.
Roques, A. (2007). “Old and new pathways for invasion of exotic forest insects in Europe,” in Alien Invasive Species and International Trade, eds H. Evans and T. Oszako (Warsaw: Forest Research Institute), 80–88.
Roques, A. (2010a). Alien forest insects in a warmer world and a globalised economy: impacts of changes in trade, tourism and climate on forest biosecurity. New Zeal. J. For. Sci. 40(Suppl.), S77–S94.
Roux, J., and Wingfield, M. J. (2009). Ceratocystis species: emerging pathogens of non-native plantations Eucalyptus and Acacia species. South. For. 71, 115–120.
Ruiz, G. M., and Carlton, J. T. (eds) (2003). Invasive Species: Vectors and Management Strategies. Washington, DC: Island Press.
Santini, A., Ghelardini, L., De Pace, C., Desprez-Loustau, M.-L., Capretti, P., Chandelier, A., et al. (2013). Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 197, 238–250. doi: 10.1111/j.1469-8137.2012.04364.x
Santini, A., Liebhold, A., Migliorini, D., and Woodward, S. (2018). Tracing the role of human civilization in the globalization of plant pathogens. ISME J. 12, 647–652. doi: 10.1038/s41396-017-0013-9
Seebens, H., Blackburn, T. M., Dyer, E. E., Genovesi, P., Hulme, P. E., Jeschke, J. M., et al. (2017). No saturation in the accumulation of alien species worldwide. Nat. Commun. 8:14435.
Sikes, B. A., Bufford, J. L., Hulme, P. E., Cooper, J. A., Johnston, P. R., and Duncan, R. P. (2018). Import volumes and biosecurity interventions shape the arrival rate of fungal pathogens. PLoS Biol. 16:e2006025. doi: 10.1371/journal.pbio.2006025
Simpson, J. A. (2006). “Wood decay fungi,” in Fungi of Australia Vol. 1B, eds K. Mallett and C. Grgurinovic (Canberra: Australian Biological Resources Study), 95–128. Biological Resources Study, Canberra), 95-136. (already in refs)
Slippers, B., Stenlid, J., and Wingfield, M. J. (2005). Emerging pathogens: fungal host jumps following anthropogenic introduction. Trends Ecol. Evol. 20, 420–421. doi: 10.1016/j.tree.2005.05.002
Smith, R. M., Baker, R. H. A., Malumphy, C. P., Hockland, S., Hammon, R. P., Ostojá-Starzewski, J. C., et al. (2007). Recent non-native invertebrate plant pest establishments in Great Britain: origins, pathways, and trends. Agric. For. Entomol. 9, 307–326. doi: 10.1111/j.1461-9563.2007.00349.x
Soper D. S. (2019). Significance of the Difference between Two Slopes Calculator [Software]. Available at: http://www.danielsoper.com/statcalc doi: 10.1111/j.1461-9563.2007.00349.x (accessed March 8, 2019).
Sopow, S., Gresham, B., and Bain, J. (2015). Exotic longhorn beetles (Coleoptera: Cerambycidae) established in New Zealand. New Zeal. Entomol. 38, 107–125. doi: 10.1080/00779962.2014.993798
Stone, C., and Carnegie, A. (2018). The Australian forest industry takes a lead role in reducing the risk from exotic pests and pathogens. Austral. For. 81, 1–2. doi: 10.1080/00049158.2018.1425965
Tobin, P. C. (2015). Ecological consequences of pathogen and insect invasions. Curr. For. Rep. 1, 25–32. doi: 10.1007/s40725-015-0008-6
Tovar, F., and Carnegie, A. J. (2019). A forest biosecurity program. Austral. Plant Conserv. 27, 27–30.
Tovar, F., Carnegie, A. J., Collins, S., Horwood, M., Lawson, S., Smith, D., et al. (2016). Framework for National Biosecurity Surveillance of Exotic Forest Pests. Canberra: Department of Agriculture and Water Resources.
Wardlaw, T., Cameron, N., Carnegie, A. J., Lawson, S. A., and Venn, T. (2018). Costs and benefits of the leaf beetle integrated pest management. I. Modelling wood volume outcomes from the IPM. Austral. For. 81, 46–52. doi: 10.1080/00049158.2018.1425969
Wingfield, M. J., Garnas, J. R., Hajek, A., Hurley, B. P., and de Beer, Z. W. (2017). Novel and co-evolved associations between insects and microorganisms as drivers of forest pestilence. Biol. Invasions 18, 1045–1056. doi: 10.1007/s10530-016-1084-7
Wingfield, M. J., Roux, J., and Wingfield, B. D. (2011). Insect pests and pathogens of Australian acacias grown as non-natives – An experiment in biogeography with far-reaching consequences. Divers. Distrib. 17, 968–977. doi: 10.1111/j.1472-4642.2011.00786.x
Wingfield, M. J., Slippers, B., and Wingfield, B. D. (2010). Novel associations between pathogens, insects and tree species threaten world forests. New Zeal. J. For. Sci. 40(Suppl.), S95–S103.
Withers, T. M. (2001). Colonization of eucalypts in New Zealand by Australian insects. Austral. Ecol. 5, 467–476. doi: 10.1046/j.1442-9993.2001.01140.x
Xu, H., Qiang, S., Han, Z., Guo, J., Huang, Z., Sun, H., et al. (2006). The status and causes of alien species invasion in China. Biodivers. Conserv. 15, 2893–2904. doi: 10.1007/s10531-005-2575-5
Keywords: invasive pests, biosecurity, impacts, biological invasions, exotic pests, alien species
Citation: Nahrung HF and Carnegie AJ (2020) Non-native Forest Insects and Pathogens in Australia: Establishment, Spread, and Impact. Front. For. Glob. Change 3:37. doi: 10.3389/ffgc.2020.00037
Received: 12 August 2019; Accepted: 10 March 2020;
Published: 01 April 2020.
Edited by:
Alberto Santini, Italian National Research Council, ItalyReviewed by:
Ignazio Graziosi, Innovation Pole of Genomics, Genetics and Biology (Polo GGB), ItalyNicolas Meurisse, New Zealand Forest Research Institute Limited (Scion), New Zealand
Michael John Wingfield, University of Pretoria, South Africa
Copyright © 2020 Nahrung and Carnegie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helen F. Nahrung, hnahrung@usc.edu.au
 Helen F. Nahrung
Helen F. Nahrung Angus J. Carnegie
Angus J. Carnegie