
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. For. Glob. Change, 20 February 2020
Sec. Forest Soils
Volume 3 - 2020 | https://doi.org/10.3389/ffgc.2020.00016
This article is part of the Research TopicCarbon-Nitrogen-Phosphorus Coupling in ForestsView all 6 articles
Forest ecosystems are subjected to global change drivers worldwide, such as increasing temperature, atmospheric carbon dioxide, nutrient pollution, as well as changes in fire and precipitation regimes. These global change drivers have greatly modified the biogeochemical cycles of carbon (C), nitrogen (N), and phosphorus (P), which has an impact on primary productivity in forest ecosystems and in turn, affect the quality and quantity of resources entering the soil food web. However, C, N, and P soil dynamics have been mostly studied without considering their coupling effects on soil organisms. This is of critical interest because changes in nutrient stoichiometry may have a strong effect on soil biota and the ecosystem functions they drive. Further, most studies have focused on global change effects on bacteria and fungi and their C:N:P stoichiometry, while neglecting other soil organisms at higher trophic levels. This has led to an incomplete understanding of how the entire soil food web drives ecosystem processes involved in organic matter turnover and nutrient cycling. Here, we review studies that investigated how global change drivers impact C:N:P stoichiometry of soil organisms at different trophic levels in forest ecosystems and identify important knowledge gaps. We propose future directions for research on global change impacts on the linkages between soil biota and C:N:P stoichiometry.
In forests around the world, soil biota play a critical role in regulating ecosystem processes involved in plant litter decomposition, soil organic matter turnover, and associated nutrient mineralization (Nielsen et al., 2011; Bardgett and van der Putten, 2014). Soil fungi and bacteria breakdown organic matter using an arsenal of hydrolytic and lignolytic enzymes that provide available nutrients for plants and other soil organisms (Chahartaghi et al., 2005). Further up in the soil food web, micro-, meso-, and macro-fauna also enhance nutrient cycling through plant litter and organic matter comminution and by grazing microbial biomass (Setälä, 1995; Briones, 2014; Medina-Sauza et al., 2019). There is increasing evidence that both soil micro- and meso-biota biomass, community structure and function are constrained by the elemental carbon:nitrogen:phosphorus (C:N:P) ratios of available litter resources (Martinson et al., 2008; Ott et al., 2014; Zechmeister-Boltenstern et al., 2015; Maaroufi et al., 2018).
Ecological stoichiometry describes how elements, particularly C, N, and P, vary based on food resource and consumers and at different levels of organization such as cellular, individual, population, community, between different trophic levels and at the ecosystem level (Figure 1, Sterner and Elser, 2002). Further, the stoichiometry of organisms reflects their degree of elemental homeostasis. Thus, a homeostatic organism is able to maintain its C:N:P ratio independently of the C:N:P of its food resource. However, soil organisms facing nutrient imbalances may need to constrain their metabolisms, growth, and reproduction, which in turn may alter population dynamics, trophic interactions and change key ecosystem processes (Frost et al., 2005 and reference therein).
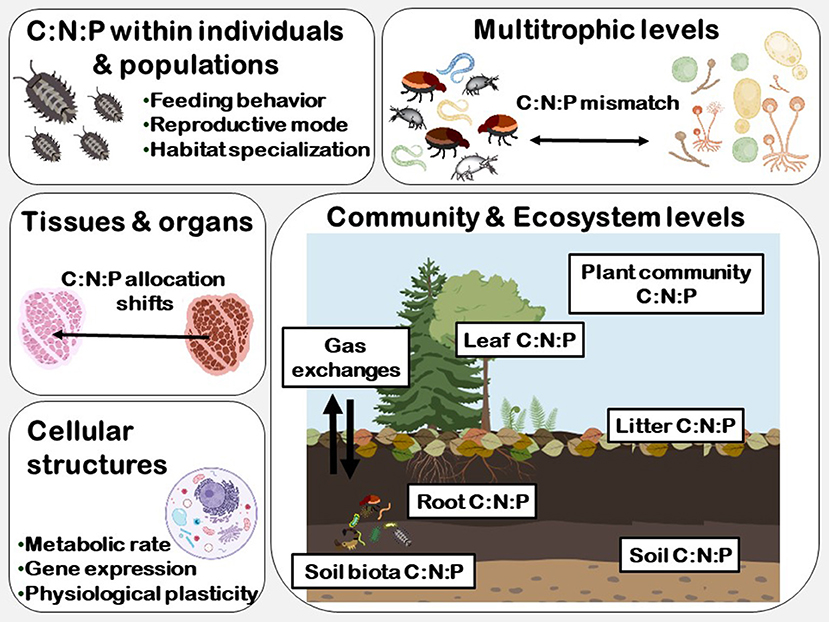
Figure 1. The different biological levels of organization and the relationships of the carbon: nitrogen: phosphorus stoichiometry of primary producers and soil biota.
Forest ecosystems are currently subjected to global change (GC), which has greatly modified the biogeochemical cycles of C, N, and P, thereby impacting on net primary productivity (NPP) and, in turn, affecting the quality and quantity of litter resources entering the soil food web. Therefore, research on soil biota should be prioritized due to the high sensitivity of most soil organisms to global change drivers (Blankinship et al., 2011) and the important role these organisms play in driving ecosystem functions related to nutrient cycling (Lavelle et al., 1997; Briones, 2014; Filser et al., 2016; Grandy et al., 2016). Below we discuss how the GC drivers of increasing temperature, changes to precipitation, elevated atmospheric carbon dioxide (CO2), fire regimes and nutrient enrichment shape relationships between forest primary resources and soil biota. We provide examples from different types of forests across the globe and highlight consistencies and discrepancies in our current understanding. Finally, we provide suggestions for future research that will close critical knowledge gaps in C-N-P coupling in forest soil biota and, thereby, improve our ability to anticipate the implications of GC drivers in forest ecosystems.
Annual global temperatures and extreme temperature events generated by advancing climate change are on the rise (Hoegh-Guldberg et al., 2018). Higher temperatures are expected to accelerate metabolic rates of organisms at different trophic levels (Walther et al., 2002), which, e.g., increase forest productivity, but this outcome depends on factors such as precipitation and soil nutrient availability (Boisvenue and Running, 2006; McMahon et al., 2010). Changes to productivity and associated shifts in plant community composition and litter quality will affect the behaviors, abundances, and nutritional requirements of forest soil organisms (Ott et al., 2012; Briones, 2014; Classen et al., 2015; Zhang and Elser, 2017). Generally speaking, litter P concentrations tend to decrease, while litter N concentrations and N:P and C:P ratios tend to increase, with higher temperatures (Yuan and Chen, 2009), which could lead to knock-on effects for soil biota via changes of nutrient ratios in the soil as the litter decomposes. For example, in boreal forest soils, high C:N and C:P ratios (i.e., lower nutrient availability) were associated with higher fungal vs. bacterial abundance, which resulted in higher temperature sensitivity in respiration rates (Briones, 2014). Interestingly, studies have identified a clear interaction between temperature, litter stoichiometry, and decomposer feeding behaviors (i.e., compensatory and avoidance strategies; see Hillebrand et al., 2009). For instance, Ott et al. (2012) reported that increasing temperature increased feeding rates and decreased ingestion time of isopods especially for high quality compared to low quality litter (i.e., higher C: nutrient ratios). Further, warmer temperatures were found to increase cast production in two forest-dwelling millipede species and thereby soil inorganic N concentrations due to increased mineralization rates, but this increased soil N did not lead to higher plant productivity in the short-term in deciduous forests (Makoto et al., 2014). Moreover, temperature had contrasting effects on the feeding behavior of the two millipede species, with one species consuming more and the other consuming less leaf litter, which may be due to differences in their metabolic requirements to maintain elemental homeostasis. These findings actually raise more questions than they answer about what species-specific temperature responses might mean in the long-term for nutrient cycling and plant productivity in forests, particularly if the N budget of the entire soil food web were to be considered in tandem. Firstly, soil fauna C:N:P ratios were never measured directly, but instead only for primary resources and soil microbes. Secondly, nutrient limitation, specifically N and P, typically varies based on whether northern boreal and temperate forests vs. tropical forests are considered, respectively (Xu et al., 2017). Such contrasts in nutrient limitation are likely to interact differently with increasing global temperatures, which will likely lead to different effects on soil biota stoichiometry that is specific to forest type and latitude.
Droughts are linked to a range of climatic conditions such as climate warming (which enhances evapotranspiration) and decreased precipitation (causing water shortages). Such drought events have important consequences in forested ecosystems, such as reduced growth and even forest die-offs (Dai, 2012; Camarero et al., 2018). Studies in temperate and Mediterranean forests showed that plant litter, root and soil C:N:P ratios respond to drought in different ways. In a meta-analysis, Sardans et al. (2017) showed that drought caused a reallocation of P, but not N, from leaves to roots in temperate forests, which may correspond to an increase of resources for enhancing root water uptake (Gargallo-Garriga et al., 2015). In a Mediterranean forest, Sardans and Peñuelas (2008) reported a decline in C:P and N:P ratios in roots, but a decline of C:P and no effect on N:P ratios in leaf litter in response to drought. These changes in C:N:P ratios between plant organs may impact on soil biota abundances and activities differently depending on if they rely directly on autotrophic C sources (plant root based compounds, e.g., mycorrhizal fungi) or on heterotrophic C sources (leaf litter based, saprotrophic fungi or detritivorous arthropods). Further, other studies focusing on the impact of drought upon soil biota reported contradicting results with a decline in soil enzymatic activities, microbial decomposition, and C and N mineralization rates in Mediterranean forests (Sardans and Peñuelas, 2005, 2008). In contrast, other studies reported a microbial community shift, as well as an increase of gene expression and enzymatic activities targeting C and N acquisitions via the decomposition of cellulose, chitin and lignin compounds, in tropical forests (Bouskill et al., 2016a,b). Regarding soil fauna, studies investigating the impact of drought only focused on abundances and species richness. On average, soil fauna abundances and species richness declined regardless trophic levels, with habitat generalist and parthenogenetic species more affected than sexually reproducing species and habitat specialists (i.e., adapted to drought; Lindberg and Bengtsson, 2005). While most studies stress the shifts of soil biota community abundances, richness and functions in response to drought, it remains to be investigated which nutrient stoichiometry strategies (i.e., techniques used to balance internal nutritional requirements) soil biota adopt to cope with increasing drought events.
Drying and rewetting cycle frequencies are predicted to increase as climate change progresses (Reichstein et al., 2013). These cycles cause an increase of C and N mineralization rates and the release of P due to changes in soil structure, an increase of nutrient desorption from soil particles and the lysis of fungal and bacterial cells also referred to as the “Birch effect” (Turner and Haygarth, 2001; Blackwell et al., 2010). While the Birch effect primarily decreases microbial biomass, episodic drying and rewetting cycles also increase nutrient availability to primary producers, e.g., by the release of P compounds from microbial origin compared to control forest soils (Fierer and Schimel, 2002; Brödlin et al., 2019). Studies reported a rather quick recovery of the microbial community after drying-rewetting events most likely due to microbial community shifts (Bouskill et al., 2013), but also due to changes in microbial community properties due to life strategy plasticity (e.g., sensitive, opportunistic, or tolerant; see Evans and Wallenstein, 2014). Other soil biota such as nematodes, Acari and Collembola were reported to be unaffected by drying and rewetting cycles (Taylor et al., 2004), suggesting that higher trophic levels may not be sensitive to rapid changes in soil moisture, redox potential, or oxygen content variation that occur in response to drying to rewetting cycles (Bouskill et al., 2013).
As humans burn ever-increasing amounts of fossil fuels on an annual basis (Le Quéré et al., 2009), the atmospheric CO2 concentration has risen from 277 ppm in 1750 to 405 ppm in 2018 (Le Quéré et al., 2018). This radical increase in CO2 has already had massive impacts on productivity (McMahon et al., 2010; Campbell et al., 2017) and litter quality (Norby et al., 2001), thereby leading to shifts in decomposition rates and overall C cycling in forest soils (Goodale et al., 2002; Hyvönen et al., 2007). But what role do soil biota and their stoichiometric requirements play? Populus plantations growing under elevated CO2 showed increased soil microbial biomass and concomitant increases in enzyme activity associated with C, N, and P foraging (Moscatelli et al., 2005). This is likely driven, in part, by both increased demand for nutrients as the Populus grew larger with CO2 fertilization and the resultant increase in exudation that allowed the microbial community to proliferate, thereby leading to further increases in nutrient demands. However, the responses of larger soil biota to elevated CO2 are not always the same. For example, in Mediterranean Quercus forests near a naturally occurring CO2 spring, litter P content was significantly lower compared to litter from control sites, which impaired decomposition probably via decreased litter quality and diminished soil fauna (Cotrufo and Raschi, 1999). In contrast, Hättenschwiler and Bretscher (2001) found that although litter produced under elevated CO2 levels was of poorer quality, it did not deter feeding by detritivorous isopods. Collectively, these findings highlight the need to consider how increasing CO2 levels affect the specific mechanisms behind the decrease in soil fauna, as well as the potentially stoichiometric reasons why certain groups of decomposers may respond differently to changes in litter quality caused by elevated CO2.
In many forest systems, especially the boreal forest, fire plays a pivotal role in shaping plant community composition and nutrient cycling (Zackrisson, 1977). This, in turn, drives soil biota community abundance, composition, and nutritional requirements (Pressler et al., 2019). However, in some regions of the world, fire frequency is expected to increase with advancing climate change (Westerling et al., 2006), while in other regions, fire suppression by humans has led to dramatic declines in fire incidences (Keeley et al., 1999; Nowacki and Abrams, 2008). What impacts have changes to forest fire regimes had on stoichiometric interactions with soil biota? As demonstrated in Mediterranean forests, fire can stimulate soil microbial activity due to nutrient rich ash deposition (Antunes et al., 2009). However, North American pine forest soils exposed to fire had lower C and N concentrations, which contributed to lower basal respiration and microbial biomass C (Choromanska and Deluca, 2002). In Swedish boreal forests, collembolans and macro-faunal predators were found to tolerate fire well, while mites and macro-faunal detritivore abundances were strongly reduced post-fire (Gongalsky et al., 2012). These disproportional impacts of fire on contrasting groups of soil biota likely have repercussions for C-N-P cycling, but these effects remain thus far unexplored. Further, ant mounds in the Canadian boreal forest were found to significantly increase soil N availability, microbially-preferred C sources and decrease P, but these effects were independent of time since fire (Lafleur et al., 2002). Critically, ants could play a role in neutralizing the effects of fire on boreal forest soil properties, leading to knock-on effects for soil biota such as, but not limited to, bacteria and fungi. Thereby, ants, as ecosystem engineers (Sanders and van Veen, 2011), may play a buffering role to the effects increasing forest fires on other soil biota and their nutrient requirements. However, despite the arguably important effects of fire on forest soil organisms, a recent review suggests that the effects of fire on forest soil biota are highly understudied and/or never published (Zaitsev et al., 2016).
Fossil fuel combustion, fertilizer production, and agricultural intensification have greatly increased the quantity of N, sulphur, and heavy metals released into the atmosphere that eventually affect forest ecosystems through deposition (Lorenz et al., 2010). These anthropogenic depositions of nutrients can directly affect soil properties by, e.g., affecting soil pH and nutrient balances or indirectly by impacting forest vegetation and thus the primary resources fuelling soil primary consumers (Binkley and Högberg, 2016). Many forests are N limited and it is now well-established that N enrichment increases NPP and soil C accumulation (Fernández-Martínez et al., 2014; Maaroufi et al., 2015), which may lead to stoichiometric imbalances in the soil and co-limitations of other nutrients. For instance, N deposition can cause P limitation in forest ecosystems (Lorenz et al., 2010; Vitousek et al., 2010; Peñuelas et al., 2012). Studies in tropical forests showed that the concomitant addition of N and P had the strongest effect on microbial activity relative to when N or P were applied alone or when the application was temporally separated (Fanin et al., 2016). The authors also reported an increase of the fungal:bacteria ratio in response to N addition, while NP or P additions reversed this increase. However, these fertilization treatments had a minor effect on the microbial C:N:P ratios, suggesting that shifts in microbial community structure and activity maintain the homeostasis of the whole microbial community might buffer against nutrient pollution (Fanin et al., 2017). It further suggests that fungi (saprotrophs) were favored by N enrichment at the expense of bacteria that are more P-demanding in tropical forests without strongly affecting their C:N:P stoichiometry (Krashevska et al., 2010).
In higher trophic levels, Barantal et al. (2014) found that stoichiometrically dissimilar (i.e., contrasting C:N:P ratios) litter mixtures decomposed more quickly than single species litter mixtures in the presence of soil Acari and detritivorous macrofauna (e.g., isoptera and diptera), probably due to complementarity in nutrient requirements between the different decomposer taxa. However, this enhanced decomposition effect disappeared when individual additions of C, N, and P were added. This highlights how nutrient pollution can disrupt litter-decomposer relationships across taxa (Mcglynn et al., 2007). Collectively, these studies highlight the critical roles of N and P availability for soil biota, but the response of soil fauna stoichiometry to nutrient enrichment still remains unexplored.
Soils and the organisms that inhabit them are the foundation of production and nutrient cycling in forests around the globe. In order to better understand stoichiometric relationships between forest soil biota and global biogeochemical cycling, further research that delves deeper into the mechanisms and controls behind these relationships is needed. Specifically, we would call for advancement in a number of areas such as: (1) Basic information is lacking about C-N-P coupling in forest soil fauna (Figure 2). This is critical because of the known role that soil organisms play in driving nutrient cycling (Lavelle et al., 1997; Briones, 2014). Future research should focus on implementing new techniques to obtain, collate and utilize information on how global change drivers affect C-N-P in forest soil fauna. Specifically, once more basic information is obtained on how the stoichiometry of individuals groups of forest soil organisms is affected by global change factors, interactive effects between taxa can be assessed. (2) Future research should merge the concepts of ecological stoichiometry and trait based ecology to further our understanding on the different mechanisms used by soil organisms to cope with stoichiometric mismatches caused by global change drivers at different levels of biological organization (Figures 1, 2, see Meunier et al., 2017). (3) Emergent research on the role of soil biota functional traits in controlling nutrient cycling offers a promising platform upon which to build more accurate biogeochemical global models (Aguilar-Trigueros et al., 2015; Moretti et al., 2017; Fry et al., 2018). This includes pulling focus on the universal mechanisms that control C:N:P ratios and on those that are unique to forested ecosystems. (4) Once more basic information on forest soil biota C:N:P coupling and their traits has been collected, this information can be used to better inform models that seek to predict biogeochemical cycling under global change (Filser et al., 2016; Grandy et al., 2016; Fry et al., 2018). More accurate understanding and predictions of nutrient cycling in forests will allow us to better anticipate the long-term ramifications of global change, both for human society and for the ecosystem processes upon which our survival depends.
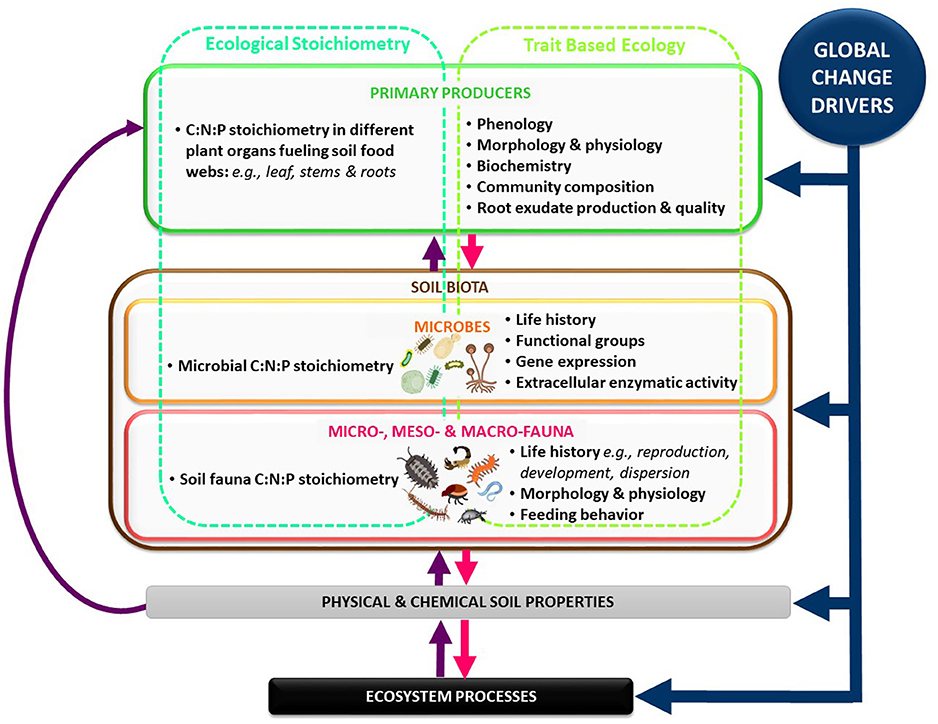
Figure 2. Simplified conceptual model of global change driver effects on soil biota in forest ecosystems. Blue arrows: effects of global change drivers on forest ecosystem compartments. Purple and fuchsia arrows: links between producers, consumers, soil properties and ecosystem processes. Turquoise column: ecological stoichiometry measurements. Green column: trait-based ecology measurements.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
NM was financed by a research fellowship from the Swedish Research Council (FORMAS 2018-00748) and JD was funded by a Vici grant from The Netherlands Organization for Scientific Research (NWO VICI grant 865.14.006) awarded to T. Martijn Bezemer.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aguilar-Trigueros, C. A., Hempel, S., Powell, J. R., Anderson, I. C., Antonovics, J., Bergmann, J., et al. (2015). Branching out: towards a trait-based understanding of fungal ecology. Fungal Biol. Rev. 29, 34–41. doi: 10.1016/j.fbr.2015.03.001
Antunes, S. C., Curado, N., Castro, B. B., and Gonçalves, F. (2009). Short-term recovery of soil functional parameters and edaphic macro-arthropod community after a forest fire. J. Soils Sediments 9, 267–278. doi: 10.1007/s11368-009-0076-y
Barantal, S., Schimann, H., Fromin, N., and Hättenschwiler, S. (2014). C, N and P fertilization in an Amazonian rainforest supports stoichiometric dissimilarity as a driver of litter diversity effects on decomposition. Proc. R. Soc. B 281:20141682. doi: 10.1098/rspb.2014.1682
Bardgett, R. D., and van der Putten, W. H. (2014). Belowground biodiversity and ecosystem functioning. Nature 515, 505–511. doi: 10.1038/nature13855
Binkley, D., and Högberg, P. (2016). Tamm review: revisiting the influence of nitrogen deposition on Swedish forests. For. Ecol. Manage. 368, 222–239. doi: 10.1016/j.foreco.2016.02.035
Blackwell, M. S. A., Brookes, P. C., Fuente-martinez, N., De Gordon, H., Murray, P. J., Snars, K. E., et al. (2010). “Phosphorus solubilization and potential transfer to surface waters from the soil microbial biomass following drying-rewetting and freezing-thawing,” in Advances in Agronomy, ed. D.J. Sparks (San Diego, CA: Academic Press; Elsevier Inc.), 1–35. doi: 10.1016/S0065-2113(10)06001-3
Blankinship, J. C., Niklaus, P. A., and Hungate, B. A. (2011). A meta-analysis of responses of soil biota to global change. Oecologia 165, 553–565. doi: 10.1007/s00442-011-1909-0
Boisvenue, C., and Running, S. W. (2006). Impacts of climate change on natural forest productivity - evidence since the middle of the 20th century. Glob. Chang. Biol. 12, 862–882. doi: 10.1111/j.1365-2486.2006.01134.x
Bouskill, N. J., Lim, H. C., Borglin, S., Salve, R., Wood, T. E., Silver, W. L., et al. (2013). Pre-exposure to drought increases the resistance of tropical forest soil bacterial communities to extended drought. ISME J. 7, 384–394. doi: 10.1038/ismej.2012.113
Bouskill, N. J., Wood, T. E., Baran, R., Hao, Z., Ye, Z., Bowen, B. P., et al. (2016a). Belowground response to drought in a tropical forest soil. II. change in microbial function impacts carbon composition. Front. Microbiol. 7:323. doi: 10.3389/fmicb.2016.00323
Bouskill, N. J., Wood, T. E., Baran, R., Ye, Z., Bowen, B. P., Lim, H. C., et al. (2016b). Belowground response to drought in a tropical forest soil. I. changes in microbial functional potential and metabolism. Front. Microbiol. 7:525. doi: 10.3389/fmicb.2016.00525
Briones, M. J. I. B. (2014). Soil fauna and soil functions: a jigsaw puzzle. Front. Environ. Sci. 2:7. doi: 10.3389/fenvs.2014.00007
Brödlin, D., Kaiser, K., Kessler, A., and Hagedorn, F. (2019). Drying and rewetting foster phosphorus depletion of forest soils. Soil Biol. Biochem. 128, 22–34. doi: 10.1016/j.soilbio.2018.10.001
Camarero, J. J., Gazol, A., Sangüesa-barreda, G., Cantero, A., Sánchez-salguero, R., Sánchez-miranda, A., et al. (2018). Forest growth responses to drought at short- and long-term scales in Spain : squeezing the stress memory from tree rings. Front. Ecol. Evol. 6:9. doi: 10.3389/fevo.2018.00009
Campbell, B. M., Beare, D. J., Bennett, E. M., Hall-spencer, J. M., Ingram, J. S. I., and Jaramillo, F. (2017). Agriculture production as a major driver of the earth system exceeding planetary boundaries. Ecol. Soc. 22:8. doi: 10.5751/ES-09595-220408
Chahartaghi, M., Langel, R., Scheu, S., and Ruess, L. (2005). Feeding guilds in collembola based on nitrogen stable isotope ratios. Soil Biol. Biochem. 37, 1718–1725. doi: 10.1016/j.soilbio.2005.02.006
Choromanska, U., and Deluca, T. H. (2002). Microbial activity and nitrogen mineralization in forest mineral soils following heating: evaluation of post-fire effects. Soil Biol. Biochem. 34, 263–271. doi: 10.1016/S0038-0717(01)00180-8
Classen, A. T., Sundqvist, M. K., Henning, J., Newman, G. S., Moore, J. A. M., Cregger, M. A., et al. (2015). Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: what lies ahead? Ecosphere 6, 1–21. doi: 10.1890/ES15-00217.1
Cotrufo, M. F., and Raschi, A. (1999). Decomposition and nutrient dynamics of quercus pubescens leaf litter in a naturally enriched CO2 mediterranean. Ecosystem 13, 343–351. doi: 10.1046/j.1365-2435.1999.00328.x
Dai, A. (2012). Increasing drought under global warming in observations and models. Nat. Clim. Chang. 3, 52–58. doi: 10.1038/nclimate1633
Evans, S. E., and Wallenstein, M. D. (2014). Climate change alters ecological strategies of soil bacteria. Ecol. Lett. 17, 155–164. doi: 10.1111/ele.12206
Fanin, N., Fromin, N., Barantal, S., and Hättenschwiler, S. (2017). Stoichiometric plasticity of microbial communities is similar between litter and soil in a tropical rainforest. Sci. Rep. 7:12498. doi: 10.1038/s41598-017-12609-8
Fanin, N., Hättenschwiler, S., Chavez Soria, P. F., and Fromin, N. (2016). (A)synchronous availabilities of N and P regulate the activity and structure of the microbial decomposer community. Front. Microbiol. 6:1507. doi: 10.3389/fmicb.2015.01507
Fernández-Martínez, M., Vicca, S., Janssens, I. A., Sardans, J., Luyssaert, S., Campioli, M., et al. (2014). Nutrient availability as the key regulator of global forest carbon balance. Nat. Clim. Chang. 4, 471–476. doi: 10.1038/nclimate2177
Fierer, N., and Schimel, J. P. (2002). Effects of drying-rewetting frequency on soil carbon and nitrogen transformations. Soil Biol. Biochem. 34, 777–787. doi: 10.1016/S0038-0717(02)00007-X
Filser, J., Faber, J. H., Tiunov, A. V., Brussaard, L., Frouz, J., De Deyn, G., et al. (2016). Soil fauna: key to new carbon models. Soil 2, 565–582. doi: 10.5194/soil-2-565-2016
Frost, P. C., Evans-white, M. A., Finkel, Z. V., Jensen, T. C., and Matzek, V. (2005). Are you what you eat? physiological constraints on organismal stoichiometry in an elementally imbalanced world. Oikos 1, 18–28. doi: 10.1111/j.0030-1299.2005.14049.x
Fry, E. L., Long, J. R., De Garrido, L. Á., Alvarez, N., Mathilde, L. C., Marta, C., et al. (2018). Using plant, microbe, and soil fauna traits to improve the predictive power of biogeochemical models. Methods Ecol. Evol. 1–12. doi: 10.1111/2041-210X.13092
Gargallo-Garriga, A., Sardans, J., Oravec, M., Urban, O., Jentsch, A., Kreyling, J., et al. (2015). Warming differentially influences the effects of drought on stoichiometry and metabolomics in shoots and roots. New Phytol. 207, 591–603. doi: 10.1111/nph.13377
Gongalsky, K. B., Malmström, A., Zaitsev, A. S., Shakhab, S. V., Bengtsson, J., and Persson, T. (2012). Do burned areas recover from inside? An experiment with soil fauna in a heterogeneous landscape. Appl. Soil Ecol. 59, 73–86. doi: 10.1016/j.apsoil.2012.03.017
Goodale, C. L., Apps, M. J., Birdsey, R., Field, C. B., Heath, L. S., Houghton, R. A., et al. (2002). Forest carbon sinks in the northern hemisphere. Ecol. Appl. 12, 891–899. doi: 10.1890/1051-0761(2002)012[0891:FCSITN]2.0.CO;2
Grandy, A. S., Wieder, W. R., Wickings, K., and Kyker-Snowman, E. (2016). Beyond microbes: are fauna the next frontier in soil biogeochemical models? Soil Biol. Biochem. 102, 40–44. doi: 10.1016/j.soilbio.2016.08.008
Hättenschwiler, S., and Bretscher, D. (2001). Isopod effects on decomposition of litter produced under elevated CO2, N deposition and different soil types. Glob. Chang. Biol. 7, 565–579. doi: 10.1046/j.1365-2486.2001.00402.x
Hillebrand, H., Borer, E. T., Bracken, M. E. S., Cardinale, B. J., Cebrian, J., Cleland, E. E., et al. (2009). Herbivore metabolism and stoichiometry each constrain herbivory at different organizational scales across ecosystems. Ecol. Lett. 12, 516–527. doi: 10.1111/j.1461-0248.2009.01304.x
Hoegh-Guldberg, O., Jacob, D., Taylor, M., Bindi, M., Brown, S., Camilloni, I., et al. (2018). “Impacts of 1.5°C global warming on natural and human systems,” in An IPCC Special Report on the Impacts of Global Warming of 1.5°C Above Preindustrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change,Sustainable Development, eds V. Masson-Delmotte, P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, and T. Waterfield. 179–311. Available online at: https://www.ipcc.ch/sr15/
Hyvönen, R., Agren, G. I., Linder, S., Persson, T., Cotrufo, M. F., Ekblad, A., et al. (2007). The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytol. 173, 463–480. doi: 10.1111/j.1469-8137.2007.01967.x
Keeley, J. E., Fotheringham, C. J., and Morais, M. (1999). Impacts on brushland fire. Science 284, 1829–1832. doi: 10.1126/science.284.5421.1829
Krashevska, V., Maraun, M., Ruess, L., Scheu, S., Krashevska, V., Maraun, M., et al. (2010). Carbon and nutrient limitation of soil microorganisms and microbial grazers in a tropical montane rain forest. Oikos 119, 1020–1028. doi: 10.1111/j.1600-0706.2009.18169.x
Lafleur, B., Bradley, R., and Francoeur, A. (2002). Soil modifications created by ants along a post-fire chronosequence in lichen-spruce woodland soil modifications created by ants along a post-fire chronosequence in lichen-spruce woodland. Écoscience 6860, 63–73. doi: 10.1080/11956860.2002.11682691
Lavelle, P., Bignell, D., Heal, W., Lepage, M., Ineson, P., and Dhillion, S. (1997). Soil function in a changing world : the role of invertebrate ecosystem engineers soil function in a changing world : the role of invertebrat ~ ecosystem engineers. Eur. J. Soil Sci. 33, 159–193.
Le Quéré, C., Andrew, R., Friedlingstein, P., Sitch, S., Hauck, J., Pongratz, J., et al. (2018). Global carbon budget 2018. Earth Syst. Sci. Data 10, 2141–2194. doi: 10.5194/essd-10-2141-2018
Le Quéré, C., Raupach, M. R., Canadell, J. G., Marland, G., Bopp, L., Ciais, P., Conway, T. J., et al. (2009). Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2, 831–836. doi: 10.1038/ngeo689
Lindberg, N., and Bengtsson, J. (2005). Population responses of oribatid mites and collembolans after drought. Appl. Soil Ecol. 28, 163–174. doi: 10.1016/j.apsoil.2004.07.003
Lorenz, M., Clarke, N., Paoletti, E., Bytnerowicz, A., Grulke, N., Lukina, N., et al. (2010). “4 Air pollution impacts on forests in a changing climate,” in Forests and Society - Responding to Global Drivers of Change, Vol. 25, eds G. Mery, P. Katila, G. Galloway, R. I. Alfaro, M. Kanninen, M. Lobovikov, and J. Varjo (IUFRO World Series), 55–74.
Maaroufi, N. I., Nordin, A., Hasselquist, N. J., Bach, L. H., Palmqvist, K., and Gundale, M. J. (2015). Anthropogenic nitrogen deposition enhances carbon sequestration in boreal soils. Glob. Chang. Biol. 21, 3169–3180. doi: 10.1111/gcb.12904
Maaroufi, N. I., Palmqvist, K., Bach, L. H., Bokhorst, S., Liess, A., Gundale, M. J., et al. (2018). Nutrient optimization of tree growth alters structure and function of boreal soil food webs. For. Ecol. Manage. 428, 46–56. doi: 10.1016/j.foreco.2018.06.034
Makoto, K., Arai, M., and Kaneko, N. (2014). Change the menu? species-dependent feeding responses of millipedes to climate warming and the consequences for plant soil nitrogen dynamics. Soil Biol. Biochem. 72, 19–25. doi: 10.1016/j.soilbio.2014.01.016
Martinson, H. M., Schneider, K., Gilbert, J., Hines, J. E., Hambäck, P. A., and Fagan, W. F. (2008). Detritivory: stoichiometry of a neglected trophic level. Ecol. Res. 23, 487–491. doi: 10.1007/s11284-008-0471-7
Mcglynn, T. P., Salinas, D. J., Dunn, R. R., Wood, T. E., Lawrence, D., and Clark, D. A. (2007). Phosphorus limits tropical rain forest litter. Fauna. Biotropica 39, 50–53. doi: 10.1111/j.1744-7429.2006.00241.x
McMahon, S. M., Parker, G. G., and Miller, D. R. (2010). Evidence for a recent increase in forest growth. Proc. Natl. Acad. Sci. U.S.A. 107, E86–E87. doi: 10.1073/pnas.0912376107
Medina-Sauza, R. M., Álvarez-Jiménez, M., Delhal, A., Reverchon, F., Blouin, M., Guerrero-Analco, J. A., et al. (2019). Earthworms building up soil microbiota, a review. Front. Environ. Sci. 7:81. doi: 10.3389/fenvs.2019.00081
Meunier, C. L., Boersma, M., El-Sabaawi, R., Halvorson, H. M., Herstoff, E. M., Van de Waal, D. B., et al. (2017). From elements to function: toward unifying ecological stoichiometry and trait-based ecology. Front. Environ. Sci. 5:18. doi: 10.3389/fenvs.2017.00018
Moretti, M., Dias, A. T. C., de Bello, F., Altermatt, F., Chown, S. L., Azcárate, F. M., et al. (2017). Handbook of protocols for standardized measurement of terrestrial invertebrate functional traits. Funct. Ecol. 31, 558–567. doi: 10.1111/1365-2435.12776
Moscatelli, M. C., Lagomarsino, A., De Angelis, P., Grego, S. (2005). Seasonality of soil biological properties in a poplar plantation growing under elevated atmospheric CO2. Appl. Soil. Eco. 30, 162–173. doi: 10.1016/j.apsoil.2005.02.008
Nielsen, U. N., Ayres, E., Wall, D. H., and Bardgett, R. D. (2011). Soil biodiversity and carbon cycling: a review and synthesis of studies examining diversity-function relationships. Eur. J. Soil Sci. 62, 105–116. doi: 10.1111/j.1365-2389.2010.01314.x
Norby, R. J., Kobayashi, K., and Kimball, B. A. (2001). Rising CO2 – future ecosystems. New Phytol. 150, 215–221. doi: 10.1046/j.1469-8137.2001.00118.x
Nowacki, G. J., and Abrams, M. D. (2008). The demise of fire and “mesophication” of forests in the Eastern United States. Bioscience 58, 123–138. doi: 10.1641/B580207
Ott, D., Digel, C., Rall, B. C., Maraun, M., Scheu, S., and Brose, U. (2014). Unifying elemental stoichiometry and metabolic theory in predicting species abundances. Ecol. Lett. 17, 1247–1256. doi: 10.1111/ele.12330
Ott, D., Rall, B. C., and Brose, U. (2012). Climate change effects on macrofaunal litter decomposition: the interplay of temperature, body masses and stoichiometry. Philos. Trans. R. Soc. B Biol. Sci. 367, 3025–3032. doi: 10.1098/rstb.2012.0240
Peñuelas, J., Sardans, J., Rivas-ubach, A., and Janssens, I. A. (2012). The human-induced imbalance between C, N and P in earth's life system. Glob. Chang. Biol. 18, 3–6. doi: 10.1111/j.1365-2486.2011.02568.x
Pressler, Y., Moore, J. C., and Cotrufo, M. F. (2019). Belowground community responses to fire: meta-analysis reveals contrasting responses of soil microorganisms and mesofauna. Oikos 128, 309–327. doi: 10.1111/oik.05738
Reichstein, M., Bahn, M., Ciais, P., Frank, D., Mahecha, M. D., Seneviratne, S. I., et al. (2013). Climate extremes and the carbon cycle. Nature 500, 287–295. doi: 10.1038/nature12350
Sanders, D., and van Veen, F. J. F. (2011). Ecosystem engineering and predation: the multi-trophic impact of two ant species. J. Anim. Ecol. 80, 569–576. doi: 10.1111/j.1365-2656.2010.01796.x
Sardans, J., Grau, O., Chen, H. Y. H., and Janssens, I. A. (2017). Changes in nutrient concentrations of leaves and roots in response to global change factors. Glob. Chang. Biol. 23, 3849–3856. doi: 10.1111/gcb.13721
Sardans, J., and Peñuelas, J. (2005). Drought decreases soil enzyme activity in a mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 37, 455–461. doi: 10.1016/j.soilbio.2004.08.004
Sardans, J., and Peñuelas, J. (2008). Drought-induced changes in C and N stoichiometry in a Quercus ilex mediterranean forest. For. Sci. 54, 513–522. doi: 10.1093/forestscience/54.5.513
Setälä, H. (1995). Growth of birch and pine seedlings in relation to grazing by soil fauna on ectomycorrhizal fungi. Ecology 76, 1844–1851. doi: 10.2307/1940716
Sterner, R. W., and Elser, J. J. (2002). Ecological Stoichiometry. Oxford: Princeton University Press. doi: 10.1515/9781400885695
Taylor, A. R., Schröter, D., Pflug, A., and Wolters, V. (2004). Response of different decomposer communities to the manipulation of moisture availability: potential effects of changing precipitation patterns. Glob. Chang. Biol. 10, 1313–1324. doi: 10.1111/j.1365-2486.2004.00801.x
Turner, B. L., and Haygarth, P. M. (2001). Phosphorus solubilization in rewetted soils. Nature 411, 258–259. doi: 10.1038/35077146
Vitousek, P. M., Porder, S., Houlton, B. Z., and Chadwick, O. A. (2010). Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 20, 5–15. doi: 10.1890/08-0127.1
Walther, G., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T. J. C., et al. (2002). Ecological responses to recent climate change. Nature 416, 389–395. doi: 10.1038/416389a
Westerling, A. L., Hidalgo, H. G., Cayan, D. R., and Swetnam, T. W. (2006). Warming and earlier spring increase Western U.S. forest wildfire activity. Science 313, 940–943. doi: 10.1126/science.1128834
Xu, Z., Yu, G., Zhang, X., He, N., Wang, Q., Wang, S., et al. (2017). Soil enzyme activity and stoichiometry in forest ecosystems along the North-South Transect in eastern China (NSTEC). Soil Biol. Biochem. 104, 152–163. doi: 10.1016/j.soilbio.2016.10.020
Yuan, Z. Y., and Chen, H. Y. H. (2009). Global trends in senesced-leaf nitrogen and phosphorus. Glob. Ecol. Biogeogr. 18, 532–542. doi: 10.1111/j.1466-8238.2009.00474.x
Zackrisson, O. (1977). Influence of forest fires on the north swedish boreal forest. Oikos 29, 22–32. doi: 10.2307/3543289
Zaitsev, A. S., Gongalsky, K. B., Malmström, A., Persson, T., and Bengtsson, J. (2016). Why are forest fires generally neglected in soil fauna research? A mini-review. Appl. Soil Ecol. 98, 261–271. doi: 10.1016/j.apsoil.2015.10.012
Zechmeister-Boltenstern, S., Keiblinger, K. M., Mooshammer, M., Peñuelas, J., Richter, A., Sardans, J., et al. (2015). The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 85, 133–155. doi: 10.1890/14-0777.1
Keywords: biogeochemical cycling, climate change, ecological stoichiometry, meso-fauna, micro-fauna, plant-microbe-soil fauna interactions, soil food webs
Citation: Maaroufi NI and De Long JR (2020) Global Change Impacts on Forest Soils: Linkage Between Soil Biota and Carbon-Nitrogen-Phosphorus Stoichiometry. Front. For. Glob. Change 3:16. doi: 10.3389/ffgc.2020.00016
Received: 12 July 2019; Accepted: 03 February 2020;
Published: 20 February 2020.
Edited by:
Marie Spohn, University of Bayreuth, GermanyReviewed by:
Ivika Ostonen, University of Tartu, EstoniaCopyright © 2020 Maaroufi and De Long. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan R. De Long, ai5kZWxvbmdAbmlvby5rbmF3Lm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.