
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 08 January 2020
Sec. Tropical Forests
Volume 2 - 2019 | https://doi.org/10.3389/ffgc.2019.00075
This article is part of the Research Topic Improving Environmental Sustainability in Oil Palm: Linking Science, Policy, & Practice across the Tropics View all 18 articles
 Sarah H. Luke1*
Sarah H. Luke1* Andreas Dwi Advento2
Andreas Dwi Advento2 Anak Agung Ketut Aryawan2
Anak Agung Ketut Aryawan2 Dwi Nugroho Adhy3
Dwi Nugroho Adhy3 Adham Ashton-Butt4,5
Adham Ashton-Butt4,5 Holly Barclay6
Holly Barclay6 Jassica Prajna Dewi2
Jassica Prajna Dewi2 Julia Drewer7
Julia Drewer7 Alex J. Dumbrell8
Alex J. Dumbrell8 Edi3
Edi3 Amy E. Eycott1,9
Amy E. Eycott1,9 Martina F. Harianja1
Martina F. Harianja1 Julie K. Hinsch1,10
Julie K. Hinsch1,10 Amelia S. C. Hood1
Amelia S. C. Hood1 Candra Kurniawan2
Candra Kurniawan2 David J. Kurz1,11
David J. Kurz1,11 Darren J. Mann12
Darren J. Mann12 Kirsty J. Matthews Nicholass8
Kirsty J. Matthews Nicholass8 Mohammad Naim2
Mohammad Naim2 Michael D. Pashkevich1
Michael D. Pashkevich1 Graham W. Prescott1,13
Graham W. Prescott1,13 Sudharto Ps2
Sudharto Ps2 Pujianto2
Pujianto2 Dedi Purnomo2
Dedi Purnomo2 Rizky Rajabillah Purwoko2
Rizky Rajabillah Purwoko2 Syafrisar Putra2
Syafrisar Putra2 T. Dzulfikar S. Rambe2
T. Dzulfikar S. Rambe2 Soeprapto2
Soeprapto2 Dakota M. Spear1
Dakota M. Spear1 Suhardi2
Suhardi2 David J. X. Tan14,15
David J. X. Tan14,15 Hsiao-Hang Tao16,17
Hsiao-Hang Tao16,17 Ribka Sionita Tarigan2
Ribka Sionita Tarigan2 Resti Wahyuningsih2
Resti Wahyuningsih2 Helen S. Waters1
Helen S. Waters1 Rudi Harto Widodo2
Rudi Harto Widodo2 Whendy3
Whendy3 Christopher R. Woodham16,18
Christopher R. Woodham16,18 Jean-Pierre Caliman2
Jean-Pierre Caliman2 Eleanor M. Slade16,19
Eleanor M. Slade16,19 Jake L. Snaddon20,21
Jake L. Snaddon20,21 William A. Foster1
William A. Foster1 Edgar C. Turner1
Edgar C. Turner1Conversion of tropical forest to agriculture results in reduced habitat heterogeneity, and associated declines in biodiversity and ecosystem functions. Management strategies to increase biodiversity in agricultural landscapes have therefore often focused on increasing habitat complexity; however, the large-scale, long-term ecological experiments that are needed to test the effects of these strategies are rare in tropical systems. Oil palm (Elaeis guineensis Jacq.)—one of the most widespread and important tropical crops—offers substantial potential for developing wildlife-friendly management strategies because of its long rotation cycles and tree-like structure. Although there is awareness of the need to increase sustainability, practical options for how best to manage oil palm plantations, for benefits to both the environment and crop productivity, have received little research attention. In this paper we introduce the Biodiversity and Ecosystem Function in Tropical Agriculture (BEFTA) Programme: a long-term research collaboration between academia and industry in Sumatra, Indonesia. The BEFTA Programme aims to better understand the oil palm agroecosystem and test sustainability strategies. We hypothesise that adjustments to oil palm management could increase structural complexity, stabilise microclimate, and reduce reliance on chemical inputs, thereby helping to improve levels of biodiversity and ecosystem functions. The Programme has established four major components: (1) assessing variability within the plantation under business-as-usual conditions; (2) the BEFTA Understory Vegetation Project, which tests the effects of varying herbicide regimes; (3) the Riparian Ecosystem Restoration in Tropical Agriculture (RERTA) Project, which tests strategies for restoring riparian habitat; and (4) support for additional collaborative projects within the Programme landscape. Across all projects, we are measuring environmental conditions, biodiversity, and ecosystem functions. We also measure oil palm yield and production costs, in order to assess whether suggested sustainability strategies are feasible from an agronomic perspective. Early results show that oil palm plantation habitat is more variable than might be expected from a monoculture crop, and that everyday vegetation management decisions have significant impacts on habitat structure. The BEFTA Programme highlights the value of large-scale collaborative projects for understanding tropical agricultural systems, and offers a highly valuable experimental set-up for improving our understanding of practices to manage oil palm more sustainably.
Agriculture has expanded and intensified rapidly in recent decades, with rates of change greatest in the tropics (Foley et al., 2005, 2011; Laurance et al., 2014). The economic benefits of these transformations have been accompanied by substantial environmental costs, largely as a result of initial deforestation (e.g., Tilman et al., 2001; Foley et al., 2005, 2011; Gibbs et al., 2010; Dislich et al., 2017). The tropics contain the majority of the world's biodiversity “hotspots” (Myers et al., 2000), and conversion of natural habitats to agriculture in these regions leads to substantial reductions in biodiversity (e.g., Fitzherbert et al., 2008; Gibson et al., 2011; Barnes et al., 2014, 2017; Laurance et al., 2014; Clough et al., 2016). Ecosystem functions and services are also heavily altered by conversion of natural habitats to agriculture. For example, changes in a range of functional metrics, including carbon storage and fluxes, soil fertility, pollination, functional diversity, and foodweb networks have been shown across multiple crop systems and regions (Kremen et al., 2002; Foley et al., 2007; Tscharntke et al., 2008; Bihn et al., 2010; Sekercioglu, 2012; Barnes et al., 2014; Allen et al., 2015; Guillaume et al., 2015; Hassler et al., 2015; Kotowska et al., 2015, 2016; Clough et al., 2016; Dislich et al., 2017; Kurniawan et al., 2018). One of the major reasons for loss of biodiversity, when natural habitat is converted to agriculture, is a reduction in structural complexity and heterogeneity of vegetation, and consequent reductions in shading, alterations to microclimate, and changes to soil physical and chemical properties (Matson et al., 1997; Benton et al., 2003; Wilson et al., 2005; Luskin and Potts, 2011; Drescher et al., 2016; Meijide et al., 2018).
Although efforts for biodiversity conservation must focus on protecting remaining natural habitats wherever possible (Green et al., 2005; Gibson et al., 2011; Phalan et al., 2011), there is also a need to maximise biodiversity within the high-yield crop matrix (Bommarco et al., 2013; Kremen, 2015). This is in part for its inherent value, but also because promoting biodiversity within agricultural areas can help sustain ecosystem functions and crop productivity (Bommarco et al., 2013). This could reduce reliance on chemical inputs to maintain soil fertility (Isbell et al., 2017) and on pesticide and herbicide applications to control pests (Pywell et al., 2015), and also reduce pressure to convert additional areas of land to agriculture. Practising biodiversity-friendly farming can also increase the availability of food, and therefore provide benefits for certain neighbouring forest species (Tscharntke et al., 2005b; Rand et al., 2006). Given the link between vegetation complexity and biodiversity and functions, management strategies that aim to increase the structural complexity of crop systems have been suggested as a potential approach to making crop systems more wildlife-friendly (e.g., Tscharntke et al., 2005a, 2007). However, the value of different management strategies can be difficult to assess because even relatively simplified tropical agricultural systems are highly complex and can contain high species numbers and complex webs of interactions which will affect levels of biodiversity, ecosystem functions, and yield (e.g., Perfecto et al., 2014; Wielgoss et al., 2014; Gras et al., 2016). Therefore, in order to properly assess the effects of change on an ecosystem and allow strategies for mitigation to be properly tested, an experimental approach is required (Fayle et al., 2015).
Oil palm (Elaeis guineensis Jacq.) is an ideal tropical agricultural system in which to conduct experiments to test the impacts of habitat management. As one of the most rapidly-expanding crops in Southeast Asia (FAO, 2019), a large planted area is available for experimental and comparative studies. As a perennial crop with a planting rotation of at least 25 years, it is suitable for long-term field studies and has high potential to develop substantial structural complexity during its lifetime (Foster et al., 2011; Luskin and Potts, 2011). In addition, the international economic importance of the crop means that there is potential financial, logistic, and intellectual support for such research (Padfield et al., 2019). There is also growing international pressure to reduce the environmental impact of oil palm (Meijaard et al., 2018), and several certification initiatives and standards are already well-established that have developed guidelines on sustainable plantation management (e.g., the Roundtable on Sustainable Palm Oil, RSPO, https://rspo.org/; Indonesian Sustainable Palm Oil, ISPO (http://www.ispo-org.or.id; and Malaysian Sustainable Palm Oil, MSPO, https://www.mpocc.org.my/mspo-certification-scheme). These include guidelines relating to soil fertility and erosion; water and riparian zone management; Integrated Pest Management (IPM); and the protection of high conservation value (HCV) areas (Roundtable on Sustainable Palm Oil, 2018).
The development of these schemes is an important step towards greater sustainability and illustrates the growing importance of environmental considerations within the oil palm sector. However, current guidelines still lack a rigorous evidence base and require additional research to underpin and improve recommendations. Few studies have specifically tested the impacts of different plantation management techniques on biodiversity, ecosystem functions or environmental conditions (Savilaakso et al., 2014) beyond a general testing of whether certified plantations performed better than non-certified plantations (e.g., Cattau et al., 2016). Even fewer have used an experimental approach or considered impacts on yield and profitability as well as biodiversity, although a study that experimentally removed epiphytes (Prescott et al., 2015) and some projects that are currently underway (e.g., within the EFForTS Programme Drescher et al., 2016; Teuscher et al., 2016; Darras et al., 2019, and the SEnSOR Project (http://www.sensorproject.net/) are notable exceptions to this. There is, therefore, a significant knowledge gap in oil palm research to inform more environmentally-friendly management within the crop itself (Foster et al., 2011; Padfield et al., 2019), and what the effect of proposed solutions may be on profitability, and hence their chances of uptake by the industry. Given the huge land area occupied by oil palm plantations [18.7 million ha worldwide in 2017 (Meijaard et al., 2018)] and continued expansion of the crop (FAO, 2019), such work has the potential to influence the biodiversity and functioning of ecosystems over vast areas.
Here, we outline the rationale and methodology of a large-scale experiment-based research programme: the Biodiversity and Ecosystem Function in Tropical Agriculture (BEFTA) Programme, which investigates management strategies to increase the structural complexity of oil palm plantations in an effort to support biodiversity and ecosystem processes in the oil palm landscape. The overarching aim of the BEFTA Programme is to demonstrate whether it is practicable to enhance the sustainability of oil palm through various management strategies that increase vegetation complexity within the crop ecosystem with the minimum possible impact on yield and profits. Uniquely, the programme is a fully-integrated collaboration between universities, research institutes, and the oil palm industry, ensuring that management practices are realistic and that the outcomes of the project are readily implemented by land managers. Results will provide a rigorous, experimentally-validated evidence base for practical management interventions and protocols that can support a more sustainable oil palm industry in the years to come.
Southeast Asia has dominated the global production of palm oil over the last few decades, with Indonesia currently the leading producer, followed by Malaysia (FAO, 2018). Our work is based in plantations within Riau Province in central Sumatra, Indonesia (0° 56′0″ N, 101°18′0″ E), a region with one of the highest percentage covers of oil palm nation-wide (Uryu et al., 2008). The plantations are owned and managed by PT Ivo Mas Tunggal (a subsidiary company of Golden Agri Resources, GAR), and run with technical advice and input from Sinar Mas Agro Resources and Technology Research Institute (SMARTRI), which is the research and development centre for upstream activities of GAR. The region has 2,300 mm y−1 average rainfall, 27°C average temperature (from SMARTRI data, 1998–2018), and lowland rainforest as the natural vegetation type. The area was selectively logged in the 1970s and cleared and converted to oil palm plantations between 1985 and 1995: little forest remains and the landscape is now dominated by oil palm agriculture. Our study sites therefore represent a tropical ecosystem that has been altered fundamentally from a pre-disturbance system. Although the potentially limited local species pool presents challenges to restoration, it also means that any benefits attributed to our restoration work are also likely to work in other, less heavily-impacted regions, and with perhaps even more marked results.
The main SMARTRI research station is surrounded by seven oil palm estates, which together cover ~15,000 ha, and are on mineral soil, with low-elevation, and a generally flat terrain. Our core experimental projects are based across three of these estates: Palapa, Ujung Tanjung, and Kandista (Figure 1), whilst additional collaborative research projects are taking place throughout the seven estates. Across the estates, palms are planted in blocks that are 1,000 m long and 300 m wide, laid out on a grid system intersected by dirt roads. Areas of open block-edge habitat are therefore intermingled with more closed block-core habitat across the whole plantation. Within blocks, palms are planted in rows ~8 m apart. Rows are staggered so that neighbouring palms are arranged in a triangular formation.
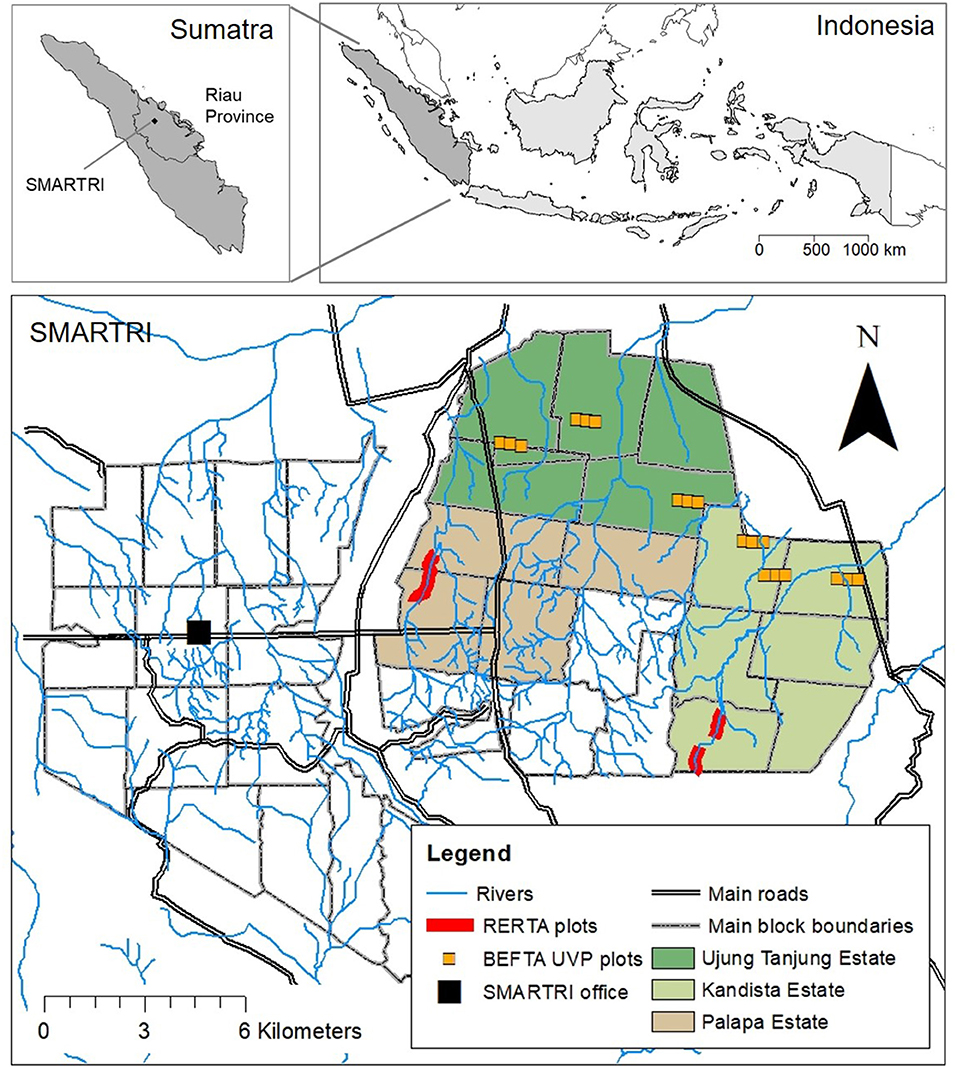
Figure 1. Maps to show the location of our experimental plots within the SMARTRI landscape and within Sumatra and Indonesia. BEFTA Understory Vegetation Project (UVP) plots are set up in triplets across Ujung Tanjung and Kandista Estates; RERTA 1 is set up in Palapa Estate; and RERTA 2 is set up in Kandista Estate. Maps were made using ArcMap 10.5.1 [Environmental Systems Research Institute (ESRI), 2017], library “maps” in R statistical package (Brownrigg, 2016; R Core Team, 2017), and with reference to maps produced by Sinar Mas.
Normal business-as-usual management involves application of a mixture of inorganic fertilisers, empty fruit bunches (EFB), and palm oil mill effluent (POME) to the soils between two and eight times per year depending on the fertiliser regime (see Ashton-Butt et al., 2018 for more details). Insecticides are routinely applied twice per month to prevent Oryctes (Coleoptera: Scarabaeidae) attacks on the immature palms (up to 2 years after planting) when this pest is present in the area, with potential additional insecticides/rodenticides added if outbreaks of other pest species are detected. On average, pest outbreaks affect <2–5% of the plantation area each year. A combination of chemical herbicides are usually applied three times per year to control vegetation in selected areas at the base of each palm and on the harvesting paths (also sometimes referred to as “interrows” Corley and Tinker, 2003a; Rambe et al., 2012). SMARTRI has a large, and well-established research centre within the study area, which houses ~80 researchers who study aspects of oil palm agronomy and sustainability (https://goldenagri.com.sg/sustainability/smart-research-institute-smartri/research-and-development/). The centre is well-equipped with extensive laboratory facilities, research nurseries, and equipment for field-based studies.
The BEFTA Programme aims to increase understanding of oil palm ecosystems, and to test the effects of management strategies to increase vegetation complexity on plantation biodiversity, ecosystem functions, yield and profitability (Figure 2). The programme currently has four major components:
1. Assessing existing variability within the plantation. Increasing understanding of the oil palm ecosystem, the long-term influence of climate on how the ecosystem functions, and the contribution of oil palm biodiversity to ecosystem processes and yield under “business-as-usual” conditions.
2. The BEFTA Understory Vegetation Project. Conducting large-scale, long-term experimental manipulations of understory vegetation within plantations to test the effects of this structural and biological complexity on oil palm ecosystems.
3. The Riparian Ecosystem Restoration in Tropical Agriculture (RERTA) Project. Conducting large-scale, long-term experiments to test different options for restoring riparian buffers along waterways, and to assess how differences in structural and biological complexity of different restoration strategies affect surrounding plantation ecosystems.
4. Additional projects and collaborations. Providing study sites, and opportunities for collaboration, to allow tests of the effects of a range of other habitat management options within the plantation ecosystem.
We introduce these key components in this paper, and provide early results from the BEFTA Understory Vegetation Project, and from baseline data collected as part of the RERTA Project. We also signpost results from other sections of the Programme that have been published so far, as well as plans for upcoming work (Figure 2).
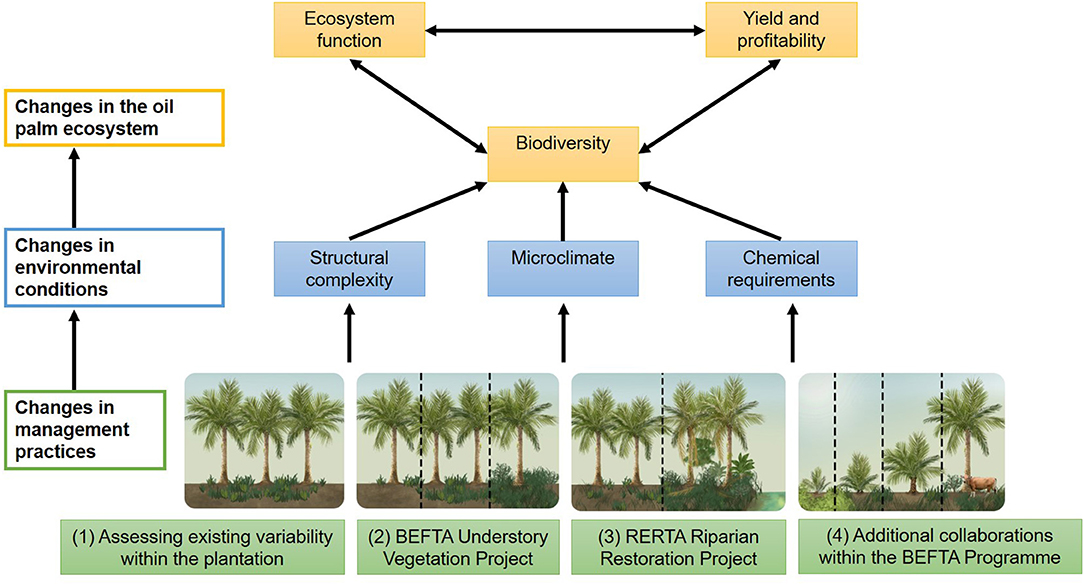
Figure 2. Schematic to show the four key research components of the BEFTA Programme: (1) Assessing existing variability within the oil palm plantation under business-as-usual practices; (2) the BEFTA Understory Vegetation Project experimental manipulation; (3) the RERTA Project experimental manipulation of riparian restoration; (4) and a suite of additional collaborative projects that test a range of other management strategies within the plantation system. We anticipate that use of different management practices will cause changes in environmental conditions (including structural complexity of the habitat, microclimate, and the requirement for chemical inputs such as fertilisers and pesticides). This, in turn, will have effects on how the oil palm ecosystem operates, with changes in biodiversity, ecosystem functions, yield and profitability, and feedbacks between all three. By gaining a better understanding of how management decisions affect the oil palm ecosystem, the BEFTA Programme aims to improve the sustainability of palm oil production. In this paper we present initial results on the effects of management on structural complexity and microclimate. Other published studies from the BEFTA Programme present results on the effects of management on a range of environmental conditions, biodiversity, and functions (Foster et al., 2014; Slade et al., 2014; Kurz et al., 2016; Tao et al., 2016, 2017, 2018; Ashton-Butt et al., 2018, 2019; Spear et al., 2018; Eycott et al., 2019; Hood et al., 2019; Luke et al., 2019a; Woodham et al., 2019). Additional studies, which are still in preparation, will further extend our understanding of these linkages, and also consider impacts on yield, profitability, and recommendations for the industry.
Although they are generally grown as monocultures, the layout of oil palm plantations and different strategies that are used for management mean that there can be substantial habitat variation within plantations. This can include areas within the core and on the edge of plantation blocks, riparian areas, areas planted with beneficial plants, or areas used for cattle grazing. In addition, the oil palm ecosystem can vary over time as a result of changes in seasonal and inter-annual changes in climate. We are assessing this existing variability within the plantation using measurements taken within “business-as-usual” treatments set-up as part of the BEFTA Understory Vegetation Project, the RERTA Project, and also as part of our additional collaborations.
Oil palm plantations often contain dense stands of understory vegetation, which establish and develop over the long lifespan of a plantation (Corley and Tinker, 2003b). This understory complexity has previously been linked to higher levels of biodiversity within plantations (Chung et al., 2000; Nájera and Simonetti, 2010), reduced numbers for some pests (Bedford, 1980), potentially improved ecosystem services (Gitau et al., 2009), and higher rates of leaf-litter decomposition (Ashton-Butt et al., 2018). However, the cover and composition of understory in oil palm is extremely variable, with management and age of the oil palm having a marked effect (Combaz et al., 2010; Luskin and Potts, 2011; Purnomo and Caliman, 2012). In particular, herbicide spraying regimes differ across plantations, especially among smallholders, and can result in a gradient from very sparse understory vegetation to widespread woody growth.
The BEFTA Understory Vegetation Project has established a large-scale, long-term experimental study to investigate the impact of understory management on plantation biodiversity, ecosystem functioning, oil palm yield, and profitability. We have established 18 study plots, distributed across two oil palm estates (Kandista and Ujung Tanjung; Figure 1, Supplementary Table 1) and arranged in six triplets. We chose flat areas of the plantation, between 10 and 30 m above sea level, in areas of mature palm (planted between 1988 and 1993; Supplementary Table 1) and not close to human settlements. Plots are 150 × 150 m (2.25 ha) in area, and were established at the ends of three neighbouring 300 × 1,000 m plantation planting blocks, such that the middle plot in each triplet is 155 m from each of the outer plots within the triplet. Plots are adjacent to plantation access roads and therefore include both core plantation areas and more open roadside edge vegetation, including a drainage ditch (Figure 3).
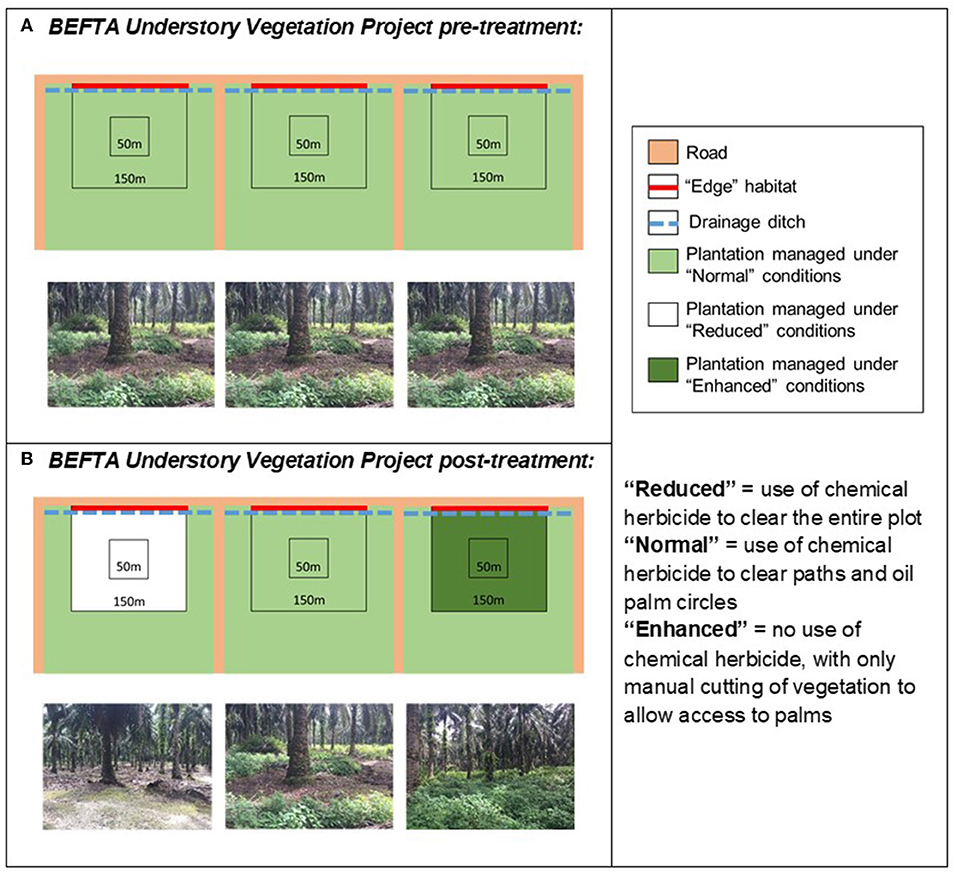
Figure 3. Schematic to show the design of the BEFTA Understory Vegetation Project. Each diagram represents a set of three experimental plots, each of which is 150 × 150 m, within which there is a central 50 × 50 m area (the Core area) where the majority of measurements take place. The roadside edge of the plot is shown as a red line and is referred to as Edge habitat. Each plot is located at the 300 m wide end of a plantation block (300 × 1,000 m; note that the plots and blocks are not drawn to the same scale). (A) Represents the habitat management that all plots received “pre-treatment,” whilst (B) shows how management was altered “post-treatment.” This triplet design is replicated six times across two estates (Kandista and Ujung Tanjung) within the SMARTRI landscape (see Figure 1). In each triplet the order in which Normal, Reduced, and Enhanced treatments were allocated was randomised. This figure is reproduced and adjusted, with permission, from Luke et al. (2019a).
Within each triplet we randomly allocated three different understory management treatments which represent the range of practices currently commonly used within the industry:
• Reduced complexity understory vegetation (hereafter referred to as “Reduced”): representing the most intense level of management used by growers. This treatment involves spraying of all understory vegetation with herbicides. Herbicides used included Glyphosate (Rollup 480 SL), metsulfuron- methyl (Erkafuron 20 WG), Fluroxypyr (Starane 290 EC), Paraquat Dichloride (Rolixone 276 SL—until 2016), and ammonium glufocinate (Rolifos 150 SL—after 2016, following GAR policy change on the use of Paraquat Dichloride). Herbicides were re-applied as necessary, three to five times per year, to maintain a clear understory throughout the plots.
• Normal complexity understory vegetation (hereafter referred to as “Normal”): representing a moderate level of management used by growers and the standard practice within GAR estates. This treatment includes an intermediate level of herbicide spraying (using the herbicides listed above) along harvesting paths and within 1.5 m circles around palms, manual removal of woody vegetation (using a machete), but other vegetation is allowed to regrow. Herbicides were re-applied as necessary, three to five times per year, to maintain clear paths and circles.
• Enhanced complexity understory (hereafter referred to as “Enhanced”): representing the lowest intensity level of management commonly used by growers. This treatment involves no herbicide spraying and only limited manual cutting of vegetation (using a machete) along harvesting paths and in the 1.5 m circle around palms. It also includes removal of large woody vegetation (also using a machete). Manual cutting in Enhanced plots began one year after treatments started, but was then carried out at the same frequency as herbicide application in the Reduced and Normal plots.
Understory management treatments were implemented across the full 150 × 150 m (2.25 ha) area of each study plot, but most data collection took place within a smaller central 50 × 50 m (0.25 ha) area, in order to buffer treatment effects from non-treatment vegetation management in the surrounding oil palm landscape (Figure 3). Plots were established and marked out in October 2012, with treatments implemented in February 2014. This timeframe allowed collection of pre-treatment data to assess the similarity of the plots before understory management was varied experimentally. The set-up therefore follows a Before After Control Impact (BACI) design.
Since October 2012, repeated sets of measurements have been taken in each of the plots to quantify aspects of environmental heterogeneity, biodiversity, ecosystem functioning, yield, and production costs. For environmental measurements, we have recorded canopy cover, soil temperature, soil physical and chemical properties, understory vegetation cover, and vegetation height. We have also recorded measurements of daily temperature and rainfall from within the wider plantation. For biodiversity, we have carried out surveys on a range of different taxonomic groups, including understory plants, canopy invertebrates, understory invertebrates, ground-dwelling invertebrates, soil microbial communities, dragonflies, butterflies, dung beetles, frogs, birds, and mammals. For ecosystem functions, we have measured predation levels, seed removal, herbivory, leaf litter decomposition, dung removal, soil invertebrate activity, and soil microbial functions. For yield and production costs, we measured number and weight of oil palm fruit bunches, recorded herbicide application, and worker-hours devoted to the plots.
In this paper we present pre-treatment data (collected March–April 2013) on vegetation structure and canopy openness to quantify the degree of heterogeneity within the existing plantation, prior to manipulating it. These data were collected using transect techniques (henceforth referred to as “transect vegetation data”), and involved walking along the road edge at the plot boundary (150 m) (“Edge”) or around the central 50 × 50 m area (“Core”) (Figure 3) and recording canopy cover, vegetation height, and vegetation type present every 10 m (therefore a total of 15 points along the road and 20 points around the box). Canopy cover and vegetation height were recorded in the same way as above, and vegetation occurrence was recorded by holding a metre ruler vertically on the ground every 10 m and recording which different understory vegetation types touched the ruler. Categories of understory vegetation were the same as above, although we also included Turnera ulmifolia L., a beneficial plant that is planted along plantation roads to attract predatory and parasitising insect species.
We also present data on the effects of the understory management treatments on canopy cover, vegetation structure, and soil temperature. Canopy cover and vegetation structure data were collected pre- and post-treatment at ~6-monthly intervals between March 2013 and September 2015, and again in May 2017 (each later referred to as “collection time blocks”). They were measured at three points, arranged 50 m apart around the centre of each plot (henceforth referred to as “point vegetation data”). We used a spherical densiometer (Lemmon, 1956) to record canopy cover, taking four measurements in each cardinal direction, before summing and converting to percentage cover. We recorded percentage cover by eye of different understory vegetation categories within a 5 × 5 m area at each point, with the categories fern cover, dead palm frond cover, other herbaceous plant cover, and bare ground. We recorded vegetation height by gently laying a clipboard (A4 Portrait WeatherWriter weighing 500 g) on the vegetation to exclude single tall fronds or grasses and reading off the height against a tape measure. Soil temperature was measured at the same three points using iButton dataloggers [DS1922L-F5 thermochrons (high capacity)]. Dataloggers, set to record temperature every 3 h (starting at 00:00), were enclosed within mesh bags and buried at 5 cm depth at the centre of each point. Temperatures were recorded pre- and post-treatment between April 2013 and August 2017.
Riparian buffers (also known as riparian reserves, margins, strips, and zones) are areas of non-cultivated habitat left beside water bodies in agricultural areas. In tropical agricultural systems, they have been found to have substantial environmental benefits including helping to maintain water quality and hydrological patterns, protecting freshwater biodiversity, providing habitat for terrestrial biodiversity, as well as having the potential to increase carbon storage within agricultural landscapes (Barclay et al., 2017; Luke et al., 2019b). The tropical research conducted to date suggests that wider buffers with higher quality natural habitat can provide the greatest environmental benefits (Gray et al., 2017; Mitchell et al., 2018; Luke et al., 2019b). In Southeast Asia, natural habitat is typically forest, and so forest cover should therefore be retained around waterways where possible, or restored in areas where it has been cleared or degraded during land use change. Riparian protection and restoration are required by national law and sustainability certification bodies such as the RSPO (Roundtable on Sustainable Palm Oil, 2018), but many plantations, particularly those established before such schemes were in place, lack these buffers. In these cases, certification bodies require that riparian areas be restored at replanting time. As a result, there is an urgent need for guidance on how to restore forest in riparian buffers currently planted with oil palm. However, very limited literature is available on strategies for riparian restoration in oil palm plantations.
The Riparian Ecosystem Restoration in Tropical Agriculture (RERTA) Project is a large-scale, long-term riparian restoration project established as part of the BEFTA Programme (Figures 1, 4). In this project, we are making use of scheduled oil palm re-planting across SMARTRI estates to set-up replicate plots of three different riparian restoration strategies that represent plausible options for the industry, along with a no restoration plot required as a control treatment. Each restoration treatment is being tested across a width of 50 m on each side of a river (the Indonesian legal standard for small rivers—defined as those with a watershed ≤ 500 km2 (The Government of Indonesia, 2011), and over a length of 400 m. The treatments are (and see Figure 4):
• Mature palm and enrichment planting buffer. Leave mature palms in place in the buffer area after re-planting, and also carry out enrichment planting with a mix of six native tree species (Treatment A on Figure 4; Supplementary Tables 2, 3).
• Mature palm-only buffer. Leave mature palms in place in the buffer area after re-planting, and allow natural vegetation regrowth (Treatment B on Figure 4; Supplementary Table 2).
• Enrichment planting only buffer. Remove all mature palms from the buffer during re-planting, and carry out enrichment planting with a mix of six native tree species (Treatment C on Figure 4; Supplementary Tables 2, 3).
• No buffer. Re-plant oil palm to the river's edge, therefore acting as a control treatment that has no restoration. (Treatment D on Figure 4; Supplementary Table 3). Such practice would apply to non-certified plantations and smallholders in countries where there are no legal restrictions on planting within river margins.
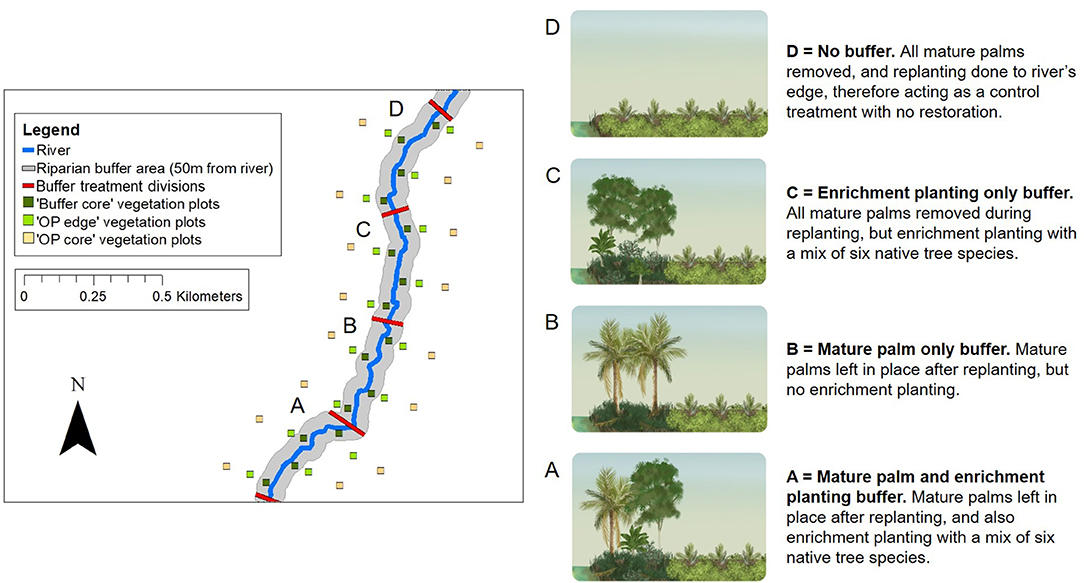
Figure 4. Schematic to show the RERTA plot layout at the RERTA 1 site in Palapa Estate, along with diagrams and explanations of broad habitat conditions in different treatments (A–D) post-treatment. On the map, 25 × 25 m vegetation plots are shown by green and yellow squares with the Buffer Core, OP Edge, and OP Core plots at each distance point along the river comprising a sampling triplet. The red lines across the river denote the treatment start and end points, whilst the grey shading represents the riparian buffer area. On the ground, each experimental riparian treatment is 50 m wide (on each side of the river, and measured perpendicular to the river) and 400 m long (measured along the path of the river), and survey measurements are taken up to 180 m away from the river on each side (measured perpendicular to the river). The study site is therefore 360 m wide, and 1,600 m long in total. The area is transected by harvesting roads, but for clarity, these are omitted from the map. The same design is used at the RERTA 2 site in Kandista Estate. Re-planting and establishment of the riparian restoration treatments was completed at RERTA 1 in 2018, and at RERTA 2 in 2019. The map was made using ArcMap 10.5.1 (Environmental Systems Research Institute (ESRI), 2017) with reference to maps produced by Sinar Mas.
The river sites where we have established the experiment have bankfull widths of ~5–20 m, wetted widths of up to about 5 m, and are mostly wadeable when not in flood. Prior to the experimental set-up, the riparian areas (50 m from the river) were all areas of mature palms, as were the surrounding plantations (planted 1987–1988; Supplementary Table 2). However, the riparian areas have been managed in a less intensive way than the surrounding plantation for the last 10 years in an attempt to bring environmental benefits via a “passive restoration” strategy. This included no use of chemical fertilisers, pesticides, herbicides, or trapping of pests, although vegetation was cut manually along paths to allow access for harvesting. There was also some limited additional planting of vetiver grass [Chrysopogon zizanioides (L.) Roberty] and bamboos to reduce erosion near the river banks.
Within the two enrichment planting treatments (A and C, Figure 4), we planted seedlings in a 4 × 4 m grid within each 50 × 400 m buffer treatment. An equal number of six native tree species (Sungkai, Peronema canescens Jack; Meranti tembaga, Shorea leprosula Miq.; Pulai, Alstonia scholaris (L.) R.Br.; Cempedak, Artocarpus integer (Thunb.) Merr.; Bintangur, Calophyllum inophyllum L.; and Sengon, Albizia chinensis (Osbeck) Merr.) were used, which were chosen from those that were readily available within regional nurseries to together provide a range of functional traits including fruiting, leguminous, and pioneer species (Supplementary Table 3). In addition, two of these species (Shorea leprosula and Peronema canescens) are the same as those used in an enrichment planting experiment in smallholder oil palm plantations in neighbouring Jambi province (EFForTS-BEE, Teuscher et al., 2016), therefore giving the potential for comparisons. The seedlings were planted in a random order across the grid, apart from in set locations where we manipulated the planting to conduct neighbourhood experiments. The neighbourhood experiments consist of focal tree seedlings surrounded by seedlings of the same species or control seedlings with a random mix of all possible species. After an initial period of seedling settlement and replacement for mortality, growth and survival of all seedlings are being monitored at 3–4 month intervals. Within the two treatments that retain mature palms, oil palm fruit is still being harvested whilst the palms continue to produce (as a discouragement to encroachment), but we do not consider them to be “cultivated” in the same sense as the surrounding plantation, because no fertilisers, herbicide, or other pesticides are being added, and so it is likely that yields will decline quickly. Harvesting is conducted by a designated team that have been trained to minimise impacts on the surrounding vegetation.
We have already established the first and second RERTA replicates (RERTA 1 and 2) across two estates (Palapa and Kandista) (Figures 1, 4), with plans for a third, as soon as a suitable site becomes available. We are taking measurements before and after treatments have been set-up, following a similar BACI experimental framework to the BEFTA Understory Vegetation Project. At RERTA 1, pre-treatment data collections were conducted 2017–18 and experimental treatments were set-up in 2018, with initial post-treatment data collection in 2019. At RERTA 2, pre-treatment data collections were conducted 2018–19 and the experimental treatments were set-up in 2019, with post-treatment data collection to follow.
Measurements are being taken at a range of locations including: within the river; within the riparian buffer (“Buffer Core”); in oil palm close to the riparian buffer that may be experiencing spillover effects from the buffer (“OP Edge”); and in oil palm that we expect to be beyond the range of effect of the riparian buffer (“OP Core”; Figure 4). At each location, a central sampling point is surrounded by a 25 × 25 m vegetation plot in which the majority of sampling is focused. Buffer Core points are located 25 m from the river edge, and therefore sit in the middle of the buffer. OP Edge points are located 28.5 m from the edge of the buffer [the distance of two oil palm planting rows (16 m) plus half of the width of the vegetation plot (12.5 m); therefore 78.5 m from the river edge]. OP Core points are located 130 m from the edge of the buffer (therefore 180 m from the river edge; see RERTA 1 plot layout, Figure 4). Buffer Core, OP Edge, and OP Core points and vegetation plots are arranged in triplets, such that one of each point/plot type is found at 16 different distance points along the river (see RERTA 1 plot layout, Figure 4). Each treatment (A, B, C, D on Figure 4) contains four Buffer Core—OP Edge—OP Core triplets of points and vegetation plots. Within each treatment, two triplets are located on each side of the river, in an alternating pattern. This leaves 100 m between replicates on opposite sides of the river, and 200 m between replicates on the same side of the river (see RERTA 1 plot layout, Figure 4).
We are assessing the baseline pre-treatment condition and effects of each riparian restoration treatment on a range of different environmental metrics, biodiversity, ecosystem functions, yield, and profitability (once the replanted palms have matured). For environmental conditions, we are recording soil physical and chemical properties, erosion rates, water quality, rainfall, wind speed and direction, humidity, photosynthetically active radiation (PAR), air temperature, soil temperature, greenhouse gas emissions (methane, nitrous oxide and carbon dioxide), and understory vegetation structure. For biodiversity, we are surveying a range of taxa including canopy invertebrates, understory invertebrates, ground-dwelling invertebrates, soil invertebrates, soil microbial communities, butterflies, dragonflies, dung beetles, Oryctes beetles, frogs, birds, rats, small carnivores, fish, and freshwater invertebrates. For ecosystem functions, we are measuring predation levels, seed removal, herbivory, leaf litter decomposition, dung removal, and soil invertebrate activity. For yield and profitability, we will assess number and weight of oil palm fruit bunches, and any costs associated with crop management and creating and maintaining the buffers.
In this paper, we present baseline pre-treatment data on vegetation structure and temperature at RERTA 1 sites. Both sets of measurements were taken within the 25 × 25 m vegetation plots centred around Buffer Core, OP Edge, and OP Core points. Percentage cover of understory vegetation was assessed by eye within each of the plots. Canopy openness was measured at the centre of the plot using a spherical densiometer (Lemmon, 1956) in the same way as in the BEFTA Understory Vegetation Project plots, but with points aligned with the edges of the vegetation plot rather than compass points. Vegetation surveys were completed in November 2017. Temperature was measured at the centre point of each Buffer Core and OP Core plot using dataloggers in the same way as for the BEFTA Understory Vegetation Project plots, and were deployed October 2017–February 2018.
The experimental and collaborative research set-up of the BEFTA Programme offers opportunities for additional research projects that test the sustainability of management practices. These include:
• Potential for conducting additional analyses using the core data. For example: The BACI design, repeat measures, and sampling of a wide range of variables is allowing us to answer additional questions about the effects of El Niño-related drought (e.g., Eycott et al., 2019).
• Additional measurements or taxonomic surveys within the plots, which were beyond the scope of the core project work. For example: additional details about spider cleptoparasites (Spear et al., 2018), body size, and prey capture behaviour, as well as surveys investigating the value of termite nests as nesting sites for other species.
• Additional experiments within the large-scale experimental framework. For example: ant suppression experiments have been established at the scale of an individual palm within the BEFTA Understory Vegetation Project plots and the seedling neighbourhood experiments that have been established within the RERTA riparian restoration set-up (the latter are described above).
• Additional studies that are within the wider landscape, and have been facilitated by the development of the BEFTA Programme research set-up and collaborations. Example studies include: testing the effectiveness of beneficial planting for herbivory control (Hinsch, 2013); considering the potential for cattle grazing within mature oil palm to provide ecosystem function benefits (Slade et al., 2014); assessing the benefits of Empty Fruit Bunch (EFB) fertilisers and other soil management techniques for soil biodiversity, function, and yield (Tao et al., 2016, 2017, 2018); and measuring ecosystem functions in mature oil palm riparian buffers (Woodham et al., 2019).
• Ability to test the impacts of oil palm replanting. Over the coming years, large areas of oil palm plantations will be reaching the end of their harvestable life and will soon be replanted. Replanting is likely to have substantial impacts on ecosystems across Malaysia and Indonesia, yet very little research has yet been done on this (Snaddon et al., 2013). Several areas of the SMARTRI estates have been replanted over recent years, creating a mosaic of oil palm areas of different ages which can be used as chronosequence to assess the effects of replanting and oil palm age (e.g., Kurz et al., 2016; Ashton-Butt et al., 2019). In addition, RERTA OP Edge and OP Core plots have been replanted since creation of the RERTA restoration treatments, and the BEFTA Understory Vegetation Project plots are in areas of mature oil palm that are soon due for replanting as part of the normal replanting cycle within the plantation. This therefore gives us the opportunity to conduct additional surveys at the same points post-replanting, and compare them to our pre-replanting data, to experimentally test the effects of this disturbance.
We carried out all analyses in R statistical program version 3.4.1 (R Core Team, 2017), using the program R Studio (R Studio Team, 2016), and plotted results using the package “ggplot2” (Wickham, 2009). For “transect vegetation data” from the BEFTA Understory Vegetation Project plots, we created overall vegetation occurrence values for Core and Edge in each plot by calculating the percentage of the 10 m sampling points in Core and Edge at which each vegetation type occurred. Similarly, vegetation heights and canopy openness values were averaged across all points to create single values for Core and Edge per plot. We used these values in subsequent analyses. For “point vegetation data” we included values for each of the three 5 × 5 m quadrats per plot separately in analyses, but accounted for multiple points per plot using random effects within models. We arcsin square root transformed percentage understory vegetation and canopy openness data for use in analyses in order to modify the distribution to better approximate continuous data (Sokal and Rohlf, 1995). For soil temperature data we calculated the mean, maximum, minimum, and daily range in temperature values for each datalogger point over the full time period, and also calculated mean values per datalogger point for each time of day, and used these values as replicates within statistical tests.
To test for differences between different microhabitats within the plantation we first compared vegetation occurrence and canopy cover data from Core and Edge areas within the BEFTA Understory Vegetation Project. We ran linear mixed effects models [lmer with Gaussian error distribution, from the “lme4” package (Bates et al., 2015)], using just the 2013 “transect vegetation data.” We included “microhabitat type” (i.e., Core or Edge) as a fixed effect, and to account for spatial autocorrelation between Core and Edge data collected from the same plots, we included “plot” as a random effect. The model formula used was: lmer(Response~MicrohabitatType+(1|Plot)). We also tested whether the current “passive restoration” practices of lower-intensity vegetation management within the buffer zone in RERTA had created significant differences in microhabitats. We focused on differences in vegetation cover, canopy cover and overall mean, minimum, maximum, and daily temperature range between buffer and non-buffer plots. For the vegetation data we compared Buffer Core, OP Edge, and OP Core points, whilst for the temperature we compared just Buffer Core and OP Core. We ran linear mixed effects models (as above), with “microhabitat type” (i.e., Buffer Core, OP Edge, OP Core) as a fixed effect. To account for spatial autocorrelation between data collected at the same point along the river we included “triplet” as a random effect. The model formula used was: lmer(Response~MicrohabitatType+(1|Triplet)). When considering differences in temperatures at RERTA plots across the day we used “mean temperature” for each time of day as the response, including “microhabitat” and “time of day” (coded as a factor) and the interaction between them as fixed effects. To account for correlations between repeated measures taken from the same sites we included “plot” as a random effect. The model formula used was: lmer(meanTemp~Microhabitat*TimeOfDay+(1|Plot)).
We checked all models for heteroscedasticity and non-normality of residuals, and used log-likelihood ratio test (LLRT) comparisons to assess significance of fixed effects. For the RERTA vegetation and canopy cover models that showed a significant effect of microhabitat, we used the glht function from the “multcomp” package (Hothorn et al., 2008) to determine which microhabitat types (Buffer Core, OP Edge, or OP Core) were significantly different. For the RERTA “time of day” temperature models that showed a significant interaction between “microhabitat” and “time of day” we used the package “lsmeans” (Lenth, 2016) to compare Buffer Core and OP Core temperatures at different time points.
To assess changes in vegetation and canopy cover in the BEFTA Understory Vegetation Project plots as a result of the experimental treatments, we ran linear mixed effects models (as above) using the full pre- and post-treatment “point vegetation data.” We included “treatment type” (i.e., Reduced, Normal, Enhanced), “pre- or post-treatment time period,” and the interaction between the two variables as fixed effects. To account for repeat measures within plots, for spatial autocorrelation of plots within triplets, and correlations between data collected at different sites but in the same time period we included “plot” nested within “triplet,” and “collection time block” as random effects. The model formula used was: lmer(Response~Treatment*PrePost + (1|Triplet/Plot) + (1|TimeBlock)). To test for differences in temperature as a result of the experimental treatments we ran similar models using mean, maximum, minimum, and daily range temperature data. When considering differences in temperatures over the course of the day we used just post-treatment “mean temperature” values for each time of day as the response, including “treatment” and “time of day” (coded as a factor) and the interaction between them as fixed effects. We included “plot” as a random effect to account for correlations between repeated measures taken from the same sites. The model formula used was: lmer(meanTemp~Treatment*TimeOfDay+(1|Plot)).
We checked all models for heteroscedasticity and non-normality of residuals, and used log-likelihood ratio test (LLRT) comparisons to assess significance of fixed effects. In the case of overall vegetation and temperature data, significance of the interaction term (Treatment:PrePost) indicated an effect of the BEFTA Understory Vegetation Project experimental treatments. When there was a significant interaction between “treatment type” and “pre- post-treatment time period” we used the package “lsmeans” to determine which treatments showed a difference between pre- and post-treatment values (i.e., which changed as a result of the experiment), and which treatments the post-treatment differences were between (i.e., which experimental treatments were significantly different from each other). For the “time of day” temperature models that showed a significant interaction between “treatment” and “time of day” we used the package “lsmeans” (Lenth, 2016) to compare Enhanced, Normal and Reduced temperatures at different time points.
In the BEFTA Understory Vegetation Project plots, we found differences in vegetation cover between Core and Edge areas before understory management treatments started, highlighting the heterogeneity existing within oil palm plantations (Figure 5). Canopy openness was about six times higher in Edge compared to Core areas (Figure 5A; LLRT comparison = 59.453, p < 0.001). Percentage cover of ferns (Figure 5B; LLRT comparison = 4.714, p = 0.030) and beneficial plants (T. ulmifolia) (Figure 5C; LLRT comparison = 17.509, p < 0.001) were substantially higher in Edge than in Core areas, whilst percentage cover of frond piles was lower (Figure 5D; LLRT comparison X2(1) = 15.226, p < 0.001). Bare ground cover was also lower in Edge than in Core habitat, but the difference was not significant at the p < 0.05 level (Figure 5E; LLRT comparison = 3.682, p = 0.055).
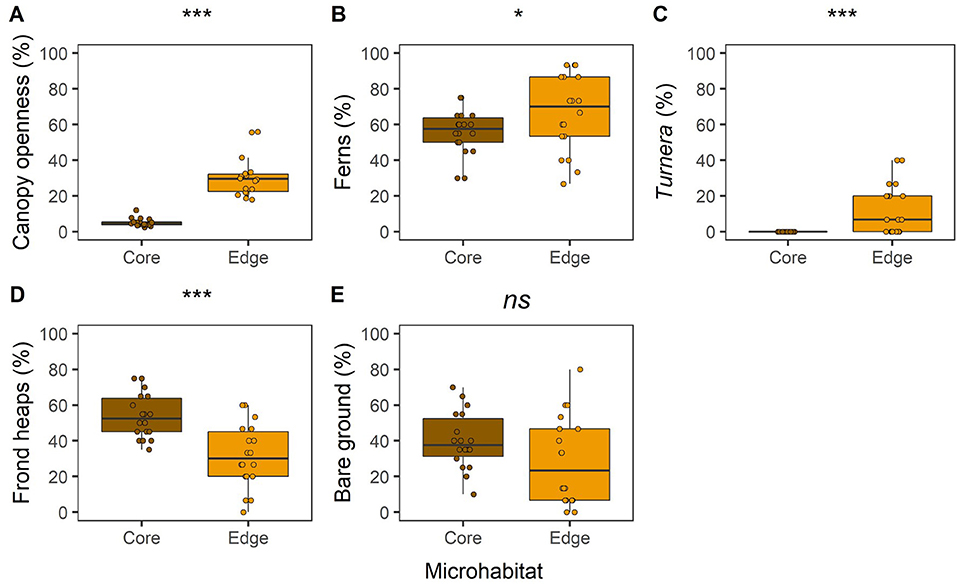
Figure 5. Boxplots to show differences in percentage canopy openness (A); fern occurrence (B); Turnera ulmifolia beneficial plant occurrence (C); dead palm frond occurrence (D); and bare ground occurrence (E), between “transect vegetation data” surveyed at Core (the 50 × 50 m recording box) and Edge (plot boundary) microhabitats of the BEFTA Understory Vegetation Project plots. Occurrence values give the percentage of the 10 m sampling points at which each vegetation type was found. In each case, data were collected from across all plots (Normal, Enhanced, and Reduced) during the pre-treatment time period, and therefore all plots were being managed in the same way (Normal, “business-as-usual”). The boxplots indicate median, and interquartile ranges across plots, with all raw data points (n = 18 plots) overlaid as coloured circles. Asterisks above boxplots indicate results of LLRT comparisons for differences between Core and Edge microhabitats: ***p < 0.001; *p < 0.01; and ns, not significant.
In the RERTA plots, understory vegetation cover was lower in OP Core than in Buffer Core and OP Edge, but the difference was not significant at the p < 0.05 level (Figure 6A; LLRT comparison = 5.562, p = 0.062). There was also no difference or clear trend in canopy openness between plots (Figure 6B; LLRT comparison = 3.695, p = 0.158). Buffer Core and OP Core sites were similar in temperature, with no differences in overall mean, maximum, or minimum temperatures recorded by underground dataloggers across the two sets of sites (Figure 6C; Mean temperature: LLRT comparison = 0.249, p = 0.618; Maximum temperature: LLRT comparison = 0.343, p = 0.558; and Minimum temperature: LLRT comparison = 0.693, p = 0.405). However, there was a tendency for Buffer Core sites to be more stable in temperature across the day than OP Core sites (Figure 6D). Differences in daily temperature range were small (Daily temperature range: LLRT comparison = 0.004, p = 0.952), and there were no substantial differences in the temperature at each different time of day (lsmeans, p > 0.05) (Figure 6D; Supplementary Table 4).
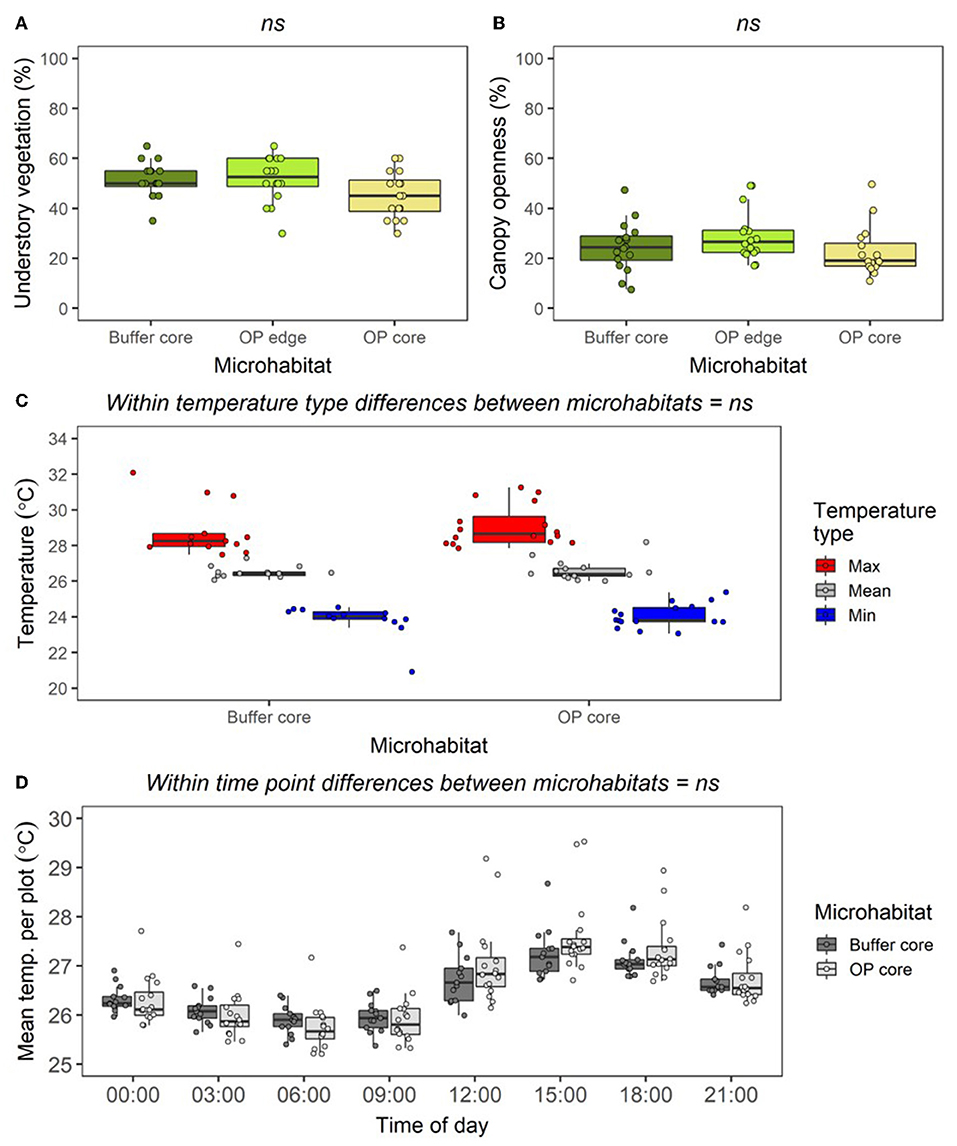
Figure 6. Boxplots to show differences in percentage cover of understory vegetation (A); percentage canopy openness (B); mean, maximum, and minimum soil temperature (at depth of 5 cm) per plot across all times combined (C); mean soil temperature per plot at different times of day (D), between plots in the Buffer Core, OP Edge, and OP Core RERTA 1 vegetation plots. All data were collected from across all RERTA 1 treatments (treatments A, B, C, and D) during the pre-treatment time period. During this period, all points were mature oil palm, but Buffer Core plots were being managed in a “lower impact” way than the OP Edge and OP Core plots, with no herbicide application. The boxplots indicate median, and interquartile ranges across vegetation quadrats, with all raw data points overlaid as coloured circles. For the vegetation data n = 16 per microhabitat; for the temperature data, n = 16 for OP Core and n = 13 for Buffer Core (owing to datalogger breakages). Text above boxplots shows results of LLRT comparisons for differences between microhabitats: ns = not significant.
Adjusting understory management as part of the BEFTA Understory Vegetation Project had substantial effects on percentage cover of ferns, other plants, bare ground, and fronds, as well as on vegetation height (shown by p < 0.05 values in LLRT comparisons for interactions between understory vegetation treatment and experimental time period (pre- and post-treatment) within our models; Figure 7; Supplementary Table 5). Vegetation height decreased in all treatments during the post-treatment period (lsmeans p < 0.001; Figure 7B; Supplementary Table 5) probably in relation to reduced rainfall during the survey period (Supplementary Figure 1). However, the post-treatment decrease in vegetation height was more substantial in the Reduced plots than in the Enhanced and Normal, leading to post-treatment differences in Reduced cf. Normal (lsmeans p < 0.001) and Reduced cf. Enhanced (lsmeans p < 0.001), but not in Normal cf. Enhanced plots (lsmeans p = 0.716) (Figure 7B; Supplementary Table 5).
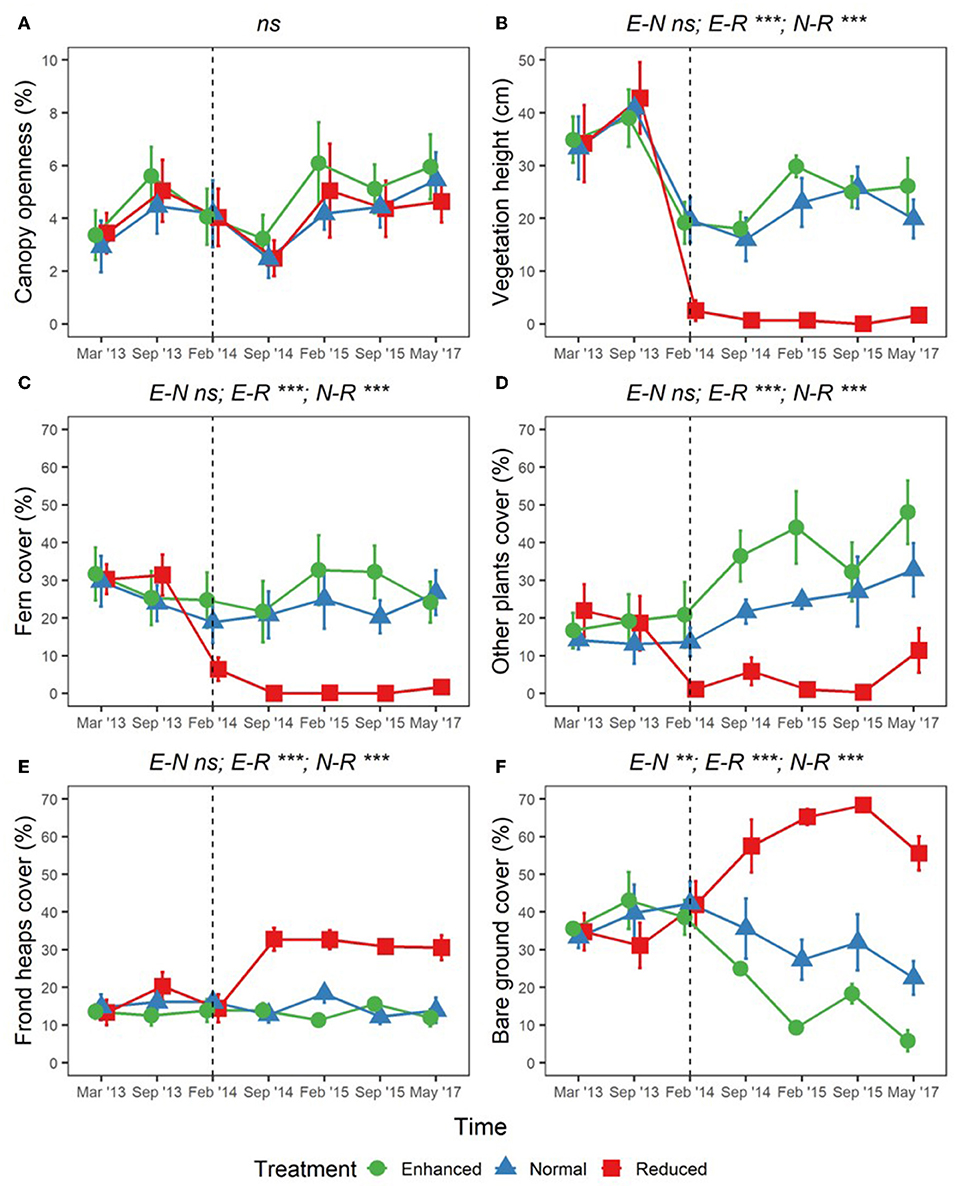
Figure 7. Changes in percentage canopy openness (A); vegetation height (B); percentage cover of ferns (C); percentage cover of other plants (D); percentage cover of frond heaps (E); and percentage cover of bare ground (F), across BEFTA Understory Vegetation Project plots 2013–2017 (“point vegetation data”). Data are split by plot treatment type (Enhanced, Normal, and Reduced) with the legend applicable to all six panels. Points represent the mean (±1SE) across six replicate plots (except February 2015 where n = 5 owing to missing data), where each plot value is the mean of three 5 × 5 m quadrats within it. The vertical dotted lines indicate the beginning of treatments. Before this point (March 2013, September 2013) all plots received the “business-as-usual” Normal understory management. From February 2014 onwards plots received different understory management according to their treatment designation (Enhanced, Normal, or Reduced). The surveys in February 2014 were conducted post-treatment. Asterisks and letters above each graph denote significance of differences between treatments [in the case of a single result, LLRT comparison results showing overall effect of Treatment:PrePost; and in the case of three results, where the significant differences lie after an overall significant result (results of lsmeans test), Supplementary Table 4]: ***p < 0.001, **p < 0.01, ns, not significant. Points have been jittered around each time point to avoid overlap and therefore aid viewing.
Post-treatment percentage cover of ferns remained similar to pre-treatment values in Enhanced and Normal plots (lsmeans p = 0.308, and p = 0.1208, respectively), but decreased substantially in Reduced plots (lsmeans p < 0.001; Figure 7C; Supplementary Table 5). This meant that there were large post-treatment differences in fern cover in Reduced cf. Normal (lsmeans p < 0.001) and Reduced cf. Enhanced (lsmeans p < 0.001), but not in Normal cf. Enhanced plots (lsmeans p = 0.889; Figure 7C; Supplementary Table 5). Percentage cover of other plants also decreased in Reduced plots post-treatment (lsmeans p = 0.009), but unlike fern cover, cover of other plants increased slightly post-treatment in the Normal plots and, to a greater extent, in Enhanced plots (lsmeans p = 0.114 and p = 0.028, respectively; Figure 7D; Supplementary Table 5). Post-treatment percentage cover of other plants was therefore substantially different for Reduced cf. Enhanced (lsmeans p < 0.001) and Reduced cf. Normal (lsmeans p < 0.001) plots, and also, to a lesser extent between Enhanced cf. Normal plots (lsmeans p = 0.059) (Figure 7D; Supplementary Table 5).
Frond heap cover remained similar in Normal and Enhanced plots pre- and post-treatment, but increased substantially in Reduced treatment plots (lsmeans p < 0.001). This meant that post-treatment frond levels were significantly higher in Reduced cf. Enhanced (lsmeans p < 0.001) and Reduced cf. Normal plots (lsmeans p < 0.001), but showed no difference in Enhanced cf. Normal plots (lsmeans p = 0.871; Figure 7E; Supplementary Table 5). Bare ground cover increased substantially in Reduced plots post-treatment compared to pre-treatment, stayed consistent in Normal plots, and decreased in Enhanced plots (Figure 7F; Supplementary Table 5). This meant that bare ground levels were different between all plot pairings post-treatment (lsmeans p < 0.010; Figure 7F; Supplementary Table 5).
We found no differences in canopy openness as a result of the experiment (LLRT comparisons p = 0.444; Figure 7A; Supplementary Table 5). We also found no differences in overall mean, maximum, minimum, or daily range of soil temperature as a result of understory experimental treatments (LLRT comparison p > 0.05) (Figures 8A–C; Supplementary Table 5), or when looking specifically at the majority of time points throughout the day (lsmeans p > 0.05) (Figure 8D; Supplementary Table 6). However, mean temperatures at 15:00 were the exception, with Reduced plots being an estimated 0.4°C warmer than Enhanced and Normal plots (lsmeans p = 0.032 and p = 0.029; Figure 8D; Supplementary Table 6). There was also a tendency for Enhanced and Normal plots to be more stable in temperature across the day than Reduced plots, but differences in daily range in temperature between plot types were not significant (LLRT comparison p = 0.496; Figure 8D; Supplementary Table 5). In all treatments, there were trends of increasing mean and maximum temperatures, and decreasing minimum temperatures 2013–2017 (lsmeans p < 0.05; Figures 8A–C; Supplementary Table 7), suggesting that, overall, soil temperatures got hotter and more variable over time during this period.
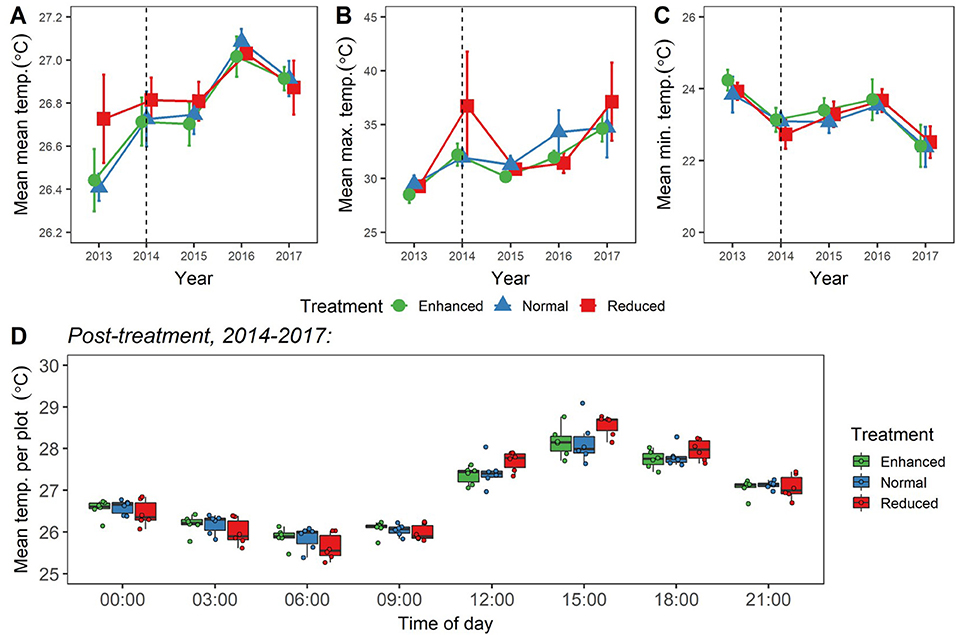
Figure 8. (A–C) Changes in values of mean (A); maximum (B); and minimum (C) soil temperature (at depth of 5 cm) for the BEFTA Understory Vegetation Project plots, 2013–2017. Data are split by plot treatment type (Enhanced, Normal, and Reduced) with the legend applicable to all three panels. Points represent the mean (±1SE) across six replicate plots (except Enhanced 2013, 2014, 2015; Normal 2013; and Reduced 2013, 2014, 2015, 2016, which—owing to datalogger breakages—are means from 2, 4, 4, 3, 3, 5, 5, 5 plots, respectively). Each plot mean is generated from >500 individual datalogger measurements, from 1 to 3 dataloggers placed within each plot. Points have been jittered around each time point to avoid overlap and therefore aid viewing. The dotted vertical line gives an indication of when experimental understory treatments began (February 2014). The points plotted for 2014 therefore represent a mixture of pre- and post-treatment data values. Although a single set of 2014 points has been presented here for clarity, whether 2014 data points were pre- or post-treatment has been fully accounted for in analyses. (D) Mean “post-treatment” temperature per plot split by plot treatment type (Enhanced, Normal, and Reduced), and time of day. Boxplots indicate median values, interquartile ranges, and show raw data points for each plot (n = 6) overlaid as coloured circles. Each plot mean is generated from >300 individual datalogger measurements taken “post-treatment,” from 1 to 3 dataloggers placed within each plot.
We found significant environmental heterogeneity within oil palm estates as a result of spatial layout of the plantation. Areas on the edge of blocks had canopies that were more open, had higher cover of ferns and beneficial plants (T. ulmifolia), and lower frond pile coverage than areas in the core of blocks. Oil palm plantations contain a high density of access roads to facilitate harvesting and collection of fruits, therefore, areas of open block-edge habitat are intermingled with more closed block-core habitat across the whole plantation, allowing both closed- and open-habitat species to have patchy, but relatively connected populations. As well as spatial heterogeneity across areas of the plantation, we observed changes in vegetation structure over time, with declines in vegetation height seen in the Normal plots over the course of the experiment, where management strategies were not varied. These changes are probably linked to decreases in rainfall that occurred in 2014 and 2015, likely in association with an El Niño-related drought (Santoso et al., 2017). We also observed increases in temperature, and in variability of temperature, over the course of our study, perhaps also in association with this El Niño event, or as a result of gradual climate warming over time. Taken together, this variability emphasises the benefits of carrying out BACI experiments that can assess the effects of treatments across the range of environmental conditions found in the system.
In addition to spatial and temporal differences in habitat across the plantation, we also found that significant heterogeneity could be introduced through different understory management practices. The BEFTA Understory Vegetation Project plots were treated with three different vegetation management strategies that represented the range of practices currently in use within plantations in Southeast Asia. As would be expected with use of different intensities of weed control, the three plot treatments showed significant differences in percentage cover of ferns, other plants, bare ground, and fronds, as well as in overall ground vegetation height, with widespread use of herbicides having more extreme effects on habitat structure than selective use of manual cutting. Our experiment therefore demonstrates how everyday management decisions that are made within plantations have a substantial effect on the vegetation complexity, indicating that there is potential for managers to select more biodiversity-friendly practices within plantations. The analyses of initial biodiversity and ecosystem functioning data from the BEFTA Understory Vegetation Project plots show that loss of vegetation reduces: plant diversity (Luke et al., 2019a); soil macrofaunal diversity and abundance and litter decomposition (Ashton-Butt et al., 2018); density and body size of Nephila spp. spiders; and leopard cat activity (Hood et al., 2019). Further upcoming analyses that bring together the biodiversity, ecosystem function, yield, and profitability data from the BEFTA Understory Vegetation Project will provide further valuable information which can be used to inform vegetation management decisions. Other recent studies have also successfully manipulated vegetation structure and diversity within plantations, for example via intercropping with fruit trees and other crops (Teuscher et al., 2016; Gérard et al., 2017; Ashraf et al., 2018), and have found substantial impacts on birds and invertebrates (Teuscher et al., 2016), arthropod diversity and ecosystem functions (Ashraf et al., 2018), and potential benefits for yield (Gérard et al., 2017). Recent early results from a study testing the effects of herbicide and fertiliser inputs on oil palm ecosystems are indicating that lower intensity management can help support biodiversity, without significant harm to yield (Darras et al., 2019).
In comparison to the significant vegetation differences achieved with active changes in herbicide and cutting regimes in the BEFTA Understory Vegetation Project, areas within 50 m of streams at the RERTA site that had reduced-intensity vegetation management—including no use of chemical herbicide—showed no significant improvements in understory vegetation levels, canopy cover, or temperature when compared to areas within the core of the block. Ecosystem functioning has also been shown to vary little between mature palm riparian buffer areas and core oil palm (Woodham et al., 2019). This indicates that the benefits of the “passive riparian restoration” strategy (reduced intensity management, no use of chemical fertilisers, pesticides, herbicides, or trapping of pests within 50 m of the river) that was used in RERTA sites prior to replanting may be limited in supporting the development of vegetation structure in the system. This could particularly be a problem in plantation-dominated landscapes where there is limited availability of native seeds and/or seed dispersers to enable natural regeneration to occur. More active restoration is likely to be needed to increase vegetation complexity beyond what is currently present within the wider plantation. This gives extra support to conclusions drawn from the literature (Luke et al., 2019b) that developing strategies for the active restoration of riparian buffers is necessary within tropical agricultural systems. Experiments such as the RERTA Project are therefore timely and important. The RERTA Project will test three different restoration strategies (mature palms only; mature palms and native tree enrichment planting; no mature palms and native tree enrichment planting) against a control (replanting palms to the river edge) and assess their feasibility, and value for biodiversity, ecosystem functions, yield, and profitability in the surrounding plantation.
The BEFTA Programme has successfully established large-scale, long-term, multidisciplinary experiments, and associated collaborative projects, to enable us to understand how the oil palm ecosystem works, and to test the effects of different plantation management strategies on a range of environmental conditions, biodiversity, ecosystem functions, and plantation productivity. It is already clear that tropical forest ecosystems are highly complex, and one of the best ways to understand them is to conduct large-scale whole-ecosystem experimental manipulations (Fayle et al., 2015). Even though oil palm plantations are structurally and taxonomically simplified compared to tropical forests, our findings highlight the complexity and variability that exists within tropical agricultural landscapes (see also Luke et al., 2019a). Large-scale manipulative experiments therefore offer a valuable tool for testing the sustainability of agricultural management options, allowing the causal links between an environmental change and its consequences within the ecosystem to be determined much more reliably than can be achieved from correlative studies alone.
We hope that the core results from the BEFTA Understory Vegetation Project and RERTA riparian restoration experiment will yield important information about how best to manage ground vegetation in oil palm plantations and to restore degraded riparian areas, in order to balance the benefits for biodiversity, ecosystem functions, and crop profitability. The BEFTA Programme also offers a valuable framework for substantial extra study. This includes sub-experiments within the existing experimental framework; surveys of additional taxonomic groups or variables; additional analyses; use of the programme infrastructure to facilitate separate related projects; and monitoring of long-term changes in relation to climate patterns or replanting cycles. Overall, therefore, the Programme offers a highly valuable long-term, experimental, interdisciplinary, and international collaborative project. It provides substantial potential to address current oil palm research priorities (Padfield et al., 2019), generating industry- and conservation-relevant research, as well as providing support for collaborations, training, and within-country development.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This study was exempt from ethics approval as according to Indonesian regulation Undang 440 undang no. 12. 1992 pest monitoring must be carried out in plantations. These data were collected as part of ongoing monitoring in the plantations, following standard industry operating procedures. No change in the monitoring procedure was made for this study. An ethics approval was not required for this study as per applicable institutional and national guidelines and regulations.
ET, WF, JS, ES, J-PC, and SL designed the experimental manipulations. ADA, AAKA, AA-B, HB, JPD, JD, AD, AE, MH, JH, AH, CK, DK, DM, KM, MN, MP, GP, SPs, P, DP, RP, SPu, TR, ES, So, DS, Su, DT, H-HT, RT, RW, HW, RHW, CW, J-PC, JS, WF, ET, and SL contributed to the design of survey protocols, and to the running of data collection. DA, E, and W facilitated data collection. SL performed the data analyses and led the writing of the manuscript. All authors commented on the manuscript.
This work was funded by The Isaac Newton Trust Cambridge, Golden Agri Resources, ICOPE (the International Conference on Oil Palm and the Environment), and the Natural Environment Research Council (grant number NE/P00458X/1). Open access publication was funded by the University of Cambridge's Open Access Service, as a result of this project's Research Council UK (RCUK) funding.
Co-authors listed with a Sinar Mas Agro Resources and Technology Research Institute (SMARTRI) affiliation were employed by SMARTRI, the research division of Golden Agri Resources (GAR), at the time of the study. Co-authors listed with a Sinar Mas Agro Resources Technology PT Ivo Mas Tunggal affiliation were estate managers employed by PT Ivo Mas Tunggal (a subsidiary company of GAR), at the time of the study. The wider BEFTA Programme is co-funded by GAR. However, there is an MOU in place that protects the intellectual freedom and data-use for all researchers working on the project. The Programme therefore represents a collaboration between the University of Cambridge and an oil palm company, ensuring that results are readily disseminated to inform best management practices, but maintaining academic independence.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Pt Ivo Mas Tunggal and Golden Agri resources for allowing us to conduct research in their plantations, and we are grateful to the staff of SMARTRI for their help with fieldwork. We thank Damayanti Buchori and Purnama Hidayat for their support of the project, and also Kevin Darras, Clara Zemp, the wider EFForTS Project team, and Tom Swinfield for their advice and discussions. We thank RISTEK for permission for ET, SL, and AE to conduct research in Indonesia to set-up the core aspects of the BEFTA Programme (ET: permit numbers 426/SIP/FRP/SM/XI/2012, 72/EXT/ SIP/FRP/SM/IX/2013, 44/EXT/SIP/FRP/SM/IX/2014; SL: permit numbers 354/SIP/FRP/E5/Dit.KI/X/2016, 66/EXT/SIP/FRP/E5/Dit.KI/IX/2017, 45/EXT/SIP/FRP/E5/Dit.KI/X/2018; AE: permit number 189/SIP/FRP/E5/Dit.KI/IV/2017). We thank two reviewers for their suggested improvements to the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2019.00075/full#supplementary-material
Allen, K., Corre, M. D., Tjoa, A., and Veldkamp, E. (2015). Soil nitrogen-cycling responses to conversion of lowland forests to oil palm and rubber plantations in Sumatra, Indonesia. PLoS ONE 10:e0133325. doi: 10.1371/journal.pone.0133325
Ashraf, M., Zulkifli, R., Sanusi, R., Tohiran, K. A., Terhem, R., Moslim, R., et al. (2018). Alley-cropping system can boost arthropod biodiversity and ecosystem functions in oil palm plantations. Agric. Ecosyst. Environ. 260, 19–26. doi: 10.1016/j.agee.2018.03.017
Ashton-Butt, A., Aryawan, A. A. K., Hood, A. S. C., Naim, M., Purnomo, D., Suhardi, et al. (2018). Understory vegetation in oil palm plantations benefits soil biodiversity and decomposition rates. Front. For. Glob. Chang. 1:10. doi: 10.3389/ffgc.2018.00010
Ashton-Butt, A., Willcock, S., Purnomo, D., Suhardi, Aryawan, A. A. K., Wahyuningsih, R., et al. (2019). Replanting of first-cycle oil palm results in a second wave of biodiversity loss. Ecol. Evol. 3.5218. doi: 10.1002/ece3.5218
Barclay, H., Gray, C. L., Luke, S. H., Nainar, A., Snaddon, J. L., and Turner, E. C. (2017). RSPO Manual on Best Management Practices (BMPs) for the Management and Rehabilitation of Riparian Reserves.
Barnes, A. D., Allen, K., Kreft, H., Corre, M. D., Jochum, M., Veldkamp, E., et al. (2017). Direct and cascading impacts of tropical land-use change on multi-trophic biodiversity. Nat. Ecol. Evol. 1, 1511–1519. doi: 10.1038/s41559-017-0275-7
Barnes, A. D., Jochum, M., Mumme, S., Haneda, N. F., Farajallah, A., Widarto, T. H., et al. (2014). Consequences of tropical land use for multitrophic biodiversity and ecosystem functioning. Nat. Commun. 5:5351. doi: 10.1038/ncomms6351
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bedford, G. O. (1980). Biolog, ecology, and control of palm rhinoceros beetles. Ann. Rev. Entomol. 25, 309–339. doi: 10.1146/annurev.en.25.010180.001521
Benton, T. G., Vickery, J. A., and Wilson, J. D. (2003). Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol. Evol. 18, 182–188. doi: 10.1016/S0169-5347(03)00011-9
Bihn, J. H., Gebauer, G., and Brandl, R. (2010). Loss of functional diversity of ant assemblages in secondary tropical forests. Ecology 91, 782–792. doi: 10.1890/08-1276.1
Bommarco, R., Kleijn, D., and Potts, S. G. (2013). Ecological intensification: harnessing ecosystem services for food security. Trends Ecol. Evol. 28, 230–238. doi: 10.1016/j.tree.2012.10.012
Brownrigg, R. (2016). Package ‘maps’: draw geographical maps. Original S code by Richard, A. Becker, Allan, R. Wilks. R version by Ray Brownrigg. Enhancements by Thomas P. Minka and Alex Deckmyn. R Packag. version 3.1.1.$$
Cattau, M. E., Marlier, M. E., and DeFries, R. (2016). Effectiveness of Roundtable on Sustainable Palm Oil (RSPO) for reducing fires on oil palm concessions in Indonesia from 2012 to 2015. Environ. Res. Lett. 11:105007. doi: 10.1088/1748-9326/11/10/105007
Chung, A. Y., Eggleton, P., Speight, M. R., Hammond, P. M., and Chey, V. K. (2000). The diversity of beetle assemblages in different habitat types in Sabah, Malaysia. Bull. Entomol. Res. 90, 475–496. doi: 10.1017/S0007485300000602
Clough, Y., Krishna, V. V., Corre, M. D., Darras, K., Denmead, L. H., Meijide, A., et al. (2016). Land-use choices follow profitability at the expense of ecological functions in Indonesian smallholder landscapes. Nat. Commun. 7:13137. doi: 10.1038/ncomms13137
Combaz, D., Wohlfahrt, J., Deddy, Verwilghen, A., and Caliman, J. P. (2010). “Vascular plant biodiversity in an established oil palm plantation,” in International Conference on Oil Palm and the Environment (Bali: ICOPE).
Corley, R., and Tinker, P. (2003a). “Site selection and land preparation,” in The Oil Palm, 4th Edn (Oxford: Blackwell Science Ltd.), 234–269.
Corley, R., and Tinker, P. (2003b). “Care and maintenance of oil palms”. in The Oil Palm, 4th Edn. (Blackwell Science Ltd.), 287–326.
Darras, K. F. A., Corre, M. D., Formaglio, G., Tjoa, A., Potapov, A., Brambach, F., et al. (2019). Reducing fertilizer and avoiding herbicides in oil palm plantations-ecological and economic valuations. Front. For. Glob. Change. doi: 10.3389/ffgc.2019.00065
Dislich, C., Keyel, A. C., Salecker, J., Kisel, Y., Meyer, K. M., Auliya, M., et al. (2017). A review of the ecosystem functions in oil palm plantations, using forests as a reference system. Biol. Rev. 92, 1539–1569. doi: 10.1111/brv.12295
Drescher, J., Rembold, K., Allen, K., Beckschäfer, P., Buchori, D., Clough, Y., et al. (2016). Ecological and socio-economic functions across tropical land use systems after rainforest conversion. Philos. Trans. R. Soc. Lond. B Biol. Sci. 231, 1–7. doi: 10.1098/rstb.2015.0275
Eycott, A. E., Advento, A. D., Waters, H. S., Luke, S. H., Aryawan, A. A. K., Hood, A. S., et al. (2019). Resilience of ecological functions to drought in an oil palm agroecosystem. Environ. Res. Commun. 1, 101004–101004. doi: 10.1088/2515-7620/ab48da
FAO (2018). “FAO,” in Food Agric. Organ. United Nations, Stat. Div. Available online at: http://www.fao.org/faostat/en/#home (accessed Sep 3, 2018).
FAO (2019). “FAO,” in Food Agric. Organ. United Nations, Stat. Div. Available online at: http://www.fao.org/faostat/en (accessed Feb 26, 2019).
Fayle, T. M., Turner, E. C., Basset, Y., Ewers, R. M., Reynolds, G., and Novotny, V. (2015). Whole-ecosystem experimental manipulations of tropical forests. Trends Ecol. Evol. 30, 1–13. doi: 10.1016/j.tree.2015.03.010
Fitzherbert, E. B., Struebig, M. J., Morel, A., Danielsen, F., Carsten A.BrühlC., A., Donald, P. F., et al. (2008). How will oil palm expansion affect biodiversity? Trends Ecol Evol 23, 538–545. doi: 10.1016/j.tree.2008.06.012
Foley, J. A., Asner, G. P., Costa, M. H., Coe, M. T., DeFries, R., Gibbs, H. K., et al. (2007). Amazonia revealed: forest degradation and loss of ecoystem goods and services in the Amazon Basin. Front. Ecol. Environ. 5, 25–32. doi: 10.1890/1540-9295(2007)5[25:ARFDAL]2.0.CO;2
Foley, J. A., Defries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R., et al. (2005). Global consequences of land use. Science 309, 570–574. doi: 10.1126/science.1111772
Foley, J. A., Ramankutty, N., Brauman, K. A., Cassidy, E. S., Gerber, J. S., Johnston, M., et al. (2011). Solutions for a cultivated planet. Nature 478, 337–342. doi: 10.1038/nature10452
Foster, W. A., Snaddon, J. L., Advento, A. D., Agung, A. A., Barclay, H., Caliman, J. P., et al. (2014). The Biodiversity and ecosystem function in tropical agriculture (BEFTA) project, Vol. 90. Kuala Lumpur: The Planter, 581–591.
Foster, W. A., Snaddon, J. L., Turner, E. C., Fayle, T. M., Cockerill, T. D., Ellwood, M. D., et al. (2011). Establishing the evidence base for maintaining biodiversity and ecosystem function in the oil palm landscapes of South East Asia. Philos. Trans. R. Soc. B Biol. Sci. 366, 3277–3291. doi: 10.1098/rstb.2011.0041
Gérard, A., Wollni, M., Hölscher, D., Irawan, B., Sundawati, L., Teuscher, M., et al. (2017). Oil-palm yields in diversified plantations: Initial results from a biodiversity enrichment experiment in Sumatra, Indonesia. Agric. Ecosyst. Environ. 240, 253–260. doi: 10.1016/j.agee.2017.02.026
Gibbs, H. K., Ruesch, A. S., Achard, F., Clayton, M. K., Holmgren, P., Ramankutty, N., et al. (2010). Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc. Natl. Acad. Sci. U.S.A. 107, 16732–16737. doi: 10.1073/pnas.0910275107
Gibson, L., Lee, T. M., Koh, L. P., Brook, B. W., Gardner, T. A., Barlow, J., et al. (2011). Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478, 378–381. doi: 10.1038/nature10425
Gitau, C. W., Gurr, G. M., Dewhurst, C. F., Fletcher, M. J., and Mitchell, A. (2009). Insect pests and insect-vectored diseases of palms. Aust. J. Entomol. 48, 328–342. doi: 10.1111/j.1440-6055.2009.00724.x
Gras, P., Tscharntke, T., Maas, B., Tjoa, A., Hafsah, A., and Clough, Y. (2016). How ants, birds and bats affect crop yield along shade gradients in tropical cacao agroforestry. J. Appl. Ecol. 53, 953–963. doi: 10.1111/1365-2664.12625
Gray, C., Slade, E., Mann, D., and Lewis, O. (2017). Designing oil palm landscapes to retain biodiversity using insights from a key ecological indicator group. BioRxiv. doi: 10.1101/204347
Green, R., Cornell, S., Scharlemann, J., and Balmford, A. (2005). Farming and the fate of wild nature. Science 307, 550–555. doi: 10.1126/science.1106049
Guillaume, T., Damris, M., and Kuzyakov, Y. (2015). Losses of soil carbon by converting tropical forest to plantations: erosion and decomposition estimated by δ 13 C. Glob. Chang. Biol. 21, 3548–3560. doi: 10.1111/gcb.12907
Hassler, E., Corre, M. D., Tjoa, A., Damris, M., Utami, S. R., and Veldkamp, E. (2015). Soil fertility controls soil–atmosphere carbon dioxide and methane fluxes in a tropical landscape converted from lowland forest to rubber and oil palm plantations. Biogeosci. Discuss 12, 9163–9207. doi: 10.5194/bgd-12-9163-2015
Hinsch, J. (2013). Substituting Pesticides With Biodiversity–The Potential for Biocontrol in Oil Palm Plantations. Copenhagen: University of Copenhagen.
Hood, A. S. C., Aryawan, A. A. K., Advento, A. D., Purnomo, D., Wahyuningsih, R., Luke, S. H., et al. (2019). Understory vegetation in oil palm plantations promotes leopard cat activity, but does not affect rats or rat damage. Front. For. Glob. Chang. 2:51. doi: 10.3389/ffgc.2019.00051
Hothorn, T., Bretz, F., and Westfall, P. (2008). Simultaneous inference in general parametric models. Biometrical. J. 50, 346–363 doi: 10.1002/bimj.200810425
Isbell, F., Adler, P. R., Eisenhauer, N., Fornara, D., Kimmel, K., Kremen, C., et al. (2017). Benefits of increasing plant diversity in sustainable agroecosystems. J. Ecol. 105, 871–879. doi: 10.1111/1365-2745.12789
Kotowska, M. M., Leuschner, C., Triadiati, T., and Hertel, D. (2016). Conversion of tropical lowland forest reduces nutrient return through litterfall, and alters nutrient use efficiency and seasonality of net primary production. Oecologia 180, 601–618. doi: 10.1007/s00442-015-3481-5
Kotowska, M. M., Leuschner, C., Triadiati, T., Meriem, S., and Hertel, D. (2015). Quantifying above- and belowground biomass carbon loss with forest conversion in tropical lowlands of Sumatra (Indonesia). Glob. Chang. Biol. 21, 3620–3634. doi: 10.1111/gcb.12979
Kremen, C. (2015). Reframing the land-sparing/land-sharing debate for biodiversity conservation. Ann. N. Y. Acad. Sci. 1335, 52–79. doi: 10.1111/nyas.12845
Kremen, C., Williams, N. M., and Thorp, R. W. (2002). Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. U.S.A. 99, 16812–16816. doi: 10.1073/pnas.262413599
Kurniawan, S., Corre, M. D., Matson, A. L., Schulte-Bisping, H., Utami, S. N. H., van Straaten, O., et al. (2018). Conversion of tropical forests to smallholder rubber and oil palm plantations impacts nutrient leaching losses and nutrient retention efficiency in highly weathered soils. Biogeosciences 15, 5131–5154. doi: 10.5194/bg-15-5131-2018
Kurz, D. J., Turner, E. C., Aryawan, A. A., Barkley, H. C., Caliman, J. P., Konopik, O., et al. (2016). Replanting reduces frog diversity in oil palm. Biotropica. 48, 483–490. doi: 10.1111/btp.12320
Laurance, W. F., Sayer, J., and Cassman, K. G. (2014). Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 29, 107–116. doi: 10.1016/j.tree.2013.12.001
Lemmon, P. E. (1956). A spherical densiometer for estimating forest overstory density. For. Sci. 2, 314–320. doi: 10.1093/forestscience/2.4.314
Lenth, R. V. (2016). Least-squares means: the R package lsmeans. J. Stat. Softw. 69, 1–33. doi: 10.18637/jss.v069.i01
Luke, S. H., Purnomo, D., Advento, A. D., Aryawan, A. A. K., Naim, M., Pikstein, R. N., et al. (2019a). Effects of understory vegetation management on plant communities in oil palm plantations in Sumatra, Indonesia. Front. For. Glob. Chang. 2:33. doi: 10.3389/ffgc.2019.00033
Luke, S. H., Slade, E. M., Gray, C. L., Annammala, K. V., Drewer, J., Williamson, J., et al. (2019b). Riparian buffers in tropical agriculture: Scientific support, effectiveness and directions for policy. J. Appl. Ecol. 56, 85–92. doi: 10.1111/1365-2664.13280
Luskin, M. S., and Potts, M. D. (2011). Microclimate and habitat heterogeneity through the oil palm lifecycle. Basic Appl. Ecol. 12, 540–551. doi: 10.1016/j.baae.2011.06.004
Matson, P. A., Parton, W. J., Power, G. A., and Swift, M. J. (1997). Agricultural intensification and ecosystem properties. Science 277, 504–509. doi: 10.1126/science.277.5325.504
Meijaard, E., Garcia-Ulloa, J., Sheil, D., Wich, S. A., Carlson, K. M., Juffe-Bignoli, D., et al. (2018). Oil Palm and Biodiversity: A Situation Analysis by the IUCN Oil PALM Task Force. Gland: IUCN, International Union for Conservation of Nature.
Meijide, A., Badu, C. S., Moyano, F., Tiralla, N., Gunawan, D., and Knohl, A. (2018). Impact of forest conversion to oil palm and rubber plantations on microclimate and the role of the 2015 ENSO event. Agric. For. Meteorol. 252, 208–219. doi: 10.1016/j.agrformet.2018.01.013
Mitchell, S. L., Edwards, D. P., Bernard, H., Coomes, D., Jucker, T., Davies, Z. G., et al. (2018). Riparian reserves in oil palm can protect forest bird communities. J. Appl. Ecol. 55, 2744–2755. doi: 10.1111/1365-2664.13233
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Nájera, A., and Simonetti, J. A. (2010). Can oil palm plantations become bird friendly? Agrofor. Syst. 80, 203–209. doi: 10.1007/s10457-010-9278-y
Padfield, R., Hansen, S., Davies, Z. G., Ehrensperger, A., Slade, E. M., Evers, S., et al. (2019). Co-producing a research agenda for sustainable palm oil. Front. For. Glob. Chang. 2:13. doi: 10.3389/ffgc.2019.00013
Perfecto, I., Vandermeer, J., and Philpott, S. M. (2014). Complex ecological interactions in the coffee agroecosystem. Ann. Rev. Ecol. Evol. Syst. 45, 137–158. doi: 10.1146/annurev-ecolsys-120213-091923
Phalan, B., Onial, M., Balmford, A., and Green, R. E. (2011). Reconciling food production and biodiversity conservation: land sharing and land sparing compared. Science 333, 1289–1291. doi: 10.1126/science.1208742
Prescott, G. W., Edwards, D. P., and Foster, W. A. (2015). Retaining biodiversity in intensive farmland: epiphyte removal in oil palm plantations does not affect yield. Ecol. Evol. 5, 1944–1954. doi: 10.1002/ece3.1462
Purnomo, D., and Caliman, J. P. (2012). “Comparison of flora diversity in oil palm plantation on two soil types in Riau-Sumatra: mineral soil and peat soil,” in International Conference on Oil Palm and the Environment (Bali: ICOPE).
Pywell, R. F., Heard, M. S., Woodcock, B. A., Hinsley, S., Ridding, L., Nowakowski, M., et al. (2015). Wildlife-friendly farming increases crop yield: evidence for ecological intensification. Proc. R. Soc. B Biol. Sci. 282:1740. doi: 10.1098/rspb.2015.1740
R Core Team (2017). R: A Language And Environment for Statistical Computing. R Foundation for Statistical Computing. Available online at: https://www.rproject.org/
R Studio Team (2016). RStudio: Integrated Development for R. RStudio, Inc. Available online at: http://www.rstudio.com/
Rambe, T. D., Lasiman, P., Naim, M., et al. (2012). “Sustainable weed management: selective and site specific spraying to minimize herbicide use in oil palm plantation,” in XVII International Oil Palm Conference (Cartagena de Indias).
Rand, T. A., Tylianakis, J. M., and Tscharntke, T. (2006). Spillover edge effects: the dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol. Lett. 9, 603–614. doi: 10.1111/j.1461-0248.2006.00911.x
Santoso, A., Mcphaden, M. J., and Cai, W. (2017). The defining characteristics of ENSO extremes and the strong 2015/2016 El Niño. Rev. Geophys. 55, 1079–1129. doi: 10.1002/2017RG000560
Savilaakso, S., Garcia, C., Garcia-Ulloa, J., Ghazoul, J., Groom, N., Guariguata, M. R., et al. (2014). Systematic review of effects on biodiversity from oil palm production. Environ. Evid. 3:4. doi: 10.1186/2047-2382-3-4
Sekercioglu, C. H. (2012). Bird functional diversity and ecosystem services in tropical forests, agroforests and agricultural areas. J. Ornithol. 153, 153–161. doi: 10.1007/s10336-012-0869-4
Slade, E. M., Burhanuddin, M. I., Caliman, J., Foster, W., Naim, M., Prawirosukarto, S., et al. (2014). Can cattle grazing in mature oil palm increase biodiversity and ecosystem service provision, Vol. 90. Plant, 655–665.
Snaddon, J. L., Willis, K. J., and Macdonald, D. W. (2013). Oil-palm replanting raises ecology issues. Nature 502, 170–171 doi: 10.1038/502170d
Sokal, R., and Rohlf, F. (1995). Biometry: The Principles and Practice of Statistics in Biological Research, 3rd Edn. New York, NY: Freeman and Company.
Spear, D. M., Foster, W. A., Advento, A. D., Naim, M., Caliman, J. P., Luke, S. H., et al. (2018). Simplifying understory complexity in oil palm plantations is associated with a reduction in the density of a cleptoparasitic spider, Argyrodes miniaceus (Araneae: Theridiidae), in host (Araneae: Nephilinae) webs. Ecol. Evol. 8, 1595–1603. doi: 10.1002/ece3.3772
Tao, H.-H., Slade, E. M., Willis, K. J., Caliman, J. P., Snaddon, J. L., et al. (2016). Effects of soil management practices on soil fauna feeding activity in an Indonesian oil palm plantation. Agric. Ecosyst. Environ. 218, 133–140. doi: 10.1016/j.agee.2015.11.012
Tao, H.-H., Snaddon, J. L., Slade, E. M., et al. (2017). Long-term crop residue application maintains oil palm yield and temporal stability of production. Agron. Sustain. Dev. 37:33. doi: 10.1007/s13593-017-0439-5
Tao, H.-H., Snaddon, J. L., Slade, E. M., Caliman, J. P., Widodo, R. H., Suhardi, et al. (2018). Application of oil palm empty fruit bunch effects on soil biota and functions: a case study in Sumatra, Indonesia. Agric. Ecosyst. Environ. 256, 105–113. doi: 10.1016/j.agee.2017.12.012
Teuscher, M., Gérard, A., Brose, U., Buchori, D., Clough, Y., Ehbrecht, M., et al. (2016). Experimental biodiversity enrichment in oil-palm-dominated landscapes in Indonesia. Front. Plant Sci. 7:1538. doi: 10.3389/fpls.2016.01538
The Government of Indonesia (2011) The Republic of Indonesia Government Ordinance No 38 Year 2011 About Rivers. Indonesia: The Government of Indonesia.
Tilman, D., Fargione, J., Wolff, B., D'Antonio, C., Dobson, A., Howarth, R., et al. (2001). Forecasting agriculturally driven global environmental change. Science 292, 281–284. doi: 10.1126/science.1057544
Tscharntke, T., Bommarco, R., Clough, Y., Crist, T. O., Kleijn, D., Rand, T. A., et al. (2007). Conservation biological control and enemy diversity on a landscape scale. Biol. Control. 43, 294–309. doi: 10.1016/j.biocontrol.2007.08.006
Tscharntke, T., Klein, A. M., Kruess, A., Steffan-Dewenter, I., and Thies, C. (2005a). Landscape perspectives on agricultural intensification and biodiversity - ecosystem service management. Ecol. Lett. 8, 857–874. doi: 10.1111/j.1461-0248.2005.00782.x
Tscharntke, T., Rand, T. A., and Bianchi, F. J. J. A. (2005b). The landscape context of trophic interactions: insect spillover across the crop-noncrop interface. Ann. Zool. Fenn. 42, 421–432.
Tscharntke, T., Sekercioglu, C. H., Dietsch, T. V., Sodhi, N. S., Hoehn, P., and Tylianakis, J. M. (2008). Landscape constraints on functional diversity of birds and insects in tropical agroecosystems. Ecology 89, 944–951. doi: 10.1890/07-0455.1
Uryu, Y., Mott, C., Foead, N., Yulianto, K., Budiman, A., Setiabudi, et al. (2008). “Deforestation, forest degradation, biodiversity loss and CO2 emissions in Riau, Sumatra, Indonesia,” in One Indonesian Province's Forest and Peat Soil Carbon Loss over a Quarter Century and its Plans for the Future. Jakarta, Indonesia
Wielgoss, A., Tscharntke, T., Rumede, A., Fiala, B., Seidel, H., Shahabuddin, S., et al. (2014). Interaction complexity matters: disentangling services and disservices of ant communities driving yield in tropical agroecosystems. Proc. R. Soc. B Biol. Sci. 281, 1–10. doi: 10.1098/rspb.2013.2144
Wilson, J. D., Whittingham, M. J., and Bradbury, R. B. (2005). The management of crop structure: a general approach to reversing the impacts of agricultural intensification on birds? Ibis 147, 453–463. doi: 10.1111/j.1474-919x.2005.00440.x
Keywords: biodiversity, habitat heterogeneity, palm oil, plantation management, sustainability, tropical agriculture, riparian buffer, understory vegetation
Citation: Luke SH, Advento AD, Aryawan AAK, Adhy DN, Ashton-Butt A, Barclay H, Dewi JP, Drewer J, Dumbrell AJ, Edi, Eycott AE, Harianja MF, Hinsch JK, Hood ASC, Kurniawan C, Kurz DJ, Mann DJ, Matthews Nicholass KJ, Naim M, Pashkevich MD, Prescott GW, Ps S, Pujianto, Purnomo D, Purwoko RR, Putra S, Rambe TDS, Soeprapto, Spear DM, Suhardi, Tan DJX, Tao H-H, Tarigan RS, Wahyuningsih R, Waters HS, Widodo RH, Whendy, Woodham CR, Caliman J-P, Slade EM, Snaddon JL, Foster WA and Turner EC (2020) Managing Oil Palm Plantations More Sustainably: Large-Scale Experiments Within the Biodiversity and Ecosystem Function in Tropical Agriculture (BEFTA) Programme. Front. For. Glob. Change 2:75. doi: 10.3389/ffgc.2019.00075
Received: 31 March 2019; Accepted: 30 October 2019;
Published: 08 January 2020.
Edited by:
Trevor F. Keenan, University of California, Berkeley, United StatesReviewed by:
Kevin Felix Arno Darras, University of Göttingen, GermanyCopyright © 2020 Luke, Advento, Aryawan, Adhy, Ashton-Butt, Barclay, Dewi, Drewer, Dumbrell, Edi, Eycott, Harianja, Hinsch, Hood, Kurniawan, Kurz, Mann, Matthews Nicholass, Naim, Pashkevich, Prescott, Ps, Pujianto, Purnomo, Purwoko, Putra, Rambe, Soeprapto, Spear, Suhardi, Tan, Tao, Tarigan, Wahyuningsih, Waters, Widodo, Whendy, Woodham, Caliman, Slade, Snaddon, Foster and Turner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah H. Luke, c2FyYWguaC5sdWtlQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.