- 1Lester E. Fisher Center for the Study and Conservation of Apes, Lincoln Park Zoo, Chicago, IL, United States
- 2Global Conservation Program, Wildlife Conservation Society, New York, NY, United States
- 3Data Services, Washington University in Saint Louis, Saint Louis, MO, United States
- 4Department of Anthropology, Washington University in Saint Louis, Saint Louis, MO, United States
- 5Congo Program, Wildlife Conservation Society, Brazzaville, Republic of Congo
- 6Faculty of Sciences and Techniques, Marien Ngouabi University, Brazzaville, Republic of Congo
- 7Initiative for Mushrooms and Plants of Congo, Brazzaville, Republic of Congo
The tropical forests of Western Equatorial Africa are home to extraordinary biodiversity, including sympatric chimpanzees (Pan troglodytes troglodytes) and western lowland gorillas (Gorilla gorilla gorilla). The region is also comprised of significant stands of Intact Forest Landscapes (IFL) that are in rapid decline. As part of a regional monitoring effort, we partnered with local government officials, conservation NGOs, and the timber company working in the region to assess ape abundances in relation to habitat characteristics and anthropogenic disturbances and compare IFL and non-IFL areas in the Sangha Trinational landscape, Republic of Congo. We found that chimpanzees and gorillas occur at high densities in IFL, as well as non-IFL. To better understand how selective logging changes floristic factors, we compared herb and tree densities from botanical surveys conducted in IFL and non-IFL. IFL had higher tree stem densities and less terrestrial herbs than logged habitats. However, few ape resources were logged in this extraction cycle and areas with tree stems removed subsequently had higher abundances of terrestrial herbs preferred by apes, which may contribute to the elevated ape abundance estimates. Floristic differences in logged forest were identified to coincide with differences in ape resource use. The chimpanzee tree nesting niche was reduced in non-IFL as night nests were constructed significantly closer to the ground than in IFL. Whereas, gorilla nest height locations did not differ significantly between IFL and non-IFL. To identify other potential anthropogenic impacts, we assessed direct and indirect impacts of road expansion and illegal hunting on wildlife in these remote areas. Increased access to IFL that facilitates illegal hunting raises concern for protecting wildlife across Western Equatorial Africa. We urge that the results of biodiversity assessments and strategic aspects of long-term protection should be taken into account when identifying conservation set-asides and maintaining diverse states of modified forests. Finally, the results of our monitoring efforts are provided as evidence of the value of long-term collaborations among local stakeholders, government officials, conservation agencies, and industrial partners to improve the implementation of certification standards and biodiversity conservation initiatives.
Introduction
Early efforts to identify the world's remaining “frontier” forests highlighted the substantial abundance of pristine habitats in the tropics (Bryant et al., 1997). Since the identification of such Intact Forest Landscapes (IFL), which are forest/mosaics at least 500 km2 (50,000 ha) lacking overt anthropogenic disturbance such as infrastructure (Potapov et al., 2008), there has been dramatic decline in such areas (Potapov et al., 2017). The startling loss of IFL is largely due to tropical nations' economies and infrastructural development being rooted in the exploitation of natural resources. Africa contains one of the three large blocks of the world's tropical forests and with the depletion of natural resources in Asia, multi-national companies have sought new outlets in timber-rich nations such as those in Western Equatorial Africa (Angola (Cabinda enclave), Cameroon, Central African Republic, mainland Equatorial Guinea, Gabon, and Republic of Congo). Selective logging is the primary extraction industry responsible for IFL loss in the region (Asner et al., 2010; Potapov et al., 2017) and Republic of Congo has been in the vanguard of this expansion. An accelerated rate of logging road construction has ensued particularly in the north of the country over the last two decades (Laporte et al., 2007; Kleinschroth and Healey, 2017). In the wake of such expansion follows considerable degradation of natural resources and increased human immigration (Geist and Lambin, 2002; Watson et al., 2018). If action is not taken to avert losses, it is estimated that all IFL outside of protected areas in Republic of Congo will have disappeared by 2050 (Potapov et al., 2017) and wildlife populations in the region will be reduced by 80% (Fa et al., 2003). These developments will assuredly have negative consequences on forest and biodiversity within protected areas including those of Natural World Heritage Sites as many are already under elevated levels of human pressure and forest conversion (DeFries et al., 2005; Laurance et al., 2012; Bailey et al., 2016; Lui and Coomes, 2016; Allan et al., 2017).
Identifying important environmental attributes of forest intactness and indicators of change in intactness are key to more informed conservation management. With 77.4% of critically endangered western lowland gorillas and 80.7% of endangered central chimpanzees existing outside of protected areas (Strindberg et al., 2018) there is a great need for management beyond protected areas to conserve these flagship species. Based on recent regional modeling, gorillas and chimpanzees occur at higher densities in IFL compared to non-IFL (Strindberg et al., 2018). The physical structure of these forests has considerable influence on great ape distributions with generally higher ape densities associated with increasing tree canopy height (Strindberg et al., 2018). The emergent and high canopy levels are the result of a few “biomass hyperdominant” tree species (Bastin et al., 2015) in a region overall typified by low tree stem densities (Lewis et al., 2013). Local-scale surveys and remote sensing indicate frequent disruption in canopy continuity even in an intact state (Devos et al., 2008). This is a product of the mixed species forest composition which is comprised of a mosaic of regenerating patches from natural disturbances with canopies varying in height and composition. The dynamic and complex nature of this habitat supports high densities of both chimpanzees and gorillas (Devos et al., 2008).
A key question remains as to how increases in canopy gaps associated with the loss of dominant canopy trees, as occurs in selective logging, affect forest composition and resources important to apes. In South America, highly disturbed forests have been shown to support elevated densities of climbers such as those of the genus Ficus, which include species bearing high-quality fruit found to predict populations of primates (Terborgh, 1986; Leighton, 1993; Wrangham et al., 1993; Marshall and Leighton, 2006). Removal of canopy trees also brings elevated light exposure to the lower understory strata which in turn bolsters the growth of non-arboreal pioneer species (Malcolm and Ray, 2000) that are likely of benefit to apes. Members of the families referred to as terrestrial herbaceous vegetation (THV) are important to gorillas and chimpanzees for both foraging and nesting (e.g., Wrangham, 1986; Rogers and Williamson, 1987; Fay, 1997). Chimpanzees are classically referred to as more of a dietary specialist with their resource use focused on fruit-bearing tree species whereas gorillas are typically considered to be more along the lines of a generalist with a diet focused mostly on herbaceous ground vegetation (Bourliere, 1985). This classification has proven to be a useful dichotomy when assessing factors shaping species responses to perturbation, with the former more often negatively impacted by forestry than the latter (Johns and Skorupa, 1987; Sodhi et al., 2010; Burivalova et al., 2014). To date, however, surveys of gorillas and chimpanzees in post-logged forests indicate increases as well as decreases in population numbers, which raises questions regarding the relationship between compositional changes in the environment and ape abundance. Overall, gorilla populations in this region are in decline (Strindberg et al., 2018) and negative impacts of anthropogenic disturbance on chimpanzee behaviors have become increasingly apparent (Kuehl et al., 2019). However, alteration in the structural complexity of IFL may not be the only or principal factor responsible for potential changes in ape abundance in logged habitats of Western Equatorial Africa.
The rise in unsustainable hunting of wildlife for meat (i.e., bushmeat) and body parts is the most severe and rapidly expanding threat facing species today (Ripple et al., 2017). Life-history traits are known to influence species-specific vulnerability to hunting pressure (Reynolds, 2003; Marshall and Leighton, 2006). Human settlements, consumption practices, and accessibility are also strong determining factors in the persistence or decline of wildlife in an area (Barnes and Lahm, 1997; Fa et al., 2000; Blake, 2002; Jerozolimski and Peres, 2003; Blake et al., 2008). We suggest that a putative pattern of hunting pressure is triggered by increasing access to IFL. Forests distant from human infrastructure have higher abundances of wildlife compared to forests with longer histories of human influence (e.g., Eves and Ruggiero, 2000; Fa et al., 2004; Dupain et al., 2012) and greater accessibility (Yackulic et al., 2011). It follows that larger and highly profitable animals are reportedly originating from more distant and less-accessible areas (Allebone-Webb et al., 2011). There are indications that declines in wildlife associated with opening of IFL are rapid. In a previously intact concession in northern Congo, (Wilkie et al., 1992) estimated that 3,140 km of primary roads, secondary roads, and transects were opened in a single year. This region has subsequently been shown to be a primary source of most ivory reaching markets, substantiated by the staggering 62% decline of forest elephants in the Congo Basin since the early 2000s (Wasser et al., 2004; Maisels et al., 2013). Understanding the temporal-spatial patterning of hunting pressure in relation to the decline of IFL is crucial to preventing species declines both in protected areas and neighboring forests through proactive conservation measures to address such threats.
As part of a regional monitoring effort, we partnered with local government officials, conservation NGOs, and the timber company working in the region to assess ape abundances in relation to habitat characteristics and anthropogenic disturbances and compare IFL and non-IFL in the Sangha Trinational landscape. Baseline estimates of great ape densities in an IFL are compared with post-logging densities to better understand population dynamics in relation to anthropogenic disturbance. We also document floristic differences between IFL and non-IFL and relate floral differences to great ape resource use needs. The study took place in and around the Goualougo Triangle which is located in the southern portion of the Nouabalé-Ndoki National Park. Initial surveys in this region conducted by the Wildlife Conservation Society which led to the creation of the Nouabalé-Ndoki National Park cited the intact nature of the Goualougo Triangle and its conservation as essential for maximizing protection of a key area of core habitat for the region (Fay et al., 1990; Fay, 1992; Blake, 1994). Among all long-term ape research sites in Africa, the Goualougo study area was found to be the least disturbed by anthropogenic disturbances (Wilson et al., 2014). As such, we provide an update on the expansion of timber harvesting in the forest surrounding this protected area and increasing anthropogenic pressures which have reached the most remote areas of the Goualougo Triangle. This provides a rare opportunity to observe the temporal patterning of increased accessibility to remote forests and how this relates to illegal poaching pressures. We use this information to provide recommendations for park management and forest certification policies, as well as to promote the potential for permanent research sites to contribute to conservation initiatives through monitoring and surveillance.
Materials and Methods
This study took place in the Nouabalé-Ndoki National Park (NNNP; 2°05′-3°03′ N; 16°51′-16°56′ E) and the adjacent Kabo Forestry Management Unit (FMU) which is an immediately neighboring logging concession in northern Republic of Congo. The National Park was established in 1993 and covers 5,000 km2 of forest and is part of the Trinational de la Sangha (TNS), a designated UNESCO Natural World Heritage Site (NWHS) which spans the Republic of Congo, Cameroon, and Central African Republic. The landscape is primarily comprised of a vast stretch of lowland Guineo-Congolian forest (White, 1983) with altitudes ranging from 330 to 600 m. The major habitat types in this region include monodominant Gilbertiodendron forest, mixed species forest, transitional Gilbertiodendron to mixed species forest, and swamp forest. This semi-deciduous mixed species forest has a diverse flora and canopy that is not always continuous. Rainfall is bimodal with a main rainy season from August through November and a short rainy season in May. Annual rainfall averaged 1,728 ± 47 mm between 2010 and 2017.
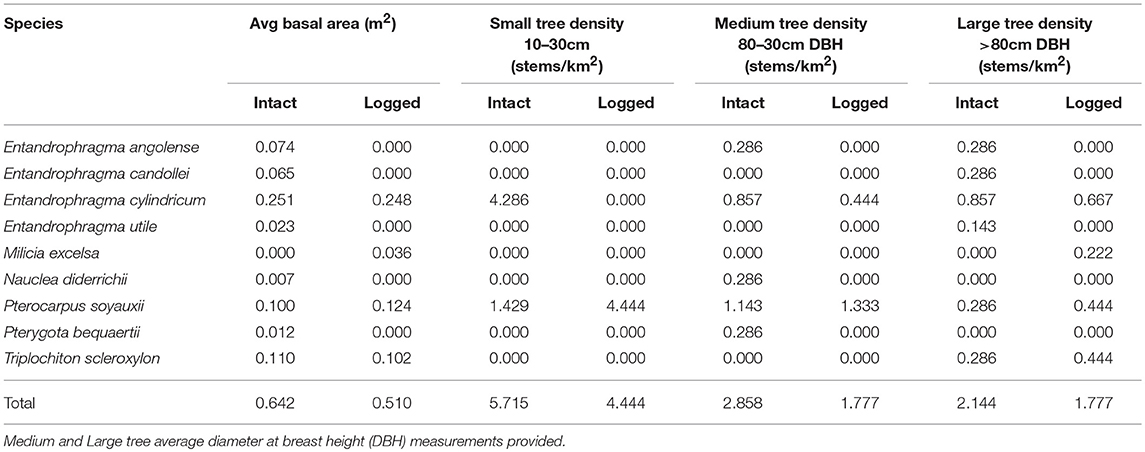
The Wildlife Conservation Society has the mandate to manage the NNNP and the FMU bordering it. In 1995, the landscape encircling the NNNP was divided into four concessions. The Kabo FMU is comprised of 2,960 km2 which surrounds the southern sector of the National Park. The western sector of the Kabo FMU was selectively logged between 1971 and 1972 by the Société Nouvelle des Bois de la Sangha (SNBS) and then harvested a second time from 2005 to 2009 by Congolaise Industrielle des Bois (CIB). The Kabo concession was among the first concessions in Western Equatorial Africa to achieve Forest Stewardship Council (FSC) certification in 2006. The majority of the volume extracted consisted of Entandrophragma species, Triplochiton scleroxylon, and Milicia excelsa (CIB 2014). The eastern sector of the Kabo FMU was an IFL until the mid-2000s (Figure 1).
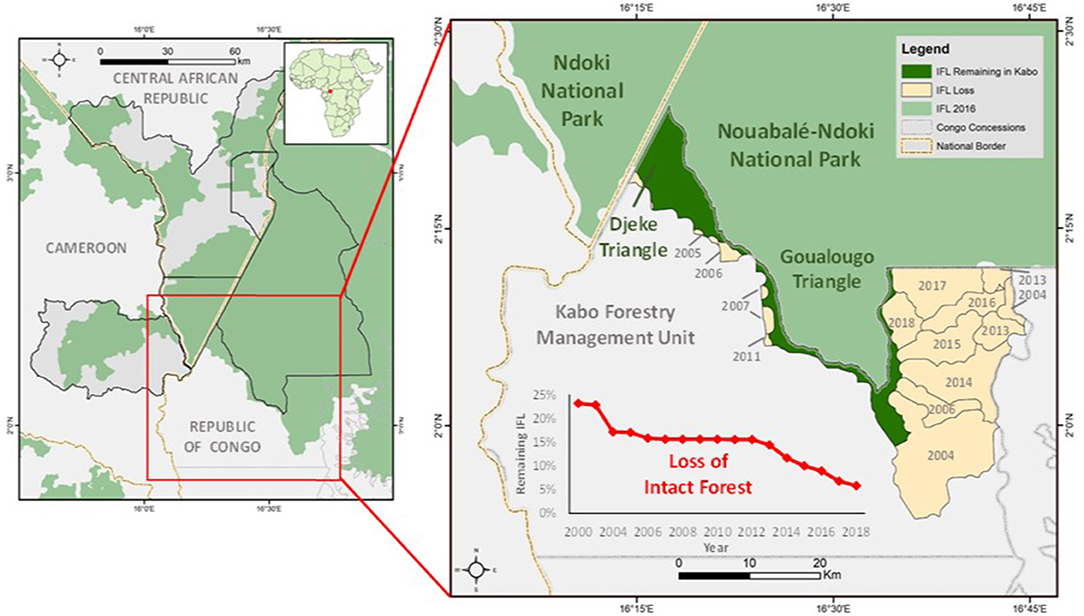
Figure 1. Location of the study area within the Sangha Trinational landscape. Inset shows Goualougo and Djeke Triangle which both consist of IFL in relation to recent declines of IFL within the adjacent Kabo Forestry Management Unit.
The Goualougo Triangle Ape Project (GTAP) was established in 1999 with the aim of conducting applied conservation research on the behavioral ecology of gorillas and chimpanzees in northern Congo. The Goualougo Triangle is an enclave of lowland forest between the Ndoki and Goualougo Rivers, which forms the southernmost section of the NNNP. It is bordered to the south, west, and east by the Kabo FMU (Figure 1). The Djeke Triangle is located between the Ndoki National Park in Central African Republic and NNNP in Republic of Congo. The GTAP and Wildlife Conservation Society research teams maintain a year-round field presence in the Goualougo and Djeke Triangles with daily reconnaissance missions to follow great apes that have been habituated to human presence. Both the Goualougo and Djeke Triangles are represented as IFL.
Forest Status
We used the IFL inventory map (Potapov et al., 2008) to define intactness of the NNNP and neighboring Kabo FMU. This map is based on the extent of roads and settlements documented from Landsat images (of 30 m resolution) up to 2013 (Potapov et al., 2017). The IFL regions are forested areas >500 km2 and >10 km wide that fall outside a 1-km buffer around such infrastructure (Potapov et al., 2008). Since the 2013 estimate of IFL, new road networks have been established within the concessions surrounding NNNP and within the IFL with updated estimates for the region provided in Morgan et al. (in press). Digital features representing new roads were provided by the local timber operating company and Kleinschroth et al. (2016). We used current Landsat 8 satellite imagery to review road features and verify placement. Duplicated roads in Kleinschroth et al. (2016) and the timber company files were removed. Once completed, the new roads were buffered and used to update the extent of IFL for areas of interest following Potapov et al. (2008). The revised extent of IFL in the Kabo concession was presented and reviewed by the local industrial logging company for verification.
Accessibility
Accessibility was assessed retrospectively based on the progression of roads within the Kabo concession from 1996 to 2018. Peres and Terborgh (1995) proposed a 10-km criterion to set how far hunter incursions into neighboring interior forests are likely to occur from roads. The 10-km criterion differs from the IFL measure that also considers the location of settlements in a given area.
Human Presence
Armed law enforcement teams began foot and vehicle patrols in NNNP and Kabo FMU in 2005. The location of human sign data, as well as other signs (carcasses, spent ammunitions, camps) observed, were recorded by either mobile research teams or patrol teams traversing the study region or on fluvial patrols. In 2015, patrol teams began using the Spatial Monitoring and Reporting Tool (SMART) (Connect version 4.1).
Since the project's inception in 1999, GTAP research teams have documented any human activities detected while following habituated apes or during surveys (ape nest transects, botanical) within or outside of NNNP. Both direct and indirect signs of illegal human activity are reported to law enforcement officials. In 2017, GTAP adopted the SMART data collection method implemented by NNNP patrol teams so as to use our field efforts to contribute to regional surveillance.
Botanical Surveys
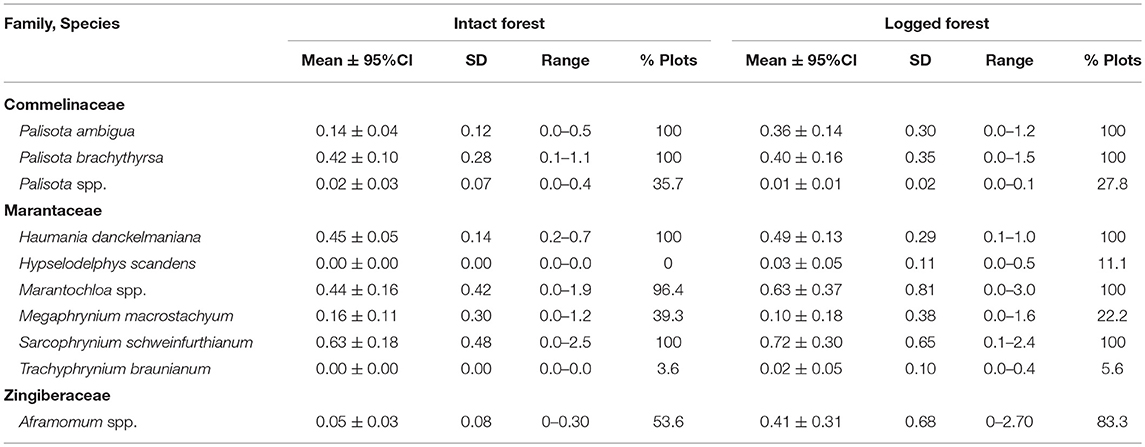
Botanical plots (50 × 50 m) were conducted in IFL and a twice selectively logged forest. To enumerate and measure stems of different size classes and growth forms within each plot, we identified and measured all trees with diameter at breast height (DBH) >80 cm with a base falling entirely within 25 m of either side of the midline, and all strangler figs with DBH ≥10 cm whose host trees had a base falling entirely within 25 m of the midline. We identified and measured all trees and free-standing Ficus spp. with a DBH 30–80 cm with bases falling entirely within 12.5 m of either side of the midline. All strangler Ficus spp. associated with trees in the survey plots were also identified and measured. We recorded all trees with a DBH of 10–30 cm with bases falling entirely within 2.5 m of either side of the midline, as well as every stem or leaf rooted in the ground for all terrestrial herbaceous vegetation. In cases where specimens were not identifiable, vouchers were collected for identification by Dr. David Harris, Royal Botanical Garden of Edinburgh. Overall stem densities were generated by averaging densities across plots within each zone type (intact, logged).
To better understand how herb density is affected by logging, as well as whether the effect is consistent across the floral families present, we used lme4 (Bates et al., 2015) in R (version 3.4.3, R Core Team, 2017) to run a linear mixed effect model with Gaussian error structure. The response variable was the square root-transformed herb density of each floral family in each plot surveyed. The fixed effect predictors were zone (logged and intact), family (Commelinaceae, Marantaceae, and Zingiberaceae), and their interaction. Plot ID was included as a random effect. The model residuals were assessed visually and were normally distributed as well as homogenous. We also assessed model stability by removing data points from each plot ID sequentially and running the model again each time. The results from the models using the reduced datasets were consistent with the results from the original model, suggesting the model was stable. The dataset for this model contained 138 total data points from 46 plots. We first established the combined significance of the fixed effects by comparing the full model, which contained all fixed effects and the random effect, to a null model (Forstmeier and Schielzeth, 2011), which contained only the random effect, using a likelihood ratio test (Dobson, 2002). We then tested the effect of the interaction using a likelihood ratio test (Barr et al., 2013), comparing the full model to a model that did not contain the interaction term.
Ape Abundance
We stratified the southern section of NNNP and Kabo FMU into study zones to systematically evaluate changes in forests and ape abundance and distribution as related to protection status, forestry activities, and other factors. The automated survey design component of the custom Distance software was used to generate systematically-spaced transects with a random start throughout the study area (Thomas et al., 2010). The total line length (and number of transects) in each zone is sufficient to calculate precise densities (and precise estimates of precision) of great apes for each zone individually (see Morgan et al., 2006). Ape nests and human signs were recorded during each survey. See Morgan et al. (2006) for a detailed description of data collection protocols and methods. Ape densities and abundance were calculated using decay rates for gorilla and chimpanzee nests from Morgan et al. (2016) a nest creation rate of 1.09 nests/day (SE = 0.05).
Species Specific Nest Heights in IFL vs. Logged Habitat
All independent gorillas and chimpanzees build one-night nest per day on average. A separate investigation of species-specific nesting heights in IFL vs. non-IFL was conducted using archived ape transect data collected by GTAP research teams in the NNNP and Kabo FMU (see Morgan et al., 2006, 2018). Nests not assigned to a particular species along transects were classified as constructed by either chimpanzee or gorilla following Sanz et al. (2007). A Wilcoxon rank sum test was used to test within species for statistically significant differences in nest heights between IFL and logged forest. This test was chosen since nest height was not normally distributed. We customized the wilcox.test function from R (version 3.4.3, R Core Team, 2017) so that the z-score from the test would be available as an output.
Results
Quantification of IFL
In 2000, 756 km2 of the Kabo logging concession (2,960 km2) consisted of IFL. Review of the IFL map from Potapov et al. (2008) revealed 69.7 km2 of terre firma forest in the Kabo concession was erroneously classified as IFL. This area was selectively logged in the 1970's and so was removed from the IFL estimate. Industrial logging activities in the 1970s focused on timber extraction on the western section of the concession. Over the last two decades 415.38 km2 of IFL in the concession was spared from timber extraction for conservation purposes. In 2012, a 310 km2 area known as the Goualougo Triangle was officially annexed to the Nouabalé-Ndoki National Park by presidential decree. In 2005, the 102 km2 consisting of the Djeke Triangle was designated as a “conservation de serie” or land-set aside to meet environmental standards for certification of the concession.
Over the last two decades the Goualougo Triangle experienced two temporally and spatially distinct periods of logging outside its boundaries. The first was in non-IFL from 2005 through 2009 when a second cycle of industrial logging along the western border of the Goualougo Triangle occurred. Starting in 2014, timber extraction began in neighboring IFL southeast of the Goualougo Triangle and progressed north along the eastern border of the study area through 2018.
From 2000 to 2018, a 68% reduction in the amount of IFL occurred in the Kabo FMU. A notable increase in the yearly percent of IFL loss started in 2013 and continued through 2018 as a result of road expansion and timber removal in the eastern section of the Kabo concession. Since 1985, nearly 1,400 km of timber extraction routes (primary, secondary roads) have been opened in the Kabo concession.
In 2017, the first illegal incursions (three instances of hunting raids) were detected inside the Goualougo Triangle after nearly two decades of surveillance. In 2018, five illegal incursions were recorded. These events coincided with timber removal in the neighboring forest which was previously IFL but being exploited for timber (Figure 1). Entry points of poachers into the IFL of the Goualougo Triangle were within 10 km of logging roads.
Botanical Surveys
A total of 28 botanical plots were surveyed within the mixed-species IFL of the Goualougo Triangle. In addition, we surveyed 18 botanical plots within the logged forests of Kabo West. We counted and identified 562 trees and 16,140 herb stems within IFL. A total of 313 trees and 14,314 herb stems were surveyed within the logged forest.
Average density of trees and figs was 389.86 stems/ha in IFL, with 38 families and 143 species represented. We documented an average density of 354.89 stems/ha in the logged forests, representing 34 families and 110 species. The average basal area for trees in IFL (4.35 m2) was higher than in logged forest (3.17 m2 per plot). As shown in Table 1, this was also the case for the subset of timber tree species. Total densities of trees across large, medium, and small size classes were lower in logged forest vs. IFL.
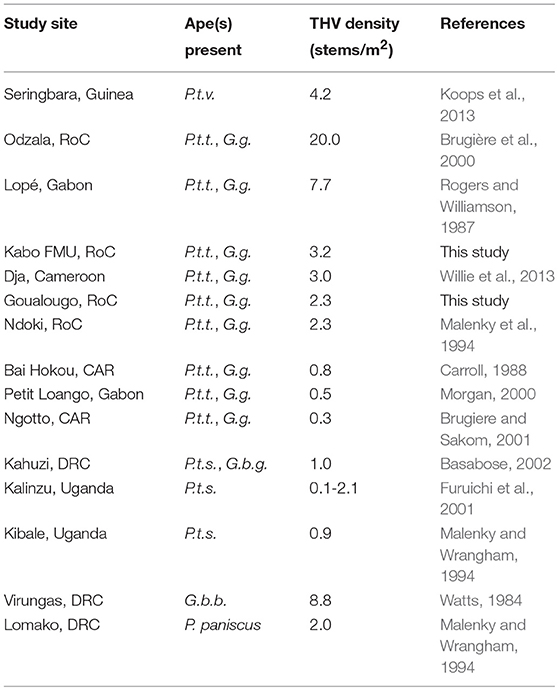
The average herb density in the intact zone, 2.31 stems/m2, was lower than the average herb density in the logged zone which was 3.18 stems/m2. Mean herbaceous stem densities ranged considerably between families and forest status (Table 2). The combined fixed effects from the linear mixed model were found to be significant (likelihood ratio test: X2 = 156.470, df = 5, p < 0.001). Specifically, the interaction between forest status (intact vs. logged) and herb family exhibited a trend (likelihood ratio test: X2 = 4.826, df = 2, p = 0.090). The nature of the interaction can be seen in Figure 2. The density by family is always larger in the logged zone, with the difference between intact and logged areas appearing largest for the Zingiberaceae family and more moderate for the Commelinaceae and Marantaceae families. These THV density estimates are placed in context of botanical surveys conducted across African ape field sites (Table 3).
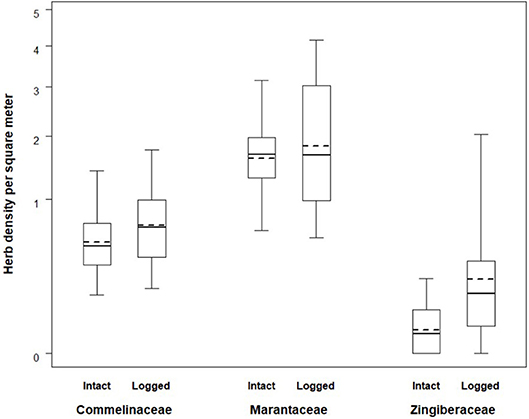
Figure 2. Herb densities across intact and logged forest. Boxes span the 25th to 75th percentile and whiskers extend to the 2.5th and 97.5th percentiles. Medians are shown as bold lines while expected values produced by the model are shown as dashed lines.
Ape Density Estimation
As reported in Table 4, line transect surveys of great ape nests were conducted in both intact and logged forests of northern Congo. A total of 272 ape nests were surveyed along 26 transects (34 km of total effort) in the Djeke Triangle which comprises the last block of IFL in the Kabo FMU. We surveyed another 332 ape nests along 10 transects (54 km of effort) in the Goualougo Triangle which comprises the southernmost section of NNNP. Surveys within logged forest were conducted within the once-logged forests located to the east of the Goualougo Triangle (henceforth referred to as Kabo East) and within forests that have been harvested twice that are immediately west of the Goualougo Triangle (henceforth referred to as Kabo West). A total of 471 ape nests were surveyed along 29 transects (107 km of effort) in Kabo East, and a total of 647 nests along 14 transects (88 km of effort) in Kabo West.
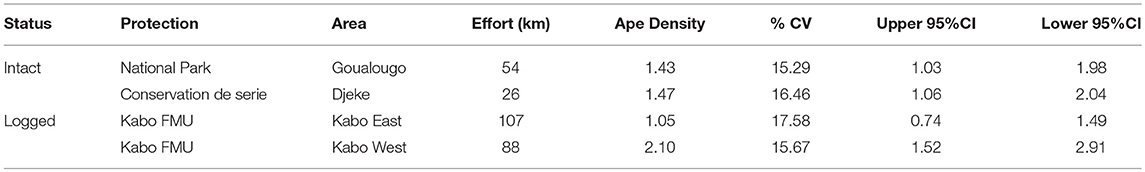
Table 4. Ape density estimates in IFL and non-IFL with percent coefficient of variation (%CV) and 95% confidence intervals (95% CI) for each survey stratum and for the landscape.
Transect surveys within IFL yielded overall density estimates of 1.43 apes/km2 within the Goualougo Triangle and 1.47 apes/km2 within the Djeke Triangle (see Table 4). In the logged forests, we documented 1.05 apes/km2 in once-logged forests located to the east of the Goualougo Triangle and 2.10 apes/km2 in the forests west of the Goualougo Triangle which have been subjected to a second cycle of timber exploitation.
Species Specific Nest Heights in IFL vs. Logged Habitat
A total of 3,902 nests representing 1,447 nest sites were identified as either built by chimpanzee or gorilla. In IFL we recorded the heights (m above ground) of 359 chimpanzee and 267 gorilla nests included within 141 and 94 nest sites, respectively. Whereas, in twice logged forests we documented 1,794 chimpanzee and 1,482 gorilla nests within 682 and 530 nest sites, respectively. This allowed nest height comparisons between these two different environmental conditions. Chimpanzee nests were built significantly closer to the ground in logged forests than IFL (Z = 13.89, p < 0.001; 95% CI, 5.0–6.0) (see Figure 3). While gorilla nest heights were also lower in logged forests than IFL, the difference was non-significant (Z = 0.36, p = 0.72) (see Figure 4).
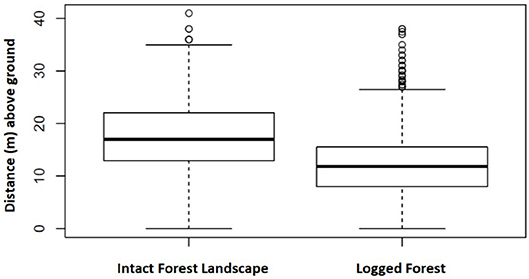
Figure 3. Chimpanzee nest height in IFL and logged differed significantly. Boxes span the 25th to 75th percentile and whiskers extend to the 2.5th and 97.5th percentiles. Medians are shown as bold lines. In IFL chimpanzee nests were constructed higher in the forest canopy compared to those documented in twice logged habitat.
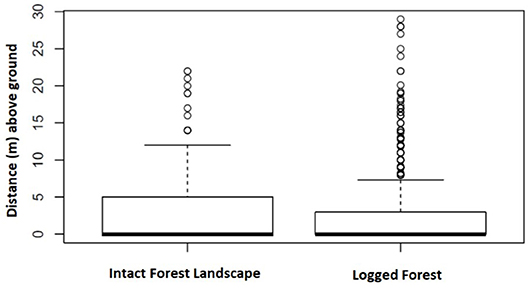
Figure 4. Gorilla nest height locations in IFL and logged forest were not significantly different. Boxes span the 25th to 75th percentile and whiskers extend to the 2.5th and 97.5th percentiles. Medians are shown as bold lines.
Discussion
Once considered a stronghold of pristine habitat, IFL loss in Western Equatorial Africa is occurring at an alarming rate. Comparisons between intact vs. disturbed habitats in the Kabo FMU confirmed that overall tree stem density and total basal area were lower in non-IFL than IFL forests. While ape food resources still persist in these forests, as selectively logged habitats contain fruit-bearing tree species and canopy gaps promote growth of high abundances of terrestrial herbs, the long-term ecological consequences of timber extraction on ape populations are not yet known. For example in this study, we documented species-specific changes in resource use in nesting between IFL and twice logged habitat. Chimpanzees nested significantly closer to the ground in logged forests suggesting that changes in forest structure associated with logging may impact resource use. In addition, nearly two decades of antipoaching surveillance in the region indicate concerning trends between proximity of new roads in IFL and increasing vulnerability of wildlife to illegal hunting in neighboring IFL including protected areas. Together, such biodiversity assessments and strategic aspects of long-term protection should be taken into account when identifying conservation set asides. For example, the majority of the remaining IFL in the Kabo concession is within the Djeke Triangle which is contiguous with the Ndoki National Park in Central African Republic and the Nouabalé-Ndoki National Park in Republic of Congo. Thus, this area is a strategic location for curbing future poaching incursions into both protected areas. It also comprises the home ranges of gorillas habituated to human presence for scientific study and tourism development. This information could be used to advocate for the formal protection of the Djeke Triangle, not only as remaining IFL but for its conservation value and role in serving as a buffer to neighboring protected areas. However, such initiatives will only be successful through collaboration of local stakeholders, government officials, conservation agencies, and industrial partners.
It has been previously asserted that chimpanzees prefer primary forest (Tutin and Fernandez, 1984; Furuichi et al., 1997), whereas primary lowland forests were thought to be insufficient in terrestrial herbaceous vegetation to support high gorilla numbers (Schaller, 1963; Groves, 1971). The intact Ndoki forest can be considered a climax forest with a high vertical canopy structure created by light-demanding pioneer species such as mahogany (Entandrophragma spp.) which were established centuries ago (Fay, 1997). Such large trees play critical ecological roles in forest dynamics (Lindenmayer and Laurance, 2017) and also influence neighboring floral communities. Natural canopy disturbance is consequential in terms of THV recruitment and can resemble structural changes similar to selectively logged forest. The impact is reflected in our botanical surveys, showing relatively high densities of THV in intact as well as logged forests. In sum, the diversity of tree species and variation in canopy coverage of IFL in northern Congo provide adequate resources to support relatively large numbers of both chimpanzees and gorillas.
Overall dietary flexibility and degree of folivory have been cited in primate species' abilities to cope with environmental disturbances (Johns, 1997; Meijaard and Sheil, 2008) and could explain high ape densities documented in non-IFL. However, it is also possible that these forests historically differed in their inherent suitability to support great apes which was not taken into account in this investigation. We found lower stem densities and total basal areas of larger trees in logged forest which has implications on carbon storage potentials and elevating environmental risks. Forest stand change was also accompanied by a successional shift to more numerous trees in the small and medium size classes. These findings are in accord with other studies on the effects of selective logging on forest composition and growth dynamics in semi-deciduous forests in Western Equatorial Africa (Gourlet-Fleury et al., 2013a,b) and support findings that logged tropical forests transition to shorter and more broken canopy stands (Felton et al., 2003). Evidence for changes in forest structure influencing ape resource use was provided by species-specific nest height selection. We found that chimpanzees preferentially nested in the middle and upper story tree stratums in IFL. Whereas, chimpanzees nested at significantly lower heights in logged forests than in IFL. Nesting options located higher in the canopy may have diminished with the loss of the large timber species. These findings are similar to reports that orangutans shift their nest locations lower in logged environments (Felton et al., 2003). Gorilla nests heights did not significantly differ between IFL and logged habitat. There was however notable variability in gorilla nest height location in logged vs. intact forests which may indicate this species is opportunistically responding to increased nesting options. Increased diversity in nest construction patterns of gorillas may be a result of the elevated availability of THV documented and associated growth in pioneer species in the exploitation zone.
Industrial logging is projected to continue at 30-year rotation cycles in most of Western Equatorial Africa and so the fate of many tree species is unknown. Repeated removal of timber even at low intensity levels can degrade the quality of habitat over time (Lindenmayer and Franklin, 2002), and could have negative consequences for apes as shown in the degraded forests of Asia (Rao and van Schaik, 1997; Felton et al., 2003; Wich et al., 2004; Husson et al., 2009). Structural changes in the logged forests in this study indicate some implications for floral climbers and epiphytes. Compared to the diverse representation of Ficus in the IFL, few figs were found in non-IFL which raises important questions about host specificity of strangler figs in relation to logging species and potential consequences to the frugivore community reliant on these resources. Ficus spp. are a critical component of the overall chimpanzee diet in IFL (Morgan and Sanz, 2006). Monitoring the direct impacts of logging on large fruit-bearing tree species preferred by chimpanzees and gorillas in future exploitation cycles will be important as such resources can influence reproduction and fitness in wild apes (Emery Thompson et al., 2007). Future studies of nesting resource use and distribution could also be informative for conservation planning, as the difference observed in chimpanzee nesting in IFL vs. logged habitat could be the result of indirect rather than direct disturbances associated with logging. Low intensity logging was practiced in the concession with off-take ranging between 0.5 and 3.0 trees/ha. Importantly, the top three marketable tree species exploited in the study area were rarely used for nest construction by chimpanzees or gorillas. Such insights on shifting resource use by apes should be considered in identification of High Conservation Value Forests (HCVF) which is an important environmental criterion of FSC certification.
Compared to IFL, apes in non-IFL are also at increased risk of synergistic interactions with other threats such as edge effects and emerging diseases. For example, areas of high disturbance and elevated undergrowth as documented in this study are likely to be more fire prone. Logging routes and their margins transform the local forest with elevated levels of disturbance (Brandt et al., 2016) which is followed by rapid growth of high densities of Marantaceae and Zingiberaceae families in the abandoned tracks and edges (Malcolm and Ray, 2000; Kleinschroth et al., 2015). Increased rates of tree mortality, leaf litter accumulation, and damage (Ferreira and Laurance, 1997; Laurance et al., 1998) likely combine with changes in microclimate conditions and combustibility of flora, elevating risks of severe fire (Leighton and Wirawan 1986; Campbell 1992; Dennis and Colfer, 2006). The recent fire that raged across IFL and non-IFL bordering the main national road accessing the north of Republic of Congo provides evidence that wildfire is now an agent of disturbance in this region (Potapov et al., 2017). Another threat that is exacerbated by reduction of IFL is considerable spatial overlap between apes and humans occurring in proximity to roads that may elevate risk for cross-species transmission of pathogens through handling of shared resources or even direct contact. Finally, dramatic reductions of great ape numbers in some regions of northern Congo have been attributed to excessive hunting levels (Bermejo et al., 2006).
Poaching remains the greatest threat to wildlife in this region, and the spatial-temporal relationship between expanding road networks and increased illegal hunting pressure within NNNP is both pervasive and instructive. Since the Park's creation, effective management of poaching pressure in the surrounding logging concessions has been a priority to protect the integrity of the core area (Elkan et al., 2006). Law enforcement patrols can lower threat levels to protected areas (Stokes et al., 2010; Tranquilli et al., 2014), but increased support for these activities is critical as expanding road networks are providing unprecedented access to previously remote areas. We documented the first instances of poacher incursions in Goualougo Triangle region of NNNP that coincided spatially and temporally with the arrival of roads and active logging in adjacent forest. The spatiotemporal patterning of high-value natural resource extraction in Africa has expanded across a gradient from high to low sourced areas (Ahrends et al., 2010). Currently, the NNNP is in the midst of experiencing the first wave of hunting pressure targeted at ivory. This supports assumptions that poachers preferentially target IFL. It is likely part of a larger spatial pattern that has typified hunting in Western Equatorial Africa since the onset of selective logging in the early 1970s, but is only now reaching the previously inaccessible forests which form the core protected areas of the Sangha Trinational NWHS. At the time of this study, the poaching profitability perimeter for ivory hunters remained spatially close to logging roads. Entry points of illegal raids into the southeastern sector of the NNNP were typically within 10 km of the nearest road which supports broader assumptions of distances traveled by hunters (Peres and Terborgh, 1995). We strongly urge research assessing whether the profitability of hunting diminishes with closure of logging roads. The arrival and sudden intensification of poaching in the Goualougo Triangle is particularly concerning given the incessant poaching pressure over the last decade in the IFL of the Dja Faunal Reserve NWHS which is another important landscape for great ape conservation (IUCN, 2014). Long-term studies in Malaysia also suggest survival prospects for species such as orangutans in production forests are mainly determined by hunting (Ancrenaz et al., 2004). If properly coordinated with regional antipoaching efforts, we assert that research outposts and field teams can serve as sentinels of such poaching pressure in remote areas and act as force multipliers in maintaining surveillance. In addition to improving coordination of ecological and behavioral data collection across research sites, technologically-enhanced monitoring with SMART has the potential to expedite reporting of regional antipoaching efforts. More importantly, this increased monitoring and collaboration can lead to the arrest of poachers, which proves that the relationship between law enforcement and research efforts can be mutually beneficial in safeguarding wildlife.
Beyond implementation of best practice guidelines in and outside production forests, the most effective way to protect the outstanding flora and fauna attributes of IFL is through policies that formally set aside such forests (Watson et al., 2016). Successful mandates to attain increased protected status require collaborative and holistic approaches (Peres, 2005; Haurez et al., 2017; Chazdon, 2018) such as those that led to the annexation of the Goualougo Triangle to the NNNP (Morgan et al., 2012). In these cases, increases in formal protected status were the result of coordination among local stakeholders, government officials, conservation agencies, and industry partners. Over the last several years the debate regarding the fate of IFL, particularly in certified logging concessions, has become a central focus of global conservation. In 2016, the IUCN World Conservation Congress adopted a motion (https://portals.iucn.org/congress/motion/048) encouraging the monitoring and sparing of IFL from degradation and loss. FSC has integrated the protection of IFL into their International Standards, urging governments and concessionaires to decrease the rate of IFL loss. The Djeke Triangle comprises the majority of remaining IFL in the Kabo concession, and is also the location of the longest-running gorilla research and tourism site in Western Equatorial Africa, which employs a large number of local people. Given its location along the international borders of two National Parks, increased protected status through annexation to the NNNP would further strengthen efforts to maintain the ecological integrity of the Sangha Trinational.
Conclusion
Our ground-based efforts to identify and verify IFL resulted in a more accurate depiction of both the amount and location of remaining intact forest in the Kabo FMU. We found that 69.7 km2 of the terre firma forest in the concession had previously been erroneously classified as lacking anthropogenic disturbance, when it had actually been logged. Botanical surveys conducted in IFL revealed higher densities of trees across all size classes, relative to non-IFL. Non-IFL forests were composed of reduced numbers of trees, but elevated herb densities. Results from our longterm studies of chimpanzees and gorillas show that ape resource use may be affected by logging, as nesting patterns differed between intact and logged forests. The height of chimpanzee nests was significantly lower in non-IFL compared to IFL, indicating that nesting niche options may be reduced in modified habitats. In contrast, there is indication that the gorilla niche may have expanded in logged habitat as a result of greater nesting material options associated with secondary growth in logged habitats. Along with the ecological changes in logged forest, we also documented that road expansion was associated with increased poaching pressure in nearby IFL. Real time indicators of forest change and associated data on resource use by endangered species are critical to promoting long-term preservation of biodiversity across different landscapes. However, such opportunities to study and protect IFL areas are quickly vanishing. As of 2013, it was estimated that only 8% of forests within Central Africa remained intact (Potapov et al., 2017).
Author Contributions
DM, SS, and CMS: designed the study and oversaw data collection. WW, WM, and FI: compiled data for this study. SS, WW, CRS, CT, and CMS: analyzed the data. CMS and DM: wrote the manuscript with input from all co-authors. DM, CA, CMS, SN, WM, DK, and FI: collected data in the field. DM, CA, and CMS provided logistical support and infrastructure for data collection.
Funding
Grateful acknowledgment of funding is due to the U.S. Fish and Wildlife Service, the Arcus Foundation, Lincoln Park Zoo, Nouabale-Ndoki Foundation, Cincinnati Zoo and Botanical Gardens, Indianapolis Zoo, Houston Zoo, the American Zoological Association, Margot Marsh Biodiversity Foundation, Columbus Zoological Park, and the International Center for Advanced Renewable Energy and Sustainability (I-CARES) of Washington University in Saint Louis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This field research was conducted in accordance with the research protocols of the Institutional Animal Care and Use Committee of Washington University in Saint Louis. We are deeply appreciative of the opportunity to work in the Nouabalé-Ndoki National Park and the Kabo Forestry Management Unit in northern Republic of Congo. This work would not be possible without the continued support of the Ministére de l'Économie Forestiére du gouvernement de la République du Congo et du Agence Congolaise de la Faune et des Aires Protégées (ACFAP). The Wildlife Conservation Society's Congo Program is an integral partner in this continuing research. We also wish to thank OLAM Ltd., and specifically recognize the efforts of D. Paget, H. Ekani, and V. Istace for facilitating access to the inventory data. Special thanks are due to J. M. Fay, B. Djoni, P. Elkan, S. Elkan, B. Curran, P. Telfer, M. Gately, E. Stokes, P. Ngouembe, D. Dos Santos, E. Arnhem, and M. Ngangoue. We would also like to recognize the tireless dedication of M. Mguessa, J. Wawa, C. Abedine, and the Goualougo tracking team.
References
Ahrends, A., Burgess, N. D., Milledge, S. A. H., Bulling, M. T., Fisher, B., Smart, J. C. R., et al. (2010). Predictable waves of sequential forest degradation and biodiversity loss spreading from an African city. Proc. Natl. Acad. Sci. U.S.A. 107, 14556–14561. doi: 10.1073/pnas.0914471107
Allan, J. R., Venter, O., Maxwell, S., Bertzky, B., Jones, K., Shi, Y., et al. (2017). Recent increases in human pressure and forest loss threaten many Natural World Heritage Sites. Biol. Conserv. 206, 47–55. doi: 10.1016/j.biocon.2016.12.011
Allebone-Webb, S. M., Kumpel, N. F., Rist, J., Cowlishaw, G., Rowcliffe, J. M., Milner-Gulland, E. J., et al. (2011). Use of market data to assess bushmeat hunting sustainability in Equatorial Guinea. Conservation Biology 25, 597–606. doi: 10.1111/j.1523-1739.2011.01681.x
Ancrenaz, M., Calaque, R., and Lackman-Ancrenaz, I. (2004). Orangutan nesting behavior in disturbed forest of Sabah, Malaysia: implications for Nest Census. Int. J. Primatol. 25, 983–1000. doi: 10.1023/B:IJOP.0000043347.84757.9a
Asner, G. P., Loarie, S. R., and Heyder, U. (2010). Combined effects of climate and land-use change on the future of humid tropical forests. Conserv. Lett. 3, 395–403. doi: 10.1111/j.1755-263X.2010.00133.x
Bailey, K. M., McCleery, R. A., Binford, M. W., and Zweig, C. (2016). Land-cover change within and around protected areas in a biodiversity hotspot. J. Land Use Sci. 11, 154–176. doi: 10.1080/1747423X.2015.1086905
Barnes, R. F. W., and Lahm, S. A. (1997). An ecological perspective on human densities in the central African forests. J. Appl. Ecol. 34, 245–260. doi: 10.2307/2404862
Barr, D. J., Levy, R., Scheepers, C., and Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lang. 68, 255–278. doi: 10.1016/j.jml.2012.11.001
Basabose, A. K. (2002). Diet composition of chimpanzees inhabiting the montane forests of Kahuzi, Democratic Republic of Congo. Am. J. Primatol. 58, 1–21. doi: 10.1002/ajp.10049
Bastin, J. F., Barbier, N., Réjou-Méchain, M., Fayolle, A., Gourlet-Fleury, S., Maniatis, D., et al. (2015). Seeing Central African forests through their largest trees. Sci. Rep. 5:13156. doi: 10.1038/srep13156
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bermejo, M., Rodriguez-Teijeiro, J. D., Illera, G., Barroso, A., Vila, C., and Walsh, P. D. (2006). Ebola outbreak killed 5000 gorillas. Science 314, 1564–1564. doi: 10.1126/science.1133105
Blake, S. (1994). A Reconnaissance Survey in the Kabo Logging Concession South of the Nouabalé-Ndoki National Park, Northern Congo. Report to USAID, WCS, Government of Congo, GTZ, and the World Bank.
Blake, S. (2002). Forest Elephant Ecology and Distribution in the Ndoki Forests, Republic of Congo. Unpublished Ph.D. thesis: Edinburgh University. Edinburgh.
Blake, S., Deem, S. L., Strindberg, S., Maisels, F., Momont, L., Isia, I. B., et al. (2008). Roadless wilderness area determines forest elephant movements in the Congo Basin. PLoS ONE 32:e03546. doi: 10.1371/journal.pone.0003546
Bourliere, F. (1985). Primate communities - their structure and role in the tropical ecosystems. Int. J. Primatol. 6, 1–26. doi: 10.1007/BF02693694
Brandt, J. S., Nolte, C., and Agrawal, A. (2016). Deforestation and timber production in Congo after implementation of sustainable forest management policy. Land Use Policy 52, 15–22. doi: 10.1016/j.landusepol.2015.11.028
Brugière, D., Bourgras, S., and Gautier-Hoin, A. (2000). Dynamique Forestière à Processus de Colonisation - Extinction: Relations Faune - Flore dans les Forêts à Marantacees du Parc National d'Odzala. Bruxelles, ECOFAC Project.
Brugiere, D., and Sakom, D. (2001). Population density and nesting behavior of lowland gorillas (Gorilla gorilla gorilla) in the Ngotta forest, Central African Republic. J. Zool. Lond. 255:251–259. doi: 10.1017/S0952836901001315
Bryant, D., Nielsen, D., and Tangley, L. (1997). The last frontier forests: ecosystems and economies on the edge: World Resources Institute.
Burivalova, Z., Sekercioglu, C. H., and Koh, L. P. (2014). Thresholds of logging intensity to maintain tropical forest biodiversity. Curr. Biol. 24, 1893–1898. doi: 10.1016/j.cub.2014.06.065
Campbell, J. (1992). Ecology of Bornean Orang-utans (Pongo pygmaeus) in drought and fire- affected lowland rainforest (Ph.D. thesis). Penn State University.
Carroll, R. (1988). Relative density, range extension and conservation potential of the lowland gorilla (Gorilla gorilla gorilla) in Dzanga-Sangha region of southwestern Central African Republic. Mammalia 53, 309–323. doi: 10.1515/mamm-1988-0302
Chazdon, R. L. (2018). Protecting intact forests requires holistic approaches. Nat. Ecol. Evol. 2, 915–915. doi: 10.1038/s41559-018-0546-y
DeFries, R., Hansen, A., Newton, A. C., and Hansen, M., C. (2005). Increasing isolation of protected areas in tropical forests over the past twenty years. Ecol. Applic. 15, 19–26. doi: 10.1890/03-5258
Dennis, R. A., and Colfer, C. P. (2006). Impacts of land use and fire on the loss and degradation of lowland forest in 1983-2000 in East Kutai District, East Kalimantan, Indonesia. Singap. J. Trop. Geogr. 27, 30–48. doi: 10.1111/j.1467-9493.2006.00238.x
Devos, C., Sanz, C., Morgan, D., Onononga, J. R., Laporte, N., and Huynen, M. C. (2008). Comparing ape densities and habitats in northern Congo: surveys of sympatric gorillas and chimpanzees in the Odzala and Ndoki regions. Am. J. Primatol. 70, 439–451. doi: 10.1002/ajp.20514
Dobson, A. J. (2002). An Introduction to Generalized Linear Models. Boca Raton, FL: Chapman and Hall/CRC. doi: 10.1201/9781420057683
Dupain, J., Nackoney, J., Mario Vargas, J., Johnson, P. J., Farfan, M. A., Bofaso, M., et al. (2012). Bushmeat characteristics vary with catchment conditions in a Congo market. Biol. Conserv. 146, 32–40. doi: 10.1016/j.biocon.2011.11.025
Elkan, P. W., Elkan, S. W., Moukassa, A., Malonga, R., Ngangoue, M., and Smith, J. L. D. (2006). “Managing threats from bushmeat hunting in a timber concession in the Republic of Congo,” in Emerging Threats to Tropical Forests, eds W. F. Laurance and C. A. Peres (Chicago, IL: University of Chicago Press, 393–415.
Emery Thompson, M., Kahlenberg, S. M., Gilby, I. C., and Wrangham, R. W. (2007). Core area quality is associated with variance in reproductive success among female chimpanzees at Kibale National Park. Anim. Behav. 73, 501–512. doi: 10.1016/j.anbehav.2006.09.007
Eves, H., and Ruggiero, R. G. (2000). “Socio-economics and the sustainability of hunting in the forests of northern Congo (Brazzaville),” in Hunting for Sustainability in Tropical Forests, eds E. Bennett and J. Robinson (New York, NY: Colombia University Press, 427–454.
Fa, J. E., Currie, D., and Meeuwig, J. (2003). Bushmeat and food security in the Congo Basin: linkages between wildlife and people's future. Environ. Conserv. 30, 71–78. doi: 10.1017/S0376892903000067
Fa, J. E., Johnson, P. J., Dupain, J., Lapuente, J., Koster, P., and Macdonald, D. W. (2004). Sampling effort and dynamics of bushmeat markets. Anim. Conserv. 7, 409–416. doi: 10.1017/S136794300400160X
Fa, J. E., Yuste, J. E. G., and Castelo, R. (2000). Bushmeat markets on Bioko Island as a measure of hunting pressure. Conserv. Biol. 14, 1602–1613. doi: 10.1111/j.1523-1739.2000.99067.x
Fay, J. M. (1992). A Survey of the Proposed Nouabalé-Ndoki National Park Conservation Area, northern Congo. Report to: Wildlife Conservation Society.
Fay, J. M. (1997). The Ecology, Social Organization, Populations, Habitat and History of the Western Lowland Gorilla (Gorilla gorilla gorilla Savage and Wyman 1847). (Ph.D. thesis), Washington University in Saint Louis.
Fay, J. M., Agnagna, M., and Moutsamboté, J. M. (1990). A Survey of the Proposed Nouabalé Conservation Area in northern Congo. Congo, WCS.
Felton, A. M., Engstrom, L. M., Felton, A., and Knott, C. D. (2003). Orangutan population density, forest structure and fruit availability in hand-logged and unlogged peat swamp forests in West Kalimantan, Indonesia. Biol. Conserv. 114, 91–101. doi: 10.1016/S0006-3207(03)00013-2
Ferreira, L. V., and Laurance, W. F. (1997). Effects of forest fragmentation on mortality and damage of selected trees in central Amazonia. Conserv. Biol. 11, 797–801. doi: 10.1046/j.1523-1739.1997.96167.x
Forstmeier, W., and Schielzeth, H. (2011). Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner's curse. Behav. Ecol. Sociobiol. 65, 47–55. doi: 10.1007/s00265-010-1038-5
Furuichi, T., Hashimoto, C., and Tashiro, Y. (2001). Extended application of a marked-nest census method to examine seasonal changes in habitat use by chimpanzees. Int. J. Primatol. 22, 913–928. doi: 10.1023/A:1012057403512
Furuichi, T., Inagaki, H., and Angoue-Ovono, S. (1997). Population density of chimpanzees and gorillas in the Petit Loango Reserve, Gabon: employing a new method to distinguish between nests of the two species. Int. J. Primatol. 18, 1029–1046. doi: 10.1023/A:1026356432486
Geist, H. J., and Lambin, E. F. (2002). Proximate causes and underlying driving forces of tropical deforestation. Bioscience 52, 143–150. doi: 10.1641/0006-3568(2002)052[0143:PCAUDF]2.0.CO;2
Gourlet-Fleury, S., Beina, D., Fayolle, A., Ouedraogo, D. Y., Mortier, F., Benedet, F., et al. (2013a). Silvicultural disturbance has little impact on tree species diversity in a Central African moist forest. For. Ecol. Manage 304, 322–332. doi: 10.1016/j.foreco.2013.05.021
Gourlet-Fleury, S., Mortier, F., Fayolle, A., Baya, F., Ouedraogo, D., Benedet, F., et al. (2013b). Tropical forest recovery from logging: a 24 year silvicultural experiment from Central Africa. Philos. Trans. R. Soc. B Biol. Sci. 368:1625. doi: 10.1098/rstb.2012.0302
Groves, C. P. (1971). Distribution and place of origin of Gorilla. Man 6, 44–51. doi: 10.2307/2798426
Haurez, B., Dainou, K., Vermeulen, C., Kleinschroth, F., Mortier, F., Gourlet-Fleury, S., et al. (2017). A look at Intact Forest Landscapes (IFLs) and their relevance in Central African forest policy. Forest Policy Econom. 80, 192–199. doi: 10.1016/j.forpol.2017.03.021
Husson, S., Wich, S., Marshall, A. J., Dennis, D., Ancrenaz, M., Brassey, R., et al. (2009). “Orangutan distribution, density, abundance and impacts of disturbance,” in Orangutans, eds S. Wich, S. S. Utami Atmoko, T. M. Setia, and C. van Schaik (Oxford: Oxford Press), 77–96. doi: 10.1093/acprof:oso/9780199213276.003.0006
IUCN (2014). Regional Action Plan for the Conservation of Western Lowland Gorillas and Central Chimpanzees 2015-2025. IUCN SSC Group. Gland, Switzerland: 1–56.
Jerozolimski, A., and Peres, C. A. (2003). Bringing home the biggest bacon: a cross-site analysis of the structure of hunter-kill profiles in Neotropical forests. Biol. Conserv. 111, 415–425. doi: 10.1016/S0006-3207(02)00310-5
Johns, A. D. (1997). Timber Production and Biodiversity Conservation in Tropical Rain Forests. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511525827
Johns, A. D., and Skorupa, J. P. (1987). Responses of rain-forest primates to habitat disturbance: a review. Int. J. Primatol. 8, 157–191. doi: 10.1007/BF02735162
Kleinschroth, F., Gourlet-Fleury, S., Sist, P., Mortier, F., and Healey, J. R. (2015). Legacy of logging roads in the Congo Basin: how persistent are the scars in forest cover? Ecosphere 6, 1–17. doi: 10.1890/ES14-00488.1
Kleinschroth, F., and Healey, J. R. (2017). Impacts of logging roads on tropical forests. Biotropica 49, 620–635. doi: 10.1111/btp.12462
Kleinschroth, F., Healey, J. R., Sist, P., Mortier, F., and Gourlet-Fleury, S. (2016). How persistent are the impacts of logging roads on Central African forest vegetation? J. Appl. Ecol. 53, 1–17. doi: 10.1111/1365-2664.12661
Koops, K., McGrew, W. C., and Matsuzawa, T. (2013). Ecology of culture: do environmental factors influence foraging tool use in wild chimpanzees, Pan troglodytes verus? Anim. Behav. 85, 175–185. doi: 10.1016/j.anbehav.2012.10.022
Kuehl, H. S., Boesch, C., Kulik, L., Haas, F., Arandjelovic, M., Dieguez, P., et al. (2019). Human impact erodes chimpanzee behavioral diversity. Science 363, 1453–1455. doi: 10.1126/science.aau4532
Laporte, N., Stabach, J. A., Grosch, R., Lin, T. S., and Goetz, S. J. (2007). Expansion of industrial logging in central Africa. Science 316:1451. doi: 10.1126/science.1141057
Laurance, W. F., Ferreira, L. V., Rankin-de Merona, J. M., and Laurance, S. G. (1998). Rain forest fragmentation and the dynamics of Amazonian tree communities. Ecology 79, 2032–2040. doi: 10.1890/0012-9658(1998)079[2032:RFFATD]2.0.CO;2
Laurance, W. F., Useche, D. C., Rendeiro, J., Kalka, M., Bradshaw, C. J. A., Sloan, S. P., et al. (2012). Averting biodiversity collapse in tropical forest protected areas. Nature 489, 290–294. doi: 10.1038/nature11318
Leighton, M. (1993). Modeling dietary selectivity by Bornean orangutans: evidence for integration of multiple criteria in fruit selection. Int. J. Primatol. 14, 257–313. doi: 10.1007/BF02192635
Leighton, M., and Wirawan, N. (1986). “Catastrophic drought and fire in Borneo tropical rain forest associated with the 1982-1983 El Nino Southern Oscillation event,” in Tropical Forests and the World Atmosphere, ed G. Prance (Washington, DC: American Association for the Advancement of Science), 75–102.
Lewis, S. L., Sonke, B., Sunderland, T., Begne, S. K., Lopez-Gonzalez, G., van der Heijden, G. M. F., et al. (2013). Above-ground biomass and structure of 260 African tropical forests. Philosoph. Trans. R. Soc. B Biol. Sci. 368:20120295. doi: 10.1098/rstb.2012.0295
Lindenmayer, D. B., and Franklin, J. F. (2002). Conserving Forest Biodiversity: A Comprehensive Multiscaled Approach. Washington, DC: Island Press.
Lindenmayer, D. B., and Laurance, W. F. (2017). The ecology, distribution, conservation and management of large old trees. Biol. Rev. 92, 1434–1458. doi: 10.1111/brv.12290
Lui, G. V., and Coomes, D. A. (2016). Tropical nature reserves are losing their buffer zones, but leakage is not to blame. Environ. Res. 147, 580–589. doi: 10.1016/j.envres.2015.11.008
Maisels, F., Strindberg, S., Blake, S., Wittemyer, G., Hart, J., Williamson, E., et al. (2013). Devastating decline of forest elephants in Central Africa. PLoS ONE 8:e59469. doi: 10.1371/journal.pone.0059469
Malcolm, J. R., and Ray, J. C. (2000). Influence of timber extraction routes on central African small-mammal communities, forest structure, and tree diversity. Conserv. Biol. 14, 1623–1638. doi: 10.1046/j.1523-1739.2000.99070.x
Malenky, R. K., Kuroda, S., Ono-Vineberg, E., and Wrangham, R. W. (1994). “The significance of terrestrial herbaceous foods for bonobos, chimpanzees, and gorillas,” in Chimpanzee Cultures, eds R. W. Wrangham, W. C. McGrew, F. B. M. de Waal, and P. G. Heltne. (Cambridge, MA: Harvard University Press), 59–75.
Malenky, R. K., and Wrangham, R. W. (1994). A quantitative comparison of terrestrial herbaceous food-consumption by Pan paniscus in the Lomako Forests, Zaire, and Pan troglodytes in the Kibale Forest, Uganda. Am. J. Primatol. 32, 1–12. doi: 10.1002/ajp.1350320102
Marshall, A. J., and Leighton, M. (2006). “How does food availability limit the population density of whitebearded gibbons?” in Primate Feeding Ecology in Apes and Other Primates: Ecological, Physiological, and Behavioural Aspects, eds G. Hohmann, M. M. Robbins, and C. Boesch (Cambridge: Cambridge University Press, 311–333.
Meijaard, E., and Sheil, D. (2008). The persistence and conservation of Borneo's mammals in lowland rain forests managed for timber: observations, overviews and opportunities. Ecol. Res. 23, 21–34. doi: 10.1007/s11284-007-0342-7
Morgan, B. (2000). Ecology of Mammalian Frugivores in the Reserve de Faune du Petit Loango, (Gabon Ph.D.), University of Cambridge.
Morgan, D., Mundry, R., Sanz, C., Ayina, C. E., Strindberg, S., Lonsdorf, E., et al. (2018). African apes coexisting with logging: comparing chimpanzee (Pan troglodytes troglodytes) and gorilla (Gorilla gorilla gorilla) resource needs and responses to forestry activities. Biol. Conserv. 218, 277–286. doi: 10.1016/j.biocon.2017.10.026
Morgan, D., and Sanz, C. (2006). “Chimpanzee feeding ecology and comparisons with sympatric gorillas in the Goualougo Triangle, Republic of Congo,” in Primate Feeding Ecology in Apes and Other Primates: Ecological, Physiological, and Behavioural Aspects, eds G. Hohmann, M. Robbins, and C. Boesch (Cambridge: Cambridge University Press, 97–122.
Morgan, D., Sanz, C., Onononga, J. R., and Strindberg, S. (2006). Ape abundance and habitat use in the Goualougo Triangle, Republic of Congo. Int. J. Primatol. 27, 147–179. doi: 10.1007/s10764-005-9013-0
Morgan, D., Sanz, C., Onononga, J. R., and Strindberg, S. (2016). Factors influencing the survival of sympatric gorilla (Gorilla gorilla gorilla) and chimpanzee (Pan troglodytes troglodytes) nests. Int. J. Primatol. 37, 718–737. doi: 10.1007/s10764-016-9934-9
Morgan, D., Sanz, C., and Stokes, E. (2012). “The importance of set-aside areas for the conservation of great apes in the Ndoki Region: Preservation of the Goualougo and Djeke triangle forests,” in Tropical Forest Conservation and Industry Partnership: An Experience from the Congo Basin. Conservation Science and Practice, eds C. J. Clark and J. R. Poulsen (Oxford: Wiley-Blackwell, 171–172.
Peres, C. A. (2005). Why we need megareserves in Amazonia. Conserv. Biol. 19, 728–733. doi: 10.1111/j.1523-1739.2005.00691.x
Peres, C. A., and Terborgh, J. W. (1995). Amazonian nature reserves: an analysis of the defensibility status of existing conservation units and design criteria for the future. Conserv. Biol. 9, 34–46. doi: 10.1046/j.1523-1739.1995.09010034.x
Potapov, P., Hansen, M. C., Laestadius, L., Turubanova, S., Yaroshenko, A., Thies, C., et al. (2017). The last frontiers of wilderness: tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 3:e1600821. doi: 10.1126/sciadv.1600821
Potapov, P., Yaroshenko, A., Turubanova, S., Dubinin, M., Laestadius, L., Thies, C., et al. (2008). Mapping the World's intact forest landscapes by remote sensing. Ecol. Soc. 13:51. doi: 10.5751/ES-02670-130251
R Core Team (2017). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Rao, M., and van Schaik, C. P. (1997). The behavioral ecology of Sumateran orangutans in logged and unlogged forest. Trop. Biodivers. 4, 173–185.
Reynolds, J. (2003). “Life histories and extinction risk,” in Macroecology, eds T. M. Blackburn and J. K. Gaston (Oxford: Blackwell, 195–217.
Ripple, W. J., Wolf, C., Newsome, T. M., Hoffmann, M., Wirsing, A. J., and McCauley, D. J. (2017). Extinction risk is most acute for the world's largest and smallest vertebrates. Proc. Natl. Acad. Sci. U.S.A. 114, 10678–10683. doi: 10.1073/pnas.1702078114
Rogers, M., and Williamson, E. (1987). Density of herbaceous plants eaten by gorillas in Gabon: some preliminary data. Biotropica 19, 278–281. doi: 10.2307/2388348
Sanz, C., Morgan, D., Strindberg, S., and Onononga, J. R. (2007). Distinguishing between the nests of sympatric chimpanzees and gorillas. J. Appl. Ecol. 44, 263–272. doi: 10.1111/j.1365-2664.2007.01278.x
Schaller, G. B. (1963). The Mountain Gorilla: Ecology and Behavior. Oxford: University of Chicago Press.
Sodhi, N. S., Koh, L. P., Clements, R., Wanger, T. C., Hill, J. K., Hamer, K. C., et al. (2010). Conserving Southeast Asian forest biodiversity in human-modified landscapes. Biol. Conserv. 143, 2375–2384. doi: 10.1016/j.biocon.2009.12.029
Stokes, E. J., Strindberg, S., Bakabana, P. C., Elkan, P. W., Elkan, F. C., Madzoke, B., et al. (2010). Monitoring great ape and elephant abundance at large spatial scales: measuring effectiveness of a conservation landscape. PLoS ONE 5:e10294. doi: 10.1371/journal.pone.0010294
Strindberg, S., Maisels, F., Williamson, E. A., Blake, S., Stokes, E. J., Aba'a, R., et al. (2018). Guns, germs, and trees determine density and distribution of gorillas and chimpanzees in Western Equatorial Africa. Science Advances 4:eaar2964. doi: 10.1126/sciadv.aar2964
Terborgh, J. (1986). “Keystone plant resources in the tropical forests,” in Conservation Biology: the Science of Scarcity and Diversity, ed M. E. Soule (Saunderland, MA: Sinauer Associates).
Thomas, L., Buckland, S. T., Rexstad, E. A., Laake, J. L., Strindberg, S., Hedley, S. L., et al. (2010). Distance software: design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 47, 5–14. doi: 10.1111/j.1365-2664.2009.01737.x
Tranquilli, S., Abedi-Lartey, M., Abernethy, K., Amsini, F., Asamoah, A., Balangtaa, C., et al. (2014). Protected areas in tropical Africa: assessing threats and conservation activities. PLoS ONE 9:e0114154. doi: 10.1371/journal.pone.0114154
Tutin, C. E. G., and Fernandez, M. (1984). Nationwide census of gorilla and chimpanzee populations in Gabon. Am. J. Primatol. 6, 313–336. doi: 10.1002/ajp.1350060403
Wasser, S. K., Shedlock, A. M., Comstock, K., Ostrander, E. A., Mutayoba, B., and Stephens, M. (2004). Assigning African elephant DNA to geographic region of origin: applications to the ivory trade. Proc. Natl. Acad. Sci. U.S.A. 101, 14847–14852. doi: 10.1073/pnas.0403170101
Watson, J. E. M., Evans, T., Venter, O., Williams, B., Tulloch, A., Stewart, C., et al. (2018). The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2, 599–610. doi: 10.1038/s41559-018-0490-x
Watson, J. E. M., Shanahan, D. F., Di Marco, M., Allan, J., Laurance, W. F., Sanderson, E. W., et al. (2016). Catastrophic declines in wilderness areas undermine global environment targets. Curr. Biol. 26, 2929–2934. doi: 10.1016/j.cub.2016.08.049
Watts, D. P. (1984). Composition and variability of mountain gorilla diets in the central Virungas. Am. J. Primatol. 7, 323–356. doi: 10.1002/ajp.1350070403
Wich, S., Buij, R., and van Schaik, C. (2004). Determinants of orangutan density in the dryland forests of the Leuser Ecosystem. Primates 45, 177–182. doi: 10.1007/s10329-004-0080-1
Wilkie, D. S., Sidle, J. G., and Boundzanga, G. C. (1992). Mechanized logging, market hunting, and a bank loan in Congo. Conserv. Biol. 6, 570–580. doi: 10.1046/j.1523-1739.1992.06040570.x
Willie, J., Petre, C. A., Tagg, N., and Lens, L. (2013). Density of herbaceous plants and distribution of western gorillas in different habitat types in south-east Cameroon. Afr. J. Ecol. 51, 111–121. doi: 10.1111/aje.12014
Wilson, M. L., Boesch, C., Fruth, B., Furuichi, T., Gilby, I. C., Hashimoto, C., et al. (2014). Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414–417. doi: 10.1038/nature13727
Wrangham, R., Conklin, N. L., Etot, G., Obua, J., Hunt, K. D., Hauser, M. D., et al. (1993). The value of figs to chimpanzees. Int. J. Primatol. 14, 243–256 doi: 10.1007/BF02192634
Wrangham, R. W. (1986). “Ecology and social relationships in two species of chimpanzees,” in Ecological Aspects of Social Evolution: Birds and Mammals, eds D. I. Rubenstein and R. W. Wrangham (Princeton, NJ: Princeton University Press), 352–378. doi: 10.2307/j.ctt7zvwgq.20
Keywords: gorilla, chimpanzee, certification, biodiversity, Congo Basin
Citation: Morgan D, Strindberg S, Winston W, Stephens CR, Traub C, Ayina CE, Ndolo Ebika ST, Mayoukou W, Koni D, Iyenguet F and Sanz CM (2019) Impacts of Selective Logging and Associated Anthropogenic Disturbance on Intact Forest Landscapes and Apes of Northern Congo. Front. For. Glob. Change 2:28. doi: 10.3389/ffgc.2019.00028
Received: 14 January 2019; Accepted: 20 May 2019;
Published: 03 July 2019.
Edited by:
Yadvinder Malhi, University of Oxford, United KingdomReviewed by:
Giuliano Maselli Locosselli, University of São Paulo, BrazilMarília Cunha-Lignon, São Paulo State University, Brazil
Copyright © 2019 Morgan, Strindberg, Winston, Stephens, Traub, Ayina, Ndolo Ebika, Mayoukou, Koni, Iyenguet and Sanz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Morgan, ZG1vcmdhbkB3Y3Mub3Jn
 David Morgan
David Morgan Samantha Strindberg2
Samantha Strindberg2