- 1Department of Biological Sciences, University of Alberta, Edmonton, AB, Canada
- 2Department of Biology, University of Virginia, Charlottesville, VA, United States
Semiochemical-baited traps are commonly used to monitor moth pests and inform management decisions. Unfortunately, bee pollinators can be unintentionally captured, which reduces monitoring system efficiency and may negatively impact pollinator biodiversity and pollination services. We assessed the abundance and diversity of wild bees captured in semiochemical-baited traps designed to capture cutworm and armyworm (Lepidoptera: Noctuidae) pests in North America. Green Unitraps were baited with semiochemicals including: (1) species-specific noctuid pheromone lures; (2) food bait lures consisting of fermentation by-products; or (3) floral volatiles. Traps were positioned in canola (Brassica napus L.) and wheat (Triticum aestivum L.) fields in Alberta, Canada. We also explored the mechanisms of bee detection of moth pheromone components using electroantennogram (EAG) assays to assess the antennal response of two Bombus species. We found that more bumble bees (Hymenoptera: Apidae), and especially Bombus rufocinctus Cresson, were captured in traps baited with moth pheromone lures than in unbaited control traps. Fermentation by-product lures captured a similar low number of bees as unbaited traps, whereas floral volatile-based food bait lures captured more bees, comparable to capture in pheromone-baited traps. In general, more Bombus spp. were captured in traps positioned at canola vs. wheat fields, but the community composition was similar among crops. EAG assays indicate that sensory receptors on the antennae of B. rufocinctus Cresson and B. impatiens Cresson detect noctuid moth pheromones. Perception of chemical signals of a different insect order may be explained by structural similarities in pheromone components produced by both moths and bumble bees.
Introduction
Monitoring pest populations is the foundation of integrated pest management (IPM) programs (Witzgall et al., 2010; Flint and Van den Bosch, 2012). The temporal and spatial distribution of lepidopteran pests in agroecosystems can be assessed through moth capture in traps that are attractive to target species (Mason et al., 1998; Mori and Evenden, 2013; Spears et al., 2016). Traps baited with synthetic copies of species-specific moth pheromones are commonly used to detect and provide a measure of density of target populations in many species (Byers and Struble, 1987; Mason et al., 1998). As pheromone signals travel far down wind and many moths are strong fliers (Schneider, 1999), the capture of moths in pheromone-baited traps may not always reflect breeding populations or predict larval densities and subsequent economic damage at the site of capture (Gerber and Walkof, 1992). Monitoring traps baited with food bait lures (e.g., fermentation products, plant volatiles, etc.) that attract both male and female moths over short distances may better reflect breeding populations (Landolt et al., 2007). Unfortunately, both pheromone-baited traps and food bait traps can also capture non-target insects, or bycatch.
Bycatch of non-target insects in monitoring traps increases trap processing time, decreases trap effectiveness for the target species, and can include beneficial arthropods that provide important ecosystem services (Cha et al., 2015; Landolt and Zhang, 2016). If beneficial species are frequently captured, monitoring traps might negatively impact these populations and the services they provide. Beneficial hymenopteran pollinators make up much of the insect bycatch in moth pheromone-baited traps positioned in a variety of agroecosystems (Adams et al., 1989; Meagher and Mitchell, 1999; Aurelian et al., 2015; Spears et al., 2016). Although the impact of bee removal from agroecosystems in monitoring traps that target moth pests is unknown (Meagher and Mitchell, 1999), it may negatively influence local bee abundance and species diversity, which could alter pollination services for both wild plants and managed crops and could reduce crop yields (Goulson, 2003; Potts et al., 2010). As there is already widespread recognition of global declines of wild bee populations (Potts et al., 2010; Goulson et al., 2015), practices that systematically remove bees from agricultural landscapes should be avoided.
Attraction of bees to semiochemical-baited traps that target moth pests might be mediated by both visual (e.g., trap style, color) and olfactory cues (Stephen and Rao, 2005; Spears et al., 2016; Sipolski et al., 2019). Bees use visual cues to locate floral resources when foraging for nectar and pollen (Chittka and Spaethe, 2007; Junker and Parachnowitsch, 2015). More bees orient to and are captured in yellow, blue, and white traps than in green traps positioned in several cropping systems (Gross and Carpenter, 1991; Mori and Evenden, 2013; Spears et al., 2016; Sipolski et al., 2019). Wild bee pollinators, especially bumble bees (Hymenoptera: Apidae), are frequently captured as bycatch in traps baited with moth pheromones, which consist of unsaturated carbon-10-18 acetates, aldehydes, and alcohols; this attraction occurs regardless of trap color (Meagher and Mitchell, 1999; Turnock et al., 2007; Mori and Evenden, 2013; Aurelian et al., 2015; Spears et al., 2016). The capture of wild bees in traps baited with pheromone signals used as mating cues in a distantly related insect assemblage raises questions about whether bees can detect and respond to these heterospecific signals, but the mechanisms driving wild bee bycatch in lepidopteran pheromone-baited monitoring traps have not yet been reported.
Here, we assess the abundance and diversity of Bombus spp. and other wild bees captured in green non-saturating Unitraps baited with synthetic noctuid pheromone or food bait lures when positioned in canola, Brassica napus L. (Brassicaceae), and wheat, Triticum aestivum L. (Poaceae), fields in Alberta, Canada. Wild bee pollination is linked with increased productivity of many crops globally and improves canola seed set in Alberta (Morandin and Winston, 2005; Dainese et al., 2019). As canola is a mass-flowering crop that provides valuable food resources for wild bees (Senapathi et al., 2017), we expect a higher abundance and diversity of bees to be captured at canola fields. We also investigate how bees respond to heterospecific semiochemical signals by measuring the electrophysiological response of two bumble bee species to moth pheromone components. Finally, we provide recommendations that may minimize the capture of non-target pollinator species in moth monitoring systems.
Materials and Methods
Study System
Cutworms and armyworms (Lepidoptera: Noctuidae) are generalist herbivores that are pestiferous in many agroecosystems including the Prairies of western Canada (Byers and Struble, 1987). In Alberta, some of the native noctuid moths are field crop pests that can cause economic loss when larval populations reach outbreak levels (Byers and Struble, 1987; Mason et al., 1998). Feeding damage ranges from plant removal causing minor patchiness to destruction of entire fields when larvae are at outbreak levels. Strategies to manage cutworms and armyworms in the Prairie Provinces include cultural practices, natural biological control, and insecticide application (Mason et al., 1998; Evenden et al., 2017). Reliable monitoring tools for this guild of noctuid pests are less well developed.
We monitored bycatch in semiochemical-baited traps targeting cutworm and armyworm pests positioned in crop fields in the Prairies of the Aspen Parkland Ecoregion in Alberta, Canada. Like most prairies, this region has been extensively modified over the past century to support agriculture (Shorthouse, 2010). This region is located in the Canadian Prairie Ecozone, which contains twenty-eight of Canada’s forty bumble bee species (Sheffield et al., 2014). The landscape is characterized by extensive agricultural plains with discontinuous clusters of trembling aspen (Populus tremuloides Michx.) (Salicaceae), balsam poplar (Populus balsamifera L.) (Salicaceae), and mixed stands (Shorthouse, 2010).
Seven sites were selected across five counties in central Alberta (Supplementary Table S1). All experimental sites were separated by at least 20 km. Each site was comprised of a paired canola and wheat field, separated by at least 500 m. All experiments were conducted at the same sites in each of 3 years from 2014 to 2016. Due to crop rotation practices, fields planted with canola in the first year were rotated to wheat in the second year and back to canola in the third year of the study.
Field Experiments
Non-saturating green Unitraps (Contech Enterprises Inc., Delta, BC, Canada) were deployed to monitor for cutworm and armyworm moths in each of the 3 years of the study. At each site, Unitraps were baited with either a synthetic sex pheromone (Contech Enterprise Inc., Delta, BC, Canada) targeting one of the focal moth pests or with a feeding attractant lure (Table 1). Moth sex pheromone lures were prepared by Contech Enterprise Inc. (Delta, BC, Canada) and loaded onto pre-extracted red rubber septa. Sex pheromone lures were placed inside the roof of the Unitrap in a basket and were replaced every 4 weeks, as recommended by the manufacturer. Feeding attractant or food bait lures consist of sugar bait fermentation products or floral volatiles and are known to attract many species of noctuid moths (Landolt et al., 2007). Food bait lures were prepared in the laboratory (Landolt et al., 2007) and dispensed in 15 mL Nalgene HDPE vials (Thermo Scientific, Rochester, NY, United States). Vials were packed with cotton balls, loaded with 10 mL of the chemical mixture, and secured inside the bucket of the Unitrap. A 3.0 mm diameter hole drilled in the center of the vial cap allowed the release of the volatile components. Food bait lures were replaced every 2 weeks (Landolt et al., 2007). An unbaited control trap was included for comparison at each field for all experiments. All Unitraps were positioned 1.5 m above ground and spaced 25 m apart in a linear transect along the field edge. Trap transects were always positioned along a road side on a random edge of the study field. An insecticidal strip of Hercon Vaportape II (10% dichlorvos; Hercon Environmental, Emigsville, PA, United States) was placed inside the bucket of each trap to kill captured insects and was replaced every 4 weeks. Traps were intentionally deployed in the field to coincide with the flight periods of the target moth species and to limit the capture of bumble bee queens.
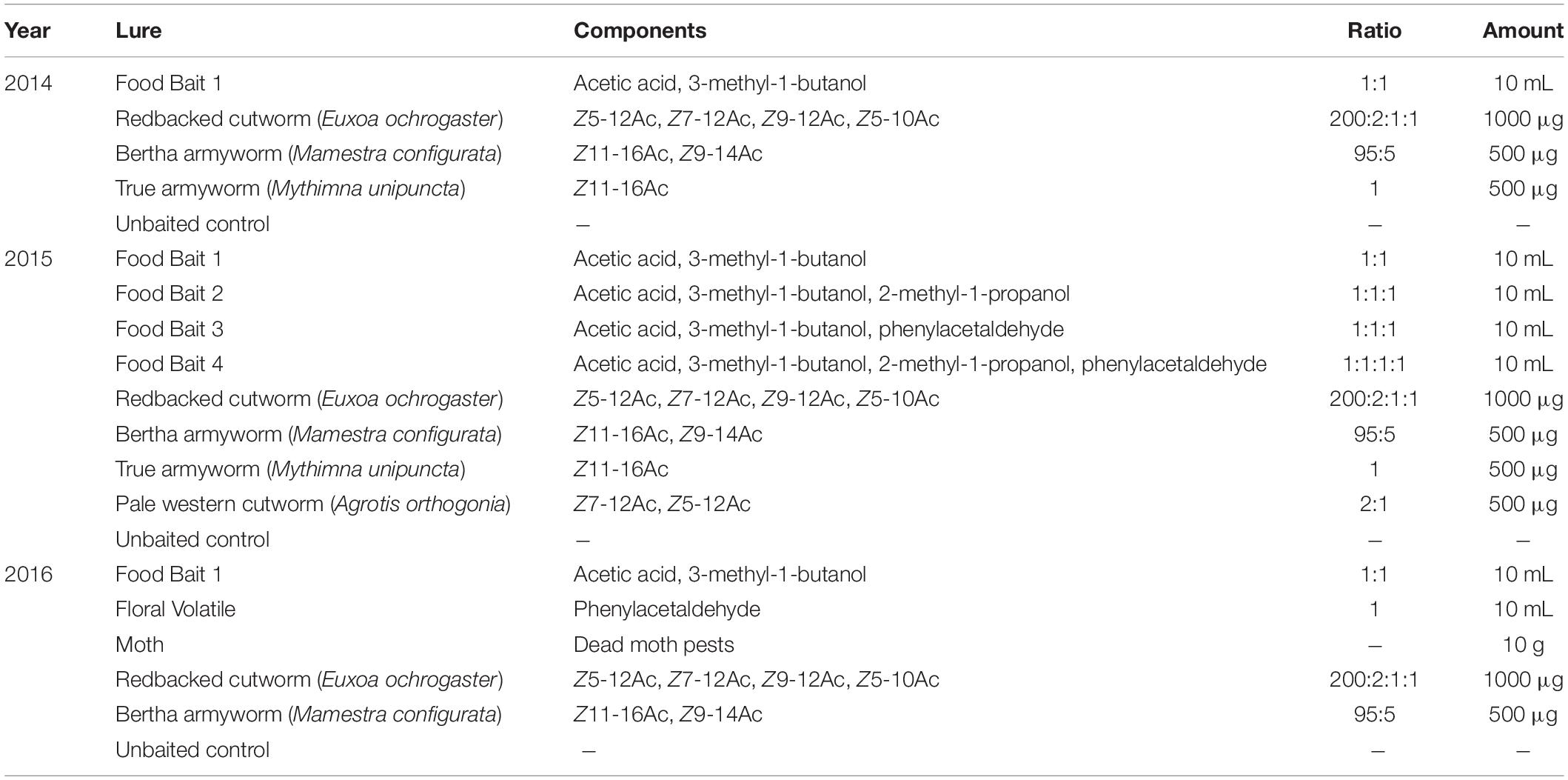
Table 1. Composition of lures deployed in non-saturating green Unitraps in the margin of canola and wheat fields in central Alberta, Canada (2014–2016).
In 2014, pollinator bycatch was compared in traps baited with a fermentation by-product based food bait lure consisting of glacial acetic acid (Fisher, Fair Lawn, NJ, United States; 99.7% purity) and 3-methyl-1-butanol (Sigma Aldrich, St. Louis, MO, United States; 98.5% purity) mixed in equal parts by weight (Table 1) or one of three commercially prepared synthetic noctuid pheromone lures targeting redbacked cutworm [Euxoa ochrogaster (Guenée)], bertha armyworm (Mamestra configurata Walker) or true armyworm [Mythimna unipuncta (Haworth)] (Table 1). All lures were present concurrently at each site from 23 June to 2 September 2014.
In 2015, bee bycatch was obtained from traps baited with one of four food bait lures that consisted of both sugar fermentation by-products and floral volatiles (Table 1). This experiment tested the effect of additional semiochemicals such as a short chain alcohol, 2-methyl-1-propanol (Acros Organics, Fair Lawn, NJ; >99% purity), and a floral volatile, phenylacetaldehyde (Acros Organics, Fair Lawn, NJ; >98% purity), in combination with the original food bait lure from the previous experiment (Table 1). The pale western cutworm [Agrotis orthogonia (Morrison)] pheromone was included in addition to the 3 moths pheromone lures tested in the first year of the study. All lures were present from 29 June to 4 August 2015.
In 2016, bycatch in traps baited with the original food bait lure, comprised of acetic acid and 3-methyl-1-butanol was compared to that in traps baited with the floral volatile phenylacetaldehyde. This lure was assessed to determine the pollinator attraction to phenylacetaldehyde in the absence of fermentation by-products. Moth pheromone-baited traps targeting the redbacked cutworm and bertha armyworm pheromone (Table 1) were also repeated for this year of the study. A final trap baited with dead moths was included to test if bumble bees could detect and respond to volatile odors released by dead moths in the traps. The dead moth treatment consisted of a mesh bag filled with 10 g of dead moths obtained from previous capture in pheromone-baited Unitraps and stored at −20°C until use. All lures were present from 7 June to 28 July 2016.
Trap-catch was collected every week and frozen at −20°C. Bee bycatch was separated from the other trap content and individual bees were pinned and dried for identification. Honey bee (Apis mellifera L.) (Hymenoptera: Apidae) and bumble bee workers and queens were identified to species (Packer and Ratti, 2009; Williams et al., 2014), but males were excluded from identification and subsequent analyses, as few were captured. All other bees were identified to family (Packer and Ratti, 2009). After identification, we used morphometric measurements to distinguish between worker and queen B. rufocinctus females captured in the first year of the study (Supplementary Material: Methods). Identifications were verified using comparisons with our own reference collections and specimens housed in the E. H. Strickland Entomological Museum (University of Alberta, Edmonton, Canada), where voucher specimens are deposited.
Electrophysiological Experiment
We tested the hypothesis that bumble bees can detect the various moth pheromones used to bait Unitraps in the field (Table 1) using laboratory-based electroantennogram (EAG) assays with bumble bee antennae as detectors. We compared antennal response of the most commonly captured bumble bee species in field studies, B. rufocinctus, with that of the commercially available species, B. impatiens Cresson, which is often used in field and laboratory studies in North America (Shipp et al., 1994; Cnaani et al., 2002). A standard colony of B. impatiens (Biobest Canada, Leamington, ON, United States) was maintained in a growth chamber (Percival Intellus Environmental Controller Model I30VL; Percival Scientific, Perry, IA, United States) at 23 ± 2°C on a 12:12 hour L:D cycle and provisioned with BIOGLUC sugar solution (Biobest Canada, Leamington, ON, United States). Similarly-sized workers were randomly selected and removed from the colony one month later, in May 2018, for use in EAG assays. In early July 2018, we collected B. rufocinctus workers from field margins of a canola field near Sunnybrook, Alberta (53°09′00.8″N 114°07′25.0″W). Bees were captured using insect nets (0.3 m diameter) and were housed in refrigerated containers for transport to the University of Alberta, where they were placed in individual containers and provided with 10% sugar solution. Bees were placed into a growth chamber, under the environmental conditions detailed above, for ∼18 h prior to EAG assays.
The EAG system consisted of an IDAC-02 data acquisition controller system, a Syntech EAG probe (Type PRG-2, internal gain 10X), and EAG 2000 software (Syntech, Hilversum, Netherlands). Bumble bees were chilled at 4°C for 10–15 min before the right antenna was excised using micro-dissecting scissors; the left antenna was used if the right was missing or damaged. The antenna was cut at the base of the flagellum and at the tip of the terminal segment. The cut antenna was attached to a stainless-steel antenna holder using a small quantity of conductive gel (Spectra 360; Parker Laboratories, Orange, NJ, United States). We tested three synthetic moth pest pheromone components, Z11-16Ac, Z5-12Ac, and Z7-12Ac (Pherobank, Wijk bij Duurstede, Netherlands), which were selected because they are the major components of the pheromone baits used in our field experiments (Table 1). Each test compound was serially diluted in hexane (Sigma Aldrich, St. Louis, MO, United States) to obtain 1, 10, and 100 μg/μL hexane solutions. 25 μL of each solution was pipetted onto 0.2 cm × 7.0 cm strips of filter paper (Whatman No. 1), which was inserted into individual Pasteur pipettes 30 min prior to the assay so that the solvent could evaporate. Hexane-treated filter paper inserts served as a negative control. Linalool (Sigma Aldrich, St. Louis, MO; 97% purity) was diluted to 10 μg/μL with hexane and was used as a positive control (Anfora et al., 2011) to ensure bumble bee antennae remained alive for the duration of the trial. Carbon-filtered and humidified air, from a Syntech CS-55 stimulus controller, flowed at 50 mL/min over each mounted antenna. Stimulus puffs were triggered by hand via the stimulus controller and had a pulse duration of 0.2 s and flow rate of 10 mL/s. Test compounds were presented in ascending order of dosage with 30 s inter-stimuli intervals (i.e. hexane, linalool, 1 μg/μL Z11-16Ac, linalool, 10 μg/μL Z11-16Ac, linalool, 100 μg/μL Z11-16Ac, hexane) and stimuli cartridges were replaced after every five antennae tested. Antennae from 10 individuals of both B. impatiens and B. rufocinctus were tested and EAG responses were recorded as the maximum amplitude of depolarization (mV) induced by the test compound.
Statistical Analysis
All statistical analyses were conducted using R in “RStudio v1.1.447” (R v3.5.0; R Core Team, 2018). To determine the effect of lure and crop type on bumble bee bycatch, we modeled data from field experiments in each year independently using generalized linear mixed models with negative binomial error term distributions (glmer.nb function, lme4 package; Bates et al., 2015). For each model, we performed an analysis of deviance using the Wald chi-square test statistic (Anova function, car package; Fox et al., 2012) to assess differences in the number of bumble bees captured in traps baited with different lures and positioned in different crop types. This was followed with post hoc means separation using the Tukey method (α = 0.05) (lsmeans package; Lenth and Hervé, 2015). For each experiment, a separate model was created specifying the total number of B. rufocinctus, the dominant species trapped throughout the season, as the dependent variable, different lure and crop types as independent variables, and site as a random effect. Date was not included as an explanatory variable as low weekly capture rates prevented analysis of un-pooled data. Models initially included all possible interactions, which were removed if they were not statistically significant (α = 0.05). In 2014, we captured a substantial number of other bumble bee species and ran an additional model which excluded B. rufocinctus, but there were too few other species captured to repeat this analysis for 2015 and 2016. Other species of wild bees (non-Bombus) were captured in low numbers in all experiments and were not included in statistical models.
We used non-metric multidimensional scaling (NMDS) ordination using Bray-Curtis pairwise distance matrices (bcdist function, ecodist package; Goslee and Urban, 2007) and ANOSIM analysis (anosim function, vegan package; Oksanen et al., 2013) to visualize and assess differences in the species richness of bumble bee bycatch in traps baited with the different lures and positioned in the different crops in each year of the study. Analyses were performed excluding B. rufocinctus to assess differences for less frequently captured species represented by >1 individual. ANOSIM analysis uses a ranked dissimilarity matrix to compare the similarity of the community within and between treatment groups. ANOSIM generates the R test statistic, which indicates treatment differences if significantly different from zero (Clarke, 1993).
For the electrophysiological experiment, EAG response data were transformed using [ln(x + 1)] to satisfy assumptions of normality. We analyzed the response to each test compound separately using linear mixed models (lmer function, lme4 package) for each bumble bee species. EAG responses were analyzed with a random intercept and slope to account for the repeated measures on the same bee antenna. For each model, we specified the transformed EAG response as the dependent variable and compound concentration as a fixed factor. Compound concentration of the stimulus was specified as the random intercept and the antenna identification number was considered as the random slope (∼ Concentration | Antenna ID). We used an ANOVA analysis (Anova function; R Core Team, 2018) to test for differences in EAG response at varying concentrations of each test compound, compared to the hexane control. This was followed with post hoc means separation using the Tukey method (α = 0.05).
Results
Field Experiments
A total of 1746 female and 59 male bumble bees representing 16 species were captured across the entire study period (Table 2). Bombus rufocinctus was the most abundant species with a total of 1429 captured during the study. We also captured 229 other bees from four families: Andrenidae, Apidae, Halictidae, and Megachilidae (Table 3).
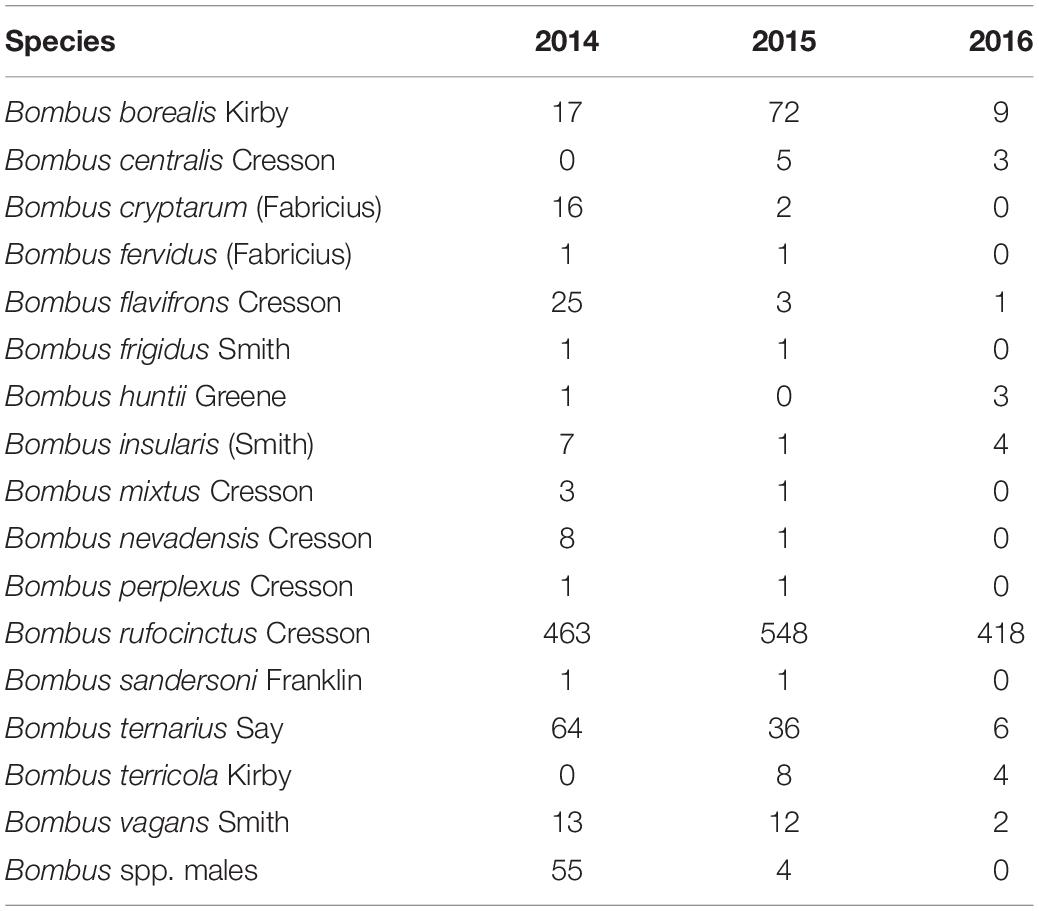
Table 2. Season-long bumble bee bycatch abundance in monitoring traps positioned in agroecosystems in central Alberta, Canada (2014–2016).
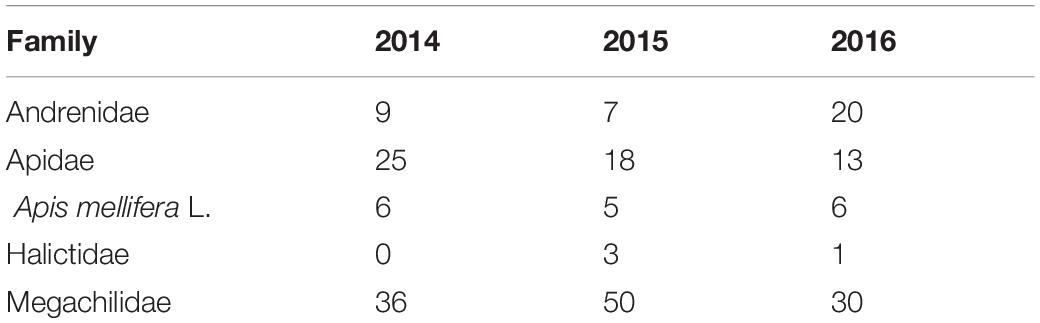
Table 3. Season-long non-Bombus bee bycatch in monitoring traps positioned in agroecosystems in central Alberta, Canada (2014–2016).
There were significant differences in the number of B. rufocinctus captured in traps baited with different lure types in 2014 (Wald χ2 = 60.949, df = 4, p<0.0001). There were significantly more B. rufocinctus per trap captured in the noctuid moth pheromone-baited Unitraps than in unbaited control traps (Figure 1). There was no difference in B. rufocinctus capture in unbaited control traps and traps baited with the food bait lure comprised of acetic acid and 3-methyl-1-butanol (Table 1). Capture of other Bombus spp. was not influenced by lure type (Wald χ2 = 4.381, df = 4, p = 0.357). On average, more B. rufocinctus were captured in Unitraps positioned along canola fields compared to wheat, but this difference was not statistically significant (Wald χ2 = 2.971, df = 1, p = 0.085). Traps positioned in canola fields captured significantly more other Bombus spp., excluding B. rufocinctus, compared to traps in wheat fields (Wald χ2 = 4.159, df = 1, p = 0.041). All morphometric measurements indicated that the majority of B. rufocinctus females captured were similarly-sized worker bees (raw data available upon request).
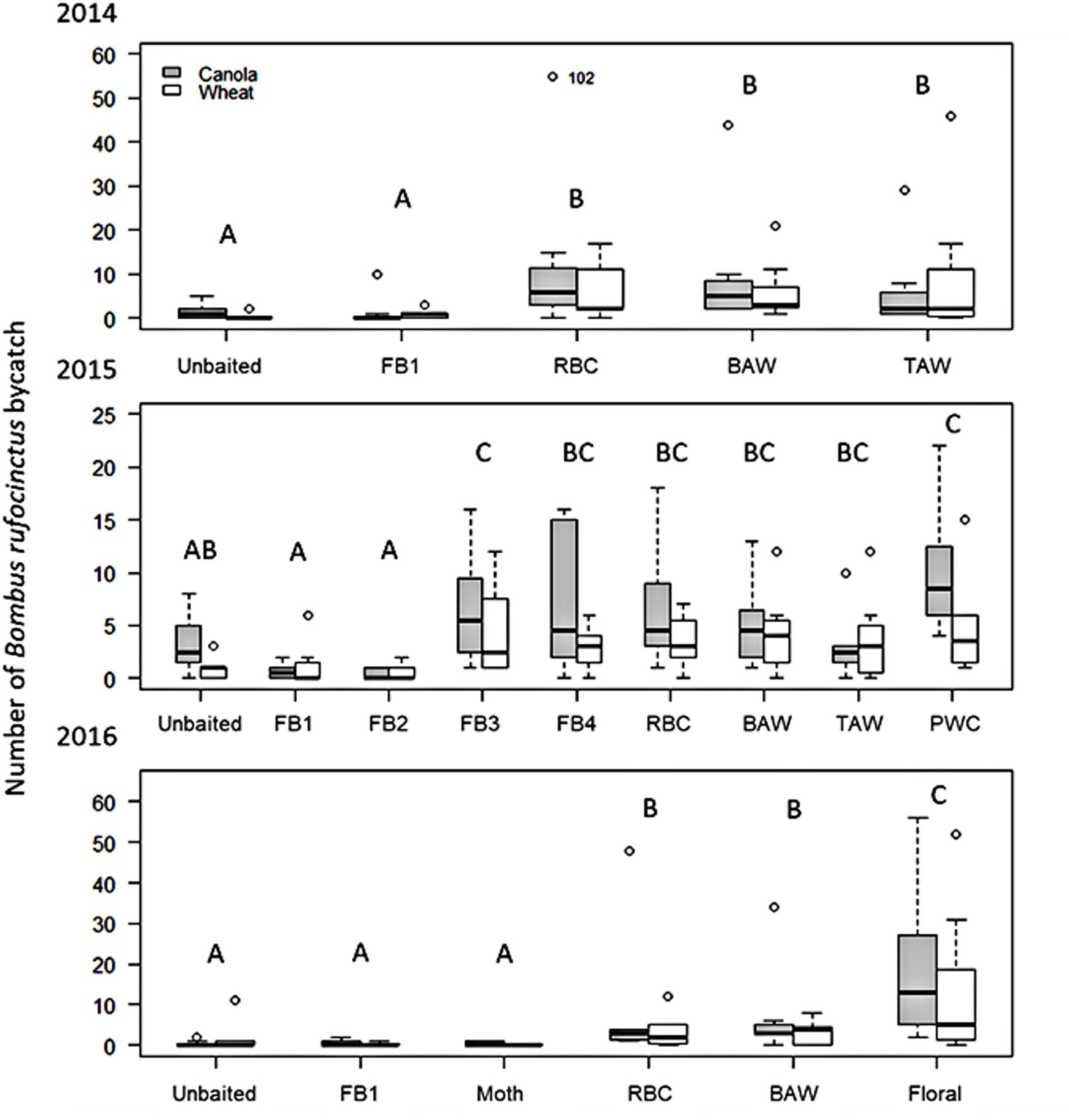
Figure 1. Boxplots of the season long capture of Bombus rufocinctus in baited Unitraps at canola and wheat crops in central Alberta, Canada between 2014 and 2016. Lure treatments varied by season. 2014: FB1 = acetic acid + 3-methyl-1-butanol; RBC = Redbacked cutworm pheromone; BAW = Bertha armyworm pheromone; TAW = true armyworm pheromone. 2015: FB1; FB2 = FB1 + 2-methyl-1-propanol; FB3 = FB1 + phenylacetaldehyde; FB4 = FB1 + 2-methyl-1-propanol + phenylacetaldehyde; RBC; BAW; TAW; PWC = Pale western cutworm pheromone. 2016: FB1; Floral = phenylacetaldehyde; Moth = 10 g previously captured dead noctuid moths; RBC; BAW. Open circles represent points more than 1.5 times the interquartile range (repositioned points are shown with the original location indicated to allow for easier visual comparison between treatments). Different letters indicate significant differences between treatments within year (Tukey’s HSD: p<0.05).
Similarly, in 2015, lure type significantly affected the number of B. rufocinctus captured in traps baited with the different lure types (Wald χ2 = 72.682, df = 8, p<0.0001). More B. rufocinctus were captured in pheromone-baited Unitraps compared to the unbaited control traps, but only traps baited with the pale western cutworm pheromone lure captured significantly more bees than the control trap (Figure 1). Capture of B. rufocinctus in traps baited with the original food bait lure and the lure containing the additional fermentation by-product, 2-methyl-1-propanol (Table 1), did not differ from capture in unbaited control traps. The addition of the floral component, phenylacetaldehyde, to the original food bait lure (Table 1) as well as to the lure composed of all tested feeding attractant components (Table 1) enhanced capture of B. rufocinctus. Only traps baited with the food bait including the floral volatile lure captured significantly more than the unbaited control trap (Figure 1). More B. rufocinctus were captured in Unitraps positioned along canola fields compared to wheat in 2015 (Wald χ2 = 7.522, df = 1, p = 0.006).
Lure type again impacted the number of B. rufocinctus captured in 2016 (Wald χ2 = 91.77, df = 5, p<0.0001). The most B. rufocinctus were captured in traps baited with the phenylacetaldehyde floral volatile lure alone (Figure 1). There were significantly more B. rufocinctus captured in traps baited with the pheromone of either redbacked cutworm or bertha armyworm than in unbaited control traps. As in previous years, traps baited with the original two-component food bait lure captured similar numbers of B. rufocinctus as the unbaited control trap. Additionally, there was no significant difference in the number of B. rufocinctus captured in the unbaited control and the dead moth treatment. More B. rufocinctus were captured in Unitraps positioned along canola fields compared to wheat, but this difference was not significant in 2016 (Wald χ2 = 2.79, df = 1, p = 0.095).
For all years of the study, there was an overlapping pattern in the NMDS plots of bumble bee species composition for both lure and crop type (Supplementary Figures S1–S6). There were no significant differences in the species composition of bumble bees captured in traps baited with any of the tested lure types (2014: ANOSIM R = −0.026, p = 0.734; 2015: ANOSIM R = 0.047, p = 0.119; 2016: ANOSIM R = −0.040, p = 0.641). Similarly, there were no differences in species composition between Bombus spp. captured in traps positioned along canola or wheat fields (2014: ANOSIM R = −0.007, p = 0.551; 2015: ANOSIM R = 0.001, p = 0.362; 2016: ANOSIM R = 0.004, p = 0.412).
Electrophysiological Experiment
Antennae of both B. rufocinctus and B. impatiens showed a dose dependent EAG response to the tested moth pheromone components. Responses to the test components varied by bumble bee species (Figure 2). The EAG response to Z11-16Ac, the major pheromone component of the bertha armyworm and the true armyworm, was only significantly higher than the hexane control at 100 μg/μL for B. impatiens (F3,27 = 4.51, p = 0.011) and not for B. rufocinctus at any concentration. Conversely, B. rufocinctus had significantly higher responses to Z5-12Ac, the major pheromone component of the redbacked cutworm pheromone, at 100 μg/μL compared to hexane (F3,27 = 10.84, p<0.0001), but the antennal response of B. impatiens to Z5-12Ac did not vary from the hexane control at any of the tested concentrations. Both bumble bee species showed significantly greater EAG responses to the main component of the pale western cutworm pheromone, Z7-12Ac, at 10 and 100 μg/μL as compared to hexane (F3,27, p<0.0001).
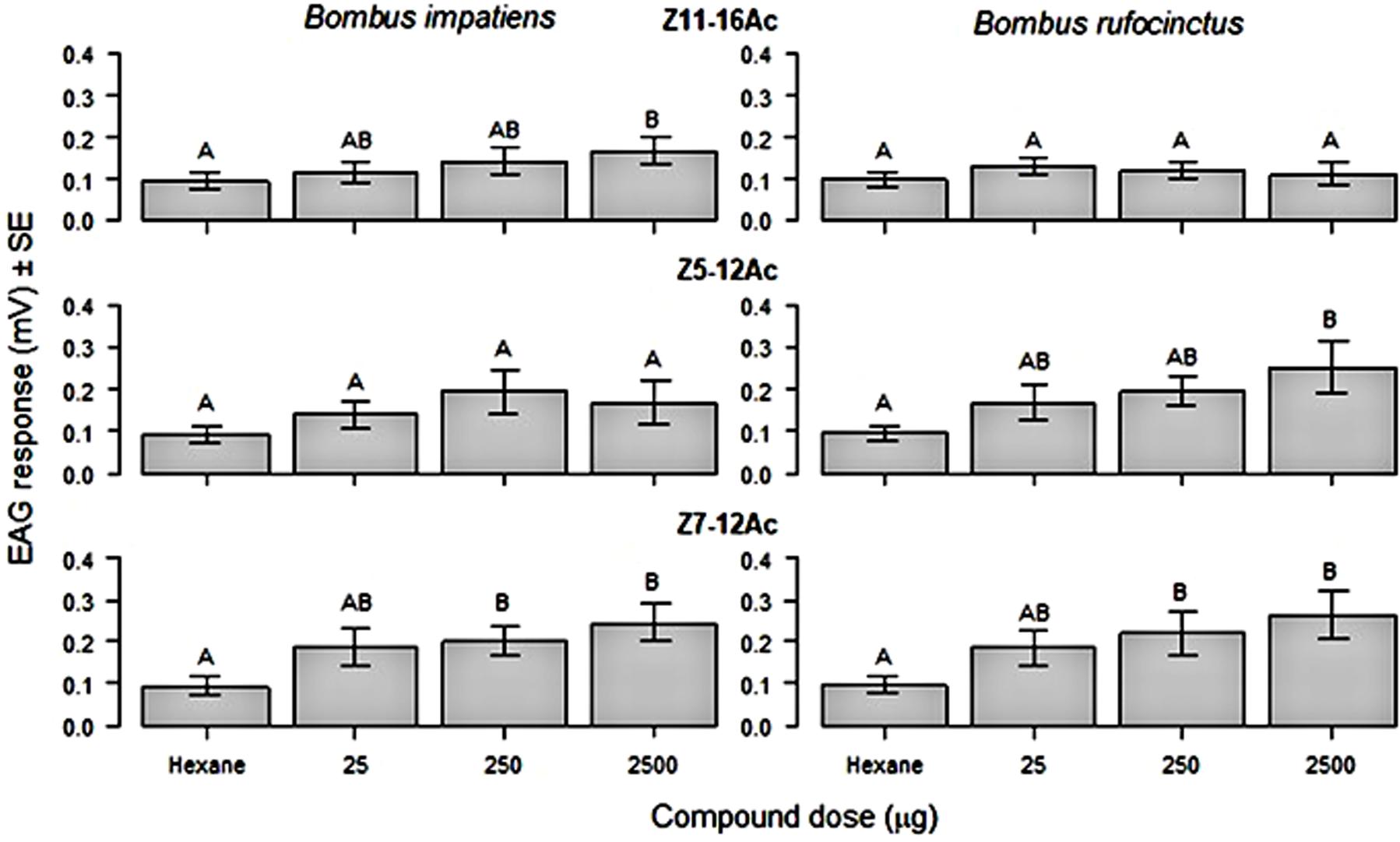
Figure 2. Mean (± SE) electroantennogram (EAG) response (mV) generated from excised antennae of Bombus impatiens (n = 10) and B. rufocinctus (n = 10) workers stimulated with a hexane control and various doses of moth pest pheromone components: Z11-16Ac, Z5-12Ac, and Z7-12Ac. Different letters indicate significant differences after means comparison (Tukey’s HSD: p<0.05).
Discussion
Trapping experiments in canola and wheat fields in Alberta, Canada show that bumble bee bycatch is prevalent in traps baited with pheromones of noctuid moth pests native to the Prairie Provinces, whereas relatively few other wild bees were captured. Bombus rufocinctus was the most commonly captured species across all 3 years of our study and were more commonly captured in traps baited with pheromone lures than the various food bait lures or unbaited control traps. Other bumble bee species were captured in numbers that reflect abundance in the sampling area (Kohler et al., 2020). There were a similar number of B. rufocinctus captured in all pheromone-baited traps in this study, whereas previous studies have found that bycatch often varies in traps baited with different pheromone lures. For example, in alfalfa and corn growing regions of Utah, traps baited with pheromone lures that target Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) moths have high bee bycatch, while traps baited with Spodoptera litura Fabricius (Lepidoptera: Noctuidae) and S. littoralis Boisduval (Lepidoptera: Noctuidae) pheromone lures do not capture more bees than unbaited control traps (Spears et al., 2016). However, it is important to note that both Spodoptera spp. lures tested in this study are exotic pests, whereas more attractive Helicoverpa lures are native to the study region (Spears et al., 2016). Traps in corn fields baited with S. frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) pheromone, however, attract more Bombus spp. than the floral volatile phenylacetaldehyde (Meagher and Mitchell, 1999), which was highly attractive in our study. Differential bumble bee attraction to pheromone lures that target closely related moth pests could be due to differences in agroecosystems, but is more likely driven by differences in the molecular structure of the pheromone components used to bait traps that target different moth species.
Long chain monounsaturated hydrocarbons are widely attractive to Bombus spp. (Meagher and Mitchell, 1999; Spears et al., 2016), but the mechanism of this attraction remains unknown. Bumble bees could be preadapted to sense these molecules because of structural similarity of moth pheromones to signals used by the bees. Male bumble bees produce species-specific pheromone blends in the cephalic portion of the labial gland (De Meulemeester et al., 2011). These pheromones include fatty acid derivatives, straight chain saturated and monounsaturated hydrocarbons, and acyclic terpenes with alcohol, aldehyde, or acetate functional groups (Appelgren et al., 1991; Bergström et al., 1996). In some North American Bombus spp., such as B. nevadensis Cresson, B. griseocollis De Geer, and B. rufocinctus, males perch on prominent landscape features and mark the perches with pheromones to attract females (O’Neill et al., 1991). The secretions of B. rufocinctus and other perching males are predominately acetate based (Bertsch et al., 2004). Interestingly, B. rufocinctus in the current study was the predominant species captured in traps baited with acetate-based pheromone lures.
Although the primary function of male-produced pheromones in Bombus spp. is to attract unmated queens for reproduction (Appelgren et al., 1991; Bergström et al., 1996; Šobotník et al., 2008), it is likely that conspecific workers and males are also pre-adapted to respond to this signal. Bumble bee workers, males, and young queens have similar EAG responses to a variety of floral volatiles and bumble bee pheromone components (Fonta and Masson, 1984). Male Bombus spp. may try to usurp the scent-marked territories of other perching males (O’Neill et al., 1991) and can use these chemicals to locate mating sites (Bertsch et al., 2004). In the present study, ∼80% of male bumble bees were captured in traps baited with noctuid moth pheromone lures. In our electrophysiological experiment, both B. impatiens and B. rufocinctus female workers had significant and similar EAG responses to the synthetic noctuid moth pheromone component Z7-12Ac, a component of the sex pheromone of the redbacked cutworm and the pale western cutworm (Table 1). Antennae from the two Bombus species responded differently to the other pheromone components tested. Bombus rufocinctus responded to Z5-12Ac but not to Z11-16Ac and the opposite was observed for B. impatiens. The pheromone blends of both the redbacked and pale western cutworm moths contain Z5-12Ac and these lures captured high numbers of B. rufocinctus in field tests. Numerically but not statistically more B. rufocinctus were captured in pheromone traps targeting the cutworm species over those targeting the two armyworm species. The main pheromone component of both the bertha armyworm and true armyworm is Z11-16Ac, which did not elicit an antennal response in B. rufocinctus. These EAG results demonstrate that workers of both B. impatiens and B. rufocinctus can perceive individual lepidopteran pheromone components and are differentially responsive to different components. Many laboratory studies use B. impatiens or B. terrestris as a focal bumble bee species (Shipp et al., 1994; Cnaani et al., 2002), but the results here indicate the importance of considering the response of multiple bumble bee species, particularly locally dominant species, to different environmental conditions or cues.
Based on this study, it appears that many species of bumble bees can perceive noctuid moth pheromones released from baited Unitraps, but only some species respond behaviorally. Antennal electrophysiological response, as measured by EAG, informs perception capability but not the resulting behavioral response, which is often highly context dependent. For example, volatiles released by the obligate nest parasite B. vestalis Geoffroy repel B. terrestris workers (Lhomme et al., 2012), whereas in our study similar, and in some cases the same, semiochemicals attract B. rufocinctus. The orientation exhibited by bumble bees to moth pheromone-baited traps in our study must be due to the recognition and response to noctuid pheromone components as fewer bees were captured in unbaited control traps and bees were not attracted to volatiles released from large numbers of dead moths alone.
The lures based on fermentation products of sugar baits were tested as potential alternatives for monitoring multiple species of noctuid moths (Landolt et al., 2007; Batallas, 2019). In general, fewer bees were captured in traps baited with feeding attractant lures than in those baited with moth pheromones. The addition of the floral volatile, phenylacetaldehyde, to baits releasing the fermentation products enhanced bee capture in traps baited with food bait lures. Bee bycatch in traps baited with the phenylacetaldehyde floral volatile alone was far greater than in food bait traps and even exceeded bycatch in pheromone-baited traps. Similarly, Landolt et al. (2007) captured more Bombus spp. in traps baited with a floral lure (phenylacetaldehyde, β-myrcene, methyl-salicylate, and methyl-2-methoxy benzoate) than in traps baited with the same type of fermentation product food bait lures tested in the current study. As such, although floral volatiles can be attractive to many noctuid pests, they are not recommended for monitoring because of high pollinator bycatch (Meagher and Mitchell, 1999; Landolt et al., 2007; Sipolski et al., 2019). Fermentation-based food bait lures may be effective for simultaneously monitoring many species of noctuid moth pests (Batallas, 2019), while reducing the impact of monitoring on beneficial pollinator populations.
As predicted, we found that traps positioned in the field margins of canola crops had higher bumble bee bycatch than traps adjacent to wheat fields, but the differences were often only marginally significant. The availability of mass-flowering crops such as canola provide a highly rewarding food resource for many pollinators and can increase bumble bee colony growth and abundance during a growing season (Westphal et al., 2009; Senapathi et al., 2017). The community composition of Bombus spp. bycatch captured in traps was not influenced by crop type. In Alberta, canola and wheat crops are often grown in close proximity, which may explain the relatively minor differences in bycatch community composition. Bumble bees are capable of long-distance flights during foraging trips, especially in agriculturally dominated landscapes (Rao and Strange, 2012). This allows them to access high quality floral resources far from nesting areas and creates opportunities for encounters with monitoring traps.
Four other families of hymenopteran pollinators (Andrenidae, Apidae, Halictidae, and Megachilidae) were captured in relatively low numbers in this study. The proportion of non-Bombus bees captured using pan trapping and active netting in Alberta was much higher than found in the current study (Kohler et al., 2020). Although other non-Bombus bees were likely present in the agroecosystems where we conducted our study, they were not frequently captured in monitoring traps. Our results differ from previous studies that report frequent capture of non-Bombus bees in monitoring traps baited with a variety of pheromone lures (Meagher and Mitchell, 1999; Spears et al., 2016). For example, Spears et al. (2016) captured significantly greater numbers of both Lasioglossum (Hymenoptera: Halictidae) and Agapostemon (Hymenoptera: Halictidae) in multicolored traps baited with H. armigera pheromone than in unbaited control traps. Overall, our results corroborate previous studies that show visual cues from green-colored monitoring traps do not attract non-Bombus pollinators (Stephen and Rao, 2005; Mori and Evenden, 2013; Spears et al., 2016).
This study demonstrates that acetate-based noctuid pheromone lures attract bumble bees, and especially B. rufocinctus, in both canola and wheat fields in central Alberta. This attraction is likely due to the similarities between the components of male-produced bumble bee pheromones and noctuid moth pheromones. This study also provides the first electrophysiological evidence that bumble bee workers can perceive the components of moth pheromone lures and that the detection of some components may be Bombus species-specific. Future work to assess the response of Bombus spp. to acetate, aldehyde, and alcohol-based pheromone lures as well as to male-produced bumble bee pheromones could provide additional understanding of the mechanisms driving this response. Although the level of bee bycatch in this study may not pose a significant threat to pollinator populations, any reduction in bycatch would be beneficial as monitoring is essential for the success of agricultural production in the Canadian Prairies. To limit the bycatch of wild bee pollinators in monitoring traps that target noctuid moth pests in the Prairie Provinces, managers should continue to use green monitoring traps that are deployed when queen and male bumble bees are not active. Additionally, lures based on fermentation by-products are attractive to multiple noctuid moth species (Landolt et al., 2007; Batallas, 2019) and do not attract Bombus species. Further research on the efficacy of using food bait lures for wide-scale monitoring and the impact of these lures on other beneficial insects such as vespid wasps (Hymenoptera: Vespidae), lady beetles (Coleoptera: Coccinellidae), and parasitoids is needed before they can be widely adopted for monitoring. Future research in this area should also investigate the environmental factors that influence wild bee bycatch in pheromone baited traps so monitoring protocol changes can be implemented to reduce bycatch and lower the impact of pest monitoring on beneficial wild pollinators.
Data Availability Statement
The data supporting the findings of this study are openly available in the University of Alberta Dataverse at doi: 10.7939/DVN/ZGXPKE.
Author Contributions
NG, RB, and ME conceived the ideas and designed the methodology. NG, RB, EM, and AS collected the data. NG and RB analyzed the data. NG and ME led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Funding
This work was supported by the Agriculture Funding Consortium (2015F014R: Development of synthetic food bait traps to monitor multiple cutworm pests and minimize bee bycatch).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all Evenden lab members who took part in field work and bee maintenance. We would also like to thank all reviewers for their valuable suggestions to improve this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.576692/full#supplementary-material
References
Adams, R. G., Murray, K. D., and Los, L. M. (1989). Effectiveness and selectivity of sex pheromone lures and traps for monitoring fall armyworm (Lepidoptera: Noctuidae) adults in Connecticut sweet corn. J. Econ. Entomol. 82, 285–290. doi: 10.1093/jee/82.1.285
Anfora, G., Rigosi, E., Frasnelli, E., Ruga, V., Trona, F., and Vallortigara, G. (2011). Lateralization in the invertebrate brain: left-right asymmetry of olfaction in bumble bee, Bombus terrestris. PLoS One 6:e18903. doi: 10.1371/journal.pone.0018903
Appelgren, M., Bergström, G., Svensson, B. G., and Cederberg, B. (1991). Marking pheromones of Megabombus bumble bee males. Acta Chem. Scand. 45, 972–974. doi: 10.3891/acta.chem.scand.45-0972
Aurelian, V. M., Evenden, M. L., and Judd, G. J. (2015). Diversity and abundance of arthropod by-catch in semiochemical-baited traps targeting apple clearwing moth (Lepidoptera: Sesiidae) in organic and conventional apple orchards in British Columbia. Canada. Can. Entomol. 147, 227–243. doi: 10.4039/tce.2014.47
Batallas, R. E. (2019). The Basis for Cutworm (Lepidoptera: Noctuidae) Integrated Pest Management: Understanding Crop-Pest Interaction and Moth Community Structure in Prairie Agroecosystems. Ph.D. dissertation, University of Alberta, Alberta, Edmonton, AB.
Bates, D., Maechler, M., Bolker, B., Walker, S., and Christensen, R. H. B. (2015). lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package Version 1.1–7.
Bergström, G., Bergman, P., Appelgren, M., and Schmidt, J. O. (1996). Labial gland chemistry of three species of bumblebees (Hymenoptera: Apidae) from North America. Bioorg. Med. Chem. 4, 515–519. doi: 10.1016/0968-0896(96)00034-x
Bertsch, A., Schweer, H., and Titze, A. (2004). Analysis of the labial gland secretions of the male bumblebee Bombus griseocollis (Hymenoptera: Apidae). Zeitschrift für Naturforschung C 59, 701–707. doi: 10.1515/znc-2004-9-1015
Byers, J. R., and Struble, D. L. (1987). Monitoring population levels of eight species of noctuids with sex-attractant traps in southern Alberta, 1978–1983: specificity of attractants and effect of target species abundance. Can. Entomol. 119, 541–556. doi: 10.4039/ent119541-6
Cha, D. H., Hesler, S. P., Park, S., Adams, T. B., Zack, R. S., Rogg, H., et al. (2015). Simpler is better: fewer non-target insects trapped with a four-component chemical lure vs. a chemically more complex food-type bait for Drosophila suzukii. Entomol. Exp. Appl. 154, 251–260. doi: 10.1111/eea.12276
Chittka, L., and Spaethe, J. (2007). Visual search and the importance of time in complex decision making by bees. Arthropod Plant Interact. 1, 37–44. doi: 10.1007/s11829-007-9001-8
Clarke, K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Austr. J. Ecol. 18, 117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Cnaani, J., Schmid-Hempel, R., and Schmidt, J. O. (2002). Colony development, larval development and worker reproduction in Bombus impatiens Cresson. Insectes Soc. 49, 164–170. doi: 10.1007/s00040-002-8297-8
Dainese, M., Martin, E. A., Aizen, M., Albrecht, M., Bartomeus, I., Bommarco, R., et al. (2019). A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 5:eaax0121.
De Meulemeester, T., Gerbaux, P., Boulvin, M., Coppée, A., and Rasmont, P. (2011). A simplified protocol for bumble bee species identification by cephalic secretion analysis. Insectes Soc. 58, 227–236. doi: 10.1007/s00040-011-0146-1
Evenden, M. L., Batallas, R. E., and Weeraddana, C. (2017). Biology and Management of the Generalist Herbivore, the Bertha Armyworm, Mamestra configurata (Lepidoptera: Noctuidae), on Canola in Western Canada. Boston, MA: cabi, 114–129.
Flint, M. L., and Van den Bosch, R. (2012). Introduction to Integrated Pest Management. Berlin: Springer Science & Business Media.
Fonta, C., and Masson, C. (1984). Comparative study by electrophysiology of olfactory responses in bumblebees (Bombus hypnorum and Bombus terrestris). J. Chem. Ecol. 10, 1157–1168. doi: 10.1007/bf00988546
Fox, J., Weisberg, S., Adler, D., Bates, D., Baud-Bovy, G., Ellison, S., et al. (2012). Package ‘car’. Vienna: R Foundation for Statistical Computing.
Gerber, G. H., and Walkof, J. (1992). Phenology and reproductive status of adult redbacked cutworms, Euxoa ochrogaster (Guenée)(Lepidoptera: Noctuidae), in southern Manitoba. Can. Entomol. 124, 541–551. doi: 10.4039/ent124541-3
Goslee, S. C., and Urban, D. L. (2007). The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw. 22, 1–19.
Goulson, D., Nicholls, E., Botías, C., and Rotheray, E. L. (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:255957.
Gross, H. R., and Carpenter, J. E. (1991). Role of the fall armyworm (Lepidoptera: Noctuidae) pheromone and other factors in the capture of bumblebees (Hymenoptera: Aphidae) by universal moth traps. Environ. Entomol. 20, 377–381. doi: 10.1093/ee/20.1.377
Junker, R. R., and Parachnowitsch, A. L. (2015). Working towards a holistic view on flower traits—how floral scents mediate plant–animal interactions in concert with other floral characters. J. Indian Inst. Sci. 95, 43–68.
Kohler, M., Sturm, A., Sheffield, C. S., Carlyle, C. N., and Manson, J. S. (2020). Native bee communities vary across three prairie ecoregions due to land use, climate, sampling method and bee life history traits. Insect Conserv. Divers. doi: 10.1111/icad.12427
Landolt, P., and Zhang, Q. H. (2016). Discovery and development of chemical attractants used to trap pestiferous social wasps (Hymenoptera: Vespidae). J. Chem. Ecol. 42, 655–665. doi: 10.1007/s10886-016-0721-z
Landolt, P. J., Pantoja, A., Hagerty, A., Crabo, L., and Green, D. (2007). Moths trapped in Alaska with feeding attractant lures and the seasonal flight patterns of potential agricultural pests. Can. Entomol. 139, 278–291. doi: 10.4039/n06-034
Lhomme, P., Ayasse, M., Valterová, I., Lecocq, T., and Rasmont, P. (2012). Born in an alien nest: how do social parasite male offspring escape from host aggression? PLoS One 7:e43053. doi: 10.1371/journal.pone.0043053
Mason, P. G., Arthur, A. P., Olfert, O. O., and Erlandson, M. A. (1998). The bertha armyworm (Mamestra configurata)(Lepidoptera: Noctuidae) in western Canada. Can. Entomol. 130, 321–336. doi: 10.4039/ent130321-3
Meagher, R. L., and Mitchell, E. R. (1999). Nontarget Hymenoptera collected in pheromone-and synthetic floral volatile-baited traps. Environ. Entomol. 28, 367–371. doi: 10.1093/ee/28.3.367
Morandin, L. A., and Winston, M. L. (2005). Wild bee abundance and seed production in conventional, organic, and genetically modified canola. Ecol. Appl. 15, 871–881. doi: 10.1890/03-5271
Mori, B. A., and Evenden, M. L. (2013). Factors affecting pheromone-baited trap capture of male Coleophora deauratella, an invasive pest of clover in Canada. J. Econ. Entomol. 106, 844–854. doi: 10.1603/ec12437
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O’hara, R. B., et al. (2013). Package ‘vegan’. Community Ecology Package, Version, 2, 1–295.
O’Neill, K. M., Evans, H. E., and Bjostad, L. B. (1991). Territorial behaviour in males of three North American species of bumblebees (Hymenoptera: Apidae. Bombus). Can. J. Zool. 69, 604–613. doi: 10.1139/z91-090
Packer, L., and Ratti, C. (2009). Key to the Bee Families of the World. Rome: United Nations Food and Agriculture Organisation.
Potts, S. G., Biesmeijer, J. C., Kremen, C., Neumann, P., Schweiger, O., and Kunin, W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. doi: 10.1016/j.tree.2010.01.007
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rao, S., and Strange, J. P. (2012). Bumble bee (Hymenoptera: Apidae) foraging distance and colony density associated with a late-season mass flowering crop. Environ. Entomol. 41, 905–915. doi: 10.1603/en11316
Schneider, J. C. (1999). Dispersal of a highly vagile insect in a heterogeneous environment. Ecology 80, 2740–2749. doi: 10.2307/177254
Senapathi, D., Goddard, M. A., Kunin, W. E., and Baldock, K. C. (2017). Landscape impacts on pollinator communities in temperate systems: evidence and knowledge gaps. Funct. Ecol. 31, 26–37. doi: 10.1111/1365-2435.12809
Sheffield, C. S., Frier, S. D., and Dumesh, S. (2014). The bees (Hymenoptera: Apoidea, Apiformes) of the Prairies Ecozone, with comparisons to other grasslands of Canada. Arthropods Can. Grasslands 4, 427–467.
Shipp, J. L., Whitfield, G. H., and Papadopoulos, A. P. (1994). Effectiveness of the bumble bee, Bombus impatiens Cr.(Hymenoptera: Apidae), as a pollinator of greenhouse sweet pepper. Sci. Horticult. 57, 29–39. doi: 10.1016/0304-4238(94)90032-9
Shorthouse, J. D. (2010). Ecoregions of Canada’s prairie grasslands. Arthropods f Can. Grasslands 1, 53–81. doi: 10.3752/9780968932148.ch3
Sipolski, S. J., Datson, S. W., Reding, M., Oliver, J. B., and Alm, S. R. (2019). Minimizing Bee (Hymenoptera: Apoidea) Bycatch in Japanese Beetle Traps. Environ. Entomol. 48, 1203–1213. doi: 10.1093/ee/nvz098
Šobotník, J., Kalinová, B., Cahlíková, L., Weyda, F., Ptáček, V., and Valterová, I. (2008). Age-dependent changes in structure and function of the male labial gland in Bombus terrestris. J. Insect Physiol. 54, 204–214. doi: 10.1016/j.jinsphys.2007.09.003
Spears, L. R., Looney, C., Ikerd, H., Koch, J. B., Griswold, T., Strange, J. P., et al. (2016). Pheromone lure and trap color affects bycatch in agricultural landscapes of Utah. Environ. Entomol. 45, 1009–1016. doi: 10.1093/ee/nvw085
Stephen, W. P., and Rao, S. (2005). Unscented color traps for non-Apis bees (Hymenoptera: Apiformes). J. Kansas Entomol. Soc. 78, 373–381. doi: 10.2317/0410.03.1
Turnock, W. J., Kevan, P. G., Laverty, T. M., and Dumouchel, L. (2007). Abundance and species of Bumble Bees (Hymenoptera: Apoidea: Bombinae) in fields of canola, Brassica rapa L., in Manitoba: an 8-year record. J. Entomol. Soc. Ontar. 137, 31–40. doi: 10.1007/s11118-011-9244-y
Westphal, C., Steffan-Dewenter, I., and Tscharntke, T. (2009). Mass flowering oilseed rape improves early colony growth but not sexual reproduction of bumblebees. J. Appl. Ecol. 46, 187–193. doi: 10.1111/j.1365-2664.2008.01580.x
Williams, P. H., Thorp, R. W., Richardson, L. L., and Colla, S. R. (2014). An Identification Guide: Bumble Bees of North America. Princeton, NJ: Princeton University Press.
Keywords: Bombus, pollinator, bycatch, non-target, pheromone, semiochemical
Citation: Grocock NL, Batallas RE, McNamara EA, Sturm AB, Manson JS and Evenden ML (2020) Bumble Bees (Hymenoptera: Apidae) Respond to Moth (Lepidoptera: Noctuidae) Pheromone Components, Leading to Bee Bycatch in Monitoring Traps Targeting Moth Pests. Front. Ecol. Evol. 8:576692. doi: 10.3389/fevo.2020.576692
Received: 26 June 2020; Accepted: 13 August 2020;
Published: 16 September 2020.
Edited by:
Aijun Zhang, United States Department of Agriculture (USDA), United StatesReviewed by:
Jason Gibbs, University of Manitoba, CanadaRobert Meagher, United States Department of Agriculture (USDA), United States
Copyright © 2020 Grocock, Batallas, McNamara, Sturm, Manson and Evenden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicholas L. Grocock, Z3JvY29ja0B1YWxiZXJ0YS5jYQ==
 Nicholas L. Grocock
Nicholas L. Grocock Ronald E. Batallas
Ronald E. Batallas Emily A. McNamara
Emily A. McNamara Ashton B. Sturm
Ashton B. Sturm Jessamyn S. Manson
Jessamyn S. Manson Maya L. Evenden
Maya L. Evenden