- Department of Biological Sciences, University of New Orleans, New Orleans, LA, United States
Selection on behavior, signaling, and morphology can be strongly affected by variation in habitat type. Consequently, populations inhabiting different environments can exhibit divergent phenotypes as a result of either habitat-specific selection or plasticity. Urban habitats in particular represent different challenges for organisms adapted for rural environments, including disparate complements of predators and competitors, resource availability, and habitat complexity. In this paper, I review work aimed at understanding the different selective challenges experienced by rural and urban populations of green anole lizards, primarily those in southeastern Louisiana. I also describe a long-term mark-recapture experiment on an urban population of green anoles in New Orleans, and consider how sex ratios and population density changes over time. Collectively, this work shows that urban and rural populations of green anoles diverge markedly in behavior and morphology driven both by differences in habitat and the presence of competitors in the urban environment; however, it also shows that the effects of urbanization on the ecology and evolution of green anoles are understudied.
Introduction
A key principle in evolutionary biology is that environmental differences among populations can drive divergent selection on morphology, behavior, and physiology. Whether mediated by plasticity or by a genetic response to selection, phenotypes of organisms can vary markedly across populations in response to local conditions (Reznick et al., 1990; Donihue et al., 2018; Lapiedra et al., 2018). Although the nature of the divergence is determined by the specific environmental challenges in conjunction with the evolutionary history of the species or population in question, we can nonetheless discern broad patterns of repeated evolutionary change in response to similar selection pressures (Langerhans and DeWitt, 2004; Losos, 2011; Moore et al., 2016; Auer et al., 2018).
Urban environments comprise a specific habitat milieu that is characterized by distinct structural habitat and environmental variables such as light or thermal regime, amongst others. These urban environments frequently differ markedly from the natural habitats and environments of the organisms inhabiting urban areas. Additionally, because urban areas are also typified by high degrees of disturbance, as well as the presence of man-made structures, urban environments tend to exhibit greater variation in both habitat type and in the thermal microhabitat compared to natural environments (Ackley et al., 2015), but also greater habitat loss concomitant with a high degree of habitat fragmentation resulting in uneven and patchy distribution of vegetation in particular (Watling and Donnelly, 2006; Liu et al., 2016). Because urbanization results in repeated environments that are more similar to each other than they are to neighboring environments, the urban environment is in many respects a replicated one that is likely to exert many similar selection pressures on organisms that inhabit them (Delaney et al., 2010; Johnson and Munshi-South, 2017). Given the pace and scale of anthropogenic induced habitat alteration, understanding how those pressures affect the integrated overall phenotype of urban animal populations should be a priority.
An increasing number of studies are documenting distinct shifts in the phenotypes of urban populations of animals relative to their rural conspecifics. For example, urban populations of Podarcis muralis, a widespread lacertid lizard, exhibit greater fluctuating asymmetry in morphology than rural populations (Lazic et al., 2013). Similarly, urbanization drives genetically based differences in swimming kinematics in the fish Semotilus atromaculatus (Kern and Langerhans, 2019). In addition to morphology and physiology, urban conditions also affect a suite of behavioral traits ranging from acoustic signaling (Slabbekoorn and Peet, 2003; Parris et al., 2009; Luther et al., 2016) to boldness (Atwell et al., 2012) and foraging behavior (Rhodes and Catterall, 2008). Behavior is of particular interest in this regard because behavioral responses of animals to novel conditions can often precede or buffer changes in performance or morphology (Huey et al., 2003; Munoz and Losos, 2018). Indeed, behavioral flexibility is considered a hallmark of urban populations, and may lead to the breakdown of behavioral syndromes seen in natural populations (Scales et al., 2011).
Lizards are commonly found in urban environments and have emerged as a useful taxon for testing the effects of urbanization on ecology, morphology, physiology, and behavior. For instance, geckos have been touted as indicators of urban pollution and habitat quality (Fletcher et al., 2008) and the distribution of some gecko species tracks urbanization because they forage close to artificial lights where insect abundance is typically high (Zozaya et al., 2015). Anolis lizards in particular have previously been suggested to be ideal organisms for understanding adaptation to urban environments at multiple levels of organization, from the within- and among-individual levels to that of the species (Lapiedra, 2018), and indeed a growing number of studies have tested for adaptation to urban habitats in several species of anoles (Campbell-Staton et al., 2020).
Green anole (Anolis carolinensis) lizards colonized the continental United States from Cuba 12–6 million years ago (Glor et al., 2005). Over the last 100,000 years in particular, green anoles have expanded northward and westward from their original site of arrival in Florida (Bourgeois and Boissinot, 2019) and today are widely distributed from Texas in the west to Tennessee in the north, along with introduced populations in southern California, and have also been introduced on Hawai’i, Guam, and Okinawa. Importantly, A. carolinensis are present in both rural and urban settings across much of their North American range, often existing in researchers’ literal backyards. A number of studies of green anoles consequently have used, and continue to use, individuals sampled from or present in urban habitats due to their availability (e.g., Ruby, 1984; Irschick et al., 2006b; Lailvaux et al., 2015). This makes them a useful species for comparing and testing aspects of behavior, morphology, and related traits in different environmental contexts. However, despite being the most well-studied member of the Anolis genus (see Lovern et al., 2004 for a historical overview), no studies yet have synthesized the literature on green anole behavioral ecology with an eye toward understanding how the urban environment in particular affects ecology, behavior, and morphology in this species.
In this paper, I review the literature on green anole urban ecology by comparison wherever possible to natural/rural populations or similar studies on such populations. I focus especially on studies comparing green anoles from two populations in southeastern Louisiana region, namely a rural and an urban population, for two main reasons. First, this body of work represents a detailed comparison of the ecology, morphology, and behavior of urban and rural green anoles in the same area and over a relatively short span of time from several diverse approaches (e.g., habitat use, sexual selection, and interspecific competition, amongst others); yet despite the broad nature of this work, it was not conducted explicitly within the emerging urban ecology framework, and was instead couched as a series of studies of intraspecific variation. Consequently, it has yet to be integrated and interpreted from an urban ecology perspective. Second, replicates of both urban and rural populations in the same region and elsewhere are for the most part unavailable, and few other studies have examined similar issues relating to morphology, performance, and (especially) behavior in other populations of green anoles, although I highlight and discuss those that have.
The ecomorphological paradigm states that morphology determines performance, and further that behavior and performance interact to determine fitness (Arnold, 1983; Garland and Losos, 1994). Consequently, I deal first with the difference in structural habitat between wild/rural and urban environments and how those differences affect morphology and performance before considering further population differences in escape and display behavior within the context of population ecology. Finally, given the paucity of long-term studies on basic aspects of urban population ecology, I present some novel data drawn from a 5-year study of another urban population in New Orleans with the aim of testing some classic hypotheses regarding temporal variation in population structure within an urban setting.
Habitat Use, Morphology, and Performance
Animals in urban areas experience clear differences in the type and availability of structural habitat compared to their natural milieu (Sol et al., 2013). These differences in habitat type can have functional consequences for both how organisms using such habitats are shaped, and how well they are able to execute dynamic, ecologically relevant tasks such as jumping, running, and biting (collectively termed whole-organism performance traits; Bennett and Huey, 1990; Lailvaux and Irschick, 2006), as well as for their behavior. Anolis lizards in general exemplify the interplay among habitat use, morphology, and performance whereby the match between limb length and perch diameter is mediated by behavior, resulting in lizards avoiding substrates on which their sprint performance is submaximal (Irschick and Losos, 1999). Given this “habitat constraint” phenomenon, one might predict that structural habitat differences in the urban environment will alter the morphology of urban green anole populations as well as, potentially, their performance.
The degree to which urban populations of animals might diverge in morphology and performance from conspecifics in wild populations likely depends in part on the degree of urbanization. All urban environments are characterized by increased disturbance, presence of man-made structures, and increased human foot traffic relative to wild or rural counterparts at a minimum, but there is likely to be variation in each of these even within an urbanized region. Similarly, different taxa could be differentially affected by or sensitive to urbanization (Markovchick-Nicholls et al., 2008). For example, a population of lizards in a city block or university campus might be considered to be highly urbanized in terms of the types of habitat they use and the availability and openness of that habitat relative to a natural or rural population, with a city park perhaps falling somewhere between those two extremes, whereas parks and campuses might be more similar to each other from the perspective of a different organism which uses available habitat in a different way (see also Battles et al., 2013 for a classification based on degree of disturbance).
Irschick et al. (2005a) studied two green anole populations in south-eastern Louisiana situated 30 km apart: Good Hope Field (GHF), a relatively undisturbed lowland freshwater swamp in St. Charles Parish, LA; and Tulane University campus (TU) in Orleans Parish, LA. The two sites differ markedly in habitat complexity and in the type of available habitat, with GHP dominated by tall and continuous closed habitat comprising primarily narrow perches, whereas the habitat at TU is fragmented and open, made up mainly of clumps of broad leafed palmetto (Aspidistra elatior) plants close to the ground but with few large trees or bushes. The habitat use of green anoles at these two sites reflected the difference in habitat availability, with 71% of TU lizards perching on palmetto leaves which are patchily distribution, as opposed to GHF lizards who perched primarily on branches (68%) and tree trunks (18%) (Irschick et al., 2005a). Importantly, however, habitat use differed significantly from random habitat availability in both populations, meaning that the anoles did not passively track the underlying habitat distribution. This difference in habitat use was also reflected in a difference in morphology, with TU lizards exhibiting a more slender body shape; longer forelimbs; shorter hindlimbs; and larger toepad areas than GHF animals (Irschick et al., 2005a). This difference in toepad area is also significant in terms of whole-organism performance, because larger toepads translated into higher clinging forces in TU lizards, possibly driven by the smoother perch substrates used by animals in this population (Irschick et al., 2005a).
The finding that green anole lizards exhibit shorter hindlimbs in habitats that have been subject to human disturbance has been replicated elsewhere in the southeastern United States. In a study comparing populations of green anoles among plots that differed in habitat type in Palmetto State Park in Texas, Dill et al. (2013) found that green anole adult females inhabiting a predominately narrow-perch habitat plot that had been subject to moderate human disturbance (i.e., the Lake plot; see Battles et al., 2013) also had shorter hindlimbs than females from natural plots, whereas males exhibited no such difference. In this case, the limb morphology difference tracks the habitat use, unlike the TU lizards which have shorter hindlimbs despite using broader perches (Irschick et al., 2005a). Dill et al. (2013) further noted that this difference arises only in adults, with juveniles showing no effects of population on limb morphology. These sex- and age-specific effects are consistent with a causal explanation of plasticity driving the population effect, as opposed to selection, particularly since green anole females are known to be more plastic in their limb morphology than males (Kolbe and Losos, 2005).
Subsequent studies of urban populations using other Anolis species have reported very similar patterns to those exhibited by green anoles. For example, urban Anolis cristatellus and Anolis sagrei lizards prefer broader perches (Battles et al., 2018) and also exhibit longer limbs (albeit again hindlimbs as opposed to forelimbs) than their rural counterparts (Marnocha et al., 2011; Winchell et al., 2016). Furthermore, urban A. cristatellus also exhibit larger toepad areas, just as urban green anoles do (Winchell et al., 2016), and thus likely higher clinging ability as well. Despite these patterns, Kolbe et al. (2016) noted that A. cristatellus lizards in urban habitats do not conform to the habitat constraint hypothesis, frequently making use of structural habitat substrates on which sprint performance is not maximized (see also Winchell et al., 2018a; Winchell et al., 2018b). It is unclear whether urban green anoles exhibit the same phenomenon, although Gilman and Irschick (2013) found that green anoles in a park in Volusia County, Florida, preferred to jump from low compliance perches, and avoided compliant perches that are known to impede jumping ability (Gilman et al., 2012). However, despite the urban setting of this population the distribution of available habitat is more similar to that of the rural GHP population in Louisiana. Furthermore, given that the performance phenotype is a multivariate one and different performance traits are supported by different morphologies it is likely that habitat choice involves trade-offs among different aspects of performance such as sprinting, jumping, and endurance (Husak and Lailvaux, 2019; Lailvaux et al., 2019), and that these trade-offs are different in urban versus natural environments (see Winchell et al., 2018b for an example). Consequently, habitat constraint could be context-dependent.
In addition to direct effects on morphology, changes in habitat due to human activity can also have indirect effects on lizard shape and size. Battles et al. (2013) found the body condition (assessed specifically via a body-mass index measure) of female green anoles to be higher in natural plots compared to disturbed ones in Palmetto State Park, TX. Even though arthropod biomass was also higher in natural plots, these differences did not appear to be driven by prey availability but likely have complex causes including, potentially, habitat sensitivity (Battles et al., 2013). This complexity appears to extend to other Anolis species as well; for example, Hall and Warner (2017) found the opposite pattern in A. cristatellus lizards in south Florida, with A. cristatellus from an urban site exhibiting higher body condition than those from a forested site, yet Chejanovski et al. (2017) found no effect at all of urbanization on body condition in A. cristatellus within their native range in Puerto Rico, but did find higher condition in urban A. sagrei lizards compared to those from natural populations. Finally, Winchell et al. (2019) found that body condition does vary between urban and natural populations of A. cristatellus in Puerto Rico, albeit not always in a consistent direction. The mechanisms underlying these changes in condition are unclear, and it is worth noting that morphological condition indices have often been criticized, and that their functional and ecological relevance is controversial (Vervust et al., 2008; but see Husak and Lailvaux, 2019).
Escape Behavior
Although lizards will not necessarily make use of all of the habitat available to them (Johnson et al., 2006), this variation can nonetheless drive differences in behavior in specific ecological contexts. One such behavior that is affected by habitat type and availability is escape behavior. The decision as to when to flee from a potential threat weighs the various opportunity and energetic costs of escape against the probability of mortality (Ydenberg and Dill, 1986; Cooper, 2015b). These costs are themselves dependent on numerous intrinsic and extrinsic factors including habitat type and openness (Martin and López, 1995); temperature (in ectotherms) (Hertz et al., 1982); sex (Lailvaux et al., 2003); and the presence of humans (Mikula, 2014). That urban populations of birds allow closer approach of humans before initiating escape compared to rural ones has been attributed to habituation to human presence (Cooke, 1980; Blumstein, 2014).
Good Hope Field and TU lizards also show significant differences in escape behavior. A finding common to both populations is that males allow closer approach to a potential predator than females before initiating escape, although there was no effect of either sex or population on how far lizards fled; however, both male and female GHP anoles showed significantly longer approach distances than TU lizards (Irschick et al., 2005a). McMillan and Irschick (2010) used clay models to measure predation pressures at these same two populations and found no evidence of attacks by predators at the urban TU site, but a much higher frequency of bites from predators on models in the GHF swamp locality. This suggests that TU green anoles likely allow closer approach of human “predators” before initiating flight because they are habituated to the presence of humans; indeed, flight initiation distance is substantially shorter in habituated compared to unhabituated populations in eleven lizard species spanning six families (Cooper, 2015a), as well as in other anoles (Avilés-Rodríguez and Kolbe, 2019). Although the TU escape behavior results are consistent with this literature, the findings of McMillan and Irschick (2010) regarding low predation pressure in the urban TU population stands in contrast to similar studies on related lizards. For example, Tyler et al. (2016) used frequency of tail autotomy in the congener Anolis cristatellus as a proxy for predation pressure in urban and natural sites in four areas of Puerto Rico, and found that urban populations exhibited consistently higher frequencies of tail autotomy and regeneration, pointing toward clear differences in the predation ecology, be it predator density or efficiency, of the two population types (a further possibility is competition; see below). McMillan and Irschick (2010) did not consider autotomy in their study, and it could be that clay models and autotomy frequency capture different aspects of predation ecology. Interspecific variation in response to the threat of predation is yet another possibility (Blumstein et al., 2005; Vanhooydonck et al., 2007).
One feature of urban ecology that might mediate results gleaned from these different approaches to estimating predation is boldness, which describes the behavioral response to threatening situations. Boldness can be selected against by predation pressure (Lapiedra et al., 2018), but may also be a common feature of urban populations of organisms (Lowry et al., 2013); indeed, there is evidence that increased boldness and exploratory behavior is associated with populations inhabiting novel urban habitat in various animals (Evans et al., 2010; Atwell et al., 2012; but see Hurtado and Mabry, 2017; Sol et al., 2018), including several species of lizards (Damas-Moreira et al., 2019; but see Putnam et al., 2020). Kuo et al. (2015) found that bolder Anolis sagrei individuals more readily autotomize their tails, and also exhibited a higher propensity to drop their tails when available food resources are abundant. If bolder individuals are more likely to persist in urban areas with ample resources, then those individuals might exhibit higher frequencies of tail autotomy independent of predation risk. For example, Itescu et al. (2017) found that tail loss was a function of intraspecific competition rather than predation in two species of Mediterranean geckos. Habitat type has also been suggested as a potential factor (Bateman and Fleming, 2009). Of course neither clay models nor autotomy frequency are perfect indices of predation, and it may well be the case that there are key differences in the predation ecology of the urban environments in Puerto Rico versus southeastern Louisiana. Furthermore, no studies to my knowledge have considered boldness and autotomy in urban green anoles. However, given the general higher population densities of lizards in urban versus natural populations (see below) as well as the general differences in structural habitat, the notion that boldness affects tail loss of anoles in urban environments via avenues other than predation is a testable hypothesis.
Display Behavior, Population Density, and Parasitism
Natural selection favors displays, signals, and receptors that maximize signal to noise ratio in a given environment (Endler, 1992). Because the physical properties of the environment can affect signal transmission and degradation, signaling behavior can also be modulated by microhabitat choice and availability (Calsbeek and Marnocha, 2006; Barker and Mennill, 2009). Consequently, differences in structural habitat can also drive divergence in display behavior between urban and natural animal populations (Fernández-Juricic et al., 2005). Anolis lizards communicate via visual displays involving stereotyped movements of the head and the dewlap, an extensible flap of colorful skin under the throat (Fleishman, 1992). Males will display toward other males, females, and potential predators but will also perform undirected displays that appear to advertise territory ownership. Although variable both within and among species, displays in green anoles are highly conserved and feature at least three distinct combinations of head bobs and dewlap extensions, termed A, B, and C displays, respectively (Lovern et al., 1999; Lovern and Jenssen, 2003; Orrell and Jenssen, 2003). Juveniles and adult females also perform versions of these displays, despite their reduced dewlap size (Lovern and Jenssen, 2003).
Although the structure of these displays is remarkably stable, both display rate and the proportions of display types can vary among populations. Bloch and Irschick (2006) compared display behavior of green anoles at the urban TU and rural GHF sites and found that Tulane anoles not only displayed roughly twice as much as GHF anoles, they also differed significantly in their relative frequencies of display types: TU lizards exhibited higher proportions of A and B display types, whereas C type displays were observed more often in rural GHF. However, there was also evidence of a previously undescribed display type, termed the Y display, in GHF but not in the TU population (Bloch and Irschick, 2006). Although this suggests that the urban environment might constrain display behavior, this difference could also arise due to some factor that is only indirectly related or entirely unrelated to urbanization. For example, Edwards and Lailvaux (2012) studied the display behaviors of two different green anole populations within the greater New Orleans area, and found that proportions of display types were altered by both habitat type and the presence of an invasive congener (A. sagrei).
Invasive species are a perennial feature of urban environments (Blair, 2001). Just as the urban habitat is a replicated one, so urbanization also leads to biotic homogenization and a loss of diversity in species assemblages (McKinney, 2006) as urban intolerant natives are replaced with successful invaders. One mechanism for this may be alteration of habitat in urban and disturbed areas, which can exclude native species from the modified urban environment (Forman, 2014). Indeed the loss of habitat complexity alone in urban areas can contribute to a decline in density of native species; for example, Petren and Case (1998) showed that experimentally increasing structural habitat complexity reduced interspecific competition between an invasive and a non-invasive gecko species. These collective effects of biotic homogenization and decreased complexity and diversity of structural habitat could exert significant selection on the behaviors of local native species.
One common characteristic of successful invasive species is that they often exist at higher population densities in invasive areas compared to within their native range. For example, population densities of Eleutherodactylus coqui frogs are estimated to be three times denser on Hawai’i, where they are invasive, compared to native populations on Puerto Rico (Woolbright et al., 2006). Consequently, a further indirect consequence of invasive species presence which can also affect native behavior is a negative effect on population density of natives. Bloch and Irschick (2006) reported a density of 0.19 males/m2 at the urban TU population at time of their study (2004). However, Edwards and Lailvaux (2012) estimated the male density of that same population in 2010 to be 0.073 males/m2; less than half of the population density 6 years earlier. Furthermore, the total display time of males at the TU population was also roughly halved compared to that reported earlier by Bloch and Irschick (2006). The major difference between the TU population of 2004 and the same population of 2010 is the presence of A. sagrei, which was absent from TU at the time of the earlier study (Edwards and Lailvaux, 2012; SPL personal observation). Indeed, Bloch and Irschick (2006) specifically noted that the TU male density was stable at the time they undertook their initial study. Furthermore, A. sagrei is known to exist at remarkably high densities elsewhere within its range, habitat permitting, even when other anoles are present (Schoener and Schoener, 1980). Taken together, the results of these two studies suggest that the establishment of A. sagrei on TU campus affected the density of green anole males in that population, either by limiting the amount of habitat available to those males which in turn altered their habitat use (Edwards and Lailvaux, 2012) or by altering the habitat use of green anole females, which prompted males to follow suit (Edwards and Lailvaux, 2013). The replicated nature of these challenges in urban environments strongly suggests that green anoles in other areas are likely to face similar challenges when faced with the presence of invasive A. sagrei in particular.
Although the presence of A. sagrei appears to have had a negative effect on the density of A. carolinensis males at TU in 2010, there is also evidence that green anole male TU density in 2004 was already artificially high - almost three times higher compared to rural GHF (Bloch and Irschick, 2006). This increased density can have implications for intraspecific behavioral interactions. For instance, in addition to a low (or absent) predator density at TU relative to GHF, the clay model approach of McMillan and Irschick (2010) also revealed that TU lizards seemed to experienced intense male-male competition, as all of the model bites on TU campus were inflicted by green anole males. This competition was significantly higher than that at the GHP locality based on the same index, and also exhibited a clear temporal component, with competition appearing more intense at both GHF and TU during the anole breeding season (spring-fall) than in the winter (McMillan and Irschick, 2010). The “credit-card hypothesis” suggests that individuals in urban populations will on average be less competitive than rural populations even if populations are denser because resources tend to be more readily available in urban habitats, easing selective for competitiveness (e.g., Hasegawa et al., 2014); in this case, however, it appears that the patchy distribution of preferred green anole habitat in the artificially-managed, lizard-dense TU location (Bloch and Irschick, 2006) forced adult males into close proximity with each other, resulting in unusually intense male-male competition.
Despite the apparently lower predation intensity at the TU locality, expansion into urban environments (or, alternatively, encroachment of urban habitat into natural species distributions) can nonetheless bring species into contact with novel pathogens and parasites or otherwise increase the risk of infection. This appears to have also occurred in the urban TU green anole population. Irschick et al. (2006a) documented infection in this population of green anoles by Lepidodexia blakeae, a sarcophagid fly. The fly larviposits on the skin, with the larvae developing inside the lizard until emerging from a wound and pupating in sediment. The open wounds in the lateral abdominal areas caused by the larvae during and immediately following active infection are large, and lizards that survive infection bear obvious scars. Irschick et al. (2006a) surveyed both the GHF and TU populations for incidences of L. blakeae infection over a 10-month period, and found no cases of either active infection or of scars in the GHF population. Urban TU lizards, however, exhibited infection rates ranging from 6.2% in the winter, to 7.6% the subsequent fall. However, the risk of infection was not distributed evenly across age/sex classes, with adult male green anole males being between 5 and 8 times more likely to be parasitized than juveniles or adult females (Irschick et al., 2006a). Despite reports elsewhere of as many as 17 larvae infesting a single green anole (Dodge, 1955), infections in the TU lizards are not always lethal, with 9.5 and 4.3% of sampled adult males bearing parasite scars in the spring and fall, respectively, of 2004 (Irschick et al., 2006a).
Previous studies have shown that the intensity of parasitism or predation can influence sexual selection by altering the conspicuousness of visual or auditory sexual displays (Zuk and Kolluru, 1998; Godin and McDonough, 2003; Zuk et al., 2006). Although the role of behavior, and display behavior in particular, in mediating parasite infection probability in green anoles is unknown, one possibility is that the overall high display rate of males in this population, coupled with the high male density (Bloch and Irschick, 2006), decreased wariness (Irschick et al., 2005a), and openness of the patchy TU green anole habitat collectively contributed to high male conspicuousness at the TU location, and thus increased male vulnerability to parasites. Green anole males with larger dewlaps also display more frequently (Johnson et al., 2011), which likely contributes further to their conspicuousness, as might the openness of the TU habitat; for example, Stroud et al. (2019) found that A. sagrei lizards in open urban environments displayed twice as frequently as those in natural environments. In addition to behavior increasing the risk of infection or parasitism, infection status might also alter behavior. For instance, Anolis brevirostris lizards heavily parasitized by ectoparasitic mites exhibited duller dewlaps and less frequent displays than individuals with fewer parasites (Cook et al., 2013). However, arguing against the conspicuousness hypothesis is the observation that active sarcophagid infections in the TU green anole population were observed only in the winter (Irschick et al., 2006a), when males are less likely to display. Indeed, individual dewlaps in TU lizards also change size over the course of a year, shrinking during the non-breeding season (Irschick et al., 2006b) due to reduced frequency of use in the fall and winter (Lailvaux et al., 2015).
Regardless of the mechanism, the main finding that the urban green anole population suffers a higher rate of infection than natural populations is consistent with results both from other Anolis species and from other lizards. Thawley et al. (2019) found that A. sagrei lizards from urban populations experienced higher intensity of parasite infection compared to conspecifics sampled from natural habitats (although they also found no effect of urbanization on infection intensity in another species, A. cristatellus). Furthermore, Lazíc et al. (2017) reported more variable but on average significantly higher blood parasite loads in urban versus rural populations of Podarcis muralis lizards. Higher rates of parasitism in urban populations might be driven by several factors, ranging from increased infection transmission at higher population densities (Cressler et al., 2016) to increased immune costs suffered by urban populations compared to natural populations. Artificial or dim light at night, for instance, can disrupt circadian rhythms, increasing susceptibility to infections and altering disease transmission dynamics (Kernbach et al., 2018). Thus far there have been no studies comparing the immune capacity of urban and rural populations of green anoles, although Husak and Lailvaux (2019) found that immunocompetence as assessed by phytohemagglutinin (PHA) challenge was not a significant predictor of mortality in an introduced and manipulated urban A. carolinensis population elsewhere in New Orleans. The seasonality of immune defenses in green anoles is also understudied (Tylan and Langkilde, 2017), and it may be that investment in immune defenses has a seasonal component as in other lizard species (e.g., Huyghe et al., 2010; see also Reedy et al., 2015).
Finally, It is also of note that the immune defenses of successful invaders tend to render them less susceptible to pathogens than natives (Lee et al., 2005); for example, invasive anoles were found to exhibit lower frequencies of malarial infection than native species in central Florida (Doan et al., 2019). Consequently, asymmetric immune strategies between green anoles and potential competitors, such as A. sagrei, could also affect green anole behavior, if only indirectly.
Population Structure and Demography
Population biology and demography are fundamental to evolutionary ecology. The number, age, and sex of animals in a given population can have consequences for behavior, life-history, and reproductive strategies. For example, operational sex ratio significantly influences the strength of sexual selection in animals, either through changes in mate choice or by affecting the strength of intrasexual competition (Janicke and Morrow, 2018). Male-biased sex ratios and resulting increased sexual competition can also lead to increased investment in body mass (Jarman, 1983), which can be achieved through adjustments in other key life history traits. Indeed, juvenile crickets that are exposed to increased numbers of adult male calls may adjust their development time such that they mature later than would otherwise be the case but at a larger body size (Kasumovic, 2013). Adult density effects on juveniles also exist in anoles; for example, A. sagrei juveniles alter their structural habitat use when adult male density is high (Delaney and Warner, 2017), which could have implications for development trajectories given the documented morphological plasticity in this species (Losos et al., 2000; Bonneaud et al., 2016). It is currently unclear how the documented higher densities of organisms in urban environments affect either their population structures and sex ratios or, in turn, other aspects of their life-history, particularly for small, cryptic species such as green anoles.
Green anoles are considered model organisms for ecology and evolution (Lovern et al., 2004), and consequently several studies have considered the population ecology of free-ranging anoles in nature. However, the structure of A. carolinensis populations in urban areas have received relatively little attention. Work on Caribbean anoles in particular offers several testable predictions regarding how urban green anole populations might be affected by population size and density in particular. For example, Schoener and Schoener (1980) found that sex ratios and population densities differed among populations with different habitat types in four species of Bahamian anoles. Muralidhar and Johnson (2017) reported similar intraspecific variation in sex ratio in some species of Caribbean anoles. Furthermore, Schoener and Schoener (1980) also present a model predicting that numbers of females should vary more than numbers of males within populations over time, such that denser populations should have greater numbers of females.
Few studies have attempted to test these predictions among populations of green anoles, and the long-term demographic data required to do so are seldom collected. Michael (1972) used mark-recapture methods to study an urban population of 181 green anoles in eastern Texas from October 1966 through to May of 1970 but focused primarily on measuring growth rates. A similar multi-year study was conducted from February 1979 to July 1980 by Ruby (1984) in Metairie, an urban residential area outside of New Orleans. This study neither estimated population density nor broke down the age/sex structure by year, but it did specifically note a stable 1:1 sex ratio over the study period (Ruby, 1984). However, given that sex ratios may also cycle over time (Uller et al., 2007), the possibility exists that a longer study period could show dynamic sex ratios.
Temporal Patterns of Sex Ratio and Density in Washington Square Park Green Anoles
As an additional test of the above intra-population predictions, and to provide data as a reference for future studies of urban green anole populations, I analyze and present here the results of a 5-year mark-recapture study of an urban A. carolinensis population located in Washington Square Park (hereafter WSP) in the Faubourg Marigny neighborhood of downtown New Orleans, LA (N29.965005°, W90.057302°). This park, one hectare in area, is bordered by an iron fence and fringed with A. elatior palmetto plants which serve as the primary green anole habitat (as in the TU population; Irschick et al., 2005a). Other potential habitat includes man-made structures such as benches, trash cans, and playground equipment, as well as oak trees (Quercus virginiana) and small bushes of various species. Although lizards have at times been seen to use all of this habitat, the vast majority of anoles are observed on the palmetto plants and the fence. This park was chosen because it is entirely surrounded by roadway on all sides, and thus comprises a discrete population with likely minimal immigration and emigration. However, the areas immediately surrounding the park were also searched during each sampling period.
Methods
The WSP population was exhaustively censused twice per year over a 5-year period from 2010 to 2014 using methods consistent with Irschick et al. (2005a, b). Briefly, lizards were captured by hand or by noose and marked permanently and uniquely with visual implant elastomer (VIE) tags (Northwest Marine Technology, Inc., Shaw Island, WA, United States) on the ventral side of the limb elements. Lizards were sexed, weighed, and measured to the nearest 0.01 mm with Rok digital calipers (Rok International Industry Co., Limited, Shenzhen, China). Following Irschick et al. (2005b) I considered adult males to be greater than 45 mm snout-vent length (SVL), and adult females to be greater than 40 mm SVL. Upon capture the GPS coordinates were recorded and point of capture marked with colored tape. The next morning the lizards were released at the exact location from where they were collected. Prior to release each lizard was marked with a permanent marker just above the dorsal tail base to prevent recapture within the same sampling period; this marking is eliminated when the lizard next molts. Sampling occurred in the spring (April–May) and fall (September–October) of each year, at approximately the beginning and the end of the green anole breeding season, respectively.
Analyses
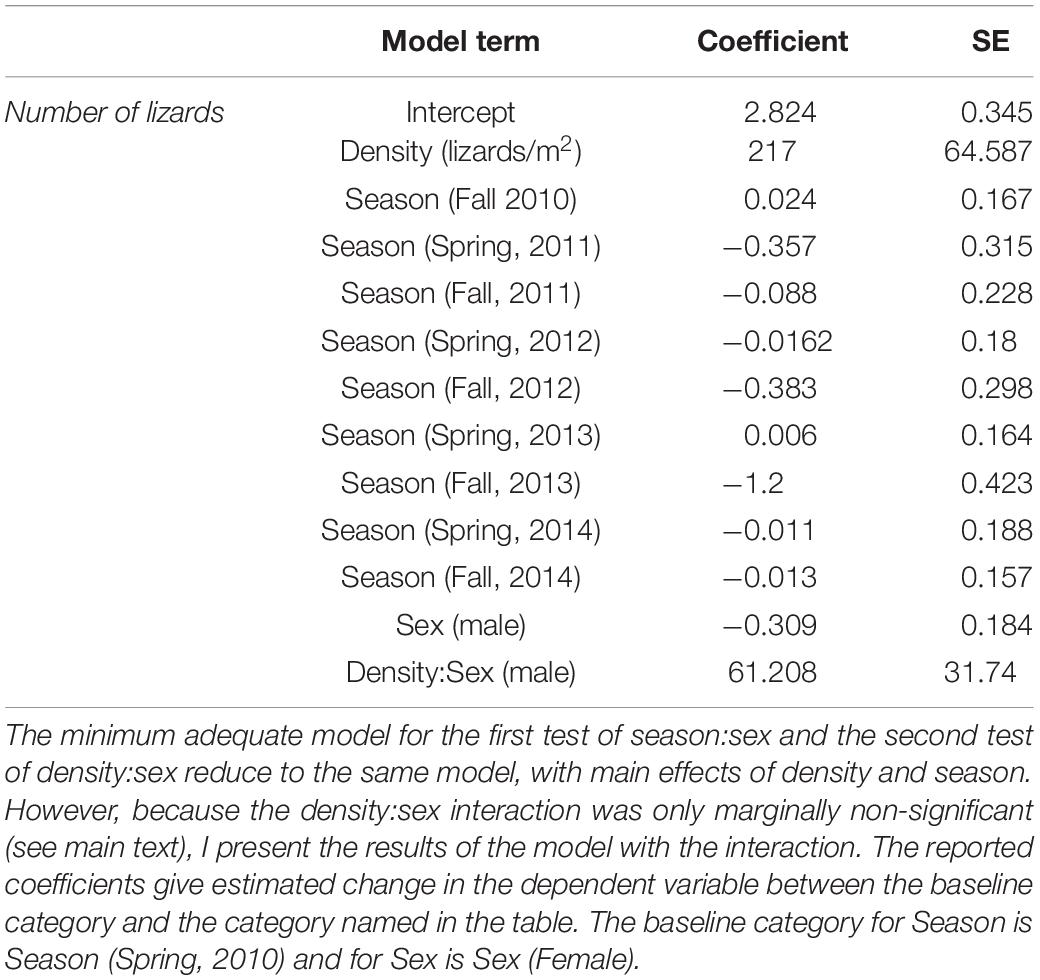
Following a recent study of sex ratios in anoles by Muralidhar and Johnson (2017) I calculated sex ratio as the proportion of males among all adults in the population. I calculated population density as density of adult lizards (lizards/m2), and as densities of each sex/age class. I used a time-series analysis to test for autocorrelation in sex ratios and densities over the 5-year period. Densities varied markedly across seasons and years, which could also affect sex ratios. To test whether numbers of males and females varied differently over each season while controlling for effects of population density, I used a generalized linear model with Poisson errors with season, sex, and an interaction between season and sex as factors and population density as a covariate. To test whether numbers of males and females changed across seasons along with density, I fit a second such model with season, sex, density, and an interaction between sex and density as factors. I did this both to avoid uninterpretable three-way interactions, and to prevent overfitting any one model. I identified the minimum adequate models for each using log-likelihood ratio tests. I do not conduct any across-population analyses on density or sex ratio data because the GHF and TU data represent snapshots of single seasons whereas the WSP data comprises multiple seasons over 5 years, and choosing any specific season for comparison would be subjective. I used R version 3.6.0 (R Core Team, 2019) for all analyses.
Results and Discussion
Sex ratios in WSP were close to 1:1 over the 5-year period, but where biased were almost always female-biased. Sex ratios showed no evidence of cycling over the 5-year period (Ljung-Box χ2 = 0.456, d.f. = 1, P < 0.5). Similarly, overall population density (χ2 = 1, d.f. = 1, P < 0.312) and densities of males (χ2 = 0.132, d.f. = 1, P < 0.716) and females (χ2 = 2.65, d.f. = 1, P < 0.104) were not autocorrelated among seasons.
The model for number of lizards retained season and density as factors, but sex and the interaction between sex and season were not significant. Thus, numbers of males and females do not change relative to each other once population density is accounted for. In the second model, the interaction between density and sex for number of animals was insignificant, but only marginally so (model with interaction compared to model without interaction: d.f. = 8, deviance = −3.723, P < 0.054). This interaction indicates larger numbers of males at higher population densities. Because these two models end up being identical with the exception of the marginal density:sex interaction, I present only the results of the second model with that interaction in Table 1.
Both Figure 1 and the coefficients in Table 1 indicate a clear reduction in population size in Fall, 2013. The reasons for this bottleneck are not immediately apparent; there were no changes in habitat nor any extreme weather events in 2013 that might account for any increased mortality. Nonetheless, the population recovered in the following sampling period (Spring, 2014) which is suggestive of some immigration, although hatchlings often appear late in the year as well.
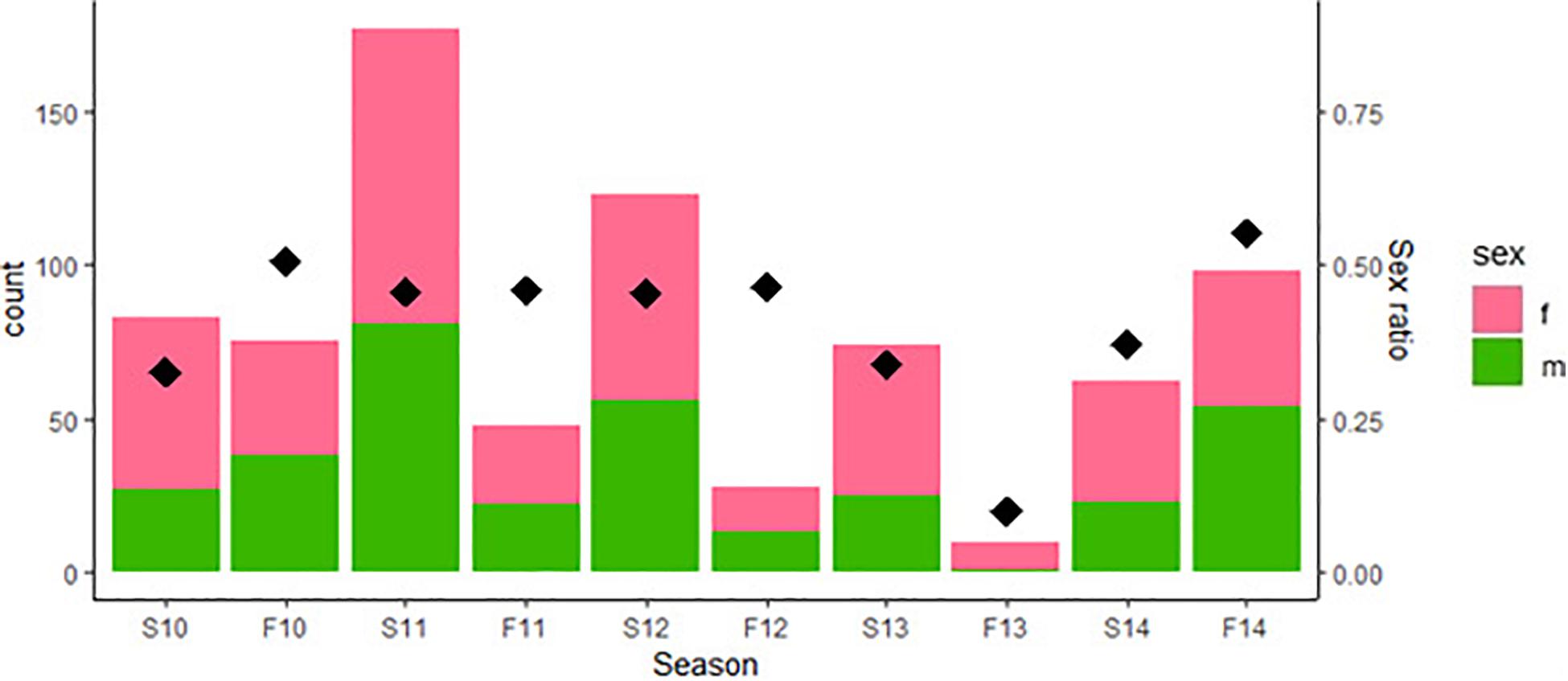
Figure 1. Counts of adult male and adult female green anole lizards in the Washington Square Park population from 2010 to 2014 broken down by census period [i.e., by spring (S) and Fall (F) of each year]. Thus “S10” denotes the spring of 2010, “F10” the fall of 2010, and so on. Diamonds represent the sex ratio, calculated as proportion of males among all adults in the population as indicated on the right axis, for each census period.
The prediction that numbers of females should be more variable than that of males as population density changes was not supported in this case, as changes in density appear to be related to number of males in the population instead (Table 1). Schoener and Schoener (1980) specifically note that the phenomenon of female variability relating to density should be apparent in good habitats. Given that urban habitats differ in so many ways from those of natural populations, one possible explanation for this discrepancy is that urban habitats are inferior to those inhabited by natural populations for this species.
Conclusion and Future Directions
Urban populations show distinct differences in morphology, performance, and behavior in a number of animal taxa. This brief review demonstrates that A. carolinensis is among those species whose behavior and ecology are altered by urban environments. Drawing inferences from population comparisons is not without potential pitfalls, and indeed, Mayr (1963) claimed that “every population of a species differs from all others.” Consequently, several of the differences noted here between urban and rural populations could be due not to effects of urbanization itself, but to any of the other myriad factors contributing to intraspecific and interpopulational variation. For example, a rigorous population comparison of green anole infestation by L. blakea should take into account the population ecology of the fly as well, and it may be that the higher infection rates in the TU population compared to the GHP population are more a function of local density effects on L. blakea as opposed to an urbanization effect on A. carolinensis. Indeed, the WSP population study I described above found only one active L. blakea infection and four individuals with parasite scars over a 5-year period despite WSP also being an urban population, indicating variation among urban populations in infection rate, mortality rate, or both. Previous studies have also indicated variation among green anole populations in such key traits as display type structure (Lovern et al., 1999) and dewlap color (Michaud and Echternacht, 1995).
Such caveats notwithstanding, these comparisons recapitulate several findings that characterize general differences between urban and rural populations in other animal species, and are thus likely to constitute real consequences of the urban habitat. Green anoles inhabiting urban environments are shaped differently; live at higher densities; are less wary; display more frequently; are more likely to suffer interference competition from invasive species; and appear to experience lower predation and higher intrasexual competition than conspecifics in natural populations. Other differences, such as disparate immune strategies or propensity for risk taking, are less supportable and suffer mainly from a lack of relevant data. In fact, a major message of this review is not how much is known about urban populations of green anoles, but rather how little is known regarding how green anoles have adapted to the urban environments which they appear to have inhabited for some time. This is particularly true for some aspects of population ecology, which have received less attention in urban populations of green anoles as compared to many Caribbean congenerics. The data presented here suggest tentatively that the temporal dynamics of populations in an urban environment could differ from those in natural habitats, although further tests in replicate populations are required before this conclusion can be reached.
Although this lack of data represents a challenge for the current paper, for the field of urban ecology it is instead an opportunity. Green anoles not only exhibit a large and environmentally heterogeneous distribution, they are also highly variable in morphology (Jaffe et al., 2016) and show substantial life-history variation across their North American range (Michaud and Echternacht, 1995). Indeed, the effects of urbanization on life-history have arguably not received the attention that they deserve, even though urban environments certainly hold the potential to affect animal life-histories (Ditchkoff et al., 2006). For example, birds appear to trade-off reproduction against survival in urban populations, exhibiting longer life spans but lower clutch sizes in urban environments and thereby adopting a slower life-history strategy compared to natural populations (Sepp et al., 2018), and underground populations of Culex pipiens mosquitoes in urban areas exhibit divergent life-cycles to aboveground populations (Byrne and Nichols, 1999; Asgharian et al., 2015). In this respect, green anoles represent an untapped resource for understanding how urbanization affects life-histories in small vertebrates given both their presence across the United States in urban environments and the variation in green anole life-history strategies across that same range. Yet another aspect of green anole urban ecology that has received less attention than it might, especially with regard to behavior, is thermal ecology. Temperature has been shown to impose selection on the green anole phenotype in the southern part of their range (Campbell-Staton et al., 2017), and there is evidence of selection on genes related to metabolism and behavior in the northern part of their range as well (Bourgeois and Boissinot, 2019). The combination of urban heat island effects (Campbell-Staton et al., 2020) with potential shifts in behavioral strategies at higher latitudes could facilitate the expansion of green anoles into urban areas in the northern extreme of the green anole range as the climate continues to warm. However, few data are currently available on the thermal preferences, tolerances, and performance curves of urban green anoles (but see Lailvaux and Irschick, 2007 for an example, again from an urban population in New Orleans), and there are potential costs to urban heat island effects that might mitigate any such benefits in reptiles (e.g., Hall and Warner, 2018).
Despite these opportunities, studies of urban populations also have shortcomings. Because patches of habitat that might harbor animal species in urban areas are often carefully managed, changes in management practice can also alter habitat structure or resource availability in such a way that overall habitat quality can be reduced. It is worth noting that the TU green anole population to which I have referred time and time again in this paper for all intents and purposes no longer exists; much of the palmetto habitat in the transect area was removed several years ago by the Tulane University groundskeepers, and that change in the physical environment, coupled with the growing numbers of A. sagrei for whom the new physical landscape is less of a deterrent, has made TU green anoles scarcer and more difficult to locate ever since. Thus, maintaining long-term study sites in urban areas can be problematic if the priorities of stakeholders - who may have less of an interest in biology than do urban ecologists - change. It is perhaps ironic that the same human activity that drives rampant urbanization can also render urban populations of certain animal species ephemeral.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the author, without undue reservation.
Ethics Statement
This animal study was reviewed and approved by the University of New Orleans Institutional Animal Care and Use Committee [IACUC protocol (UNO-11-004)].
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
I do not currently have external funding, so any funds which are due will be paid by my institution.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I thank J. Edwards, S. Gipson, W. del Corral, C. Steele, A. Cespedes, W. Weber, C. Policastro, and the many undergraduates who assisted with the WSP surveys over the years. All WSP research was approved under IACUC protocol (UNO-11-004).
References
Ackley, J. W., Angilletta, M. J., DeNardo, D., Wu, B. S. J., and Wu, J. G. (2015). Urban heat island mitigation strategies and lizard thermal ecology: landscaping can quadruple potential activity time in an arid city. Urban Ecosyst. 18, 1447–1459. doi: 10.1007/s11252-015-0460-x
Arnold, S. J. (1983). Morphology, performance, and fitness. Am. Zool. 23, 347–361. doi: 10.1093/icb/23.2.347
Asgharian, H., Chang, P. L., Lysenkov, S., Scobeyeva, V. A., Reisen, W. K., and Nuzhdin, S. V. (2015). Evolutionary genomics of Culex pipiens: global and lcoal adaptations associated with climate, life-history traits and anthropogenic factors. Proc. R. Soc. B Biol. Sci. 282:20150728. doi: 10.1098/rspb.2015.0728
Atwell, J. W., Cardoso, G. C., Whittaker, D. J., Campbell-Nelson, S., Robertson, K. W., and Ketterson, E. D. (2012). Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav. Ecol. 23, 960–969. doi: 10.1093/beheco/ars059
Auer, S. K., Dick, C. A., Metcalfe, N. B., and Reznick, D. N. (2018). Metabolic rate evolves rapidly and in parallel with the pace of life history. Nat. Commun. 9:6.
Avilés-Rodríguez, K. J., and Kolbe, J. J. (2019). Escape in the city: urbanization alters the escape behavior of Anolis lizards. Urban Ecosyst. 22, 733–742. doi: 10.1007/s11252-019-00845-x
Barker, N. K. S., and Mennill, D. J. (2009). Song perch height in Rufous-and-White Wrens: does behaviour enhance efective communication in a tropical forest? Ethology 115, 897–904. doi: 10.1111/j.1439-0310.2009.01674.x
Bateman, P. W., and Fleming, P. A. (2009). To cut a long tail short: a review of lizard caudal autotomy studies carried out over the last 20 years. J. Zool. 277, 1–14. doi: 10.1111/j.1469-7998.2008.00484.x
Battles, A. C., Moniz, M., and Kolbe, J. J. (2018). Living in the big city: preference for broad substrates results in niche expansion for urban Anolis lizards. Urban Ecosyst. 21, 1087–1095. doi: 10.1007/s11252-018-0787-1
Battles, A. C., Whittle, T. K., Stehle, C. M., and Johnson, M. A. (2013). Effects of human land use on prey availability and body condition in the green anole lizard, Anolis carolinensis. Herpetol. Conserv. Biol. 8, 16–26.
Bennett, A. F., and Huey, R. B. (1990). “Studying the evolution of physiological performance,” Oxford Surveys in Evolutionary Biology, eds D. J. Futuyma and J. Antonovics (Oxford: Oxford University Press), 251–284.
Blair, R. B. (2001). “Birds and butterflies along urban gradients in two ecoregions of the United States: is urbanization creating a homogeneous fauna?,” in Bioltic Homogenization, eds J. L. Lockwood and M. L. McKinney (Boston, MA: Springer).
Bloch, N., and Irschick, D. J. (2006). An analysis of inter-population divergence in visual display behavior of the green anole lizard (Anolis carolinensis). Ethology 112, 370–378. doi: 10.1111/j.1439-0310.2006.01162.x
Blumstein, D. T. (2014). “Attention, habituation, and anti-predator behaviour: implications for urban birds,” in Avian Urban Ecology eds D. Gil and H. Brumm (Oxford: Oxford University Press), 41–53.
Blumstein, D. T., Fernández-Juricic, E., Zollner, P. A., and Garity, S. C. (2005). Inter-specific variation in avian responses to human disturbance. J. Appl. Ecol. 42, 943–953. doi: 10.1111/j.1365-2664.2005.01071.x
Bonneaud, C., Marnocha, E., Herrel, A., Vanhooydonck, B., Irschick, D. J., and Smith, T. B. (2016). Developmental plasticity affects sexual size dimorphism in an anole lizard. Funct. Ecol. 30, 235–243. doi: 10.1111/1365-2435.12468
Bourgeois, Y., and Boissinot, S. (2019). Selection at behavioural, developmental and metabolic genes is associated with the northward expansion of a successful tropical colonizer. Mol. Ecol. 28, 3523–3543. doi: 10.1111/mec.15162
Byrne, K., and Nichols, R. A. (1999). Culex pipiens populations in London Underground tunnels: differentiation between surface and subterranean populations. Heredity 82, 7–15. doi: 10.1038/sj.hdy.6884120
Calsbeek, R., and Marnocha, E. (2006). Context dependent territory defense: the importance of habitat structure in Anolis sagrei. Ethology 112, 537–543. doi: 10.1111/j.1439-0310.2006.01194.x
Campbell-Staton, S. C., Cheviron, Z. A., Rochette, N. C., Catchen, J., Losos, J. B., and Edwards, S. V. (2017). Winter storms drive rapid phenotypic, regulatory, and genomic shifts in the green anole lizard. Science 357, 495–498. doi: 10.1126/science.aam5512
Campbell-Staton, S. C., Winchell, K. M., Rochette, N. C., Fredette, J., Maayan, I., Schweizer, R. M., et al. (2020). Parallel selection on thermal physiology facilitates repeated adaptation of city lizards to urban heat islands. Nat. Ecol. Evol. 4, 652–658.
Chejanovski, Z. A., Aviles-Rodriguez, K. J., Lapiedra, O., Preisser, E. L., and Kolbe, J. J. (2017). An experimental evaluation of foraging decisions in urban and natural forest populations of Anolis lizards. Urban Ecosyst. 20, 1011–1018. doi: 10.1007/s11252-017-0654-5
Cook, E. G., Murphy, T. G., and Johnson, M. A. (2013). Colorful displays signal male quality in a tropical anole lizard. Naturwissenschaften 100, 993–996. doi: 10.1007/s00114-013-1095-5
Cooke, A. S. (1980). Observations on how close certain passerine species will tolerate an approaching human in rural and suburban areas. Biol. Conserv. 18, 85–88. doi: 10.1016/0006-3207(80)90072-5
Cooper, W. E. (2015a). “Reptiles. Escaping From Predators,” in An Integrative View of Escape Decisions eds W. E. Cooper and D. T. Blumstein (Cambridge: Cambridge University Press).
Cooper, W. E. (2015b). “Theory: models of escape behavior and resource use,” in Escaping From Predators: An Integrative View of Escape Decisions eds W. E. Cooper and D.T. Blumstein (Cambridge: Cambridge University Press).
Cressler, C. E., Mcleod, D. V., Rozins, C., Van Den Hoogen, J., and Day, T. (2016). The adaptive evolution of virulence: a review of theoretical predictions and empirical tests. Parasitology 143, 915–930. doi: 10.1017/s003118201500092x
Damas-Moreira, I., Riley, J. L., Harrias, D. J., and Whiting, M. J. (2019). Can behaviour explain invasion success? A comparison between sympatric invasive and native lizards. Anim. Behav. 151, 195–202. doi: 10.1016/j.anbehav.2019.03.008
Delaney, D. M., and Warner, D. A. (2017). Adult male density influences juvenile microhabitat use in a territorial lizard. Ethology 123, 157–167. doi: 10.1111/eth.12586
Delaney, K. S., Riley, S. P. D., and Fisher, R. N. (2010). A rapid, strong, and convergent genetic response to urban habitat fragmentation in four divergent and widespread vertebrates. PLoS One 5:e12767. doi: 10.1371/journal.pone.0012767.t001
Dill, A. K., Sanger, T. J., Battles, A. C., and Johnson, M. A. (2013). Sexual dimorphisms in habitat-specific morphology and behavior in the green anole lizard. J. Zool. 290, 135–142. doi: 10.1111/jzo.12020
Ditchkoff, S. S., Saalfield, S. T., and Gibson, C. J. (2006). Animal behavior in urban ecosystems: modifications due to human-induced stress. Urban Ecosyst. 9, 5–12. doi: 10.1007/s11252-006-3262-3
Doan, T. M., Devlin, B. G., and Greene, K. C. (2019). Malaria infection is lower in invasive anoles than aative anoles in Central Florida, USA. J. Herpetol. 53, 22–26. doi: 10.1670/18-056
Dodge, H. R. (1955). Sarcophagid flies parasitic on reptiles. Proc. Entomol. Soc. Washington 57, 183–187.
Donihue, C. M., Herrel, A., Fabre, A. C., Kamath, A., Geneva, A. J., Schoener, T. W., et al. (2018). Hurricane-induced selection on the morphology of an island lizard. Nature 560:88. doi: 10.1038/s41586-018-0352-3
Edwards, J. R., and Lailvaux, S. P. (2012). Display behavior and habitat use in single and mixed populations of Anolis carolinensis and Anolis sagrei lizards. Ethology 118, 494–502. doi: 10.1111/j.1439-0310.2012.02037.x
Edwards, J. R., and Lailvaux, S. P. (2013). Do interspecific interactions between females drive shifts in habitat use? A test using the lizards Anolis carolinensis and A. sagrei. Biol. J. Linn. Soc. 110, 843–851. doi: 10.1111/bij.12180
Endler, J. A. (1992). Signals, signal conditions, and the direction of evolution. Am. Nat. 139, S125-S153.
Evans, J., Boudreau, K., and Hyman, J. (2010). Behavioural syndromes in urban and rural populations of song sparrows. Ethology 116, 588–595.
Fernández-Juricic, E., Poston, R., De Collibus, K., Morgan, T., Bastain, B., Martin, C., et al. (2005). Microhabitat selection and singing behavior patterns of male house finches (Carpodacus mexicanus) in urban parks in a heavily urbanized landscape in the western U.S. Urban Habit. 3, 49–69.
Fleishman, L. J. (1992). The influence of the sensory system and the environment on motion patterns in the visual displays of anoline lizards and other vertebrates. Am. Nat. 139, S36-S61.
Fletcher, D. E., Hopkins, W. A., Standora, M. M., Arribas, C., Baionna-Parikh, J. A., Saldaña, T., et al. (2008). “Geckos as indicators of urban pollution,” in Urban Herpetology eds J. C. Mitchell, R. E. J. Brown, and B. Bartholomew (Humlebæk: Socoety for the Study of Amphibians and Reptiles).
Garland, T., and Losos, J. B. (1994). “Ecological morphology of locomotor performance in squamate reptiles,” in Ecological Morphology: Integrative Organismal Biology, eds P.C. Wainwright and S. Reilly (Chicago, CL: University of Chicago Press), 240–302.
Gilman, C. A., Bartlett, M. D., Gillis, G. B., and Irschick, D. J. (2012). Total recoil: perch compliance alters jumping performance and kinematics in green anole lizards (Anolis carolinensis). J. Exp. Biol. 215, 220–226. doi: 10.1242/jeb.061838
Gilman, C. A., and Irschick, D. J. (2013). Foils of flexion: the effects of perch compliance on lizard locomotion and perch choice in the wild. Funct. Ecol. 27, 374–381. doi: 10.1111/1365-2435.12063
Glor, R. E., Losos, J. B., and Larson, A. (2005). Out of Cuba: overwater dispersal and speciation among lizards in the Anolis carolinensis subgroup. Mol. Ecol. 14, 2419–2432. doi: 10.1111/j.1365-294x.2005.02550.x
Godin, J. G. J., and McDonough, H. E. (2003). Predator preference for brightly colored males in the guppy: a viability cost for a sexually selected trait. Behav. Ecol. 14, 194–200. doi: 10.1093/beheco/14.2.194
Hall, J. M., and Warner, D. A. (2018). Thermal spikes from the urban heat island increase mortality and alter physiology of lizard embryos. J. Exp. Biol. 221:jeb181552. doi: 10.1242/jeb.181552
Hall, J. W., and Warner, D. A. (2017). Body size and reproduction of a non-native lizard are enhanced in an urban environment. Biol. J. Linn. Soc. 122, 860–871. doi: 10.1093/biolinnean/blx109
Hasegawa, M., Ligon, R. A., Giraudeau, M., Watanabe, M., and McGraw, K. J. (2014). Urban and colorful male house finches are less aggressive. Behav. Ecol. 25, 641–649. doi: 10.1093/beheco/aru034
Hertz, P. E., Huey, R. B., and Nevo, E. (1982). Fight versus flight - body temperature influences defensive responses of lizards. Anim. Behav. 30, 676–679. doi: 10.1016/s0003-3472(82)80137-1
Huey, R. B., Hertz, P. E., and Sinervo, B. (2003). Behavioral drive versus behavioral inertia in evolution: a null model approach. Am. Nat. 161, 357–366. doi: 10.1086/346135
Hurtado, G., and Mabry, K. E. (2017). Aggression and boldness in Merriam’s kangaroo rat: an urban-tolerant species? J. Mammol. 98, 410–418. doi: 10.1093/jmammal/gyw199
Husak, J. F., and Lailvaux, S. P. (2019). Experimentally enhanced performance decreases survival in nature. Biol. Lett. 15:20190160. doi: 10.1098/rsbl.2019.0160
Huyghe, K., Van Oystaeyen, A., Pasmans, F., Tadiæ, Z., Vanhooydonck, B., and Van Damme, R. (2010). Seasonal changes in parasite load and a cellular immune response in a colour polymorphic lizard. Oecologia 163, 867–874. doi: 10.1007/s00442-010-1646-9
Irschick, D. J., Carlisle, E., Elstrott, J., Ramos, M., Buckley, C. R., Vanhooydonck, B., et al. (2005a). A comparison of habitat use, morphology, clinging performance and escape behaviour among two divergent green anole lizard (Anolis carolinensis) populations. Biol. J. Linn. Soc. 85, 223–234. doi: 10.1111/j.1095-8312.2005.00487.x
Irschick, D. J., Gentry, G., Herrel, A., and Vanhooydonck, B. (2006a). Effects of sarcophagid fly infestations on green anole lizards (Anolis carolinensis): an analysis across seasons and age/sex classes. J. Herpetol. 40, 107–112. doi: 10.1670/132-05a.1
Irschick, D. J., and Losos, J. B. (1999). Do lizards avoid habitats in which performance is submaximal? The relationship between sprinting capabilities and structural habitat use in Caribbean anoles. Am. Nat. 154, 298–305.
Irschick, D. J., Ramos, M., Buckley, C., Elstrott, J., Carlisle, E., Lailvaux, S. P., et al. (2006b). Are morphology-performance relationships invariant across different seasons? A test with the green anole lizard (Anolis carolinensis). Oikos 114, 49–59. doi: 10.1111/j.2006.0030-1299.14698.x
Irschick, D. J., Vanhooydonck, B., Herrel, A., and Meyers, J. J. (2005b). Intraspecific correlations among morphology, performance and habitat use within a green anole lizard (Anolis carolinensis) population. Biol. J. Linn. Soc. 85, 211–221. doi: 10.1111/j.1095-8312.2005.00486.x
Itescu, Y., Schwartz, R., Meiri, S., and Pafillis, P. (2017). Intraspecific competition, not predation, drives lizard tail loss on islands. J. Anim. Ecol. 86, 66–74. doi: 10.1111/1365-2656.12591
Jaffe, A. L., Campbell-Staton, S. C., and Losos, J. B. (2016). Geographical variation in morphology and its environmental correlates in a widespread North American lizard, Anolis carolinensis (Squamata: Dactyloidae). Biol. J. Linn. Soc. 117, 760–774. doi: 10.1111/bij.12711
Janicke, T., and Morrow, E. H. (2018). Operational sex ratio predicts the opportunity and direction of sexual selection across animals. Ecol. Lett. 21, 384–391. doi: 10.1111/ele.12907
Jarman, P. (1983). Mating system and sexual dimorphism in large, terrestrial, mammalian herbivores. Biol. Rev. 58, 485–520. doi: 10.1111/j.1469-185X.1983.tb00398.x
Johnson, M. A., Cohen, R. E., Vandecar, J. R., and Wade, J. (2011). Relationships among reproductive morphology, behavior, and testosterone in a natural population of green anole lizards. Physiol. Behav. 104, 437–445. doi: 10.1016/j.physbeh.2011.05.004
Johnson, M. A., Kirby, R., Wang, S., and Losos, J. B. (2006). What drives variation in habitat use by Anolis lizards: habitat availability or selectivity? Can. J. Zool. 84, 877–886. doi: 10.1139/z06-068
Johnson, M. T. J., and Munshi-South, J. (2017). Evolution of life in urban environments. Science 358:eaam8327. doi: 10.1126/science.aam8327
Kasumovic, M. M. (2013). The multidimensional consequences of the juvenile environment: towards an integrative view of the adult phenotype. Anim. Behav. 85, 1049–1059. doi: 10.1016/j.anbehav.2013.02.009
Kern, E. M. A., and Langerhans, R. B. (2019). Urbanization alters swimming performance of a stream fish. Front. Ecol. Evol. 6:12. doi: 10.3389/fevo.2018.00229
Kernbach, M. E., Hall, R. J., Burkett-Cadena, N. D., Unnasch, T. R., and MArtinf, L. B. (2018). Dim light at night: physiological effects and ecological consequences for infectious disease. Integr. Comp. Biol. 58, 995–1007.
Kolbe, J. J., Battles, A. C., and Aviles-Rodriguez, K. J. (2016). City slickers: poor performance does not deter Anolis lizards from using artificial substrates in human-modified habitats. Funct. Ecol. 30, 1418–1429.
Kolbe, J. J., and Losos, J. B. (2005). Hind-limb length plasticity in Anolis carolinensis. J. Herpetol. 39, 674–678. doi: 10.1670/87-05n.1
Kuo, C. Y., Irschick, D. J., and Lailvaux, S. P. (2015). Trait compensation between boldness and the propensity for tail autotomy under different food availabilities in similarly aged brown anole lizards. Funct. Ecol. 29, 385–392. doi: 10.1111/1365-2435.12324
Lailvaux, S. P., Alexander, G. J., and Whiting, M. J. (2003). Sex-based differences and similarities in locomotor performance, thermal preferences, and escape behaviour in the lizard Platysaurus intermedius wilhelmi. Physiol. Biochem. Zool. 76, 511–521. doi: 10.1086/376423
Lailvaux, S. P., Cespedes, A. M., and Houslay, T. M. (2019). Conflict, compensation, and plasticity: sex-specific, individual-level trade-offs in green anole (Anolis carolinenis) performance. J. Exp. Zool. A 331, 280–289.
Lailvaux, S. P., and Irschick, D. J. (2006). A functional perspective on sexual selection: insights and future prospects. Anim. Behav. 72, 263–273. doi: 10.1016/j.anbehav.2006.02.003
Lailvaux, S. P., and Irschick, D. J. (2007). Effects of temperature and sex on jump performance and biomechanics in the lizard Anolis carolinensis. Funct. Ecol. 21, 534–543. doi: 10.1111/j.1365-2435.2007.01263.x
Lailvaux, S. P., Leifer, J., Kircher, B. K., and Johnson, M. A. (2015). The incredible shrinking dewlap: signal size, skin elasticity, and mechanical design in the green anole lizard (Anolis carolinensis). Ecol. Evol. 5, 4400–4409. doi: 10.1002/ece3.1690
Langerhans, R. B., and DeWitt, T. J. (2004). Shared and unique features of evolutionary diversification. Am. Nat. 164, 335–349. doi: 10.1086/422857
Lapiedra, O. (2018). Urban behavioral ecology: lessons from Anolis lizards. Integr. Comp. Biol. 58, 939–947.
Lapiedra, O., Schoener, T. W., Leal, M., Losos, J. B., and Kolbe, J. J. (2018). Predator-driven natural selection on risk-taking behavior in anole lizards. Science, 360:1017. doi: 10.1126/science.aap9289
Lazíc, M. M., Carretero, M. A., Živkovíc, U., and Crnobrnja-Isailovíc, J. (2017). City life has fitness costs: reduced body condition and increased parasite load in urban common wall lizards, Podarcis muralis. Salamandra 53, 10–17.
Lazic, M. M., Kaliontzopoulou, A., Carretero, M. A., and Crnobrnja-Isailovic, J. (2013). Lizards from urban areas are more asymmetric: using fluctuating asymmetry to evaluate environmental disturbance. PLos One 8:e84190. doi: 10.1371/journal.pone.0084190
Lee, K. A., Martin, L. B., and Wikelski, M. C. (2005). Responding to inflammatory challenges is less costly for a successful avian invader, the house sparrow (Passer domesticus), than its less-invasive congener. Oecologia. 143, 243–250.
Liu, Z., He, C., and Wu, J. (2016). The relationship between habitat loss and fragmentation during urbanization: an empirical evaluation from 16 world cities. PLoS One. 11:e0154613. doi: 10.1371/journal.pone.0154613
Losos, J. B. (2011). Convergence, adaptation, and constraint. Evolution 65, 1827–1840. doi: 10.1111/j.1558-5646.2011.01289.x
Losos, J. B., Creer, D. A., Glossip, D., Goellner, R., Hampton, A., Roberts, G., et al. (2000). Evolutionary implications of phenotypic plasticity in the hindlimb of the lizard Anolis sagrei. Evolution 54, 301–305. doi: 10.1554/0014-3820(2000)054[0301:eioppi]2.0.co;2
Lovern, M., and Jenssen, T. A. (2003). Form emergence and fixation of head bobbing displays in the green anole lizard (Anolis carolinensis): a reptilian model of signal ontogeny. J. Comp. Physiol. 117, 133–141. doi: 10.1037/0735-7036.117.2.133
Lovern, M. B., Holmes, M. M., and Wade, J. (2004). The green anole (Anolis carolinensis): a reptilian model for laboratory studies of reproductive morphology and behavior. ILAR J. 45, 54–64. doi: 10.1093/ilar.45.1.54
Lovern, M. B., Jenssen, T. A., Orrell, K. S., and Tuckak, T. (1999). Comparisons of temporal display structure across contexts and populations in male Anolis carolinensis: signal stability or lability? Herpetologica 55, 222–234.
Lowry, H., Lill, A., and Wong, B. B. M. (2013). Behavioural responses of wildlife to urban habitats. Biol. Rev. 88, 537–549. doi: 10.1111/brv.12012
Luther, D. A., Phillips, J., and Derryberry, E. P. (2016). Not so sexy in the city: urban birds adjust songs to noise but compromise voval performance. Behav. Ecol. 27, 332–340. doi: 10.1093/beheco/arv162
Markovchick-Nicholls, L., Regan, H. M., Deutschman, D. H., Widyanata, A., Martin, B., Noreke, L., et al. (2008). Relationships between human disturbance and wildlife land use in urban habitat fragments. Conserv. Biol. 22, 99–109. doi: 10.1111/j.1523-1739.2007.00846.x
Marnocha, E., Pollinger, J., and Smith, T. B. (2011). Human-induced morphological shifts in an island lizard. Evol. Appl. 4, 388–396. doi: 10.1111/j.1752-4571.2010.00170.x
Martin, J., and López, P. (1995). Influence of habitat structure on the escape tactics of the lizard Psammodromus algirus. Can. J. Zool. 73, 129–132. doi: 10.1139/z95-014
McKinney, M. L. (2006). Urbanization as a major cause of biotic homogenization. Biol. Conserv. 127, 247–260. doi: 10.1016/j.biocon.2005.09.005
McMillan, D. M., and Irschick, D. J. (2010). Experimental test of predation and competition pressures on the green anole (Anolis carolinensis) in varying structural habitats. Journal of Herpetology, 44, 272–278. doi: 10.1670/08-196.1
Michael, E. D. (1972). Growth rates in Anolis carolinensis. Copeia 1972, 575–577. doi: 10.2307/1442932
Michaud, E. J., and Echternacht, A. C. (1995). Geographic variation in the life history of the lizard Anolis carolinensis and support for the pelvic constraint model. J. Herpetol. 29, 86–97. doi: 10.2307/1565090
Mikula, P. (2014). Pedestrian density influences flight distances of urban birds. ARDEA 102, 53–60. doi: 10.5253/078.102.0105
Moore, M. P., Riesch, R., and Martin, R. A. (2016). The predictability and magnitude of life-history divergence to ecological agents of selection: a meta-analysis in livebearing fishes. Ecol. Lett. 19, 435–442. doi: 10.1111/ele.12576
Munoz, M. M., and Losos, J. B. (2018). Thermoregulatory behavior simultaneously promotes and forestalls evolution in a tropical lizard. Am. Nat. 191, E15-E26.
Muralidhar, P., and Johnson, M. A. (2017). Sexual selection and sex ratios in Anolis lizards. J. Zool. 302, 178–183. doi: 10.1111/jzo.12446
Orrell, K. S., and Jenssen, T. A. (2003). Heterosexual signalling by the lizard Anolis carolinensis, with intersexual comparisons across contexts. Behaviour 140, 603–634. doi: 10.1163/156853903322149469
Parris, K. M., Velik-Lord, M., and North, J. M. A. (2009). Frogs call at a higher pitch in traffic noise. Ecol. Soc. 14:25.
Petren, K., and Case, T. J. (1998). Habitat structure determines competition intensity and invasion success in gecko lizards. Proc. Natl. Acad. Sci. U.S.A. 95, 11739–11744. doi: 10.1073/pnas.95.20.11739
Putnam, B. J., Pauly, G. P., and Blumstein, D. T. (2020). Urban invaders are not bold risk-takers: a study of 3 invasive lizards in Southern California. Curr. Zool. doi: 10.1093/cz/zoaa015
Reedy, A. M., Cox, C. L., Chung, A. K., Evans, W. J., and Cox, R. M. (2015). Both sexes suffer increased parasitism and reduced energy storage as costs of reproduction in the brown anole. Anolis sagrei. Biol. J. Linn. Soc. 117, 516–527. doi: 10.1111/bij.12685
Reznick, D. A., Bryga, H., and Endler, J. A. (1990). Experimentally induced life-history evolution in a natural population. Nature 346, 357–359. doi: 10.1038/346357a0
Rhodes, M., and Catterall, C. (2008). Spatial foraging behavior and use of an urban landscape by a fast-flying bat, the Molossid Tadarida australis. J. Mammal. 89, 34–42. doi: 10.1644/06-mamm-a-393.1
Ruby, D. E. (1984). Male breeding success and differential access to females in Anolis carolinensis. Herpetologica 40, 272–280.
Scales, J., Hyman, J., and Hughes, M. (2011). Behavioral syndromes break down in urban song sparrow populations. Ethology 117, 887–895. doi: 10.1111/j.1439-0310.2011.01943.x
Schoener, T., and Schoener, A. (1980). Densities, sex rations, and population structure in four species of Bahamian Anolis lizards. J. Anim. Ecol. 49, 19–53. doi: 10.2307/4276
Sepp, T., McGraw, K. J., Kaasik, A., and Giraudeau, M. (2018). A review of urban impacts on avian life-history evolution: does city living lead to a slower pace-of life? Glob. Change Biol. 24, 1452–1469. doi: 10.1111/gcb.13969
Slabbekoorn, H., and Peet, M. (2003). Birds sing at higher pitch in urban noise. Nature 424:267. doi: 10.1038/424267a
Sol, D., Lapiedra, O., and González-Lagos, C. (2013). Behavioral adjustments for a life in the city. Anim. Behav. 85, 1101–1112. doi: 10.1016/j.anbehav.2013.01.023
Sol, D., Maspons, J., Gonzalez-Voyer, A., Morales-Castilla, I., Garamszegi, L. Z., and Møller, A. P. (2018). Risk-taking behavior, urbanization, and the pace of life in birds. Behav. Ecol. Sociobiol. 72. doi: 10.1007/s00265-018-2463-0
Stroud, J. T., Colom, M., Ferrer, P., Palermo, N., Vargas, V., Cavallini, M., et al. (2019). Behavioral shifts with urbanization may facilitate biological invasion of a widespread lizard. Urban Ecosyst. 22, 425–434. doi: 10.1007/s11252-019-0831-9
R Core Team. (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Thawley, C. J., Moniz, H. A., Merritt, A. J., Battles, A. C., Michaelides, S. N., and Kolbe, J. J. (2019). Urbanization affects body size and parasitism but not thermal preferences in Anolis lizards. J. Urban Ecol. 5, 1–9.
Tylan, C., and Langkilde, T. (2017). Local and systemic immune responses to different types of phytohemagglutanin in the green anole: lessons for field ecoimmunologists. J. Exp. Zool. Part A Ecol. Integr. Physiol. 327, 322–332. doi: 10.1002/jez.2108
Tyler, R. K., Winchell, K. M., and Revell, L. J. (2016). Tails of the city: caudal autotomy in the tropical lizard, Anolis cristatellus, in urban and natural areas of Puerto Rico. J. Herpetol. 50, 435–441. doi: 10.1670/15-039
Uller, T., Pen, I., Wapstra, E., Beukeboom, L. W., and Komdeur, J. (2007). The evolution of sex ratios and sex-determining systems. Trends Ecol. Evol. 22, 292–297. doi: 10.1016/j.tree.2007.03.008
Vanhooydonck, B., Herrel, A., and Irschick, D. J. (2007). Determinants of sexual differences in escape behavior in lizards of the genus Anolis: a comparative approach. Integr. Comp. Biol. 47, 200–210. doi: 10.1093/icb/icm018
Vervust, B., Lailvaux, S. P., Grbac, I., and Van Damme, R. (2008). Do morphological condition indices predict locomotor performance in the lizard Podarcis sicula? Acta Oecol. 34, 244–251. doi: 10.1016/j.actao.2008.05.012
Watling, J. I., and Donnelly, M. A. (2006). Fragments as islands: a synthesis of faunal responses to habitat patchiness. Conserv. Biol. 20, 1016–1025. doi: 10.1111/j.1523-1739.2006.00482.x
Winchell, K. M., Briggs, D., and Revell, L. J. (2019). The perils of city life: patterns of injury and fluctuating asymmetry in urban lizards. Biol. J. Linn. Soc. 126, 276–288. doi: 10.1093/biolinnean/bly205
Winchell, K. M., Carlen, E. J., Puente-Rolón, A. R., and Revell, L. J. (2018a). Divergent habitat use of two urban lizard species. Ecol. Evol. 2018, 25–35. doi: 10.1002/ece3.3600
Winchell, K. M., Maayan, I., Fredette, J. R., and Revell, L. J. (2018b). Linking locomotor performance to morphological shifts in urban lizards. Proc. R. Soc. B Biol. Sci. 285:10.
Winchell, K. M., Reynolds, R. G., Prado-Irwin, S. R., Puente-Rolon, A. R., and Revell, L. J. (2016). Phenotypic shifts in urban areas in the tropical lizard Anolis cristatellus. Evolution 70, 1009–1022. doi: 10.1111/evo.12925
Woolbright, L. L., Hara, A. H., Jacobsen, C. M., Mautz, W. M., and Benevides, F. L. (2006). Population densities of the coqui, Eleutherodactylus coqui (Anura: Leptodactylidae) in newly invaded Hawaii and in native Puerto Rico. J. Herpetol. 40, 122–126. doi: 10.1670/79-05w.1
Ydenberg, R. C., and Dill, L. M. (1986). The economics of fleeing from predators. Adv. Stud. Anim. Behav. 16, 229–249. doi: 10.1016/s0065-3454(08)60192-8
Zozaya, S. M., Alford, R. A., and Schwarzkopf, L. (2015). Invasive house geckos are more willing to use artificial lights than are native geckos. Austr. Ecol. 40, 982–987. doi: 10.1111/aec.12287
Zuk, M., and Kolluru, G. R. (1998). Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 73, 415–443. doi: 10.1086/420412
Keywords: Anolis, urban ecology, behavior, morphology, habitat
Citation: Lailvaux SP (2020) It’s Not Easy Being Green: Behavior, Morphology, and Population Structure in Urban and Natural Populations of Green Anole (Anolis carolinensis) Lizards. Front. Ecol. Evol. 8:570810. doi: 10.3389/fevo.2020.570810
Received: 09 June 2020; Accepted: 31 August 2020;
Published: 25 September 2020.
Edited by:
Elizabeth Perrault Derryberry, The University of Tennessee, Knoxville, United StatesReviewed by:
Zachary Chejanovski, College of Idaho, United StatesKristin M. Winchell, Washington University in St. Louis, United States
Copyright © 2020 Lailvaux. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simon P. Lailvaux, slailvaux@gmail.com http://orcid.org/0000-0002-2737-8682
 Simon P. Lailvaux
Simon P. Lailvaux