- Dipartimento di Scienze AgroAlimentari, Ambientali e Animali, Università Degli Studi di Udine, Udine, Italy
A Commentary on
Engineered symbionts activate honey bee immunity and limit pathogens
by Leonard, S. P., Powell, J. E., Perutka, J., Geng, P., Heckmann, L. C., Horak, R. D., et al. (2020). Science 367, 573–576. doi: 10.1126/science.aax9039
In a recently published paper, Leonard et al. (2020) described a genetically modified gut bacterium for the delivery of dsRNA to adult honey bees. Furthermore, by engineering the symbiont to interfere with the expression of some Varroa genes, the authors showed the potential of this method for the control of the most important threat to honey bees: the parasitic mite Varroa destructor. The paper, beyond presenting a powerful, novel tool for the study of functional genomics in bees, rekindled hopes that an effective method to control the Varroa mite could be developed (Paxton, 2020). However, a careful evaluation of the proposed method in light of ecological and evolutionary principles reveals that it may be less promising than hoped.
The need to consider honey bee diseases in the framework of ecological and evolutionary principles has been recognized (Brosi et al., 2017); however, some peculiar features of the biological cycle of V. destructor, have been overlooked so far. Importantly, little attention has been paid to the possible adaptation of the mite (Eliash and Mikheyev, 2020).
The Varroa mite, together with the pathogenic deformed wing virus, causes huge losses of honey bee colonies in the northern hemisphere (Carreck and Neumann, 2010), threatening the pollination service provided by Apis mellifera (Potts et al., 2010). The mite's life cycle includes a phoretic phase, on adult bees, and a reproductive phase, within the brood cells (Nazzi and Le Conte, 2016). Only mites entering a brood cell before sealing can reproduce while phoretic mites are exposed to high mortality, including that caused by control methods applied by beekeepers. In fact, most available chemical control methods for the mite, including both synthetic chemicals (e.g., the pyrethroid Tau-fluvalinate), essential oil components (e.g., Thymol), and widely used organic acids (e.g., Oxalic acid), act only upon phoretic mites (Rosenkranz et al., 2010). Actually, even the possible novel methods based upon engineered symbionts (Leonard et al., 2020), would act primarily upon phoretic mites; in fact, differently from adult bees, the preimaginal stages, where mite reproduction takes place, are colonized only by an erratic gut community (Kwong and Moran, 2016) and may therefore be not suited for a method based on an engineered gut bacterium.
Therefore, we can conclude that the majority of control methods, by impacting the parasites on adult bees, likely select for mite strains with a reduced phoretic phase, resulting in a high proportion of mites in brood, and an accelerated population growth.
Any possible hereditary trait influencing mite's cell invasion, such as, for example, a differential response to semiochemicals from brood cells (Nazzi et al., 2004), would determine the proportion of mites in brood and thus reproduction. Therefore, such traits play a critical role in the development of infestation and are likely under intense selective pressure. However, some interventions can influence the mortality of mites within brood cells, thus attenuating that pressure (Figure 1). These methods include the selection of hygienic bees that recognize and remove infested brood, biotechnical control methods based upon brood removal and possible chemicals capable of crossing the cell sealing (Rosenkranz et al., 2010). As shown in figure legend, these latter control methods could balance the increase in the mite's invasion rate caused by the repeated use of currently available control methods; this, in turn, may have an important impact on the growth of the mite population. In fact, a selection program based on brood removal actually lead to the selection of an hypo-virulent mite strain (Milani et al., 1999).
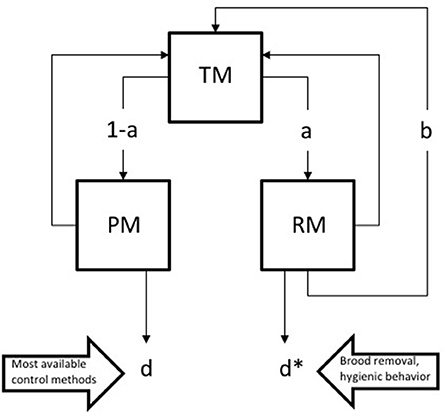
Figure 1. Some dynamic implications of the life cycle of Varroa destructor. The life cycle of the parasite includes a phoretic phase on adult bees and a reproductive phase in brood cells; phoretic mites enter brood cells containing a bee larva, reproduce and emerge with the eclosing bee; after some time on adult bees, another reproductive phase is initiated. Therefore, the mite's life cycle can be schematized as follows. A proportion “a” of the total mite population (“TM”) can enter the reproductive phase within brood cells (i.e., reproducing mites: “RM”) and reproduce at a rate “b” or die at a rate “d*”. Mites not entering a brood cell (i.e., phoretic mites: “PM”) die at a rate “d” or can later enter a brood cell to reproduce. The increase of the mite population can be summarized as: dTM/dt = TMa(b − d*) − TM(1 − a)d. The mite population grows if dTM/dt > 0, which is obtained if TMa(b − d*) > TM(1 − a)d, thus for any a > d/(b + d − d*). Therefore, any intervention increasing “d*”, such as, for example, brood removal, can increase the ratio “d/(b+d-d*)”, mitigating the effects of the increase of “a” determined by the selective pressure exerted by most currently used control methods.
Clearly, mite transfer between hives could hinder the development of such hypo-virulent mite populations and care should be taken to minimize this risk, for example by preventing robbing which represents a common cause of mite importation from collapsing hives into colonies with limited mite infestation (Greatti et al., 1992).
Indeed reinfestation, through which mites leading bee colonies to collapse can escape this dead end and get transferred to a new healtier colony, represents a critical point to be carefully considered under the evolutionary perspective outlined here.
Only sustainable beekeeping based upon an efficient control of Varroa mite, grounded on the evolutionary interpretation of this host-parasite interaction, will guarantee pollination continuity in a changing world.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I gratefully acknowledge Robert J. Paxton for the constructive discussion.
References
Brosi, B. J., Delaplane, K. S., Boots, M., and de Roode, J. C. (2017). Ecological and evolutionary approaches to managing honeybee disease. Nat. Ecol. Evol. 1, 1250–1262. doi: 10.1038/s41559-017-0246-z
Carreck, N., and Neumann, P. (2010). Honey bee colony losses. J. Apic. Res. 49, 1. doi: 10.3896/IBRA.1.49.1.01
Eliash, N., and Mikheyev, A. (2020). Varroa mite evolution: a neglected aspect of worldwide bee collapses? Curr. Opin. Insect Sci. 39, 21–26. doi: 10.1016/j.cois.2019.11.004
Greatti, M., Milani, N., and Nazzi, F. (1992). Reinfestation of an acaricide-treated apiary by Varroa jacobsoni Oud. Exp. Appl. Acarol. 16, 279–286. doi: 10.1007/BF01218569
Kwong, W. K., and Moran, N. A. (2016). Gut microbial communities of social bees. Nat. Rev. Microbiol. 14, 374–384. doi: 10.1038/nrmicro.2016.43
Leonard, S. P., Powell, J. E., Perutka, J., Geng, P., Heckmann, L. C., Horak, R. D., et al. (2020). Engineered symbionts activate honey bee immunity and limit pathogens. Science 367, 573–576. doi: 10.1126/science.aax9039
Milani, N., Pechhacker, H., and Della Vedova, G. (1999). Reduced fertility in a European population of Varroa jacobsoni Oudemans. Apidologie 30, 435–436. doi: 10.1051/apido:19990211
Nazzi, F., and Le Conte, Y. (2016). Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annu. Rev. Entomol. 61, 417–432. doi: 10.1146/annurev-ento-010715-023731
Nazzi, F., Milani, N., and Della Vedova, G. (2004). A semiochemical from larval food influences the entrance of Varroa destructor into brood cells. Apidologie 35, 403–410. doi: 10.1051/apido:2004023
Paxton, R. J. (2020). A microbiome silver bullet for honey bees. Science 367, 504–506. doi: 10.1126/science.aba6135
Potts, S. G., Biesmeijer, J. C., Kremen, C., and Neumann, P. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. doi: 10.1016/j.tree.2010.01.007
Keywords: Varroa destructor, control, evolution, honey bee (Apis mellifera L.), virulence
Citation: Nazzi F (2020) Commentary: Engineered symbionts activate honey bee immunity and limit pathogens. Front. Ecol. Evol. 8:538520. doi: 10.3389/fevo.2020.538520
Received: 28 February 2020; Accepted: 21 August 2020;
Published: 22 September 2020.
Edited by:
David Baltrus, University of Arizona, United StatesReviewed by:
Samuel Ramsey, United States Department of Agriculture (USDA), United StatesCopyright © 2020 Nazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Nazzi, ZnJhbmNlc2NvLm5henppQHVuaXVkLml0
 Francesco Nazzi
Francesco Nazzi