- 1Department of Biology, University of Saskatchewan, Saskatoon, SK, Canada
- 2Instituto de Biología Subtropical (IBS), CONICET–UNaM, Puerto Iguazú, Argentina
- 3Department of Forest and Conservation Sciences, University of British Columbia, Vancouver, BC, Canada
- 4Science & Technology Branch, Environment and Climate Change Canada, Delta, BC, Canada
Many animals require tree cavities for breeding and these sites may be reused by a diversity of secondary cavity nesters over a timespan of decades. It is unknown whether the reuse of holes changes their desirability as nest sites. We hypothesized that some species, “cavity destroyers,” degrade the quality of holes by filling them with coarse nest material or waste whereas excavating species, “cavity cleaners,” might prolong the use of a hole by removing debris or enlarging the hole. Using data gathered during 22 years from a field study in central British Colombia, we analyzed long-term patterns of cavity occupancy in relation to their sequential use by bird and mammal species, grouped by traits. Patterns of cavity occupancy were variable with 49% of 875 large-sized holes (excavated by northern flickers Colaptes auratus and pileated woodpeckers Dryocopus pileatus) and 19% of 652 smaller-sized holes incorporating runs of sequential use that lasted to 18 years. About 11% of large and 25% of small cavities also had gaps of 3–13 years between occupancies. Mammals, raptors and European starlings, consistent with the hypothesis, were cavity destroyers, occupying cavities as terminal users and before gaps more often than expected by chance. The pattern of occupancy by northern flickers was random in relation to gaps or prior use by other species. Although flickers did not target old holes to clean, neither did they avoid them. Small cavities that were renovated by flickers into larger cavities were reused at twice the rate after renovation. Runs of cavity occupancy that involved only cavity-destroying species were shorter than runs that involved periodic use by flickers, suggesting the woodpecker, through its cleaning and renovation, prolonged the use of such holes. Our study contributes insights on additional ecological factors, namely previous users, that can influence the use and availability of cavities over time.
Introduction
Tree cavities are a multi-annual resource that is often in high demand among forest vertebrates for breeding or roosting; individual cavities may be used more than 20 times over greater than three decades (Cockle et al., 2019). Thus, understanding the dynamics of cavity use and availability over time is important to maintain biodiversity in forest communities (Lindenmayer et al., 1993; Aitken and Martin, 2004). In North America, excavators (especially woodpeckers) create most of the cavities that are subsequently reused by a diverse assemblage of secondary cavity nesters which cannot excavate their own holes (Martin et al., 2004; Cockle et al., 2011). Depending on rates of cavity formation, the presence of landscape disturbances and the population densities of secondary cavity users, there may be high demand and competition for these breeding locations (Newton, 1994; Wiebe, 2011). Consequently, the same hole may be used repeatedly by multiple species (Raphael and White, 1984; Aitken et al., 2002; Blanc and Walters, 2008; Pakkala et al., 2017).
Long-term studies monitoring the use of individual cavities over time allow detailed analysis of the dynamics of these “nestweb” systems (Martin et al., 2004). Several studies have documented the longevity of cavities in trees of different species and decay classes (Sedgwick and Knopf, 1992; Lindenmayer and Wood, 2010; Wesołowski, 2011). For example, cavities in trembling aspen Populus tremuloides trees in central British Columbia lasted on average seven years in dead trees and more than 15 years in living trees (Edworthy et al., 2012). During their lifespan, cavities may change in size and decay state and different species of secondary cavity nesters may prefer cavities of different ages. In central British Columbia, European starlings Sturnus vulgaris and red-breasted nuthatches Sitta canadensis preferred relatively new cavities whereas mountain bluebirds Sialia currucoides and tree swallows Tachycineta bicolor used older cavities more frequently (Edworthy et al., 2018). The changing physical properties of cavities as a result of their aging and decay (Edworthy and Martin, 2014) may thus affect the identity of the species that occupy them over long timespans; however, it is unknown how the sequential use of holes from 1 year to the next may be affected by the use by various types secondary cavity nesters.
Several hypotheses predict that the repeated (annual) reuse of cavities, i.e., “runs” of cavity occupancy (see Figure 1), would be of short duration. Birds and mammals often bring nest material into cavities and this might accumulate over time causing cavities to become shallower and unusable (Perrins, 1979). Cavity-nesting raptors like owls and falcons do not add nest material but, unlike most passerine parents which remove their nestlings’ fecal sacs during at least part of the nestling period, raptor chicks excrete inside the cavity and so this material and the bulkier remains of vertebrate prey may accumulate on cavity bottoms. Such refuse in the cavity may prevent drainage of moisture. Sequential reuse of cavities may also cause a buildup of parasites (Short, 1979) or, in the case of prey remains and excrement left by raptors and mammals, a buildup of bacteria and pathogens that could deter reuse (Mazgajski, 2007a). Finally, experiments suggest that passerines may avoid cavities with visual cues of use or visitation by mammalian predators (Ekner and Tryjanowski, 2008; Mönkkönen et al., 2009) and these songbirds may or may not avoid holes with mammalian scent (review in Amo et al., 2018). Cavities with such cues may signal a high predation risk and discourage subsequent reuse of that nest site by other species.
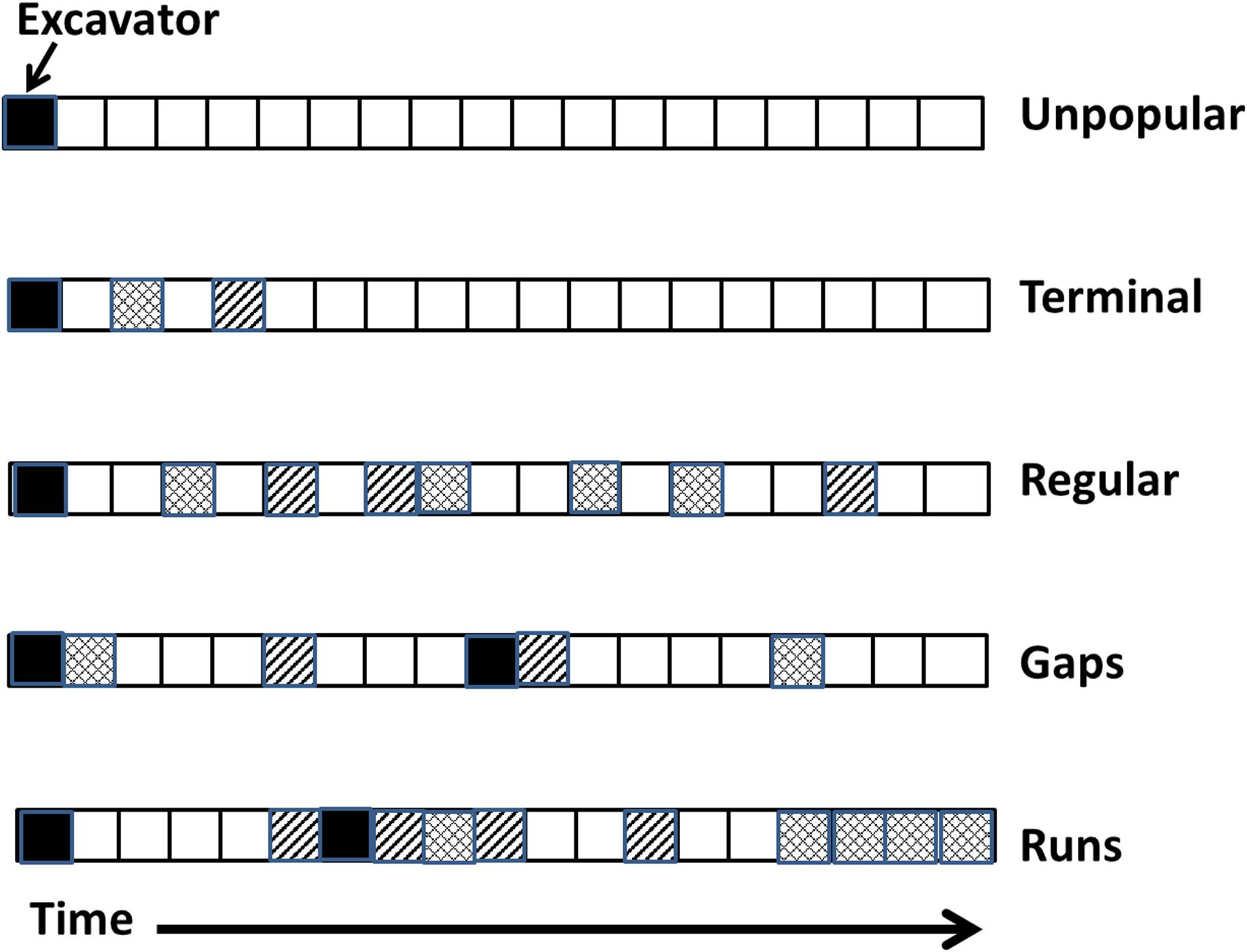
Figure 1. Five patterns of tree cavity reuse observed during long-term monitoring of cavities. The different patterning within squares indicates different hypothetical species. Not all patterns were mutually exclusive. Whereas the regular pattern of use never involved gaps, runs, or terminal use, other cavities might have both gaps and runs and terminal use.
On the other hand, added nest material may not prevent reuse if it decomposes rapidly or if animals remove debris (Wesołowski, 2000). Hebda et al. (2013) found that experimentally added mesh bags filled with paper/cellulose and hair (replicating material comprising nests of titmice) emptied substantially after being placed in nest cavities of tits for 9.5 months overwinter. The experiment indicated that the structural decomposition of such material may be rapid although any particulate debris that fell through the mesh bag and may have remained on the cavity bottom was not measured. Mazgajski (2007a) noted that old nesting material of tits and starlings lasted at least 1 year in tree cavities so decomposition rates may vary between geographic regions. Other passerine species such as the European starling and the house wren Troglodytes aedon use sturdier nest materials with lignin (e.g., thick straw or small sticks and twigs) and these nest substrates may take longer to decay and disappear (Hebda et al., 2017). Red squirrels Tamiasciurus hudsonicus, in addition to building bulky nests of shredded bark and grass (Patterson et al., 2007), may use cavities to store food caches of conifer cones or dried mushrooms overwinter and the remains of such larders may last several years, rendering the cavity unusable for nesting until cleaned.
Of course, any direct cavity-cleaning behavior of secondary users may prolong the usability of holes and enable many sequential years of breeding. Different degrees of nest-cleaning are associated with different species groups. Limited nest preparation or cleaning has been observed in the pied flycatcher Ficedula hypoleuca (Merino and Potti, 1995), the house wren, and tit species (Hebda et al., 2013) during which the birds sometimes remove leaves and other light debris from cavity bottoms. The larger European starling has a greater ability to clean away the remains of its old straw nests, but cavity-cleaning costs time and energy and starlings usually only remove the top layers, leaving old nest bottoms to potentially accumulate (Mazgajski, 2013). Woodpeckers, if they reuse cavities, may have the greatest ability to clean and renovate interiors by removing any accumulated material with their strong bills and adding a fresh layer of woodchips to the cavity floor (Wiebe et al., 2007).
Here, we examined sequential reuse of tree cavities by the community of cavity-nesting vertebrates over 22 breeding seasons in central British Columbia, Canada. We categorized patterns of occupancy into five types (Figure 1) and limited our study to avian-excavated holes (versus non-excavated, e.g., cavities created by branch fall) because 92% of nests in our system are in excavated cavities (Martin et al., 2004). “Unpopular” holes, after being used by the excavator for breeding in the first year, were never reused by a secondary nester. One can not rule out that the interior of some unpopular holes was still clean and of high quality but the location of the tree or other reason may have caused secondary nesters difficulty in finding the cavity. Because about 60% of cavities in this system are empty each year (Edworthy et al., 2018; Cockle et al., 2019), 1-year “gaps” in use are very probable by chance and would not necessarily indicate that the interior of a cavity was degraded. Therefore, we define a “gap” here as a sequence of at least 3 years of non-use [a probability of (0.6)3 = 0.22] bracketed between two cases of occupancy. Gaps indicate that the cavity’s physical size and structure was still usable across that duration although the hole was not occupied in the interim, perhaps because the interior was unsuitable. “Runs” were a sequence of at least 3 years of consecutive use. “Terminal” patterns occurred when a secondary nester reused a cavity at least once (irrespective of any gaps or runs) but then the cavity was never used again, falling into long-term disuse for a minimum of 3 years before the cavity disappeared, usually when the tree fell. Note that some of these patterns are not mutually exclusive as a single tree cavity may show gaps, runs and/or terminal use over its lifespan. Finally, a “regular” pattern of reuse was defined as having no runs or gaps, i.e., < 3 years of sequential occupancy alternating with < 3 years of non-use throughout the lifespan of the cavity (Figure 1).
We were particularly interested in whether occupancy by certain species degrades cavity interiors, rendering them less likely to be reused in subsequent years and, conversely, whether cavity-cleaning species, particularly excavating species with strong, excavating, bill morphology might rejuvenate cavities after a gap in use or facilitate a long run of use. We hypothesized that cavity-degrading or “destroying” species would include mammals, raptors, and European starlings, the latter because of their thick straw nests and fecal deposits in cavities that accumulate once parents stop removing fecal sacs after the first week (Cabe, 2020). Species that degrade cavities would be expected to be terminal users or should occur before gaps in cavity occupancy more often than expected by chance. We predicted that excavating species (woodpeckers and nuthatch), which we also called “cleaners,” would occupy cavities more often after gaps than random because they could remove a buildup of old material and refresh the holes. We also predicted that the length of runs would be longest for sequences involving only excavators/cleaners, of moderate length if involving only “neutral” species (i.e., ducks, bluebirds, tree swallows and chickadees, that use fine nest material such as grass and feathers, and shortest for sequences with only destroyer species.
Materials and Methods
We studied a cavity-nesting community of birds and mammals during 1995–2016 in central British Columbia, Canada, in the vicinity of Riske Creek and Williams Lake (52°09′N, 122°09’W). The landscape consisted of native grassland with groves of trembling aspen (Populus tremuloides) and patches of continuous forest dominated by lodgepole pine (Pinus contorta) or Douglas-fir (Pseudotsuga menziesii), with hybrid spruce (Picea spp.) and trembling aspen (Martin et al., 2004; Fisher and Wiebe, 2006b). Each year, we searched for active nests by following adult birds (excavators and secondary cavity-nesters) and inspecting tree holes using mirrors and flashlights or video cameras mounted on extendable poles. In this way, we could directly assess the contents of cavities up to 15 m high. For higher cavities, we inferred breeding if behavior of adults indicated the presence of eggs or young. Cavities were checked regularly for signs of use, about every 4–5 days during the breeding season. After a cavity was recorded as being occupied, we continued to check it every subsequent breeding season until it was destroyed, either because the tree itself fell (90% of cases) or because the cavity walls collapsed or we stopped monitoring the site. About one fifth of cavities were still standing when the study ended and not followed to the end of the lifespan.
Statistical Analysis
Because species of secondary cavity users tend to assort into cavities that match their body size (Bai et al., 2005), we analyzed patterns of nest reuse within two categories. “Large” holes were those excavated by the two largest woodpeckers northern flickers Colaptes auratus or pileated woodpeckers Dryocopus pileatus and, as such, had an entrance diameter of at least 5.2 cm and a vertical depth (entrance rim to cavity floor) averaging 38 cm (see Wiebe, 2001 for average interior and exterior dimensions of flicker cavities). “Small” holes were excavated by other smaller-bodied woodpeckers (hairy woodpecker Picoides villosus, American three-toed woodpecker Picoides dorsalis, red-naped sapsucker Sphyrapicus nuchalis, downy woodpecker Picoides pubescens) or red-breasted nuthatch or black-capped chickadee Poecile atricapillus. A subset of small cavities was later reused, and hence enlarged, by northern flickers. These “renovated” cavities were not included in analyses of either the small or large cavities but were treated as a separate group in which reuse rates were compared before versus after renovation with Wilcoxon matched-pair tests.
For meaningful sequences of runs and gaps in cavity reuse we analyzed only those cavities that had been checked for at least four consecutive breeding seasons subsequent to the first year they were found active (see Supplementary Figure 1 for sample sizes). To simplify analyses and increase statistical power, we pooled some similar secondary cavity users into taxon groups. Ducks included Barrow’s goldeneye Bucephala clangula, bufflehead Bucephala albeola and hooded merganser Lophodytes cucullatus. Raptors included American kestrel Falco sparverius, northern saw-whet owl Aegolius acadicus and flammulated owl Psiloscops flammeolus. Mammals included red squirrel, northern flying squirrel Glaucomys sabrinus, short-tailed weasel Mustela ermina, bushy-tailed woodrat Neotoma cinerea and American pine marten Martes americana. Chickadees included black-capped Poecile atricapillus and mountain chickadee P. gambeli. The length and composition of runs was calculated including the first year the cavity was found active.
For each cavity, we categorized the pattern(s) of use (Figure 1) and recorded which species comprised runs, which occupied the cavity immediately before and after any gap(s) and immediately before a terminal use. It is more likely to find nests of abundant cavity nesters simply because they are more common and so we needed to test whether the likelihood of certain species reusing cavities before or after gaps differed from that expected from their proportional abundance in the community of cavity nesters. Therefore, we calculated “expected by chance” probabilities of each species/guild of cavity users according to their proportional composition of the total nests on the study area during the entire study period. For example, if mountain bluebirds accounted for 8% of all cavity nests in the long-term dataset, we assumed they would occur before a gap with a frequency of 0.08 by chance. We then compared the frequencies of the various species/taxon groups expected by chance to the frequencies actually observed before or after gaps, or before terminal usage with Chi-square tests.
In a different set of analyses, we looked at transitions from one species/taxon group to another user in the next year, or from one type of user to non-use in the subsequent year (t + 1). Here again we used the proportional abundance of the species in the community as the transitional frequency expected by chance but for this suite of analyses we calculated the abundances separately for each year of the study. For example, if 60% of cavities were unused and 10% were used by mammals in a given year (t + 1), the expected transition from any particular species of cavity user in year (t) to an empty hole in the year (t + 1) would be 0.6 and the expected transition of that cavity to a mammal user in year (t + 1) would be 0.10. From 1995 to 2016 we had 21 such “cavity cohort” transitions from year (t) to (t + 1) and so we had 21 comparisons between an “expected transition frequency” and the “observed transition frequency” for each type of transition (e.g., from a bluebird to a mammal). Thus, we treated the 21 years of transitions as independent sampling events and used paired t-tests (with sample size n = 21) to see whether the expected and observed frequencies differed significantly.
Given the many species in the community, there were many types of possible transitions between one species and the next, so to minimize the number of statistical tests, we only examined transitions to non-use in the subsequent year (to test the hypothesis of cavity degradation) and transitions to a conspecific user which visual inspection of the data (see Figure 5) suggested was a prevalent pattern, i.e., usually accounting for at least 25% of cases. For illustration, the frequency of all types of transitions between secondary cavity users was shown in the nest-web diagram. The length of runs in relation to categories of taxa comprising the runs (i.e., destroyer, cleaner, or neutral) was compared using ANOVA. Statistical tests were conducted using SPSS v.26. If residuals were not normally distributed, Wilcoxon matched-pairs tests instead of paired t-tests were used. Significance was set at alpha = 0.05 and all tests were two-tailed.
Results
Among cavities monitored at least five consecutive years, 79 of 875 (9%) of large-sized cavities and 287 of 652 (44%) small cavities fit the “unpopular” pattern (never reused; Figure 1). Other cavities (41% of the large holes and 23% of small ones) were used in a regular pattern and 32% of large and 27% of small cavities had a terminal pattern of use (Figure 1). During the timespan we monitored cavities (see Supplementary Figure 1) an average of 2.08 ± 0.95 species used a large hole and 1.67 ± 0.78 used a small one. A run of at least three years of occupancy occurred in 429 of 875 (49%) large cavities and in 124 of 652 (19%) small cavities. Consistent with higher rates of reuse and greater species diversity in large holes, the average length of runs and the number of species comprising a run was also greater in large holes (Table 1). We pooled flicker and pileated woodpecker holes in the large-size class although the latter accounted for only 36 (4.1%) of the large holes and were reused less frequently; 13 (36%) of pileated woodpecker cavities were unpopular and only 3 (8%) had a run of occupancy.
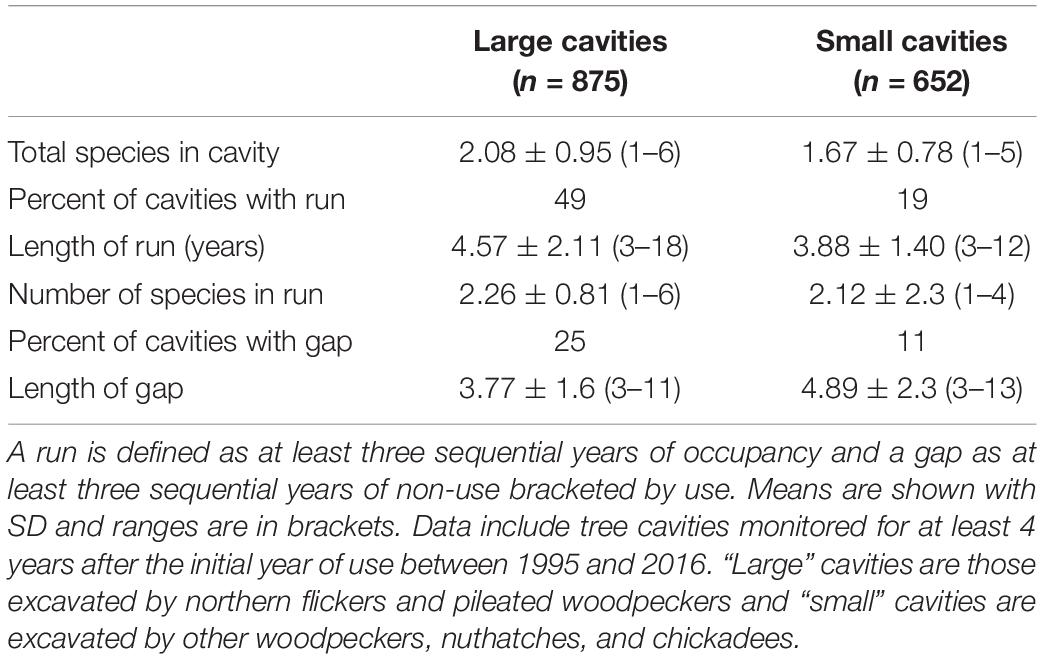
Table 1. Attributes of gaps and runs within the reuse patterns of tree cavities in central British Columbia.
Species as Terminal Users and in Relation to Gaps
The frequency of the seven species/guilds that were terminal users differed from their composition of the cavity-nesting community as a whole for large cavities (Chisquare X2 = 168, df = 6, p < 0.001) and small cavities (Chisquare X2 = 78.1, df = 6, p < 0.001). Raptors and starlings (large holes) and mammals (large and small holes) were the last species to use a hole more often than expected by chance (Figure 2). Likewise, the proportional representation of species immediately before a gap differed from random for large cavities (n = 236 gaps; Chisquare X2 = 60.5, df = 6, p < 0.001) and small holes (n = 69 gaps; Chisquare X2 = 103.1, df = 6, p < 0.001). Starlings, mammals and raptors tended to occur immediately before gaps in large cavities, and nuthatches and smaller woodpeckers tended to occur before gaps in small cavities (Figure 2).
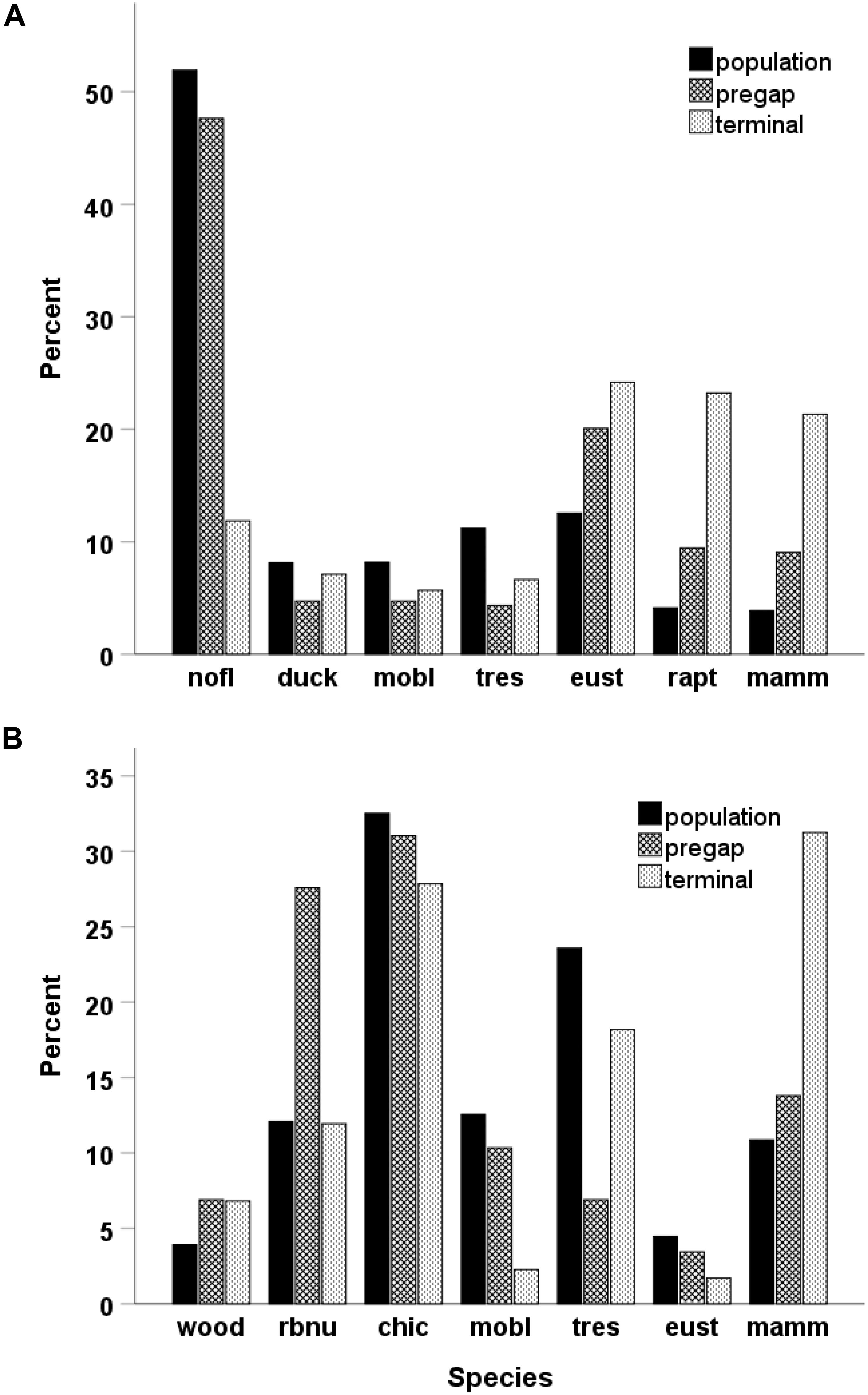
Figure 2. Distribution of secondary cavity nesters in the community as a whole (expected frequencies of occurrence based on abundance) versus their distribution before a gap or as terminal users of a cavity for large-sized cavities (A) and small-sized cavities (B). The species are abbreviated and categorized as (c)leaners, (d)estroyers or (n)eutral as follows: nofl = northern flicker (c), mobl = mountain bluebird (n), tres = tree swallow (n), eust = European starling (d), rapt = raptor (d), mamm = mammal (d), wood = small woodpecker (c), rbnu = red-breasted nuthatch (c), chic = chickadee (n). See text for scientific names of species involved in each taxon group.
Gaps lasted as long as 11 years in large cavities and up to 13 years in small ones but frequently lasted 4–5 years (Table 1). Within a given cavity, we also compared the taxon of user immediately before and after a gap, expecting the proportions to be the same if use of the cavity was random in relation to gaps. However, the proportion of species/guilds differed when comparing the users before and after gaps (large holes: Chisquare X2 = 52.1, df = 6, p < 0.001, n = 236; small holes: Chisquare X2 = 34.7, df = 6, p < 0.001, n = 69). Within the community of large-cavity users, tree swallows and mountain bluebirds occurred more often immediately after gaps than immediately before (Figure 3) whereas northern flickers and ducks occurred with about equal frequency immediately before and after gaps. Starlings, mammals and raptors occurred more often before gaps, consistent with the idea they were cavity destroyers. In the smaller cavities, starlings and small woodpeckers occurred more often before gaps than after, whereas tree swallows and mammals occurred more often immediately after a gap than before it (Figure 3).
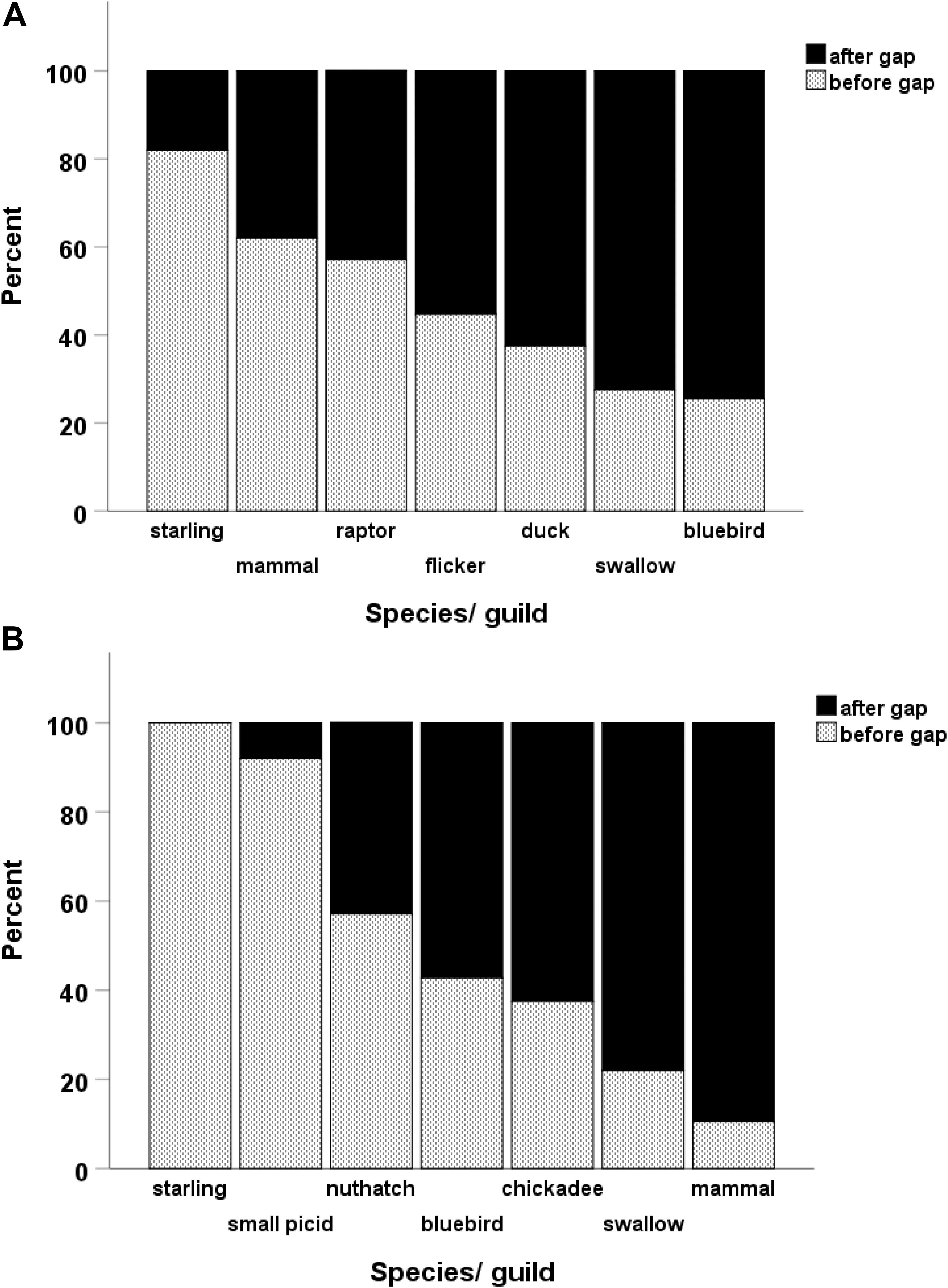
Figure 3. Of the nests that bracketed a gap in cavity use, a breakdown within each species showing the proportion of its nests that occurred either before or after the gap. A gap was defined as at least three consecutive years when the cavity was empty. Large-sized cavities in (A) and smaller cavities in (B). See legend of Figure 2 for the categorization of species.
Length and Composition of Runs
Of 429 runs in large-sized cavities, 333 (77%) involved sequences of two or more species. Of 96 single-species runs in large-sized cavities, 62 (65%) were flickers reusing holes previously excavated by flickers. Runs lasted up to 18 years in large cavities (which was the longest time we monitored a hole) and up to 12 years in small holes (Table 1). The length of runs depended on the category of species in the sequence (destroyer, neutral or excavator/cleaner) for large cavities [ANOVA: F(5, 411) = 7.97, p < 0.001] and for small ones [ANOVA: F(5, 124) = 5.34, p = 0.001; Figure 4]. In both sizes of holes, Bonferroni post hoc tests indicated runs that were comprised of a sequence that included both cavity destroying and cavity excavating species were longer than other categories (Figure 4). In both sizes of cavities runs comprised only of excavating species (woodpeckers and nuthatches) were the shortest. There were no runs of purely destroyer species among small cavities but in large holes, such runs were of intermediate length.
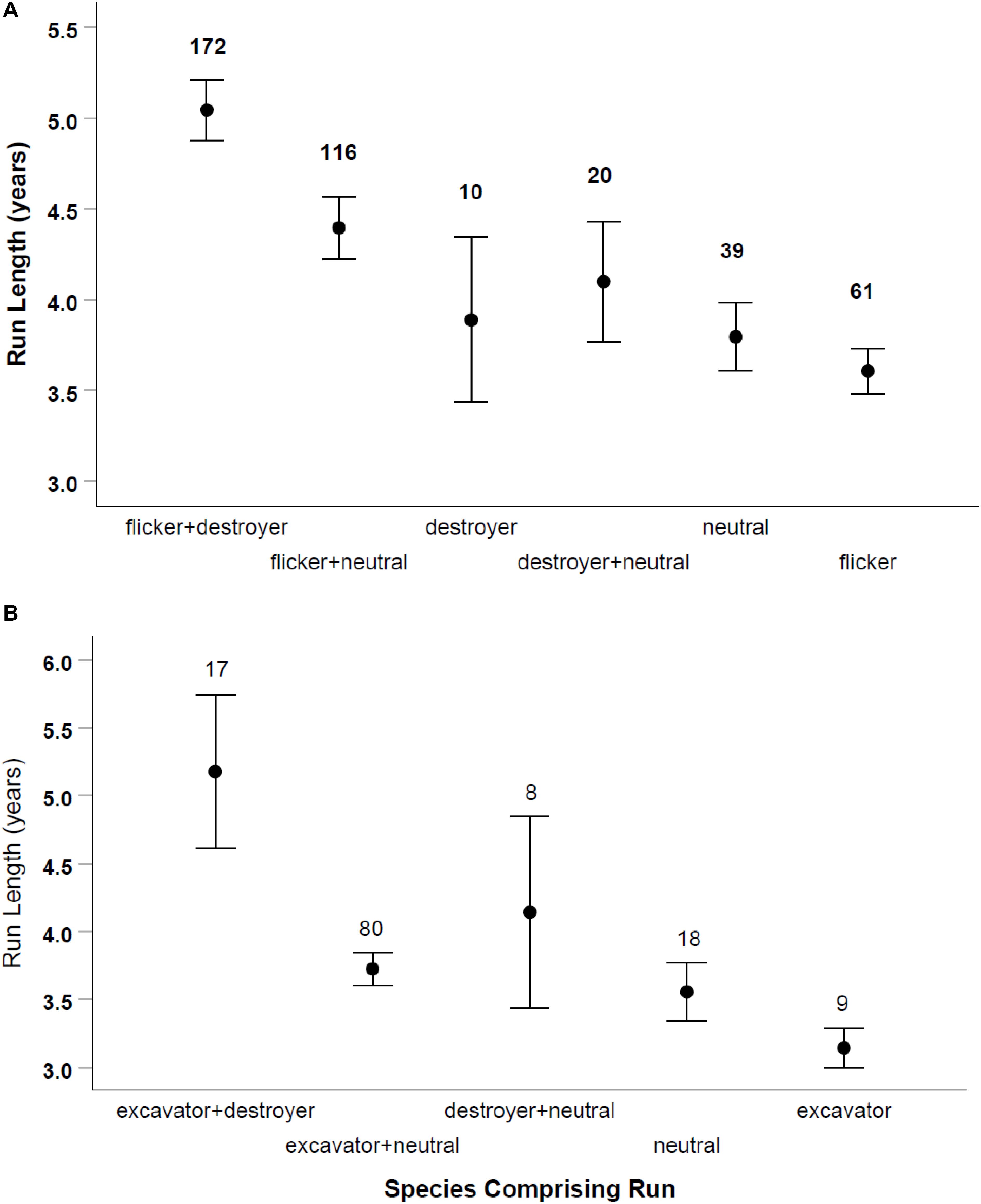
Figure 4. Length of runs of cavity reuse according to the type of species using the hole. Mammals, raptors and starlings were defined as destroyer species, woodpeckers and nuthatches were defined as excavator/cleaning species, and tree swallows, chickadees, bluebirds and ducks were defined as neutral species. Large-sized cavities on (A) and small holes on (B). Means and SE are shown with sample size of runs above each bar.
Transitions Between Secondary Cavity Users
We also examined pairwise transitions occurring between secondary cavity users in 1 year and the next (Figure 5). Among the users of large-sized holes, most cavities occupied by putative “destroying” species (mammals and raptors) transitioned to empty cavities in the subsequent year and this rate was greater than expected by chance (Table 2). The transition rate of cavities occupied by the putative destroyer European starling to empty the following year also differed from random but surprisingly in the opposite direction from that predicted – rather than being empty more starling holes were reused in a subsequent year, mainly due to repeated use of the hole by starlings. The transition rates of cavities occupied by putative “cleaning” or “neutral” species to empty holes in the subsequent year did not differ from chance (Table 2). Sequential reuse of holes was often within a single species, indeed, the frequency of transition to a conspecific user was greater than expected by chance for all species except northern flickers (Table 2). Holes excavated by flickers thus appeared to be reoccupied by a wide variety of secondary users in proportion to the abundance of the various users in the community.
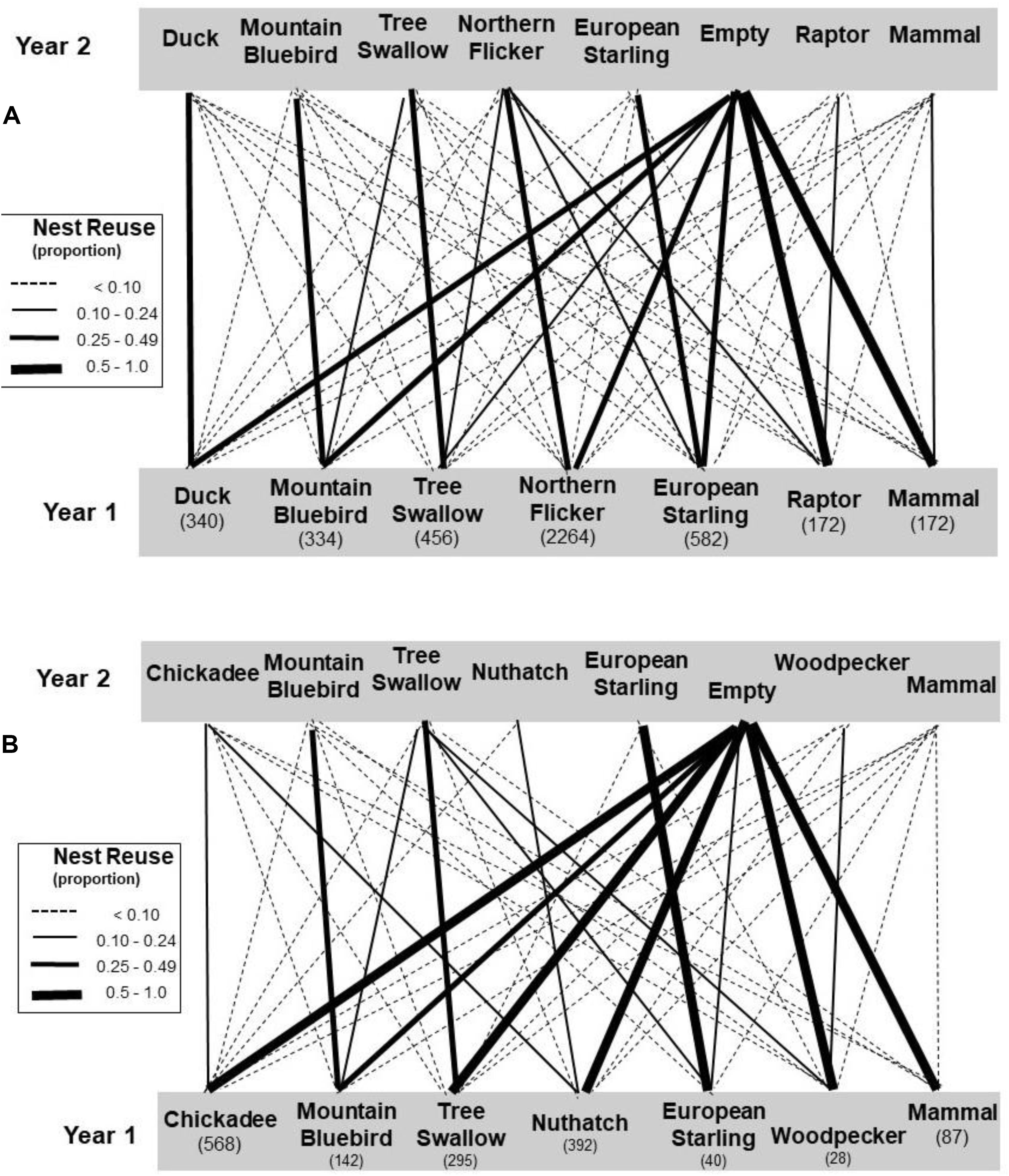
Figure 5. Transition rates (proportion of cavities moving from secondary cavity users in year one to various users in the subsequent year). The community using large holes is in (A) and small holes in (B). Sample sizes of cavities in each case are shown under the users. Only secondary use of cavities is considered – woodpeckers are included only when they reused existing holes. See legend of Figure 2 for categorizations of the species.
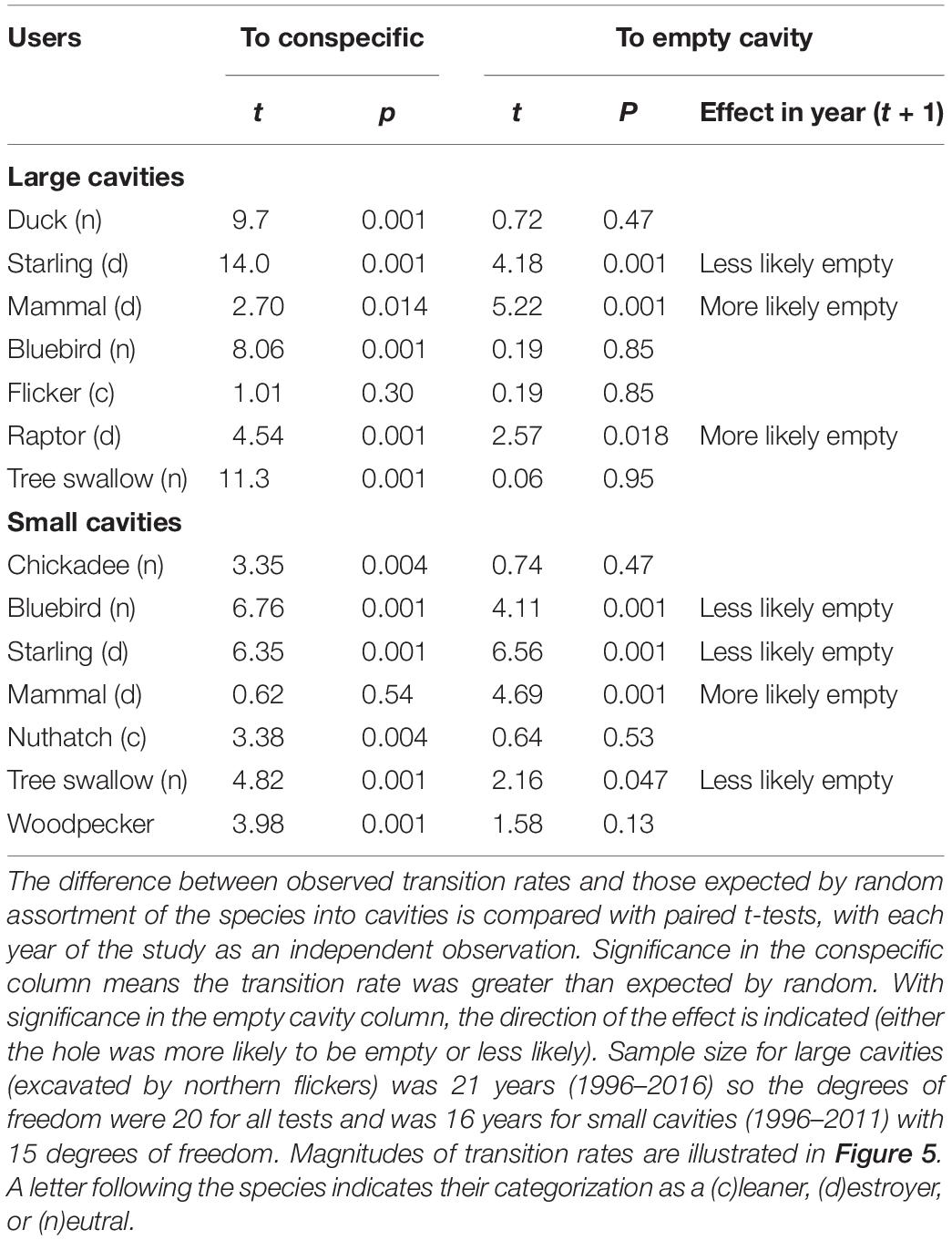
Table 2. Transitions from secondary cavity-nesting taxa to a conspecific or to an unused hole in the subsequent year.
Overall, small holes were reused less frequently than large holes (Chisquare: X2 = 250, df = 1, P < 0.001) and this was reflected in greater transition rates to empty holes, typically over 50% for most taxa, compared to the large holes (Figure 5). As with large cavities, small cavities were more likely to be empty the following year than expected by chance if they were used by mammals, whereas small cavities used by starlings were more likely to be reoccupied, mostly by starlings (Table 2). Small cavities used by bluebirds were also more likely to be reoccupied than expected. In general, if small holes were reused the next year, the transition was more likely to be to a conspecific than expected by chance in all cases, except when the earlier user was a mammal (Table 2). An overview of transition frequencies between all types of users is summarized in Figure 5.
Renovations of Cavities
Large-bodied species are physically too large to fit into small holes, but northern flickers renovated and enlarged 47 cavities initially created by small-bodied excavators (29 red-naped sapsucker holes, 9 hairy woodpecker, 2 three-toed woodpecker, 4 black-capped chickadee, 3 red-breasted nuthatch). Renovation by flickers doubled the median rate of cavity occupancy with 0.25 nests/yr before renovation and 0.50 nests/year after (Wilcoxon matched-pair test: Z = 1.96, p = 0.05). We did not observe any cases of pileated woodpeckers renovating holes in the “small” size class.
Discussion
Tree cavities are valuable and contested resources (Newton, 1994; Wiebe, 2011); the rate at which they are reused may depend on structural features of the cavity itself such as decay state or age (Bai et al., 2005; Koch et al., 2008; Cockle et al., 2019) and the location of the cavity tree in relation to landscape features such as forest edges or habitat types (Aitken and Martin, 2004; Remm et al., 2006). Here, we document for the first time that patterns of sequential occupancy were also associated with the species of secondary cavity users themselves. Consistent with our hypothesis, a few types of species “cavity destroyers,” mainly mammals (typically red squirrels and bushy-tailed woodrats) raptors (American kestrels and saw-whet owls) and starlings occurred more often as the terminal users of cavities or before a multi-annual gap in use than expected by chance. Contrary to our predictions, use of a cavity by a putative cavity cleaner (woodpecker or nuthatch) did not reduce the probability of a subsequent gap in cavity reuse, however, flickers did increase cavity occupancy after they renovated or enlarged small cavities and the length of runs was prolonged when the sequence involved excavators for both sizes of cavities. Thus, there is evidence that some species appear to degrade cavity quality, shortening or inhibiting use by other species, and limited evidence that cleaning species increased the length of time a cavity was reused.
Patterns of Reuse in Relation to Cavity Size and Age
Most cavities that we monitored for at least five consecutive years were reused at least once by a secondary cavity nester although occupancy was higher for large holes created by northern flickers and pileated woodpeckers than for small holes. Several other studies of cavity-nesting communities have reported higher occupancy and species richness of secondary cavity users in larger compared to smaller holes (Gibbons et al., 2002; Cockle et al., 2019) perhaps because these can physically accommodate a greater diversity of species with larger body sizes like mammals, ducks and raptors. In our system, the greater demand for large (mainly flicker-excavated) holes was reflected in the pattern of more and longer-lasting runs and shorter gaps in use compared to the holes excavated by smaller woodpecker species and nuthatches. Although occupancy of small holes on our study area declined rapidly by the third year post-excavation (Edworthy et al., 2018), the northern flicker sometimes renovated small cavities for its own use. The immediate increase in size that resulted apparently allowed the holes to be used by the more diverse community of larger-bodied vertebrates and triggered a reuse rate that doubled after renovation.
Repeated measurements of cavities in aspen trees on our study area showed that their entrance diameter and chamber size increased gradually over a timespan of 17 years, especially in live trees with decay, and the decay stage of a tree advanced one stage from alive to increasing decay stages about every 12.5 years (Edworthy and Martin, 2014). Furthermore, different species of secondary cavity user were associated with different ages and decay stages of cavities (Edworthy et al., 2018), so one might expect a gradual turnover of the species using a certain cavity that could be explained by its changing physical properties (size and decay) unrelated to the former users of that hole, per se. Regular aging and decay of cavities might explain some of the distribution of species occurring before gaps versus after gaps, especially within the community using small holes for which structural size is a probable constraint. For example, for small holes but not large ones, mammals occurred frequently after gaps, perhaps because it was only when trees had aged and decayed for several years that many of these holes became large enough to even accommodate mammals. However, our analysis of transition rates focused on use between subsequent summers, i.e., over a span of = 12 months, and so it largely controlled for natural decay progression and would reflect instead the state of the interior of the cavity resulting from use by certain species. As well, the progression of decay stages of cavities is relatively slow compared to the duration of our 22 years field study (e.g., the median survival of a cavity in a tree that was still alive but with some decay was greater than 15 years, Edworthy et al., 2012).
Degradation in Cavity Quality
The “cavity-destroyer” species in our study might have degraded holes in several ways that are not mutually exclusive. On our study area, cavity depth, at least in freshly excavated small holes, became shallower by 4.2 cm after the first reuse by a secondary cavity nester, presumably as a result of added nest material (Edworthy and Martin, 2014). In contrast, Hebda et al. (2013) found that cellulose and hair that comprises the nest material of small passerines like tits and flycatchers decayed and broke down over the span of a year in tree cavities in a temperate deciduous woodland. Additional comparative studies would help elucidate the effects of microclimate and type of nest material on decomposition rates of old nest material in tree cavities. In general, the use of holes by small passerines such as chickadees, swallows and bluebirds did not appear to deter subsequent occupancy and runs lasting 4–5 years were common among these species. Anecdotally, we noted that the greater volume and coarseness of material and wastes in mammal, starling and raptor holes persisted longer than a year. Whether the feces or prey remains in former mammalian or raptor nests acted as an additional visual or scent-based deterrent for prospecting birds needs further experimentation (see Amo et al., 2018).
We also observed that cavity entrances were sometimes gnawed and enlarged by mammals, and raptors sometimes cracked the cavity walls or entrance holes with their talons such that the structural integrity, and possibly the attractiveness, of these cavities to other potential users may be reduced more rapidly than holes that age without use. Consistent with the hypothesis that European starlings degraded cavities, they often occurred as the terminal users or before gaps. However, their effect on cavities was more nuanced because many cavities used by starlings were reused by starlings in the subsequent year, consistent with a study in Europe where 57% of cavities occupied by starlings in 1 year were used by starlings in the next (Mazgajski, 2007b). Mazgajski (2013) noted the ability of starlings to clean out old nest material which probably enables these birds to reuse particular cavities for multiple years in succession. Starlings are aggressive competitors for nest holes (Dobkin et al., 1995; Wiebe, 2004) and they prefer relatively new cavities with good structural integrity (Edworthy et al., 2018). Thus, they appear able to secure quality cavities on the landscape and reuse these intensively until the cavities become too old or dirty.
Cavity Rejuvenation
Small cavities that were renovated to a larger size class by flickers certainly became more productive but the hypothesis that cavity excavators prolong the use of cavities by cleaning material from old holes received mixed support. It initially seems counterintuitive that runs involving destroying species were longer than runs comprised of only cleaners/excavators (Figure 4) but excavators have the option of creating a new cavity; they differ in the propensity to reuse old holes (Wiebe et al., 2006; Pakkala et al., 2017) in relation to a suite of costs and benefits associated with nest reuse (Wiebe et al., 2007). Northern flickers reuse cavities at a higher rate than most other woodpeckers and are generalists in terms of the age and decay stage of cavities used (Blanc and Martin, 2012; Edworthy et al., 2018). Flickers occurred before and after gaps with a frequency predicted by random use; hence, they did not select degraded holes that had been vacant for several years to rejuvenate but neither did they avoid such holes. Within the community of small cavity users, however, the species of smaller picids and nuthatch reused holes less frequently than the flicker and their occupancy of cavities after 4 years post-excavation declined rapidly (Edworthy et al., 2018). These smaller excavators occurred more often before gaps than after, and their role in cleaning or modifying cavity interiors would not be significant after about 5 years post-excavation. Nevertheless, we found that runs in both large and small cavities were longest when there was alternating use between cavity destroyers and cavity excavators, suggesting that periodic cleaning and renovation by the latter maintained the quality of holes longer than otherwise.
Except for flickers, most transitions between secondary cavity users were to a conspecific. Thus, our data imply that even “cavity-destroying” species were able to cope with their own accumulated nest material if the cavity was in demand. In contrast, Hayward and Rosentreter (1994) noted that nests of northern flying squirrels and red squirrels lasted longer than 1 year in nestboxes and that the squirrels preferred cleaned boxes, only rarely reusing a site with old squirrel material from the previous year. Providing an abundance of clean boxes probably reduced the demand and use of dirty nest sites in that study and so patterns of reuse in nestboxes cannot be easily compared to that in natural cavities in forests. Hebda et al. (2013) reported that one third of the bags filled with nest material and experimentally placed in small tree cavities in Poland were physically removed by vertebrates (perhaps mice or woodpeckers which used the cavities for roosting) during the overwinter period.
Because we did not individually mark most of the secondary cavity nesters, future studies at the population level are needed to determine whether transitions between conspecifics were actually the same individual using the same cavity over time. Any benefits of breeding philopatry might cause an individual to be more tolerant of its own dirty or degraded cavity and hence foster longer runs of reuse. An intensive study of color-marked northern flickers on the site revealed that about 40% of individuals reused the same cavity they had occupied the previous year but this reuse rate depended on whether or not the nest had been depredated (Fisher and Wiebe, 2006a).
Patterns of Use
The probability of reuse often declines as a cavity ages, sometimes precipitously. For example, among cavities excavated by three-toed woodpeckers in a Finnish boreal forest, reuse rates declined exponentially with cavities > 5 years old rarely used (Pakkala et al., 2018). In contrast, regular patterns of use (Figure 1) were common in our system (41% of large holes and 23% of small ones) showing that many cavities were used consistently up to the time the tree blew down (i.e., the cavity was productive throughout its lifespan). Accordingly, the transition from use to non-use was often not abrupt like that of a terminal pattern. Indeed, up to a quarter of large-sized cavities had gaps, so holes that were empty for three or more years were still usable in our system and thus valuable to retain on the landscape.
A number of abiotic and biotic factors may influence patterns of use in cavities over time and these factors might vary at continental scales and among forest biomes. Regular patterns of use might be predicted to be more common in nestweb systems lacking many cavity-cleaning vertebrates. In such systems, only one or two sequential uses might be possible before a period of one or 2 years in which insects or micro-organisms digest accumulated material and wastes in the cavity and render it usable again. The rate of decomposition of this accumulated waste is likely to increase with temperature and humidity (e.g., in hot and humid tropical forest) so gaps might be shorter, and runs longer in tropical compared to temperate forests. In temperate systems, the number of times a cavity can be used in succession might also depend on its initial depth because more old nest material can accumulate in large and deep cavities until they become too shallow and susceptible to predation (Mazgajski, 2007a). Hence, reuse patterns of a particular cavity could depend on body size of the initial excavator.
Several studies involving nestboxes have shown that secondary cavity nesters avoid boxes with old nest material (e.g., Loye and Carroll, 1998) because they may harbor more parasites (Rendell and Verbeek, 1996). Hence, the length of gaps in nest reuse may be related to the survival rate of parasites in cavities over time, or to their transferability among species. However, we showed elsewhere that ectoparasites of flickers were not more prevalent in old versus freshly-excavated holes (Wiebe, 2009) and others have suggested parasite levels in nest boxes may overestimate those in natural cavities (Wesołowski, 2001). Because there have been few studies of parasites in natural cavities and the life histories of parasites are diverse, the relationship between nest reuse and parasitism certainly deserves more detailed study in other ecosystems.
To compliment the non-random patterns of sequential cavity use we document in this study, studies that quantify parasites and measure annual changes in cavity dimensions are needed to confirm mechanisms. Nest-web communities with few cavity-destroying species may show few long gaps or terminal uses of tree holes when those holes can retain their size and structure over time. To refine our understanding of which types of trees and cavities are most useful to retain in forests, we recommend documenting long-term patterns of reuse across a variety of forest systems that vary in microclimate and in the community composition of secondary cavity users – potential cavity destroyers and cleaners. Such information would help us to understand cavity selection and cavity limitation for diverse communities of cavity-using vertebrates.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the University of Saskatchewan Animal Care Committee.
Author Contributions
KW, AE, and KM collected the data. KW, KC, and MT analyzed the data. KW conceived the idea and wrote the manuscript with editing and suggestions from the other authors. All authors contributed to the article and approved the submitted version.
Funding
The research was funded by the Natural Sciences and Engineering Research Council in discovery grant 20317 to KW and a special Strategic Grant to KM. Additional funding came from the provincial government of British Columbia and Environment and Climate Change Canada. Tolko Industries Limited provided logistical and financial support in the field during 1996–2003.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer, ML, declared a shared affiliation, with no collaboration, with one of the authors, KC, to the handling editor at the time of review.
Acknowledgments
We thank the many field assistants over the years who helped find and monitor nests.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.00205/full#supplementary-material
References
Aitken, K. E. H., and Martin, K. (2004). Nest cavity availability and selection in aspen-conifer groves in a grassland landscape. Can. J. Forest Res. 34, 2099–2109. doi: 10.1139/x04-086
Aitken, K. E. H., Wiebe, K. L., and Martin, K. (2002). Nest-site reuse patterns for a cavity-nesting bird community in interior British Columbia. Auk 19, 391–402. doi: 10.1093/auk/119.2.391
Amo, L., Tomas, G., Saavedra, I., and Visser, M. E. (2018). Wild great and blue tits do not avoid chemical cues of predators when selecting cavities for roosting. PLoS One 13:e0203269. doi: 10.1371/journal.pone.0203269
Bai, M.-L., Wichmann, F., and Mûhlenberg, M. (2005). Nest-site characteristics of hole-nesting birds in a primeval boreal forest of Mongolia. Acta Ornithol. 40, 1–14. doi: 10.3161/068.040.0105
Blanc, L. A., and Martin, K. (2012). Identifying suitable woodpeckers nest trees using decay selection profiles in trembling aspen (Populus tremuloides). Forest Ecol. Manag. 286, 192–202. doi: 10.1016/j.foreco.2012.08.021
Blanc, L. A., and Walters, J. R. (2008). Cavity nest-webs in a longleaf pine ecosystem. Condor 110, 80–92. doi: 10.1525/cond.2008.110.1.80
Cabe, P. R. (2020). “European starling Sturnus vulgaris, version 1.0,” in Birds of the World, ed. S. M. Billerman (Ithaca, NY: Cornell Lab of Ornithology).
Cockle, K. L., Martin, K., and Wesołowski, T. (2011). Woodpeckers, decay, and the future of cavity-nesting vertebrate communities worldwide. Front. Ecol. Environ. 9, 377–382. doi: 10.1890/110013
Cockle, K. L., Trzcinski, M. K., Wiebe, K. L., Edworthy, A. B., and Martin, K. (2019). Lifetime productivity of tree cavities used by cavity-nesting animals in temperate and subtropical forests. Ecol. Appl. 29, e01916.
Dobkin, D. S., Rich, A. C., Pretare, J. A., and Pyle, W. H. (1995). Nest-site relationships among cavity-nesting birds of riparian and snowpocket aspen woodlands in the northwestern Great Basin. Condor 97, 694–707. doi: 10.2307/1369178
Edworthy, A. B., and Martin, K. (2014). Long-term dynamics of the characteristics of tree cavities used for nesting by vertebrates. Forest Ecol. Manag. 334, 122–128. doi: 10.1016/j.foreco.2014.09.001
Edworthy, A. B., Trzcinski, M. K., Cockle, K. L., Wiebe, K. L., and Martin, K. (2018). Tree cavity occupancy by nestling vertebrates across cavity age. J. Wildl. Manag. 82, 639–648. doi: 10.1002/jwmg.21398
Edworthy, A. B., Wiebe, K. L., and Martin, K. (2012). Survival analysis of a critical resource for cavity-nesting communities: patterns of tree cavity longevity. Ecol. Appl. 22, 1733–1742. doi: 10.1890/11-1594.1
Ekner, A., and Tryjanowski, P. (2008). Do small hole nesting passerines detect cues left by a predator? A test on winter roosting sites. Acta Ornithol. 43, 107–111. doi: 10.3161/000164508x345392
Fisher, R. J., and Wiebe, K. L. (2006a). Breeding dispersal of northern flickers in relation to natural nest predation and experimentally increased perception of predation risk. Ibis 148, 772–781. doi: 10.1111/j.1474-919x.2006.00582.x
Fisher, R. J., and Wiebe, K. L. (2006b). Nest site attributes and temporal patterns of northern flicker nest loss: effects of predation and competition. Oecologia 147, 744–753. doi: 10.1007/s00442-005-0310-2
Gibbons, P., Lindenmayer, D. B., and Barry, S. C. (2002). Hollow selection by vertebrate fauna in forests of southeastern Australia and implications for forest management. Biol. Cons. 103, 1–12. doi: 10.1016/s0006-3207(01)00109-4
Hayward, G. D., and Rosentreter, R. (1994). Lichens as nesting material for northern flying squirrels in the northern Rocky Mountains. J. Mammology 75, 663–673. doi: 10.2307/1382514
Hebda, G., Pochrzast, K., Mitrus, S., and Wesolowski, T. (2013). Disappearance rates of old nest material from tree cavities: an experimental study. Scand. J. Forest Res. 28, 445–450. doi: 10.1080/02827581.2013.783100
Hebda, G. A., Kandziora, A., and Mitrus, S. (2017). Decomposition of nest material in tree holes and nest-boxes occupied by European starlings Sturnus vulgaris: an experimental study. Acta Ornithol. 52, 119–125. doi: 10.3161/00016454ao2017.52.1.011
Koch, A., Munks, S., and Driscoll, D. (2008). The use of hollow-bearing trees by vertebrate fauna in wet and dry Eucalyptus obliqua forest, Tasmania. Wildl. Res. 35, 727–746.
Lindenmayer, D. B., Cunningham, R. B., Donnelly, C. F., Tanton, M. T., and Nix, H. A. (1993). The abundance and development of cavities in Eucalyptus trees: a case study in the montane forests of Victoria, southeastern Australia. Forest Ecol. Manag. 60, 77–104. doi: 10.1016/0378-1127(93)90024-h
Lindenmayer, D. B., and Wood, J. T. (2010). Long-term patterns in the decay, collapse, and abundance of trees with hollows in the mountain ash (Eucalyptus regnans) forests of Victoria, southeastern Australia. Can. J. Forest Res. 40, 48–54. doi: 10.1139/x09-185
Loye, J. E., and Carroll, S. P. (1998). Ectoparasite behavior and its effects on avian nest site selection. Ann. Ent. Soc. Am. 91, 159–163. doi: 10.1093/aesa/91.2.159
Martin, K., Aitken, K. E. H., and Wiebe, K. L. (2004). Nest sites and nest webs for cavity-nesting communities in interior British Columbia, Canada: nest characteristics and niche partitioning. Condor 106, 5–19. doi: 10.1093/condor/106.1.5
Mazgajski, T. D. (2007a). Effect of old nest material on nest site selection and breeding parameters in secondary hole nesters–a review. Acta Ornithol. 42, 1–14. doi: 10.3161/068.042.0107
Mazgajski, T. D. (2007b). Nest hole age decreases nest site attractiveness for the European starling Sturnus vulgaris. Ornis Fenn. 84, 32–38.
Mazgajski, T. D. (2013). Nest site preparation and reproductive output of the European starling (Sturnus vulgaris). Avian Biol. Res. 6, 119–126. doi: 10.3184/175815513x13612057571673
Merino, S., and Potti, J. (1995). Pied flycatchers prefer to nest in clean nest boxes in an area with detrimental nest ectoparasites. Condor 97, 828–831. doi: 10.2307/1369195
Mönkkönen, M., Forsman, J. T., Kananoja, T., and Ylönen, H. (2009). Indirect cues of nest predation risk and avian reproductive decisions. Biol. Lett. 5, 176–178. doi: 10.1098/rsbl.2008.0631
Newton, I. (1994). The role of nest sites in limiting the numbers of hole-nesting birds: a review. Biol. Cons. 70, 265–276. doi: 10.1016/0006-3207(94)90172-4
Pakkala, T., Tiainen, J., and Kouki, J. (2017). The importance of nesting cavity and tree reuse in the three-toed woodpecker Picoides tridactylus in dynamic forest landscapes. Ann. Zool. Fenn. 54, 175–191. doi: 10.5735/086.054.0116
Pakkala, T., Tianen, J., Piha, M., and Kouki, J. (2018). How important are nest cavities made by the three-toed woodpecker Picoides tridactylus for cavity-nesting forest bird species? Acta Ornithol. 53, 69–79. doi: 10.3161/00016454AO2018.53.1.007
Patterson, J. E. H., Patterson, S. J., and Malcolm, J. R. (2007). Cavity nest materials of northern flying squirrels Glaucomys sabrinus and North American red squirrels Tamiasciurus hudsonicus in a secondary hardwood forest of southern Ontario. Can. Field Nat. 121, 303–307.
Raphael, M. G., and White, M. (1984). Use of snags by cavity-nesting birds in the Sierra Nevada. Wildl. Monogr. 86, 1–66.
Remm, J., Lohmus, A., and Remm, K. (2006). Tree cavities in riverine forests: what determines their occurrence and use by hole-nesting passerines? Forest Ecol. Manage. 221, 267–277. doi: 10.1016/j.foreco.2005.10.015
Rendell, W. B., and Verbeek, N. A. M. (1996). Are avian ectoparisites more numerous in nest boxes with old nest material? Can. J. Zool. 74, 1819–1825. doi: 10.1139/z96-203
Sedgwick, J. A., and Knopf, F. L. (1992). Cavity turnover and equilibrium cavity densities in a cottonwood bottomland. J. Wildl. Manag. 56, 477–484.
Wesołowski, T. (2000). What happens to old nests in natural cavities? Auk 117, 498–500. doi: 10.1093/auk/117.2.498
Wesołowski, T. (2001). High ectoparasite loads in hole-nesting birds: a nestbox bias? J. Avian Biol. 32, 281–285. doi: 10.1111/j.0908-8857.2001.320313.x
Wesołowski, T. (2011). Lifespan of woodpecker-made holes in a primeval temperate forest: a thirty year study. Forest Ecol. Manag. 262, 1846–1852. doi: 10.1016/j.foreco.2011.08.001
Wiebe, K. L. (2001). Microclimate of tree cavity nests: is it important for reproductive success in northern flickers? Auk 118, 412–421. doi: 10.1093/auk/118.2.412
Wiebe, K. L. (2004). Innate and learned components of defence by flickers against a novel nest competitor, the European starling. Ethology 110, 779–791. doi: 10.1111/j.1439-0310.2004.01016.x
Wiebe, K. L. (2009). Nest excavation does not reduce harmful effects of ectoparasitism: an experiment with a woodpecker, the northern flicker Colaptes auratus. J. Avian Biol. 50, 166–172. doi: 10.1111/j.1600-048X.2009.04481.x
Wiebe, K. L. (2011). Nest sites as limiting resources for cavity-nesting birds in mature forest ecosystems: a review of the evidence. J. Field Ornith. 82, 239–248. doi: 10.1111/j.1557-9263.2011.00327.x
Wiebe, K. L., Koenig, W. D., and Martin, K. (2006). Evolution of clutch size in cavity-excavating birds: the nest site limitation hypothesis revisited. Am. Nat. 167, 343–353. doi: 10.1086/499373
Keywords: cavity-nester, tree cavity, woodpecker, nest reuse, competition
Citation: Wiebe KL, Cockle KL, Trzcinski MK, Edworthy AB and Martin K (2020) Gaps and Runs in Nest Cavity Occupancy: Cavity “Destroyers” and “Cleaners” Affect Reuse by Secondary Cavity Nesting Vertebrates. Front. Ecol. Evol. 8:205. doi: 10.3389/fevo.2020.00205
Received: 27 January 2020; Accepted: 04 June 2020;
Published: 26 June 2020.
Edited by:
Patrick S. Fitze, National Museum of Natural Sciences (MNCN), SpainReviewed by:
Gustavo Tomás, Estacion Experimental de Zonas Aridas (EEZA), SpainMartjan Lammertink, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) Diamante, Argentina
Copyright © 2020 Wiebe, Cockle, Trzcinski, Edworthy and Martin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karen L. Wiebe, a2FyZW4ud2llYmVAdXNhc2suY2E=
†ORCID: Karen L. Wiebe, orcid.org/0000-0002-3148-8278
 Karen L. Wiebe
Karen L. Wiebe Kristina L. Cockle2,3
Kristina L. Cockle2,3 Amanda B. Edworthy
Amanda B. Edworthy Kathy Martin
Kathy Martin