- 1Department of Natural Resources and Environmental Science, University of Nevada, Reno, Reno, NV, United States
- 2Mojave National Preserve, National Park Service, Barstow, CA, United States
- 3Sierra Nevada Bighorn Sheep Recovery Program, California Department of Fish and Game, Bishop, CA, United States
Large herbivores exhibit relatively slow-paced life histories, and allocate resources toward maintaining high rates of adult survival, while juvenile survival has greater variability. Maternal females make decisions throughout life stages of reproduction to meet their nutritional demands while simultaneously ensuring survival and recruitment of young to maximize fitness. We investigated tradeoffs associated with resource selection by mule deer (Odocoileus hemionus) surrounding stages of reproduction in Mojave National Preserve, CA, United States. To understand potential tradeoffs associated with offspring survival and maternal nutritional condition, we measured differences in patterns of resource selection among pre-parturient females, females provisioning young, and females following the loss of young. The third trimester of gestation and lactation are considered the most nutritionally demanding stages of reproduction. We hypothesized that energetic costs would change rapidly throughout those stages of reproduction, especially after the loss of an offspring. Further, we hypothesized that lactating females would balance the acquisition of nutritional sources with safety of young. We used radio-collar and randomly generated locations to model resource selection in a hierarchical approach utilizing machine learning algorithms and traditional resource selection functions (RSFs). We also monitored recruitment of young born to GPS-collared females using VHF radio-collars equipped with mortality indicators. During all three stages of reproduction, adult females selected greater NDVI, less rugged terrain, areas close to water (especially while provisioning offspring), and higher elevations. Selection for greater levels of NDVI was stronger pre-parturition and following the loss of offspring compared to when females were provisioning offspring. We also observed high variation toward the selection of NDVI among individual females while provisioning young, which was less pronounced during the other reproductive stages. Offspring survival during our study was positively associated with females that selected greater levels of NDVI. Further, we were not able to detect a tradeoff between safety of young (ruggedness) and nutrient acquisition (NDVI). Perhaps predation risk and nutritional resources are not mutually exclusive in this ecosystem; and, females may be able to balance reproductive investment with the ability to select for water and nutrition while simultaneously ensuring lower risk of predation for themselves and their offspring.
Introduction
Life-history strategies and associated decisions are tightly linked with survival and reproductive success, which are directly linked to population performance (Clutton-Brock and Sheldon, 2010; Bonte and Dahirel, 2017). Variation in life-history strategies among species result from selective pressures caused by a variety of factors including: climatic stochasticity, predation, disease, or food availability (Ricklefs and Wikelski, 2002). Animals exhibit variation in pace of their life-histories; specifically, the amount of investment to the care of young versus survival or future reproduction during the reproductive cycle (Clutton-Brock and Sheldon, 2010). Species with fast-paced life histories typically invest time and energy into producing many young in a short lifetime (Promislow and Harvey, 1990). In contrast, species that exhibit a slow-paced life history typically invest a larger proportion of resources into longer gestation, greater investment in individual offspring, and successful recruitment of young over a longer lifetime (Van Noordwijk and de Jong, 1986).
Animals are commonly forced to make tradeoffs to maximize certain demographic parameters to enhance overall fitness (Ricklefs, 1994). Whether or not individuals are forced to make those reproductive tradeoffs is often driven by somatic reserves or availability of nutritional resources on the landscape. When a large amount of maternal time and effort is allocated to producing and recruiting young, somatic reserves can be greatly diminished, and availability of resources on the landscape may determine if reproduction will be successful (Stearns, 1989). If females are in a poor nutritional state with limited opportunity to successfully reproduce, species with fast-paced life histories may be forced to trade off their own survival for reproduction. Conversely, species that exhibit slow-pace of life strategies are more likely to tradeoff current for future reproductive effort rather than trading survival. Both strategies have the goal of maximizing reproductive fitness over their lifetime (Williams, 1966; Stearns, 1989; Gaillard et al., 1998, 2000).
Large, herbivorous mammals exhibit a slow pace of life strategy, and individuals in poor nutritional condition may tradeoff investment in current reproduction for future reproductive success (Clutton-Brock et al., 1983). Nevertheless, maternal females that consistently maintain a higher nutritional plane between breeding seasons rarely exhibit signs of reproductive costs and limitations on opportunities for future reproduction (Hamel et al., 2009). Conversely, females occupying nutritionally limiting environments, or individuals unable to maintain a continuous high plane of nutrition are often tightly linked to environmental stochasticity, especially relative to forage availability to drive year-to-year reproductive success (Therrien et al., 2008; Monteith et al., 2014; Heffelfinger et al., 2018). Even in instances where females are not nutritionally limited, an additional cost to reproduction is keeping young safe from predation by maintaining vigilance and using areas that are safer for young (White and Berger, 2001). Thus, in areas where current and future reproductive costs may be more dependent on landscape level or environmental attributes (arid environments, harsh winters, etc.), maternal females may be faced with decisions about selection or use of resources that directly affect their ability to care and provision young, while attempting to maintain a high enough plane of nutrition to invest in future reproduction.
Animal location data can be used to investigate patterns of resource use on the landscape at both the individual and population levels (Johnson, 1980; Manly et al., 2007). Those patterns can, in turn, reflect behavioral influence on acquisition and allocation of resources toward survival and reproductive success. Selection of high-quality resources can directly result in higher fitness for an animal regardless of their life-history strategy. Therefore investigation into selection of resources is assumed to be tied directly to reproductive fitness (Kawecki and Stearns, 1993; Aldridge and Boyce, 2007; Dzialak et al., 2011). The stages of reproduction, however, induce different limitations and requirements on the nutritional state or potential to increase fitness of individuals (Barboza et al., 2009). For example, adults may select habitats with lower quality food resources if the habitat affords offspring increased protection from predators (White and Berger, 2001; Hebblewhite and Merrill, 2009). Conversely, late-gestation and lactation are the most energetically and nutritionally demanding periods throughout the life-cycle of females, and selection of resources should be directed toward those with the greatest nutrition (Barboza et al., 2009). Therefore, understanding resource selection during reproductive stages such as gestation, early provisioning of young (lactation), and post-provisioning (following juvenile mortality), may prove to be important in understanding potential tradeoffs throughout the reproductive cycle (McLoughlin et al., 2006). A sudden shift to a non-provisioning state from the energetically costly state of lactation (via loss of offspring) may result in a shift in behavior and resource use. With the loss of an offspring, females likely shift to a strategy of resource selection that maximizes body condition to recover from the high demands of lactation prior to the start of the next reproductive cycle. Further, adult females must also maintain vigilance to mitigate predation risk among variable nutritional constraints throughout the reproductive life stages (Cristescu et al., 2019). Few studies, however, have explored differences in resource selection associated with specific stages of reproduction using location data (Barten et al., 2001; Long et al., 2009; Shuman et al., 2018). Even fewer studies have attempted to assess the fitness consequences (e.g., individual probability of successful recruitment of young into the population) resulting from variation in selection of resources between reproductive stages.
Large, herbivorous mammals typically exhibit high and relatively stable survival rates of adults throughout their geographical range (Bishop et al., 2009; Hurley et al., 2011; Bender et al., 2012; Monteith et al., 2014). Therefore, population performance of ungulates is most often regulated by successful reproduction and recruitment of young (Gaillard et al., 1998, 2000). We used a non-migratory population of mule deer (Odocoileus hemionus) as a representative species of large herbivores to test how selection of resources varies during different stages of reproduction as a result of tradeoffs between nutritional requirements for the mother and safety of offspring. Our objective was to identify factors that influence space use of individual females and selection of resources within their annual home range, indicated by landscape characteristics, during three stages of reproduction, (1) late gestation and just prior to parturition, (2) while the maternal female is provisioning young (i.e., during lactation), and (3) after an abrupt halt in allocating resources to young (i.e., following mortality of offspring). Further, we seek to evaluate how selection of resources by females while provisioning young may influence the chance of successfully recruiting their young into the population. Previous work in our study area indicates that individuals select landscape features, including areas close to water and at high elevations, typical for mule deer populations occupying arid environments prior to parturition (McKee et al., 2015). We hypothesized that female mule deer trade off high-quality resources for safety of offspring, by selecting areas within their annual home range that are more conducive to survival and recruitment of offspring. Therefore, we predicted that females with dependent young select and occupy areas more suitable to the safety of young compared to selection of resources prior to parturition. Further, we hypothesized that after a sudden transition to a non-reproductive state following loss of offspring, mule deer selected resources suitable for recovery of nutrient stores to improve nutritional condition prior to the next reproductive cycle.
Materials and Methods
Study Site
Mojave National Preserve is located in San Bernardino County, in southeastern California, United States (35° 00′ N 115° 28′ W). Mojave National Preserve covers nearly 650,000 ha of extensive bajadas and playas in the valley floors between rugged mountain ranges of granite, basalt, and igneous rock (McKee et al., 2015). Elevation ranges from 270 m in the valleys to 2417 m at the peak of Clark Mountain. Vegetative communities in Mojave National Preserve vary by elevation and with temperature and precipitation (National Park Service, 2017). Vegetative assemblages represent typical Mojave Desert ecosystems with small influences of Great Basin and Sonoran Desert vegetation in transition zones (McKee et al., 2015; National Park Service, 2017).
Mojave National Preserve has high temperatures during the summer months and the precipitation pattern is bi-modal, with peaks during summer and winter (McKee et al., 2015). Mean annual precipitation at mid- to upper elevations is 18 cm (SD = 26; 1992-present, Meso West Weather Station, Operated by the University of Utah, Salt Lake City) and 9.3 cm (SD = 12) at low elevations (1980-present, Soda Springs, northern Mojave National Preserve). Mean maximum temperatures during the winter are 19 and 13°C, and 40.5 and 33°C during the summer at low and high elevations respectively.
We established three study sites that best characterized suitable habitat based on movements of adult female mule deer from a previous study (Figure 1; McKee et al., 2015). The New York Mountains study site is 27,195 ha and has four permanent water sources. The New York Mountains study site consists of steep, rocky pinyon-juniper woodland (Pinus monophylla and Juniperus osteosperma) in the upper elevations, a scrub live oak (Quercus turbinella) and bitterbrush (Purshia glandulosa) shrubland in mid-elevations, and yucca (Yucca schidigera) and creosote (Larrea tridentata) in desert shrublands at lower elevations. The Midhills study site consists of 39,368 ha with 19 water sources, and experienced an extensive wildfire in 2005. The burned portion of the study site is currently dominated by globemallow (Sphaeralcea spp.), bitterbrush, and desert almond (Prunus fasciculata) within the rolling hills but still has patches of unburned Great Basin sagebrush (Artemisia tridentata) and pinyon-juniper woodland in the upper elevations (McKee et al., 2015). Cima Dome, the third study area, consists of 40,404 ha, has seven permanent water sources with little elevation change, and is dominated by Joshua tree (Yucca brevifolia) woodland, blackbrush (Coleogyne ramosissima), bitterbrush, creosote bush, and sparse patches of juniper (Figure 1; McKee et al., 2015).
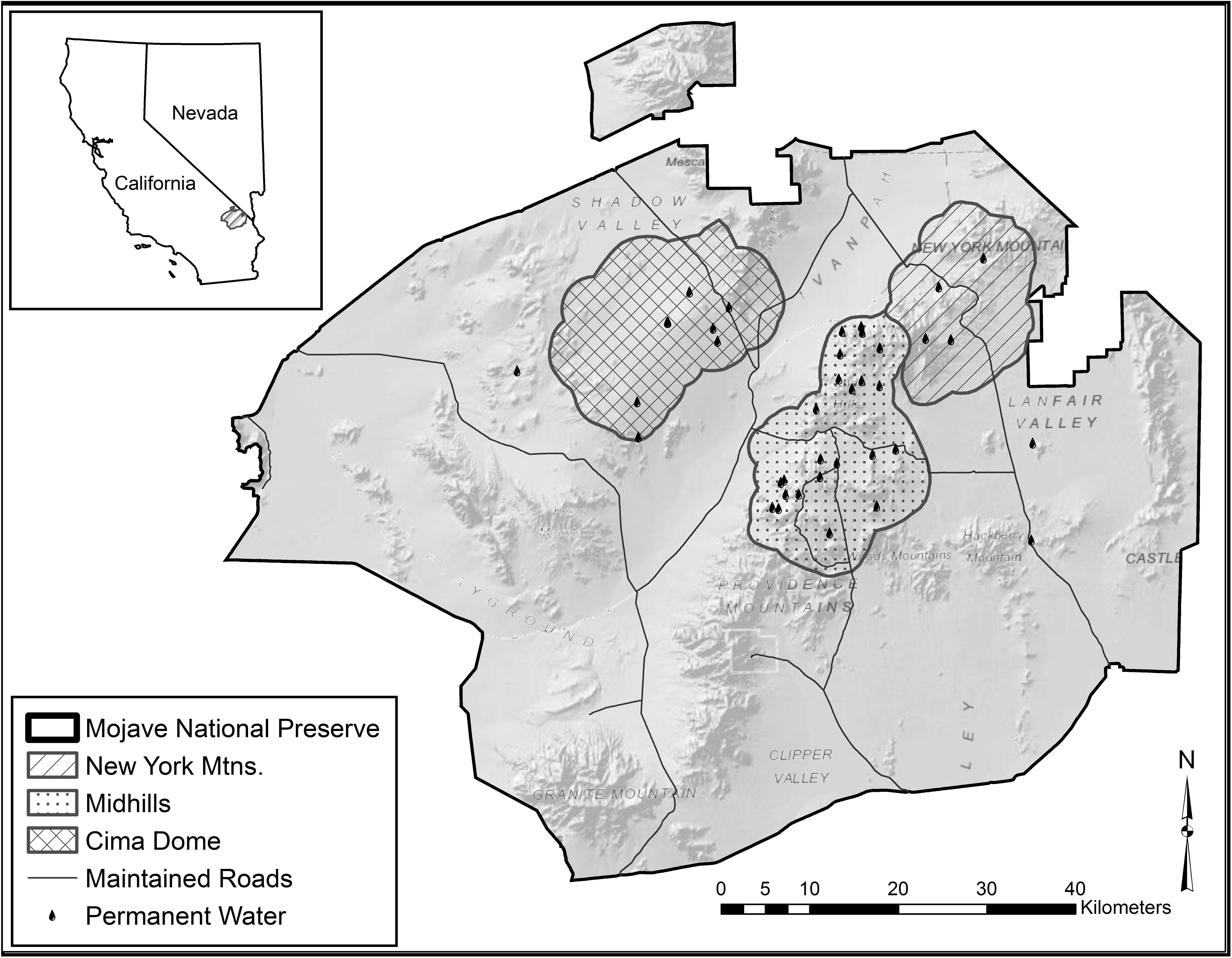
Figure 1. Map of the Mojave National Preserve, CA, United States, with study area delineations and permanent water occurrences exhibited in the Mojave Desert. Inset map shows location relative to Nevada and California, United States (shaded). Figure recreated from McKee et al. (2015).
Field Data Collection
In late February or early March from 2013 to 2016, we captured adult female mule deer via net gun from a helicopter (Krausman et al., 1985). Only one female was captured from each observed social group encountered to maintain independence of sampling. Each deer was brought to a central processing station where they were marked with uniquely colored and numbered ear tags and fitted with GPS radio collars programmed to collect one location every 90 min (collar model G2110D, Advanced Telemetry Systems, Isanti, MN, United States). After each adult female was processed we released them from the central processing station. Movement data were censored for the first 2 weeks following capture to discount the effects of handling. We programmed radio collars to drop off about 1 year after deployment; collars also had a mortality switch with a Very High Frequency (VHF) transmitter so ground crews could locate collars following a mortality event or after the collar was dropped.
We used ultrasonography to determine pregnancy status for each adult female (Stephenson et al., 1995, 2002). A vaginal implant transmitter (VIT, model M3930L, Advanced Telemetry Systems, Isanti, MN, United States) was inserted into the birth canal of all females that were pregnant (Bishop et al., 2007). We used modified VITs described by Bishop et al. (2011) that were also equipped with a temperature sensor, a photo sensor, and Precise Event Timing (PET) as described by Carstensen et al. (2003), Bishop et al. (2007), and Heffelfinger et al. (2018). When data collection was completed, individuals were then released from the central processing station, or if moved more than 4 km from the capture site, were returned to the original capture location and released.
Concurrent with this study, juvenile survival was investigated with 110 neonates that were captured, collared, and monitored every 1–3 days for survival (see Heffelfinger et al., 2018 for a more detailed description of neonate handling methodology). Using the PET coding from each VIT, used to capture a neonate, timing of parturition for the mother was known. For those juveniles caught in instances where the PET coding failed (n = 44), parturition date was estimated using at least two of the following indices: neonate’s hoof condition, behavior, umbilicus condition, size, and date the mother was last known to be pregnant (Haugen and Speake, 1958; Haskell et al., 2007; Monteith et al., 2014). Juveniles were monitored via telemetry following capture. Thus, the date of mortality for each neonate was known and was then linked with GPS data for the associated maternal female.
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Nevada, Reno (IACUC Protocol #: 00538) and were in keeping with guidelines established by the American Society of Mammologists for research on wild mammals (Sikes, 2016). We also complied with capture and handling procedures developed by the California Department of Fish and Wildlife.
Dataset Preparation
Our ability to assess resource selection during various stages of reproduction was dependent on capturing and monitoring neonates from collared adults. Thus, for this analysis, we used locations of collared females with associated captured and marked neonates from 2013 to 2016. We eliminated any major outlying points associated with short-term, exploratory movements by visually evaluating locations in ArcGIS (Moen et al., 1997; ArcGIS 10.3, Environmental Systems Research Institute, Redlands, CA, United States). We calculated annual kernel density estimates with a 99.9% isopleth using Geospatial Modeling Environment (Beyer, 2015). Those annual home ranges were used to quantify availability of resources within the home range (i.e., 3rd order selection, Johnson, 1980). We buffered the estimated kernel by the average movement between points from marked individuals (200 m; SD = 196.63) to prevent exclusion of space that was available to study animals beyond the outermost used locations in the 99.9% kernel. This method of defining the available space familiar to females resulted in a mean kernel estimate of availability of 24.34 km2 (SD = 16.48; Supplementary Figure S1). Spatial familiarity regarding resources within an animal’s annual home range can influence demographic parameters (Forrester et al., 2015; Gehr et al., 2020). Thus, we used those estimates of annual home ranges as our designation for availability in order to draw inference in how maternal females select areas for reproduction from a spatial area in which they are familiar. Thus, our goal was to understand what features in individual home ranges influenced resource selection throughout phases of reproduction.
We separated locations from each individual into time periods associated with pre-parturition, provisioning of young, and for some individuals, post-juvenile mortality. We classified locations using the following criteria. For pre-parturition, we included locations 30 days prior to the date of parturition for each individual from which we had captured a neonate. We expected that individuals would most likely exhibit behaviors associated with the preparation of giving birth during this timeframe that could be compared with the subsequent periods of interest. For the timeframe of provisioning young, locations were included from parturition to either the time of juvenile mortality or through 30 days of provisioning young. We used 30 days post-parturition, because the quantity of milk produced and frequency of nursing drastically diminishes after 30 days, and resource requirements for the mother decline substantially (Sadlier, 1980; Gauthier and Barrette, 1985). Thus, to compare the change from extreme provisioning of resources to no longer allocating resources to offspring, we only included individuals who had lost young prior to 30 days post-parturition for our post-juvenile mortality period. Inherently, our strict rule of only including females who lost young within the first 30 days resulted in a lower sample size of individuals, but the first 30 days is when female investment in provisioning young is greatest and most appropriate to test our hypothesis.
Our movement data set consisted of many unique maternal females throughout the 4 years of the study within reproductive timeframes (pre-parturition, provisioning young, and post-juvenile mortality). We obtained movement data during the pre-parturition timeframe for 12 females in 2013, 15 females in 2014, 19 females in 2015, and 22 females in 2016 (n = 68; 32,297 locations). During the provisioning young timeframe, we obtained movement data for 12 females in 2013, 15 females in 2014, 18 females in 2015, and 22 females in 2016 (n = 67; 26,655 locations). Lastly, during the post-juvenile mortality timeframe we gathered location data for 7 females in 2013, 8 females in 2014, 3 females in 2015, and 2 females in 2016 (n = 20; 9,214 locations).
We included multiple landscape characteristics, shown to be important in selection of resources by mule deer (McKee et al., 2015), as covariates to identify patterns of resource selection during the three time periods (Table 1). Those covariates included distance to the nearest water source (m), elevation (m), slope (%), tree cover (%), and shrub cover (%) using LANDFIRE remote imagery products (Landfire, 2014). We also estimated a measure of ruggedness with the vector ruggedness metric (VRM; Sappington et al., 2007) and a transformation of aspect by cosine (north-south) and sine function (east-west), which was calculated from a Digital Elevation Model (DEM) of the study area (Landfire, 2014).
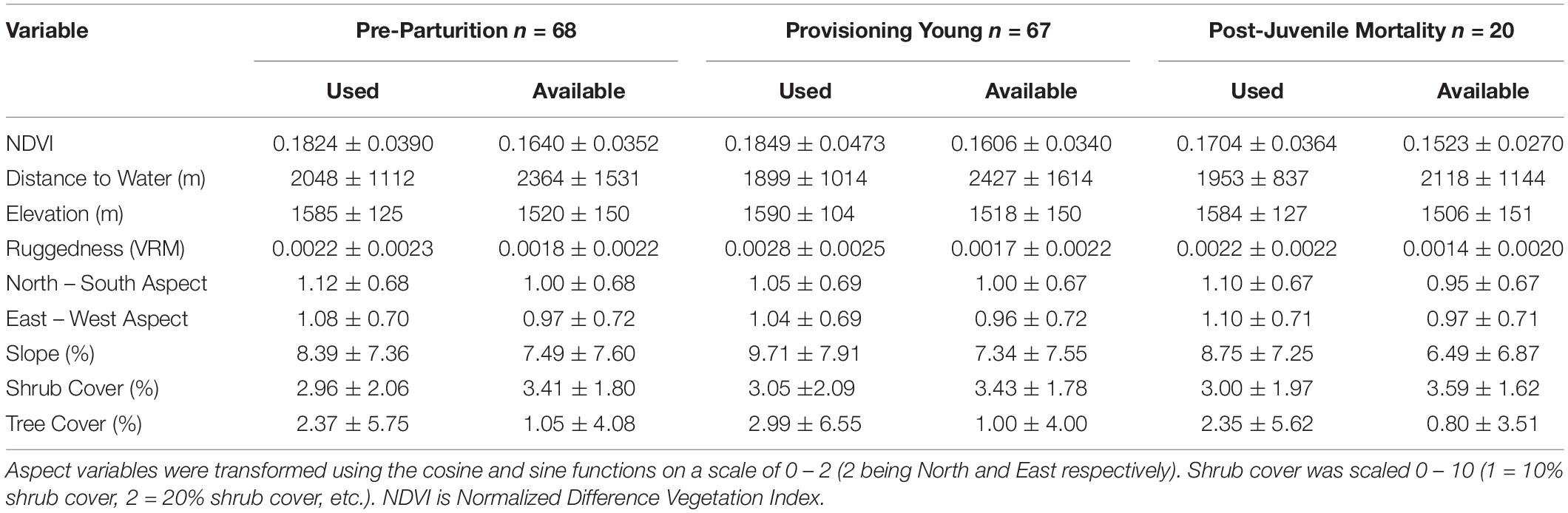
Table 1. Descriptive statistics (mean ± SD) of available (random) and used (mule deer locations) data points for individuals in each reproductive time period on the Mojave National Preserve, California, United States, 2013–2016.
We retrieved monthly Normalized Difference Vegetation Index (NDVI) values for the duration of the study from Landsat 8 imagery to include as a covariate to assess selection based on vegetation phenology (Climate Engine, 2017). The Mojave National Preserve has very little hardwood canopy cover. Thus, NDVI is a relative measure of both shrub and annual forb “greenness.” Finally, we retrieved land cover data to assign dominant vegetative communities throughout the study area using the United States Geological Survey (USGS) Central Mojave Vegetation Database derived in 2011 and amended in 2014 (Thomas et al., 2004; McKee et al., 2015). Dominant vegetation types were divided into the following categories: juniper wooded shrubland, Joshua tree wooded shrubland, pinyon wooded shrubland, Mojave yucca shrubland, desert wash, low elevation blackbrush-creosote shrubland, big-sagebrush shrubland, and burn area from the Hackberry Complex fire in 2005. Collinearity was assessed for all predictor variables using a correlation matrix in R (3.3.2, R Core Team) and we did not include any variables in the same model if highly correlated (| r | > 0.65 with one another; Stewart et al., 2002; Long et al., 2014).
We determined the number of ‘available’ points to sample randomly within the complete home range for each deer following the sensitivity analysis approach suggested by Renner et al. (2015), which is designed to ensure an appropriate sample size of background points such that resource selection coefficients derived from logistic regression (e.g., generalized linear mixed-effects model; Gillies et al., 2006) matches the ‘selection intensity’ coefficients derived from a point-process modeling approach (Warton and Shepherd, 2010; Renner et al., 2015). Specifically, we first fitted down-weighted Poisson regression (DWPR) models for each individual deer using the full set continuous and categorical covariates hypothesized to influence resource selection (see below) to a wide range of alternative background point densities (‘glm’ function in R). We varied background point densities from 50 to 2500 points per km2, repeating the sampling and fitting algorithm 25 times across 8 different background point densities. We then identified the approximate threshold beyond which model performance (log likelihood) became insensitive to the specific set of background points sampled (‘likelihood convergence’; Renner et al., 2015). Using this method, we found that an average density of 500 random background points per km2 was sufficient for characterizing the distribution of environmental conditions available to each deer in our study (Supplementary Figure S2). Therefore, we constructed our resource selection models (both Random Forest and GLMM; see below) such that each unique deer/status combination was paired with an appropriate number of random background points (500 per km2).
Identifying Variables for Resource Selection by Reproductive Stage
We used Random Forest, a machine learning approach (Breiman, 2001; Cutler et al., 2007; Shoemaker et al., 2018, implemented in the ‘ranger’ package in R), to identify those features on the landscape that were most important in explaining resource selection across our population of female mule deer for each reproductive stage. Those features identified as important by the Random Forest algorithm were used to fit a generalized linear mixed-effects model (GLMM) designed to test for differences among reproductive timeframes (see below). RF is a machine-learning algorithm commonly used by ecologists to perform feature elimination and to discover relationships between a response variable and numerous predictor variables without imposing constraints such as linear responses and interactions (Cutler et al., 2007; Shoemaker et al., 2018). Our RF models (one model for each reproductive status) were fitted using 1000 trees, with each splitting criterion chosen from a sample of 5 (out of 9) predictor variables (we removed the ‘slope’ variable prior to model fitting due to high correlation with ‘ruggedness’). The RF settings were optimized via cross-validation using the ‘caret’ package in R (Kuhn, 2019). We computed the relative importance (RI) of predictor variables as the average degree to which out-of-bag prediction error increased when information about each predictor variable was removed from the analysis (Cutler et al., 2007). Importance rankings therefore account for both the main influence of the predictor on selection as well as inclusion of the variables in identified interactions (Shoemaker et al., 2018). For ease of interpretation we rescaled the reported relative importance values by normalizing them from 0 (least) to 1 (most) important variables. We generated partial dependence plots to visualize univariate relationships for those variables with the highest importance indices (Shoemaker et al., 2018). Finally, we used cross-validation to evaluate how well our RF model built at the individual level would predict resource use at a population scale; instead of the traditional method where each “fold” (n = 3) represented a random subset of the entire dataset (De’ath and Fabricius, 2000), we generated each fold as a subset of individuals from the study.
Testing Differences in Resource Selection by Reproductive Stage
After characterizing important variables and general trends in resource selection across our population via our RF modeling process, we used a generalized linear mixed-effect modeling framework (GLMM, for which we assumed a binomial error distribution and a logit link) to test for differences in resource selection patterns among reproductive stages. To ensure that selection coefficients and interactions terms were generalizable to the population and not an artifact of individual variation in selection patterns (Gillies et al., 2006; Aarts et al., 2013), we included random intercept and slope terms for each unique individual. Specifically, variation in resource selection patterns among individuals was modeled with a random-intercept term and random coefficients for each main effect and interaction term in the full model (Gillies et al., 2006) (analogous to 3rd order selection; Johnson, 1980). The full model included main effect terms for each of the top four environmental gradients identified in the RF analysis (NDVI, elevation, ruggedness, and distance to water; see “Results” section), a main effect term for reproductive stage, and interaction terms for each of these environmental gradients with reproductive stage (testing differences in resource selection patterns by reproductive stage). To ensure resource selection coefficient estimates were unbiased and analogous to coefficients derived from a point-process model (PPM; Warton and Shepherd, 2010), we fixed the random intercept term with a high variance and “infinitely weighted” available vs. used points following Muff et al. (2019).
Effects of Resource Selection on Reproductive Output
After identifying population level patterns in selection of resource (RF modeling) and differences in reproductive timeframes at the individual scale (GLMM tests), we sought to identify a potential tradeoff between juvenile safety (rugged terrain) and nutrition acquisition (NDVI). We tested for a linear relationship between an individual’s selection for ruggedness (individually based selection coefficients (from our GLMM exercise) and the available NDVI within a female’s home range to potentially illustrate a tradeoff. Additionally, we sought to assess influences of habitat selection on increasing an individual’s reproductive output (i.e., successful juvenile recruitment). To test for a relationship between habitat characteristics and reproductive success (juvenile recruitment), we conducted a known-fate analysis using juvenile recruitment data derived from telemetry data which was censored after the first 120 days of life (DeCesare et al., 2016). We summarized the data in a capture history format, with each row representing a unique juvenile and each column representing whether each juvenile was known to be alive or dead during each 1-week interval. Weekly juvenile survival probability was modeled as a logit-linear function of the maternal female’s use of the top three environmental gradients identified in the RF analysis (see above), NDVI, and a logit-normal random intercept term for year. We fitted known-fate models in a Bayesian framework using JAGS (Plummer, 2003), which was called from R using the “jagsUI” package (Kellner, 2019).
Results
Identifying Variables for Resource Selection by Reproductive Stage
In all three reproductive periods, the RF model indicated a clear top four variables that explain resource selection patterns within our system. In order of importance, mule deer tended to select greater values of NDVI, a variation in rugged terrain, areas closer to water sources, and higher elevations, than were available (Figure 2). The order and magnitude of these four variables switched slightly among time periods (Figure 3), with distance to water becoming more important than ruggedness for the provisioning period. Using these identified important variables, directed tests for differences between reproductive timeframes are outlined in our GLMM procedure (below). Predictor variables that did not prove to be especially important in explaining resource selection during our reproductive timeframes included vegetation type, North-South aspect, East-West Aspect, shrub cover, and tree cover (Figure 3). Cross-validation of our RF models resulted in AUC values of 0.65, 0.65, and 0.63 for pre-parturition, provisioning, and post-juvenile mortality stages, respectively (0.96, 0.97, and 0.97 when cross-validated with standard threefold cross-validation). Low cross-validation performance when folds were comprised of entire deer likely reflects substantial variation in resource selection patterns among individuals in our study population (also detected in our GLMM models; Figure 4).
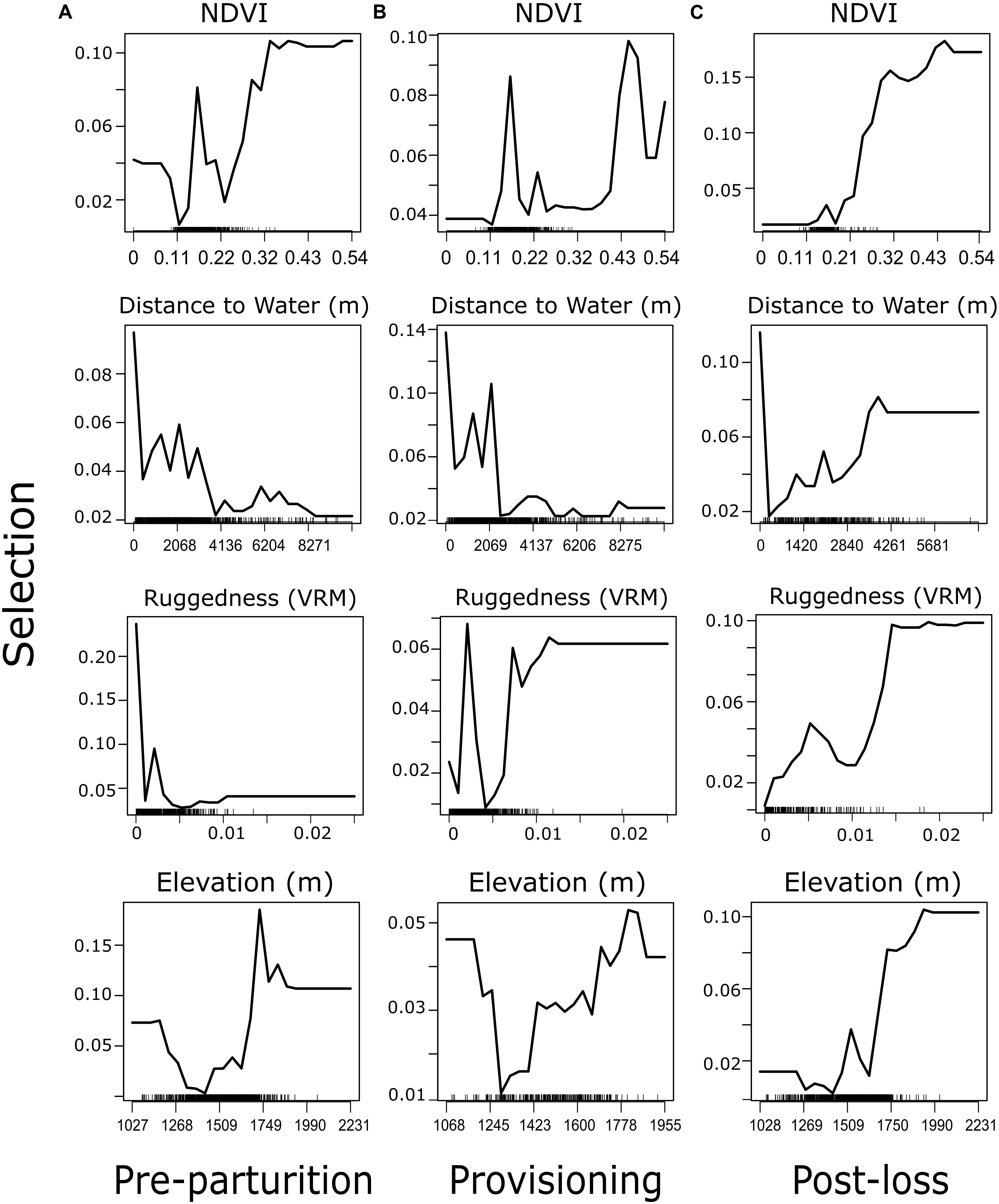
Figure 2. Partial dependence plots of the top four variables explaining resource selection from random forest analysis of female mule deer during (A) pre-parturition (n = 68), (B) while provisioning offspring (n = 67), and (C) post-juvenile mortality (n = 20) on the Mojave National Preserve, California, United States, 2013 – 2016. Selection represents the density of expected occurrences associated with a point on the landscape at the respective value with a point process model (analogous to the probability of being a used location).
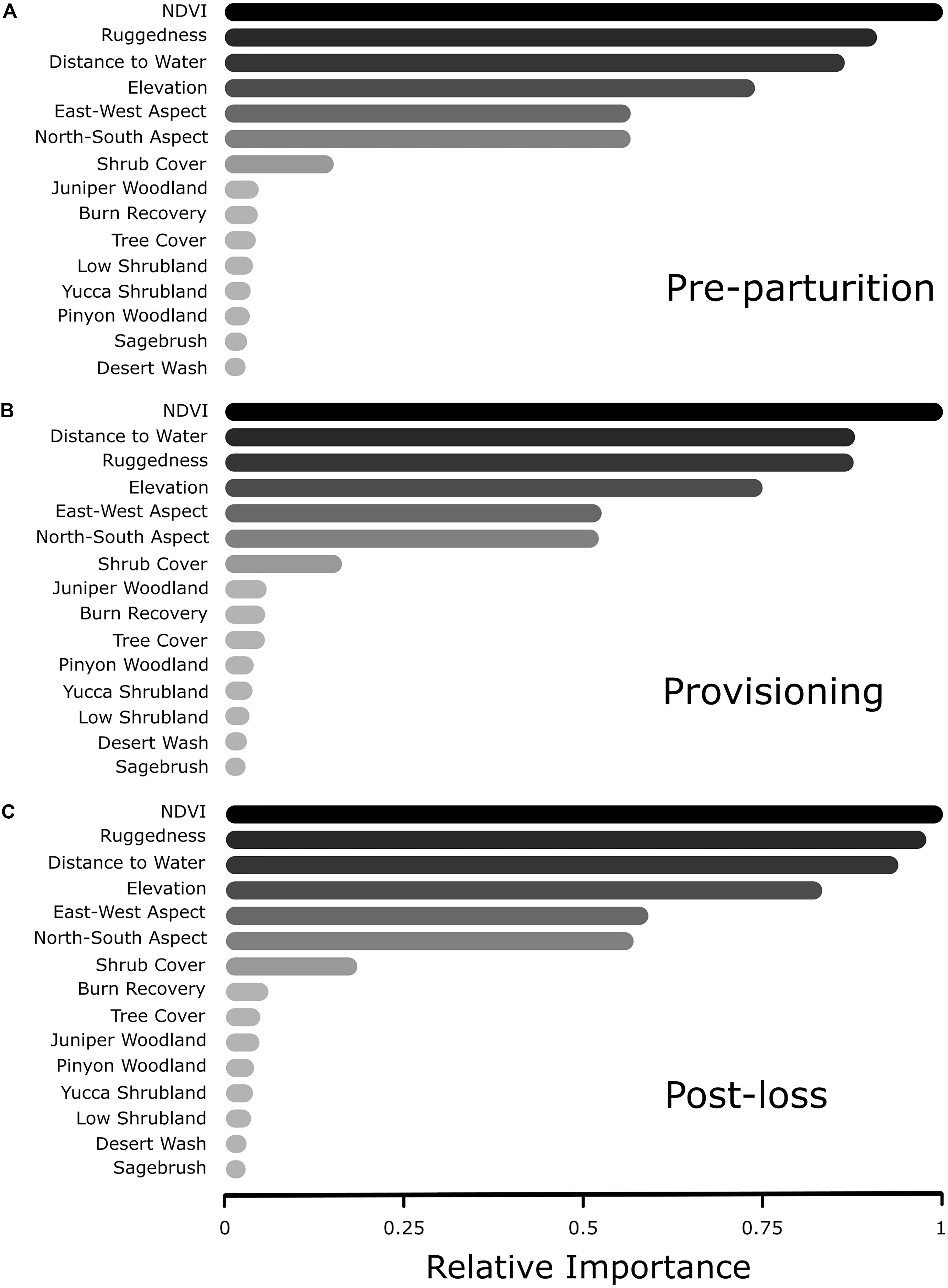
Figure 3. Relative importance rankings of resource selection from random forest analysis of female mule deer during (A) pre-parturition (n = 68), (B) while provisioning offspring (n = 67), and (C) post-juvenile mortality (n = 20) on the Mojave National Preserve, California, United States, 2013 – 2016. Relative importance illustrates the influence of the predictor on resource selection estimated through rigorous conditional inference trees via a random forest algorithm.
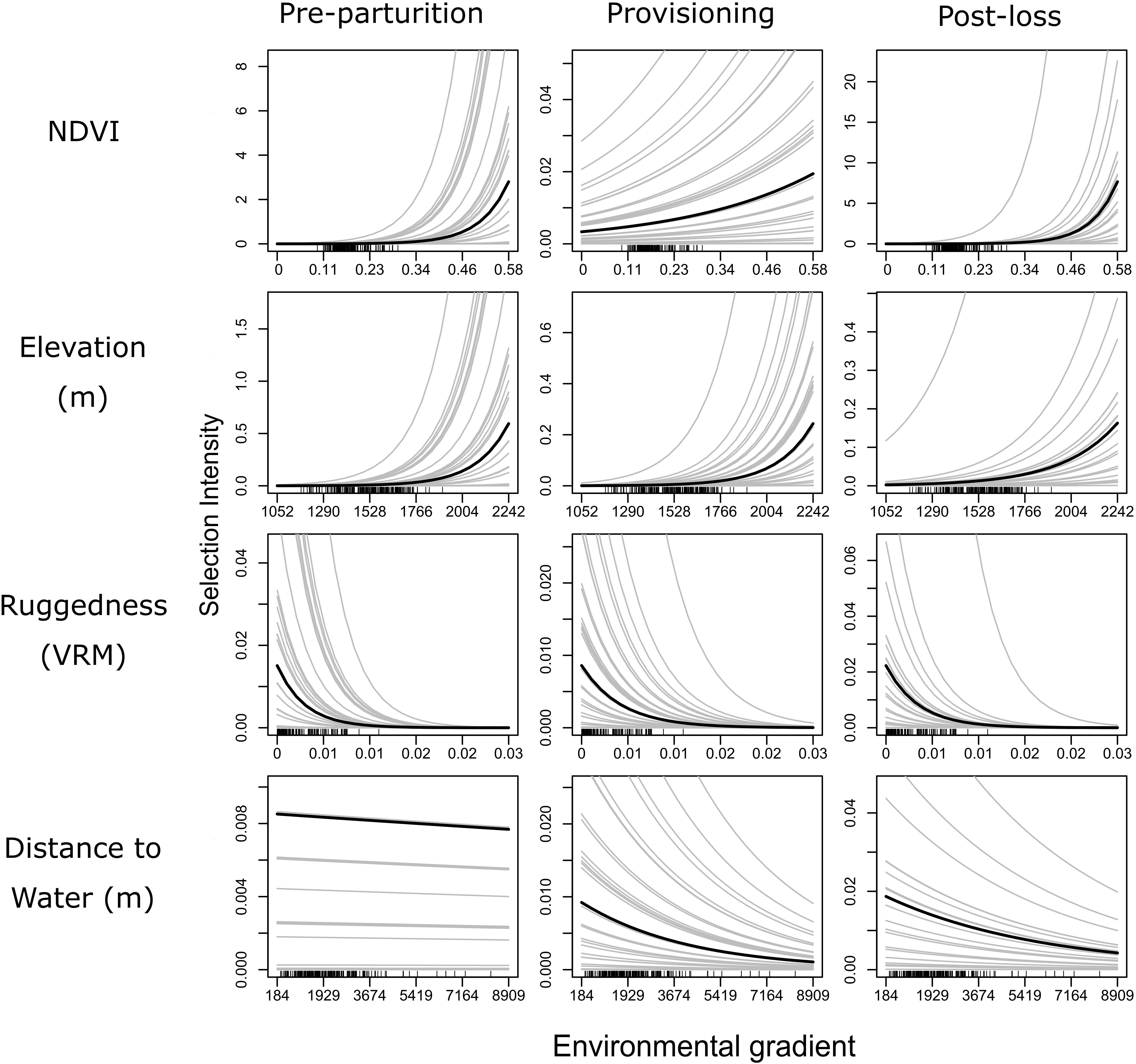
Figure 4. Partial dependence plots illustrating the expected influence of each of four environmental gradients (rows of panel grid) on relative resource selection of female mule deer during pre-parturition (n = 68), while provisioning young (n = 67), and post-juvenile mortality (n = 20) on the Mojave National Preserve, California, United States, 2013 – 2016. Solid black lines indicate population level patterns, while gray lines represent patterns of selection for individual mule deer. All predictions were derived from a GLMM model fitted to GPS collar data, with random intercepts and slope terms fitted to individual deer. This figure illustrates the extreme variation in patterns of selection among individuals and the varying degree of differences in patterns of resource selection among reproductive states.
Testing Differences in Resource Selection by Reproductive Stage
Our GLMM models further confirmed our important variable identification (RF modeling) by demonstrating a selection for greater values of NDVI, less rugged terrain, areas closer to water sources, and higher elevations, than were available (Table 2 and Figure 4). Our test of differences between reproductive stages also indicated that selection coefficients moderately differed by reproductive status along resource gradients (Table 2). Interestingly, the GLMM models suggested a less prominent role for NDVI as a predictor of resource selection while females were provisioning young, though, population level inference indicates general selection for higher NDVI (Table 2 and Figure 4). Our GLMM results also indicate a stronger selection for areas closer to permanent water sources while females were provisioning young (Table 2). Additionally, our GLMM modeling results highlighted the high individual heterogeneity in selection coefficients within our study (Figure 4), which generally exceeded variation by reproductive status (with the exception of NDVI and distance to water). Estimated among-individual heterogeneity in selection, reported as standard deviations on the logit scale, was 0.40 for NDVI, 1.54 for ruggedness, 1.34 elevation, and 1.53 for distance to water.
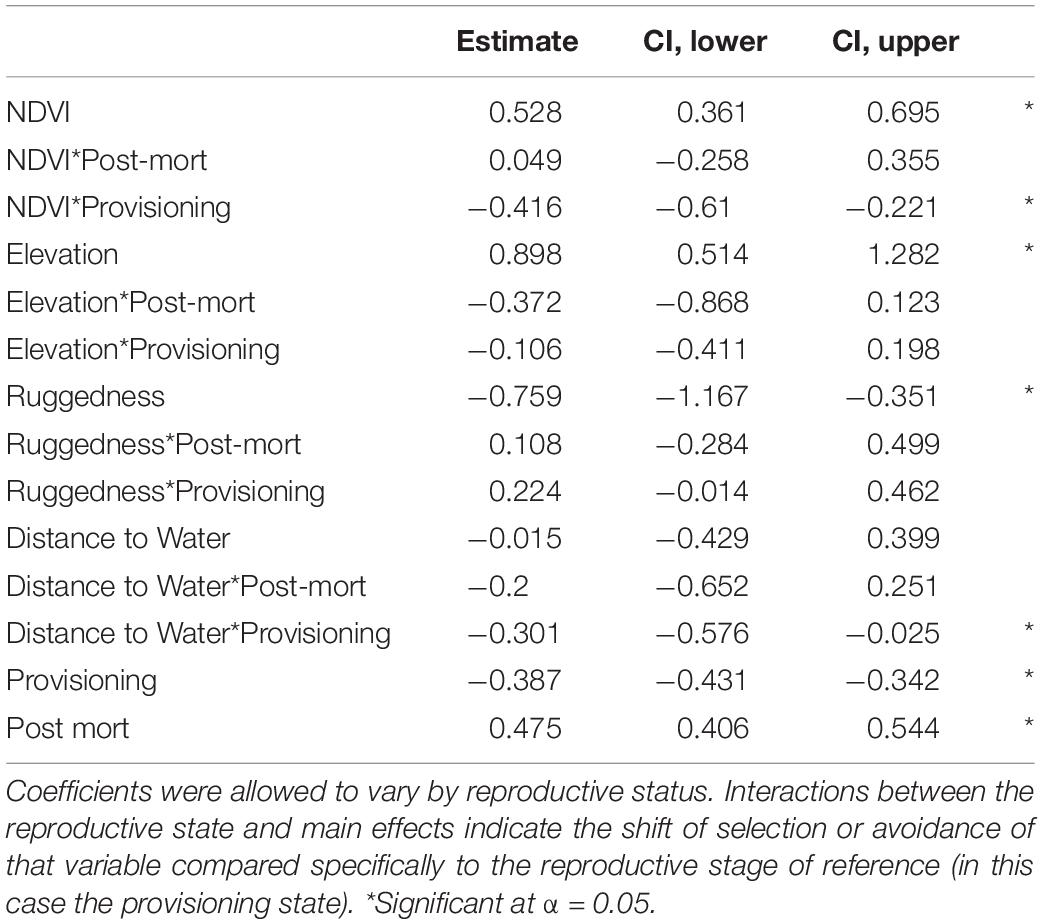
Table 2. Coefficients from a generalized linear mixed model in which resource selection was modeled as a function of (standardized) NDVI, elevation, ruggedness, and distance to water.
Effects of Resource Selection on Reproductive Output
Our test whether females traded off nutritional acquisition for juvenile safety indicated no statistical relationship between an individual’s selection for ruggedness and available NDVI (Figure 5). Our known-fate survival analyses indicated that juvenile recruitment was positively influenced by the maternal females use of areas with greater NDVI while provisioning (mean = 0.56, 95% CI 0.09 to 1.04; Figure 6). We also noted a weak effect of elevation on juvenile survival, with higher elevations corresponding to lower recruitment success (mean = −0.27, 95% CI −0.72 to 0.13; Figure 6). The use of other environmental gradients (terrain ruggedness and distance to water) by maternal females had no detectable effect on juvenile recruitment (ruggedness: mean = −0.13, 95% CI −0.5 to 0.23; distance to water: mean = −0.03, 95% CI −0.39 to 0.34; Figure 6).
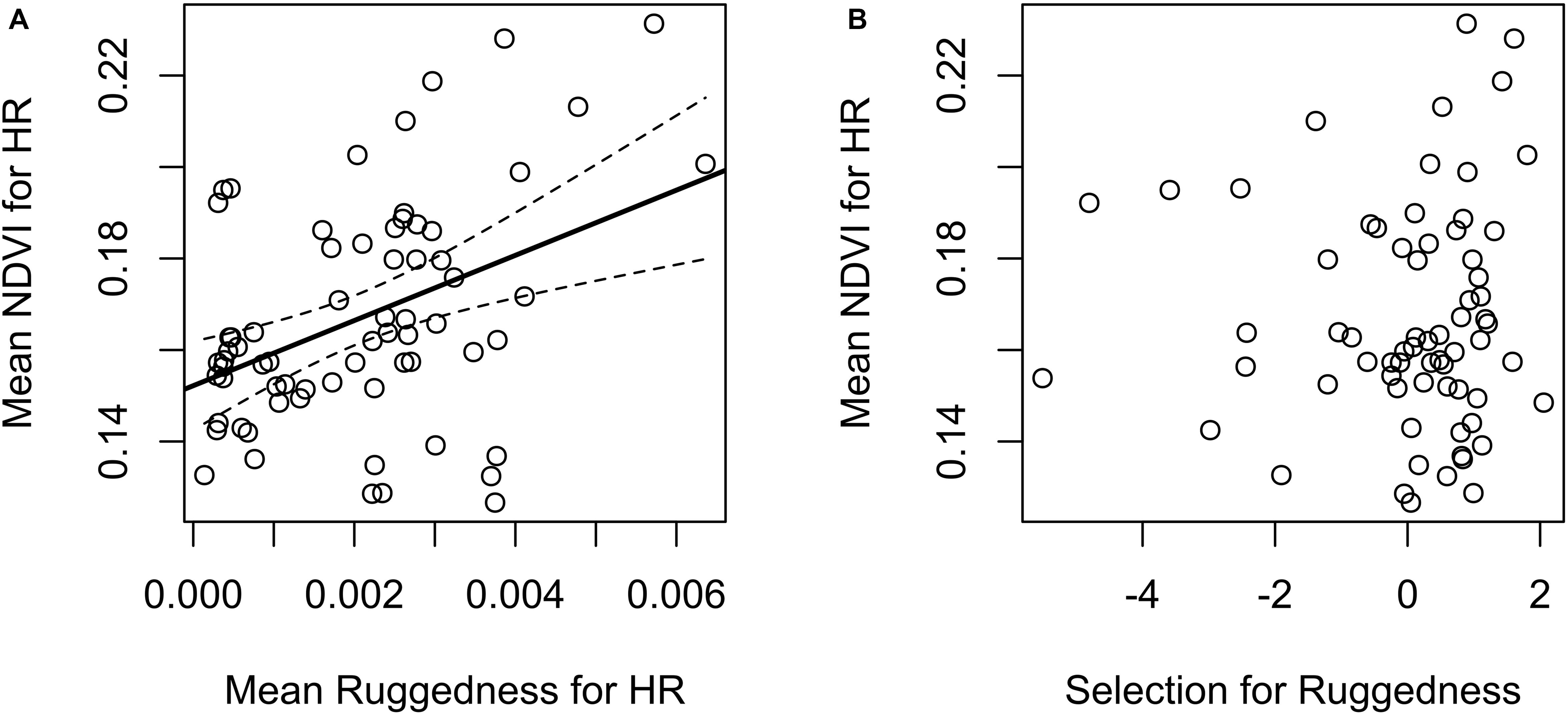
Figure 5. Potential trade-offs (or lack thereof) between nutritional resources and offspring safety for female mule deer on the Mojave National Preserve (2013–2016) while provisioning young (n = 67). (A) The left figure reveals a general positive relationship between habitat regions likely to confer offspring safety (topographic ruggedness, averaged across individual home ranges) and the availability of nutritional resources (NDVI, averaged across individual home ranges). Black solid line and dashed curve were derived from ordinary linear regression, and represent a fitted regression line and 95% confidence interval, respectively. (B) The right figure illustrates the relationship between individual-level selection coefficients for rugged terrain (logit-scale, derived from a generalized linear mixed-effects model of resource selection) vs. the availability of nutritional resources (NDVI), averaged across individual home ranges (no statistical relationship was detected).
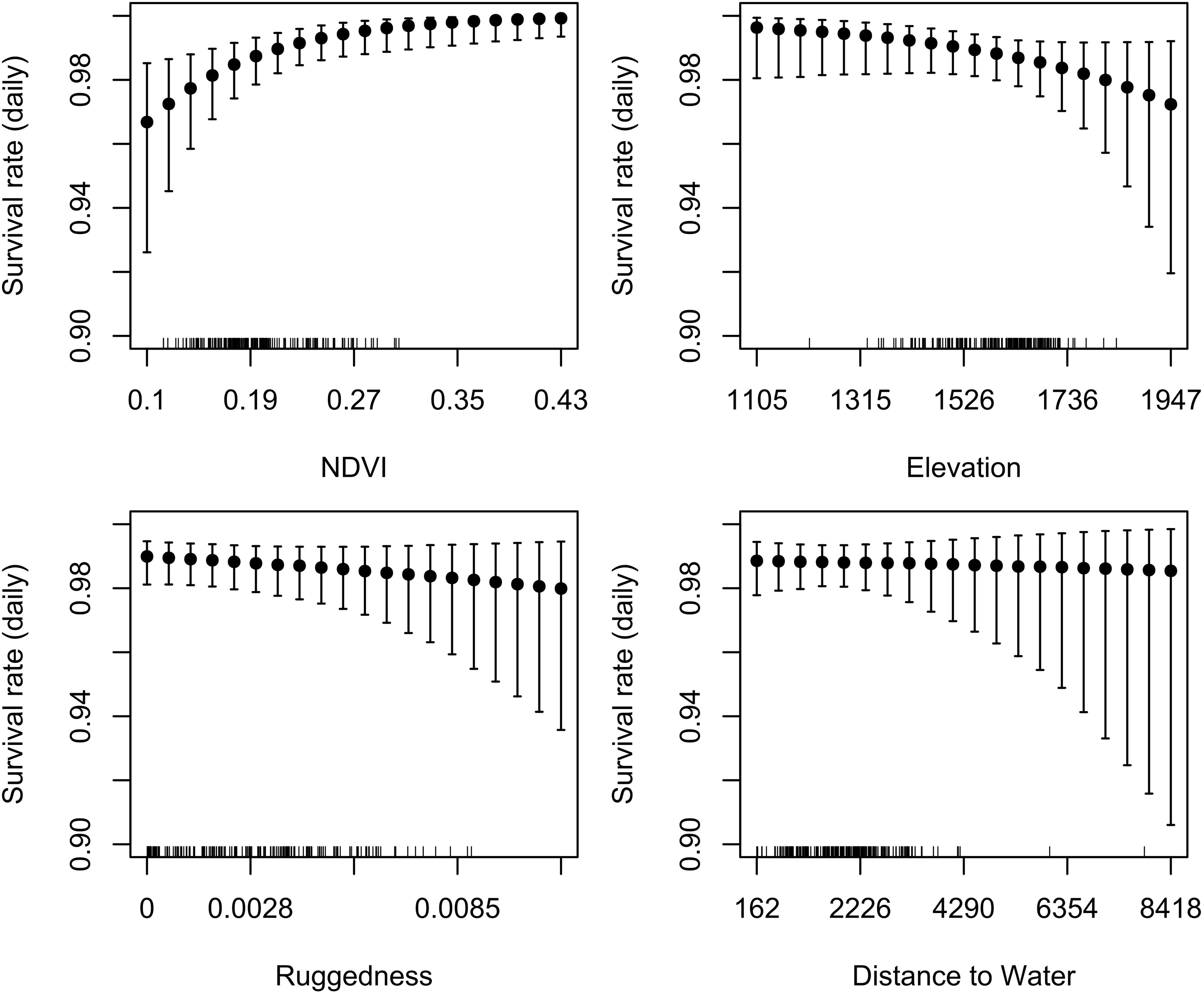
Figure 6. Partial dependence plots illustrating the relationship between daily offspring survival rate and corresponding mean environmental attributes (extracted from 30 m rasters) of habitats used by maternal female mule deer while provisioning young (n = 67 fitted with GPS collars) on the Mojave National Preserve (2013–2016), determined on the basis of a Bayesian known-fate survival analysis. Models were fit using data from up to 120 days post-parturition; after 120 days offspring were considered to be successfully recruited. There was strong evidence for a positive effect of NDVI on offspring survival, weak evidence for a negative effect of elevation, and little to no evidence for effects of terrain ruggedness or distance to water.
Discussion
Our hypothesis that female mule deer in the Mojave National Preserve would trade off nutritional intake for selection of areas to increase safety of young by shifting to a risk averse strategy was not well supported. In fact, we observed few instances where patterns of selection differed substantially among reproductive stages, primarily because of the large amount of individual variation in selection among our study animals. Overall, there were four main variables that explained the majority of resources selected by mule deer within this population across reproductive stages; vegetation greenness (NDVI), terrain ruggedness, distance to water, and elevation. Females that were provisioning young appeared to select lower values of NDVI relative to other reproductive timeframes (albeit with much more individual heterogeneity), but also selected areas close to sources of water. Though selection for more rugged terrain seemed to vary between our population level modeling (RF) and individual based procedure (GLMM) analyses, there were no significant shifts in selection between reproductive stages. Further, our tests of a direct tradeoff between nutritional intake (NDVI) and safety of young (rugged terrain) was not well supported. Selection of rugged terrain, however, did not decrease while females were provisioning young. Thus, females may have reduced concentration on nutrient acquisition (NDVI), while staying close to water and maintaining a minimum threshold level of safety for young through moderate use of rugged terrain among other landscape features.
Our GLMM results suggest that females tended to select spatial areas to maximize nutritional intake (selecting habitat with higher NDVI) during all reproductive stages. Though, there was a shift in selection toward lower values of NDVI while females were provisioning young, the overall population level effect remained positive. We also demonstrated that terrain ruggedness and NDVI were positively correlated in our study area suggesting that females were able to select areas that were relatively safe for offspring while also allowing provisioning mothers to meet their nutritional and water demands. This observation is consistent with our result that successful recruitment of young, indicated by survival to 120 days after parturition, was highest in habitats with higher NDVI. Those results suggest that the nutritional value of habitat areas with relatively high NDVI, were not especially risky for mothers with dependent young. A more effective test for a tradeoff between personal nutrition acquisition and offspring safety would require a study site where patches of high value for resource acquisition and offspring protection were mutually exclusive.
We expected female mule deer to use shrub and tree cover in addition to rugged terrain to increase safety of young, but those variables were not selected strongly by females while provisioning young; in fact, shrub and tree cover were not meaningful predictors of habitat use during any reproductive stage. Interestingly, mule deer are known to defend their young from small or mid-sized predators (Lingle et al., 2005). Potentially for this reason, mule deer typically prefer habitats with high visibility, in addition to a lower perceived risk of predation while foraging or resting (Altendorf et al., 2001; Esparza-Carlos et al., 2016; Bose et al., 2018). Our GLMM analyses did not confirm a shift to stronger selection for offspring safety while provisioning young, indeed; the overall population level effect for selection of rugged terrain was negative. Further, we observed constant selection of higher elevations in addition to lesser degrees of ruggedness, all of which could be assumed to enhance the ability for maternal vigilance toward predation risk. Our survival analysis, however, failed to confirm that ruggedness or elevation was positively correlated with offspring survival at our study site, and even indicated a trend toward lower recruitment success at higher elevations.
Forage quality, as indicated by higher NDVI, had a strong positive effect on the probability of successfully recruiting young into the population, while the effect of other environmental characteristics (e.g., ruggedness and distance to water) was much weaker. Indeed, NDVI has been directly linked to availability of high-quality forage for large herbivores (Marshal et al., 2005; Pettorelli et al., 2005; Creech et al., 2016). Additionally, lactation is nutritionally demanding for females (Oftedal, 2000; Barboza et al., 2009), and the month immediately following parturition is when quantity of milk and the number of suckling bouts by the neonate are greatest (Sadlier, 1980; Gauthier and Barrette, 1985). When rich nutritional sources are available to a female while provisioning offspring, the quality and quantity of milk increases and she is able to invest those resources in her young (Scornavacca et al., 2016). In addition to nutrient acquisition to support lactation, the increased requirements for water during lactation is paramount to successfully supply milk to young (Barboza et al., 2009). Throughout all reproductive timeframes, females selected areas closer to permanent water sources, however, we observed stronger selection for areas closer to water while females were provisioning young. Water resources are essential for preparation for parturition and meeting the physiological demands of late gestation and lactation (Barboza et al., 2009; Bleich et al., 2010; McKee et al., 2015). Both nutrient acquisition (via areas of greater NDVI) and access to water sources are important to maintain quality and quantity of milk for growth and survival of young. Thus, the nutritional quality of the landscape (through accessibility to forage and water) is directly linked to investment in offspring by maternal females (Scornavacca et al., 2016). Therefore, the ability of a female to keep her young safe while acquiring quality nutrients and then investing them in offspring directly affects her fitness and also population performance through survival and recruitment of young (Heffelfinger et al., 2018). Therefore, the ability of large herbivores to provision nutritious milk to their young (i.e., current investment), while maintaining the mother’s nutritional plane during lactation for future reproductive effort and survival (i.e., future investment) is likely to be an optimum strategy in instances where females are not forced to make tradeoffs between nutrition and safety of young.
Our prediction that females would prioritize resource acquisition (i.e., NDVI) over other landscape characteristics after loss of their offspring was mildly supported by our results. Indeed, females selected for greater values of NDVI in all reproductive timeframes. However, our GLMM models indicated stronger selection for areas of greater NDVI following mortality of juveniles compared to the period when females were provisioning young. Those females that lost young immediately shifted to strong selection for higher nutritional sources likely to recover body condition following peak lactation. The ability to recover from the reproductive effort may be crucial to rebuild resources for the upcoming reproductive period. Therefore, change in selection of resources between provisioning young and recovery to a higher nutritional state may enable females to restore lost energy reserves and be more likely to successfully rear offspring to recruitment the following year. Nevertheless, our observation of increased selection for high NDVI habitats after offspring loss was either a result of release from the need to protect offspring or an increased need to replenish somatic reserves after the nutritional demands of lactation to further future investment in reproduction the following year.
Selection for NDVI was strong and consistent before parturition and following the loss of young compared to when females were provisioning young. Further, individual heterogeneity was greater while females were provisioning. Caring for young imposes many constraints on individual behavior for maternal mule deer. They must obtain nutritional resources, access sources of free-standing water, and maintain vigilance and defense of young from predation. The suite of constraints imposed on maternal females may result in many short term tradeoffs in resource use that are difficult to detect at a longer temporal scale. Heterogeneity among individuals that we observed during the provisioning stage, however, may indicate that individuals exhibited varying behaviors based on their individual needs, differences in nutritional condition, and the requirements of their young. Indeed, we demonstrated that among the high heterogeneity for selection of NDVI during this timeframe, females that successfully utilized areas of greater NDVI were more successful at recruiting young into the population. Therefore, weak selection of resources at the population level toward greater values of NDVI during the provisioning stage may be indicative of a high variance of differing short-term behaviors exhibited by maternal females rather than an indication of nutritional tradeoffs to increase survival of young. Furthermore, the lack of high variance in selection of NDVI pre-parturition and following the loss of young may indicate that females are no longer making short term behavioral decisions toward caring for young and avoiding predators of young, and therefore shift to more risk prone strategies to directly provision themselves to recover from the nutritional constraints of late gestation and lactation.
There are very few studies investigating tradeoffs associated with selection of resources during different periods of the reproductive cycle, and how potentially differing needs of individuals affected overall selection of resources to enhance individual fitness (Barten et al., 2001; Long et al., 2009; Shuman et al., 2018). Long et al. (2009) investigated selection of resources and movements of female mule deer before and after parturition in a montane environment. Compared to our findings, they observed a varying relationship before and after estimated parturition in respect to the selection of distance to water sources. During pre-parturition, Long et al. (2009) reported that mule deer selected locations further from water compared to post-parturition. Nevertheless, they investigated movement patterns of mule deer without information on survival of young in a temperate forest region of northeastern Oregon, United States (Long et al., 2009). Mule deer are known to be more closely tied to water in arid environments than in cooler, wetter environments, but plants in the montane environment generally have lower preformed water content than many of the succulent plants in our study (Hervert and Krausman, 1986; McKee et al., 2015). Therefore, availability of water, both temporally and spatially, may differ too much to directly compare our results to Long et al. (2009). Barten et al. (2001) investigated habitat selection by caribou before and after parturition. They reported that caribou mothers switched habitat types when transitioning from pre-parturition to provisioning of young, whereas females that did not reproduce did not exhibit habitat switching. They also reported that females with young preferred high-elevation terrain, similar to our results and likely indicating selection of areas that balance nutrient acquisition and safety of young. Their study area also had more efficient, larger-bodied predators [e.g., wolves (Canis lupus) and brown bears (Ursus arctos)] than occur in our study area, which consist of coyotes (Canis latrans), bobcats (Lynx rufus), and occasionally cougars (Puma concolor). Finally, Shuman et al. (2018) evaluated resource selection of female white-tailed deer (Odocoileus virginianus) during both pre-parturition and while provisioning young collectively. Their findings are counterintuitive in that females selected regions generally closer to nutritional sources (agriculture) to give birth while avoiding those same resources given availability within the region. Further, they conclude that females may be balancing both nutrition acquisition and care for young via predator avoidance, similar to the heterogeneity we observed during the provisioning time period. Although our investigation compared to Barten et al. (2001); Long et al. (2009), and Shuman et al. (2018) occurred in dramatically different ecosystems, the evidence that large, female herbivores select habitats to increase safety of young while acquiring resources for nutritional maintenance during the reproductive cycle is compelling (Bleich et al., 1997; Bowyer, 2004). Depending on habitat quality and resources available, ungulates must constantly balance reproductive output via protection and safety of young with maintenance of their own nutritional needs for future investment in offspring to maximize their reproductive fitness.
We utilized RF as a means of feature selection and to identify potential differences in resource selection across reproductive timeframes. Because the RF models did not account for autocorrelation of observations within individuals, these models were prone to overfitting and therefore we only interpreted broad resource selection patterns and we caution against interpreting any fine-scale patterns identified by these models (Figure 2). Performance of our RF models for resource selection, measured via a rigorous cross-validation whereby we sequentially withheld all data associated with individual deer from model-fitting, was generally poor (AUC of 0.65 for pre-parturition, 0.65 while provisioning young, and 0.63 following mortality of young). This unimpressive relationship, though better than random performance (Hernandez et al., 2006), likely resulted from high among-individual variation in patterns of resource selection, which we also detected via our GLMM modeling approach when modeled at the individual scale (3rd order selection; Johnson, 1980). Indeed, when we compare univariate relationships between our RF models (Figure 2) and our GLMM models (Figure 4) several key differences are apparent. Both modeling approaches suggest a strong and positive effect of NDVI on resource selection propensity by females across all reproductive stages and a general tendency to select habitat areas nearer to water and higher in elevation. However, the GLMM models suggest a general tendency to avoid rugged habitats whereas the RF models suggest (albeit weakly) a tendency toward selection of habitats with high or intermediate ruggedness during provisioning and after loss of offspring. In general, these differences could be due to the ability of RF models to capture non-linear relationships, the fact that RF models implicitly incorporate complex (potentially over fitted) interactions that can cloud the interpretation of partial dependence plots, or due simply to increased overfitting tendency of RF and other machine learning methods. Overall, our study underscores the benefits of coupling exploratory machine-learning methods (e.g., RF) with model-based inference (e.g., GLMM) to make inferences about selection of resources in wild populations (Shoemaker et al., 2018).
In our study and in future studies evaluating tradeoffs in resource selection around reproduction, a potential difficulty is the lower number of individuals that are included in the reproductive timeframe following the loss of an offspring. Indeed, we imposed a strict rule to only include females that had lost young within the first 30 days, because our objectives were to evaluate shifts in resource selection following a sudden shift in nutritional requirements (e.g., cessation of lactation). Energetic demands of lactation via quantity of milk produced to provide for dependent young is greatest during the first 30 days post-parturition (Sadlier, 1980; Gauthier and Barrette, 1985). Thus, by imposing a strict rule of including females that lost young in the first 30 days, we had greater potential to identify shifts in resource selection as a result in life-history stage changes. Indeed, loss of young is not a desired outcome, but understanding how maternal females shift from investment in current to future reproduction to maximize lifetime fitness is also important. Likely the only way to overcome the low sample size of this reproductive stage without an experiment to remove young from maternal females is to maximize the initial sample size of maternal females at capture. Nevertheless, we observed less variation among individuals after the loss of young so perhaps landscape scale movement and resource selection is more predictable and can be understood with lower sample sizes than understanding variation among individuals in selection of resources while provisioning young. Thus, the lower sample size during this timeframe may still result in reasonable inferences. Further research is needed toward examining tradeoffs surrounding reproductive stages in large herbivores. Additionally, monitoring individuals for greater than a single year to understand longitudinal shifts in reproductive strategies over time also would be beneficial (Festa-Bianchet et al., 2017). Our study was limited to 1 year of location data per unique individual. Incorporating multi-year movement data for individuals may shed additional light on how reproductive strategies shift temporally.
In many ecosystems, maternal females are faced with potentially conflicting decisions to maintain their nutritional condition, care for young, and recover from the costly life-history stages of reproduction. We show that females invest energy in selection of habitats that cater to the survivorship of their young, thereby investing in current reproduction. Further, the assumption that females may have to trade safety of offspring for nutritional requirements may not always be necessary. If females are able to select resources that allow for safety of offspring, usually a risk averse strategy, and also allow for females to obtain resources that support their nutritional needs (usually a risk prone strategy), they may not be forced to make those reproductive tradeoffs. Our study area appears to have areas where safety of young and availability of nutritional resources are not mutually exclusive. Indeed, we observed that females selected areas of higher NDVI, indicating green forage, while caring for and provisioning young, likely a strategy to increase individual fitness. However, there was a large amount of individual variation toward the selection of NDVI while provisioning young. We show that among this variation, those females that select greater levels of NDVI were more successful at recruiting young into the population. Further, after a transition to a non-provisioning state (i.e., post-juvenile mortality), females still shifted selection to areas with even higher quality forage to recover from the rearing of young, and likely to begin replenishing energetic stores necessary for reproduction the next year. Expanding our knowledge of factors that influence behavioral decisions during reproduction will prove to be of high importance moving forward. As environments fluctuate and landscape dynamics shift, understanding strategies of reproductive investment at the individual level that animals make to increase fitness, and then how those decisions relate to population performance, will further our understanding of large mammal ecology.
Data Availability Statement
Code and data for all analyses presented in this paper are available on GitHub: https://github.com/kevintshoemaker/desert_deer_rsf. The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Nevada, Reno Protocol # 00538 and were in keeping with guidelines established by the American Society of Mammologists for research on wild mammals (Sikes, 2016).
Author Contributions
LH was a graduate student and KMS was the graduate advisor on this project. KMS and VB obtained the funding for the project and designed the study with input from ND. KTS and LH performed the statistical analyses with input from KMS. All authors contributed to writing the manuscript.
Funding
We appreciate the financial and technical support provided by the Safari Club International Foundation (Grant # 1321-153-523F), the Golden Gate Chapter of Safari Club International (Grant #1321-153-52VY), the California Department of Fish and Wildlife (in-kind support), National Park Service (1320-153-52XV), and the Boone and Crockett Club. The research presented here was supported by a Hatch Grant from the Agricultural Experiment Station at the University of Nevada, Reno.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We received invaluable assistance with adult animal capture and handling from B. Gonzalez, B. Pierce, L. Konde, J. Villipique, A. Adams, M. Blum, J. Merrell, D. Huggins, N. Jackson, B. Regan, T. Allen, and all others who helped with data collection for this manuscript. We graciously thank J. Sedinger, B. Sedinger, and A. Foley for their guidance in improving our manuscript, and A. Bush for his exhaustive help with field methodologies and collecting some of the data used herein. This is Professional Paper 122 from the Eastern Sierra Center for Applied Population Ecology.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.00163/full#supplementary-material
FIGURE S1 | Distribution of buffered annual home range sizes used for quantifying availability across reproductive time frames for 68 (pre-parturition), 67 (provisioning young), and 20 (post juvenile mortality), female mule deer on the Mojave National Preserve, California, United States from 2013–2016. Home ranges were estimated with 99.9% isopleths from a kernel density estimate (KDE) and then buffered by mean step length (200 m) to account for available space on the outer edge of the home range.
FIGURE S2 | Representative results from our ‘likelihood convergence’ procedure for ensuring that GLMM models were supplied with a sufficient density of background points to capture the full range of environmental variation. Specifically, we fitted down-weighted Poisson regression (DWPR) models (fitted as a standard GLM in R using the full set of continuous and categorical covariates) to a wide range of alternative background point densities for each individual, and identified the minimum threshold beyond which model performance become largely independent of the specific set of background points sampled. Using this technique, we found that 500 random background points per km2 was sufficient for our purposes.
References
Aarts, G., Fieberg, J., Brasseur, S., and Matthiopoulos, J. (2013). Quantifying the effect of habitat availability on species distributions. J. Animal Ecol. 82, 1135–1145. doi: 10.1111/1365-2656.12061
Aldridge, C. L., and Boyce, M. S. (2007). Linking occurrence and fitness to persistence: habitat−based approach for endangered greater sage−grouse. Ecol. Appl. 17, 508–526. doi: 10.1890/05-1871
Altendorf, K. B., Laundré, J. W., López González, C. A., and Brown, J. S. (2001). Assessing effects of predation risk on foraging behavior of mule deer. J. Mammal. 82, 430–439. doi: 10.1093/jmammal/82.2.430
Barten, N. L., Bowyer, R. T., and Jenkins, K. J. (2001). Habitat use by female caribou: tradeoffs associated with parturition. J. Wildl. Manag. 65, 77–92.
Bender, L. C., Hoenes, B. D., and Rodden, C. L. (2012). Factors influencing survival of desert mule deer in the greater San Andres Mountains, New Mexico. Hum. Wildl. Interact. 6, 245–260.
Beyer, H. L. (2015). Geospatial Modelling Environment (Version 0.7.4.0). (Software). Available online at: http://www.spatialecology.com/gme (accessed March 3, 2017).
Bishop, C. J., Anderson, C. R., Walsh, D. P., Bergman, E. J., Kuechle, P., and Roth, J. (2011). Effectiveness of a redesigned vaginal implant transmitter in mule deer. J. Wildl. Manag. 75, 1797–1806. doi: 10.1002/jwmg.229
Bishop, C. J., Freddy, D. J., White, G. C., Watkins, B. E., Stephenson, T. R., and Wolfe, L. L. (2007). Using vaginal implant transmitters to aid in capture of mule deer neonates. J. Wildl. Manag. 71, 945–954. doi: 10.2193/2006-123
Bishop, C. J., White, G. C., Freddy, D. J., Watkins, B. E., and Stephenson, T. R. (2009). Effect of enhanced nutrition on mule deer population rate of change. Wildl. Monogr. 172, 1–28. doi: 10.2193/2008-107
Bleich, V. C., Bowyer, R. T., and Wehausen, J. D. (1997). Sexual segregation in mountain sheep: resources or predation? Wildl. Monogr. 97, 1–50.
Bleich, V. C., Marshal, J. P., and Andrew, N. G. (2010). Habitat use by a desert ungulate: predicting effects of water availability on mountain sheep. J. Arid Environ. 74, 638–645. doi: 10.1016/j.jaridenv.2009.10.019
Bonte, D., and Dahirel, M. (2017). Dispersal: a central and independent trait in life history. Oikos 126, 472–479. doi: 10.1111/oik.03801
Bose, S., Forrester, T. D., Cassady, D. S., and Wittmer, H. U. (2018). Effect of activity states on habitat selection by black-tailed deer. J. Wildlife Manag. 82, 1711–1724. doi: 10.1002/jwmg.21529
Bowyer, R. T. (2004). Sexual segregation in ruminants: definitions, hypotheses, and implications for conservation and management. J. Mammal. 85, 1039–1052. doi: 10.1644/bbl-002.1
Carstensen, M., DelGiudice, G. D., and Sampson, B. A. (2003). Using doe behavior and vaginal-implant transmitters to capture neonate white-tailed deer in north-central Minnesota. Wild. Soc. Bull. 31, 634–641.
Climate Engine. (2017). Desert Research Institute and University of Idaho. Available online at: http://climateengine.org (accessed March 3, 2017)Google Scholar
Clutton-Brock, T., and Sheldon, B. C. (2010). Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 25, 562–573. doi: 10.1016/j.tree.2010.08.002
Clutton-Brock, T. H., Guinness, F. E., and Albon, S. D. (1983). The costs of reproduction to red deer hinds. J. Anim. Ecol. 52, 367–383.
Creech, T. G., Epps, C. W., Monello, R. J., and Wehausen, J. D. (2016). Predicting diet quality and genetic diversity of a desert-adapted ungulate with NDVI. J. Arid Environ. 127, 160–170. doi: 10.1016/j.jaridenv.2015.11.011
Cristescu, B., Bose, S., Elbroch, L. M., Allen, M. L., and Wittmer, H. U. (2019). Habitat selection when killing primary versus alternative prey species supports prey specialisation in an apex predator. J. Zool. 309, 259–268. doi: 10.1111/jzo.12718
Cutler, D. R., Edwards, T. C., Beard, K. H., Cutler, A., Hess, K. T., Gibson, J., et al. (2007). Random forests for classification in ecology. Ecology 88, 2783–2792. doi: 10.1890/07-0539.1
De’ath, G., and Fabricius, K. E. (2000). Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81, 3178–3192. doi: 10.1890/0012-9658(2000)081[3178:cartap]2.0.co;2
DeCesare, N. J., Hebblewhite, M., Lukacs, P. M., and Hervieux, D. (2016). Evaluating sources of censoring and truncation in telemetry−based survival data. J. Wildl. Manag. 80, 138–148. doi: 10.1002/jwmg.991
Dzialak, M. R., Harju, S. M., Osborn, R. G., Wondzell, J. J., Hayden-Wing, L. D., Winstead, J. B., et al. (2011). Prioritizing conservation of ungulate calving resources in multiple-use landscapes. PLoS One 6:14597.
Esparza-Carlos, J. P., Laundré, J. W., Hernández, L., and Íñiguez-Dávalos, L. I. (2016). Apprehension affecting foraging patterns and landscape use of mule deer in arid environments. Mammal. Biol. 81, 543–550. doi: 10.1016/j.mambio.2016.07.006
Festa-Bianchet, M., Douhard, M., Gaillard, J. M., and Pelletier, F. (2017). Success and challenges of long-term field studies of marked ungulates. J. Mammalo. 98, 612–620. doi: 10.1093/jmammal/gyw227
Forrester, T. D., Casady, D. S., and Wittmer, H. U. (2015). Home sweet home: fitness consequences of site familiarity in female black-tailed deer. Behav. Ecol. Sociobiol. 69, 603–612. doi: 10.1007/s00265-014-1871-z
Gaillard, J. M., Festa-Bianchet, M., and Yoccoz, N. G. (1998). Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends Ecol. Evol. 13, 58–63. doi: 10.1016/s0169-5347(97)01237-8
Gaillard, J. M., Festa-Bianchet, M., Yoccoz, N. G., Loison, A., and Toigo, C. (2000). Temporal variation in fitness components and population dynamics of large herbivores. Ann. Rev. Ecol. Syst. 31, 367–393. doi: 10.1146/annurev.ecolsys.31.1.367
Gauthier, D., and Barrette, C. (1985). Suckling and weaning in captive white-tailed and fallow deer. Behaviour 94, 128–149. doi: 10.1163/156853985x00307
Gehr, B., Bonnot, N. C., Heurich, M., Cagnacci, F., Ciuti, S., Hewison, A. M., et al. (2020). Stay home, stay safe—Site familiarity reduces predation risk in a large herbivore in two contrasting study sites. J. Anim. Ecol. doi: 10.1111/1365-2656.13202
Gillies, C. S., Hebblewhite, M., Nielsen, S. E., Krawchuk, M. A., Aldridge, C. L., Frair, J. L., et al. (2006). Application of random effects to the study of resource selection by animals. J. Anim. Ecol. 75, 887–898. doi: 10.1111/j.1365-2656.2006.01106.x
Hamel, S., Côté, S. D., Gaillard, J. M., and Festa−Bianchet, M. (2009). Individual variation in reproductive costs of reproduction: high−quality females always do better. J. Anim. Ecol. 78, 143–151. doi: 10.1111/j.1365-2656.2008.01459.x
Haskell, S. P., Ballard, W. B., Butler, D. A., Tatman, N. M., Wallace, M. C., Kochanny, C. O., et al. (2007). Observations on capturing and aging deer fawns. J. Mammal. 88, 1482–1487. doi: 10.1644/07-mamm-a-004r.1
Haugen, A. O., and Speake, D. W. (1958). Determining age of young fawn white-tailed deer. J. Wildl. Manag. 22, 319–321.
Hebblewhite, M., and Merrill, E. H. (2009). Trade−offs between predation risk and forage differ between migrant strategies in a migratory ungulate. Ecology 90, 3445–3454. doi: 10.1890/08-2090.1
Heffelfinger, L. J., Stewart, K. M., Bush, A. P., Sedinger, J. S., Darby, N. W., and Bleich, V. C. (2018). Timing of precipitation in arid environments: effects on population performance of a large herbivore. Ecol. Evol. 8, 3354–3366. doi: 10.1002/ece3.3718
Hernandez, P. A., Graham, C. H., Master, L. L., and Albert, D. L. (2006). The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 29, 773–785. doi: 10.1111/j.0906-7590.2006.04700.x
Hervert, J. J., and Krausman, P. R. (1986). Desert mule deer use of water developments in Arizona. J. Wildl. Manag. 50, 670–676.
Hurley, M. A., Unsworth, J. W., Zager, P., Hebblewhite, M., Garton, E. O., Montgomery, D. M., et al. (2011). Demographic response of mule deer to experimental reduction of coyotes and mountain lions in southeastern Idaho. Wildl. Monogr. 178, 1–33. doi: 10.1002/wmon.4
Johnson, D. H. (1980). The comparison of usage and availability measurements for evaluating resource preference. Ecology 61, 65–71. doi: 10.2307/1937156
Kawecki, T. J., and Stearns, S. C. (1993). The evolution of life histories in spatially heterogeneous environments: optimal reaction norms revisited. Evol. Ecol. 7, 155–174. doi: 10.1007/bf01239386
Kellner, K. (2019). jagsUI: A Wrapper Around ‘rjags’ to Streamline ‘JAGS’ Analyses. R Package Version 1.5.1. Available online at: https://CRAN.R-project.org/package=jagsUI (accessed July 30, 2019).
Krausman, P. R., Hervert, J. J., and Ordway, L. L. (1985). Capturing deer and mountain sheep with a net-gun. Wildl. Soc. Bull. 13, 71–73.
Kuhn, M. (2019). caret: Classification and Regression Training. R Package Version 6.0-82.. Available online at: https://CRAN.R-project.org/package=caret (accessed April 22, 2020).
Landfire. (2014). Existing Vegetation Type Layer, LANDFIRE 1.1.0, U.S. Department of the Interior, Geological Survey. Available online at: https://www.landfire.gov/vegetation.php (accessed February 28, 2017)Google Scholar
Lingle, S., Pellis, S. M., and Wilson, W. F. (2005). Interspecific variation in antipredator behaviour leads to differential vulnerability of mule deer and white−tailed deer fawns early in life. J. Anim. Ecol. 74, 1140–1149. doi: 10.1111/j.1365-2656.2005.01014.x
Long, R. A., Bowyer, R. T., Porter, W. P., Mathewson, P., Monteith, K. L., and Kie, J. G. (2014). Behavior and nutritional condition buffer a large-bodied endotherm against direct and indirect effects of climate. Ecol. Monogr. 84, 513–532. doi: 10.1890/13-1273.1
Long, R. A., Kie, J. G., Bowyer, R. T., and Hurley, M. A. (2009). Resource selection and movements by female mule deer Odocoileus hemionus: effects of reproductive stage. Wildl. Biol. 15, 288–298. doi: 10.2981/09-003
Manly, B. F. J., McDonald, L., Thomas, D., McDonald, T. L., and Erickson, W. P. (2007). Resource Selection By Animals: Statistical Design And Analysis For Field Studies. Berlin: Springer Science & Business Media.
Marshal, J. P., Krausman, P. R., and Bleich, V. C. (2005). Rainfall, temperature, and forage dynamics affect nutritional quality of desert mule deer forage. Rangel. Ecol. Manag. 58, 360–365. doi: 10.2111/1551-5028(2005)058[0360:rtafda]2.0.co;2
McKee, C. J., Stewart, K. M., Sedinger, J. S., Bush, A. P., Darby, N. W., Hughson, D. L., et al. (2015). Spatial distributions and resource selection by mule deer in an arid environment: Responses to provision of water. J. Arid Environ. 22, 76–84. doi: 10.1016/j.jaridenv.2015.06.008
McLoughlin, P. D., Boyce, M. S., Coulson, T., and Clutton-Brock, T. (2006). Lifetime reproductive success and density-dependent, multi-variable resource selection. Proc. R. Soc. B Biol. Sci. 273, 1449–1454. doi: 10.1098/rspb.2006.3486
Moen, R. A., Pastor, J., and Cohen, Y. (1997). Accuracy of GPS telemetry collar locations with differential correction. J. Wildl. Manag. 61, 530–539.
Monteith, K. L., Bleich, V. C., Stephenson, T. R., Pierce, B. M., Conner, M. M., Kie, J. G., et al. (2014). Life-history characteristics of mule deer: Effects of nutrition in a variable environment. Wildl. Monogr. 186, 1–62. doi: 10.1002/wmon.1011
Muff, S., Signer, J., and Fieberg, J. (2019). Accounting for individual-specific variation in habitat-selection studies: Efficient estimation of mixed-effects models using Bayesian or frequentist computation. J. Anim. Ecol. 19, 1–13.
National Park Service (2017). Nature and Science of Mojave National Preserve. Available online at: <http://www.nps.gov/moja/naturescience/index.htm> (accessed March 3, 2017)Google Scholar
Oftedal, O. T. (2000). Use of maternal reserves as a lactation strategy in large mammals. Proc. Nutr. Soc. 59, 99–106. doi: 10.1017/s0029665100000124
Pettorelli, N., Vik, J. O., Mysterud, A., Gaillard, J. M., Tucker, C. J., and Stenseth, N. C. (2005). Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 20, 503–510. doi: 10.1016/j.tree.2005.05.011
Plummer, M. (2003). “JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling,” in Proceedings of the 3rd International Workshop on Distributed Statistical Computing, Vol. 124, Vienna, 10.
Promislow, D. E., and Harvey, P. H. (1990). Living fast and dying young: A comparative analysis of life−history variation among mammals. J. Zool. 220, 417–437. doi: 10.1111/j.1469-7998.1990.tb04316.x
Renner, I. W., Elith, J., Baddeley, A., Fithian, W., Hastie, T., Phillips, S. J., et al. (2015). Point process models for presence−only analysis. Methods Ecol. Evol. 6, 366–379. doi: 10.1111/2041-210x.12352
Ricklefs, R. E. (1994). Life history invariants: some explorations of symmetry in evolutionary biology. Science 264, 116–118.
Ricklefs, R. E., and Wikelski, M. (2002). The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468. doi: 10.1016/s0169-5347(02)02578-8
Sappington, J. M., Longshore, K. M., and Thompson, D. B. (2007). Quantifying landscape ruggedness for animal habitat analysis: a case study using bighorn sheep in theMojave Desert. J. Wildl. Manag. 71, 1419–1426. doi: 10.2193/2005-723
Scornavacca, D., Lovari, S., Cotza, A., Bernardini, S., Brunetti, C., Pietrocini, V., et al. (2016). Pasture quality affects juvenile survival through reduced maternal care in a mountain−dwelling ungulate. Ethology 122, 807–817. doi: 10.1111/eth.12530
Shoemaker, K. T., Heffelfinger, L. J., Jackson, N. J., Blum, M. E., Wasley, T., and Stewart, K. M. (2018). A machine-learning approach for extending classical wildlife resource selection analyses. Ecol. Evol. 8, 3556–3569. doi: 10.1002/ece3.3936
Shuman, R. M., Cherry, M. J., Dutoit, E. A., Simoneaux, T. N., Miller, K. V., and Chamberlain, M. J. (2018). Resource selection by parturient and postparturient white-tailed deer and their fawns. J. South. Assoc. Fish Wildl. Agen. 5, 78–84.
Sikes, R. S. (2016). Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 97, 663–688. doi: 10.1093/jmammal/gyw078
Stephenson, T. R., Bleich, V. C., Pierce, B. M., and Mulcahy, G. P. (2002). Validation of mule deer body composition using in vivo and post-mortem indices of nutritional condition. Wildl. Soc. Bull. 30, 557–564.
Stephenson, T. R., Ward Testa, J., Adams, G. P., Garth Sasser, R., Schwartz, C. C., and Hundertmark, K. J. (1995). Diagnosis of pregnancy and twinning in moose by ultrasonography and serum assay. Alces 31, 167–172.
Stewart, K. M., Bowyer, R. T., Kie, J. G., Cimon, N. J., and Johnson, B. K. (2002). Temporospatial distributions of elk, mule deer, and cattle: resource partitioning and competitive displacement. J. Mammalo. 83, 229–244. doi: 10.1644/1545-1542(2002)083<0229:tdoemd>2.0.co;2
Therrien, J. F., Côté, S. D., Festa-Bianchet, M., and Ouellet, J. P. (2008). Maternal care in white-tailed deer: trade-off between maintenance and reproduction under food restriction. Anim. Behav. 75, 235–243. doi: 10.1016/j.anbehav.2007.04.030
Thomas, K., Keeler-Wolf, T., and Stine, P. (2004). Mojave Desert Ecosystem Program: Central Mojave Vegetation Database. USGS Final Report for Mojave Desert Ecosystem Program. Sacramento, CA: USGS.
Van Noordwijk, A. J., and de Jong, G. (1986). Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142. doi: 10.1086/284547
Warton, D. I., and Shepherd, L. C. (2010). Poisson point process models solve the “pseudo-absence problem” for presence-only data in ecology. Ann. Appl. Stat. 4, 1383–1402. doi: 10.1214/10-aoas331
White, K. S., and Berger, J. (2001). Antipredator strategies of Alaskan moose: are maternal trade-offs influenced by offspring activity? Canad. J. Zool. 79, 2055–2062. doi: 10.1139/z01-170
Keywords: fitness, Odocoileus hemionus, machine learning, Mojave desert, life history, mule deer, random forest, resource selection
Citation: Heffelfinger LJ, Stewart KM, Shoemaker KT, Darby NW and Bleich VC (2020) Balancing Current and Future Reproductive Investment: Variation in Resource Selection During Stages of Reproduction in a Long-Lived Herbivore. Front. Ecol. Evol. 8:163. doi: 10.3389/fevo.2020.00163
Received: 20 December 2019; Accepted: 11 May 2020;
Published: 18 June 2020.
Edited by:
Andrew James Jonathan MacIntosh, Kyoto University, JapanReviewed by:
Heiko U. Wittmer, Victoria University of Wellington, New ZealandChuck Anderson, Colorado Parks and Wildlife, United States
Copyright © 2020 Heffelfinger, Stewart, Shoemaker, Darby and Bleich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelley M. Stewart, S2VsbGV5U0B1bnIuZWR1; a3N0ZXdhcnRAY2FibnIudW5yLmVkdQ==
†Present address: Levi J. Heffelfinger, Caesar Kleberg Wildlife Research Institute, Texas A&M University-Kingsville, Kingsville, TX, United States
 Levi J. Heffelfinger
Levi J. Heffelfinger Kelley M. Stewart
Kelley M. Stewart Kevin T. Shoemaker
Kevin T. Shoemaker Neal W. Darby
Neal W. Darby Vernon C. Bleich
Vernon C. Bleich