- 1Forestry College, Research Center of Forest Ecology, Guizhou University, Guiyang, China
- 2Systems Ecology, Department of Ecological Science, Faculty of Earth and Life Sciences, VU University (Vrije Universiteit) Amsterdam, Amsterdam, Netherlands
- 3College of Horticulture and Forestry Sciences / Hubei Engineering Technology Research Center for Forestry Information, Huazhong Agricultural University, Wuhan, China
Eupatorium adenophorum is an alien species that threatens community stability and diversity in karst areas. Arbuscular mycorrhizal (AM) fungi form interconnected mycorrhizal network, connecting adjacent plants and plant species. How mycorrhizal networks affect the competition for nutrients between invasive and native plants in karst habitat remains unclear at present. An experiment was conducted using a compartmental growing device, which was composed of two planting compartments (for the invasive E. adenophorum or native Artemisia annua) and a competitive compartment (for the interconnected mycorrhizal network). The experiment contained mycorrhizal fungus treatments, with AM fungi (M+) and without AM fungi (M−) using the species Claroideoglomus etunicatum, and the nutrient utilization treatments using nylon mesh to interconnective mycorrhizal networks, including common utilization (Cu), single utilization (Su), and non-utilization (Nu). The results showed as follows: AM fungi differentially increased biomass, nitrogen (N) acquisition and phosphorus (P) acquisition and significantly reduced N/P ratio of the invasive E. adenophorum and native A. annua under Cu, Su, and Nu conditions. Additionally, the biomass, N acquisition and P acquisition of E. adenophorum was greater than A. annua and the N/P ratio of E. adenophorum was significantly lower than A. annua under Cu condition, which AM fungi promoted the accumulation of biomass, N and P for E. adenophorum and A. annua, and E. adenophorum experienced a greater reduction of P limitation than A. annua via the interconnected mycorrhizal network. In conclusion, we suggest that AM fungi endow invasive plants greater alleviation of P limitation and enhancement of nutrient competition than native plants via mycorrhizal network in low-P karst soil.
Introduction
The karst landform is composed of mainly of carbonate rock and covers an area of 22 million km2 globally (Liu and Zhao, 2000). The largest karst region in China is 1.3 million km2 (Wang et al., 2004; Liu et al., 2009), characterized by nutrients (especially phosphorus) and water deficit, fragile habitat and severe soil erosion especially in southwest China (Li et al., 2002), Some invasive plants, such as Eupatorium adenophorum, have successfully invaded karst habitats in southwest China, and have expanded rapidly, affecting the establishment of native plant communities (Jia et al., 2009). However, the invasion mechanisms of these alien plants within the fragile karst habitats remain unclear at present. Based on previous studies, when invasive species enter new habitats they may gradually outcompete native species to withdraw from their original habitat (Smith and Knapp, 2001; Wang Y. J. et al., 2017; Wang et al., 2019; Chen D. et al., 2019). Alien plant invasion is possibly facilitated by a lack of natural enemies including pathogens and insect herbivores (Van der Putten et al., 2013). Callaway and Aschehoug (2000) argued that while natural enemies may have not evolved specificity to invasive plants, symbiotic fungi in soils are ubiquitous and associate with both native and invasive species. Research shows that soil microbes in some invaded ecosystems may promote the successful invasion of exotic plants (Callaway et al., 2004). In addition, invasive plants can also change soil microbial communities in invasive areas, shift soil nutrient availability, and create a positive soil-plant feedback for their invasion (Rodgers et al., 2008). Thus, the role of soil microbes cannot be ignored in successful plant invasions.
Arbuscular mycorrhizal (AM) fungi are a class of soil microbes that can form symbiotic relationships with the roots of 80% of the terrestrial plant species in the world (Smith and Read, 2010). A mycorrhizal network is formed when fungal mycelia colonize and link the roots of two or more plants (Selosse et al., 2006). The mycorrhizal network can influence competition between plants (Merrild et al., 2013). Research suggests that AM fungi can promote uptake of N and P, and change the N/P ratio (Johnson, 2010; Mei et al., 2019) and (likely as a result) increase plant biomass (Wagg et al., 2011). Wang et al. (2015) argued that invasive species may have a higher N/P ratio than native species, which may contribute to their invasiveness in soils where N is not a strong constraints on plant growth. In addition, AM fungi can also transfer the carbon from native plants to invasive plants through the interconnected mycorrhizal networks, conferring a competitive advantage for invasive plants (Carey et al., 2004). Meanwhile, AM fungi may promote the uptake of P by host plants after obtaining carbon from them (Ryan et al., 2012). In karst habitats, where the soil is usually deficient in nutrients, competition for soil nutrients between invasive plants and native plants is inevitable (Vila and Weiner, 2004). Jiang et al. (2019) showed that the diversity of the soil microbial community around E. adenophorum roots increase significantly with increased of invasion level, this diverse soil microbial community could potentially form a micro-environment conducive to the competitive advantage of invasive plants in karst areas. Additionally, AM fungi can promote exotic plants in competition for nutrients with native plants in southwest China (Li et al., 2016). Mycorrhizal networks connecting plant roots can transfer the disease-resistant signaling between plants (Zhang et al., 2019, 2020), as well as to transfer mineral nutrients from one plant to another (He et al., 2019). However, the role that this mechanism plays in plant invasions in karst habitats has not been well documented.
Ba et al. (2018) found invasion changes mycorrhizal fungi community in the soil, which could contribute to increase competitive advantage of exotic plant over native plant. Mycorrhizal networks connecting plant roots have been shown to transfer mineral nutrients between invasive and native plants, preferentially to invasive species (Awaydul et al., 2019). Therefore, mycorrhizal networks affect the competition for nutrients between invasive and native plants in karst habitat, and thus the aim of this study is to elucidate the role of AM fungi in the invasion mechanism of alien plants. In our previous field survey, the exotic plant E. adenophorum already invaded the karst area in a large area and coexists with native A. annua, which distributes as a common species in the karst area of southwest China. Especially these two species are Asteraceae herbaceous plants with a high niche overlap (Liu et al., 2010). Therefore, we speculated that E. adenophorum competing with A. annua might be caused by the regulation of nutrients by mycorrhizae roles. We hypothesize that: (1) Interconnected arbuscular mycorrhizal networks can enhance P uptake and reduce the N/P ratio of invasive and native species in karst soil (H1); (2) AM fungi confer a competitive advantage for nutrients to invasive species over native species in karst soil (H2).
Materials and Methods
The Experimental Growth Microcosm
Experimental microcosms consisting of three compartments were constructed using polypropylene plastic in the greenhouse of Forestry College of western campus in Guizhou university, Guiyang, China (106°22′E, 29°49′N, 1120 m above the sea level). The thickness of the material was 2 mm (Figure 1). Each microcosm was composed of two planting compartments on opposite sides and a competition compartment in the center. Five circular holes of 5mm diameter were drilled in the baffle plate between the planting compartments (Pc) and competition compartment (Cc). Depending on the treatment, nylon meshes of 20 μm or 0.45 μm were attached to either side of the baffle plate to form an air gap to prevent the flow of nutrients among compartments. The 20 μm nylon mesh can allow mycelium to pass through but not for plant roots, while 0.45 μm nylon mesh can neither allow mycelium nor roots to pass through (Yang et al., 2017). The size of each of the three compartments was about 10 cm × 10 cm × 10 cm (length × width × height).
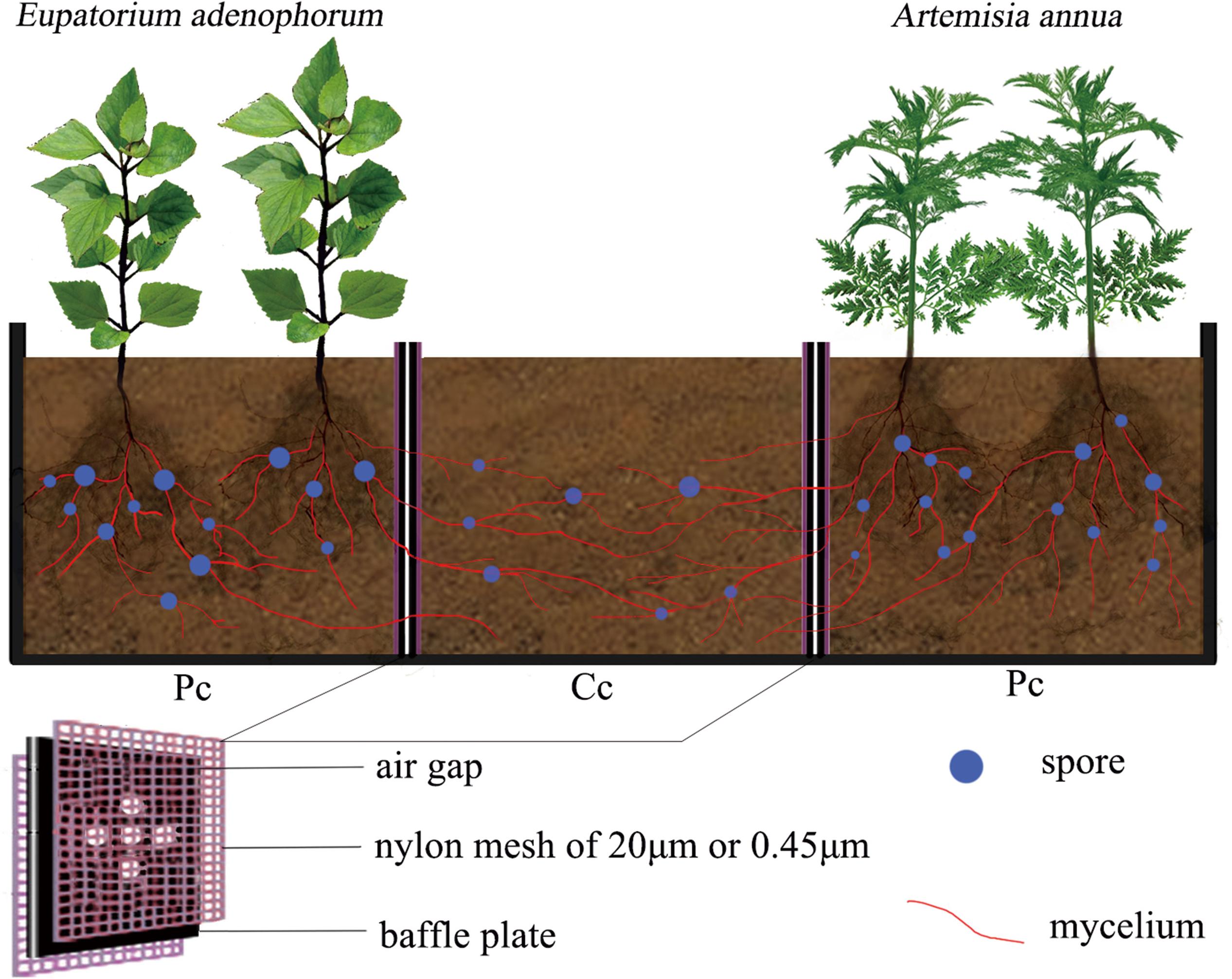
Figure 1. The compartmentalized microcosm growing device. The setup consists of three compartments, two plant compartments on either end, one for the native species and one for the invasive species, and one competition compartment in the center. Plates containing five holes separate the compartments. Depending on the experimental treatment, one of two different meshes line the plates separating the plant compartments from the competition compartment. 20 μm nylon mesh allows mycelium to pass through but not the plant root system; the 0.45 μm nylon mesh blocks both mycelium and the plant root system from passing through the baffle plates into the competition compartment. An air gap within the baffle plate, described in more detail in the text, prevents the flow of nutrients between the three compartments. E. adenophorum = invasive plant; Artemisia annua = native plant; Pc = planting compartment; Cc = competition compartment.
The experiment was conducted using the mycorrhizal fungus treatments with or without C. etunicatum (purchased from the Institute of Nutrition Resources, Beijing Academy of Agricultural and Forestry Sciences, BGA0046) and the nutrient utilization treatments for competition compartment through mycorrhizal networks interconnecting two planting compartments, were constructed using meshes of 20 μm for mycelium connectivity and of 0.45 μm for no mycelium connectivity. The mycorrhizal fungus treatments included the inoculation with AM fungi (M+), and the control without AM fungi (M−); The nutrient utilization treatments were the following: (1) the common nutrient competition (common utilization, Cu) treatment in which the competition compartment was allowed to be connected to the planting compartments of E. adenophorum and A. annua via a mycorrhizal network (20 μm of double-nylon meshes were used on both sides of two baffle plates, allowing mycelium to grow freely from both side compartments into the competition compartment); (2) the single nutrient utilization (Su) treatment in which the competition compartment could be connected by mycelium to one planting compartment of E. adenophorum or A. annua only (20 μm of double-nylon mesh used on the one baffle plate, with 0.45 μm mesh on the other plate, allowing AM mycelium to pass through from one planting compartment to the competition compartment, but preventing in-growth from the other planting compartment); (3) the nutrient non-utilization (Nu) as a control (0.45 μm nylon meshes against the two baffle plates on both sides, to prevent the growth of AM mycelium into competition compartments). The mycelium and spore existed in competition compartment soil by checking under Cu and Su of M+ treatments, which showing that mycelium passed through planting compartment to competition compartment through mycelial expansion, and formed interconnective mycorrhizal networks between Pc and Ac.
A 2.5 kg mixture containing limestone soil and sand (3:1 by volume) was placed into each compartment; this mixture had first been sterilized at 0.14 Mpa at 126°C for 1 h. The soil, which had been collected from a typical karst habitat close to Guiyang city, had pH 7.45, total nitrogen (N) concentration 2.27 g kg–1, available N 127.48 mg kg–1, total phosphorus(P) 0.90 g kg–1, available P 11.48 mg kg–1, total potassium (K) 4.99 g kg–1, available K 287.30 mg kg–1, by the measurement refering to Tan (2005). The seeds of E. adenophorum and A. annua were collected from a karst mountain experiencing severe soil degradation in Guanling county, Guizhou province, China. Five seeds of each species were planted into the soil in their respective planting compartments and two plants per compartment were kept after germination. 50g of inoculum was added after seeding, consisting of spores, mycelium and plant roots. The inoculum had been propagated with Trifolium repens for 4 months which had been grown in limestone soil by the pre-sterilized treatment at 0.14 Mpa, at 126°C for 1 h before inoculation with C. etunicatum. Each treatment was repeated six times. Thus, the overall factorial experimental design contained three nutrient access treatments times two mycorrhizal treatments (M+, M−) times six replicates as 36 samples. From germination, the experimental plants were cultured in a greenhouse for 3 months and were arranged in a randomized block design in western campus of Guizhou university, then were harvested for measurement.
Measurements of the Mycorrhizal Colonization Rate, Biomass, Nitrogen, and Phosphorus
The determination of mycorrhizal colonization rate adopted methods described by He et al. (2012). The plant biomass of E. adenophorum and A. annua was determined by weighing root, stem and leaf material after drying at 80°C for 24 h. The determinations of N and P concentration of plant tissues followed the traditional Kjeldahl method and the Molybdenum-antimony anti-colorimetric method (Bao, 2000). The N acquisition and P acquisition were calculated by multiplying each plant nutrient concentration by each plant biomass.
Statistical Analysis
Statistical analyses were performed using the SPSS 23.0 software. All of the data were tested for normality and homogeneity of variance before analysis. First, two- or three-way or three ANOVAs were applied to test for the overall effects of plant species (E. adenophorum, A. annua) mycorrhizal fungus (M+, M−), nutrient utilization treatments (Cu, Su, and Nu) and their interactions on plant biomass, N acquisition and P acquisition and N/P ratio, respectively. Then the single factor differences between Cu, Su, and Nu on biomass, N and P acquisition and the N/P ratio, respectively, were analyzed by the least significant difference (LSD) test, and a t-test was applied to compare the differences in N acquisition and P acquisition and the N/P ratio between treatments of M+ and M−. All results graphics were produced using the Origin 2018 software.
Results
The Mycorrhizal Colonization Rate of E. adenophorum and A. annua Seedlings
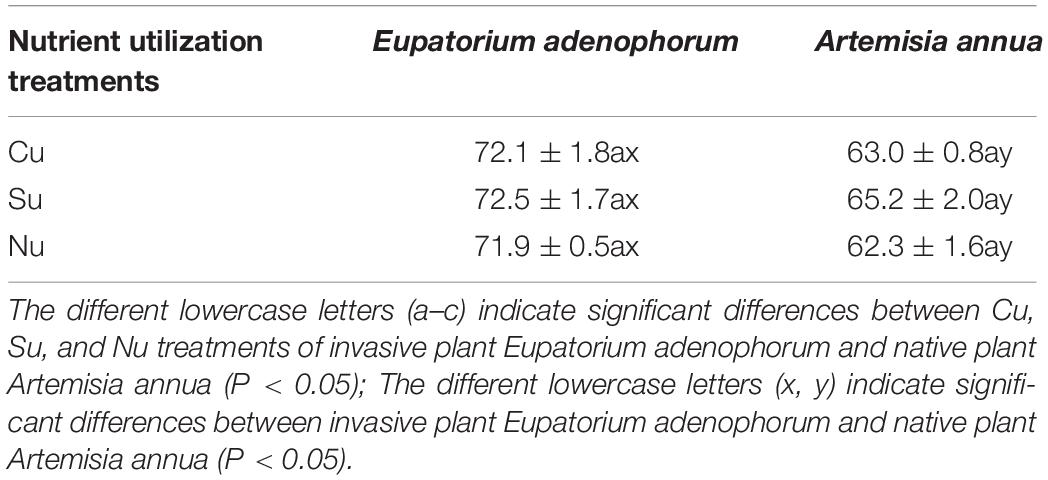
The mycorrhizal colonization rates of E. adenophorum were significantly higher than those of A. annua under Cu, Su and Nu treatments (Table 1). For the mycorrhizal colonization rates of E. adenophorum and A. annua, there were non-significant differences among the Cu, Su, and Nu treatments. Both species had the same pattern of mycorrhizal colonization rate as follows: Su > Cu > Nu.
The Biomass of E. adenophorum and A. annua Seedlings and Its Allocation to Root, Stem, and Leaf
For E. adenophorum seedlings, the mycorrhizal fungus treatments significantly affected the individual, root and leaf biomass but did not affect stem biomass (Table 2). Significantly larger biomass in M+ than in M− was measured for whole, plant individuals under Nu, for root biomass under Cu, Su, and Nu and for leaf biomass under Su, while an opposite significant response (M+ < M−) was seen for stem biomass under Su (Figures 2A–D). The nutrient utilization treatments significantly affected stem biomass (Table 2); the stem biomass of Su was significantly lower than that of Cu and Nu under M+ treatment (Figure 2C). The interaction of M × U significantly affected stem biomass but did not significantly affect root, leaf or individual biomass (Table 2). On the other hand, for A. annua seedlings, the mycorrhizal fungus treatments significantly affected the stem biomass but did not significantly affect individual plant, root or leaf biomass (Table 2). A significant M+ > M− effect was present in individual and stem biomass under Su treatment. Adversely (M+ < M−) affected individual biomass and leaf biomass under Cu treatment (Figures 2A,C,D). Nutrient utilization treatments significantly affected leaf biomass (Table 2). Under M+ treatment, the stem biomass under Cu and Su was significantly greater than under Nu treatment, and under M− treatment, the individual and stem biomass under Cu and Su was also significantly greater than under Nu treatment, the root and leaf showed no significant difference among Cu, Su, and Nu treatments in this study (Figures 2A–D). The interaction of M × U did not significantly affect individual, root, stem and leaf biomass of A. annua (Table 2). Besides, the mycorrhizal fungus treatments significantly affected the individual biomass. However, the plant species and nutrient utilization were significantly affected seedling accumulations via three-way ANOVAs analysis, including their interactions (Table 6). Overall, the results indicated that AM fungi differentially increased the biomass accumulation of the invasive plant E. adenophorum and native plant A. annua, even though an adverse non-significant trend (M+ < M−) was observed in individual biomass of A. annua under the Cu treatment. After inoculation with AM fungi, the biomass of A. annua was greater than that of E. adenophorum under Su treatment. However, E. adenophorum biomass was even larger in comparison to A. annua under the Cu and Nu treatments. This result indicates that the biomass of E. adenophorum was higher than that of A. annua when E. adenophorum competed with A. annua for nutrients, but compared to E. adenophorum, when native A. annua utilized nutrients singly via its mycelium, it accumulated more biomass than the invasive E. adenophorum.
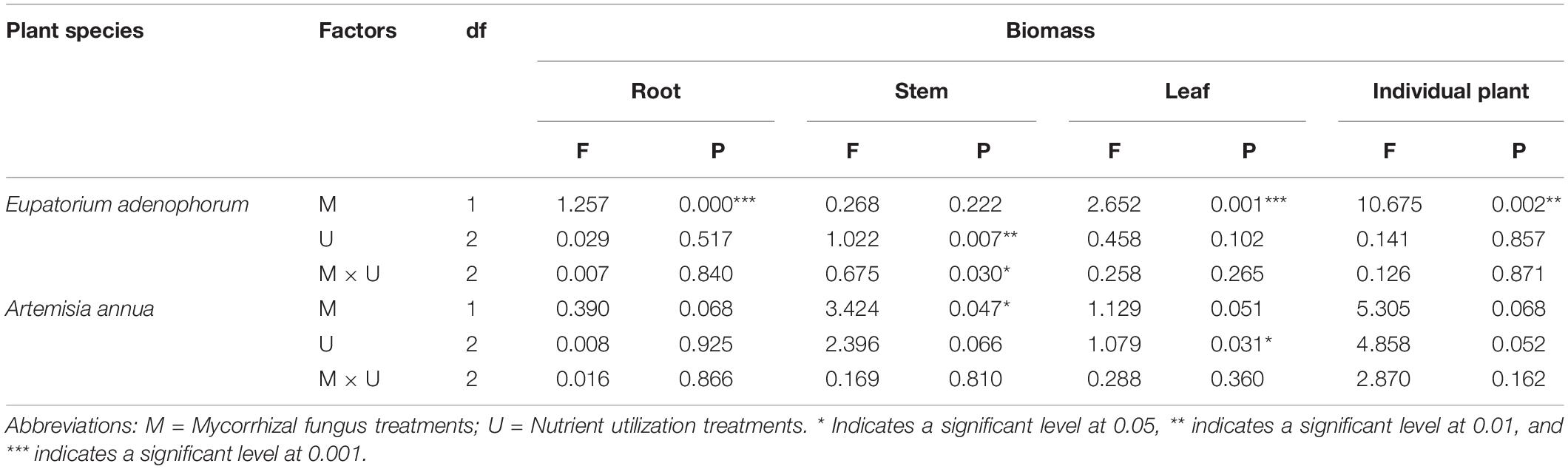
Table 2. Two-Way ANOVAs for the effects of mycorrhizal fungus (M+ vs M−) and nutrient utilization treatments (Cu vs Su vs Nu) on biomass of the invasive plant Eupatorium adenophorum and the native plant Artemisia annua.
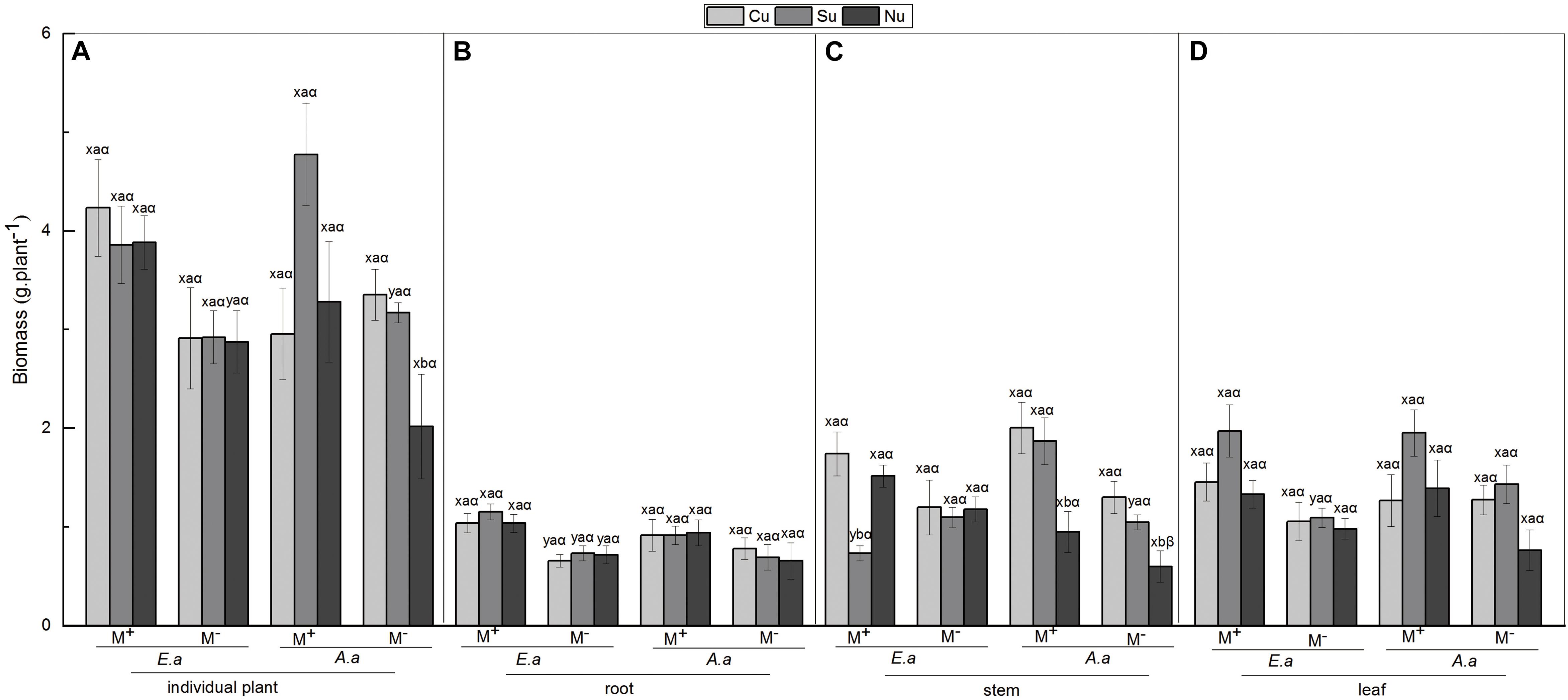
Figure 2. Biomass of E. adenophorum and A. annua seedlings and its allocation to root, stem and leaf. Abbreviations: E. a = E. adenophorum; A. a = A. annua; M+ = with AM fungus; M− = without AM fungus; Cu (Common utilization) = invasive plant and native plant can access the competition compartment due to their mycorrhizal networks passing through the 20 μm nylon mesh; Su (Single utilization) = the invasive plant or the native plant singly have access the competition compartment due to their mycelium passing through the 20 μm nylon mesh; Nu (non-utilization) = Neither the invasive plant nor the native plant have access the competition compartment due to their mycelium both being blocked by 0.45 μm nylon mesh. The different lowercase letters (x, y) above the bars indicate significant differences between M+ and M− treatments of invasive plant E. adenophorum and native plant A. annua (P < 0.05); The different lowercase letters (a, b, c) above the bars indicate significant differences between Cu, Su and Nu treatments of invasive plant E. adenophorum and native plant A. annua (P < 0.05); The different Greek letters (α, β) above the bars indicate significant differences between invasive plant E. adenophorum and native plant A. annua (P < 0.05).
The N Acquisition of E. adenophorum and A. annua Seedlings and Its Allocation to Root, Stem and Leaf
For the invasive plant E. adenophorum, the mycorrhizal fungus treatments significantly affected root N acquisition and did not significantly affect stem, leaf or individual N acquisition (Table 3). A significant M+ > M− effect was observed in root N acquisition under Cu, Su, and Nu treatments, but a significant M+ < M− effect was observed in stem N acquisition under the Su treatment (Figures 3B,C). The nutrient utilization treatments had a significant effect on root, leaf and individual N acquisition, but did not significantly affect stem N acquisition (Table 3). Stem N acquisition was observed as Cu > Su > Nu under the M+ treatment, while the N acquisition of individual, root and leaf was observed as Su > Cu > Nu under M+ and M− treatments (Figures 3A–D). The interaction of M × U significantly affected stem N acquisition but did not significantly affect N acquisition of root, leaf or individual (Table 3). For the native plant A. annua, there were no significant effects of the mycorrhizal fungus treatments on N acquisition of root, stem, leaf or individual plant (Table 3). A significant difference between M+ and M− treatments was observed in individual and stem N acquisition under Su, while M+ and M− did not significantly affect root or leaf N acquisition under Cu, Su and Nu treatments (Figures 3A–D). The nutrient utilization treatments significantly affected stem, leaf and individual N acquisition, but the effect on root N acquisition was non-significant (Table 3). Under the M+ treatment, the root N acquisition was observed as Cu > Su > Nu, the individual, stem and leaf N acquisition was observed as Su > Cu > Nu; Under M− treatment, the stem N acquisition was observed as Cu > Su > Nu, the individual, root and leaf N acquisition was observed as Su > Cu > Nu (Figures 3A–D). The interaction of M × U did not significantly affect N acquisition of root, stem, leaf or individual plant (Table 3).
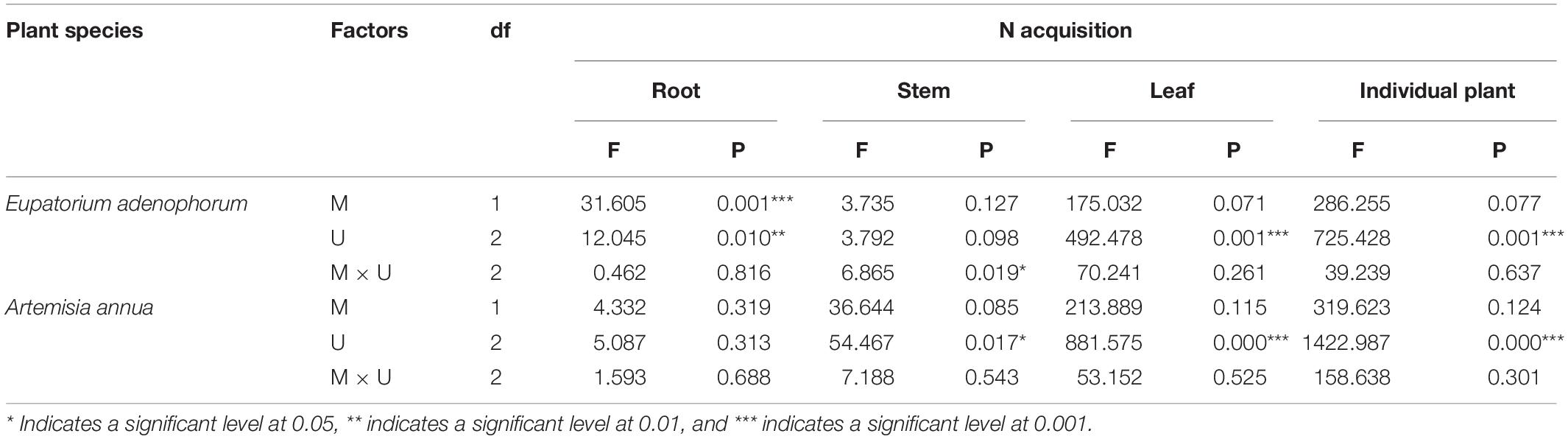
Table 3. Two-Way ANOVAs for the effects of mycorrhizal fungus (M+ vs M−) and nutrient utilization treatments (Cu vs Su vs Nu) on N acquisition of the invasive plant Eupatorium adenophorum and the native plant Artemisia annua.
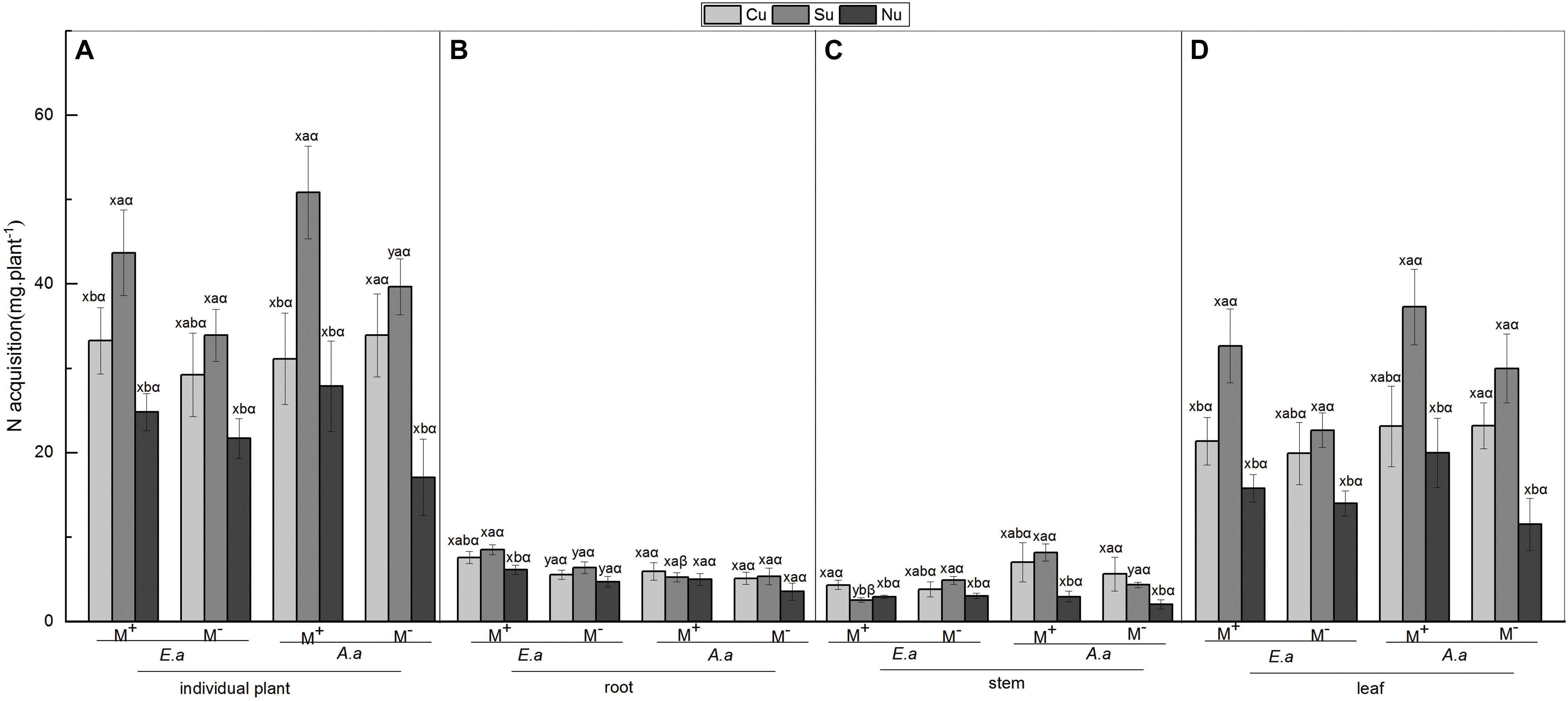
Figure 3. N acquisition of E. adenophorum and A. annua seedlings and its allocation to root, stem and leaf. Abbreviations: E. a and A. a; M+ and M−; Cu and Su and Nu, meaning same as in Figure 2. The lowercase letters x and y, (a, b, c). The Greek letters α and β above the bars, they mean the same as in Figure 2.
For individual plants, under M− treatment, the N acquisition of A. annua was higher than that of E. adenophorum under Su and Cu treatments. In addition, under M+ treatment, N acquisition of A. annua was also greater than E. adenophorum under Su but that of A. annua was lower than E. adenophorum under Cu treatment (Figure 3A). Furthermore, the mycorrhizal fungus treatments and the nutrient utilization treatments significantly affected individual plants N acquisition but not for plant species and their interactions via further three-way ANOVAs analysis (Table 6). This result suggests that AM fungi differentially enhanced the N acquisition for the invasive E. adenophorum and the native A. annua, even though a non-significant adverse effect (M+ < M−) was observed in individual N acquisition for the native A. annua under Cu treatment. After inoculation with AM fungi, when the native A. annua absorbed nutrients from the competitive compartment through it mycelium singly under Su treatment, the N acquisition was higher than that of E. adenophorum, but when both species had access to the competitive compartment through mycelium, the N acquisition of A. annua was lower than that of E. adenophorum.
The P Acquisition of E. adenophorum and A. annua Seedlings and Its Allocation to Root, Stem, and Leaf
For E. adenophorum, the mycorrhizal fungus treatments significantly affected P acquisition of root, stem, leaf and individual plant (Table 4). A significant difference between M+ and M− was observed in individual, root, and leaf P acquisition under Cu, Su, and Nu treatments, and in stem P acquisition under Cu and Nu treatments (Figures 4A–D). The nutrient utilization treatments significantly affected stem, leaf and individual P acquisition (Table 4). Under M+ treatment, the individual and stem P acquisition under Cu was significantly higher than under Su and Nu treatment, and leaf P acquisition under Cu and Su was significantly greater than under Nu treatment; Under M− treatment, the stem P acquisition under Cu and Su treatments was significantly lower than Nu, and leaf P acquisition under Su was significantly higher than Su and Nu treatments (Figures 4A,C,D). The interaction of M × U significantly affected P acquisition of stem and leaf (Table 4). For A. annua, the mycorrhizal fungus treatments significantly affected P acquisition of stem, leaf and individual plant (Table 4). A significant difference between M+ and M− was observed in individual and stem P acquisition under the Su treatment (Figures 2A,C). The nutrient utilization treatments significantly affected leaf and individual P acquisition (Table 4). A significant Su > Nu was observed in individual P acquisition under the M+ and M− treatments, and stem P acquisition in Cu and Su was significantly higher than in Nu under M+ and M− treatments, and leaf P acquisition in Cu and Su was significantly higher than in Nu under the M− treatment (Figures 4A,C,D). The interaction of M × U did not significantly affect P acquisition in root, stem, leaf or individual plant (Table 4).
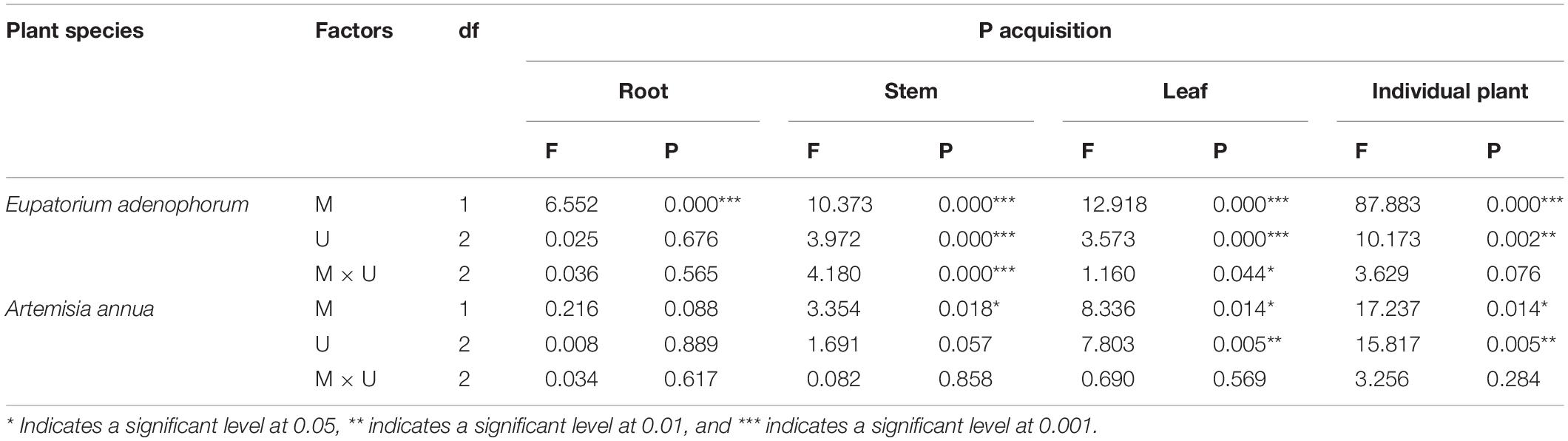
Table 4. Two-Way ANOVAs for the effects of mycorrhizal fungus (M+ vs M−) and nutrient utilization treatments (Cu vs Su vs Nu) on P acquisition of the invasive plant Eupatorium adenophorum and the native plant Artemisia annua.
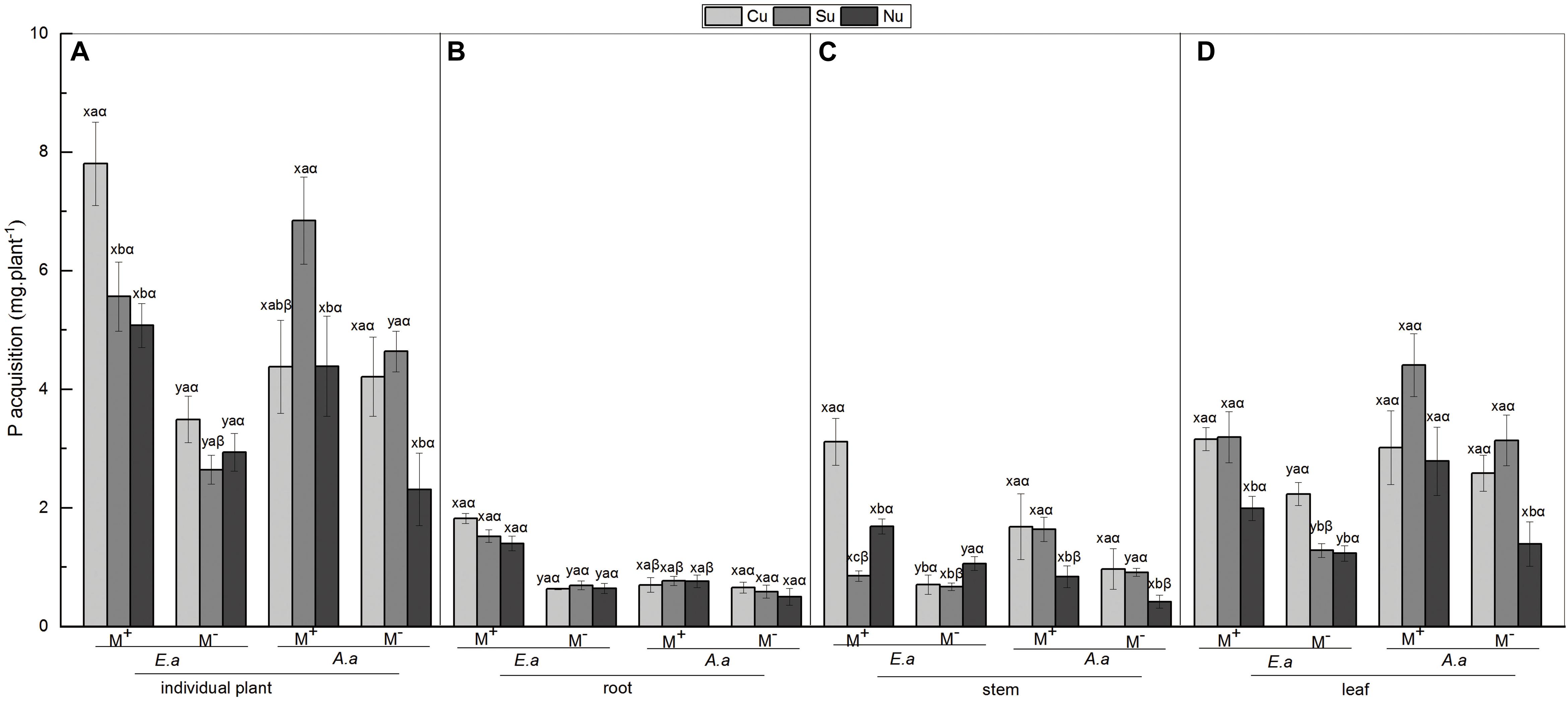
Figure 4. P acquisition of E. adenophorum and A. annua seedlings and its allocation to root, stem and leaf. Abbreviations: E. a and A. a; M+ and M−; Cu and Su and Nu, meaning same as in Figure 2. The lowercase letters x and y, (a, b, c). The Greek letters α and β above the bars, they mean the same as in Figure 2.
For individual plants, under M− treatment, the P acquisition of A. annua was higher than E. adenophorum under Su and Cu treatments, but the P acquisition of A. annua was lower than E. adenophorum under Nu treatment. In addition, under M+ treatment, A. annua was also greater than E. adenophorum under Su but was lower than E. adenophorum under Cu and Nu treatments (Figure 4A). Besides, the mycorrhizal fungus treatments and the nutrient utilization treatments significantly affected individual plants P acquisition, as well as the interaction of S × M, S × U, and S × M × U for plants P acquisition, but not for plant species (Table 6). It showed AM plays a crucial role in P utilization of two species, even though biomass was not affected significantly by species. The intrinsic differences were evident in the ability to accumulate biomass for these two species. These results illustrate that AM fungi significantly increased P acquisition of the invasive E. adenophorum, and differentially enhanced the P acquisition of the native plant A. annua. Moreover, when E. adenophorum competed with A. annua for nutrients (under Cu condition), the AM fungi enabled the invasive plant E. adenophorum to accumulate more P than the native plant A. annua.
The N/P Ratio of E. adenophorum and A. annua Seedlings Including to Root, Stem, and Leaf
Firstly, for E. adenophorum seedlings, the mycorrhizal fungus treatments significantly affected root, stem, leaf and individual plant N/P ratio (Table 5). A significant M+ < M− effect was observed in individual plant, root, and leaf N/P ratio under Cu, Su, and Nu treatments, and in stem N/P ratio under Cu and Su treatments (Figures 5A–D). The nutrient utilization treatments significantly affected root, stem, leaf and individual N/P ratio (Table 5). The root N/P ratio was observed as Cu > Su > Nu under M− treatment, but it was observed as Su > Cu > Nu under the M+ treatment, and the N/P ratio of individual, stem and leaf was observed as Su > Cu > Nu under M+ and M− treatments (Figures 5A–D). The interaction of M × U significantly affected stem, leaf and individual plant N/P ratio (Table 5). Secondly, for A. annua seedlings, the mycorrhizal fungus treatments was significantly affected stem and individual plant N/P ratio, but did not significantly affect root or leaf N/P ratio (Table 5). A significant M+ < M− effect was observed in individual N/P ratio under Cu, Su and Nu treatments, and in root N/P ratio under the Su treatment, and in stem N/P ratio under the Cu treatment (Figures 5A–C). The nutrient utilization treatments significantly affected stem and individual plant N/P ratio (Table 5). The N/P ratio of individual plants was observed as Su > Cu > Nu under M+ and M− treatments, and the stem N/P ratio under Cu treatment was significantly greater than Su and Nu under M− treatment (Figures 5A,C). The interaction of M × U significantly affected the stem N/P ratio (Table 5). Finally, for individual plant N/P ratio, under M− treatment, the N/P ratio of invasive E. adenophorum was significantly higher than that of native A. annua under Su treatment; Under M+ treatment, the individual plant N/P ratio of invasive E. adenophorum was significantly lower than that of native A. annua under Cu and Nu treatments (Figure 5A). The mycorrhizal fungus treatments significantly affected individual plants N/P ratio, but not for plant species and mycorrhizal fungus through further three-way ANOVAs analysis (Table 6). Overall, AM fungi significantly reduced the N/P ratio of both the invasive plant E. adenophorum and the native plant A. annua.
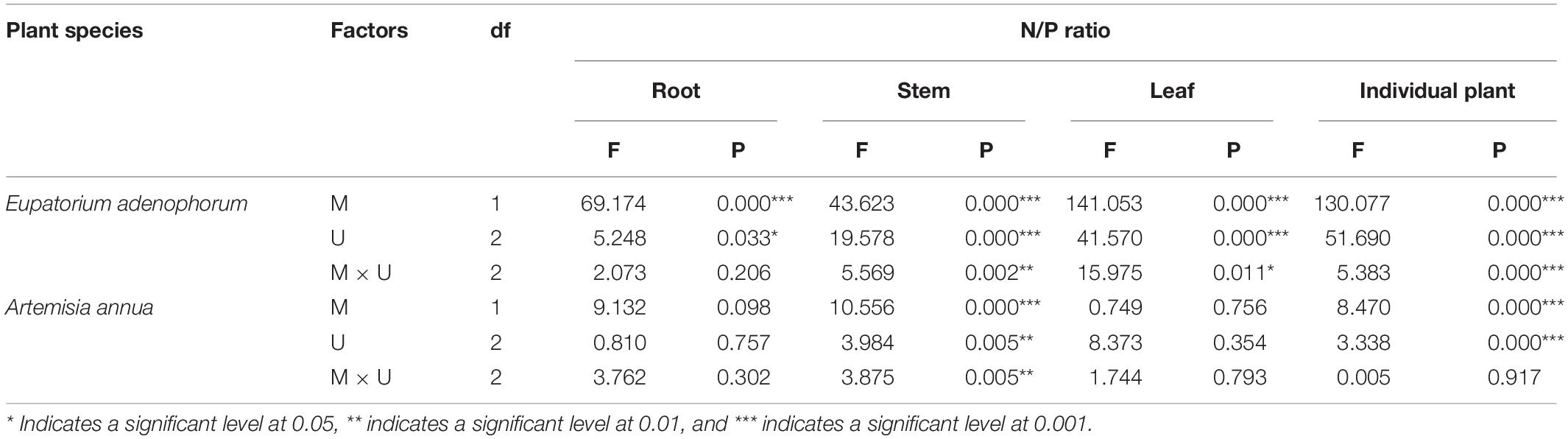
Table 5. Two-Way ANOVAs for the effects of mycorrhizal fungus (M+ vs M−) and nutrient utilization treatments (Cu vs Su vs Nu) on N/P ratio of the invasive plant Eupatorium adenophorum and the native plant Artemisia annua.
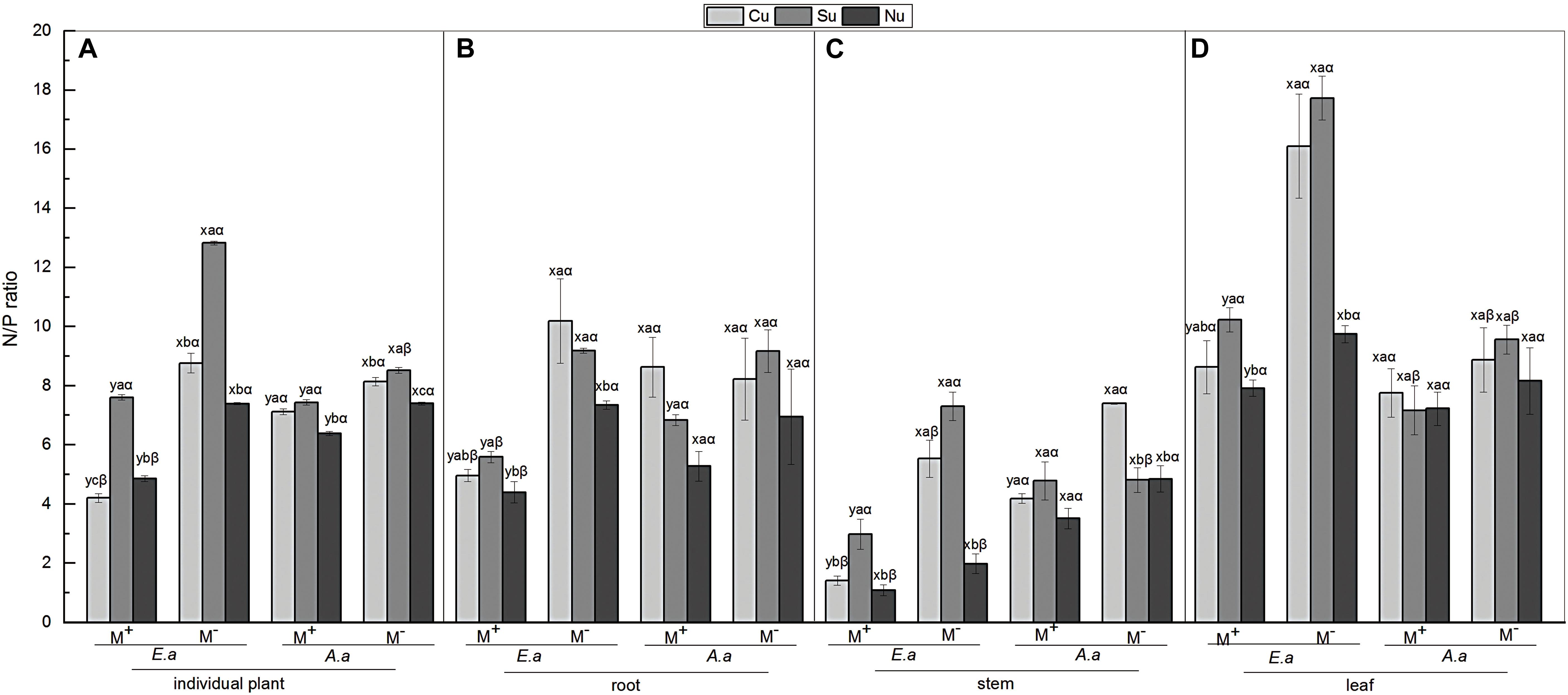
Figure 5. N/P ratio of E. adenophorum and A. annua seedlings including in root, stem and leaf. Abbreviations: E. a and A. a; M+ and M−; Cu and Su and Nu, meaning same as in Figure 2. The lowercase letters x and y, (a, b, c). The Greek letters α and β above the bars, they mean the same as in Figure 2.
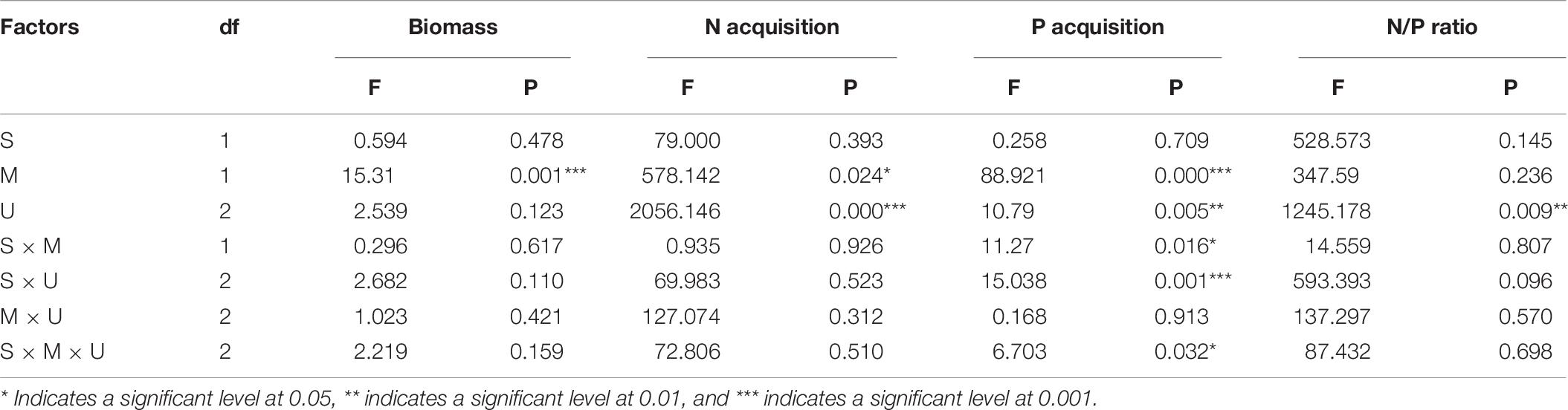
Table 6. Three-Way ANOVAs for the effects of species (Eupatorium adenophorum vs Artemisia annua), mycorrhizal fungus (M+ vs M−) and nutrient utilization (Cu vs Su vs Nu) on biomass, N acquisition, P acquisition and N/P ratio.
Discussion
AM Fungi Differentially Affected the Biomass Accumulation and Nutrient Utilization Between Invasive and Native Species
AM fungi differentially increased the biomass, and the acquisitions of N and P for the invasive E. adenophorum and native plant A. annua in this experiment (Figures 2A, 3A, 4A). Previous studies demonstrated that soil microbes affected nutrient transformation and plant growth (Lugtenberg et al., 2013; Leff et al., 2015), for instance, AM fungi facilitated plants’ uptake of mineral nutrients by extending the spatial absorption scale and thereby promoting plant growth (Lin et al., 2017; Wang W. X. et al., 2017). He et al. (2007) revealed that the AM fungi promoted the accumulation of biomass, N and P of the host plants in karst soil. In this study our results broadly illustrate that AM fungi increases biomass, N and P acquisition through expanding the surface area of absorbed nutrients by mycelium in both a native and invasive plant species. Plant resource allocation patterns can reflect the adaptation of plant growth and development to environmental conditions (Aoyagi and Akimoto, 2010). In our study, both plant species accumulated more biomass, N and P in the stem and leaf than root (Figures 2–4), which indicating that both plants preferentially allocate resources to photosynthetic organ leaves and stems, and thereby producing more photosynthetic products for their growth (Saxena and Ramakrishnan, 2010). He and Han (2010) showed that the N/P ratio of plant leaves can be used to assess the nutrient status of soil. Plants with an N/P ratio that is less than 14 are thought to be mainly limited in growth by N, while plants with an N/P ratio greater than 16 are mainly limited by P, and plants with a ratio between 14 and 16 are co-limited by both N and P (Koerselman and Meuleman, 1996). In this study, the N/P ratio of the invasive E. adenophorum decreased from above 16 to below 14 via AM fungi under Cu and Su treatments (Figure 5D). This indicates that the growth of the invasive plant E. adenophorum may be limited by P in karst areas of southwest China, and that AM fungi alleviates this limitation. AM fungi have been shown to promote P uptake by plants, resulting in P no longer being the plant’s main limiting factor (Yang et al., 2017). This phenomenon is of great significance to plant growth in the low P areas of karst regions. In addition, Cu treatment symbolizing mycorrhizal networks connecting both species reduced the N/P ratio of invasive and native species in karst soil in this experiment (Figure 5A), and this is consistent with our hypothesis (H1). Invasive E. adenophorum had stronger P absorption than A. annua via interconnected mycorrhizal networks in this study (Figure 4A). Therefore, the N/P ratio of the invasive E. adenophorum was lower than that of the native A. annua. Plants in karst areas are characterized by low levels of P (Wen et al., 2018), and AM fungi can promote absorption by host plants of P in the soil (Zhang et al., 2018). Additionally, Hu et al. (2016) argued that typical alien invasive plants have higher P uptake in southwestern China, which is consistent with the results of this study. This means that E. adenophorum would be more invasive due to successful competition for nutrients in the fragile lower-phosphorus karst habitats.
Nutrient Competition Between Invasive and Native Species via Interconnected Mycorrhizal Networks
When invasive plants competed with native plants, AM fungi tend to have a positive impact on invasive plants (Neuenkamp et al., 2019). Yu et al. (2014) argued that AM fungi enhanced the competitiveness of invasive E. adenophorum against native species. Higher mycorrhizal colonization rate gives a competitive advantage to invader over native species in nutrient resources (Zhang et al., 2017), which supporting that E. adenophorum had greater competitive advantage due to higher mycorrhizal colonization rate, when comparing with A. annua by Table 1. Our data indicated that when the invasive E. adenophorum competed with native A. annua for nutrients in karst areas, the AM fungi conferred the invasive plant E. adenophorum a competitive advantage in obtaining nutrients as compared to the native plant A. annua, and E. adenophorum had higher mycorrhizal colonization rate than A. annua. This was consistent with our hypothesis (H2). In the karst regions of southwest China, the AM fungi composition differed significantly between vegetation types (Liang et al., 2016), and the invasion of alien plants will shift the species composition and diversity of AM fungi, change the preference of AM fungi to the original host, and establish a symbiotic relationship favorable to the invasive plant (Vogelsang and Bever, 2009). Additionally, AM fungi can facilitate the invasion of alien species by decreasing the competitiveness of the native plants (Yang et al., 2014), and promoting the growth of invasive species in a nutrient-poor environment (Chen Q. et al., 2019), which might contribute to their successful invasion into karst habitats. Moreover, AM fungi conferred invasive plants with greater advantage in competition with native plants, and AM fungi had a negative impact on the growth of native plants (Batten et al., 2008; Zubek et al., 2016). Fellbaum et al. (2014) showed that mycorrhizal networks of AM fungi in the soil simultaneously provide multiple host plants with nutrients, and AM fungi can preferentially allocate nutrients to high-quality hosts. Yuan et al. (2014) argued that invasive plant may produce substances in the soil that inhibit native plants. It may explain our results that interconnected mycorrhizal network may give priority to nutrients to the invasive plant E. adenophorum, while biomass and N acquisition of the native plant A. annua decreased via an interconnected mycorrhizal network (Figures 2A, 3A). AM fungi only had a very significant effect on the root biomass accumulation and N and P acquisition of the invasive plant E. adenophorum, but had no any significant effect on the root of native plant A. annua (Tables 2–4). The root system of E. adenophorum has strong vegetative reproductive ability, and can occupy the soil habitat quickly, and its root system can enrich AM fungi in the rhizosphere and break the reciprocal symbiotic relationship between AM fungi and native plants (Jiang et al., 2014). Additionally, Hawkes et al. (2006) argued that invasive plants influence the availability of the mycorrhizal fungi network to native plants in soil via earlier root activity compared to native plants. Therefore, in the karst habitats of southwest China, earlier root activity of E. adenophorum compared to the native plant A. annua may be one of the important reasons for the former’s invasiveness. In summary, the invasive E. adenophorum can successfully invade in the karst soil, possibly through a competitive advantage in nutrient uptake in comparison to native plants, and the effect of AM fungi on the invasiveness of E. adenophorum may be mediated by earlier root activity.
Conclusion
In conclusion, AM fungi can promote the accumulation of biomass, N and P of E. adenophorum and A. annua, and conferred the invasive plant E. adenophorum a competitive advantage in nutrient acquisition in comparison to the native plant A. annua. Finally, the interconnected mycorrhizal networks conferred the invasive E. adenophorum greater alleviation effect of phosphorus limitation than the native A. annua in karst areas, potentially contributing to the former’s invasiveness. Further study is needed to test whether the interactions between invasive and native plant nutrient acquisition and growth performance, as mediated by AM mycorrhizal networks as reported here are commonplace in other species combinations and soil environments.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
All authors contributed to the formulation, writing, and revision of this manuscript and made direct intellectual contributions. They all approve of its publication.
Funding
This study was supported by the national Natural Science Foundation of China (NSFC: 31660156; 31360106; 31560223), the First-class Disciplines Program on Ecology of Guizhou Province (GNYL[2017]007), the Natural Science Foundation of China and the Karst Science Research Center of Guizhou Province (Grant No. U1812401), the Guizhou High-level (hundred-levels) Innovative Talents Project (Qiankehe talent-platform [2020] 6004), the Provincial Key Technologies R&D Program of Guizhou Province of China (NY[2014]3029; [2016] Zhi-cheng 2805), the Special Program Foundation on Training the Young Talents for Science & Technology by Guizhou Province (Qian-ke-he-ren [2013]10), the Talent-platform Program of Guizhou Province ([2017]5788; [2018]5781).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aoyagi, Y., and Akimoto, M. (2010). Reactive shifts in the pattern of resource allocation in three Lolium species with different levels of persistency under clipping disturbance. Grassl. Sci. 55, 181–186. doi: 10.1111/j.1744-697X.2009.00157.x
Awaydul, A., Zhu, W. Y., Yuan, Y. G., Xiao, J., Hu, H., Chen, X., et al. (2019). Common mycorrhizal networks influence the distribution of mineral nutrients between an invasive plant, Solidago canadensis, and a native plant, kummerowa striata. Mycorrhiza 29, 29–38. doi: 10.1007/s00572-018-0873-5
Ba, L., Facelli, E., and Facelli, J. M. (2018). Plant-mycorrhizal fungi feedbacks: potential accomplices of avena barbata’s high invasiveness. Plant Ecol. 219, 1045–1052. doi: 10.1007/s11258-018-0857-8
Batten, K. M., Scow, K. M., and Espeland, E. K. (2008). Soil microbial community associated with an invasive grass differentially impacts native plant performance. Microb. Ecol. 2, 220–228. doi: 10.1007/s00248-007-9269-3
Callaway, R. M., and Aschehoug, E. T. (2000). Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290, 521–523. doi: 10.1126/science.290.5491.521
Callaway, R. M., Thelen, G. C., Rodriguez, A., and Holben, W. E. (2004). Soil biota and exotic plant invasion. Nature 427, 731–733. doi: 10.1038/nature02322
Carey, E. V., Marler, M. J., and Callaway, R. M. (2004). Mycorrhizae transfer carbon from a native grass to an invasive weed: evidence from stable isotopes and physiology. Plant Ecol. 172, 133–141. doi: 10.1023/B:VEGE.0000026031.14086.f1
Chen, D., Ali, A., Lin, C. G., Yong, X. H., Niu, X. H., Cai, A. M., et al. (2019). A multi-species comparison of selective placement patterns of ramets in invasive alien and native clonal plants to light, soil nutrient and water heterogeneity. Sci. Total Environ. 657, 1568–1577.
Chen, Q., Wu, W. W., Qi, S. S., Cheng, H., Li, Q., Ran, Q., et al. (2019). Arbuscular mycorrhizal fungi improve the growth and disease resistance of the invasive plant Wedelia trilobata. J. Appl. Microbiol. doi: 10.1111/jam.14415
Fellbaum, C. R., Mensah, J. A., Cloos, A. J., Strahan, G. E., Pfeffer, P. E., Kiers, E. T., et al. (2014). Fungal nutrient allocation in common mycorrhizal networks is regulated by the carbon source strength of individual host plants. New Phytol. 203, 646–656. doi: 10.1111/nph.12827
Hawkes, C. V., Belnap, J., D’Antonio, C., and Firestone, M. K. (2006). Arbuscular mycorrhizal assemblages in native plant roots change in the presence of invasive exotic grasses. Plant Soil 281, 369–380. doi: 10.1007/s11104-005-4826-3
He, J. S., and Han, X. G. (2010). Ecological stoichiometry: searching for unifying principles from individuals to ecosystems. Chin. J. Plant Ecol. 34, 2–6. doi: 10.3724/SP.J.1142.2010.40521
He, Y. J., Cornelissen, J. H. C., Wang, P. P., Dong, M., and Ou, J. (2019). Nitrogen transfer from one plant to another depends on plant biomass production between conspecific and heterospecific species via a common arbuscular mycorrhizal network. Environ. Sci. Pollut. R 26, 8828–8837. doi: 10.1007/s11356-019-04385-x
He, Y. J., Zhong, Z. C., and Dong, M. (2012). Nutrients transfer for host plant and litter decomposition by amf in karst soil. Acta. Ecol. Sin. 32, 2525–2531. doi: 10.5846/stxb201111101702
He, Y. J., Zhong, Z. C., Liu, J. M., Liu, J. C., Jin, J., and Song, H. X. (2007). Response of n and p absorption on Broussonetia papyrifera seedlings to inoculate vesicular-arbuscular mycorrhizal fungus. Acta Ecol. Sin 27, 4840–4847. doi: 10.1016/S1872-2032(07)60072-9
Hu, C. C., Liu, X. Y., Lei, Y. B., Tan, Y. H., Zhang, P., Dong, Y. P., et al. (2016). Foliar nitrogen and phosphorus stoichiometry of alien invasive plants and co-occurring natives in xishuangbanna. Chin. J. Plant Ecol. 40, 1145–1153. doi: 10.17521/cjpe.2016.0052
Jia, H. J., Li, X. K., Tnag, S. C., Tang, S. Q., and Xu, X. L. (2009). Allelopathic effects of Eupatorium adenophorum on seed germination of three woody plants in karst region. Guihaia 29, 631–639.
Jiang, C., Shui, W., Jian, X. M., Guo, P. P., and Chen, Y. P. (2019). Soil microbial community characteristics in degraded karst tiankeng invaded by Eupatorium adenophorum. Chin. J. Appl. Ecol. 30, 2002–2010. doi: 10.13287/j.1001-9332.201906.03
Jiang, Z. L., Wang, W. Y., Lei, G. S., Gui, F. R., Liu, W. X., and Li, Z. Y. (2014). Root growth characteristics and competitive effects of Ageratina adenophora and four functional type herbaceous plants. Chin. J. Appl. Ecol. 25, 2833–2839. doi: 10.13287/j.1001-9332.2014.0152
Johnson, N. C. (2010). Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 185, 631–647. doi: 10.1111/j.1469-8137.2009.03110.x
Koerselman, W., and Meuleman, A. F. M. (1996). The vegetation N: P ratio: a new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 33, 1441–1450.
Leff, J. W., Jones, S. E., Prober, S. M., Barberán, A., Borer, E. T., Firn, J. L., et al. (2015). Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. U.S.A. 112, 10967–10972. doi: 10.1073/pnas.1508382112
Li, L. Q., Zhang, M. S., Liang, Z. P., Xiao, B., Wan, F. H., and Liu, W. X. (2016). Arbuscular mycorrhizal fungi enhance invasive plant, ageratina Adenophora growth and competition with native plants. Chin. J. Ecol. 35, 79–86. doi: 10.13292/j.1000-4890.201601.011
Li, Y. B., Hou, J. J., and Xie, D. T. (2002). The recent development of research on karst ecology in southwest china. Sci. Geogra Sin. 22, 365–370. doi: 10.3969/j.issn.1000-0690.2002.03.019
Liang, Y. M., Pan, F. J., He, X. Y., Chen, X. B., and Su, Y. R. (2016). Effect of vegetation types on soil arbuscular mycorrhizal fungi and nitrogen-fixing bacterial communities in a karst region. Environ. Sci. Pollut. R 23, 18482–18491. doi: 10.1007/s11356-016-7022-5
Lin, J. X., Wang, Y. N., Sun, S. N., Mu, C. S., and Yan, X. F. (2017). Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 576, 234–241. doi: 10.1016/j.scitotenv.2016.10.091
Liu, C. Q., Lang, Y. C., Li, S. L., Piao, H. C., Tu, C., Liu, Z., et al. (2009). Researches on biogeochemical processes and nutrient cycling in karstic ecological systems, southwest china: a review. Earth Sci. Front. 16, 1–12. doi: 10.1016/S1003-6326(09)60084-4
Liu, S. C., Liao, Z. Y., He, L., Zhang, Z. G., and Zhang, X. (2010). Allelopathic effects of associated herbs Artemisia carvifolia and Eulaliopsis binata on Eupatorium adenophorum sprengel. J. Anhui Agric. Sci. 38, 6167–6168. doi: 10.3969/j.issn.0517-6611.2010.12.036
Liu, Z. H., and Zhao, J. (2000). Contribution of carbonate rock weathering to the atmospheric co2 sink. Environ. Geol. 39, 1053–1058. doi: 10.1007/s002549900072
Lugtenberg, B. J. J., Malfanova, N., Kamilova, F., and Berg, G. (2013). Plant growth promotion by microbes. Mol. Microb. Ecol. Rhizosphere 2, 561–573. doi: 10.1002/9781118297674.ch53
Mei, L. L., Yang, X., Cao, H. B., Zhang, T., and Guo, J. X. (2019). Arbuscular mycorrhizal fungi alter plant and soil C: N: P stoichiometries under warming and nitrogen input in a semiarid meadow of china. Int. J. Environ. Res. Pub He 16:397. doi: 10.3390/ijerph16030397
Merrild, M. P., Ambus, P., Rosendahl, S., and Jakobsen, I. (2013). Common arbuscular mycorrhizal networks amplify competition for phosphorus between seedlings and established plants. New Phytol. 200, 229–240. doi: 10.1111/nph.12351
Neuenkamp, L., Zobel, M., Lind, E., Gerz, M., and Moora, M. (2019). Arbuscular mycorrhizal fungal community composition determines the competitive response of two grassland forbs. PLoS ONE 14:e0219527. doi: 10.1371/journal.pone.021952
Rodgers, V. L., Wolfe, B. E., Werden, L. K., and Finzi, A. C. (2008). The invasive species Alliaria petiolata (garlic mustard) increases soil nutrient availability in northern hardwood-conifer forests. Oecologia 157, 459–471. doi: 10.1007/s00442-008-1089-8
Ryan, M. H., Tibbett, M., Edmonds-Tibbett, T., Suriyagoda, L. D. B., Lambers, H., Cawthray, G. R., et al. (2012). Carbon trading for phosphorus gain: the balance between rhizosphere carboxylates and arbuscular mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ. 35, 2170–2180. doi: 10.1111/j.1365-3040.2012.02547.x
Saxena, K. G., and Ramakrishnan, P. S. (2010). Growth and patterns of resource allocation in Eupatorium odoratum l. In the secondary successional environments following slash and burn agriculture (jhum). Weed Res. 24, 127–134. doi: 10.1111/j.1365-3180.1984.tb00580.x
Selosse, M. A., Richard, F., He, X. H., and Simard, S. W. (2006). Mycorrhizal networks: des liaisons dangereuses? Trends Ecol. Evol. 21, 621–628. doi: 10.1016/j.tree.2006.07.003
Smith, M. D., and Knapp, A. K. (2001). Physiological and morphological traits of exotic, invasive exotic, and native plant species in tallgrass prairie. Int. J. Plant Sci. 162, 785–792. doi: 10.1086/320774
Van der Putten, W. H., Bardgett, R. D., Bever, J. D., Bezemer, T. M., Casper, B. B., Fukami, T., et al. (2013). Plant–soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265–276. doi: 10.1111/1365-2745.12054
Vila, M., and Weiner, J. (2004). Are invasive plant species better competitors than native plant species?–evidence from pair-wise experiments. Oikos 105, 229–238. doi: 10.1111/j.0030-1299.2004.12682.x
Vogelsang, K. M., and Bever, J. D. (2009). Mycorrhizal densities decline in association with nonnative plants and contribute to plant invasion. Ecology 90, 399–407. doi: 10.1890/07-2144.1
Wagg, C., Jansa, J., Stadler, M., Schmid, B., and Van Der Heijden, M. G. A. (2011). Mycorrhizal fungal identity and diversity relaxes plant–plant competition. Ecology 92, 1303–1313. doi: 10.1890/10-1915.1
Wang, S. J., Liu, Q. M., and Zhang, D. F. (2004). Karst rocky desertification in southwestern china: geomorphology, landuse, impact and rehabilitation. Land Degrad. Dev. 15, 115–121. doi: 10.1002/ldr.592
Wang, W. Q., Sardans, J., Wang, C., Zeng, C. S., Tong, C., Asensio, D., et al. (2015). Ecological stoichiometry of c, n, and p of invasive Phragmites australis and native Cyperus malaccensis species in the minjiang river tidal estuarine wetlands of china. Plant Ecol. 216, 809–822. doi: 10.1007/s11258-015-0469-5
Wang, W. X., Shi, J. C., Xie, Q. J., Jiang, Y., Yu, N., and Wang, E. T. (2017). Nutrient exchange and regulation in arbuscular mycorrhizal symbiosis. Mol. Plant 10, 1147–1158. doi: 10.1016/j.molp.2017.07.012
Wang, Y. J., Chen, D., Yan, R., Yu, F. H., and van Kleunen, M. (2019). Invasive alien clonal plants are competitively superior over co-occurring native clonal plants. Perspect. Plant Ecol. Evol. Syst. 40:125484.
Wang, Y. J., Müller-Schärer, H., van Kleunen, M., Cai, A. M., Zhang, P., Yan, R., et al. (2017). Invasive alien plants benefit more from clonal integration in heterogeneous environments than natives. New Phytol. 216, 1072–1078.
Wen, P. C., Wang, L. J., and Sheng, M. Y. (2018). Research progress in ecological stoichiometry of karst forest ecosystem in southwest china. World For. Res. 31, 66–71. doi: 10.13348/j.cnki.sjlyyj.2017.0091.y
Yang, R. Y., Zhou, G., Zan, S. T., Guo, F. Y., Su, N. N., and Li, J. (2014). Arbuscular mycorrhizal fungi facilitate the invasion of Solidago canadensis l. In southeastern china. Acta Oecol. 61, 71–77. doi: 10.1016/j.actao.2014.10.008
Yang, Y., Jiang, C. H., He, Y. J., Qu, J., and Lin, Y. (2017). Effects of arbuscular mycorrhizal networks on the n and p contents and stoichiometry of three plants species from karst area. Plant Physiol. J. 53, 2078–2090. doi: 10.13592/j.cnki.ppj.2017.0144
Yu, W. Q., Wan, F. H., He, X. H., Liu, W. Z., Liu, W. X., and Zhang, L. L. (2014). Soil microbes enhance competition ability of the exotic Ageratina Adenophora sprengel against native plant species. J. Biosaf. 23, 156–164. doi: 10.3969/j.issn.2095-1787.2014.03.004
Yuan, Y. G., Tang, J. J., Leng, D., Hu, S. J., Yong, J. W. H., and Chen, X. (2014). An invasive plant promotes its arbuscular mycorrhizal symbioses and competitiveness through its secondary metabolites: indirect evidence from activated carbon. PLoS ONE 9:e97163. doi: 10.1371/journal.pone.0097163
Zhang, F., Zou, Y. N., Wu, Q. S., and Kuca, K. (2020). Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environ. Exp. Bot. 171, 103926. doi: 10.1016/j.envexpbot.2019.103926
Zhang, F. J., Li, Q., Chen, F. X., Xu, H. Y., and Wan, F. H. (2017). Arbuscular mycorrhizal fungi facilitate growth and competitive ability of an exotic species Flaveria bidentis. Soil Biol. Biochem. 115, 275–284. doi: 10.1016/j.soilbio.2017.08.019
Zhang, L., Shi, N., Fan, J. Q., Wang, F., George, T. S., and Feng, G. (2018). Arbuscular mycorrhizal fungi stimulate organic phosphate mobilization associated with changing bacterial community structure under field conditions. Environ. Microbiol. 20, 2639–2651. doi: 10.1111/1462-2920.14289
Zhang, Y. C., Zou, Y. N., Liu, L. P., and Wu, Q. S. (2019). Common mycorrhizal networks activate salicylic acid defense responses of trifoliate orange (poncirus trifoliata). J. Integr. Plant Biol. 61, 1099–1111. doi: 10.1111/jipb.12743
Zubek, S., Majewska, M. L., Błaszkowski, J., Stefanowicz, A. M., Nobis, M., and Kapusta, P. (2016). Invasive plants affect arbuscular mycorrhizal fungi abundance and species richness as well as the performance of native plants grown in invaded soils. Biol. Fert Soils 52, 879–893. doi: 10.1007/s00374-016-1127-3
Keywords: arbuscular mycorrhizal, mycorrhizal network, invasive plants, nutrient competition, karst
Citation: Shen K, Cornelissen JHC, Wang Y, Wu C, He Y, Ou J, Tan Q, Xia T, Kang L, Guo Y and Wu B (2020) AM Fungi Alleviate Phosphorus Limitation and Enhance Nutrient Competitiveness of Invasive Plants via Mycorrhizal Networks in Karst Areas. Front. Ecol. Evol. 8:125. doi: 10.3389/fevo.2020.00125
Received: 11 March 2020; Accepted: 17 April 2020;
Published: 13 May 2020.
Edited by:
Zhi-Cong Dai, Jiangsu University, ChinaReviewed by:
Asad Shabbir, University of Sydney, AustraliaQiang-Sheng Wu, Yangtze University, China
Copyright © 2020 Shen, Cornelissen, Wang, Wu, He, Ou, Tan, Xia, Kang, Guo and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuejun He, hyj1358@163.com
 Kaiping Shen
Kaiping Shen J. Hans C. Cornelissen
J. Hans C. Cornelissen Yongjian Wang3
Yongjian Wang3 Yuejun He
Yuejun He