- 1Department of Environmental Sciences, Informatics and Statistics, Ca’ Foscari University of Venice, Venice, Italy
- 2Department of Chemical and Pharmaceutical Sciences, University of Ferrara, Ferrara, Italy
Aquatic angiosperms favor the development of ecosystems services, the welfare of marine organisms and people. Generally, the presence of angiosperms in transitional water systems (TWS) are indicators of good ecosystem status. Presently, these environments are densely populated and often are so highly degraded that angiosperms have almost disappeared, replaced by tionitrophilic macroalgae responsible of anoxic events that deteriorate the environment furtherly. Although this trend is hardly reversible because the anthropogenic impact is increasing and the restoring of damaged environments within a reasonable time is difficult, recent studies have shown that by managing the harvesting of the natural algal species of commercial interest a progressive environmental recovery is achievable. Biomass-harvesting can contribute both to the removal of high amounts of nutrients and the generation of economic revenues for a sustainable, self-financed environmental restoration. In fact, unlike clam-farming which destroys the seabed and re-suspends large amounts of sediments, the proper management of the macroalgal biomass, can favor the nutrient abatement and the recolonization of aquatic angiosperms which help restore the conditions necessary for the conservation of the benthic and fish fauna and birds, and produce valuable economic resources.
Introduction
Primary producers in transitional water systems (TWS) are mainly represented by macrophytes: aquatic angiosperms and macroalgae. The former usually prevail over the others, but that depends on the ecological status of the study area. Aquatic angiosperms are considered the most important producers under pristine conditions (Orfanidis et al., 2001; Sfriso et al., 2007; Viaroli et al., 2008). They colonize environments with a low trophic status and provide a variety of ecosystem services (ES) whose most common meaning is:
• The benefits people obtain from ecosystems (Millenium Ecosystem Assessment, 2005);
• Natural processes and components that benefit human needs directly or indirectly (Nordlund et al., 2016).
A conceptual framework for ES classification was established in the studies of the Millennium Ecosystem Assessment and the Economics of Ecosystems and Biodiversity (Arico et al., 2005; Barker et al., 2010) that define four major classes, inclusive of numerous subclasses, based on the kind of service provided by ecosystems for human well-being (Liquete et al., 2013; Hattam et al., 2015; Haines-young and Potschin, 2018), i.e., Regulation services, Habitat services, Provisioning services, and Cultural services.
The ES supplied by the macrophytes, both aquatic angiosperms and macroalgae, may belong to all those categories. In fact, aquatic angiosperms reduce sediment resuspension, favor clear waters, contrast sediment erosion, and contribute to permanent CO2 sequestration (Regulation services); provide shelter and nursery areas for benthic and fish fauna and pasture areas for birds (Habitat services); increase recreational activities (Cultural services); and sustain traditional fishing activities (Provisioning services) as a consequence of a general improvement of the environment. In contrast, macroalgae are an important source of biomass which is exploitable for the production of compost, fertilizer, human and animal food, pharmaceuticals, and cosmetics (Provisioning services). In pristine environments the calcareous species trap CO2, whereas in degraded environments other taxa accumulate nutrients (Sfriso and Marcomini, 1994; Sfriso et al., 1994) and contaminants (Maroli et al., 1993) (Habitat services).
Recent studies have shown that macrophytes can be used as water quality indicators and bio-ecological sentinels. They play a key role in the indices of ecological status set up for the assessment of TWS (Ecological Evaluation Index continuous – EEI-c, Orfanidis et al., 2011; Macrophyte Quality Index – MaQI, Sfriso et al., 2014) and coastal areas (Posidonia oceanica Rapid Easy Index – PREI, Buia et al., 2003; EEI-c, Orfanidis et al., 2011; CARLIT, Ballesteros et al., 2007; Sfriso and Facca, 2011) according to the requirements of the Water Framework Directive (2000/60/EC) (European. Union [Eu], 1992; European Union [Eu], 2000).
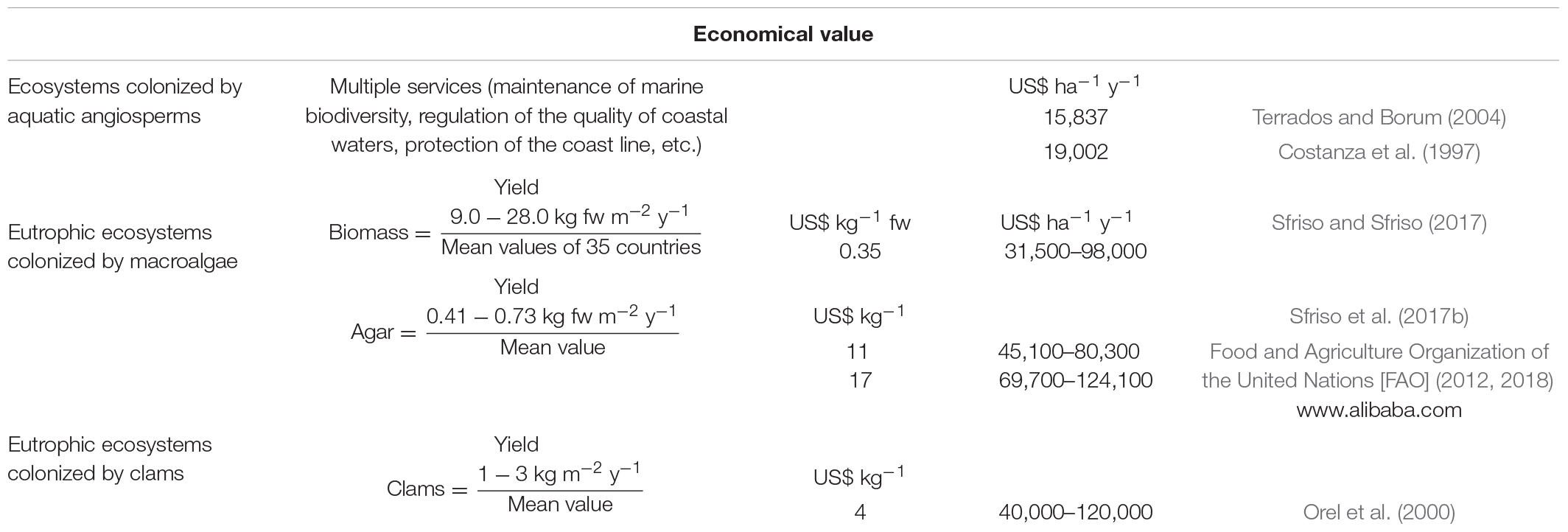
Terrados and Borum (2004) estimated that environments colonized by aquatic angiosperms produced a minimum revenue of US$ 15,837 ha–1 y–1, which is nearly twice the revenue from croplands. Costanza et al. (1997) have come to the same conclusions estimating for these plants a revenue of US$ 19,002 ha–1 y–1. Generally, nutrient recycling and water quality were the most important ES considered by those authors. However, the economic value calculated per single ES is also very important. By considering the value of large-sized, commercially targeted, fish species Tuya et al. (2014) estimated the areas covered by Cymodocea nodosa in Canary islands to be worth €866 ha–1 y–1. Other researchers as Guerry et al. (2012) reported that the value of multiple services resulting from seagrass beds reached €4228 ha–1 y–1. If we consider the carbon storage and sequestration both in the aquatic plants and surface sediments, the ES is worth ca. US$ 30.5 ha–1 y–1 (Barbier et al., 2011); but even more important is the organic-rich materials which favors the rise of the seafloor at rates from 0.6 to 6 mm y–1 (Duarte, 2013) and can be preserved over millennia.
However, the increase of anthropogenic impacts worldwide has led to a progressive degradation of the marine coasts, especially transitional areas where human pressure is remarkable (Sfriso et al., 2017a) with the consequence that often aquatic angiosperms have been replaced by fast-growing macroalgae (Morand and Briand, 1996; Ménesguen, 2018). The same changes have been observed in the Italian TWS where in the worst conditions phytoplankton or cyanobacteria blooms have prevailed (Sfriso and Facca, 2007; Cecere et al., 2009; Munari and Mistri, 2012). Under eutrophic conditions, the benefits due to the presence of aquatic angiosperms are lost, but recent studies focused on the production of macroalgae for food, cosmetics, or pharmaceuticals (Francavilla et al., 2013, 2015; Sfriso and Sfriso, 2017; Sfriso et al., 2017b) showed that the management of the biomass of some macroalgae may be economically very interesting and provide more ES (in terms of nutrient abatement and commodities) than aquatic angiosperms. In addition, the harvesting of macroalgae, which in eutrophic areas accumulate high amounts of phosphorus (P) and nitrogen (N) (Sfriso and Marcomini, 1994; Sfriso et al., 1994), may contribute to the reduction of nutrients in the environment attenuating eutrophication. By the present paper the authors intend to show that highly degraded TWS dominated by macroalgae can be recovered by a good management, and the cause of degradation transformed into a valuable resource for both the environment and people welfare, notwithstanding the fact that it is not always possible to reduce the trophic status of a water body in a short time. The studied areas are the lagoons of the Po Delta, some chocked areas of the Venice Lagoon and Pialassa della Baiona where trophic conditions are high and suffer from intense clam-farming and/or a rich growth of macroalgal biomass.
Materials and Methods
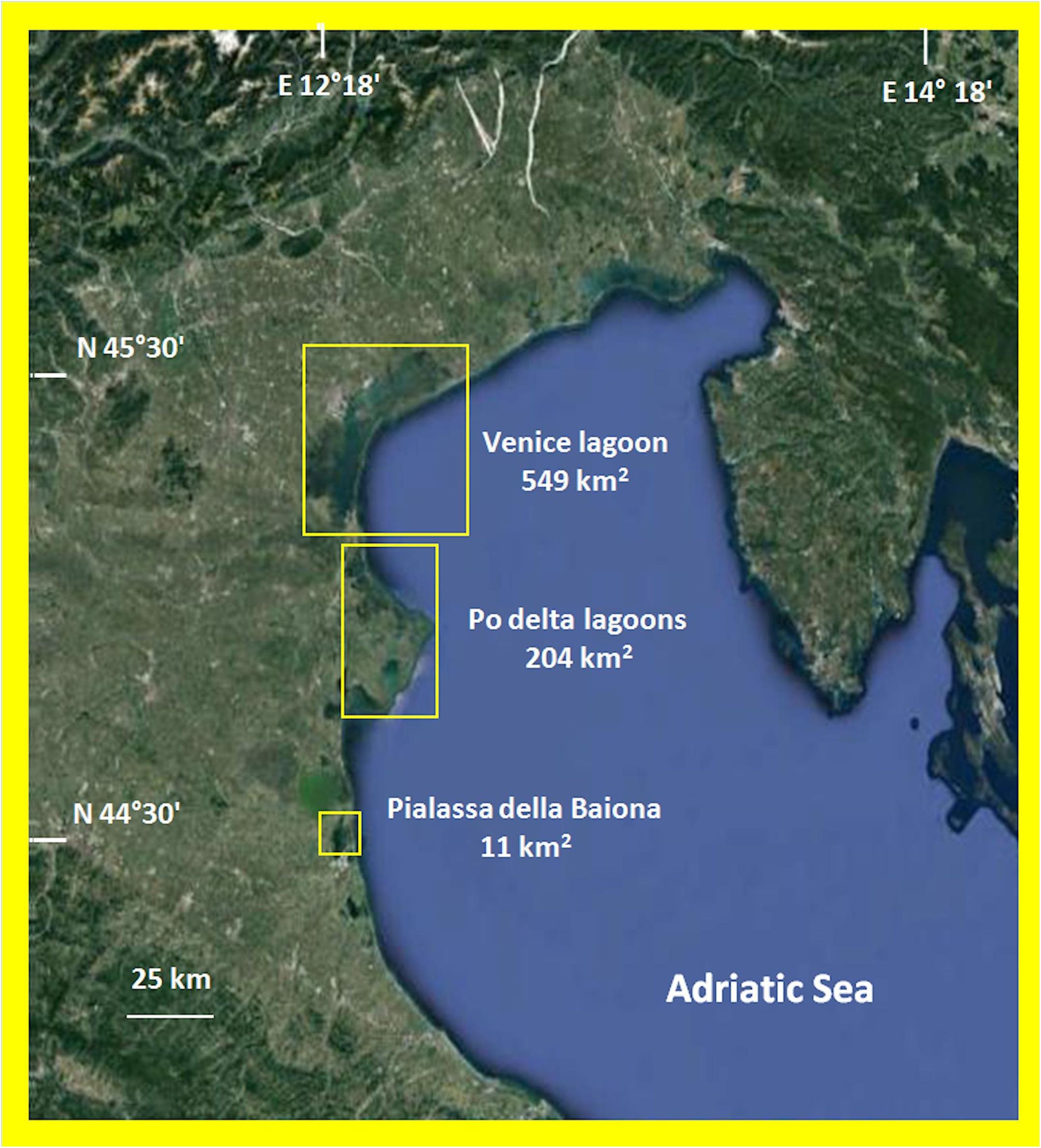
The data on macroalgal distribution come from researches carried out in various Italian TWS (Figure 1) over the last 10 years for different purposes: to apply the Water Framework Directive (2000/60/EC) (European. Union [Eu], 1992; European Union [Eu], 2000) in the lagoons of Venice (114 sites), Pialassa della Baiona (three sites) and the Po Delta (21 sites); and to study the macroalgal production of some Gracilariaceae and Ulva rigida C. Ag. over 1 year (Sfriso and Sfriso, 2017; Sfriso et al., 2017a) as part of the COST Action FA1406 (2015–2019) (Phycomorph, 2015).
The information obtained by those studies allows to assess the water surfaces colonized by aquatic angiosperms or macroalgae, and the areas which are potentially exploitable for macroalgal harvesting and nutrient abatement following the removal of the biomass.
In addition, a comparison between the potential revenue from macroalgal harvesting and the one from clam breeding/harvesting, which is a highly destructive activity for the environment (Pranovi and Giovanardi, 1994; Sfriso et al., 2005), highlights the benefits that might come from a sustainable management of the environment.
Study Areas
The Venice Lagoon is the largest TWS of the Mediterranean Sea. It has a surface of ca. 549 km2, which totals 432 km2, if islands, fishing ponds, and salt marshes are excluded. It is a very polyhedral shallow (mean depth ca. 1.2 m) environment with wide areas colonized by five different aquatic angiosperms and an almost equivalent surface colonized by a biomass of macroalgae among which Gracilariaceae, Ulvaceae, and Cladophoraceae prevail. For a more complete representation of the environmental variability of the Venice Lagoon, 114 sampling sites were selected among the 118 identified by the Regional Agency for Environmental Prevention and Protection of Veneto (ARPAV) for macrophytes monitoring according to WFD (2000/60/EC).
The Po Delta exhibits a high number of lagoons and ponds scattered on a surface of 204 km2. The environment is eutrophic, macrophyte biodiversity is low and aquatic angiosperms are missing, whereas macroalgae, especially Gracilariaceae, Solieriaceae, and Ulvaceae are abundant. These rather uniform lagoons were studied by monitoring 20 sites. Pialassa della Baiona in the Emilia Romagna Region is a small eutrophic lagoon of ca. 11 km2 which is mainly colonized by Gracilariaceae and aquatic angiosperms are absent. Its variability was tested by monitoring three sites. The sites selected in the Po Delta and Pialassa della Baiona Lagoons were also the same monitored by the Regional Agencies for Environmental Prevention and Protection of Veneto and Emilia Romagna.
On the whole, these TWS have a surface of 764 km2 and represent ca. 76% of TWS of the Northern Adriatic Sea (1008 km2) and ca. 55% of the total TWS in Italy (1398 km2, Sfriso et al., 2017a).
Macroalgal Composition Analysis
Samples of three Gracilariaceae: Agarophyton vermiculophyllum (Ohmi) Gurgel, J. N. Norris et Fredericq; Gracilaria gracilis (Stackhouse) Steentoft, L. M. Irvine & Farnham; and Gracilariopsis longissima (S. G. Gmelin) Steentoft, L. M. Irvine & Farnham (12 samples per 4 species) were collected monthly for chemical analyzes from March 2014 to February 2015 in two stations of the Venice Lagoon choked area, one with clear waters (st. a) and the other with turbid waters (st. b). Macroalgae were wet weighed, lyophilized, and re-weighed to calculate the dry weight and to determine the dry/wet-weight ratio. Ashes were measured after 2 h combustion at 440°C. The organic matter content was calculated by weight difference. The mean and standard deviation values determined in the two stations were presented.
Nutrient Analysis in Algal Tissues
The concentration of P was determined by applying the phosphomolybdenum blue method according to Strickland and Parsons (1972) after a 2 h digestion of 0.3 g of dry biomass in teflon bombs at 130°C with 4 ml of Milli-Q, 3 ml of HNO3, and 3 ml of HClO4 (Kornfeldt, 1982). Analyzes were replicated in different days and the result uncertainty was <5%. The total N was determined by using a Flash 2000 CHNS Elemental Analyzer of Thermo Fisher Scientific (Monza, Italy). Measurements were replicated in different days and precision was >3%.
Polysaccharide Determination
The native sulfated agar was extracted from a 50 mg algal sample using 10 ml of Milli-Q water at 100°C for 2 h, vortexing for few seconds every 15 min for 1.5 h. The extracts were analyzed only after the solution became clear by the colorimetric method of Soedjak (1994), adding a methylene blue solution to a diluted hydrocolloid shifting absorbance to 559 nm. The calibration curves were calculated from the native sulfated agar of the respective algae after they had been purified using the method of Gonzalez-Leija et al. (2009). This native sulfated agar can be converted to a valuable commercial agar by means of pre-treatments that removes the sulfated groups and reduces the yield by approx. 55% (Wakibia et al., 2001) improving the chemical and physical properties of the gel. All reagents were purchased from Sigma–Aldrich, SRL, Milano (Italy). All the chemical analyzes were done in triplicate and repeated until the analytical reproducibility (coefficient of variation) was within 5%.
Biomass Harvesting and Production
The biomass level recorded during a sampling period must be taken into account before selecting the areas intended for production. The highest yield of Gracilariaceae is obtained from a biomass of approx. 3–4 kg fw m–2 (Friedlander and Levy, 1995; Sfriso and Sfriso, 2017). Accordingly, in the areas where Gracilariaceae prevail it is important to keep the biomass around that value by collecting only the exceeding macroalgae every 7–15 days, depending on the season and growth rate. In addition, the biomass should be hand-harvested, since bottoms are usually lower than 1 m on the mean tide level. Flat boats should be used to avoid sediment re-suspension and reduce impacts on the environment. A similar procedure was followed in the 1980s when up to 7000 tonnes y–1 of Gracilariaceae were harvested in the Venice Lagoon for agar production (Orlandini and Favretto, 1987; Sfriso et al., 1994).
In our production measurements 200 g of A. vermiculophyllum, G. gracilis, and G. longissima were inserted in green plastic-coated cubical wire-cages (25 × 25 × 25 cm with 1-cm net mesh), one species per cage. Two cages per species were placed on the bottom where, seasonally, other species could be present. On the whole, between March 2014 to February 2015, 39 sampling campaigns were carried out. The sampling interval ranged between 7 and 11 days, depending on the weather conditions. On each sampling day, in-cage biomass was collected, dripped with a salad spinner to remove excess water (ca. 10 s), and weighed (precision: 1 g). Then, the in-cage biomass was restored to 200 g in order to obtain productions suitable to be compared with the samples from the other campaigns. The biomass of 200 g per cage used to estimate algal production throughout 1 year was selected by comparison with the mean biomass present in the two areas during preliminary surveys (ca. 3 kg fw m–2). This value was in the optimal range (3–4 kg fw m–2) for intensive Gracilaria production (Friedlander and Levy, 1995).
The relative growth rate (RGR) between two consecutive sampling days was calculated using the formula reported by Lignell et al. (1987): %RGR = 100 × [(Bt/Bo)1/t−1] where B0 = initial biomass, Bt = final biomass at time t expressed in days.
Results
Macrophyte Cover
The macrophyte distribution in the studied lagoons showed that their diversity was strongly related to the ecological conditions of the considered areas. By considering the Venice Lagoon, aquatic angiosperms colonized 23 stations out of 114 (20.2% of the total), accounting for ca. 87 km2. Conversely, the number of stations with a mean macroalgal biomass ≥3 kg fw m–2 was 18 out of 114 (16% of the total), accounting for a surface of ca. 68 km2. The lagoons of the Po Delta showed 3 stations out of 20 with a biomass ≥3 kg fw m–2, accounting for ca. 30.6 km2.
Pialassa della Baiona showed two stations covered with a high macroalgal biomass, but excluding salt marshes and canals only ca. 3 km2 showed a biomass ≥3 kg fw m–2. The dominant species was the non-indigenous species (NIS) A. vermiculophyllum.
Macroalgal Composition
The concentration of nutrients in the macroalgal tissues of the dominant taxa was studied in many areas of the Venice Lagoon (Sfriso and Marcomini, 1994; Sfriso et al., 1994). The attention was particularly focused on U. rigida and G. gracilis that represented a large portion of the lagoon biomass (Sfriso and Facca, 2007). The nutrient concentrations in tissues were strongly related to the sampling areas and seasons; the highest values were recorded in eutrophic areas where the trophic level was high and the biomass abundant (Sfriso et al., 1994). Table 1A shows the nutrients and carbon content of G. gracilis found in three very different areas in 1994, Table 1B the composition of different species of Gracilariaceae recorded in the choked area in 2014.
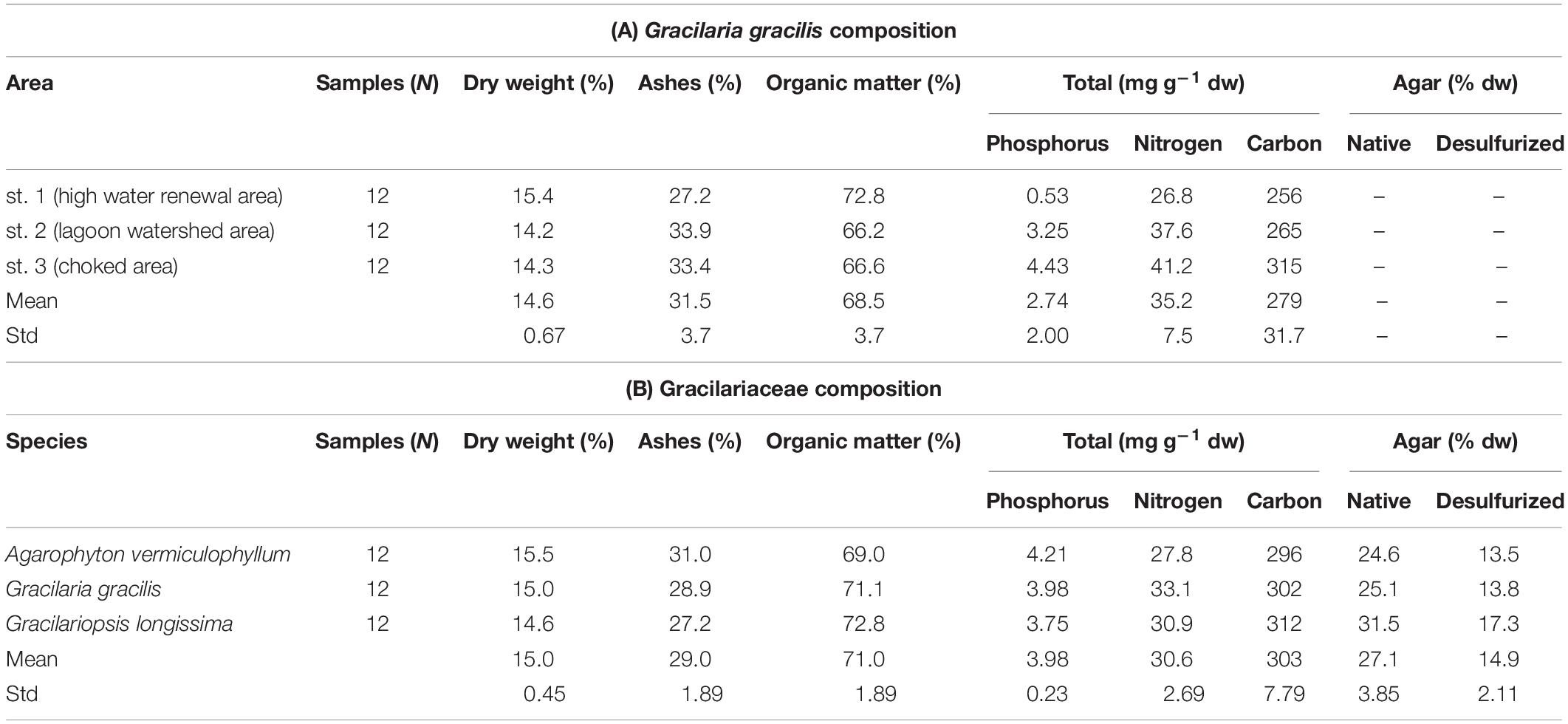
Table 1. Composition of Gracilariaceae in different areas of the Venice Lagoon: (A) composition of Gracilaria gracilis found in 1994 in three very different areas: st. 1 (high water renewal area), st. 2 (watershed area), and st. 3 (choked area) (Sfriso et al., 1994); (B) mean composition of different Gracilariaceae recorded at sts. a and b in the choked area.
The dry weight/wet weight (dw/wt) ratio of Gracilariaceae was approximately 15%, ranging between 14.2 (Table 1A) and 15.5% (Table 1B). The ash percentage fluctuated around 30% whereas, on average, the organic matter was approximately 70%. The tissue concentrations of P and N were significantly affected by the harvesting area. At st. 3 (choked area, Table 1A) Gracilaria P and N concentrations were ca. 4.43 and 41.2 mg g–1, respectively (high water renewal area), i.e., ca. 8 and 0.5 times higher than those recorded at st. 1, respectively. No significant differences were found by considering the three Gracilariaceae. The native agar concentration of these species recorded in 2014, on average, was 27.1% of the dry weight (Table 1B), with slightly higher, values in G. longissima (31.5%) accounting for a commercial (desulfurized agar) of ca.14–17% dw (Sfriso et al., 2017b).
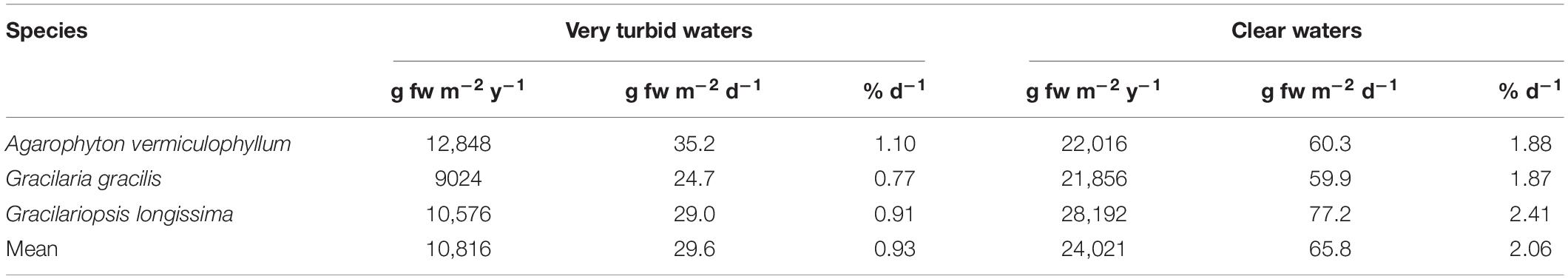
The annual net biomass production of the three Gracilariaceae ranged from 9.0 to 12.8 kg fw m–2 y–1 in very turbid waters, accounting for 24.7–35.2 g fw m–2 d–1 and a percent growth rate of 0.77–1.10 d–1. The highest net production was recorded in clear waters with 21.9–28.2 kg fw m–2 y–1 (Table 2).
The potential economical value of these biomasses is reported in Table 3 together with the estimation for the ecosystems colonized by clams and aquatic angiosperms. The potential revenue of the raw macroalgal biomass, in accordance with the estimate made by Food Agriculture Organization of the United Nations Food and Agriculture Organization of the United Nations [FAO] (2012) in 35 countries (US$ 0.35 kg–1 of fresh biomass) could vary between US$ 31,500 and 98,000 ha–1 y–1, depending on the biomass production. Specifically, if we consider the production of commercial agar and the price of US$ 11–17 kg–1, the revenue can vary from US$ 45,100–124,100 ha–1 y–1. This value is very similar to that obtained from clam production which ranged between US$ 40,000 and 120,000 ha–1 y–1. Instead the revenue obtainable from ecosystems colonized by aquatic angiosperms ranged between US$ 15,837 and 19,002 ha–1 y–1 (Costanza et al., 1998; Terrados and Borum, 2004).
Table 4 shows an estimation of the amount of nutrient abatement due to Gracilariaceae harvesting in the considered lagoons. These amounts are between 1521–4737 tonnes y–1 for N and 198–615 tonnes y–1 for P.
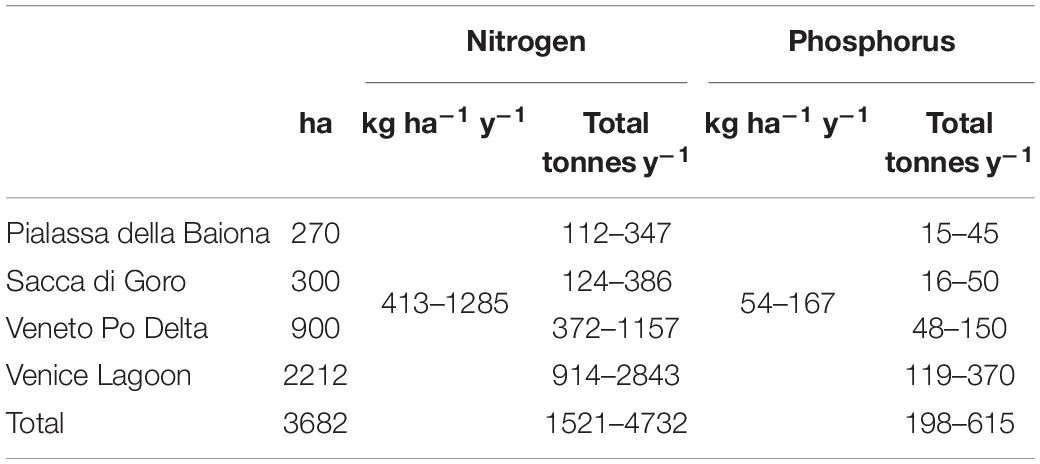
Table 4. Estimate of the potential nutrient removal by macroalgal harvesting taking into account the mean nutrient concentrations.
Finally, the potential revenue obtainable from the production of agar in areas predominantly covered by Gracilariaceae is reported in Table 5. In a total lagoon surface of approx. 3682 ha prevalently colonized by Gracilariaceae, approx. 14,985–26,731 tonnes of commercial agar per year could be produced with a total potential revenue ranging from US$ 165 to 454 million. However, this potential profit should be evaluated taking into account the production costs that have been calculated from the literature and can vary greatly depending on various factors that must be taken into due consideration.
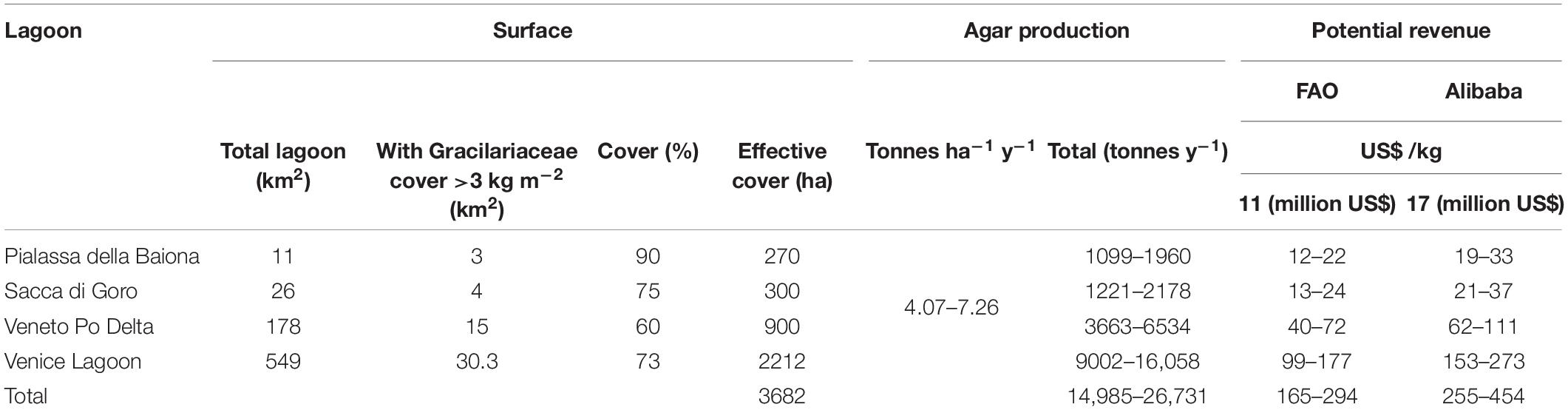
Table 5. Estimate of the potential revenue obtainable from the production of agar in areas with Gracilariaceae biomass >3 kg m–2.
Discussion
Pristine TWS are predominantly colonized by aquatic angiosperms which have a high ecological value and affect people’s recreational activities and their life positively. Unfortunately, most TWS are strongly eutrophic and plants have been replaced by opportunistic fast-growing macroalgae such as Ulvaceae, Cladophoraceae, Gracilariaceae, and Solieriaceae.
In some cases, especially in wide TWS, pristine and eutrophic conditions can coexist in the same basin and its surface is year by year sensitive to changes related to the weather variations or different anthropogenic impacts. The efforts of local administrations, national environmental agencies, and the European Community (see LIFE projects) aim at the recovery and conservation of natural and semi-natural habitats, wild flora and fauna (Habitat Directive 92/43/EEC, water Directive 2000/60/EC). However, it is not always possible to bring back pristine conditions and/or the efforts to do that are judged economically unreasonable. In Italy, there are many deltaic systems or confined areas such as the Po Delta, some areas of the Venice Lagoon, Pialassa della Baiona, and the “Valli di Comacchio” in the Veneto and Emilia-Romagna Regions where the ecological conditions are strongly eutrophic and the abatement of nutrients has not been successful. Under such conditions aquatic angiosperms have disappeared, clam farming (breading or free harvesting of Ruditapes philippinarum Adams & Reeve) activities are intense and macroalgae grow massively, often triggering dystrophic conditions (Morand and Briand, 1996; Ménesguen, 2018).
Despite the situation an alternative to reduce the trophic level and at the same time have an economic return is possible. Although the estimation of ES provided by aquatic plants is high (Costanza et al., 1997; Terrados and Borum, 2004; Short et al., 2011) the value of eutrophic environments employed for clam-farming or macroalgal harvesting is considered higher especially in terms of provisioning services at the expense of regulation and habitat services. Additionally, the well-being produced from an environment of high ecological quality cannot always be easily estimated in economic terms.
In 2011 the production of macroalgae in 35 countries worldwide was estimated to be 21 million tonnes fresh weight, corresponding to US$ 7.35 billion revenue, that is US$ 0.35 per kg (Food and Agriculture Organization of the United Nations [FAO], 2012). In 2016 the production increased to 31.2 million tonnes (Food and Agriculture Organization of the United Nations [FAO], 2018) showing the importance of this resource. Recent studies on the natural productivity of the most common macroalgae carried out in the Venice Lagoon (Sfriso and Sfriso, 2017) showed that the annual net production of Gracilariaceae ranged between 9.0 and 28 kg fw m–2 y–1, depending on water turbidity (Table 2). According to FAO quotation this biomass would account from US$ 31,500 to 98,000 ha–1 (Table 3).
Significant results may be also obtained by the production of commercial agar as it occurred in the Venice Lagoon in the 1980s where up to 7000 tonnes of Gracilariaceae were collected to produce this phytocolloid (Sfriso et al., 1994). In the following years the biomass of macroalgae declined in the whole lagoon (Sfriso and Facca, 2007) and the harvesting of Gracilariaceae was no longer economically feasible. Currently, Gracilariaceae biomass has increased significantly, also thanks to the introduction of the NIS A. vermiculophyllum which prefers degraded environments, and the harvesting of these species for agar production could be re-evaluated.
A study by Sfriso et al. (2017b) showed that the production of native agar by Gracilariaceae ranged between 7.4 and 13.2 tonnes ha–1 y–1, accounting for 4.07–7.26 tonnes ha–1 y–1 of commercial product (desulfurized agar). By considering that the price of this product can vary between US$ 11 kg–1 (FAO) and US$ 15–19 kg–1 with a mean value of US$ 17 kg–1 (mean of 50 suppliers in “Alibaba Group Holding Limited” a Chinese multinational holding specialized in e-commerce1), the potential revenue from one ha of lagoon surface is similar to or even higher than the revenue obtainable from the raw biomass (Table 3).
In addition, taking into account the mean nutrient concentrations in the dry biomass recorded in Sfriso et al. (1994) (on average 30.6 and 3.98 g kg–1 dw for N and P, respectively) in the choked area (mean of sts. a and b) of the Venice Lagoon, the amount of nutrients that could be removed by macroalgal harvesting ranges between 413–1285 and 54–167 kg ha–1 y–1 for N and P, respectively (Table 4). These amounts account for a removal of 1521–4732 tonnes y–1 of N and 198–615 tonnes y–1 of P if related to the entire harvesting area of 3682 ha. In the case of the Venice Lagoon, the nutrients removed would be almost equal or even higher than the nutrients released into this basin on a yearly basis (Solidoro et al., 2010). However, the continuous supply of nutrients both from the rivers that flow into this area and from the urban centers of Mestre and the historic center of Venice would guarantee long-term production.
The potential revenue of macroalgal-harvesting is about the same as clam-farming by considering a clam yield of 2–6 kg m–2 during a 2-year period, as it occurs in the most productive environments such as some areas of the Venice Lagoon and the Po Delta2 (Orel et al., 2000).
However, in both cases the economical assessment didn’t consider the harvesting and processing costs therefore the final profit would be actually lower. But, while the activities of clam-farming and clam-harvesting are highly destructive and endangers the environment (Sfriso et al., 2005), the macroalgal harvesting can be managed without risks favoring a constant improvement of the environment through the removal of considerable amounts of nutrients.
Pialassa della Baiona, with a surface of 11 km2 out of whom ca. 3 km2 are colonized by a dense biomass of Gracilariaceae, especially A. vermiculophyllum, is the smallest TWS, but its potential yield ranges between 1099 and 1960 tonnes of commercial agar accounting for US$ 12.1–33.3 million (Table 5), depending on nutrient availability and water transparency. Sacca di Goro, which is invaded by the same species, shows a similar revenue (US$ 13.4–37.0 million) whereas in the Veneto lagoons of the Po Delta the potential revenue reaches US$ 40.3–111.1 million. This value could be reached in only 7% (ca. 30 km2) of the Venice Lagoon surface in the areas prevalently colonized by Gracilariaceae [especially: A. vermiculophyllum, G. gracilis, Gracilaria bursa-pastoris (S. G. Gmelin) P.C. Silva and G. longissima], where a minimum of 9002–16,058 tonnes of commercial agar could be produced achieving a potential revenue in the range of US$ 99–177 million. The four considered environments have altogether a surface of ca. 37 km2 (3682 ha) suitable for algal exploitation where no aquatic angiosperms are present or clam-harvesting can occur. These areas have a potential yield of ca. 15–27 ktonnes y–1 of commercial agar, accounting for US$ 165–454 million, depending on the different market surveys.
As for clams, this analysis does not take into account the production costs, which can be very variable, and depend on the location, the size of the plant, the costs of the biomass production/harvesting and processing, and the country tax rates.
Herrera-Rodriguez et al. (2018) by a techno-economic analysis of industrial agar production from Gracilaria sp. in North Colombia pointed out the role of plant size for a profitable agar production. They found that for a plant with a processing capacity of 8,640 tonnes y–1 and life of 15 years, the break-even production capacity was around 4,200 tonnes of red algae per year.
Delgado et al. (2018) analyzed two different routes (freeze-thaw and evaporation) for agar extraction at industrial scale in order to select the process that offers greater profitability under defined criteria. The effect of raw material cost and plant location on profitability of both routes was also evaluated through sensibility analysis. It was found that evaporation route showed a higher profitability due to its lower fixed capital, operating and utilities costs. But they indicated that raw material cost as the most influential factor in seaweed profitability and this depended on plant location and total tax rate applied by the Government to the industrial sector.
On the other hand, an analysis of costs for clam production in the lagoon of Venice and the Po Delta showed that the revenue depended on the type of company, the means used: small boat, fishing gear with vibrating rake or manual rake. For example, a company that harvest 100 kg of clams per day with a small boat selling them at 3 euros per kg would reach the break-even production after 186 days of work, while a fishing gear with vibrating rake that collects 150 kg per day would reach a balance after 139 days. In the Po Delta the clam fishing with manual rake that harvests 25 kg of clam per day selling them at 4 euros per kg would reach the break-even production after 119 days (Mauracher et al., 2011).
Therefore, in both cases: macroalgal and clam exploitation, the net gain depends on many factors which are difficult to predict as they depend on the environment considered. However, these sources, if adequately managed, can guarantee a profitable exploitation of these eutrophic environments, especially the abundant macroalgal biomass naturally produced in the lagoons of the Northern Adriatic Sea, whose oculate exploitation can promote environmental recovery with nutrient abatement. Indeed, the management of Gracilariaceae (Sfriso and Sfriso, 2017; Sfriso et al., 2017b), but also of invasive allochthonous Laminariales such as Undaria pinnatifida (Harvey) Suringar and Fucales such as Sargassum muticum (Yendo) Fensholt (Armeli-Minicante et al., 2016; Sfriso et al., 2020) that have colonized the lagoon of Thau (France, Verlaque, 2001) and the lagoon of Venice (Italy, Sfriso and Facca, 2013) could be a sustainable solution to restore the environment and at the same time complement traditional fishing or replace mollusk-farming which has severe environmental impacts. In addition, the biomass control could help avoid anoxic crises and their environmental and socio-economic repercussions (Morand and Briand, 1996; Ménesguen, 2018). Even though, harvest should be conducted under strict protocols to avoid environmental impacts as sediment disturbances and resuspension, as well as other disturbances on aquatic flora and fauna.
Conclusion
Transitional water systems have a significant impact on the quality of life, the well-being, and the economy of riparian populations. However, environmental quality is often in strong contrast with anthropogenic activities that affect it and trigger negative effects also of an economic nature. The high economic value provided by ES of poorly impacted basins colonized by aquatic angiosperms is well known. However, degraded environments characterized by a significant macroalgal biomass, if carefully managed, could provide and create complementary resources, even higher value than aquatic angiosperms do. They could integrate the revenues from other activities such as traditional fishing, a good cultural heritage to maintain, or progressively replace clam harvesting/farming activities which are negative for the environment, and at the same time contributing to the recovery of eutrophic areas by self-financing the removal of high amounts of nutrients.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
All the authors have participated to the sampling efforts, the data analysis, and manuscript preparation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor is currently co-organizing a Research Topic with one of the authors, MM, and confirms the absence of any other collaboration.
Acknowledgments
We thank Orietta Zucchetta for reviewing the English language and the reviewers for improving the manuscript with their useful suggestions. A heartfelt thanks also goes to the Regional Agencies for Environmental Protection (ARPAs) of Veneto and Emilia-Romagna regions that by funding the monitoring of macrophytes in the TWS of the north-western Adriatic Sea made the preparation of this work possible.
Footnotes
- ^ https://www.alibaba.com/showroom/prices-agar-agar.html. (last access March 10, 2019).
- ^ http://www.gral.venezia.it/attachments/159_Piano_uso_sostenibile.pdf (last access March 10, 2019).
References
Arico, S., Bridgewater, P., El-Beltagy, A., Harms, E., Program, S., Hepworth, R., et al. (2005). Ecosystems and Human Well-Being. Washington: Island Press.
Armeli-Minicante, S. A., Michelet, S., Bruno, F., Castelli, G., Vitale, F., Sfriso, A., et al. (2016). Bioactivity of phycocolloids against the mediterranean protozoan leishmania infantum: an inceptive study. Sustainability 8, 1–9.
Ballesteros, E., Torras, X., Pinedo, S., García, M., Mangialajo, L., and de Torres, M. (2007). A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European water framework directive. Mar. Poll. Bull. 55, 172–180. doi: 10.1016/j.marpolbul.2006.08.038
Barbier, E. B., Hacker, S. D., Kennedy, C., Koch, E. W., Stier, A. C., and Silliman, B. R. (2011). The value of estuarine and coastal ecosystem services. Ecol. Monograph. 81, 169–193.
Barker, T., Mortimer, M., and Perrings, C. (2010). Biodiversity, ecosystems and ecosystem services. Econ. Ecosyst. Biodivers. Ecol. Econ. Found 41–104.
Buia, M. C., Gambi, M. C., and Dappiano, M. (2003). Seagrass systems, in: mediterranean marine benthos: a manual of methods for its sampling and study, ed. M.C. Gambi, M. Dappiano Biol. Mar. Mediterr. 11, 133–183.
Cecere, E., Petrocelli, A., Izzo, G., and Sfriso, A. (2009). Flora and Vegetation of the Italian Transitional Water Systems. Venezia: CoRiLa, Stampa Multigraf Spinea.
Costanza, R., d’Arge, R., de Groot, R., Farber, S., Grasso, M., Hannon, B., et al. (1997). The value of the world’s ecosystem services and natural capital. Nature 387, 253–260.
Costanza, R., d’Arge, R., de Groot, R., Farber, S., Grasso, M., Hannon, B., et al. (1998). The value of ecosystem services: putting the issues in perspective. Ecol. Econ. 25, 67–72. doi: 10.1016/s0921-8009(98)00019-6
Delgado, ÁG., León-Pulido, J., and Peralta-Ruíz, Y. Y. (2018). Economic evaluation and comparison of two topologies for agar production from red algae. Contemp. Eng. Sci. 11, 659–667. doi: 10.12988/ces.2018.7994
Duarte, C. M. (2013). “The role of Seagrass in Climate Change Mitigation and Adaptation,” in The University of Western Australia and Spanish National Research Council (Bonn), 24–25.
European. Union [Eu]. (1992). Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Offi. J. L 206, 0007–0050.
European. Union [Eu]. (2000). Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. L 327, 0001–0073.
Food and Agriculture Organization of the United Nations [FAO] (2012). Fisheries, and Aquaculture Department. The State of the World’s Fisheries and Aquaculture. FAO: Rome, 209.
Food and Agriculture Organization of the United Nations [FAO] (2018). The State of World Fisheries and Aquaculture 2018 - Meeting the Sustainable Development Goals. Rome: Licence.
Francavilla, M., Franchi, M., Monteleone, M., and Caroppo, C. (2013). The red seaweed gracilaria gracilis as a multi products source. Mar. Drugs 11, 3754–3776. doi: 10.3390/md11103754
Francavilla, M., Manara, P., Kamaterou, P., Monteleone, M., and Zabaniotou, A. (2015). Cascade approach of red macroalgae Gracilaria gracilis sustainable valorization by extraction of phycobiliproteins and pyrolysis of residue. Bioresour. Technol. 184, 305–313. doi: 10.1016/j.biortech.2014.10.147
Friedlander, M., and Levy, I. (1995). Cultivation of gracilaria in outdoor tanks and ponds. J. Appl. Phycol. 7, 315–324. doi: 10.1007/bf00004005
González-Leija, J.A., Hernández-Garibay, E., Pacheco-Ruíz, I., Espinoza-Avalos, J., López-VivasJosé, J. M., Bautista-Alcantar, J., et al. (2009). Optimization of the yield and quality of agar from Gracilariopsis lemaneiformis (Gracilariales) from the Gulf of California using an alkaline treatment. J. Appl. Phycol. 21, 321–326. doi: 10.1007/s10811-008-9370-0
Guerry, A. D., Ruckelshaus, M. H., Arkema, K. K., Bernhardt, J. R., Guannel, G., Kim, C.-K., et al. (2012). Modeling benefits from nature: using ecosystem services to inform coastal and marine spatial planning. Int. J. Biodivers. Sci.Ecosyst. Serv. Manag. First 2012, 1–15.
Haines-young, R., and Potschin, M. (2018). Common International Classification of Ecosystem Services Guidance on the Application of the Revised Structure. Nottingham: Fabis Consulting Ltd.
Hattam, C., Atkins, J., Beaumont, N., Börger, T., Böhnke-Henrichs, A., Burdon, D., et al. (2015). Marine ecosystem services: Linking indicators to thei classification. Ecol. Indic. 49, 61–75. doi: 10.1016/j.ecolind.2014.09.026
Herrera-Rodriguez, T., Parejo-Palacio, V., and González-Delgado, ÁD. (2018). Technoeconomic sensibility analysis of industrial agar production from red Algae. Chem. Eng. Transactions 70, 2029–2034.
Kornfeldt, R. A. (1982). Relation between nitrogen and phosphorus content of macroalgae and the waters of Northern Oresund. Bot. Mar. 25, 197–201.
Lignell, A., Ekman, P., and Pedersèn, M. (1987). Cultivation technique for marine seaweeds allowing controlled and optimized conditions in the laboratory and on a pilotscale. Bot. Mar. 30, 417–424.
Liquete, C., Piroddi, C., Drakou, E. G., Gurney, L., Katsanevakis, S., Charef, A., et al. (2013). Current status and future prospects for the assessment of marine and coastal ecosystem services: a systematic review. PLoS One 8:e67737. doi: 10.1371/journal.pone.0067737
Maroli, L., Pavoni, B., Sfriso, A., and Raccanelli, S. (1993). Concentrations of polychlorinated biphenyls and pesticides in different species of macroalgae from the lagoon of Venice. Mar. Pollut. Bull. 26, 553–558. doi: 10.1016/0025-326x(93)90405-9
Mauracher, C., Pellizzato, M., and Trevisan, G. (2011). “Una Valutazione Tecnico-Economica Del Comparto Veneto Della Vongola,”in Le vongole dell’alto Adriatico tra Ambiente e mercato, ed C. Trevisan (Milano: Franco Angeli), 102–136.
Ménesguen, A. (2018). Les Marées Vertes. 40 Clés Pour Comprendre. Édition Quae RD 10. France: Versailles,.
Millenium Ecosystem Assessment, (2005). Ecosystems and Human Well-Being: Current States and Trends. Washington, D.C: Island Press.
Morand, P., and Briand, X. (1996). Excessive growth of macroalgae. A Symptom of environmental disturbance. Bot. Mar. 39, 491–516.
Munari, C., and Mistri, M. (2012). Ecological status assessment and response of benthic communities to environmental variability: the Valli di Comacchio (Italy) as a study case. Mar. Environ. Res. 81, 53–61. doi: 10.1016/j.marenvres.2012.08.008
Nordlund, L. M., Koch, E. W., Barbier, E. B., and Creed, J. C. (2016). Seagrass ecosystem services and their variability across genera and geographical regions. PLoS One 11:e0163091. doi: 10.1371/journal.pone.0163091
Orel, G., Boatto, V., Sfriso, A., and Pellizzato, M. (2000). Piano Per la Gestione Delle Risorse Alieutiche Delle Lagune Della Provincia di Venezia. Benevento: Sannioprint.
Orfanidis, S., Panayotidis, P., and Stamatis, N. (2001). Ecological evaluation of transitional and coastal waters: a marine benthic macrophytes-base model. Mediterr. Mar. Sci. 2/2, 45–65.
Orfanidis, S., Panayotidis, P., and Ugland, K. I. (2011). Ecological evaluation index continuous formula (EEI-c) application: a step forward for functional groups, the formula and reference condition values. Mediterr. Mar. Sci. 12, 199–231.
Orlandini, M., and Favretto, L. (1987). “Utilisation of macroalgae in Italy for pollution abatement and as source of energy and chemicals,” in COST-48, Symposium of Sub-Group III, Crema-L’Homeau (France: CNRS-IFREMER), 25–28.
Phycomorph, (2015). “Advancing knowledge on seaweed growth and development,” in COST Action FA1406 (2015–2019). Brussels: European Commission
Pranovi, F., and Giovanardi, O. (1994). The impact of hydraulic dredging for shortnecked clams, Tapes spp., on an infaunal community in the lagoon of Venice. Sci. Mar. 58, 345–353. doi: 10.1016/j.marenvres.2015.05.003
Sfriso, A., Buosi, A., Wolf, M. A., and Sfriso, A. A. (2020). Invasion of alien macroalgae in the Venice Lagoon, a pest or a resource? Aquat. Invasion 15, (in press).
Sfriso, A., Buosi, A., Facca, C., and Sfriso, A. A. (2017a). Role of environmental factors in affecting macrophyte dominance in transitional environments: the Italian Lagoons as a study case. Mar. Ecol. 38, e12414. doi: 10.1111/maec.12414
Sfriso, A., and Facca, C. (2007). Distribution and production of macrophytes in the lagoon of Venice. Comparison of actual and past abundance. Hydrobiologia 577, 71–85. doi: 10.1007/s10750-006-0418-3
Sfriso, A., and Facca, C. (2011). Macrophytes in the anthropic constructions of the Venice littorals and their ecological assessment by an integration of the “CARLIT” index. Ecol. Indic. 11, 772–781. doi: 10.1016/j.ecolind.2010.10.002
Sfriso, A., and Facca, C. (2013). Annual growth and environmental relationships of the invasive species Sargassum muticum and Undaria pinnatifida in the lagoon of Venice. Est. Coast. Shelf Sci. 129, 162–172. doi: 10.1016/j.ecss.2013.05.031
Sfriso, A., Facca, C., Bonometto, A., and Boscolo, R. (2014). Compliance of the macrophyte quality index (MaQI) with the WFD (2000/60/EC) and ecological status assessment in transitional areas: The Venice lagoon as study case. Ecol. Indic. 46, 536–547. doi: 10.1016/j.ecolind.2014.07.012
Sfriso, A., Facca, C., and Ghetti, P. F. (2007). Rapid quality index (R-MaQI), based mainly on macrophyte associations, to assess the ecological status of mediterranean transitional environments. Chem. Ecol. 23, 1–11.
Sfriso, A., Facca, C., and Marcomini, A. (2005). Sedimentation rates and erosion processes in the lagoon of Venice. Environ. Int. 31, 983–992. doi: 10.1016/j.envint.2005.05.008
Sfriso, A., and Marcomini, A. (1994). Gross primary production and nutrient behaviours in shallow lagoon waters. Bioresour. Technol. 47, 59–66. doi: 10.1016/0960-8524(94)90029-9
Sfriso, A., Marcomini, A., and Pavoni, B. (1994). Gracilaria distribution, production and composition in the lagoon of Venice. Bioresour. Technol. 50, 165–173. doi: 10.1016/0960-8524(94)90069-8
Sfriso, A. A., Gallo, M., and Baldi, F. (2017b). Seasonal variations and yields of sulfated polysaccharides in seaweeds from the Venice Lagoon. Bot. Mar. Special Issue Phycomorph 60, 339–349.
Sfriso, A. A., and Sfriso, A. (2017). In field gracilariaceae and Ulva biomass production: the venice lagoon as study case. Bot. Mar. Special Issue Phycomorph 60, 271–283.
Short, F. T., Polidoro, B., Livingstone, S. R., Carpenter, K. E., Bandeira, S., and Bujang, et al. (2011). Extinction risk assessment of the world’s seagrass species. Biol. Conserv. 144, 1961–1971.
Soedjak, H. S. (1994). Colorimetric determination of carragenans and other anionic hydrocolloids with methylene blue. Anal. Chem. 66, 4514–4518. doi: 10.1021/ac00096a018
Solidoro, C., Bandelj, V., Bernardi, F. A., Camatti, E., Ciavatta, S., Cossarini, G., et al. (2010). “Response of the Venice Lagoon Ecosystem to Natural and Anthropogenic Pressures over the last 50 years,” in Coastal Lagoons - Critical Habitats of Environmental Change, eds M. J. Kennish and H. W. Paerl (Boca Raton FL: CRC Press), 483–511. doi: 10.1201/ebk1420088304-c19
Strickland, J. D. G., and Parsons, T. R. (1972). A Practical Handbook of Seawater Analyses. Ottawa: Fish. Res. Board of Canada.
Terrados, J., and Borum, J. (2004). “Why are seagrasses important? Goods and services provided by seagrass meadows,” in European Seagrasses: An Introduction to Monitoring and Management. The M & MS Project, eds J. Borum, C. M. Duarte, D. Krause-Jensen, and T. N. Greve (Brussels: European Community).
Tuya, F., Haroun, R., and Espino, F. (2014). Economic assessment of ecosystem services: Monetary value of seagrass meadows for coastal fisheries. Ocean Coast.Manage 96, 181–187. doi: 10.1016/j.ocecoaman.2014.04.032
Verlaque, M. (2001). Check-list of the macroalgae of Thau lagoon (Hérault, France): a hot spot of marine species introduction in Europe. Oceanol. Acta 24, 29–49. doi: 10.1016/s0399-1784(00)01127-0
Viaroli, P., Bartoli, M., Giordani, G., Naldi, M., Orfanidis, S., and Zaldívar, J. M. (2008). Community shifts, alternative stable states, biogeochemical controls and feed- backs in eutrophic coastal lagoons: a brief overview. Aquat. Conserv. 18, S105–S117.
Keywords: macrophytes, Agarophyton vermiculophyllum, Gracilariopsis longissima, Gracilaria gracilis, nutrient removal, agar production, ecosystem services, transitional water systems
Citation: Sfriso A, Mistri M, Munari C, Buosi A and Sfriso AA (2020) Management and Exploitation of Macroalgal Biomass as a Tool for the Recovery of Transitional Water Systems. Front. Ecol. Evol. 8:20. doi: 10.3389/fevo.2020.00020
Received: 28 March 2019; Accepted: 24 January 2020;
Published: 14 February 2020.
Edited by:
Angel Pérez-Ruzafa, University of Murcia, SpainReviewed by:
Lorena Rodríguez-Gallego, Universidad de la República, UruguayMarc Verlaque, Centre National de la Recherche Scientifique (CNRS), France
Copyright © 2020 Sfriso, Mistri, Munari, Buosi and Sfriso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adriano Sfriso, c2ZyaXNvYWRAdW5pdmUuaXQ=
 Adriano Sfriso
Adriano Sfriso Michele Mistri
Michele Mistri Cristina Munari
Cristina Munari Alessandro Buosi1
Alessandro Buosi1