- 1Department of Biological Sciences, University of Notre Dame, Notre Dame, IN, United States
- 2Odum School of Ecology, University of Georgia, Athens, GA, United States
Organisms exert multiple, and often contrasting, influences on ecosystems. During their spawning runs, Pacific salmon (Oncorhynchus spp.) deliver nutrients to freshwater ecosystems, but also disturb benthic sediments during upstream migration and nest building. The relative importance of these contrasting roles is not well understood, especially in relation to the dynamics of other environmental drivers. To assess the influence of salmon-mediated enrichment and disturbance, we measured stream biofilm metrics (production, respiration, chlorophyll a [chla], ash-free dry mass, stable isotope signatures, ∼ every 8 days) and stream variables (spawner and carcass abundance, dissolved nutrients, temperature, discharge, light, daily to every few days) from July through September (salmon arrived in August), in multiple habitats of a southeast Alaska (USA) stream. Biofilm production and biomass increased around the start of the salmon run, but declined later in the run. Biofilm stable isotope composition indicated incorporation of salmon-derived carbon and nitrogen (N) during the latter part of the run. Biofilm biomass differed among benthic habitat types (i.e., riffles, pools, stream edges) but temporal patterns were generally similar, suggesting that salmon and environmental influences were not habitat-specific. We used these high-frequency field data to parameterize an ordinary differential equation model for dissolved inorganic N, chla, and cellular N, and estimated model parameters using Markov chain Monte Carlo. Posterior distributions indicated that (1) habitats and locations were generally similar in model parameters, (2) removing the effect of salmon resulted in no change in biofilm chla early in the run (mid-August), but higher chla biomass for some habitats later in the run (September), and (3) the overall integrated salmon effect over the run was one of biofilm loss. Only by combining high frequency biofilm and environmental data with a process-based model could we determine how environmental context dynamics interact with salmon run dynamics to modulate the biofilm response in natal spawning streams.
Introduction
The physical and chemical template of ecosystems combined with biological processes provide the environmental context (after Janetski et al., 2009) that influences ecosystem structure and dynamics, including the response to resource subsidies (sensu Polis et al., 2004). Resource subsidies (Polis et al., 1997) take on many forms, such as organic matter provided to streams through leaf-litter fall (Wallace et al., 1997) or marine nutrients deposited on islands by birds (Anderson and Polis, 1999). Most research on resource subsidies has focused on the influence of material quantity versus quality (Marcarelli et al., 2011; Sitters et al., 2015) or the timing and duration of influence (Subalusky and Post, 2019). In fact, the influence of subsidies on a recipient ecosystem varies in relation to the available endogenous resources and the environmental context (Marczak et al., 2007; Subalusky and Post, 2019). Both the subsidy and environmental context can be spatially and temporally dynamic, creating complex patterns in responses, and currently limiting our understanding of the overall importance of resources subsidies.
Pacific salmon (Oncorhynchus spp.) are an important resource subsidy in their native range (Gende et al., 2002) and are often viewed as ecosystem engineers due to their pronounced influences on stream ecosystems (Moore, 2006; Flecker et al., 2010). Salmon provide nutrients through excretion and decomposition of their carcasses, but they also disturb the benthos by scouring substrates during redd construction. The nutrient enrichment and physical disturbance of the benthos can potentially drive the dynamics of benthic biofilm (i.e., the complex of algae, bacteria and fungi on submerged surfaces), whose responses may propagate throughout the entire food web and influence whole stream ecosystem dynamics. The net ecological effect of salmon on benthic biofilms is one of nutrient enrichment, that may enhance biofilm productivity, and its offset by benthic disturbance. In addition, the effect of salmon likely changes over time related to spatial and temporal dynamics in the size, number, and behavior of salmon (i.e., the subsidy dynamics) and also in the environmental context. For example, finer sediments are more easily disturbed (Tiegs et al., 2008; Janetski et al., 2009), and heavy canopy shading may prevent salmon enrichment (Ambrose et al., 2004). Understanding the effects of salmon is therefore challenging because it requires explicit quantification of the enrichment and disturbance effects in the context of subsidy and environmental dynamics.
Quantifying salmon enrichment and disturbance effects has been approached with field studies and modeling approaches. Salmon exclusion experiments have shown that disturbance of benthic biofilms due to nest excavation counteracts benefits to biofilm from nutrients excreted by salmon (Tiegs et al., 2011). However, such experiments cannot directly control for water-column nutrient enrichment, except by making comparisons between the periods before and during the salmon run, or to upstream reaches where salmon do not spawn. Each of these approaches has limitations, such as varying environmental conditions either in time or space that are confounded with the salmon run. Bellmore et al. (2014) developed a system dynamics model for biofilm, parameterized it with values from the literature, and performed simulations to address the dual roles of salmon in relation to the environmental context. They concluded that enrichment from the presence of salmon only occurs under specific environmental contexts, namely if background nutrient concentrations are low and the portion of the stream bed suitable for spawning is small. Most empirical studies on salmon effects have limited inferential power because they consist only of single sampling points before and during the run (e.g., Rüegg et al., 2012; Harding and Reynolds, 2014; but see Moore and Schindler, 2008), precluding parameterization of a dynamic model of salmon influence on benthic biofilms. In addition, studies of salmon subsidy effects often focus on stream riffles where salmon typically spawn and, therefore, exert the most disturbance (e.g., Tiegs et al., 2008, 2011). Other stream habitats that are less suitable to spawning (e.g., pools) remain understudied but may respond differently (Bellmore et al., 2014). To better understand the importance of salmon on the productivity of their natal spawning streams, we need to address habitat variability in response to salmon spawners and uniquely identify the salmon effects in relation to both subsidy dynamics (e.g., run size and timing) and other environmental variables (e.g., discharge, irradiance).
We evaluated the ecological influences of salmon spawners on biofilms in a southeast Alaska (USA) stream by combining high-resolution field data on salmon, biofilm, and environmental context with a dynamic process-based model. We measured multiple biofilm metrics approximately weekly across multiple locations for three different habitat types. We also surveyed salmon and environmental characteristics at least every few days, starting prior to and over the course of an annual salmon run. We then parameterized the model with the field data for all locations separately, and performed simulation experiments to infer separate enrichment and disturbance effects, and therefore the net salmon effect on biofilm.
Materials and Methods
Study Site
We conducted our study in Twelve Mile Creek, Prince of Wales Island, in southeastern Alaska, USA. The Twelve Mile Creek watershed is 31 km2 and our 300-m study reach had an average discharge of 1320 L s–1 (range, 25 to 6450 L s–1) during the study (early July – late September 2008). Salmon spawners, which were dominated by pink salmon (Oncorhynchus gorbuscha) with low numbers of dog salmon (O. keta), typically appear in the stream at the beginning of August and their density peaks in early September (Rüegg et al., 2012).
We identified three habitat types that we predicted would differ in their response to salmon (see Supplementary Figure S1). First, pools were considered a low-disturbance location, especially at their upstream end, as salmon tend to spawn in faster flowing areas (e.g., riffles and pool tail-outs; Quinn, 2005). Second, riffle/run habitats were selected as high-disturbance areas based on flow and suitability as high-quality spawning habitat (for simplicity we will refer to this habitat type as riffle). Third, edges were habitats within 0.5 m of the water’s edge on the sampling day, located adjacent to riffle habitats, and disturbance-prone as either potential spawning habitat at high discharge or due to emersion/drying at low discharge. For each habitat, we haphazardly selected three replicate locations (i.e., three separate pools, riffles, or edges) within the 300 m reach, for a total of nine sampling locations.
Environmental Context
We measured multiple biological, chemical, and physical variables to quantify the environmental context before and during the salmon run. Salmon spawners and carcasses were counted every 2–3 days in 4 m belt transects every 10 m along the 300 m reach during the salmon run (5 August to 24 Sept 2008) (Tiegs et al., 2009). Because the transects covered 40% of the stream benthic area (i.e., 4 m belt transects every 10 m along the stream), we multiplied the number of salmon counted by 2.5 to estimate total numbers (i.e., 100%), and then converted to density using reach area (length × mean width). Dissolved nutrient concentrations were measured from water samples collected at least weekly before (1 July to 4 August 2008) and during the salmon run (5 August to 20 September 2008). Samples were filtered through Whatman® GF/F filters and frozen at −20°C until analyzed in the laboratory. A Lachat QC-8500 Flow Injection Autoanalyzer (Lachat Instruments, Loveland, Colorado, USA) was used to determine soluble reactive phosphorous (SRP) with the ascorbic acid method and nitrate (NO3–-N) with the cadmium reduction method. Ammonium (NH4+-N) was determined on a Shimadzu UV-1601 spectrophotometer (Shimadzu Corporation, Columbia, MA, USA) using the phenol-hypochlorite method (see Levi et al., 2011 for detailed section “Materials and Methods”). Water temperature (°C) was measured hourly using HOBO data loggers (Onset Corporation, Pocasset, MA, USA). Discharge (L s–1) was estimated from water level changes recorded every 30 min by an Odyssey capacitance meter (Dataflow Systems, Inc., Christchurch, New Zealand) and a standard rating curve (Levi et al., 2011). Daily means were calculated for both temperature and discharge. Light intensity (μE cm–2 d–1) was measured using an Odyssey light meter (Dataflow Systems, Inc., Christchurch, New Zealand) and integrated to obtain total daily photosynthetically active radiation (PAR).
Biofilm
Benthic biofilm was sampled every 8 days from 7 July to 20 September 2008. In each of the nine locations, we haphazardly collected three representative rocks touched blindly that fell within the gravelometer size classes of 32–90 mm. From these rocks, we measured net community production (NCP), community respiration (CR), gross community production (GCP = NCP + CR), chlorophyll a (chla), and ash-free dry mass (AFDM). The three rocks per location were averaged and one value for each location was used for statistical analyses. Additionally, we measured the stable isotope composition (δ13C and δ15N) of biofilm using the aggregate of the three rocks per location.
In the field, we used a light/dark chamber method (Bott, 2006) to measure NCP and CR. Rocks were placed into 960-mL clear plastic cups (Mold-Rite Plastics, Plattsburgh, NY, USA). Cups were filled with stream water and closed underwater to eliminate air bubbles. Streamwater dissolved oxygen (DO) was recorded using a Hach Luminescent DO probe (Model HQ30d, Hach Company, Loveland, CO, USA) along with the closing time for each cup. Cups were then placed on the sediments and exposed to in situ light and temperature conditions. Light absorption by cups (37% of light available at the water surface) was similar to the absorption by stream water at depth of incubation (53%). After a minimum of 2.5 h, cups were opened and DO and time recorded. Water was then replaced with fresh stream water, cups closed, and placed in black plastic bags to simulate night-time conditions. Protocols followed those of the light incubations. Following the incubations, rocks were placed in individual plastic bags and transported to the laboratory in a cooler.
In the laboratory, each rock was scrubbed (all surfaces) with a stiff brush to measure chla and AFDM. A known sub-sample of the resulting biofilm slurry was filtered onto a Whatman® GF/F filter. Filters were analyzed sequentially for chla and AFDM using standard methods (see Tiegs et al., 2008). Chlorophyll a was determined fluorometrically after extraction in ethanol. Ash-free dry mass was determined after drying for at least 48 h at 60°C, followed by ashing for 3 h at 500°C. The remaining slurries of the three replicate rocks per location (e.g., pool 1) were combined, centrifuged, and dried at 60°C for analysis of stable isotopes. The dried biofilm was acidified to remove carbonates, redried, ground, and analyzed for nitrogen and carbon stable isotope composition using a Carlo Erba Elemental Analyzer coupled to a Finnegan Delta + Mass Spectrometer (Chaloner et al., 2002).
Scrubbed rocks were measured for length, width, and height; surface area of the entire rock was calculated assuming an ellipsoid. Water displacement by rocks in sampling cups was also measured. From the changes in DO and the volume of water used for incubations, we determined net community production (NCP; light incubation) and community respiration (CR; dark incubation) assuming a linear change and expressed rates per unit surface area (mg O2 m–2 h–1) (after Johnson et al., 2009; Rüegg et al., 2011). Gross community production was determined as the sum of NCP and CR fluxes for a specific rock. Due to low CR relative to NCP, GCP and NCP were very similar and only GCP and CR were used in statistical analyses. Chlorophyll a and AFDM were also calculated on a per unit area basis (mg m–2 and g m–2, respectively). Stable isotopes are presented as their isotopic ratios (δ15N, δ13C). Isotopic analyses also provided percent nitrogen in samples (see modeling below).
Statistical Analyses
To examine habitat and time effects on biofilm metabolism, biomass, and stable isotope composition, we used a generalized additive model (GAM; Zuur et al., 2007). We treated habitat type as a fixed factor with three levels: pools, riffles, and edges, as we expected them to differ in their biofilm characteristics. We used Julian day as the continuous time variable, as we expected biofilm characteristics to change over the course of the study period, in response to changes in the environmental context (i.e., seasonal trends in light, temperature, salmon run dynamics). We used a smoother for the time variable as data followed non-linear patterns. We also included interaction effect between time and habitat type as we expected the influence of salmon and potentially other environmental characteristics to be habitat-specific, and the temporal patterns to differ among the habitats [GAM model: Dependent variable ∼ Habitat type + s(Julian Day) + s(Julian Day, by = Habitat Type)]. The response variables were the six biofilm metrics (GCP, CR, chla, AFDM, δ15N, and δ13C) and we included the specific location (nine locations) as random effects (N = 90). We used the GAM analyses to support the description of temporal patterns detected while we use the dynamic model presented below to detect various aspects of the salmon effects and make predictions about what would have occurred without salmon (for the chla metric). All analyses were conducted using R 2.11.2 (R Core Team, 2019), with the mcgv package used for the GAM models.
Modeling Biofilm Dynamics in Relation to the Environmental Context
We examined the influence of salmon density on biofilm accrual (as inferred from chla concentrations) using a process-based model to disentangle the salmon influence from changes in the other environmental characteristics, such as discharge which is generally higher during salmon runs in our study system. As in Bellmore et al. (2014), who developed a single equation for chla dynamics, our objective was to model the major influences on the dynamics of DIN, N concentration in benthic biofilm, and chla with established formulations describing these processes. We explicitly modeled the linked dynamics of inorganic nitrogen (N) in the water column with biofilm N and chla to predict inorganic N and chla in the absence of salmon. Our main goal was to capture the influence of salmon and abiotic variables (i.e., light, temperature, and discharge) on biofilm chla in a reasonable fashion by estimating parameters for different locations in the stream, and therefore wanted our model to be flexible enough to match location-specific dynamics. The stream system modeled here showed nutrient limitation and its alleviation by salmon in a previous year (Rüegg et al., 2011), as well as location-specific differences in sediment size and thus potential salmon disturbance (e.g., Tiegs et al., 2008; Rüegg et al., 2012), allowing us to address our goals.
The dynamics of dissolved inorganic N (i.e., DIN, N in the equations), chla, and N in the biofilm (i.e., NB in the equations) are described in eqs. 1–3. Here, the units for N are nitrogen mass per unit volume (m3) or concentration in the water column, whereas the units for chla and NB are mass per benthic area (m2). To derive the dynamics of DIN concentration, we considered a parcel of water over 1 m2 of benthic area and treated the flow into and out of the parcel as a chemostat (Smith and Waltman, 1995). We used a mass balance approach, incorporating chemostat-like advective flow of DIN into and out of the water column (Smith and Waltman, 1995), and Michaelis-Menten uptake kinetics (e.g., Kim et al., 1990, 1992) to describe the loss of DIN from the water column due to algal uptake. We assumed that salmon-derived DIN is proportionally to salmon density. Combing the chemostat-like advective inputs and losses, the salmon fertilization effect, and DIN removal by biofilm, we arrive at,
in which the first two terms describe changes in DIN mass due to advection, the third term describes the salmon subsidy to the DIN pool, and the fourth term prescribes DIN uptake by biofilm as a saturating function of DIN (see eq. 2 below). The first two terms can be combined to yield a simpler expression,
In eqs. 1a and b, I represents the input from upstream DIN, and N is the DIN concentration in the column, both of which are multiplied by the volume of the water parcel (d = depth multiplied by the 1 m2 benthic area) and by discharge (v). Again, this component of eqs. 1a and 1b corresponds to advective flow. The salmon nutrient subsidy of N into the DIN pool, the second term in eqs. 1a and 1b, is the product of salmon density (S) for 1 m2 benthic surface area, and the rate of N production via excretion and decomposition (η). Biofilm uptake of DIN, the third term in eqs. 1a and 1b, is described by a saturating Michaelis-Menten function of DIN, with the maximum uptake rate, αN, with units of N mass per unit chla per unit time. Thus, total DIN losses from the parcel above the 1 m2 benthic area result from advection moving DIN downstream and from uptake by biofilms, whereas inputs result from upstream flow and salmon. To model DIN concentration on an areal basis, we divided the entire equation by the water parcel volume (i.e., 1 m2 × d) to obtain the equation for DIN concentration in the parcel,
To model biofilm dynamics, we employ an approach similar to Bellmore et al. (2014), assuming that chla production adheres to Liebig’s law of the minimum. Specifically, the maximum production rate (μ) is multiplied by the minimum of Droop functions of light (as described by PARmin) and cellular nitrogen (as described by NBmin). We added temperature-dependence of chla production by multiplying the production rate by an Arrhenius function, as in Brown et al. (2004) (Ea = activation energy, κ = Boltzman constant, and T = water temperature in Kelvin), while chla loss occurs due to flow or salmon-induced disturbance and other mortality (m) (eq. 2). We assumed that disturbance-related loss was a threshold phenomenon (i.e., critical discharge for biofilm loss, Qcrit) and used a sigmoidal function of discharge, the Hill function, in which β controls the steepness of the threshold (e.g., Eggert et al., 2012). We also assumed that salmon density (S) could be linearly translated into discharge (γ) so that salmon disturbance effects could be directly incorporated into the flow-induced loss of chla from the benthic surface underlying the water column. Combining all these effects on chla production yields the following equation for chla dynamics,
To link the dynamics of DIN with the growth of biofilm, we require a third equation describing the dynamics of intracellular N (i.e., cell quota) because chla production is explicitly a function of the N quota (Legović and Cruzado, 1997). Cellular N quota increases as a function of uptake from the water column, and decreases as a function of chla production rate, described by,
in which the first term describes cellular uptake from DIN in the water column and the second term describes loss from the biofilm N pool.
We used the discharge, light, temperature, and salmon density data collected over the course of the study to drive the dynamics of the above model, by linearly interpolating each between the observed data points. For a given set of parameters, we simulated the above system to obtain trajectories for all three state variables (N, chla, NB) and assumed that differences between the simulated (model predicted) trajectories and the observed data were the result of independent and identically distributed normal observation error at each time point. We used uniform priors for all parameters (I, η, αN, KN, PARmin, NBmin, Ea, m, β, γ, and Qcrit), and used an adaptive random walk Metropolis-Hastings algorithm (Haario et al., 2001) using the metrop() function in R to obtain the posterior distributions of all the parameters. Multiple Markov chains were run for several hundred thousand iterations for each location, and trace plots were visually inspected to ensure convergence and stationarity. Posterior distributions were generated from traces that were thinned after an initial burn-in period. Separate models were fit to each of the locations to account for potential differences among habitats and locations within habitats. To assess the enrichment, disturbance, and net effects of salmon, we simulated the model for all sets of parameters from the joint posterior, but with salmon abundance set to zero for the entire duration of data collection. We then created envelopes defined by the 2.5th and 97.5th quantiles (i.e., 95% confidence interval), for each location and state variable combination in the presence and absence of salmon. This allowed us to use the uncertainty in parameters that resulted from variability in our measurements to infer statistical significance of salmon effects. Thus, spans of time during which trajectory envelopes for salmon and no-salmon do not overlap correspond to times when the salmon effect is unlikely to occur by random chance. Furthermore, model parameters were used to estimate the seasonal gain of chla biomass and loss (integration under modeled curves), as well as N uptake, again both in the presence and absence of salmon spawners.
Results
Environmental Context
The first live salmon (primarily pink salmon, O. gorbuscha) in Twelve Mile Creek were observed on 5 August, and abundance then increased to >1000 salmon in the 300 m reach within 2 weeks, before declining near the end of September (Figure 1A). Salmon carcasses began to accumulate in the stream channel on September 5 and were nearly as abundant as live spawners at the end of September (>300 carcasses in the 300 m reach).
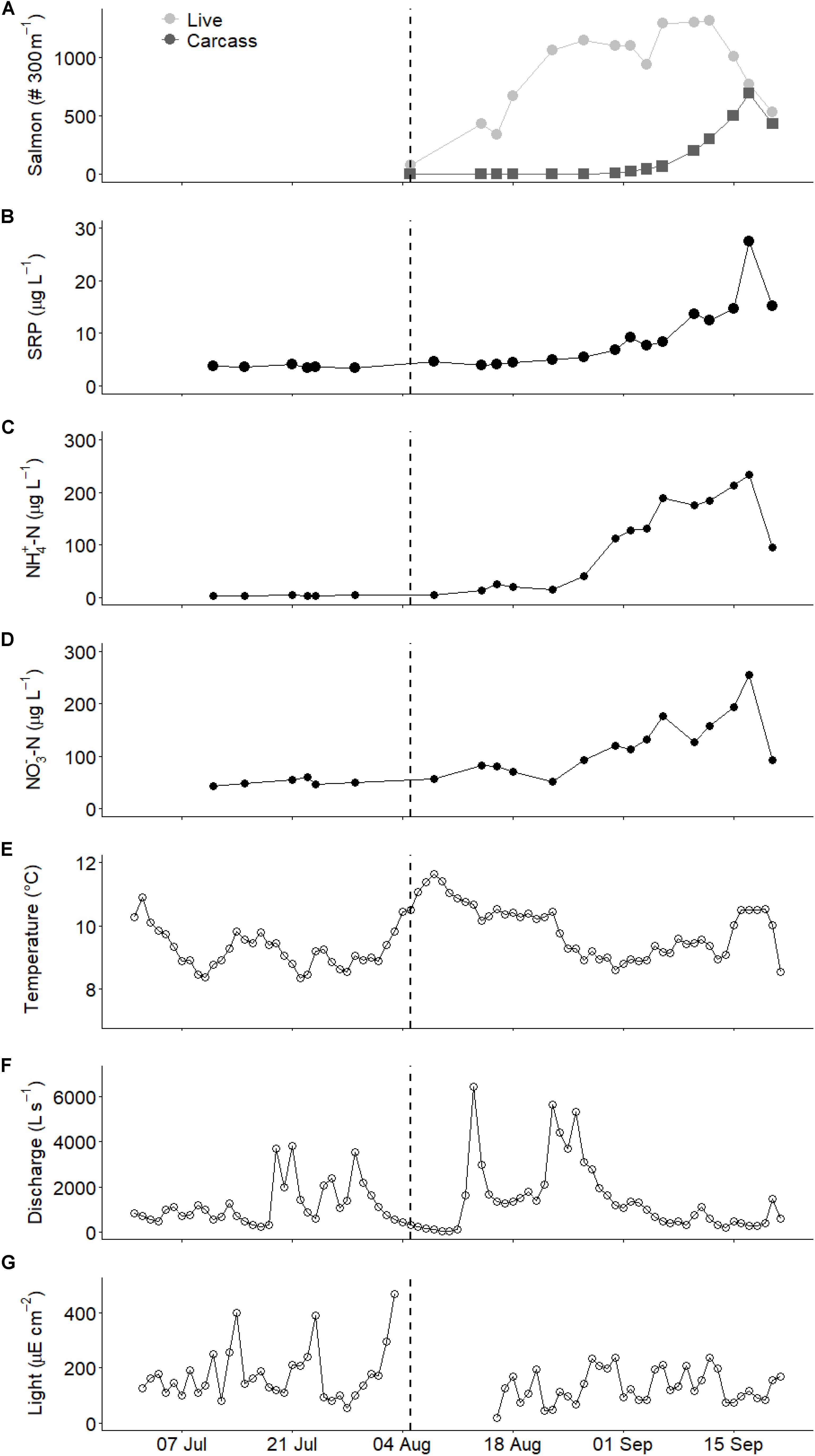
Figure 1. Temporal patterns in environmental variables during the study period: (A) Salmon spawner and carcass abundances, (B) soluble reactive phosphorous (SRP), (C) ammonium (NH4+), and (D) nitrate (NO3–) concentrations, (E) streamwater temperature, (F) discharge, and (G) light intensity. Panels (E–G) are shown as mean daily values. Dashed vertical line represents the arrival of salmon spawners.
Nutrient concentrations increased concomitantly with salmon presence. Soluble reactive phosphorous (SRP) concentrations increased from fairly constant levels prior to and during the initial stages of the run (∼5 μg L–1) to much higher levels as carcasses became very abundant (up to 28 μg L–1; Figure 1B). Ammonium concentrations increased from fairly constant and low levels of ∼2 μg L–1 prior to salmon arrival to over 20 μg L–1 within 2 weeks of the arrival of live salmon spawners (Figure 1C). Toward the end of the run (September), ammonium concentration declined with live salmon abundance. Nitrate concentrations also increased in the presence of salmon, but more slowly than ammonium concentrations and peaked at 250 μg L–1 in mid-September (Figure 1D).
Physical conditions in the stream varied during the study. Streamwater temperature varied from 8.0 to 11.6°C with no pronounced association with salmon abundance (Figure 1E). Discharge varied from 25 to 3825 L s–1 before the salmon run, with most of the high discharge attributable to four events of >2000 L s–1 (Figure 1F). Discharge during the run generally remained below 2000 L s–1, at a slightly higher base flow than before the run, with four high flow events exceeding 5000 L s–1 occurring during the early run (mid- to late-August). Light ranged from 20 to 470 μE cm–2 d–1 before the salmon run compared with 20 to 237 μE cm–2 d–1 during the run, reflecting the transition from summer to autumn (Figure 1G).
Biofilm
Biofilm functional metrics of gross community production (GCP) and community respiration (CR) showed clear temporal patterns (GAM Time effect, p < 0.001) and were consistent among the riffle, edge, and pool habitat sampled (GAM Interaction n.s.). Before the salmon run, GCP was relatively constant (7.2 to 9.6 mg O2 m–2 h–1), but increased shortly before the arrival of salmon and remained higher early in the salmon run (9.1 to 13.6 mg O2 m–2 h–1; Figure 2A). As the salmon run progressed, production decreased to the lowest recorded levels (0 to 6.0 mg O2 m–2 h–1). Respiration was low and at times undetectable (range −0.01 to 0.02 mg m–2 h–1) without a clear seasonal pattern (Figure 2B). None of the metrics differed among habitat types (GAM Habitat n.s.).
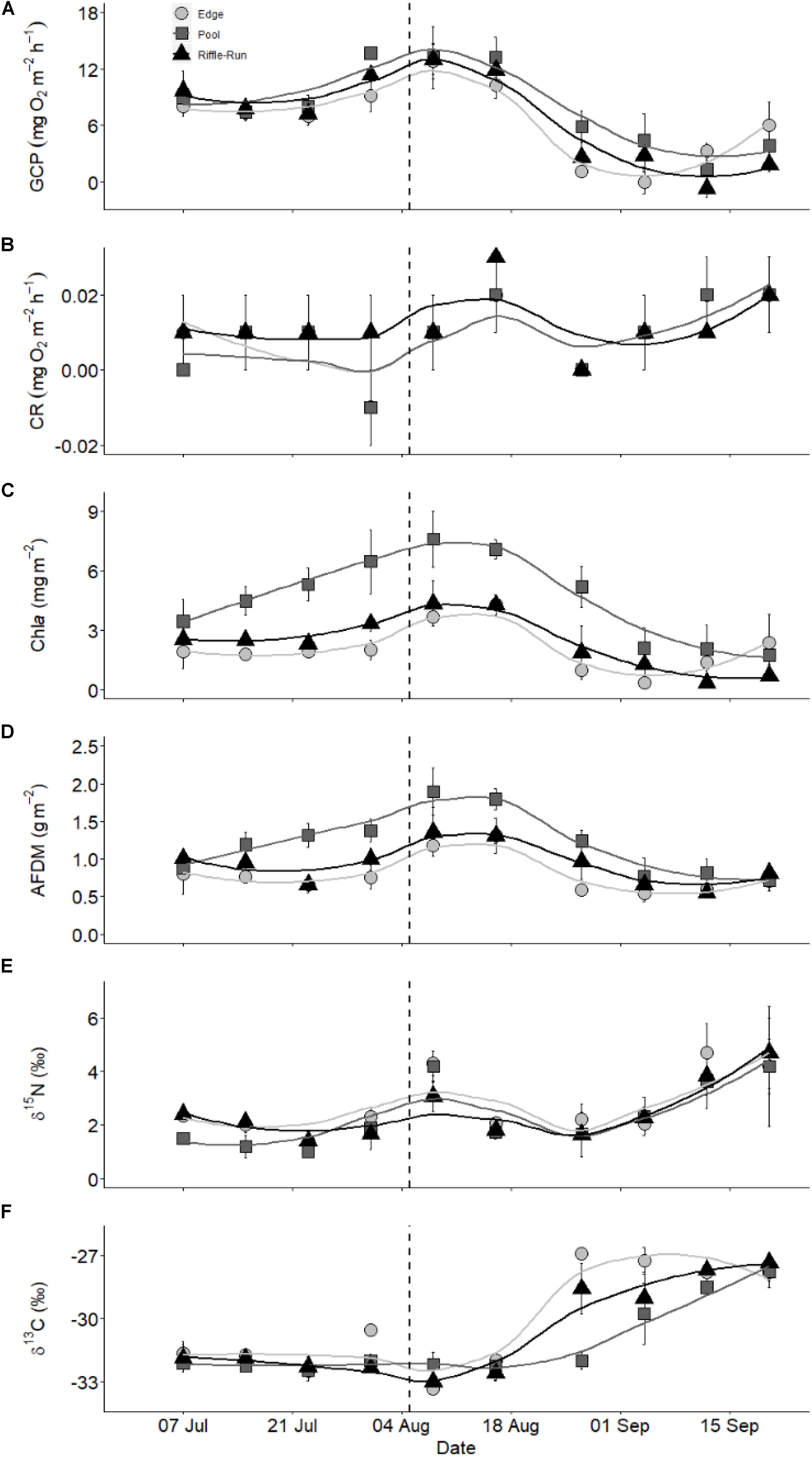
Figure 2. Temporal patterns of biofilm characteristics across three habitat types ( pools, ▲ riffles,
pools, ▲ riffles,  edges): (A) gross community production (GCP), (B) community respiration (CR), (C) chlorophyll a (chla), (D) ash-free dry mass (AFDM), (E) nitrogen stable isotope ratio (δ15N), and (F) carbon stable isotope ratio (δ13C). Dashed vertical line represents the arrival of salmon spawners. Trend lines were smoothed with loess (locally weighted smoothing).
edges): (A) gross community production (GCP), (B) community respiration (CR), (C) chlorophyll a (chla), (D) ash-free dry mass (AFDM), (E) nitrogen stable isotope ratio (δ15N), and (F) carbon stable isotope ratio (δ13C). Dashed vertical line represents the arrival of salmon spawners. Trend lines were smoothed with loess (locally weighted smoothing).
Temporal patterns in biofilm biomass, namely chlorophyll a (chla) and ash-free dry mass (AFDM), reflected those of production (GAM Time effect p < 0.001), but habitat types differed in biomass (GAM Habitat p < 0.001). Biofilm chla increased over time before the salmon run (ranges among habitats: 1.8 to 6.5 mg m–2) and reached the highest levels early in the salmon run (3.7 to 7.6 mg m–2; Figure 2C). As with GCP, chla declined rapidly to levels lower than before salmon (0.3 to 2.1 mg m–2) later in the run. Chlorophyll a was greater in pool habitats than in edge and riffle habitats (Figure 2C). Biofilm AFDM patterns were similar to chla, ranging from 0.7 to 1.4 g m–2 before the salmon run, 1.2 to 1.9 g m–2 during early in the salmon run, and 0.5 to 1.2 g m–2 later in the run (Figure 2D). Pools had the greatest AFDM. Temporal patterns did not differ among habitats. Differences among habitats did not change over time for either chla or AFDM, except for AFDM biomass in pools which increased and then decreased more rapidly than in the other habitats (GAM Time × Pool Habitat Interaction, p = 0.022).
Biofilm stable isotope ratios varied significantly over time (GAM Time effect p < 0.001) and showed some variation among habitat types. Nitrogen stable isotope ratios were relatively constant before the salmon run (δ15N 1.0 to 2.4‰) but increased shortly after the arrival of salmon (δ15N 3.0 to 4.5%; Figure 2E). However, in all habitat types δ15N then declined to pre-salmon levels (1.6 to 2.4%) for 3 weeks before increasing again toward the end of the salmon run (3.6 to 4.7%), with signatures similar among habitat types (GAM Habitat effect n.s.). Carbon stable isotope ratios were relatively constant before and early into the salmon run (δ13C −33.3 to −30.5%) before increasing to relatively constant levels (−28.5 to −27.3%) by the end of September (Figure 2F). Habitats were significantly different (GAM Habitat type p < 0.001), likely due to the fact that each habitat showed a significantly different temporal pattern (GAM interaction between Time effect and pool, riffle, and edge habitat type all p < 0.001) with edge habitats increasing earliest, followed by riffles, and pools with the slowest C isotopic enrichment.
Influence of Environmental Context on Biofilm
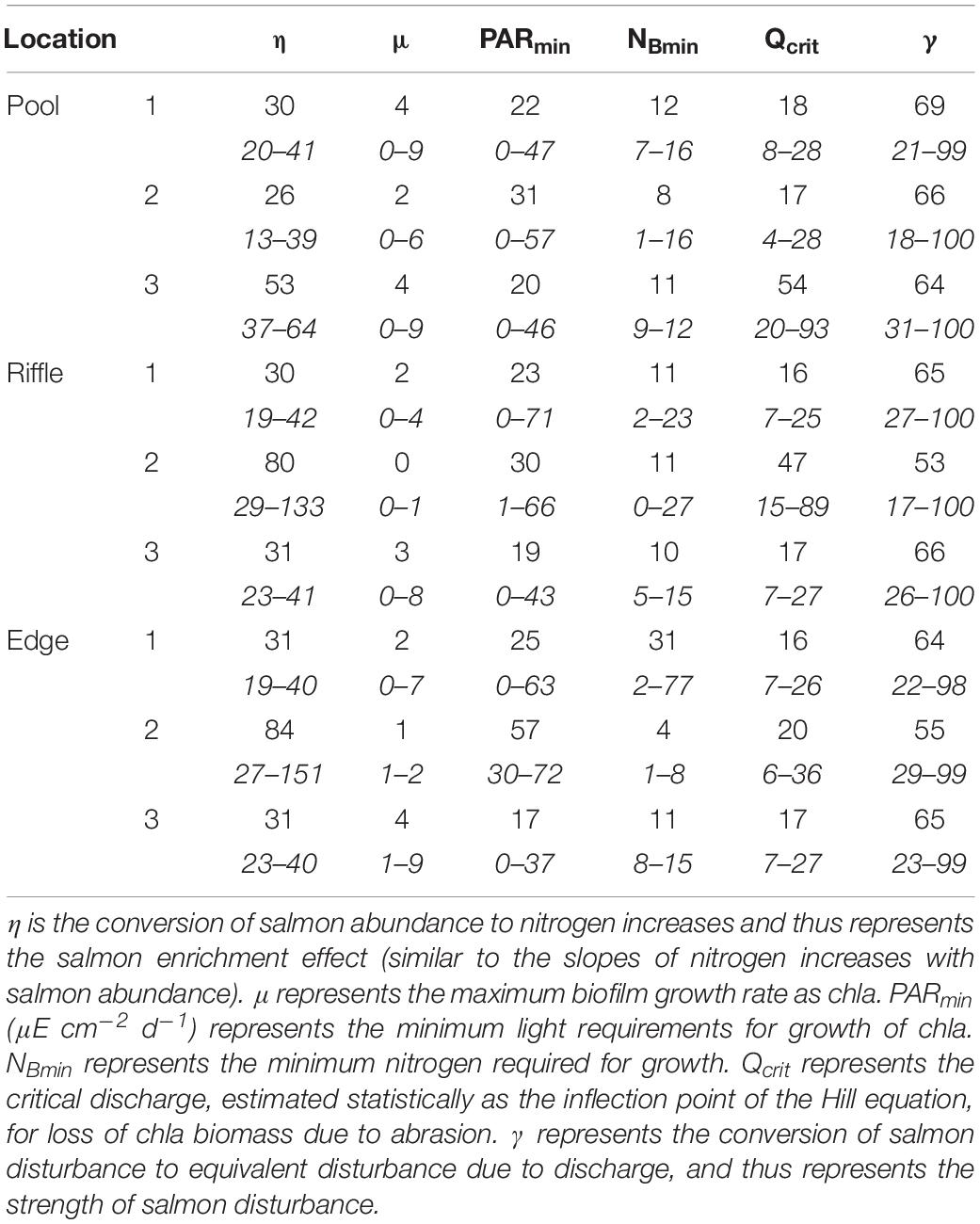
Model parameters corresponding to maximum biofilm growth rate (μ), salmon enrichment rate (η), and discharge-based disturbance (Qcrit) did not vary systematically across habitat types, but some locations, rather than habitats, appeared to stand out. For the contribution of salmon to dissolved nitrogen (N), one location of each habitat (η of Pool 3, Riffle 2, Edge 2) was higher than average across all locations (Table 1). Biofilm growth rates were similar among habitat types and locations, as were minimum light and nutrient requirements. One notable exception was the minimum N quota of edge habitats, which had both the highest (Edge 1) and lowest modal values (Edge 2). Two locations showed some resistance (Pool 3, Riffle 2) to discharge disturbance, as evidence by higher values of Qcrit. Surprisingly, salmon disturbance effects, as indicated by the conversion factor (γ), were consistent across all locations. Also, the salmon-based disturbance effect was unrelated to the critical discharge, which would be expected if sediment stability were the most critical factor for salmon disturbance. Model parameter values suggest that variation in biofilm response to salmon response is not related to common habitat types but is the result of dynamics occurring at relatively small scales.
Model trajectories for all state variables approximate the observed dynamics well (Figure 3 and Supplementary Figure S2), suggesting that the model does a reasonable job of capturing the essence of the biofilm-environmental context relationship. Our main goal in the modeling was to use reasonable formulations of the most important factors influencing biofilm growth to recreate the dynamics of DIN, chla, and biofilm N. Using a model that is a reasonable reflection of reality and parameterized with field data allowed us to separate the salmon effect from the remaining environmental context by simulating the dynamics of the three state variables in the absence of salmon. Chlorophyll a, which is proportional to biofilm mass, exhibited short windows of increased accrual or loss with the presence of salmon. Removing the salmon effect, by setting salmon abundance to zero for the entire duration of data collection and simulating location-specific (in terms of parameters) models, indicated certain periods when biofilm accrual could exceed biomass expected if salmon were absent during a few days early in the run (around mid-August) (Figure 3). However, the generally overlapping confidence envelopes show that this enrichment effect is typically weak or non-existent. On the other hand, certain periods exhibited significantly lower biomass than expected if salmon were absent, based on the absence of overlap of the 95% confidence envelopes for trajectories in the presence and absence of salmon. These apparent disturbance effects by salmon spawner presence were evident in almost all habitat types and locations in September. Thus, the balance between salmon enrichment and disturbance may shift from minimal enrichment earlier in the run to net disturbance later in the run.
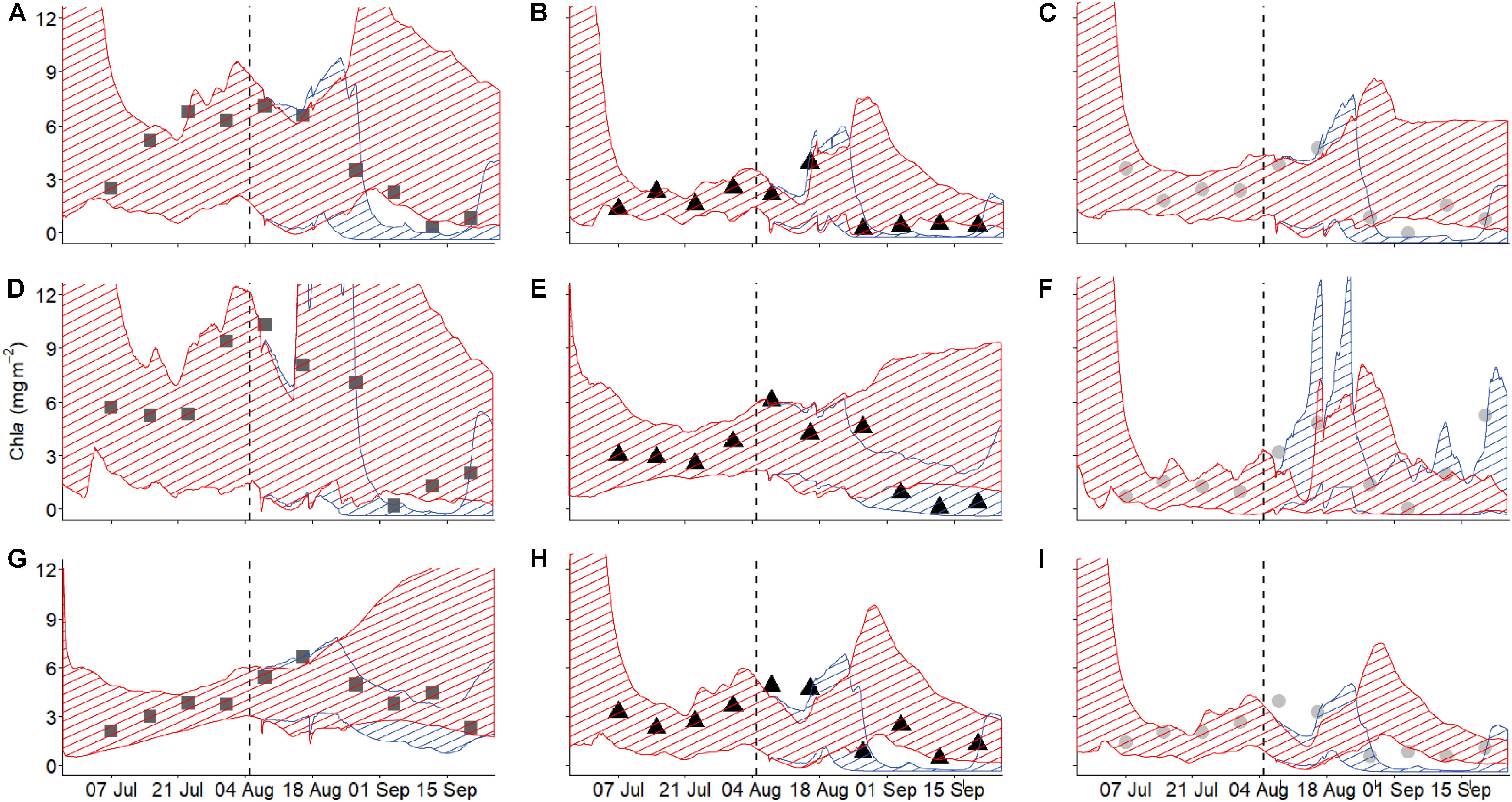
Figure 3. Modeled (shaded area) and measured (symbols) biofilm chla over the course of the salmon run at the nine habitat locations studied. Different columns show the different habitat types [left column (A,D,G) = pools ( ), middle column (B,E,H)] = riffles (▲), right column (C,F,I)] = edges (
), middle column (B,E,H)] = riffles (▲), right column (C,F,I)] = edges ( )], while the rows depict the three locations studied for each habitat type [top = 1 (A,B,C), middle = 2 (D,E,F), bottom = 3 (G,H,I)]. Blue shaded area indicates the confidence interval of the model including salmon while the red shaded area indicates the model with salmon abundance artificially set to zero (i.e., salmon were stopped).
)], while the rows depict the three locations studied for each habitat type [top = 1 (A,B,C), middle = 2 (D,E,F), bottom = 3 (G,H,I)]. Blue shaded area indicates the confidence interval of the model including salmon while the red shaded area indicates the model with salmon abundance artificially set to zero (i.e., salmon were stopped).
Comparing seasonal accrual and loss of biofilm chla and N uptake indicated that salmon presence acted predominantly as a source of disturbance during the study in our stream reach. Biofilm chla produced over the study was similar whether salmon were present or absent, suggesting that environmental variation beyond salmon presence may swamp salmon enrichment effects (e.g., decreasing light later in the season/run) (Figure 4A). Salmon clearly increased the loss of biofilm chla, as losses in the presence of salmon were much higher than those in the absence of salmon (Figure 4B). While the uptake of nitrogen per unit chla was much higher in the presence of salmon spawners than in their absence (Figure 4C), the higher biomass losses due to salmon disturbance limited the absolute amount of nitrogen retained in the stream ecosystem. Together, these results suggest that salmon have minimal enrichment effects, and that disturbance predominates, especially later in the run, which translates into a net negative cumulative impact on biofilm over the course of the run.
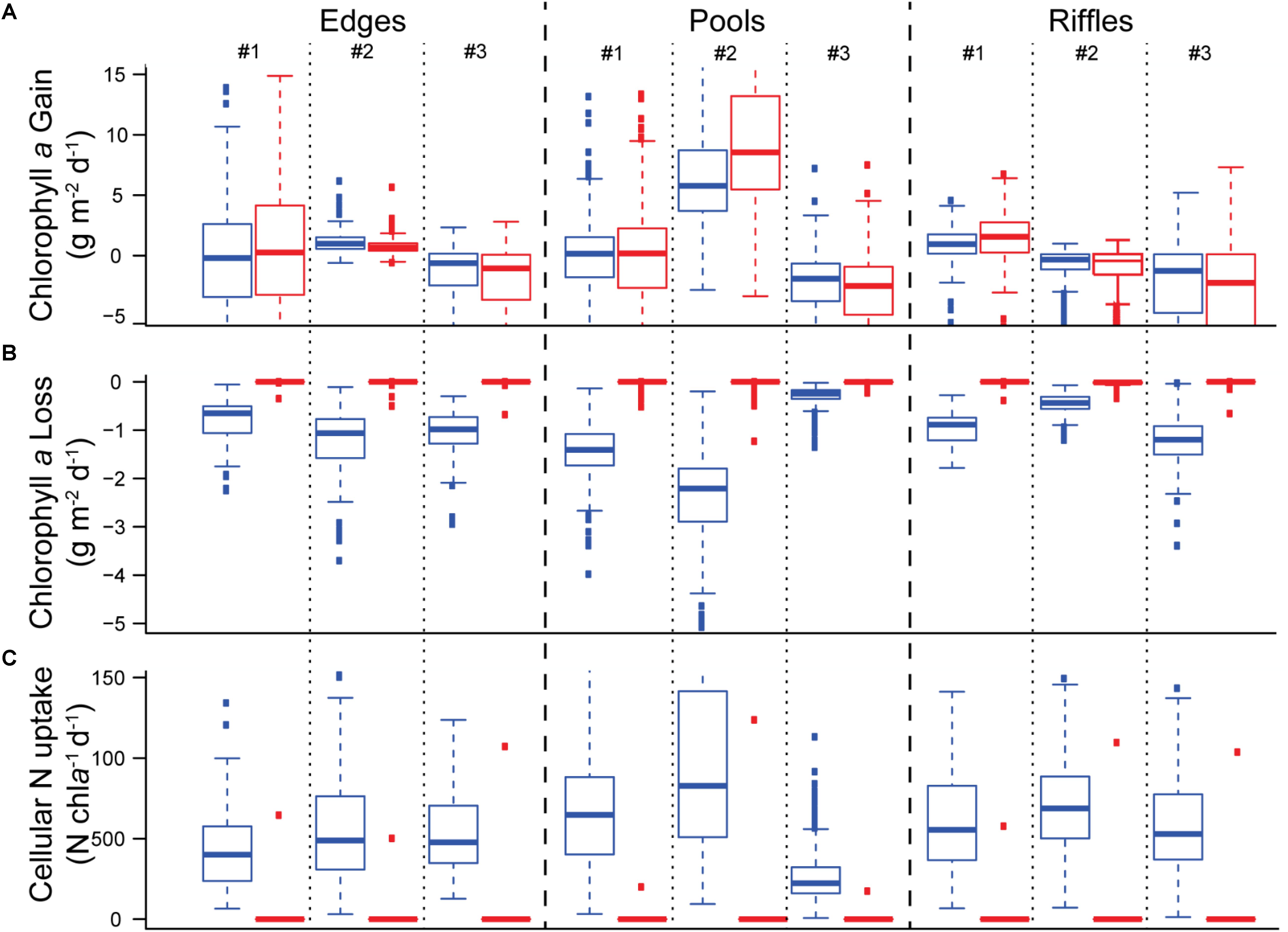
Figure 4. Boxplots showing: daily (A) chla production, (B) chla losses, and (C) nitrogen uptake per unit chla over the course of the run. Blue boxes indicate modeled values including the presence of salmon while red boxes indicate modeled values where salmon were excluded by setting salmon abundance to zero (i.e., salmon were stopped).
Discussion
Our high-frequency sampling approach, encompassing the biological response metrics and the environmental context, combined with a dynamic process model allowed us to address the question of the relative importance of salmon nutrient subsidies and physical disturbance on biofilm dynamics over the course of a run and across multiple locations within a stream. The magnitude of the salmon resource pulse (sensu Anderson et al., 2008) depends on the abundance of spawners and carcasses, which varies over time based on run dynamics. However, environmental conditions can also interact with the salmon run dynamics to modify the biotic responses to this nutrient pulse (Janetski et al., 2009; Subalusky and Post, 2019). For example, sediment size can strongly influence the extent of spawner-mediated enrichment versus disturbance (Tiegs et al., 2008; Holtgrieve et al., 2010) and nutrient limitation determines subsidy use (Rüegg et al., 2011). Theoretical models have supported the role of the environmental context (Bellmore et al., 2014), and spatio-temporal variation in stream environmental context could thereby modulate the ecological responses to salmon-mediated enrichment and disturbance (Rüegg et al., 2012; Subalusky and Post, 2019). However, no previous study has combined a high-frequency field data set such as ours with a dynamic model to separate the dual salmon effects from the general environmental context. Our model identified a small net disturbance effect of salmon that was fairly universal across habitat types. Pronounced salmon effects only occurred during small windows of time (a few days to 2 weeks), as evidenced by biofilm biomass being higher or lower than expected had the salmon run not occurred in the given environmental context. In other words, the effects of salmon as a keystone organism (sensu Willson and Halupka, 1995), be it as a resource subsidy or a physical disturbance (Gende et al., 2002; Moore, 2006; Flecker et al., 2010), may be most intense during limited periods for algal and nutrient dynamics related to species phenology such as timing and size of runs or spawning requirements (e.g., sucker, Childress and McIntyre, 2016). However, a sequence of salmon runs by different species, as occurs in many systems, may have an aggregate effect on ecosystem structure and function (Lamberti et al., 2010).
In Twelve Mile Creek, temporal changes in environmental context had stronger influences than did spatial difference among habitats (cf. Flecker et al., 2002; Geddes and Trexler, 2003), potentially due to changes to the stream context that lead to pervasive spawning throughout the reach. Namely, sediment sizes were generally smaller throughout the entire stream than those of similar streams with comparable run sizes in the area (Tiegs et al., 2008; Rüegg et al., 2012), reducing differences among the selected habitat types. Large salmon returns, as witnessed for pink salmon (O. gorbuscha) in Twelve Mile Creek, can force spawners to use all available space, resulting in spawner-mediated disturbance in lower-quality spawning habitat (Quinn, 2005). We realize that this study represents one season in a 300-m stream reach of one stream in southeast Alaska. Unfortunately, we were not able to extend the sampling past the end of the salmon run, but an earlier study in the region suggests that biofilms in some streams recover from salmon-mediated disturbance (Tiegs et al., 2008) and that salmon nutrients provide a limiting resource (Rüegg et al., 2011) that can persist beyond the actual run (Reisinger et al., 2013). Before-during comparison of salmon effects in this stream were consistent over multiple years (Rüegg et al., 2012), suggesting that the model may be applicable beyond the temporal scope of the study. Unreplicated study designs can provide valuable information on potential mechanisms, such as the one applied to this stream reach, but their applicability to other streams needs to be tested in further high-frequency studies. However, despite simplifications in both the study design and the representation of the environmental context (e.g., environmental characteristics only measured at reach scale), we were able to evaluate the relative roles of salmon enrichment and disturbance on biofilms. Our integrative approach indicates that salmon contribute more to biofilm losses than enrichment and, therefore, that their overall effect may be that of an ecosystem disturbance (Moore and Schindler, 2008; Bellmore et al., 2014). However, the short windows of enrichment may still be critical for overall stream ecosystem productivity, especially if salmon nutrients are integrated and propagated in stream food webs (e.g., macroinvertebrates or fish) and persist past the presence of salmon spawners (e.g., Reisinger et al., 2013; Harding and Reynolds, 2014).
Small-scale temporal responses of biofilm were evident for all metrics. Biofilm production, biomass, and nitrogen isotopes increased early in the run. Biofilm exhibited rapid δ15N enrichment (within a few days) similar to the isotopic signature of salmon material (cf. Chaloner et al., 2002), likely reflecting uptake of salmon-derived nitrogen by otherwise nutrient-limited biofilms (Rüegg et al., 2011). The absence of increased streamwater nutrient concentrations during that same period may be due to rapid uptake of salmon nutrients by biofilms. Later in the run, the increased dissolved nitrogen concentration due to salmon may have saturated the capacity for biofilm uptake (Levi et al., 2011; Rüegg et al., 2011). Most biofilm metrics exhibited sharp changes a few weeks into the salmon spawning run when the beginning of spawning activity would have scoured biofilm from sediments, thereby negating any enrichment effects of salmon (i.e., no net biofilm response; cf. Molinos and Donohue, 2010). This disturbance prevailed until the end of the study when disturbance dominated over enrichment and prevented biofilm biomass recovery. However, stable isotope signatures (i.e., isotopic enrichment) suggest that biofilms were actively growing while taking up salmon-derived dissolved nutrients during these periods of disturbance, assimilating more nitrogen per unit biomass than early in the run. Thus, salmon provide resources over the entire run, but physical disturbance may negate most of the enrichment effect at high salmon spawner densities and widespread disturbance (cf. Moore and Schindler, 2008). However, biofilm with low biomass but rapid turnover rates can still take up salmon nutrients and transfer that production to higher trophic levels (Lamberti and Resh, 1983; Morley et al., 2016), suggesting that enrichment needs to be defined both in terms of productivity and the amount of resources incorporated by direct (e.g., nutrient uptake by biofilms, consumption of eggs by resident fish) and indirect pathways (e.g., trophic transfers).
Our results show that salmon spawner effects may manifest at different time points of the salmon run, but that biofilm responses were similar with and without the presence of salmon over most of the run, supporting the theoretical findings of Bellmore et al. (2014) that an early period of enrichment is followed by predominantly disturbance later in the run, while the integrated effects of salmon are relatively balanced. Biofilm production can respond rapidly (within days to 2–3 weeks) to changes in environmental conditions (Biggs, 1996), potentially before live salmon abundances peak (3–4 weeks in this study). Thus, environmental conditions in the 1–2 weeks prior to sampling might be more critical determinants of biofilm responses than conditions at the time of sampling (cf. Holtgrieve and Schindler, 2011), potentially explaining why environmental conditions at the time of sampling often have low explanatory power (e.g., Rüegg et al., 2012). Salmon run dynamics may have contributed to observed patterns as salmon spawners reached peak densities within 2 weeks of the start of the spawning run. The period leading up to peak densities corresponds to when males that excrete nutrients but do not dig nests in sediment (Quinn, 2005) arrive en masse. Females that both excrete nutrients and excavate redds, thus disturbing the sediment, attained peak density after approximately 4 weeks; this potential ecological effect of sequencing of male and female salmon has not yet been considered. The early part of the salmon run likely provides enrichment but little disturbance, which enables biofilm to respond with increased productivity during a time when other environmental conditions are also favorable (Bellmore et al., 2014).
An early window of subsidy use suggests that seasonal changes over the course of the run, such as declining light and temperature, may have little effect on the magnitude of salmon subsidy use because maximal response to salmon enrichment happens early in the run. Delayed arrival of male salmon into streams (Quinn, 2005), such as when low discharge prevents upstream migration, may therefore have important consequences because the period of net enrichment may decline relative to spawning disturbance. Our study suggests that spawner enrichment and disturbance are restricted to a narrow time window of about 6 weeks in this stream, whereas environmental factors such as flood disturbance can persist much longer (e.g., 3–6 months in coastal Alaska streams, Oswood et al., 2006). However, over the course of the run we infer that biofilm accrual is balanced by the dual effects of salmon, as daily biofilm growth was similar for model trajectories with or without salmon. Biofilm losses were clearly driven by the presence of salmon while nitrogen uptake increased in the presence of salmon, suggesting that despite the overall biomass loss, relative nutrient enrichment may still occur (e.g., Holtgrieve et al., 2010; Reisinger et al., 2013). While the relative importance of salmon enrichment and disturbance has been studied (e.g., Moore et al., 2004; Tiegs et al., 2009), this has rarely involved differentiating between enrichment and disturbance at daily time steps over the salmon run. Determining the salmon spawner effect with only limited sampling frequency may miss critical “windows of effects” or overemphasize the magnitude of the effect, especially considering the large spatial variability in responses among ecosystems (Chaloner et al., 2004; Tiegs et al., 2008; Rüegg et al., 2012).
Previous studies have rarely considered dynamic environmental variables, such as discharge and temperature (but see Chaloner et al., 2007; Rüegg et al., 2012), that could enhance or diminish salmon effects (Tiegs et al., 2011). A recent framework on animal subsidies argues that the context of the donor and recipient ecosystems determines the quality, quantity, timing, and duration of the resource subsidy, which also modulate the ecosystem’s response to the subsidy (Subalusky and Post, 2019). Our field and modeling results suggest that considering salmon run dynamics in conjunction with dynamics of other environmental characteristics is key to understanding salmon as a subsidy to their natal streams. Variation in biofilm production and biomass during the salmon run is likely due to the synergistic effects of several environmental variables (cf. Wipfli et al., 1999; Hill et al., 2011), including increasing water temperature, low discharge, and high irradiance during high biofilm biomass periods (i.e., baseflow) at the onset of the salmon run. Our model further suggests that concurrent changes in light, temperature, nutrients, discharge, and salmon abundance all contribute to biofilm dynamics, and have implications for the interpretation of salmon effects (cf. Stevenson, 1997; Hill et al., 2011). Quantifying only the subsidy effect will overestimate salmon’s positive bottom-up influence given their countering role as agents of disturbance. Considering the multiple roles of salmon, in conjunction with environmental conditions, is therefore needed to accurately predict the net impacts that salmon have on their natal streams.
Conclusion
By combining high frequency field data with a process-based model, we showed that changes in environmental context can interact with salmon run dynamics to modulate the response in stream biofilm. Early in the run, salmon nutrient enrichment was favored by low discharge, increasing temperature, and high light that enhanced biofilm. Later in the run, high discharge events combined with intense spawning activity and declining temperature and light slowed biofilm recovery, leading to a reduction in biofilm biomass. Overall, the net effect of salmon on benthic biofilm accrual was slightly negative, and driven by late-run disturbance. Our study demonstrates that the resource subsidy and sediment disturbance imparted by salmon are dynamic and interactive over the course of a salmon run. Thus, studies that target the period of peak spawner abundance are unlikely to capture the full variation in biologically important responses, be they those of basal resources such as biofilm or higher trophic levels, even if undertaken over a broad spatial scale encompassing multiple salmon streams. As such, high frequency sampling in multiple streams may be needed to sufficiently capture the complex ecological influence of salmon spawners on streams ecosystems.
Data Availability Statement
The datasets generated for this study are available on request from the corresponding author.
Author Contributions
JR, DC, and ST conceived and designed the study with inputs from JT and GL. JR, PL, and ST performed the sampling. JR and PL analyzed all samples. JR, CS, and FB analyzed the data. JR and DC wrote the manuscript. All authors provided major inputs and revisions to the manuscript.
Funding
This research was supported by the USDA-CSREES National Research Initiative Competitive Grants Program (Managed Ecosystems Program 2006-35101-16566) and the Pacific Northwest Research Station (USDA Forest Service) Aquatic-Land Interactions Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ann Marie Larquier, Sarah Winikoff, Angeline Kosnik, Mollie McIntosh, and Emily Campbell for assistance in the field and laboratory. Aaron Prussian, Katherine Prussian, and Steve McCurdy provided insights about streams on Prince of Wales Island. Dave D’Amore, Rick Edwards, Jacob Berkowitz, Erik Norberg, Mike Brueseke, and Susanne Hebbeler provided logistical support. We thank members of the Lamberti and Tank Laboratories as well as the reviewers for improvements to this manuscript. We also thank the Thorne Bay and Craig Ranger Districts as well as the Juneau Forestry Science Laboratory (USDA Forest Service) for their collaboration and support of this project. Stable isotope analyses were performed at the University of Notre Dame Center for Environmental Science and Technology.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.00019/full#supplementary-material
References
Ambrose, H. E., Wilzbach, M. A., and Cummins, K. W. (2004). Periphyton response to increased light and salmon carcass introduction in northern California streams. J. North Am. Benthol. Soc. 23, 701–712. doi: 10.1899/0887-3593(2004)023<0701:prtila>2.0.co;2
Anderson, W. B., and Polis, G. A. (1999). Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California islands. Oecologia 118, 324–332. doi: 10.1007/s004420050733
Anderson, W. B., Wait, D. A., and Stapp, P. (2008). Resources from another place and time: responses to pulses in a spatially subsidized system. Ecology 89, 660–670. doi: 10.1890/07-0234.1
Bellmore, J. R., Fremier, A. K., Mejia, F., and Newsom, M. (2014). The response of stream periphyton to P acific salmon: using a model to understand the role of environmental context. Freshw. Biol. 59, 1437–1451. doi: 10.1111/fwb.12356
Biggs, B. J. F. (1996). “Patterns in benthic algae of streams,” in Algal Ecology, eds R. J. Stevenson, M. L. Bothwell, and R. L. Lowe (Cambridge, MA: Academic Press), 31–58.
Bott, T. L. (2006). “Primary production and community respiration,” in Methods in Stream Ecology, eds F. R. Hauer, and G. A. Lamberti (Cambridge, MA: Academic Press), 663–690. doi: 10.1016/b978-012332908-0.50040-1
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M., and West, G. B. (2004). Toward a metabolic theory of ecology. Ecology 85, 1771–1789. doi: 10.1890/03-9000
Chaloner, D. T., Lamberti, G. A., Cak, A. D., Blair, N. L., and Edwards, R. T. (2007). Inter-annual variation in responses of water chemistry and epilithon to Pacific salmon spawners in an Alaskan stream. Freshw. Biol. 52, 478–490. doi: 10.1111/j.1365-2427.2006.01715.x
Chaloner, D. T., Lamberti, G. A., Merritt, R. W., Mitchell, N. L., Ostrom, P. H., and Wipfli, M. S. (2004). Variation in responses to spawning Pacific salmon among three southeastern Alaska streams. Freshw. Biol. 49, 587–599. doi: 10.1111/j.1365-2427.2004.01213.x
Chaloner, D. T., Martin, K. M., Wipfli, M. S., Ostrom, P. H., and Lamberti, G. A. (2002). Marine carbon and nitrogen in southeastern Alaska stream food webs: evidence from artificial and natural streams. Can. J. Fish. Aquat. Sci. 59, 1257–1265. doi: 10.1139/f02-084
Childress, E. S., and McIntyre, P. B. (2016). Life history traits and spawning behavior modulate ecosystem-level effects of nutrient subsidies from fish migrations. Ecosphere 7:e01301.
Eggert, S. L., Wallace, J. B., Meyer, J. L., and Webster, J. R. (2012). Storage and export of organic matter in a headwater stream: responses to long-term detrital manipulations. Ecosphere 3, 1–25.
Flecker, A. S., McIntyre, P. B., Moore, J. W., Anderson, J. T., Taylor, B. W., and Hall, R. O. Jr. (2010). Migratory fishes as material and process subsidies in riverine ecosystems. Am. Fish. Soc. 73, 559–592.
Flecker, A. S., Taylor, B. W., Bernhardt, E. S., Hood, J. M., Cornwell, W. K., Cassatt, S. R., et al. (2002). Interactions between herbivorous fishes and limiting nutrients in a tropical stream ecosystem. Ecology 83, 1831–1844. doi: 10.1890/0012-9658(2002)083
Geddes, P., and Trexler, J. C. (2003). Uncoupling of omnivore-mediated positive and negative effects on periphyton mats. Oecologia 136, 585–595. doi: 10.1007/s00442-003-1294-4
Gende, S. M., Edwards, R. T., Willson, M. F., and Wipfli, M. S. (2002). Pacific salmon in aquatic and terrestrial ecosystems. Bioscience 52, 917–928.
Haario, H., Saksman, E., and Tamminen, J. (2001). An adaptive Metropolis algorithm. Bernoulli 7, 223–242.
Harding, J. N., and Reynolds, J. D. (2014). Opposing forces: evaluating multiple ecological roles of Pacific salmon in coastal stream ecosystems. Ecosphere 5, 1–22.
Hill, W. R., Roberts, B. J., Fancoeur, S. N., and Fanta, S. E. (2011). Resource synergy in stream periphyton communities. J. Ecol. 99, 454–463.
Holtgrieve, G. W., and Schindler, D. E. (2011). Marine-derived nutrients, bioturbation, and ecosystem metabolism: reconsidering the role of salmon in streams. Ecology 92, 373–385. doi: 10.1890/09-1694.1
Holtgrieve, G. W., Schindler, D. E., Gowell, C. P., Ruff, C. P., and Lisi, P. J. (2010). Stream geomorphology regulates the effects on periphyton of ecosystem engineering and nutrient enrichment by Pacific salmon. Freshw. Biol. 55, 2598–2611. doi: 10.1111/j.1365-2427.2010.02489.x
Janetski, D. J., Chaloner, D. T., Tiegs, S. T., and Lamberti, G. A. (2009). Pacific salmon effects on stream ecosystems: a quantitative synthesis. Oecologia 159, 583–595. doi: 10.1007/s00442-008-1249-x
Johnson, L. T., Tank, J. L., and Dodds, W. K. (2009). The influence of land use on stream biofilm nutrient limitation across eight North American ecoregions. Can. J. Fish. Aquat. Sci. 66, 1081–1094. doi: 10.1139/f09-065
Kim, B. K., Jackman, A. P., and Triska, F. J. (1990). Modeling transient storage and nitrate uptake kinetics in a flume containing a natural periphyton community. Water Resour. Res. 26, 505–515. doi: 10.1029/wr026i003p00505
Kim, B. K., Jackman, A. P., and Triska, F. J. (1992). Modeling biotic uptake by periphyton and transient hyporrheic storage of nitrate in a natural stream. Water Resour. Res. 28, 2743–2752. doi: 10.1029/92wr01229
Lamberti, G. A., Chaloner, D. T., and Hershey, A. E. (2010). Linkages among aquatic ecosystems. J. North Am. Benthol. Soc. 29, 245–263. doi: 10.1899/08-166.1
Lamberti, G. A., and Resh, V. H. (1983). Stream periphyton and insect herbivores: an experimental study of grazing by a caddisfly population. Ecology 64, 1124–1135. doi: 10.2307/1937823
Legović, T., and Cruzado, A. (1997). A model of phytoplankton growth on multiple nutrients based on the Michaelis-Menten-Monod uptake, Droop’s growth and Liebig’s law. Ecol. Model. 99, 19–31. doi: 10.1016/s0304-3800(96)01919-9
Levi, P. S., Tank, J. L., Tiegs, S. D., Rüegg, J., Chaloner, D. T., and Lamberti, G. A. (2011). Does timber harvest influence the dynamics of marine-derived nutrients in southeast Alaska streams? Can. J. Fish. Aquat. Sci. 68, 1316–1329. doi: 10.1139/f2011-067
Marcarelli, A. M., Baxter, C. V., Mineau, M. M., and Hall, R. O. Jr. (2011). Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92, 1215–1225. doi: 10.1890/10-2240.1
Marczak, L. B., Thompson, R. M., and Richardson, J. S. (2007). Meta-analysis: trophic level, habitat, and productivity shape the food web effects of resource subsidies. Ecology 88, 140–148. doi: 10.1890/0012-9658(2007)88
Molinos, J. G., and Donohue, I. (2010). Interactions among temporal patterns determine the effects of multiple stressors. Ecol. Appl. 20, 1794–1800. doi: 10.1890/10-0018.1
Moore, J. W., and Schindler, D. E. (2008). Biotic disturbance and benthic community dynamics in salmon-bearing streams. J. Anim. Ecol. 77, 275–284. doi: 10.1111/j.1365-2656.2007.01336.x
Moore, J. W., Schindler, D. E., and Scheuerell, M. D. (2004). Disturbance of freshwater habitats by anadromous salmon in Alaska. Oecologia 139, 298–308. doi: 10.1007/s00442-004-1509-3
Morley, S. A., Coe, H. J., Duda, J. J., Dunphy, L. S., McHenry, M. L., Beckman, B. R., et al. (2016). Seasonal variation exceeds effects of salmon carcass additions on benthic food webs in the Elwha River. Ecosphere 7:e01422. doi: 10.1002/ecs2.1422
Oswood, M. W., Irons, J. G., and Milner, A. M. (2006). “River and stream ecosystems of Alaska,” in River and Stream Ecosystems of the World, eds C. E. Cushing, K. W. Cummins, and G. W. Minshall (Berkeley, CA: University of California Press), 9–32.
Polis, G. A., Anderson, W. B., and Holt, R. D. (1997). Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Syst. 28, 289–316. doi: 10.1146/annurev.ecolsys.28.1.289
Polis, G. A., Power, M. E., and Huxel, G. R. (2004). Food Webs at the Landscape Level. Chicago, IL: University of Chicago Press.
Quinn, T. P. (2005). The Behavior and Ecology of Pacific Salmon and Trout. Seattle: University of Washington Press.
R Core Team, (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Reisinger, A. J., Chaloner, D. T., Rueegg, J., Tiegs, S. D., and Lamberti, G. A. (2013). Effects of spawning Pacific salmon on the isotopic composition of biota differ among southeast Alaska streams. Freshw. Biol. 58, 938–950. doi: 10.1111/fwb.12098
Rüegg, J., Chaloner, D. T., Levi, P. S., Tank, J. L., Tiegs, S. D., and Lamberti, G. A. (2012). Environmental heterogeneity and the ecological effects of spawning Pacific salmon. Freshw. Biol. 57, 129–142. doi: 10.1111/j.1365-2427.2011.02703.x
Rüegg, J., Tiegs, S. D., Chaloner, D. T., Levi, P. S., Tank, J. L., and Lamberti, G. A. (2011). Salmon subsidies alleviate nutrient limitation of benthic biofilms in southeast Alaska streams. Can. J. Fish. Aquat. Sci. 68, 277–287. doi: 10.1139/f10-145
Sitters, J., Atkinson, C. L., Guelzow, N., Kelly, P., and Sullivan, L. L. (2015). Spatial stoichiometry: cross-ecosystem material flows and their impact on recipient ecosystems and organisms. Oikos 124, 920–930. doi: 10.1111/oik.02392
Smith, H., and Waltman, P. (1995). The Theory of the Chemostat: Dynamics of Microbial Competition (Cambridge Studies in Mathematical Biology). Cambridge, MA: Cambridge University Press.
Stevenson, R. J. (1997). Scale-dependent determinants and consequences of benthic algal heterogeneity. J. North Am. Benthol. Soc. 16, 248–262. doi: 10.2307/1468255
Subalusky, A. L., and Post, D. M. (2019). Context dependency of animal resource subsidies. Biol. Rev. 94, 517–538. doi: 10.1111/brv.12465
Tiegs, S. D., Campbell, E. Y., Levi, P. S., Rüegg, J., Benbow, M. E., Chaloner, D. T., et al. (2009). Separating physical disturbance and nutrient enrichment caused by Pacific salmon in stream ecosystems. Freshw. Biol. 54, 1864–1875. doi: 10.1111/j.1365-2427.2009.02232.x
Tiegs, S. D., Chaloner, D. T., Levi, P., Rüegg, J., Tank, J. L., and Lamberti, G. A. (2008). Timber harvest transforms ecological roles of salmon in Southeast Alaska rain forest streams. Ecol. Appl. 18, 4–11. doi: 10.1890/07-0655.1
Tiegs, S. D., Levi, P. S., Rüegg, J., Chaloner, D. T., Tank, J. L., and Lamberti, G. A. (2011). Ecological effects of live salmon exceed those of carcasses during an annual spawning migration. Ecosystems 14, 598–614. doi: 10.1007/s10021-011-9431-0
Wallace, J. B., Eggert, S. L., Meyer, J. L., and Webster, J. R. (1997). Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277, 102–104. doi: 10.1126/science.277.5322.102
Willson, M. F., and Halupka, K. C. (1995). Anadromous fish as keystone species in vertebrate communities. Conserv. Biol. 9, 489–497. doi: 10.1046/j.1523-1739.1995.09030489.x
Wipfli, M. S., Hudson, J. P., Chaloner, D. T., and Caouette, J. P. (1999). Influence of salmon spawner densities on stream productivity in Southeast Alaska. Can. J. Fish. Aquat. Sci. 56, 1600–1611. doi: 10.1139/f99-087
Keywords: marine-derived nutrients, epilithon, periphyton, environmental context, salmon runs, Oncorhynchus
Citation: Rüegg J, Chaloner DT, Ballantyne F, Levi PS, Song C, Tank JL, Tiegs SD and Lamberti GA (2020) Understanding the Relative Roles of Salmon Spawner Enrichment and Disturbance: A High-Frequency, Multi-Habitat Field and Modeling Approach. Front. Ecol. Evol. 8:19. doi: 10.3389/fevo.2020.00019
Received: 31 August 2019; Accepted: 23 January 2020;
Published: 07 February 2020.
Edited by:
J. Ryan Bellmore, Pacific Northwest Research Station, USDA Forest Service, United StatesReviewed by:
Matthew J. Kaylor, Oregon State University, United StatesJonathan Moore, Simon Fraser University, Canada
Copyright © 2020 Rüegg, Chaloner, Ballantyne, Levi, Song, Tank, Tiegs and Lamberti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janine Rüegg, amFuaW5lLnJ1ZWdnQHVuaWwuY2g=
†Present address: Janine Rüegg, Interdisciplinary Center for Mountain Research (CIRM), University of Lausanne (UNIL), Lausanne, Switzerland Peter S. Levi, Environmental Science and Sustainability, Drake University, Des Moines, IA, United States Chao Song, Department of Fisheries and Wildlife, Michigan State University, East Lansing, MI, United States Scott D. Tiegs, Department of Biological Sciences, Oakland University, Rochester, MI, United States
 Janine Rüegg
Janine Rüegg Dominic T. Chaloner
Dominic T. Chaloner Ford Ballantyne
Ford Ballantyne Peter S. Levi1†
Peter S. Levi1† Chao Song
Chao Song Scott D. Tiegs
Scott D. Tiegs Gary A. Lamberti
Gary A. Lamberti