- 1Department of Biology, Wake Forest University, Winston-Salem, NC, United States
- 2Department of Zoology, Milwaukee Public Museum, Milwaukee, WI, United States
Many aposematic animals are well-known to exhibit generally sluggish movements. However, less is known about their escape responses when under direct threat of predation. In this study, we characterize the anti-bat escape responses of 5 species of tiger moth (Erebidae: Arctiinae), a subfamily of Lepidoptera which possess ultrasound-sensitive ears. These ears act as an early-warning system which can detect the ultrasonic cries of nearby echolocating bats, allowing the moths to enact evasive flight behaviors in an effort to escape predation. We examine the role that unpalatability plays in predicting the likelihood that individuals of a given species will enact escape behaviors in response to predation. We hypothesized that more unpalatable species would be less likely to exhibit escape maneuvers (i.e., more nonchalant) than their less unpalatable counterparts. Our results demonstrate significant interspecific variation in the degree to which tiger moths utilize evasive flight behaviors to escape bat predators as well as in their degree of unpalatability. We provide evidence for the existence of a nonchalance continuum of anti-bat evasive flight response among tiger moths and show that species are arrayed along this continuum based on their relative unpalatability to bat predators. Relatively unpalatable prey more often exhibit nonchalant flight behaviors whereas palatable prey more often employ evasive dives. Our findings demonstrate that the degree to which certain animals are protected by potent chemical defenses can influence the likelihood that they will exhibit evasive escape behaviors. Further, we argue that the bat-moth predator-prey system is an ideal model for future studies of escape behaviors of prey which overcomes some of the limitations inherent to current model systems.
Introduction
Aposematic animals have long been recognized to exhibit sluggish movements. Bates was the first to observe this, describing several strikingly colored butterflies as possessing “weak, slow flight” (Bates, 1862; Poulton, 1890). Wallace later wrote of the chemically defended, black-and-white striped skunk, “Its consciousness that it needs only to be seen to be avoided gives it that slowness of motion and fearlessness of aspect which are characteristic of most creatures so protected.” (Wallace, 1889). The phenomenon of sluggishness was later incorporated into the “chemical defense syndrome” (CDS), a general suite of characteristics commonly exhibited by chemically protected animals (Whitman et al., 1985). There are several hypotheses which seek to explain why aposematic animals exhibit these sluggish behaviors. One such hypothesis is that these slow movements evolved because they avoid triggering the attack responses of motion-oriented, ambush predators like frogs and praying mantids (Hatle and Faragher, 1998; Hatle et al., 2002). Another possibility is that these conspicuous, slow movements are themselves aposematic, acting as an additional signal of unprofitability to would-be predators. Expanding on Bates' early observations, a comparative study of butterfly species has quantitatively shown that distasteful species fly relatively more slowly than palatable species, indicating that sluggish flight can honestly signal prey defenses (i.e., “locomotor mimicry”; Chai and Srygley, 1990; Srygley, 1994, 2004). Another non-mutually exclusive hypothesis posits that these slow movements may make visual aposematic signals more apparent to predators, increasing the effectiveness of such signals (i.e., “raised exposure”; Speed et al., 2010).
We reserve “sluggishness” as a description of general locomotory movements, with “more sluggish” behavior indicating slower, seemingly careless movements, as it has traditionally been defined (Hatle and Faragher, 1998; Hatle and Whitman, 2001). However, animals can alter their movements in different contexts. When animals are actively pursued by predators they will often exhibit escape maneuvers. We define a seemingly careless demeanor in response to an immediate threat as “nonchalant.” “More nonchalant” indicates slower, less complex, delayed, and/or more rarely enacted escape responses in response to a potential predatory threat (Dowdy and Conner, 2016). Because of inherent costs of fleeing, prey are expected to balance perceived predation risk with the costs of fleeing such that they initiate escape at a distance which maximizes their fitness at the end of an encounter with a predator (i.e., “Optimal Escape Model”; Ydenberg and Dill, 1986; Cooper and Frederick, 2007). Flight initiation distance (FID) is known to vary depending on a variety of factors (e.g., body condition, distance to refugia) which likely influence prey assessment of predation risk and/or the costs associated with escape (Stankowich and Blumstein, 2005; Samia et al., 2016; Cooper, 2018). Chemical defenses may be one such factor influencing FID, however the escape responses of chemically defended prey have not been well-studied, and it is not clear whether sluggish animals should be expected to remain so when directly threatened. To our knowledge, the escape responses of aposematic animals in response to the threat of predation have only been investigated among dendrobatid frogs. Generally, these studies have found that aposematic frogs retain escape responses but delay fleeing relative to cryptic species, though variation in FID exists between aposematic species and based on the context of the encounter (Cooper et al., 2009a,b; Ozel and Stynoski, 2011; Dugas et al., 2015; Blanchette et al., 2017). Unfortunately, due to methodological limitations, the results of these studies confound escape decisions based on predation avoidance with trample avoidance (i.e., avoidance of being crushed), limiting the usefulness of their conclusions.
The bat-moth predator-prey system is an ideal system in which to study the escape behaviors of aposematic prey. Many moth species are equipped with ultrasound-sensitive ears which they use to detect bat predators. The ability of insects to detect the ultrasonic cries of bats led to their development of avoidance behaviors such as negative phonotaxis, spiraling erratic flight, and powered dives (Roeder, 1962). These maneuvers have been shown to be an effective means of dodging bat attacks (Acharya and Fenton, 1999; Triblehorn et al., 2008). Additionally, some moths possess secondary defenses which may alter their risk of predation. Many tiger moths (Erebidae: Arctiinae) sequester defensive toxins from their host plants and produce ultrasound in response to bat echolocation, advertising their unpalatability (i.e., “acoustic aposematism”; Acharya and Fenton, 1992; Dunning et al., 1992; Hristov and Conner, 2005; Barber et al., 2009; Dowdy and Conner, 2016). Unpalatability varies between species and may relate to the concentration and type of chemical compounds they have sequestered.
Interestingly, some tiger moths have been noted to lack any significant evasive flight response to bat attacks, even though they possess and utilize ultrasonic hearing (Goldman and Henson, 1977; Acharya and Fenton, 1992; Dunning et al., 1992; Dowdy and Conner, 2016). Field experiments with certain species of tiger moths have uncovered variation in escape behaviors in response to bat attacks (Dowdy and Conner, 2016). Two sympatric tiger moth species, Pygarctia roseicapitis and Cisthene martini, differed in both the likelihood of enacting evasive dives as well as in their unpalatability. The more unpalatable C. martini was significantly more nonchalant, rarely performing diving escape maneuvers. In contrast, the less unpalatable P. roseicapitis was less nonchalant, diving much more frequently when attacked by bats, indicating that a nonchalance continuum among aposematic species may exist.
In this study we examine the role that unpalatability plays in predicting the likelihood that individuals of a given species will enact escape behaviors in response to the threat of predation. Chemical defenses have not yet been recognized as a key factor affecting escape behaviors (Stankowich and Blumstein, 2005; Samia et al., 2016; Cooper, 2018). We hypothesized that more unpalatable tiger moth species would be less likely to exhibit escape maneuvers (i.e., more nonchalant) than their less unpalatable counterparts. This is the first study to comparatively examine the escape behaviors of aposematic insects in a natural context, compare the likelihood of escape between both aposematic and mimetic species, and construct a predictive model of escape behavior.
Materials and Methods
Ethics Statement
Most of the data presented here involves free-flying bats in their natural habitats, apart from some additional palatability data for P. rosecapitis and C. martini. Bats included in palatability tests were captured and released under USFWS permit TE01690A-0. The methods of this study were approved by the Wake Forest University Institutional Animal Care and Use Committee (protocol #A12-048). This work was performed with permission on private property.
Field Site
Field experiments were conducted at the Southwestern Research Station (SWRS) operated by the American Museum of Natural History. SWRS is located in Cochise County approximately 7 km southwest of Portal, Arizona, United States. The GPS coordinates of the field site are: 31°53'00.30” N 109°12'27.20” W; elevation: 1,650 m. This site was chosen for its high diversity of both bats and moths. The field trials were performed between July 18th and August 10th during 2011, 2012, and 2013.
Moth Collection and Manipulation
Moths were collected on station grounds from sheets illuminated with 15 Watt ultraviolet “quantum” lights (Leptraps.com; F15T8QBL). Moths identified as either Carales arizonensis, C. martini, Pygarctia murina, or P. roseicapitis were stored individually for up to 24 h in 30 mL plastic containers at ambient temperatures. Moths were randomly placed into one of three treatment groups: Tymbals Intact (T+), Tymbals Removed (T–), and Sham Control (S). Individuals from all three treatment groups were chilled for 5 min in an ice bath prior to surgery. Individuals assigned to the T+ group were removed from the ice bath and no further manipulations were performed. Individuals assigned to the T- group had their tymbal organs ablated with curved forceps by removing the cuticular surface of the organ. This ablation does not cause any discernable injury or loss of haemolymph. Impairment of sound production was verified by manipulating the individual while monitoring with an ultrasonic detector (Pettersson Model D-100). Individuals in the S group had their tymbals left intact, however scales near the tymbal organs were removed using curved forceps to simulate experimental manipulation.
Outdoor Flight Arena
Two ultraviolet lights were placed ~5 m off the ground and set 4 m apart in the center of a large, open field (~600 m2). These lights increased the general insect abundance within the flight arena, luring in free-flying bats which then began to forage reliably at this location. Moths included in this study were released at this site, one at a time, starting after sunset (21:00) for 6 h (03:00) or until we ran out of moths to run in trials. Most releases involved the moths taking off shortly after being released from their containers. In a few cases we released the moths by tossing them up in the air. In these cases, we did not collect data for 5–10 s or until it was clear the moth was flying under its own power and would be able to react normally to any bat attacks. Bat-moth interactions were recorded using high-speed, infrared cameras and ultrasound-sensitive microphones. Using these tools, we were able to document the evasive and acoustic responses of moths in response to natural interactions with their bat predators. To reduce the effects of pseudo-replication, we used only the first interaction for each individual moth included in our study. The number of individual bats within the calibrated space at any given time varied from one to six. This resulted in some unavoidable pseudo-replication across individual bats which could not be accounted for. However, this study was conducted over a period of 3 years, increasing the likelihood that unique bats were included.
Audio Recording and Analysis
Audio of the bat-moth interactions was recorded using three Avisoft Bioacoustics CM16/CMPA ultrasonic microphones (Berlin, Germany) with an Avisoft Ultrasound Gate 416H recording interface. Two microphones were placed ~1.5 m high and 4 m apart, near the edges of the recording volume, pointed up toward the interaction space. A third microphone was placed on the end of a pole which was held close (between 1 and 3 m) to the moth's location.
Video Recording
In 2011–2012, three Basler AG Scout infrared cameras (Model scA640-120gc; Ahrensburg, Germany) were used to record bat-moth interactions at 60 frames*sec-1 with 640 × 480 resolution. In 2013, three Basler Ace acA-2000-50 gm infrared cameras capable of recording up to 80 frames*sec-1 with 1,024 × 720 resolution were substituted. The cameras were synchronized with the audio recordings using custom hardware (Innovision Systems, Columbiaville, MI, USA). Video was acquired with MaxTraq2D software (Innovision Systems) and two Intel PRO/1000 PT Dual Server Adapters (Intel, Model: EXPI9402PT) installed in a PC running Windows 7. Six Wildlife Engineering IR-Lamp6 lights (Tucson, AZ, USA), two Bosch UFLED20-8BD illuminators (Farmington Hills, MI, USA) and two Raytec Raymax 200 platinum illuminators (Ashington, UK) provided infrared illumination in the flight arena.
Evasive Flight Behavior
We recorded interactions of moths with free-flying bats to determine how frequently they utilized evasive flight maneuvers. We included data from 345 bat-moth interactions across 5 species of tiger moth, P. murina, Bertholdia trigona, P. roseicapitis, C. arizonensis, and C. martini. Field trials included moths from experimental groups which could normally produce sound (i.e., T+, S) as well as individuals which had their sound-producing structures removed via ablation (i.e., T-). We included data about evasive flight responses from both clicking and silenced moths in this study. We manually classified each interaction and scored them as either “Turn Away,” “Dives,” or “No Evasion” as defined in classic studies of moth evasive flight (Roeder, 1962). However, we restricted our analysis to include only moths exhibiting “Dives” (renamed “Evasion”) or “No Evasion,” as we observed “Turn Away” flight in fewer than 10% of interactions. Examples of these behaviors can be seen in Supplemental Videos 1–3 as well as Figures 6A,B from Dowdy and Conner (2016).
Unpalatability
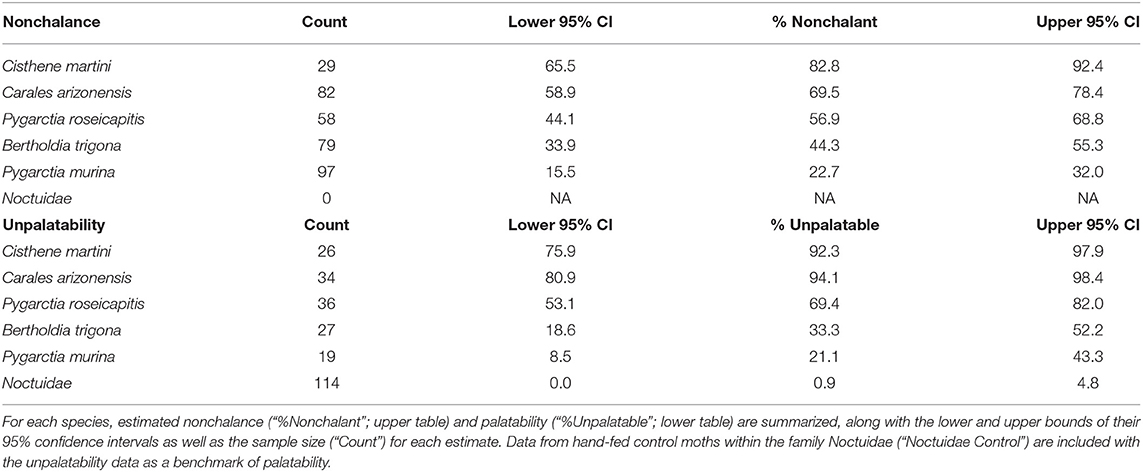
All moths species included in this study were previously known to produce ultrasound in response to bat echolocation (Corcoran et al., 2010; Dowdy, unpublished). Because of this, all of the palatability data reported in this study was measured from moths which were experimentally silenced (i.e., T–) to avoid confounding palatability with potentially deterrent effects of moth clicks. This data was derived from 118 interactions with wild, free-flying bats, which are also represented in the evasive flight behavior dataset. When captured by bats, moths were either dropped immediately (“Capture, Drop”—unpalatable) or not at all (“Consume”—palatable). In addition, we included data from 14 hand-feedings of silenced P. roseicapitis and 10 hand-feedings of silenced C. martini to captive big brown bats (Eptesicus fuscus). These 24 moths were presented to captive bats in a random order, interspersed with palatable moth species from the family Noctuidae. When moths were presented to these bats they were either rejected (unpalatable) or consumed (palatable) within 5–30 s of their first presentation. Trials continued until bats showed signs of satiation, which was defined as two sequential and complete rejections of control moths. Trials began again after 1–2 h until all tiger moths had been tested.
Bertholdia Trigona Data
Data for B. trigona (79 of our total 349 interactions) come from a previous study using the same experimental methods and location as those used in this study (Corcoran and Conner, 2012). Palatability and evasive diving flight data for B. trigona come from Figures 2B, 4A in Corcoran and Conner (2012), respectively. We included only data about diving evasive behavior from this study. Palatability data was taken only for silenced moths and was measured as the number of captured individuals that were not consumed.
Pygarctia roseicapitis and Cisthene martini Data
Data for P. roseicapitis and C. martini (87 of our total 349 interactions) come from a previous publication using the same experimental methods and location as those used in this study (Dowdy and Conner, 2016). Palatability and evasive diving flight data for these species come from Figures 2, 7 in Dowdy and Conner (2016), respectively, excluding some additional, new palatability data for these species which is presented here for the first time.
Statistics
Statistical analyses were performed in R version 3.3.2 (R Core Team, 2016) utilizing the dplyr package to prepare the data (Wickham et al., 2019). We implemented the glm function of the base R stats package to create a generalized linear model (GLM) with a binomial variance function and logit link function. This approach was used to compare the proportion of nonchalant flight behavior between species as well as the proportion of unpalatability between species. Using the relevel function within the base R stats package, we constructed a set of level contrasts for the species factor and applied them to our model in order to more easily compare the model results among all species. For each species, 95% confidence intervals for the binomial probabilities of nonchalance and unpalatability were constructed using the binconf function of the Hmisc package (Harrell, 2019) using the Wilson interval method. We used these proportions as a property of each species and examined how unpalatability predicted nonchalant flight using linear regression. Because we were interested in predicting the frequency of nonchalant evasive responses exhibited by species given their unpalatability, we constructed 95% prediction intervals for nonchalant flight using the predict function in the base R stats package. LaTeX tables were prepared using the texreg package (Leifeld, 2013). Plots were generated using ggplot2 (Wickham, 2016) with additional functions from the scales (Wickham, 2018) and gridExtra packages (Auguie, 2017). All R scripts are available via online repository (see Data Availability Statement).
Results
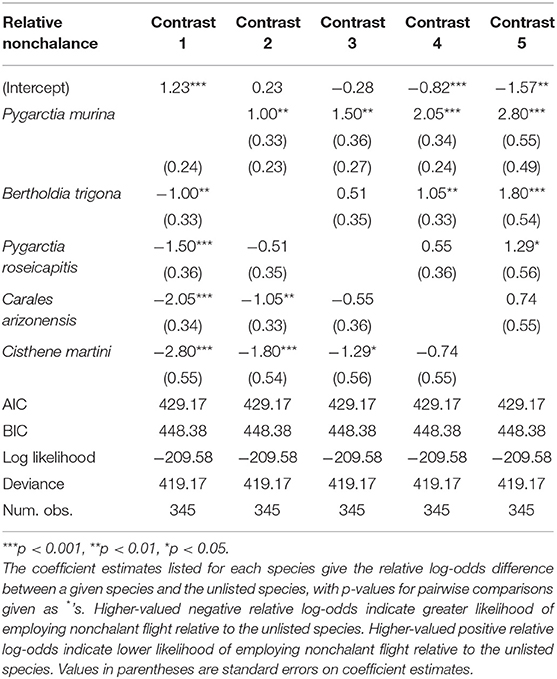
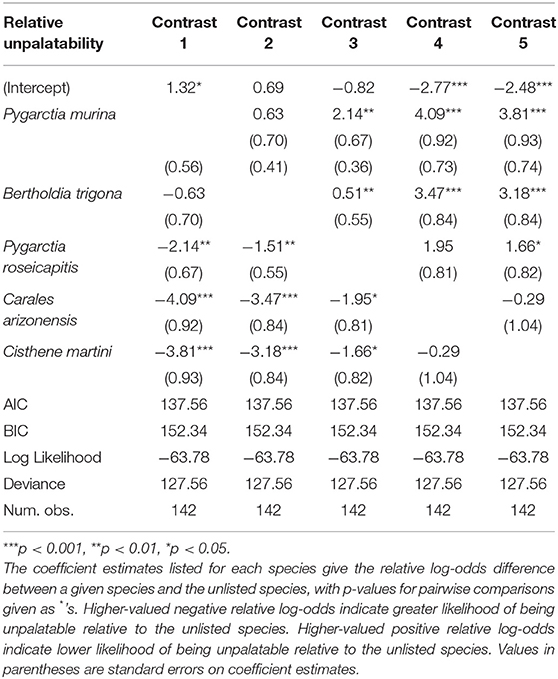
We found significant intraspecific variation in nonchalance and unpalatability (Figure 1). Statistical significance, level contrasts, and relative coefficients are given in Tables 1, 2. P. murina was significantly less nonchalant than all other species in our analysis. The prevalence of nonchalance between B. trigona and P. roseicapitis was not significantly different, though B. trigona was significantly less nonchalant as compared to C. arizonensis and C. martini. P. roseicapitis and C. arizonensis were not significantly different, however P. roseicapitis was significantly less nonchalant than C. martini. Finally, C. arizonenesis and C. martini were not significantly different.
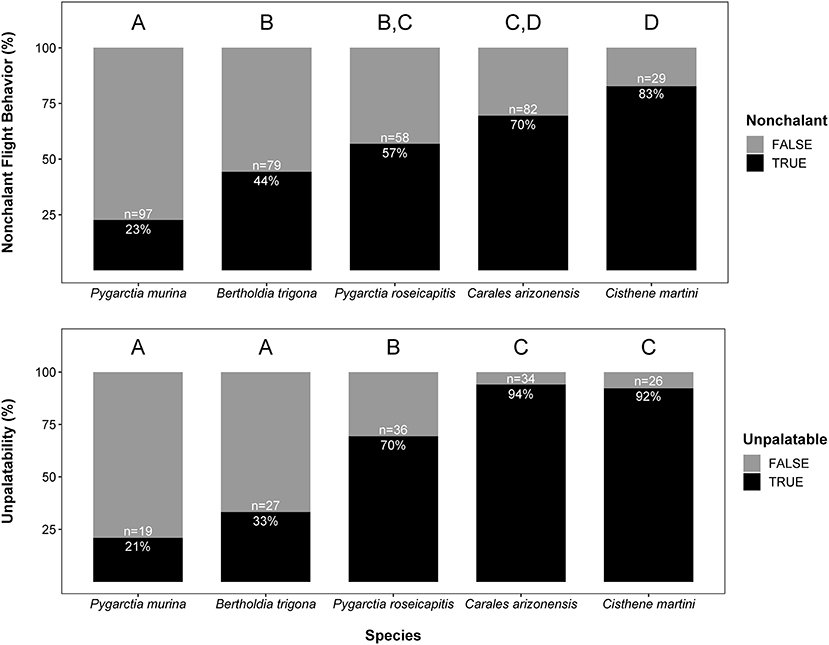
Figure 1. Interspecific variation in nonchalant flight behavior and unpalatability. Sample sizes for each species and the percentage exhibiting either nonchalant flight or unpalatability are given within each bar. Significantly different groups within each plot are indicated by different letters (see Tables 1, 2 for more info).
Compared to all other species in our analysis, P. murina and B. trigona were significantly less unpalatable, whereas C. arizonensis and C. martini were significantly more unpalatable than all other species in our analysis. P. roseicapitis was significantly different from both of these groups, exhibiting an intermediate level of unpalatability.
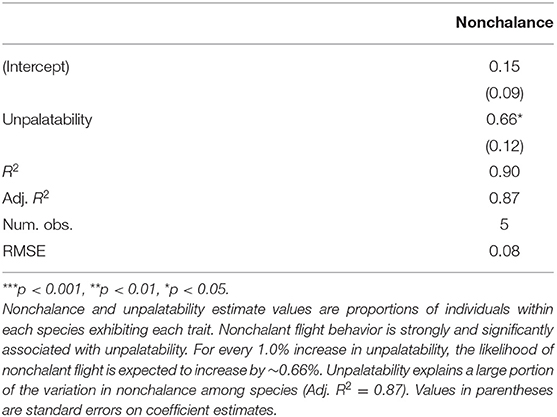
Modeling nonchalance from unpalatability yielded a significantly strong, positive relationship (p < 0.05, Adj. R2: 0.87; Table 3). However, because we could only sample 5 species, our 95% prediction intervals are large, limiting our current ability to confidently estimate nonchalance from unpalatability (Figure 2). A summary of the estimated nonchalance and unpalatability for each species, along with their 95% confidence intervals, can be found in Table 4.
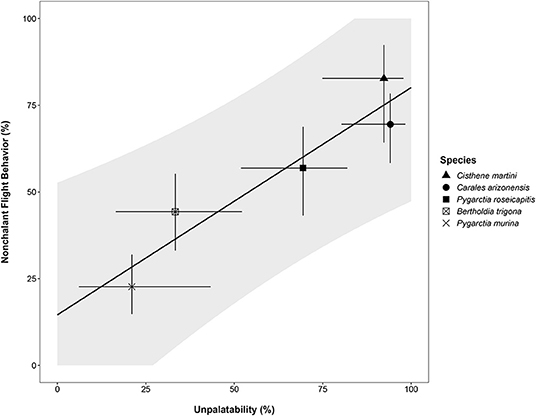
Figure 2. Plot of linear model of nonchalance. Gray ribbon represents the 95% prediction interval for the linear model relative nonchalant flight behavior and unpalatability (Table 3). For a species with a given Unpalatability, Nonchalance is expected to fall within these intervals in 95% of cases. For each species, point estimates (unique symbols) and 95% confidence intervals (line segments) for the binomial probabilities for both nonchalance and unpalatability are plotted.
Discussion
Our results demonstrate that, at least among tiger moths, significant interspecific variation in the likelihood of enacting evasive maneuvers in response to bat echolocation exists. This phenomenon forms a nonchalance continuum, with some species enacting escape maneuvers often, some rarely, and others lying somewhere between. In addition, we found significant interspecific differences in palatability among tiger moths. Variation in palatability has been noted in other insects, driven mainly by the host plants which they have fed on, and is generally known as a “palatability spectrum” (Brower et al., 1968, 1972; Turner, 1984). Further, we have shown that the position of tiger moth species along the nonchalance continuum and the palatability spectrum are strongly correlated. In other words, moths with more potent chemical defenses are more likely to display nonchalant flight. Interestingly, this is apparently independent of anti-bat strategy. Two species included in our analysis, B. trigona and C. arizonensis, produce clicks at very high rates and have been hypothesized or demonstrated to be capable of jamming bat sonar (Corcoran et al., 2010; Conner and Corcoran, 2012). Despite this, they each occupy significantly different places along the nonchalance continuum. However, consistent with our model, the large difference in unpalatability between these two species appears to be an important factor in determining their anti-bat evasive flight responses, with the more unpalatable C. arizonensis exhibiting nonchalant responses more frequently. Less unpalatable moths face an increased risk of consumption if captured by bat predators, and so they utilize evasive diving maneuvers more frequently, likely offsetting some of this risk. Additionally, more unpalatable prey may benefit from nonchalant flight by avoiding some of the potential costs of evasive maneuvers, including energy expenditure, opportunity costs related to hostplant- or mate-finding, or even increased exposure to terrestrial or aquatic predators (Guignion and Fullard, 2004; Yager, 2011). Our findings lend further support to an earlier study which demonstrated that palatability relates to evasive behaviors enacted from rest on substrates (e.g., flying away, dropping from vegetation) when exposed to tactile stimulation (Evans, 1983). These observations describe unpalatable prey exhibiting behaviors that could also be considered more nonchalant.
The relatively palatable P. murina produces anti-bat ultrasound with acoustic characteristics similar to those of its more unpalatable, sympatric congener P. roseicapitis (Dowdy and Conner, 2016; Dowdy, unpublished). This is similar to the relationship between Cycnia tenera and Euchaetes egle, two sympatric tiger moths native to eastern North America. Lab experiments with naïve bats have shown that the palatable E. egle was protected from predation by Eptesicus fuscus bats, acting as an acoustic Batesian mimic of C. tenera (Barber and Conner, 2007). It is likely that P. murina is an acoustic Batesian mimic of P. roseicapitis. It is intriguing then, that P. murina is less nonchalant than its more toxic model. It is possible that Batesian mimics will often “play it safe” by enacting escape responses more frequently than their more honestly signaling counterparts. This should be explored further with experiments confirming model-mimic relationships followed by a comparison of the frequency of evasive behaviors between members of these groups.
Of course, tiger moths are not the only chemically protected insects that employ evasive flight to contend with bat predation. Ears tuned to ultrasonic frequencies can be found in at least 5 orders of insects (i.e., Coleoptera, Lepidoptera, Mantodea, Neuroptera, and Orthoptera) and have evolved independently at least 14 times (Dawson et al., 2004; Greenfield, 2016). All 5 orders of ultrasound-sensitive insects have been reported to exhibit evasive flight responses in response to bat echolocation (Roeder, 1962; Miller and Oleson, 1979; Spangler, 1988; Yager et al., 1990; Dawson et al., 2004). Each of these orders, with the exception of Mantodea, contains examples of species utilizing a chemical defense of some kind (Eisner et al., 2005). Intraspecific variation in host plant chemical defenses as well as multiple host plant affiliations in some groups could generate intraspecific differences in chemical protection, potentially explaining some of the variation in nonchalance both among and within a variety of insect species, such as the recently reported nonchalant flight in fireflies in response to foraging bats (Leavell et al., 2018).
By gathering more evasive flight and palatability data for more species, we could also better predict nonchalance from palatability data. This is useful, as measuring palatability can be much simpler than making detailed observations of evasive flight. Additionally, if palatability could be confidently inferred from preserved museum specimens using analytical chemistry methods such as mass spectroscopy, then these traits could be determined on an unprecedented scale (Bowers, 2009; Anderson et al., 2016). Gaining a better understanding of the variation in nonchalance will help us better characterize how animals balance the potential costs of escape maneuvers with the potential benefits gained from eluding predators.
The bat-moth predator-prey system is a useful model for examining the escape behaviors of prey, both aposematic and non-aposematic. The sensory system that moths use to detect bat predators and their acoustic aposematic signals can be ablated by simple surgical impairment without compromising their overall physical integrity, their remaining behavioral repertoire, nor other cues produced by the specimen. Additionally, moth unpalatability can be modulated by rearing individuals on diets that are lacking defensive compounds. With this approach, the nonchalance, unpalatability, and/or aposematic signals of individual moths can be experimentally manipulated to yield greater insights into the costs, benefits, and factors influencing escape behavior. We believe the bat-moth predator-prey system is a promising new modality for understanding how the escape behaviors of prey are enacted and evolve.
Data Availability Statement
The datasets analyzed for this study and the associated R scripts can be found in the FigShare repositories: Evasive Flight Data (10.6084/m9.figshare.9705788), Palatability Data (10.6084/m9.figshare.9706187), R Script (10.6084/m9.figshare.9706280), Figures and Tables (10.6084/m9.figshare.10318622), Tables in LaTeX format (10.6084/m9.figshare.10318604), and Supplemental Videos (10.6084/m9.figshare.10115180; 10.6084/m9.figshare.10115204; 10.6084/m9.figshare.10115207).
Author Contributions
ND conceived and designed experiments and performed and analyzed experiments. ND and WC wrote the manuscript.
Funding
This work was supported by a National Science Foundation grant to WC (IOS 0951160) and an NSF PRFB grant (DBI-1811897) to ND.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Wake Forest undergraduates Kaitlyn Roman, Deanna Margius, Meredith Shaw, and Celia Spell and Dr. Aaron J. Corcoran for their assistance in the lab and field. We also thank the staff of The Southwestern Research Station of the American Museum of Natural History. Finally, we would like to thank Yohami Fernández Delgado and two reviewers for their constructive comments and suggestions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00480/full#supplementary-material
References
Acharya, L., and Fenton, M. B. (1992). Echolocation behaviour of vespertilionid bats (Lasiurus cinereus and Lasiurus borealis) attacking airborne targets including arctiids moths. Can. J. Zool. 70, 1292–1298. doi: 10.1139/z92-180
Acharya, L., and Fenton, M. B. (1999). Bat attacks and moth defensive behavior around street lights. Can. J. Zool. 77, 27–33. doi: 10.1139/z98-202
Anderson, T. J., Wagner, D. L., Cooper, B. R., McCarty, M. E., and Zaspel, J. M. (2016). HPLC-MS analysis of Lichen-derived metabolites in the life stages of Crambidia cephalica (Grote and Robinson). J. Chem. Ecol. 43, 66–74. doi: 10.1007/s10886-016-0799-3
Auguie, B. (2017). gridExtra: Miscellaneous Functions for “Grid” Graphics. R package version 2.3. Available online at: https://CRAN.R-project.org/package=gridExtra (accessed November 18, 2019).
Barber, J. R., Chadwell, B. A., Garrett, N., Schmidt-French, B., and Conner, W. E. (2009). Naïve bats discriminate arctiid moth warning sounds but generalize their aposematic meaning. J. Exp. Biol. 212, 2141–2148. doi: 10.1242/jeb.029991
Barber, J. R., and Conner, W. E. (2007). Acoustic mimicry in a predator-prey interaction. Proc. Nat. Acad. Sci. USA. 104, 9331–9334. doi: 10.1073/pnas.0703627104
Bates, H. W. (1862). XXXII. Contributions to an Insect Fauna of the Amazon Valley. Lepidoptera: Heliconidæ. Trans. Linn. Soc. Lond. 23, 495–566. doi: 10.1111/j.1096-3642.1860.tb00146.x
Blanchette, A., Becza, N., and Saporito, R. A. (2017). Escape behavior of aposematic (Oophaga pumilio) and cryptic (Craugastor sp.) frogs in response to simulated predator approach. J. Trop. Ecol. 33, 165–169. doi: 10.1017/S0266467417000037
Bowers, M. D. (2009). “Chemical defenses in woolly bears: sequestration and efficacy against predators and parasitoids”, in Tiger Moths and Wooly Bears: Behavior, Ecology, and Evolution of the Arctiidae, ed W. E. Conner (New York: Oxford University Press), 83–101.
Brower, L. P., McEvoy, P. B., Williamson, K. L., and Flannery, M. A. (1972). Variation in cardiac glycoside content of monarch butterflies from natural populations in eastern North America. Science 177, 426–429. doi: 10.1126/science.177.4047.426
Brower, L. P., Ryerson, W. N., Coppinger, L. L., and Glazier, S. C. (1968). Ecological chemistry and the palatability spectrum. Science 161, 1349–1350. doi: 10.1126/science.161.3848.1349
Chai, P., and Srygley, R. B. (1990). Predation and the flight, morphology, and temperature of neotropical rain-forest butterflies. Am. Nat. 135, 748–765. doi: 10.1086/285072
Conner, W. E., and Corcoran, A. J. (2012). Sound strategies: the 65-million-year-old battle between bats and insects. Annu. Rev. Entomol. 57, 21–39. doi: 10.1146/annurev-ento-121510-133537
Cooper, W. E. Jr. (2018). “Escape from predators,” in Encyclopedia of Animal Behavior, 2nd Edn, ed J. Choe (San Diego, CA: Academic Press), 349–360.
Cooper, W. E. Jr., Caldwell, J., and Vitt, L. (2009a). Conspicuousness and vestigial escape behaviour by two dendrobatid frogs, Dendrobates auratus and Oophaga pumilio. Behaviour 146, 325–349. doi: 10.1163/156853909X410946
Cooper, W. E. Jr., Caldwell, J., and Vitt, L. (2009b). Risk assessment and withdrawal behavior by two species of aposematic poison frogs, Dendrobates auratus and Oophaga pumilio, on forest trails. Ethology 115, 311–320. doi: 10.1111/j.1439-0310.2009.01615.x
Cooper, W. E. Jr., and Frederick, W. G. (2007). Optimal flight initiation distance. J. Theor. Biol. 244, 59–67. doi: 10.1016/j.jtbi.2006.07.011
Corcoran, A.J., Conner, W. E., and Barber, J. R. (2010). Anti-bat tiger moth sounds: form and function. Curr. Zool. 56, 358–369. doi: 10.1093/czoolo/56.3.358
Corcoran, A. J., and Conner, W. E. (2012). Sonar-jamming in the field: effectiveness and behavior of a unique prey defense. J. Exp. Biol. 215, 4278–4287. doi: 10.1242/jeb.076943
Dawson, J. W., Kutsch, W., and Robertson, R. M. (2004). Auditory-evoked evasive manoeuvres in free-flying locusts and moths. J. Comp. Physiol. A 190, 69–84. doi: 10.1007/s00359-003-0474-3
Dowdy, N. J., and Conner, W. E. (2016). Acoustic aposematism and evasive action in select chemically defended arctiine (Lepidoptera: Erebidae) species: nonchalant or not? PLoS ONE 11:e0152981. doi: 10.1371/journal.pone.0152981
Dugas, M., Halbrook, S. R., Killius, A. M., del Sol, J. F., and Richards-Zawacki, C. L. (2015). Colour and escape behaviour in polymorphic populations of an aposematic poison frog. Ethology 121, 813–822. doi: 10.1111/eth.12396
Dunning, D. C., Acharya, L., Merriman, C. B., and Ferro, L. D. (1992). Interactions between bats and arctiid moths. Can. J. Biol. 70, 2218–2223. doi: 10.1139/z92-298
Eisner, T., Eisner, M., and Siegler, M. (2005). Secret Weapons: Defenses of Insects, Spiders, Scorpions, and Other Many-Legged Creatures. Cambridge: Belknap Press of Harvard University Press.
Evans, D. L. (1983). Relative defensive behavior of some moths and the implication to predator-prey interactions. Entomol. Exp. Appl. 33, 103–111. doi: 10.1111/j.1570-7458.1983.tb03240.x
Goldman, L. J., and Henson, O. W. (1977). Prey recognition and selection by the constant frequency bat, Pteronotus p. parnellii. Behav. Ecol. Sociobiol. 2, 411–419. doi: 10.1007/BF00299509
Greenfield, M. D. (2016). “Evolution of Acoustic Communication in Insects”, in Insect Hearing, eds G. S. Pollack, A. C. Mason, A. N. Popper, and R. R. Fay (Switzerland: Springer), 17–47.
Guignion, C., and Fullard, J. H. (2004). A potential cost of responding to bats for moths flying over water. Can. J. Zool. 82, 529–532. doi: 10.1139/z04-015
Harrell, F. E. Jr. (2019). Hmisc: Harrell Miscellaneous. R package version 4.2-0. Available online at: https://CRAN.R-project.org/package=Hmisc (accessed November 18, 2019).
Hatle, J. D., and Faragher, S. G. (1998). Slow movement increases the survivorship of a chemically defended grasshopper in predatory encounters. Oecologia 115, 260–267. doi: 10.1007/s004420050515
Hatle, J. D., Salazar, B. A., and Whitman, D. W. (2002). Survival advantage of sluggish individuals in aggregations of aposematic prey, during encounters with ambush predators. Evol. Ecol. 16, 415–431. doi: 10.1023/A:1020814110102
Hatle, J. D., and Whitman, D. W. (2001). “Sluggish movement of conspicuous insects as a defense mechanism against motion-oriented predators,” in Insects and Plant Defence Dynamics, ed T. N. Ananthakrishnan (Enfield, NH: Science Publishers), 209–228.
Hristov, N. I., and Conner, W. E. (2005). Sound strategy: acoustic aposematism in the bat-moth arms race. Naturwiss 92, 164–169. doi: 10.1007/s00114-005-0611-7
Leavell, B. C., Rubin, J. J., McClure, C. J. W., Miner, K. A., Branham, M. A., and Barber, J. R. (2018). Fireflies thwart bat attack with multisensory warnings. Sci. Adv. 4:eaat6601. doi: 10.1126/sciadv.aat6601
Leifeld, P. (2013). texreg: conversion of Statistical Model Output in R to LaTeX and HTML Tables. J. Stat. Softw. 55, 1–24. doi: 10.18637/jss.v055.i08
Miller, L. A., and Oleson, J. (1979). Avoidance behavior in green lacewings – I. Behavior of free flying green lacewings to hunting bats and ultrasound. J. Comp. Physiol. A 131, 113–120. doi: 10.1007/BF00619071
Ozel, L. D., and Stynoski, J. L. (2011). Differences in escape behavior between a cryptic and an aposematic litter frog. J. Herp. 45, 395–398. doi: 10.1670/10-249.1
Poulton, E. B. (1890). The Colours of Animals: Their Meaning and Use, Especially Considered in the Case Of Insects. (New York, NY: D. Appleton), 1–360. doi: 10.5962/bhl.title.11353
R Core Team (2016). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Roeder, K. D. (1962). The behaviour of free flying moths in the presence of artificial ultrasonic pulses. Anim. Behav. 10, 300–304. doi: 10.1016/0003-3472(62)90053-2
Samia, D. S. M., Blumstein, D. T., Stankowich, T., and Cooper, W. E Jr. (2016). Fifty years of chasing lizards: new insights advance optimal escape theory. Biol. Rev. 91, 349–366. doi: 10.1111/brv.12173
Spangler, H. G. (1988). Hearing in tiger beetles (Cicindelidae). Physiol. Entomol. 13, 447–452. doi: 10.1111/j.1365-3032.1988.tb01129.x
Speed, M. P., Brockhurst, M. A., and Ruxton, G. D. (2010). The dual benefits of aposematism: predator avoidance and enhanced resource collection. Evolution 64, 1622–1633. doi: 10.1111/j.1558-5646.2009.00931.x
Srygley, R. B. (1994). Locomotor mimicry in butterflies? The associations of positions of centres of mass among groups of mimetic, unprofitable prey. Philos. Trans. R. Soc. Lond. B 343, 145–155. doi: 10.1098/rstb.1994.0017
Srygley, R. B. (2004). The aerodynamic costs of warning signals in palatable mimetic butterflies and their distasteful models. Proc. R. Soc. Lond. B 271, 589–594. doi: 10.1098/rspb.2003.2627
Stankowich, T., and Blumstein, D. T. (2005). Fear in animals: a meta-analysis and review of risk assessment. Proc. R. Soc. B 272, 2627–2634. doi: 10.1098/rspb.2005.3251
Triblehorn, J. D., Ghose, K., Bohn, K., Moss, C. F., and Yager, D. D. (2008). Free-flight encounters between praying mantids (Parasphendale agrionina) and bats (Eptesicus fuscus). J. Exp. Biol. 211, 555–562. doi: 10.1242/jeb.005736
Turner, J. R. G. (1984). “Mimicry: the palatability spectrum and its consequences”, in The Biology of Butterflies, eds R. I. Vane-Wright and P. R. Ackery (London: Academic Press), 141–161.
Wallace, A. R. (1889). Darwinism – An Exposition of the Theory of Natural Selection With Some of Its Applications. London: MacMillan and Co.
Whitman, D. W., Blum, M. S., and Jones, C. G. (1985). Chemical defense in Taeniopoda eques (Orthoptera: Acrididae): role of the metathoracic secretion. Ann. Entomol. Soc. Am. 78, 451–455. doi: 10.1093/aesa/78.4.451
Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis, 2nd Edn. New York, NY: Springer-Verlag.
Wickham, H. (2018). scales: Scale Functions for Visualization. R package version 1.0.0. Available online at: https://CRAN.R-project.org/package=scales (accessed November 18, 2019).
Wickham, H., François, R., Henry, L., and Müller, K. (2019). dplyr: A Grammar of Data Manipulation. R package version 0.8.3. Available online at: https://CRAN.R-project.org/package=dplyr (accessed November 18, 2019).
Yager, D. D. (2011). Predator detection and evasion by flying insects. Curr. Opin. Neurobiol. 22, 1–7. doi: 10.1016/j.conb.2011.12.011
Yager, D. D., May, M. L., and Fenton, M. B. (1990). Ultrasound-triggered, flight-gated evasive maneuvers in the praying mantis Parasphendale agrionina I. free flight. J. Exp. Biol. 152, 17–39.
Keywords: nonchalance, escape behavior, anti-predator defense, palatability, Lepidoptera
Citation: Dowdy NJ and Conner WE (2019) Nonchalant Flight in Tiger Moths (Erebidae: Arctiinae) Is Correlated With Unpalatability. Front. Ecol. Evol. 7:480. doi: 10.3389/fevo.2019.00480
Received: 22 August 2019; Accepted: 25 November 2019;
Published: 16 December 2019.
Edited by:
Piotr Jablonski, Seoul National University, South KoreaReviewed by:
Changku Kang, Carleton University, CanadaRyo Nakano, National Agriculture and Food Research Organization (NARO), Japan
Copyright © 2019 Dowdy and Conner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicolas J. Dowdy, bmpkb3dkeSYjeDAwMDQwO2dtYWlsLmNvbQ==
 Nicolas J. Dowdy
Nicolas J. Dowdy William E. Conner1
William E. Conner1