- Department of Biological Sciences, University of Alberta, Edmonton, AB, Canada
Loss of migratory behavior in ungulates often occurs with habituation to people to cause several challenges for wildlife managers, particularly in protected and urban areas. Aversive conditioning to increase ungulate wariness toward people could be an important tool for managing wildlife conflicts, but it is frequently thwarted by variation in responsiveness among individuals, an aspect of personality that is currently little understood by managers. In our paper, we describe the potential role of personality in the ecological progression associated with habituation, loss of migration, and human-wildlife conflict in ungulates. We do so by (a) synthesizing our prior work on two populations of wild elk (Cervus canadensis) living in national parks in the Canadian Rocky Mountains, (b) using it to articulate a conceptual model to explain how anthropogenic changes in landscapes favor bolder individuals, and (c) showing how targeted use of aversive conditioning could limit the advantages to bold individuals that promote residency. Our review showed how bolder elk, defined by a combination of seven separate personality metrics on a bold-shy continuum, are three times more likely to forego migration, but are also quicker to learn by association, whether via the provision or cessation of aversive conditioning. Differences in personality may relate to cognitive flexibility, which we measured with limb use preferences, to imbue bolder elk with more rapid responses to changing environments. In our conceptual model, we show how four ecological drivers comprised by interactions with humans, predators and conspecifics, in addition to changes in forage, favor bolder elk that are more likely to adopt a resident migratory tactic. We also explain how bold personalities could result from behavioral flexibility, genetic differences, or gene-environment interactions, each of which could be moderated by frequency-dependent payoffs to individuals. We suggest that managers could limit the prevalence of bold, resident ungulates by targeting bolder individuals with active and specific aversive conditioning, while minimizing anthropogenic food sources in predator refugia. A better understanding of personality in wildlife could support more proactive strategies to limit habituation and encourage migration and other keystone behaviors in changing landscapes.
Diverse species of ungulates exhibit seasonal migration with potential benefits of increased foraging opportunities (Albon and Langvatn, 1992), reduced predation (Hebblewhite and Merrill, 2009) and avoidance of parasites (Altizer et al., 2011). Recently, ungulate populations around the world demonstrate reduced tendencies to migrate with profound effects on associated ecosystems (Bolger et al., 2008; Tucker et al., 2018). Such declines have been documented for wildebeest (Conochaetes spp.) in Africa (Morrison and Bolger, 2012), Mongolian gazelles (Procapra gutturosa) in Asia (Ito et al., 2005), moose (Alces alces) in Europe (Singh et al., 2012), and elk (Cervus canadensis) in North America (Hebblewhite et al., 2006). As ungulates become more sedentary, they often become hyperabundant, over-consume vegetation, disrupt historic predator-prey relationships, and otherwise alter ecosystem functions (White and Ward, 2010). Problems with overabundance are often greatest in protected areas, where humans present little risk, but ample reward in the form of anthropogenic food (Thompson and Henderson, 1998) and protection from predators (Berger, 2004). The combination of sedentary behavior and the absence of predators has been recognized as a threat to the health and sustainability of ungulate populations for almost 100 years (Murie, 1934), but it increasingly characterizes populations of wild ungulates around the world (Bolger et al., 2008; Polfus and Krausman, 2012; Tucker et al., 2018).
In the sequence of ecosystem change that occurs when ungulate prey are decoupled from their predators, habituation by ungulates to people appears to play a pivotal role (Thompson and Henderson, 1998; Whittaker and Knight, 1998). The process of habituation is described simply as a learned behavioral response or waning physiological response to stimuli that lack fitness consequences (Blumstein, 2016). Habituation was extensively studied by learning theorists over the past century and is a well-known contributor to human-wildlife conflict in diverse settings that range from crop damage by birds to urbanizing carnivores and ungulates (Conover, 2001). Habituation by ungulates frequently results in conflict, particularly in protected areas where it can compromise human safety (Thompson and Henderson, 1998; Kloppers et al., 2005). In those areas, ungulates can rapidly lose their historical wariness to people, allowing them to reduce energetically costly responses, such as escape behavior (Gates and Hudson, 1978) and vigilance (Shannon et al., 2014). Increasing habituation by deer species is occurring around the world in urban or urbanizing areas that exclude human hunting and predators (Honda et al., 2018).
The capacity to distinguish between aversive and benign forms of similar stimuli appears to be a critical component of the habituation process (Bejder et al., 2009) with fitness consequences suggested by its occurrence in examples as diverse as carnivores (Ohta et al., 2012), birds (Mackay et al., 2014) and insects (Davis and Heslop, 2004). Although habituated behavior occurs in diverse taxa, prey seem to be more prone to habituation than predators, which generally show a greater innate avoidance of people (Thompson and Henderson, 1998; Berger, 2004), perhaps because habituation helps prey species escape predation. In many ungulate species, proclivity to habituate often results in a positive feedback loop whereby predator refugia formed in human-disturbed areas select for individuals that habituate more readily, increasing, in turn, their capacity to exploit the benefits of the refugium (Polfus and Krausman, 2012). Even in songbirds, there appears to be a positive feedback between the tendency to habituate to people and the tendency to exploit urban areas (Atwell et al., 2012).
Exploitation of urban areas by wildlife is often addressed with the management tools of hazing, which is short-term deterrence, and aversive conditioning, which relies upon associative learning (Hopkins et al., 2014). Aversive conditioning is a systematic method of modifying an individual's behavior by imposing evolutionarily-relevant negative consequences on individuals exhibiting undesirable behaviors (Domjan, 2014), such as a willingness to tolerate close approaches by people or use of human-dominated spaces. Aversive conditioning may consist of chasing animals, firing projectiles, or emitting loud noises and has been used to restore wariness in black bears (Ursus americanus; Mazur, 2010), elk (Kloppers et al., 2005), wolves (Canis lupus; Hawley et al., 2009), and coyotes (Canis latrans; Bonnell and Breck, 2017). Human hunting may not lead to avoidance behavior, especially when it is highly targeted and immediately lethal, if it limits negative consequences experienced by other animals. However, the efficacy of aversive conditioning can also be limited by substantial variation among individuals in responsiveness to the aversive stimuli and duration of learned responses. This variation in responses along with subtle differences in context can determine whether individuals sensitize or habituate to stimuli that are intended by managers to be aversive (Blumstein, 2016).
Similar inter-individual variation in behavioral tendencies across contexts has been described for hundreds of species and is defined as personality (Gosling, 2001), behavioral syndromes (Sih et al., 2004), or temperament (Réale et al., 2007), all of which relate to the degree of plasticity in behavior that stems from a combination of genes, development, environment, and experience (Stamps, 2016). Individual variation in the tendency to habituate to people has high relevance to the management of migratory ungulates, but also to wildlife management more generally. For example, habituated individuals are often associated with hyper-abundance, ecological damage, and human-wildlife conflict (Polfus and Krausman, 2012), but they may also be more likely to spread zoonotic diseases (Murray et al., 2015) or lead conspecifics into adopting similar behavior (Modlmeier et al., 2014).
Relationships between the tendency to habituate and more general responsiveness to environmental stimuli suggest an underlying difference in behavioral flexibility. Such variation might stem partly from the degree of cerebral lateralization, defined as specialization of neural tasks to different brain hemispheres to speed neural processing and reaction times (Rogers, 2000; Vallortigara, 2006). Lateralization is familiar to humans as handedness; the strength of hand preference correlates with cognitive speed and efficiency in domains ranging from athletics to academics (Bisazza et al., 1998). In many animals, greater lateralization translates into greater speed in detecting and responding to predators (Brown et al., 2007). However, the greater speed of cognitive processing for familiar behavioral routines comes at a price, because strongly lateralized animals appear to have less flexibility in adjusting behavior to changing environments (Porac and Searleman, 2006; Carlier et al., 2011). Associations with response times potentially make the measurement of laterality a powerful complement to studies of personality, particularly in animals that express routine behaviors with which it can be easily quantified.
Behavioral variation consistent with definitions of personality have been described in both wild ungulates (Réale et al., 2000) and domesticated species (Wesley et al., 2012), but there has been no generalized effort to explore how personality traits affect ungulate migration and management. That application is overdue considering that livestock owners have recognized and used this variation for centuries to select for more docile animals. In the first section below, we synthesize our past work showing that behavioral syndromes can be quantified in wild, habituated elk (Found and St. Clair, 2016) that reside in the protected areas and mountain townsites of Banff and Jasper, Canada. Next, we develop a conceptual model to show how environmental changes wrought by four ecological drivers increase the benefits of bolder behavior, which favors the individuals that are more likely to use the resident migratory tactic. We explain the mechanisms by which directional selection for bolder individuals could occur and how it is limited by frequency dependence, which may also limit the correlation between personality and migratory tactic. We conclude by showing how greater acknowledgment of behavioral variation and explicit targeting of bold behaviors could increase the efficacy of aversive conditioning to manage both habituation and migration in wild ungulates.
Review of Personality and Migration in Wild Elk
Between 2010 and 2013, we studied the impacts of individual behavioral variation on habituation and migratory choices in wild, adult, female elk near the townsites of Banff 51°10′N 115°34′W pop. est. 7,850) and Jasper (52°52′N, 118°04′W, pop. est. 4,500) AB, Canada, each contained in a national park of the same name. Both townsite areas exhibit high levels of human disturbance that reduce predatory activity in their vicinity (Paquet et al., 1996; Goldberg et al., 2014), while providing anthropogenic and natural foraging opportunities (McKenzie, 2001). Elk in Jasper are partially separated into three herds with only one making extensive use of the townsite each winter (Found, 2015). The elk in Banff annually form a single large over-wintering herd comprised of both migrant and resident elk that is centered on the townsite. It forms a predator refugium that is readily apparent when comparing the locations of radio-collared elk with the snow-tracked paths of wolves (Canis lupus) and their known kill sites of elk (Found, 2015). Wolves are the main predator of adult elk in Jasper in winter (Dekker et al., 1995) although cougar (Puma concolor) also hunt near the townsite of Banff (Kortello et al., 2007) and grizzly bears (Ursus arctos) are an additional important predator of elk fawns in late spring (Hamer and Herrero, 1991).
Populations of wild elk in both Banff and Jasper exhibited behavioral syndromes that we quantified with a composite metric of personality based on seven different traits that were inter-correlated and consistently expressed within individuals and among years (Found and St. Clair, 2016). We used multivariate statistical techniques to delineate these behavioral types along intra-population gradients we defined as “shy” to “bold.” We use these terms as labels to connote broad suites of traits (Wilson et al., 1994), but acknowledge there is high variation in their use and interpretation by others (Carter et al., 2013). In our system, bolder elk were characterized by lower flight response distances, reduced responsiveness to sounds, occupancy of more peripheral positions within groups, greater exploration of novel objects, increased vigilance, social dominance over shyer conspecifics, and a greater frequency of leading other elk to new habitats (Found and St. Clair, 2016). We used several of these metrics with captive elk of known birthdates to show that personality was not influenced by age (Found and St. Clair, 2016).
Using these metrics to define behavioral types made it possible to determine that elk with bolder personalities were also more likely to adopt non-migratory, resident strategies. Specifically, resident elk were more exploratory, had lower flight response distances, and higher mean dominance rankings (Found and St. Clair, 2016). In both years and both study populations, individual personality scores from a multi-variate gradient measured in winter were a significant predictor of migratory status in the following summer. In fact, after dividing the elk population at median values for our composite metric into bold and shy halves, bold residents outnumbered shy residents with a 3:1 ratio, whereas bold migrants were outnumbered by shy migrants with a ratio of 1:3. The difference in ratios remained stable throughout the 3 years of our study (Found and St. Clair, 2016) and similar between parks, despite the occurrence of sub-populations in Jasper.
There is no comparable literature with which to compare ungulate personality to migratory patterns, but personality appears to contribute to the choice of migratory tactics in moose (Alces alces; Rolandsen et al., 2017). Personality is likely involved in the variable expression of migration, also known as partial migration, that occurs in all migratory taxa, but the vast movements involved with migration make personality very difficult to study in this context (Nilsson et al., 2014). Moreover, the few studies that directly link personality and migration are difficult to generalize. For example, in a freshwater fish (roach, Rutilus rutilus), boldness was positively correlated with migratory movement away from predators (Chapman et al., 2011b). But in blue tits (Cyanistes caeruleus), migrants were usually sub-dominant birds that expressed boldness via neophilia, partly to overcome exclusion from better habitat by dominant birds (Chapman et al., 2011b).
In our study populations of elk, the apparent importance of predation risk to the occurrence of behavioral types made it surprising that we found a similar gradient of shy through bold individuals in a captive elk population, where predators were effectively absent and forage was uniformly available (Found and St. Clair, 2016). Evidence of personality is similarly apparent for several domestic species (Finkemeier et al., 2018), many of which are also protected from natural predators. More generally, behavioral types appear to result from complex gene-environment interactions that involve multiple, pleiotropic genes (Bouchard and Loehlin, 2001; Krueger et al., 2008) that persist in all populations owing to variable or changing environments (Dingemanse et al., 2007) and both frequency and density-dependent effects (Aplin et al., 2014; Nicolaus et al., 2016). Later, we explore how those factors might contribute to the maintenance of bold and shy elk and their relevance to migration and habituation.
We interpret our results from elk to suggest that the relative advantages of migration for an individual depend on its inherent personality, which interacts with development, learning, and environmental context. The environment includes interactions with people, predators, and conspecifics, as well as forage type and availability. Despite so many sources of variation, average personality-based pay-offs are likely to emerge. In our study system, a bold, neophilic individual, with high tolerance to sound disturbance, a tendency to occur on the periphery of the herd, and low vigilance is presumably more likely to discover novel food sources in a human-dominated area, more likely to be able to exploit them quickly, and more likely to dominate conspecifics. Contrastingly, a shy individual with higher vigilance that seeks natural forage that is abundant, but widely distributed, may have lower likelihood of predation owing to neophobia and greater vigilance. Those shy individuals may also be better able to escape competition via seasonal migration or through reduced population density (Hebblewhite et al., 2002).
Similar interactions between individuals and their environments correspond to predictable advantages for well-known behavioral types in many other species, such as producers and scroungers (Giraldeau and Beauchamp, 1999), fast vs. slow explorers (Dingemanse et al., 2003), or proactive vs. reactive coping styles (Coppens et al., 2010). Each of these dichotomies may also extend to pace-of-life strategies that correlate with physiological and life history traits (Réale et al., 2010; Careau and Garland, 2012). All of these contrasting types potentially impose evolutionary and strategic constraints on individuals via fitness costs that occur as interacting effects of behavioral types and environmental context (Smith and Blumstein, 2008). Based on our observations of behavioral types in elk, we speculated that several anthropogenic changes to our study landscapes increase selection for bolder personalities, largely through habituation to people. In turn, those tendencies increase the fitness benefits of a resident tactic, but those benefits might be curtailed with management actions, especially aversive conditioning.
Personality-Dependent Responses to Aversive Conditioning
A core purpose of our work to identify behavioral syndromes in habituated, town-dwelling elk was to determine whether that information could be used to increase the efficacy of aversive conditioning as a management technique. Aversive conditioning has been used primarily to increase human safety, but previously targeted town-dwelling animals that are disproportionately likely to be involved in human-wildlife conflict (Kloppers et al., 2005). We now know these animals have consistently bolder personality types and are less likely to migrate (Found and St. Clair, 2016). However, aversive condition has also been used in an effort to increase migratory tendency (Spaedtke, 2009). Accordingly, we compared elk of different behavioral types in Jasper before, during and after being exposed to aversive conditioning consisting of active chases by people and benign stimuli consisting of slow, non-targeted walking (Found and St. Clair, 2017). Our aversive conditioning consisted of high-speed foot pursuits of targeted elk with 10-min durations to create an energetic consequence of being pursued (Found, 2015) that might mimic pursuit by coursing predators like wolves (Kloppers et al., 2005). Human hunting can also change prey behavior, but the lacking impact of pursuit may influence space use more than wariness (Bateson and Bradshaw, 1997).
Somewhat counter intuitively, we discovered that bolder elk in Jasper responded more strongly to aversive stimuli with increases in their average flight response distances that were up to five times greater than those expressed by the shyest elk Found and St. Clair, 2018). However, bolder animals also returned to their (originally lower) baseline measures of flight response distances when the aversive stimuli ceased. In combination, bolder elk appeared to be more responsive to approaches by humans whether they were negative or neutral. One year after aversive conditioning treatment, migrants had retained about half of their conditioned increases in wariness, whereas residents had lost all conditioned gains (Found and St. Clair, 2018). The more rapid loss of conditioned responses suggest that aversive conditioning programs need to be targeted and consistent to achieve their desired outcomes, a topic we return to below.
We used the metric of flight response distance to explore individual variation in responsiveness to the frequency of aversive conditioning events for an older dataset collected from elk in Banff (Found et al., 2018). There, elk subjected to more frequent aversive conditioning exhibited greater increases in their flight response distances, but those elk also exhibited more rapid returns to baseline flight response distances when conditioning ceased. As for the Jasper population, the Banff elk with the lowest flight response distances at the beginning of the study (i.e., those exhibiting greater habituation) exhibited the greatest changes in flight response distances during both the conditioning and extinction periods. Additional work with a captive population demonstrated that elk can habituate rapidly with either of food-based conditioning or benign approaches by people, which was also more rapid for the bolder individuals (Found, 2019).
Other study systems have revealed similar evidence that more habituated individuals exhibit greater responsiveness to human activity (Bejder et al., 2009). For example, house sparrows (Passer domesticus) demonstrated considerable individual variation in neophobia, measured as a latency to explore a novel object, while exhibiting consistent tendencies within individuals to habituate to human disturbance (Ensminger and Westneat, 2012). Similarly, when yellow baboons (Papio cynocephalus) were introduced to an area with accessible human food, only some individuals were bold enough to became crop raiders (Strum, 2010). Juncos (Junco hyemalis) with greater behavioral flexibility appear to be pre-adapted to thrive in urban areas (Atwell et al., 2012). We know of only one other study that attempted to relate existing habituation behavior to responses to aversive conditioning. In black bears (Ursus americanus), more habituated animals were more responsive to aversive conditioning, although it was the less habituated animals that exhibited greater recidivism (Mazur, 2010).
To better understand the sources of behavioral variation among individuals, we studied lateralization in elk by quantifying the proportion of times each marked individual used its left vs. right forelimb to dig in the snow to expose edible vegetation (Found and St. Clair, 2017). In both the Jasper and Banff study populations resident elk were more ambidextrous (less lateralized) than migrants in their use of forelimbs, which we interpreted as equating to increased cognitive flexibility (Found and St. Clair, 2017). Further evidence that laterality reflects cognitive flexibility stems from the congruence in the Jasper population between the conditioning experiments (above) and laterality. It was the bolder elk, comprised mostly by the ambidextrous residents, that expressed the most rapid increases in wariness during aversive conditioning and the most rapid losses in that response when it was removed. Together, these results suggest that behavioral flexibility manifested in weakly lateralized animals contributed to their ability to rapidly identify benign interactions with humans and habituate accordingly.
Taken together, our studies of elk in the mountain parks of Banff and Jasper, plus a nearby captive population, firmly establish the presence of a definable personality gradient in each population that correlates with migratory tendency (Found and St. Clair, 2016), responsiveness to aversive conditioning (Found and St. Clair, 2018; Found et al., 2018), and the process of habituation (Found, 2019). A further source of this variation appears to relate to cerebral lateralization (Found and St. Clair, 2017), which may be especially relevant to predation risk (Found, 2019). Relationships among different components of behavior have undeniable management implications (that we explore below), but they do not reveal the causative agents that maintain this behavioral variation in populations. Many others suggest that personality persists as a frequency-dependent function of changing environments (Dingemanse et al., 2004; Smith and Blumstein, 2008; Réale et al., 2010). A dependency on environmental variation makes personality an especially likely contributor to the dynamics of ungulate migration, which are also known to respond to environmental change and anthropogenic habitat (Bolger et al., 2008; Tucker et al., 2018).
The conceptual model we develop in the next section stems partly from what we know about changes in our study landscapes over the past century related to elk, human use, and predator distribution. In Jasper, elk were absent from the park when it was founded in 1907, introduced from Yellowstone National Park (US) in 1920, had become hyper-abundant by the 1940's, were extensively culled by wardens until 1970, and stabilized at about 1,000 animals in the 1990's (Dekker et al., 1995). Meanwhile, wolves that were abundant in the 1800's were also effectively absent when Jasper was established, but gradually increased until the 1940's when wolf control began, rebounded when it ceased (1966), and exhibited stable populations in the 1990's (Dekker et al., 1995). During their era of high population abundance, elk migrated extensively throughout the park, and the tactic for year-round residency at low elevations first appeared only in about 1980, on the heels of increasing elk mortality from wolves (Dekker et al., 1995; Beschta and Ripple, 2007). Banff's history is similar, but occurred a little later, with rebounding wolf populations in the 1980's and 90's gradually increasing the tendency for elk to congregate near town sites (Hebblewhite et al., 2002), which may have intensified with the cessation of lethal control of grizzly bears by about 2000 (St. Clair et al., 2019).
A history of predator control in our study areas amply demonstrate the capacity elk have to adjust their migratory tactics to changing environmental circumstances. Indeed, migration does not appear to be a genetically-fixed strategy in any ungulate species (reviewed by Berg et al., 2019) although the degree of plasticity may vary even within species (Cagnacci et al., 2011). In our Banff study population, between 2010 and 2011, 16% (8 of 50 marked animals) switched tactics and a similar rate occurred annually in a population adjacent to Banff National Park, most often by migrants switching to residency (Eggeman et al., 2016). Rates of switching migratory tactics appear to be 10–20% in many other ungulate populations (Berg et al., 2019). A capacity to switch tactics begs a question: how do individuals determine which migratory tactic optimizes fitness for their own personality and environment? We develop a model below to show how (a) how environments undergoing anthropogenic change might increase selection for bolder behavior via (b) four interacting ecological drivers, to (c) increase the benefits and, consequently prevalence, of a resident tactic. Later we describe how aversive conditioning might be used to increase selection for migration by increasing the costs of residency. We propose that selection for boldness results secondarily in the resident migratory tactic, but with frequency-dependent limits.
A Conceptual Model Relating Behavioral Types, Ecological Drivers, and Migratory Tactics in Elk
Most biologists are familiar with the competitive and frequency-dependent dynamic between hypothetical hawks and doves in the classic game theory (Maynard Smith and Price, 1973). As behavioral types, aggressive hawks and docile doves persist in a population as a mixed evolutionarily stable strategy via specific proportions that equalize the costs and benefits of the two strategies. Stable equilibria in mixed strategies of this sort require that individuals play the tactic suited to their morphological type (Gross, 1984), but strategies can be flexible within individuals according to ecological context (Maynard Smith and Price, 1973). Our system lacks information on the fitness pay-offs that would support development of a formal ESS, but similar concepts apply to the conceptual model we develop below and present schematically (Figure 1). In it, we show how behavioral types on a bold-shy continuum respond differently, on average, to several ecological drivers to change the relative fitness of migrant vs. resident strategies. We follow Chapman et al. (2011a) by identifying ecological drivers in the environment that promote different migratory tactics, but differ in the specific drivers we name.
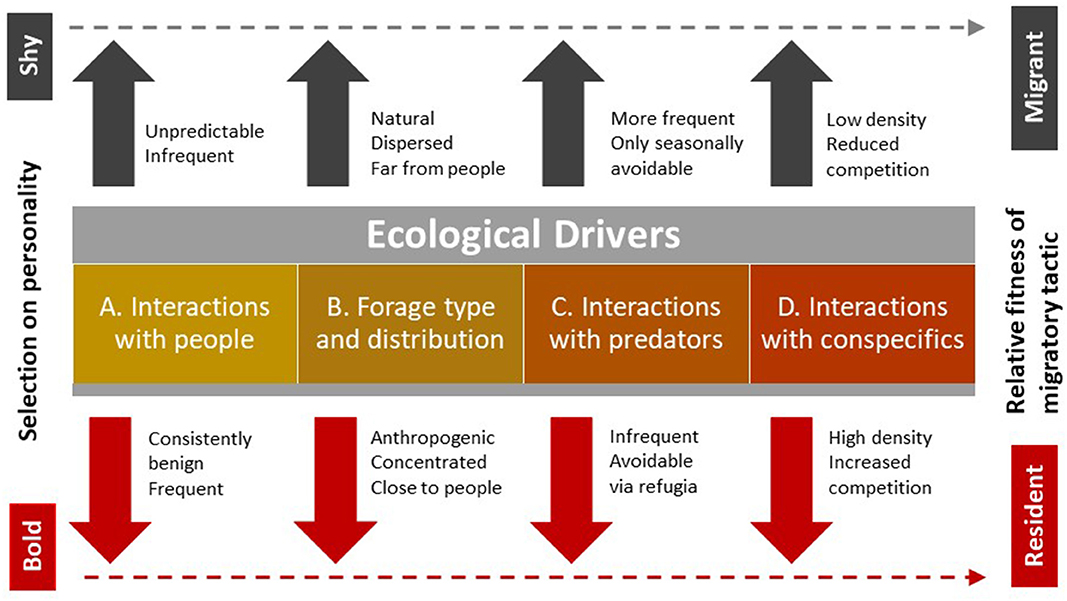
Figure 1. Selection for animal personality, which we have previously described in elk on a shy-bold gradient, stems from four types of ecological drivers to influence the relative fitness of migrant vs. resident strategies. We suggest that bolder animals typically accrue more benefits than shy animals when (A) human activity is prevalent but generates consistently benign encounters, (B) novel types of anthropogenic forage are concentrated close to people, (C) predators are excluded from areas of high human density to create refugia, and (D) there is increasing competition among individuals, typically because of increased density. All of these conditions tend to favor a residency tactic (dashed line), but directional selection for bolder personalities is also mediated by the frequency of bold and shy individuals to explain the presence of a few bold migrants and shy residents. Managers could use these drivers to reduce conflict, that results disproportionately from bolder individuals, by targeting them with aversive conditioning to limit their habituation to humans, and removing or securing sources of anthropogenic forage, particularly where encounters with humans are frequent and predators are excluded. The other drivers are less amenable to manipulation because human safety limits tolerance for predators close to high densities of people and public opinion opposes culling to reduce elk populations.
The first of our ecological drivers is human activity, which begins the cascade of landscape changes that favor bolder individuals. In protected areas, including town sites, where hunting is not permitted, ungulates have little need to fear or avoid people, as they do outside of protected areas where humans sometimes act as predators. As human activity and density increased in our study areas, the frequency of close, but benign encounters with humans necessarily also increased. In this and similar urban contexts, habituation by ungulates to people occurs rapidly (Honda et al., 2018). Captive elk with bolder personalities showed a greater tendency to habituate that began when they were still calves (Found, 2019).
Habituation to people increased opportunity for selection on personality by the second of our ecological drivers; forage availability. In the mountain parks of North America, elk historically foraged on natural vegetation that was widely distributed and migrated to higher elevations each spring to follow greening vegetation (Boyce, 1991). Others have suggested that finding such forage and avoiding competition for it have been core contributors to migration in ungulates (Fryxell and Sinclair, 1988; Chapman et al., 2011a). As anthropogenic development proceeded, novel forage sources in our study area included mowed parks, school yards, playing fields, golf courses, palatable ornamental vegetation, and spilled grain from train cars. Bolder elk are more likely to explore these novel food sources and more likely to tolerate people or infrastructure near them.
The third driver is predator distribution and activity. Historically, predators were well-dispersed in the landscape and difficult to avoid completely. Migration by small groups of ungulates lessens aggregation to reduce both detectability and attractiveness for predators (Hebblewhite et al., 2002). Predator refugia occur in areas where wildlife have high rates of benign encounters with people because carnivores are typically more wary around people than their ungulate prey (Muhly et al., 2011), and because they are actively excluded by managers to support human safety (Lennox et al., 2018). Such refugia would be expected to favor bolder individuals that can tolerate closer proximity to people and their infrastructure, such as townsites both inside and outside the boundaries of protected areas and, more generally, in landscapes where predators are consistently persecuted. Outside of protected areas, human hunters exhibit similar selection on ungulates (Bateson and Bradshaw, 1997), but the effect of personality may differ. There, bolder animals may be more likely to be shot by human hunters (Ciuti et al., 2012), even if they are also more likely to learn how to avoid hunters over time (Thurfjell et al., 2017).
The final driver is interactions with conspecifics, which would favor bolder animals because they can outcompete shyer animals that are more submissive. Intraspecific competition necessarily also interacts with each of the other drivers to provide a competitive advantage to bolder elk that are also able to share close proximity with people, physically dominate shyer elk at concentrated novel food sources, and better compete to exploit the best opportunities to avoid predators while accessing food.
Many other authors have shown how these four ecological drivers are among the factors associated with the loss of migration in ungulates (Berger, 2004, 2007; Bolger et al., 2008; Tucker et al., 2018), but our model differs by positing that these changes may result secondarily from selection on personality to favor bolder individuals. Two important parts of our explanation are that (a) directional selection for boldness is limited by frequency-dependent pay-offs and (b) bolder individuals are described by phenotypes, with little knowledge of the genetic basis of this behavioral type. The limitations imposed by frequency dependence are familiar enough from hypothetical example of hawks and doves, wherein the advantages of bold behavior are greater amid shy individuals. But another purpose of the hawk-dove game was to demonstrate the multiple mechanisms that could produce alternative behavioral phenotypes. Individuals might exhibit bolder behavior (hawks) as a fixed genetic strategy, as a context-dependent tactic with ongoing flexibility, or as a mix of the two via gene-environment interactions during development. By any of these mechanisms, higher proportions of bold-acting individuals within a group necessarily increase per capita competition for food, costs of agonistic behavior, poorer detection (via vigilance) but higher attraction (via aggregation) of predators, and higher susceptibility to parasites and disease. Despite these frequency-dependent limits, it is easy to imagine that anthropogenic changes to environments increase the stable proportion of bold individuals in a population.
The consequences of such directional selection on boldness could explain why a majority of bold individuals are residents and a majority of shy ones are migrants, while frequency-dependent limits to that selection could explain why some animals exhibit the opposing relationship as bold migrants or shy residents. As a minority, shy residents might exploit the food-finding ability of the bolder animals, while increasing herd-level security near humans. Shy elk might also benefit from associating with large groups that attract cleaner birds into mutualistic interactions to remove ectoparasites that favor shyer elk almost exclusively (Found, 2017a). Conversely, bold migrants would be at a competitive advantage in groups of shyer individuals when they encounter concentrated, but limited, sources of natural foods. For example, in winter it is quite common for shy and submissive animals to dig through the snow to access underlying forage, only to then have bolder animals physically displace them and eat the forage without the effort of finding or exposing it (R. Found, personal observation). The mapping of personality to migratory tactic might be further blurred by the ongoing interactions between types. For example, habituation by shyer elk might be accelerated via imitation in the presence of bolder elk (Found, 2019). Despite the frequency-dependent limits to directional selection and diversity of interactions among conspecifics, environmental change has often favored bolder animals to increase the proportion of residents and create several challenges for wildlife managers.
Management Implications of Personality in Ungulates
Our conceptual model (Figure 1) reveals how the best way to limit the positive feedback between resident migratory strategies and boldness behavior that is otherwise favored by anthropogenic landscape change is to exert contrary effects on the drivers themselves. Of the four drivers we present, eliminating predation refugia through predator redistribution is presumably off the table; managers can educate the public to avoid worsening human-carnivore conflict in a variety of ways (Baruch-Mordo et al., 2011), but they cannot invite predators into town sites and other areas with concentrated use by people. Similarly, anthropogenic forage is already recognized as a contributor to human-wildlife conflict (Newsome et al., 2015) and it is increasingly managed to limit associated ecological problems (Nyhus, 2016). Its availability to elk is limited in Banff and Jasper via ungulate fencing (Shepherd and Whittington, 2006), exclosures around palatable vegetation, and efforts to reduce grain spills on railways near townsites (St. Clair et al., 2019), although more could be done. Predator-resembling aversive conditioning is a tool that can manipulate the first driver by making encounters with people less benign, but also influences the third driver by decreasing the benefits afforded to animals using refugia. However, refinement is needed in the way it is practiced and its goals need to be articulated clearly.
To be effective at restoring wariness to increase human safety, aversive conditioning would need to increase the costs of proximity to people via association with aversive stimuli that are immediate, initially intense, consistently applied, evolutionarily relevant, and unpredictable in space or time (after Conover, 2001; Domjan, 2014). To limit adoption of the resident tactic that results from boldness, aversive conditioning needs to be even more specific. It was possible to shift the short-term distribution of elk by targeting individual animals repeatedly with evolutionarily-relevant chases (Kloppers et al., 2005) and we expanded that approach by identifying bold animals (Found and St. Clair, 2016), using isolation to increase the costs of being targeted (Found and St. Clair, 2018), and determining the frequency of conditioning that minimizes extinction of learned wariness (Found et al., 2018). Others have shown that learned wariness by habituated animals will gradually disappear if the aversive stimulus is removed (Lattal and Lattal, 2012) and that frequent, low-intensity conditioning can generate habituation (Powell et al., 2016). Synthesis of laboratory studies predict that the products of aversive conditioning can be maintained with lesser effort than is required to initiate a conditioned response (Domjan, 2014), but more work will be needed to know what types and frequency of aversive conditioning are needed to achieve long term management objectives for public safety, migratory behavior, and ecological goals.
Ideally and more generally, aversive conditioning opposes directional selection on bold personality types, reducing the benefits of a resident tactic in ungulates by restoring a “landscape of fear,” which is especially likely if perceived risks apply to calves (Laundre et al., 2001). By increasing the need for vigilance and other energetically costly anti-predator behaviors, aversive conditioning could reduce the benefits of a predator refugia provided by people in urban areas. Effective aversive conditioning could have a similar effect to the presence of wolves which, even days previously, can cause profound increases in the wariness of elk (Creel et al., 2005; Found and St. Clair, 2016). By contrast, culling animals via shooting removes the target animal, but does not seem to alter the distribution or behavior of any of the surviving animals, even if they witnessed the death of a conspecific (R. Found, personal observation). Aversive conditioning is also widely viewed as more ethical than either of lethal management (Koval and Mertig, 2004) or translocation (Whitwell et al., 2012).
Despite the uncertain effect and frequent public opposition to lethal management of urban ungulates (Dandy et al., 2012), it has been promoted as a necessary consequence of selection for bold, habituated deer that inhabit urban areas around the world (Honda et al., 2018). These authors acknowledged that inadvertent selection by humans for bolder behavior by ungulates can occur rapidly via behavioral flexibility to cause human-wildlife conflict and other management problems. Many managers see the situation similarly, partly because human injury by wildlife is both grave and potentially litigious. In Banff, managers culled 10–20 resident individuals annually for most of the last two decades and a similar practice was common in Jasper in the 1960's and 70's. In effect, they were attempting to oppose the directional selection on bold behavior that supports the switch by migrants, typically shyer, to a resident tactic. Beyond the problem of public opposition, our conceptual model suggests that culling cannot solve the problem of selection for bolder personalities because boldness itself is relative phenotype in this (and perhaps any) population that results from a combination of behavioral flexibility, genetic differences in temperament, and gene-environment interactions, such as developmental plasticity. Culled individuals are easily and rapidly replaced by the next boldest individuals in the population. These predictions are supported by the fact that the ratio of residents:migrants in Banff, along with mean metrics of boldness, did not change during our study despite ongoing culling of resident elk, which were almost exclusively the bolder individuals.
Conclusions
In our paper, we reviewed our past work addressing the contribution of animal personality to the problem of non-migratory, habituated elk in mountain town sites. We showed that personality metrics can be developed for wild ungulates (Found and St. Clair, 2016) and used to interpret responses to management actions that include aversive conditioning (Found and St. Clair, 2018; Found et al., 2018). In elk, personality appears to relate to expressions of behavioral laterality, which may signal cognitive flexibility (Found and St. Clair, 2017). A suite of personality metrics influence social dynamics in elk (Found and St. Clair, 2016), herd-level behaviors (Found, 2017b), and may also extend to inter-specific interactions (Found, 2017a). Similar personality gradients have been found in diverse species and ecological contexts (Sih et al., 2012) and certainly occur in other ungulates that could be similarly studied in the wild.
We synthesized our past work on habituated elk to develop a conceptual model to show how changes in human behavior, forage availability, predator distribution, and conspecific interactions have favored bolder individuals to contribute to the loss of migration. Specifically, individuals with bolder personalities are more likely to exploit human-dominated areas because they are quicker to discover novel food resources and learn that predators avoid these areas. The same personality and laterality characteristics make these animals more likely to habituate to people, further amplifying their benefits and reducing their costs of co-occurrence. We described how managers might limit this selection on boldness with aversive conditioning that consistently imposes costs on targeted, bold individuals. We also suggested that culling the bolder individuals is unlikely to solve management challenges stemming from boldness because it is partially caused by behavioral flexibility that responds rapidly to changing circumstances that include the distribution of personalities in a population.
Similar implications of inadvertent selection on ungulate personality may apply to other populations of migratory ungulates and extend well-beyond the problems caused by habituation and residency. For example, selection of animals to support captive breeding or enhance the genetic diversity of declining populations could also inadvertently target the bolder, neophilic individuals that are easier to catch or maintain in captivity (McDougall et al., 2006). The subset of bolder individuals and their descendants might be less likely to survive if they are translocated or reintroduced to landscapes containing predators or hunters (Smith and Blumstein, 2008; Ciuti et al., 2012). Similarly, the prevalence of conservation-relevant research based on GPS-collared animals may impose a systematic bias toward individuals with personalities, not just age and sex distributions, that are more likely to be captured in the first place (Merrick and Koprowski, 2017).
A major limitation of using personality in wildlife management is the paucity of studies that combine those contexts, despite rapid increases in personality research on many species (Dingemanse et al., 2012). Behavioral studies of animal personality are urgently needed for ungulates, whose large size and gregarious tendency can cause rapid changes to habitat (Polfus and Krausman, 2012). Personality-mediated choices of migratory tactics in our study populations may offer some general insights for the loss of migratory behavior in ungulates around the world (Berger, 2004; Bolger et al., 2008; Tucker et al., 2018). Similar selection for bold and behaviourally flexible individuals is likely occurring for hundreds of other synanthropic species owing to the rapid rate of human population growth and urbanization (Walter et al., 2010). For ungulates, additional interacting effects include climate change (Tucker et al., 2018), urbanization and habituation of predators (Bateman and Fleming, 2012), and declines in predators overall (Ripple et al., 2014). Wildlife managers of the Anthropocene urgently need more tools, which should include better understanding and use of animal personality (Sih et al., 2012; Merrick and Koprowski, 2017).
Author Contributions
All authors are equal lead authors, reflecting equal contributions to this manuscript.
Funding
This work was supported by Natural Sciences and Engineering Research Council, via a Discovery Grant (RGPIN-2017-05915) to CC.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Albon, S. D., and Langvatn, R. (1992). Plant phenology and the benefits of migration in a temperate ungulate. Oikos 65, 502–513. doi: 10.2307/3545568
Altizer, S., Bartel, R., and Han, B. A. (2011). Animal migration and infectious disease risk. Science 331, 296–302. doi: 10.1126/science.1194694
Aplin, L. M., Farine, D. R., Mann, R. P., and Sheldon, B. C. (2014). Individual-level personality influences social foraging and collective behaviour in wild birds. Proc. R. Soc. B Biol. Sci. 281:20141016. doi: 10.1098/rspb.2014.1016
Atwell, J. W., Cardoso, G. C., Whittaker, D. J., Campbell-Nelson, S., Robertson, K. W., and Ketterson, E. D. (2012). Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav. Ecol. 23, 960–969. doi: 10.1093/beheco/ars059
Baruch-Mordo, S., Breck, S. W., Wilson, K. R., and Broderick, J. (2011). The carrot or the stick? Evaluation of education and enforcement as management tools for human-wildlife conflicts. PLoS ONE 6:e15681. doi: 10.1371/journal.pone.0015681
Bateman, P. W., and Fleming, P. A. (2012). Big city life: carnivores in urban environments. J. Zool. 287, 1–23. doi: 10.1111/j.1469-7998.2011.00887.x
Bateson, P., and Bradshaw, E. L. (1997). Physiological effects of hunting red deer (Cervus elaphus). Proc. R. Soc. B Biol. Sci. 264, 1707–1714. doi: 10.1098/rspb.1997.0237
Bejder, L., Samuels, A., Whitehead, H., Finn, H., and Allen, S. (2009). Impact assessment research: use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar. Ecol. Prog. Ser. 395, 177–185. doi: 10.3354/meps07979
Berg, J. E., Hebblewhite, M., St, Clair, C. C., and Merrill, E. H. (2019). Prevalence and mechanisms of partial migration in ungulates. Front. Ecol. Evol. 7:325. doi: 10.3389/fevo.2019.00325
Berger, J. (2004). The last mile: how to sustain long-distance migration in mammals. Conserv. Biol. 18, 320–331. doi: 10.1111/j.1523-1739.2004.00548.x
Berger, J. (2007). Fear, human shields and the redistribution of prey and predators in protected areas. Biol. Lett. 3, 620–623. doi: 10.1098/rsbl.2007.0415
Beschta, R. L., and Ripple, W. J. (2007). Wolves, elk, and aspen in the winter range of Jasper National Park, Canada. Can. J. Forest Res. 37, 1873–1885. doi: 10.1139/X07-017
Bisazza, A., Rogers, L. J., and Vallortigara, G. (1998). The origins of cerebral asymmetry: a review of evidence of behavioural and brain lateralization in fishes, reptiles and amphibians. Neurosci. Biobehav. Rev. 22, 411–426. doi: 10.1016/S0149-7634(97)00050-X
Blumstein, D. T. (2016). Habituation and sensitization: new thoughts about old ideas. Anim. Behav. 120, 255–262. doi: 10.1016/j.anbehav.2016.05.012
Bolger, D. T., Newmark, W. D., Morrison, T. A., and Doak, D. F. (2008). The need for integrative approaches to understand and conserve migratory ungulates. Ecol. Lett. 11, 63–77. doi: 10.1111/j.1461-0248.2007.01109.x
Bonnell, M. A., and Breck, S. W. (2017). Using resident-based hazing programs to reduce human-coyote conflicts in urban environments. Hum. Wildl. Interact. 11, 146–155. doi: 10.26077/ab7k-6j25
Bouchard, T. J., and Loehlin, J. C. (2001). Genes, evolution, and personality. Behav. Genet. 31, 243–273. doi: 10.1023/A:1012294324713
Boyce, M. S. (1991). Migratory behavior and management of elk (Cervus elaphus). Appl. Anim. Behav. Sci. 29, 239–250.
Brown, C., Western, J., and Braithwaite, V. A. (2007). The influence of early experience on, and inheritance of, cerebral lateralization. Anim. Behav. 74, 231–238. doi: 10.1016/j.anbehav.2006.08.014
Cagnacci, F., Focardi, S., Heurich, M., Stache, A., Hewison, A. J. M., Morellet, N., et al. (2011). Partial migration in roe deer: migratory and resident tactics are end points of a behavioural gradient determined by ecological factors. Oikos 120, 1790–1802. doi: 10.1111/j.1600-0706.2011.19441.x
Careau, V., and Garland, T. (2012). Performance, personality, and energetics: Correlation, causation, and mechanism. Physiol. Biochem. Zool. 85, 543–571. doi: 10.1086/666970
Carlier, M., Desplanches, A. G., Philip, N., Stefanini, S., Vicari, S., Volterra, V., et al. (2011). Laterality preference and cognition: cross-syndrome comparison of patients with trisomy 21 (Down), del7q11.23 (Williams-Beuren) and del22q11.2 (DiGeorge or Velo-Cardio-Facial) syndromes. Behav. Genet. 41, 413–422. doi: 10.1007/s10519-011-9465-2
Carter, A. J., Feeney, W. E., Marshall, H. H., Cowlishaw, G., and Heinsohn, R. (2013). Animal personality: what are behavioural ecologists measuring? Biol. Rev. 88, 465–475. doi: 10.1111/brv.12007
Chapman, B. B., Bronmark, C., Nilsson, J. A., and Hansson, L. A. (2011a). The ecology and evolution of partial migration. Oikos 120, 1764–1775. doi: 10.1111/j.1600-0706.2011.20131.x
Chapman, B. B., Hulthen, K., Blomqvist, D. R., Hansson, L. A., Nilsson, J. A., Brodersen, J., et al. (2011b). To boldly go: individual differences in boldness influence migratory tendency. Ecol. Lett. 14, 871–876. doi: 10.1111/j.1461-0248.2011.01648.x
Ciuti, S., Muhly, T. B., Paton, D. G., McDevitt, A. D., Musiani, M., and Boyce, M. S. (2012). Human selection of elk behavioural traits in a landscape of fear. Proc. R. Soc. B Biol. Sci. 279, 4407–4416. doi: 10.1098/rspb.2012.1483
Conover, M. R. (2001). Resolving Human-Wildlife Conflicts: The Science of Wildlife Damage Management. Boca Raton, FL: CRC Press.
Coppens, C. M., de Boer, S. F., and Koolhaas, J. M. (2010). Coping styles and behavioural flexibility: towards underlying mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 365, 4021–4028. doi: 10.1098/rstb.2010.0217
Creel, S., Winnie, J., Maxwell, B., Hamlin, K., and Creel, M. (2005). Elk alter habitat selection as an antipredator response to wolves. Ecology 86, 3387–3397. doi: 10.1890/05-0032
Dandy, N., Ballantyne, S., Moseley, D., Gill, R., Quine, C., and Van Der Wal, R. (2012). Exploring beliefs behind support for and opposition to wildlife management methods: a qualitative study. Eur. J. Wildl. Res. 58, 695–706. doi: 10.1007/s10344-012-0619-1
Davis, H., and Heslop, E. (2004). Habituation of hissing by Madagascar hissing cockroaches (Gromphadorhina portentosa): evidence of discrimination between humans? Behav. Processes 67, 539–543. doi: 10.1016/j.beproc.2004.08.003
Dekker, D., Bradford, W., and Gunson, J. R. (1995). “Elk and wolves in Jasper National Park, Alberta, from historical times to 1992,” in Ecology and Conservation of Wolves in a Changing World. Occasional Publication No. 35, eds L. Carbyn, S. H. Fritts, and D. R. Seip (Edmonton, AB: Canadian Circumpolar Institute), 85–94.
Dingemanse, N. J., Both, C., Drent, P. J., and Tinbergen, J. M. (2004). Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. B Biol. Sci. 271, 847–852. doi: 10.1098/rspb.2004.2680
Dingemanse, N. J., Both, C., van Noordwijk, A. J., Rutten, A. L., and Drent, P. J. (2003). Natal dispersal and personalities in great tits (Parus major). Proc. R. Soc. B Biol. Sci. 270, 741–747. doi: 10.1098/rspb.2002.2300
Dingemanse, N. J., Dochtermann, N. A., and Nakagawa, S. (2012). Defining behavioural syndromes and the role of ‘syndrome deviation' in understanding their evolution. Behav. Ecol. Sociobiol. 66, 1543–1548. doi: 10.1007/s00265-012-1416-2
Dingemanse, N. J., Wright, J., Kazem, A. J., Thomas, D. K., Hickling, R., and Dawnay, N. (2007). Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138. doi: 10.1111/j.1365-2656.2007.01284.x
Eggeman, S. L., Hebblewhite, M., Bohm, H., Whittington, J., and Merrill, E. H. (2016). Behavioural flexibility in migratory behaviour in a long-lived large herbivore. J. Anim. Ecol. 85, 785–797. doi: 10.1111/1365-2656.12495
Ensminger, A. L., and Westneat, D. F. (2012). Individual and sex differences in habituation and neophobia in house sparrows (Passer domesticus). Ethology 118, 1085–1095. doi: 10.1111/eth.12009
Finkemeier, M. A., Langbein, J., and Puppe, B. (2018). Personality research in mammalian farm animals: concepts, measures, and relationship to welfare. Front. Vet. Sci. 5:131. doi: 10.3389/fvets.2018.00131
Found, R. (2015). Ecological implications of personality in elk (Ph.D. dissertation), University of Alberta, Edmonton, AB, Canada.
Found, R. (2017a). Interactions between cleaner-birds and ungulates are personality dependent. Biol. Lett. 13:20170536. doi: 10.1098/rsbl.2017.0536
Found, R. (2017b). Lateral posture biases, habituation, and risk monitoring by wild ungulates. Laterality 22, 521–540. doi: 10.1080/1357650X.2016.1223091
Found, R. (2019). Personality influences habituation behaviour in ungulates. J. Ethol. 37, 47–58. doi: 10.1007/s10164-018-0567-7
Found, R., Kloppers, E. L., Hurd, T. E., St, and Clair, C. C. (2018). Intermediate frequency of aversive conditioning best restores wariness in habituated elk (Cervus canadensis). PLoS ONE 13:e0199216. doi: 10.1371/journal.pone.0199216
Found, R., St, and Clair, C. C. (2016). Behavioural syndromes predict loss of migration in wild elk. Anim. Behav. 115, 35–46. doi: 10.1016/j.anbehav.2016.02.007
Found, R., St, and Clair, C. C. (2017). Ambidextrous ungulates have more flexible behaviour, bolder personalities and migrate less. R. Soc. Open Sci. 4:160958. doi: 10.1098/rsos.160958
Found, R., St, and Clair, C. C. (2018). Personality influences wildlife responses to aversive conditioning. J. Wildl. Manage. 82, 747–755. doi: 10.1002/jwmg.21449
Fryxell, J. M., and Sinclair, A. R. E. (1988). Causes and consequences of migraion by large herbivores. Trends Ecol. Evol. 3, 237–241. doi: 10.1016/0169-5347(88)90166-8
Gates, C., and Hudson, R. J. (1978). Energy costs of locomotion in wapiti. Acta Theriol. 23, 365–370. doi: 10.4098/AT.arch.78-27
Giraldeau, L. A., and Beauchamp, G. (1999). Food exploitation: searching for the optimal joining policy. Trends Ecol. Evol. 14, 102–106. doi: 10.1016/S0169-5347(98)01542-0
Goldberg, J. F., Hebblewhite, M., and Bardsley, J. (2014). Consequences of a refuge for the predator-prey dynamics of a wolf-elk system in Banff National Park, Alberta, Canada. PLoS ONE 9:e91417. doi: 10.1371/journal.pone.0091417
Gosling, S. D. (2001). From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127, 45–86. doi: 10.1037//0033-2909.127.1.45
Gross, M. R. (1984). “Sunfish, salmon, and the evolution of alternative reproductive strategies and tactics in fishes,” in Fish Reproduction: Strategies and Tactics, eds R. Wooton and G. Potts (London: Academic Press), 55–75.
Hamer, D., and Herrero, S. (1991). Elk, Cervus elaphus, calves as food for grizzly bears, Ursus arctos, in Banff National Park, Alberta Canadian. Field Nat. 105, 101–103.
Hawley, J. E., Gehring, T. M., Schultz, R. N., Rossler, S. T., and Wydeven, A. P. (2009). Assessment of shock collars as nonlethal management for wolves in Wisconsin. J. Wildl. Manage. 73, 518–525. doi: 10.2193/2007-066
Hebblewhite, M., and Merrill, E. H. (2009). Trade-offs between predation risk and forage differ between migrant strategies in a migratory ungulate. Ecology 90, 3445–3454. doi: 10.1890/08-2090.1
Hebblewhite, M., Merrill, E. H., Morgantini, L. E., White, C. A., Allen, J. R., Bruns, E., et al. (2006). Is the migratory behavior of montane elk herds in peril? The case of Alberta's Ya Ha Tinda elk herd. Wildl. Soc. Bull. 34, 1280–1294. doi: 10.2193/0091-7648(2006)34[1280:ITMBOM]2.0.CO;2
Hebblewhite, M., Pletscher, D. H., and Paquet, P. C. (2002). Elk population dynamics in areas with and without predation by recolonizing wolves in Banff National Park, Alberta. Can. J. Zool. 80, 789–799. doi: 10.1139/z02-058
Honda, T., Iijima, H., Tsuboi, J., and Uchida, K. (2018). A review of urban wildlife management from the animal personality perspective: the case of urban deer. Sci. Total Environ. 644, 576–582. doi: 10.1016/j.scitotenv.2018.06.335
Hopkins, J.B III, Whittington, J., Clevenger, A. P., Sawaya, M. A., St, and Clair, C. C. (2014). Stable isotopes reveal rail-associated behavior in a threatened carnivore. Isotopes Environ. Health Stud. 50, 322–331. doi: 10.1080/10256016.2014.922555
Ito, T. Y., Miura, N., Lhagvasuren, B., Enkhbileg, D., Takatsuki, S., Tsunekawa, A., et al. (2005). Preliminary evidence of a barrier effect of a railroad on the migration of Mongolian gazelles. Conserv. Biol. 19, 945–948. doi: 10.1111/j.1523-1739.2005.004364.x
Kloppers, E. L., St, Clair, C. C., and Hurd, T. E. (2005). Predator-resembling aversive conditioning for managing habituated wildlife. Ecol. Soc. 10:31. doi: 10.5751/ES-01293-100131
Kortello, A. D., Hurd, T. E., and Murray, D. L. (2007). Interactions between cougars (Puma concolor) and gray wolves (Canis lupus) in Banff National Park, Alberta. Ecoscience 14, 214–222. doi: 10.2980/1195-6860(2007)14[214:IBCPCA]2.0.CO;2
Koval, J. H., and Mertig, A. G. (2004). Attitudes of the Michigan public and wildlife agency personnel toward lethal wildlife management. Wildl. Soc. Bull. 32, 232–243. doi: 10.2193/0091-7648(2004)32[232:AOTMPA]2.0.CO;2
Krueger, R. F., South, S., Johnson, W., and Iacono, W. (2008). The heritability of personality is not always 50%: gene-environment interactions and correlations between personality and parenting. J. Pers. 76, 1485–1521. doi: 10.1111/j.1467-6494.2008.00529.x
Lattal, K. M., and Lattal, K. A. (2012). Facets of Pavlovian and operant extinction. Behav. Processes 90, 1–8. doi: 10.1016/j.beproc.2012.03.009
Laundre, J. W., Hernandez, L., and Altendorf, K. B. (2001). Wolves, elk, and bison: reestablishing the “landscape of fear” in Yellowstone National Park, USA. Can. J. Zool. 79, 1401–1409. doi: 10.1139/z01-094
Lennox, R. J., Gallagher, A. J., Ritchie, E. G., and Cooke, S. J. (2018). Evaluating the efficacy of predator removal in a conflict-prone world. Biol. Conserv. 224, 277–289. doi: 10.1016/j.biocon.2018.05.003
Mackay, B., Little, R. M., Amar, A., and Hockey, P. A. R. (2014). Incorporating environmental considerations in managing Egyptian geese on golf courses in South Africa. J. Wildl. Manage. 78, 671–678. doi: 10.1002/jwmg.711
Maynard Smith, J., and Price, G. R. (1973). The logic of animal conflict. Nature 246:15. doi: 10.1038/246015a0
Mazur, R. L. (2010). Does aversive conditioning reduce human—black bear conflict? J. Wildl. Manage. 74, 48–54. doi: 10.2193/2008-163
McDougall, P. T., Reale, D., Sol, D., and Reader, S. M. (2006). Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced, and wild populations. Anim. Conserv. 9, 39–48. doi: 10.1111/j.1469-1795.2005.00004.x
McKenzie, J. A. (2001). The demographic and nutritional benefits of urban habitat use by elk (M.Sc. thesis), University of Guelph, Guelph, ON, Canada.
Merrick, M. J., and Koprowski, J. L. (2017). Should we consider individual behavior differences in applied wildlife conservation studies? Biol. Conserv. 209, 34–44. doi: 10.1016/j.biocon.2017.01.021
Modlmeier, A. P., Keiser, C. N., Watters, J. V., Sih, A., and Pruitt, J. N. (2014). The keystone individual concept: an ecological and evolutionary overview. Anim. Behav. 89, 53–62. doi: 10.1016/j.anbehav.2013.12.020
Morrison, T. A., and Bolger, D. T. (2012). Wet season range fidelity in a tropical migratory ungulate. J. Anim. Ecol. 81, 543–552. doi: 10.1111/j.1365-2656.2011.01941.x
Muhly, T. B., Semeniuk, C., Massolo, A., Hickman, L., and Musiani, M. (2011). Human activity helps prey win the predator-prey space race. PLoS ONE 6:e17050. doi: 10.1371/journal.pone.0017050
Murie, A. (1934). The Moose of Isle Royale. University of Michigan Musuem of Zoology. Miscellaneous Publications No. 25. Ann Arbor, MI: University of Michigan Press.
Murray, M., Edwards, M. A., Abercrombie, W., St, and Clair, C. C. (2015). Poor health is associated with use of anthropogenic resources in an urban carnivore. Proc. R. Soc. B Biol. Sci. 282:20150009. doi: 10.1098/rspb.2015.0009
Newsome, T. M., Dellinger, J. A., Pavey, C. R., Ripple, W. J., Shores, C. R., Wirsing, A. J., et al. (2015). The ecological effects of providing resource subsidies to predators. Glob. Ecol. Biogeogr. 24, 1–11. doi: 10.1111/geb.12236
Nicolaus, M., Tinbergen, J. M., Ubels, R., Both, C., and Dingemanse, N. J. (2016). Density fluctuations represent a key process maintaining personality variation in a wild passerine bird. Ecol. Lett. 19, 478–486. doi: 10.1111/ele.12584
Nilsson, J.-Å., Bronmark, C., Hansson, L.-A., and Chapman, B. B. (2014). “Individuality in movement: the role of animal personality,” in Animal Movement Across Scales, eds L.-A. Hansson and S. Akesson (Oxford: Oxford University Press), 90–109. doi: 10.1093/acprof:oso/9780199677184.003.0006
Nyhus, P. J. (2016). Human-wildlife conflict and coexistence. Annu. Rev. Environ. Resour. 41, 143–171. doi: 10.1146/annurev-environ-110615-085634
Ohta, U., Jusup, M., Mano, T., Tsuruga, H., and Matsuda, H. J. E. M. (2012). Adaptive management of the brown bear population in Hokkaido, Japan. Ecol. Modell. 242, 20–27. doi: 10.1016/j.ecolmodel.2012.05.011
Paquet, P. C., Wierzchowski, J., and Callaghan, C. (1996). “Summary report on the effects of human activity on gray wolves in the Bow River Valley, Banff National Park, Alberta,” in Chapter 7: A Cumulative Effects Assessment and Futures Outlook for the Banff Bow Valley. Prepared for the Banff Bow Valley Study. Effects of human activity on gray wolves in the Bow River Valley, Banff National Park, Alberta, eds J. Green, C. Pacas, S. Bayley, and L. Cornwell (Ottawa, ON: Department of Canadian Heritage), 74–120.
Polfus, J. L., and Krausman, P. R. (2012). Impacts of residential development on ungulates in the rocky mountain west. Wildl. Soc. Bull. 36, 647–657. doi: 10.1002/wsb.185
Porac, C., and Searleman, A. (2006). The relationship between hand preference consistency, health, and accidents in a sample of adults over the age of 65 years. Laterality 11, 405–414. doi: 10.1080/13576500600677823
Powell, R. A., Honey, P. L., and Symbaluk, D. G. (2016). Introduction to Learning and Behavior. Boston, MA: Cengage Learning.
Réale, D., Gallant, B. Y., Leblanc, M., and Festa-Bianchet, M. (2000). Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Anim. Behav. 60, 589–597. doi: 10.1006/anbe.2000.1530
Réale, D., Garant, D., Humphries, M. M., Bergeron, P., Careau, V., and Montiglio, P. O. (2010). Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos. Trans. R. Soc. B Biol. Sci. 365, 4051–4063. doi: 10.1098/rstb.2010.0208
Réale, D., Reader, S. M., Sol, D., McDougall, P. T., and Dingemanse, N. J. (2007). Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. doi: 10.1111/j.1469-185X.2007.00010.x
Ripple, W. J., Estes, J. A., Beschta, R. L., Wilmers, C. C., Ritchie, E. G., Hebblewhite, M., et al. (2014). Status and ecological effects of the world's largest carnivores. Science 343:1241484. doi: 10.1126/science.1241484
Rogers, L. J. (2000). Evolution of hemispheric specialization: advantages and disadvantages. Brain Lang. 73, 236–253. doi: 10.1006/brln.2000.2305
Rolandsen, C. M., Solberg, E. J., Saether, B. E., Van Moorter, B., Herfindal, I., and Bjorneraas, K. (2017). On fitness and partial migration in a large herbivore–migratory moose have higher reproductive performance than residents. Oikos 126, 547–555. doi: 10.1111/oik.02996
Shannon, G., Cordes, L. S., Hardy, A. R., Angeloni, L. M., and Crooks, K. R. J. P. O. (2014). Behavioral responses associated with a human-mediated predator shelter. PLoS ONE 9:e94630. doi: 10.1371/journal.pone.0094630
Shepherd, B., and Whittington, J. (2006). Response of wolves to corridor restoration and human use management. Ecol. Soc. 11:1. doi: 10.5751/ES-01813-110201
Sih, A., Bell, A., and Johnson, J. C. (2004). Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. doi: 10.1016/j.tree.2004.04.009
Sih, A., Cote, J., Evans, M., Fogarty, S., and Pruitt, J. (2012). Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. doi: 10.1111/j.1461-0248.2011.01731.x
Singh, N. J., Borger, L., Dettki, H., Bunnefeld, N., and Ericsson, G. (2012). From migration to nomadism: movement variability in a northern ungulate across its latitudinal range. Ecol. Appl. 22, 2007–2020. doi: 10.1890/12-0245.1
Smith, B. R., and Blumstein, D. T. (2008). Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455. doi: 10.1093/beheco/arm144
Spaedtke, H. R. (2009). Aversive conditioning on horse back: A management alternative for grassland systems threatened by sedentary elk populations (M.Sc. thesis), University of Alberta, Edmonton, AB, Canada.
Stamps, J. A. (2016). Individual differences in behavioural plasticities. Biol. Rev. 91, 534–567. doi: 10.1111/brv.12186
St, Clair, C. C., Friesen, A., Gangadharan, A., Gilhooly, P., Murray, M., and Pollock, S. (2019). Animal learning may contribute to both problems and solutions for wildlife–train collisions. Philos. Trans. R. Soc. B Biol. Sci. 374:20180050. doi: 10.1098/rstb.2018.0050
Strum, S. C. (2010). The development of primate raiding: implications for management and conservation. Int. J. Primatol. 31, 133–156. doi: 10.1007/s10764-009-9387-5
Thompson, M. J., and Henderson, R. E. (1998). Elk habituation as a credibility challenge for wildlife professionals. Wildl. Soc. Bull. 26, 477–483.
Thurfjell, H., Ciuti, S., and Boyce, M. S. (2017). Learning from the mistakes of others: How female elk (Cervus elaphus) adjust behaviour with age to avoid hunters. PLoS ONE 12:20. doi: 10.1371/journal.pone.0178082
Tucker, M. A., Bohning-Gaese, K., Fagan, W. F., Fryxell, J. M., Van Moorter, B., Alberts, S. C., et al. (2018). Moving in the anthropocene: global reductions in terrestrial mammalian movements. Science 359, 466–469. doi: 10.1126/science.aam9712
Vallortigara, G. (2006). The evolutionary psychology of left and right: costs and benefits of lateralization. Dev. Psychobiol. 48, 418–427. doi: 10.1002/dev.20166
Walter, W. D., Lavelle, M. J., Fischer, J. W., Johnson, T. L., Hygnstrom, S. E., and VerCauteren, K. C. (2010). Management of damage by elk (Cervus elaphus) in North America: a review. Wildl. Res. 37, 630–646. doi: 10.1071/WR10021
Wesley, R. L., Cibils, A. F., Mulliniks, J. T., Pollak, E. R., Petersen, M. K., and Fredrickson, E. L. (2012). An assessment of behavioural syndromes in rangeland-raised beef cattle. Appl. Anim. Behav. Sci. 139, 183–194. doi: 10.1016/j.applanim.2012.04.005
White, P. C. L., and Ward, A. I. (2010). Interdisciplinary approaches for the management of existing and emerging human-wildlife conflicts. Wildl. Res. 37, 623–629. doi: 10.1071/WR10191
Whittaker, D., and Knight, R. L. (1998). Understanding wildlife responses to humans. Wildlife Soc. Bull. 26, 312–317.
Whitwell, S. M., Amiot, C., McLean, I. G., Lovegrove, T. G., Armstrong, D. P., Brunton, D. H., et al. (2012). Losing anti-predatory behaviour: a cost of translocation. Austral Ecol. 37, 413–418. doi: 10.1111/j.1442-9993.2011.02293.x
Keywords: behavioral flexibility, habituation, human-wildlife conflict, personality, ungulates
Citation: Found R and St. Clair CC (2019) Influences of Personality on Ungulate Migration and Management. Front. Ecol. Evol. 7:438. doi: 10.3389/fevo.2019.00438
Received: 01 May 2019; Accepted: 25 October 2019;
Published: 27 November 2019.
Edited by:
Brett K. Sandercock, Norwegian Institute for Nature Research (NINA), NorwayReviewed by:
Paul Cross, United States Geological Survey (USGS), United StatesIvar Herfindal, Norwegian University of Science and Technology, Norway
Copyright © 2019 Found and St. Clair. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Colleen Cassady St. Clair, Y3N0Y2xhaXJAdWFsYmVydGEuY2E=
†Present address: Robert Found, Parks Canada, Elk Island National Park, Fort Saskatchewan, AB, Canada
 Robert Found
Robert Found Colleen Cassady St. Clair
Colleen Cassady St. Clair