- 1Department of Fisheries, Wildlife and Conservation Biology, University of Minnesota, St. Paul, MN, United States
- 2U.S. Geological Survey, St. Paul, MN, United States
- 3Polistes Foundation, Inc., St. Paul, MN, United States
- 4Department of Entomology, University of Minnesota, St. Paul, MN, United States
- 5University of Wisconsin Arboretum, University of Wisconsin–Madison, Madison, WI, United States
Much of the remaining suitable habitat for monarchs (Danaus plexippus) in Minnesota is found in tallgrass prairies. We studied the association of adult monarch abundance with use of fire or grazing to manage prairies. Sites (n = 20) ranged in size from 1 to 145 hectares and included land owned and managed by the Minnesota DNR, U.S. Fish and Wildlife Service, The Nature Conservancy, and private landowners. We measured Asclepias spp. (milkweeds, monarch host plants) and forb frequency in 0.5 × 2-m plots located along randomly-placed transects that were stratified to sample wet, mesic, and dry prairie types at each site. Adult butterfly surveys took place three times at each site during the summers of 2016 and 2017, using a standardized Pollard Walk (400 m). Data were analyzed using mixed effects models. Monarchs were more abundant at sites managed with prescribed fire than with grazing. We found no difference in milkweed and forb frequency between burned and grazed prairies. There was no relationship between monarch abundance and the other predictor variables tested: milkweed frequency, site area, forb frequency, and percent prairie in a 1.5 km buffer area surrounding each site. Monarch abundance was lowest at grazed sites with high stocking rates. Our findings suggest that milkweed and forb frequency do not vary between burned and grazed sites, although we only considered land management practices for the 12 years before the study and the most recent burns occurred in 2014, 2 years prior to the start of our study. They also suggest that heavy grazing may have negative impacts on monarchs.
Introduction
The current decline in eastern North American monarch (Danaus plexippus) numbers (Rendón-Salinas et al., 2018), the risk this decline poses for the long-term survival of the population (Semmens et al., 2016), the strong evidence that breeding habitat in the Upper Midwestern U.S. is a key factor driving the decline (Oberhauser et al., 2016; Pleasants et al., 2016; Pleasants, 2017; Thogmartin et al., 2017b), and the interest that people have in preserving monarchs (Diffendorfer et al., 2014), behooves us to understand best management practices for potential monarch breeding habitat. While we focus on management of remnant tallgrass prairie, where the ground has never been plowed, our findings are likely to be applicable to most Midwestern grasslands. Understanding how management affects monarch use of grasslands is important because they have the potential to contribute more to monarch conservation goals than any landcover category except if current cropland is restored to grassland (Thogmartin et al., 2017a).
The tallgrass prairie evolved with natural disturbances such as fire, grazing, and drought, without which woody plant encroachment is inevitable (Axelrod, 1985; Anderson, 2006). Today, many land managers use prescribed burns and grazing, often by cattle, to mimic these historical processes and maintain remnant prairie (Brudvig et al., 2007). Prairies are one of the most critically endangered habitats in North America and tallgrass prairie alone once covered over 100 million acres; <2% of these grasslands remain (Samson et al., 2004; Anderson, 2006). Minnesota, once home to 18 million acres of tallgrass prairie, has suffered comparable losses (Samson et al., 2004). Until recently, the agricultural lands that now dominate the fragmented landscape supported more milkweeds and monarchs than other habitat types (Oberhauser et al., 2001). With the advent and wide-spread use of transgenic herbicide-resistant crops and increased herbicide use, however, the Midwest has suffered a 40% loss in milkweed stems (Pleasants and Oberhauser, 2013; Pleasants et al., 2016). It is critical that we understand the capacity of our remaining grasslands to support monarchs so that land managers can take this information into consideration when setting conservation and management goals.
There has been considerable attention given to the effects of burning on arthropods, which may be killed directly by fire, or which could be affected positively or negatively by changes to the vegetation or soil. In many cases, prairie dependent, less mobile insect species, and species in less mobile life stages are more likely to exhibit negative post-fire responses in isolated grassland sites, where recolonization is presumably less likely (Panzer, 2002), unless refugia are maintained (Swengel and Swengel, 2007). In Minnesota, however, monarchs are generally not present in any life stage during the early- to mid-spring (Prysby and Oberhauser, 2004), when most prescribed fires occur (Emery and Gross, 2005; Towne and Craine, 2014), so direct impacts are likely to be minimal. Indirect impacts due to ways in which fire affects monarch host plants or nectar plants are more likely. The effects of fire on common milkweed (Asclepias syriaca) are unclear; Towne and Kemp (2008) reported that A. syriaca declined in frequency after summer fire, but a recent study in Kansas found that it increased after fire and decreased with grazing, while seven other species of milkweeds increased with grazing (Ricono et al., 2018). In the southern Great Plains, Asclepias viridis density and re-growth were significantly higher immediately after summer burns, leading to an increase in observed monarch eggs and larvae in burned areas (Baum and Sharber, 2012). Impacts of grazing could include direct consumption of immature monarchs or removal of their host plants or nectar sources. However, milkweed, especially the most toxic species, can harm vertebrate herbivores if they consume it (Holmgren, 1971; Panter et al., 2011). If more desirable forage is available, vertebrates usually do not consume milkweed (personal observations; Holmgren, 1971; Panter et al., 2011).
Conservation grazing, the use of grazing by domestic animals to achieve conservation goals, is seen as an attractive management alternative to reduce potential threats of fire to insect communities (Panzer, 2002). Prior to European settlement, bison were the dominant grazers in the tallgrass prairie. Today, however, conservation grazing is done almost exclusively with domesticated cattle, which preferentially graze different vegetation, prefer wetter areas, and move with different herd patterns than bison (Plumb and Dodd, 1993; Allred et al., 2011; Kohl et al., 2013). The impacts of grazing include the removal of plant material (thus making grazers potential competitors of herbivore arthropods) and effects on plant communities. Grazer impacts also include soil disturbance, nutrient concentration, and direct consumption of arthropods as they consume plant material. Because ungulates are grass specialists, they can increase plant diversity (Collins et al., 1998) and can also increase habitat heterogeneity (Knapp et al., 1999). Grazing can also alter plant quality and abundance (Joern, 2005; Moran, 2014) leading to enhancement of herbivorous arthropod abundance (Moran, 2014). Both herbivorous arthropods and large ungulates can consume considerable amounts of plant biomass, suggesting that interactions between them could be important (e.g., Pringle et al., 2007). However, most work on impacts of grazing has focused on extreme levels (e.g., comparing no grazing to high levels of grazing), and there has been less work on levels between these extremes (van Klink et al., 2015; Neilly et al., 2016).
While there have been several studies that examined the responses of butterflies, including monarchs, to fire, grazing, and patch-burn grazing (e.g., Vogel et al., 2007; Moranz et al., 2012), no individual studies focus on the impacts of these management strategies on both monarchs and their host plants. Vogel et al. (2007) found higher adult monarch abundance at grazed sites than at burned sites, as well as sites with higher floral resources, while Moranz et al. (2012) found adult monarchs in highest abundance at burned sites compared to sites that were burned-and-grazed or patch-burn-grazed. Vogel et al. (2010) found a positive correlation between time since burn and butterfly richness and abundance in Iowa tallgrass prairie; although they did not report specific results for monarch butterflies, they found that habitat generalists [which monarchs are characterized as by Vogel et al. (2010)] were more influenced by the direct effects of fire whereas habitat-specialist butterflies were more influenced by vegetation responses to fire. Migrating monarch and nectar resource abundance in the Ouachita Mountains of Arkansas increased following frequent prescribed fires after an extended period of fire suppression (Rudolph et al., 2006).
Most existing analyses of adult monarch numbers look at regional or population-level trends, even those that use data from individual surveys (e.g., Semmens et al., 2016; Thogmartin et al., 2017b). Few have looked at the importance of site-specific characteristics (but see Saunders et al., 2018), including management. As part of a larger study of the impacts of vegetation management through fire and grazing on plants, butterflies, and bees, we collected data on adult monarch abundance at 20 remnant prairie sites in western Minnesota, 10 managed with burning, and 10 with grazing.
Our objective was to estimate the effect of prairie management by fire vs. grazing on adult monarchs in Minnesota tallgrass prairie remnants. We hypothesized that if prairie management by fire or grazing positively influenced the frequency of nectar plants (forbs) and monarch host plants (milkweeds), then that management type would positively affect monarch abundance. If prairie management by fire and grazing did not influence nectar and host plant frequency, we expected to find no difference in monarch response to management, although patterns in site area or prairie habitat in the surrounding area may still influence site occupancy.
Materials and Methods
Site Selection
The land-use legacy of fire and grazing management can take years to become apparent and is known to affect prairie plants and butterfly communities (Debinski et al., 2011; Moranz et al., 2012). To account for this, we selected remnant prairies that had never been plowed and that had documented management histories of either grazing or prescribed fire from 2005 through the completion of our study in 2017. We chose 20 sites, all within the Prairie Parkland Province of Minnesota (Figure 1). Sites ranged in size from 1.13 to 144.7 hectares with a median of 10.6 hectares (see Table S1 for additional details). We obtained management histories and permission to survey the sites from owners and land managers: private landowners (5 sites) and multiple agencies including The Nature Conservancy (TNC) (2 sites), US Fish and Wildlife Service (10 sites), and the MN Department of Natural Resources (MN DNR) (3 sites). To minimize variability, the grazed (n = 10) and burned (n = 10) sites in which we surveyed monarchs were selected to represent similar geographical distributions and size ranges. Sites managed with fire were burned during the spring, 1–3 times since 2005; none were burned during 2016 or 2017. All grazed sites were rotationally grazed by domesticated cattle and stocking rates ranged from 0.52 to 2.9 Animal Unit Months/acre (AUM), representing a range of stocking rates used for rotational grazing in the Midwest (McCollum et al., 1999; Derner et al., 2008); privately-owned sites were grazed every year from 2005 to 2017 and public and TNC sites were grazed 2–5 years since 2005. Presence of milkweeds was not considered during site selection because we were interested in adult monarch use of sites and the extent to which this may or may not correlate with larval host plants.
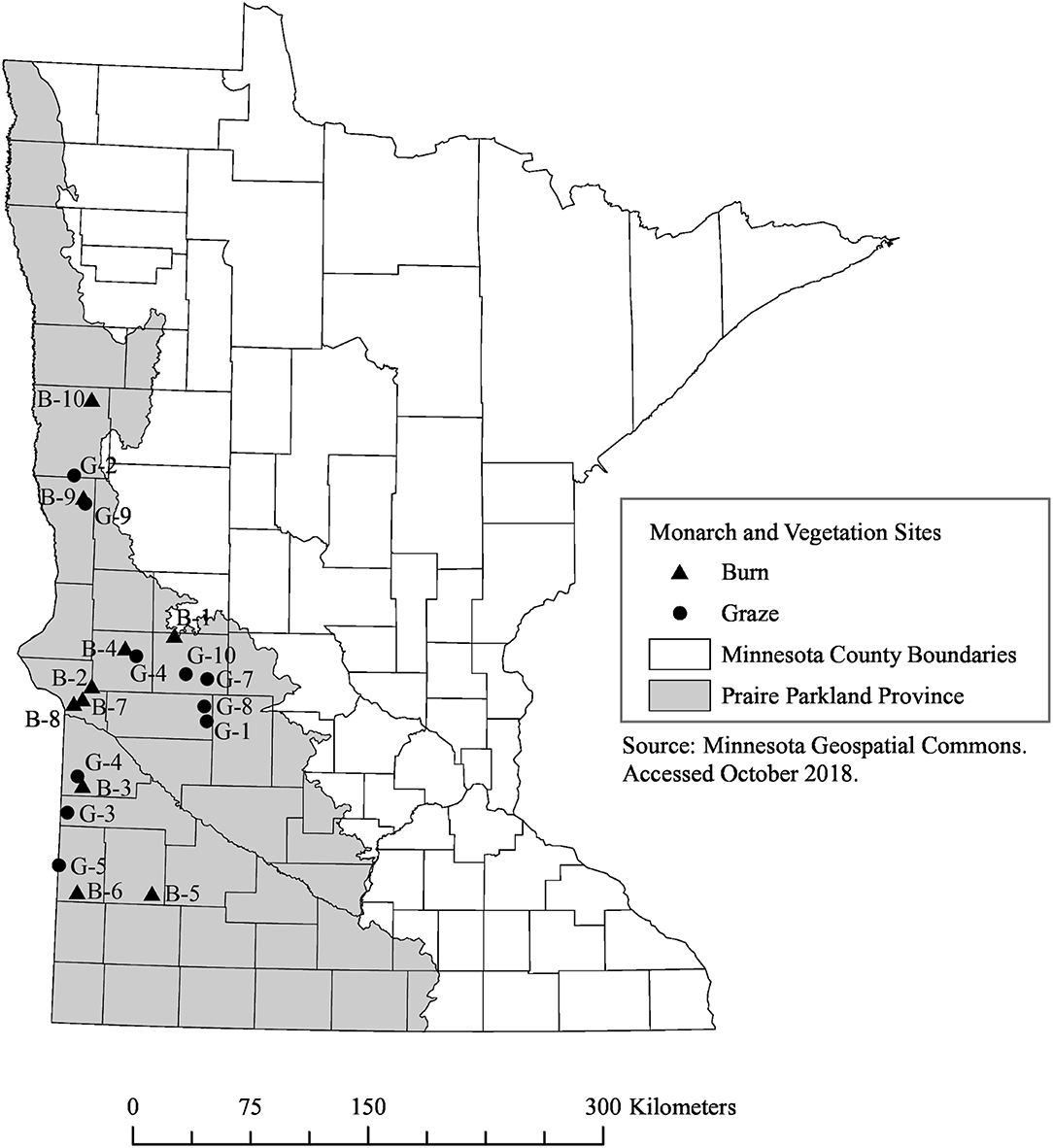
Figure 1. Map of 10 burned (triangles, B 1–10) and 10 grazed (circles, G 1–10) remnant prairie sites where vegetation and monarchs were surveyed in Minnesota in 2016 and 2017. Bounding coordinates for map are West: −97.02026367158, East: −95.372314452892, North: 46.754616856587, South: 43.627806711488.
For each site, we determined the percent of prairie in the surrounding landscape by first creating a 1.5 km buffer around each site using ArcMap (v 10.5.1) and overlaying this buffer onto landscape data obtained from the US Department of Agriculture, MN DNR, and South Dakota State University. We then calculated the percentage of the land within the buffer that was classified as prairie.
Vegetation Sampling and Analysis
We surveyed vegetation twice at each site, once in 2016 and again in 2017 (May 31 through August 28 in 2016 and June 1 through August 24 in 2017). Vegetation and monarch surveys were not concurrent. Water retention properties of soils were used to stratify sampling within all potential prairie types (wet, mesic, dry) at each site. Within each prairie type, transects were delineated on maps prior to the field season and were parallel to the elevation gradient; vegetation was sampled in each prairie type in proportion to its area at a site. Sites contained 1 to 10 transects, depending on prairie type, distribution, and site shape, and transects ranged in length from 45 to 792 m. We used 0.5 x 2-m plots arrayed equidistantly on transects to estimate the frequency (proportion of plots occupied) of Asclepias and forb species (Elzinga et al., 1998). Asclepias species were included in the measure of forb frequency because milkweeds are both important host plants and nectar resources for monarch butterflies. Frequency was estimated whether or not plants were in bloom. Plots were distributed along transects in proportion to the transect length and were at least 10 m from the ends of transects to avoid potential edge effects. Plots were oriented perpendicular to the transect, and the number of plots per site was proportional to the size of the site, with a maximum of 30 plots and a minimum of 5 plots in any given site. The number of plots at each site was determined using the equation:
where a = 30 (the maximum number of plots per site), b = 0.163, and x = site area(ha). See Table S1 for an index of vegetation plots at each site and Figure S1 for an example of transect and plot distribution at a site.
We also conducted botanist-directed meandering walks through each prairie type within a site in both 2016 and 2017 to scout for additional species not seen along transect surveys, which is especially relevant for patchy species such as Asclepias. Effort, or time spent searching, was recorded and proportional to the size of the search area. Observations from the meandering walks are included in the summary statistics of milkweed presence at each site and Table 2 but not in the forb and Asclepias frequency analyses.
We summed the frequency (n occupied plots/total plots) of all forb species combined, and Asclepias species only by year for each study site. Vegetation data were analyzed using analysis of variance models [proc mixed in SAS software, Version 9.4 SAS Institute Inc., 2015]. With management type as the predictor variable of interest, we built two models, one with forb frequency as the response variable, and one with Asclepias frequency as the response variable. Models were developed separately for 2016 and 2017 data because different people conducted the vegetation surveys in each year. Site nested within management type was the random effect in all models.
Butterfly Sampling and Analysis
Monarchs were surveyed three times each summer for two summers (June 15 through August 31 in 2016 and May 14 through August 18 in 2017), with each round of surveying beginning in the south and moving north. Sites sampled in the morning during one visit were sampled in the afternoon during the subsequent visit, and vice versa. Prior to surveying, 400 meters of transects were randomly selected from pre-established vegetation transects at each site, with transects selected to proportionally represent the prairie types (wet, mesic, dry) present at each site. If multiple transects were required due to the size and shape of the site, they were at least 20 meters apart to avoid counting redundancy. Monarchs were surveyed concurrently with bees and other butterfly species.
We used a modification of the standardized Pollard Walk for relative abundance (Pollard, 1977; Thomas, 2005; Swengel and Swengel, 2013; Smith and Cherry, 2014). During each site visit, the observer walked 400 m of transects at a steady pace of 10 m/min and recorded all individuals seen within 2.5 m on both sides, 5 m ahead, and 5 m above. Monarchs were sampled by sight identification, and sex was not recorded. Surveys were conducted, when possible, between 09:30 h and 18:30 h when temperatures were above 18°C, sustained winds <17 km/h, and cloud cover was <50% with no precipitation (Shepherd and Debinski, 2005; Moranz et al., 2012). Butterfly surveys were all conducted by the same observer. Adult monarch abundances from three survey visits per site per year were summed separately for summer 2016 and summer 2017 to create an index of monarch abundance for each year, hereafter referred to simply as monarch abundance. One grazed site, G-1, was only surveyed in 2017. Sixty surveys were conducted at burned sites and 57 at grazed sites.
Monarch data were analyzed using Poisson distributed generalized linear mixed effects models (GLMMs). With number of monarchs as the response variable, we used a two-step modeling process. First, we chose five predictor variables of interest as fixed effects to examine based on study design and a priori knowledge: (1) management type, (2) milkweed frequency, (3) forb frequency, (4) site area, and (5) percent prairie in a 1.5 km buffer surrounding each site. Year was included as a sixth predictor. We built six univariate GLMMs to look at the individual effect of each of these predictor variables on monarch abundance. Second, we built a single global GLMM including all six predictor variables listed above and three interaction terms and then used backward elimination, eliminating least significant variables one at a time (alpha = 0.05); the final model was determined to be the one with only significant terms remaining and the lowest AIC value (ΔAIC > 2, Arnold, 2010). We included the interactions between management type and milkweed frequency, management type and forb frequency, and milkweed frequency and site area (an index of resource availability). Site was included as a random effect. Prior to review, year was analyzed as a nested random effect instead of a fixed effect; final model results did not differ. The variable for site area was log10 transformed to normalize overdispersed size data.
To examine the possibility of management-specific effects on monarchs, we built additional management-specific GLMMs. First, univariate responses to management-specific predictors were modeled for monarch abundance at burned sites (the number of years each site was burned between 2005 and 2017 and time since the last burn) and grazed sites (the number of years each site was grazed between 2005 and 2017, time since last grazing, and stocking rate). Next, we built two additional sets of GLMMs, one with monarch abundance at burned sites as the response variable and one with monarch abundance at grazed sites as the response variable. We replaced management type in these models with management-specific predictors: time since last burn in the burn-only model and stocking rate and the number of years a site had been grazed between 2005 and 2017 in the graze-only model. The number of years each site was burned (2005–2017) was not included in the burn-only models due to its collinearity with time since last burn and time since last grazing was not included in the graze-only models due to its collinearity with the number of years a site had been grazed. All other predictor variables and random effects remained the same.
All monarch analyses were conducted in R 3.4.1 (R Core Team, 2017), RStudio 1.0.153 (R Studio Team, 2016) using the glmmTMB function from the glmmTMB package (Magnusson et al., 2017). Multicollinearity was tested using Pearson's correlation in the ggscatter function from the ggpubr package in R (Kassambara, 2017).
Results
Vegetation
The frequency of Asclepias did not differ between burned and grazed sites in 2016 or 2017, nor did the frequency of all forbs combined (Table 1). Seven species of milkweeds were observed in at least one site during the course of this study: Asclepias incarnata, A. ovalifolia, A. speciosa, A. syriaca, A. tuberosa, A. verticillata, and A. viridiflora. Five of these were observed in transect plots and included in frequency analyses: A. incarnata, A. speciosa, A. syriaca, A. verticillata, and A. viridiflora (Table 2).
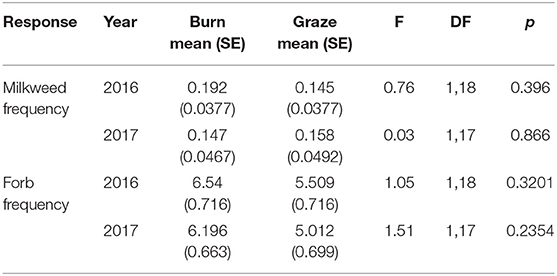
Table 1. Comparison of the frequency of Asclepias and forb species at burned vs. grazed sites in 2016 and 2017.
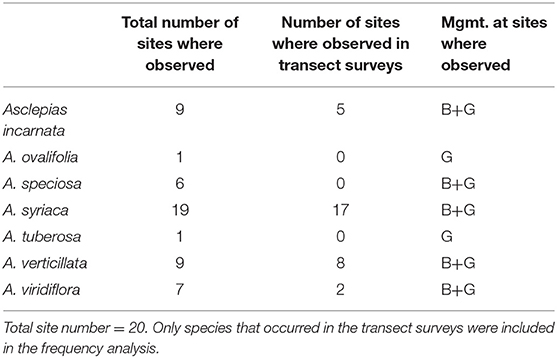
Table 2. Summary of the total number of sites where each milkweed species was observed (transect surveys + meandering walk), the number of sites where each milkweed species was observed in transect surveys only and the management (B = burned, G = grazed) at sites where milkweed species were observed.
For transect survey data used in frequency analyses, we only observed one species of Asclepias in eight sites, another eight sites had two species observed, and two sites had three species observed. At two sites (G-3 and G-5) no Asclepias species were observed in our transect plots during either year of the study, although three species were observed in meandering walks at both sites. At three sites (G-2, B-3, and B-9), Asclepias species were observed in transect surveys during only 1 year of the study, although Asclepias species were observed at G-2 and B-3 in 2016 and 2017 during meandering walks. All milkweed species were observed at both burned and grazed sites with the exception of A. ovalifolia and A. tuberosa, which were seen at only one grazed site each during meandering walks.
Monarchs
Adult monarchs were observed at all 20 study sites (Figure 2A). One hundred ninety-eight adult monarchs were observed during Pollard transect walks in 2016 and 2017 (99 in 2016 and 99 in 2017). One hundred forty-eight monarchs were observed at sites managed with fire and 50 monarchs were observed at sites managed with grazing. The majority of monarch observations were made during the third round of monarch surveys in late July and August in both years (Figures 2B,C).
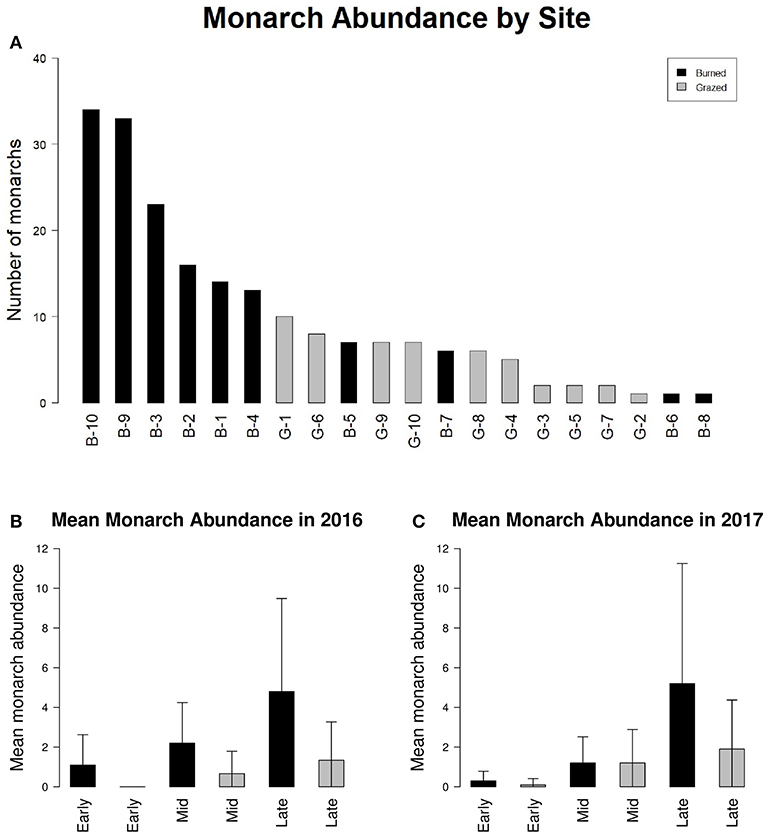
Figure 2. (A) Index of monarch abundance at burned sites (black bars) and grazed sites (gray bars) and (B) mean monarch abundance at burned sites (black bars) and grazed sites (gray bars) for early season (May-June), mid season (June-July), and late season (July-August) survey periods during 2016 and (C) 2017. Error bars in (B,C) represent standard deviation between sites.
The number of adult monarchs found at sites that had been burned was approximately three times as high as the number found at grazed sites (z = −2.076, p = 0.0379) (Figure 2). Table 3 shows the univariate responses of predictor variables to monarch abundance and Figure 3 the correlations between monarch abundance and milkweed frequency, forb frequency, percent prairie, and site area in 2016 and 2017. In univariate models, only management type was a significant predictor of monarch abundance (Table 3); monarch abundance was not correlated with milkweed frequency (Figure 3A), nor with the other predictor variables tested.
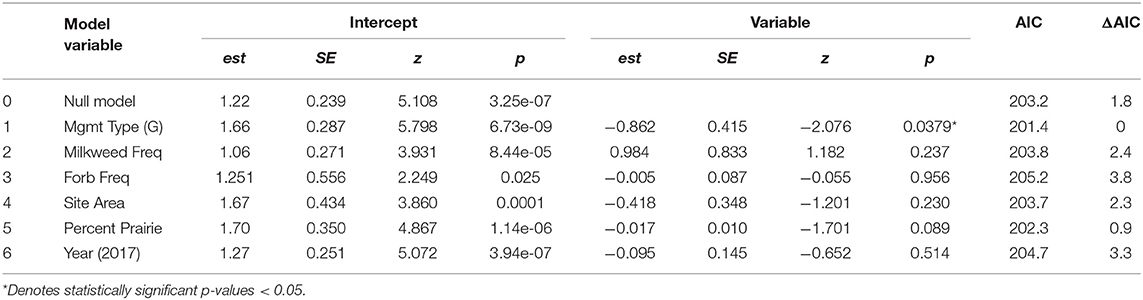
Table 3. Univariate models showing the effects of predictor variables of interest on monarch abundance.
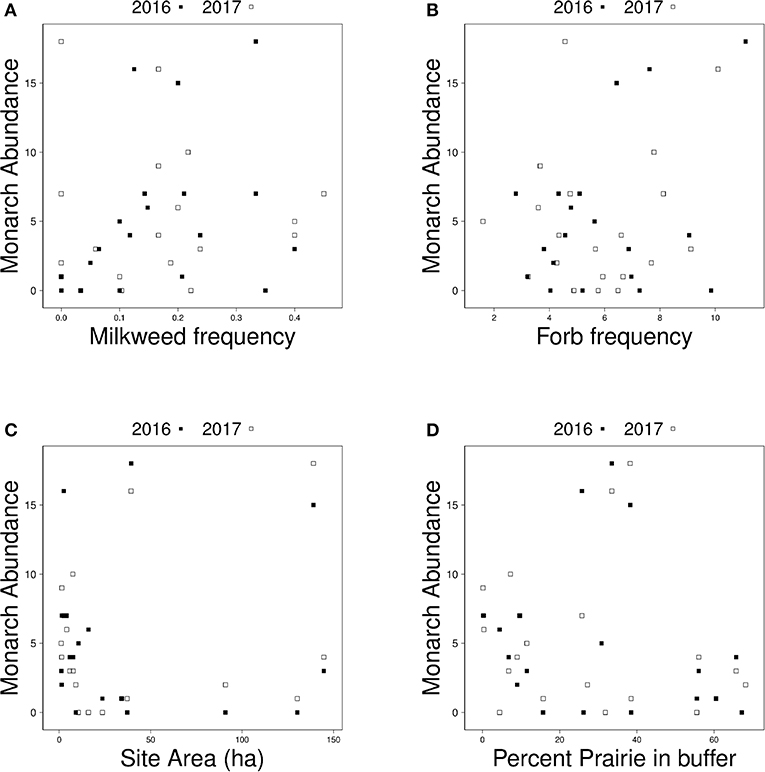
Figure 3. Relationship between monarch abundance and (A) milkweed frequency, (B) forb frequency, (C) site area, and (D) percent prairie in a surrounding 1.5 km buffer around each site in 2016 (black squares) and 2017 (white squares).
In the global model, none of the interaction terms tested were significant. There was also no relationship between monarch abundance and forb frequency, a surrogate for nectar resource availability, site area, or the percent of prairie habitat in a surrounding 1.5 km buffer around sites, The final model after backward elimination of all non-significant predictors included management type as the only predictor variable and was the same as the univariate model 1 in Table 3 (est = −0.862, SE = 0.415, z = −2.076, p = 0.0379, AIC = 201.4). The next best-fit model included milkweed frequency as a second, although non-significant, predictor variable (est = 1.036, SE = 0.817, z = 1.269, p = 0.205, AIC = 201.8). Additional predictors did not improve model fit and were removed from the model in the following order: (1) the interaction between forb frequency and management type, (2) the interaction between site area and milkweed frequency, (3) site area, (4) the interaction between management type and milkweed frequency, (5) year, (6) forb frequency, (7) percent prairie, and (8) milkweed frequency. Global model results can be found in Table S2. Adult monarchs and milkweeds were observed at all sites during at least one survey year, indicating that sites are potential breeding habitat or possess some other resource sought after by adult monarchs.
In management specific models, there was no relationship between monarch abundance at grazed sites and the number of years a site had been grazed (Figure 4A) or monarch abundance at burned sites and time since last fire (Figure 4D). The best-fit model for grazed sites after backwards elimination of all non-significant predictors included only stocking rate as a predictor variable; monarch abundance was higher at grazed sites with lower average stocking rates (est = −0.923, SE = 0.417, z = −2.212, p = 0.027, AIC = 80.7) (Figure 4E). No predictors explained variation in monarch abundance at burned sites; the null model was the best fit (est = 1.625, SE = 0.335, z = 4.852, p = 1.22e-06, AIC = 118.7) Although not included in analyses due to collinearity, time since grazing and the number of years each site was burned are shown in Figures 4B,C, respectively. Univariate responses of management-specific predictors are presented in Table 4. Full model results for burn-only and graze-only models can be found in Table S2.
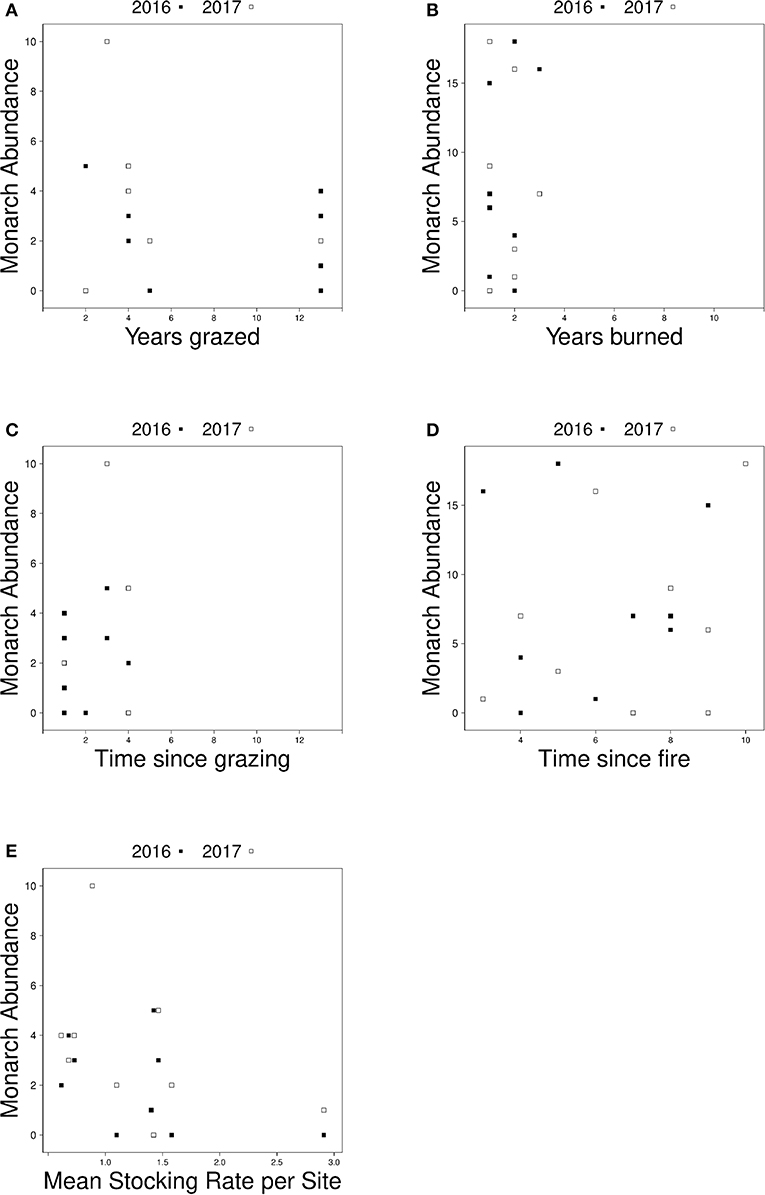
Figure 4. Relationship between monarch abundance and (A) the number of years grazed sites were grazed from 2005 to 2017, (B) the number of years burned sites were burned from 2005 to 2017, (C) the number of years since grazing management took place at grazed sites, (D) the number of years since fire management took place at burned sites, and (E) the average stocking rate at grazed sites in 2016 (black squares) and 2017 (white squares).
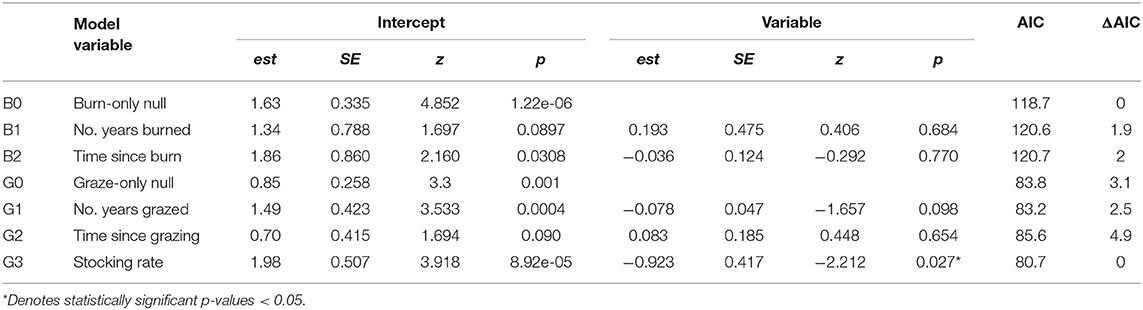
Table 4. Univariate models showing the effects of fire- (B0 – B2) and grazing- (G0 – G3) specific predictor variables on monarch abundance at burned or grazed sites, respectively.
Data are archived and available (Leone et al., 2019).
Discussion
Adult monarchs in the Minnesota tallgrass prairie remnants that we surveyed were significantly more abundant in sites that had been managed with prescribed fire than those managed with grazing. Adult monarchs, milkweeds, or both monarchs and milkweeds were observed at all sites during at least one survey year, indicating that sites are potential breeding habitat or possess some other resource sought after by adult monarchs. Monarch abundance was independent of milkweed and forb frequency, as well as other site variables and the amount of grassland habitat within 1.5 km of the site. None of the variables that we measured allowed us to pinpoint a mechanism for the association between burning or grazing and monarch abundance. Because this association was across all sites on which burning is used as a management tool, and no sites were burned in 2015, 2016, or 2017, we do not think that it is due to qualitative differences in host or nectar plants due to immediate effects of burning.
We had hypothesized that if prairie management by fire or grazing influenced the frequency of forbs and milkweeds, then these management types would affect monarch abundance. We also hypothesized that if prairie management by fire and grazing did not influence nectar and host plant frequency, then we would find no difference in monarch response to management, unless that response was driven by patterns in site area or prairie habitat in the surrounding area. It is surprising, therefore, that we found no difference between forb and milkweed frequency at burned vs. grazed sites and yet observed significantly higher abundance of monarchs at burned sites. This correlation between monarch abundance and management type is apparently not driven by the indirect effect of management on the vegetation, nor is it the result of an interaction between management type and host plant frequency or forb frequency; host plant resource availability, measured as the interaction between milkweed frequency and site area, also was not predictive. The lack of patterns in the monarch response to the vegetation may be due in part to the scale of sampling (frequency). A study with more exhaustive milkweed sampling methods (for example, density or abundance), including a study of the chemical response of milkweed to fire over multiple years, may help explain this finding. Similarly, nectar plant frequency ignores important variation in flower density and floral traits.
The fact that we found no pattern in monarch response to time since burn or frequency of fire management does not necessarily mean that no such pattern exists. Given the limitations in a sample size of 10 burned sites that were not explicitly selected to encompass a range of additional fire variables, more studies with a larger sample size and a range of times since fire and years of management might provide additional insight. Several studies have found significant post-burn butterfly and host plant responses to fire within 1–2 years of fire (e.g., Fleishman, 2000; Rudolph et al., 2006; Baum and Sharber, 2012); however, few studies examine long-term trends in butterfly or monarch abundance after fire. Vogel et al. (2010) found that floral resource availability was negatively correlated with time since burn and suggests that post-fire recovery may exceed 5 years for some butterfly species. Extrapolation from studies of other insect responses to fire should be approached with caution, since multi-taxa studies have found that different insect taxa respond differently to time since fire (New et al., 2010; Pryke and Samways, 2012a; Yekwayo et al., 2018). For example (Pryke and Samways, 2012b), found that aerial assemblages, including lepidoptera, showed little difference in species composition immediately after fire but significant differences 3 years post-fire, contrary to other insect taxa. There is still much to learn about how time since fire may impact butterflies and monarchs in particular, and more research that encompasses 1–5+ year post-burn responses would help contextualize our findings and guide best management practices for monarchs.
Our observation that there were fewer monarchs at sites with higher stocking rates of cattle suggests that grazing, or at least heavy grazing, may have a negative impact on monarchs. It is possible that monarch eggs, larvae, and pupae may suffer incidental mortality from cattle grazing and trampling. It is also possible that there are fewer nectar resources available at grazed sites. While the frequency of forb plants did not differ between burned and grazed sites, we did observe cattle consuming flowers during our surveys, and also made the anecdotal observation that sites with cattle present tended to have fewer plants in flower. In addition, frequent fire has been shown to increase nectar resources and migrating monarch abundance compared to unburned controls (Rudolph et al., 2006). We did not quantify this impact, which is a limitation of our study. A study that directly measures nectar resources, not just forb frequency, would help elucidate the potential impacts of cattle on monarchs and their nectar plants.
Heavier grazing rates by cattle can also reduce the height of the vegetation, which may be detrimental to monarchs by limiting host plant and nectar plant biomass. Multiple studies have documented positive correlations between butterflies and taller vegetation in grasslands (Poyry et al., 2006; Berg et al., 2013). We made anecdotal observations that the vegetation at many grazed sites was much shorter than at burned sites, however, we are unable to quantify its effect on monarch abundance. This is an area for further study.
Our study found no relationship between the size of our sites and monarch abundance. Other studies examining the relationship between site area and butterfly abundance and diversity have observed similar patterns (e.g., Krämer et al., 2012), although in a study in the United Kingdom, butterfly abundance and diversity increased with an increase in grassland habitat area, with additional effects of surrounding habitat diversity and the larger landscape context (Botham et al., 2015). It is possible that the range of site areas in our study (many small and a few large; Figure 3C) was insufficient to capture a pattern in monarch response to site area. Landscape and local variables, including site area, have been found to affect the butterfly community in the fragmented tallgrass prairie (Davis et al., 2007). We know that monarchs can travel fairly long distances, up to 15 km/day (Zalucki et al., 2016). Their mobility is another possible explanation for why larger sites do not correlate with higher abundance of monarchs. Grant et al. (2018), Zalucki and Lammers (2010), and Zalucki et al. (2016) all suggest that monarchs will be more likely to encounter habitat patches when small patches are dispersed at distances within the monarchs' perceptual range than when a few large sites are more dispersed, but empirical data to parameterize and test these models are lacking. Additionally, we have little understanding of the characteristics of sites that are used by monarchs and there are no published studies that selected monitoring sites in a way that would allow comparisons of the relative importance of site and landscape characteristics. We attempted to account for the potential patchiness of host plant distribution within sites by including the interaction between milkweed frequency and site area as a surrogate for host plant resource availability in our analyses and found no effect.
Grazed and burned sites were chosen to represent management history in this part of the monarch breeding range and to be roughly equivalent in size; due to constraints in the number of grazed sites that met these criteria, more of our grazed sites are closer to the eastern edge of the Prairie Parkland Province (Figure 1). We do not believe that this has biased our results, as many of these sites (G-10, G-8, G-1) have some of the highest monarch counts compared to grazed sites in closer proximity to other burned sites along the western edge of the Prairie Parkland Province. Our study was set up to test management effects on insects and plants in remnant prairies, so our site selection process did not include consideration of the surrounding habitat. Our finding that monarch abundance was not affected by the amount of other grassland habitat in the surrounding 1.5 km buffer could mean that the surrounding habitat is not important, or that the spatial scale of our buffer was too small to account for monarch perceptual range. More research is needed to detect the mechanism(s) driving these observations.
We chose a retrospective study design using sites with known management history instead of implementing our own experimental design because management can take many years to become apparent on the landscape and our study was constrained to 2 years. While there are benefits to this design, there are also disadvantages and potential for bias; for example, management has not been applied randomly to the landscape and there may have been initial bias in the decisions of managers to burn or graze certain prairies. Because of the inherent challenges in a retrospective study design, given sufficient time for implementation, we recommend experimental fire and grazing management studies to eliminate confounding variables. Long-term experimental research of this kind would provide valuable insight into understanding the mechanisms underlying the patterns we observed. Another challenge in this system is that there are no undisturbed or unmanaged prairies to serve as controls to compare to fire and grazing management; this is inherent in a disturbance-dependent ecosystem that evolved with fire, grazing, and drought (Anderson, 2006). Unmanaged prairie succeeds quickly to shrubland and experimental “controls” no longer represent the ecosystem they are designed to study.
Conclusions
Remnant tallgrass prairies and other grasslands in the Midwestern U.S.A. provide important habitat for eastern monarchs during the breeding season (Oberhauser et al., 2016). In the fragmented landscape of our present day, natural disturbances such as unchecked wildfire and roaming herds of bison are no more, and these grasslands would succeed to shrub and woodland without management (Gibson and Hulbert, 1987; McClain and Elzinga, 1994; Anderson, 2006). Although grazing has been suggested as a tool for minimizing potential negative effects of fire on invertebrates, our study suggests that we should treat grazing, or at least grazing at high stocking rates, with caution as a management tool for monarchs, until we can understand a mechanism for lower numbers of monarchs at grazed sites. It is clear that additional research is needed to fully understand the effects of fire and grazing management on monarch butterflies. To this end, we present our recommendations for further research in Table 5. Our research is an important step toward understanding the effects of fire and grazing management practices on monarch butterflies and informing additional studies that will guide future conservation and management decisions for monarch butterflies.
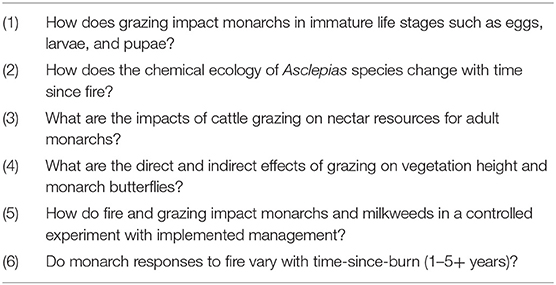
Table 5. Recommendations for further research into the effects of fire and grazing on monarch butterflies.
Data Availability Statement
Data and metadata are available on ScienceBase.gov through a U.S. Geological Survey data release, https://doi.org/10.5066/P940ICLS.
Author Contributions
DL, KO, and JLe conceived the ideas for the study. All authors designed the methodology. JLe collected the monarch data. JLa supervised vegetation data collection and analyzed GIS and landscape data. JLe and DL analyzed monarch and vegetation data, respectively. JLe led manuscript writing and revisions. KO led writing of the introduction and discussion. All authors contributed critically to the drafts and gave final approval for publication.
Funding
Funding for this project came from the Minnesota Environment and Natural Resources Trust Fund (ENRTF), M.L. 2015, Chp. 76, Sec. 2, Subd. 03o; Prairie Biotic Research, Inc.; National Science Foundation Graduate Research Fellowship (GRFP).
Conflict of Interest
JLa is a research affiliate of the Polistes Foundation, Inc., a 501-c-3 non-profit organization.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thank you to scientists and land managers at the U.S. Fish and Wildlife Service, The Minnesota Department of Natural Resources, The Nature Conservancy, and private land owners for sharing resources, land, permits, site access, and management history. Thank you to the botanists and field technicians who helped collect data. We are grateful to Brian Aukema and Deb Buhl for providing valuable advice and feedback on statistical analyses, Erin Treiber for reviewing an earlier version of the manuscript, and Alex Leone for providing database and programming expertise and support. This paper has been improved through conversation with Myron Zalucki. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00435/full#supplementary-material
Figure S1. Example site map with transects and vegetation plots distributed proportionally in each prairie type. Mesic prairie is shown in white (5.9 ha) and wet prairie is shown in gray (1.53 ha). Total site area is 7.43 ha. Transects run perpendicular to elevation gradients and vegetation plots (not to scale) are oriented perpendicular to the transects they are on. The three transects in mesic prairie are M1 (103 m; 6 plots), M2 (236 m; 7 plots) and M3 (170 m; 6 plots). Wet transects are W1 (53 m; 2 plots) and W2 (64 m; 2 plots). Monarchs were surveyed on 400 meters of randomly selected vegetation transects in proportion to wet and mesic prairie types: all of transect M2 and 84 m of M3 were surveyed to equal 320 m in mesic prairie (~80%) and all of W1 and 27 m of W2 were surveyed to equal 80 m in wet prairie (~20%).
Table S1. Index of study sites including forb, Asclepias, and monarch survey data.
Table S2. Full model results for monarch abundance at burned and grazed sites, burned-only sites, and grazed-only sites.
References
Allred, B. W., Fuhlendorf, S. D., and Hamilton, R. G. (2011). The role of herbivores in Great Plains conservation: comparative ecology of bison and cattle. Ecosphere 2, 1–17. doi: 10.1890/ES10-00152.1
Anderson, R. C. (2006). Evolution and origin of the Central Grassland of North America: climate, fire, and mammalian grazers. J. Torrey Bot. Soc. 133, 626–647. doi: 10.3159/1095-5674(2006)133[626:EAOOTC]2.0.CO;2
Arnold, T. W. (2010). Uninformative parameters and model selection using Akaike's information criterion. J. Wildlife Manage. 74, 1175–1178. doi: 10.1111/j.1937-2817.2010.tb01236.x
Axelrod, D. I. (1985). Rise of the grassland biome, central North America. Bot. Rev. 51, 163–201. doi: 10.1007/BF02861083
Baum, K. A., and Sharber, W. V. (2012). Fire creates host plant patches for monarch butterflies. Biol. Lett. 8, 968–971. doi: 10.1098/rsbl.2012.0550
Berg, Å., Ahrné, K., Öckinger, E., Svensson, R., and Wissman, J. (2013). Butterflies in semi-natural pastures and power-line corridors - effects of flower richness, management, and structural vegetation characteristics. Insect. Conserv. Divers. 6, 639–657. doi: 10.1111/icad.12019
Botham, M. S., Fernandez-Ploquin, E. C., Brereton, T., Harrower, C. A., Roy, D. B., and Heard, M. S. (2015). Lepidoptera communities across an agricultural gradient: how important are habitat area and habitat diversity in supporting high diversity? J. Insect. Conserv. 19, 403–420. doi: 10.1007/s10841-015-9760-y
Brudvig, L. A., Mabry, C. M., Miller, J. R., and Walker, T. A. (2007). Evaluation of central North American prairie management based on species diversity, life form, and individual species metrics. Conserv. Biol. 21, 864–874. doi: 10.1111/j.1523-1739.2006.00619.x
Collins, S. L., Knapp, A. K., Briggs, J. M., Blair, J. M., and Steinauer, E. M. (1998). Modulation of diversity by grazing and mowing in native tallgrass prairie. Science 280, 745–747. doi: 10.1126/science.280.5364.745
Davis, J. D., Debinski, D. M., and Danielson, B. J. (2007). Local and landscape effects on the butterfly community in fragmented Midwest USA prairie habitats. Landsc. Ecol. 22, 1341–1354. doi: 10.1007/s10980-007-9111-9
Debinski, D. M., Moranz, R. A., Delaney, J. T., Miller, J. R., Engle, D. M., Winkler, L. B., et al. (2011). A cross-taxonomic comparison of insect responses to grassland management and land-use legacies. Ecosphere 2:131. doi: 10.1890/ES11-00226.1
Derner, J. D., Hart, R. H., Smith, M. A., and Waggoner, J. W. (2008). Long-term cattle gain responses to stocking rate and grazing systems in northern mixed-grass prairie. Livest. Sci. 117, 60–69. doi: 10.1016/j.livsci.2007.11.011
Diffendorfer, J. E., Loomis, J. B., Ries, L., Oberhauser, K., Lopez-Hoffman, L., Semmens, D., et al. (2014). National valuation of monarch butterflies indicates an untapped potential for incentive-based conservation. Conserv. Lett. 7, 253–262. doi: 10.1111/conl.12065
Elzinga, C. L., Willoughby, J. W., and Salzer, D. W. (1998). Measuring and Monitoring Plant Populations. Denver, CO: Bureau of Land Management.
Emery, S. M., and Gross, K. L. (2005). Effects of timing of prescribed fire on the demography of an invasive plant, Spotted Knapweed Centaurea maculosa. Br. Ecol. Soc. 42, 60–69. doi: 10.1111/j.1365-2664.2004.00990.x
Fleishman, E. (2000). Monitoring the response of butterfly communities to prescribed fire. Env.Manage. 26, 685–695. doi: 10.1007/s002670010125
Gibson, D. J., and Hulbert, L. C. (1987). Effects of fire, topography and year-to-year climate variation on species composition in tallgrass prairie. Vegetation 72, 175–185.
Grant, T. J., Parry, H. R., Zalucki, M. P., and Bradbury, S. P. (2018). Predicting monarch butterfly (Danaus plexippus) movement and egg-laying with a spatially-explicit agent-based model: the role of monarch perceptual range and spatial memory. Ecol. Model. 374, 37–50. doi: 10.1016/j.ecolmodel.2018.02.011
Holmgren, A. (1971). Weeds of Utah. Utah Agric. Exp. Station. Available online at: https://www.worldcat.org/title/weeds-of-utah/oclc/605528
Joern, A. (2005). Disturbance by fire frequency and bison grazing modulate grasshopper assemblages in tallgrass prairie. Ecology 86, 861–873. doi: 10.1890/04-0135
Kassambara, A. (2017). ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.1.6. Available online at: https://cran.r-project.org/bin/windows/base/
Knapp, A. K., Blair, J. M., Briggs, J. M., Collins, S. L., Hartnett, D. C., Johnson, L. C., et al. (1999). The keystone role of bison in North American tallgrass prairie. Bioscience 49:39. doi: 10.2307/1313492
Kohl, M. T., Krausman, P. R., Kunkel, K., Williams, D. M., Boone, C., Fellow, B., et al. (2013). Bison versus cattle: are they ecologically synonymous? Rangel. Ecol. Manage. 66, 721–731. doi: 10.2111/REM-D-12-00113.1
Krämer, B., Poniatowski, D., and Fartmann, T. (2012). Effects of landscape and habitat quality on butterfly communities in pre-alpine calcareous grasslands. Biol. Conserv. 152, 253–261. doi: 10.1016/j.biocon.2012.03.038
Leone, J. B., Larson, D. L., Larson, J. L., Pennarola, P., and Oberhauser, K. (2019). Monarch Densities in Burned or Grazed Minnesota Remnant Prairie, 2016-2017. U.S. Geological Survey data release.
Magnusson, A., Skaug, H. J., Nielsen, A., Berg, C. W., Kristensen, K., Maechler, M., et al. (2017). glmmTMB: Generalized Linear Mixed Models Using Template Model Builder. R package version 0.1.3. Available online at: https://github.com/glmmTMB
McClain, W., and Elzinga, S. (1994). The occurrence of prairie and forest fires in Illinois and other Midwestern states, 1679 to 1854. Erigenia 13, 79–90.
McCollum, F. T. Jr., Gillen, R. L., Karges, B. R., and Hodges, M.E. (1999). Stocker cattle response to grazing management in tallgrass prairie. J. Range Manage. 52, 120–126. doi: 10.2307/4003504
Moran, M. D. (2014). Bison grazing increases arthropod abundance and diversity in a tallgrass prairie. Environ. Entomol. 43, 1174–1184. doi: 10.1603/EN14013
Moranz, R. A., Debinski, D. M., McGranahan, D. A., Engle, D. M., and Miller, J. R. (2012). Untangling the effects of fire, grazing, and land-use legacies on grassland butterfly communities. Biodiv. Conserv. 21, 2719–2746. doi: 10.1007/s10531-012-0330-2
Neilly, H., Vanderwal, J., and Schwarzkopf, L. (2016). Balancing biodiversity and food production: a better understanding of wildlife response to grazing will inform off-reserve conservation on Rangelands. Rangel. Ecol. Manage. 69, 430–436. doi: 10.1016/j.rama.2016.07.007
New, T. R., Yen, A., Sands, D. P. A., Greenslade, P., Neville, P. J., York, A., et al. (2010). Planned fires and invertebrate conservation in south east Australia. J Insect Conserv. 14, 567–574. doi: 10.1007/s10841-010-9284-4
Oberhauser, K., Prysby, M. D., Mattila, H. R., Stanley-Horn, D. E., Sears, M. K., Dively, G., et al. (2016). A trans-national monarch butterfly population model and implications for regional conservation priorities. Ecol. Entomol. 42, 51–60. doi: 10.1111/een.12351
Oberhauser, K. S., Prysby, M. D., Mattila, H. R., Stanley-Horn, D. E., Sears, M. K., Dively, G., et al. (2001). Temporal and spatial overlap between monarch larvae and corn pollen. Proc. Natl. Acad. Sci. U.S.A. 98, 11913–11918. doi: 10.1007/s10841-010-9284-4
Panter, K. E., Ralphs, M. H., Pfister, J. A., Gardner, D. R., Stegelmeier, B. L., Lee, S. T., Welch, K. D., et al. (2011). Plants Poisonous to Livestock in the Western States. U.S. Department of Agriculture, Agriculture Information Bulletin 415. Available online at: https://www.ars.usda.gov/pacific-west-area/logan-ut/poisonous-plant-research/docs/milkweed-asclepias-spp/ (accessed December 17, 2018).
Panzer, R. (2002). Compatibility of prescribed burning with the conservation of insects in small, isolated prairie reserves. Conserv. Biol. 16, 1296–1307. doi: 10.1046/j.1523-1739.2002.01077.x
Pleasants, J. (2017). Milkweed restoration in the Midwest for monarch butterfly recovery: estimates of milkweeds lost, milkweeds remaining and milkweeds that must be added to increase the monarch population. Insect Conserv. Divers. 10, 42–53. doi: 10.1111/icad.12198
Pleasants, J. M., and Oberhauser, K. S. (2013). Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conserv. Divers. 6, 135–144. doi: 10.1111/j.1752-4598.2012.00196.x
Pleasants, J. M., Williams, E. H., Brower, L. P., Oberhauser, K. S., and Taylor, O. R. (2016). Conclusion of no decline in summer monarch population not supported. Ann. Entomol. Soc. Am. 109, 169–171. doi: 10.1093/aesa/sav115
Plumb, G. E., and Dodd, J. L. (1993). Foraging ecology of bison and cattle on a mixed prairie: Implications for Natural Area Management. Ecol. Appl. 3, 631–643. doi: 10.2307/1942096
Pollard, E. (1977). Method for assessing changes in abundance of butterflies. Biol. Conserv. 12, 115–134. doi: 10.1016/0006-320790065-9
Poyry, J., Luoto, M., Paukkunen, J., Pykala, J., Raatikainen, K., and Kuussaari, M. (2006). Different responses of plants and herbivore insects to a gradient of vegetation height: an indicator of the vertebrate grazing intensity and successional age. Oikos 115, 401–412. doi: 10.1111/j.2006.0030-1299.15126.x
Pringle, R. M., Young, T. P., Rubenstein, D. I., and McCauley, D. J. (2007). Herbivore-initiated interaction cascades and their modulation by productivity in an African savanna. Proc. Natl. Acad. Sci. U.S.A. 104, 193–7. doi: 10.1073/pnas.0609840104
Pryke, J. S., and Samways, M. J. (2012a). Importance of using many taxa and having adequate controls for monitoring impacts of fire for arthropod conservation. J. Insect Conserv. 16, 177–185. doi: 10.1007/s10841-011-9404-9
Pryke, J. S., and Samways, M. J. (2012b). Differential resilience of invertebrates to fire. Aust. Ecol. 37, 460–469. doi: 10.1111/j.1442-9993.2011.02307.x
Prysby, M., and Oberhauser, K. S. (2004). “Temporal and geographical variation in monarch densities: citizen scientists document monarch population patterns,” in The Monarch Butterfly: Biology and Conservation, eds K. S. Oberhauser and M. J. Solensky (Ithaca, NY: Cornell University Press), 9–20.
R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.r-project
R Studio Team (2016). RStudio: Integrated Development for R. Boston, MA: R Studio, Inc. Available online at: http://www.rstudio.com/
Rendón-Salinas, E., Martínez-Meza, F., Martínez-Pacheco, A., and Cruz-Piña, M. (2018). Superficie Forestal Ocupada por las Colonias de Hibernación de la Mariposa Monarca en México Durante Diciembre de 2017. Available online at: http://www.wwf.org.mx/?uNewsID=324152 (accessed December 9, 2018).
Ricono, A., Dixon, R., Eaton, I., Brightbill, C. M., Yaziji, Y., Puzey, J. R., et al. (2018). Long- and short-term responses of Asclepias species differ in respect to fire, grazing, and nutrient addition. Am. J. Bot. 105, 2008–2017. doi: 10.1002/ajb2.1197
Rudolph, D. C., Ely, C. A., Schaefer, R. R., Williamson, J. H., and Thill, R. E. (2006). Monarch (Danaus plexippus L. Nymphalidae) migration, nectar resource and fire regimes in the Ouachita Mountains of Arkansas. J. Lepid. Soc. 60, 165–170.
Samson, F. B., Knopf, F. L., and Ostlie, W. R. (2004). Great Plains ecosystems: past, present, and future. Wildl. Soc. Bull. 32, 6–15. doi: 10.2193/0091-7648(2004)32[6:GPEPPA]2.0.CO;2
Saunders, S. P., Ries, L., Oberhauser, K. S., Thogmartin, W. E., and Zipkin, E. F. (2018). Local and cross-seasonal associations of climate and land use with abundance of monarch butterflies Danaus plexippus. Ecography 41, 278–290. doi: 10.1111/ecog.02719
Semmens, B. X., Semmens, D. J., Thogmartin, W. E., Wiederholt, R., López-Hoffman, L., Diffendorfer, J. E., et al. (2016). Quasi-extinction risk and population targets for the Eastern, migratory population of monarch butterflies (Danaus plexippus). Sci. Rep. 6, 1–7. doi: 10.1038/srep23265
Shepherd, S., and Debinski, D. M. (2005). Evaluation of isolated and integrated prairie reconstructions as habitat for prairie butterflies. Biol. Conserv. 126, 51–61. doi: 10.1016/j.biocon.2005.04.021
Smith, L. M., and Cherry, R. (2014). Effects of management techniques on grassland butterfly species composition and community structure. Am. Midl. Nat. 172, 227–235. doi: 10.1674/0003-0031-172.2.227
Swengel, A. B., and Swengel, S. R. (2007). Benefit of permanent non-fire refugia for Lepidoptera conservation in fire-managed sites. J. Insect Conserv. 11, 263–279. doi: 10.1007/s10841-006-9042-9
Swengel, A. B., and Swengel, S. R. (2013). Decline of Hesperia ottoe (Lepidoptera: Hesperiidae) in Northern tallgrass prairie preserves. Insects 4, 663–682. doi: 10.3390/insects4040663
Thogmartin, W. E., López-Hoffman, L., Rohweder, J., Diffendorfer, J., Drum, R., Semmens, D., et al. (2017a). Restoring monarch butterfly habitat in the Midwestern US: ‘all hands on deck.’ Environ. Res. Lett. 12:074005. doi: 10.1088/1748-9326/aa7637
Thogmartin, W. E., Wiederholt, R., Oberhauser, K., Drum, R. G., Diffendorfer, J. E., Altizer, S., et al. (2017b). Monarch butterfly population decline in North America: identifying the threatening processes. R. Soc. Open Sci. 4:170760. doi: 10.1098/rsos.170760
Thomas, J. A. (2005). Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 360, 339–57. doi: 10.1098/rstb.2004.1585
Towne, E. G., and Craine, J. M. (2014). Ecological consequences of shifting the timing of burning tallgrass prairie. PLoS ONE 9:e103423. doi: 10.1371/journal.pone.0103423
Towne, E. G., and Kemp, K. E. (2008). Long-term response patterns of tallgrass prairie to frequent summer burning. Rangel. Ecol. Manage. 61, 509–520. doi: 10.2111/08-043.1
van Klink, R., van der Plas, F., van Noordwijk, C. G., WallisDeVries, M. F., and Olff, H. (2015). Effects of large herbivores on grassland arthropod diversity. Biol. Rev. 90, 347–366. doi: 10.1111/brv.12113
Vogel, J. A., Debinski, D. M., Koford, R. R., and Miller, J. R. (2007). Butterfly responses to prairie restoration through fire and grazing. Biol. Conserv. 140, 78–90. doi: 10.1016/j.biocon.2007.07.027
Vogel, J. A., Koford, R. R., and Debinski, D. M. (2010). Direct and indirect responses of tallgrass prairie butterflies to prescribed burning. J Insect Conserv. 14, 663–677. doi: 10.1007/s10841-010-9295-1
Yekwayo, I., Pryke, J. S., Gaigher, R., and Samways, M. J. (2018). Only multi-taxon studies show the full range of arthropod responses to fire. PLoS ONE 13:e0195414. doi: 10.1371/journal.pone.0195414
Zalucki, M. P., and Lammers, J. H. (2010). Dispersal and egg shortfall in Monarch butterflies: what happens when the matrix is cleaned up? Ecol. Entomol. 35, 84–91. doi: 10.1111/j.1365-2311.2009.01160.x
Keywords: monarch butterfly, Danaus plexippus, tallgrass prairie, prescribed fire, conservation grazing, milkweeds, Asclepias, prairie management
Citation: Leone JB, Larson DL, Larson JL, Pennarola N and Oberhauser K (2019) Adult Monarch (Danaus plexippus) Abundance Is Higher in Burned Sites Than in Grazed Sites. Front. Ecol. Evol. 7:435. doi: 10.3389/fevo.2019.00435
Received: 29 December 2018; Accepted: 25 October 2019;
Published: 14 November 2019.
Edited by:
Cheryl Schultz, Washington State University Vancouver, United StatesReviewed by:
Victoria Pocius, Pennsylvania State University (PSU), United StatesErica Henry, North Carolina State University, United States
At least a portion of this work is authored by Diane L. Larson on behalf of the U.S. Government and, as regards Diane L. Larson and the U.S. Government, is not subject to copyright protection in the United States. Foreign and other copyrights may apply. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julia B. Leone, bGVvbmUwNTBAdW1uLmVkdQ==
 Julia B. Leone
Julia B. Leone Diane L. Larson
Diane L. Larson Jennifer L. Larson
Jennifer L. Larson Nora Pennarola
Nora Pennarola Karen Oberhauser5
Karen Oberhauser5